Graphical abstract
Keywords: Pitahaya peel, Ultrasound-alkaline extraction, Bound polyphenols, UPLC-QTOF-MS/MS, Antioxidant capacity, Enzyme inhibitory activity
Highlights
-
•
Bound polyphenol was obtained by ultrasound-alkaline extraction from pitahaya.
-
•
A great difference in component content of free polyphenol and bound polyphenol.
-
•
Bound polyphenol showed a better bioactivity than free polyphenol.
-
•
Ultrasound-assisted extraction is crucial to the release of bound polyphenols.
Abstract
In this study, ultrasound-assisted alkaline hydrolysis was used to extract polyphenols from pitahaya peel. The effects of sonication time, ultrasonic density, NaOH concentration and the liquid–material ratio on the total phenolic content (TPC), total flavonoid content (TFC) and antioxidant activity of the extracts were studied. The composition and content difference of the extracts were analyzed and the inhibitory effect of α-amylase and α-glucosidase was measured. The results of single-factor analysis showed that when the sonication time was 45 min, the ultrasonic density was 32 W/L, the NaOH solution concentration was 6 M and the liquid–material ratio was 30 mL/g, the release of phenolic compounds was the largest and the antioxidant activity was the strongest. An UPLC-QTOF-MS/MS method was used to analyze the components and contents of the extracts. We found that there was a great difference in the component content of the free polyphenol extract and the bound polyphenol extract. From the results, we concluded that there was a strong correlation between the type and content of phenolic compounds and antioxidant activities, indicating that phenolic compounds were the main compounds of these biological activities. Moreover, the bound polyphenol extracts showed a significant inhibitory effect on α-amylase and α-glucosidase was stronger than that of the free polyphenol extracts. In addition, scanning electron microscopy showed that ultrasound-assisted extraction is crucial to the destruction of the cell wall and the release of bound polyphenols. Therefore, the pitahaya peel has the potential for therapeutic, nutritional, and functional food applications, and ultrasound-assisted alkaline hydrolysis is an effective means to release phenolic compounds.
1. Introduction
Pitahaya (Hylocereus undatus 'Foo-Lon') is a typical tropical plant, belonging to the family Cactaceae. Pitahaya peel is the main by-product of pitahaya processing, accounting for about 30 %–45 % of the whole fruit. Pitahaya peel is rich in natural pigments, polysaccharides, dietary fiber and phenolic compounds, which is a rich source of natural substances. However, most pitahaya peels are directly thrown away as waste. This is not only a waste of resources but also environmentally damaging [1]. Thus, recycling fruit and vegetable peels is a major strategy to save resources and protect the environment.
Polyphenols are a class of bioactive substances, which are often extracted by organic solvents, although this neglect non-extractable phenolic compounds that bind to the cell wall matrix. This type of non-extractable phenolic compound is also called a bound polyphenol because of its covalent bonds with the cell wall. Many studies have shown that bound polyphenols have a variety of good biological activities, such as antioxidant activity and hypoglycemic activity [2].
Mechanical-assisted technology is the most common physical extraction method in the extraction process. The working principle of mechanical assisted technology is to transfer energy to the food matrix through mechanical waves, break various interactions between phenolic compounds and the food matrix and release phenolic compounds. Ultrasound-assisted technology is one of the most common mechanical auxiliary technologies, which is often used as an auxiliary extraction method for functional active substances. It is well known that the effects produced in the ultrasonic extraction process mainly include the cavitation effect, thermal effect and mechanical effect [3]. These effects can promote the destruction of the cell wall, and the target compounds in the cell can increase the dissolution efficiency by using the mass transfer effect of the cell wall, without causing changes in the structure and function of the target. Among them, the cavitation effect is the most important [4]. This technology uses the cavitation effect to produce instantaneous high temperature and high pressure to damage the cell wall, increase the contact between the solvent and the target compound and promote the dissolution of the target compound. These effects present in ultrasonic technology not only increase the yield of the target compounds but also reduce the solvent, save energy and protect the environment [5]. The extraction effect of polyphenol compounds from brewer's spent grain by ultrasound-assisted technology and mechanical stirring was compared, and it was found that for the ground brewer's spent grain, it took 24 h to achieve complete extraction by mechanical stirring, but only 1200–1800 s by ultrasound-assisted technology [6]. Coincidentally, Wu et al. explored the optimal conditions for ultrasound-assisted extraction of polyphenols from pomegranate flowers, in which the ultrasonic density was 30 W/L and the sonication time was only 10 min [7]. Therefore, ultrasound-assisted technology is a promising potential method as it is a green, simple and rapid process for extracting bioactive compounds.
Acid hydrolysis, alkaline hydrolysis and enzymatic hydrolysis are common methods for extracting bound polyphenols. Polyphenols can be released because acid hydrolysis can break glycosidic bonds [8]. However, strong acid solution and high temperature treatment will cause the degradation of some phenolic compounds, especially flavonoids [9]. Alkaline hydrolysis can hydrolyze the ester bond and ether bond between the phenolic compounds and the cell wall, to release phenolic compounds more effectively [10]. Enzymatic hydrolysis has mild reaction conditions and high specificity, which can effectively release phenolic substances without destroying their structure. It is often used to improve the bioavailability of phenolic compounds in agricultural by-products [11]. But the cost of enzyme reagents is high and they are difficult to preserve. In contrast, the advantages of alkaline hydrolysis are highlighted. It was found that the alkaline hydrolysis method is better than the enzymatic hydrolysis method and acid hydrolysis method for the bound polyphenols in jackfruit pulp [12]. Therefore, alkaline hydrolysis has been widely used in the release of bound polyphenols from food substrates such as grains, beans and seeds [8].
It was found that ultrasound-assisted alkaline hydrolysis extraction method was more effective in releasing bound polyphenols than alkaline hydrolysis and ultrasound-assisted technology alone [13]. Therefore, in this study, bound polyphenols from pitahaya peel were extracted by ultrasound-assisted alkaline hydrolysis. The effects of different factors (sonication time, ultrasonic density, NaOH concentration and liquid–material ratio) on the antioxidant effect of bound polyphenols were observed and their hypoglycemic activity was explored. The effects of the components and contents of the extract on the antioxidant activity and enzyme inhibitory activity were analyzed.
2. Materials and methods
2.1. Chemicals and reagents
2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid (ABTS), 1,1-diphenyl-2-picrylhydrazyl (DPPH), 2,4,6-tri(pyridin-2-yl)-1,3,5-triazine (TPTZ), 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), ammonium acetate (NH4AC) and ammonium hydroxide (NH4OH) were purchased from Sigma Aldrich. 3,5-dinitrosalicylic acid (DNS), α-amylase (1500 U/g) and α-glucosidase (50,000 U/g) were obtained from Shanghai Yuanye Bio-Technology Co. ltd. Acetonitrile and 4-nitrophenyl α-d-glucopyranoside (p-NPG) were supplied by Merck. All other chemicals and solvents used in this study were analytical grade.
2.2. Plant materials collection and preparation
2.2.1. Pretreatment of pitahaya peel
Pitahaya fruits were purchased from a local supermarket (Dongguan, China) without mildew and deterioration. Pitahaya peel was separated from the fruit and then freeze-dried. Pitahaya peel was stored at −20 °C after crushing into powder.
2.2.2. Removal of free polyphenols
The method proposed by Gonzales et al. [14] was slightly modified. Briefly, approximately 6 g of the dried pitahaya peel power and 45 mL of methanol were added to a 100 mL tube. The material was homogenized at 10,000 rpm by a high-speed disperser for 1 min. The mixture was centrifuged at 5000 rpm for 10 min after placing on ice for 1 min. The above steps were repeated with 80 % methanol to extract free polyphenols again. The supernatant was collected and diluted to 100 mL with methanol. The residue was vacuum-dried and stored at −20 °C until further analysis.
2.3. Extraction of bound polyphenols
Ultrasonic combined with alkaline hydrolysis was used to extract bound polyphenols from pitahaya peel in this study. Briefly, the dried residue was hydrolyzed with NaOH solution at 60 °C and then was put into an ultrasonic bath for ultrasonic treatment. Four factors were considered in the above experiments, including sonication time (15, 30, 45, 60 and 75 min), ultrasonic density (24, 28, 32, 36 and 40 W/L), NaOH concentration (2, 4, 6, 8 and 10 M) and liquid–material ratio (10, 20, 30, 40 and 50 mL/g).
Subsequently, the mixture was extracted with an equal volume of ethyl acetate after adjusting the pH range to 1–3 using 12 M HCl. The mixture was centrifuged at 4000 rpm for 10 min after stirring at 1000 rpm for 10 min. The above steps were repeated three times. All the supernatants were concentrated with a rotary evaporator at 40 °C. MeOH (4 mL) was added to redissolve the extract and the extract was stored at −20 °C until analysis.
2.4. Analyses of total phenolic and total flavonoid content
The Folin–Ciocalteau method was used to determine the bound polyphenol content from pitahaya peel [12]. The bound polyphenol extracts (100 µL) were oxidized with 10 % Folin–Ciocalteau reagent (500 µL) for 10 min at room temperature. Sodium carbonate (7.5 %, 500 µL) was added to the mixture. After incubation at room temperature for 60 min, the absorbance of the mixture was measured at 765 nm. Thus, the standard curve (R2 = 0.99) was drawn with gallic acid (0–300 µg/mL) as the standard and the results were expressed as mg of gallic acid equivalents/g dry weight (mg GAE/g DW).
The aluminum nitrate colorimetric method was slightly modified to detect the total flavonoid content (TFC) according to Cao et al. [15]. The extracts (500 µL) were mixed with 5 % NaNO2 solution (w/v, 30 µL) for 5 min. Then, 10 % Al(NO3)3 solution (30 µL) was added to the mixture for 6 min. Finally, NaOH solution (1 M, 400 µL) and 75 % ethanol (40 µL) were successively added to the reaction solution. Before taking the absorbance at 510 nm, the reaction solution was placed at room temperature for 10 min. the standard curve (R2 = 0.99) was drawn with rutin (0–400 µg/mL) as the standard and the results were expressed as mg of rutin equivalents/g dry weight (mg RE/g DW).
2.5. Antioxidant capacities
2.5.1. ABTS radical scavenging capacity
ABTS radical scavenging capacity of the extracts was evaluated according to study provided by Re et al. [16] and appropriately modified. Briefly, ABTS stock solution (7.4 mmol/L) and potassium persulfate stock solution (2.6 mmol/L) were mixed in equal quantities and left in the dark for 12–16 h before use. The ABTS radical solution was diluted with water to an absorbance of 0.70 ± 0.02 at 734 nm and 1 mL solution was withdrawn to mix with the extracts (20 µL). The absorbance of the mixture was measured at 734 nm after being stored in the dark for 5 min. The results were expressed as μmol of Trolox equivalents/g dry weight (μmol TE/g DW).
2.5.2. DPPH radical scavenging capacity
DPPH radical scavenging assay of the extract was detected with slight adjustment to the method of Dong et al. [17]. The bound polyphenol extracts and 12 mg/mL DPPH solution were mixed in a ratio of 1:9.5, and left in the dark for 30 min. The absorbance of the mixture was measured at 517 nm and the results were expressed as μmol Trolox equivalents/g dry weight (μmol TE/g DW).
2.5.3. Ferric reducing antioxidant power (FRAP)
This assay was performed with minor modifications following the method of Benzie et al. [18]. The FRAP reagent was a mixture of 10 mmol/L TPTZ solution in 40 mmol/L HCl, 20 mmol/L iron (III) chloride solution and 300 mmol/L sodium acetate buffer (pH 3.6) in the volume ratio of 1:1:10. The extract (50 µL) was mixed with the FRAP reagent (1 mL). After 60 min, the absorbance of the mixture was determined at 593 nm. The results were expressed as mmol of Fe2+ equivalents/g dry weight (mmol Fe2+/g DW).
2.6. UPLC-QTOF-MS/MS analysis
Ultra-performance liquid chromatography (UPLC, 1290 Infinity LC, Agilent Technologies) coupled to a quadrupole time-of-flight (AB Sciex TripleTOF 6600) was used to elute the extract components. The samples were separated by UPLC on a C18 column. The column temperature was 40 °C; the flow rate was set at 0.4 mL/min; and the injection volume was 2 μL. Mobile phase A consisted of 25 mM ammonium acetate and 0.5 % formic acid in water, mobile phase B was methanol. The gradient elution procedure was as follows: 0–0.5 min, 5 % B; then B changed to 100 % linearly from 0.5 to 10 min; 10–12.0 min, B was maintained at 100 %; From 12.0 to 12.1 min, B changed linearly from 100 % to 5 %; 12.1–16 min, B was maintained at 5 %. During the whole analysis, the sample was placed in an automatic sampler at 4 °C.
The electrospray ionization (ESI) source conditions were set as follows: curtain gas as 30 psi, source temperature: 600 °C, IonSpray Voltage Floating (ISVF) ± 5500 V. Only in mass spectrometry (MS) acquisition, the instrument was set to acquire over the m/z range 60–1000 Da and in auto tandem mass spectrometry (MS/MS) acquisition, the instrument was set to acquire over the m/z range 25–1000 Da. The product ion scan was acquired using information dependent acquisition (IDA) with high sensitivity mode selected.
2.7. Inhibition of α-amylase and α-glucosidase activities
2.7.1. α-Amylase inhibitory activity
The α-amylase inhibition experimental method was revised slightly from that in the literature [19]. Bound polyphenol extracts were dissolved in 0.02 mol/L phosphate buffer (pH 6.9) and stored at 4 °C until analysis. The extracts were diluted to different concentrations (0–25 mg/mL). Different concentration extracts (400 µL) were mixed with 0.1 U/mL α-amylase solution (50 µL) respectively at 37 °C for 10 min. 1 % Soluble starch solution (1 %, 100 µL) was added to the mixture for 6 min. Subsequently, the reaction was terminated by DNS solution (500 µL). The reaction tubes were heated in a boiling water bath for 10 min and then cooled to room temperature. The reaction solutions were diluted with 1 mL water and determined at 540 nm.
2.7.2. α-Glucosidase inhibitory activity
The method of α-glucosidase inhibition activity assay was measured with a slight change from that of Zheng et al. [20]. Initially, 1 U/mL α-glucosidase solution (50 µL) was mixed with inhibitor (50 µL) at 37 °C for 10 min. PNPG solution (10 mmol/L, 100 µL) was added to the mixture for 6 min. Finally, 1 mol/L sodium carbonate solution (500 µL) was used to terminate the reaction and the mixed solutions were detected at 400 nm.
2.8. Surface microstructure of residues
To compare the scanning electron microscopy (SEM) images of the residues, a desk-type scanning electron microscope was utilized at 1000 and 5000 magnifications. In advance, the insoluble solid residues after extraction treatment were separated and freeze-dried. The freeze-dried residues were paved on the sample table with conductive adhesive, the powder not fixed on the conductive adhesive was blown off with washing ear ball, and the samples were sprayed with gold for one minute before observation.
2.9. Statistical analysis
All tests mentioned above were performed in triplicate and repeated at least three times. The data were expressed as the mean ± SD, and p-values<0.05 were considered statistically significant. After normalizing the processed data to the total peak intensity, the R package was used for correlation analysis.
3. Results and discussion
3.1. Effect of ultrasound on the total phenolic and total flavonoid content in the extracts
The single-factor experiment clarified the influence of each factor on the extraction results. Among the parameters related to the ultrasonic procedure, the factors affecting the extraction efficiency of the ultrasonic technology include ultrasonic density, sonication frequency, sonication amplitude and sonication time [21]. The sonication time and ultrasonic density are discussed in this study.
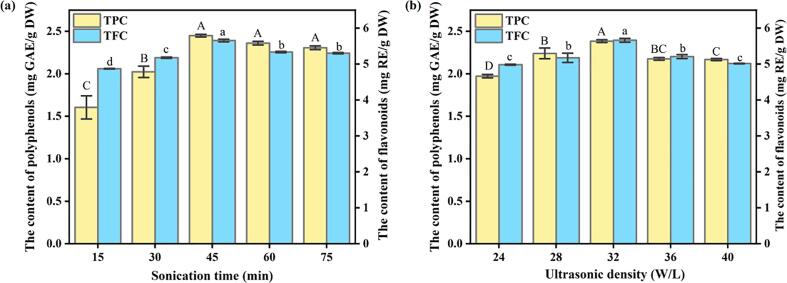
Firstly, Fig. 1a shows the trend for the total phenolic and total flavonoid contents of pitahaya peel extracts under different sonication times. Sonication time ranged from 15 min to 45 min, resulting in increased extract yield. The extract yield reached the maximum at 45 min. However, when the sonication time was further prolonged, the total phenolic content (TPC) of the extract did not fluctuate significantly, while the total flavonoid content (TFC) decreased slightly. This could be attributed to the mechanical shearing effect of ultrasound. This effect could destroy the cell wall, promote the dissolution of active components in the cell, and improve the extraction rate of the target compounds. However, too strong a mechanical effect may lead to the degradation and destruction of the polyphenol structure and the reduction of active ingredients [22]. This phenomenon also exists in the process of extracting total phenols from pomegranate flowers [7] and total flavonoids from asparagus tender stems [15].
Fig. 1.
(a) Effect of different sonication times on the extraction rate of TPC and TFC; (b) Effect of different ultrasonic density on the extraction rate of TPC and TFC.
There are also great differences in the release of phenolic substances with different ultrasonic density as shown in Fig. 1b. When the ultrasonic density reached 32 W/L, the released amount of the target substances reached the maximum output. At this time, a negative pressure is generated in the liquid due to the action of ultrasound, forming many small bubbles. With an increase in the ultrasonic density, the sonication energy also increases. When the energy reaches the threshold value, the small bubbles in the liquid collapse rapidly, generating instantaneous high temperature and high pressure, destroying plant cells and thus promoting the dissolution of the target substances. This effect is known as the “cavitation effect”. When the ultrasonic density was < 32 W/L, the cavitation effect was weak, the target could not be completely dissolved and the extraction rate was low. When the ultrasonic density exceeded 32 W/L, the extraction rate decreased. This may be attributed to the excessive cavitation effect caused by the extremely high power, and the instantaneous high temperature and pressure lead to the reduction in the activity of the target substance and the decrease in the release of the extract. Similarly, in the extraction of total phenols from pomegranate flowers, with the increase of ultrasonic intensity within a certain range, the content of total phenols increased from 130 mg GAE/g to 240 mg GAE/g [7].
The factors affecting the extraction efficiency of ultrasonic technology also include solvent type, solvent concentration and solvent usage. In this study, the NaOH solution concentration and the liquid-to-material ratio also have a certain impact on the results.
The influence of NaOH solution concentration on the antioxidant activity of the extract can be seen in Fig. S1a. The extraction yield increased at the NaOH concentration from 2 M to 6 M and the best extraction effect was achieved at a concentration of 6 M. It may be because the higher concentration of NaOH solution can more quickly and efficiently break the ester bond and ether bond between the target extract and the cell wall matrix. When the NaOH solution concentration exceeded 6 M, the TPC of the extract decreased slightly, while the total flavonoid content fluctuated within the error margin, and the extraction quantity became stable. In cauliflower waste, it was also found that the polyphenol release amount of 2 M and 4 M NaOH solution was significantly higher than that of 1 M NaOH solution [13].
The influence of liquid–material ratio is shown in Fig. S1b. The ratio of liquid to material significantly affects the extraction efficiency. With the gradual increase in the liquid–material ratio, the release of phenolic compounds showed an upward trend. The reason is that with an increase in solvent usage, the contact area between the sample powder and the solvent increases, so that the target substance forms a larger concentration difference inside and outside the cell, thereby contributing to the dissolution of the target substances. However, when the liquid–material ratio exceeded 30 mL/g, the contribution of this factor to the extraction effect could be almost negligible. At this time, the extraction rate of the target substance did not continue to rise after reaching a certain liquid–material ratio and further increasing the solvent usage would lead to waste. The extraction of total flavonoids from the tender stems of asparagus also showed a similar result, when the ratio of liquid to material increased from 10 mL/g to 25 mL/g, the total flavonoid content increased by about 30 % [15].
Based on the single-factor experiment, we could conclude that the TPC and TFC of the extract reached a maximum under the conditions of 45 min sonication time, ultrasonic density 32 W/L, 6 M NaOH concentration and 30 mL/g liquid–material ratio. Under this condition, the TPC is in the range of 2.38–2.45 mg GAE/g DW, and the total flavonoid content is in the range of 5.65–5.72 mg RE/g DW. It is worth noting that the TPC (2.14 mg GAE/g DW) and total flavonoid content (2.72 mg RE/g DW) of the free polyphenol extracts were less than the corresponding values of the extract under the above optimal conditions.
3.2. Effect of ultrasound on the antioxidant activity of the extracts
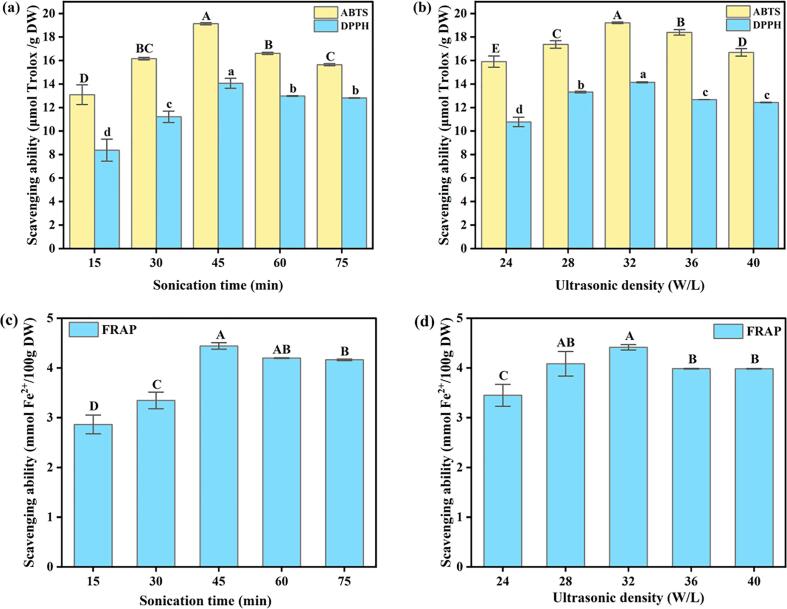
Due to the differences in the types and contents of the different phenolic compounds, their ability to exert a certain biological activity also differs. The antioxidant performance of the target substances cannot be objectively evaluated by using a single test method, but it is more scientific to use a variety of experimental methods for comprehensive evaluation [23], [24]. Therefore, the antioxidant activity of pitahaya peel bound polyphenol extracts were comprehensively evaluated by the ABTS and DPPH radical scavenging ability tests and the ferric reducing antioxidant power assay.
Ultrasonic treatment had a great effect on the ABTS radical scavenging activity of the extract. The effect of sonication time on the ABTS radical scavenging activity of the extracts is shown in Fig. 2a. With the extension of sonication time, the ABTS radical scavenging activity of the extract firstly increased, reached the maximum at 45 min, and then decreased. This may be because the shearing effect of ultrasound promotes the dissolution of the active substance and enhances the ABTS radical scavenging ability of the extract, while the extremely strong shearing effect will cause the destruction of the active substance structure and a decrease in the ABTS radical scavenging ability of the extract. As shown in Fig. 2b, the ultrasonic density also had a significant effect on the ABTS radical scavenging activity of the extract. When the ultrasonic density was 32 W/L, the ABTS radical scavenging activity of the extract reached a maximum. However, when the ultrasonic density was < 32 W/L or greater than 32 W/L, the ABTS radical scavenging activity of the extract decreased due to insufficient or excessive ultrasonic cavitation effect. Besides, the alkaline solution concentration and the liquid-to-material ratio also had a certain impact on the results, as shown in Figure S2. The ABTS radical scavenging activity of the extract was significantly increased when the NaOH solution concentration was between 2 M and 6 M. When the NaOH solution concentration was 6 M, the ABTS radical scavenging capacity reached the maximum. The ABTS radical scavenging activity decreased slightly with increasing solution concentration. When the liquid–material ratio was < 30 mL/g, the sample was not fully contacted with the solvent and the dissolution of the target substance was insufficient. When the liquid–material ratio was 30 mL/g, the target substance was fully extracted and the ABTS radical scavenging activity of the extract was optimized. However, the ABTS radical scavenging activity of the extracts decreased with a continuous increase in the liquid-to-material ratio. At the same time, this would also lead to a waste of resources. Therefore, sonication time, ultrasonic density, solvent concentration and liquid-material ratio all had significant effects on the extraction efficiency of the ultrasound-assisted technology. The optimal extraction conditions for the ABTS radical scavenging activity of the extract are consistent with the release of the TPC and TFC, that is, when the sonication time was 45 min, the ultrasonic density was 32 W/L, the NaOH solution concentration was 6 M, and the liquid–material ratio was 30 mL/g.
Fig. 2.
The effect of different (a) sonication times and (b) ultrasonic density on scavenging activity of ABTS and DPPH radicals, and the effect of different (c) sonication times and (d) ultrasonic density on the ferric reducing antioxidant ability.
The trend and conclusion in the determination of DPPH radical scavenging capacity and ferric reducing antioxidant power were approximately the same as those in the above ABTS radical scavenging activity determination. It was worth noting that under the optimal conditions, the scavenging ability of the extract on the ABTS radical (18.83–19.22 μmol TE/g DW) was greater than that of the DPPH radical (13.93–14.14 μmol TE/g DW). It indicated that the bound polyphenol extract had a stronger scavenging ability on the ABTS radical than on the DPPH radical. In addition, the variation trend on the antioxidant activity for the extract was similar to its TPC and TFC, because the content of bound polyphenol was positively correlated with the antioxidant activity [25]. Moreover, the ABTS radical scavenging ability (9.78 μmol TE/g DW), DPPH radical scavenging capacity (6.23 μmol TE/g DW) and ferric reducing antioxidant power (2.01 mmol Fe2+/g DW) of the free polyphenol extract were lower than the bound polyphenol extract under the optimal conditions (4.36–4.44 mmol Fe2+/g DW) (Fig. 2c-d and S3c-d).
Higher equivalent values of Trolox and Fe2+ correspond to stronger ABTS and DPPH radical scavenging ability and ferric reducing antioxidant power, and thus stronger antioxidant activity. The above results showed that under 45 min sonication conditions, 32 W/L ultrasonic density, 6 M NaOH solution concentration and liquid–material ratio of 30 mL/g, the equivalent value of the compound is the highest, corresponding to the strongest antioxidant capacity. Therefore, the bound polyphenol extract obtained under this condition was selected for enzyme inhibition test.
3.3. Component analysis of pitahaya peel extract
An UHPLC-Q-TOF MS method was used to identify and analyze the components in different extracts. Both the free polyphenol and bound polyphenol extracts were shown to contain a large number of organic compounds, which could be roughly divided into benzenoids, organic acids and derivatives and phenylpropanoids and polyketides three superclasses (Table 1 and S1). There were many phenolic compounds in the three superclasses and here we focus on phenolic compounds.
Table 1.
Identification of the compositions of pitahaya peel extracts.
| ID | Compounds | Formula | m/z | Superclass | Class | Log2(BP/FP) |
|---|---|---|---|---|---|---|
| 1 | 7-[3,5-Dihydroxy-4-(4-hydroxyphenyl)-2-methoxyphenyl]-3-methoxy-3,4-dihydrooxepine-2,5-dione | C20H18O8 | 385.092 | Benzenoids | Benzene and substituted derivatives | 8.24 |
| 2 | Daphnetin | C9H6O4 | 179.032 | Phenylpropanoids and polyketides | Coumarins and derivatives | 6.91 |
| 3 | Orsellinic acid | C8H8O4 | 167.035 | Benzenoids | Benzene and substituted derivatives | 6.38 |
| 4 | 3-(4-Hydroxy-3-methoxyphenyl)-2-propenoic acid | C10H10O4 | 193.049 | Phenylpropanoids and polyketides | Cinnamic acids and derivatives | 6.32 |
| 5 | Sinapoyl malate | C15H16O9 | 207.064 | Phenylpropanoids and polyketides | Cinnamic acids and derivatives | 5.75 |
| 6 | Homogentisic acid | C8H8O4 | 167.033 | Benzenoids | Benzene and substituted derivatives | 5.63 |
| 7 | Phenylpyruvic acid | C9H8O3 | 163.040 | Benzenoids | Benzene and substituted derivatives | 5.61 |
| 8 | 4,6,7-Trihydroxy-5-methoxy-1,8,8,9-tetramethyl-8,9-dihydro-3H-phenaleno[1,2-b]furan-3-one | C20H20O6 | 355.116 | Benzenoids | Phenalenes | 5.49 |
| 9 | Sinapic acid | C11H12O5 | 223.061 | Phenylpropanoids and polyketides | Cinnamic acids and derivatives | 5.25 |
| 10 | 4-Hydroxyphenyl acetate | C8H8O3 | 151.040 | Benzenoids | Phenol esters | 5.15 |
| 11 | 4-Vinylphenol | C8H8O | 119.051 | Benzenoids | Benzene and substituted derivatives | 5.02 |
| 12 | Ferulic acid | C10H10O4 | 195.063 | Phenylpropanoids and polyketides | Cinnamic acids and derivatives | 4.99 |
| 13 | Medicarpin | C16H14O4 | 269.082 | Phenylpropanoids and polyketides | Isoflavonoids | 4.86 |
| 14 | 3-(4-Hydroxyphenyl)-2-propenoic acid | C9H8O3 | 163.040 | Phenylpropanoids and polyketides | Cinnamic acids and derivatives | 4.78 |
| 15 | Drofenine | C20H31NO2 | 318.240 | Benzenoids | Benzene and substituted derivatives | 4.56 |
| 16 | Eupatilin | C18H16O7 | 343.082 | Phenylpropanoids and polyketides | Flavonoids | 4.49 |
| 17 | 3,4-Dihydroxybenzoic acid | C7H6O4 | 153.020 | Benzenoids | Benzene and substituted derivatives | 4.47 |
| 18 | [3,4,5-Trihydroxy-6-(hydroxymethyl)oxan-2-yl] 3-hydroxy-4,5-dimethoxybenzoate | C15H20O10 | 359.096 | Phenylpropanoids and polyketides | Tannins | 4.42 |
| 19 | (E)-1-(3,4-Dihydroxy-2,6-dimethoxyphenyl)-3-phenylprop-2-en-1-one | C17H16O5 | 299.091 | Phenylpropanoids and polyketides | Linear 1,3-diarylpropanoids | 4.35 |
| 20 | 3-Hydroxysebacic acid | C10H18O5 | 217.108 | Organic acids and derivatives | Hydroxy acids and derivatives | 4.28 |
Note:BP, bound polyphenol extract; FP, free polyphenol extract; Log2 (BP/FP), a logarithmic value based on 2 for fold change (FC) of component content in the bound polyphenol and free polyphenol extracts.
7-[3,5-Dihydroxy-4-(4-hydroxyphenyl)-2-methoxyphenyl]-3-methoxy-3,4-dihydrooxepine-2,5-dione is a complex high molecular weight phenolic compound with the largest content difference between the two extracts. Followed by daphnetin (7,8-dihydroxycoumarin), whose content in the bound polyphenol extract was about 120 times more abundant than that in the free polyphenol extract. Daphnetin is a typical coumarin derivative with various biological activities, such as anticancer, anti-inflammatory, antibacterial and anti-arthritic [26]. Some studies have found that the antioxidant activity of daphnetin is closely related to the catechol group [27]. In addition, it has been reported that daphnetin can improve lung tissue damage [28] and kidney injury [29] by activating or inhibiting the corresponding signaling pathway and regulating cell apoptosis.
Orsellinic acid is a phenolic acid compound with the largest content difference between the two extracts, which has certain antioxidant activity. Moreover, there are also significant differences in the contents of some common phenolic acids in the two extracts, such as sinapic acid, ferulic acid and 3,4-dihydroxybenzoic acid (protocatechuic acid). Sinapic acid and ferulic acid are the most abundant hydroxycinnamic acid compounds in nature, including p-coumaric acid and caffeic acid [30]. These compounds exist mainly through ester or ether bonds in combination with other components, such as quinic acid, tartaric acid or carbohydrate derivatives [31]. It has been proven that sinapic acid and ferulic acid can achieve antioxidant effects by scavenging excess free radicals [32], [33]. Both are potent antioxidants, but sinapic acid has been considered to be more effective than ferulic acid [34]. 3,4-Dihydroxybenzoic acid belongs to the hydroxybenzoic acid compounds, which are effective antioxidants and anti-hyperglycemic agents [35].
Medicarpin was found to be the flavonoid with the largest content difference between the two extracts. Medicarpin can be used as a chemopreventive or sensitizer related to cancer [36]. The contents of isorhamnetin, kaempferol and quercetin in the two extracts were also significantly different. These three flavonoids usually exist in herbal medicines and have good anti-inflammatory, analgesic and antitussive activities [37]. Isorhamnetin, kaempferol and quercetin also appear to be the main active ingredients in traditional Chinese medicines for the management COVID-19 [38].
The content difference of common phenolic compounds, such as catechol and vanillin, was more than 10 times between the two extracts. These results indicate that there is a big difference in the content of components between the free polyphenol extract and the combined polyphenol extract, which is also the main reason for the difference in antioxidant activity of the extracts.
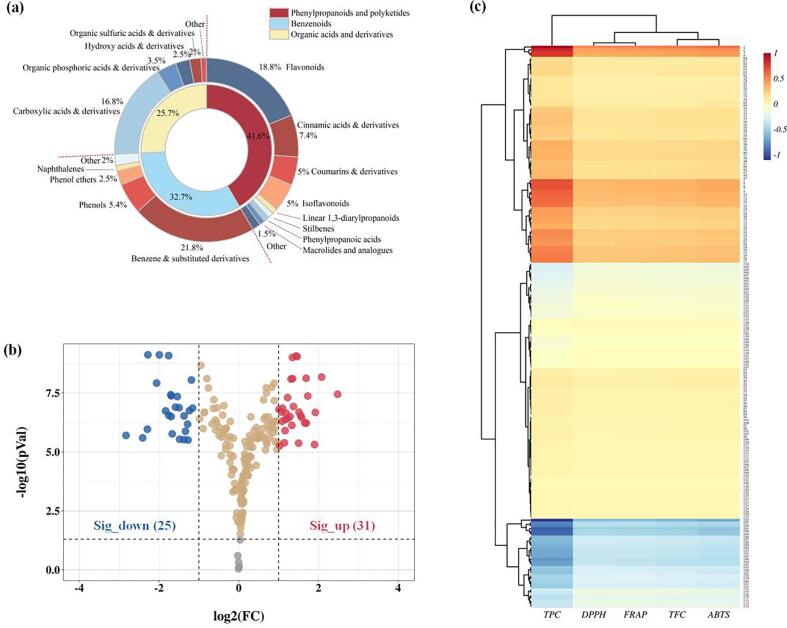
3.4. Correlation between phenolic compounds and antioxidant activity
Fig. 3a shows the main types of bound polyphenol extracts, including phenylpropanoids and polyketides, benzenoids and organic acids and derivatives, accounting for 41.6 %, 32.7 % and 25.7 %, respectively. Among them, phenolic compounds, such as flavonoids accounted for 18.8 %, isoflavonoids 5 %, simple phenols 5.4 %, stilbenes and phenylpropanoid acids 1 %. In addition, cinnamic acids, coumarins, benzene, phenol ethers and their derivatives, in full or in part, also belong to phenolic compounds. The components in the sample of free polyphenols and bound polyphenols mentioned above were visualized using a volcano plot (Fig. 3b). In the volcano plot, each point represents a product. Through comparison, 56 products had statistical significance in the separation of the two samples. Among them, 31 products were found to be upregulated (red points), and 25 products were found to be downregulated (blue points). The upregulated fraction may be the reason for improved antioxidant or enzyme inhibition.
Fig. 3.
(a) The main types of bound polyphenol extracts. (b) Volcano plot of component comparison of free polyphenol and bound polyphenol extracts. (c) Correlation heat map of components with TPC, TFC and antioxidant activity. And the numbers represent the ID number of the component in the extracts, corresponding to Table 1 and S1.
The correlation between the composition content and the antioxidant activity in the extract is shown in Fig. 3c. Fig. 3c shows that the TPC, TFC, ABTS and DPPH radical scavenging activity and ferric reducing antioxidant power of pitahaya peel extract were significantly related to the components and their contents.
7-[3,5-dihydroxy-4-(4-hydroxyphenyl)-2-methoxyphenyl]-3-methoxy-3,4-dihydrooxepine-2,5-dione, daphnetin, orsellinic acid, 3-(4-hydroxy-3-methoxyphenyl)-2-propenoic acid and sinapoyl malate, whose content and antioxidant activity had the most significant positive correlation. They were all phenolic compounds, which preliminarily indicated that phenolic compounds were the main contributors to the antioxidant activity of pitahaya peel extract. In addition, the content of phenolic compounds such as homogentisic acid, sinapic acid, ferulic acid, medicarpin, 3-(4-hydroxyphenyl)-2-propanoic acid and eupatilin also had a strong correlation with the antioxidant activity of the extract. However, the content of some phenolic compounds showed a negative correlation with the antioxidant activity of the extract, such as orientanol E, xanthorhamnin, psoralidin, rhamnetin 3-sophoroside and procyanidin B2. This indicated that these substances did not show a contribution to the antioxidant activity. It has been reported that the antioxidant activity of phenolic compounds is attributed to their structure, especially the number and positions of hydroxyl groups and the nature of substitutions on the aromatic rings [39]. Notably, phenolic compounds can interact with each other, thereby affecting the total antioxidant capacity of the mixture [40], [41]. In fact, the interactions between phytochemicals can be additive, synergistic or antagonistic in terms of antioxidant activity. The antagonistic effect between phenolic compounds may be aggravated by the influence of concentration, ambient temperature and the interaction between different substances [42]. Therefore, the total antioxidant activity of the extract mixture system in this study is not only affected by the structure of phenolic compounds but also by the interactions between phenolic compounds and between phenolic compounds and other compounds.
These results emphasize the correlation between the composition and content of phenolic compounds from pitahaya peel and the antioxidant activity, and they contribute greatly to the antioxidant activity of polyphenolic extract analyzed in this study due to the structure of phenolic compounds and their additive and (or) synergistic effects. Therefore, ultrasound-assisted alkaline hydrolysis is an effective way to release polyphenols.
3.5. The inhibitory effect on α-amylase and α-glucosidase of the extracts
Dietary polyphenol can pass through the insulin dependent approaches (such as the protection and proliferation of the pancreatic islet β-cell) and insulin independent approaches (such as inhibition of digestive enzymes and glucose absorption) to reduce blood glucose and improve diabetes and its complications [43]. In the present study, the hypoglycemic activity of the extract was determined by the enzyme inhibition test.
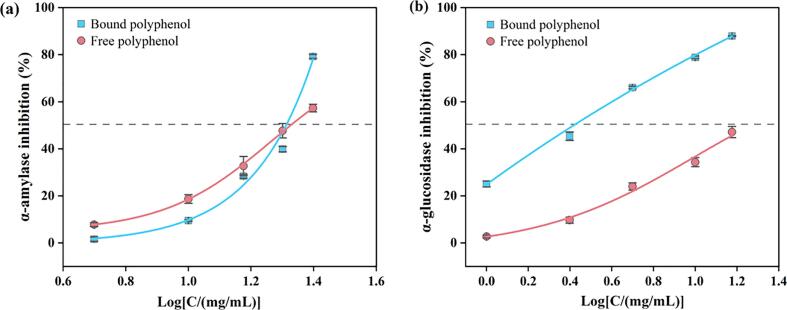
Fig. 4a shows the inhibitory effect of bound polyphenol extract on α-amylase and α-glucosidase. With an increase in the concentration of the extract, the inhibitory effect of the extract on the two enzymes increased. When the extract concentration was 25 mg/mL, the α-amylase inhibition rate was 79.23 %. For α-glucosidase, the inhibition rate could reach 87.91 % at an extract concentration of 15 mg/mL. The IC50 values of the extracts against α-amylase and α-glucosidase were 19.95 mg/mL and 2.83 mg/mL, respectively. The inhibition effect of the two enzymes could not be compared due to the inconsistent enzyme dosage. Notably, the bound polyphenol extract has inhibitory activity on both α-amylase and α-glucosidase, and this effect was better than that of the free polyphenol extract. Since the enzyme inhibitory activity of the extract is expressed by the IC50 value, a smaller IC50 value corresponds to a stronger enzyme inhibitory activity, which also has a stronger hypoglycemic activity. The results showed that the IC50 of the bound polyphenol extract was smaller than that of the free polyphenol extract, so the hypoglycemic activity of the bound polyphenol extract was stronger than that of the free polyphenol extract. This is consistent with the results of the release of phenolic compounds and the determination of antioxidant activity. It seems to be attributed to the direct relationship between antioxidant, TPC and anti-diabetes activity [44], [45].
Fig. 4.
Inhibition rate of the bound polyphenol extract and the free polyphenol extract from pitahaya peel against (a) α-amylase inhibition activity and (b) α-glucosidase inhibition activity.
3.6. Effect of ultrasound-assisted alkaline hydrolysis on the surface microstructure of residues
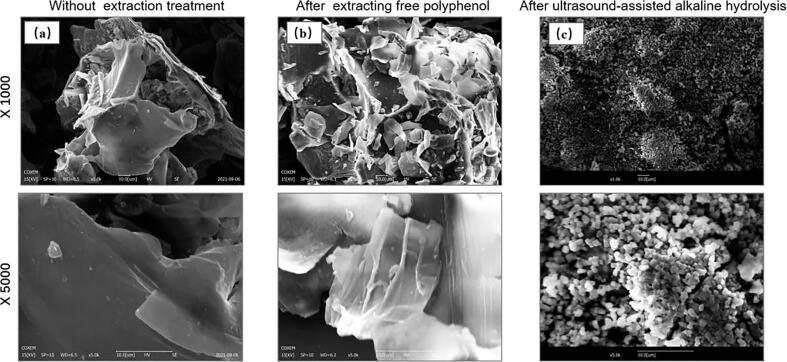
The surface structure of pitahaya peel without any extraction treatment, after extraction of free polyphenols and after ultrasound-assisted alkaline hydrolysis can be observed by SEM.
The microstructure of pitahaya peel without any extraction treatment was compact, flat and without cracks (Fig. 5a). The surface of the residue after extraction of free polyphenols was brittle, rough and uneven, and obvious cracks began to appear (Fig. 5b). This indicates that mechanical-assisted techniques can disrupt the dense fibrous network structure of the cell wall. Therefore, the organic solvent can extract the internal substances of the cell. After ultrasound-assisted alkaline hydrolysis extraction, the surface structure of the residue changed significantly, and a porous honeycomb structure appeared on the surface (Fig. 5c). The reason is that ultrasound-assisted technology aggravates the damage of the cell wall. In the ultrasonic treatment process, the cavitation effect, mechanical effect and thermal effect of the ultrasonic wave act on the plant cell wall together, causing the destruction and collapse of the network structure of the cell wall, increasing the contact area between the solvent and the residue, thus promoting the release of bound substances.
Fig. 5.
Effect of ultrasound-assisted alkaline hydrolysis on the surface microstructure of residues (a) without any extraction treatment, (b) after extracting free polyphenol and (c) after ultrasound-assisted alkaline hydrolysis.
4. Conclusion
This study represents a further contribution to the investigation of the bound polyphenols from pitahaya peel. In the combined alkaline hydrolysis and ultrasound-assisted extraction method, four influencing factors were investigated: sonication time, ultrasonic density, NaOH concentration and the liquid–material ratio. Each influencing factor had a great impact on each index. We noticed that the extract under optimal extraction conditions (45 min, 32 W/L, 6 M NaOH, 30 mL/g) had the highest content of bound polyphenols and the strongest antioxidant activity. On the basis of our analysis, we can conclude that phenolic compounds are major contributors to antioxidant activity, and the extract released by ultrasound-assisted alkaline hydrolysis had stronger enzyme inhibitory activity than the free polyphenol extract. Therefore, ultrasound-assisted alkaline hydrolysis is an effective method of releasing phenolic compounds and offers the possibility of further separating these active ingredients and developing of functional foods and new drugs.
CRediT authorship contribution statement
Xuanyu Zhong: Investigation, Writing – original draft. Shuyan Zhang: Investigation, Writing – original draft. Hong Wang: Conceptualization, Methodology. Jinyi Yang: Visualization, Writing – review & editing. Lin Li: Validation, Supervision. Jie Zhu: Investigation, Writing – review & editing, Funding acquisition. Yujia Liu: Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No. 31901682), and the program from Institute of Science and Technology Innovation, DGUT (No. KCYCXPT2017007), the Foundation for Innovation Team in Higher Education of Guangdong Province (2021KCXTD035).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ultsonch.2022.106213.
Contributor Information
Jie Zhu, Email: Zhujie@dgut.edu.cn.
Yujia Liu, Email: Yujialiu@dgut.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
No data was used for the research described in the article.
References
- 1.Sagar N.A., Pareek S., Sharma S., Yahia E.M., Lobo M.G. Fruit and vegetable waste: bioactive compounds, their extraction, and possible utilization. Compr. Rev. Food Sci. F. 2018;17(3):512–531. doi: 10.1111/1541-4337.12330. [DOI] [PubMed] [Google Scholar]
- 2.Giambanelli E., Gómez-Caravaca A.M., Ruiz-Torralba A., Guerra-Hernández E.J., Figueroa-Hurtado J.G., García-Villanova B., Verardo V. New advances in the determination of free and bound phenolic compounds of banana passion fruit pulp (passiflora tripartita, var Mollissima (kunth) l.h. Bailey) and their in vitro antioxidant and hypoglycemic capacities. Antioxidants. 2020;9(7):628. doi: 10.3390/antiox9070628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raza H., Ameer K., Ren X., Liang Q., Chen X., Chen H., Ma H. Physicochemical properties and digestion mechanism of starch-linoleic acid complex induced by multi-frequency power ultrasound. Food Chem. 2021;364 doi: 10.1016/j.foodchem.2021.130392. [DOI] [PubMed] [Google Scholar]
- 4.Kumar K., Srivastav S., Sharanagat V.S. Ultrasound assisted extraction (uae) of bioactive compounds from fruit and vegetable processing by-products: a review. Ultrason. Sonochem. 2021;70:105325. doi: 10.1016/j.ultsonch.2020.105325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dash D.R., Pathak S.S., Pradhan R.C. Improvement in novel ultrasound-assisted extraction technology of high value-added components from fruit and vegetable peels. J. Food Process Eng. 2021;44(4) [Google Scholar]
- 6.Alonso-Riaño P., Sanz Diez M.T., Blanco B., Beltrán S., Trigueros E., Benito-Román O. Water ultrasound-assisted extraction of polyphenol compounds from brewer's spent grain: kinetic study, extract characterization, and concentration. Antioxidants. 2020;9(3):265. doi: 10.3390/antiox9030265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu W., Jiang S., Liu M., Tian S. Simultaneous process optimization of ultrasound-assisted extraction of polyphenols and ellagic acid from pomegranate (punica granatum l.) flowers and its biological activities. Ultrason. Sonochem. 2021;80 doi: 10.1016/j.ultsonch.2021.105833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Z., Li S., Ge S., Lin S. Review of distribution, extraction methods, and health benefits of bound phenolics in food plants. J. Agr. Food Chem. 2020;68(11):3330–3343. doi: 10.1021/acs.jafc.9b06574. [DOI] [PubMed] [Google Scholar]
- 9.Moussa-Ayoub T.E., El-Samahy S.K., Kroh L.W., Rohn S. Identification and quantification of flavonol aglycons in cactus pear (opuntia ficus indica) fruit using a commercial pectinase and cellulase preparation. Food Chem. 2011;124(3):1177–1184. [Google Scholar]
- 10.Acosta-Estrada B.A., Gutiérrez-Uribe J.A., Serna-Saldívar S.O. Bound phenolics in foods, a review. Food Chem. 2014;152:46–55. doi: 10.1016/j.foodchem.2013.11.093. [DOI] [PubMed] [Google Scholar]
- 11.Radenkovs V., Juhnevica-Radenkova K., Górnaś P., Seglina D. Non-waste technology through the enzymatic hydrolysis of agro-industrial by-products. Trends Food Sci. Tech. 2018;77:64–76. [Google Scholar]
- 12.Zhang X., Zhu K., Xie J., Chen Y., Tan L., Liu S., Dong R., Zheng Y., Yu Q. Optimization and identification of non-extractable polyphenols in the dietary fiber of jackfruit (artocarpus heterophyllus lam.) Pulp released by alkaline, acid and enzymatic hydrolysis: content, composition and antioxidant activities. LWT. 2021;138 [Google Scholar]
- 13.Gonzales G.B., Smagghe G., Raes K., Van Camp J. Combined alkaline hydrolysis and ultrasound-assisted extraction for the release of nonextractable phenolics from cauliflower (brassica oleracea var.botrytis) waste. J. Agr. Food Chem. 2014;62(15):3371–3376. doi: 10.1021/jf500835q. [DOI] [PubMed] [Google Scholar]
- 14.Gonzales G.B., Raes K., Coelus S., Struijs K., Smagghe G., Van Camp J. Ultra(high)-pressure liquid chromatography–electrospray ionization-time-of-flight-ion mobility-high definition mass spectrometry for the rapid identification and structural characterization of flavonoid glycosides from cauliflower waste. J. Chromatogr. A. 2014;1323:39–48. doi: 10.1016/j.chroma.2013.10.077. [DOI] [PubMed] [Google Scholar]
- 15.Cao Q., Yan J., Sun Z., Gong L., Wu H., Tan S., Lei Y., Jiang B., Wang Y. Simultaneous optimization of ultrasound-assisted extraction for total flavonoid content and antioxidant activity of the tender stem of triarrhena lutarioriparia using response surface methodology. Food Sci. Biotechnol. 2021;30(1):37–45. doi: 10.1007/s10068-020-00851-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice-Evans C. Antioxidant activity applying an improved abts radical cation decolorization assay. Free Radical Bio. Med. 1999;26(9–10):1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 17.Dong R., Liu S., Xie J., Chen Y., Zheng Y., Zhang X., Zhao E., Wang Z., Xu H., Yu Q. The recovery, catabolism and potential bioactivity of polyphenols from carrot subjected to in vitro simulated digestion and colonic fermentation. Food Res. Int. 2021;143 doi: 10.1016/j.foodres.2021.110263. [DOI] [PubMed] [Google Scholar]
- 18.Benzie I.F.F., Strain J.J. The ferric reducing ability of plasma (frap) as a measure of “antioxidant power”: the frap assay. Anal. Biochem. 1996;239(1):70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 19.Spanier A.M., McMillin K.W., Miller J.A. Enzyme activity levels in beef: effect of postmortem aging and end-point cooking temperature. J. Food Sci. 1990;55(2):318–322. [Google Scholar]
- 20.Zheng Y., Liu S., Xie J., Chen Y., Dong R., Zhang X., Liu S., Xie J., Hu X., Yu Q. Antioxidant, α-amylase and α-glucosidase inhibitory activities of bound polyphenols extracted from mung bean skin dietary fiber. LWT. 2020;132 [Google Scholar]
- 21.Yusoff I.M., Mat Taher Z., Rahmat Z., Chua L.S. A review of ultrasound-assisted extraction for plant bioactive compounds: phenolics, flavonoids, thymols, saponins and proteins. Food Res. Int. 2022;157 doi: 10.1016/j.foodres.2022.111268. [DOI] [PubMed] [Google Scholar]
- 22.Papoutsis K., Vuong Q.V., Golding J.B., Hasperué J.H., Pristijono P., Bowyer M.C., Scarlett C.J., Stathopoulos C.E. Pretreatment of citrus by-products affects polyphenol recovery: a review. Food Rev. Int. 2018;34(8):770–795. [Google Scholar]
- 23.Liang Q., Sun X., Raza H., Aslam Khan M., Ma H., Ren X. Fabrication and characterization of quercetin loaded casein phosphopeptides-chitosan composite nanoparticles by ultrasound treatment: Factor optimization, formation mechanism, physicochemical stability and antioxidant activity. Ultrason. Sonochem. 2021;80:105830. doi: 10.1016/j.ultsonch.2021.105830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pérez-Jiménez J., Arranz S., Tabernero M., Díaz- Rubio M.E., Serrano J., Goñi I., Saura-Calixto F. Updated methodology to determine antioxidant capacity in plant foods, oils and beverages: extraction, measurement and expression of results. Food Res. Int. 2008;41(3):274–285. [Google Scholar]
- 25.Chandrasekara A., Shahidi F. Content of insoluble bound phenolics in millets and their contribution to antioxidant capacity. J. Agr. Food Chem. 2010;58(11):6706–6714. doi: 10.1021/jf100868b. [DOI] [PubMed] [Google Scholar]
- 26.S. Hang, W. Wu, Y. Wang, R. Sheng, Y. Fang, R. Guo, Daphnetin, a coumarin in genus stellera chamaejasme linn: chemistry, bioactivity and therapeutic potential, Chem. Biodivers. (2022). [DOI] [PubMed]
- 27.Xia Y., Chen C., Liu Y., Ge G., Dou T., Wang P. Synthesis and structure-activity relationship of daphnetin derivatives as potent antioxidant agents. Molecules. 2018;23(10):2476. doi: 10.3390/molecules23102476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang S., Song Y., Wang Q., Liu Y., Wu Z., Duan X., Zhang Y., Bi X., Geng Y., Chen S., Zhu C. Daphnetin ameliorates acute lung injury in mice with severe acute pancreatitis by inhibiting the jak2–stat3 pathway. Sci. Rep.-Uk. 2021;11(1) doi: 10.1038/s41598-021-91008-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fan X., Gu W., Gao Y., Ma N., Fan C., Ci X. Daphnetin ameliorated gm-induced renal injury through the suppression of oxidative stress and apoptosis in mice. Int. Immunopharmacol. 2021;96 doi: 10.1016/j.intimp.2021.107601. [DOI] [PubMed] [Google Scholar]
- 30.Sova M., Saso L. Natural sources, pharmacokinetics, biological activities and health benefits of hydroxycinnamic acids and their metabolites. Nutrients. 2020;12(8):2190. doi: 10.3390/nu12082190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.El-Seedi H.R., El-Said A.M.A., Khalifa S.A.M., Göransson U., Bohlin L., Borg-Karlson A., Verpoorte R. Biosynthesis, natural sources, dietary intake, pharmacokinetic properties, and biological activities of hydroxycinnamic acids. J. Agr. Food Chem. 2012;60(44):10877–10895. doi: 10.1021/jf301807g. [DOI] [PubMed] [Google Scholar]
- 32.Zduńska K., Dana A., Kolodziejczak A., Rotsztejn H. Antioxidant properties of ferulic acid and its possible application. Skin Pharmacol. Phys. 2018;31(6):332–336. doi: 10.1159/000491755. [DOI] [PubMed] [Google Scholar]
- 33.Nićiforović N., Abramovič H. Sinapic acid and its derivatives: natural sources and bioactivity. Compr. Rev. Food Sci. F. 2014;13(1):34–51. doi: 10.1111/1541-4337.12041. [DOI] [PubMed] [Google Scholar]
- 34.Yang J., Chen J., Hao Y., Liu Y. Identification of the dpph radical scavenging reaction adducts of ferulic acid and sinapic acid and their structure-antioxidant activity relationship. LWT. 2021;146 [Google Scholar]
- 35.Harini R., Pugalendi K.V. Antihyperglycemic effect of protocatechuic acid on streptozotocin-diabetic rats. J. Basic Clin. Physiol. Pharmacol. 2010;21(1) doi: 10.1515/jbcpp.2010.21.1.79. [DOI] [PubMed] [Google Scholar]
- 36.Gatouillat G., Magid A.A., Bertin E., El Btaouri H., Morjani H., Lavaud C., Madoulet C. Medicarpin and millepurpan, two flavonoids isolated from medicago sativa, induce apoptosis and overcome multidrug resistance in leukemia p388 cells. Phytomedicine. 2015;22(13):1186–1194. doi: 10.1016/j.phymed.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 37.Zhu J., Wen L., Zhong W., Xiong L., Liang J., Wang H. Quercetin, kaempferol and isorhamnetin inelaeagnus pungensthunb. Leaf: pharmacological activities and quantitative determination studies. Chem. Biodivers. 2018;15(8):e1800129. doi: 10.1002/cbdv.201800129. [DOI] [PubMed] [Google Scholar]
- 38.Huang Y., Bai C., He F., Xie Y., Zhou H. Review on the potential action mechanisms of chinese medicines in treating coronavirus disease 2019 (covid-19) Pharmacol. Res. 2020;158 doi: 10.1016/j.phrs.2020.104939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fernandez-Panchon M.S., Villano D., Troncoso A.M., Garcia-Parrilla M.C. Antioxidant activity of phenolic compounds: fromin vitro results toin vivo evidence. Crit. Rev. Food Sci. 2008;48(7):649–671. doi: 10.1080/10408390701761845. [DOI] [PubMed] [Google Scholar]
- 40.Palafox-Carlos H., Gil-Chávez J., Sotelo-Mundo R., Namiesnik J., Gorinstein S., González-Aguilar G. Antioxidant interactions between major phenolic compounds found in ‘ataulfo’ mango pulp: chlorogenic, gallic, protocatechuic and vanillic acids. Molecules. 2012;17(11):12657–12664. doi: 10.3390/molecules171112657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hidalgo M., Sánchez-Moreno C., de Pascual-Teresa S. Flavonoid–flavonoid interaction and its effect on their antioxidant activity. Food Chem. 2010;121(3):691–696. [Google Scholar]
- 42.Wang S., Meckling K.A., Marcone M.F., Kakuda Y., Tsao R. Synergistic, additive, and antagonistic effects of food mixtures on total antioxidant capacities. J. Agr. Food Chem. 2011;59(3):960–968. doi: 10.1021/jf1040977. [DOI] [PubMed] [Google Scholar]
- 43.Sun C., Zhao C., Guven E.C., Paoli P., Simal Gandara J., Ramkumar K.M., Wang S., Buleu F., Pah A., Turi V., Damian G., Dragan S., Tomas M., Khan W., Wang M., Delmas D., Portillo M.P., Dar P., Chen L., Xiao J. Dietary polyphenols as antidiabetic agents: advances and opportunities, Food. Frontiers. 2020;1(1):18–44. [Google Scholar]
- 44.Ramkumar K.M., Thayumanavan B., Palvannan T., Rajaguru P. Inhibitory effect of gymnema montanum leaves on α-glucosidase activity and α-amylase activity and their relationship with polyphenolic content. Med. Chem. Res. 2010;19(8):948–961. [Google Scholar]
- 45.Xiao J., Kai G., Ni X., Yang F., Chen X. Interaction of natural polyphenols with α-amylase in vitro: molecular property–affinity relationship aspect. Mol. Biosyst. 2011;7(6):1883. doi: 10.1039/c1mb05008g. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data was used for the research described in the article.