Abstract
Hyperhidrosis is a condition in which the cholinergic receptors on the eccrine glands are overstimulated, resulting in excessive sweating. It is considered a serious cosmetic and psychological problem that affects the patient’s quality of life. Searching for novel treatment modalities is required to minimize the side effects and to attain better patient satisfaction.
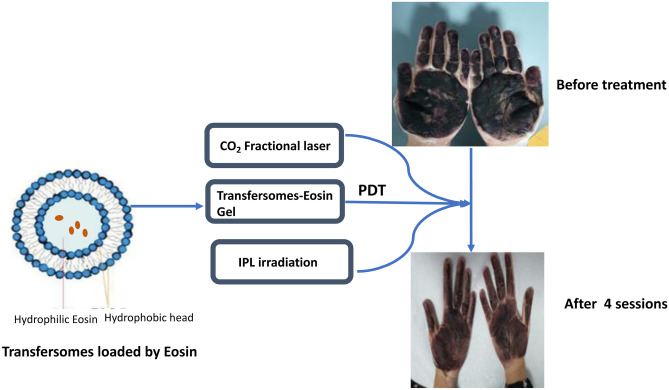
Photodynamic therapy (PDT), using eosin as a photosensitizer, is developed as a promising modality of the treatment of palmar and axillary hyperhidrosis. In this study, we treated six cases suffering palmar hyperhidrosis by applying the fractional CO2 laser prior to PDT session. For PDT, a hydrogel of eosin loaded in a transfersomes as a nano-delivery carrier was applied for 5 min, followed by irradiation by intense pulsed light (IPL). The prepared transfersomes loaded by eosin were spherical in shape with encapsulation efficiency of 33 ± 3.5%, particle size 305.5 ± 5.7 nm, average zeta potential of − 54 ± 7.6 mV with 80 ± 4% of the loaded eosin was released after 3 h. Two cases achieved 90% improvement after four sessions, three patients needed six sessions to show 75% improvement, while one patient showed only 25% improvement after six sessions. This resulted in shortening the time of PS application and decreasing the number of sessions required to achieve acceptable improvement. More clinical studies on large number of patients are required to optimize the results.
Graphical abstract
Keywords: Hyperhidrosis, Photodynamic therapy, Eosin yellow photosensitizer, Transfersomes
Introduction
Hyperhidrosis is a condition in which the cholinergic receptors on the eccrine glands are overstimulated, resulting in excessive sweating. It could be either primary or secondary, can be generalized or localized in certain areas such as the axilla, feet, and palms [1]. Consequently, it is considered a serious cosmetic and psychological problem that affects the patient’s quality of life [2].
Several treatment strategies are available; however, most of them possess serious side effects. Topical therapy with antiperspirants and botulinum toxin may develop contact reactions and intolerable pain. Systemic therapy with anticholinergic drugs is associated with several systemic side effects such as mouth dryness, ocular, and GIT disorders. Surgical treatment is available but it is an invasive technique that lacks patient satisfaction [3, 4]. Therefore, searching for novel treatment modalities is required to overwhelm the aforementioned drawbacks.
Photodynamic therapy (PDT) is developed as a promising modality for the treatment of various malignant and non-malignant skin diseases. A chemical compound called photosensitizer (PS) is activated by light with a certain wavelength which excites the electrons of the PS. In the presence of oxygen, the excited molecule is reverted to its ground state by means of either two mechanisms: type I reaction producing free radicals (FR), or type II reaction producing reactive oxygen species (ROS) and singlet oxygen (1O2). The produced compounds are cytotoxic and result in the devastation of the target tissues. The PS itself is non-toxic, and it becomes toxic only upon radiation with an appropriate wavelength light source. The main advantage of the topical PDT is the specificity which is achieved by application of the PS to the desired site of action and the irradiation of this site only. Consequently, the production of FR, ROS, and /or 1O2 is confined only to the diseased tissue, while the normal tissues remained unaffected [5].
In topical PDT, the stratum corneum (SC) is the main obstacle that prevents PS penetration, especially hydrophilic ones. There are many physical approaches to overcome the SC barrier properties, such as ultrasound, iontophoresis, microneedles, and electroporation. These techniques are invasive, need sophisticated devices, and have many limitations in the clinical applications [6, 7].
Nanotechnology has emerged as a strategy to facilitate dermal and transdermal drug delivery. Encapsulating PS in a lipid nano-vesicular system such as liposomes, transfersomes, niosomes, and ethosomes could be a promising approach to enhance the passage of the PS through the SC, hence improving the penetration of drugs to deep skin layers [8].
The proper choice of the PS and the use of the appropriate delivery system to carry it across the SC are crucial to enhance the topical PDT efficacy.
Eosin yellow (EY) is a hydrophilic dye that was clinically approved for many clinical applications and exhibited marked efficiency as a photosensitizer in PDT of many skin disorders [9]. PDT using EY has been used successfully for the treatment of axillary hyperhidrosis by the author [10] and for the treatment of palmar hyperhidrosis [11]; however, palmar hyperhidrosis is still challenging due to the increased thickness of the palmar skin.
Regarding the delivery system, transfersomes have been widely studied as an efficient delivery system to enhance the topical application of PS. They are phospholipid bilayer vesicles with an edge activator in their membrane. The lipidic membrane imparts the required hydrophobicity to penetrate through the SC, while an edge activator, a surfactant, imparts the required elasticity and deformability to the vesicle membrane [12, 13].
In previous work, we succeeded to treat plantar warts by topical PDT using EY loaded in transfersomes [14]. This encouraged us to use this formula to treat another challenging skin disorder, palmar hyperhidrosis. Moreover, in this study, we used a CO2 fractional laser prior to the application of EY to facilitate its penetration, hence improving the clinical outcome of the PDT. Collectively, in this study, we provided a new protocol for the treatment of palmar hyperhidrosis. This protocol combines the fractional CO2 laser and PDT using EY loaded in transfersomes as a nano-delivery carrier.
Materials
Eosin Y (EY) 90% dye content (Mwt 691.88) was purchased from Loba Chemie, India. Sodium deoxycholate (SDC), lecithin from soybean oil, phosphate-buffered saline (PBS), and chloroform were purchased from Sigma Aldrich. Carboxymethyl cellulose Na (Na-CMC) was purchased from Normest Company, Egypt, for scientific development. Methanol and absolute ethanol (99%) were purchased from El Nasr Pharmaceutical Chemicals Co., Adwic, Egypt.
Methods
Preparation of transfersomes loaded by eosin
Transfersomes loaded by EY were prepared as previously described in our previous work [14]. Briefly, the lipid (lecithin from soybean oil) and the edge activator (sodium deoxycholate) were dissolved in a mixture of chloroform: methanol 2:1. The organic solvents were then evaporated under vacuum in a rotary evaporator (Heidolph-Elektro GmbH + Co KG, Germany) rotating at 90 rpm at 45 °C. The rotation is continued till complete evaporation of the solvents and the formation of a uniform thin lipid film. The obtained lipid film was then hydrated by phosphate-buffered EY solution (PBS-EY) and left in the rotary evaporator for a further 1 h. The lipid: edge activator: EY ratio was set as 10:1:1. The transfersomes dispersion was sonicated for 5 min in a water bath sonicator and was stored in the refrigerator for further use.
Characterization of the prepared transfersomes
I. Morphology and shape
The diluted transfersomal dispersion was examined by a fluorescence microscope (Olympus BX51), supplied by a 75-W xenon lamp as an excitation source.
Moreover, the shape of the prepared transfersomes was examined under transmission electron microscopy (TEM, Jeol, Ltd., Tokyo, Japan) after negative staining.
II. UV–visible spectroscopy
The absorption spectrum of (PBS-EY) solution and EY-transfersomes dispersion were recorded by double beam spectrophotometer (RayLeigh UV-2601) at wavelength range 400–700 nm, using PBS solution as a blank reference.
III. Encapsulation efficiency (EE)
The unloaded EY was separated from the loaded transfersomes by centrifugation at 10,000 rpm at 5–6 °C (Centrikon T-42 K, Kontron Instruments, UK). The precipitated loaded transfersomes were dissolved in ethanol, to extract EY from them, and diluted by PBS (pH 7.4). The concentration of the extracted EY was measured spectrophotometrically at 520 nm (Rayleigh UV-2601) from a previously established standard calibration curve in PB-ethanol solution. Finally, the EE was determined as the following: EE % = (the calculated EY concentration extracted from the transfersomes/initial EY concentration used in preparation) X100.
IV. Particle size and zeta potential
The mean particle size and zeta potential of the prepared transfersomes were measured by the Zetasizer system (Malvern Instruments Ltd., Malvern, UK) after ten folds dilution with double distilled water at laser wavelength 632 nm, a temperature of 23 °C, scattering angle of 14.1°, and a refractive index of 1.33.
V. In-vitro release of EY from the prepared transfersomes
Three aliquots (n = 3) of the transfersomes suspension were dispersed in PBS (pH 7.4) and incubated separately at 37 °C under continuous stirring in a water bath. Each of the incubated dispersions was centrifuged for 10 min at 10,000 rpm at preset intervals. The precipitates were re-dispersed in fresh PBS and the concentration of released EY in the supernatants was measured by measuring the absorbance at 520 nm. Finally, the cumulative EY released percentage was determined as a function of time.
Preparation of transfersomal eosin hydrogel
The prepared EY-loaded transfersomes were incorporated into 5% Na-CMC hydrogel. A volume of transfersomal suspension, corresponding to 20 mg EY, was completed to 100 ml distilled water. Then, 5 gm of Na-CMC was added portion-wise with continuous stirring till full dispersion. Methylparaben (0.1%) and propylparaben (0.2%) were then added with continuous stirring to serve as preservatives. The gel was left for 24 h for complete swelling and to ensure the absence of any aggregates or air bubbles.
Clinical cases
Patients
The study was conducted at the dermatology clinic at the National Institute of Laser Enhanced Sciences (NILES), Cairo University, Egypt, after the approval of the board of the research ethics committee of the Institute (approval no: CU-NILES/24/21). The study protocol conformed to the guidelines of the Declaration of Helsinki. This case study was conducted on 6 patients, representing 6 case reports. The demographic data of the patients are listed in Table 1. A written informed consent was obtained from every patient. The authors affirm that one of the participants provided informed consent for publication of the images in Fig. 4a and b.
Table 1.
Patients characteristic
| Patients’ characteristics | Number/percentage |
|---|---|
| Number of patients | 6 patients |
| Sex | Females |
| Site | Bilateral palmar hyperhidrosis |
| Age | Range: 20–38 years (23,20,21,22,38,21) |
| Duration of suffering hyperhidrosis | Range: 2–6 years (3,2,5,3,2,3) |
| Affecting factors | Hot weather in 3, stress in 3 patients |
Fig. 4.
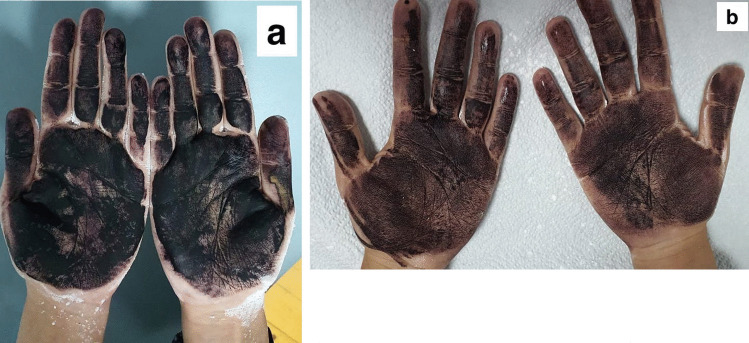
(a) Iodine starch test before treatment showing dark blue color and (b) iodine starch test after treatment showing light blue color
Exclusion criteria included children, pregnant or lactating females.
Treatment protocols
The palms were subjected to 2 passes of fractional CO2 laser (Bison, Italy) with the following parameters; scanning area 3 ~ 20 mm, Y: 3 ~ 20 mm, the fluence 7.5 mJ/cm2, pulse duration 500 µs and dot density 0.8. Afterward, the transfersomal EY gel was applied for 5 min.
The affected sites were then irradiated using an intense pulsed light (IPL, EPI-C PLUS; Espansione Group, Bologna, Italy), as a light source to activate the photosensitizer, fitted with a 550 nm filter that matches the maximum absorption peak of EY (2.5 × 4.5 cm spot area, total area 11.25 cm2, 20 ms pulse duration, and 25 J/cm2 fluence). Sessions were performed once per week, for 6 weeks. The assessment was done by starch iodine test to determine the area of hyperhidrosis before and after treatment. The sweating intensity was determined based on the color intensity of the starch iodine test according to the 5-grades Sweating Intensity Visual Scale [11], where Grade 0 indicates no sweating, grade 1 indicates minimal sweating, grade 2 indicates mild sweating, grade 3 indicates moderate sweating, grade 4indicates intense sweating, and grade 5 indicates over-sweating.
Moreover, patient satisfaction was measured by a scoring system: 0 (unsatisfied), 1 (partially satisfied), and 2 (very satisfied).
Results and discussion
Preparation and characterization of transfersomes loaded by eosin
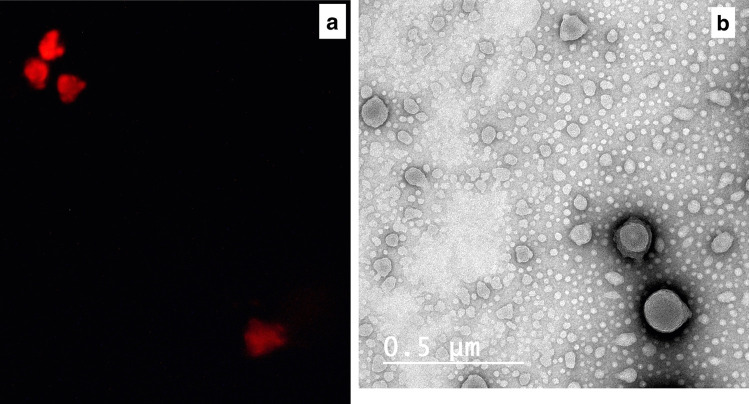
Transfersomal vesicles loaded by EY were spontaneously formulated upon hydration of the lipid film with PBS-EY solution. Being a hydrophilic drug, EY was incorporated in the aqueous core of the transfersomal vesicles, as indicated by the fluorescence micrograph (Fig. 1a). TEM image (Fig. 1b) proved the formation of multilayered spherical vesicles with no aggregation or irregularities.
Fig. 1.
(a) Fluorescence microscope micrograph and (b) TEM image of the prepared eosin-loaded transfersomes
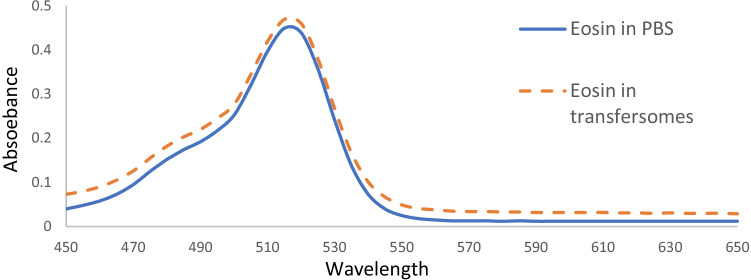
The peaks of the maximum absorption of both PBS-EY and EY loaded in transfersomes were found to be at 520 nm (Fig. 2), indicating that the loading of EY in transfersomes did not affect its spectroscopic properties.
Fig. 2.
Spectroscopic properties of eosin in PBS solution and in the prepared transfersomes
The calculated EE was 33 ± 3.5% which is considered high for a hydrophilic drug as EY.
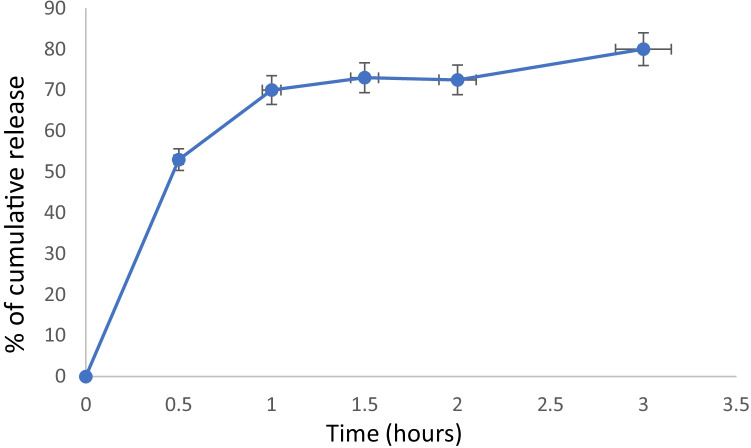
The mean diameter was found to be 305.5 ± 5.7 nm with a polydispersity index (P.I.) of 0.5 indicating homogenous particle size distribution. The prepared transfersomes exhibited an average zeta potential of − 54 ± 7.6 mV indicating good stability. The release profile (Fig. 3) showed that 80 ± 4% of EY was released from the transfersomes after 3 h.
Fig. 3.
In vitro release of eosin from the prepared transfersomes
Transfersomes are lipid vesicles that resemble the liposomes in their structure, but their lipid membrane’s composition is altered by the addition of an edge activator[12].
Previous studies on hyperhidrosis treatment used liposomes, to enhance the drug delivery through palmar skin [11, 15]. In this case study, we used transfersomes as it was proved that transfersomes were superior to liposomes in the dermal and transdermal delivery [16]. The presence of edge activator, as SDC, in their lipid membrane improved the membrane deformability and flexibility, permits the passage of the vesicles between the narrow skin pores, thus delivering the drug to deeper skin layers without being cracked [17].
Clinical cases
The results of our case studies revealed that two patients achieved 90% improvement after four sessions, three patients needed six sessions to show 75% improvement, and one patient showed only 25% improvement after six sessions (Table 2). The improvement was determined based on the sweating intensity visual scale and the patient satisfaction scoring system.
Table 2.
Patient’s outcome
| Degree of improvement | Number of sessions | Relapse |
|---|---|---|
|
3 patients: 75% 2 patients: 90% 1 patient: 25% |
6 sessions 4 sessions 6 sessions |
- - After 1 year |
There was a marked improvement as stated by the patients (80% at the palm and 70% at the fingers, Fig. 4a, b).
The pain experienced throughout the treatment sessions was manageable, and no topical anesthetics were required.
The treatment mechanism of hyperhidrosis by PDT relies on ROS production upon excitation of the PS by light, thus affecting the eccrine glands and reducing their sweat production [10].
PDT using EY as a PS (PDT-EY) was used previously for the treatment of both axillary [10] and palmar hyperhidrosis [11]. The results obtained from those studies revealed that the management of palmar hyperhidrosis was more difficult and took a longer time and higher number of sessions (8 sessions). Moreover, the EY gel was applied for 1 h prior to irradiation, which caused patients dissatisfaction. This is because the palms are characterized by thick skin, thus it is difficult for the topically applied drug to reach the eccrine glands.
However, in this case study, the patients needed a smaller number of sessions (4–6 sessions) with no relapse (Table 2). We applied the EY transfersomal gel for 5 min only, which was more convenient to the patients.
This may be attributed to two reasons; the first is the loading of EY in transfersomes which facilitate the passage and the penetration of EY through the palmar skin as described above. The second reason is the combination between CO2 fractional laser and PDT.
CO2 fractional laser was used as trans-epidermal drug delivery to facilitate the penetration of the PS. It causes ablation of the upper skin layer creating minute vertical channels that extend to the upper dermis. Through these channels, the topically applied drugs can penetrate to deeper epithelial layers [18]. It has been reported that the use of CO2 fractional laser prior to PDT was beneficial in the treatment of actinic keratoses [19, 20], high-risk basal cell carcinomas [21], Bowen disease [22], onychomycosis [23], and actinic cheilitis [24]. However, the combination between CO2 fractional laser and PDT did not impart a significant effect in the treatment of vulvar Paget’s disease [25]. All of the aforementioned studies were done using 5-aminolevulinic acid and its derivative methyl aminolevulinate as a photosensitizer. Up to our knowledge, no previous studies investigated the combined effect of CO2 fractional laser with EY-PDT for the treatment of palmar hyperhidrosis.
Conclusion
Continuous endeavors for the treatment of hyperhidrosis revealed that PDT can be a promising modality of treatment. In this study, we tried to improve the clinical outcome of PDT treatment by two approaches. The first one is the loading of EY (PS) in transfersomes which are deformable vesicles that improve penetration of drugs through skin layers. The second one is the use of a CO2 fractional laser prior to PDT treatment to assist the penetration of the photosensitizer. This resulted in shortening the time of PS application and decreasing the number of sessions required to achieve acceptable improvement. More clinical studies on a larger number of patients are required to optimize the results.
Author contribution
All authors contributed to the study’s conception and design. Transfersomes preparation and characterization were performed by DAAF, MF, and YO. The clinical experiment was performed by AT. All authors contributed equally in writing and reviewing the manuscript. All authors read and approved the final manuscript.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Availability of data and materials
All data can be available.
Declarations
Ethics approval and consent to participate
The study was approved by the board of the research ethics committee of the National Institute of Laser Enhanced Sciences, Cairo University (approval no: CU-NILES/24/21). The study protocol conformed to the guidelines of the Declaration of Helsinki. Written informed consent was obtained from every patient.
Consent for publication
The authors affirm that one of the participants provided informed consent for publication of the images in Fig. 4a, b.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Brackenrich J, Fagg C. Hyperhidrosis. Lewis Gale - Montgomery: StatPearls Publishing, Treasure Island (FL); 2020. [PubMed] [Google Scholar]
- 2.Kamudoni P, Mueller B, Halford J, Schouveller A, Stacey B, Salek MS. The impact of hyperhidrosis on patients’ daily life and quality of life: a qualitative investigation. Health Qual Life Outcomes. 2017;15(1):1–10. doi: 10.1186/s12955-017-0693-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stuart ME, Strite SA, Gillard KK. A systematic evidence-based review of treatments for primary hyperhidrosis. J drug Assess. 2021;10(1):35–50. doi: 10.1080/21556660.2020.1857149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gregoriou S, Sidiropoulou P, Kontochristopoulos G, Rigopoulos D. Management strategies of palmar hyperhidrosis: challenges and solutions. Clin Cosmet Investig Dermatol. 2019;12:733. doi: 10.2147/CCID.S210973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Juarranz Á, Jaén P, Sanz-Rodríguez F, Cuevas J, González S. Photodynamic therapy of cancer. Basic principles and applications. Clin Transl Oncol. 2008;10(3):148–154. doi: 10.1007/s12094-008-0172-2. [DOI] [PubMed] [Google Scholar]
- 6.Alkilani AZ, McCrudden MTC, Donnelly RF. Transdermal drug delivery: innovative pharmaceutical developments based on disruption of the barrier properties of the stratum corneum. Pharmaceutics. 2015;7(4):438–470. doi: 10.3390/pharmaceutics7040438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramadon D, McCrudden MTC, Courtenay AJ, Donnelly RF. Enhancement strategies for transdermal drug delivery systems: current trends and applications. Drug Deliv Transl Res. 2022;12(4):758–791. doi: 10.1007/s13346-021-00909-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fadel M, Kassab K, Fadeel DA, Farag MA. Topical photodynamic therapy of tumor bearing mice with meso-tetrakis (N-methyl-4-pyridyl) porphyrin loaded in ethosomes. Photodiagnosis Photodyn Ther. 2020;30:101789. 10.1016/j.pdpdt.2020.101789. [DOI] [PubMed]
- 9.Shamali N, et al. In vitro photodynamic inactivation (PDI) of pathogenic germs inducing onychomycosis. Photodiagnosis Photodyn Ther. 2018;24(October):358–365. doi: 10.1016/j.pdpdt.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Salah M, Attia A. New topical photodynamic therapy for management of primary axillary hyperhidrosis: a single-blinded, placebo-controlled study. J Egypt Women’s Dermatologic Soc. 2011;8(1):36–42. doi: 10.1097/01.EWX.0000392816.83337.a4. [DOI] [Google Scholar]
- 11.Shabaik AHA, Shaheen MA, Soltan MY. Efficacy of photodynamic therapy for treatment of primary palmar hyperhidrosis. Dermatol Ther. 2021;34(1):1–6. doi: 10.1111/dth.14659. [DOI] [PubMed] [Google Scholar]
- 12.Romero EL, Morilla MJ. Highly deformable and highly fluid vesicles as potential drug delivery systems: theoretical and practical considerations. Int J Nanomedicine. 2013;8:3171. doi: 10.2147/IJN.S33048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Witika BA, et al. Vesicular drug delivery for the treatment of topical disorders: current and future perspectives. J Pharm Pharmacol. 2021;73(11):1427–1441. doi: 10.1093/jpp/rgab082. [DOI] [PubMed] [Google Scholar]
- 14.Ibrahim NA, Fadeel DA, Sadek A, Fadel M, Tawfik A. D3 versus new topical photodynamic therapy in recalcitrant palmoplanter warts randomized comparative controlled study,” Photodiagnosis Photodyn Ther. 2020;32:101979. 10.1016/j.pdpdt.2020.101979. [DOI] [PubMed]
- 15.Ibrahim IM, Abdel Kareem IM, Alghobashy MA. Evaluation of topical liposome incorporated clove oil in the treatment of idiopathic palmar hyperhidrosis: Single‐blinded placebo‐controlled study. Journal of cosmetic dermatology. 2018;17(6):1084–9. 10.1111/jocd.12471. [DOI] [PubMed]
- 16.Kassab K, Fadeel E, Abd D, Fadel M. Topical photodynamic therapy using transfersomal aluminum phthalocyanine tetrasulfonate: in vitro and in vivo study. Lasers Med Sci. 2013;28:5. 10.1007/s10103-012-1256-3. [DOI] [PubMed]
- 17.Fadel M, Kassab K, Samy N, Abdelfadeel D, Yassin G, Nasr M. Nanovesicular photodynamic clinical treatment of resistant plantar warts. Curr Drug Deliv. 2020;17(5):396–405. doi: 10.2174/1567201817666200324142221. [DOI] [PubMed] [Google Scholar]
- 18.Hædersdal M, et al. Enhanced uptake and photoactivation of topical methyl aminolevulinate after fractional CO 2 laser pretreatment. Lasers Surg Med. 2011;43(8):804–813. doi: 10.1002/lsm.21096. [DOI] [PubMed] [Google Scholar]
- 19.Falkenberg C, Schmitz L, Dicke K, Dervenis V, Szeimies RM, Dirschka T. Pretreatment with ablative fractional carbon dioxide laser improves treatment efficacy in a synergistic PDT protocol for actinic keratoses on the head. Photodiagnosis Photodyn Ther. 2021;34:102249. 10.1016/j.pdpdt.2021.102249. [DOI] [PubMed]
- 20.Togsverd-Bo K, Haak CS, Thaysen-Petersen D, Wulf HC, Anderson RR, Haedesdal M. Intensified photodynamic therapy of actinic keratoses with fractional CO2 laser: a randomized clinical trial. Br J Dermatol. 2012;166(6):1262–1269. doi: 10.1111/j.1365-2133.2012.10893.x. [DOI] [PubMed] [Google Scholar]
- 21.Haak CS, et al. Fractional laser-mediated photodynamic therapy of high-risk basal cell carcinomas - a randomized clinical trial. Br J Dermatol. 2015;172(1):215–222. doi: 10.1111/bjd.13166. [DOI] [PubMed] [Google Scholar]
- 22.Kim SK, Park JY, Song HS, Kim YS, Kim YC. Photodynamic therapy with ablative carbon dioxide fractional laser for treating Bowen disease. Ann Dermatol. 2013;25(3):335–339. doi: 10.5021/ad.2013.25.3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Oliveira GB, Antonio JR, Antonio CR, Tomé FA. The association of fractional CO2 laser 10.600nm and photodynamic therapy in the treatment of onychomycosis. An Bras Dermatol. 2015;90(4):468–471. doi: 10.1590/abd1806-4841.20153588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garofalo V, et al. Combination of laser therapy and photodynamic therapy with 5-aminolevulinic acid patch for the treatment of actinic cheilitis. Photobiomodulation, Photomedicine, Laser Surg. 2021;39(4):303–307. doi: 10.1089/photob.2020.4853. [DOI] [PubMed] [Google Scholar]
- 25.Ferrara F, Bardazzi F, Messori S, Abbenante D, Barisani A, Vaccari S. Photodynamic therapy following fractional CO2 laser for treatment of primary vulvar Paget’s disease: does it really work? J Dermatolog Treat. 2019;1–3. 10.1080/09546634.2019.1707755. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data can be available.