Abstract
In multiple system atrophy (MSA), the protein α-synuclein misfolds into a prion conformation that self-templates and causes progressive neurodegeneration. While many point mutations in the α-synuclein gene, SNCA, have been identified as the cause of heritable Parkinson’s disease (PD), none have been identified as causing MSA. To examine whether MSA prions can transmit disease to mice expressing wild-type (WT) human α-synuclein, we inoculated transgenic (Tg) mice denoted TgM20+/− with brain homogenates prepared from six different deceased MSA patients. All six samples transmitted CNS disease to the mice, with an average incubation period of ~ 280 days. Interestingly, TgM20+/− female mice developed disease > 60 days earlier than their male counterparts. Brains from terminal mice contained phosphorylated α-synuclein throughout the hindbrain, consistent with the distribution of α-synuclein inclusions in MSA patients. In addition, using our α-syn–YFP cell lines, we detected α-synuclein prions in brain homogenates prepared from terminal mice that retained MSA strain properties. To our knowledge, the studies described here are the first to show that MSA prions transmit neurological disease to mice expressing WT SNCA and that the rate of transmission is sex dependent. By comparison, TgM20+/− mice inoculated with WT preformed fibrils (PFFs) developed severe neurological disease in ~ 210 days and exhibited robust α-synuclein neuropathology in both limbic regions and the hindbrain. Brain homogenates from these animals exhibited biological activities that are distinct from those found in MSA-inoculated mice when tested in the α-syn–YFP cell lines. Differences between brains from MSA-inoculated and WT PFF-inoculated mice potentially argue that α-synuclein prions from MSA patients are distinct from the PFF inocula and that PFFs do not replicate MSA strain biology.
Keywords: α-Synuclein strains, α-Synuclein mouse models, Multiple system atrophy, Synucleinopathies
Introduction
Multiple system atrophy (MSA) is a progressive, invariably fatal neurodegenerative disease. While MSA is neuropathologically defined by the presence of glial cytoplasmic inclusions (GCIs) in oligodendrocytes [9, 29], the contribution of neuronal cytoplasmic inclusions to the disease has been increasingly recognized and investigated [33]. In 1998, Spillantini et al. first identified the protein α-synuclein as a major component of GCIs [29]; the previous year, α-synuclein was reported in Lewy bodies from the brains of Parkinson’s disease (PD) patients [30]. These findings linked the two disorders as synucleinopathies.
Following these discoveries, the transmissibility of human MSA brain homogenates to transgenic (Tg) mice was identified [34]. Watts et al. used Tg mice that express human α-synuclein carrying the A53T mutation, which were designated TgM83+/− mice. Brain homogenates prepared from two different MSA patient samples were intracranially inoculated into the TgM83+/− mice and were found to induce neurological disease with an incubation period of ~ 120 days. Subsequent studies testing a total of 17 homogenates prepared from 14 different MSA patients were collected from 3 different continents. Consistent with our earlier findings, all MSA samples transmitted disease to the mice [26]. This work led us to argue that α-synuclein becomes a prion in MSA patients. We subsequently demonstrated that α-synuclein prions in MSA samples are resistant to inactivation by formalin fixation, can transmit disease to mice following exposure to contaminated stainless steel wires (modeling iatrogenic transmission from surgical instruments), and can spread from the periphery into the brain following extraneural challenge in mice [36]. Moreover, MSA prions can propagate among multiple hosts, including two different Tg mouse models and cultured cells [35, 37].
While transmission of MSA prions from brains of patients was readily accomplished using Tg mice expressing mutant human α-synuclein [26], transmission to non-Tg mice has failed to induce neurological disease. Notably, species barriers in prion diseases are thought to arise from differences in the amino acid sequences of the prion protein (PrP). For example, of the 253 amino acids in PrP, the human and mouse sequences differ by 28 amino acids [31]. Similarly, among the 140 amino acids in α-synuclein, the human and mouse amino acid sequences differ at 7 residues, creating a species barrier that prevents MSA from propagating using mouse α-synuclein as substrate [20]. While these findings were used to argue that MSA is not a prion disease, they are consistent with several decades of research on Creutzfeldt–Jakob disease (CJD) prions showing that a species barrier prevents disease transmission to non-Tg mice. Efficient CJD transmission requires the use of Tg animals that express either human [32] or human–mouse chimeric PrPs that do not prevent the species barrier from interfering with PrP misfolding [13, 31]. In contrast, the Tg mouse models used for MSA studies typically express human α-synuclein with the A53T mutation. This particular mutation was first identified 25 years ago in the Contursi kindred of PD patients [25], and as a result, it is commonly used in rodent models of synucleinopathy. The absence of any mutation in the human α-synuclein gene (SNCA) causing familial MSA has raised concerns about whether the TgM83+/− mice are a relevant model for research on MSA.
In comparison with the TgM83+/− mouse model, the TgM20+/− mouse line expresses WT human α-synuclein [7]. In 2019, Dhillon et al. tested the ability of MSA patient samples to transmit disease to the TgM20+/− mice by inoculating postnatal day 0 (P0) pups with lysates prepared from MSA samples [5]. The authors noted a remarkable lack of phosphorylated α-synuclein neuropathology when brains from the mice were collected 5 months later. In contrast, MSA-inoculated TgM83+/− mice exhibited robust neuropathological lesions, leading to the conclusion that MSA prions cannot transmit to TgM20+/− mice over the same time course. More recently, Lloyd et al. inoculated adult TgM83+/− and TgM20+/− mice with brain homogenates from two MSA patient samples and assessed the presence of α-synuclein neuropathology either postmortem in TgM83+/− mice or 6 months after injection in TgM20+/− mice [18]. The MSA patient samples induced α-synuclein inclusions in the brains of both mouse models, although to a remarkably lower extent than in mice inoculated with recombinant WT preformed fibrils (PFFs). Both the PFFs and MSA patient samples induced less phosphorylated α-synuclein pathology in the TgM20+/− mice compared to the terminal TgM83+/− mice. Importantly, studies investigating the effect of the A53T mutation on α-synuclein fibrillization kinetics have shown that the mutation accelerates α-synuclein misfolding and fibrillization [3, 8, 21]. We, therefore, hypothesized that MSA transmission to TgM20+/− mice occurs over a longer incubation period than transmission to TgM83+/− mice.
Testing this hypothesis, we report for the first time that MSA prions induce neurological disease characterized by hind limb clasping, hind limb paralysis, loss of grip strength, bradykinesia, circling, ataxia, and kyphosis in mice expressing WT human α-synuclein. TgM20+/− mice inoculated with six different MSA patient samples developed neurological signs ~ 280 days postinoculation (dpi), or 9.5 months, whereas control-inoculated mice remained asymptomatic through 475 dpi. Moreover, we found that the female TgM20+/− mice developed disease more than 60 days earlier, on average, than male mice following MSA inoculation. Brains from terminal animals contained pathological deposits of α-synuclein in the hindbrain, particularly in the midbrain and pons, which is likely responsible for the progressive degeneration observed in the mice. In addition, α-synuclein prions isolated from brain homogenates prepared from symptomatic mice propagated in α-syn140–YFP cells expressing WT, A53T, and A30P,A53T α-synuclein. However, as we previously demonstrated using MSA patient samples [35], the mouse-passaged samples could not infect cells expressing full-length α-synuclein with the E46K mutation or α-synuclein with the A53T mutation truncated at residue 95. These findings show that MSA prions retain their strain properties when passaged in TgM20+/− mice. Critically, with the exception of the longer incubation period, transmission of MSA patient samples to TgM83+/− mice yields a similar neuropathological lesion profile and an identical infectivity profile in the α-syn140–YFP cells [35, 38]. These findings suggest that the A53T mutation in TgM83+/− mice accelerates α-synuclein misfolding kinetics without altering α-synuclein biology.
In contrast, TgM20+/− mice inoculated with recombinant WT PFFs developed neurological disease ~ 210 dpi, or 7 months. Brains from terminal mice contained robust neuropathological inclusions throughout the hindbrain that spread into several limbic brain regions. This difference in lesion profile suggests that the WT PFFs are composed of different α-synuclein strain(s) than those found in MSA patient samples. Consistent with this interpretation, insoluble α-synuclein aggregates isolated from TgM20+/− mice inoculated with WT PFFs were capable of propagating in cells expressing α-syn*A53T–YFP with a truncation at residue 95. These findings demonstrate that WT PFFs exhibit critical differences in biological activity compared to MSA patient samples, and, therefore, should not be used to investigate MSA strain biology.
Materials and methods
Human tissue samples
Frozen brain tissue samples from neuropathologically confirmed cases of MSA were provided by the Parkinson’s UK Brain Bank at Imperial College London and the Massachusetts Alzheimer’s Disease Research Center. We used tissue from the basal ganglia from four of the MSA patient samples and the substantia nigra and surrounding midbrain from two of the MSA patient samples. Control patient tissue was provided by Dr. Deborah Mash (Miami Brain Bank). Demographic information about samples used are included in Table S1, provided as an online resource.
Mice
Animals were maintained in an AAALAC-accredited facility in compliance with the 8th edition of the Guide for the Care and Use of Laboratory Animals. All procedures used in this study were approved by the University of California San Francisco Institutional Animal Care and Use Committee. All animals were housed under ABSL-2 conditions in an environmentally controlled room (10–15 air changes per hour) at a temperature of 22.5 ± 1.4 °C, a relative humidity of 45% ± 15%, and with a 12-h light/dark cycle. Animals had free access to a Tekland diet from Envigo (Indianapolis, IN) and tap water. Mice were group housed unless an animal’s health status necessitated individual housing. The B6;C3-Tg(Prnp-SNCA)20Vle (referred to here as TgM20) mice [7] were kindly provided by Dr. Benoit Giasson (University of Florida).
Inoculations
Fresh-frozen human tissue was used to create a 10% (wt/vol) homogenate using calcium- and magnesium-free 1 × Dulbecco’s phosphate buffered saline (DPBS) using the Omni Tissue Homogenizer (Omni International). Homogenates were diluted to 1% using 5% (wt/vol) bovine serum albumin in 1 × DPBS. Recombinant WT α-synuclein (Sigma-Aldrich) was aggregated in 1 × DPBS as previously described [37]. Recombinant WT PFFs were diluted in 1 × DPBS to a final concentration of 1 mg/mL.
Eight-week-old TgM20+/− mice were anesthetized with isoflurane prior to inoculation. Freehand inoculations were performed using 30 μL of the 1% brain homogenate or 1 mg/mL WT PFFs into the thalamus. Following inoculation, all mice were assessed twice each week for the onset of neurological signs based on standard diagnostic criteria for prion disease [2]. Uninoculated mice were euthanized at 546 days of age. Control-inoculated TgM20+/− mice were euthanized 475 dpi. MSA-inoculated animals were euthanized 500 dpi or following the onset of progressive neurological signs. Following euthanasia, the brain was removed and bisected down the midline. The left hemisphere was frozen for reporter cell assays and biochemical analysis, and the right hemisphere was fixed in formalin for neuropathological assessment.
Immunohistochemistry and neuropathology
Formalin-fixed mouse half-brains were cut into four sections prior to processing through graded alcohols, clearing with xylene, infiltrating with paraffin, and embedding. Thin sections (8 μm) were cut, collected on slides, deparaffinized, and exposed to heat-mediated antigen retrieval with citrate buffer (0.1 M; pH 6) for 20 min. Slides were stained using the Thermo Fisher 480S Autostainer with 30 min blocking in 10% (vol/vol) normal goat serum and incubating with primary and secondary antibodies (2 h each). Primary antibody binding of phosphorylated (S129) α-synuclein (EP1536Y; 1:1,000; Abcam) and glial fibrillary acidic protein (GFAP; 1:500; Abcam) were detected using secondary antibodies conjugated to AlexaFluor 488 or 647 (1:500; Thermo Fisher Scientific). Slides were imaged using the Zeiss AxioScan. Z1 and were then analyzed using the ZEN Analysis software package (Zeiss). To quantify α-synuclein neuropathology, a pixel intensity threshold was determined using a positive control slide. Regions of interest were drawn around the caudoputamen (Cd), hippocampus (HC), piriform cortex and amygdala (PC), thalamus (Thal), hypothalamus (HTH), midbrain (Mid), and pons. The percentage of positively stained pixels in each region was determined and averaged across inoculation groups.
α-Synuclein prion bioassay
Frozen brain tissue was used to make a 10% (wt/vol) brain homogenate in calcium- and magnesium-free 1 X DPBS× using the Omni Tissue Homogenizer with disposable soft tissue tips (Omni International). Aggregated protein was isolated from the homogenates using phosphotungstic acid (PTA; Sigma-Aldrich) as described [28, 39]. Isolated protein pellets were diluted 1:10 in 1 × DPBS before testing in the α-synuclein prion bioassays. HEK293T parent cell line was obtained from ATCC (line CRL-3216). Transfection to establish α-syn-YFP cell lines was done using a low passage number. HEK293T cells expressing α-syn140–YFP (WT), α-syn140*E46K–YFP (E46K), α-syn140*A53T-YFP (A53T), α-syn140*A30P,A53T–YFP (A30P,A53T), α-syn140*E46K,A53T–YFP (E46K,A53T), and α-syn95*A53T–YFP (1–95) were generated as previously described; culture and bioassay conditions were also used as previously described [35]. Cells incubated with isolated protein pellets were imaged using the IN Cell Analyzer 6000 (GE Healthcare). DAPI and FITC images from five different regions in each well were analyzed using IN Cell Developer software with an algorithm designed to quantify intracellular aggregates, represented as total fluorescence per cell (× 103, arbitrary units [A.U.]). One measurement was generated for each well across the five regions imaged, and each sample was tested in six replicate wells.
Immunoblotting
To visualize soluble α-synuclein in TgM20+/− mouse brain homogenates, samples were clarified by centrifugation for 5 min at 1000 ×g. The supernatant was collected, and total protein was measured using the bicinchoninic acid (BCA) assay (Pierce). A total of 2.5 μg total protein was prepared in 1 × NuPAGE LDS loading buffer and boiled for 10 min. Samples were loaded onto a 10% Bis–Tris gel, and SDS–PAGE was performed using the MES buffer system. Protein was transferred to a polyvinylidene fluoride (PVDF) membrane. The membrane was fixed in 0.4% formalin for 30 min at room temperature prior to incubating in blocking buffer (5% [wt/vol] nonfat milk in 1 × Tris-buffered saline containing 0.05% [vol/vol] Tween 20 [TBST]) for 30 min at room temperature. Blots were incubated with primary antibodies for phosphorylated α-synuclein (EP1535Y; 1:4000; Abcam) and the loading control vinculin (1:10,000; Abcam) in blocking buffer overnight at 4 °C in a vacuum-sealed pouch. Membranes were washed three times with 1 × TBST before incubating with horseradish peroxidase–conjugated goat anti-rabbit secondary antibody (1:10,000; Abcam) diluted in blocking buffer for 1 h at 4 °C in a vacuum-sealed pouch. After washing blots three times in 1 × TBST, membranes were developed using the enhanced chemiluminescent detection system (Pierce) for exposure to X-ray film.
To detect insoluble α-synuclein, protein aggregates were isolated via PTA precipitation, and resuspended pellets were diluted 1:5 in 1 × NuPAGE LDS loading buffer and 1 × DPBS. Samples were boiled for 20 min before loading on a 10% Bis–Tris gel. Using the protocol described above, PVDF membranes were probed for phosphorylated α-synuclein (EP1536Y; 1:4000; Abcam).
Statistical analysis
Data are presented as mean ± standard deviation. Data were analyzed using GraphPad Prism software. Kaplan–Meier curves and differences in incubation period for female and male mice were analyzed using a log-rank Mantel–Cox test. Analysis of data collected from the α-syn–YFP cell assays and neuropathology data comparing control-inoculated and PFF-inoculated samples was performed using a two-way ANOVA with a Tukey multiple comparison post hoc test. Cell assay and neuropathology data comparing control- and MSA-inoculated mice were analyzed using a two-way ANOVA with a Dunnett multiple comparison post hoc test. Neuropathology and cell assay data comparing MSA- and PFF-inoculated mice were analyzed using a two-way ANOVA with a Bonferroni multiple comparison post hoc test. Significance was determined with a P value < 0.05.
Results
Incubation times following MSA transmission to TgM20+/− mice are sex dependent
Initial studies using the TgM20 mouse model reported that neither the hemizygous nor the homozygous animals developed spontaneous neurological disease [5-7, 18]. To confirm this in our TgM20+/− colony, we assessed hemizygous mice for neurological signs twice each week through 546 days (78 weeks) of age and found no indication of spontaneous synucleinopathy (Supplementary Fig. S1a, online resource). We dissected brains from the 546-day-old TgM20+/− mice, flash-freezing one half (cut sagittally) and fixing the other half in formalin. We homogenized the frozen brain tissue and isolated protein aggregates by PTA precipitation. We next tested the resulting pellets for the presence of α-synuclein prions using our α-syn–YFP prion bioassays [35, 39]. We found that none of the samples propagated in any of the cell lines tested (Supplementary Fig. S1b, online resource). Immunostaining of the fixed half-brain for phosphorylated α-synuclein (EP1536Y primary antibody) showed an absence of α-synuclein prion inclusions in the brains of the aged mice (Supplementary Fig. S1c, online resource). Together, these results confirmed that the TgM20+/− mice do not develop spontaneous α-synuclein prion disease, eliminating a possible confounding factor in our studies.
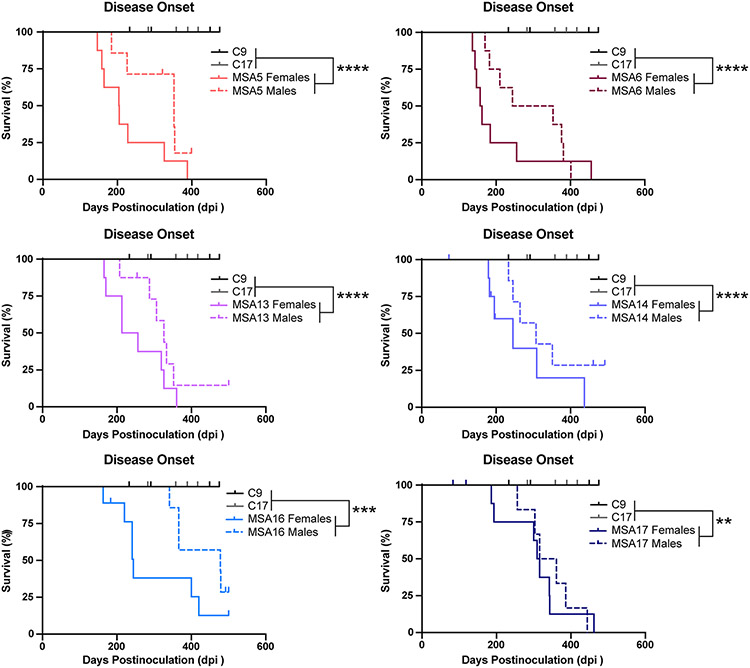
Recent studies by others [18] demonstrated that TgM20+/− mice develop α-synuclein neuropathology 6 months after inoculation with MSA prions from patient samples. To our knowledge, the ability of MSA samples to induce neurological disease in TgM20+/− mice has not been reported. To determine if MSA prions can transmit disease to TgM20+/− mice, 8-week-old animals were inoculated with 30 μL of 1% brain homogenate prepared from either two control patient samples or six different MSA patient samples (Fig. 1; Table 1). While the control-inoculated mice remained healthy through 475 days postinoculation (dpi), MSA-inoculated animals developed progressive neurological signs 281 ± 93 dpi (P < 0.001), indicating that MSA transmission to Tg mice requires expression of the human α-synuclein protein. Notably, this average incubation period is more than twice the length observed in the TgM83+/− mouse model (~ 120 dpi) [26]. While the TgM20+/− mice actually express more human α-synuclein than the TgM83+/− line [7], the A53T mutation is known to accelerate α-synuclein misfolding [3, 8, 21], which likely explains the decreased incubation periods observed in the inoculated TgM83+/− mice.
Fig. 1.
MSA patient samples transmit neurological disease to TgM20+/− mice. Eight-week-old TgM20+/− mice were inoculated with either 30 μL of two control patient samples (C9 and C17) or six MSA patient samples (MSA5, MSA6, MSA13, MSA14, MSA16, and MSA17). Kaplan–Meier plots show disease onset in female (solid line) and male (dotted line) TgM20+/− mice. Control-inoculated mice did not develop disease by 475 days postinoculation (dpi), but mice inoculated with all six MSA samples developed progressive neurological signs. Incubation times shown in Table S2, online resource (** = P < 0.01; *** = P < 0.001; **** = P < 0.0001). Female mice inoculated with MSA patient samples developed neurological disease ~ 63 days earlier than male mice (P = 0.0005)
Table 1.
Incubation period and attack rates
| Sample | All mice |
Female mice |
Male mice |
P value | |||
|---|---|---|---|---|---|---|---|
| Incubation period Avg ± SD (dpia) |
n/n0b | Incubation period Avg ± SD (dpia) |
n/n0b | Incubation period Avg ± SD (dpia) |
n/n0b | ||
| C9 | > 475 | 0/8 | n.d. | n.d. | |||
| C17 | > 475 | 0/8 | n.d. | n.d. | |||
| WT PFFs | 210 ± 37 | 7/7 | n.d. | n.d. | |||
| MSA5 | 263 ± 93 | 14/15 | 227 ± 86 | 8/8 | 311 ± 85 | 6/7 | 0.08 |
| MSA6 | 247 ± 109 | 16/16 | 205 ±108 | 8/8 | 290 ± 98 | 8/8 | 0.31 |
| MSA13 | 289 ± 88 | 14/16 | 253 ± 75 | 8/8 | 302 ± 52 | 6/8 | 0.18 |
| MSA14 | 287 ± 99 | 11/15 | 258 ± 101 | 6/7 | 280 ± 49 | 5/8 | 0.20 |
| MSA16 | 363 ± 117 | 12/16 | 275 ± 96 | 7/9 | 406 ± 67 | 5/7 | 0.13 |
| MSA17 | 323 ± 79 | 14/16 | 306 ± 88 | 8/8 | 344 ± 67 | 6/8 | 0.65 |
| MSA (all) | 281 ± 93 | 81/94 | 254 ± 93 | 45/48 | 317 ± 80 | 36/46 | 0.0005 |
n.d. not done
Days postinfection
Number of symptomatic animals (n)/number of mice inoculated (n0)
While MSA appears to afflict roughly an equal number of men and women, recent work has shown that median survival for males is significantly shorter than for females. Survival from diagnosis is almost 1 year shorter for males compared to females [4]. To determine if similar sex-specific effects were present in our experiments, we compared the onset of neurological signs in female and male TgM20+/− mice inoculated with each MSA patient sample (Fig. 1; Table 1). Notably, we found that the female mice developed disease faster than the male mice; however, our studies were too underpowered to support statistical analysis within an inoculation group. When we compared the difference in incubation period across mice inoculated with all 6 MSA patient samples, we found females developed disease > 60 days before the males (Females: 254 ± 93 dpi with 45 of 48 mice becoming symptomatic; Males: 317 ± 80 dpi with 36 of 46 mice becoming symptomatic; P = 0.0005). Importantly, both female and male mice developed the same neurological signs, despite this difference in incubation period. Efforts to determine why female TgM20+/− mice develop disease earlier than male animals will be the focus of future studies.
Symptomatic MSA-inoculated TgM20+/ mice develop pathogenic α-synuclein inclusions
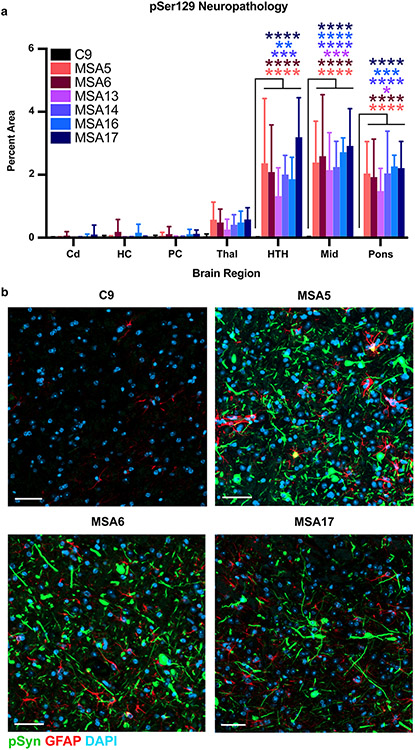
To determine the neurological changes driving the onset of disease in symptomatic MSA-inoculated TgM20+/− mice, we first analyzed fixed half-brains for the presence of phosphorylated α-synuclein inclusions (Fig. 2). The MSA patient samples induced significant neuropathology in the hypothalamus, midbrain, and pons (P < 0.05), but the changes in the thalamus were not significant (Fig. 2a). By comparison, control-inoculated mice did not develop α-synuclein neuropathology. Consistent with the use of the Prnp promoter to express the SNCA transgene, TgM20+/− mice appear to primarily develop neuronal inclusions that fill both the neurite and cell body. Representative images of immunostaining in the pons from mice inoculated with C9, MSA5, MSA6, and MSA17 patient samples also indicate an increase in GFAP immunostaining (astrogliosis) around the α-synuclein lesions in MSA-inoculated animals (Fig. 2b). The distribution of pathological lesions in these brain regions is consistent with the onset of overt motor signs that include hind limb clasping, difficulty rearing, weak grip strength, circling, and bradykinesia.
Fig. 2.
MSA patient samples induce phosphorylated α-synuclein neuropathology in terminal TgM20+/− mice. Eight-week-old TgM20+/− mice were inoculated with 30 μL of C9 control sample or six MSA patient samples (MSA5, MSA6, MSA13, MSA14, MSA16, and MSA17). a Quantification of stained brain slices showed no phosphorylated α-synuclein inclusions were present in the caudate (Cd), hippocampus (HC), piriform cortex and amygdala (PC), thalamus (Thal), hypothalamus (HTH), midbrain (Mid), or pons of C9-inoculated mice. However, the MSA patient samples induced neuropathological lesions in the HTH, Mid, and pons (* = P < 0.05; ** = P < 0.01; *** = P < 0.001; **** = P < 0.0001). b Representative images of the Mid from TgM20+/− mice inoculated with C9, MSA5, MSA6, or MSA17 brain homogenate. Phosphorylated α-synuclein in green, GFAP in red, and DAPI in blue. Scale bar: 50 μm
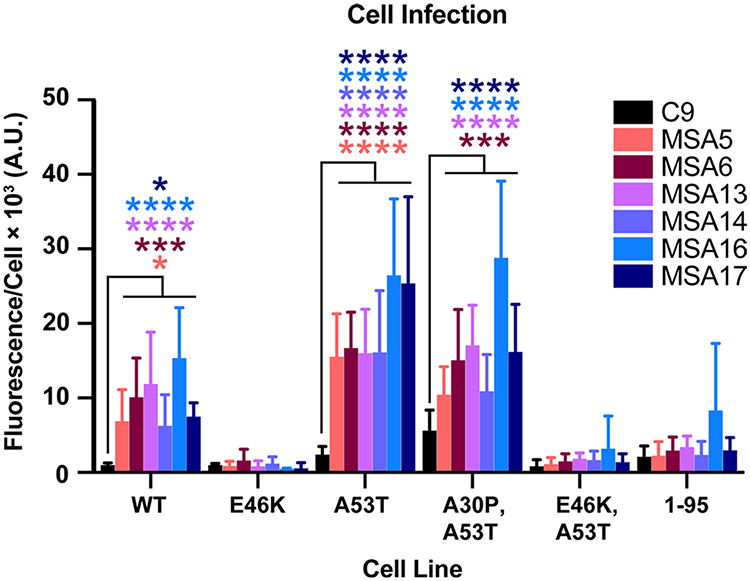
Using our cultured cell bioassay for α-synuclein prions, we measured the levels of MSA prions in the brains of inoculated TgM20+/− mice (Fig. 3). Consistent with our previous findings [35], mouse-passaged MSA samples infected α-syn–YFP cells expressing WT, A53T, or the double mutation A30P and A53T (P < 0.05 for MSA samples compared to C9). Unsurprisingly, the human MSA prions could not propagate in cells expressing the E46K single mutation or the E46K and A53T double mutation. Notably, α-synuclein prions could not propagate in cells expressing α-syn*A53T truncated at residue 95 (P > 0.05; Fig. 3) either. These findings demonstrate that MSA α-synuclein prions retain their strain-specific properties during passaging in the TgM20+/− mice, yielding similar findings to our TgM83+/− transmission studies [35, 37]. We confirmed the presence of pathogenic α-synuclein aggregates in the terminal mouse brains by probing for detergent-insoluble phosphorylated α-synuclein using Western blots (Supplementary Fig. S2a, online resource). Control-inoculated mouse samples lacked insoluble α-synuclein, while phosphorylated insoluble α-synuclein was present in MSA-inoculated samples (top blots). All mouse samples contained soluble phosphorylated α-synuclein (middle blots). Together, these results show that MSA prions transmit neurological disease and pathological inclusions to mice expressing WT human α-synuclein.
Fig. 3.
MSA patient samples transmit α-synuclein prions to TgM20+/− mice. Eight-week-old TgM20+/− mice were inoculated with 30 μL of control patient sample (C9) or six different MSA patient samples (MSA5, MSA6, MSA13, MSA14, MSA16, and MSA17). Brain homogenates from inoculated TgM20+/− mice were assessed for α-synuclein prion transmission using our cell-based assay [35, 39]. α-Synuclein prions were isolated via phosphotungstic acid precipitation and were incubated with α-syn–YFP cells for 4 d. The cell lines tested express wild-type (WT) α-syn–YFP, single mutations (E46K and A53T), double mutations (A30P,A53T and E46K,A53T), or A53T truncated at residue 95 (1–95). None of the homogenates from C9-inoculated TgM20+/− mice infected the cells, but MSA-inoculated mouse samples infected WT, A53T, and A30P,A53T cells (* = P < 0.05; *** = P < 0.001; **** = P < 0.0001)
TgM20+/− mice inoculated with WT preformed fibrils (PFFs) develop artificial α-synuclein prions with biological activities distinct from MSA prions
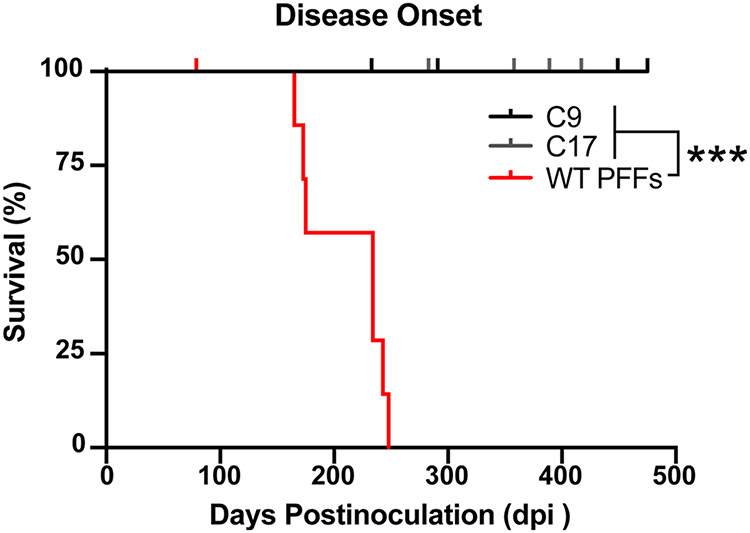
Using our α-syn–YFP cell-based assay, we previously showed that WT PFFs exhibit a distinct biological activity compared to the α-synuclein prions isolated from MSA patient samples [35]. To determine if similar strain differences could be detected using mouse bioassay in TgM20+/− animals, we tested the ability of WT PFFs to induce neurological disease in the mice. TgM20+/− mice inoculated with 30 μL of 1% brain homogenate from two control patient samples (C9 and C17) remained asymptomatic through 475 dpi (Fig. 4). However, mice inoculated with 30 μg of WT PFFs developed progressive neurological signs 210 ± 37 dpi (P < 0.001). This incubation period is notably shorter than the ~ 280 days following MSA inoculation; however, it is important to note that this difference may be due to differences in the titer of the inoculum and not the strain.
Fig. 4.
Recombinant WT PFFs induce neurological disease in TgM20+/− mice. Eight-week-old TgM20+/− mice were inoculated with either control patient samples (C9 and C17) or recombinant WT PFFs. Kaplan–Meier plot of disease onset in TgM20+/− mice shows control-inoculated mice did not develop disease by 475 days postinoculation (dpi), but WT PFF-inoculated mice developed overt motor deficits 210 ± 37 dpi (*** = P < 0.001)
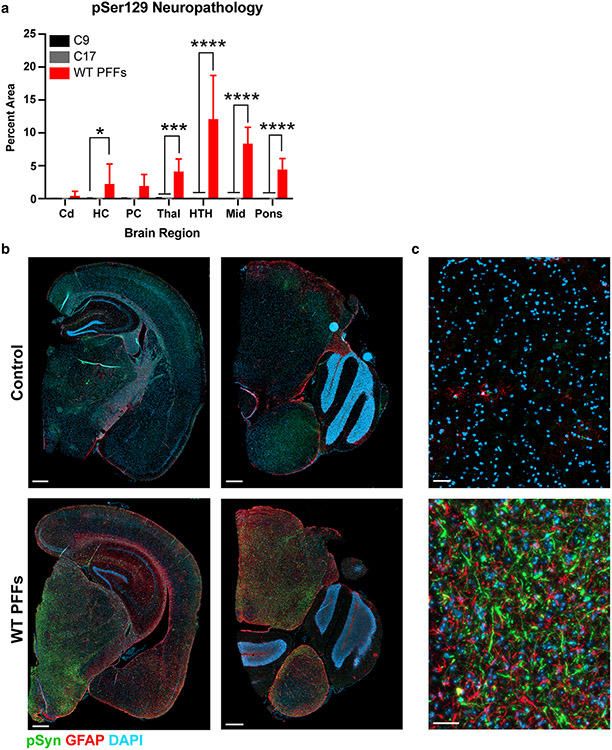
Fixed half-brains from the control-inoculated and WT PFF-inoculated TgM20+/− mice were immunostained for phosphorylated α-synuclein and GFAP (Fig. 5). WT PFFs induced robust and widespread neuropathology (Fig. 5a), with significant accumulation in the hippocampus (P < 0.05), thalamus (P < 0.001), hypothalamus (P < 0.0001), midbrain (P < 0.0001), and pons (P < 0.0001). While α-synuclein pathology was observed in the piriform cortex and amygdala of some WT PFF-inoculated mice, it was not present in all and was, therefore, non-significant (P = 0.1). We also observed an increase in GFAP staining around these inclusions compared to control-inoculated animals, indicating that the α-synuclein inclusions coincide with astrogliosis in the mice (Fig. 5b, c).
Fig. 5.
WT PFFs induce robust phosphorylated α-synuclein pathology in terminal TgM20+/− mice. Eight-week-old TgM20+/− mice were inoculated with either 30 μL of two control patient samples (C9 and C17) or 30 μg of WT PFFs. a Quantification of stained brain slices showed no phosphorylated α-synuclein inclusions were present in the caudate (Cd), hippocampus (HC), piriform cortex and amygdala (PC), thalamus (Thal), hypothalamus (HTH), midbrain (Mid), or pons of control-inoculated mice. However, WT PFFs induced robust neuropathological lesions in the HC, Thal, HTH, Mid, and pons (* = P < 0.05; *** = P < 0.001; **** = P < 0.0001). b, c Representative images from either C9-inoculated (top row) or WT PFF-inoculated mice (bottom row). Phosphorylated α-synuclein in green, GFAP in red, and DAPI in blue. b Images on the left include the HC, PC, Thal, and HTH. Images on the right include the pons and a portion of the cerebellum. Scale bar: 500 μm. c Higher magnification of Mid α-synuclein neuropathology. Scale bar: 50 μm
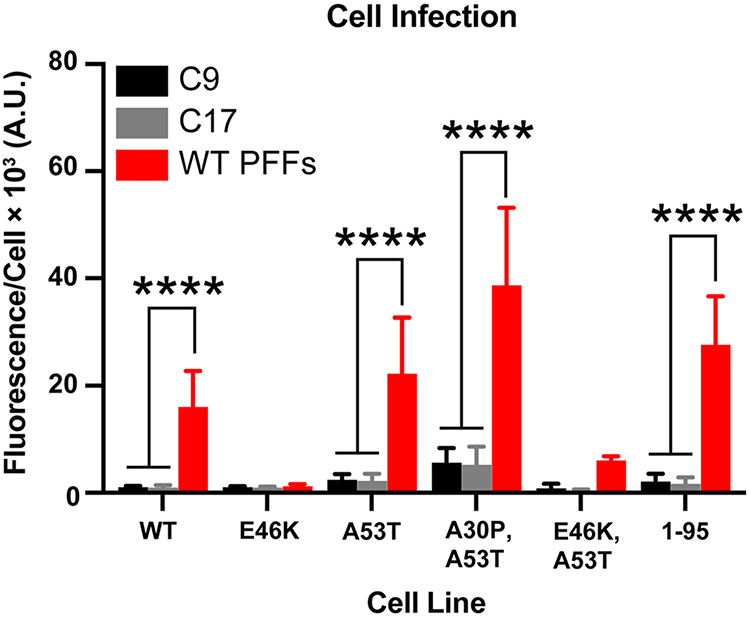
Next, we assayed frozen half-brains collected from the control- and PFF-inoculated mice in the α-syn–YFP cell lines for the presence of pathogenic α-synuclein (Fig. 6). The α-synuclein prions isolated from the PFF-inoculated animals infected cells expressing WT, A53T, and A30P,A53T α-synuclein as well as α-syn*A53T truncated at residue 95 (P < 0.0001). Interestingly, we previously reported that WT PFFs propagate in cell lines expressing E46K and E46K,A53T α-synuclein; however, their ability to do so after passaging in TgM20+/− mice is diminished compared to our other cell lines [35]. Here, we find that WT PFF conformations that cannot use E46K α-synuclein as substrate selectively propagated in TgM20+/− mice. In addition to cell infectivity studies, we also analyzed the frozen brain samples for the presence of insoluble phosphorylated α-synuclein (Supplementary Fig. S2b, online resource). Samples from two control-inoculated animals did not contain insoluble α-synuclein, whereas samples from three PFF-inoculated mice did (top blot; EP1536Y primary antibody). In comparison, all five samples contained soluble phosphorylated α-synuclein (middle blot).
Fig. 6.
WT PFFs induce α-synuclein prion formation in TgM20+/− mice. Eight-week-old TgM20+/− mice were inoculated with either control patient samples (C9 and C17) or recombinant WT PFFs. Brain homogenates were assayed for α-synuclein prion transmission using our cell-based assay [35, 39]. α-Synuclein prions were isolated from brain homogenates via phosphotungstic acid precipitation and were incubated with α-syn–YFP cells for 4 d. The cell lines tested express wild-type (WT) α-syn–YFP, single mutations (E46K and A53T), double mutations (A30P,A53T and E46K,A53T), or A53T truncated at residue 95 (1–95). None of the homogenates from control-inoculated TgM20+/− mice infected the cells, but WT PFF-inoculated mouse samples infected WT, A53T, A30P,A53T, and 1–95 cells (**** = P < 0.0001)
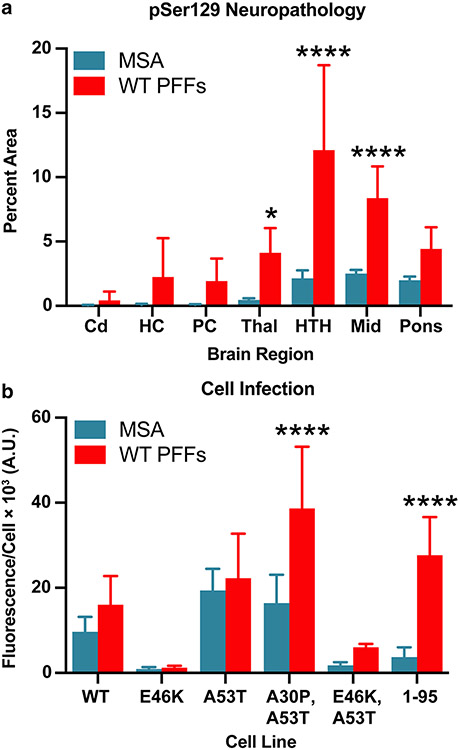
To determine if we could resolve strain differences between MSA-inoculated and WT PFF-inoculated TgM20+/− mice, we compared the distribution of phosphorylated α-synuclein inclusions between the two groups of mice (Fig. 7a). Here, we compared the average measurement made across all MSA-inoculated mice with that of the WT PFF-injected animals. Unlike the MSA inocula, WT PFFs induced α-synuclein inclusions in the hippocampus and piriform cortex and amygdala, but this change was not significantly different. We did detect a significant increase in the intensity of α-synuclein staining in the thalamus (P < 0.05), hypothalamus (P < 0.0001), and midbrain (P < 0.0001). In addition, when we compared cell infectivity data between MSA- and PFF-inoculated mice, we found that brain samples from PFF-inoculated mice propagated in the α-syn95*A53T–YFP cells, while MSA-inoculated mouse samples did not (Fig. 7b; P < 0.0001). Combined, these findings indicate that while WT PFFs are capable of inducing the formation of pathogenic α-synuclein prions in TgM20+/− mice, the strain differences between MSA prions and WT PFFs result in distinct distributions of neuropathological inclusions and biological activities in a cell-based assay. We must, therefore, conclude that WT PFFs should not be used as an alternative to MSA patient samples to investigate MSA disease pathogenesis.
Fig. 7.
MSA and WT PFFs contain distinct α-synuclein prion strains. Eight-week-old TgM20+/− mice were inoculated with either MSA patient samples or recombinant WT PFFs. Measurements from MSA-inoculated mice were averaged across experiments testing all 6 MSA patient samples. a Quantification of stained brain slices showed a significant increase in phosphorylated α-synuclein lesions in the thalamus (Thal), hypothalamus (HTH), and midbrain (Mid). Cd caudate, HC hippocampus, and PC piriform cortex and amygdala (* = P < 0.05; **** = P < 0.0001). b Brain homogenates were assayed for α-synuclein prion transmission using our cell-based assay [35, 39]. α-Synuclein prions were isolated from brain homogenates via phosphotungstic acid precipitation and were incubated with α-syn–YFP cells for 4 d. The cell lines tested express wild-type (WT) α-syn–YFP, single mutations (E46K and A53T), double mutations (A30P,A53T and E46K,A53T), or A53T truncated at residue 95 (1–95). WT PFF-inoculated mouse samples showed greater infectivity in the A30P,A53T and 1–95 cell lines than the MSA-inoculated samples (**** = P < 0.0001)
Discussion
Over the past decade, we and others found that α-synuclein prions in MSA patient samples propagate in two different synucleinopathy mouse models expressing human α-synuclein with the A53T mutation [1, 5, 15, 26, 34-39]. These studies have been criticized for using animal models harboring a PD-causing SNCA mutation, because no SNCA mutations have been identified in MSA patients. That MSA patient samples cannot induce neurological signs in mice expressing endogenous WT α-synuclein has been used as an argument against recognizing MSA as a prion disease. Here, for the first time, we show that six different MSA patient samples collected from two different continents transmit neurological disease and induce α-synuclein prion formation in mice expressing WT human α-synuclein.
Initial MSA transmission experiments relied on the TgM83+/− mouse model, which uses the Prnp promoter to express human α-synuclein with the A53T mutation [7]. Because the Prnp promoter limits α-synuclein expression to neurons in the mouse brain, terminal mice in these experiments developed neuronal inclusions. Though these neuronal α-synuclein deposits differ from the GCIs in oligodendrocytes [24], they are consistent with the presence of neuronal cytoplasmic inclusions in MSA patient samples [33]. With the goal of inducing glial pathology upon MSA transmission, we inoculated Tg(SNCA*A53T+/+)Nbm mice with MSA patient samples [37]. The Tg(SNCA*A53T+/+) Nbm line was generated using a P1 artificial chromosome containing the full human SNCA gene with its normal intron and exon structure as well as 35 kb of upstream regulatory elements [14]. As a result, transgene expression in these mice is much more widespread than in the TgM83+/− animals. While MSA patient samples did not induce neurological signs in any of the inoculated Tg(SNCA*A53T+/+) Nbm mice due to the lack of hindbrain pathology, they did propagate and form both neuronal and glial inclusions in the limbic system [37]. Given that MSA pathology in human patients is located in both glial and neuronal cells [11, 12, 23, 24], these findings were important for demonstrating that we can replicate the neuropathological hallmarks of MSA in vivo using this particular model. Moreover, when we used the MSA-inoculated Tg(SNCA*A53T+/+)Nbm mouse brain samples for second passage experiments in TgM83+/− mice, we found that MSA prions can propagate between hosts while faithfully maintaining strain properties, as determined using our α-syn–YFP cell lines [37].
Having established that we can replicate MSA pathology in vivo, we next sought to determine if MSA patient samples can transmit disease to mice expressing WT human α-synuclein. We chose to use the TgM20+/− line for these experiments because it was generated using the same promoter and methods as the TgM83+/− mice [7]. While we cannot perfectly control for differences in transgene expression or incorporation, the TgM20+/− line is the closest matched comparison to the TgM83+/− mouse model, allowing us to test the effect of host genotype on MSA α-synuclein prion transmission. Using a similar rationale, Dhillon et al. reported in 2019 that MSA inoculations in P0 TgM20+/− mouse pups failed to induce α-synuclein neuropathology within 5 months of inoculation [5]. The authors selected the 5-month timepoint based on the average incubation periods reported for the TgM83+/− mice (~ 120 days). However, because the A53T mutation accelerates protein misfolding kinetics [3, 8, 21], we hypothesized that MSA transmission to TgM20+/− mice would require a longer incubation period. Consistent with this prediction, Lloyd et al. recently reported the presence of α-synuclein neuropathology in adult TgM20+/− mice terminated 6 months after inoculation with MSA patient samples [18]. However, none of the mice in these studies developed neurological signs.
In the studies reported here, we found that MSA patient samples transmit neurological disease to TgM20+/− mice with an average incubation period of ~ 280 days, or roughly 9.5 months (Fig. 1; Table 1). This extended incubation period is beyond the scope of previous studies and more than double the incubation time observed in TgM83+/− mice, indicating that the misfolding kinetics for WT α-synuclein are much slower than the A53T mutant. In addition, we observed that MSA inoculations in female TgM20+/− mice induce disease > 60 days faster than male mice inoculated with the same patient samples. While the mechanism responsible for these sex differences is currently unclear, it is important that future studies using these models are well-powered to account for sex as a biological variable. It is also quite likely that by investigating the molecular mechanisms that drive these differences in incubation period, we will identify disease-modifying factors that may ultimately become therapeutic targets for patients living with MSA.
Analyzing brain samples collected from the terminal TgM20+/− mice, we detected the presence of pathogenic α-synuclein prions using three different methods: immunostaining for phosphorylated α-synuclein inclusions, α-syn–YFP cell assays to test prion propagation, and Western blots to detect detergent-insoluble phosphorylated α-synuclein. Notably, the distribution of neuropathology in the midbrain and brainstem (Fig. 2) is consistent with the regional distribution of GCIs in MSA patients, as well as in TgM83+/− mice inoculated with MSA patient samples. The selective ability of the mouse-passaged MSA samples to propagate in some α-syn–YFP cell lines, but not those expressing the E46K mutation or a truncation at residue 95 (Fig. 3), is consistent with the biological strain profile we previously reported for MSA prions [35]. These results indicate that MSA prions retain their strain properties following transmission to TgM20+/− mice. Moreover, when comparing data reported here with our previous studies transmitting MSA to TgM83+/− mice, the only measurable effect of the A53T mutation is the decrease in the incubation period following inoculation. These findings suggest that even though the A53T mutation is not present in MSA patients, animal and cell models expressing this particular mutation can and should be used to accelerate research on α-synuclein misfolding and spreading in MSA without altering strain properties. The finding that incubation times in the TgM20+/− model are more than double those of the TgM83+/− model highlights the importance of mutant Tg mouse models to the acceleration of MSA research.
Bolstering this interpretation, we detected strain differences in TgM20+/− mice inoculated with WT PFFs compared to those inoculated with MSA patient samples. First, while the MSA patient samples yielded an average incubation period of 281 ± 93 days, the average incubation period for WT PFF-inoculated mice was 210 ± 37 dpi (Fig. 4). It is important to note, though, that this difference in incubation period may be a reflection of differences in titer rather than strain. Second, we found that the WT PFF-induced α-synuclein neuropathology spanned additional brain regions from those observed in either TgM20+/− or TgM83+/− mice following MSA inoculation (Fig. 7). As mentioned previously, MSA transmission to TgM20+/− and TgM83+/− mice induces phosphorylated α-synuclein inclusions that are limited to hindbrain areas, with the most predominant inclusions in the midbrain and pons. However, the WT PFF inoculations reported here induced α-synuclein pathology in the hippocampus and piriform cortex and amygdala in addition to the hindbrain regions (Fig. 5). The density of staining was also substantially greater than that measured following MSA inoculation (comparing percent area measurements in Fig. 7). Finally, brain samples from mice inoculated with the two strains exhibited distinct biological activities when assayed in the α-syn–YFP cells. Unlike the MSA patient samples, the mouse-passaged WT PFFs were able to replicate in α-syn95*A53T–YFP cells (Fig. 6), consistent with our previous findings [35]. However, these samples could not replicate in cells expressing the E46K mutation. Several cryo-electron microscopy studies have shown that many WT fibril structures contain a salt bridge between residues E46 and K80, which stabilizes α-synuclein misfolding into a Greek key conformation [10, 16, 17, 22]. As a result, the E46K mutation would disrupt this misfolded structure, preventing the WT fibrils from being able to propagate using E46K α-synuclein as a substrate. While we observed this phenomenon using an in vitro model here, Long et al. obtained similar results using a cell-free cross-seeding approach [19]. Altogether, these differences in incubation period, neuropathological distribution, and α-syn–YFP cell assay infectivity demonstrate that the recombinant WT PFFs and MSA patient samples contain distinct α-synuclein strains. Future studies should investigate the effect of strain titer on these findings.
The studies reported here include pivotal discoveries for the synucleinopathy field for two reasons. First, while non-Tg mice are resistant to MSA transmission [26], this is the first demonstration that MSA prions transmit neurological disease to mice expressing WT human α-synuclein. This is consistent with results from the PrP prion literature showing that, to overcome the human–mouse species barrier, transmission of CJD prions to mice requires the expression of either human PrP or a human–mouse chimeric PrP sequence [13, 31, 32]. These important results add to the known similarities between MSA and CJD prions. Second, these studies identify the third Tg mouse model capable of propagating multiple α-synuclein prion strains, including those found in MSA patient samples. The ability to transmit α-synuclein prion strains to both the TgM20+/− and the TgM83+/− mouse models reveals a unique opportunity to investigate the effect of host genotype on disease pathogenesis. Until now, transmission models relied on either the TgM83+/− or the Tg(SNCA*A53T+/+)Nbm mouse models, both of which harbor the A53T mutation. However, while the A53T mutation is the most common SNCA mutation [27], the vast majority of synucleinopathy patients have WT SNCA genes. Future experiments enabled by the discoveries reported here will allow us to determine how disease pathogenesis differs in patients with and without familial SNCA mutations.
Supplementary Material
Acknowledgements
We thank Dr. Benoit Giasson (University of Florida) for kindly providing the TgM20 mice, Jeffrey Lau for his assistance with experiments, and the Hunters Point animal facility staff for breeding and caring for the animals used in this study. We also thank Dr. Deborah Mash (Miami Brain Bank) for providing control tissue. This work was supported by grants from the National Institutes of Health (NIH) (P01AG002132) as well as support by the Sergey Brin Foundation and the Sherman Fairchild Foundation (S.B.P.). It was also supported by grants from the NIH (R01NS121294) and the CurePSP Foundation (668-2020-06), and by the University of Massachusetts Amherst (A.L.W.). The Parkinson’s UK Brain Bank at Imperial College London is funded by Parkinson’s UK, a charity registered in England and Wales (948776) and in Scotland (SC037554). The Massachusetts Alzheimer’s Disease Research Center is supported by the NIH (AG005134).
Footnotes
Conflict of interest S.B.P is the founder of Prio-Pharma, which has not contributed financial or any other support to these studies. A.L.W. is a member of Acta Neuropathologica’s Editorial Board. They were not involved in the assessment or decision-making process for this manuscript.
Ethical approval Animals were maintained in an AAALAC-accredited facility in compliance with the 8th Guide for the Care and Use of Laboratory Animals. All procedures used in this study were approved by the University of California San Francisco Institutional Animal Care and Use Committee.
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s00401-022-02476-7.
References
- 1.Bernis ME, Babila JT, Breid S, Wüsten KA, Wüllner U, Tamgüney G (2015) Prion-like propagation of human brain-derived alpha-synuclein in transgenic mice expressing human wild-type alpha-synuclein. Acta Neuropathol Commun 3:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carlson GA, Kingsbury DT, Goodman PA, Coleman S, Marshall ST, DeArmond S et al. (1986) Linkage of prion protein and scrapie incubation time genes. Cell 46:503–511 [DOI] [PubMed] [Google Scholar]
- 3.Conway KA, Harper JD, Lansbury PT (1998) Accelerated in vitro fibril formation by a mutant alpha-synuclein linked to early-onset Parkinson disease. Nat Med 4:1318–1320 [DOI] [PubMed] [Google Scholar]
- 4.Coon EA, Nelson RM, Sletten DM, Suarez MD, Ahlskog JE, Benarroch EE et al. (2019) Sex and gender influence symptom manifestation and survival in multiple system atrophy. Auton Neurosci 219:49–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dhillon J-S, Trejo-Lopez JA, Riffe C, Levites Y, Sacino AN, Borchelt DR et al. (2019) Comparative analyses of the in vivo induction and transmission of α-synuclein pathology in transgenic mice by MSA brain lysate and recombinant α-synuclein fibrils. Acta Neuropathol Commun 7:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Emmer KL, Waxman EA, Covy JP, Giasson BI (2011) E46K human alpha-synuclein transgenic mice develop Lewy-like and tau pathology associated with age-dependent, detrimental motor impairment. J Biol Chem 286:35104–35118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giasson BI, Duda JE, Quinn SM, Zhang B, Trojanowski JQ, Lee VM (2002) Neuronal α-synucleinopathy with severe movement disorder in mice expressing A53T human α-synuclein. Neuron 34:521–533 [DOI] [PubMed] [Google Scholar]
- 8.Giasson BI, Uryu K, Trojanowski JQ, Lee VM (1999) Mutant and wild type human alpha-synucleins assemble into elongated filaments with distinct morphologies in vitro. J Biol Chem 274:7619–7622 [DOI] [PubMed] [Google Scholar]
- 9.Gilman S, Wenning GK, Low PA, Brooks DJ, Mathias CJ, Trojanowski JQ et al. (2008) Second consensus statement on the diagnosis of multiple system atrophy. Neurology 71:670–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guerrero-Ferreira R, Taylor NM, Mona D, Ringler P, Lauer ME, Riek R et al. (2018) Cryo-EM structure of alpha-synuclein fibrils. eLife 7:e36402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hass EW, Sorrentino ZA, Lloyd GM, McFarland NR, Prokop S, Giasson BI (2021) Robust α-synuclein pathology in select brainstem neuronal populations is a potential instigator of multiple system atrophy. Acta Neuropathol Commun 9:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jellinger KA, Lantos PL (2010) Papp-Lantos inclusions and the pathogenesis of multiple system atrophy: an update. Acta Neuropathol 119:657–667 [DOI] [PubMed] [Google Scholar]
- 13.Korth C, Kaneko K, Groth D, Heye N, Telling G, Mastrianni J et al. (2003) Abbreviated incubation times for human prions in mice expressing a chimeric mouse—human prion protein transgene. Proc Natl Acad Sci USA 100:4784–4789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuo YM, Li Z, Jiao Y, Gaborit N, Pani AK, Orrison BM et al. (2010) Extensive enteric nervous system abnormalities in mice transgenic for artificial chromosomes containing Parkinson disease-associated alpha-synuclein gene mutations precede central nervous system changes. Hum Mol Genet 19:1633–1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lau A, So RWL, Lau HHC, Sang JC, Ruiz-Riquelme A, Fleck SC et al. (2020) α-Synuclein strains target distinct brain regions and cell types. Nat Neurosci 23:21–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li B, Ge P, Murray KA, Sheth P, Zhang M, Nair G et al. (2018) Cryo-EM of full-length α-synuclein reveals fibril polymorphs with a common structural kernel. Nat Commun 9:3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Y, Zhao C, Luo F, Liu Z, Gui X, Luo Z et al. (2018) Amyloid fibril structure of α-synuclein determined by cryo-electron microscopy. Cell Res: 10.1038/s41422-018-0075-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lloyd GM, Sorrentino ZA, Quintin S, Gorion KM, Bell BM, Paterno G et al. (2022) Unique seeding profiles and prion-like propagation of synucleinopathies are highly dependent on the host in human α-synuclein transgenic mice. Acta Neuropathol 143:663–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Long H, Zheng W, Liu Y, Sun Y, Zhao K, Liu Z et al. (2021) Wild-type α-synuclein inherits the structure and exacerbated neuropathology of E46K mutant fibril strain by cross-seeding. Proc Natl Acad Sci USA 118:e2012435118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luk KC, Covell DJ, Kehm VM, Zhang B, Song IY, Byrne MD et al. (2016) Molecular and biological compatibility with host alpha-synuclein influences fibril pathogenicity. Cell Rep 16:3373–3387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Narhi L, Wood SJ, Steavenson S, Jiang Y, Wu GM, Anafi D et al. (1999) Both familial Parkinson’s disease mutations accelerate alpha-synuclein aggregation. J Biol Chem 274:9843–9846 [DOI] [PubMed] [Google Scholar]
- 22.Ni X, McGlinchey RP, Jiang J, Lee JC (2019) Structural insights into α-synuclein fibril polymorphism: effects of Parkinson’s disease-related C-terminal truncations. J Mol Biol 431:3913–3919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nishie M, Mori F, Yoshimoto M, Takahashi H, Wakabayashi K (2004) A quantitative investigation of neuronal cytoplasmic and intranuclear inclusions in the pontine and inferior olivary nuclei in multiple system atrophy. Neuropathol Appl Neurobiol 30:546–554 [DOI] [PubMed] [Google Scholar]
- 24.Papp MI, Kahn JE, Lantos PL (1989) Glial cytoplasmic inclusions in the CNS of patients with multiple system atrophy (striatonigral degeneration, olivopontocerebellar atrophy and Shy-Drager syndrome). J Neurol Sci 94:79–100 [DOI] [PubMed] [Google Scholar]
- 25.Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A et al. (1997) Mutation in the α-synuclein gene identified in families with Parkinson’s disease. Science 276:2045–2047 [DOI] [PubMed] [Google Scholar]
- 26.Prusiner SB, Woerman AL, Mordes DA, Watts JC, Rampersaud R, Berry DB et al. (2015) Evidence for α-synuclein prions causing multiple system atrophy in humans with parkinsonism. Proc Natl Acad Sci USA 112:E5308–E5317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosborough K, Patel N, Kalia LV (2017) α-Synuclein and parkinsonism: updates and future perspectives. Curr Neurol Neurosci Rep 17:31. [DOI] [PubMed] [Google Scholar]
- 28.Safar J, Wille H, Itri V, Groth D, Serban H, Torchia M et al. (1998) Eight prion strains have PrPSc molecules with different conformations. Nat Med 4:1157–1165 [DOI] [PubMed] [Google Scholar]
- 29.Spillantini MG, Crowther RA, Jakes R, Cairns NJ, Lantos PL, Goedert M (1998) Filamentous α-synuclein inclusions link multiple system atrophy with Parkinson’s disease and dementia with Lewy bodies. Neurosci Lett 251:205–208 [DOI] [PubMed] [Google Scholar]
- 30.Spillantini MG, Schmidt ML, Lee VM- Y, Trojanowski JQ, Jakes R, Goedert M (1997) α-Synuclein in Lewy bodies. Nature 388:839–840 [DOI] [PubMed] [Google Scholar]
- 31.Telling GC, Scott M, Hsiao KK, Foster D, Yang S-L, Torchia M et al. (1994) Transmission of Creutzfeldt-Jakob disease from humans to transgenic mice expressing chimeric human-mouse prion protein. Proc Natl Acad Sci USA 91:9936–9940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Telling GC, Scott M, Mastrianni J, Gabizon R, Torchia M, Cohen FE et al. (1995) Prion propagation in mice expressing human and chimeric PrP transgenes implicates the interaction of cellular PrP with another protein. Cell 83:79–90 [DOI] [PubMed] [Google Scholar]
- 33.Wakabayashi K, Miki Y, Tanji K, Mori F (2022) Neuropathology of multiple system atrophy, a glioneuronal degenerative disease. Cerebellum. 10.1007/s12311-022-01407-2 [DOI] [PubMed] [Google Scholar]
- 34.Watts JC, Giles K, Oehler A, Middleton L, Dexter DT, Gentleman SM et al. (2013) Transmission of multiple system atrophy prions to transgenic mice. Proc Natl Acad Sci USA 110:19555–19560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Woerman AL, Kazmi SA, Patel S, Aoyagi A, Oehler A, Widjaja K et al. (2018) Familial Parkinson’s point mutation abolishes multiple system atrophy prion replication. Proc Natl Acad Sci USA 115:409–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Woerman AL, Kazmi SA, Patel S, Freyman Y, Oehler A, Aoyagi A et al. (2018) MSA prions exhibit remarkable stability and resistance to inactivation. Acta Neuropathol 135:49–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woerman AL, Oehler A, Kazmi SA, Lee J, Halliday GM, Middleton LT et al. (2019) Multiple system atrophy prions retain strain specificity after serial propagation in two different Tg(SNCA*A53T) mouse lines. Acta Neuropathol 137:437–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Woerman AL, Patel S, Kazmi SA, Oehler A, Lee J, Mordes DA et al. (2020) Kinetics of α-synuclein prions preceding neuropathological inclusions in multiple system atrophy. PLOS Pathog 16:e1008222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Woerman AL, Stöhr J, Aoyagi A, Rampersaud R, Krejciova Z, Watts JC et al. (2015) Propagation of prions causing synucleinopathies in cultured cells. Proc Natl Acad Sci USA 112:E4949–E4958 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.