Abstract
This study was undertaken to review fatal cases of insulin overdose in South Australia (SA) over a 20-year period to assess rates and characteristics of insulin-related deaths among insulin-dependent diabetics and non-diabetics for all manners of death. Records from the National Coronial Information System (NCIS) and Forensic Science SA (FSSA) were searched for all cases of fatal insulin overdose in South Australia (SA) between 2000 and 2019. Collected variables included age, sex, cause of death, scene findings, manner of death, decedent medical and personal histories, biochemistry, toxicology, histopathology, and autopsy findings. Statistical analyses were performed using R (version 4.1.2). Forty cases of insulin overdose were identified in SA between 2000 and 2019. Twenty-nine cases (72.5%) were suicides, with the remaining cases classified as accidental or undetermined intent. Thirteen of the 22 insulin-dependent diabetics (59%) had a history of depression, 10 of whom had previously demonstrated suicidal ideation. The current study has shown that suicides using insulin among insulin-dependent diabetics are equally as prevalent, if not more so than fatal accidental insulin overdoses. This can largely be attributed to insulin-dependent diabetic access to a potentially lethal substance. Suicide prevention strategies should focus on insulin-dependent diabetics with a history of depression, particularly for those with access to rapid-acting insulin.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12024-022-00511-3.
Keywords: Coronial, Insulin, Diabetes, Suicide, Toxicology
Introduction
Synthetic insulin has been an effective drug for the maintenance of normal blood glucose levels for type I and type II diabetics since its discovery and isolation in 1921 [1]. Excessive insulin administration may, however, cause profound hypoglycaemia, subsequent brain damage, and even death, if not correctly diagnosed and promptly treated. While non-fatal accidental insulin overdoses are relatively common among diabetics [2–5], fatal suicidal insulin overdoses are much less frequent. Such cases present significant difficulties for forensic toxicologists and pathologists in the death investigation process sometimes due to a lack of medical history at the time of autopsy and analytical limitations in the laboratory. While there are numerous case reports and reviews of fatal and non-fatal insulin overdoses including accidents [6–8], homicides [9–17], and suicides [18–66], there are few longitudinal retrospective reviews of fatal insulin overdoses from a single institution. Therefore, a study was undertaken to review fatal cases of insulin overdose in South Australia (SA) over a 20-year period, from 2000 to 2019 to assess rates and characteristics of insulin-related deaths among insulin-dependent diabetics and non-diabetics.
Materials and methods
Records from the National Coronial Information System (NCIS) and Forensic Science SA (FSSA) were searched for all cases of insulin overdose in South Australia (SA) over a 20-year period (2000–2019). An initial comprehensive search was conducted in the NCIS for cases from 1 July 2000 to 31 December 2019 for all pharmaceutical substance-related deaths using the search terms “insulin” and “hypoglycaemia” which returned results for all cases where insulin or hypoglycaemia was found to cause or contribute to death. Only cases where the cause of death was recorded as “insulin toxicity” or “insulin overdose” (or other comparable terminology) were included in the current study. Cases where insulin administration or hypoglycaemia were recognized but not related to the cause of death were excluded. Relevant cases (n = 40) were then cross-referenced against autopsy and toxicology reports from FSSA to retrieve missing and additional information. Cases which occurred prior to 1 July 2000 were sourced from FSSA records only, as NCIS records prior to 1 July 2000 are unavailable. Collected variables included age, sex, cause of death, scene findings, manner of death, decedent medical and personal histories, biochemistry, toxicology, histopathology, and autopsy findings. Age was categorized into the following groups: young person (15–24 years), adult (25–64 years), and elderly (65 + years).
Routine toxicological analyses (for alcohol and common drugs) were conducted at FSSA. However, FSSA does not currently have a validated method for the analysis of insulin in post-mortem specimens. Therefore, post-mortem samples were sent to an external laboratory (SA Pathology, Royal Adelaide Hospital, North Terrace, Adelaide, South Australia) for quantitative analysis of insulin and C-peptide.
Statistical analyses were performed using R (version 4.1.2). A Poisson regression was used to characterize trends over time. While statistical analysis was performed on the number of deaths in each year, the number of deaths is presented as 5-year bins in Fig. 1 to ensure individual cases within small cohorts are not identifiable. A Welsh two-sample t-test was used to determine significance in the age and sex distribution of the study population. Cohorts of fewer than five are reported as “less than five” within the text to ensure small cohorts are not identifiable.
Fig. 1.
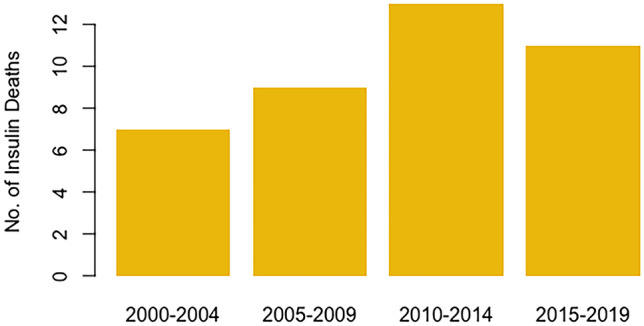
Number of insulin overdoses in South Australia between 2000 and 2019, divided into 5-year bins
Ethics approval for the data used in this study was granted by the University of Adelaide Human Research Ethics Committee (H-2020–033) and the Justice Human Research Ethics Committee (CF/21/2821).
Results
Overview
Rate of deaths
Forty cases of fatal insulin overdose were identified in SA between 2000 and 2019 with an average of two deaths per year. Poisson regression demonstrated no significant change in the rate of deaths over this period (p = 0.427); i.e., the rate of deaths per year across the 20-year study period was consistent (Fig. 1, see Appendix A for full statistical details).
Demographic profile
A total of 87.5% (n = 35) of decedents were aged 25–64 years; the remaining five decedents were either younger or older. The average age of all decedents was 49.5 years and included 19 females (mean 48 years) and 21 males (mean 51 years) with no significant difference in the age/sex distribution of the study population (p = 0.489).
Scene findings and external examination
Twenty-three deaths occurred at the home address of the decedent, nine in hospital, less than five at places of accommodation (hotel, motel etc.), and less than five on public property. Of those found on public property, all were found inside their motor vehicle. At the death scene, a suicide note was found in 19 cases. Evidence of insulin administration (syringes, needles, insulin vials, etc.) was documented in 26 cases (65%). While the type of insulin administered was not recorded in a large proportion of cases (61%), Novorapid, Actrapid, and Lantus were frequently encountered, either alone or in combination. External examination of the deceased revealed injection sites in 23 cases (57.5%), most frequently on the abdomen or thighs.
Histopathology
Of the 40 cases, a full autopsy with histopathological examination of tissue specimens was only performed in 31 cases. An autopsy was not performed in six cases as the cause of death was ascertained from hospital admission records in conjunction with a pathology review; histopathological examination of tissue specimens was not undertaken in two cases as the cause of death could be ascertained from the toxicology and biochemistry findings alone and severe putrefaction prevented accurate assessment of tissue morphology in the final case. Histopathology revealed evidence of lung oedema and congestion with or without the presence of early bronchopneumonia in 30 cases (97%). Neuropathology revealed evidence of hypoglycaemia-induced neuronal necrosis in 17 cases (54%), with normal neuronal architecture in the remaining 14 cases. For the cases with region-specific neuropathology findings, neuronal necrosis was preferentially observed in the cerebral cortex and hippocampus (n = 10), followed by the cerebellum (n = 6), basal ganglia (n = 5), and brainstem (n = 2).
Toxicology
Of the 40 cases where insulin overdose was implicated in the cause of death, 33 were attributed to insulin toxicity alone (82.5%) and the remaining seven cases were attributed to mixed drug toxicity. For cases where the cause of death was recorded as mixed drug toxicity, the drugs that were co-administered and considered contributory to the death were most often benzodiazepine sedatives (e.g., diazepam and alprazolam), antidepressants (e.g., amitriptyline and venlafaxine), and one case with co-administration of the semi-synthetic narcotic analgesic, hydromorphone. The full toxicology and biochemistry findings for the 7 cases of mixed drug toxicity are presented in Appendix B.
Biochemistry (SA pathology)
Post-mortem blood samples were submitted to an external laboratory for quantitative analysis of insulin and C-peptide. Insulin and C-peptide levels were reported either in full or in part for 31 cases (Table 1). The C-peptide level was reported without a corresponding insulin level in one case, and three cases only reported an insulin level. The range for insulin and C-peptide levels for these cases were 1–3200 mU/L (mean 594 mU/L) and 12–553 pmol/L (mean 131 pmol/L), respectively.
Table 1.
Summary of case findings for all fatal insulin overdoses
| Case no. | Cause of death | Scene findings | External autopsy findings | Histopathology | Insulin level (mU/L) | C-peptide level (pmol/L) | PMI (days) | Comment |
|---|---|---|---|---|---|---|---|---|
| 1 | Fresh water drowning associated with insulin toxicity | Nil | Nil |
Lungs: oedema/congestion Kidneys: no diabetic glomerular/vascular changes Brain: normal neuronal architecture |
1100 | NA | 1 | - |
| 2 | Hypoglycaemia and asphyxia | Found with plastic bag over their head, IV line in arm with insulin | Nil |
Lungs: oedema/congestion Kidneys: no diabetic glomerular/vascular changes Brain: normal neuronal architecture |
83 | NA | 2 | - |
| 3 | Bronchopneumonia complicating hypoglycaemic and anoxic/ischaemic brain damage due to insulin toxicity | Nil | Injection marks on lower abdomen |
Lungs: oedema/congestion with early bronchopneumonia Kidneys: no diabetic glomerular/vascular changes Brain: widespread necrosis of cerebral cortex and basal ganglia |
NA | NA | 1 | Hospital admission blood sample not available for testing |
| 4 | Acute bronchopneumonia due to insulin toxicity | Syringes, a large quantity of medication and several suicide notes | Nil |
Lungs: oedema/congestion with acute bronchopneumonia Kidneys: markedly autolysed, focal areas of interstitial chronic inflammation/scarring Brain: normal neuronal architecture |
6.7 | NA | 1 | Insulin degraded during period of coma* |
| 5 | Insulin toxicity | Nil | Injection site on left lower thigh |
Lungs: oedema/congestion Kidneys: diffuse cortical atrophy, diffuse diabetic glomerulosclerosis Brain: normal neuronal architecture |
> 300 | < 200 | 2 | - |
| 6 | Consistent with insulin overdosage | Nil | Multiple injection sites on upper abdomen |
Lungs: oedema/congestion Kidneys: no diabetic glomerular/vascular changes Brain: normal neuronal architecture |
NA | NA | 4 | Blood specimen not suitable for analysis |
| 7 | Insulin toxicity | Used insulin syringes and suicide note | Multiple injection sites across upper abdomen and dorsal aspect of left hand |
Lungs: oedema/congestion with focal early acute bronchopneumonia Kidneys: mildly autolytic, mild vascular sclerosis Brain: normal neuronal architecture |
3100 | < 200 | 3 | - |
| 8 | Oxazepam and insulin toxicity | Nil | Injection site on lower abdomen |
Lungs: oedema/congestion Kidneys: marked vascular sclerosis, diffuse/nodular glomerulosclerosis Brain: normal neuronal architecture |
2200 | < 200 | 3 | - |
| 9 | Insulin toxicity | Nil | Multiple injection sites on upper abdomen |
Lungs: oedema/congestion with focal early bronchopneumonia Kidneys: mild diabetic microvascular and glomerular changes Brain: possible early red cell change in Purkinje cells |
1600 | < 200 | 3 | - |
| 10 | Insulin toxicity | Nil | Autopsy not performed | Autopsy not performed | NA | NA | NA | Hospital admission blood sample not available for testing |
| 11 | Insulin toxicity | Empty medication packets, empty insulin syringes and a suicide note | Seven injection sites on abdomen |
Lungs: oedema/congestion with focal early bronchopneumonia Kidneys: mild glomerulomegaly with diffuse/focal nodular glomerulosclerosis Brain: microscopic focus of axonal and neuronal loss in basal ganglia and evidence of neuronal injury in cortex and cerebellum |
190 | < 200 | 4 | - |
| 12 | Insulin toxicity | Suicide notes and several empty vials of insulin | Injection site on left side of abdomen |
Lungs: oedema/congestion Kidneys: enlarged glomeruli Brain: normal neuronal architecture |
15 | < 100 | 4 | - |
| 13 | Attributed to insulin overdose | Nil | Multiple injection sites on abdomen |
Lungs: oedema/congestion Kidneys: scattered sclerotic glomeruli, patchy lymphocytic infiltrate Brain: normal neuronal architecture |
2.6 | 100 | 4 | Gross haemolysis of blood specimen, results unreliable* |
| 14 | Hypoxic brain injury due to benzodiazepine and insulin toxicity | Several empty medication packets and an insulin syringe | Nil |
Lungs: oedema/congestion Kidneys: no diabetic glomerular/vascular changes Brain: severe and diffuse anoxic encephalopathy of cerebellum |
NA | 553 | 5 | Insulin level not quantitated |
| 15 | Insulin toxicity | A suicide note and several empty insulin syringes | Multiple injection sites on lower abdomen and both thighs |
Lungs: oedema/congestion Kidneys: focal nodular glomerulosclerosis Brain: normal neuronal architecture |
160 | < 166 | 2 | - |
| 16 | Presumed insulin toxicity | Found with empty insulin syringes | Marked putrefaction and decomposition | Marked putrefaction and decomposition prevented accurate assessment of tissue morphology | NA | NA | 11 | Marked putrefaction inhibited analysis of a post-mortem blood specimen |
| 17 | Toxic effects of insulin | Suicide note | Injection site in right antecubital fossa |
Lungs: oedema/congestion Kidneys: no diabetic glomerular/vascular changes Brain: prominent focal red degeneration-type change of nerve cells in hippocampus |
410 | < 100 | 4 | - |
| 18 | Insulin toxicity | Suicide note | Autopsy not performed | Autopsy not performed | NA | NA | NA | Hospital admission blood sample not available for testing |
| 19 | Attributed to insulin toxicity | 19 empty vials of Humalog insulin and syringes | Nil |
Lungs: early haemorrhagic acute pneumonia Kidneys: no diabetic glomerular/vascular changes Brain: normal neuronal architecture |
2.5 | < 100 | 5 | Extended PMI* |
| 20 | Insulin toxicity | Empty Actrapid insulin vial and syringe |
Lungs: oedema/congestion with early bronchopneumonia Kidneys: no diabetic glomerular/vascular changes Brain: prominent focal red degeneration-type change of nerve cells in hippocampus and cortex |
260 | < 100 | 2 | - | |
| 21 | Hypoxic-ischaemic encephalopathy and toxic effects of insulin | Empty insulin vials in bin | Injection mark on lateral thigh |
Lungs: oedema/congestion Kidneys: no diabetic glomerular/vascular changes Brain: extensive ischaemic necrosis of cortex, cerebellum, pons, midbrain and medulla |
350 | 113 | 4 | - |
| 22 | Insulin toxicity | Suicide note | Injection site on abdomen |
Lungs: oedema/congestion Kidneys: consistent with diabetic change Brain: normal neuronal architecture |
290 | < 100 | 4 | - |
| 23 | Insulin and temazepam toxicity complicated by aspiration pneumonia | Suicide notes, a will, empty insulin syringes and an empty packet of temazepam | Multiple injection sites over lower abdomen |
Lungs: oedema/congestion, patchy aspiration pneumonia Kidneys: no diabetic glomerular/vascular changes Brain: possible early neuronal red cell changes |
29 | < 100 | 3 | Early putrefaction, gross haemolysis of blood sample* |
| 24 | Insulin and venlafaxine toxicity | 5 empty insulin vials and empty medications packets in kitchen bin | Injection site on left lower abdomen |
Lungs: oedema/congestion with early bronchopneumonia Kidneys: consistent with diabetic change Brain: early neuronal red cell change in hippocampus |
920 | < 100 | 2 | - |
| 25 | Mixed drug toxicity (insulin and hydromorphone) | Numerous empty medication packets, insulin vials and a suicide note | Multiple injection sites on lower abdomen | Histological examination was not undertaken since the cause of death could be determined from the toxicological and biochemical findings | 93 | < 100 | 3 | - |
| 26 | Toxic effects of insulin | Several insulin syringes and a suicide note | Histological examination was not undertaken since the cause of death could be determined from the toxicological and biochemical findings | 120 | < 100 | 2 | - | |
| 27 | Insulin toxicity | Diabetic testing kits, insulin vials and a suicide note | Autopsy not performed | Autopsy not performed | NA | NA | NA | Hospital admission blood sample not available for testing |
| 28 | Insulin and alprazolam toxicity | Insulin vials and syringes, will and suicide note | Nil |
Lungs: oedema/congestion Kidneys: mild intimal hyperplasia Brain: extensive red degeneration of nerve cells of hippocampus, cerebellum and cerebral cortex |
1700 | 0 | 4 | - |
| 29 | Toxic effects of insulin | Lantus and NovoRapid insulin pens | Nil |
Lungs: oedema/congestion Kidneys: no diabetic glomerular/vascular changes Brain: red degenerative change of neuronal cells in hippocampus and cortex |
3200 | < 100 | 2 | - |
| 30 | Hypoxic, ischaemic and hypoglycaemic encephalopathy due to insulin overdose | Suicide note | Multiple injection sites on abdomen and both thighs |
Lungs: oedema/congestion Kidneys: chronic interstitial nephritis, focal sclerosis Brain: neuronal necrosis in hippocampus, basal ganglia and parietal cortex |
310 | < 100 | 8 | Extended PMI* |
| 31 | Insulin toxicity | Insulin vials | Autopsy not performed | Autopsy not performed | NA | NA | NA | Hospital admission blood sample not available for testing |
| 32 | Insulin toxicity/overdosage | Suicide note, Lantus and NovoRapid insulin pens |
Lungs: oedema/congestion Kidneys: sclerotic glomeruli Brain: early neuronal dark cell change within hippocampus |
26 | < 100 | 3 | - | |
| 33 | Consistent with insulin toxicity | Syringe and empty Lantus and Actrapid insulin vials | Injection site on right abdomen |
Lungs: oedema/congestion Kidneys: no diabetic glomerular/vascular changes Brain: evidence of neuronal necrosis in hippocampus and basal ganglia |
6 | < 100 | 3 | - |
| 34 | Attributed to insulin toxicity | 15 empty Humalog insulin pens, Last Will and Testament nearby | Seven injection sites on lower abdomen |
Lungs: oedema/congestion, focal acute pneumonia Kidneys: no diabetic glomerular/vascular changes Brain: potential dark-cell change in dentate nuclei, Purkinje cells, cortex and basal ganglia |
22 | < 100 | 3 | - |
| 35 | Insulin toxicity | One empty Lantus and seven empty NovoRapid insulin pens | Several injection sites on right abdomen |
Lungs: oedema/congestion Kidneys: consistent with diabetes Brain: normal neuronal architecture |
1300 | < 100 | 4 | - |
| 36 | Hypoglycaemic brain injury due to insulin toxicity complicated by aspiration pneumonia | Suicide note and 8 empty NovoRapid FlexPen’s | Several injection sites on lower abdomen and upper thighs |
Lungs: oedema/congestion with patchy early aspiration pneumonia Kidneys: consistent with diabetes Brain: early neuronal necrosis in hippocampus and superficial cortical neurons |
1.9 | < 100 | 4 | Rapid acting (3–5 h) insulin administered, rapidly degraded* |
| 37 | Insulin toxicity | Numerous Novorapid insulin vials and an empty syringe | Injection site on lower right abdomen |
Lungs: nil Kidneys: consistent with diabetes Brain: neuronal necrosis in cerebellum, dentate nucleus, cerebral cortex and hippocampi |
< 1 | 47 | 4 | Insulin degraded during period of coma* |
| 38 | Insulin toxicity | Suicide note detailing insulin overdose | Autopsy not performed | Autopsy not performed | NA | NA | NA | Hospital admission blood sample not available for testing |
| 39 | Hypoxic ischaemic encephalopathy following insulin and benzodiazepine overdose | Empty insulin syringe, suicide note | Autopsy not performed | Autopsy not performed | NA | NA | NA | Hospital admission blood sample not available for testing |
| 40 | Attributed to insulin toxicity | 15 empty Lantus insulin pens, 2 empty NovoRapid insulin pens and a suicide note | Several injection sites on abdomen |
Lungs: oedema/congestion Kidneys: consistent with diabetes Brain: red cell change in frontal cortex and temporal cortices and cerebellum |
12.8 | < 12 | 7 | Extended PMI* |
NA not available
*Insulin/C-peptide results unreliable
Insulin and C-peptide analyses were not performed in the remaining nine cases due to the unavailability of a hospital blood sample for analysis in seven cases and two cases where the blood sample was deemed unsuitable for analysis.
Identification and interpretation of insulin and C-peptide levels are dependent on the integrity of the post-mortem blood sample. Insulin levels may be artificially lowered due to a prolonged post-mortem interval (PMI) which is associated with haemolysis of post-mortem blood samples and subsequent insulin degradation. The PMI ranged from 1 to 11 days across all 40 cases (mean = 3.6 days). Of the 31 cases where insulin and C-peptide levels were reported, the results in five cases were considered to be unreliable due to a significant PMI (i.e., 5 days or longer) or evident haemolysis of the blood sample. Furthermore, three cases demonstrated a prolonged period of survival (coma) between insulin administration and death allowing time for insulin to be degraded, or the administration of rapid-acting insulin which is also rapidly degraded. Therefore, the insulin and C-peptide levels reported in the eight cases highlighted above were significantly lower than would be reflective of a death due to insulin toxicity.
Average (mean) insulin and C-peptide levels were calculated for the remaining 23 cases where the biochemistry results were considered accurate and reliable. The average blood level of insulin and C-peptide for these cases was 816 mU/L and 144 pmol/L, respectively, with an approximate ratio of 6:1. The C-peptide level was frequently denoted as “ < 100 pmol/L”; therefore, this is a significant underestimate of the real ratio. For reference, a normal fasting insulin level is between 0 and 12 mU/L and a normal C-peptide level is between 300 and 1600 pmol/L.
Decedent histories
The medical history of decedents revealed a history of depression in 23 cases (57.5%), 12 of whom had previously demonstrated suicidal ideation. However, fewer than five decedents did not have a recorded history of depression but had previously demonstrated suicidal ideation according to the personal histories available. Proximal risk factors for intentional self-harm noted in decedent histories included family/relationship issues in seven cases, significant health issues in five cases, and financial/employment/legal issues in three cases. More than half of decedents were insulin-dependent diabetics (n = 22, 55%) and five were not. The diabetic status of the remaining decedents was unknown. Thirteen of the 22 insulin-dependent diabetics (59%) had a history of depression, 10 of whom had previously demonstrated suicidal ideation.
Manner of death
The manner of death was suicide in 29 cases (72.5%) with the remaining 11 cases being of accidental or undetermined intent (27.5%).
Accidental/undetermined deaths
Rate of deaths
Eleven cases of accidental or undetermined intent were identified with an average rate of 0.58 deaths per year. Poisson regression demonstrated no significant change in the rate of deaths over this period (p = 0.379).
Demographic profile
The average age of all decedents was 46.4 years and included 5 females (mean 48 years) and 6 males (mean 45 years) with no significant difference in the age/sex distribution of the cohort (p = 0.733).
Decedent histories
Decedent histories revealed a history of depression in seven cases, five of whom had previously demonstrated suicidal ideation. Financial/employment/legal issues were noted in one case. Six of the decedents were insulin-dependent diabetics (55%); the diabetes status of the remaining decedents was unknown.
Suicide
Rate of deaths
Twenty-nine cases of suicide were identified with an average rate of 1.38 deaths per year. Poisson regression demonstrated no significant change in the rate of deaths over this period (p = 0.120).
Demographic profile
The average age of all decedents was 50.8 years and included 14 females (mean 48 years) and 15 males (mean 53.3 years) with no significant difference in the age/sex distribution of the cohort (p = 0.294).
Decedent histories
Decedent histories revealed a history of depression in 16 cases (55.2%), seven of whom had previously demonstrated suicidal ideation. Previous suicidal ideation was noted in less than five cases where there was no recorded history of depression. Family/relationship issues were noted in seven cases, significant health issues in five cases and financial/employment/legal issues in two cases. A suicide note was found in 19 cases (65.5%). Sixteen decedents were insulin-dependent diabetics (55.2%), three were not, and the diabetes status of the remaining decedents was unknown. Of those that were not insulin-dependent diabetics, the insulin was sourced either from their workplace or a family member.
Discussion
Since its discovery and isolation, insulin has been an effective drug for the maintenance of normal blood glucose levels for type I and type II diabetics. However, self-administration dosing errors have been identified as a frequent cause of hospital admission among insulin-dependent diabetics, particularly among the young [2–5]. Excessive insulin administration can cause profound hypoglycaemia, subsequent brain damage, and even death if not correctly diagnosed and promptly treated. There are an abundance of case reports and literature reviews on fatal and non-fatal insulin overdoses involving accidents [6–8], suicides [18–66], and homicides [9–17]. In comparison to other compounds, insulin has, however, been considered a somewhat ineffective lethal agent due to the long period of time required to produce permanent damage and the ease with which an overdose may be diagnosed and treated [67–69]. Unfortunately, there is a lack of longitudinal reviews in the literature to assess long-term trends in fatal insulin overdoses for all manners of death.
Case reports and literature reviews of fatal insulin overdoses have identified likely underreporting due to laboratory and pathology limitations. Suspicion may not be aroused without a comprehensive medical or personal history, which is often not available at the time of autopsy. Cases may also be incorrectly classified as accidents due to an undiagnosed history of depression [8]. The literature has identified an association between diabetes and an increased prevalence of psychiatric disorders such as depression or suicidality, particularly in those with a long history of diabetes and those on insulin therapy [70–72]. At a poisons unit in Germany, the incidence of intentional insulin overdoses was significantly higher (85.3%) than accidental cases compared to other anti-diabetic compounds [73]. More than half (59%) of insulin-dependent diabetics in the current study had a medical history of depression, the majority of which had demonstrated suicidal ideation (77%). However, if the decedent has not been prescribed insulin or is not known to be an insulin-dependent diabetic, as was highlighted in this study, examination for evidence of exogenous insulin administration may not be considered. Even if examination is performed, puncture wounds may not be evident as the needle used to administer subcutaneous insulin is very fine which may further obscure cases [74]. At autopsy, evidence of subcutaneous insulin injection was only observed in 57.5% of cases in the current study. Case reports of insulin-related deaths often report the presence of insulin administration equipment at the death scene. In the current study, evidence of insulin administration (i.e., used syringes, insulin vials) was documented in just over half (65%) of cases. There also remains the possibility that such material may be removed or hidden by the decedent or by family/friends. Therefore, a presumptive cause of death of insulin toxicity should be based on a multitude of factors including the decedent’s medical history integrated with scene, autopsy, toxicology, biochemistry, and histology findings.
While cases of fatal insulin overdose are thought to be a rare occurrence, the current study suggests that while in low numbers, they continue to occur consistently over time. Furthermore, suicidal insulin overdoses are just as frequent, if not more so than accidental overdoses or those of undetermined intent. While significant sex/gender disparities are often observed in suicidal deaths with a male predominance [75], no differences were shown in age or sex distribution of the current study population. For insulin-dependent diabetics, an undiagnosed history of depression with or without suicidal ideation is particularly problematic as those on insulin therapy have access to a potentially lethal pharmacological suicide agent. As several decedents within this cohort had previously attempted suicide using insulin, it is important that appropriate medical monitoring is directed towards repeat-attempters with access to insulin [71]. In the current study, those who were not insulin-dependent diabetics and utilized insulin as a pharmacological suicide agent were either health professionals or had a relative/partner who had been prescribed insulin. However, for several of the cases deemed to be of accidental or undetermined intent, it is unclear how the decedent gained access to insulin.
In addition to difficulties in ascertaining manner of death, there are also limitations in the detection and confirmation of exogenous insulin administration which may contribute to the underreporting of cases. As noted, there are significant analytical limitations associated with post-mortem insulin and C-peptide detection and quantification. Insulin is known to be unstable in post-mortem blood samples due to the activity of insulin-degrading enzyme which is concentrated within red blood cells and released with haemolysis [76–78]. Traditional methodologies utilize radioimmunoassay and enzyme-linked immunoassays which are associated with limitations in identification, differentiation, and quantification [64]. For traditional immunoassays, insulin analogues have also been shown to be cross-reactive which has been a major obstacle in developing an immunoassay to effectively identify analogues or metabolites, with significant variation between assays [79–81]. Newer methods using liquid chromatography with tandem mass spectrometry (LC–MS-MS) have been developed in recent years with success in both quantification of insulin and differentiation between analogues [5, 64, 65, 67]. However, it is yet to be seen how accessible these new methodologies will be in terms of availability and cost.
For cases where reliable insulin and C-peptide levels were obtained, these were significantly outside the normal ranges. Some authors also consider the ratio of insulin to C-peptide (I:C) to be a useful measure to distinguish between exogenous and biological hypoglycaemia as C-peptide is only formed with release of endogenous insulin, and should not be > 1 in a healthy individual [82]. The approximate ratio of insulin to C-peptide was shown to far exceed the normal, healthy level in all decedents where reliable biochemistry results were available. However, the ratio of insulin to C-peptide has also been shown to change significantly in the post-mortem interval, even within 24 h [11]. If post-mortem blood is to be used, samples should be taken within 48 h of death (if possible) and from a peripheral site to limit the effects of post-mortem redistribution [11, 83]. Several other biological sample sites have been evaluated, with vitreous humor found to be the most promising as it is less susceptible to diffusion and the effects of decomposition, and thus is more reliable for determination of insulin and C-peptide [67].
At autopsy, pathological changes indicative of insulin overdose and hypoglycaemia are often nonspecific although hypoglycaemic neuronal necrosis may be observed [84–87]. Neuropathological animal studies show that blood glucose must persist at a level below 1 mM for at least 30 min for neuronal death to occur which has been confirmed by human autopsy studies [84–88]. While normal neuronal architecture was observed in 35% of cases, this could be indicative of a short survival period post-insulin administration, and thus, the presence or absence of neuronal pathology cannot be considered diagnostic of fatal hypoglycaemia without a detailed timeline of events. For decedents in the current study where extensive neuronal death was observed, this was generally correlated with a prolonged period of survival between insulin administration and death. Previous studies show that hypoglycaemic neuronal death occurs sequentially by brain region, first evident in the cerebral cortex and hippocampus, followed by the basal ganglia and thalamus as was shown in the present report [9, 87, 89].
This study has shown a consistent although low rate of insulin-related deaths over a two-decade period in a single Australian state. Further investigations are required to determine whether this trend is observed in other states and countries. Problems with pathological assessments and toxicological/biochemical evaluations may indicate, however, that the true number of these deaths may be higher and that some of the deaths considered to be accidental or undetermined may in fact be occult suicides. Therefore, suspicion and confirmation of insulin toxicity as the cause of death should be based on a multitude of factors including the decedent’s medical history integrated with scene, autopsy, toxicology, biochemistry, and histology findings.
Key points
A low, yet consistent number of fatal insulin overdoses occurred in South Australia between 2000 and 2019
Suicidal insulin overdoses were significantly more frequent than accidental insulin overdoses, with more than two-thirds of cases found to be suicides
A large proportion of insulin-dependent diabetics who died due to fatal insulin overdose had a history of depression, often with previous suicidal ideation
There are significant analytical limitations associated with quantification of insulin and C-peptide in post-mortem samples
A finding of insulin toxicity as the cause of death should be based on a multitude of factors including the decedent’s medical history integrated with scene, autopsy, toxicology, biochemistry, and histology findings
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank Forensic Science South Australia and the National Coronial Information System of the Department of Justice and Community Safety for granting data access and Drug and Alcohol Services South Australia for their financial support.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Drug and Alcohol Services South Australia (Project ID: 18124993).
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rosenfeld L. Insulin: discovery and controversy. Clin Chem. 2002;48:2270–2288. [PubMed] [Google Scholar]
- 2.Cox AR, Ferner RE. Prescribing errors in diabetes. Br J Diabetes Vasc Dis. 2009;9:84–88. [Google Scholar]
- 3.Bronstein AC, Spyker DA, Cantilena LR, Jr, Green JL, Rumack BH, Heard SE. 2007 annual report of the American association of poison control centers’ national poison data system (NPDS): 25th annual report. Clin Toxicol (Phila) 2008;46:927–1057. doi: 10.1080/15563650802559632. [DOI] [PubMed] [Google Scholar]
- 4.Lai MW, Klein-Schwartz W, Rodgers GC, Abrams JY, Haber DA, Bronstein AC, Wruk KM. 2005 annual report of the American association of poison control centers’ national poisoning and exposure database. Clin Toxicol (Phila) 2006;44:803–932. doi: 10.1080/15563650600907165. [DOI] [PubMed] [Google Scholar]
- 5.Hess C, Madea B, Daldrup T, Musshoff F. Determination of hypoglycaemia induced by insulin or its synthetic analogues post mortem. Drug Test Anal. 2013;5:802–807. doi: 10.1002/dta.1500. [DOI] [PubMed] [Google Scholar]
- 6.Thordarson H, Søvik O. Dead in bed syndrome in young diabetic patients in Norway. Diabet Med. 1995;12:782–787. doi: 10.1111/j.1464-5491.1995.tb02080.x. [DOI] [PubMed] [Google Scholar]
- 7.Tattersall RB, Gill GV. Unexplained deaths of type 1 diabetic patients. Diabet Med. 1991;8:49–58. doi: 10.1111/j.1464-5491.1991.tb01516.x. [DOI] [PubMed] [Google Scholar]
- 8.Batalis NI, Prahlow JA. Accidental insulin overdose. J Forensic Sci. 2004;49:1117–1120. [PubMed] [Google Scholar]
- 9.Tong F, Wu R, Huang W, Yang Y, Zhang L, Zhang B, Chen X, Tang X, Zhou Y. Forensic aspects of homicides by insulin overdose. Forensic Sci Int. 2017;278:9–15. doi: 10.1016/j.forsciint.2017.06.015. [DOI] [PubMed] [Google Scholar]
- 10.Marks V. Murder by insulin: suspected, purported and proven-a review. Drug Test Anal. 2009;1:162–176. doi: 10.1002/dta.38. [DOI] [PubMed] [Google Scholar]
- 11.Iwase H, Kobayashi M, Nakajima M, Takatori T. The ratio of insulin to C-peptide can be used to make a forensic diagnosis of exogenous insulin overdosage. Forensic Sci Int. 2001;115:123–127. doi: 10.1016/s0379-0738(00)00298-x. [DOI] [PubMed] [Google Scholar]
- 12.Haibach H, Dix JD, Shah JH. Homicide by insulin administration. J Forensic Sci. 1987;32:208–216. [PubMed] [Google Scholar]
- 13.Levy WJ, Gardner D, Moseley J, Dix J, Gaede SE. Unusual problems for the physician in managing a hospital patient who received a malicious insulin overdose. Neurosurgery. 1985;17:992–996. doi: 10.1227/00006123-198512000-00023. [DOI] [PubMed] [Google Scholar]
- 14.Uezono T, Shiono H, Shimizu K, Ogawa K, Saito O, Yoshida M, Mizukami H, Matsubara K. Simultaneous analyses of hypoglycemic agents and C-peptide are essential in a homicide case with the combined dosing insulin and insulin-releasing drug. Leg Med (Tokyo) 2002;4:34–36. doi: 10.1016/s1344-6223(01)00042-6. [DOI] [PubMed] [Google Scholar]
- 15.Koskinen PJ, Nuutinen HM, Laaksonen H, Klossner JA, Irjala KM, Kalimo H, Viikari JS. Importance of storing emergency serum samples for uncovering murder with insulin. Forensic Sci Int. 1999;105:61–66. doi: 10.1016/s0379-0738(99)00111-5. [DOI] [PubMed] [Google Scholar]
- 16.Birkinshaw VJ, Gurd MR, Randall SS, Curry AS, Price DE, Wright PH. Investigations in a case of murder by insulin poisoning. Br Med J. 1958;2:463–468. doi: 10.1136/bmj.2.5094.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Green RP, Hollander AS, Thevis M, Thomas A, Dietzen DJ. Detection of surreptitious administration of analog insulin to an 8-week-old infant. Pediatrics. 2010;125:e1236–e1240. doi: 10.1542/peds.2009-2273. [DOI] [PubMed] [Google Scholar]
- 18.Tofade TS, Liles EA. Intentional overdose with insulin glargine and insulin aspart. Pharmacotherapy. 2004;24:1412–1418. doi: 10.1592/phco.24.14.1412.43147. [DOI] [PubMed] [Google Scholar]
- 19.Walsh SM, Sage RA. Depression and chronic diabetic foot disability. A case report of suicide. Clin Podiatr Med Surg. 2002;19:493–508. doi: 10.1016/s0891-8422(02)00019-8. [DOI] [PubMed] [Google Scholar]
- 20.Bugelli V, Campobasso CP, Angelino A, Focardi M, Pinchi V. CLEIA of humor vitreous in a case of suicidal insulin overdose. Leg Med (Tokyo) 2019;40:22–25. doi: 10.1016/j.legalmed.2019.06.007. [DOI] [PubMed] [Google Scholar]
- 21.Birngruber CG, Krüll R, Dettmeyer R. Verhoff MA [Alleged suicide by insulin] Arch Kriminol. 2015;235:43–52. [PubMed] [Google Scholar]
- 22.Sunderland N, Wong S, Lee CK. Fatal insulin overdoses: case report and update on testing methodology. J Forensic Sci. 2016;61(Suppl 1):S281–S284. doi: 10.1111/1556-4029.12958. [DOI] [PubMed] [Google Scholar]
- 23.Nikolić S, Atanasijević T. Popović V [A suicide by insulin injection–case report] Srp Arh Celok Lek. 2006;134:444–447. doi: 10.2298/sarh0610444n. [DOI] [PubMed] [Google Scholar]
- 24.Rao NG, Menezes RG, Nagesh KR, Kamath GS. Suicide by combined insulin and glipizide overdose in a non-insulin dependent diabetes mellitus physician: a case report. Med Sci Law. 2006;46:263–269. doi: 10.1258/rsmmsl.46.3.263. [DOI] [PubMed] [Google Scholar]
- 25.Winston DC. Suicide via insulin overdose in nondiabetics: the New Mexico experience. Am J Forensic Med Pathol. 2000;21:237–240. doi: 10.1097/00000433-200009000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Junge M, Tsokos M, Püschel K. Suicide by insulin injection in combination with beta-blocker application. Forensic Sci Int. 2000;113:457–460. doi: 10.1016/s0379-0738(00)00283-8. [DOI] [PubMed] [Google Scholar]
- 27.Patel F. Successful suicide by insulin injection in a non-diabetic. Med Sci Law. 1995;35:181–182. doi: 10.1177/002580249503500216. [DOI] [PubMed] [Google Scholar]
- 28.Patel F. Fatal self-induced hyperinsulinaemia: a delayed post-mortem analytical detection. Med Sci Law. 1992;32:151–159. doi: 10.1177/106002809203200210. [DOI] [PubMed] [Google Scholar]
- 29.Hänsch CF, De Roy G. An extraordinary case: suicide with insulin in a grave dug by the victim himself. Z Rechtsmed. 1977;79:319–320. doi: 10.1007/BF00201175. [DOI] [PubMed] [Google Scholar]
- 30.Beastall GH, Gibson IH, Martin J. Successful suicide by insulin injection in a non-diabetic. Med Sci Law. 1995;35:79–85. doi: 10.1177/002580249503500116. [DOI] [PubMed] [Google Scholar]
- 31.Arem R, Zoghbi W. Insulin overdose in eight patients: insulin pharmacokinetics and review of the literature. Medicine (Baltimore) 1985;64:323–332. doi: 10.1097/00005792-198509000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Kernbach-Wighton G, Püschel K. On the phenomenology of lethal applications of insulin. Forensic Sci Int. 1998;93:61–73. doi: 10.1016/s0379-0738(98)00032-2. [DOI] [PubMed] [Google Scholar]
- 33.Cooper AJ. Attempted suicide using insulin by a non diabetic: a case study demonstrating the acute and chronic consequences of profound hypoglycemia. Can J Psychiatry. 1994;39:103–107. doi: 10.1177/070674379403900207. [DOI] [PubMed] [Google Scholar]
- 34.Behera C, Swain R, Mridha AR, Pooniya S. Suicide by injecting lispro insulin with an intravenous cannula. Med Leg J. 2015;83:147–149. doi: 10.1177/0025817215573171. [DOI] [PubMed] [Google Scholar]
- 35.Kaminer Y, Robbins DR. Attempted suicide by insulin overdose in insulin-dependent diabetic adolescents. Pediatrics. 1988;81:526–528. [PubMed] [Google Scholar]
- 36.Thewjitcharoen Y, Lekpittaya N, Himathongkam T. Attempted suicide by massive insulin injection: a case report and review of the literature. J Med Assoc Thai. 2008;91:1920–1924. [PubMed] [Google Scholar]
- 37.Dickson SJ, Cairns ER, Blazey ND. The isolation and quantitation of insulin in post-mortem specimens–a case report. Forensic Sci. 1977;9:37–42. doi: 10.1016/0300-9432(77)90063-2. [DOI] [PubMed] [Google Scholar]
- 38.Critchley JA, Proudfoot AT, Boyd SG, Campbell IW, Brown NS, Gordon A. Deaths and paradoxes after intentional insulin overdosage. Br Med J (Clin Res Ed) 1984;289:225. doi: 10.1136/bmj.289.6439.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Given BD, Ostrega DM, Polonsky KS, Baldwin D, Jr, Kelley RI, Rubenstein AH. Hypoglycemia due to surreptitious injection of insulin. Identification of insulin species by high-performance liquid chromatography. Diabetes Care. 1991;14:544–7. doi: 10.2337/diacare.14.7.544. [DOI] [PubMed] [Google Scholar]
- 40.Scarlett JA, Mako ME, Rubenstein AH, Blix PM, Goldman J, Horwitz DL, Tager H, Jaspan JB, Stjernholm MR, Olefsky JM. Factitious hypoglycemia. Diagnosis by measurement of serum C-peptide immunoreactivity and insulin-binding antibodies. N Engl J Med. 1977;297:1029–32. doi: 10.1056/NEJM197711102971903. [DOI] [PubMed] [Google Scholar]
- 41.Martin FI, Hansen N, Warne GL. Attempted suicide by insulin overdose in insulin-requiring diabetics. Med J Aust. 1977;1:58–60. doi: 10.5694/j.1326-5377.1977.tb130503.x. [DOI] [PubMed] [Google Scholar]
- 42.Price DE. Some observations on another case of insulin poisoning, with complications. Med Sci Law. 1965;5:101–109. doi: 10.1177/002580246500500208. [DOI] [PubMed] [Google Scholar]
- 43.Uchida D, Ohigashi S, Hikita S, Kitamura N, Motoyoshi M, Tatsuno I. Acute pulmonary edema caused by hypoglycemia due to insulin overdose. Internal medicine (Tokyo, Japan) 2004;43:1056–1059. doi: 10.2169/internalmedicine.43.1056. [DOI] [PubMed] [Google Scholar]
- 44.Doğan FS, Onur OE, Altınok AD, Göneysel O. Insulin glargine overdose J Pharmacol Pharmacother. 2012;3:333–335. doi: 10.4103/0976-500X.103694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lu M, Inboriboon PC. Lantus insulin overdose: a case report. J Emerg Med. 2011;41:374–377. doi: 10.1016/j.jemermed.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 46.Guclu M, Ersoy C, Imamoglu S. Suicide attempt of a physician with 3600 units of insulin and rapid onset acute hepatitis. Intern Med J. 2009;39:e5–7. doi: 10.1111/j.1445-5994.2009.02090.x. [DOI] [PubMed] [Google Scholar]
- 47.Brvar M, Mozina M, Bunc M. Prolonged hypoglycaemia after insulin lispro overdose. Eur J Emerg Med. 2005;12:234–235. doi: 10.1097/00063110-200510000-00007. [DOI] [PubMed] [Google Scholar]
- 48.Bobzien WF., 3rd Suicidal overdoses with hypoglycemic agents Jacep. 1979;8:467–470. doi: 10.1016/s0361-1124(79)80062-3. [DOI] [PubMed] [Google Scholar]
- 49.Mork TA, Killeen CT, Patel NK, Dohnal JM, Karydes HC, Leikin JB. Massive insulin overdose managed by monitoring daily insulin levels. Am J Ther. 2011;18:e162–e166. doi: 10.1097/MJT.0b013e3181f4eadb. [DOI] [PubMed] [Google Scholar]
- 50.Svingos RS, Fernandez EM, Reeder DN, Parker JJ. Life-threatening hypoglycemia associated with intentional insulin ingestion. Pharmacotherapy. 2013;33:e28–33. doi: 10.1002/phar.1207. [DOI] [PubMed] [Google Scholar]
- 51.Tunbridge WM. Factors contributing to deaths of diabetics under fifty years of age. On behalf of the medical services study group and british diabetic association. Lancet. 1981;2:569–72. doi: 10.1016/s0140-6736(81)90950-8. [DOI] [PubMed] [Google Scholar]
- 52.Campbell IW, Ratcliffe JG. Suicidal insulin overdose managed by excision of insulin injection site. Br Med J (Clin Res Ed) 1982;285:408–409. doi: 10.1136/bmj.285.6339.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gin H, Larnaudie B, Aubertin J. Attempted suicide by insulin injection treated with artificial pancreas. Br Med J (Clin Res Ed) 1983;287:249–250. doi: 10.1136/bmj.287.6387.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Blotner H. Attempted suicide with insulin. Am J Med Sci. 1954;227:387–390. doi: 10.1097/00000441-195404000-00003. [DOI] [PubMed] [Google Scholar]
- 55.Creutzfeldt W, Frerichs H, Perings E. Serum-insulin levels in hypoglycaemic shock due to attempted suicide with tolbutamide and with insulin. Ger Med Mon. 1969;14:14–6. [PubMed] [Google Scholar]
- 56.Jefferys DB, Volans GN. Self poisoning in diabetic patients. Hum Toxicol. 1983;2:345–348. doi: 10.1177/096032718300200228. [DOI] [PubMed] [Google Scholar]
- 57.Jordan H. Unusual sequel of a large overdose of insulin. Br Med J. 1946;1:276. [PubMed] [Google Scholar]
- 58.Levine DF, Bulstrode C. Managing suicidal insulin overdose. Br Med J (Clin Res Ed) 1982;285:974–975. doi: 10.1136/bmj.285.6346.974-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lindgren L. Enormous dose of insulin with suicidal intent. Acta Med Scand. 1960;167:297–300. doi: 10.1111/j.0954-6820.1960.tb03550.x. [DOI] [PubMed] [Google Scholar]
- 60.Samuels MH, Eckel RH. Massive insulin overdose: detailed studies of free insulin levels and glucose requirements. J Toxicol Clin Toxicol. 1989;27:157–168. doi: 10.3109/15563658909038579. [DOI] [PubMed] [Google Scholar]
- 61.Stapczynski JS, Haskell RJ. Duration of hypoglycemia and need for intravenous glucose following intentional overdoses of insulin. Ann Emerg Med. 1984;13:505–511. doi: 10.1016/s0196-0644(84)80513-2. [DOI] [PubMed] [Google Scholar]
- 62.Brouhard BH. Surreptitious insulin administration. Am J Dis Child. 1987;141:28–29. doi: 10.1001/archpedi.1987.04460010028016. [DOI] [PubMed] [Google Scholar]
- 63.Logemann E, Pollak S, Khalaf AN. Petersen KG [Postmortem diagnosis of exogenous insulin administration] Arch Kriminol. 1993;191:28–36. [PubMed] [Google Scholar]
- 64.Legg KM, Labay LM, Aiken SS, Logan BK. Validation of a fully automated immunoaffinity workflow for the detection and quantification of insulin analogs by LC-MS-MS in postmortem vitreous humor. J Anal Toxicol. 2019;43:505–511. doi: 10.1093/jat/bkz014. [DOI] [PubMed] [Google Scholar]
- 65.Thevis M, Thomas A, Schänzer W, Ostman P, Ojanperä I. Measuring insulin in human vitreous humour using LC-MS/MS. Drug Test Anal. 2012;4:53–56. doi: 10.1002/dta.368. [DOI] [PubMed] [Google Scholar]
- 66.Kaminer Y, Robbins DR. Insulin misuse: a review of an overlooked psychiatric problem. Psychosomatics. 1989;30:19–24. doi: 10.1016/S0033-3182(89)72313-6. [DOI] [PubMed] [Google Scholar]
- 67.Ojanperä I, Sajantila A, Vinogradova L, Thomas A, Schänzer W, Thevis M. Post-mortem vitreous humour as potential specimen for detection of insulin analogues by LC-MS/MS. Forensic Sci Int. 2013;233:328–332. doi: 10.1016/j.forsciint.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 68.MacLeod K, Hepburn D, Frier BM. Frequency and morbidity of severe hypoglycaemia in insulin-treated diabetic patients. Diabet Med. 1993;10:238–245. doi: 10.1111/j.1464-5491.1993.tb00051.x. [DOI] [PubMed] [Google Scholar]
- 69.Marks V. Murder by insulin Medico-legal journal. 1999;67:147–163. doi: 10.1258/rsmmlj.67.4.147. [DOI] [PubMed] [Google Scholar]
- 70.Conti C, Mennitto C, Di Francesco G, Fraticelli F, Vitacolonna E, Fulcheri M. Clinical characteristics of diabetes mellitus and suicide risk. Front Psych. 2017;8:40. doi: 10.3389/fpsyt.2017.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Myers AK, Trivedi MH. Death by insulin: management of self-harm and suicide in diabetes management. Curr Diabetes Rev. 2017;13:251–262. doi: 10.2174/1573399812666161005163618. [DOI] [PubMed] [Google Scholar]
- 72.Sarkar S, Balhara YP. Diabetes mellitus and suicide. Indian journal of endocrinology and metabolism. 2014;18:468–474. doi: 10.4103/2230-8210.137487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.von Mach MA, Gauer M, Meyer S, Omogbehin B, Schinzel H, Kann PH, Weilemann LS. Antidiabetic medications in overdose: a comparison of the inquiries made to a regional poisons unit regarding original sulfonylureas, biguanides and insulin. Int J Clin Pharmacol Ther. 2006;44:51–56. doi: 10.5414/cpp44051. [DOI] [PubMed] [Google Scholar]
- 74.Marks V, Wark G. Forensic aspects of insulin. Diabetes Res Clin Pract. 2013;101:248–254. doi: 10.1016/j.diabres.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 75.Australian Institute of Health Welfare. Suicide and intentional self-harm. Canberra: AIHW. 2020.
- 76.Brodal BP. Evidence of an enzymatic degradation of insulin in blood in vitro. Eur J Biochem. 1971;18:201–206. doi: 10.1111/j.1432-1033.1971.tb01231.x. [DOI] [PubMed] [Google Scholar]
- 77.Wunder C, Kauert GF, Toennes SW. Factors leading to the degradation/loss of insulin in postmortem blood samples. Forensic Sci Int. 2014;241:173–177. doi: 10.1016/j.forsciint.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 78.Steinke J, Gries F, Driscoll S, Grinbush M. In vitro studies of insulin inactivation with reference to erythroblastosis fetalis. Blood. 1967;30:359–363. [PubMed] [Google Scholar]
- 79.Heurtault B, Reix N, Meyer N, Gasser F, Wendling MJ, Ratomponirina C, Jeandidier N, Sapin R, Agin A. Extensive study of human insulin immunoassays: promises and pitfalls for insulin analogue detection and quantification. Clin Chem Lab Med. 2014;52:355–362. doi: 10.1515/cclm-2013-0427. [DOI] [PubMed] [Google Scholar]
- 80.Owen WE, Roberts WL. Cross-reactivity of three recombinant insulin analogs with five commercial insulin immunoassays. Clin Chem. 2004;50:257–259. doi: 10.1373/clinchem.2003.026625. [DOI] [PubMed] [Google Scholar]
- 81.Manley SE, Stratton IM, Clark PM, Luzio SD. Comparison of 11 human insulin assays: implications for clinical investigation and research. Clin Chem. 2007;53:922–932. doi: 10.1373/clinchem.2006.077784. [DOI] [PubMed] [Google Scholar]
- 82.Mitchell R, Stanley A. The molar ratio of insulin to C peptide. Arch Int Med. 1993;153:650–655. [PubMed] [Google Scholar]
- 83.Lindquist O, Rammer L. Insulin in post-mortem blood. Z Rechtsmed. 1975;75:275–277. doi: 10.1007/BF00201181. [DOI] [PubMed] [Google Scholar]
- 84.Hess C, Musshoff F, Madea B. Disorders of glucose metabolism-post mortem analyses in forensic cases: part I. Int J Legal Med. 2011;125:163–170. doi: 10.1007/s00414-010-0509-6. [DOI] [PubMed] [Google Scholar]
- 85.Auer RN, Olsson Y, Siesjö BK. Hypoglycemic brain injury in the rat: correlation of density of brain damage with the EEG isoelectric time: a quantitative study. Diabetes. 1984;33:1090–1098. doi: 10.2337/diab.33.11.1090. [DOI] [PubMed] [Google Scholar]
- 86.Auer R, Kalimo H, Olsson Y, Siesjö B. The temporal evolution of hypoglycemic brain damage. Acta Neuropathol. 1985;67:13–24. doi: 10.1007/BF00688120. [DOI] [PubMed] [Google Scholar]
- 87.Suh SW, Hamby AM, Swanson RA. Hypoglycemia, brain energetics, and hypoglycemic neuronal death. Glia. 2007;55:1280–1286. doi: 10.1002/glia.20440. [DOI] [PubMed] [Google Scholar]
- 88.Suh SW, Aoyama K, Chen Y, Garnier P, Matsumori Y, Gum E, Liu J, Swanson RA. Hypoglycemic neuronal death and cognitive impairment are prevented by poly (ADP-ribose) polymerase inhibitors administered after hypoglycemia. J Neurosci. 2003;23:10681–10690. doi: 10.1523/JNEUROSCI.23-33-10681.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Auer R, Hugh J, Cosgrove E, Curry B. Neuropathologic findings in three cases of profound hypoglycemia. Clin Neuropathol. 1989;8:63–68. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


