Version Changes
Revised. Amendments from Version 2
We expanded the methods section to show how study eligibility was determined. We have included some data on the frequency of SARs in different geographical regions. We also note in our limitations, the effect of seasonality on the transmission of SARS-CoV-2. In addition, we have revised Figure 3a.
Abstract
Background: SARS-CoV-2 transmission has been reported to be associated with close contact with infected individuals. However, the mechanistic pathway for transmission in close contact settings is unclear. Our objective was to identify, appraise and summarise the evidence from studies assessing the role of close contact in SARS-CoV-2 transmission.
Methods: This review is part of an Open Evidence Review on Transmission Dynamics of SARS-CoV-2. We conduct ongoing searches using WHO Covid-19 Database, LitCovid, medRxiv, PubMed and Google Scholar; assess study quality based on the QUADAS-2 criteria and report important findings on an ongoing basis.
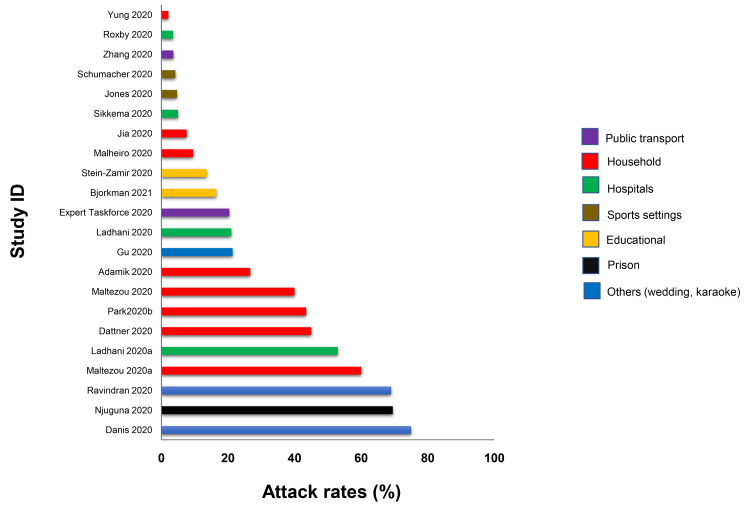
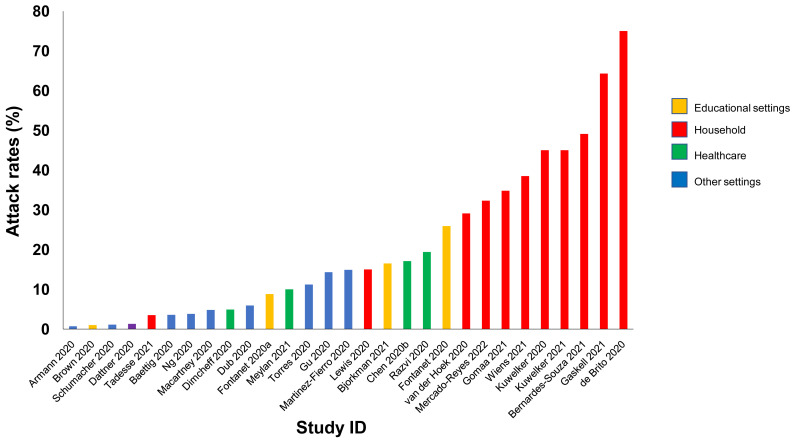
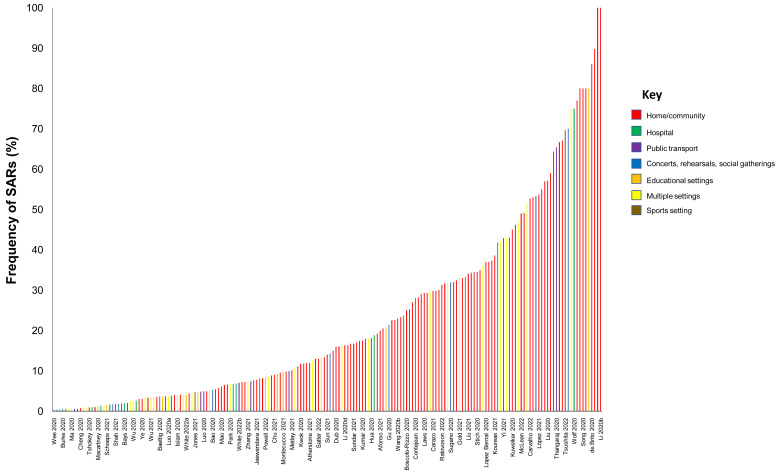
Results: We included 278 studies: 258 primary studies and 20 systematic reviews. The settings for primary studies were predominantly in home/quarantine facilities (39.5%) and acute care hospitals (12%). The overall reporting quality of the studies was low-to-moderate. There was significant heterogeneity in design and methodology. The frequency of attack rates (PCR testing) varied between 2.1-75%; attack rates were highest in prison and wedding venues, and in households. The frequency of secondary attack rates was 0.3-100% with rates highest in home/quarantine settings. Three studies showed no transmission if the index case was a recurrent infection. Viral culture was performed in four studies of which three found replication-competent virus; culture results were negative where index cases had recurrent infections. Eighteen studies performed genomic sequencing with phylogenetic analysis – the completeness of genomic similarity ranged from 77-100%. Findings from systematic reviews showed that children were significantly less likely to transmit SARS-CoV-2 and household contact was associated with a significantly increased risk of infection.
Conclusions: The evidence from published studies demonstrates that SARS-CoV-2 can be transmitted in close contact settings. The risk of transmission is greater in household contacts. There was a wide variation in methodology. Standardized guidelines for reporting transmission in close contact settings should be developed.
Keywords: Close contact, transmission, COVID-19, systematic review
Introduction
The SARS-CoV-2 (COVID-19) pandemic is a major public health concern. Based on WHO data, there have been over 533 million confirmed cases and over two and a half million deaths globally as of 15th June 2022 1 . Many national governments have implemented prevention and control measures and vaccines are now being approved and administered; the overall global spread of the virus now appears to be slowing 2 , but the virus continues to evolve. Current evidence from epidemiologic and virologic studies suggest SARS-CoV-2 is primarily transmitted via exposure to infectious respiratory fluids such as fine aerosols and respiratory droplets, and to a lesser extent through fomites; however, the relative contributions of the different modes of transmission is not completely understood 3– 5 . Controversy still exists about how the virus is transmitted and the relative frequency of the modes of transmission and if these modes may be altered in specific settings 6, 7 .
Although close contact is thought to be associated with transmission of SARS-CoV-2, there is uncertainty about the thresholds of proximity for “close contact” and the factors that may influence the transmission in a “close contact”. Furthermore, there is lack of clarity about how research should be conducted in the setting of transmission with close contact which may include transmission via any one of or the combination of respiratory droplets, direct contact, or indirect contact.
Several studies investigating the role of close contact in SARS-CoV-2 transmission have been published but the pathways and thresholds for transmission are not well established. The objective of this review was to identify, appraise and summarize the evidence from primary studies and systematic reviews investigating the role of close contact in the transmission of SARS-CoV-2. Terminology for this article can be found in Box 1.
Box 1. Terminology.
Close contact: Someone who was within 6 feet of an infected person for a cumulative total of 15 minutes or more over a 24-hour period starting from 2 days before illness onset (or, for asymptomatic patients, 2 days prior to a positive test result) until the time the patient is isolated 1 ; The World Health Organization (WHO) additionally includes direct physical contact with a probable or confirmed case, direct care for a patient with probable or confirmed COVID-19 disease without using proper personal protective equipment (PPE), and other situations as indicated by local risk assessments.
Attack rate: The proportion of those who become ill after a specified exposure 2 .
Secondary attack rate: The probability that an infection occurs among susceptible persons within a reasonable incubation period after known contact with an infectious person in household or other close-contact environments 3 .
Cycle threshold: The number of cycles required for the fluorescent signal to cross the threshold. Ct levels are inversely proportional to the amount of target nucleic acid in the sample 4 .
2 https://www.who.int/foodsafety/publications/foodborne_disease/Annex_7.pdf
Methods
We are undertaking an open evidence review examining the factors and circumstances that impact on the transmission of SARS-CoV-2, based on our published protocol last updated on the 1 December 2020 (Version 3: 1 December 2020, Extended data: Appendix 1 8 ). This review aims to identify, appraise, and summarize the evidence (from peer-reviewed studies or studies awaiting peer review) examining the role of close contact in the transmission of SARS-CoV-2 and the factors that influence transmissibility. We are conducting an ongoing search in WHO Covid-19 Database, LitCovid, medRxiv, and Google Scholar for SARS-CoV-2 for keywords and associated synonyms. For this review, we also conducted searches on PubMed. The searches for this update were initially conducted up to 20th December 2020 ( Extended data: Appendix 2 8 ). The searches were further updated till 30th April 2022. We did not impose any language restrictions. Two reviewers (IJO and JB) independently screened articles to determine eligibility. A third reviewer (EAS) independently cross-checked the data. Any disagreements were resolved through discussion.
We included studies of any design that investigated transmission associated with close contact but excluded predictive or modelling studies. We reviewed the results for relevance and for articles that appeared particularly relevant, we undertook forward citation matching to identify relevant results. We assessed the risk of bias of included primary studies using five domains from the QUADAS-2 criteria 9 ; we adapted this tool because the included studies were not primarily designed as diagnostic accuracy studies. We examined the following domains in each included study: 1 – Did the authors describe the study methods in sufficient detail to allow for replication of the study? 2 – Were the sample studies clearly described? 3 – Was the reporting of the results and the analysis of the findings appropriate? 4 – Did the study authors account for any limitations due to bias? 5 – Are the study results applicable to the study population? We did not perform formal assessments of the quality of included systematic reviews but summarized their findings, including quality of their included studies as reported by the authors. We extracted the following information from included studies: study design characteristics including the definition used for “close contact”, population, main methods, and associated outcomes including the number of swab samples taken with frequency and timing of samples, and cycle thresholds. We also extracted information on viral cultures including the methods used. One reviewer (IJO) assessed the risk of bias from primary studies, and these were independently verified by a second reviewer (EAS). One reviewer (IJO) extracted data from the included primary studies, and these were independently checked by a second reviewer (CJH). One reviewer (CJH) extracted data from the included systematic reviews, and these were independently checked by a second reviewer (IJO). Disagreements in the data extraction or bias assessments were resolved by consensus. We presented the results in tabular format, and bar charts used to present the frequency of positive tests. We reported results of specific subgroups of studies where relevant. Because of substantial heterogeneity across the included studies, we considered meta-analyses inappropriate.
Results
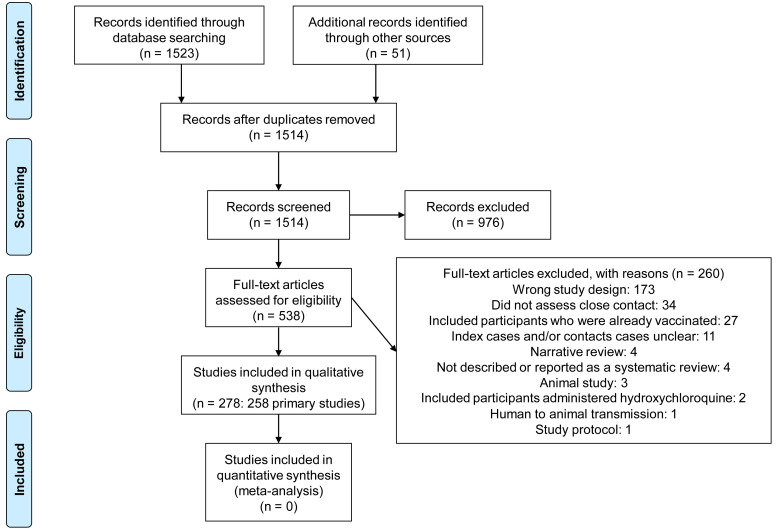
We identified 1514 non-duplicate citations of which 538 were considered eligible ( Figure 1). We excluded 260 full-text studies for various reasons (see Extended data: Appendix 3 8 for the list of excluded studies and reasons for exclusion). Finally, we included 278 studies: 258 primary studies and 20 systematic reviews (see Extended data: Appendix 4 for references to included studies). The main characteristics of the included primary studies and systematic reviews are shown in Table 1 and Table 2, respectively.
Figure 1. Flow diagram showing the process for inclusion of studies assessing close contact transmission in SARS-CoV-2.
Table 1. Main Characteristics of Included Studies Conducted in Close Contact Settings.
| Study ID | Country | Study Design/
Setting |
Type of
transmission |
Population/
environment |
Test method | Timing of
sample collection |
Viral
culture |
Cycle
threshold |
Other information |
|---|---|---|---|---|---|---|---|---|---|
| Abdulrahman 2020 | Bahrain | Observational
comparative Country-wide 09/2020 |
Community | Before and after study
of subjects attending 2 religious events |
PCR | Not reported | No | >40 was
considered negative |
A 10-day period before the event was compared to a 10-
day period beginning 10 days after the event. All symptomatic individuals and close contacts to a confirmed case were tested. Positive and negative controls were included for quality control purposes. |
| Adamik 2020 | Poland | Observational
Home |
Household | 9756 index cases;
3553 secondary cases |
Not reported | Not reported | No | No | Only cases for which clear epidemiological links were
registered as household transmission together with their source cases were included. Cases in social care units and households of minimum 15 inhabitants were removed from the analysis, as an initial analysis revealed that those were not representative for the overall population, due to over-represented comorbidities and severe cases. |
| Afonso 2021 | Brazil | Observational
- cross-sectional Homes June 15 to October 28, 2020 |
Household | 267 children and
adolescents who were household contacts of parents and/or relatives who were essential workers (index cases) |
RT-PCR | Within 10-12
days of contact with index case |
No | <25, 25–30,
or >30 |
Ct cut-offs corresponded to high, moderate, or low viral
load respectively. Essential workers included HCWs, public security workers, university staff and others. |
| Agergaard 2020 | Denmark | Home
quarantine with 1 asymptomatic index case 11/03/2020 to 01/04/2020 |
Household | Family cluster of 5:
Index case arranged a self-imposed 2-week home quarantine along with family of four |
PCR
Serology |
Not reported
for PCR |
No | Not specified
for PCR |
Index case recently returned from skiing trip in Austria
iFlash SARS-CoV-2 N/S IgM/IgG cut-off: ≥12 AU/ml = positive. DiaSorin SARS-CoV-2 S1/S2 IgG cut-off: ≥15 AU/ml = positive |
| Akaishi 2021 | Japan | Observational
Homes Community July 2020 to March 2021 |
Household
Community |
2179 participants
with recent history of close contact in home, dormitory, school, workplace, hotels, restaurants, bars, cars, or other places |
RT-PCR | Unclear | No | Not specified | 4550 participants had reliable data regarding the place of
contact; however, only 2179 of these were documented as close contacts. The study period was before the replacement of major viral strains spreading in the locality from the original strains to N501Y mutant strains in May 2021. |
|
Angulo-Bazán
2021 |
Peru | Observational
retrospective Household 23/04/2020 to 02/05/2020 |
Household | 52 households in
Metropolitan Lima with only one member with COVID-19 Contacts cohabited in same home with index case |
RT-PCR
(index) Serology |
Not reported | No | Not specified | Evaluation was conducted 13.6 ± 3.7 days after the
diagnostic test |
| Armann 2021 | Germany | Observational
- cross-sectional Schools, homes May to October 2020 |
Local
Household |
1538 students and
507 teachers were initially enrolled, and 1334 students and 445 teachers completed both study visits. |
Serology | Week 0 and
Week 16 |
No | N/A | an index (S/C) of < 1.4 was considered negative whereas
one >/= 1.4 was considered positive) and an ELISA detecting IgG against the S1 domain of the SARS-CoV-2 spike protein (Euroimmun® Anti-SARS CoV-2 ELISA) (a ratio < 0.8 was considered negative, 0.8–1.1 equivocal, >1.1 positive) |
| Arnedo-Pena 2020 | Spain | Retrospective
cohort Homes February-May 2020 |
Household | 347 index cases: 745
household contacts |
RT-PCR | Not reported | No | Not specified | COVID-19 cases of community outbreaks and from
institutions as nursing homes were excluded. Secondary attack rate was defined as the proportion of secondary cases from the total of contacts that live in the household of index case. |
| Atherstone 2021 | USA | Observational
Community December 2020 |
Community | 441 close contacts of
COVID-19 patients at 2 high school wrestling tournaments |
RT-PCR | Unclear | No | Not specified | 5 close contacts excluded because of previous SARS-CoV-
2 positive test |
| Baettig 2020 | Switzerland | Retrospective
case series Military canton March 2020 |
Local | 1 index case; 55
contacts |
RT-PCR
Serology |
PCR: Within
24 hrs of index case for symptomatic subjects Serology: 14 days post- exposure |
No | Not reported | Positive cases were defined with two positive PCR testing
for SARS-CoV-2 from nasopharyngeal swabs. |
| Baker 2020 | USA | Observational
Acute-care hospital |
Nosocomial | 44 HCWs who
provided care for a hospitalized patient with COVID-19 without PPE due to delayed diagnosis of COVID-19 |
RT-PCR | Not reported | No | Not specified | Contact and droplet precautions (including eye
protection) were instituted |
| Bao 2020 | China | Observational
Entertainment venue January and February 2020 |
Community | Potentially exposed
workers, customers and their family members potentially exposed to COVID-19 subject at a swimming pool |
RT-PCR | Not reported | No | Not specified | Men and women exhibited different usage behaviour in
that male bathers occupied the entire area, but mainly stayed at the lounge hall, while female bathers always went home after a bath. The temperature and humidity were significantly higher than what they would have been in an open air-conditioning environment. |
| Basso 2020 | Norway | Observational
study Hospital |
Nosocomial | Quarantined HCWs
exposed to COVID-19 patient |
PCR
Serology |
Approximately
2 weeks after viral exposure; 3 weeks for serology |
No | N/A
S/CO ratio ≥1 is positive for antibody |
The HCWs were quarantined for 2 weeks due to
participation in aerosol-generating procedures (AGPs) with insufficient personal protective equipment (PPE), or close contact viral exposure (defined as ≤2 m for ≥15 min). |
| Bays 2020 | USA | Observational
study Community hospital and university medical centre February and March, 2020 |
Nosocomial | Two index patients
and 421 exposed HCWs |
RT-PCR | Not reported | No | Not specified | Exposed staff were identified by analyzing the EMR
and conducting active case finding in combination with structured interviews. They wore neither surgical masks nor eye protection, and were risk stratified based on examination of the medical record and subsequent phone interviews as follows: high risk: nose or mouth exposed during intubation or bronchoscopy; moderate: nose or mouth exposed and for over 2 minutes; and low: nose or mouth exposed under 2 minutes. Ct was 25 for 1 index case - day 15 |
| Bender 2021 | Germany | Observational
- cohort Homes Community February to March 2020 |
Community | 59 index cases; 280
contacts |
Not specified | Not specified | No | Not specified | |
|
Bernardes-Souza
2021 |
Brazil | Observational-
case-control Homes May and June 2020 |
Household | 95 cases and 393
controls Index cases were logistics workers |
RT-PCR
Serology |
Beginning of
each visit |
No | N/A | Logistics worker was defined as an individual with an
occupation focused on the transportation of people or goods and whose job involves traveling outside the town of residence at least once a week. A sample was considered positive if IgM or IgG antibodies were detectable. |
| Bhatt 2022 | Canada | Observational
- cohort Home Sept 2020 to March 2021 |
Household | 180 index participants;
515 household contacts |
RT-PCR
Serology |
At the study
visit: within 14 days of patient screening or consent |
No | Not specified | Samples were considered antibody positive for a
particular isotype (IgG, IgA or IgM) when both antispike and anti-nucleocapsid antibodies were detected above the cut-off values (signal-to-cut-off value ≥ 1) for that isotype. Samples were considered positive for SARS-CoV- 2 antibody if they were positive for IgG or for both IgA and IgM. |
| Bi 2020 | China | Retrospective
cohort Home or quarantine facility January-February 2020 |
Local
Household Community |
391 SARS-CoV-2
cases and 1286 close contacts |
RT-PCR | RT-PCR | No | Not reported | Close contacts were identified through contact tracing of
a confirmed case and were defined as those who lived in the same apartment, shared a meal, travelled, or socially interacted with an index case 2 days before symptom onset. Casual contacts (e.g., other clinic patients) and some close contacts (e.g., nurses) who wore a mask during exposure were not included in this group. |
| Bi 2021 | Switzerland | Observational
- cross-sectional Homes April 3rd to June 30th 2020 |
Household | 4534 household
members |
Serology | N/A | No | N/A | IgG antibodies by ELISA |
| Bistaraki 2021 | Greece | Observational
- cohort Homes Community October to December 2020 |
Household
Community |
29,385 index cases;
64,608 contacts |
Not specified | Not specified | No | Not specified | Various social distancing measures were imposed
depending on the COVID-19 risk of each regional unit in Greece. Lockdown in place |
| Bjorkman 2021 | USA | Observational
Residence halls in university August 17 – November 25 2020 |
Local | 6408 residential
students |
RT-qPCR | Not specified | No | N/A | |
| Blaisdell 2020 | USA | Observational
study 4 overnight camps June–August 2020 |
Community | Multi-layered
prevention and mitigation strategy 642 children and 380 staff members, aged 7–70 years |
RT-PCR | 4.1 to 9.1 days
after camp arrival |
No | Not specified | Hygiene measures: Precamp quarantine, pre- and
postarrival testing and symptom screening, cohorting, and physical distancing between cohorts. In addition, camps required use of face coverings, enhanced hygiene measures, enhanced cleaning and disinfecting, maximal outdoor programming, and early and rapid identification of infection and isolation. |
| Böhmer 2020 | Germany | Observational
Workplace, home January-February 2020 |
Local
Household |
1 index case; 241
contacts |
RT-PCR
WGS |
3-5 days post-
exposure |
No | Not reported | |
|
Boscolo-Rizzo
2020 |
Italy | Cross-sectional
Homes March to April 2020 |
Household | 179 primary cases;
296 household contacts |
RT-PCR | Unclear | No | Not reported | |
| Brown 2020 | USA | Survey - cross-
sectional Classroom February to March, 2020 |
Local | Students exposed
to an index case (teacher) |
Serology | 2 weeks post-
exposure to index case |
No | Reciprocal
titres of >400 considered positive Reciprocal titres of >100 but <400 considered indeterminate |
|
| Burke 2020 | USA | Observational
prospective Homes February to March 2020 |
Household | 10 primary cases; 445
close contacts |
Not reported | Within 2 weeks
of exposure to infected case |
No | Not reported | 19 (4%) of the 445 contacts were members of a patient’s
household, and five of these 19 contacts continued to have household exposure to the patient with confirmed COVID-19 during the patient’s isolation period; 104 (23%) were community members who spent at least 10 minutes within 6 feet of a patient with confirmed disease; 100 (22%) were community members who were exposed** to a patient in a health care setting; and 222 (50%) were health care personnel |
| Calvani 2021 | Italy | Observational
- case-control Homes Schools October to December 2020 |
Local
Household |
162 children (81 SARS-
CoV-2 positive and 81 Controls) |
Antigen rapid
detection test (Ag RDT) NAAT |
Not specified | No | Ag RDT < 10 UI
was confirmed by a positive SARS-CoV-2 NAAT result |
|
| Canova 2020 | Switzerland | Observational
case series Primary care setting |
Nosocomial | 1 index case; 21 HCWs
who interacted with index case without PPE |
RT-PCR | 7 days after
the initial exposure |
No | Not reported | |
| Carazo 2021 | Canada | Observational
- cross-sectional Homes May 6 to June 22 2020 |
Household | 9,096 household
contacts of 4,542 SARS-CoV-2 infected HCWs |
PCR | Not specified | No | Not specified | Secondary household attack rates were estimated in a
subsample of 3,823 participants who lived in households with ≥2 members where the HCW was the first case. |
| Cariani 2020 | Italy | Retrospective
Hospital March to April 2020 |
Nosocomial | HCWs in close contact
with SARS-CoV- 2-positive cases (patients, co-workers, or relatives), or with symptoms of RTI |
RT-PCR | Not reported | No | <40
considered positive |
|
| Carvalho 2022 | Brazil | Observational
Homes 16 April to 3 November 2020 |
Household | 60 family clusters:
household contacts of HCWs |
RT-qPCR | Not specified | No | Not specified | |
| Cerami 2021 | USA | Observational
- cohort Homes April to October 2020 |
Household | 100 index cases and
208 of their household members |
PCR
WGS Phylogenetic analysis |
Within 3 weeks | No | Not specified | |
| Charlotte 2020 | France | Retrospective
Indoor choir rehearsal March 2020 |
Community | Nonventilated room;
sitting less close to one another than usual, but at <1.82m |
RT-PCR | Not reported | No | Not reported | |
| Chaw 2020 | Brunei | Observational
Various March 2020 |
Local
Community |
Primary cases:
Presumably infected at religious event in Malaysia Secondary cases: Epidemiologic link to a primary case |
RT-PCR | Not reported | No | Not reported | Household, workplace, social, and a local religious
gathering. Initial cluster of SARS-CoV-2 cases arose from 19 persons who had attended the Tablighi Jama’at gathering in Malaysia, resulting in 52 locally transmitted cases. |
| Chen 2020 | China | Aircraft
24 January 2020 |
Aircraft | Close contact to 2
passengers presenting with a fever and URTI symptoms |
RT-PCR | Not reported | No | Not reported | The aircraft was equipped with air handling systems. |
| Chen 2020a | China | Retrospective
observational Home or workplace January-March 2020 |
Local
Household |
69 recurrent-positive
patients; 209 close contacts |
RT-PCR | Every 3 days | No | Not specified | |
| Chen 2020b | China | Prospective
cohort Hospital January-February 2020 |
Nosocomial | 5 index patients; 105
HCWs |
RT-PCR
Serology |
From 14 days
post-exposure: 1st & 14th day of quarantine |
No | <40
considered positive |
|
| Chen 2020c | China | Observational
Various January to March 2020 |
Local
Household Community Nosocomial |
157 locally reported
confirmed cases, 30 asymptomatic infections: 2147 close contacts |
Not reported | Unclear | No | Not reported | Family members, relatives, friends/pilgrims, colleagues/
classmates, medical staff, and general personnel judged by the investigator. |
| Cheng 2020 | Taiwan | Observational
Homes, hospital January to March 2020 |
Household
Nosocomial |
100 confirmed cases
of confirmed: 2761 close contacts |
RT-PCR | Unclear | No | Not reported | |
| Chu 2020 | USA | Observational
Various January 2020 |
Community | Close contacts for an
early confirmed case of COVID-19 |
RT-PCR
Serology |
Unclear | No | Antibody
titres >400 considered seropositive. |
Office, community, Urgent care clinic identified via contact
tracing |
| Chu 2021 | USA | Retrospective
cohort study Household |
Household | Household contacts
of primary cases defined as children and adolescents with lab-confirmed COVID- 19 (n=224) |
Not reported | Not reported | No | Not reported | Did not distinguish between confirmed and probable
cases among household contacts. A “primary case” is camp attendee with the earliest onset date in the household and a “secondary case” as a household contact with confirmed or probable COVID-19. |
| Contejean 2020 | France | Observational
Comparative Tertiary-care university hospital Feb-Mar 2020 |
Nosocomial | HCW exposed to
COVID-19 patients |
RT-PCR | Not reported | No | Not reported:
result was +ve if 3/5 of gene targets amplified |
Hygiene measures: All employees were encouraged
to wear a face mask as often as possible in hospital (particularly in the presence of other persons), to wash/ disinfect their hands regularly (and after every contact with other persons), to stay at least 2 meters away from others, to cover their mouth and nose with a tissue or sleeve when coughing or sneezing, to put used tissues in the bin immediately and wash hands afterwards, to avoid touching eyes, mouth. Educational messages were released on the internal website and on posters placed in all hospital premises. |
| Cordery 2021 | UK | Observational
Schools Homes September 2020 |
Local
Household |
5 symptomatic cases
13 bubble contacts 8 child household contacts 15 adult household contacts |
PCR | Days 7, 14,
and 21 |
No | Not specified | |
|
COVID-19 National
Emergency Response Center 2020 |
S. Korea | Observational
Various January to March 2020 |
Local
Household Nosocomial |
30 cases; 2,370
contacts |
RT-PCR | Not reported | No | Not reported | Homes, work, hospitals |
| Craxford 2021 | UK | Observational
- cohort Homes April to July 2020 |
Household | 178 household
contacts of 137 HCWs |
Serology | Within 5
months of tracking HCWs |
No | N/A | |
| Danis 2020 | France | Observational
case series Chalet, school January to February 2020 |
Local
Household |
I adult case with 15
contacts in chalet; 1 paediatric case with 172 school contacts |
RT-PCR | Within 5 days
of diagnosis of cases |
No | Not reported | The index case stayed 4 days in the chalet with 10 English
tourists and a family of 5 French residents. One paediatric case, with picornavirus and influenza A coinfection, visited 3 different schools while symptomatic. |
| Dattner 2020 | Israel | Observational
Home March to June 2020 |
Household | 637 households,
average household size of 5.3 |
RT-PCR
Serology |
Serology: 4
weeks post PCR testing |
No | Not reported | |
| de Brito 2020 | Brazil | Observational
descriptive Household April-May 2020 |
Household | Socially distanced
household contacts of index case |
RT-PCR
Serology |
Serology: 4
weeks post- exposure PCR unclear |
No | Not reported | Index case: First member of the cluster who had
symptoms and who had a known risk of exposure outside the household during the family's stay in the same condominium; secondary case: Contacts with the index case. Asymptomatic patients: Those who had household contact and positive serology but no symptoms. Probable cases corresponded to confirmed case contacts who developed symptoms compatible with COVID despite negative serology and/or negative RT-PCR results. |
| Deng 2020 | China | Observational
Home January to February 2020 |
Household | 27 cases; 347 close
contacts |
Not reported | Not reported | No | Not reported | |
| Desmet 2020 | Belgium | Observational
- cross-sectional School November 2019 to March 2020 |
Local | 84 aged between
6 and 30 months attending day-care |
RT-PCR | First weeks of
the epidemic in Belgium |
No | Not reported | |
| Dimcheff 2020 | USA | Survey: cross-
sectional Tertiary-care referral facility June 8 to July 8, 2020 |
Community
Nosocomial Household |
HCW exposed to
COVID-19 patients either in or outside hospital |
Serology | 8 weeks post-
exposure |
No | Not reported | Hygiene measures: Daily COVID-19 symptom screening
upon building entry, exclusion of visitors from the facility, and institution of telework in remote offices or at home, isolation of confirmed COVID-19 patients, conversion of COVID-19 wards to negative pressure environments, use of PAPRs) or N95 respirators along with PPE by staff. |
| Dong 2020 | China | Observational
Homes |
Household | 135 cases; 259 close
contacts |
Not reported | Not reported | No | Not reported | |
| Doung-ngern 2020 | Thailand | Retrospective
case-control Various March to April 2020 |
Local | 3 large clusters in
nightclubs, boxing stadiums, and a state enterprise office |
RT-PCR | Not reported | No | Not reported | Hygiene measures: Consistent wearing of masks,
handwashing, and social distancing in public. |
| Draper 2020 | Australia | Observational
Various March to April 2020 |
Local
Household Nosocomial |
28 cases; 445 close
contacts |
RT-PCR | Within 2 weeks
of exposure to infected case |
No | Not reported | Cruise ship, homes, aircraft, hospital |
| Dub 2020 | Finland | Retrospective
cohort (2) School and Household |
Local
Household |
School and household
contacts of 2 index cases who contracted COVID-19 at school |
RT-PCR
Serology |
Serology: >4
weeks post- exposure |
No | MNT titre of
≥ 6 considered positive FMIA titre 3·4 U/ml considered positive |
|
|
Expert Taskforce
2020 |
Japan | Observational
prospective Cruise ship February 2020 |
Local | 3,711 persons in
cruise ship |
RT-PCR | Not reported | No | Not reported | Passengers were allowed a 60-minute period on an
exterior deck each day, during which they were instructed to wear masks, refrain from touching anything, and maintain a 1-meter distance from others. Monitors observed these periods. After each group came a 30- minute period in which the areas were disinfected. Room cleaning was suspended. Food and clean linens were delivered to cabin doors by crew, and dirty dishes and linens were picked up at cabin doors by crew. Only symptomatic close contacts were tested initially. |
| Farronato 2021 | Italy | Observational
Homes June 2020 to August 2020 |
Household | 49 child contacts of
52 cases |
Serology | 22 and 152
days of diagnosis |
No | N/A | Anti-S1 and anti-nucleocapsid ELISA. CBC buffer as
controls. |
|
Fateh-Moghadam
2020 |
Italy | Observational
Various March to April 2020 |
Community | 2,812 cases; 6,690
community contacts |
Not reported | Not reported | No | Not reported | Institutional settings including nursing homes, hospitals,
day and residential centers for the disabled and similar structures, and convents |
| Firestone 2020 | USA | Observational
retrospective Motorcycle rally August- September 2020 |
Local | 51 primary event-
associated cases, and 35 secondary or tertiary cases |
RT-PCR
WGS Phylogenetic analysis |
Unclear | No | Not reported |
Secondary cases: Laboratory-confirmed infections in
persons who did not attend the rally but who received SARS-CoV-2–positive test results after having contact with a person who had a primary case during their infectious period. Tertiary cases were laboratory-confirmed cases in persons who had contact with a person who had a secondary case during their infectious period. SARS-CoV-2 RNA-positive clinical specimens were obtained from clinical laboratories, and |
| Fontanet 2021 | France | Retrospective
cohort study School March to April 2020 |
Local | 2004 participants:
pupils, their parents and siblings, as well as teachers and non- teaching staff of a high school |
Serology | 10 weeks | No | N/A | |
| Galow 2021 | Germany | Observational
Homes June 2020 |
Household | 143 index cases; 248
household contacts |
Serology | Not specified | No | N/A | |
|
Gamboa Moreno
2021 |
Spain | Observational
Schools Homes Sept. 7 to Oct. 31, 2020 |
Household
Community |
Exposures:
School - 729 Home - 974 |
PCR | Not specified | No | Not specified | There were strict non-pharmaceutical measures at school
settings and proper epidemiological surveillance. |
| Gan 2020 | China | Observational
retrospective survey Various January-February 2020 |
Local
Household Community |
1 052 cases in 366
epidemic clusters |
Not reported | Not reported | No | Not reported | Family living together, gathering dinner, collective work,
ride-thy-car, other aggregation exposure, |
| Gaskell 2021 | UK | Observational
- cross-sectional Homes October to December 2020 |
Household | 343 households with
1242 participants |
Serology | Within 10
days of completing the questionnaire |
No | N/A | Strictly orthodox Jewish Community. |
| Ge 2021 | China | Observational
- cohort Homes Community January to August 2020 |
Household
Community |
730 index patients
8852 close contacts |
RT-PCR | Unclear: index
patients and their contacts received regular testing |
No | Not specified | Close contacts were centrally quarantined for at least 14
days except in areas with limited resources where home self-quarantine was alternatively suggested. |
| Ghinai 2020 | USA | Observational
2 Social gatherings January-March 2020 |
Community | 16 cases (7 confirmed
and 9 probable) (1 index case) |
RT-PCR | Not reported | No | Not reported | A birthday party, funeral, and church attendance. |
| Gold 2021 | USA | Observational
School Homes Dec. 1, 2020–Jan. 22, 2021 |
Local
Household |
9 school clusters
(n=45); 69 household members |
RT-PCR | Within
5–10 days of their last documented in-school exposure |
No | Not specified | Students and staff members exposed to a COVID-19
patient were advised to quarantine for a minimum of 7 days if a specimen collected ≥5 days after exposure was negative for SARS-CoV-2 and they remained asymptomatic or for 10 days if they were not tested and remained asymptomatic. |
| Gomaa 2021 | Egypt | Observational
- cohort Homes April to October 2020 |
Household | 23 index cases; 98
household contacts |
RT-PCR
Serology |
Days 1, 3, 6, 9
and 14 |
No | Not specified | Microneutralization Assay used to test for antibodies |
| Gonçalves 2021 | Brazil | Observational
- case-control Homes April–June 2020 |
Household | 271 case-patients and
1,396 controls |
RT-PCR
Serology |
Not specified | No | Not specified | Controls were the seronegative persons in 3
representative community surveys of SARS-CoV-2 antibody prevalence. |
| Gong 2020 | China | Observational
Various January-February 2020 |
Household
Community |
3 clusters: 5 index
cases; 9 close contacts |
RT-PCR | Not reported | No | Not reported | Travelling and dining, or were living together. |
| Gu 2020 | China | Observational
Karaoke room January 2020 |
Local | 14 people exposed
to 2 index cases in a karaoke room |
RT-PCR
Serology |
PCR: Within
72 hrs post- exposure Serology: 6 weeks post- exposure |
No | Not reported | |
| Hamner 2020 | USA | Observational
Choir practice March 2020 |
Local | 1 index case; 60 close
contacts |
RT-PCR | Within 2 weeks
of index case |
No | Not reported | |
| Han 2020 | S. Korea | Observational
Spa facility Mar-April 2020 |
Community | Contacts for 10 index
cases from Spa facility |
RT-PCR | Not reported | No | Not reported | |
| Hast 2022 | USA | Observational
School December 2020–January 2021 |
Local | 90 index cases; 628
school contacts |
RT-PCR | 5 to 7 days
post-exposure; up to 10 days where necessary |
No | Not specified | |
| Heavey 2020 | Ireland | Observational
School March 2020 |
Local | 6 index cases; 1155
contacts |
Not reported | Not reported | No | No | Three paediatric cases and three adult cases of COVID-
19 with a history of school attendance were identified. Exposed at school in the classroom, during sports lessons, music lessons and during choir practice for a religious ceremony, which involved a number of schools mixing in a church environment. |
| Helsingen 2020 | Norway | RCT
Training facilities May-June 2020 |
Local | Members of the
participating training facilities age 18 years or older who were not at increased risk for severe Covid-19 |
RT-PCR
Serology |
Serology: 4
weeks after start of study |
No | Not reported | Hygiene measures: Avoidance of body contact; 1
metre distance between individuals at all times; 2 metre distance for high intensity activities; provision of disinfectants at all workstations; cleaning requirements of all equipment after use by participant; regular cleaning of facilities and access control by facility employees to ensure distance measures and avoid overcrowding. Changing rooms were open, but showers and saunas remained closed. All participants were mailed a home-test kit including two swabs and a tube with virus transport medium for SARS-CoV-2 RNA. |
| Hendrix 2020 | USA | Observational
Hair salon May 2020 |
Local | Contacts for 2 stylists
who tested positive for COVID-19 |
PCR | Not reported | No | Not reported | Hygiene measures: During all interactions with clients
at salon A, stylist A wore a double-layered cotton face covering, and stylist B wore a double-layered cotton face covering or a surgical mask. |
| Hirschman 2020 | USA | Observational
study Home and social gatherings June 2020 |
Household
Community |
2 index cases;
58 primary and secondary contacts |
RT-PCR | Unclear | No | Not reported | |
| Hobbs 2020 | USA | Case-control
study University Medical Centre September- November 2020 |
Local
Household Community |
397 children and
adolescents: Cases 154; controls 243 |
RT-PCR | Not reported | No | Not reported | |
| Hoehl 2021 | Germany | Observational
Daycare Centre 12 weeks (June- Sept 2020) |
Local
Community |
Attendees and staff
from 50 day-care centres |
RT-PCR | Not reported | No | Not reported | Hygiene measures: Barring children and staff with
symptoms of COVID-19, other than runny nose, from entering the facilities, as well as denying access to individuals with known exposure to SARS-CoV-2. Access to the facilities was also denied to children if a household member was symptomatic or was in quarantine due to contact with SARS-CoV-2. Wearing of masks was not mandatory for children or nor staff. The access of caregivers to the facilities was limited. |
| Hong 2020 | China | Observational
prospective Home January-April 2020 |
Household | 9 patients with
recurrent infection; 13 close contacts |
RT-PCR
Serology NGS |
After re-
admission of index patients. |
No | Not reported | |
| Hsu 2021 | Taiwan | Observational
Homes Jan. 28/01/2020 to 28/02/2021 |
Household | 18 index cases, 145
household contacts |
RT-PCR | Testing
was done if contacts showed symptoms |
No | Not specified | |
| Hu 2020 | China | Observational
Train travellers 19 Dec. 2019 to 6 Mar. 2020 |
Local | 2334 index patients
and 72 093 close contacts who had co-travel times of 0-8 hours |
Not specified | Not specified | No | Not specified | |
| Hu 2021 | China | Observational
retrospective Various January to April 2020 |
Household
Community |
1178 cases; 15,648
contacts |
Not reported | Not reported | No | Not reported | Homes, social events, travel, other settings. |
| Hu 2021 | China | Observational
Aircrafts Jan. 4 to Mar. 14, 2020 |
Local | 175 index cases; 5622
close contacts |
RT-PCR | Not specified | No | Not specified | |
| Hua 2020 | China | Observational
retrospective Home January to April 2020 |
Household | Children and adult
contacts from the 314 families |
RT-PCR | Not reported | No | Not reported | |
| Huang 2020 | China | Prospective
contact-tracing study Restaurant, home January 2020 |
Household
Community |
1 index case; 22 close
contacts |
RT-PCR | Within 3 days
of index cases |
No | Not reported | Close contacts quarantined at home or hospital. |
| Huang 2020a | Taiwan | Retrospective
case series Various January-April 2020 |
Local
Household Community Nosocomial |
15 primary cases:
3795 close contacts |
RT-PCR | Not reported | No | Not reported | Aircraft, home, classroom, workplace, hospital. |
| Huang 2021 | Taiwan | Observational
Hospital Feb. to Mar. 2020 |
Nosocomial | 181 close contacts:
HCWs (n=127), in-patients (n=27), persons accompanying hospital patients (n=27) |
RT-PCR
WGS Phylogenetic analysis |
Not specified | No | 21.3 on day
9 and 16.7 on day 12 for index case |
The index case was admitted due to heart failure and
cellulitis. |
| Islam 2020 | Bangladesh | Observational
Various March to June 2020 |
Local
Household Community Nosocomial |
181 cases; 391 close
contacts |
Not reported | Not reported | No | Not reported | Household, health care facility, funeral ceremony, public
transportation, family members, and others. |
| Jashaninejad 2021 | Iran | Observational
- cohort Homes Mid-May to mid- July, 2020 |
Household | 323 index cases and
989 related close contacts |
RT-PCR | Unclear: after
identification through contact tracing |
No | Not specified | |
| Jeewandara 2021 | Sri-Lanka | Observational
- cohort Homes Community 15 April 2020 to 19 May 2020 |
Household
Community |
3 cases; 1093 close
contacts |
RT-PCR
Serology WGS Phylogenetic analysis |
Within 14 days | No | Not specified | All RT-qPCR positive, close contacts were classified as
cases and were hospitalized. RT-qPCR negative contacts were directed to a quarantine facility for 14 days to ensure that they stay isolated under observation of health staff. -COV-2 Total antibody responses were assessed using ELISA. |
| Jia 2020 | China | Observational
Home January to February 2020 |
Household | 11 clusters (n=583) | RT-PCR | Not reported | No | <37
considered positive |
A
close contact was defined as a person who did
not take effective protection against a suspected or confirmed case 2 d before the onset of symptoms or an asymptomatic infected person 2 d before sampling. Ct-value of 40 or more was defined as negative. |
| Jiang 2020 | China | Observational
Home January to February 2020 |
Household
Community |
8 index cases, 300
contacts |
rRT-PCR
WGS Phylogenetic analysis |
Every 24 hours
for 2 weeks |
No | <37
considered positive |
Ct value ≥40 was considered negative. The maximum
likelihood phylogenetic tree of the complete genomes was conducted by using RAxML software with 1000 bootstrap replicates, employing the general time- reversible nucleotide substitution mode. |
| Jing 2020 | China | Retrospective
cohort study Homes January-February 2020 |
Household | 195 unrelated close
contact groups (215 primary cases, 134 secondary or tertiary cases, and 1964 uninfected close contacts) |
RT-PCR | Days 1 and 14
of quarantine |
No | Not reported | |
| Jing 2020a | China | Observational
study Homes, public places February 2020 |
Household
Community |
68 clusters involving
217 cases |
RT-PCR | Not reported | No | Not reported | |
| Jones 2021 | UK
France |
Observational
Super League Rugby August to October 2020 |
Local | 136: 8 index cases:
28 identified close contacts and 100 other players |
RT-PCR | Within 14 days
of match day |
No | Not specified:
Ct for index cases 17.8 to 27 |
Close contacts were defined by analysis of video footage
for player interactions and microtechnology (GPS) data for proximity analysis. All participants were within a ≤7- day RT-PCR screening cycle. |
| Jordan 2022 | Spain | Observational
Schools 29 June to 31 July 2020 |
Local | 2 index cases; 253
close contacts |
RT-PCR
Serology |
Days 0, 7, 14
for PCR; 0 and 5 weeks for serology |
No | Not specified | Stringent infection control measures were in place. IgG
serology. |
| Kang 2020 | S. Korea | Observational
Night clubs April-May 2020 |
Local | 96 primary cases and
150 secondary cases; 5,517 visitors |
Not reported | Not reported | No | Not reported | |
| Kant 2020 | India | Retrospective
(contact tracing) Regional Medical Research Centre May 2020 |
Local
Community Nosocomial |
1 index case
diagnosed post- mortem: number of exposures unclear |
RT-PCR | Unclear | No | Not reported | Contacts traced: People from the market where the index
case had his shop, his treating physicians, people who attended his funeral, family members and friends. |
|
Karumanagoundar
2021 |
India | Observational
- cohort Homes Community March–May 2020 |
Household
Community |
931 primary cases; 15
702 contacts |
RT-PCR | Not specified | No | Not specified | |
| Katlama 2022 | France | Observational
Homes July to September 2020 |
Household | 87 index cases and
255 contacts |
Serology | Prior to a
potential second wave of the epidemic that emerged in France in early October 2020 |
No | N/A | The presence of IgG antibodies against the nucleocapsid
protein was measured and interpreted using commercially available chemiluminescent microparticle immunoassay (CMIA) kits. |
| Kawasuji 2020 | Japan | Case-control
study University Hospital April-May 2020 |
Nosocomial | 28 index cases: 105
close contacts |
RT-PCR | Unclear | No | Not reported | Index patients and those with secondary transmission
were estimated based on serial intervals in the family clusters. |
| Khanh 2020 | Vietnam | Retrospective
Aircraft March 2020 |
Community | 1 index case: 217
close contacts |
PCR | 4 days after
positive test result of index case |
No | Not reported | Successfully traced passengers and crew members were
interviewed by use of a standard questionnaire, tested for SARS-CoV-2. |
| Kim 2020 | S. Korea | Retrospective
observational Home setting January-April 2020 |
Household | 107 paediatric index
cases: 248 household members of which 207 were exposed |
RT-PCR | Within 2 days
of COVID-19 diagnosis of the index case |
No | Ct value of ≤35
is positive and >40 is negative |
Guardian wore a KF94 (N95 equivalent) mask, gloves, full
body suit (or waterproof long-sleeve gowns) and goggles. |
| Kim 2020a | S. Korea | Case series
Various January-February 2020 |
Household
Community |
1 index case; 4 close
contacts |
RT-PCR | 4 days post-
exposure |
No | N/A | 2 household contacts, 1 church contact, 1 restaurant |
| Kim 2020b | S. Korea | Retrospective
observational University hospital February 2020 |
Nosocomial | 4 confirmed cases:
290 contacts |
RT-PCR | Within 8 days
of index case diagnosis |
No | Ct <35 was
considered positive |
Medical staff in the triage room used level-D PPE and
everyone in the hospital was encouraged to wear masks and follow hand hygiene practices. Contact with confirmed COVID-19 cases was frequent among inpatients and medical support personnel. |
| Kim 2021 | S. Korea | Observational
Dental clinic May 2020 |
Local | 1 index case, 8 close
contacts (HCWs) |
RT-PCR
Serology |
Start and
the end of a two-week quarantine. Serologic tests were performed one to two months post- quarantine |
No | 22.38 for RdRp
and 22.52 for E genes |
All HCWs wore particulate filtering respirators with 94%
filter capacity and gloves, but none wore eye protection or gowns. Patient (index case) did not wear a face mask. |
| Kitahara 2022 | Japan | Observational
- cohort Homes Community Aug. 1 to Sept. 6 2020 |
Household
Community |
20 index cases; 114
close contacts |
RT-PCR | 1-3 days after
identification and symptoms onset |
No | Not specified | |
| Klompas 2021 | USA | Observational
- case-control Hospital (acute care) September 2020 |
Nosocomial | 1 large cluster with
1 index case; 1457 direct and associated contacts |
RT-PCR
WGS Phylogenetic analysis |
Every 3 days | No | Not specified | |
| Kolodziej 2022 | Netherlands | Observational
- cohort Homes October to December 2020 |
Household | 85 index cases; 241
household contacts |
RT-PCR
Serology WGS Phylogenetic analysis |
Saliva samples
by self- sampling at day 1, 3, 5, 7, 10, 14, 21, 28, 35, and 42; NPS and OPS sample day 7; Capillary blood day 42 |
No | Not specified | |
| Koureas 2021 | Greece | Observational
Homes 8 April–4 June 2020 |
Household | 40 infected
households: 135 cases and 286 contacts |
RT-PCR | Day 0, day 7,
day 14 |
No | Not specified | |
| Kumar 2021 | India | Observational
Community March-May 2020 |
Community | 144 source cases: | RT-PCR | Unclear | No | Not reported | Persons with symptoms of ILI and SARI as well as known
high-risk contacts of a confirmed COVID-19 patient were included. |
| Kuwelker 2021 | Norway | Prospective
case-ascertained study Homes Feb-April 2020 |
Household | 112 index cases; 179
household members |
Serology | 6-8 weeks
after symptom onset in the index case. |
No | N/A | Single-person households were excluded from the
analysis. Serum samples from index cases and household members were collected 6-8 weeks after symptom onset in the index case. |
| Kuwelker 2021 | Norway | Observational
- cohort Homes 28th February to 4th April 2020 |
Household | 112 households (291
participants) |
Serology | 6–8 weeks
after NP sampling of index patient |
No | Individuals
with titres ≥100 were defined as positive |
ELISA was used for detecting SARS-CoV-2-specific
antibodies. IgG antibody. |
| Kwok 2020 | Hong Kong | Retrospective
observational Quarantine or isolation February 2020 |
Local
Household |
53 cases; 206 close
contacts |
Not reported | Not reported | No | Not reported | A
secondary case referred to the first generation of
infection induced by an index case following contact with this case. |
| Ladhani 2020 | UK | Prospective
Care homes April 2020 |
Nosocomial | 6 London care homes
reporting a suspected outbreak (2 or more cases); 254 staff members |
RT-PCR | Not reported | No | Not reported | 254 of 474 (54%) staff members provided a nasal self-
swab; 12 were symptomatic at the time of swabbing. |
| Ladhani 2020a | UK | Prospective
Care homes April 2020 |
Nosocomial | 6 London care
homes reporting a suspected outbreak (2 or more cases); 254 staff members; 264 residents |
RT-PCR | Not reported | Yes | Unclear: Ct
values <35 were cultured |
254 of 474 (54%) staff members provided a nasal self-
swab; 12 were symptomatic at the time of swabbing. |
| Laws 2020 | USA | Prospective
cohort Home setting March-May 2020 |
Household | 1 paediatric index
case: 188 household contacts |
RT-PCR | Study
enrolment (day 0); study close- out (day 14) |
No | Not reported | Index case: household member with earliest symptom
onset (and positive SARS-CoV-2 RT-PCR test result). Community prevalence in the 2 metropolitan areas was low during this time, and both were under stay-at-home orders. All enrolled index case patients and household contacts were followed prospectively for 14 days. Five households were selected for intensive swabbing requiring collection of respiratory specimens from all household members during four interim visits regardless of symptom presence. |
| Laws 2021 | USA | Observational
- cohort Homes March to May 2020 |
Household | 188 household
contacts |
RT-PCR | Days 0 and 14 | No | Not specified | |
|
Laxminarayan
2020 |
India | Observational
Various April to August 2020 |
Local
Household Community |
3,084,885 known
exposed contacts |
Not reported | Not reported | No | Not reported | Individual-level epidemiological data on cases and
contacts, as well as laboratory test results, were available from 575,071 tested contacts of 84,965 confirmed cases. |
| Lee 2020 | S. Korea | Observational
Hospital February-June 2020 |
Household | 12 paediatric cases;
12 guardians as close contact. All guardians used PPE |
Not reported | Not reported | No | Not reported | |
| Lee 2020a | S. Korea | Observational
Homes February to March 2020 |
Household | 23 close contacts | PCR | Unclear | No | Not reported | |
| Lewis 2020 | USA | Observational
Homes March to April 2020 |
Household | 58 households (Utah,
n = 34; Wisconsin, n = 24), 58 primary patients and 188 household contacts |
RT-PCR
Serology |
Not reported | No | Not reported | |
| Li 2020 | China | Observational
Home setting Feb 2020 |
Household | Family cluster of
1 index case: 5 household contacts |
RT-PCR | One day after
index case tested positive |
No | Not reported | Unknown when index case started shedding virus. |
| Li 2020a | China | Observational
case series Home, hospital January-February 2020 |
Household
Nosocomial |
2-family cluster of 1
index case: 7 close contacts |
Not reported | Not reported | No | Not reported | |
| Li 2020b | China | Retrospective
observational Home January-February 2020 |
Household | 3-family cluster of 3
index cases: 14 close contacts |
RT-PCR | Every 2–3 days
until hospital discharge. |
No | <38
considered positive |
|
| Li 2020c | China | Retrospective
observational Home January-March 2020 |
Household | 30 cases from 35
cluster-onset families (COFs) and 41 cases from 16 solitary-onset families (SOFs) |
Not reported | Not reported | No | Not reported | |
| Li 2020d | China | Observational
Household February to March 2020 |
Household | 105 index patients;
392 household contacts |
RT-PCR | Within 2 weeks
of exposure to infected case |
No | Not reported | |
| Li 2021a | China | Observational
- cohort Homes Dec 2, 2019 to April 18, 2020 |
Household | 24985 primary cases
and 52822 household contacts |
RT-PCR | Not specified | No | Not specified | |
| Li 2021b | China | Observational
Homes Community January 23- February 25, 2020. |
Household
Community |
476 symptomatic
persons; 2,382 close contacts |
PCR | Not specified | No | Not specified | |
| Lin 2021 | China | Observational
Home January 2020 |
Household | 1 paediatric index
case; 5 household contacts |
RT-PCR
Serology |
Not specified | No | Serology: Test
result ≥ 10.0 AU/mL was reported as positive |
|
| Liu 2020 | China | Retrospective
observational Home setting Feb 2020 |
Household | Family cluster of
1 index case: 7 household contacts |
RT-PCR | Immediately
after index case tested positive |
No | If both the
nCovORF1ab and nCoV-NP showed positive results, COVID-19 infection was considered |
Unclear whether the index case was actually the first case |
| Liu 2020a | China | Retrospective
case series Hospital January 2020 |
Nosocomial | 30 HCWs with direct
contact with patients |
RT-PCR | Not reported | No | <40
considered positive |
30 cases have a history of direct contact with patients
with neo-coronary pneumonia (within 1 m), 1 to 28 contacts, an average of 12 (7,16) contact times, contact time of 0.5 to 3.5 h, the average cumulative contact time of 2 (1.5, 2.7) h. |
| Liu 2020b | China | Retrospective
cohort study Various January-March 2020 |
Household
Community Nosocomial |
1158 index cases:
11,580 contacts |
RT-PCR | Every several
days |
No | Not reported | Homes, social venues, various types of transportations |
| Liu 2020c | China | Prospective
observational |
Unclear | 147 asymptomatic
carriers: 1150 close contacts |
RT-PCR | Not reported | No | Not reported | RT-PCR for asymptomatic carriers - testing method not
described for close contacts |
| Liu 2021 | USA | Observational
- cohort Homes Dec. 2020 to Feb. 2021 |
Household | 15 index cases; 50
household contacts |
RT-PCR | Every 3 days
for 14 days after index positivity. |
No | Not specified | |
| López 2020 | USA | Retrospective
contact tracing School setting April-July 2020 |
Local
Household |
12 index paediatric
cases: 101 facility contacts; 184 overall contacts |
RT-PCR | Not reported | No | Not reported | Index case: first confirmed case identified in a person at
the childcare facility Primary case: Earliest confirmed case linked to the outbreak. Overall attack rates include facility-associated cases, nonfacility contact cases and all facility staff members and attendees and nonfacility contacts. |
| López 2021 | Spain | Observational
- cohort Homes April to June 2020 |
Household | 89 index cases; 229
household members |
PCR | Not specified | No | Not specified | |
| Lopez Bernal 2020 | UK | Observational
Homes January to March 2020 |
Household
Community |
233 households with
two or more people; 472 contacts. |
PCR | Unclear | No | Not reported | Healthcare workers, returning travellers and airplane
exposures were excluded. |
| Lopez Bernal 2022 | UK | Observational
Homes Community January to March 2020 |
Household
Community |
233 households with
472 contacts |
PCR | If and when
contacts developed symptoms |
No | Not specified | |
| Lucey 2020 | Ireland | Observational
Hospital March-May 2020 |
Nosocomial | 5 HCWs in cluster 1;
2 HCWs in cluster 3; HCW in cluster 2 not specified; 52 patients infected with SARS- CoV-2; |
RT-PCR
WGS Phylogenetic analysis |
Not reported | No | Not reported | SARS-CoV-2 RNA was extracted from nasopharyngeal
swabs obtained from COVID-19 cases and their corresponding HCWs were sequenced to completion. HA COVID-19 was classified into two groups according to the length of admission: >7 days and >14 days. Majority of patients required assistance with mobility (65%) and selfcare (77%). |
| Luo 2020 | China | Observational
retrospective Public transport January 2020 |
Community | 1 index case; 243
close contacts |
RT-PCR | Within 2 weeks
of exposure to index case |
No | Not reported | The tour coach was with 49 seats was fully occupied with
all windows closed and the ventilation system on during the 2.5-hour trip. |
| Luo 2020a | China | Prospective
cohort study Various January to March 2020 |
Household
Community Nosocomial |
391 index cases; 3410
close contacts |
RT-PCR
Serology |
Every 24 hours. | No | Not reported | Homes, public transport; healthcare settings,
entertainment venues, workplace, multiple settings |
| Lyngse 2020 | Denmark | Retrospective
Homes February to July 2020 |
Household | 990 primary cases;
2226 household contacts |
Not reported | Within 14 days
of exposure to primary case |
No | Not reported | Secondary cases: those who had a positive test within 14
days of the primary case being tested positive. 3 phases of epidemic examined. Assumed that the secondary household members were infected by the household primary case, although some of these secondary cases could represent co-primary cases. A longer cut-off time period could result in misclassification of cases among household members with somewhere else being the source of secondary infections. |
| Ma 2020 | China | Observational
Medical isolation |
Unclear | 1665 close contacts | RT-PCR | Not reported | No | Not reported | |
| Macartney 2020 | Australia | Prospective
cohort study Educational settings April to May 2020 |
Local | 27 primary cases; 633
contacts |
RT-PCR,
serology, or both |
PCR: 5–10
days after last case contact if not previously collected Serology: day 21 following last case contact. |
No | Not reported |
Index case: The first identified laboratory-confirmed
case who attended the facility while infectious. A school or ECEC setting primary case was defined as the initial infectious case or cases in that setting, and might or might not have been the index case. Primary case: Initial infectious case or cases in that setting, and might or might not have been the index case Secondary case: Close contact with SARS-CoV-2 infection (detected through nucleic acid testing or serological testing, or both), which was considered likely to have occurred via transmission in that educational setting. |
| Malheiro 2020 | Portugal | Retrospective
cohort study Homes March to April 2020 |
Household | Intervention group
(n=98), Control (n=453) |
Not reported | Not reported | No | Not reported | The intervention group comprised all COVID-19
confirmed cases that were either identified as close contacts of an index caseor returned from affected areas and placed under mandatory quarantine, with daily follow-up until laboratory confirmation of SARS-CoV- 2 infection. The control group included all COVID-19 confirmed cases that were not subject to contact tracing nor to quarantine measures preceding the diagnosis. |
| Maltezou 2020 | Greece | Retrospective
observational Home setting February to June 2020 |
Household | 203 SARS-CoV-2-
infected children; number of index cases and close contacts unclear |
RT-PCR | Not reported | No | Ct >38
considered negative |
A
family cluster was defined as the detection of at least
2 cases of SARS-CoV-2 infection within a family. First case was defined as the first COVID-19 case in a family. High, moderate, or low viral load (Ct <25, 25–30 or >30, respectively). |
| Maltezou 2020a | Greece | Retrospective
observational Home setting February to May 2020 |
Household | 23 family clusters
of COVID-19; 109 household members |
RT-PCR | Not reported | No | <25, 25– 30
or >30 |
A
family cluster was defined as the detection of at least
2 cases of SARS-CoV-2 infection within a family. Index case was defined as the first laboratory-diagnosed case in the family. |
| Mao 2020 | China | Cross-sectional
study Home, family gatherings January-March 2020 |
Household
Local |
67 clusters with 226
cases confirmed cases |
RT-PCR | Not reported | No | Not reported | |
| Martínez-Baz 2022 | Spain | Observational
- cohort Homes Community 11 May to 31 December 2020 |
Household
Community |
20,048 index cases;
59,900 close contacts |
RT-qPCR | 0 and 10 days
after the last contact |
No | Not specified | |
|
Martinez-Fierro
2020 |
Mexico | Cross-sectional
June-July 2020 |
Unclear | 19 asymptomatic
index cases; 81 contacts |
RT-PCR
Serology |
Not reported | No | Not reported | |
| McLean 2022 | USA | Observational
Homes April 2020 to April 2021 |
Household | 226 primary cases,
404 household contacts |
rRT-PCR | Daily | No | Not specified | |
|
Mercado-Reyes
2022 |
Colombia | Observational
- cross-sectional Homes Sept. 21 to Dec. 11 2020. |
Household | 17863 participants | Serology | Not specified | No | N/A | |
| Metlay 2021 | USA | Observational
- cohort Homes March 4 and May 17, 2020 |
Household | 7262 index cases;
17917 household contacts |
RT-PCR | Not specified | No | N/A | |
| Meylan 2021 | Switzerland | Observational
- cross-sectional Hospital 18 May and 12 June 2020. |
Nosocomial | 1872 HCWs | Serology | Over a 4-week
period |
No | N/A | |
| Miller 2021 | UK | Observationa
- cohort Homes May 2020 |
Household | 431 contacts of 172
symptomatic index cases |
PCR
Serology |
PCR: days 0
and 7 Serology: day 35 |
Yes | ≤39 | |
| Montecucco 2021 | Italy | Observational
University October 2020 – March 2021 |
Local
Household Community |
53 cases; 346 close
contacts. |
RT-PCR | Not specified | No | Not specified | |
| Mponponsuo 2020 | Canada | Observational
Hospital March-April 2020 |
Nosocomial | 5 HCWs were index
cases; 39 HCWs (16 underwent testing) and 33 patients were exposed (22 underwent testing) |
RT-PCR | Not reported | No | Not reported | All 5 HCWs had E gene cycle threshold (Ct) values
between 10.9 and 30.2. Those exposed to the index HCWs were followed for 30 days. |
| Musa 2021 | Bosnia and
Herzegovina |
Observational
Homes August– December 2020 |
Household | 383 households and
793 contacts |
RT-PCR | Within 2–14
days |
No | Not specified | |
| Ng 2020 | Singapore | Retrospective
cohort study Various January-April 2020 |
Household
Local Community |
1114 PCR-confirmed
COVID-19 index cases in the community in Singapore. 13 026 close contacts (1863 household, 2319 work, and 3588 social) |
RT-PCR
Serology |
If contacts
reported symptoms |
No | Not reported |
Lower risk contacts: Other contacts who were with the
index case for 10–30 min within 2 m Contacts who reported symptoms were admitted to the hospital for COVID-19 testing by PCR. |
| Ng 2021 | Malaysia | Observational
Homes 1 Feb. to 31 Dec. 2020 |
Household | 185 index patients;
848 household contacts |
RT-PCR | Within 0–14
days |
No | Not specified | |
| Ning 2020 | China | Observational
study Various January-February 2020 |
Household
Local Community |
Local cases: 3,435
close contacts Imported cases: 3,666 close contacts |
Not reported | Not reported | No | Not reported | Imported cases, farmers' markets, malls, and wildlife
exposure. |
| Njuguna 2020 | USA | Observational
Prison May 2020 |
Local | 98 incarcerated and
detained persons |
RT-PCR | Not reported | No | Not reported | Unclear how many index or close contacts. |
| Nsekuye 2021 | Rwanda | Observational
Homes Night clubs 14 March to 4 May 2020 |
Local
Household Community |
40 cases; 1035
contacts |
RT-PCR | Not specified | No | N/A | |
| Ogata 2021 | Japan | Observational
- cross-sectional Homes August 2020– February 2021 |
Household | 236 index cases; 496
household contacts |
RT-PCR | Not specified | No | N/A | |
| Ogawa 2020 | Japan | Observational
Hospital |
Nosocomial | 1 index patient; 15
HCWs were contact |
RT-PCR
Serology |
RT-PCR: 10th
day after exposure Serology: Before isolation |
No | Not specified | Viral culture performed for only the index patient. |
| Paireau 2022 | France | Retrospective
observational Various January to March 2020 |
Household
Local Nosocomial |
735 index cases; 6,082
contacts |
RT-PCR | Not reported | No | Not reported | Family, home, work, hospital.
Index case: A case whose detection initiated an investigation of its contacts through contact tracing Only contacts who developed symptoms compatible with COVID-19 were tested for SARS-CoV-2 |
| Pang 2022 | Singapore | Observational
- cohort Nursing home March 2020 |
Local | 164 participants: 108
residents and 56 healthcare staff |
PCR
WGS Phylogenetic analysis |
Not specified | No | N/A | |
| Park 2020 | S. Korea | Retrospective
observational Various February 2020 |
Local
Household Community |
2 index cases; 328
contacts |
RT-PCR | 24 hrs for 37
first contacts; others within 2 weeks |
No | <40
considered positive |
Aircraft, home, restaurant, clinic, pharmacy.
Contact tracing of COVID-19 cases was conducted from 1 day before symptom onset or 1 day before the case was sampled. |
| Park 2020a | S. Korea | Observational
study Homes January to March 2020 |
Household
Non-household |
5,706 COVID-19 index
patients; 59,073 contacts |
Not reported | Not reported | No | Not reported | |
| Park 2020b | S. Korea | Observational
study Workplace, home March 2020 |
Local
Household |
216 employees, 225
household contacts |
RT-PCR | Within 2 weeks
of report of infected case |
No | Not reported | Employees do not generally go between floors, and they
do not have an in-house restaurant for meals. Sent a total of 16,628 text messages to persons who stayed >5 minutes near the building X; we tracked these persons by using cell phone location data. |
| Passarelli 2020 | Brazil | Observational
Hospital August 2020 |
Nosocomial | 6 index cases; 6 close
contacts |
RT-PCR | Not reported | No | <40
considered positive |
All index cases were asymptomatic hospital visitors. |
| Patel 2020 | UK | Retrospective
observational Hospital, community March to April 2020 |
Household | 107 cases; 195
household contacts |
RT-PCR | Not tested | No | Not reported | |
| Pavli 2020 | Greece | Observational
contact tracing Aircraft February to March 2020 |
Aircraft | 6 index cases; 891
contacts |
RT-PCR | Not reported | No | Not reported | A COVID-19 case was defined at that time as a case with
signs and symptoms compatible with COVID-19 in a patient with laboratory-confirmed SARS-CoV-2 infection, recent travel history to a country with evidence of local transmission of SARS-CoV-2 or close contact with a laboratory-confirmed case. |
| Petersen 2021 | Faroe Islands | Observational
- cohort Homes March 3–April 22 |
Household | 584 close contacts | Serology | Within 16
weeks |
No | N/A | |
| Pett 2021 | UK | Observational
Home Community 26 Feb. to 26 April 2020 |
Household
Community |
27 cases; 392 contacts | Not specified | Not specified | No | N/A | |
| Phiriyasart 2020 | Thailand | Observational
Homes April 2020 |
Household | 471 household
contacts |
RT-PCR | Within 5 days
of exposure |
No | Not reported | |
| Poletti 2020 | Italy | Observational
February-April 2020 |
Unclear | 5,484 close contacts
from clusters |
RT-PCR
Serology |
Not reported | No | Not reported | Only contacts belonging to clusters (i.e., groups of
contacts identified by one positive index case) were included. 1,364 (25%) were tested with only RT-PCR, 3,493 (64%) with only serology at least a month after the reporting date of their index case and 627 (11%) were tested both by RT-PCR and serology. |
| Powell 2022 | UK | Observational
Schools November to December 2020 |
Local | 183 school contacts | RT-PCR
Serology WGS Phylogenetic analysis |
Days 0 and 7
PCR Days 0 and 30 serology |
No | Not specified | |
| Pung 2020 | Singapore | Observational
Various February 2020 |
Local
Community |
425 close contacts
from 3 clusters; index case unclear |
PCR
WGS Phylogenetic analysis |
Not reported | No | Not reported | Company conference, church, tour group.
Close contacts under quarantine for 14 days from last exposure to the individual with confirmed COVID-19, either at home or at designated government quarantine facilities. |
| Pung 2020a | Singapore | Observational
Homes Up till March 2020 |
Household | 277 were primary or
co-primary cases: 875 household contacts |
Not reported | Not reported | No | Not reported | Household contacts were tested if they showed
symptoms of SARS-CoV-2 infection, or if aged 12 years or below. |
| Qian 2020 | Hong Kong | Observational
retrospective Various January to February 2020 |
Local
Household Community |
Unclear | Not reported | Not reported | No | Not reported | Homes, transport, restaurants, shopping and
entertainment venues. Four categories of infected individuals were considered based on their relationship: family members, family relatives, socially connected individuals, and socially non-connected individuals |
| Ratovoson 2022 | Madagascar | Observational
Homes March to June 2020. |
Household | 96 index cases and
179 household contacts. |
RT-qPCR
Serology |
First visit and
every 7 days until 21 days. |
No | Not specified | |
| Ravindran 2020 | Indonesia | Retrospective
cohort Wedding March 2020 |
Local | 41 guests; no. of index
cases unclear |
RT-PCR | Not reported | No | Not reported |
Primary case: Any person who attended the wedding
events in Bali Indonesia during 15–21 March 2020 and who tested positive. Secondary case: any person who tested positive on SARS-CoV-2 after the 14-day period and who was a close contact of a COVID-19 case from the wedding events. |
| Razvi 2020 | UK | Observational
study Hospital May to June 2020 |
Nosocomial | 2,521 HCWs | Serology | Voluntary
first-come, first-served basis |
No | N/A | |
| Reukers 2021 | Netherlands | Observational
cohort Homes March to May 2020 |
Household | 55 index cases with
187 household contacts |
RT-qPCR
Serology |
Serology: 4–6
weeks |
No | Not specified | |
|
Robles Pellitero
2021 |
Spain | Observational
- case-control Homes September 2020 |
Household | Case: 96 cases
Controls: 182 Cohabitants: 586 |
Not specified | Not specified | No | Not specified | Case: person whose address was recorded more than
one cohabiting person diagnosed of COVID-19 declared in the SIVE during the study period. Control: person in whose home there were no more persons diagnosed with COVID-19 disease in the study period. |
| Rosenberg 2020 | USA | Observational
retrospective Homes March 2020 |
Household | 229 cases; 498
household contacts |
RT-PCR | Not reported | No | Not reported | |
| Roxby 2020 | USA | Observational
- cross-sectional Nursing home March 2020 |
Nosocomial | 80 residents and 62
staff members; no index case |
RT-PCR | Day 1 and 7
days late |
No | No | Residents isolated in their rooms; no communal meals or
activities, no visitors allowed in the facility, staff member screening and exclusion of symptomatic staff members implemented. Enhanced hygiene practices were put into effect, including cleaning and disinfection of frequently touched surfaces and additional hand hygiene stations in hallways for workers to use. All residents were tested again 7 days later. |
| Sakamoto 2022 | Japan | Observational
Hospital April to early May 2020 |
Nosocomial | 2 clusters with 517
contacts (HCWs) |
RT-PCR | Day 0, then
if patients developed symptoms |
No | Not specified | Some surgeons reported not wearing masks during their
biweekly conferences in a small conference room and other HCWs reported using the small break room without masks. |
| Sang 2020 | China | Case series
Home February 2020 |
Household | 1 index case; 6 family
members |
Not reported | Within 24 hrs
of index case |
No | Not reported | Central air conditioner was always running at home. |
| Sarti 2021 | Italy | Observational
- retrospective Workplace Nov. to Dec. 2020 |
Local | 1 index case; 5
contacts |
RT-PCR | Within 2 weeks
of contact with index case |
No | Not specified | Six workers were working together at full time regimen
for 5 days a week for an average of 8 h daily. Prevention measures were in place. |
| Satter 2022 | Bangladesh | Observational
- cohort Homes Community 27 June to 26 September 2020 |
Local
Community |
37 index cases; 684
contacts |
RT-PCR
Serology |
RT-PCR: days 1,
7, 14, and 28 Serology: day 1 and day 28 |
No | Not specified | |
| Schoeps 2021 | Germany | Observational
- cohort Educational institutions August to December 2020 |
Local | 441 index cases;
14,591 contacts |
PCR | Between seven
and 10 days after their last contact with the index case |
No | Not reported | |
| Schumacher 2021 | Qatar | Prospective
cohort study Football team June to September 2020 |
Local | 1337; no index cases | RT-PCR
Serology |
RT-PCR: Every
3–5 days Serology: Every 4 weeks |
No | ≤30 positive | Strict hygiene measures and regular testing.
Two phases, the quarantine phase (entry until exit) and the training and match phase (after quarantine exit until the first test done during the week after the last match. Ct >30 but <40 reactive. 1337 subjects were tested at least once; however, some players and staff joined their team and were gradually included in (or left) the programme during the study period. |
| Schwierzeck 2020 | Germany | Observational
Hospital paediatric dialysis unit |
Nosocomial | 1 index case; 48
contacts |
RT-PCR | 24 hrs after
index case |
No | Not specified |
Outbreak was defined as two or more COVID-19
infections resulting from a common exposure. |
| Semakula 2021 | Rwanda | Observational
Homes Community 14 March 2020 to 20 July 2020 |
Household
Community |
2216 index cases;
11809 contacts |
PCR | Not specified | No | Not specified | |
| Shah 2020 | India | Observational
Homes March to July 2020 |
Household | 74 primary cases; 386
household contacts |
RT-PCR | Not reported | No | Not reported | |
| Shah 2021 | India | Observational
Homes March to July 2020 |
Household | 72 paediatric index
cases; 287 household contacts |
Not specified | Not specified | No | Not specified | |
| Shen 2020 | USA | Observational
Social gathering January to February 2020 |
Household
Community |
1 index case: 539
social and family contacts |
RT-PCR | If contact had
symptoms |
No | Not specified | |
| Sikkema 2020 | Netherlands | Cross-sectional
Hospital March 2020 |
Nosocomial | 1796 HCWs; index
case not specified |
RT-PCR
WGS Phylogenetic analysis |
N/A | No | <32
considered positive |
HCWs across 3 hospitals. |
| Son 2020 | S. Korea | Observational
study Homes January to March 2020 |
Household | 108 primary cases;
3223 contacts |
RT-PCR | Unclear | No | Not reported | |
| Song 2020 | China | Observational
case series Home January 2020 |
Household | 4 family clusters. 4
index cases: 18 close contacts |
RT-PCR | 0 to 72 hrs
after index case tested positive |
No | Not reported | |
| Sordo 2022 | Australia | Observational
- cohort Homes July-October 2020 |
Household | 229 primary cases and
659 close contacts. |
PCR
Serology |
Not specified | No | Serology: 4-
fold or greater increase in a SARS-CoV-2 antibody of any subclass |
|
|
Soriano-Arandes
2021 |
Spain | Observational
Homes July-October 2020 |
Household | 3392 household
contacts linked to 1040 paediatric index cases |
RT-PCR | Not specified | No | Not specified | NPIs were applied in all schools, including face masks in
classrooms and school buildings in children older than 6 years. |
| Speake 2020 | Australia | Observational
retrospective Aircraft March 2020 |
Aircraft | 241 passengers
some of whom had disembarked from 1 of 3 cruise ships that had recently docked in Sydney Harbour. 6 primary cases initially |
RT-PCR
WGS Phylogenetic analysis |
Within 2 weeks
of primary cases |
Yes | Not specified |
Primary cases as passengers with SARS-CoV-2 who had
been on a cruise ship with a known outbreak in the 14 days before illness onset and whose specimen yielded a virus genomic sequence closely matching that of the ship’s outbreak strain Secondary cases: Passengers with PCR-confirmed SARS- CoV-2 infection who had not been on a cruise ship with a known SARS-CoV-2 outbreak within 14 days of illness onset and in whom symptoms developed >48 hours after and within 14 days of the flight; or international passengers who had not been on a cruise ship in the 14 days before illness and whose specimens yielded a WGS lineage not known to be in circulation at their place of origin but that closely matched the lineage of a primary case on the flight. |
| Stein-Zamir 2020 | Israel | Observational
- cross-sectional Schools May 2020 |
Local | 1,190 students aged
12–18 years (grades 7–12) and 162 staff members. |
PCR | Unclear | No | Not reported | |
| Stich 2021 | Germany | Observational
Homes May–August 2020 |
Household | 1,625 study
participants from 405 households |
RT-PCR
Serology |
PCR: Within 24
hours |
No | Not specified | |
| Sugano 2020 | Japan | Observational
retrospective Music concerts February 2020 |
Local | 1 index case; 72
exposures |
RT-PCR | Not reported | No | Not specified | |
| Sun 2020 | China | Observational
Homes |
Household | Family clusters | Not reported | Not reported | No | Not reported | |
| Sun 2021 | China | Observational
Homes May 2020 |
Household | 50 household contacts | RT-PCR | Not specified | No | Not specified | |
| Sundar 2021 | India | Observational
Homes Community August 2020 |
Household
Community |
496 contacts of 18
cases |
RT-PCR | Day 3 or day
4 of symptom onset. Days 6 to 10 for asymptomatic contacts |
No | Not specified | |
| Tadesse 2021 | Ethiopia | Observational
- cross-sectional Homes July 2020 |
Household | 40 households | Serology | Not specified | No | Not specified | |
| Tanaka 2021 | Japan | Observational
Homes April to May 2020 |
Household | 687 household
contacts of 307 index cases |
RT-PCR | Not specified | No | Not specified | Assessed transmissibility of the SARS-CoV-2 Alpha Variant. |
| Tanaka 2022 | USA | Observational
Homes June to December 2020. |
Household | 101 households with
477 individuals |
RT-PCR | Every 3–7 days
for up to 4 weeks |
No | Not specified | |
| Taylor 2020 | USA | Observational
Skilled nursing facilities April-June 2020 |
Nosocomial | 259 tested residents,
and 341 tested HCP |
RT-PCR
WGS Phylogenetic analysis |
Weekly serial
testing (every 7–10 days) |
No | Not specified | |
| Teherani 2020 | USA | Observational
Homes March to June 2020 |
Household | 32 paediatric cases;
144 household contacts |
PCR | Within 2 weeks
of exposure to infected case |
No | Not reported | Only children who presented with symptoms concerning
for COVID-19 infection were included. |
| Thangaraj 2020 | India | Observational
Tourist group February 2020 |
Community | 1 index case; 26 close
contacts |
RT-PCR | Within 24 hrs
of index case |
No | Not reported | |
| Torres 2020 | Chile | Cross-sectional
Community March-May 2020 |
Community | 1009 students and
235 staff |
Serology | 8–10 weeks
after school outbreak |
No | N/A | The school was closed on March 13, and the entire
community was placed in quarantine. |
| Tsang 2022 | China | Observational
- retrospective Homes Community 22 January to 30 May, 2020 |
Household
Community |
97 laboratory-
confirmed index cases and 3158 close contacts |
RT-PCR | Days 1, 4, 7
and 14 |
No | Not specified | |
| Tshokey 2020 | Bhutan | Observational
Tourists May 2020 |
Local
Community |
27 index cases; 75
high-risk contacts, 1095 primary contacts; 448 secondary contacts |
RT-PCR | High-risk
contacts: minimum of three times with RT-PCR |
No | ≤ 40
considered positive |
|
| Tsushita 2022 | Japan | Observational
Special training venue May 2020 |
Local | 1 index case; 23
contacts |
RT-PCR | Not specified | No | Not specified | Training comprised individual physical fitness training
in the first week, basic movement training conducted by two people in addition to this in the second week, and practical training conducted by two people while randomly changing the combination from the third week. |
| van der Hoek 2020 | Netherlands | Observational
Household March to April 2020 |
Household | 231 cases; 709 close
contacts. 54 families have 239 participants, 185 of whom are family members. |
RT-PCR
Serology |
Not reported | No | Not reported | |
| Vičar 2021 | Czech
Republic |
Observational
Homes March to October 2020 |
Household | 226 household
contacts |
RT-PCR | Not specified | No | Not specified | |
| Wang 2020 | China | Observational
Home January-February 2020 |
Nosocomial
Household |
25 HCWs, 43 family
members |
RT-PCR
WGS Phylogenetic analysis |
Not reported | No | Not reported | |
| Wang 2020a | China | Retrospective
observational Home February 2020 |
Household | 85 primary cases: 155
household contacts in 78 households |
RT-PCR | Not reported | No | <37
considered positive |
|
| Wang 2020b | China | Retrospective
cohort study Homes February to March 2020 |
Household | 124 primary cases;
335 close contacts |
RT-PCR | Within 2 weeks
of symptom onset of the primary case |
No | Not reported | |
| Wee 2020 | Singapore | Observational
Tertiary Hospital February to May 2020 |
Nosocomial | 28 index cases; 253
staff close-contacts and 45 patient close- contacts |
RT-PCR | If patient
close-contacts or staff close-contacts developed symptoms |
No | Not specified | Infection control bundle was implemented comprising
infrastructural enhancements, improved PPE, and social distancing between patients. Patients were advised to wear surgical masks, to remain within their room or cohorted cubicle at all times, and to avoid mingling with each other. |
| Wendt 2020 | Germany | Observational
Hospital March 2020 |
Nosocomial | 1 index case physician;
187 contacts with HCWs and 67 contacts with patients - 23 high-risk contacts in total |
RT-PCR
Serology |
5-days post
exposure (5- & 10-days post exposure for high-risk contacts |
No | <36 or <39
considered positive |
All high-risk contacts and the index physician were
examined serologically on days 15 or 16 and days 22 or 23 after exposure. |
| White 2022a | Ireland | Observational
Schools August to October 2020 |
Local | 56 index cases; 485
school close contacts |
PCR | Within the
14-day period after the last exposure to the index case |
No | Not specified | |
| White 2022b | Ireland | Observational
Aircrafts November to December 2020 |
Local | 165 infectious cases;
899 flight close contacts |
PCR | Within the
14-day period after the last exposure to the index case |
No | Not specified | |
| Wiens 2021 | South Sudan | Observational
- cross-sectional Homes August to September 2020 |
Household | 435 households with
2,214 participants |
Serology | Not specified | No | N/A | |
| Wolf 2020 | Germany | Observational
case series Hospital quarantine January-February 2020 |
Household | Family cluster: 1 index
case, 4 close contacts |
RT-PCR | 5-days after
index case tested positive |
No | Not reported | The parents were asked to wear masks; wearing masks
was not practical for the children. |
| Wong 2020 | Hong Kong | Observational
Hospital February 2020 |
Nosocomial | 1 index case in AIIR:
71 staff and 49 patients |
RT-PCR | End of 28-day
surveillance |
No | Not specified | |
| Wood 2021 | UK | Retrospective
cohort HCW homes |
Household | 241,266 adults did not
share a household with young children; 41,198, 23,783 and 3,850 shared a household with 1, 2 and 3 or more young children |
PCR | Not reported | No | Not reported | Primary exposure was the number of children aged 0 to
11 years in each household. |
| Wu 2020 | China | Retrospective
cohort study Various January-February 2020 |
Household
Local Community |
144 cases, 2994 close
contacts |
Not reported | Not reported | No | Not reported | Shared transport, visit, medical care, household, brief
contact. |
| Wu 2020a | China | Prospective
observational Homes February to March 2020 |
Household | 35 index cases; 148
household contacts |
Not reported | Not reported | No | Not reported | All consecutive patients with probable or confirmed
COVID-19 admitted to the Fifth Affiliated Hospital of Sun Yat-sen University from 17 January to 29 February 2020 were enrolled. All included patients and their household members were interviewed. |
| Wu 2021 | China | Observational
- retrospective Home Workplace Community January to April 2020 |
Local
Household Community |
578 index cases and
4214 close contacts |
RT-PCR | Within the
14-day period after the last exposure to the index case |
No | N/A | 393 symptomatic index cases with 3136 close contacts
and 185 asymptomatic index cases with 1078 close contacts |
| Xie 2020 | China | Cross-sectional
Home January-February 2020 |
Household | 2 family clusters with
61 residents (5 cases) |
RT-PCR | 7 days after
primary or index cases diagnosed |
No | Not reported | |
| Xie 2021 | China | Observational
- cohort Homes January to February 2020 |
Household | 79 household contacts
of hospitalised patients |
RT-PCR | Not specified | No | N/A | |
| Xin 2020 | China | Prospective
cohort study Homes January to March 2020 |
Household | 31 primary cases; 106
household contacts |
RT-PCR | Not reported | No | Not reported | |
| Yang 2020 | China | Observational
cohort study Home quarantine February-May 2020 |
Household
Local |
93 recurrent-positive
patients; 96 close contacts and 1,200 candidate contacts |
RT-PCR
Serology |
Within 14 days
post-exposure |
Yes | ≤ 40
considered positive |
|
| Yau 2020 | Canada | Retrospective
cohort study Hospital dialysis unit April 2020 |
Nosocomial | 2 index cases; 330
contacts (237 patients and 93 staff) |
RT-PCR | Not reported | No | Not reported | All symptomatic contacts were referred for testing but
asymptomatic household contacts were not routinely tested as per public health protocols at the time. |
| Ye 2020 | China | Observational
Religious gathering January-February 2020 |
Local
Community |
66 confirmed cases
and 15 asymptomatic infections: 1,293 close contacts |
RT-PCR | Not reported | No | Not reported | All close contacts were quarantined |
| Yi 2021 | China | Observational
- cohort Homes January to February 2020 |
Household | 96 families; 475 close
contacts |
RT-PCR | Not specified | No | <37 positive
>40 negative |
|
| Yoon 2020 | S. Korea | Observational
Childcare Centre February-March 2020 |
Local | 1 index case: 190
persons (154 children and 36 adults) were identified as contacts; 44 were defined as close contacts (37 children and 7 adults) |
PCR | 8–9 days
after the last exposure |
No | <37
considered positive |
Wearing masks, more frequent hand hygiene, and
disinfection of the environment were required before the child index case tested positive. |
| Yousaf 2020 | USA | Survey: cross-
sectional Tertiary-care referral facility June 8 to July 8, 2020 |
Household | 198 household
contacts; index cases not specified |
RT-PCR | Day 1 of study | No | Not reported | |
| Yu 2020 | China | Observational
study Homes January to February 2020 |
Household | 560 index cases; 1587
close contacts |
Not reported | Within 2 weeks
of exposure to primary case |
No | Not reported | Exposure environments included workplace, medical
centre, etc. Contact methods included eating or living together, sleeping together, living in same house, etc. |
| Yung 2020 | Singapore | Observational
prospective Homes March to April 2020 |
Household | 137 households, 213
paediatric contacts |
Not reported | Unclear | No | Not reported | |
| Zhang 2020 | China | Retrospective
Observational Aircraft March-April 2020 |
Aircraft | 4462 passengers
screened for COVID-19 based on close contact |
RT-PCR | Not reported | No | Not reported | All passengers were quarantined after arrival. |
| Zhang 2020a | China | Retrospective
observational Various January-March 2020 |
Household
Local Community |
359 cases: 369 close
contacts |
Not reported | Not reported | No | Not reported | Households, social contact, workplace. |
| Zhang 2020b | China | Observational
study Hospital April 2020 |
Household | 3 index cases; 10 close
contacts |
RT-PCR
Serology |
Not reported | No | <37
considered positive |
Ct value of 40 or more was defined as a negative test. |
| Zhang 2020c | China | Observational
Quarantine January-February 2020 |
Local
Household |
Multi-family cluster
of 22 cases: 93 close contacts |
RT-PCR | Not specified | No | Not reported | All close contacts were quarantined in centralized
facilities. |
| Zhang 2020d | China | Observational
Supermarket January-February 2020 |
Local | 1 index case: 8437
contacts |
RT-PCR | Not reported | No | Not reported | |
| Zhang 2021 | China | Observational
Homes Workplace February 2020 |
Local
Household |
1 index case; 178
close contacts |
qRT-PCR
WGS Phylogenetic analysis |
Not specified | No | ≤38 positive | |
| Zhuang 2020 | China | Observational study
Various January to February 2020 |
Household
Community |
Cluster outbreaks;
8363 close contacts |
Not reported | Not reported | No | Not reported | Family and non-family cases. |
Table 2. Main characteristics of included Systematic Reviews.
| Study ID
(n=20) |
Fulfils
systematic review methods |
Research question (search date up
to) |
No. of included studies
(No. of participants) |
Main results | Key conclusions |
|---|---|---|---|---|---|
| Chen 2021 | Yes | To estimate seroprevalence by different
types of exposures, within each WHO region, we categorized all study participants into five groups: 1) close contacts, 2) high-risk healthcare workers, 3) low-risk healthcare workers, 4) general populations, and 5) poorly defined populations (Search from Dec 1, 2019, to Sep 25, 2020). |
230 studies involving
1,445,028 participants were included in our meta-analysis after full-text scrutiny: Close contacts 16 studies 2901 positives out of 9,349 participants. |
Estimated seroprevalence of all infections,
22.9% [95% CI, 11.1-34.7] compared to relatively low prevalence of SARS-CoV-2 specific antibodies among general populations, 6,5% (5.8-7.2%) The overall risk of bias was low. |
There were a very limited
number of high-quality studies of exposed populations, especially for healthcare workers and close contacts, and studies to address this knowledge gap are needed. Pooled estimates of SARS-CoV-2 seroprevalence based on currently available data demonstrate a higher infection risk among close contacts and healthcare workers lacking PPE. |
| Chu 2020 | Yes | To investigate the effects of physical
distance, face masks, and eye protection on virus transmission in healthcare and non-healthcare (e.g., community) settings (Searched up to March 26, 2020) |
Identified 172 studies; 44
studies included in the meta-analysis which 7 were Covid-19. |
A strong association was found of proximity of
the exposed individual with the risk of infection (unadjusted n=10 736, RR 0·30, 95% CI 0·20 to 0·44; adjusted n=7782, aOR 0·18, 95% CI 0·09 to 0·38; absolute risk [AR] 12·8% with shorter distance vs 2·6% with further distance, risk difference. There were six studies on COVID-19, the association was seen irrespective of causative virus (p value for interaction=0·49). The risk of bias was generally low-to-moderate. |
Physical distancing of at least
1m is strongly associated with protection, but distances of up to 2m might be more effective. |
| Fung 2020 | Yes | To review and analyze available studies
of the household SARs for SARS-CoV-2. Searched PubMed, bioRxiv, and medRxiv on 2 September 2020 for published and prepublished studies reporting empirical estimates of household SARs for SARS-CoV-2. Considered only English-language records posted on or after 1 January 2019. |
22 papers met the eligibility
criteria: 6 papers reported results of prospective studies and 16 reported retrospective studies. The number of household contacts evaluated per study ranged from 11 to 10592. |
The 22 studies considered 20 291 household
contacts, 3151 (15.5%) of whom tested positive for SARS-CoV-2. Household secondary attack rate estimates ranged from 3.9% in the Northern Territory, Australia to 36.4% in Shandong, China. The overall pooled random-effects estimate of SAR was 17.1% (95% confidence interval [CI], 13.7–21.2%), with significant heterogeneity (p<0.0001). The household secondary attack rate was highest for index cases aged 10–19 years (18.6%; 95% CI, 14.0–24.0%) and lowest for those younger than 9 (5.3%; 95% CI, 1.3–13.7%). Four of the studies were judged to be of high quality; 14 as moderate quality; and 4 as low quality. Between-study variation could not be explained by differences in study quality. |
Secondary attack rates reported
using a single follow-up test may be underestimated and testing household contacts of COVID-19 cases on multiple occasions may increase the yield for identifying secondary cases. There is a critical need for studies in Africa, South Asia, and Latin America to investigate whether there are setting- specific differences that influence the household SAR. |
|
Goodwin
2021 |
Yes | What evidence is there for the
transmission in indoor residential settings? What evidence is there for transmission in indoor workplace settings? What evidence is there for transmission in other indoor settings (social, community, leisure, religious, public transport)? Do particular activities convey greater risk (e.g. shouting, singing, eating together, sharing bedrooms)? What evidence is there for the appropriate length of distancing between people? Searches were conducted in May 2020 in PubMed, medRxiv, arXiv, Scopus, WHO COVID-19 database, Compendex & Inspec. |
58 articles were included. | Pooled secondary attack rate within households
was 11% (95%CI = 9, 13). There were insufficient data to evaluate the transmission risks associated with specific activities. |
The overall quality of the
evidence was low. |
| Irfan 2021 | Yes | To assess transmission and risks for
SARS-CoV-2 in children (by age- groups or grades) in community and educational-settings compared to adults. Searches conducted in PubMed, EMBASE, Cochrane Library, WHO COVID-19 Database, China National Knowledge Infrastructure (CNKI) Database, WanFang Database, Latin American and Caribbean Health Sciences Literature (LILACS), Google Scholar, and preprints from medRixv and bioRixv) covering a timeline from December 1, 2019, to April 1, 2021. |
90 studies were included. | In educational-settings, children attending
daycare/preschools (OR = 0.53, 95% CI = 0.38- 0.72) were observed to be at lower-risk when compared to adults, with odds of infection among primary (OR = 0.85, 95% CI = 0.55-1.31) and high-schoolers (OR = 1.30, 95% CI = 0.71- 2.38) comparable to adults. 28/29 prevalence studies were of good quality while one was of fair quality. 25/31 of contact- tracing studies were of good quality while six were of fair quality. 22/30 of studies conducted in educational settings were good quality while eight were of fair quality. |
Children and adolescents
had lower odds of infection in educational settings compared to community and household clusters. |
| Koh 2020 | Yes | The secondary attack rate (SAR) in
household and healthcare settings. Search between Jan 1 and July 25, 2020. |
118 studies, 57 were
included in the meta- analyses. |
Pooled household secondary attack rate
was 18.1% (95% CI: 15.7%, 20.6%) significant heterogeneity (P<0.001). No significant difference in secondary attack rates in terms of the definition of household close contacts, whether based on living in the same household (18.2%; 95% CI: 15.3%, 21.2%) or on relationships such as family and close relatives (17.8%; 95% CI: 13.8%, 21.8%) In three studies, the household secondary attack rates of symptomatic index cases (20.0%; 95% CI: 11.4%, 28.6%) was higher than asymptomatic ones (4.7%; 95% CI: 1.1%, 8.3%) Secondary attack rate from 14 studies showed close contacts adults were more likely to be infected compared to children (<18), relative risk 1.71 (95% CI: 1.35, 2.17). 43 high-quality studies were included for meta- analysis. |
There was variation in the
definition of household contacts; most included only those who resided with the index case, some studies expanded this to include others who spent at least a night in the same residence or a specified duration of at least 24 hours of living together, while others included family members or close relatives. |
| Li 2020 | No (quality
assessment not performed) |
Carriage and transmission potential
of SARS-CoV-2 in children in school and community settings (Search performed on 21 June 2020 with entry date limits from late 2019) |
33 studies were included for
this review. Four new studies on SARS-CoV-2 transmission in school settings were identified. |
There is a lack of direct evidence on the
dynamics of child transmission, however the evidence to date suggests that children are unlikely to be major transmitters of SARS-CoV-2. |
The balance of evidence
suggests that children play only a limited role in overall transmission, but it is noted that the relative contribution of children to SARS-CoV-2 transmission may change with reopening of society and schools. |
|
Ludvigsson
2020 |
No (quality
assessment not performed) |
Are children the main drivers of the
COVID-19 pandemic (Search to 11 May 2020) |
47 full texts studied in detail. | This review showed that children constituted a
small fraction of individuals with COVID-19. |
Children are unlikely to be the
main drivers of the pandemic. Data on viral loads were scarce but indicated that children may have lower levels than adults. |
|
Madewell
2020 |
Yes | What is the household secondary
attack rate for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)? ( Searched through Oct 19, 2020) single database assessed |
54 studies with 77,758
participants were included. |
Household secondary attack rate was 16.6%;
restricted index cases to children (<18 years), lower SAR of 0.5%. SAR for household and family contacts was 3 times higher than for close contacts (4.8%; 95% CI, 3.4%-6.5%; P<0.001). Estimated mean household secondary attack rates from symptomatic index cases was significantly higher than from asymptomatic or presymptomatic index cases (18% vs 0.7%, P<0.001). Estimated mean household secondary attack rates to spouses (37.8%; 95% CI, 25.8%-50.5%) was higher than to other contacts (17.8%; 95% CI, 11.7%-24.8%). Significant heterogeneity was found among studies of spouses (I2 = 78.6%; P<0 .001) and other relationships (I2 = 83.5%; P<0.001). Contact frequency with index case associated with higher odds of infection. At least 5 contacts during 2 days before the index case was confirmed; at least 4 contacts and 1 to 3 contacts, or frequent contact within 1 meter. Secondary attack rates for households with 1 contact was significantly higher than households with at least 3 contacts (41.5% vs 22.8%, P<0.001) but not different than households with 2 contacts. There was significant heterogeneity in secondary attack rates between studies with 1 contact (I2 = 52.9%; P = .049), 2 contacts (I2 = 93.6%; P<0.001), or 3 or more contacts (I2 = 91.6%; P<0 .001). 16 of 54 studies (29.6%) were at high risk of bias, 27 (50.0%) were moderate, and 11 (20.4%) were low. |
Secondary attack rates were
higher in households from symptomatic index cases than asymptomatic index cases, to adult contacts than to child contacts, to spouses than to other family contacts, and in households with 1 contact than households with 3 or more contacts. Our study had several limitations. The most notable is the large amount of unexplained heterogeneity across studies. This is likely attributable to variability in study definitions of index cases and household contacts, frequency and type of testing, sociodemographic factors, household characteristics (e.g., density, air ventilation), and local policies (e.g., centralized isolation). The findings of this study suggest that households are and will continue to be important venues for transmission, even where community transmission is reduced. |
|
Madewell
2021 |
No (quality
assessed in previous review) |
To further the understanding of SARS-
CoV-2 transmission in the household. PubMed and reference lists of eligible articles were used to search for records published between October 20, 2020, and June 17, 2021. |
A total of 87 studies
representing 1 249 163 household contacts from 30 countries. |
The estimated household secondary attack rate
for all 87 studies was 18.9% (95% CI, 16.2%- 22.0%). Quality of included studies not reported. |
Household remains an
important site of SARS-CoV-2 transmission. |
| Qiu 2021 | Yes | To critically appraise available data
about secondary attack rates from people with asymptomatic, pre- symptomatic and symptomatic SARS- CoV-2 infection. Medline, EMBASE, China Academic Journals full-text database (CNKI), and pre-print servers were searched from 30 December 2019 to 3 July 2020. |
80 studies were included. | Majority of studies identified index cases with
a clear diagnosis, had an acceptable case definition and sufficiently followed up close contacts (for a minimum of 14 days). However, in some studies the definition of close contact and setting of transmission was not provided. The overall reporting quality was uncertain. Summary secondary attack rate estimate for asymptomatic cases was 1% (95% CI 0%–2%). The summary secondary attack rate estimate for presymptomatic index subjects was 7% (95% CI 3%–11%). The summary estimate of secondary attack rate from symptomatic index subjects was 6% (95% CI 5%–8%). |
Asymptomatic patients can
transmit SARS-CoV-2 to others, but such individuals are responsible for fewer secondary infections than people with symptoms. |
| Shi 2022 | Yes | To examine the transmissibility and
pathogenicity of COVID-19 reflected by the secondary infection rate (SIR), secondary attack rate (SAR), and symptomatic infection ratio. Searches were conducted in Web of Science and PubMed, and Chinese databases, including China National Knowledge Infrastructure, WANFANG Database, and the VIP Database for Chinese Technical Periodicals. 17 August 2020 |
A total of 105 studies were
identified, with 35042 infected cases and 897912 close contacts. |
28 studies were of high quality, 66 studies were
of moderate quality, and 11 were of low quality. The secondary attack rate was 6.6% (95% CI, 5.7%−7.5%). Household contact had significantly higher secondary attack rate (19.6%, 95% CI [15.4–24.2%]) than community contact (SAR, 8.1%, 95% CI [5.2–11.5%]; P=0.013) and medical contact (SAR, 3.8%, 95% CI [0.9–8.4%]; P<0.001). |
There is a higher risk of infection
among household contacts. |
|
Silverberg
2022 |
Yes | To identify the role of children in SARS-
CoV-2 transmission to other children and adults. MEDLINE, EMBASE, CINAHL, Cochrane Central Register of Controlled Trials, and Web of Science were electronically searched for articles published before March 31, 2021. |
40 articles were included.
357 paediatric index cases. |
The overall SAR was 8.4% among known
contacts (5.7% in children and 26.4% in adults). Children were significantly less likely to be infected than adults: OR 0.21 (95% CI 0.05-0.91), with no heterogeneity (I2=0%) Ten were deemed to be of good quality and have low risk of bias, while 22 were of fair quality and 8 were of poor quality. |
Children transmit COVID-19 at
a lower rate to children than to adults. Household adults are at highest risk of transmission from an infected child. |
|
Thompson
2021 |
Yes | To estimate SAR of SARS-CoV-2 in
households, schools, workplaces, healthcare facilities, and social settings. Searches were conducted in MEDLINE, Embase, MedRxiv, BioRxiv, arXiv, and Wellcome Open Research with no language restrictions up to July 6, 2020. |
45 studies were included for
meta-analysis. |
Household SAR was 21.1% (95%CI: 17.4–24.8).
The SAR increased with longer durations of exposure (14.2% [95% CI: 5.8–22.5] with ≤5 days of exposure to an index case vs 34.9% [95%CI 16.3–53.6] with >5 days of exposure; P=0.05. SARs were significantly higher for presymptomatic and symptomatic index cases, estimated at 9.3% (95% CI: 4.5–14.0, P=0.01) and 13.6% (95%CI 9.7–17.5, P<0.001), respectively. Articles that met the inclusion criteria for meta- analysis all had high quality scores. |
Exposure in settings with
familiar contacts increases SARS- CoV-2 transmission potential. |
| Tian 2020 | Yes | Searched published literatures and
preprints in international databases of PubMed and medRxiv, and in five major Chinese databases as of 20 April 2020 |
18 studies were included
for meta-analysis. A total of 32,149 close contacts were documented. |
The pooled SAR was 0.07 (95%CI 0.03-0.12).
Household setting and social gatherings were associated with significantly elevated SARs (P<0.01). 17 studies were high quality, and one was moderate quality using the AHRQ criteria. |
The transmission risk of
SARS-CoV-2 is much higher in households than in other scenarios. |
| Viner 2021 | Yes | To assess child and adolescent
susceptibility to SARS-CoV-2 compared with adults. Searched 2 electronic databases, PubMed and the medical preprint server medRxiv, on May 16, 2020, and updated this on July 28, 2020 |
32 studies comprising 41 640
children and adolescents and 268 945 adults met inclusion criteria. |
The pooled odds ratio of being an infected
contact in children compared with adults was 0.56 (95% CI, 0.37-0.85), with substantial heterogeneity (I2=94.6%). Two studies were high quality, 22 were medium quality, 7 were low quality, and 1 was uncertain quality. |
Children have a lower
susceptibility to SARS-CoV-2 infection compared with adults |
| Viner 2022 | Yes |
Research questions:
(a) To what extent do CYP under 20 years of age transmit SARS-CoV- 2 to other CYP and to adults in household and child-specific (e.g. educational) settings?; (b) how does transmission differ between household and educational settings?; and (c) is community infection incidence associated with prevalence of or transmission of infection within educational settings? Searched four electronic databases (PubMed; medRxiv; COVID-19 Living Evidence database; Europe PMC) to 28 July 2021. |
37 studies were included. | The pooled estimates of SAR were 7.6% (3.6,
15.9) for household studies, significantly higher than the pooled estimate for school studies of 0.7% (0.2, 2.7), P=0.002)). Across all studies, pooled risk of transmission was lower from child index cases than adults (OR 0.49 (0.25, 0.98). 24 studies had high quality, and 13 were medium quality. |
SAR were markedly lower
in school compared with household settings, suggesting that household transmission is more important than school transmission in this pandemic. |
| Xu 2020 | Yes | Evidence for transmission of COVID-
19 by children in schools ( search in MEDLINE up to 14 September 2020. Further hand-searched reference lists of the retrieved eligible publications to identify additional relevant studies). Included children (defined as ≤18 years old) who were attending school, and their close contacts (family and household members, teachers, school support staff) during the COVID-19 pandemic |
11 studies were included: 5
cohort studies and 6 cross- sectional studies. |
Overall infection attack rate (IAR) in cohort
studies: 0.08%, 95% CI 0.00%-0.86%. IARs for students and school staff were 0.15% (95% CI 0.00%-0.93%) and 0.70% (95% CI = 0.00%-3.56%) respectively (p<0.01). Six cross-sectional studies reported 639 SARS-CoV-2 positive cases in 6682 study participants tested [overall SARS-CoV-2 positivity rate: 8.00% (95% CI = 2.17%-16.95%). SARS-CoV-2 positivity rate was estimated to be 8.74% (95% CI = 2.34%-18.53%) among students, compared to 13.68% (95% CI = 1.68%- 33.89%) among school staff (p<0.01). Overall study quality was judged to be poor with risk of performance and attrition bias. |
There is limited high-quality
evidence to quantify the extent of SARS-CoV-2 transmission in schools or to compare it to community transmission. Emerging evidence suggests lower IAR and SARS-CoV-2 positivity rate in students compared to school staff. |
|
Yanes-Lane
2020 |
Yes | Proportion of asymptomatic infection
among coronavirus disease 2019 (COVID-19) positive persons and their transmission potential. (Search up to up to 22 June 2020) |
28 studies were included. | Asymptomatic COVID-19 infection at time
of testing ranged from 20% – 75%; among three studies in contacts it was 8.2% to 50%. Asymptomatic infection in obstetric patients pooled proportion was 95% (95% CI, 45% to 100%) of which 59% (49% to 68%) remained asymptomatic through follow-up; Among nursing home residents, the proportion of asymptomatic was 54% (42% to 65%) of which 28% (13% to 50%) remained asymptomatic through follow-up. The overall quality of included studies was moderate-to-high. |
The proportion of asymptomatic
infection among COVID-19 positive persons appears high and transmission potential seems substantial. |
| Zhu 2021 | Meta-
analysis: Quality assessment not reported |
Role of children in SARS-CoV-2 in
household transmission clusters ( Search between Dec 2019 & Aug 2020). |
57 articles with 213 clusters
were included. |
8 (3.8%) transmission clusters were identified as
having a paediatric index case. Asymptomatic index cases were associated with lower secondary attack rates in contacts than symptomatic index cases [RR] 0.17 (95% CI,0.09- 0.29). SAR in paediatric household contacts was lower than in adult household contacts (RR, 0.62; 95% CI, 0.42-0.91). |
The data suggest that should
children become infected at school during this period, they are unlikely to spread SARS- CoV-2 to their co-habiting family members. |
Quality of included studies
None of the included primary studies reported a published protocol except one (Helsingen 2020). The risk of bias of the included primary studies is shown in Table 3. One hundred and twenty-four studies (48.1%) adequately reported the methods used, and 158 (61.2%) adequately described the sources of sample collection. Only nine studies (3.5%) adequately reported methods used to address biases. The overall reporting across the studies was judged as low to moderate (see the risk of bias graph in Figure 2).
Figure 2. Risk of bias graph in primary studies of close contacts in SARS-CoV-2.
Table 3. Risk of Bias.
| Study ID | Description of
methods and sufficient detail to replicate? |
Sample
sources clear? |
Analysis &
reporting appropriate? |
Is bias
dealt with? |
Results
applicable? |
Notes |
|---|---|---|---|---|---|---|
| Abdulrahman 2020 | Unclear | Yes | Yes | No | Yes | |
| Adamik 2020 | Unclear | Unclear | Yes | No | Unclear | |
| Afonso 2021 | Yes | Yes | Yes | Unclear | Yes | |
| Agergaard 2020 | No | Yes | Yes | No | Yes | |
| Akaishi 2021 | Yes | Yes | Yes | Unclear | Yes | |
| Angulo-Bazán 2020 | Yes | No | Yes | Unclear | Yes | |
| Armann 2020 | Unclear | Yes | Yes | No | Yes | |
| Arnedo-Pena 2020 | Yes | Yes | Yes | Unclear | Yes | |
| Atherstone 2021 | No | No | Yes | Unclear | Yes | |
| Baettig 2020 | Unclear | Yes | Yes | Unclear | Yes | |
| Baker 2020 | Unclear | Yes | Yes | Unclear | Yes | |
| Bao 2020 | Unclear | Yes | Yes | No | Yes | |
| Basso 2020 | Unclear | Yes | Yes | Unclear | Yes | |
| Bays 2020 | Unclear | Yes | Yes | No | Yes | |
| Bender 2021 | Unclear | No | Yes | Unclear | Unclear | Evidence was obtained from
a single outbreak and might not be applicable to other settings. Recall bias |
| Bernardes-Souza 2021 | Yes | Yes | Yes | Unclear | Yes | Recall bias |
| Bhatt 2022 | Yes | Yes | Yes | Unclear | Yes | |
| Bi 2020 | Yes | Yes | Yes | Unclear | Yes | |
| Bi 2021 | Yes | Yes | Yes | Unclear | Yes | |
| Bistaraki 2021 | Yes | Unclear | Yes | Unclear | Yes | |
| Bjorkman 2021 | Unclear | Yes | Yes | Unclear | Yes | |
| Blaisdell 2020 | Yes | No | Yes | Unclear | Yes | |
| Böhmer 2020 | Yes | Yes | Yes | Unclear | Yes | |
| Boscolo-Rizzo 2020 | Unclear | Yes | Yes | No | Yes | |
| Brown 2020 | Yes | Yes | Yes | Unclear | Unclear | |
| Burke 2020 | Unclear | No | Yes | No | Yes | |
| Calvani 2021 | Yes | Yes | Yes | Unclear | Yes | Recall bias |
| Canova 2020 | Unclear | Yes | Yes | Unclear | Yes | |
| Carazo 2021 | Yes | Unclear | Yes | Unclear | Yes | |
| Cariani 2020 | Unclear | Yes | Unclear | Unclear | Yes | |
| Carvalho 2022 | Unclear | Yes | Yes | Unclear | Yes | |
| Cerami 2021 | Yes | Yes | Yes | Unclear | Yes | |
| Charlotte 2020 | Unclear | Yes | Yes | Unclear | Yes | |
| Chaw 2020 | Unclear | Yes | Yes | Unclear | Yes | |
| Chen 2020 | Unclear | Unclear | Yes | No | Unclear | |
| Chen 2020a | Unclear | Yes | Yes | Unclear | Yes | |
| Chen 2020b | Yes | Yes | Yes | Unclear | Yes | |
| Chen 2020c | Unclear | No | Yes | No | Yes | |
| Cheng 2020 | Yes | No | Yes | Unclear | Yes | |
| Chu 2020 | Yes | Yes | Yes | Unclear | Yes | |
| Chu 2020a | Unclear | Unclear | Unclear | No | Yes | |
| Contejean 2020 | Unclear | Yes | Yes | Unclear | Yes | |
| Cordery 2021 | Yes | Yes | Yes | Unclear | Yes | |
|
COVID-19 National
Emergency Response Center 2020 |
Unclear | No | Yes | No | Yes | |
| Craxford 2021 | Yes | Yes | Yes | Unclear | Yes | |
| Danis 2020 | Yes | Yes | Yes | No | Yes | |
| Dattner 2020 | Yes | Yes | Yes | Unclear | Yes | |
| de Brito 2020 | Yes | Yes | Unclear | Unclear | Yes | |
| Deng 2020 | Unclear | No | Unclear | Unclear | Unclear | |
| Desmet 2020 | Yes | Yes | Yes | No | Unclear | |
| Dimcheff 2020 | Yes | Unclear | Yes | Unclear | Unclear | |
| Dong 2020 | Unclear | No | Unclear | No | Yes | |
| Doung-ngern 2020 | Yes | Yes | Yes | Unclear | Yes | |
| Draper 2020 | Yes | Yes | Yes | No | Yes | |
| Dub 2020 | Yes | Yes | Yes | Unclear | Yes | |
| Expert Taskforce 2020 | Unclear | Unclear | Yes | Unclear | Unclear | |
| Farronato 2021 | Yes | Yes | Yes | Unclear | Yes | |
| Fateh-Moghadam 2020 | Unclear | No | Yes | No | Yes | |
| Firestone 2020 | Unclear | Unclear | Yes | Unclear | Yes | |
| Fontanet 2021 | Yes | Yes | Yes | No | Yes | |
| Galow 2021 | No | Yes | Yes | Unclear | Unclear | |
| Gamboa Moreno 2021 | Unclear | Unclear | Unclear | Unclear | Unclear | no explanation of sample
taking |
| Gan 2020 | Unclear | Unclear | Unclear | Unclear | Unclear | |
| Gaskell 2021 | Unclear | Yes | Yes | Unclear | Yes | |
| Ge 2021 | Yes | Yes | Yes | Yes | Yes | Conducted sensitivity
analyses restricting the study population to household and nonhousehold contacts |
| Ghinai 2020 | Unclear | Unclear | Unclear | Unclear | Unclear | |
| Gold 2021 | Unclear | Yes | Yes | Unclear | Yes | Method used to identify close
contacts not clearly described |
| Gomaa 2021 | Unclear | Yes | Yes | Unclear | Yes | |
| Gonçalves 2021 | Yes | Yes | Yes | Unclear | Yes | Recall bias |
| Gong 2020 | Yes | Yes | Unclear | Unclear | Unclear | |
| Gu 2020 | Unclear | Unclear | Unclear | No | Unclear | |
| Hamner 2020 | Unclear | Unclear | Yes | No | Yes | |
| Han 2020 | Yes | Yes | Yes | Unclear | Yes | |
| Hast 2022 | Yes | Yes | Yes | Unclear | Yes | |
| Heavey 2020 | Unclear | No | Yes | No | Yes | |
| Helsingen 2020 | Yes | Yes | Yes | Yes | Yes | |
| Hendrix 2020 | Yes | Yes | Yes | No | Yes | |
| Hirschman 2020 | Unclear | Unclear | Unclear | No | Yes | |
| Hobbs 2020 | Yes | Yes | Yes | Unclear | Yes | |
| Hoehl 2020 | Yes | Yes | Yes | Unclear | Yes | |
| Hong 2020 | Yes | Yes | Yes | Unclear | Yes | |
| Hsu 2021 | Unclear | Unclear | Yes | Unclear | Yes | Asymptomatic patients could
have been missed |
| Hu 2020 | Unclear | No | Yes | No | Yes | |
| Hu 2020 | Yes | Unclear | Yes | Unclear | Yes | |
| Hu 2021 | Yes | Yes | Unclear | Unclear | Yes | Criteria for categorizing times
into 0-1.5, 1.5-2.5, and >2.5 hrs unclear |
| Hua 2020 | Yes | Unclear | Yes | Unclear | Yes | |
| Huang 2020 | Unclear | Unclear | Yes | No | Unclear | |
| Huang 2020a | Unclear | Unclear | Yes | Unclear | Unclear | |
| Huang 2021 | Yes | Yes | Yes | Unclear | Yes | |
| Islam 2020 | Yes | No | Yes | No | Yes | |
| Jashaninejad 2021 | Yes | Unclear | Yes | Unclear | Yes | |
| Jeewandara 2021 | Yes | Yes | Yes | Unclear | Yes | |
| Jia 2020 | Unclear | Unclear | Yes | No | Unclear | |
| Jiang 2020 | Yes | Yes | Unclear | No | Yes | |
| Jing 2020 | Yes | Yes | Yes | Unclear | Yes | |
| Jing 2020a | Unclear | Yes | Unclear | Unclear | Unclear | |
| Jones 2020 | Unclear | Yes | Yes | Unclear | Unclear | |
| Jordan 2022 | Yes | Yes | Yes | Unclear | Yes | |
| Kang 2020 | Unclear | Unclear | Unclear | Unclear | Unclear | |
| Kant 2020 | Unclear | Yes | Unclear | No | Unclear | |
| Karumanagoundar 2021 | Yes | Yes | Yes | Unclear | Yes | |
| Katlama 2022 | Yes | Yes | Unclear | Unclear | Yes | No formal statistical analysis
was planned |
| Kawasuji 2020 | Unclear | Yes | Unclear | Unclear | Unclear | |
| Khanh 2020 | Yes | Yes | Yes | No | Yes | |
| Kim 2020 | Unclear | Yes | Yes | Unclear | Yes | |
| Kim 2020a | Unclear | Yes | Yes | No | Unclear | |
| Kim 2020b | Yes | Yes | Yes | No | Yes | |
| Kim 2021 | N/A | Yes | Yes | N/A | Yes | |
| Kitahara 2022 | Unclear | Unclear | Yes | Unclear | Yes | |
| Klompas 2021 | Yes | Yes | Yes | Unclear | Yes | |
| Kolodziej 2022 | Yes | Yes | Yes | Yes | Yes | Conducted sensitivity analyses
excluding households with a possible other primary case than the defined index case |
| Koureas 2021 | Unclear | Unclear | Yes | Unclear | Yes | Identification of the index
case was not possible in 10/40 households since two or more household members were simultaneously found to be positive. |
| Kumar 2020 | Unclear | Yes | Unclear | No | Unclear | |
| Kuwelker 2020 | Unclear | Yes | Yes | Unclear | Yes | |
| Kuwelker 2021 | Yes | Yes | Yes | Unclear | Yes | |
| Kwok 2020 | Unclear | Unclear | Yes | Unclear | Unclear | |
| Ladhani 2020 | No | Unclear | Unclear | No | Yes | |
| Ladhani 2020a | Unclear | Unclear | Yes | Unclear | Yes | |
| Laws 2020 | Unclear | Unclear | Yes | Unclear | Yes | |
| Laws 2021 | Yes | Yes | Yes | Unclear | Yes | |
| Laxminarayan 2020 | Yes | No | Yes | No | Yes | |
| Lee 2020 | Unclear | Unclear | Yes | Unclear | Unclear | |
| Lee 2020a | Unclear | No | Yes | No | Yes | |
| Lewis 2020 | Yes | Yes | Yes | No | Yes | |
| Li 2020 | Unclear | Yes | Unclear | No | Unclear | |
| Li 2020a | Unclear | Unclear | Unclear | Unclear | Unclear | |
| Li 2020b | Unclear | Yes | Unclear | Unclear | Unclear | |
| Li 2020c | Unclear | No | Unclear | Unclear | Unclear | |
| Li 2020d | Yes | Yes | Yes | No | Yes | |
| Li 2021a | Yes | Unclear | Yes | Unclear | Yes | |
| Li 2021b | Yes | Yes | Yes | Unclear | Yes | |
| Lin 2021 | Yes | Yes | Yes | Unclear | Yes | |
| Liu 2020 | Unclear | Unclear | Unclear | No | Yes | |
| Liu 2020a | Yes | Yes | Yes | Unclear | Unclear | |
| Liu 2020b | Unclear | Yes | Yes | Unclear | Yes | |
| Liu 2020c | Unclear | Unclear | Unclear | No | Unclear | |
| Liu 2021 | Yes | Yes | Yes | Unclear | Yes | |
| López 2020 | Unclear | Unclear | Yes | Unclear | Yes | |
| López 2021 | Yes | Unclear | Yes | Unclear | Yes | |
| Lopez Bernal 2020 | Yes | Unclear | Yes | No | Yes | |
| Lopez Bernal 2022 | Yes | Unclear | Yes | Unclear | Yes | |
| Lucey 2020 | Unclear | Yes | Yes | No | Yes | |
| Luo 2020 | Unclear | Yes | Yes | Unclear | Yes | |
| Luo 2020a | Unclear | Yes | Yes | Yes | Yes | They use multiple imputation
to minimise inferential bias, and they discuss recall bias, selection bias and regression to the mean. |
| Lyngse 2020 | Yes | Unclear | Yes | Yes | Yes | They investigate bias within
their data and discuss this fairly fully |
| Ma 2020 | Unclear | Unclear | Unclear | Unclear | Unclear | |
| Macartney 2020 | Yes | Unclear | Yes | Unclear | Yes | |
| Malheiro 2020 | Yes | Unclear | Yes | Unclear | Yes | |
| Maltezou 2020 | Unclear | Unclear | Unclear | Unclear | Yes | |
| Maltezou 2020a | Unclear | Unclear | Unclear | No | Yes | |
| Mao 2020 | Unclear | Unclear | Yes | No | Unclear | |
| Martínez-Baz 2022 | Yes | Yes | Yes | Unclear | Yes | |
| Martinez-Fierro 2020 | Unclear | Yes | Yes | No | Yes | |
| McLean 2022 | Yes | Yes | Yes | Unclear | Yes | |
| Mercado-Reyes 2022 | Yes | Yes | Yes | Unclear | Yes | |
| Metlay 2021 | Unclear | Unclear | Yes | Unclear | Yes | |
| Meylan 2021 | Yes | Unclear | Yes | Unclear | Yes | |
| Miller 2021 | Yes | Yes | Yes | Unclear | Yes | |
| Montecucco 2021 | Yes | Yes | Yes | Unclear | Yes | |
| Mponponsuo 2020 | Unclear | Yes | Yes | Yes | Yes | Recall bias was minimized
by examining multiple data sources for both index cases and exposed persons |
| Musa 2021 | Yes | Unclear | Yes | Unclear | Yes | |
| Ng 2020 | Unclear | Yes | Yes | Yes | Yes | Authors looked at differences
that could have led to bias |
| Ng 2021 | Yes | Yes | Yes | Unclear | Yes | |
| Ning 2020 | Unclear | Unclear | Unclear | Unclear | Unclear | |
| Njuguna 2020 | Unclear | Unclear | Yes | Unclear | Yes | |
| Nsekuye 2021 | Unclear | Yes | Yes | Unclear | Yes | |
| Ogata 2021 | Unclear | Unclear | Yes | Unclear | Yes | |
| Ogawa 2020 | Unclear | Unclear | Yes | No | Yes | |
| Paireau 2020 | Unclear | Yes | Yes | Unclear | Yes | |
| Pang 2022 | Unclear | Unclear | Yes | Unclear | Yes | |
| Park 2020 | Unclear | Yes | Yes | Unclear | Yes | |
| Park 2020a | Unclear | No | Yes | No | Yes | |
| Park 2020b | Unclear | Yes | Yes | No | Unclear | |
| Passarelli 2020 | Unclear | No | Unclear | Unclear | Yes | |
| Patel 2020 | Yes | Yes | Yes | Unclear | Unclear | |
| Pavli 2020 | Unclear | Yes | Yes | No | Yes | |
| Petersen 2021 | Yes | Yes | Yes | Unclear | Yes | |
| Pett 2021 | Yes | Yes | Yes | Unclear | Yes | |
| Phiriyasart 2020 | Yes | Yes | Yes | No | Yes | |
| Poletti 2020 | Unclear | Yes | Yes | Yes | Unclear | |
| Powell 2022 | Yes | Yes | Yes | Unclear | Yes | |
| Pung 2020 | Yes | Unclear | Yes | Unclear | Yes | |
| Pung 2020a | Unclear | No | Unclear | Unclear | Unclear | |
| Qian 2020 | Unclear | Unclear | Unclear | No | Unclear | |
| Ratovoson 2022 | Yes | Yes | Yes | Unclear | Yes | |
| Ravindran 2020 | Unclear | Unclear | Unclear | Unclear | Unclear | |
| Razvi 2020 | Unclear | Yes | Yes | No | Yes | |
| Reukers 2021 | Yes | Yes | Yes | Unclear | Yes | |
| Robles Pellitero 2021 | Yes | No | Yes | Unclear | Yes | Recall bias |
| Rosenberg 2020 | Yes | Yes | Yes | No | Yes | |
| Roxby 2020 | Yes | Yes | Yes | Unclear | Yes | |
| Sakamoto 2022 | Unclear | Yes | Yes | Unclear | Yes | Use of protection is
unclear; testing only done is participants were symptomatic |
| Sang 2020 | Unclear | Yes | Unclear | No | Unclear | |
| Sarti 2021 | Yes | Yes | Yes | Unclear | Yes | |
| Satter 2022 | Yes | Yes | Yes | Unclear | Yes | |
| Schoeps 2021 | Yes | Yes | Yes | Yes | Yes | Advised DPHAs to report
consecutive index cases over at least a 4-week period or longer, thus reducing the chance of systematic under- or over-reporting |
| Schumacher 2020 | Unclear | Yes | Unclear | Unclear | Yes | |
| Schwierzeck 2020 | Unclear | Yes | Yes | Unclear | Yes | |
| Semakula 2021 | Yes | Yes | Yes | Unclear | Yes | |
| Shah 2020 | Unclear | No | Unclear | No | Yes | |
| Shah 2021 | Unclear | No | Yes | Unclear | Yes | |
| Shen 2020 | Yes | Yes | Yes | Unclear | Yes | |
| Sikkema 2020 | Unclear | Yes | Yes | Unclear | Yes | |
| Son 2020 | Unclear | Unclear | Yes | No | Yes | |
| Song 2020 | Unclear | Yes | Yes | Unclear | Yes | |
| Sordo 2022 | Yes | No | Yes | Unclear | Yes | |
| Soriano-Arandes 2021 | Yes | Yes | Yes | Unclear | Yes | To avoid selection bias in case
recruitment, paediatricians recorded all positive cases seen in daily practice |
| Speake 2020 | Unclear | Yes | Yes | Unclear | Yes | |
| Stein-Zamir 2020 | Yes | Unclear | Yes | No | Yes | |
| Stich 2021 | Yes | Yes | Yes | Unclear | Yes | |
| Sugano 2020 | Unclear | Unclear | Yes | Unclear | Yes | |
| Sun 2020 | Unclear | Unclear | Unclear | Unclear | Unclear | |
| Sun 2021 | Unclear | Yes | Yes | Unclear | Yes | |
| Sundar 2021 | Yes | Yes | Yes | Unclear | Yes | |
| Tadesse 2021 | Unclear | Yes | Yes | Unclear | Yes | |
| Tanaka 2021 | Unclear | Yes | Yes | Unclear | Yes | |
| Tanaka 2022 | Unclear | Yes | Yes | Unclear | Yes | Used a convenience
recruitment strategy. |
| Taylor 2020 | Yes | Yes | Yes | Unclear | Yes | |
| Teherani 2020 | Unclear | Yes | Yes | Unclear | Yes | |
| Thangaraj 2020 | Unclear | Yes | Yes | Unclear | Unclear | |
| Torres 2020 | Yes | Unclear | Yes | Unclear | Yes | |
| Tsang 2022 | Yes | Unclear | Yes | Unclear | Yes | |
| Tshokey 2020 | Unclear | Yes | Yes | Unclear | Yes | |
| Tsushita 2022 | Yes | Unclear | Yes | Unclear | Yes | |
| van der Hoek 2020 | Unclear | Yes | Yes | No | Yes | |
| Vičar 2021 | Yes | Unclear | Yes | Unclear | Yes | |
| Wang 2020 | Unclear | Yes | Unclear | Unclear | Yes | |
| Wang 2020a | Yes | Unclear | Yes | Unclear | Yes | |
| Wang 2020b | Yes | Yes | Yes | No | Yes | |
| Wee 2020 | Yes | Yes | Yes | Unclear | Yes | |
| Wendt 2020 | Yes | Yes | Yes | Unclear | Yes | |
| White 2022a | Yes | Unclear | Yes | Unclear | Yes | |
| White 2022b | Yes | Unclear | Yes | Unclear | Yes | |
| Wiens 2021 | Yes | Yes | Yes | Unclear | Yes | For households with more
than 10 people, only the first- degree relatives of the head of household were eligible for study inclusion. |
| Wolf 2020 | Yes | Yes | Yes | Unclear | Yes | |
| Wong 2020 | Yes | Yes | Yes | Unclear | Yes | |
| Wood 2020 | Unclear | No | Yes | Unclear | Yes | |
| Wu 2020 | Yes | Unclear | Yes | Unclear | Yes | |
| Wu 2020a | Yes | Unclear | Yes | Unclear | Yes | |
| Wu 2021 | Yes | Yes | Yes | Unclear | Yes | |
| Xie 2020 | Unclear | Yes | Yes | Unclear | Yes | |
| Xie 2021 | Yes | Yes | Yes | Unclear | Yes | |
| Xin 2020 | Yes | No | Yes | No | Yes | |
| Yang 2020 | Unclear | Yes | Unclear | Unclear | Yes | |
| Yau 2020 | Unclear | Yes | Unclear | Unclear | Unclear | |
| Ye 2020 | Unclear | Unclear | Unclear | Unclear | Unclear | |
| Yi 2021 | Unclear | Yes | Yes | Unclear | Yes | |
| Yoon 2020 | Yes | Yes | Yes | Unclear | Yes | |
| Yousaf 2020 | Unclear | Yes | Unclear | Unclear | Unclear | |
| Yu 2020 | Yes | No | Yes | No | Yes | |
| Yung 2020 | Unclear | Yes | Yes | No | Yes | |
| Zhang 2020 | Unclear | Unclear | Unclear | No | Unclear | |
| Zhang 2020a | Yes | Unclear | Yes | Unclear | Unclear | |
| Zhang 2020b | Unclear | Yes | Unclear | Unclear | Yes | |
| Zhang 2020c | Unclear | Unclear | Unclear | Unclear | Unclear | |
| Zhang 2020d | Unclear | Yes | Unclear | Unclear | Unclear | |
| Zhang 2021 | Yes | Yes | Yes | Unclear | Yes | |
| Zhuang 2020 | Unclear | No | Yes | No | Unclear |
Reviews
We included 20 systematic reviews investigating the role of close contact in SARS-CoV-2 transmission ( Table 2). The studies included in the reviews were primarily observational. In one review (Chen 2020), there was a higher risk of infection in close contacts and healthcare workers without PPE compared to the general population. A second review (Chu 2020) found a significant association between proximity of exposure (distance <1m), absence of barriers (not using face covering or eye protection) and the risk of infection. Two reviews (Shi 2022, Tian 2020) showed that the risk of infection was significantly higher in household settings compared to other settings. The authors of four reviews (Li 2020, Ludvigsson 2020, Silverberg 2022, Zhu 2020) concluded that children were unlikely to be the main conduit for transmission of SARS-CoV-2, and results of two reviews (Koh 2020, Viner 2021) showed that adults with close contact exposure were significantly more likely to be infected compared with children. In one review (Xu 2020), the attack rates were significantly less in students compared with staff (p<0.01), and two (Irfan 2021, Viner 2022) showed that children in educational settings (mainly schools) had lower odds of infection compared to children in household or community clusters. One review (Fung 2020) reported household SARs ranging from 3.9% to 36.4%, but also highlighted the lack of SARS-CoV-2 research in Africa, South Asia, and Latin America. Three reviews (Madewell 2020, Qiu 2021, Thompson 2021) found that SARs were higher in households from symptomatic index cases than asymptomatic index cases; a further update (Madewell 2021) concluded that household transmission remained an important setting for SARS-CoV-2 transmission. One review (Yanes-Lane 2020) concluded that the proportion of asymptomatic infection was high (20–75%).
In two reviews (Koh 2020, Yanes-Lane 2020), studies judged to be of low quality were excluded from their meta-analyses. In one review (Chen 2020, Goodwin 2021), the overall quality was reported as low, while ≥80% of included studies were reported as moderate or high quality in another seven (Fung 2020, Irfan 2021, Madewell 2020, Shi 2022, Silverberg 2022, Tian 2020, Viner 2021). In one review (Thompson 2021), all included studies had high quality scores. Another review (Chu 2020) reported the overall risk of bias as low-to-moderate, and one (Xu 2020) rated the overall quality as low. The overall reporting quality in one review (Qiu 2021) was reported as uncertain, while 69% of included studies in one review (Viner 2021) were of moderate quality. Four reviews did not assess study quality (see Table 2).
Primary studies
We found 258 primary studies ( Table 1). In general, the studies did not report any hypothesis but assessed epidemiological or mechanistic evidence for transmission associated with close contact settings. One hundred and twenty-two studies (47.3%) were conducted in Asia, 77 (29.8%) in Europe, 40 (15.5%) in North America, 10 (3.9%) in South America, six (2.3%) in Africa and four (1.6%) in Australasia.
Study settings
The study settings included home/quarantine facilities (n=102), hospital (n=31), social/religious gatherings (n=13), public transport (n=10) care homes (n=5), and educational settings (n=15). Nineteen studies used two settings (home plus one other setting). In 41 studies (15.9%), the settings were multiple (3 or more different settings). Three studies were conducted in professional sports settings: one Super League Rugby (Jones 2020), one football team (Schumacher 2020), and one special training venue (Tsushita 2022).
Study designs
All the included studies were observational in design except one RCT (Helsingen 2020): 52 studies were described as cohort, nine were case series and 21 cross-sectional. One study used a before and after study design. The number of close contact participants included ranged from 4 to 72093. Three studies (Chen 2020a, Hong 2020, Yang 2020) examined transmission dynamics in close contacts of index or primary cases with recurrent SARS-CoV-2 infections.
Definition of close contacts
One hundred and one studies (39.1%) reported definitions of close contacts ( Table 4). There was a variation in the definitions across the studies. Twenty-four studies (9.3%) defined close contact as exposure to the index or primary case within two metres for at least 15 minutes while four defined it as being within 2m for at least 10 minutes. In 30 studies, there was no specified distance reported - close contact definitions included unprotected exposure, face-to-face contact, living in the same household or bedroom, sharing a meal, having same postal address, or having repeated and prolonged contact. In seven studies of airline passengers, close contact was defined as all passengers on the flight (Chen 2020), seated within two rows of the index case (Draper 2020, Pavli 2020, Speake 2020, White 2020b), seated within three rows of the index case (Hu 2021), or being within 2m for at least 15 minutes (Khanh 2020). One hundred and sixty studies (62.1%) did not define close contact and the definition was unclear in four studies. Fifty-five studies (21.3%) defined other types of contacts including primary contact, secondary contact, high-risk contact, household contact, social contact, and work contact (see Table 4).
Table 4. Definition of Close Contacts and Other Contacts.
| Study ID | Definitions of close contacts | Definition of other contacts | Contact duration & proximity |
|---|---|---|---|
| Abdulrahman 2020 | Not defined | Not reported | Not reported |
| Adamik 2020 | Not defined | Other cases in each of the infected households were
regarded as secondary cases |
Not reported |
| Afonso 2021 | Not defined | Essential activity workers with COVID‐19, confirmed by the
RT‐PCR, were considered the primary case (index cases) at their households. Their respective infected household contacts were defined as secondary case or secondary transmission cases. |
Not reported |
| Agergaard 2020 | Not defined | Not reported | 2 weeks |
| Akaishi 2021 | 1) Contact with a COVID-19 patient between 2 days
before and 14 days after the onset of symptoms, 2) no usage of masks, 3) distance less than 1 m, and 4) more than 15 min of contact |
Lower-risk contact was defined as being in the same place
as the COVID-19 patients, but without fulfilling the above- described criteria for close contact. |
<1m for >15 min |
| Angulo-Bazán 2021 | Not defined | Not reported | Not reported |
| Armann 2021 | Not defined | Not reported | Not reported |
| Arnedo-Pena 2020 |
Close contacts living in the same household of the
index case and no other sources of transmission apart from the index case could be found. |
Closed contacts from work, social events, relatives live in
other household were excluded and index cases live alone. |
Not reported |
| Atherstone 2021 | CDC definition: <6 feet away from someone with
suspected or confirmed COVID-19 for a cumulative total of 15 minutes or more over a 24-hour period |
Not reported | <1m for >15 min |
| Baettig 2020 |
Close contact: Less than 2 m for more than 15 min in
the last 48 hours before onset symptom of the COVID-19 positive index patient. |
Not reported | <2m for >15 mins 48 hours
before onset symptom of the COVID-19 positive index patient |
| Baker 2020 | Not defined | Not reported | Median cumulative time spent
with the patient 45 mins (10–720 mins) |
| Bao 2020 | Not defined | Not reported | Average stay duration of 2.5
hr daily before the COVID‐19 outbreak. |
| Basso 2020 |
Close contact: ≥15 min at ≤2 m, or during AGPs,
between HCWs and the non-isolated COVID-19 patient |
Not reported | ≤2 m for ≥15 min or during AGP |
| Bays 2020 | Not defined | Not reported | Not specified |
| Bender 2021 | Person with >15 minutes face-to-face contact within a
maximum distance of 2 m to the index case |
Not reported | <2m for >15min |
|
Bernardes-Souza
2021 |
Not defined | Not reported | Not reported |
| Bhatt 2022 | Not defined | Household members: people residing in the same dwelling
as the index participant. |
Not reported |
| Bi 2020 |
Close contacts were identified as those who lived
in the same apartment, shared a meal, travelled, or socially interacted with an index case 2 days before symptom onset. |
Casual contacts (eg, other clinic patients) and some close
contacts (eg, nurses) who wore a mask during exposure were not included in this group. |
Not specified |
| Bi 2021 | Not defined | Not reported | Not reported |
| Bistaraki 2021 | Not defined | Household contacts were defined as people who lived in
the same house with the confirmed SARS-CoV-2 patient. |
Not reported |
| Bjorkman 2021 | Not defined | Not reported | Not reported |
| Blaisdell 2020 | Not defined | Not reported | 1 week |
| Böhmer 2020 | High risk if they had cumulative face-to-face contact
with a patient with laboratory-confirmed SARS-CoV-2 infection for at least 15 min, had direct contact with secretions or body fluids of a patient with confirmed COVID-19, or, in the case of health-care workers, had worked within 2 m of a patient with confirmed COVID-19 without PPE |
All other contacts were classified as low-risk contacts. | Face-to-face for at least 15
minutes, direct contact without PPE |
| Boscolo-Rizzo 2020 | Not defined | Not reported | Not reported |
| Brown 2020 | Not defined | Not reported | Mean in-class time = 50 minutes |
| Burke 2020 | Either at least 10 minutes spent within 6 feet of the
patient with confirmed COVID-19 (e.g., in a waiting room) or having spent time in the same airspace (e.g., the same examination room) for 0–2 hours after the confirmed COVID-19 patient. |
Not reported | Within 6 feet for at least 10
minutes |
| Calvani 2021 | Not defined | Not reported | Not reported |
| Canova 2020 | Not defined | Not reported | 5 HCWs: >30 minutes
5 HCWs: >15-30 mins 6 HCWs: 5-15 mins 5 HCWs: ≤5 mins |
| Carazo 2021 | Not defined | Not reported | Not reported |
| Cariani 2020 | Not defined | Not reported | Not reported |
| Carvalho 2022 | Not defined | Not reported | Not reported |
| Cerami 2021 | Not defined | Not reported | Not reported |
| Charlotte 2020 | Not defined | Not reported | 2-hours |
| Chaw 2020 |
Close contact: Any person living in the same
household as a confirmed case-patient or someone who had been within 1 m of a confirmed case-patient in an enclosed space for >15 minutes |
Not reported | Within 1m for >15 mins |
| Chen 2020 |
Close contact: All passengers were regarded as close
contacts |
Not reported | Flight duration 5 hours approx |
| Chen 2020a |
Close contacts are persons who have had close
contact with re-positive patients without effective protection with masks, such as living and working together |
Not reported | Not specified |
| Chen 2020b | Not defined | Not reported | Not specified |
| Chen 2020c |
Community contact: Any close contact (being within
6 feet of the case-patient) for a prolonged time (>10 minutes); being an office co-worker of the case- patient with close contact of any duration; contact with infectious secretions from the case-patient; or sharing a healthcare waiting room or area during the same time and up to 2 hours after the case-patient was present. |
Healthcare contact: Face-to-face interaction between
healthcare personnel (HCP) and the case-patient without wearing the full PPE that was recommended at the time of the investigation or potential contact with the case- patient’s secretions by HCP without wearing full PPE. |
>10 mins to 2 hours |
| Cheng 2020 | Not defined | Not reported | Not reported |
| Chu 2020 | Close contact was a person who did not wear
appropriate PPE while having face-to-face contact with a confirmed case for more than 15 minutes during the investigation period. A contact was listed as a household contact if he or she lived in the same household with the index case. Those listed as family contacts were family members not living in the same household. For health care settings, medical staff, hospital workers, and other patients in the same setting were included; close contact was defined by contacting an index case within 2 m without appropriate PPE and without a minimal requirement of exposure time |
Those listed as family contacts were family members not
living in the same household. |
Within 2 m without PPE, face-to-
face contact for >15 minutes |
| Chu 2021 | Not defined | Not reported | Stayed ≥1 night in the household
during case’s infectious period |
| Contejean 2020 |
Close contact: Distance <2 meters for >10 minutes
was defined as close contact |
Not reported | <2 metres for >10 minutes |
| Cordery 2021 | Not defined | Pupil contacts were children who were either in the same
bubble as the Case (Bubble Contact, BC) or in a class within the same school that was adjacent in terms of age-group or proximity (School Contact, SC). Household contacts (HC) were adults and children of any age normally resident with the Case |
Not reported |
|
COVID-19 National
Emergency Response Center 2020 |
Close contact (or high risk exposure)” was being within
2 meters of a COVID-19 case |
Daily contact (or low risk exposure) was defined as having
proximity with a person who was a confirmed COVID-19 case, without having had close contact. |
Not reported |
| Craxford 2021 | Not defined | Not reported | Not reported |
| Danis 2020 | All children and teachers who were in the same class
as the symptomatic pediatric case were considered as high risk contacts and were isolated at home. Moderate/high risk: Person who had prolonged (> 15 min) direct face-to-face contact within 1 m with a confirmed case, shared the same hospital room, lived in the same household or shared any leisure or professional activity in close proximity with a confirmed case, or travelled together with a COVID-19 case in any kind of conveyance, without appropriate individual protection equipment. |
Low risk: Person who had a close (within 1 m) but short
(< 15 min) contact with a confirmed case, or a distant (> 1 m) but prolonged contact in public settings, or any contact in private settings that does not match with the moderate/ high risk of exposure criteria. Negligible risk: Person who had short (< 15 min) contact with a confirmed case in public settings such as in public transportation, restaurants and shops; healthcare personnel who treated a confirmed case while wearing appropriate PPE without any breach identified. |
4 days in chalet |
| Dattner 2020 | Not defined | Not reported | Not reported |
| de Brito 2020 |
Close contact: Close and prolonged contact in the
same room |
Not reported | Not specified |
| Deng 2020 | Not defined | Not reported | Not reported |
| Desmet 2020 | Not defined | Not reported | Not reported |
| Dimcheff 2020 |
Close contact: Within 2 m or 6 feet) with an individual
with confirmed COVID-19 for >15 minutes with the example being exposed to a family member at home who has had a positive COVID-19 nasal swab |
Not reported | Within 2m for >15 mins |
| Dong 2020 | Not defined | Not reported | Not reported |
| Doung-ngern 2020 |
High-risk if they were family members or lived in the
same household as a COVID-19 patient, if they were within a 1-meter distance of a COVID-19 patient longer than 15 minutes; if they were exposed to coughs, sneezes, or secretions of a COVID-19 patient and were not wearing protective gear, such as a mask; or if they were in the same closed environment within a 1-meter distance of a COVID-19 patient longer than 15 minutes and were not wearing protective gear |
Not reported | <15 min vs >15 min, <1m vs >1m |
| Draper 2020 | Close contact was defined as anyone who had face-
to-face contact with a confirmed COVID-19 case for more than 15 minutes cumulatively or continuously (e.g., household setting or healthcare setting without appropriate use of personal protective equipment) or who was in the same room with an infectious case for more than 2 hours (e.g., school room, workplace) while a case was symptomatic or during the 24 hours preceding symptom onset. Aircraft close contacts included passengers seated in the same row as, or in the two rows in front of or behind, an infectious case. If the case was a crew member, the passengers in the area in which the crew member worked were classified as close contacts. Passengers disembarking from cruise ships with high incidence of COVID-19 were also classified as close contacts for surveillance purposes. |
Not reported | Not reported |
| Dub 2020 |
Close household contact, i.e., an individual sharing
the main residence of the secondary case |
A regular household contact, i.e., an individual who would
regularly host or stay in the same residence of a secondary case (stepsibling, divorced parent and new partner). Extended contact, i.e., an individual who would have frequent contact with the secondary case around and after the exposure, for example, grandparents who were involved in caring of the secondary case, according to parents’ reports. |
<2 meters for >10 minutes |
| Expert Taskforce 2020 | Close contact: Cabinmates of confirmed case-patients | Not reported | Not specified |
| Farronato 2021 | Not defined | Not reported | Not reported |
| Fateh-Moghadam 2020 | Contact of a COVID-19 case has been considered any
person who has had contact with a COVID-19 case within a time frame ranging from 48 hours before the onset of symptoms of the case to 14 days after the onset of symptoms |
Not reported | Not reported |
| Firestone 2020 |
Close contact: Being within 6 feet of a patient with
laboratory-confirmed COVID-19 infection for ≥15 minutes |
Not reported | Within 2m for >15 mins |
| Fontanet 2021 | Not defined | Not reported | Not reported |
| Galow 2021 | Not defined | Not reported | Not reported |
| Gamboa Moreno 2021 | Not defined | School contact was a close contact that originated from an
exposure to a case within the school environment. Family or social contact was a contact with a case outside the school environment. |
Not reported |
| Gan 2020 | Not defined | Not reported | Not reported |
| Gaskell 2021 | Not defined | Not reported | Not reported |
| Ge 2021 | A close nonhousehold contact was defined as an
individual exposed (within 1 meter) to an index patient |
A household contact was defined as an individual in the
same household or an individual who dined together with the index patient. |
Within 1m |
| Ghinai 2020 | Not defined | Not reported | Not reported |
| Gold 2021 | Persons exposed to an index patient at school within
6 ft for >15 minutes per day during a 24-hour period while the index patient was infectious (48 hours before to 10 days after symptom onset or, if asymptomatic, 48 hours before to 10 days after specimen collection). |
N/A | <1m for >15 min |
| Gomaa 2021 | Not defined | Not reported | Not reported |
| Gonçalves 2021 | Not defined | Not reported | Not reported |
| Gong 2020 |
Close contact: Anyone who was closely in contact with
a suspected, confirmed and asymptomatic case without effective personal protection (classified protection according to the contact situation, including gloves, medical protective masks, protective face screens, isolation clothing, etc.) since onset of symptoms in the suspected case and confirmed case or the day asymptomatic case’s specimens were collected. The close contact included: (i) living, working, or studying in one house or classroom, (ii) diagnosing, treating, or visiting cases in hospital ward, (iii) being within short distance in the same vehicle, (iv) other situations assessed by the field investigators. |
Not reported | Not reported |
| Gu 2020 | Not defined | Not reported | 5 hrs, no natural ventilation or
face masks; distance between each other <0.5 m |
| Hamner 2020 | Close contact: Within 6 feet of infected case | Not reported | 2.5 hrs within 2 m |
| Han 2020 |
Close contact: Travel was defined as someone who
was in close contact with a confirmed case for over three hours as they travelled to another region aside from Region A. Close contact: meal was defined as someone who was in close contact with a confirmed case for over 30 minutes after having a meal together. |
A casual contact was defined as someone who spent
several minutes with a confirmed case within the same space without any mask on (or a person was established as a contact by an Epidemic intelligence Officer). |
30 mins to 3 hours |
| Hast 2022 | Not defined | Not reported | Not reported |
| Heavey 2020 |
Close contact: Any individual who has had greater
than 15 minutes face-to-face (<2 meters distance*) contact with a case, in any setting. |
Casual contact: Any individual who has shared a closed
space with a case for less than two hours. |
Up to 2 hours in duration |
| Helsingen 2020 | Not defined | Not reported | Not reported |
| Hendrix 2020 | Not defined | Not reported | Not reported |
| Hirschman 2020 |
Close contact: Within 6 feet of an infected person for
at least 15 minutes starting from 2 days before illness onset. |
Not reported | "Hours" |
| Hobbs 2020 |
Close contact: Within 6 feet for ≥15 minutes) with a person with
known COVID-19, school or childcare attendance, and family or community exposures ≤14 days before the SARS-CoV-2 test |
Not reported | Within 2 m for ≥15 minutes |
| Hoehl 2021 | Not defined | Not reported | Not reported |
| Hong 2020 | Anyone who ever came within 2 m of a diagnosed
patient without the use of effective personal protective equipment |
Not reported | 258 person-days |
| Hsu 2021 | Not defined | Not reported | Not reported |
| Hu 2020 | A person who had co-travelled on a train within a three-
row seat distance of a confirmed case (index patient) within 14 days before symptom onset. |
Not reported | Not reported |
| Hu 2021 |
Close contacts were defined as individuals who had
close-proximity interactions (within 1 meter) with clinically suspected and laboratory-confirmed SARS- CoV-2 cases, for the period from 2 days before, to 14 days after, the potential infector’s symptom onset. For those exposed to asymptomatic subjects, the contact period was from 2 days before, to 14 days after, a respiratory sample was taken for real-time RT-PCR testing. Close contacts included, but were not limited to, household contacts (i.e., household members regularly living with the case), relatives (i.e., family members who had close contacts with the case but did not live with the case), social contacts (i.e., a work colleague or classmate), and other close contacts (i.e., caregivers and patients in the same ward, persons sharing a vehicle, and those providing a service in public places, such as restaurants or movie theatres) |
Not reported | Not reported |
| Hu 2021 | Passengers within 3 rows of the index case seat were
considered to be close contacts for estimating the upper bound of risk detailed below |
Lower bound of risk: passengers assumed to be travelling
with their family members or friends if a small group of passengers included one index COVID-19 patient, and passengers seated immediately adjacent to this index patient shared the same departure and destination. |
Within 3 rows of the index case seat |
| Hua 2020 | Not defined | Not reported | |
| Huang 2020 | Close contacts quarantined at home or hospital | Not reported | Not reported |
| Huang 2020a | Not defined | Not reported | Not reported |
| Huang 2021 | Contact with the index case within 2 m without using
appropriate personal protective equipment (PPE) and without a minimum requirement of exposure time in hospital settings. |
Not reported | Not reported |
| Islam 2020 | Close contact was defined as individuals who were
closely linked by contact tracing and were considered a close contact group provided that no PPE was worn having direct face to face contacts. |
Household contacts were defined as individuals who lived
and were sharing the same room and same apartment in the same household. Family contacts were those who are the members of the same family but not living in the same household. |
Face-to-face |
| Jashaninejad 2021 | Person who had exposure or lived with a probable or
confirmed case or had direct and face-to-face contact 2 days before and 14 days after exposure with the index case. |
Not reported | Not reported |
| Jeewandara 2021 | Close contacts were defined as individuals living in
Bandaranayaka watta and who had direct physical contact or associated with cases (distance of 1m) within a period of 2 days from identification of the index case. |
Non-close contacts were defined as those who were living
within the CMC region as the cases, or those who worked with cases in the same causal occupations but were not qualified to be defined as close contacts. |
Within 1m |
| Jia 2020 | A
close contact was defined as a person who did
not take effective protection against a suspected or confirmed case 2 d before the onset of symptoms or an asymptomatic infected person 2 d before sampling. |
Not reported | Not reported |
| Jiang 2020 |
Close contacts: Lived with the patients and individuals
who had contact with the patients within 1 meter without wearing proper personal protection. Ct value ≥40 was considered negative. The maximum likelihood phylogenetic tree of the complete genomes was conducted by using RAxML software with 1000 bootstrap replicates, employing the general time- reversible nucleotide substitution mode |
Not reported | 1 m |
| Jing 2020 | A
close contact was defined as an individual who
had unprotected close contact (within 1 m) with a confirmed case within 2 days before their symptom onset or sample collection. Individuals who were linked by contact tracing were considered a close contact group |
Not reported | Not reported |
| Jing 2020a | Not defined | Not reported | Not reported |
| Jones 2021 |
Close contacts were defined by analysis of video
footage for player interactions and microtechnology (GPS) data for proximity analysis. |
Not reported | Within 1 m, face-to-face for ≥3
secs |
| Jordan 2022 | Not defined | Not reported | Not reported |
| Kang 2020 | Not defined | Not reported | Not reported |
| Kant 2020 | Not defined | Not reported | Not reported |
| Karumanagoundar 2021 | High-risk contact is defined as any person who was in
proximity with individuals positive for COVID-19 within 2 m of proximity for 15 min. |
Contact: any individual comes in proximity with individuals
positive for COVID-19. Low-risk contact is defined as any person who was in proximity with individuals positive for COVID-19 and sharing same environment but not having high exposure. Household contact: any individual living in the same household and comes in proximity with the individual with COVID-19 confirmed. Community contact: any individual other than living in the same household and comes in proximity with the individual with COVID-19 confirmed. Congregation exposure: individual who have attended the religious congregation event held during February and March 2020 (newspaper reference) |
<2m for >15min |
| Katlama 2022 | Not defined | Not reported | Not reported |
| Kawasuji 2020 | Not defined | Not reported | Not reported |
| Khanh 2020 |
Close contact: <2 m distance for >15 minutes.
Successfully traced passengers and crew members were interviewed by use of a standard questionnaire, tested for SARS-CoV-2 |
Not reported | <2 m distance for >15 minutes. |
| Kim 2020 | Not defined | Household contact: Occurring at least 1 day after but
within 14 days from the last point of exposure. |
2 days during the
presymptomatic period and 1 day during the symptomatic period of the index case. |
| Kim 2020a | Not defined | Not reported | 2 hrs to 4 days |
| Kim 2020b |
Contact was defined as presence in the same room
with COVID-19 confirmed patients, or in the same outpatient clinic or examination room, 30 minutes before and after COVID-19 confirmed patients. Within 2 m of confirmed patients (via CCTV) |
Not reported | Within 2 m of confirmed patients
for 30 mins |
| Kim 2021 | Not defined | Not reported | Face-to-face |
| Kitahara 2022 | Contact with confirmed case for >15 mins without
wearing proper PPE |
Not reported | >15 mins |
| Klompas 2021 | Shared a room with an infected patient, and employees
who had face-to-face contact within 6 feet of an infected employee or patient for at least 15 minutes during which either party was not wearing a mask |
Direct contact: spending ≥15 minutes interacting with staff
or patients on cluster units |
<1m for >15 min |
| Kolodziej 2022 | Not defined | Not reported | Not reported |
| Koureas 2021 | Any person who had unprotected close contact (<2 m,
more than 15 min) with a confirmed case from 2 days before symptom onset (if not available from sample collection) until the patient’s isolation. |
Household Secondary Contact: Any person residing in the
same house/apartment with a Household Index Case. |
<2m for >15min |
| Kumar 2021 | Not defined | Not reported | Not reported |
| Kuwelker 2021 | Not defined | Household members were defined as individuals who resided in the
same household as the index case. |
Not reported |
| Kuwelker 2021 | Not defined | Household members were defined as individuals who
resided in the same household as an index patient. |
Not reported |
| Kwok 2020 |
Close contacts referred to anyone who: (i) provided
care to the case (including a family member or healthcare worker) or had other close physical contact; or (ii) stayed at the same place (including household members or visitors) while the case was ill. |
Not reported | Not reported |
| Ladhani 2020 | Not defined | Not reported | Not reported |
| Ladhani 2020a | Not defined | Not reported | Not reported |
| Laws 2020 | Not defined | Not reported | Unclear |
| Laws 2021 | Not defined | Not reported | Not reported |
| Laxminarayan 2020 | High-risk contacts had close social contact or direct
physical contact with index cases without protective measures High-risk travel exposures—defined as close proximity to an infected individual in a shared conveyance for ≥6 hours |
Low-risk contacts were in the proximity of index cases but
did not meet criteria for high-risk exposure |
Not reported |
| Lee 2020 | Not defined: Frequent close contact | Not reported | >1 m |
| Lee 2020a | Close contact (household contact) | Not reported | Mean contact period was
calculated to be 7.7 days. |
| Lewis 2020 | Not defined | Household contacts were defined as all persons living in
the same household as the primary patient. |
Not reported |
| Li 2020 | Not defined | Not reported | Unclear |
| Li 2020a | Not defined | Not reported | Not reported |
| Li 2020b |
Close contact was defined as an act of sharing a meal,
party, vehicle or living room with a confirmed or latently infected patient within 14 days. |
Not reported | Not reported |
| Li 2020c |
Close contacts were mainly those who have not
taken effective protection from close contact with the suspected and confirmed cases 2 days before symptoms appeared, or the asymptomatic infected persons 2 days before the specimen collection. |
Not reported | Not reported |
| Li 2020d | Not defined | Not reported | Not reported |
| Li 2021a | Not defined | Household contact of an identified case was broadly
defined as a family member or close relative who had unprotected contact with the case within 2 days before the symptom onset or test-positive specimen collection of the case but did not necessarily live at the same address. |
Not reported |
| Li 2021b | Someone who had contact with an index case-patient
without effective protection and within 1 meter, regardless of contact duration. Persons who had close contact with the index case- patient during or 2 days before the index case patient’s illness onset were counted as close contacts |
Not reported | Within 1m regardless of duration |
| Lin 2021 | Those in proximity to cases within 2 days before the
onset of symptoms of suspected and confirmed cases or 2 days before the sampling of asymptomatic infected persons when effective protection or distancing measures were not in effect. Close contacts also included those in proximity to cases in aggregated epidemic settings within 2 weeks before diagnosis such as homes, offices, school classes, and so forth with 2 or more cases of fever and/or respiratory symptoms. |
Not reported | Not reported |
| Liu 2020 | Not defined | Not reported | Unclear |
| Liu 2020a | Direct contact with patients with neo-coronary
pneumonia (within 1 m) |
Not reported | Within 1m for 2.5 hrs |
| Liu 2020b |
Close contacts were defined by the China Prevention
and Control Scheme of COVID-19. |
Not reported | 7.8 (95%CI: 7.0–8.7) close contacts per index case. |
| Liu 2020c | Not defined | Not reported | Not reported |
| Liu 2021 | Not defined | Household contacts were broadly defined as any individual
residing with the index case during the infectious period. |
Not reported |
| López 2020 |
Close contact: Anyone who was within 6 feet of a
person with COVID-19 for at least 15 minutes ≤2 days before the patient’s symptom onset. |
Not reported | ≤1.83m of a person with COVID-
19 for at least 15 minutes ≤2 days before the patient’s symptom onset |
| López 2021 | Not defined | Not reported | Not reported |
| Lopez Bernal 2020 | Household contacts were defined as those living or
spending significant time in the same household. Household contacts, others with direct face to face contact and healthcare workers who had not worn recommended PPE |
Not reported | Not reported |
| Lopez Bernal 2022 | Not defined | Household contacts were defined as those living or
spending substantial time (overnight) in the same household. Other contacts: not classified as close contacts Community contacts: |
Not reported |
| Lucey 2020 |
Close contact: HCW or patient who spent more than
15 minutes face-to-face within 2 metres of a confirmed case or patients who shared a multi-bedded room with a confirmed case for more than 2 hours. |
Not reported | Not reported |
| Luo 2020 | The tour coach was with 49 seats was fully occupied
with all windows closed and the ventilation system on during the 2.5-hour trip. |
Not reported | 1 to 4.5m; up to 2.5 hours on
a bus |
| Luo 2020a |
Close contacts: Anyone who has had contact, without
effective protection regardless of duration of exposure, with 1 or more persons with suspected or confirmed COVID-19 any time starting 2 days before onset of symptoms in persons with a suspected or confirmed case, or 2 days before sampling for laboratory testing of asymptomatic infected persons. |
Not reported | Not reported |
| Lyngse 2020 | Not defined | Not reported | Not reported |
| Ma 2020 | Not defined | Not reported | Longest contact time: 8 days
Shortest contact time: 0 days |
| Macartney 2020 |
Close contacts: Children or staff with face-to-face
contact for at least 15 min, or who shared a closed indoor space for at least 40 min with a case during their infectious period. |
Not reported | Face-to-face contact for at least
15 min, or who shared a closed indoor space for at least 40 min |
| Malheiro 2020 |
Close contacts (high risk)were defined as individuals
who have spent 15 min or more in closeproximity (2 m or less) to, or in a closed space with, a case. |
Not reported | Not reported |
| Maltezou 2020 |
Close contact was defined as a contact of >15 minutes
within a distance of <2 m with a COVID-19 case. |
Household members were defined as persons living in the
same residence. |
>15 minutes within <2 m |
| Maltezou 2020a |
Close contact was defined as a contact of >15 minutes
within a distance of <2 meters with a COVID-19 case |
Household contacts were defined as persons either living
in the same residence or having close contacts with a family member for >4 hours daily in the family residence. |
Household: >4 hours daily
Close contact: >15 minutes within <2 m |
| Mao 2020 | Not defined | Not reported | Not reported |
| Martínez-Baz 2022 | Any person who had face-to-face contact with a
confirmed COVID-19 infected individuals within 2 m for more than a total of 15 minutes without personal protection within a timeframe ranging from two days before to 10 days after the onset of symptoms in the case, or in the two days before 10 days after the sample which led to confirmation was taken from asymptomatic cases |
Not reported | Within 2m for >15 mins |
| Martinez-Fierro 2020 | Individual who has had closer than <6 feet for ≥15 min
with people with a positive diagnosis for COVID-19 , whether they were symptomatic or asymptomatic according to the CDC definition |
Not reported | ≥15 min at a distance of <1.83m |
| McLean 2022 | Not defined | Not reported | Not reported |
| Mercado-Reyes 2022 | Not defined | Not reported | Not reported |
| Metlay 2021 | Not defined | Not reported | Not reported |
| Meylan 2021 | Not defined | Not reported | Not reported |
| Miller 2021 | Not defined | Not reported | Not reported |
| Montecucco 2021 | A person who had exposure or lived with a probable or
confirmed case or had direct and face-to-face contact exposure with the index case in the period between two days before the positive PCR test or two days preceding the onset of COVID-19 symptoms and end of isolation after infection resolution. |
Not reported | Not reported |
| Mponponsuo 2020 | An interaction of >15 minutes at a distance of <1 m | Not reported | >15 minutes at a distance of <1 m |
| Musa 2021 | Not defined | A household contact was defined as any person living
in the same household as the index case at the time of recruitment. |
Not reported |
| Ng 2020 |
Close contacts were individuals who had contact for
at least 30 min within a 2 m distance from the index case. |
Work contacts were defined as individuals who came
into close contact with the index case at work, from 2 days before the onset of symptoms to isolation of the case, to account for pre-symptomatic transmission. Social contacts were defined as individuals who came into close contact with the index case, from 2 days before onset of symptoms to isolation of the case, through social activities. Transport contacts were excluded Lower risk contacts: Other contacts who were with the index case for 10–30 min within 2 m |
At least 30 min within a 2 m |
| Ng 2021 | Not defined | Household contact was defined as all persons living in
the same household of the index patient at diagnosis, regardless of duration or proximity of contact. |
Not reported |
| Ning 2020 | Not defined | Not reported | Unclear |
| Njuguna 2020 | Not defined | Not reported | Unclear |
| Nsekuye 2021 | High risk contacts (red-ring) were those who had come
into unprotected face-to-face contact (within 2 m) or having been in a closed environment (e.g., household members) with a COVID-19 case for >15 min. Unprotected direct contact with infectious secretions of a COVID-19 case was also considered high risk (red-ring: these are immediate family, friends, relatives or co-workers that were more likely to have received exposure to transmission). |
A contact of a COVID-19 case was defined as any person
who had contact with a COVID-19 case within a timeframe ranging from 72 h before the onset of symptoms of the case to 14 days after the onset of symptoms. Low risk contacts were those who had come into contact while masked, within more than 2 m or for less than 15 min. |
<2m for >15min |
| Ogata 2021 | Not defined | Not reported | Not reported |
| Ogawa 2020 | Not defined | Not reported | Not reported |
| Paireau 2022 | Not defined | Not reported | Not reported |
| Pang 2022 | Not defined | Not reported | Not reported |
| Park 2020 | Not defined | Not reported | Not reported |
| Park 2020a | High-risk contact (household contacts of COVID-19
patients, healthcare personnel) |
Household contact was a person who lived in the
household of a COVID-19 patient and a nonhousehold contact was a person who did not reside in the same household as a confirmed COVID-19 patient. |
Not reported |
| Park 2020b | Not defined | Not reported | Not reported |
| Passarelli 2020 | Not defined | Not reported | Not reported |
| Patel 2020 | Not defined | Not reported | Not reported |
| Pavli 2020 |
Close contacts were defined as persons sitting within
a distance of <2 m for >15 min, including passengers seated two seats around the index case and all crew members and persons who had close contact with the index case. |
Not reported | <2 m for >15 min |
| Petersen 2021 | Household members and contacts who were within 2
meters of an infected person for >15 minutes, who had direct physical contact or provided caregiving without using personal protective equipment, or who had similar exposures, were defined as close contacts. |
Not reported | <2m for >15min |
| Pett 2021 | Not defined | Household contact: living or spending significant time in
the same household. High risk: persons in healthcare settings (e.g., healthcare workers, cleaners, visitors) who have not worn recommended PPE OR laboratory workers who have not used appropriate laboratory precautions during the following exposures to the patient. OR Direct contact with the case or their body fluids or their laboratory specimens OR presence in the same room of a healthcare setting when an aerosol generating procedure is undertaken on the case Lower risk: Persons in healthcare settings (e.g., healthcare workers, cleaners) who have worn recommended PPE during exposures to the patient. |
Not reported |
| Phiriyasart 2020 | Close contact was defined as a person who had at
least one of these following criteria : (i) a person who came into close (within 1 meter) contact with, or had a conversation with any patient for >5 minutes, or was coughed or sneezed on by any patient when he/she did not wear appropriate personal protective equipment (PPE), e.g. a face mask, (ii) a person who was in an enclosed space without proper ventilation, e.g. in the same air-conditioned bus/air-conditioned room as any patient , and was within one meter of any patient for >15 minutes without wearing appropriate PPE. High-risk close contact was defined as a close contact who was likely to contract the virus from any patient through exposure to respiratory secretions of any patient while not wearing PPE according to standard precautions. |
A low-risk close contact was defined as a close contact who
was less likely to contract the virus from any patient. This includes close contacts who have not met the definition for high-risk close contacts. |
Not reported |
| Poletti 2020 | Not defined | Not reported | Not reported |
| Powell 2022 | Not defined | Direct contacts are defined as the staff and students who
were asked to self-isolate. Indirect contacts refer to household members of these staff and students. |
Not reported |
| Pung 2020 |
Close contacts: People who spend a prolonged time
within 2 m of a confirmed case |
Other contacts: People who had some interactions with
the case. |
Unclear |
| Pung 2020a | Close household contacts | Not reported | Unclear |
| Qian 2020 | Four categories of infected individuals were considered
based on their relationship: family members, family relatives, socially connected individuals, and socially non-connected individuals |
Not reported | Not reported |
| Ratovoson 2022 | Defined as those who lived in the same house of a
symptomatic index case up to 4 days before symptom onset or of an asymptomatic index case up to 4 days prior to the collection date of the first positive test result. |
Not reported | |
| Ravindran 2020 |
Close contact: Face-to-face contact for greater than
15 minutes cumulative in the period extending from 48 hours before onset of symptoms in a confirmed case; or sharing of closed space with a confirmed case for a prolonged period of time in the period extending from 48 hours before onset of symptoms in a confirmed case. |
Not reported | Face-to-face contact for at least
15 min, or who shared a closed indoor space for prolonged period 48 hrs before onset of symptoms |
| Razvi 2020 | Not defined | Not reported | Not reported |
| Reukers 2021 | Not defined | Not reported | Not reported |
| Robles Pellitero 2021 | Not defined | Not reported | Not reported |
| Rosenberg 2020 | Not defined | Not reported | |
| Roxby 2020 | Not defined | Not reported | Not reported |
| Sakamoto 2022 | Not defined | Not reported | Not reported |
| Sang 2020 | Not defined | Not reported | Not reported |
| Sarti 2021 | Not defined | Not reported | Not reported |
| Satter 2022 | Not defined | A contact was defined as an individual who experienced
any of the following exposures during the 2 days before and the 14 days after the onset of symptoms of a laboratory-confirmed COVID-19 case: (1) face-to-face contact with a confirmed case within 1 m and for more than 15 min (including travel, gossips, tea stall) or (2) direct physical contact with a confirmed COVID-19 case. |
<1m for >15 min |
| Schoeps 2021 | A category-I contact is defined as a person who either
stayed face-to-face (<1·5 meters) with a COVID-19-case for 15 minutes or longer, or in the same room (i.e., irrespective of distance) for 30 minutes or longer |
Not reported | <1.5m for >15 min |
| Schumacher 2021 | Close contact: Approximately 30–90 seconds in close
proximity (<1.5 m) of other players |
Close social contacts (including sharing a car) | 30–90 seconds in close proximity
(<1.5 m) |
| Schwierzeck 2020 | Not defined | Not reported | Not reported |
| Semakula 2021 | Not defined | A contact of a COVID-19 case was defined as any person
who had contact with a COVID-19 case within a timeframe ranging from 72 hours before the onset of symptoms of the case to 14 days after the onset of symptoms |
Not reported |
| Shah 2020 | Household contact was defined as contact sharing
same residential address. |
Not reported | Not reported |
| Shah 2021 | Not defined | Household contact was defined as an individual sharing
shame postal address, and secondary case was defined as individual developing infection within 14 days from last contact with the index case. |
Not reported |
| Shen 2020 |
Close contacts defined as individuals who had close,
prolonged, and repeated interactions with the 2 source cases (Cases 2 and 3). |
All other contacts are defined as casual contacts. | Not reported |
| Sikkema 2020 | Not defined | Not reported | Not reported |
| Son 2020 | Not defined | A contact was defined as anyone who was in contact with a
confirmed case from a day before the symptoms occurred, in a manner that offered the potential for transmission through respiratory droplets |
Not reported |
| Song 2020 | Shared the same bedroom, had dinner together | Not reported | Not reported |
| Sordo 2022 | Not defined | Close contacts were considered to be part of the same
household if they had the same address as the case. |
Not reported |
| Soriano-Arandes 2021 | Not defined | Household contacts were defined as all persons living
in the same household as the first patient diagnosed, regardless of the duration or proximity of the contact |
Not reported |
| Speake 2020 | 2 rows in front and behind infectious passenger on an
airplane |
Not reported | Unclear |
| Stein-Zamir 2020 | Not defined | Not reported | Not reported |
| Stich 2021 | Not defined | Not reported | Not reported |
| Sugano 2020 | Not defined | Not reported | Unclear |
| Sun 2020 | Not defined | Not reported | Not reported |
| Sun 2021 | Not defined | Not reported | Not reported |
| Sundar 2021 | Not defined | Contacts were those who were exposed to the index case
in the pre-symptomatic (2 days prior to symptom onset) or symptomatic period and satisfied at least one of the following: a) persons at residence of index case, b) persons at workplace who were exposed to the index case at close range (less than 6 feet) for ≥15 minutes, and c) persons outside the index case residence or workplace with close range contact ≥15 minutes who are traceable |
<1m for >15 min |
| Tadesse 2021 | Not defined | Not reported | Not reported |
| Tanaka 2021 | Not defined | Not reported | Not reported |
| Tanaka 2022 | Not defined | Not reported | Not reported |
| Taylor 2020 | Not defined | Not reported | Unclear |
| Teherani 2020 | Household contacts (HCs) were defined as an adult
(18 years) or a child (<18 years) who resided in the home with the SIC at the time of diagnosis. |
Not reported | Not reported |
| Thangaraj 2020 | Not defined | Not reported | Unclear |
| Torres 2020 | Not defined | Not reported | Unclear |
| Tsang 2022 | A close contact of a case is defined as any person
who was in close proximity to the case without any personal protection equipment, starting 2 days before the symptom onset of the case or specimen collection if the case was asymptomatic. Close contact settings include but are not limited to (1) living, working, dining or taking classes with the case in the same closed space or in proximity; (2) providing health care to or visiting the case at a hospital; and (3) sharing transportation with and in close proximity to the case (in flights, passengers within 3 rows of seats in the front and back of a case as well as crew members who had been in proximity to a case were considered as close contacts); and (4) other individuals in close proximity to the cases as determined by field investigators. |
Not reported | Not reported |
| Tshokey 2020 | Close friends, roommates, flight seat partner, spouse
or partner, cousin, physician, tour driver |
Primary contacts: Individuals coming in some form of
contact with the confirmed cases such as conveyance in the same cars/flights, encounter in clinics, serving meals, or providing housekeeping services in hotels. Secondary contacts: Individuals coming in contact with the primary contacts |
Unclear |
| Tsushita 2022 | Not defined | Not reported | Not reported |
| van der Hoek 2020 | Not defined | Not reported | |
| Vičar 2021 | Not defined | Not reported | Not reported |
| Wang 2020 | Not defined | Not reported | Unclear |
| Wang 2020a | Not defined | Not reported | Unclear |
| Wang 2020b | Close contact was defined as being within 1 m or 3 feet
of the primary case, such as eating around a table or sitting together watching TV. |
Not reported | Unclear |
| Wee 2020 | Not defined | Not reported | Within 2 m of the index case
for a cumulative time of ≥15 minutes, or who had performed AGPs without appropriate PPE. |
| Wendt 2020 |
High-risk contacts: >15 min face-to-face contact,
sitting in a row behind physician for 45 mins, transfer in an ambulance (45-min drive). |
Not reported | >15 min face-to-face contact |
| White 2022a | A close contact was defined as an individual who was
within 1 m of a case of COVID-19 while wearing a mask, or within 2 m if unmasked, in an indoor or outdoor setting for a cumulative total of 15 min or more over a 24-hour period during the case’s infectious period. |
Not reported | <2m for >15 min |
| White 2022b | A close contact was defined as an individual sitting
within a two-seat radius of an infectious case, where one infectious case was identified on a flight. If any close contact sitting within a two-seat radius of an infectious case tested positive, all passengers on board were then considered close contacts. If there were two or more unrelated infectious cases on board the same flight, all passengers were considered close contacts. |
Not reported | Within a two-seat radius of an
infectious case |
| Wiens 2021 | Not defined | Households were defined as a group of individuals that
sleep under the same roof most nights and share a cooking pot. |
Not reported |
| Wolf 2020 | Not defined | Not reported | Not specified |
| Wong 2020 |
Contact case was defined as a patient or staff who
stayed or worked in the same ward as the index patient. Patients who shared the same cubicle with the index case were considered as ‘patient close contact’. Staff close contact: Staff who had contact within 2 m of the index case for a cumulative time of >15 min, or had performed AGPs, without ‘appropriate’ PPE. |
Casual contacts: All staff and patients who did not fulfil the
pre-defined criteria for close contacts. Casual/low-risk contact: HCW wearing a facemask or respirator only and have prolonged close contact with a patient who was wearing a facemask, or HCW using all recommended PPE or HCW (not using all recommended PPE) who have brief interactions with a patient regardless of whether patient was wearing a facemask. Patient close contacts were quarantined into an AIIR (or quarantine camp if the patient was deemed clinically stable to be discharged from hospital) for 14 days. |
Within 2 m of the index case for
a cumulative time of >15 min |
| Wood 2021 | Not defined | Not reported | Not reported |
| Wu 2020 |
Close contact: Been within 1 metre of a confirmed
case, without effective PPE, within the period for 5 days before the symptom onset in the index case or 5 days before sampling if the index case was asymptomatic. |
Not reported | Within 1 metre of a confirmed case,
without effective PPE |
| Wu 2020a | Household contacts were defined as person who spent
at least 1 night in the house after the symptom onset of the index patient. A household was defined as ≥2 people living together in the same indoor living space. A household index was the first person to introduce SARS-CoV-2 into the household. |
Not reported | At least 1 night |
| Wu 2021 | Close contacts of symptomatic cases were individuals
who had exposed to a confirmed patient of SARSCoV-2 infection without wearing proper PPE (including practising optimal hand hygiene or wearing gloves, and wearing surgical facemasks and gowns) and/or stayed with the case in close proximity (<1m) in a close/ semi-close environment such as household, office, elevator, etc., which should have occurred within two days before the onset of the symptomatic case until when the symptomatic index case was isolated. Close contacts of asymptomatic SARS-CoV-2 infections were people who had a close contact (same definition as above) with the confirmed asymptomatic index case within two days before the asymptomatic case provided specimens to test for SARS-CoV2 to the time when the index case was isolated. |
Not reported | <1m for >15 min |
| Xie 2020 |
Close contact: An individual who has not taken
effective protection when in proximity of suspected or confirmed cases 2 days before the onset of symptoms or 2 days before the collection of asymptomatic specimens. |
Not reported | Unclear |
| Xie 2021 | Not defined | Not reported | Not reported |
| Xin 2020 | Close contacts were defined as persons who had a
short-range contact history for 2 days before the onset of symptoms in COVID-19-suspected and -confirmed cases, or 2 days before the collection of samples from asymptomatic cases without taking effective protective measures, such as family members in the same house, direct caregivers, and medical staff who provided direct medical care, colleagues in the same office or workshop, etc. |
The effective contact duration for the close contacts
was defined as the contact days with index patients with confirmed COVID‐19, which was calculated as the last contact date minus the start contact date, and all dates were corresponding to the definition of close contacts |
The median effective contact
duration with patients with COVID‐19 was 4 (IQR: 1–6) days, with 57 (53.8%) experiencing effective contact between 3 and 11 days, and 9 (8.5%) with effective contact duration > 11 days |
| Yang 2020 | Close contacts: Unprotected exposure. | Candidate contacts: Teachers and classmates | Not reported |
| Yau 2020 | Close unprotected contact with someone who has
tested positive for COVID-19 in the last 14 days |
Not reported | Unclear |
| Ye 2020 | Not defined | Not reported | Not reported |
| Yi 2021 | Not defined | Not reported | Not reported |
| Yoon 2020 |
Close contact was defined as a person who had
face-to-face contact for >15 minutes or who had direct physical contact with the index case-patient. Persons who used the same shuttle bus were also considered to be close contacts. |
Not reported | Face-to-face contact for
>15 minutes or direct physical contact |
| Yousaf 2020 | Not defined | Not reported | Not reported |
| Yu 2020 |
Close contacts were defined as those who lived in the
same household, shared meals, travelled or had social interactions with a confirmed case two days before the onset of COVID-19 symptoms |
Not reported | Not reported |
| Yung 2020 | Not defined | Not reported | Not reported |
| Zhang 2020 | Not defined | Not reported | Not reported |
| Zhang 2020a |
Close contact: Refers to a person who had contact
with index case without using proper protection during 2 days before the index case was tested. |
Not reported | Not reported |
| Zhang 2020b | Not defined | Not reported | Not reported |
| Zhang 2020c |
Close contacts were individuals who lived with a PCR-
confirmed case or interacted with a case within 1 metre from the case without any personal protections. |
Not reported | Within 1m of case |
| Zhang 2020d | Not defined | Not reported | Not reported |
| Zhang 2021 | Not defined | Not reported | Not reported |
| Zhuang 2020 | Not defined | Not reported | Not reported |
Eighteen studies (7%) reported data on the contact duration between close contacts and the index or primary cases ( Table 4). The average contact duration ranged from 30 minutes to 8 days across 16 studies that investigated transmission rates using RT-PCR. In two studies that examined transmission using serology (Agergaard 2020, Hong 2020), the durations of contact were two weeks and 258 person-days, respectively. The mean contact duration was either unclear or not reported in 236 studies (91.2%).
Test methods
A total of 163 studies (63.2%) used RT-PCR as a test method for confirming SARS-CoV-2 positivity, while 20 studies (7.8%) exclusively investigated transmission using serology (see Table 1). In 40 studies (15.5%), both PCR and serology were used to investigate close contact in SARS-CoV-2 transmission. Thirty-seven studies (14.3%) did not report the test method used. For PCR, the timing of sample collection varied from within 24 hours to 14 days after exposure to the index or primary case; for serology, this ranged from 2–22 weeks post-exposure. In total, 118 studies (45.7%) reported the timing of sample collection. The timing of sample collection was either not reported or unclear in 141 studies (54.7%).
Twenty-six out of 163 studies (17.2%) reported Ct values for determining PCR test positivity: ≤40 (eight studies), <37 (six studies), ≤35 (three studies), <38 (three studies), one each for <25, ≤30, <32, <36 (or 39) and <39. One study (Afonso 2021) used three different Ct values: <25, 25-30, or >30. Only 12 studies (7.4%) reported the Ct values for close contacts in their results – these ranged from 16.03 to 40.
Sixty studies reported conducting serological tests to assess transmission of SARS-CoV-2 ( Table 5). There was variation in the description of the tests. Twenty-eight studies determined the antibody responses to SARS-CoV-2 spike proteins using Immunoglobulin G (IgG) and/or IgM while 22 used only IgG. In 21 studies, the threshold for serological positivity was not reported. Nine studies (Craxford 2021, Farronato 2021, Gomaa 2021, Jeewandara 2021, Kuwelker 2020, Kuwelker 2021, Ng 2020, Stich 2021, Yang 2020) performed neutralisation assays to confirm positive serologic samples. In one study (Torres 2020), study participants self-administered the serological tests.
Four studies (Ladhani 2020a, Miller 2021, Speake 2020, Yang 2020) performed viral culture, while 18 studies (Böhmer 2020, Cerami 2021, Firestone 2020, Huang 2021, Jeewandara 2021, Jiang 2020, Klompas 2021, Kolodzeij 2022, Ladhani 2020a, Lucey 2020, Pang 2022, Powell 2022, Pung 2020, Sikkema 2020, Speake 2020, Taylor 2020, Wang 2020, Zhang 2021) performed genome sequencing (GS) plus phylogenetic analysis.
Table 5. Description of Serological Tests in Included Studies Conducted in Close Contact Settings.
| Study ID | Serological
test |
Description of test | Thresholds for serological positivity |
|---|---|---|---|
|
Agergaard
2020 |
IgG and IgM | iFlash and DiaSorin | iFlash SARS-CoV-2 N/S IgM/IgG cut-off:
≥12 AU/ml = positive. DiaSorin SARS-CoV-2 S1/S2 IgG cut-off: ≥15 AU/ml = positive, 12 < x < 15 AU/ml = equivocal, and ≤12 AU/ml = negative. |
|
Angulo-Bazán
2021 |
IgG and IgM | Coretests ® COVID-19 IgM / IgG Ab Test (Core Technology Co. Ltd), a lateral flow
immunochromatographic test that qualitatively detects the presence of antibodies against SARS-CoV-2, with a sensitivity and specificity reported by the manufacturer for IgM / IgG of 97.6% and 100%, respectively |
Not reported |
| Armann 2021 | IgG | Diasorin LIAISON® SARS-CoV-2 S1/S2 IgG Assay). All samples with a positive or equivocal LIAISON®
test result, as well as all samples from participants with a reported personal or household history of a SARS-CoV-2 infection, were re-tested with two additional serological tests: These were a chemiluminescent microparticle immunoassay (CMIA) intended for the qualitative detection of IgG antibodies to the nucleocapsid protein of SARS-CoV-2 (Abbott Diagnostics® ARCHITECT SARS-CoV-2 IgG ) (an index (S/C) of < 1.4 was considered negative whereas one >/= 1.4 was considered positive) and an ELISA detecting IgG against the S1 domain of the SARS-CoV-2 spike protein (Euroimmun® Anti-SARS-CoV-2 ELISA) (a ratio < 0.8 was considered negative, 0.8–1.1 equivocal, > 1.1 positive) Participants whose positive or equivocal LIAISON® test result could be confirmed by a positive test result in at least one additional serological test were considered having antibodies against SARS-CoV2. |
Antibody levels > 15.0 AU/ml were
considered positive and levels between 12.0 and 15.0 AU/ml were considered equivocal. |
| Baettig 2020 | IgG and IgM | Used commercially available immunochromatography rapid test with SARS-CoV-2 protein-specific
IgM and IgG. This test was performed according to the manufacturers’ instructions with a reported sensitivity and specificity of 93% and 95%, respectively. |
Not reported |
| Basso 2020 | IgG and IgM | Sera were collected approximately 3 weeks following exposure for the detection of antibodies against
SARS-CoV-2. EDI Novel Coronavirus COVID-19 lgG and IgM ELISA (Epitope Diagnostics, Inc., San Diego, CA, USA) were used for initial testing, and supplemented with tests from DiaSorin (LIAISON SARS-CoV-2 S1/S2 IgG test), Abbott (Alinity i SARS-CoV-2 IgG), Roche (Elecsys Anti-SARS-CoV-2) and Wantai (WANTAI SARS-CoV-2 Ab ELISA). |
Not reported |
|
Bernardes-
Souza 2021 |
IgM or IgG | Participant’s peripheral blood (3 mL) was collected by puncture of the brachiocephalic vein by a
trained nurse and then transferred to a serum-separating tube. The tube was stored between 2 °C to 8 °C and transported within 2 hours to the public laboratory of the town Department of Health, where it was immediately centrifuged (2000xg for 10 minutes) and the separate serum was tested for SARS- CoV-2 antibodies using a lateral flow immunoassay according to the manufacturer’s instructions. |
The sample was considered positive if
IgM or IgG antibodies were detectable. |
| Bhatt 2022 | IgG, IgA or
IgM |
ELISA adapted and optimized from the assay were used to evaluate SARS-CoV-2-specific IgA, IgM and
IgG against the spike-trimer and nucleocapsid protein. |
Samples were considered antibody
positive for a particular isotype (IgG, IgA or IgM) when both antispike and anti- nucleocapsid antibodies were detected above the cut-off values (signal-to-cut- off value ≥ 1) for that isotype. Samples were considered positive for SARS-CoV- 2 antibody if they were positive for IgG or for both IgA and IgM. |
| Bi 2021 | IgG | ELISA targeting the S1 domain of the spike protein of SARS-CoV-2; sera diluted 1:101 were processed
on a EuroLabWorkstation ELISA. |
Seropositivity was defined based
on the cutoff recommended by the manufacturer and explored a higher cut- off of 1.5 (>1.5) in sensitivity analyses. |
| Brown 2020 | IgG and IgM | ELISA (authors referenced another study) | Reciprocal titers of >400 to be positive
and reciprocal titers of >100 but <400 to be indeterminate. |
| Chen 2020b | IgG and IgM | In-house enzyme immunoassay (EIA). 96-well plates were coated with 500 ng/mL of recombinant RBD
or NP protein overnight, incubating with diluted serum samples at 1:20. Plates were incubated with either anti-human IgM or IgG conjugated with HRP. Optical density (OD) value (450nm-620nm) was measured. |
Preliminary cut-off values were
calculated as the mean of the negative serum OD values plus 3 standard deviations (SD) from 90 archived healthy individuals in 2019. A close contact was considered seropositive if OD of 1:20 diluted serum was above the cut-off values for either IgM or IgG against both RBD and NP protein |
| Chu 2020 | IgG and IgM | Serum samples were tested at CDC using a SARS-CoV-2 ELISA with a recombinant SARS-CoV-2 spike
protein (courtesy of Dr. Barney Graham, National Institutes of Health, Bethesda, MD, USA) as an antigen. Protein ELISA 96-well plates were coated with 0.15 μg/mL of recombinant SARS-CoV-2 spike protein and ELISA was carried out as previously described. An optimal cutoff optical density value of 0.4 was determined for >99% specificity and 96% sensitivity. Serum samples from the case-patient were used as a positive control and commercially available serum collected before January 2020 from an uninfected person as a negative control. |
Total SARS-CoV-2 antibody titers >400
was considered seropositive. |
|
Craxford
2021 |
IgG | ELISA. Serum samples were serially diluted in 3% skimmed milk powder in PBS containing
0.05% Tween 20 and 0.05% sodium azide. All assays were performed on Biotek Precision liquid handling robots in a class II microbiological safety cabinet. For endpoint dilution ELISAs, sera were progressively 4-fold diluted from 1:150 to 1;38,400. |
Participants found to be seropositive
for SARS-CoV-2 were assessed for the presence of neutralising antibodies. |
| Dattner 2020 | IgG | Abbott SARS-CoV-2 IgG, whose specificity was estimated as ∼100% and whose sensitivity at ≥ 21 days
was estimated as ∼85% |
Not reported |
| de Brito 2020 | IgG and IgM | Chemiluminescence 4 weeks after contact with the index case | Not reported |
|
Dimcheff
2020 |
IgG | Serum IgG to thD4:D12e nucleoprotein of SARS-CoV-2 was measured using a Federal Food and Drug
Administration (FDA) emergency-use–authorized chemiluminescent microparticle immunoassay performed on an automated high throughput chemistry immunoanalyzer (Architect i2000SR, Abbott Laboratories, Abbott Park, IL). The sensitivity of this assay is reported to be 100% with a specificity of 99% at >14 days after symptom onset in those infected with SARS-CoV-2.1 At 5% prevalence, the positive predictive value is 93.4% and the negative predictive value is 100% |
Results are reported in a relative
light units (RLU) index; a value ≥1.4 RLU is considered a positive antibody response. |
| Dub 2020 | IgG | IgG antibodies to SARS-CoV-2 nucleoprotein (The Native Antigen Company, United Kingdom) were
measured with a fluorescent bead-based immunoassay (manuscript in preparation). Antigen was conjugated on MagPlex Microspheres and bound IgG antibodies were identified by a fluorescently labeled conjugated antibody (RPhycoerythrin-conjugated Goat Anti-Human IgG, Jackson Immuno Research, USA). The plate was read on Luminex® MAGPIX® system. xPONENT software version 4.2 (Luminex®Corporation, Austin, TX) was used to acquire and analyze data. Median fluorescent intensity was converted to U/ml by interpolation from a 5- parameter logistic standard curve. The specificity and sensitivity of the assay was assessed using receiver operator curve (ROC) with 100% specificity and 97.9% sensitivity |
MNT titre of ≥ 6 considered positive
FMIA titre 3·4 U/ml considered positive |
|
Farronato
2021 |
IgM or IgG | Rapid lateral flow chromatographic test. If the test sample contains IgM or IgG antibodies to SARS-
CoV-2, the test displays two different visible bands (test line and control line); however, if these antibodies are absent, only the control line appears. |
Participants found to be seropositive
for SARS-CoV-2 were assessed for the presence of neutralising antibodies. |
|
Fontanet
2021 |
IgG | ELISA N assay, detecting antibodies binding to the nucleocapsid (N) protein; a S-Flow assay, which is a
flow-cytometry based assay detecting anti-spike (S) IgG; and a luciferase immunoprecipitation system (LIPS) assay, which is an immunoprecipitation-based assay detecting anti-N, anti-S1 and anti-S2 IgG. Samples were also tested for neutralisation activity using a viral pseudotype-based assay. |
In the high school study, participants
were considered seropositive for SARS-CoV-2 antibodies if any of the serological assay tests were positive. |
| Galow 2021 | IgG | SARS-CoV-2 IgG antibodies were detected via Diasorin LIAISON® SARS-CoV-2 S1/S2 IgG Assay and
positive or equivocal results were confirmed via Abbott Diagnostics® ARCHITECT SARS-CoV-2 and Euroimmun® Anti-SARS-CoV-2 ELISA. |
Participants whose positive or equivocal
LIAISON® test result could be confirmed by an additional serological test were considered seropositive for SARS-CoV-2. |
| Gaskell 2021 | IgG | Serum samples were analysed for the presence of IgG specific for SARS CoV-2 trimeric spike
protein (S), Receptor Binding Domain (RBD) and nucleocapsid (N) antigens using a multiplex chemiluminescence immunoassay. |
Not reported |
| Gomaa 2021 | Unclear | Microneutralization Assay was conducted to measure the nAb titre in human sera using Vero-E6
(ATCC, CRL-1586) cell monolayers using SARS-CoV-2/Egypt/NRC-03/2020 under biological safety level 3. The plates were then incubated for three more days at 37°C in 5% CO2 in a humidified incubator. A virus back-titration was performed without immune serum to confirm TCID50 viral titre used. Cytopathic effect (CPE) was observed post 72 hrs of infection. |
The reciprocal of the serum dilution
that protected cells from CPE was considered the nAb titre. Negative sera were given a value of 1:5. |
|
Gonçalves
2021 |
IgM or IgG | Seropositivity was determined by a point-of-care rapid antibody test. The assays were carried out
according to the manufacturers protocol: a 10 μl sample (whole blood or serum) was applied to the sample well, followed by the addition of 2-3 drops or 80μl of diluent. The test was developed for 15 minutes at room temperature and the results (positive or negative) were read by independent experienced readers blinded to the sample status. |
Control line threshold |
| Gu 2020 | IgG | Not described | Not reported |
|
Helsingen
2020 |
IgG | Measurement of IgG antibodies was performed with a multiplex flow cytometric assay known as
microsphere affinity proteomics (MAP) |
Not specified. Referenced |
| Hong 2020 | IgG and IgM | Qualitative colloidal gold assay (Innovita (Tangshan) Biological Technology, Co., Ltd, Tangshan, China),
following manufacturers’ instructions. The sensitivity of the assay was 87.3% (95%CI 80.4–92.0%), and the specificity was 100% (95%CI 94.20–100%) according to the instructions of the assay. |
Not reported |
|
Jeewandara
2021 |
Unclear | Due to the limitations in using a BSL-3 facility to carry out assays to measure neutralizing antibodies,
the Nabs were measured using a surrogate virus neutralization test (sVNT). ELISA was used to assess antibody responses. |
Inhibition percentage ≥ 25% in a
sample was considered as positive for Nabs. |
| Jordan 2022 | IgG | Not reported | Not reported |
| Katlama 2022 | IgM or IgG | ach index case and all contact individuals were tested using the rapid immunochromatographic lateral
flow assay (LFA) COVID-PRESTO manufactured by AAZ detecting total SARS-CoV-2 IgG, IgM, or both antibodies targeting the N-protein with a sensitivity of 78.4% and 92.0% and a specificity of 100% and 92% for IgM and IgG. The presence of IgG antibodies against the nucleocapsid protein was measured and interpreted using commercially available chemiluminescent microparticle immunoassay (CMIA) kits. |
Not specified |
| Kim 2021 | IgM or IgG | IgM and IgG and ELISA total antibody testing. The FIA IgM and IgG kit used the automated
fluorescent lateral flow immunoassay method, using the AFIAS-6 analyzer system. |
FIA kit Specimens with a relative cut-off
index (COI) value ≥ 1.1 were considered positive. ELISA: An optical density (OD) ratio < 1.0 was interpreted as negative, ≥0.9 to <1.0 as borderline, and ≥1.0 as positive. |
|
Kolodziej
2022 |
IgG | Sera were tested for the presence of immunoglobulin G antibodies reactive with the SARS-CoV-2
spike trimer, S1, and N antigens in a protein microarray, in duplicate 2-fold serial dilutions starting at 1:20. For each antigen, a 4-parameter log logistic calibration curve was generated and effective concentration 50, mid-point antibody titres were calculated. |
Not specified |
|
Kuwelker
2020 |
IgG | A two-step ELISA was used for detecting SARS-CoV-2-specific antibodies, initially by screening with
receptor-binding domain (RBD) and then confirming seropositivity by spike IgG. Endpoint titres were calculated as the reciprocal of the serum dilution giving an optical density (OD) value=3 standard deviations above the mean of historical pre-pandemic serum samples. Individuals with no antibodies were assigned a titre of 50 for calculation purposes. Neutralisation assays were used to quantify SARS-CoV-2-specific functional antibodies. VN titres were determined as the reciprocal of the highest serum dilution giving no CPE. Negative titres (<20) were assigned a value of 10 for calculation purpose. |
Not specified. |
|
Kuwelker
2021 |
IgG | A two-step ELISA was used for detecting SARS-CoV-2-specific antibodies, initially by screening with
receptor-binding domain (RBD) and then confirming seropositivity by spike IgG. The neutralisation assays were used to quantify SARS-CoV-2-specific functional antibodies. |
ELISA: Individuals with titres ≥100 were
defined as positive and those with no antibodies were assigned a titre of 50 for calculation purposes. Neutralisation assays: Negative titres (<20) were assigned a value of 10 for calculation purpose. |
| Lewis 2020 | Not specified | ELISA (authors referenced another study) | Not specified |
| Lin 2021 | IgM or IgG | SARS-CoV-2 IgM and IgG antibodies were detected by Chemiluminescence and GICA. The test results
were expressed in relative light units (RLU), and the IgM or IgG levels were positively correlated with RLU. The instrument automatically calculated IgM or IgG antibody levels (AU/mL) based on RLU and the built-in calibration curve. |
Test result ≥ 10.0 AU/mL was reported
as positive. |
| Luo 2020a | IgG and IgM | Not described | Asymptomatic: Specific IgM detected
in serum. Symptomatic: Detectable SARS-CoV- 2–specific IgM and IgG in serum, or at least a 4-fold increase in IgG between paired acute and convalescent sera. |
|
Macartney
2020 |
IgA, IgG, IgM | SARS-CoV-2-specific IgG, IgA, and IgM detection was done using an indirect immunofluorescence
assay (IFA) that has a sensitivity compared with nucleic acid testing of detecting any of SARS-CoV-2- specific IgG, IgA, or IgM when samples were collected at least 14 days after illness onset of 91·3% (95% CI 84·9–95·6) and specificity of 98·9% (95% CI 98·4–99·3%; MVNO, personal communication). |
Not specified |
|
Martinez-
Fierro 2020 |
IgG and IgM | IgM and IgG against SARS-CoV-2 were determined using a total blood sample through a 2019 nCov
IgG/IgM rapid test (Genrui Biotech, Shenzen, China) |
Not specified |
|
Mercado-
Reyes 2022 |
IgM or IgG | Prior infection by SARS-CoV2 was ascertained by measuring total antibodies (IgM+ IgG) using the
SARS-CoV-2 Total (COV2T) Advia Centaur – Siemens chemiluminescent immunoassay (CLIA). Sera from 149 patients with SARS-CoV-2 infection, confirmed by RT-PCR and obtained less than 14 days after the onset of symptoms, were used as positive controls. |
Not specified |
| Meylan 2021 | IgG | Serum samples were analysed for SARS-CoV-2 serology (IgG), using a previously described Luminex-
based assay quantifying antibody binding to the trimeric form of the SARS-CoV-2 S-protein. |
This assay has shown a sensitivity and
specificity of 97% and 98%, respectively, on hospitalised patients for the chosen cut-off of positivity defined at a ratio >5.90. |
| Miller 2021 | IgG | Blood samples were tested for IgG antibody to the nucleocapsid protein (NP) by a commercial NP
assay and also by an in-house ELISA that used the receptor binding domain (RBD) as antigen. |
The cut-off for antibody positivity used
in the analyses were ≥0.8 for the Abbott assay and ≥5.0 for the RBD ELISA. |
| Ng 2020 | Not specified | human ACE-2 (hACE2) protein (Genscript Biotech, New Jersey, United States) was coated at 100
ng/well in 100 mM carbonate-bicarbonate coating buffer (pH 9.6). 3ng of horseradish peroxidase (HRP)-conjugated recombinant receptor binding domain (RBD) from the spike protein of SARS-CoV- 2 (GenScript Biotech) was pre-incubated with test serum at the final dilution of 1:20 for 1 hour at 37°C, followed by hACE2 incubation for 1 h at room temperature. Serum samples were tested with a surrogate viral neutralising assay for detection of neutralising antibodies to SARS-CoV-2. |
A positive serological test result
was concluded if the surrogate viral neutralising assay for a particular sample resulted in inhibition of 30% or greater (98·9% sensitivity and 100·0% specificity) |
| Ogawa 2020 | IgG | Abbott ® (Abbott ARCHITECT SARS-CoV-2 IgG test, Illinois, USA) | Not specified |
|
Petersen
2021 |
Unclear | SARS-CoV-2 Ab ELISA kit was used to determine serologic status | Not specified |
| Poletti 2020 | IgG | Not described | Not specified |
| Powell 2022 | Unclear | Oral fluid (OF) swabs tested for antibodies against the SARS-CoV-2 Nucleoprotein using an
Immunoglobulin G capture-based enzyme immunoassay. |
Not specified |
|
Ratovoson
2022 |
IgM or IgG | ELISA for the detection of total antibodies (including IgM and IgG) to SARS-CoV-2. | Not specified |
| Razvi 2020 | IgG and IgM | Blood samples were analysed on the day of collection using the Roche Elecsys Anti-Sars-CoV-2
serology assay. This electro chemiluminescent immunoassay is designed to detect both IgM and IgG antibodies to SARS-CoV-2 in human serum and plasma and has been shown to have a high sensitivity and specificity |
Not specified |
| Reukers 2021 | IgM or IgG | ELISA for the detection of total antibodies (including IgM and IgG) to SARS-CoV-2. | Not specified |
| Satter 2022 | IgM or IgG | ELISA for the detection of total antibodies (including IgM and IgG) to SARS-CoV-2. The Receptor
Binding Domain (RBD) of the spike protein of SARS-CoV-2 was used as an antigen to detect antibody responses |
Using serum from pre-pandemic
healthy controls, the concentration of 500 ng/mL (0.5 µg/mL) was determined as a cut-off value for seropositivity for both RBD-specific IgG and IgM antibodies. |
|
Schumacher
2020 |
IgG and IgM | SARS-CoV-2-specific antibodies were measured in serum samples using an
electrochemiluminescence immunoassay (Elecsys® Anti-SARS-CoV-2, Roche Diagnostics, Rotkreuz, Switzerland). |
Cut-off indices ≤1 reported as negative
and indices >1 as positive. |
| Sordo 2022 | IgG | Not reported | 4-fold or greater increase in a
SARS-CoV-2 antibody of any subclass. |
| Stich 2021 | IgM or IgG | ELISA for the detection of total antibodies (including IgM and IgG) to SARS-CoV-2. Antibodies reactive
to the N protein were measured either with the Elecsys Anti-SARS-CoV-2 IgG/IgM ECLIA test kit. Neutralisation assays were performed. |
Serum samples with a positive reaction
in the additional assay were classified as seropositive. |
| Tadesse 2021 | IgM or IgG | ELISA for the detection of total antibodies (including IgM and IgG) to SARS-CoV-2. Chemiluminescent
microparticle immunoassay (CMIA) was used to determine seroprevalence. |
Not specified |
| Torres 2020 | IgG and IgM | Novel Coronavirus (2019-nCoV) IgG/IgM Test Kit (Colloidal gold) from Genrui Biotech Inc. The study
nurse and/or technician viewed the photo provided by the participant along with the participant’s self-report as to the visibility of the three bands, and determined whether the tests were IgG+, IgM+, IgG & IgM+, Negative, Invalid, or Indeterminate. Participants were asked to attach a photo of the test after 15 minutes had elapsed and self-report the appearance of the three lines, G (IgG), M (IgM), and C (test control) |
Colour-coded - self-administered test:
self-reporting the appearance of the three lines, G (IgG), M (IgM), and C (test control) |
|
van der Hoek
2020 |
IgG | Fluorescent bead-based multiplex-immunoassay. Referenced | A cut-off concentration for seropositivity
(2.37 AU/mL; with specificity of 99% and sensitivity of 84.4%) was determined by ROC-analysis of 400 pre-pandemic control samples |
| Wendt 2020 | IgA and IgG | ELISA (Euroimmun, Lübeck, Germany), following the manufacturer’s instructions. | Inconclusive (≥0.8 and <1.1) or Positive
(≥1.1 |
| Wiens 2021 | IgG | ELISA for IgG antibodies. This assay quantifies RBD-specific antibody concentrations (μg/mL) using
IgG-specific anti-RBD monoclonal antibodies. To help decide on an appropriate positivity threshold and assess assay specificity, the authors measured background antibody reactivity using 104 dried blood spot samples collected in Juba in 2015. |
Seropositivity threshold (0.32 μg/mL)
that corresponded to 100% specificity in these pre-pandemic samples (i.e., their highest value) and 99.7% in the pre-pandemic samples collected from the USA. |
| Yang 2020 | IgA, IgG, IgM | Serum immunoglobulin (Ig) antibody against the SARS-CoV-2 surface spike protein receptor-binding
domain (RBD) was measured using a chemiluminescence kit (IgM, IgG, and total antibody, Beijing Wantai Biotech, measured by cut-off index [COI]) or ELISA kit (IgA, Beijing Hotgen Biotech, measured by optical density at 450/630 nm [OD450/630]). The cut-off for seropositivity was set according to the manufacturer’s instruction, verified using positive (169 serum specimens from confirmed COVID-19 patients) and negative (128 serum specimens from healthy persons) controls, and both of sensitivity and specificity were 100%. Virus neutralization assays were performed using SARS-CoV-2 virus strain 20SF014/vero-E6/3 (GISAID accession number EPI_ISL_403934) in biosafety level 3 (BSL-3) laboratories. Neutralizing antibody (NAb) titer was the highest dilution with 50% inhibition of cytopathic effect, and a NAb titer of ≥1:4 was considered positive. |
Specimens with COI>1 (IgM, IgG, or
total antibody), OD450/630 > 0.3 (IgA) were considered positive. |
| Zhang 2020b | IgG and IgM | SARS-CoV-2-specific IgM and IgG were tested by paramagnetic particle chemiluminescent
immunoassay using iFlash-SARS-CoV-2 IgM/IgG assay kit (Shenzhen YHLO Biotech Co., Ltd) and iFlash Immunoassay Analyzer (Shenzhen YHLO Biotech Co., Ltd). The specificity and sensitivity of SARS-CoV-2 IgM and IgG detection were also evaluated |
Not specified |
Frequency of SARS-CoV-2 attack rates (ARs)
Twenty-four studies reported data on attack rates using RT-PCR ( Table 6). The settings included healthcare (n=3), household (n=8), public transport (n=2), educational settings (n=4). In one study of 84 children in daycare centres during the first few weeks of the pandemic (Desmet 2020), the AR was 0%; similar results were reported in another study of hospital healthcare workers (Basso 2020). The frequency of ARs in the remaining 22 studies ranged from 2.1 to 75% ( Figure 3a). The ARs were highest in weddings (69%), prison (69.5%) and households (75%). Attack rates appeared lower in healthcare settings; two healthcare settings with higher ARs (Ladhani 2020, Ladhani 2020a) included nursing home residents – the definition of SARS-CoV-2 infection in both studies did not include the full constellation of respiratory and non-respiratory symptoms. In sports settings, the AR during matches was between 4.2% and 4.7%.
Figure 3a. Primary attack rates of SARS-CoV-2 in close contacts (PCR).
Table 6. Main Results of Included Studies Investigating SARS-CoV-2 Transmission in Close Contact Settings.
| Study ID | Type of
transmission |
Total number of
contacts |
Cycle
threshold |
Attack rates and/or secondary attack
rates (SAR) |
Notes |
|---|---|---|---|---|---|
| Abdulrahman 2020 | Community |
Eid Alfitr
Pre-: 71,553; Post-: 76,384 Ashura Pre-: 97,560; Post-: 118,548 |
Not reported |
Eid Alfitr
Pre-: 2990 (4.2%); Post-: 4987 (6.7%); p <0.001 Ashura Pre-: 3571 (3.7%); Post-: 7803 (6.6%); p <0.001 |
The rate of positive tests was significantly greater
after religious events. |
| Adamik 2020 | Household | Unclear | Not reported | Unclear: 3553 (AR 26.7%) | |
| Afonso 2021 | Household | 267 | < 25, 25–30,
or >30 |
19.9% (95% CI 15.5–25.1; 53/267) | |
| Agergaard 2020 | Household | PCR: 5
Serology: 5 |
Not reported | Index case plus 1 family member tested
positive-PCR All 5 displayed a serological SARS-CoV-2 N/S IgG response |
|
| Akaishi 2021 | Household
Community |
2179 | Not specified | 11.9% (95% CI 10.6–13.3%; 259/2179) | |
| Angulo-Bazán 2021 | Household | 52 households
(n=236 people) 4.5±2.5 members per household |
Not reported | Serology: Amongst cohabitants, SAR was
53.0% (125 cases): 77.6% of cases were symptomatic |
Convenience sampling, no component of
temporality, selection bias. |
| Armann 2021 | Local
Household |
2045 in Phase 1
1779 in Phase 2 |
N/A | Serology: 12/2045 (0.6%)
Serology: 12/1779 (0.7%) |
|
| Arnedo-Pena 2020 | Household | 745 | Not reported | 11.1% (95% CI 9.0-13.6) | |
| Atherstone 2021 | Community | 441 | Not specified | 9.3% (41/441) | |
| Baettig 2020 | Local | 55 | Not reported | Serologic attack rates: 2/55 (3.6%) | Serological testing was positive for the 2 contacts
14 days after index case |
| Baker 2020 | Nosocomial | 44 | Not reported | 3/44 (6.8%): 1 of these was also exposed to a
household member with COVID-19. |
Recall error and bias, report is limited to a single
exposure, change in mask policy partway through the exposure period |
| Bao 2020 | Community | 57 index cases
1895 exposed |
Not reported | SAR was 3.3% at the bathing pool, 20.5% in
the colleagues’ cluster and 11.8% in the family cluster. |
Delayed detection of the activity trajectory of the
primary case, reporting bias, overlap of close contacts |
| Basso 2020 | Nosocomial | 60 HCWs - ≥106
unique high-risk contacts |
Not reported | Attack rate: 0/60 (0%)
Serology: 0/60 (0%) |
Delay in diagnosing index case, recall bias |
| Bays 2020 | Nosocomial | 421 HCWs | Not reported | 8/421 (1.9%) | In all 8 cases, the staff had close contact with the
index patients without sufficient PPE. Hospital staff developing ILI symptoms were tested for SARS- CoV-2, regardless of whether they had contact with an index patient |
| Bender 2021 | Community | 280 | Not specified | 13.3% (24/180) | |
|
Bernardes-Souza
2021 |
Household | 112 | Not specified | AR 49.1% (55/112) | |
| Bhatt 2022 | Household | 487 | N/A | 49.1% (95% CI 42.9–55.3%; 239/487) | |
| Bi 2020 | Local
Household Community |
1,296 | Not reported | 98/1286 (7.6%) | |
| Bi 2021 | Household | 4534 | Not specified | 6.6% (298/4534) | |
| Bistaraki 2021 | Household
Community |
64608 | Not specified | 17.4% (95% CI 17.0–17.8; 11232/64608). | |
| Bjorkman 2021 | Local | 6408 | Not specified | AR 16.5% (1058/6408) | |
| Blaisdell 2020 | Community | 1,022 | Not reported | 1.8% of camp attendees (10 staff members
and 8 campers) |
Travel was assumed to be from home state, but
intermediate travel might have occurred |
| Böhmer 2020 | Local
Household |
241 | Not reported | 75·0% (95% CI 19·0–99·0; three of four
people) among members of a household cluster in common isolation, 10·0% (1·2–32·0; two of 20) among household contacts only together until isolation of the patient, and 5·1% (2·6–8·9; 11 of 217) among non- household, high-risk contacts. |
|
| Boscolo-Rizzo 2020 | Household | 296 | Not reported | 74/296 (25.0%, 95% CI 20.2–30.3%) | The prevalence of altered sense of smell or taste
was by far lower in subjects negative to SARS-CoV-2 compared to both positives (p < 0.001) and non- tested cases (p < 0.001). |
| Brown 2020 | Local | 21 | Not reported | Serologic attack rate: 2/21 (1%) | Social desirability bias likely |
| Burke 2020 | Household | 445 | Not reported | 0.45% (95% CI = 0.12%–1.6%) among all close
contacts, and a symptomatic secondary attack rate of 10.5% (95% CI = 2.9%–31.4%) among household members. |
2 persons who were household members of
patients with confirmed COVID-19 tested positive for SARS-CoV-2. |
| Calvani 2021 | Local
Household |
162 children
(81 SARS-CoV-2 positive and 81 Controls) 142 contacts |
Used NAAT | School contacts 40% (28/70)
Family members 30.6% (95%CI 20.2–42.5; 22/72) |
School contacts: 70 children, 219 family members |
| Canova 2020 | Nosocomial | 21 | Not reported | 0/21 (0%) | |
| Carazo 2021 | Household | 9096 | Not specified | 29.8% (2718/9096) | |
| Cariani 2020 | Nosocomial | Unclear | 33.6 to 38.03 | 182 out of 1683 (10.8%) tested positive; 27 of
whom had close contact with COVID-positive patients |
Unclear how many HCWs had close contact;
likelihood of recall bias |
| Carvalho 2022 | Household | 182 | Not specified | 52.7% (96/182) | |
| Cerami 2021 | Household | 103 | Not specified | 32% (95% CI 22%-44%; 33/103) | |
| Charlotte 2020 | Community | 27 | Not reported | 19 of 27 (70%) tested positive | High risk of selection bias: The index case-patients
were not identified. A majority of patients were not tested for SARS-CoV-2 |
| Chaw 2020 | Local
Community |
1755 | Not reported | Close contact: 52/1755 (29.6%)
Nonprimary attack rate: 2.9% (95% CI 2.2%–3.8%) |
Potential environmental factors were not
accounted for: relative household size, time spent at home with others, air ventilation, and transmission from fomites. |
| Chen 2020 | Aircraft | 335 | Not reported | 16/335 (4.8%) | Recall bias. Did not perform virus isolation and
genome sequencing of the virus, which could have provided evidence of whether viral transmission occurred during the flight. |
| Chen 2020a | Local
Household |
209 | Not reported | 0/209 (0%) | |
| Chen 2020b | Nosocomial | 105 | Not reported | Serology: 18/105 (17.1%) | |
| Chen 2020c | Local
Community Household Nosocomial |
2147 | Not reported | 110/2147 (5.12%) | |
| Cheng 2020 | Household
Nosocomial |
2761 | Not reported | 0.70% | |
| Chu 2020 | Community | 50 exposed | Not reported | None for antigen or antibody: 0/50 (0%) | Testing was biased toward contacts who knew
the case-patient personally (office co-workers) or provided direct care for the case-patient (HCP). |
| Chu 2021 | Household | 526 exposed | Not reported | 48 (9%) (CI 7-12%) | Very high risk of selection bias |
| Contejean 2020 | Nosocomial | 1344 exposed | Not reported | 373 (28%) | |
| Cordery 2021 | Local
Household |
65 | Not specified | Overall 12.3% (8/65)
Child bubble contacts 0% (0/13) School contacts 10.3% (3/29) Child household contacts 0% (0/8) Adult household contacts 26.7% (4/15) |
|
|
COVID-19 National
Emergency Response Center 2020 |
Local
Household Nosocomial |
2370 | Not reported | 13/2370 (0.6%) | There were 13 individuals who contracted COVID-
19 resulting in a secondary attack rate of 0.55% (95% CI 0.31–0.96). There were 119 household contacts, of which 9 individuals developed COVID- 19 resulting in a secondary attack rate of 7.56% (95% CI 3.7–14.26). |
| Craxford 2021 | Household | 178 | N/A | 7.2% (13/178) | |
| Danis 2020 | Local
Household |
Chalet: 16
School: 172 |
Not reported | Attack rate: 75% in chalet
Attack rate: 0% in school |
Only 73 of 172 school contacts were tested - all
tested negative |
| Dattner 2020 | Household | 3353 | Not reported | Attack rates: 25% in children and 44% adults
(45% overall) Serology: 9/714 (1.3%) |
|
| de Brito 2020 | Household | 24 exposed | Not reported | RT-PCR: 6/7 (86%); Seropositivity: 18/24 (75%) | |
| Deng 2020 | Household | 347 | Not reported | 25/347 (7.2%) | |
| Desmet 2020 | Local | 84 | 38.8 | Attack rate: 0/84 (0%) | Ct reported for only one test result |
| Dimcheff 2020 | Community
Nosocomial Household |
1476 | Not reported | Seroprevalence 72/1476: 4.9% (95% CI,
3.8%–6.1%) |
|
| Dong 2020 | Household | 259 | Not reported | 53/259 (20.5%) | |
| Doung-ngern 2020 | Local | 211 cases plus
839 non- matched controls |
Not reported | ||
| Draper 2020 | Local
Household Nosocomial |
445 | Not reported | 4/445 (0.9%) | None of the 326 aircraft passengers or 4
healthcare workers who were being monitored close contacts became cases. |
| Dub 2020 | Local
Household |
121 | Not reported | Child index case: No positive cases
Adult index case: 8/51 (16%) Serology: 6/101 (5.9%) |
|
|
Expert Taskforce
2020 |
Local | Unclear | Not reported | Attack rate 20.4% | Attack rates were highest in 4-person cabins
(30.0%; n = 18), followed by 3-person cabins (22.0%; n = 27), 2-person cabins (20.6%; n = 491), and 1- person cabins (8%; n = 6). |
| Farronato 2021 | Household | 49 | N/A | 16.3% (8/49) | |
|
Fateh-Moghadam
2020 |
Community | 6690 | Not reported | 890/6690 (13.3%) | |
| Firestone 2020 | Local | Unclear | Not reported | 41 (80%) interviewed patients with primary
event-associated COVID-19 reported having close contact with others during their infectious period, with an average of 2.5 close contacts per patient. 36 (75%) of 48 interviewed patients with primary event-associated cases reported having close contact with persons in their household while infectious, and 17 (35%) reported having other (social/workplace) close contacts while infectious. |
|
| Fontanet 2021 | Local | 2004 | N/A | Serology: 15.3% (306/2004)
10.4% (139/1,340) - primary schools 25.1% (167/664) - high schools |
|
| Galow 2021 | Household | 248 | N/A | 34.3% (85/248) | |
|
Gamboa Moreno
2021 |
Household
Community |
Unclear | Not specified | 2.9% in preschools to 7.1% in high schools | |
| Gan 2020 | Local
Household Community |
Unclear | Not reported | Not reported | Family clusters accounted for 86.9% (914/1 050) of
cases, followed by party dinners (1.1%) |
| Gaskell 2021 | Household | 1242 | N/A | AR 64.3% (95% CI 61.6-67.0%, 799/1,242). | |
| Ge 2021 | Household
Community |
8852 | Not specified | 3.6% (95% CI 3.3%-4.0%; 327/8852) | |
| Ghinai 2020 | Community | Unclear | Not reported | Unclear | |
| Gold 2021 | Local
Household |
31 school
69 household |
Not specified | School: 48% (15/31)
Household: 26% (18/69) |
|
| Gomaa 2021 | Household | 98 | Not specified | AR 6.9% (95%CI: 5.8–8.3)
89.8% (95% CI: 82.2–94.3; 88/98) AR Serology 34.8% (95% CI: 32.2–37.4; 438/1260) |
|
| Gonçalves 2021 | Household | 271 case-
patients and 1,396 household controls |
Not specified | Not reported | |
| Gong 2020 | Household
Community |
Unclear | Not reported | Unclear | |
| Gu 2020 | Local | 14 | Not reported | RT-PCR - 3/14 (21.4%)
Serology - 2/14 (14.3%) |
|
| Hamner 2020 | Local | 60 | Not reported | Confirmed: 32/60 (53.3%)
Probable: 20/60 (33.3%) |
|
| Han 2020 | Community | 192 | Not reported | 7/192 (3.7%) | |
| Hast 2022 | Community | 628 | Not specified | 9.2% (58/628) | |
| Heavey 2020 | Local | 1155 | Not reported | 0/1155 (0%) | |
| Helsingen 2020 | Local | Training arm:
1,896 Nontraining arm: 1,868 |
Not reported | 11/1896 (0.8%) vs 27/1868 (2.4%); P=0.001 | |
| Hendrix 2020 | Local | 139 exposed | Not reported | 0% | Six close contacts of stylists A and B outside of
salon A were identified: four of stylist A and two of stylist B. All four of stylist A’s contacts later developed symptoms and had positive PCR test results for SARS-CoV-2. These contacts were stylist A’s cohabitating husband and her daughter, son-in- law, and their roommate, all of whom lived together in another household. None of stylist B’s contacts became symptomatic. |
| Hirschman 2020 | Household
Community |
58 | Not reported | 27/58 (47%) | |
| Hobbs 2020 | Local
Household Community |
397 | Not reported | Not reported | |
| Hoehl 2021 | Local
Community |
825 children and
372 staff: 7,366 buccal mucosa swabs and 5,907 anal swabs |
Not reported | 0% viral shedding in children; 2/372
(0.5%) shedding for staff. No inapparent transmissions were observed |
Study was conducted in the summer of 2020, when
activity of other respiratory pathogens was also low |
| Hong 2020 | Household | 431 tests | Not reported | 0/13 (0%) | Index cases had lived with their family members
without personal protections for a total of 258 person-days. |
| Hsu 2021 | Household | 145 | Not specified | 46.2% (47/145) | |
| Hu 2020 | Community | 72093 | Not specified | 0.32% (95%CI 0.29% –0.37%; 234/72093) | |
| Hu 2021 | Household
Community |
15648 | Not reported | 471/15648 (3%) | |
| Hu 2021 | Local | 5622 | Not specified | 0.6% (95% CI 0.43 - 0.84%; 34/5622) | |
| Hua 2020 | Household | 835 | Not reported | 151/835 (18.1%) | |
| Huang 2020 | Household
Community |
22 | Not reported | 7/22 (31.8%) | |
| Huang 2020a | Local
Household Community Nosocomial |
3795 | Not reported | 32/3795 (0.84%) | |
| Huang 2021 | Nosocomial | 211 | Not specified | 3.8% (8/211) | |
| Islam 2020 | Household
Local Community Nosocomial |
391 | Not reported | The overall secondary clinical attack rate was
4.08 (95% CI 1.95-6.20) |
|
| Jashaninejad 2021 | Household | 989 | Not specified | 31.7% (95% CI: 28.8-34.7) | |
| Jeewandara 2021 | Household
Community |
1093 | Not specified | 7.8% (85/1093) - PCR
1.7% (7/439) - antibodies |
|
| Jia 2020 | Household | Unclear | Not reported | Attack rate 44/583 (7.6%) | |
| Jiang 2020 | Household
Community |
300 | Not reported | 6/300 (2%) | |
| Jing 2020 | Household | Unclear | Not reported | Household contacts 13·2%
Non-household contacts 2·4% |
The risk of household infection was significantly
higher in the older age group (≥60 years) |
| Jing 2020a | Household
Community |
Unclear | Not reported | Close contacts 17.1% to 19%
Family members 46.1% to 49.6% |
|
| Jones 2021 | Local | 128 | Not reported | 6/128 (4.7%) | |
| Jordan 2022 | Local | 253 | Not specified | 4.7% (12/253) | |
| Kang 2020 | Local | 5517 | Not reported | 96/5517 (1.7%) | |
| Kant 2020 | Local
Community Nosocomial |
Not reported | Not reported | Not reported | No details on number of contacts for index case |
|
Karumanagoundar
2021 |
Household
Community |
15702 | Not specified | 4% (599/14 002) | |
| Katlama 2022 | Household | 255 | N/A | 37.3% (95%CI 31.3–43.5%; 95/255) | |
| Kawasuji 2020 | Nosocomial | 105 | Not reported | 14/105 (1.33%) | |
| Khanh 2020 | Community | 217 | Not reported | 16/217 (7.4%) | |
| Kim 2020 | Household | 207 | 17.7 to 30 | 1/207 (0.5%) | |
| Kim 2020a | Household
Community |
4 | 18.7 to 32.1 | N/A | |
| Kim 2020b | Nosocomial | 3,091 respiratory
samples from 2,924 individuals |
Not reported | 3/290 (1%) | |
| Kim 2021 | Local | 8 | N/A | 0% RT-PCR
0% Serology |
Tests for anti-SARS-CoV-2 antibodies were
performed on quarantined HCWs on the 52nd day from exposure. All serologic test results, including FIA IgM and IgG and ELISA total antibody, were negative. |
| Kitahara 2022 | Household
Community |
114 | Not specified | 15% (17/114) | |
| Klompas 2021 | Nosocomial | 1457 | Not specified | 2.6% (38/1457) | |
| Kolodziej 2022 | Household | 241 | Not specified | 64.3% (155/241) | SAR for household was 75/85 (88.6%) |
| Koureas 2021 | Household | 286 | Not specified | 38.6% (95% CI: 32.50–45.01%) | |
| Kumar 2021 | Community | 822 | Not reported | 144/822 17.5%) | Spread of infection within the state was significantly
higher from symptomatic cases, p=0.02 |
| Kuwelker 2021 | Household | 179 | N/A | 45% | The elderly (>60 years old) had a significantly
higher attack rate (72%) than adults< 60years old (46%, p=0·045) |
| Kuwelker 2021 | Household | 291 | N/A | AR 45% (95% CI 38–53) | |
| Kwok 2020 | Local
Household |
206 | Not reported | 24/206 (11.7%) | |
| Ladhani 2020 | Nosocomial | 254 | Not reported | Unclear: 53/254 (21%) tested positive. | Staff working across different care homes (14/27,
52%) had a 3.0-fold (95% CI, 1.9–4.8; P<0.001) higher risk of SARS-CoV-2 positivity than staff working in single care homes (39/227, 17%). |
| Ladhani 2020a | Nosocomial | Residents: 264
Staff members: 254 |
Not specified | Unclear: 105/264 (53%) residents tested
positive |
Infectious virus recovery in asymptomatic staff and
residents emphasises their likely importance as silent reservoirs and transmitters of infection and explains the failure of infection control measures which have been largely based on identification of symptomatic individuals. |
| Laws 2020 | Household | 188 | Not reported | 55/188 (29.3%) | |
| Laws 2021 | Household | 188 | Not specified | 29.3% (55/188); | |
| Laxminarayan 2020 | Local
Household Community |
575,071 | Not reported | 10.7% (10.5 to 10.9%) for high-risk contacts
4.7% (4.6 to 4.8%) for low-risk contacts 79.3% (52.9 to 97.0%) for high-risk travel exposure |
|
| Lee 2020 | Household | 12 | Not reported | 0/12 (0%) | |
| Lee 2020a | Household | 23 | Not reported | 1/23 (4.4%) | |
| Lewis 2020 | Household | 188 | Not reported | RT-PCR: 55/188 (29%)
Serology: 8/52 (15%) |
|
| Li 2020 | Household | 5 | 19.66 to 26.16 | 4/5 (80%) | |
| Li 2020a | Household
Nosocomial |
7 | Not reported | 7/7 (100%) | During January 14–22, the authors report that
index patient had close contact with 7 persons |
| Li 2020b | Household | 14 | Not reported | 14/14 (100%) | |
| Li 2020c | Household | Unclear | Not reported | Unclear | In COFs, the transmission rates of respiratory
droplets in secondary and non-infected patients were 11.9 % and 66.7 %, respectively, while the transmission rates of respiratory droplets with close contacts were 88.1 % and 33.3 %, respectively. In SOFs, the proportion of respiratory droplet and respiratory droplet transmission with close contacts was 40 % and 60 %, respectively |
| Li 2020d | Household | 392 | Not reported | 64/392 (16.3%) | |
| Li 2021a | Household | 52822 | Not specified | 16·0% (15·7–16·3; 8447/52822) | |
| Li 2021b | Household
Community |
2382 | Not specified | 6.50% | |
| Lin 2021 | Household | 5 | Not specified | PCR 80%
Serology 80% |
|
| Liu 2020 | Household | 7 | Not reported | 4/7 (57.1%) | |
| Liu 2020a | Nosocomial | 30 | Not reported | N/A | |
| Liu 2020b | Household
Community Nosocomial |
11580 | Not reported | 515/11580 (4.4%) | |
| Liu 2020c | Unclear | 1150 | Not reported | 47/1150 (4.1%) | The 16 confirmed cases who had previously been
asymptomatic accounted for 236 close contacts, with a second attack rate of 9.7%, while the remaining 131 asymptomatic carriers accounted for 914 close contacts, with a second attack rate of 2.6% (p<0.001) |
| Liu 2021 | Household | 50 | Not specified | 34% (95% CI: 22%–48%; 17/50) | |
| López 2020 | Local
Household |
285 | Not reported | Facility SAR: 22/101 (21.8%)
Overall SAR: 38/184 (20.7%) |
Variation in hygiene procedures across 3 facilities.
Facility A required daily temperature and symptom screening for the 12 staff members and children and more frequent cleaning and disinfection; staff members were required to wear masks. Facility B: temperatures of the five staff members and children were checked daily, and more frequent cleaning was conducted; only staff members were required to wear masks. Facility C: 84 staff members and children check their temperature and monitor their symptoms daily; masks were not required for staff members or children. |
| López 2021 | Household | 229 | Not specified | 53.7% (123/229) | |
| Lopez Bernal 2020 | Household
Community |
472 | Not reported | 37% (95% CI 31-43%) | |
| Lopez Bernal 2022 | Household | 472 | Not specified | 37% (95% confidence interval (CI): 31–43) | |
| Lucey 2020 | Nosocomial | Not specified | N/A | Not reported | |
| Luo 2020 | Community | 243 | Not reported | 12/243 (4.9%) | No viral genetic sequence data were available
from these cases to prove linkage; and some of the secondary and tertiary cases could have been exposed to unknown infections, especially asymptomatic ones, before or after the bus trips. |
| Luo 2020a | Household
Community Nosocomial |
3410 | Not reported | 127/3410 (3.7%) | |
| Lyngse 2020 | Household | 2226 | Not reported | 371/2226 (16.7%) | |
| Ma 2020 | Unclear | 1665 | Not reported | 10/1/1665 (0.6%) | Only close contacts who fell ill were tested (n=10) |
| Macartney 2020 | Local | 633 | Not reported | 18/633 (1.2%)
Serologic attack rates: 8/171 (4.8%) |
|
| Malheiro 2020 | Household | 1627 | Not reported | Overall AR 154/1627 (9.5%) | |
| Maltezou 2020 | Household | Unclear | <25 (28.1%)
25-30 (26.8%) >30 (45.1%) |
Median attack rate 40% (range: 11.1%–100%)
per family. |
|
| Maltezou 2020a | Household | Unclear | Not reported | Median attack rate: 60% (range: 33.4%-100%) | Adults were more likely to develop a severe clinical
course compared to children (8.8% versus 0%, p- value=0.021) |
| Mao 2020 | Household
Local |
Unclear | Not reported | 6.10% | Average attack rate was 8.54% (1.02–100%) |
| Martínez-Baz 2022 | Household
Community |
59900 | Not specified | 34.9% (20905/59900) | |
|
Martinez-Fierro
2020 |
Unclear | 81 | Not reported | 34/81 (42%)
Serologic attack rates: 13/87 (14.9%) |
16% of contact showed positive serology after >2
weeks |
| McLean 2022 | Household | 404 | Not specified | 49% (198/404) | |
|
Mercado-Reyes
2022 |
Household | 17863 | N/A | AR Serology 32.3% (5811/17,863) | |
| Metlay 2021 | Household | 17917 | Not specified | 10.1% (1809/17 917) | |
| Meylan 2021 | Nosocomial | 1874 | N/A | AR Serology 10.0% (95%CI 8.7% to 11.5%;
188/1874) |
|
| Miller 2021 | Household | 431 | <20 to 40 | PCR 21.1% (91/431)
Serology 46.9% (180/431) |
|
| Montecucco 2021 | Local | 346 | Not specified | 9.8% (34/346) | |
| Mponponsuo 2020 | Nosocomial | 38 | N/A | 0/38 (0%) | |
| Musa 2021 | Household | 793 | Not specified | 17% (95%CI 14–21) | |
| Ng 2020 | Household
Local Community |
13026 | Not reported | 188/7770 (2.4%)
Household: 5·9% Work contacts: 1.3% Social contacts: 1.3% Serology: 44/1150 (3.8%) |
Serology results were positive for 29 (5·5%) of
524 household contacts, six (2·9%) of 207 work contacts, and nine (2·1%) of 419 social contacts. |
| Ng 2021 | Household | 848 | Not specified | 55% (466/848) | |
| Ning 2020 | Household
Local Community |
Unclear | Not reported | Imported cases: 69/3435 (0.8%)
Local cases: 31/3666 (2.0%) |
|
| Njuguna 2020 | Local | 98 | Not reported | Attack rate 57% to 82% | |
| Nsekuye 2021 | Local
Household Community |
1035 | Not specified | 3.5% (36/1035) | |
| Ogata 2021 | Household | 496 | Not specified | 25.2% (21.6–29.2; 125/496) | |
| Ogawa 2020 | Nosocomial | 30 PCR/serology | 33.53 to
36.83 |
0/15 (0%) for both PCR and serology | |
| Paireau 2022 | Household
Local Nosocomial |
6028 | Not reported | 248/6028 (4.1%) | Family contacts, index case was 60-74, or older
than 75 years old were significantly associated with increased odds of transmission. The proportion of nosocomial transmission was significantly higher than in contact tracing (14% vs 3%, p<0.001) |
| Pang 2022 | Local | 164 | Not specified | 9.8% (16/164) | |
| Park 2020 | Local
Household Community |
328 | 17.7 to 35 | 22/328 (6.7%) | |
| Park 2020a | Household
Non- household |
59,073 | Not reported | Household contacts: 11.8% (95% CI 11.2%–
12.4%) Non-household contacts: 1.9% (95% CI 1.8%–2.0%) |
|
| Park 2020b | Local
Household |
441 | Not reported | Attack rate 43.5% (95% CI 36.9%–50.4%)
Secondary attack rate 16.2% (95% CI 11.6%– 22.0%) |
|
| Passarelli 2020 | Nosocomial | 6 | Not reported | 2/6 (33.3%) | |
| Patel 2020 | Household | 185 | Not reported | 79/185 (43%) | Contacts not reported as tested |
| Pavli 2020 | Aircraft | 891 | Not reported | 5/891 (0.6%) | |
| Petersen 2021 | Household | 584 | N/A | 19.2% (11/584) | |
| Pett 2021 | Household
Community |
392 | Not specified | 3.3% (13/392) | |
| Phiriyasart 2020 | Household | 471 | Not reported | 27/471 (5.7%) | |
| Poletti 2020 | Unclear | 2484 | Not reported | 2824/5484 (51.5%) | |
| Powell 2022 | Local | 183 | Not specified | 8.2% (15/183) | |
| Pung 2020 | Local
Community |
425 | Not reported | 36/425 (8.5%) | |
| Pung 2020a | Household | Unclear | Not reported | 43/875 (4.9%) | |
| Qian 2020 | Local
Household Community |
Not reported | Not reported | Not reported | Home‐based outbreaks were the dominant
category (254 of 318 outbreaks; 79.9%), followed by transport‐based outbreaks (108; 34.0%) |
| Ratovoson 2022 | Household | 179 | Not specified | 31.3% (56/179) | |
| Ravindran 2020 | Local | Not reported | Not reported | Attack rate 61% to 77% | All attendees participated in activities resulting
in potential exposure, such as shaking hands, kissing, dancing, sharing drinks and sharing shisha (smoking water pipes). |
| Razvi 2020 | Nosocomial | 2521 | Not reported | Serologic attack rate 19.4% | |
| Reukers 2021 | Household | 187 | Not specified | 43% (95% CI, 33%–53%) | |
|
Robles Pellitero
2021 |
Household | Not specified | Not specified | 29.8% (SAR/family) | |
| Rosenberg 2020 | Household | 498 | Not reported | 286/498 (57%) | |
| Roxby 2020 | Nosocomial | 142 | Not reported | Attack rate in 1st round: 5/142 (3.5%) | One additional positive test result was reported for
an asymptomatic resident who had negative test results on the first round. |
| Sakamoto 2022 | Nosocomial | 517 | Not specified | 8.1% (42/517) | |
| Sang 2020 | Household | 6 | Not reported | 4/6 (66.7%) | |
| Sarti 2021 | Local | 5 | Not specified | 80% (4/5) | |
| Satter 2022 | Local
Household |
684 | Not specified | RT-PCR 13% (87/684) | |
| Schoeps 2021 | Local | 14591 (13,005
PCR-tested) |
Not specified | 1.51 (95% CI 1.30–1.73) | |
| Schumacher 2021 | Local | Quarantine
phase: 757 tests Match phase: 1167 tests |
Unclear | Quarantine phase AR: 3.6%
Match phase AR: 4.2% Serology: 1.1% |
|
| Schwierzeck 2020 | Nosocomial | 48 | 16.03 to 32.98 | 9/48 (18.8%) | Ct values of symptomatic cases were significantly
lower compared to asymptomatic cases 22.55 vs 29.94, p<0.007 (approximately 200-fold higher viral load) |
| Semakula 2021 | Household
Community |
11809 | Not specified | Overall 1.77% (95% CI 1.55% to 2.02%;
209/11809) SAR of households 2.93% (95% CI 1.85% to 4.60%) |
|
| Shah 2020 | Household | 386 | Not reported | 34/386 (8.8%) | |
| Shah 2021 | Household | 287 | Not specified | 1.7% (95%CI 0.7–4%) | |
| Shen 2020 | Household
Community |
480 | Not reported | Close contact: 2/7 (29%)
Casual contact: 3/473 (0.6%) |
|
| Sikkema 2020 | Nosocomial | 1796 | Not
specified. WGS for Ct <32 |
Attack rate 96/1796 (5%) | 46 (92%) of 50 sequences from health-care workers
in the study were grouped in three clusters. Ten (100%) of 10 sequences from patients in the study grouped into the same three clusters: |
| Son 2020 | Household | 3223 | Not reported | 8.2% (95% CI, 4.7 to 12.9) | |
| Song 2020 | Household | 20 | Not reported | 16/20 (80%) | |
| Sordo 2022 | Household | 659 | Not specified | 22.5% (148/659). | |
|
Soriano-Arandes
2021 |
Household | 283 | Not specified | 59% (167/283) | |
| Speake 2020 | Aircraft | 111 | Not reported | 11/111 (9.9%) | |
| Stein-Zamir 2020 | Local | 1312 | Not reported | Attack rate 178/1312 (13.6%) | |
| Stich 2021 | Household | 1,220 | Not specified | RT-PCR 32.8% (400/1220)
Serology 36.1% (393/1,090) |
|
| Sugano 2020 | Local | 72 | Not reported | 23/72 (31.9%) | |
| Sun 2020 | Household | Unclear | Not reported | 34.43% | |
| Sun 2021 | Household | 50 | Not specified | 14.0% (7/50) | |
| Sundar 2021 | Household
Community |
496 | Not specified | 16.7% (83/496) | |
| Tadesse 2021 | Household | 40 households | N/A | AR 3.5% (95% CI: 3.2%-3.8%) | |
| Tanaka 2021 | Household | 687 | Not specified | 28.2% (194/687) | |
| Tanaka 2022 | Household | 101 households
with 477 individuals |
Not specified | 77.0% (95% CI: 69.4-84.6%) | |
| Taylor 2020 | Nosocomial | 600 | Not reported | Resident attack rate: 137/259 (52.9%) 1st round
HCW Attack rate: 114/341 (33.4%) |
|
| Teherani 2020 | Household | 144 | Not reported | 67/144 (46.5%) | Of the total number of household contacts, at least
29 (20%) had known SARS-CoV2 testing. Child-to-adult transmission was suspected in 7/67 cases (10.5%). |
| Thangaraj 2020 | Community | 26 | Not reported | 17/26 (65.4%) | |
| Torres 2020 | Community | 1244 | N/A | Overall serologic attack rate: 139/1244
(11.2%) |
|
| Tsang 2022 | Household
Community |
3158 | Not specified | 3.5% (95%CI 2.9–4.3) | |
| Tshokey 2020 | Local
Community |
1618 | Not reported | 14/1618 (0.9%) | SAR: High-risk contacts was 9.0% (7/75), and that
among the primary contacts was 0.6% (7/1,095), and none (0/448) among the secondary contacts. |
| Tsushita 2022 | Local | 23 | Not specified | 69.6% (16/23) | |
| van der Hoek 2020 | Household | 174 | 25.1 to 35.1 | 47/174 (27%)
Serology on day 3 - family members: 43/148 (29.1%) |
|
| Vičar 2021 | Household | 226 | Not specified | 22.6% (51/226) | |
| Wang 2020 | Nosocomial
Household |
43 | Not reported | 10/43 (23.3%) | |
| Wang 2020a | Household | 155 | Not reported | 47/155 (30%) | |
| Wang 2020b | Household | 335 | Not reported | 77/335 (23%) | |
| Wee 2020 | Nosocomial | 298 | Not reported | 1/298 (0.3%) | |
| Wendt 2020 | Nosocomial | 254 | Not reported | 0/254 (0%)
Serologic attack rates 0/23 (0%) |
|
| White 2022a | Local | 485 | Not specified | 4.1% (20/485) | |
| White 2022b | Local | 859 | Not specified | 7% (60/859) | |
| Wiens 2021 | Household | 435 households | N/A | AR 38.5% (32.1 - 46.8) | |
| Wolf 2020 | Household | 4 | Not reported | 3/4 (75%) | 7-month-old female who was breastfed, was
asymptomatic throughout the observation period and never developed fevers or any other symptoms, despite continuous exposure to her parents and siblings. She remained SARS-CoV-2 PCR-negative in repeat testing of pharyngeal swab and stool specimens over the entire observation period. |
| Wong 2020 | Nosocomial | 76 tests were
performed on 52 contacts |
Not reported | 0/52 (0%) | Findings suggest that SARS-CoV-2 is not spread
by an airborne route. Ct value for throat and tracheal aspirate of index case were 22.8 and 26.1 respectively. |
| Wood 2021 | Household | Not reported | Not reported | Not reported | |
| Wu 2020 | Household
Local Community |
2994 | Not reported | 71/2994 (2.4%) | |
| Wu 2020a | Household | 148 | Not reported | 48/148 (32.4%) | |
| Wu 2021 | Local
Household Community |
4214 | Not specified | 3.3% (140/4214) | |
| Xie 2020 | Household | 56 | Not reported | 0/56 (0%) | |
| Xie 2021 | Household | 79 | Not specified | 67.1% (53/79) | |
| Xin 2020 | Household | 187 | Not reported | 19/187 (17.9%) | |
| Yang 2020 | Household
Local |
1296 | Not reported | 0/1296 (0%)
Serologic attack rates: 0/20 (0%) |
Viral cultures of 4 specimens with Ct <30 were
negative. |
| Yau 2020 | Nosocomial | 330 | Not reported | 22/330 (6.7%) | |
| Ye 2020 | Local
Community |
1293 | Not reported | 39/1,293 (3.02%) | |
| Yi 2021 | Household | 475 | <37 | 42.9% (204/475) | |
| Yoon 2020 | Local | 190 | N/A | 0/190 (0%) | |
| Yousaf 2020 | Household | 198 | Not reported | 47/198 (23.7%) | |
| Yu 2020 | Household | 1587 | Not reported | 150/1587 (9.5%) | |
| Yung 2020 | Household | 213 | Not reported | Attack rate 6.1% | |
| Zhang 2020 | Aircraft | 4492 | Not reported | Attack rate 161/4492 (3.6%) | The authors report attack rate of 0.14% based on
94 flights (n=14 505); however, only 4492 people were screened. |
| Zhang 2020a | Household
Local Community |
369 | Not reported | 12/369 (3.3%, 95% CI 1.9%–5.6%) | |
| Zhang 2020b | Household | 10 | Not reported | 0/10 (0%)
Serologic attack rates: 0/10 (0%) |
|
| Zhang 2020c | Local
Household |
93 | Not reported | 5/93 (5.4%) | |
| Zhang 2020d | Local | 8437 | Not reported | 25/8437 (0.3%) | |
| Zhang 2021 | Local
Household |
178 | ≤38 | 7.3% (13/178) | |
| Zhuang 2020 | Household
Community |
8363 | Not reported | 239/8363 (2.9%) |
Thirty-seven studies reported data on ARs using serology ( Table 6). The settings included educational (n=4), households (n=11) and healthcare (n=4). In eight studies, the frequency of attack was 0%. The frequency of attacks in the remaining 29 studies ranged from 0.7% to 75% ( Figure 3b). The frequency of attacks was highest in households but lower in educational settings - especially daycare centres.
Figure 3b. Primary attack rates of SARS-CoV-2 in close contacts (serology).
Frequency of SARS-CoV-2 secondary ARs
Overall, 204 studies (79.1%) reported data on secondary ARs ( Table 6). The studies reported the rates based on RT-PCR tests, except for one study (Angulo-Bazán 2020) that used serology, and another (Calvani 2021) that used rapid antigen test. In 16 of these studies, the SAR was 0%. The secondary ARs in the remaining 188 studies ranged from 0.3 to 100% (see Figure 4). The highest frequencies of secondary ARs (75–100%) occurred in household or quarantine settings; similar findings were observed when studies with higher reporting quality were examined. In the three studies of index or primary cases with recurrent infections, there was no positive case amongst the 1518 close contacts across the studies. Across geographical regions, the median secondary ARs were 5.4% in Asia (IQR 16.585, n=77), 16.7% in Europe (IQR 37.4), n=24), 6.8% in N. America (IQR 34.8, n=17), and 47.5% (IQR 31.9, n=4) in S. America. We were unable to compute the data for Africa and Australasia because of insufficient information.
Figure 4. Frequency of secondary attack rates of SARS-CoV-2 with Close Contacts.
Risk of infection
One hundred and twelve studies (43.4%) reported results on the risk of infection ( Table 7). One study of airline passengers (Khanh 2020) showed that seating proximity was significantly associated with the risk of contracting SARS-CoV-2 (RR 7.3, 95% CI 1.2–46.2); a second study (Speake 2020) reported that not sitting by the window was associated with a significantly increased risk of infection (RR 5.2; 95% CI 1.6–16.4; p<0.007), and a third (White 2022b) reported that flights with longer duration significantly increased the risk of infection (P=0.0008). The results of nine studies (Chen 2020b, Doung-ngern 2020, Gonçalves 2021, Hast 2022, Hobbs 2020, Montecucco 2021, Robles Pellitero 2021, Wang 2020b, Wu 2020) showed that use of face covering during close contact with infected cases was associated with significantly lower risks of infection compared with no face covering; findings from one of these studies (Doung-ngern 2020) showed that wearing masks all the time during contact was not significantly different from wearing masks sometimes. Findings from six studies (Bjorkman 2021, Katlama 2022, Koureas 2021, López 2021, Lopez Bernal 2022, Mercado-Reyes 2022) showed that higher number of household occupants was significantly associated with increased risk of infection. The results of three studies (Poletti 2020, Rosenberg 2020, Zhang 2020a) showed that the risk of infection was significantly increased in older population groups. One study (Zhang 2020a) reported that elderly close contacts (≥60 years) had a higher SAR compared with younger age groups. Findings from nine studies (Bi 2020, Hu 2020a, Islam 2020, Luo 2020a, Petersen 2021, Pett 2021, Sundar 2021, Tsang 2022, Wu 2020, Zhang 2020a) showed that household contact settings had significantly higher risks of infection compared with other types of contact settings, e.g., social, healthcare, workplace, and public transport. Seven studies (Akaishi 2021, Bi 2021, Galow 2021, Laws 2021, Liu 2021, Reukers 2021, Stich 2021) showed that the risk of infection was significantly lower in children compared to adults. One study (Lewis 2020) showed that the risk of infection was significantly increased amongst household contacts who were immunocompromised (OR 15.9, 95% CI 2.4–106.9). Finally, three studies (Bi 2020a, Wu 2020, Zhang 2020a) showed that more frequent contacts with the index case significantly increased the risk of infection.
Table 7. Risk of Infection with SARS-CoV-2 in Close Contact Settings.
| Study ID | Type of
transmission |
Risk of infection |
|---|---|---|
| Abdulrahman 2020 | Community | Eid Alfitr: Pre-: 2990 (4.2%); Post-: 4987 (6.7%); p <0.001; Ashura: Pre-: 3571 (3.7%); Post-: 7803 (6.6%); p <0.001 |
| Afonso 2021 | Household | SAR in families that had more than one infected adult, in addition to the index case, it was 1.50 times higher than those without
this feature (RR: 1.50; 95.0% CI: 1.55–4.06). SAR in symptomatic contacts was 4.87 times higher when compared to that of the nonsymptomatic group (RR: 4.87; 95.0% CI: 2.49–9.53). |
| Akaishi 2021 | Household
Community |
The rate of RT-PCR test positivity was significantly higher in those with a close contact than in those with a lower risk contact
(p<0.0001). Household secondary transmission rate was significantly similar lower in children aged <10 years compared to other groups (7.3% vs. 13.5%, p=0.02). |
| Arnedo-Pena 2020 | Household | The health profession of index case was a significant protective factor (p<0.007). Older age of secondary cases, two household
members, and higher age of index case were significantly associated with elevated risk of infection: p<0.001 in each case |
| Atherstone 2021 | Community | The odds of receiving a positive test result were highest among household contacts (odds ratio = 2.7; 95% confidence interval =
1.2–6.0) |
| Bender 2021 | Household | There was no significant difference in the SARs between household contacts of presymptomatic versus asymptomatic cases (P=0.23).
Presymptomatic transmission was more frequent than symptomatic transmission. |
| Bernardes-Souza 2021 | Household | Being a logistics worker (OR 18.0, 95%CI 8.4-38.7), living with a logistics worker (OR 6.9, 95%CI 3.3-14.5), close contact with a
confirmed COVID-19 case (OR 13.4, 6.6-27.3), living with four or more people (OR 2.7, 95% CI 1.4-5.4), and being a current smoker (OR 0.2, 0.1-0.7) were significantly associated with an increased risk of SARS-CoV-2 infection. |
| Bhatt 2022 | Household | Adults were more likely than children to transmit SARS-CoV-2 (OR 2.2, 95% CI 1.3–3.6). |
| Bi 2020 | Local
Household Community |
Household contact (OR 6·3; 95% CI 1·5–26·3) and travelling together (OR 7·1; 1·4–34·9) were significantly associated with infection.
Reporting contact that occurred often was also associated with increased risk of infection compared with moderate-frequency contact (OR 8·8; 95% CI 2·6–30·1) |
| Bi 2021 | Household | The risk of being infected by a household member was the lowest among 5–9 years old and highest among those 65 years and
older, with teenagers and working age adults sharing similar risks. Compared with 5–9-year-olds, 65 years and older had nearly three times the odds (OR=2.7, 95%CrI 0.9–7.9) |
| Bistaraki 2021 | Household
Community |
The odds of infection [95% CI] were higher in contacts exposed within the household (1.71 [1.59–1.85] vs. other) and in cases with
cough (1.17 [1.11–1.25] vs. no cough). |
| Bjorkman 2021 | Local | Students in multiple occupancy rooms were significantly twice as likely to be infected compared to students in single rooms (19.1%
vs 10.3%). Higher viral load significantly increased the risk of infection (P<0.0001) |
| Calvani 2021 | Local
Household |
The probability of being positive to SARS-CoV-2 was significantly lower in children who had school contacts or who had flu symptoms
compared to children who had household contacts (56.8% vs 2.5%, P<0.0001) |
| Carvalho 2022 | Household | The odds of SARS-CoV-2 transmission when the index case was an adult were 13.98 (4.09 to 47.77) and 11.25 (1.91 to 66.4) times
higher when compared to HCW and children as index cases, respectively |
| Cerami 2021 | Household | Households with non-white index cases were significantly more likely to experience incident transmission in the household, 51% vs
19% (p=0.008) |
| Chen 2020b | Nosocomial | In multivariate analysis, there existed higher risk of seroconversion for close contacts with patient 2 (OR, 6.605, 95% CI, 1.123,
38.830) and doctors exposed to their patient (OR, 346.837, 95% CI 8.924, 13479.434), while the lower risk of seroconversion was closely related to direct contact with COVID-19 patients wearing face mask (OR, 0.127, 95% CI 0.017, 0.968). |
| Chen 2020c | Local
Community Household Nosocomial |
Infection rate is highest when living with the case (13.26%), followed by taking the same means of transportation (11.91%). After
removing the influence factors of the "super spreader" incident, the infection rate of vehicle contact dropped to 1.80%. The infection rate (7.18%) of entertainment activities such as gatherings, meeting guests, and playing cards was also relatively high, as was short- term face-to-face unprotected conversations or doing errands (6.02%). There was a statistically significant difference in the infection rate among the four categories of life contact, transportation contact, medical contact, and other contact (p<0.005). Participation in Buddhist gatherings caused transmission. A total of 28 people were diagnosed as confirmed cases of new coronavirus pneumonia, 4 were asymptomatic infections, and the infection rate of close contacts reached 32.99% (32/97), which was much higher than the average infection rate (6.15). %), the difference is statistically significant (p<0.005). |
| Cheng 2020 | Household
Nosocomial |
The overall secondary clinical attack rate was 0.7% (95% CI, 0.4%-1.0%). The attack rate was higher among the 1818 contacts whose
exposure to index cases started within 5 days of symptom onset (1.0% [95% CI, 0.6%-1.6%]) compared with those who were exposed later (0 cases from 852 contacts; 95% CI, 0%-0.4%). The 299 contacts with exclusive presymptomatic exposures were also at risk (attack rate, 0.7% [95% CI, 0.2%-2.4%]). The attack rate was higher among household (4.6% [95% CI, 2.3%-9.3%]) and nonhousehold (5.3% [95% CI, 2.1%-12.8%]) family contacts than that in health care or other settings. The attack rates were higher among those aged 40 to 59 years (1.1% [95% CI, 0.6%-2.1%]) and those aged 60 years and older (0.9% [95% CI, 0.3%-2.6%]). |
| Chu 2021 | Household | Five (10%) of 48 secondary cases compared with 130 (33%) of 398 non-case household contacts reported potential community
exposures: unadjusted OR 0.24 (95%CI 0.09 to 0.62), p=0.003 |
| Craxford 2021 | Household | Cohabitees of seropositive HCW had a seropositive rate of 16%, compared to 2.5% of cohabitees without a seropositive HCW
(P=0.003) |
| Dattner 2020 | Household | PCR: 44% of adults were infected compared to 25% of the children (n=3353: 1809 children and 1544 adults)
Serology: 34% of these children and 48% of the adults tested serologically positive (n=705: 417 children and 288 adults |
| Dimcheff 2020 | Community
Nosocomial Household |
HCWs exposed to a known COVID-19 case outside work had a significantly higher seroprevalence at 14.8% (23 of 155) compared to
those who did not 3.7% (48 of 1,296; OR, 4.53; 95% CI, 2.67–7.68; P < 0.0001) |
| Doung-ngern 2020 | Local | Wearing masks all the time during contact was independently associated with lower risk of COVID-19 infection compared to
not wearing masks (aOR 0.23, 95% CI 0.09–45 0.60), while wearing masks sometimes during contact was not (aOR 0.87, 95% CI 0.41–1.84). Maintaining at least 1m distance from a COVID patient (aOR 0.15, 95% CI 0.04–0.63) and duration of close contact ≤15 minutes versus longer (aOR 0.24, 95% CI 0.07–0.90) were significantly associated with lower risk of infection transmission |
| Farronato 2021 | Household | Subjects tested more than 73 days after the adult negativization showed a lower probability of receiving a positive result (p = 0.059) |
|
Fateh-Moghadam
2020 |
Community | Workplace exposure was associated with higher risk of becoming a case than cohabiting with a case or having a non-cohabiting
family member or friend who was a case. The greatest risk of transmission to contacts was found for the 14 cases <15 years of age (22.4%); 8 of the 14, who ranged in age from <1 to 11 years) infected 11 of 49 contacts. |
| Fontanet 2021 | Local | Infection rates were significantly lower amongst school pupils, teachers, non-teaching staff compared to pupils' parents and relatives
(P<0.001) |
| Galow 2021 | Household | The SAR of the 17 index-cases <18 years was significantly lower compared to the 126 adult index-cases: 15% vs 38% p=0.004). |
| Gaskell 2021 | Household | The seroprevalence varied by age between 27.6% (95%CI 20.8 - 35.6%) for children aged under 5 years of age to 74% (95%CI 70.0
-77.6%) in adults |
| Ge 2021 | Household
Community |
Attack rates were highest among household members of index patients (260 of 2565 [10.1%; 95% CI, 9.0%-11.4%]) and contacts
exposed in multiple settings to the same index patient (3 of 44 [6.8%; 95% CI, 1.4%-18.7%]) |
| Gonçalves 2021 | Household | Mask use reduced odds of infection by 87% (OR 0.13, 95%CI 0.04–0.36). Persons who reported they were practically isolated from
everyone were 59% (OR 0.41, 95% CI 0.24–0.70) less likely to become infected. |
| Hast 2022 | Local | Participation in school sports was significantly associated with increased risks of infection: P=0.0004 and P=0.007 for elementary and
middle/high school students respectively. Use of masks during sports activities was associated with significant reduction in risk of infection: P=0.005 and P=0.001 for elementary and middle/high school students respectively. |
| Helsingen 2020 | Local | 11 individuals in the training arm (0.8% of those tested) and 27 in the non-training arm (2.4% of those tested) tested positive for
SARS-CoV-2 antibodies (p=0.001) |
| Hobbs 2020 | Local
Household Community |
Case-patients were significantly more likely to have had close contact with a person with known COVID-19 than control participants
(aOR = 3.2, 95% CI = 2.0–5.0) Case-patients were significantly more likely to have attended gatherings with persons outside their household, including social functions (aOR = 2.4, 95% CI = 1.1–5.5), activities with children (aOR = 3.3, 95% CI = 1.3–8.4), or to have had visitors at home (aOR = 1.9, 95% CI = 1.2–2.9) during the 14 days before the SARS-CoV-2 test. Parents of 64% of case-patients and 76% of control participants reported that their child and all staff members wore masks inside the facility (aOR = 0.4, 95% CI = 0.2–0.8). |
| Hu 2020 | Household
Community |
Household contacts were associated with a significantly larger risk of SARS-CoV-2 infection than other types of contact (P<0.001).
The transmission risk in the first generation was significantly higher than the later generations (p<0.001), possibly due to improved case isolation and contacts quarantine that deplete the number of susceptible individuals in the cluster. |
| Hu 2020 | Local | Travelers adjacent to the index patient had the highest attack rate (3.5% [95% CI, 2.9%-4.3%]) of COVID-19 infection (RR, 18.0 [95%
CI, 13.9-23.4]) among all seats. |
| Hu 2021 | Local | There was no significant difference between the estimated upper and lower bounds of ARs (p=0.06) |
| Hua 2020 | Household | Incidence of infection in child close contacts was significantly lower than that in adult contacts: 13.2% vs 21.2%, p=0.004 |
| Islam 2020 | Household
Local Community Nosocomial |
The secondary attack rate among household contacts was at the highest risk of attack (13.04%, 95% CI 9.67-16.41) followed by
funeral ceremonies (8.33%, 95% CI 3.99-12.66) and family contacts (6.52%, 95% CI 4.02-9.02). The attack rate was higher in age groups 50-59 (10.89%, 95% CI 7.05-14.66) and 60-69 (9.09%, 95% CI 5.08-13.09) |
| Jashaninejad 2021 | Household | Contacts who had more than one-hour daily contact with the index case, before the diagnosis of the disease in index cases had a
higher risk of infection (adjusted OR=2.44, 95% CI: 1.52, 3.93), compared to contacts who had one-hour and less close contact |
| Jordan 2022 | Local | Frequent hand washing was the only variable that was associated with a lower SAR, P=0.02. |
|
Karumanagoundar
2021 |
Household
Community |
Of the 599 contacts who tested positive, more than three-fourths (78%) were household contacts.
Being a household contact of a primary case with congregation exposure had a fourfold increased risk of getting COVID-19 (RR: 16.4; 95% CI: 13 to 20) than contact of primary case without congregation exposure. |
| Katlama 2022 | Household | Independent predictors of virus transmission from index to contacts were housing surface area < 60 m2 (OR: 5.6 [1.1; 28.2] and a
four-member family compared to five (OR: 3.6 [1.2; 10.3]). |
| Kawasuji 2020 | Nosocomial | Among symptomatic patients (n =18), the estimated viral load at onset was higher in the index than in the non-index patients
(median [95% confidence interval]: 6.6 [5.2–8.2] vs. 3.1 [1.5–4.8]. In adult (symptomatic and asymptomatic) patients (n = 21), median viral load at the initial sample collection was significantly higher in the index than in the non-index patients (p = 0.02) |
| Khanh 2020 | Community | Seating proximity was strongly associated with increased infection risk (RR 7.3, 95% CI 1.2–46.2). |
| Kitahara 2022 | Household
Community |
Attack rates peaked 1 day before symptom onset: 26% (95%CI 10-48) |
| Klompas 2021 | Nosocomial | Potential contributing factors included high viral loads, nebulization, and positive pressure in the index patient's room. Risk factors
for transmission to staff included presence during nebulization, caring for patients with dyspnea or cough, lack of eye protection, at least 15 minutes of exposure to case patients, and interactions with SARS-CoV-2–positive staff in clinical areas. |
| Koureas 2021 | Household | Household size was significantly associated with the risk of infection (OR: 2.65, 95% CI: 1.00–7.07). |
| Kuwelker 2021 | Household | The risk of household transmission was higher when the index patient had fever (aOR 3.31 [95% CI 1.52–7.24]; p = 0.003) or
dyspnoea (aOR 2.25 [95% CI 1.80–4.62]; p = 0.027) during acute illness. |
| Laws 2020 | Household | There were no significant differences in secondary infection rates between adult and paediatric contacts among all households (OR:
1.11; 95% CI: 0.56 to 2.21) or among households with children (OR: 0.99; 95% CI: 0.51 to 1.90). |
| Laws 2021 | Household | Children of primary patients had increased odds of acquiring infection compared with children in households in which the primary
patient was not their parent (OR: 17.28; 95% CI: 2.36 to 126.8). |
| Laxminarayan 2020 | Local
Household Community |
Secondary attack rate estimates ranged from 1.2% (0.0 to 5.1%) in health care settings to 2.6% (1.6 to 3.9%) in the community and
9.0% (7.5 to 10.5%) in the household. |
| Lewis 2020 | Household | Household contacts to COVID-19 patients with immunocompromised conditions and household contacts who themselves had
diabetes mellitus had increased odds of infection with ORs 15.9 (95% CI, 2.4–106.9) and 7.1 (95% CI: 1.2–42.5). Household contacts of a male primary patient were more likely to have secondary infection than those of a female primary patient (SIR, 36% vs 18%; OR, 2.4; 95% CI, 1.1–5.3). |
| Li 2020d | Household | The secondary attack rate to children (aged <18 years) was 4% compared with 20.5% for adult members (odds ratio [OR], .18;
95% confidence interval [CI], .06–.54; P = 0.002). The secondary attack rate to the contacts in the household with index patients quarantined at home immediately since onset of symptoms was 0% compared with 18.3% for the contacts in the households without index patients quarantined during the period between initiation of symptoms and hospitalization (OR, 0; 95% CI, .00–.00; p=0.000). The secondary transmission rate for individuals who were spouses of index cases was 27.8% compared with 17.3% for other members in the households (OR, 2.27; 95% CI, 1.22–4.22; p=0.010). |
| Li 2021a | Household | Children and adolescents younger than 20 years of age were more likely to infect others than were adults aged 60 years or older
(1·58, 1·28–1·95). Asymptomatic individuals were much less likely to infect others than were symptomatic cases (0·21, 0·14–0·31). Symptomatic cases were more likely to infect others before symptom onset than after (1·42, 1·30–1·55). |
| Li 2021b | Household
Community |
Factors associated with significantly increased SAR were living together (P<0.01), being a spouse (P<0.01), and being >60 years of
age (P=0.01). |
| Liu 2020b | Household
Community Nosocomial |
Compared to young adults aged 20–29 years, the infected risk was higher in children (RR: 2.59, 95%CI: 1.79–3.76), and old people
aged 60–69 years (RR: 5.29, 95%CI: 3.76–7.46). People having close relationship with index cases encountered higher infected risk (RR for spouse: 20.68, 95%CI: 14.28–29.95; RR for non-spouse family members: 9.55, 95%CI: 6.73–13.55; RR for close relatives: 5.90, 95%CI: 4.06–8.59). Moreover, contacts exposed to index case in symptomatic period (RR: 2.15, 95%CI: 1.67–2.79), with critically severe symptoms (RR: 1.61, 95%CI: 1.00–2.57) |
| Liu 2021 | Household | SAR among paediatric household contacts was significantly lower than among adult household contacts (P = 0.04).
Transmission was significantly lower in households with 4+ bedrooms compared with those with 3 or fewer [17% (95% CI: 7–36%) vs. 47% (95% CI: 32–68%), P = 0.03], for contacts where the index case was masked compared with those unmasked [17% (7–37%) vs. 48% (31–66%), P = 0.02] and with increased hand washing or use of hand sanitizer compared with those who did not report increased use [19% (9–36%) vs. 58% (36–77%), P = 0.01]. |
| López 2021 | Household | Higher risk of infection was found in the household members of domicile-isolated patients isolated and in those reporting
overcrowding at home, (odds ratio [OR] 1.67, 95% confidence interval [CI] 0.89–3.12) and (OR 1.44, 95% CI 0.81; 2.56), respectively. |
| Lopez Bernal 2020 | Household
Community |
Secondary attack rates were highest where the primary case was aged <18 years with a significantly higher odds of secondary
infection (OR 61, 95% CI 3.3-1133). Where the primary case was admitted to hospital there was a significantly lower odds of secondary infection in the household (OR 0.5, 95% CI 0.2-0.8). Secondary attack rates were lower in larger households. |
| Lopez Bernal 2022 | Household | There was an inverse relationship between household size and SAR, with the highest SAR in households with two people
(SAR = 0.48; 95% CI: 0.36–0.60) and the lowest in households of five or more (SAR = 0.22; 95% CI: 0.11–0.33). |
| Luo 2020a | Household
Community Nosocomial |
Household contacts had a significantly higher risk for secondary infection than did persons who were exposed in health care
settings (OR, 0.09, 95%CI 0.04 to 0.20) or those who were exposed on public transportation (OR, 0.01, 95%CI, 0.00 to 0.08). |
| Macartney 2020 | Local | The rate of staff member to child transmission was lower (1·5%) than staff to staff transmission (4·4%). |
| Malheiro 2020 | Household | Among the intervention cohort,16 of 132 close contacts tested positive during the follow-up period (attack rate:12.1%, 95%
confidence interval [CI]: 7.1-18.9). In the control cohort,138 of 1495 participants tested positive (attack rate: 9.2%, 95% CI:7.8-10.8) |
| Martínez-Baz 2022 | Household
Community |
The infectivity of the index case was lower in those aged 5–14 years and increased with age up to those aged 70 years or older (aOR
2.81, 95% CI 2.56–3.08). Infectivity was higher from immigrants (aOR 1.44, 95% CI 1.36–1.52) and from symptomatic index cases (aOR 1.50, 95% CI 1.43–1.58). |
| McLean 2022 | Household | Compared to when the primary case was age 18 to 49 years, SIR in household contacts was significantly lower when the primary
case was age 12 to 17 years (RR, 0.42; 95% CI, 0.19–0.91) |
| Mercado-Reyes 2022 | Household | The number of rooms per household and the number of people per household were significantly associated with risks of
seropositivity |
| Metlay 2021 | Household | Independent factors significantly associated with higher transmission risk included age greater than 18 years (eg, adjusted odds
ratio [OR] for those aged 50-64 years, 3.66; 95% CI, 2.92-3.66; P<0.001) and multiple comorbid conditions (eg, adjusted OR for individuals with hypertension, 1.93; 95% CI, 1.58-2.44; P<0.001) |
| Miller 2021 | Household | SARs from index cases with respiratory or systemic symptoms were significantly higher than in those without such symptoms. |
| Montecucco 2021 | Local
Household Community |
Wearing respiratory protections by both the case and the close contact resulted an effective measure compared with no use (IRR =
0.08; 95% CI: 0.03-0.2; P<0.0001). Fatigue (IRR= 17.1; 95% CI: 5.2-55.8; P<0.001), gastrointestinal symptoms (IRR= 6.6; 95% CI: 2.9-15.2; P<0.001) and cough (IRR= 8.2; 95% CI: 3.7-18.2; P<0.001) were found to be significantly associated with transmission of infection. |
| Musa 2021 | Household | Contacts were at a significantly higher risk for infection if the primary case had both cough and runny nose (OR 4.31, 95% CI
1.60–11.63), if the contact was aged 18–49 years (OR 4.67, 95% CI 1.83–11.93), if the contact kissed the primary case (OR 3.16, 95% CI 1.19–8.43), or if the contact shared a meal with the primary case (OR 3.10, 95% CI 1.17–8.27). |
| Ng 2021 | Household | Independent risk factors that were significantly associated with higher transmission risk in the household included an index case
who was symptomatic (aOR 1.5; 95% CI 1.1–2.2), and household index aged greater than 18 years (aOR 7.0; 95% CI 4.4–11.3) |
| Ogata 2021 | Household | Spouses of index patients were significantly more likely to be infected compared to other household contacts: OR 2.85 (95%CI
1.25–6.5) |
| Park 2020a | Household
Non- household |
With index patients 30–39 years of age as reference, detection of COVID-19 contacts was significantly higher for index patients >40
years of age in nonhousehold settings. |
| Petersen 2021 | Household | The risk for seropositivity was significantly higher for household contacts compared with other contacts (adjusted odds ratio [aOR]
5.4, 95% CI 1.9–15.2). |
| Pett 2021 | Household
Community |
SAR among household contacts (15.9%, 95% CI 6.6%–30.1%) was more than 6 times higher compared to high-risk contacts (2.5%,
95% CI 0.9%–5.4%) |
| Phiriyasart 2020 | Household | Locally religious and household contacts of confirmed cases had significantly higher risks of SARS-CoV-2 infection than other
community members. |
| Poletti 2020 | Unclear | Individuals younger than 70 years were at a significantly lower risk of death after infection than older patients (p<0.001). The risk of
death was 62% lower (95% CI: 31–80%; p<0.001) during the second phase of the epidemic. |
| Ratovoson 2022 | Household | In both the univariate and multivariate analyses, there was a relationship between the age of contacts and SAR, with the highest SAR
in contacts aged 35 years old or more. |
| Razvi 2020 | Nosocomial | HCWs in patient facing roles had a significantly higher frequency of positive COVID-19 antibody tests (295/1302 [22.7%]) than those
in non-patient facing roles (88/669 [13.2%]), p<0.0001) |
| Reukers 2021 | Household | Being a child was strongly associated with decreased probability of infection (P=0.006) |
| Robles Pellitero 2021 | Household | Wearing a mask during quarantine was significantly associated with reduced risk of infection: 30.5% vs 45.7% P<0.001). |
| Rosenberg 2020 | Household | Prevalence significantly increased with age, ranging from 23% among those aged <5 years to 68% among those 65 years or older
(p<0.0001) |
| Satter 2022 | Household
Community |
People living in high-density areas with high SES had significantly higher levels of SARS-CoV-2-specific IgG antibodies on both study
day 1 (P=0.011) and study day 28 (P=0.005) compared to the people with low SES. |
| Schoeps 2021 | Local | Teacher index cases caused on average more secondary cases (169/157, risk=1·08) than students/children (145/591, risk=0·25;
IRR 4·39, p<0·001). The average number of student/child-to-teacher transmission was 0·04 (corresponding to about one teacher secondary case in 25 student/child index cases) compared to 0.56 for teacher-to-teacher transmission (one teacher secondary case in 2 teacher index cases, IRR 13·25, p<0.0001). |
| Shah 2021 | Household | The family size of the index cases causing secondary infection was comparatively larger than index cases without secondary
household infection (6.75 ± 2.3 versus 4.9 ± 1.9; P=0.03). |
| Sordo 2022 | Household | The odds of secondary transmission were lower in primary cases who were asymptomatic at diagnosis than in symptomatic cases
(odds ratio, OR: 0.13; 95% 0.04-0.48); and higher in primary cases aged 60 years and over than in those aged 19-39 years (OR: 3.45; 95%CI: 1.53- 7.75). Being a spouse of the primary case was also associated with increased transmission compared to non-spouses (OR: 1.93; 95% CI: 1.24-3.02). |
| Soriano-Arandes 2021 | Household | The SAR was significantly lower in households with COVID-19 paediatric index cases during the school period relative to summer
(P=0.02) and compared to adults (P=0.006). |
| Speake 2020 | Aircraft | The risk for secondary infections among passengers seated in the mid cabin was significantly greater than for those seated in the aft
cabin (p<0.005). The SAR among mid-cabin passengers in window seats was significantly greater than among those not in window seats (RR 5.2; 95% CI 1.6–16.4; p<0.007). |
| Stich 2021 | Household | The overall secondary attack rate was was significantly higher in exposed adults (37.5%) than in children (24.6%-29.2%; P<0.015). The
risk of infection was also significantly higher when the index case-patient was >60 years of age (72.9%; P=0.04) |
| Sun 2020 | Household | The family recurrence rate of spouses who introduced cases from the family was 63.87%, which was higher than the recurrence rate
of children (30.53%), parents (28.37%) and other family members (20.93%), and the difference was statistically significant ( P <0.001) . |
| Sundar 2021 | Household
Community |
The risk of infection was significantly higher in household contacts compared to open environmental work contacts (RR 30.9, 95%CI
9.7-98.3, P<0.001), or closed environmental work contacts (RR1.68, 95%CI 1.15-2.44, P=0.006). The risk was significantly higher among closed environmental work contacts compared to open environmental work contacts (RR 18.3, 95%CI 5.8-58.2, P<0.001). |
| Tadesse 2021 | Household | Persons aged 41–65 years were significantly more likely to be infected than people above the age of 65 years: OR 2.5 (95%CI 1.1-
5.5). Employed population groups have increased risk for infection by compared to unemployed groups: OR 1.3 (95% CI 1.0-1.6). |
| Tanaka 2021 | Household | SARS-CoV-2 Alpha variant had an approximately 1.9–2.3-fold higher transmissibility than the pre-existing virus (P<0.001) |
| Tanaka 2022 | Household | Fewer children were symptomatic compared with adults [91 (51.4%) vs. 142 (65.7%), P=0.004]. Children index cases were associated
with periods of lower community case rates while adult index cases were associated with periods of high community transmission and rapid incidence rise of COVID-19 cases (P=0.006). |
| Torres 2020 | Community | Antibody positivity rates were 9.9% (95%CI: 8.2-11.8) for 1,009 students and 16.6% (95%CI: 12.1-21.9) for 235 staff. Among students,
positivity was significantly associated with history of contact with a confirmed case (p<0.0001). The greater the number of contacts, the greater the probability that a child was antibody positive (p=0.05). |
| Tsang 2022 | Household
Community |
Compared to within households, the odds of infection was much lower during air travel, OR= 0.08 (95% CrI: 0.01, 0.34), and in other
settings, OR= 0.04 (95% CrI: 0.01, 0.09). |
| van der Hoek 2020 | Household | In families of a confirmed COVID-19 patient, children between 1 and 11 years were less often positive in PCR and serology than older
children and adults. |
| Vičar 2021 | Household | There was no significantly higher SAR in families with an adult primary case compared to those with children (77.1% vs. 65.8%,
P=0.05). |
| Wang 2020b | Household | Face mask use by the primary case and family contacts before the primary case developed symptoms was 79% effective in reducing
transmission (OR=0.21, 95% CI 0.06 to 0.79). Daily use of chlorine or ethanol-based disinfectant in households was 77% effective (OR=0.23, 95% CI 0.07 to 0.84). Wearing a mask after illness onset of the primary case was not significantly protective. The risk of household transmission was 18 times higher with frequent daily close contact with the primary case (OR=18.26, 95% CI 3.93 to 84.79), and four times higher if the primary case had diarrhoea (OR=4.10, 95% CI 1.08 to 15.60). Household crowding was not significant. |
| White 2022b | Local | The SAR in flights of ≥5 h duration was significantly higher than shorter flights (P=0.008) |
| Wiens 2021 | Household | The risk of seropositivity was lowest among participants 20 to 49 years old |
| Wood 2020 | Household | Households without children had a significantly lower rate of COVID-19: HR per child 0.89; 95% CI 0.84-0.95. Households with
children had higher rates of COVID-19 tests (9.2% vs 6.1%) Compared to those in households without children, the risk of COVID-19 requiring hospitalisation was lower in those with one child and lower still in those with two or more children: HR 0.72 per child (95% CI 0.60-0.85, p<0.001); adjusted for age - HR 0.83 per child (95% CI 0.70-0.99) |
| Wu 2020 | Household
Local Community |
Contacts living in the same household as the index case had significantly higher risk of infection vs those who had only had brief
contact with the index case: RR 41.7 (17.7–98.5), p<0.001). Contacts who had visited, or had contact with the index case in a medical institution had significantly higher risk of acquiring infection vs brief contact with the index case: RR 3.6 (1.42–8.98), p=0.004. Family members who had contact with an index case had significantly higher risk of infection vs healthcare providers or other patients who had been exposed to an index case: RR 31.6 (7.69–130.01), p<0.001. Those who had contact with the index case through work, through study, or in a place of entertainment had a significantly higher risk of infection vs those who had contact with the index case in a medical institution: RR 6.7 (1.34–33.25), p=0.01. Those who had contact with the index case in or near his/her home had a significantly higher risk of infection vs those who had contact with the index case in a medical institution: RR 17.3 (4.20–70.77), p<0.001. The incidence rate among those who wore face masks was significantly lower than that among those who did not use protective measures (0.3% vs. 4.7%, respectively, p<0.001). The incidence rate of contacts with data collected by field investigation was significantly higher than that of contacts with data collected by big data (5.35% versus 0.07%, p<0.001). |
| Wu 2020a | Household | Contacts with >72 hours of exposure (SIR, 41.7%; [95% CI: 26.8%–58.3%]) had a higher SIR compared with those without (SIR, 23.2%;
[95% CI: 11.4%–41.5%]). One household-level factor was significantly associated with SIR: household members without protective measures after illness onset of the index patient (odds ratio [OR], 4.43; [95% CI: 1.37–14.34]). |
| Wu 2021 | Local
Household Community |
The SARs among close contacts of symptomatic and asymptomatic index cases were 4.1% (128 of 3136) and 1.1% (12 of 1078),
respectively, corresponding to a significantly higher transmission risk from symptomatic cases (OR 3.79; 95%CI 2.06-6.95). |
| Xie 2021 | Household | Handwashing ≥ 5 times/day was associated with significantly reduced infection risk (52.8% vs. 76.9%, P=0.04). |
| Xin 2020 | Household | Increasing risk of infection among household contacts with female index patients (adjusted hazard ratio [aHR] = 3.84, 95% CI =
1.07–13.78), critical disease index patients (aHR = 7.58, 95% CI = 1.66–34.66), effective contact duration with index patients > 2 days (aHR = 4.21, 95% CI = 1.29–13.73), and effective contact duration > 11 days (aHR = 17.88, 95% CI = 3.26–98.01) |
| Yi 2021 | Household | The frequency of exposure to positively SARS-CoV-2 cases was significantly higher in index patients (20.7% vs. 6.8%, P=0.01). |
| Yu 2020 | Household | Family members, colleagues/classmates/travel companions, and doctors-patients accounted for 88.1% (1398), 10.7% (170), and 0.3%
(5), respectively. Following this order, the infection rate was 10.2%, 1.8% and 40.0%, respectively. |
| Yung 2020 | Household | Young children <5 years old were at lowest risk of infection (1.3%). Children were most likely to be infected if the household index
case was the mother. |
| Zhang 2020a | Household
Local Community |
SAR among household contacts was 16.1% vs 1.1% for social contacts, and 0% for workplace contacts.
Older close contacts had the highest SAR compared with other age groups; 8.0% in persons >60 years of age compared with 1.4%–5.6% in persons <60 years of age. Close contacts that lived with an index case-patient had 12 times the risk for infection and those who had frequent contact with an index case-patient, >5 contacts during 2 days before the index case was confirmed, had 29 times the risk for infection. |
| Zhuang 2020 | Household
Community |
The main sources of secondary infection were family exposure (74.5%, 178 cases), transportation exposure accounted for 8.4% (20
cases), friend/colleague meal exposure accounted for 5.9% (14 cases). Shopping malls, markets, pharmacies, and other public place exposure accounted for 5.0% (12 cases), workplace exposure accounted for 3.8% (9 cases), and community exposure accounted for 2.5% (6 cases). |
Viral culture
Four studies (Ladhani 2020a, Miller 2021, Speake 2020, Yang 2020) performed viral culture ( Table 8). Three studies utilised Vero E6 cells for viral culture; one study (Miller) did not describe the methods used for culture. In Ladhani 2020a (a study of elderly nursing home residents), positive samples with a Ct of <35 were incubated on Vero E6 cells and confirmed by cytopathic effect (CPE) up to 14 days post-inoculation. Positive culture results were obtained for symptomatic, post-symptomatic, pre-symptomatic and asymptomatic cases (21 residents and 12 staff); higher Ct values was significantly associated with decreasing ability to recover the virus (p<0.001). Among residents the virus was isolated 12 days before symptom onset and up to 13 days after and in staff up to 6 days before and 7 days after symptom onset. In Miller 2021 (household contacts of index cases), 9/48 (19%) of samples were culture positive – none of the samples collected after seven days of symptom-onset yielded positive cultures. In Speake 2020, specimens were inoculated in Vero-E6 cells and inspected for CPE daily for up to 10 days with identity confirmed using “in-house” PCRs. The primary cases had boarded the flight from a cruise ship and had SARS-CoV-2 with the strain A2-Ruby Princess (A2-RP). Nine of 17 (53%) of PCR-positive samples grew SARS-CoV-2 in culture. Eight secondary cases who were in the same flight cabin with the infected travellers from the cruise ship all had viruses of the A2-RP strain (3 by full and 1 by partial sequence) ( Table 8). In the Yang 2020 study of index patients with recurrent infection, swab specimens were also inoculated on Vero cells and monitored for CPE daily for 10 days. All four viral cultures were negative (0%).
Table 8. Results of Viral Culture.
| Study ID | Types of participants | Method used for viral culture | Results of viral culture |
|---|---|---|---|
| Ladhani 2020a | Staff and residents of 6
London care homes |
All SARS-CoV-2 positive samples with a Ct
value of <35 were incubated on Vero E6 mammalian cells and virus detection was confirmed by cytopathic effect (CPE) up to 14 days post-inoculation |
87 samples with Ct values <35 were cultured and infectious virus was
recovered from all (21 residents and 12 staff). Live virus was isolated up to 13 days after and 12 days before symptom onset among residents and up to 6 days before and 7 days after symptom onset among staff. Higher Ct values was significantly associated with decreasing ability to recover infectious virus (p<0.001). There were no significant differences in virus recovery rates between symptomatic and asymptomatic residents (5/17 [29.4%] vs. 14/33 [42.4%]; P=0.37) and staff (2/6 [33.3%] vs. 10/31 [32.3%]; P=0.96) at the time of testing. |
| Miller 2021 | Household contact of PCR-
positive index cases |
Not described | Virus culture was carried out for 48 PCR positive swabs.
9 samples were culture positive (6/8 with a Ct value <25, 2/10 with a Ct value 25-<32 and 1/30 with a higher Ct value >32); no PCR positive swabs taken more than 7 days after symptom onset yielded viable virus. |
| Speake 2020 | 241 airline passengers
some of whom had disembarked from 1 of 3 cruise ships that had recently docked in Sydney Harbour. 6 primary cases initially |
Virus culture was attempted for primary
samples. Clinical specimens were inoculated in triplicate wells with Vero-E6 cells at 80% confluency, incubated at 37°C in 5% CO2, and inspected for cytopathic effect daily for up to 10 days. Identity was confirmed by in-house PCRs as described for previous sequences. |
9/17 of PCR positive samples grew SARS-CoV-2 on viral culture. Sufficient
viral RNA was available to generate an adequate sequence for 25 of the 29 samples positive by PCR. 11 passengers had PCR-confirmed SARS-CoV-2 infection and symptom onset within 48 hours of the flight. All 11 passengers had been in the same cabin with symptomatic persons who had culture-positive A2-RP virus strain. |
| Yang 2020 | Home quarantine: 93
recurrent-positive patients; 96 close contacts and 1,200 candidate contacts |
Vero-E6 cells were used for virus isolation in
a BSL-3 laboratory. |
Viral cultures of 4 specimens with Ct <30 were negative |
Genome sequencing (GS) and phylogenetic analysis
Eighteen studies (Böhmer 2020, Cerami 2021, Firestone 2020, Huang 2021, Jeewandara 2021, Jiang 2020, Klompas 2021, Kolodziej 2022, Ladhani 2020a, Lucey 2020, Pang 2022, Powell 2022, Pung 2020, Sikkema 2020, Speake 2020, Taylor 2020, Wang 2020, Zhang 2021) performed GS and phylogenetic analysis ( Table 9). The studies were primarily conducted in outbreak clusters and methods used for performing these investigations were essentially similar across the studies. The completeness of genomic similarity ranged from 77–100% across 10 studies (Cerami 2021, Firestone 2020, Huang 2021, Jiang 2020, Lucey 2020, Pang 2022, Sikkema 2020, Speake 2020, Wang 2020, Zhang 2021). Transmission from one case to a contact was demonstrated by nonsynonymous nucleotide polymorphism in SARS-CoV-2 from these two cases onwards, but not in any cases detected prior to this instance (Böhmer 2020). Genomic sequencing of viral isolates confirmed household transmission in two studies (Cerami 2021, Kolodziej 2022). In one study of skilled nursing home facilities (Taylor 2020), samples from 75 residents and five healthcare staff shared genetically related strains. In another study of care homes (Ladhani 2020a), reported nine separate introductions of SARS-CoV-2 into care homes by healthcare staff. In one study which used multiple settings (Pung 2020), the viral genomic sequences for four cases in one cluster shared identical sequences over the full genome length and shared a common base difference relative to the earlier sequences (see Table 9).
Table 9. Results of Genome Sequencing and Phylogenetic Analyses.
| Study ID | Study Setting | Method used for WGS | Phylogenetic analysis | Results |
|---|---|---|---|---|
| Böhmer 2020 | Home,
workplace |
Whole genome sequencing involved Roche KAPA
HyperPlus library preparation and sequencing on Illumina NextSeq and MiSeq instruments as well as RT-PCR product sequencing on Oxford Nanopore MinION using the primers described in Corman and colleagues. Patient 1 was sequenced on all three platforms; patients 2–7 were sequenced on Illumina NextSeq, both with and without RT- PCR product sequencing with primers as in Corman and colleagues; and patients 8–11, 14, and 16 were sequenced on Oxford Nanopore MinION. Sequencing of patient 15 was not successful. Sequence gaps were filled by Sanger sequencing. |
Not reported | Presymptomatic transmission from patient 4 to patient
5 was strongly supported by virus sequence analysis: a nonsynonymous nucleotide polymorphism (a G6446A substitution) was found in the virus from patients 4 and 5 onwards but not in any cases detected before this point (patients 1–3). Later cases with available specimens, all containing this same substitution, were all traced back to patient 5. The possibility that patient 4 could have been infected by patient 5 was excluded by detailed sequence analysis: patient 4 had the novel G6446A virus detected in a throat swab and the original 6446G virus detected in her sputum, whereas patient 5 had a homogeneous virus population containing the novel G6446A substitution in the throat swab. |
| Cerami 2021 | Household | cDNA libraries were generated using ARTIC Network
amplicons to generate cDNA followed by library construction with a QIAGEN® (Hilden, Germany) QIAseq FX kit. Paired-end libraries were sequenced on an Illumina MiSeq at the UNC High-Throughput Sequencing Facility. Following demultiplexing, libraries underwent adapter and quality trimming according to default parameters for paired-end reads in Trim Galore!. Trimmed fastq files were converted to unaligned BAM format, trimmed of primer sequences, aligned to the Wuhan reference sequence, and assembled into fasta format using the Broad Institute viral NGS pipelines implemented in Docker Desktop. The resulting fasta files were aligned via MAFFT v7.450 implemented in Geneious Prime® 2021. |
Relatedness between viral sequences was
assessed via phylogenomic analysis in MrBayes v3.2.6 implemented in Geneious Prime® 2021 using default parameters and setting the Wuhan reference sequence as the outgroup. Samples from the same household were considered to be related if they were assigned to the same larger clade by Nextclade as well as the same clade in MrBayes. All sequences included in this analysis are available on GISAID under the accession numbers EPI_ISL_3088340 to EPI_ISL_3088373. |
High density amplicon sequencing of viral isolates from
these late secondary cases and others in their household confirmed that 4/5 were indeed due to household transmission |
| Firestone 2020 | Motorcycle
rally |
WGS was conducted at the MDH Public Health Laboratory
on 38 specimens using previously described methods. |
Phylogenetic relationships, including distinct
clustering of viral whole genome sequences, were inferred based on nucleotide differences via IQ-TREE using general time reversible substitution models as a part of the Nextstrain workflow. |
38 (73%) specimens (23 [61%] from primary and 15 [39%]
from secondary and tertiary cases) were successfully sequenced, covering at least 98% of the SARS-CoV-2 genome. Six genetically similar clusters with known epidemiologic links were identified (i.e., cases in patients who were close contacts or who had common exposures at the rally), five of which demonstrated secondary or secondary and tertiary transmission. |
| Huang 2021 | Local | Not described | Not described | WGS revealed that all 5 isolates belong to the same
clade, with only four nucleotide changes in two, while the remaining three showing identical viral genome |
|
Jeewandara
2021 |
Household
Community |
Library preparation was attempted using the AmpliSeq
for Illumina SARS-CoV-2 Community Panel, in combination with AmpliSeq for Illumina library prep, index, and accessories (Illumina, San Diego, USA) and targeted RNA/cDNA amplicon assay was used. The representative lineage sequences were downloaded from https://github. com/cov-lineages/lineages (anonymised.encrypted.aln. safe.fasta) |
Sequence lineage, nucleotide mutations and
amino acid replacements were generated using the CoV-GLUE graphical user interface. GISAID database used. |
Two viruses (only 2/89 samples had RT-qPCR Ct values
<25) were sequenced from this cohort which revealed that they were of clades B.4 and B.1, suggesting that many different virus strains were circulating within the Bandaranayaka watta during this time. One of the viruses had the D614G mutation. |
| Jiang 2020 | Home | Positive samples were sequenced directly from the
original specimens as previously described. *Reference virus genomes were obtained from GenBank using Blastn with 2019-nCoV as a query. The open reading frames of the verified genome sequences were predicted using Geneious (version 11.1.5) and annotated using the Conserved Domain Database. Pairwise sequence identities were also calculated using Geneious. Potential genetic recombination was investigated using SimPlot software and phylogenetic analysis. |
The maximum likelihood phylogenetic tree
of the complete genomes was conducted by using RAxML software with 1000 bootstrap replicates, employing the general time- reversible nucleotide substitution model. |
The full genome of 8 patients were >99.9% identical
across the whole genome. Phylogenetic analysis showed that viruses from patients were clustered in the same clade and genetically similar to other SARS-CoV-2 sequences reported in other countries. |
| Klompas 2021 | Local | Total nucleic acid from respiratory specimens was
extracted using the Roche MagNA Pure 96 DNA and Viral NA Small Volume Pack. Presence and abundance estimates of SARS-CoV-2 RNA were evaluated by the CDC 2019-Novel Coronavirus Real-Time RT-PCR Diagnostic Panel. Tiled, whole-genome amplicon sequencing was performed using an adapted ARTIC V3 SARS-CoV-2 protocol and a common protocol developed by a collaborative group of state public health laboratories, and the CDC. The samples were combined after PCR tiling, screened, and quantified for Illumina DNA Prep. |
The Cecret pipeline (
https://github.com/
UPHL-BioNGS/Cecret) was used, with minor modifications for our local environment, to generate consensus genomes for each sample. To ensure accuracy of results, we only considered highly complete (≥95% coverage) genomes in downstream analyses. These sequences were aligned and computed pairwise distances between sample genomes. Resultant SNP distances were discussed within the context of epidemiologic linkage to rule in or rule out individuals from this particular cluster. |
Whole-genome sequencing confirmed that 2 staff
members were infected despite wearing surgical masks and eye protection. |
| Kolodziej 2022 | Household | Sequences were obtained from saliva samples with
the highest viral load and are labelled per household. Amplicon-based SARS-CoV-2 sequencing for was performed on the positive saliva sample with the highest viral load for each individual using the Nanopore protocol “PCR tiling of COVID-19 virus (Version: PTC_9096_v109_ revE_06FEB2020)” which is based on the ARTIC v3 amplicon sequencing protocol. Several modifications were made to the protocol as primer concentrations were increased from 0.125 to 1 pmol for the following amplicon primer pairs. AMPure XP beads purification was only performed on clinical samples with an initial Cp-value <32. Both libraries were generated using native barcode kits from Nanopore SQK-LSK109 (EXP-NBD104, EXP-NBD114 and EXP-NBD196) and sequencing was performed on a R9.4.1 flow cell multiplexing 48–96 samples per sequence run. |
Not described | Each household shows a distinct cluster in phylogenetic
analyses with minimal sequence differences indicative of a single introduction within each household. For certain households only a single genome could be determined, for which no conclusions could be drawn. |
| Ladhani 2020a | Care homes | Whole genome sequencing (WGS) was performed on all
RT-PCR positive samples. Viral amplicons were sequenced using Illumina library preparation kits (Nextera) and sequenced on Illumina short-read sequencing machines. Raw sequence data was trimmed and aligned against a SARS-CoV-2 reference genome (NC_045512.2). A consensus sequence representing each genome base was derived from the reference alignment. |
Consensus sequences were assessed for
quality, aligned using MAFFT (Multiple Alignment using Fast Fourier Transform, version 7.310), manually curated and maximum likelihood phylogenetic trees derived using IQtree (version 2.04). |
All 158 PCR positive samples underwent WGS analysis
and 99 (68 residents, 31 staff) distributed across all the care homes yielded sequence sufficient for WGS analysis. Phylogenetic analysis identified informal clusters, with evidence for multiple introductions of the virus into care home settings. All care home clusters of SARS-CoV-2 genomes included at least one staff member, apart from care home B with no PCR positive staff and high rates of staff self-isolation. Care home A exhibited three distinct sequence clusters and six singletons, potentially representing up to nine separate introductions. Genomic analysis did not identify any differences between asymptomatic/symptomatic residents/staff. The 10 sequences from residents who died were distributed across the lineages identified and were closely matched to sequences derived from non-fatal cases in the same care homes. |
| Lucey 2020 | Hospital | Complementary DNA was obtained from isolated
RNA through reverse transcription and multiplex PCR according to the protocol provided by the Artic Network initiative. Libraries were prepared using the NEBNext Ultra II kit (New England Biolabs) and sequenced on an Illumina MiSeq using 300-cycle v2 reagent kits (Illumina). Bowtie 2 was used for aligning the sequencing reads to the reference genome for SARS-CoV-2 (GenBank number, MN908947.3) and SAMtools for manipulating the alignments. |
SNPs were used to define clusters and a
median-joining network was generated including these data from this study and an additional 1,000 strains collected from GISAID available on May 22nd. Clade annotation was included for the Pangolin, GISAID and NextStrain systems. |
WvGS identified six clusters of nosocomial SARS-CoV-2
transmission. The average sequence quality per samples was > 99% for 46 samples, and between 92 and 94% for 4 samples. Phylogenetic analysis identified six independent groups of which clusters 1–3 were related to 39 patients. |
| Pang 2022 | Local | Three specific real-time RT-PCR methods targeting the
N, S, and ORF1ab genes were designed to detect the presence of SARS-CoV-2 in clinical samples. Thermal cycling for N gene real-time RT-PCR assays was performed at 50°C for 20 min for reverse transcription, 95°C for 15 min, 50 cycles of 94°C for 5 s, 55°C for 1 min. |
Residual RNA was subjected to tiled
amplicon PCR using ARTIC nCoV-2019 version 3 panel, where One-Step RT-PCR was performed using the SuperScript™ III One-Step RT-PCR System with Platinum™ Taq DNA Polymerase (Thermo Fisher Scientific, MA, USA). Sequencing libraries were prepared using the Nextera XT and sequenced on MiSeq (Illumina, CA, USA) to generate 300 bp paired-end reads. The reads were subjected to a hard-trim of 50 bp on each side to remove primer artifacts using BBMap prior to consensus sequence generation. The generated consensus sequences were shared via a global initiative on sharing avian flu data (GISAID). Closely related representative strains from other countries (99.99% identity and matching the time window) were identified in the GISAID database using BLASTN. |
With the exception of sequence, phylogenetic analysis of
SARS-CoV-2 genome sequences obtained from all cases (13/14; 92.9%), including H1, was grouped into a single cluster. This cluster was supported by a single mutation (T27588A) not found in other sequences in the database before the nursing home outbreak. |
| Powell 2022 | Local | Not described | Not described | Whole genome sequencing was successful in two of
five index cases (the initial confirmed case that led to the bubble self-isolating) and all nine positive direct contacts. Overall, four of the nine sequences available for comparison identified different SARS-CoV-2 strains, therefore, ruling out transmission between affected individuals. |
| Pung 2020 | Multiple:
Company conference, church, tour group. |
Strain names, GISAID EpiCoV accession numbers used for
genomic sequencing |
Phylogenetic tree utilised the Neighbor-
Joining method and confirmed using Maximum Likelihood approaches. Replicate trees with bootstrap used. All ambiguous positions were removed for each sequence pair (pairwise deletion option). Evolutionary analyses were conducted in MEGA X. Strain names, GISAID EpiCoV accession numbers and collection dates are shown, followed by the case number if available. |
Cluster A: Viral genomic sequences were available for
four cases (AH1, AH2, AH3, and AT1) and phylogenetic analysis confirmed their linkage, as suggested by the epidemiological data. |
| Sikkema 2020 | Hospital | Samples were selected based on a Ct <32. A SARS-CoV-2-
specific multiplex PCR for nanopore sequencing was done. The resulting raw sequence data were demultiplexed using qcat. Primers were trimmed using cutadapt,17 after which a reference-based alignment to the GISAID (Global Initiative on Sharing All Influenza Data) sequence EPI_ISL_412973 was done using minimap2. The consensus genome was extracted and positions with a coverage less than 30 reads were replaced with N using a custom script using biopython software (version 1.74) and the python module pysam (version 0.15.3). Mutations in the genome were confirmed by manually checking the alignment, and homopolymeric regions were manually checked and resolved, consulting the reference genome. Genomes were included when having greater than 90% genome coverage. All available full-length SARS-CoV-2 genomes were retrieved from GISAID20 on March 20, 2020 (appendix 1 pp 8–65), and aligned with the newly obtained SARS-CoV-2 sequences in this study using the multiple sequence alignment software MUSCLE (version 3.8.1551). Sequences with more than 10% of N position replacements were excluded. The alignment was manually checked for discrepancies, after which the phylogenomic software IQ-TREE (version 1.6.8) was used to do a maximum- likelihood phylogenetic analysis, with the generalised time reversible substitution model GTR+F+I+G4 as best predicted model.The ultrafast bootstrap option was used with 1000 replicates. Clusters were ascertained based on visual clustering and lineage designations. |
The code to generate the minimum
spanning phylogenetic tree was written in the R programming language. Ape24 and igraph software packages were used to write the code to generate the minimum spanning tree, and the visNetwork software package was used to generate the visualisation. Pairwise sequence distance (used to generate the network) was calculated by adding up the absolute nucleotide distance and indel-block distance. Unambiguous positions were dealt with in a pairwise manner. Sequences that were mistakenly identified as identical, because of transient connections with sequences containing missing data, were resolved. |
46 (92%) of 50 sequences from health-care workers in the
study were grouped in three clusters. Ten (100%) of 10 sequences from patients in the study grouped into the same three clusters: |
| Speake 2020 | Aircraft | Processed reads were mapped to the SARS-CoV-2
reference genome (GenBank accession no. MN908947). Primer-clipped alignment files were imported into Geneious Prime version 2020.1.1 for coverage analysis before consensus calling, and consensus sequences were generated by using iVar version 1.2.2. |
Genome sequences of SARS-CoV-2
from Western Australia were assigned to lineages by using the Phylogenetic Assignment of Named Global Outbreak LINeages (PANGOLIN) tool ( https://github. com/cov-lineages/pangolinExternal Link). On July 17, 2020, we retrieved SARS-CoV-2 complete genomes with corresponding metadata from the GISAID database. The final dataset contained 540 GISAID whole- genome sequences that were aligned with the sequences from Western Australia generated in this study by using MAFFT version 7.467. Phylogenetic trees were visualized in iTOL (Interactive Tree Of Life, https://itol.embl.deExternal Link) and MEGA version 7.014. |
100% coverage was obtained for 21 and partial coverage
(81%–99%) for 4 samples. The phylogenetic tree for the 21 complete genomes belonged to either the A.2 (n = 17) or B.1 (n = 4) sublineages of SARS-CoV-2 |
| Taylor 2020 | Skilled nursing
facilities |
WGS was conducted by MDH-PHL on available specimens
using previously described methods. |
Phylogenetic relationships, including distinct
clustering of viral whole genome sequences, were inferred based on nucleotide differences via IQ-TREE, using general time reversible substitution models |
Specimens from 18 (35%) residents and seven (18%) HCP
at facility A were sequenced - Strains from 17 residents and five HCP were genetically similar. At facility B, 75 (66%) resident specimens and five (7%) HCP specimens were sequenced, all of which were genetically similar. |
| Wang 2020 | Home | Full genomes were sequenced using the BioelectronSeq
4000. WGS integrated information from 60 published genomic sequences of SARS-CoV-2. Full-length genomes were combined with published SARS-CoV-2 genomes and other coronaviruses and aligned using the FFT-NS-2 model by MAFFT. |
Maximum-likelihood phylogenies were
inferred under a generalised-time-reversal (GTR)+ gamma substitution model and bootstrapped 1000 times to assess confidence using RAxML. |
The phylogenetic tree of full-length genomes showed
that SARS-CoV-2 strains form a monophyletic clade with a bootstrap support of 100%. Sequences from six HCWs in the Department of Neurosurgery and one family member were closely related in the phylogenetic tree. 33 family members of the HCWs were not secondarily infected, due to the strict self-quarantine strategies taken by the HCWs immediately after their onset of illness, including wearing a facial mask when they came home, living alone in a separated room, never eating together with their families. |
| Zhang 2021 | Local
Household |
Sequencing raw reads were trimmed to remove
sequencing adaptors and low-quality bases. Clean reads were aligned to the reference genome of the SARS-CoV-2 (GenBank: NC_045512.2) using the Bowtie2 v.2.2.537 with default parameters. Duplicate reads were removed with Picard Tools. Samtools (v.1.10) “mpileup” was used to call SNPs using mpileup files as input with parameter -Q 20. Each site was re-calculated, and variants were screened using perl script with the following parameters: (ia) depth of alternate allele ≥ 5, (ii) alternate allele frequency ≥70%, and (iii) discarding the sites only supported by a single strand. The C337T variant in P4 were also considered as an SNP that was supported by sequencing reads (with 67% frequency) and validated by Sanger sequencing. Consensus sequences were called using BCFtools based on reference sequence. |
Phylogenomic analysis of 13 high-quality
(coverage: ≥70%) viral genomes was performed together with 72 strains circulating in Beijing during the same period, including 33 public viral genomes (from global initiative on sharing all influenza data [GISAID]) and 39 viral genomes from local centre. Viral genome was obtained from all 14 patients. 72 viral genomes were obtained, 33 were from the GISAID ( https://gisaid.org), 39 were from Beijing Ditan Hospital (GenBank: PRJNA667180). Consensus sequences were trimmed to 5ʹ and 3ʹ untranslated regions due to their poor quality. Multiple sequence alignment was conducted with parameters --auto --keeplength --addfragments using MAFFT v.7.45324.39. The maximum likelihood tree was constructed using IQ- TREE v.1.6.12 with 1000 bootstrap replicates. The substitution model GTR+F+R2 was selected based on Bayesian information criteria score. TreeTime v.0.7.6 was used for time-resolved phylogenomic analysis.41 iTOL (itol.embl.de) was applied for displaying topology of phylogenomic tree. The nucleotide frequency of each genomic locus was calculated with the 85 viral genomes of circulating strains in Beijing, including 13 genomes (P1–P13) from the outbreak cluster and 72 local genomes mentioned above (Figure S1). A median joining network was constructed using NETWORK v.10.1.0.0 on the Fluxus Technology website ( https://www.fluxus- engineering.com/). |
Twelve viral genomes from this outbreak were tightly
clustered into two clades with bootstrap values of at least 77%. |
Discussion
Summary of main findings
We identified 258 primary studies and 20 systematic reviews assessing the role of close contact in transmission of SARS-CoV-2. The evidence from primary studies suggest that the risk of transmission is significantly increased through close contact with an infected case - the greater the frequency of contact, the greater the risk. Household contact setting is significantly more likely to result in transmission of SARS-CoV-2 compared to other types of contact settings. This risk of transmission appears to decrease with use of face masks (by index cases only or by both index cases and close contacts) and in cases where the index or primary cases are in the paediatric age group. The risk of close contact transmission is significantly increased in the elderly. Enclosed environments and social gatherings appear to increase the likelihood of close contact transmission. Close contact with persons having recurrent infection with SARS-CoV-2 is unlikely to result in transmission of the virus. There is wide heterogeneity in study designs and methods and the overall quality of evidence from published primary studies is low to moderate. The results of systematic reviews also suggest that household contact setting increases the risk of transmission, and the risk of transmission appears greater with symptomatics and presymptomatics compared to asymptomatics.
The positive results of viral cultures observed in two studies support the results of PCR and serologic tests showing that close contact setting was associated with transmission of SARS-CoV-2. The failure to successfully isolate the virus in the third study supports the view that individuals who are re-infected are unlikely to transmit the virus in close contact settings. The positive findings from studies that performed GS and phylogenetic analysis with identical strains supports the hypothesis that SARS-CoV-2 transmission occurs in close contact settings. The routes of transmission are unclear but may include direct and indirect contact and/or large droplet or short-range aerosol transmission as possible explanations for the identified identical strains in close contacts 3– 5 . The failure of the majority of studies to report Ct values casts doubts on the strengths of any reported associations because of the likelihood of false positives, as is the lack of (and variation in) reporting of the timelines for sample collections. The variations observed in the definitions of close contacts also cast further doubts on the validity of overall results.
Comparison with the existing literature
The results of our review are consistent with several guidelines suggesting that close contact with index cases can result in transmission of SARS-CoV-2 10– 12 . However, these guidelines could change as more evidence emerges. The results from nine primary studies suggesting that face masks may reduce the risk of SARS-CoV-2 transmission support the findings from a systematic review which concluded that face masks are effective as adjuncts for preventing transmission of respiratory viruses 13 . However, several confounders make the strengths of the association unclear, e.g., type of face mask, setting, severity of illness, and duration of exposure. However, our review contains a greater number of studies compared to each of the included individual reviews and shows evidence demonstrating positive culture of virus as well as genomic evidence of SARS-CoV-2 transmission in close contact settings. This differs from the findings from our reviews of fomite, orofecal and airborne transmission that failed to show evidence of either positive culture or genomic sequences demonstrating SARS-CoV-2 transmission 14– 16 .
Strengths and limitations
To our knowledge, this is the most comprehensive review to date investigating the role of close contact in the transmission of SARS-CoV-2. We extensively searched the literature for eligible studies, accounted for the quality of included studies and have reported outcomes (viral culture and GS) that were previously unreported in previous reviews. However, we recognize some limitations. We may not have identified all relevant studies examining the role of close contact in transmission - this is especially true for unpublished studies. The QUADAS-2 checklist we adapted to assess the quality of included primary studies has not been validated for all types of study designs included in our review. The variation in the definition of close contact across the studies could also have resulted in identification of secondary cases in the included primary studies. Furthermore, we did not assess the impact of seasonality on the risk of transmission – it has been shown that humidity and temperature can affect the transmission of SARS-CoV-2 17, 18 . We did not assess the quality of the included reviews; however, we documented the overall reporting quality of primary studies as reported by the review authors. We included results from non-peer reviewed studies which may affect the reliability of our results. However, such studies could potentially be of research benefit because of the ongoing pandemic; in addition, we performed forward citation search of relevant studies.
Implications for research
Viral load measures should be linked to symptoms and epidemiological chain of transmission and should be repeated in multiple time windows in relation to the course of illness thus providing evidence of changing infectiousness. Future studies should endeavour to include Ct values (or preferably convert the Ct values to number of genome copies using standard curves) when reporting research results and should describe the timing and methods of sample collection. Details surrounding the proximity, timing, and activities within the context of close contact need to be described. In studies of elderly subjects, more detailed description of baseline demographics should be reported. Further studies showing virus isolation in close contact settings should be conducted to strengthen the current evidence base; this could include performing serial cultures. Similarly, more research examining genomic sequences and phylogenetic trees in suspected close contact transmissions should be conducted - this should also extend to research examining other modes of transmission. The variation in methods and thresholds of the serological tests add to the confusion about diagnostic accuracy of testing; indeed, some authors have questioned the value of serological tests for diagnosing SARS-CoV-2 19 . To overcome the challenge of interpreting antibody responses, guidelines for better reporting of serological tests and results should be developed; this has previously been emphasized by other authors. Internationally recognized research dictionary of terms defining and describing close contact settings should be developed. Standardized guidelines for reporting research results should be a priority. Local, national, and international health organisations should promote good hygiene measures including hand hygiene and avoidance of overcrowded spaces. Interventions to improve the uptake of vaccinations should be encouraged 20 . In addition, the use of PPE in high-risk settings (e.g., ICU, COVID-19 wards) should be a priority.
Conclusion
The evidence from published observational studies and systematic reviews indicate that SARS-CoV-2 can be transmitted in close contact settings. Household contact and increased frequency of contact with infected cases are associated with significantly increased risks of transmission. The reporting quality of published primary studies is low-to-moderate. Variations in study designs and methodology restrict the comparability of findings across studies. Standardized guidelines for the reporting of future research (and including the definitions of close contact) should be developed.
Acknowledgements
This work was commissioned and paid for by the World Health Organization (WHO). Copyright on the original work on which this article is based belongs to WHO. The authors have been given permission to publish this article. The author(s) alone is/are responsible for the views expressed in the publication. They do not necessarily represent views, decisions, or policies of the World Health Organization.
Funding Statement
The review was funded by the World Health Organization: Living rapid review on the modes of transmission of SARs-CoV-2 reference WHO registration No2020/1077093. CH and ES also receive funding support from the NIHR SPCR Evidence Synthesis Working Group project 390.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 3; peer review: 2 approved
Data availability
Underlying data
All data underlying the results are available as part of the article and no additional source data are required.
Extended data
Figshare: Extended data: SARS-CoV-2 and the Role of Close Contact in Transmission: A Systematic Review, https://doi.org/10.6084/m9.figshare.14312630.v1 8 .
This project contains the following extended data:
Updated Protocol
Revised Search Strategy
Revised List of Referenced to Excluded Studies
Revised List of References to Included Studies
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
References
- 1. World Health Organization: WHO Coronavirus Disease (COVID-19) Dashboard. [Last Accessed 20/03/2021]. Reference Source [Google Scholar]
- 2. Office for National Statistics: Coronavirus (COVID-19) latest insights.[Accessed 15/06/2022]. Reference Source [Google Scholar]
- 3. World Health Organization: Modes of transmission of virus causing COVID-19: implications for IPC precaution recommendations. [Accessed 16/01/2021]. Reference Source [Google Scholar]
- 4. World Health Organization: Coronavirus disease 2019 (COVID-19) Situation Report – 73. [Accessed 28 February 2021]. Reference Source [Google Scholar]
- 5. Centers for Disease Control and Prevention: COVID-19. Scientific Brief: SARS-CoV-2 Transmission.[Accessed 15/06/2022]. Reference Source [PubMed] [Google Scholar]
- 6. Tanne JH: Covid-19: CDC publishes then withdraws information on aerosol transmission. BMJ. 2020;370:m3739. 10.1136/bmj.m3739 [DOI] [PubMed] [Google Scholar]
- 7. Tang JW: SARS-CoV-2 and aerosols-Arguing over the evidence. J Virol Methods. 2021;289:114033. 10.1016/j.jviromet.2020.114033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Onakpoya I, Heneghan C, Spencer E, et al. : Extended data: SARS-CoV-2 and the Role of Close Contact in Transmission: A Systematic Review. figshare. Figure.2021. 10.6084/m9.figshare.14312630.v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Whiting PF, Rutjes AWS, Westwood ME, et al. : QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529–36. 10.7326/0003-4819-155-8-201110180-00009 [DOI] [PubMed] [Google Scholar]
- 10. World Health Organization: Q&A: How is COVID-19 transmitted?[Accessed 06/04/2021]. Reference Source [Google Scholar]
- 11. Center for Disease Control and Prevention: COVID-19 Overview and Infection Prevention and Control Priorities in non-US Healthcare Settings.Updated Feb. 26, 2021. Reference Source [Google Scholar]
- 12. Public Health England: Guidance 12. COVID-19 infection prevention and control guidance: glossary of terms. Updated 21 January 2021. Reference Source [Google Scholar]
- 13. Liang M, Gao L, Cheng C, et al. : Efficacy of face mask in preventing respiratory virus transmission: A systematic review and meta-analysis. Travel Med Infect Dis. 2020;36:101751. 10.1016/j.tmaid.2020.101751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Onakpoya IJ, Heneghan CJ, Spencer EA, et al. : SARS-CoV-2 and the role of fomite transmission: a systematic review [version 3; peer review: 2 approved]. F1000Res. 2021;10:233. 10.12688/f1000research.51590.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Heneghan CJ, Spencer EA, Brassey J, et al. : SARS-CoV-2 and the role of orofecal transmission: a systematic review [version 2; peer review: 2 approved]. F1000Res. 2021;10:231. 10.12688/f1000research.51592.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Heneghan C, Spencer EA, Brassey J, et al. : SARS-CoV-2 and the role of airborne transmission: a systematic review [version 2; peer review: 1 approved with reservations, 2 not approved]. F1000Res. 2021;10:232. 10.12688/f1000research.52091.2 [DOI] [Google Scholar]
- 17. Lin G, Hamilton A, Gatalo O, et al. : Investigating the effects of absolute humidity and movement on COVID-19 seasonality in the United States. Sci Rep. 2022;12(1):16729. 10.1038/s41598-022-19898-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Landier J, Paireau J, Rebaudet S, et al. : Cold and dry winter conditions are associated with greater SARS-CoV-2 transmission at regional level in western countries during the first epidemic wave. Sci Rep. 2021;11(1):12756. 10.1038/s41598-021-91798-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bastos ML, Tavaziva G, Abidi SK, et al. : Diagnostic accuracy of serological tests for covid-19: systematic review and meta-analysis. BMJ. 2020;370:m2516. 10.1136/bmj.m2516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Halperin DT, Hearst N, Hodgins S, et al. : Revisiting COVID-19 policies: 10 evidence-based recommendations for where to go from here. BMC Public Health. 2021;21(1):2084. 10.1186/s12889-021-12082-z [DOI] [PMC free article] [PubMed] [Google Scholar]