Abstract
Previous studies from our laboratory established that C-ASWS, an alkali-soluble, water-soluble extract from cell walls of Coccidioides immitis, protects mice against lethal challenge with this fungus. The C-ASWS extract contains a glycosylated protein, designated antigen 2 (Ag2), and a polysaccharide antigen. We recently cloned Ag2 cDNA and showed that the recombinant fusion protein elicited strong delayed-type hypersensitivity responses in immunized mice. This investigation was undertaken to determine if the recombinant Ag2 protein, expressed as an Ag2-glutathione S-transferase (GST) fusion protein, or Ag2 cDNA would protect mice against lethal challenge with C. immitis. The recombinant Ag2-GST protein protected BALB/c mice against intraperitoneal challenge with 250 arthroconidia, as assessed by a decrease in fungal CFU in tissues. The Ag2-GST-immunized mice did not show, however, an increased survival during a 30-day period postinfection. By contrast, immunization of mice with Ag2 cDNA ligated into the pVR1012 plasmid engendered protection against intraperitoneal challenge with 2,500 arthroconidia and against pulmonary challenge with 50 arthroconidia. Vaccine efficacy paralleled the development of delayed-type hypersensitivity responses to C. immitis antigen. Whereas mice vaccinated with the recombinant Ag2-GST protein did not mount footpad hypersensitivity to C-ASWS or the recombinant Ag2-GST protein, mice vaccinated with the pVR1012-Ag2 construct mounted a strong footpad hypersensitivity and their spleen cells secreted gamma interferon upon in vitro stimulation with the Ag2-containing C-ASWS extract. This is the first investigation to show that genetic immunization can protect against lethal challenge with C. immitis.
Coccidioidomycosis (Valley Fever) is a fungal disease caused by Coccidioides immitis. The disease is endemic in the semiarid areas of Texas, Arizona, New Mexico, and Southern California, where the fungus propagates in the soil in a mycelium phase (35). The mycelia produce arthroconidia, measuring approximately 4 by 6 μm, which become aerosolized when the soil is disturbed. When inhaled by a susceptible host, the arthroconidia undergo a morphogenetic conversion into endosporulating spherules. The spherules rupture at maturity, releasing the endospores, each of which has the capacity to develop into a mature, endosporulating spherule.
Approximately 100,000 cases of primary coccidioidal infections occur each year in the areas of the United States where the disease is endemic (21, 40). Marked increases have occurred during sporadic epidemics, such as the one that recently occurred in California (36). The disease has protean manifestations, which range from a primary, asymptomatic, or benign pulmonary infection to a progressive pulmonary or extrapulmonary disease involving the skin, bones and/or joints, central nervous system, and other organ systems (21, 40). Cumulative studies have documented that Asians and blacks are genetically predisposed to developing the disseminated form of the disease (21, 35, 40). There is also an increased incidence in the morbidity and mortality of the disease in immunocompromised persons, for example in patients with acquired immunodeficiency disease and those receiving cytotoxic therapy (10, 21, 40).
Recovery from primary asymptomatic or benign infection with C. immitis confers lifelong immunity to exogenous reinfection. The acquired resistance strongly correlates with the development of delayed-type hypersensitivity (DTH) skin test response and the production of T-helper 1 (Th1)-associated cytokines to coccidioidal antigens, such as gamma interferon (IFN-γ) and interleukin-2 (IL-2) (2, 8, 10). The immunizing capacity of the fungus, together with the well-defined target populations, document the feasibility of developing a vaccine for use in regions endemic to C. immitis. Early investigations established that formalin-killed spherules (FKS) were highly effective in protecting mice against lethal challenge with C. immitis arthroconidia (28, 31, 32). Clinical trials in humans, however, established that the FKS vaccine was toxic, necessitating that the dose be reduced to a level that was ineffective in inducing immunity to the disease (38).
In an effort to identify the immunogenic component of C. immitis, we extracted an alkali-soluble, water-soluble fraction, designated C-ASWS, and showed that the extract protected mice against lethal intraperitoneal and pulmonary challenge with C. immitis (30) and elicited Th1 responses in experimentally infected animals and patients with active coccidioidomycosis (13, 16, 44). Antigenic analyses by crossed immunoelectrophoresis showed that C-ASWS is a large-molecular-weight polysaccharide-protein complex containing a polymeric antigen designated antigen 2 (Ag2) and the serodiagnostically important polysaccharide that reacts with the immunoglobulin M (IgM) tube precipitin antibody to C. immitis (11, 12). Since we were unable to purify Ag2 from the polysaccharide antigen, we cloned the cDNA that encodes Ag2 from a spherule-derived cDNA lambda expression library (48). The recombinant Ag2 protein was shown to elicit delayed-type footpad hypersensitivity responses in mice immunized with killed spherules and to detect IgG antibody in sera from coccidioidomycosis patients (46, 48). We undertook this investigation to evaluate and compare the vaccine efficacy of the recombinant Ag2 protein and Ag2 cDNA.
MATERIALS AND METHODS
Expression and purification of rAg2.
Details of the procedure for the expression and purification of recombinant Ag2 (rAg2) have been published elsewhere (48). Briefly, the 582-bp Ag2 cDNA fragment was isolated from C. immitis Silveira (ATCC 28868) and inserted in frame into the EcoRI and XhoI sites of the pGEX-4-T3 expression vector (Pharmacia Biotech, Piscataway, N.J.) downstream from the gene that encodes glutathione S-transferase (GST). The parental pGEX-4T-3 plasmid served as a control for the GST peptide alone. The Ag2 fusion protein and GST peptide control were expressed in Escherichia coli TG-1 cells and affinity purified by adsorption on glutathione-Sepharose 4B beads (Pharmacia).
Construction and purification of pVR1012-Ag2 cDNA plasmid.
The full-length Ag2 cDNA was amplified from the pGEX4T-3 construct by PCR with oligonucleotide primers that included the recognition sites for the restriction endonucleases XbaI and BamHI. The Ag2 open reading frame was cloned into the eukaryotic expression vector plasmid pVR1012, which was generously provided by Vical, Inc. (San Diego, Calif.).
E. coli XL-Blue cells were transformed with the pVR1012-Ag2 construct or the pVR1012 plasmid alone and then cultured at 37°C for 16 h in Luria broth supplemented with kanamycin (50 μg/ml). Plasmid DNA was isolated by using an EndoFree plasmid purification kit (Qiagen, Santa Clara, Calif.). DNA was resuspended in USP saline (Baxter Healthcare Corp., Deerfield, Ill.) and stored at −20°C until used.
Immunization.
Five-week-old female BALB/c (H-22) mice were purchased from Jackson Laboratory (Bar Harbor, Maine). The mice were maintained for at least 1 week before use.
For the recombinant Ag2 vaccine, mice were immunized with 200 μl of Ag2-GST (100 μg) or GST peptide alone (100 μg), each diluted in sterile, endotoxin-free saline and admixed with an equal volume of Ribi adjuvant (RIBI ImmunoChem Research, Inc., Hamilton, Mont.). The first injection was given in RIBI 730 adjuvant containing MPL (monophosphoryl lipid A), synthetic trehalose dicorynomycolate (TDM), and cell wall skeleton via an intramuscular route. The second and third injections were given in RIBI 700 adjuvant (MPL plus TDM) via the intramuscular (i.m.) and subcutaneous (s.c.) routes, respectively.
Genetic immunization was performed by injecting mice i.m. with 50 μg of pVR1012-Ag2 or the pVR1012 plasmid alone (17, 34). Before each injection, the mice were lightly anesthetized via inhalation of Metofane (Mallinckrodt Veterinary, Inc., Mundelein, Ill.). Injections were given in the tibialis anterior muscle in a site which had been treated with Nair (Carter-Wallace, Inc., New York, N.Y.) 1 day before administering the first injection. A total of three immunizations were given at weekly intervals in alternating sites on the left and right legs.
The FKS vaccine, prepared as reported previously (9, 39), was administered over a 3-week period by the protocol reported by Levine et al. (32). The first two injections were given i.m. in the left and right legs, and the third injection was given s.c. in the nape of the neck. Each injection consisted of 0.7 mg of FKS suspended in sterile physiologic saline, for a total of 2.1 mg per mouse.
Infection and assessment of disease severity.
Arthroconidia were harvested from 4- to 8-week-old mycelial-phase cultures of C. immitis Silveira or CC, a recent isolate from a patient with disseminated coccidioidomycosis. The arthroconidial suspensions were passed over a nylon column to remove hyphal elements, and the cells were enumerated by hemacytometer counts. Mice were infected by an intraperitoneal (i.p.) injection with 2,500 arthroconidia suspended in 0.5 ml of pyrogen-free saline or by intranasal (i.n.) instillation of 50 arthroconidia in 30 μl of saline. The viability of the inocula was confirmed by plate counts on 1% glucose–2% yeast extract agar.
Vaccine efficacy was evaluated by determining the number of C. immitis CFU in the lungs, livers, and spleens at 10 to 12 days postinfection and by monitoring survival over a 30- to 35-day period as described previously (14, 33).
Footpad hypersensitivity tests.
Delayed-type footpad hypersensitivity was evaluated by testing mice in the footpad with C-ASWS (100 μg [dry weight]) prepared from spherule-phase cells of C. immitis Silveira. In brief, mice were injected in the right or left hind footpads with either 50 μl of spherule-phase C-ASWS diluted in nonpyrogenic saline or saline alone. Footpad thickness was measured with a dual caliper (Mitutoyo, Tokyo, Japan), and the results were calculated as the difference in footpad thickness of antigen- and saline-injected pads at 18 to 24 h minus the difference in footpad thickness of antigen- and saline-injected pads before challenge (14).
Cytokine induction and analyses.
Spleens were harvested, gently teased to obtain a single-cell suspension, suspended in cold Hank’s balanced salt solution, and treated with isotonic ammonium chloride to lyse the erythrocytes. The splenocytes were washed by centrifugation, resuspended in Dulbecco’s minimal essential medium (GIBCO, Grand Island, N.Y.) containing 10% fetal bovine serum, and dispensed to wells on a microtiter plate at a concentration of 2 × 106 mononuclear cells per well. The cultures were stimulated with gradient doses of C-ASWS, 2 μg of concanavalin A (ConA; Sigma Chemical Co., St. Louis, Mo.), or medium alone. After a 48-h incubation at 37°C under 5% CO2, supernatants were collected for assays of IFN-γ and IL-4 with reagents available from PharMingen (San Diego, Calif.).
IFN-γ protein was assayed by a two-site sandwich enzyme-linked immunosorbent assay (ELISA) with affinity-purified rat IgG1 anti-mouse IFN-γ monoclonal antibody from clones R4-6A2 and XMG1.2. In brief, supernatants from stimulated spleen cells were added to wells precoated with anti-IFN-γ antibody (clone R4-6A2; 100 ng). After overnight incubation at 4°C, nonreactive sites were blocked by washing the wells with PBS containing 3% bovine serum albumin. Biotinylated rat anti-mouse IFN-γ monoclonal antibody (clone XMG1.2; 1 μg/ml) was added and, after a 1-h incubation at room temperature, the plates were washed and further incubated with horseradish peroxidase-conjugated streptavidin for 30 min. The reactions were visualized by the addition of the substrate, 2,2′-azino-bis-(3-ethylbenzthiazoline-6-sulfonic acid) with hydrogen peroxide for 30 min, and the A410 values were read. Recombinant mouse IFN-γ (PharMingen) was used to establish a standard curve.
The ELISA for IL-4 was performed as described above except that rat anti-mouse IL-4 (clone 11B11) was used to capture IL-4 and biotinylated rat anti-mouse IL-4 (clone BVD6-24G2) was used to detect captured IL-4. The standard curve was prepared with recombinant murine IL-4.
Statistical analyses.
Differences in the mean responses of the groups were analyzed by the Wilcoxin rank sums test. Differences in the survival of mice postinfection were analyzed by using the Kaplan-Meier procedure. Probability values of <0.05 were considered significant.
RESULTS
Vaccine efficacy of recombinant Ag2 protein.
Figure 1 depicts the fungus load in tissues from Ag2-GST-immunized and GST-immunized BALB/c mice (15 animals/group) 12 days after i.p. challenge with 250 arthroconidia. Mice immunized with Ag2-GST showed a significant reduction in the number of CFU recovered from their livers and spleens compared to the control group (P < 0.002 and P < 0.025, respectively) (Fig. 1A). The fungus burden was also reduced in the lungs of Ag2-GST-immunized mice, but not to a significant level when compared to GST-immunized mice (P > 0.05). To determine if the Ag2-GST vaccine would reduce the mortality of the disease, BALB/c mice (15 animals/group) were immunized with Ag2-GST or GST alone and then monitored for a period of 30 days after i.p. challenge with 250 arthroconidia. No differences were observed in the survival rates of the two groups of mice (Fig. 1B).
FIG. 1.
Vaccine efficacy of the recombinant Ag2 protein in BALB/c mice challenged by an i.p. route with 250 arthroconidia. Results are expressed as the number of CFU (mean ± standard error) in tissues at day 12 postinfection (A) and the mortality in mice at days 1 through 30 postinfection (B). The results in panel A are representative of those obtained in three separate experiments with groups of 13 or more mice immunized with the Ag2-GST protein or GST alone.
Induction of immune response in mice immunized with the recombinant Ag2 protein.
Resistance to C. immitis correlates strongly with DTH to coccidioidal antigens (2, 10, 21, 40). To determine if immunization with the Ag2-GST vaccine induces a DTH response to native Ag2, mice were immunized with Ag2-GST or GST alone and then footpad tested with C-ASWS 2 weeks after the third immunization. The Ag2-GST-immunized group showed a mean footpad response of (7.60 ± 1.9) × 10−2 mm, which was not statistically significant when compared to the footpad response of mice immunized with GST alone ([5.76 ± 1.7] × 10−2 mm). Nor were differences detected in the Ag2-GST mice and GST controls when footpad tests were performed with the recombinant fusion protein (data not shown).
Vaccine efficacy of Ag2 cDNA.
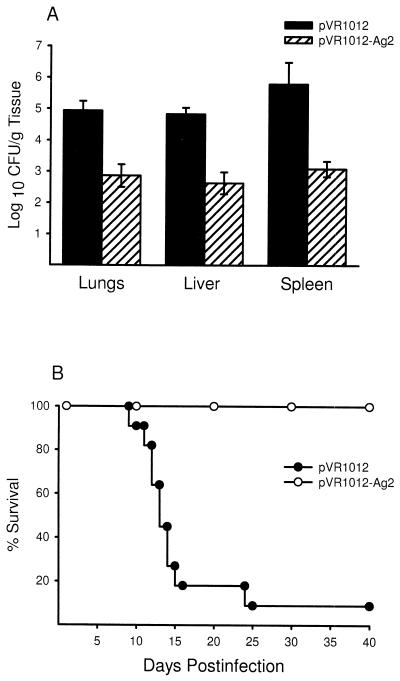
On the basis of the low level of protection obtained with the recombinant protein and the lack of induction of delayed-type footpad hypersensitivity immune response in Ag2-GST-vaccinated mice, we reasoned that the E. coli-expressed recombinant protein may lack a posttranslational modification(s) required for the induction of protective immunity. Since genetic immunization would lead to the production of the native antigen (29, 42), we redirected our studies towards examining the protective effects of the cloned Ag2 cDNA. Mice were given three weekly i.m. immunizations of the pVR1012-Ag2 plasmid or the pVR1012 plasmid alone and then challenged 2 weeks later with 2,500 arthroconidia via an i.p. route. As shown in Fig. 2A, recipients of the gene vaccine showed significant decreases in the numbers of C. immitis CFU in the lungs (P < 0.0001), livers (P < 0.0001), and spleens (P < 0.0001) compared to mice given the pVR1012 plasmid alone. Genetic immunization also effected an increase in the survival rate (Fig. 2B). Whereas only 1 (9%) of 11 mice given the plasmid alone survived a 40-day period postinfection, all 11 of the pVR1012-Ag2 vaccinated group survived this time period (P < 0.001). The foregoing experiment established that the gene vaccine effected a reduction in the fungal load in tissues and increased the survival of mice challenged with a 10-fold greater dose of arthroconidia than that used in mice immunized with recombinant Ag2.
FIG. 2.
Vaccine efficacy of the pVR1012-Ag2 construct in BALB/c mice challenged by an i.p. route with 2,500 arthroconidia, as measured by CFU in tissues from groups of 20 mice immunized with pVR1012-Ag2 or pVR1012 alone (A) and mortality at days 1 through 40 postinfection in groups of 11 mice immunized with the Ag2 gene or the pVR1012 plasmid alone (B). The results shown in panel A are representative of those obtained in three separate experiments.
Pulmonary challenge is the natural route of infection with C. immitis, and investigators have documented that pulmonary infection is a more rigorous route of challenge with this fungal pathogen (32). Experiments were conducted, therefore, to assess the capacity of the gene vaccine to protect mice against pulmonary challenge. Since FKS are considered to be the “gold standard” for vaccinating mice against C. immitis (37), the efficacy of the Ag2 gene vaccine was compared with that of FKS. The results are shown in Fig. 3. The FKS vaccine effected a significant reduction in the number of CFU units recovered from the lungs (P < 0.001), livers (P < 0.0001), and spleens (P < 0.0001) of immunized mice compared to the control mice. Genetic immunization with pVR1012-Ag2 effected a reduction in the dissemination of the fungus to the livers (P < 0.003) and spleens (P < 0.015) of mice, but did not reduce the fungus load in the lungs. To determine if mice vaccinated with the Ag2 gene vaccine were able to survive pulmonary challenge, mice were vaccinated with pVR1012-Ag2 or pVR1012 alone and monitored for survival over a 30-day period. Two (22%) of nine mice vaccinated with the pVR1012-Ag2 vaccine survived, compared to none of the nine mice vaccinated with the vector alone (P > 0.05). These results are in agreement with the finding that the gene vaccine did not effect a reduction in the fungal load in the lungs; that is, we have consistently observed a direct correlation between increased survival and ability to control the fungal disease at the lung level.
FIG. 3.
Vaccine efficacy of the pVR1012-Ag2 construct in BALB/c mice challenged with 50 arthroconidia via the pulmonary route, as assessed by measuring the fungal CFU in tissues. Results depict mean ± the standard error obtained in one of two experiment with groups of 9 to 10 mice.
Induction of immune response in Ag2 gene-vaccinated mice.
The preceding results established the Ag2 gene vaccine effected a significant decrease in the number of CFU in the livers and spleens of mice challenged via the pulmonary route. To determine if protection was accompanied by the induction of a delayed-type footpad hypersensitivity response to native Ag2, the mice were footpad tested with C-ASWS 2 weeks after the third immunization. The results, shown in Fig. 4, established that the footpad responses of mice vaccinated with the pVR1012-Ag2 construct were significantly increased compared to mice immunized with the vector alone (P < 0.001).
FIG. 4.
Footpad hypersensitivity response of mice immunized with the pVR1012-Ag2 construct or the pVR1012 plasmid alone. Results depict mean ± the standard error obtained in groups of 19 mice footpad tested with C-ASWS (10 μg) 12 days after the third immunization. The results are representative of those obtained in two separate experiments.
As another measure of the induction of a Th1 response, spleen cells were collected 12 days after i.n. challenge of mice immunized with the pVR1012-Ag2 construct, pVR1012 alone, or FKS, and the cells were stimulated in vitro with C-ASWS or the mitogen ConA. At 48 h after incubation, the supernatants were assayed for IFN-γ and IL-4 by ELISA. Spleen cells from the pVR1012-Ag2-immunized mice secreted a mean level of 320 pg of IFN-γ/ml in response to 100 μg of C-ASWS (Table 1). This level was markedly increased compared to the 15 pg of IFN-γ production by splenocytes from vector control mice. Spleen cells from FKS-immunized mice secreted even higher levels of IFN-γ in response to C-ASWS, with a mean level of 1,000 pg/ml. It is also noted that splenocytes from the FKS-immunized mice produced higher levels of IFN-γ in response to stimulation with ConA. In contrast to the induction of the Th1-associated IFN-γ response, mice vaccinated with pVR1012-Ag2 or FKS did not secrete the Th2-cytokine IL-4 in response to C-ASWS. When assayed for production of IL-4 in response to ConA, spleen cells from mice vaccinated with the vector alone secreted 260 pg/ml, whereas ConA-stimulated spleen cells from pVR1012-Ag2 or FKS secreted 170 pg/ml or less.
TABLE 1.
Production of IFN-γ and IL-4 by in vitro-stimulated spleens cells from immunized micea
| Stimulant | IFN-γ and IL-4 production (pg/ml) in vaccine group:
|
|||||
|---|---|---|---|---|---|---|
| pVR1012
|
pVR1012-Ag2
|
FKS
|
||||
| IFN-γ | IL-4 | IFN-γ | IL-4 | IFN-γ | IL-4 | |
| Medium | <15b | <15 | <15 | <15 | <15 | <15 |
| ConA (2 μg) | 1,500 | 260 | 1,700 | 140 | 4,500 | 170 |
| C-ASWS (100 μg) | 15 | <15 | 320 | <15 | 1,000 | <15 |
Depicts quantity of IFN-γ and IL-4 in supernatants from splenocytes obtained from groups of 10 BALB/c mice 12 days after vaccination with pVR1012, pVR1012-Ag2, or FKS.
Denotes lower limits of assay sensitivity.
DISCUSSION
This investigation established that genetic immunization with the Ag2 gene protects the highly susceptible BALB/c mouse strain against i.p. or pulmonary challenge with C. immitis. The protective effects of the gene vaccine correlated with the acquisition of a delayed-type footpad hypersensitivity response and the production of IFN-γ. In contrast to the efficacy of the Ag2 gene vaccine, vaccination of mice with recombinant Ag2 protein induced a low level of protection and failed to induce DTH.
The direct correlation between the level of protection and the induction of Th1 responses to the Ag2 gene vaccine is concordant with the protective effects of cell-mediated immune responses in coccidioidomycosis (3–5, 14, 15, 33). Using the murine model, Beaman et al. (5) showed that resistance to C. immitis could be adoptively transferred to syngeneic mice by using splenic T cells but not B cells or serum from FKS-immunized mice. These investigators further showed that FKS-immune spleen cells secreted IFN-γ which activated macrophages in vitro to an anticoccidioidal state (3, 4). An in vivo role for IFN-γ in host defense against C. immitis was recently established by Magee and Cox (33). A comparison of IFN-γ production in the highly susceptible BALB/c mice and DBA/2 mice, which are relatively resistant to C. immitis (15), showed that IFN-γ production was decreased in the susceptible mouse strain (33). Treatment of the susceptible mouse strain with recombinant murine IFN-γ ameliorated the course of the disease (33) and led to an increased state of macrophage anticoccidioidal activity (15). Conversely, treatment of the resistant DBA/2 mouse strain with a neutralizing anti-mouse IFN-γ potentiated the severity of the disease (33). These collective results and those obtained in this investigation suggest that measurement of IFN-γ production might serve as a surrogate marker of vaccine efficacy.
The recombinant Ag2 vaccine showed a reduced efficacy in protecting mice, compared with the Ag2 gene vaccine, and was ineffective in inducing a detectable footpad hypersensitivity response. These differences could be attributable to differences in the processing and presentation of the recombinant and gene vaccines. The recombinant protein was expressed in E. coli cells and thus would not have posttranslational modifications that may be present on native Ag2. We have previously reported that the deduced amino acid sequence of the Ag2 gene suggested that the native antigen has conformational structure, possibly attributable to glycosylation, disulfide bonding, and other putative posttranslational modifications (49). Consistent with these predictions, earlier reports have shown that C-ASWS and other Ag2-enriched extracts contain 3-O-methylmannose residues (6, 9). While glycosyl residues have not been shown to bind major histocompatibility complex (MHC) molecules, they can impart conformational structure that is requisite to the binding of the peptide epitope to MHC molecules (22, 23, 24). Future studies are needed to define the structural composition of the native Ag2 and the in vivo-expressed Ag2 gene product.
Although the Ag2 gene vaccine was significantly more protective than the recombinant Ag2 protein, it was not as effective as the FKS vaccine. There are several possible explanations that could account for these results. One is that the FKS vaccine contains other immunogen(s), in addition to Ag2, which induce protective immunity. This interpretation is consistent with the multiplicity of T-cell-reactive antigens that have been isolated from C. immitis (7, 20, 26, 41, 44, 45). Another explanation would be that the FKS have an adjuvant-like effect, inducing nonspecific responses which may enhance the development of the antigen-specific response. This possibility is supported by studies showing that FKS activate normal macrophages (from nonimmune mice and healthy, skin test-negative persons) to secrete the proinflammatory cytokines tumor necrosis factor alpha, IL-1, and IL-6 (1, 8, 19, 39). These cytokines could, in turn, accelerate the influx of antigen processing cells, T cells, and other immune effector cells. Alternatively, the reduced protective effect of the Ag2 gene vaccine could be attributable to a problem of gene delivery. While the pVR1012 vector has been used for gene delivery in other diseases, we do not know if it is an effective vector system for vaccination against pulmonary challenge. Studies are needed, therefore, to evaluate the efficacy of other plasmid DNA expression vectors which would target the Ag2 gene to the lungs.
Although several T-cell-reactive antigens have been isolated from C. immitis, only four have been evaluated as potential vaccines. Pappagianas et al. (37) reported that extraction of spherule cell walls with PBS (containing 2% chloroform as preservative) yielded a wall fraction that protected mice against pulmonary challenge when administered in alum adjuvant. Zimmermann et al. (50) recently isolated a 27-kDa subcellular fraction from mechanically disrupted spherules which induced protection against pulmonary challenge when given in alum adjuvant. The subcellular fraction was heterogeneous, as evidenced by the multiple bands on immunoblots and sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Kirkland et al. (26) cloned a gene that encodes a 48-kDa T-cell-reactive cytoplasmic protein which afforded a modest but significant level of protection against i.p. challenge. More recently, Kirkland and coworkers (25) reported that a recombinant proline-rich antigen (PRA), expressed as a pET fusion protein in E. coli, protected BALB/c mice against i.p. challenge with 50 arthroconidia when administered with complete Freund adjuvant. The investigators did not evaluate the protective effect, if any, of the vaccine against pulmonary challenge. However, given that the gene that encodes the PRA (20) is identical to that which we reported for Ag2 (48, 49), it seems improbable that the E. coli-expressed recombinant PRA protein engenders protection against pulmonary challenge.
DNA vaccination represents a novel strategy for efficient generation of CD4+ Th1 cells, CD8+ cytotoxic T cells, and humoral immune responses (17, 42). Cumulative studies have shown that gene vaccination has been applied successfully in experimental models of viral, bacterial, and protozoan infections (17, 18, 29, 34, 43, 47). To our knowledge, this is the first study to show that genetic vaccination with a C. immitis gene will induce protective immunity against challenge with this fungus. The results are extremely encouraging and offer real promise that Ag2 cDNA, alone or in combination with DNA fragments encoding Th1-associated cytokines or other C. immitis antigens, could be an effective vaccine against coccidioidomycosis.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grant AI21431 from the National Institute of Allergy and Infectious Disease and a grant from the California Health Care Foundation.
We are grateful to Vical, Inc., for providing the pVR1012 expression vector and to Yufan Zhu, Elizabeth Casanova, and Yiqiang Zhang for valuable assistance and advice.
REFERENCES
- 1.Ampel N. In vitro production of tumor necrosis factor-α by adherent human peripheral blood mononuclear cells incubated with killed coccidioidal arthroconidia and spherules. Cell Immunol. 1994;153:248–255. doi: 10.1006/cimm.1994.1022. [DOI] [PubMed] [Google Scholar]
- 2.Ampel N M, Bejarano G C, Salas S D, Galgiani J N. In vitro assessment of cellular immunity in human coccidioiodmycosis: relationship between dermal hypersensitivity, lymphocyte transformation, and lymphokine production by peripheral blood mononuclear cells from healthy adults. J Infect Dis. 1992;165:710–715. doi: 10.1093/infdis/165.4.710. [DOI] [PubMed] [Google Scholar]
- 3.Beaman L. Fungicidal activation of murine macrophages by recombinant gamma interferon. Infect Immun. 1987;55:2951–2955. doi: 10.1128/iai.55.12.2951-2955.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beaman L, Benjamini E, Pappagianis D. Activation of macrophages by lymphokines: enhancement of phagosome-lysosome fusion and killing of Coccidioides immitis. Infect Immun. 1983;39:1201–1207. doi: 10.1128/iai.39.3.1201-1207.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beaman L, Pappagianis D, Benjamini E. Significance of T cells in resistance to experimental murine coccidioidomycosis. Infect Immun. 1977;17:580–585. doi: 10.1128/iai.17.3.580-585.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cole G T, Chinn J W, Jr, Pope L M, Starr P. Characterization and distribution of 3-O-methylmannose in Coccidioides immitis. In: Einstein H E, Catanzaro A, editors. Proceedings of the Fourth International Conference on Coccidiodomycosis. Washington, D.C: The National Foundation for Infectious Disease; 1985. pp. 130–145. [Google Scholar]
- 7.Cole G T, Kirkland T N. Identification of antigens of Coccidioides immitis which stimulate immune T lymphocytes. Arch Med Res. 1993;24:281–291. [PubMed] [Google Scholar]
- 8.Corry D B, Ampel N M, Christian L, Locksley R M, Galgiani J N. Cytokine production by peripheral blood mononuclear cells in human coccidioidomycosis. J Infect Dis. 1996;174:440–443. doi: 10.1093/infdis/174.2.440. [DOI] [PubMed] [Google Scholar]
- 9.Cox R A. Antigenic structure of Coccidioides immitis. In: Kurstak E, Marquis G, Auger P, de Repentigny L, Montplaisir S, editors. Immunology of fungal diseases. New York, N.Y: Marcel Dekker, Inc.; 1989. pp. 133–1770. [Google Scholar]
- 10.Cox R A. Coccidioidomycosis. In: Murphy J W, Friedman H, Bendinelli M, editors. Fungal infections and immune responses. New York, N.Y: Plenum Press, Inc.; 1993. pp. 173–211. [Google Scholar]
- 11.Cox R A, Britt L A. Antigenic heterogeneity of an alkali-soluble, water-soluble cell wall extract of Coccidioides immitis. Infect Immun. 1985;50:365–369. doi: 10.1128/iai.50.2.365-369.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cox R A, Britt L A. Isolation of a coccidioidin component that reacts with immunoglobulin M precipitin antibody. Infect Immun. 1986;53:449–453. doi: 10.1128/iai.53.3.449-453.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cox R A, Brumer E, Lecara G. In vitro lymphocyte responses of coccidioidin skin-test-positive and -negative persons to coccidioidin, spherulin, and a Coccidioides immitis cell wall antigen. Infect Immun. 1977;15:751–755. doi: 10.1128/iai.15.3.751-755.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cox R A, Kennell W, Boncyk L, Murphy J W. Induction and expression of cell-mediated immune responses in inbred mice infected with Coccidioides immitis. Infect Immun. 1988;56:13–17. doi: 10.1128/iai.56.1.13-17.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cox R A, Magee D M. Protective immunity in coccidioidomycosis. Res Immunol. 1998;149:417–428. doi: 10.1016/s0923-2494(98)80765-7. [DOI] [PubMed] [Google Scholar]
- 16.Cox R A, Vivas J R. Spectrum of in vivo and in vitro immune responses in coccidioidomycosis. Cell Immunol. 1977;31:130–141. doi: 10.1016/0008-8749(77)90012-0. [DOI] [PubMed] [Google Scholar]
- 17.Davis H L. DNA-based immunization. Mol Cell Biol Hum Dis Ser. 1995;5:368–387. doi: 10.1007/978-94-011-0547-7_18. [DOI] [PubMed] [Google Scholar]
- 18.Davis H L, Whalen R G, Demeneix B A. Direct gene transfer into skeletal muscle in vivo: factors affecting efficiency of transfer and stability of expression. Hum Gene Ther. 1993;4:151–159. doi: 10.1089/hum.1993.4.2-151. [DOI] [PubMed] [Google Scholar]
- 19.Dooley D P, Cox R A, Hestilow K L, Dolan M J, Magee D M. Cytokine induction in human coccidioidomycosis. Infect Immun. 1994;62:3980–3983. doi: 10.1128/iai.62.9.3980-3983.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dugger K O, Villareal K M, Ngyuen A, Zimmermann C R, Law J H, Galgiani J N. Cloning and sequence analysis of the cDNA for a protein from Coccidioides immitis with immunogenic potential. Biochem Biophys Res Commun. 1996;218:485–489. doi: 10.1006/bbrc.1996.0086. [DOI] [PubMed] [Google Scholar]
- 21.Galgiani J N. Coccidioidomycosis. West J Med. 1993;159:153–171. [PMC free article] [PubMed] [Google Scholar]
- 22.Harding C V, Kihlberg J, Elofsson M, Magnusson G, Unanue E R. Glycopeptides bind MHC molecules and elicit specific T cell responses. J Immunol. 1993;151:2419–2424. [PubMed] [Google Scholar]
- 23.Ishioka G Y, Lamont A G, Thomson D, Bulbow N, Gaeta F C A, Sette A, Grey H M. MHC interaction and T cell recognition of carbohydrates and glycopeptides. J Immunol. 1992;148:2446–2451. [PubMed] [Google Scholar]
- 24.Jentoft N. Why are proteins O-glycosylated? Trends Biochem Sci. 1990;15:291–294. doi: 10.1016/0968-0004(90)90014-3. [DOI] [PubMed] [Google Scholar]
- 25.Kirkland T N, Finley F, Orsborn K I, Galgiani J N. Evaluation of the proline-rich antigen of Coccidioides immitis as a vaccine candidate in mice. Infect Immun. 1998;66:3519–3522. doi: 10.1128/iai.66.8.3519-3522.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kirkland T N, Thomas P W, Finley F, Cole G T. Immunogenicity of a 48-kilodalton recombinant T-cell reactive protein of Coccidioides immitis. Infect Immun. 1998;66:424–431. doi: 10.1128/iai.66.2.424-431.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kirkland T N, Zhu S W, Cruse D, Hsu L L, Seshan K R, Cole G T. Coccidioides immitis fractions which are antigenic for immune T lymphocytes. Infect Immun. 1991;59:3952–3961. doi: 10.1128/iai.59.11.3952-3961.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kong Y M, Levine H B, Smith C E. Immunogenic properties of nondisrupted and disrupted spherules of Coccidioides immitis in mice. Sabouraudia. 1963;2:131–142. [PubMed] [Google Scholar]
- 29.Kumar V, Sercarz E. Genetic vaccination: the advantages of going naked. Nat Med. 1996;2:857–859. doi: 10.1038/nm0896-857. [DOI] [PubMed] [Google Scholar]
- 30.Lecara G, Cox R A, Simpson R B. Coccidioides immitis vaccine: potential of an alkali-soluble, water-soluble cell wall antigen. Infect Immun. 1983;39:473–475. doi: 10.1128/iai.39.1.473-475.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levine H B, Cobb J M, Smith C E. Immunogenicity of spherule-endospore vaccines of Coccidioides immitis for mice. J Immunol. 1961;87:218–227. [PubMed] [Google Scholar]
- 32.Levine H B, Kong Y C M, Smith C E. Immunization of mice to Coccidioides immitis: dose, regimen and spherulation stage of killed spherule vaccines. J Immunol. 1965;94:132–142. [PubMed] [Google Scholar]
- 33.Magee D M, Cox R A. Roles of gamma interferon and interleukin-4 in genetically determined resistance to Coccidioides immitis. Infect Immun. 1995;63:3514–3519. doi: 10.1128/iai.63.9.3514-3519.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manthorpe M, Cornefert-Jensen F, Hartikka J, Felgner J, Rundell A, Margalith M, Dwarki V. Gene therapy by intramuscular injection of plasmid DNA: studies on firefly luciferase gene expression in mice. Hum Gene Ther. 1993;4:419–431. doi: 10.1089/hum.1993.4.4-419. [DOI] [PubMed] [Google Scholar]
- 35.Pappagianis D. Epidemiology of coccidioidomycosis. In: Steven D A, editor. Coccidioidomycosis. New York, N.Y: Plenum Publishing Corp.; 1980. p. 63. [Google Scholar]
- 36.Pappagianis, D. 1994. Marked increase in cases of coccidioidomycosis in California: 1991, 1992, and 1993. Clin. Infect. Dis. 19(Suppl. 1):S14–S18. [DOI] [PubMed]
- 37.Pappagianis D, Hector R, Levine H B, Collins M S. Immunization of mice against coccidioidomycosis with a subcellular vaccine. Infect Immun. 1979;25:440–445. doi: 10.1128/iai.25.1.440-445.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pappagianis D the Valley Fever Vaccine Study Group. Evaluation of the protective efficacy of the killed Coccidioides immitis spherule vaccine in humans. Am Rev Respir Dis. 1993;148:656–660. doi: 10.1164/ajrccm/148.3.656. [DOI] [PubMed] [Google Scholar]
- 39.Slagle D C, Cox R A, Kuruganti U. Induction of tumor necrosis factor alpha by spherules of Coccidioides immitis. Infect Immun. 1989;57:1916–1922. doi: 10.1128/iai.57.7.1916-1921.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stevens D A. Current concepts: coccidioidomycosis. N Engl J Med. 1995;332:1077–1082. doi: 10.1056/NEJM199504203321607. [DOI] [PubMed] [Google Scholar]
- 41.Thomas P W, Wyckoff E E, Pishko E J, Yu J J, Kirkland T N, Cole G T. The hsp60 gene of the human pathogenic fungus Coccidioides immitis encodes a T-cell reactive protein. Gene. 1997;199:83–91. doi: 10.1016/s0378-1119(97)00351-x. [DOI] [PubMed] [Google Scholar]
- 42.Torres C A, Iwasaki A, Barber B H, Robinson H L. Differential dependence on target site tissue for gene gun and intramuscular DNA immunizations. J Immunol. 1997;158:4529–4532. [PubMed] [Google Scholar]
- 43.Ulmer J B, Donnelly J J, Parker S E, Rhodes G H, Felgner P L, Dwarki V J, Gromkowski S H, Deck R R, DeWitt C M, Friedman A, Hawe L A, Leander K R, Martinez D, Perry H C, Shiver J W, Montgomery D L, Liu M A. Heterologous protection against influenza by injection of DNA encoding a virus protein. Science. 1993;259:1745–1749. doi: 10.1126/science.8456302. [DOI] [PubMed] [Google Scholar]
- 44.Ward E R, Jr, Cox R A, Schmitt J A, Jr, Huppert M, Sun S H. Delayed-type hypersensitivity responses to a cell wall fraction of the mycelial phase of Coccidioides immitis. Infect Immun. 1975;12:1093–1097. doi: 10.1128/iai.12.5.1093-1097.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wyckoff E, Pishko E J, Kirkland T N, Cole G T. Cloning and expression of a gene encoding a T-cell reactive protein from Coccidioides immitis: homology to 4-hydroxyphenylpyruvate dioxygenase and the mammalian F antigen. Gene. 1995;161:107–111. doi: 10.1016/0378-1119(95)00250-a. [DOI] [PubMed] [Google Scholar]
- 46.Zhu Y, Tryon V, Magee D M, Cox R A. Identification of a Coccidioides immitis antigen 2 domain that expresses B-cell-reactive epitopes. Infect Immun. 1997;65:3376–3380. doi: 10.1128/iai.65.8.3376-3380.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu X, Venkataprasad N, Thangaraj H S, Hill M, Singh M, Ivanyi J, Vordermeier H M. Functions and specificity of T cells following nucleic acid vaccination of mice against Mycobacterium tuberculosis. J Immunol. 1997;158:5921–5926. [PubMed] [Google Scholar]
- 48.Zhu Y, Yang C, Magee D M, Cox R A. Molecular cloning and characterization of Coccidioides immitis antigen 2 cDNA. Infect Immun. 1996;64:2695–2699. doi: 10.1128/iai.64.7.2695-2699.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu Y, Yang C, Magee D M, Cox R A. Coccidioides immitis antigen 2: analysis of gene and protein. Gene. 1996;181:121–125. doi: 10.1016/s0378-1119(96)00486-6. [DOI] [PubMed] [Google Scholar]
- 50.Zimmermann C R, Johnson S M, Martens G W, White A G, Zimmer B L, Pappagianis D. Protection against lethal murine coccidioidomycosis by a soluble vaccine from spherules. Infect Immun. 1998;66:2342–2345. doi: 10.1128/iai.66.5.2342-2345.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]