Abstract
Goal Management Training® (GMT) teaches strategies to reduce cognitive load and improve focus in everyday tasks. The aim of this study was to ascertain feasibility, acceptability, and efficacy potential of GMT for people (≥50 years) with stable HIV infection scoring low on tests of cognitive ability. A two-sample, parallel, controlled trial was carried out. Feasibility was demonstrated, as 21/30 participants in the GMT group attended ≥8 of the 9 sessions and completed at least half of the homework. There was no change on the primary performance-based cognitive outcomes in the GMT group or in the control group (n = 23). There was a meaningful improvement in self-reported cognition in those adherent to the intervention. GMT is a promising intervention for people aging with HIV who are dealing with cognitive difficulties affecting their everyday life and should be further investigated.
Keywords: HIV, Cognition, Rehabilitation, Goal management training
1. Introduction
People living with HIV worry about their memory, and with good reason as the prevalence of cognitive impairment may be as high as 30–50% [1,2]. In those receiving effective antiretroviral therapy, the most common profile is mild difficulties with attention, executive function and memory, with impact on everyday function in up to half of those with cognitive difficulties. Rehabilitation is a well-established approach to maintain function in the face of limitations resulting from a chronic disease, and holds promise for addressing the health challenges faced by older people with HIV [3]. Goal Management Training® (GMT) is a cognitive rehabilitation program that teaches self-management principles, stress management, and mindfulness, and trains participants in the use of several explicit strategies to reduce cognitive load in everyday tasks and methods to cue attention to maintain focus on specific tasks. GMT trains individuals to periodically stop automatic behaviours and bring their overarching goal to mind, subdivide that overall goal into sub-goals, and monitor performance. In principle, this training intervention might directly influence specific brain systems underpinning executive function and attentional control through learning and neuroplasticity, optimize the use of adaptive strategies in real-life situations, and improve confidence and self-efficacy. These mechanisms could interact in a virtuous cycle with positive effects on cognitive function.
In practice, there is evidence that GMT can improve cognitive performance and function, and lead to brain network changes measuring with functional neuroimaging: This intervention has been shown to improve cognitive function in a variety of neurological conditions, including mild traumatic brain injury [[4], [5], [6], [7]], stroke [4,5,8,9] and spina bifida [7,9], as well as in healthy older people with self-reported cognitive difficulties [4,5,8]. Evidence from randomized trials shows that this intervention leads to (i) improvements in performance on cognitive tests, including naturalistic tests of real life tasks, (ii) reduced self-reported cognitive difficulties [10], and (iii) improvements in everyday function [4,5,8,9] compared to either a wait-list condition or a general brain health education control. These improvements have been shown to last at least 6 months in some studies [7,8] and to be accompanied by changes in the brain networks underlying executive function [10].
A 2019 review of 21 trials, one of an HIV sample [11], with a total pooled sample size of 300 people, reported modest effect sizes for performance (i.e. neuropsychological) tests of executive function measured post-intervention (0.227 from 15 studies; 95% CI: 0.103–0.352) with the largest effect size (0.549 from 6 studies; 95% CI: 0.049–1.049) reported at follow-up (mostly at 6 months). Smaller effect sizes were noted for self-report measures of executive function immediately post-intervention (0.136 from 16 studies; 95% CI: 0.018–0.255) and at follow-up (0.128 from 6 studies; 95% CI: −0.128 - 0.385). Using a meta-regression, the greatest proportion of variability in effect was explained by the number of training hours attended, more was consistently better. GMT is thus a well-validated cognitive rehabilitation intervention with potential to improve cognitive ability in older HIV + people with cognitive difficulties. The specific objectives of this study were to identify challenges and solutions to feasibility and acceptability of GMT in older people with stable, treated HIV infection who report cognitive difficulties and to provide preliminary evidence of the effect of GMT on cognitive ability, compared to a waitlist control group, as measured by (i) cognitive performance tests and (ii) self-report questionnaires.
2. Methods
This study was part of a larger study, a multiple cohort randomized controlled trial (mcRCT) [12], a design in which a fully characterized cohort is used as a sampling base for multiple trials providing a common measurement platform. The protocol for the Positive Brain Health Now (+BHN) cohort of older people living with HIV in Canada has been previously described [13] including the multiple pilot trials embedded within. The study presented here is one of these pilot trials, a two-sample, parallel, controlled pilot study. The project followed the reporting guidelines for pilot and feasibility studies (PAFS) [14] The study was registered on http://www.clinicaltrials.gov/(No NCT03168724).
Study participants meeting eligibility criteria were identified from among participants in the two sites in Montreal of the +BHN [15] study (The Montreal Chest Institute and Clinique médicale (L'Actuel). People were eligible if over age 50, with self-reported cognitive difficulties or performance on objective computerized cognitive tests that fell at or below the 50th percentile of performance in the cohort as a whole (n = 856), as measured by the cohort study's primary outcome, the Brief Cognitive Ability Measure [B-CAM] [16,17] (n = 856) and by the C3Q [19] (n = 703). Details of these measures will be presented in the measurement section. Recruitment ended when 30 people had been enrolled in GMT. People who were participating as the untreated control group for another pilot RCT on cognitive training also embedded in the +BHN study served as a comparison group for GMT. Both pilot studies had similar inclusion and exclusion criteria and to some extent competed for the same study participants during the same time frame although they could only enter one.
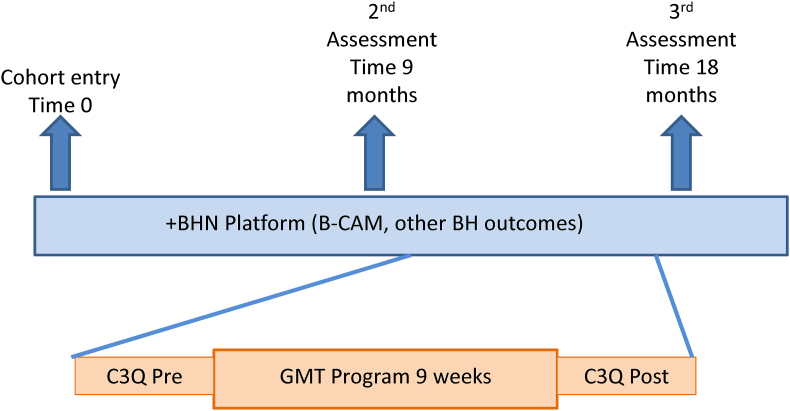
Fig. 1 shows the relationship between the timelines for the GMT trial and the +BHN cohort. GMT is a fully manualized in-person therapy delivered in small groups. Four GMT groups, each consisting of 5–8 participants, met on a weekly basis for 9 consecutive weeks. Each training session lasted 120 minutes. Sessions were delivered using the GMT training toolkit (GMT leader manual and GMT participant workbook available in English and French). A typical session consisted of a summary of the previous session, interactive discussion on homework, education on goal management and mindfulness concepts and skills, followed by “mental laboratory” and mindfulness exercises to practice the materiel covered in the session.
Fig. 1.
Relationship between the +BHN platform assessments and the GMT pilot trial
Recruitment into the GMT trial occurred between the 2nd and 3rd assessment visit in the +BHN cohort. C3Q was administered pre and post the 10 weeks of the GMT intervention period (1 week for introductions and 9 weeks of intervention). The B-CAM and platform BH measures were assessed 9 months apart.
2.1. Measures
Feasibility outcomes were adherence with the protocol, principally attendance and completion of homework. Two measures of cognitive ability developed specifically for people with HIV were the cognitive outcomes. The B-CAM) [16,17] is a short battery of computerized tests of processing speed, attention, memory and executive function developed to measure cognitive ability in older people with HIV. Although multiple domains are assessed, empirical work guided by Rasch Measurement Theory has established that these tests reflect a single underlying latent variable in HIV. Rasch analysis demonstrated that a single score measuring overall cognitive ability is a legitimate representation of the construct [18]. Higher scores indicate higher cognitive ability. An additional performance test, Tower of London, was also assessed as it had shown positive effects in an earlier study on GMT(9) although in a sample of younger people (mean age 32 years) with Spina Bifida. The Tower of London assesses working memory and attention as applied to multi-step planning and problem-solving. As such, it might be expected to be responsive to the GMT intervention, which teaches techniques to sustain attention to accomplish multi-step everyday goals. These measures were administered at baseline and at completion of the intervention.
Communicating Cognitive Concerns (C3Q) [19] is an 18-item measure of self-reported cognitive ability, developed with input from people living with HIV, simultaneously in English and French. It comprises questions related to memory (forgetting tasks to be done, what to buy at store; food cooking on the stove, as examples), attention (losing focus on instructions, conversation, reading), and executive function (making decisions, being organized, doing new things), rated on a 3-point ordinal scale for frequency (Frequently - Almost every day; Sometimes – Once a week; Rarely – Once a month or less). The items have been shown to fit the unidimensional Rasch Model, equivalently in the English and French, yielding a legitimate total score ranging from 0 to 36 with higher values indicating more cognitive ability. Meaningful change for these two measures were based on the distributional criterion of ½ standard deviation [20]. The comparison group was not assessed on the C3Q.
The groups were also characterized on other brain health outcomes which are fully described in the protocol paper for the +BHN cohort [15].
2.2. Statistical methods
In accordance with guidelines for pilot and feasibility studies (PAFS) [14], the within group effects are the focus. Change scores were calculated for each of the cognitive outcome measures for the GMT group and for the comparison group, when available, separately for those with high and low adherence to the GMT intervention. The difference in change scores (baseline and follow-up) between sub-groups defined by adherence status was also estimated. Results are presented using point estimates and 95% confidence intervals (CI). The reliable change index (RCI), which is the ratio of the difference pre-post to the variability and correlation in pre- and post-scores [21], was also calculated for each participant. For a single group, an RCI that exceeds 1.65 is considered to reflect reliable change.
In accordance with guidelines from the American Statistical Association, this study will avoid presenting p-values unless helpful for interpretation and will avoid referring to results as statistically significant [22]. Instead, effects will be reported using point estimates and 95% CI. There are no specific guidelines for sample size for PAFS. Thirty participants are often suggested as the distribution for variables that are normally distributed in the population are likely normal with 30 subjects [23].
3. Results
A total of 30 people were selected for the GMT group and 23 people served for comparison purposes. Table I presents information on the participants in the GMT and comparison groups. There were some meaningful differences between the GMT and the comparison group, with the GMT group living longer with HIV by some 4 years and with fewer hours spent volunteering. Table II provides information on feasibility/acceptability. In the GMT group, three people completed 1 to 4 of the 9 sessions and did none or only one of the 9 homework assignments; 6 people attended 8 or 9 sessions but completed less than half of the homework assignments. These 9 participants were classified as low adherence. The other 21 (70%) attended 8 or 9 sessions and completed more than half the assignments (5–8); this group was classified as high adherence.
Table 1.
Characteristics of the GMT and comparison participants.
| Variable | Group |
|
|---|---|---|
| GMT | Comparison | |
| Number | 30 | 23 |
| Age | 58.1 (7.0) | 55.7 (9.8) |
| Sex: M/F | 29/1 | 20/3 |
| Years of education | 14.0 (2.4) | 14.2 (3.2) |
| Years with HIV | 21.9 (6.2) | 17.6 (8.0) |
| Nadir CD4 | 201.9 (176.3) | 195.3 (107.8) |
| Hours of work | 12.4 (20.6) | 14.3 (18.4) |
| Hours of volunteering | 2.3 (3.2) | 6.4 (14.0) |
| Mental health (MHI)a | 64.3 (18.1) | 56.3 (17.2) |
| Depression (WHO5)b | 53.6 (21.3) | 48.9 (15.8) |
| Depression (HADS)c | 6.0 (4.3) | 6.7 (3.4) |
| Anxiety (HADS)c | 8.1 (3.9) | 9.0 (3.9) |
| Vitalityd | 51.2 (19.3) | 44.3 (16.6) |
| Motivatione | 63.5 (29.5) | 60.1 (28.3) |
| B-CAM©f | 20.1 (4.1) | 18.4 (4.8) |
| Tower of London (mean correct) | 10.7 (4.3) [n = 17] | 11.9 (4.1) [22] |
| C3Qg | 63.4 (18.0) | Not assessed |
Table 2.
Results (means and SD) on cognitive performance and self-report cognitive difficulties.
| GMT n = 30 |
Comparison (n = 23) | ||
|---|---|---|---|
| Adherence status | |||
| High (n = 21) | Low (n = 9) | ||
| Age | 57.9 (6.0) | 58.4 (9.5) | 55.7 (9.8) |
| Sex (men/women) | 21/0 | 8/1 | 20/3 |
| Education (yrs) | 14.0 (2.5) | 14.1 (2.0) | 14.2 (3.2) |
| Duration of HIV infection (years) | 21.7 (6.7) | 22.6 (4.8) | 17.6 (8.0) |
| Nadir CD4 count (cells/mm3) | 155.2 (117.0)a | 310.9 (243.5)a | 195.3 (107.8) |
| Sessions/9 [Mean: SD] | 8.2/9; range:1-9 | ||
| 1 | 0 | 1 | 0 |
| 4 | 0 | 2 | 0 |
| 8 | 3 | 4 | 0 |
| 9 | 18 | 2 | 0 |
| Homework assignments/9 | 0 | ||
| Missing | 0 | 2 | |
| 0–4 | 0 | 7 | |
| 5–8 | 21 | 0 | |
| B-CAM (higher is better)b | n = 19 | n = 9 | n = 22 |
| Baseline | 20.3 (4.3) | 19.3 (3.1) | 17.8 (4.5) |
| Post-training | 20.8 (5.0) | 20.1 (4.5) | 19.1 (3.4) |
| Change | 0.5 (2.5) | 0.8 (2.8) | 1.3 (3.2) |
| 95% CI | −0.6 to 1.6 | −1.0 to 2.8 | |
| Between group difference | −0.3 (−5.6 to 5.0) | ||
| RCI +/− (n) | 1/1 | 0/0 | |
| C3Q (higher is better) | n = 21 | n = 9 | not assessed |
| Baseline | 62.4(19.4) | 69.0 (14.0) | |
| Post-training | 71.5 (14.9) | 59.0 (18.9) | |
| Change | +9.1 (13.4) | −10.0 (16.0) | |
| 95% CI | 3.4 to 12.0 | −20.5 to 0.5 | |
| Between group difference | −19.1 (−32.4 to −5.8) | ||
| RCI +/− (n) | 3/0 | 0/1 | |
RCI: Reliable Change Index [21]; critical value for one group is + or - 1.65 for positive or negative change.
p = 0.093.
Assessed as platform measure at regularly scheduled visits 9 month apart.
The low and high adherence groups were similar at study start. Two people had missing data post-intervention on the B-CAM and two other people had data missing on C3Q. There was no average change on the measure of cognitive performance (B-CAM) at follow-up in any of the three groups (high adherence, low adherence, controls). Only one person made reliable positive change (RCI: 1.80) with a B-CAM score changing from 20 to 24.4. One person made a similar magnitude of change on B-CAM (19–23) with an RCI of 1.63). One person made negative reliable change (RCI: −2.28) with scores changing from 17.6 to 12.
There was no difference between the GMT and the comparison group on the Tower of London test at baseline (mean correct 10.7 vs. 11.9; SD: 4.3) or on change after intervention. In the comparison group only 11 of the original 23 participants completed this test.
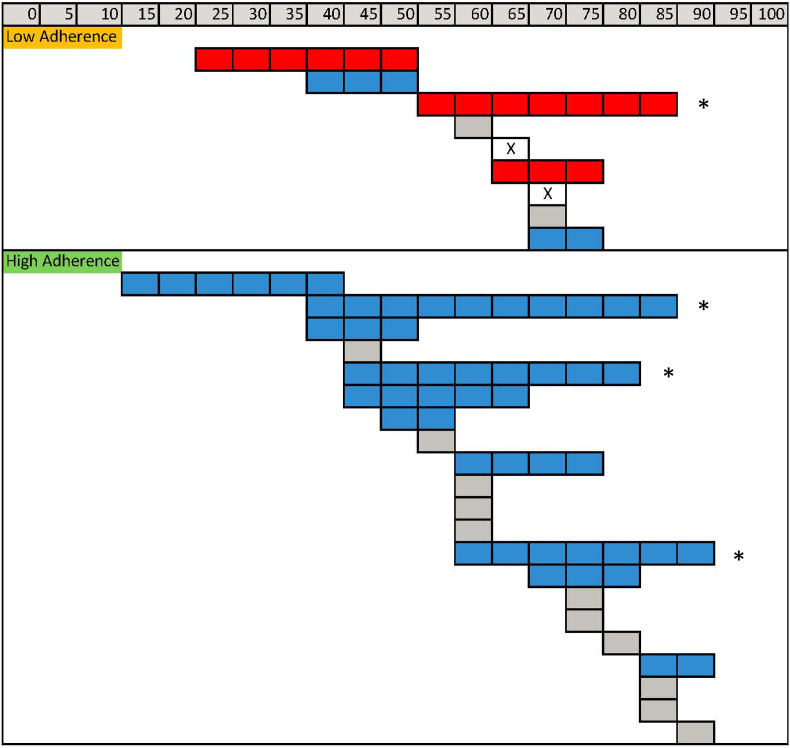
On average, there was a relevant increase in self-reported cognitive ability measured using the C3Q (+9.1; 95%CI:+3.0 to +15.2) in the high adherence sample, but in the low adherence group the average magnitude of change was negative (difference −10; 95%CI: −35.9 - +5.6), although with only 9 subjects, the 95% CI was very wide and included the null (0). As the sample size was small, average change can mask important observations that would be relevant for identifying areas related to feasibility of a full scale trial. Fig. 2 presents individual data on the C3Q from those classified as low adherence and high adherence. Of the 9 participants with low adherence, three made negative absolute change, two made smaller positive absolute changes, and two each made no change or had missing data post-intervention. Of the 21 adherent participants, 10 made positive absolute change and 11 made no change. There was no indication that positive or negative absolute change was explained by regression to the mean as change did not depend on starting values. Much fewer people made reliable change: three in the group with high adherence and none in the group with low adherence. The average changes in those with high and low adherence were large and meaningful (i.e. greater than ½ SD) but the high adherence group improved and the low adherence group declined, yielding a between sub-group difference (−19.1; 95% CI: −32.4 to-5.8).
Fig. 2.
Fig. 2. Pre and post values (scale 0 to 100; higher is better) on the C3Q for those with low and high adherence.
Blue bars indicates positive absolute change with pre and post data presented left to right; red bars indicate negative positive change with pre and post data presented right to left; grey bars indicate no meaningful absolute change either positive or negative; and clear bars with X indicate missing data. Bars with * represent people with reliable change, positive or negative.
4. Discussion
GMT was feasible and acceptable in older people with cognitive difficulties in the context of chronic HIV infection, as demonstrated by the high rate of adherence with this group-based cognitive rehabilitation program. The combination of the group effect, peer support, and perceived cognitive benefit likely contributed to the high rate of adherence. Participants often mentioned that sharing their experience in a safe space, speaking freely about their anxiety regarding their cognitive difficulties, and learning from others was of great benefit. This may be particularly important for people with HIV as they often face stigma and can be reluctant to access services.
Ten of the 21 people who received GMT as intended showed meaningful absolute changes in the self-reported measure of cognitive ability, which is a criterion for feasibility if considering a full-scale trial. Only two of 9 people in the low adherence group made positive changes and these were small, between +5.5 and + 8.3 (out of 100) on the C3Q (see Table II). However, analysis based on absolute change does not consider standard error of measurement or that the pre- and post-test variances may not be equal. RCI(21) takes both these factors into consideration. Using that approach, we found that few people made reliable change. The most responsive measure was the C3Q in the high adherence group, with 3 of 21 showing reliable change. Our findings show that self-reported cognitive performance is sensitive to the impact of GMT, in line with this intervention's therapeutic focus on cognitive difficulties experienced by people in everyday life.
While the adherent group improved on C3Q, the non-adherent group worsened by about the same amount. It is possible that those who did not adhere to the therapy may have found the activities and the homework too difficult and realized that they had more cognitive difficulties than first reported. They may also have referenced their own experience against others participating in this group-based therapy, perhaps leading to a more negative view of their cognitive capacities at post-test. We think such a recalibration response shift [24] is more likely than a true deterioration due to GMT in this sub-group.
Of note, there were no changes on the B-CAM nor the Tower of London test, the two performance-based cognitive outcomes. The relationship between performance and self-report cognition is often not strong, owing to the differences in the way these types of measures are constructed and tested [25]. As explained by Dang et al. [25], performance-based (behavioral) measures quantify responses to unusual stimuli in highly controlled testing situations, whereas self-report measures ask participants to reflect on their behaviors across a variety of unstructured real-life situations. Second, behavioral measures are based on performance such as reaction time and accuracy, whereas self-report measures are based on perceptions of performance, which reflects subjective judgments about performance rather than performance itself. As such, these two types of measures are of different constructs.
The present study is limited as the groups were not assigned at random. This is not a necessity for a PAFS but strengthens the feasibility. As a result the comparison group, which was participating as a control group for another pilot study, did not have all the same study measures as the GMT group. This is a pilot study and is not designed for making inference about efficacy or about differential response by participant characteristics. Pilot studies are designed to test all aspects of the protocol and identify whether a proportion of the participants respond to justify a larger study. Sample sizes are small for this reason.
5. Conclusion
The data presented here support pursuing GMT in future studies as a method of helping people with HIV with cognitive challenges to better focus on their goals during everyday activities. This fully manualized therapy is available in English and French and could be readily implemented in HIV clinics or in the community. Future studies should also estimate the effect of GMT on accomplishing everyday tasks and healthful activities, rather than the intermediate outcome of cognition that was the focus here. GMT holds promise as a means to improve people's everyday functioning and self-management skills.
Funding
This work was supported by a Canadian Institutes of Health Research (CIHR) Team Grant [TCO-125272] and a grant from CIHR HIV Clinical Trials Network (CTN 273).
Compliance with ethical standards
The project was approved by the Research Ethics Board of the McGill University Health Center Research Institute (REB 2104–1049) and each of the participating institutions.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Declaration of competing interest
Nancy Mayo declares she has no conflict of interest.
Dr. Brian Levine is the developer of Goal Management Training®
Marie-Josée Brouillette declares she has no conflict of interest.
Delphine Bélanger declares she has no conflict of interest.
Lesley Fellows declares she has no conflict of interest.
Contributor Information
Nancy E. Mayo, Email: nancy.mayo@mcgill.ca.
Brian Levine, Email: blevine@research.baycrest.org.
Marie-Josée Brouillette, Email: marie-josee.brouillette@mcgill.ca.
Delphine Bélanger, Email: delphinebelanger@gmail.com.
Lesley K. Fellows, Email: lesley.fellows@mcgill.ca.
Data availability
Data will be made available on request.
References
- 1.Clifford D.B., Ances B.M. HIV-associated neurocognitive disorder. Lancet Infect. Dis. 2013 Nov;13(11):976–986. doi: 10.1016/S1473-3099(13)70269-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robertson K.R., Su Z., Margolis D.M., Krambrink A., Havlir D.V., Evans S., et al. Neurocognitive effects of treatment interruption in stable HIV-positive patients in an observational cohort. Neurology. 2010 Apr 20;74(16):1260–1266. doi: 10.1212/WNL.0b013e3181d9ed09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Worthington C., Myers T., O'Brien K., Nixon S., Cockerill R. Rehabilitation in HIV/AIDS: development of an expanded conceptual framework. AIDS Patient Care STDS. 2005 Apr;19(4):258–271. doi: 10.1089/apc.2005.19.258. [DOI] [PubMed] [Google Scholar]
- 4.Levine B., Schweizer T.A., O'Connor C., Turner G., Gillingham S., Stuss D.T., et al. Rehabilitation of executive functioning in patients with frontal lobe brain damage with goal management training. Front. Hum. Neurosci. 2011;5:9. doi: 10.3389/fnhum.2011.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levine B., Stuss D.T., Winocur G., Binns M.A., Fahy L., Mandic M., et al. Cognitive rehabilitation in the elderly: effects on strategic behavior in relation to goal management. J. Int. Neuropsychol. Soc. 2007 Jan;13(1):143–152. doi: 10.1017/S1355617707070178. [DOI] [PubMed] [Google Scholar]
- 6.Levine B., Robertson I.H., Clare L., Carter G., Hong J., Wilson B.A., et al. Rehabilitation of executive functioning: an experimental-clinical validation of goal management training. J. Int. Neuropsychol. Soc. 2000 Mar;6(3):299–312. doi: 10.1017/s1355617700633052. [DOI] [PubMed] [Google Scholar]
- 7.Stubberud J., Langenbahn D., Levine B., Stanghelle J., Schanke A.K. Goal management training of executive functions in patients with spina bifida: a randomized controlled trial. J. Int. Neuropsychol. Soc. 2013 Jul;19(6):672–685. doi: 10.1017/S1355617713000209. [DOI] [PubMed] [Google Scholar]
- 8.Spikman J.M., Boelen D.H., Lamberts K.F., Brouwer W.H., Fasotti L. Effects of a multifaceted treatment program for executive dysfunction after acquired brain injury on indications of executive functioning in daily life. J. Int. Neuropsychol. Soc. 2010 Jan;16(1):118–129. doi: 10.1017/S1355617709991020. [DOI] [PubMed] [Google Scholar]
- 9.Stubberud J., Langenbahn D., Levine B., Stanghelle J., Schanke A.K. Goal Management Training improves everyday executive functioning for persons with spina bifida: self-and informant reports six months post-training. Neuropsychol. Rehabil. 2014;24(1):26–60. doi: 10.1080/09602011.2013.847847. [DOI] [PubMed] [Google Scholar]
- 10.Chen A.J., Novakovic-Agopian T., Nycum T.J., Song S., Turner G.R., Hills N.K., et al. Training of goal-directed attention regulation enhances control over neural processing for individuals with brain injury. Brain. 2011 May;134(Pt 5):1541–1554. doi: 10.1093/brain/awr067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stamenova V., Levine B. Effectiveness of goal management training(R) in improving executive functions: a meta-analysis. Neuropsychol. Rehabil. 2019 Dec;29(10):1569–1599. doi: 10.1080/09602011.2018.1438294. [DOI] [PubMed] [Google Scholar]
- 12.Relton C., Torgerson D., O'Cathain A., Nicholl J. Rethinking pragmatic randomised controlled trials: introducing the "cohort multiple randomised controlled trial" design. BMJ. 2010;340:c1066. doi: 10.1136/bmj.c1066. [DOI] [PubMed] [Google Scholar]
- 13.Mayo N.E., Brouillette M.J., Fellows L.K. Understanding and optimizing brain health in HIV now: protocol for a longitudinal cohort study with multiple randomized controlled trials. BMC Neurol. 2016 Jan 14;16:8. doi: 10.1186/s12883-016-0527-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eldridge S.M., Chan C.L., Campbell M.J., Bond C.M., Hopewell S., Thabane L., et al. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. Pilot Feasibility Stud. 2016;2:64. doi: 10.1186/s40814-016-0105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mayo N.E., Brouillette M.J., Fellows L.K. Understanding and optimizing brain health in HIV now: protocol for a cohort multiple randomized controlled trial. BMC Neurol. 2016;16(8) doi: 10.1186/s12883-016-0527-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brouillette M.J., Fellows L.K., Palladini L., Finch L., Thomas R., Mayo N.E. Quantifying cognition at the bedside: a novel approach combining cognitive symptoms and signs in HIV. BMC Neurol. 2015 Nov 13;15:224. doi: 10.1186/s12883-015-0483-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brouillette M.J., Fellows L.K., Finch L., Thomas R., Mayo N.E. Properties of a brief assessment tool for longitudinal measurement of cognition in people living with HIV. PLoS One. 2019;14(3) doi: 10.1371/journal.pone.0213908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koski L., Brouillette M.J., Lalonde R., Hello B., Wong E., Tsuchida A., et al. Computerized testing augments pencil-and-paper tasks in measuring HIV-associated mild cognitive impairment(*) HIV Med. 2011 Sep;12(8):472–480. doi: 10.1111/j.1468-1293.2010.00910.x. [DOI] [PubMed] [Google Scholar]
- 19.Askari S., Fellows L., Brouillette M.J., Mayo N.E. Development and validation of a voice-of-the-patient measure of cognitive concerns experienced by people living with HIV. Qual. Life Res. 2020 Oct doi: 10.1007/s11136-020-02679-z. [DOI] [PubMed] [Google Scholar]
- 20.Norman G.R., Sloan J.A., Wyrwich K.W. The truly remarkable universality of half a standard deviation: confirmation through another look. Expert Rev. Pharmacoecon. Outcomes Res. 2004 Oct;4(5):581–585. doi: 10.1586/14737167.4.5.581. [DOI] [PubMed] [Google Scholar]
- 21.Estrada E., Ferrer E., Pardo A. Statistics for evaluating pre-post change: relation between change in the distribution center and change in the individual scores. Front. Psychol. 2018;9:2696. doi: 10.3389/fpsyg.2018.02696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wasserstein R.L., Lazar N.A. The ASA's statement on p-values: context, process, and purpose. Am. Statistician. 2016;70(2):129–133. [Google Scholar]
- 23.Kaur N., Figueiredo S., Bouchard V., Moriello C., Mayo N. Where have all the pilot studies gone? A follow-up on 30 years of pilot studies in Clinical Rehabilitation. Clin. Rehabil. 2017 Sep;31(9):1238–1248. doi: 10.1177/0269215517692129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sprangers M.A., Schwartz C.E. Integrating response shift into health-related quality of life research: a theoretical model. Soc. Sci. Med. 1999 Jun;48(11):1507–1515. doi: 10.1016/s0277-9536(99)00045-3. [DOI] [PubMed] [Google Scholar]
- 25.Dang J., King K.M., Inzlicht M. Why are self-report and behavioral measures weakly correlated? Trends Cognit. Sci. 2020 Apr;24(4):267–269. doi: 10.1016/j.tics.2020.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.