Abstract
Allergic-type immune responses, particularly immunoglobulin E (IgE), correlate with protective immunity in human schistosomiasis. To better understand the mechanisms of parasite elimination we examined the immune correlates of protection in baboons (Papio cynocephalus anubis), which are natural hosts for Schistosoma mansoni and also develop allergic-type immunity with infection. In one experiment, animals were exposed to a single infection (1,000 cercariae) or were exposed multiple times (100 cercariae per week for 10 weeks) and subsequently were cured with praziquantel prior to challenge with 1,000 cercariae. Singly and multiply infected animals mounted 59 and 80% reductions in worm burden, respectively (P < 0.01). In a second experiment, animals were inoculated with S. mansoni ova and recombinant human interleukin 12 (IL-12). This produced a 37 to 39% reduction in adult worm burden after challenge (P < 0.05). Parasite-specific IgG, IgE, IgM, and peripheral blood cytokine production were evaluated. The only immune correlate of protection in both experiments was levels of soluble adult worm antigen (SWAP)-specific IgE in serum at the time of challenge infection and/or 6 weeks later. Baboons repeatedly infected with cercariae or immunized with ova and IL-12 developed two- to sixfold-greater levels of SWAP-specific IgE in serum than did controls, and this correlated with reductions in worm burden (r2, −0.40 to −0.64; P, <0.01). Thus, in baboons and unlike mice, adult worm-specific IgE is uniquely associated with acquired immunity to S. mansoni infection. This similar association of parasite-specific IgE and protection among primates infected with schistosomiasis, along with similar pathology, anatomy, and genetic make-up, indicates that baboons provide an excellent permissive experimental model for better understanding the mechanisms of innate and acquired immunity to schistosomiasis in humans.
Partial resistance to human schistosomiasis becomes apparent in early adolescence. Both age-dependent changes in innate susceptibility and development of partial acquired immunity have been postulated to limit the intensity of infection (4). However, the extent and mechanisms of acquired immunity to natural infections remain poorly understood. The capacity for study of natural immunity (in contrast to vaccination with irradiated cercariae) has been hampered by the inability of rodent models to sustain repeated exposures to cercariae without development of severe pathology or death. Cost and suitable immune reagents have limited similar studies in nonhuman primates. Instead, most of our knowledge derives from epidemiological studies of human schistosomiasis. These results indicate that allergic-type immune responses correlate with protection based on observations that elevated levels of parasite-specific IgE in serum and parasite antigen-induced interleukin 4 (IL-4) and IL-5 production in peripheral blood mononuclear cell (PBMC) cultures correlate with resistance to reinfection after treatment (12, 20, 31, 36, 37). Whether immunoglobulin E (IgE), IL-4, and/or IL-5 actually participate in parasite elimination has been controversial because of conflicting results from detailed studies of rodents infected with human schistosome species (5–8, 24, 26, 27, 38, 39).
As an animal model, nonhuman primates and particularly the olive baboon (Papio cynocephalus anubis) have several advantages over rodents. They become infected with human schistosomes both experimentally and in the wild (9, 10, 15, 32, 41, 42). They develop partial protection with natural infection (10, 41), form pathologies that closely resemble human infection (44), and acquire immediate hypersensitivity responses to schistosome antigens (11, 17, 18, 25). However, studies of immune responses in nonhuman primates have been limited. Investigation of the correlates of acquired resistance in these animals may help resolve contradictory findings concerning acquired immunity in rodent models of schistosomiasis and human disease.
In East Africa, Schistosoma mansoni infects wild populations of olive baboons and can sustain transmission removed from human contact (15). Baboons are highly susceptible to experimental infections. The proportion of penetrating cercariae that mature to adult worms often exceeds 90% (14), whereas cercarial infectivity in mice rarely surpasses 50%. Natural infection (10, 41), immunization with irradiated cercariae (45, 53), or recombinant schistosome antigens (3) also produce partial resistance to challenge infection in baboons. This protection has been shown to correlate in some studies with levels of parasite-specific IgG in sera (40, 53). Whether protection correlates with levels of IgE in serum directed toward adult worm antigens or levels of parasite-specific cytokine production has not been previously investigated in baboons.
The present study analyzed two experimental approaches to identify correlates with acquisition of protective immunity in baboons. The first series of experiments was based on studies showing that repeated infection induces partial protection and augments parasite-specific immunoglobulin production (11, 46). In the second series of experiments, we observed that repeated inoculation with schistosome eggs and IL-12 induced partial immunity along with a selective increase in adult worm-specific IgE. This report examines the cellular and humoral immune responses that correlate with protection in these independent experimental approaches.
MATERIALS AND METHODS
Animals and parasites.
Juvenile male baboons (6 to 8 kg), P. cynocephalus anubis, were captured by personnel from the Institute of Primate Research in schistosome-free areas of Kenya for use in the study and were maintained in open enclosures based on international standards for primates. Upon capture, animals were screened for common bacterial infections and intestinal helminths. To further eliminate the possibility that animals had been previously exposed to schistosomiasis, serum was obtained from each animal and adult worm antigen-specific IgG was measured as previously described (14). If antibody levels exceeded 22 U/ml (mean plus 3 standard deviations [SD] above serum antibody levels obtained from colony-born animals), the animals were considered to have had prior exposure to schistosomiasis and were excluded from further study. Animals used for study with intestinal helminth infection were treated with mebendazole (50 mg twice daily for 3 days) until stools became parasite free. The baboons were subsequently quarantined for 3 months prior to further study. Cercariae for infecting baboons were shed from Biophalaria pfeifferi snails, as previously described (10).
Experiment 1: single or multiple infections, praziquantel treatment, and challenge.
Experiment 1 involved three groups of seven juvenile baboons each, infected percutaneously as follows: (i) 100 cercariae weekly for a total of 10 weeks, (ii) 1,000 cercariae once, or (iii) uninfected (control group). Infected animals were treated orally with praziquantel (60 mg/kg of body weight) on weeks 19, 27, and 29 postinfection until no S. mansoni eggs were detected in the stools of all animals for at least 2 weeks. Animals from all groups were challenged percutaneously with 1,000 S. mansoni cercariae at week 34 postinfection and were perfused 16 weeks later to recover adult worms as described previously (14).
Peripheral blood for both serum and PBMC assays was obtained prior to infection, at 6 and 18 weeks after primary infection, 5 weeks after the final praziquantel treatment (week 34 after initial infection and just prior to challenge infection), and at 6, 9, and 13 weeks postchallenge infection.
Experiment 2: immunizations with eggs and IL-12.
Baboons were divided into three groups of seven animals each, injected as follows: (i) 50,000 viable S. mansoni eggs, (ii) recombinant human IL-12 (1 μg per kg per dose, reconstituted in sterile normal saline [pH 7.4], kindly provided by Genetics Institute, Cambridge, Mass.), or (iii) 50,000 eggs plus IL-12. An additional control group of three animals received saline injections only. Schistosome eggs and/or IL-12 were administered subcutaneously in 1 ml of normal saline followed by two booster immunizations at weekly intervals. Sera were obtained by peripheral venipuncture at the beginning of the experiment and every 2 weeks thereafter. Sera were assayed for soluble adult worm antigen (SWAP)-specific IgG, IgE, and IgM, as described below. Twenty-one days following initial priming, each of the 21 experimental and the 3 control baboons were challenged percutaneously in the groin area with 1,000 S. mansoni cercariae. Cumulative stool collections were obtained weekly to determine egg output by the Kato technique.
Animals were perfused 13 weeks postchallenge to recover adult worms from the mesenteric blood vessels, as previously described (14). Following perfusion, 10% (by weight) of the liver and small and large intestines were sampled separately and digested in 5% KOH to recover and count the ova (13).
Lymphocyte proliferation and cytokine assays.
To determine whether recombinant human IL-12 was biologically active in baboons, fresh PBMCs were obtained from uninfected baboons and stimulated with concanavalin A (ConA) (0.5 μg/ml) for 72 h. Cells were then washed once and resuspended at 5 × 104 activated cells per well. Cells were cultured in triplicate in 96-well microtiter plates. Different concentrations of IL-12 were added to cultures. Cultures were incubated for 18 h at 37°C and then pulsed with [3H]thymidine for an additional 6 h prior to harvest on microfiber filters and counting in a beta scintillation counter.
PBMCs were cultured for cytokine production at 2 × 106/ml in complete RPMI (RPMI 1640, 10% fetal calf serum, 4 mM l-glutamine, 25 mM HEPES, and 80 μg of gentamicin per ml) in 48-well tissue culture plates (Falcon; Becton Dickinson and Co., Franklin Lakes, N.J.) in the presence of SWAP (50 μg/ml), soluble egg antigen (SEA) (5 μg/ml), streptolysin O (SLO) (1:200 dilution), phorbol myristate acetate (50 ng/ml; Sigma, St. Louis, Mo.) and ionomycin (1 μg/ml; Behring Corp., San Diego, Calif.) or complete RPMI alone. The cells were cultured at 37°C in a humidified atmosphere with 5% CO2 in air. Supernatants were harvested after 36 h for the measurement of IL-4 and on day 5 for the measurement of IL-5 and gamma interferon (IFN-γ) production. Cytokine measurements were performed by using capture enzyme-linked immunosorbent assays (ELISAs), as has been described previously (30), although different human reagents were used in order to detect baboon cytokines. ELISA plates (Immulon 4; Dynatech, Sterling, Va.) were coated with the various antibodies in a buffer of pH 9.6. For IFN-γ, the coating antibody was monoclonal antibody (MAb) MD-1 (Biosource International, Camarillo, Calif.) at 1 μg/ml, followed by the detecting biotinylated MAb 7-B6-1 (0.2 μg/ml; Diapharma Group Inc., Franklin, Ohio). For IL-4 and IL-5, the same reagents as previously described for humans (30) were used, as were capture MAbs IL-4 (25D2; Pharmingen, San Diego, Calif.) and IL-5 (TRFK5; Pharmingen) and detecting MAbs biotinylated IL-4 (8D2; Pharmingen) and biotinylated IL-5 (5A10; Pharmingen). The conjugate was streptavidin-alkaline phosphatase (Jackson ImmunoResearch, West Grove, Pa.), used at 1:2,000. Values were obtained from standard curves by using human recombinant cytokines and are expressed in picograms per milliliter. Limits of detection are as follows: IFN-γ, 10 pg/ml; IL-4, 20 pg/ml; IL-5, 22 pg/ml.
Antibody assays.
Levels of SWAP-specific IgG and IgE in serum were measured by ELISA. Immulon 4 plates (Dynatech) were coated with SWAP at either 10 μg/ml (for IgG) or 20 μg/ml (for IgM and IgE). Plates were coated with 5 μg of SEA/ml for detection of all immunoglobulin isotypes. A carbonate-bicarbonate coating buffer (pH 9.6) was used for all antigens. All assays were performed with 50 μl of sample per well except during blocking with phosphate-buffered saline plus 5% bovine serum albumin, when 100 μl per well was used. Serum samples were diluted differently for each assay, from 1:25 to 1:100,000 for IgG and from 1:5 to 1:10,000 for IgE. Antibody levels were determined based upon at least two dilutions, interpolated from a standard curve of pooled baboon serum. Baboon immunoglobulins were detected with peroxidase-conjugated anti-human IgG diluted 1:1,000 (Jackson ImmunoResearch) and with an affinity-purified rabbit anti-human IgE (at 1:2,000) that had been used extensively in previous studies (23). The secondary antibody was a goat anti-rabbit IgG conjugated to peroxidase (1:1000; Bio-Rad, Richmond, Calif.). All plates were developed for the peroxidase reaction with ABTS [2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid)] (Kirkegaard & Perry Laboratories, Gaithersburg, Md.).
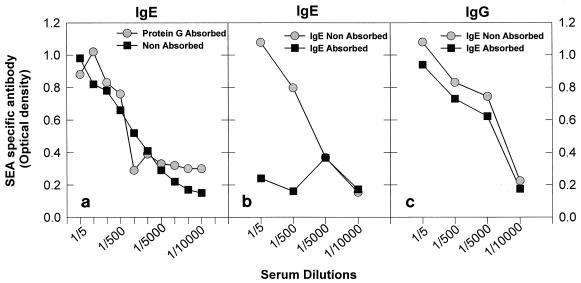
To determine whether the affinity-purified rabbit anti-human serum used specifically recognized baboon IgE and did not cross-react with immunoglobulin isotypes, the following experiments were performed. First, pooled immune baboon serum from repeatedly infected animals was absorbed to protein G (Calbiochem-Novabiochem Corp., La Jolla, Calif.) to remove baboon IgG (1). Serum depleted of IgG reactivity to SEA (optical density [OD] of 0.113 ± 0.02 at a wavelength of 405 μm at 1:100 dilution) was equivalent to that observed in nonimmune sera (OD, 0.094 ± 0.03). The depleted sera had titers of SEA-specific IgE similar to sera not depleted of IgG (see Fig. 1a). The reciprocal experiment was performed to remove IgE from immune sera. To accomplish this, rabbit anti-human IgE was biotinylated and bound to streptavidin-linked Sepharose beads (Pierce Chemical Co., Rockford, Ill.), according to the company’s protocol. Immune serum was added to the column in a binding buffer of pH 7.4, and the bound IgE was subsequently eluted with a buffer of pH 3.5 (Pierce Chemical Co.). As shown in Fig. 1b, absorption of sera on the IgE column significantly reduced the titers of SEA-specific IgE compared to unabsorbed sera but had less effect on SEA-specific IgG (Fig. 1c). The eluate from the IgE column contained SEA-specific IgE but no SEA-specific IgG (data not shown). SWAP-specific IgE and IgG levels were measured in parallel to those for SEA and showed the same pattern of depletion (data not shown). Depletion studies of SEA-specific antibody are shown because the ODs recorded were much higher than those observed for SWAP. Taken together, these results indicate that the polyclonal rabbit anti-human IgE used does not have significant cross-reactivity with baboon IgG. These experiments also show that parasite-specific IgG does not significantly block detection of parasite-specific IgE. Therefore, immune serum was not preabsorbed prior to examination of parasite-specific IgE antibody levels. Values were expressed as units per milliliter obtained from a standard curve created from a pool of baboon serum from animals in both the acute and chronic stages of S. mansoni infection.
FIG. 1.
Rabbit anti-human IgE does not show significant cross-reactivity with baboon IgG. Depletion of baboon sera with protein G did not alter levels of SEA-specific IgE in serum (a). Removal of baboon IgE from serum by using an affinity column conjugated with rabbit anti-human IgE markedly reduced levels of SEA-specific IgE (b) but not SEA-specific IgG (c). Pooled serum was used from two baboons with high SEA-specific IgE titers that had been infected, treated, and reinfected with S. mansoni.
Statistical analysis.
All data were analyzed for significant differences between experimental groups by Student’s t test of log-transformed data. Differences between the groups were considered significant at P of <0.05. Simple linear regression of log-transformed data was used to determine the correlation of parasite-specific immunoglobulin and cytokine levels with worm burden after challenge infection (SSPS software; SSPS, Inc., Chicago, Ill.).
RESULTS
Experiment 1: protective immunity induced by repeated cercarial exposure correlates with SWAP-specific IgE.
Prior infection followed by cure with praziquantel induced protection to challenge infection. In singly infected (SI) animals, this produced a 59% reduction in worm burden (P < 0.01), (Table 1), and in multiply infected (MI) animals, there was an 80% reduction (P < 0.001). MI animals had a 65% further reduction in worm burden than did SI animals P < 0.01) (Table 1). No decrease in fecundity was observed. The numbers of eggs per adult worm pair were equivalent in all treatment groups (challenge control mean, 4,526 ± 1,042; MI mean, 3,443 ± 347; SI mean, 4,059 ± 781).
TABLE 1.
Effects of multiple lower doses compared to a single mass infection followed by praziquantel treatment on resistance to challenge infection
| Mode of infectiona | n | Challenge cercariae | Mean worm burden ± SD (% protection)b | Serum SWAP-specific IgG (104 U/ml)c |
|---|---|---|---|---|
| None | 6 | 1,000 × 1 | 794 ± 97 | <0.1 |
| SI | 7 | 1,000 × 1 | 466 ± 182* (59) | 9.1 (2.4–28) |
| MI | 7 | 1,000 × 1 | 161 ± 89* (80)d | 12.8 (4.8–24) |
SI, single infection of 1,000 cercariae; MI, multiple percutaneous infections of 100 cercariae per week for 10 weeks. Animals were cured with praziquantel (60 mg/kg given as a single dose on weeks 19, 27, and 29) prior to challenge infection at week 34.
*, P < 0.01 (compared to challenge control group).
Serum was obtained after treatment and prior to challenge infection. Values are geometric means and ranges.
65.5% fewer worms than in the SI group.
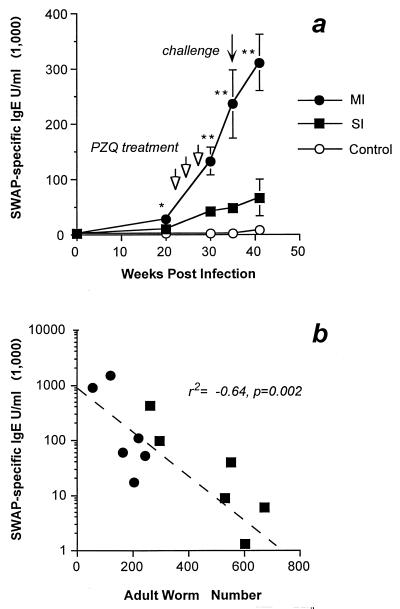
To examine the humoral correlates of protection, SWAP- and SEA-specific IgG and IgE were examined throughout the course of infection (Fig. 2a and Table 1). SWAP-specific IgE levels were significantly higher in the MI than in the SI group throughout the experiment (Fig. 1a). Treatment with praziquantel (three treatments were required to effect a complete cure) boosted the SWAP-specific IgE levels in both groups but to a much greater degree in the MI animals. At the time of challenge infection, the MI group had sixfold-higher levels of SWAP-specific IgE (P < 0.001). In contrast, levels of SWAP-specific IgG (Table 1) and SEA-specific IgE were equivalent at the time of challenge infection (SI geometric mean, 7.3 × 103 U/ml; MI geometric mean, 12.7 × 103 U/ml [P = 0.33]) and throughout the remainder of the experiment (data not shown). The levels of protection correlated with the level of serum SWAP-specific IgE prior to challenge infection (P = 0.002) (Fig. 2b) but not with SWAP-specific IgG (P = 0.23) or SEA-specific IgE (P = 0.4). Levels of SEA-specific IgG in serum failed to correlate with reductions in worm burden.
FIG. 2.
(a) Effects of praziquantel (PZQ) treatment on SWAP-specific IgE levels in MI, SI, and challenge control animals. Each point indicates the mean ± standard error for six animals. Arrows indicate points at which animals received PZQ or challenge infection. Asterisks indicate significant differences between the MI and SI experimental groups, as follows: ∗, P < 0.05; ∗∗, P < 0.01. (b) Relationship of adult worm-specific IgE at the time of challenge infection (week 34) to worm burden. Each point represents a single animal; symbols are as in panel a.
Adult worm and egg antigen-driven lymphocyte proliferation responses and cytokine production (IL-2, IL-4, IL-5, and IFN-γ) bore no relationship to levels of protection (data not shown).
Experiment 2: inoculation with eggs and IL-12. Human recombinant IL-12 induces lymphocyte proliferation by baboon PBMCs.
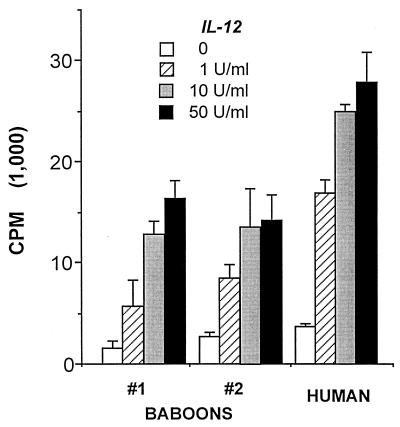
To show that recombinant human IL-12 activates baboon lymphocytes, this cytokine was added to ConA-activated baboon PBMC. IL-12 induced a dose-dependent increase in lymphocyte proliferation (Fig. 3). The magnitude of lymphocyte proliferation paralleled that observed by IL-12 stimulation of phytohemagglutinin (PHA)-activated human PBMCs.
FIG. 3.
Recombinant human IL-12 stimulates proliferation of activated PBMCs from baboons compared to that observed in humans. IL-12 was added 72 h after PBMCs (5 × 104/ml) were activated with ConA (0.5 μg/ml in two baboons) or PHA (1 μg/ml in humans), and the mixtures were incubated an additional 18 h prior to addition of [3H]thymidine. Values are means ± SD of triplicate cultures.
Immunization with eggs and IL-12 stimulates partial protection.
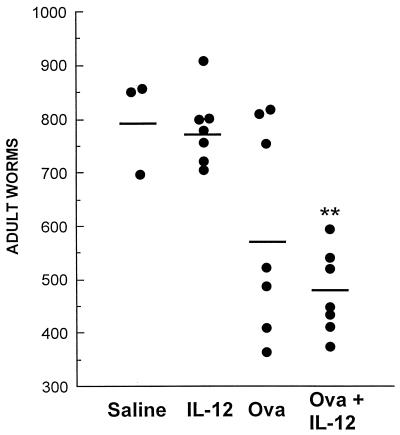
Animals immunized with schistosome eggs plus IL-12 produced significant reductions, 39 and 37%, in adult worm burden compared to unimmunized animals and those receiving IL-12 alone, respectively (P < 0.01) (Fig. 4). Immunization with schistosome eggs with or without IL-12 had no effect on fecundity. The numbers of eggs per worm pair recovered from tissues were equivalent between the treatment groups (mean for animals receiving saline alone, 6,433 ± 1,606 ova per worm pair [n = 3]; mean for IL-12 alone, 7,026 ± 2,180 [n = 7]; mean for eggs alone, 6,849 ± 4,538 [n = 7]; mean for eggs plus IL-12, 6,557 ± 341 [n = 7]).
FIG. 4.
Numbers of adult worms recovered from baboons 13 weeks after challenge infection with 1,000 cercariae in the first experiment. Animals were challenged with cercariae 21 days after the final immunization with various regimens, as indicated in the figure. Asterisks denote P < 0.01 compared to control groups with saline or IL-12 administered alone (Student’s t test). Each point represents a single animal.
IL-12 and schistosome egg inoculation augments SWAP-specific IgE that correlates with protection.
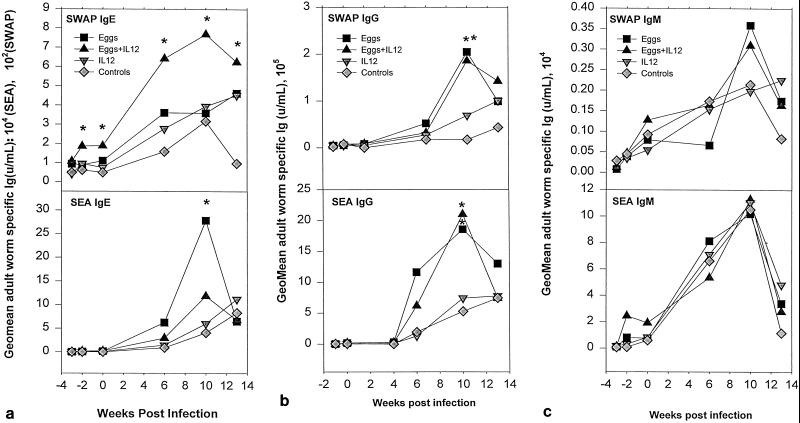
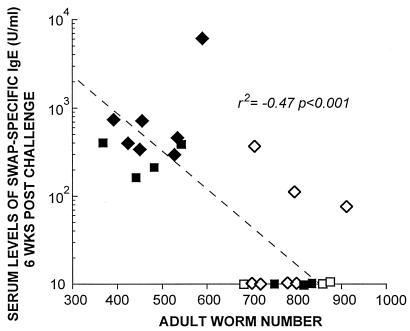
Immunization with schistosome eggs and IL-12 stimulated a twofold increase in SWAP-specific IgE prior to infection compared to the other experimental groups (Fig. 5a). Infection boosted levels of SWAP-specific IgE in animals immunized with ova plus IL-12 that remained significantly higher than in the other experimental groups (Fig. 5a). Infection also boosted levels of SWAP-specific IgG in the other experimental groups (Fig. 5b). However, only levels of SWAP-specific IgE in serum prior to infection (r2, −0.40; P, 0.01) and 6 weeks after infection (r2, −0.47; P, 0.001) (Fig. 6) correlated with a reduction in adult worms recovered after challenge infection, based on data shown in Fig. 4. No significant correlation with a reduction in worm burden was observed with SWAP-specific IgG or IgM.
FIG. 5.
Levels of parasite-specific IgE (a), IgG (b), and IgM (c) in serum after immunization and during the course of acute S. mansoni infection of baboons. Each point represents the geometric mean antibody titer for six baboons (except for the three baboons in the saline control group). Asterisks (∗, P < 0.05; ∗∗, P < 0.01) indicate serum antibody titers that are significantly different between groups of animals at a particular time after primary infection.
FIG. 6.
Inverse relationship between levels of SWAP-specific IgE in serum at week 6 postinfection and numbers of adult worms recovered from mesenteric blood vessels. Animals were immunized with 50,000 viable ova plus IL-12 (1 μg/kg) administered weekly for a total of three immunizations. Serum was obtained 6 weeks after infection. Filled diamonds represent ova plus IL-12, filled squares represent ova alone, open diamonds represent IL-12 alone, and open squares represent saline alone.
Immunization with eggs and IL-12 failed to stimulate a detectable rise in levels of SEA-specific IgE or IgG in serum prior to challenge infection, though an increase in SEA-specific IgM was observed at this time (Fig. 5c). Of note, at 10 weeks postinfection the levels of SEA-specific IgE decreased in the group inoculated with eggs and IL-12 compared to animals immunized with eggs alone (P = 0.02) (Fig. 5a). SEA-specific IgE, IgG, and IgM all failed to correlate with protection either before or after challenge infection.
Relationship of worm burden to adult worm antigen-induced cytokine production after immunization with ova and/or IL-12.
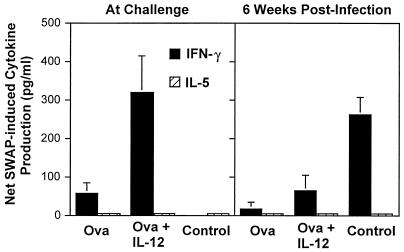
Immunization with schistosome eggs plus IL-12 stimulated significantly increased levels of SWAP-induced IFN-γ production before challenge compared to animals immunized with ova alone, with IL-12 alone, or with saline (the latter two groups are combined in Fig. 7 as controls) (P < 0.01). Levels of SWAP-induced IFN-γ prior to challenge failed to correlate with levels of protection (P = 0.31). No SWAP-induced IL-5 (or IL-4 [data not shown]) production was observed in PBMC culture supernatants at any time point (Fig. 7). At 6 weeks postinfection, SWAP-induced IFN-γ production was diminished in animals immunized with ova or ova and IL-12 compared to control animals (Fig. 7). Egg antigen-induced IFN-γ, IL-4, and IL-5 were present in culture supernatants at the time of challenge or 6 weeks postinfection, but the levels of these cytokines bore no relationship to levels of protection (data not shown).
FIG. 7.
SWAP-induced IFN-γ cytokine production by PBMCs obtained from baboons before and after challenge. Bars represent mean ± standard error net cytokine production (cultures with 50 μg of SWAP per ml added minus cultures with media alone) of six baboons.
DISCUSSION
Baboons are natural hosts for S. mansoni and, like humans, develop elevated levels of parasite-specific IgE when infected. The present study shows that natural experimental infection or immunization with schistosome antigens generates significant levels of protection that correlate with adult worm-specific IgE and not with other markers of humoral or cellular immunity directed to parasite antigens. This suggests an important role for IgE in protection.
The greatest levels of protection were induced by repeated natural infection followed by cure. This produced significantly higher protection than in animals infected once with a similar number of cercariae. Previous studies in baboons have also shown that repeated natural infection with S. mansoni or Schistosoma haematobium generates higher levels of immunity than does a single exposure (43, 51). Repeated exposure to irradiated S. haematobium cercariae in baboons has also been shown to augment protection compared to the same number of cercariae given once (34). The mechanisms by which repeated infection boosts parasite-specific IgE levels and protection may be related to the frequent exposure to proteases released by cercariae and schistosomula (49). These enzymes are necessary for digestion of the different structures of the skin, connective tissue, basement membranes, and extracellular matrix during penetration and migration within the host and have been shown to be potent inducers of type 2 immunity and IgE responses (29, 50). These multiple infections may be analogous to repeated light exposures to naturally developing cercariae in humans and may account for the slow acquisition of partial protection in human schistosomiasis. This is consistent with epidemiological findings in humans that peak prevalence and intensity of infection occur at younger ages in villages with higher rates of transmission (19). Treatment itself with praziquantel may also boost protection. SWAP-specific IgE (but not IgG) levels rose markedly after treatment, particularly in the MI animals. This has also been observed with praziquantel treatment in human schistosomiasis (52). Indeed, the treatment may also contribute to partial protection observed in treatment reinfection studies in humans (33).
Inoculation with eggs and IL-12 also augmented SWAP-specific IgE production that correlated with levels of protection. This contrasted to a decrease in levels of SEA-specific IgE in the same animals compared to those inoculated with ova alone. The failure of SEA-specific IgE or any other antigen-specific isotype to correlate with resistance to reinfection suggests a unique role of IgE directed to epitopes shared by ova and adult worms in this process. MAbs recognizing shared epitopes on ova and schistosomula have been previously shown to reduce the number of lung worms recovered (up to 85%) on day 4 postchallenge when passively transferred to naive mice (21). The levels of protective immunity induced by immunization with eggs relative to that observed with natural infection may have been limited because additional non-cross-reactive antigens expressed on schistosomula or adult worms may also contribute to protection. This might account for the greater levels of protection observed in animals repeatedly infected with cercariae.
Administration of IL-12 with eggs suppressed the egg antigen-specific IgE response, as predicted. IL-12 stimulates IFN-γ, which in turn inhibits the outgrowth of type 2 T cells and switching to IgE that typically occurs in response to egg antigens (42). Similar results have been shown in previous experiments in which mice immunized with eggs and IL-12 produced a decrease in levels of polyclonal IgE in serum compared to animals inoculated with eggs alone (48). The ability of IL-12 to boost SWAP-specific IgE in the same animals suggests that shared molecules between adult worms and eggs are processed and presented differently from non-cross-reactive egg antigens. For example, the oligosaccharides that contain the Lewis X trisaccharide are immunoreactive epitopes shared between developing larvae, adult worms, and ova (28). The Lewis X trisaccharide can directly activate B cells to produce IL-10 and PGE2, two molecules known to down-regulate Th1 responses (48). In this context, IL-12 may be less likely to expand Th1 responses and thus favor a Th2 cytokine response to these antigens. Alternatively, other shared molecules might stimulate an early IL-4 response that is augmented in the presence of IL-12 (47).
Other studies have also shown that in vivo administration of IL-12 can enhance synthesis of antigen-specific IgE (16). With doses comparable to those used in baboons, repeated administration of IL-12 with bee venom allergen PLA2 or keyhole limpet hemocyanin enhanced specific IgE levels 4- to 10-fold (35). Even in circumstances where IL-12 administration suppresses the allergen-specific T-cell repertoire to a Th1-like pattern, this effect may be only transient (2). Repeated administration of the allergen in the absence of IL-12 can augment Th2-associated recall response.
The unique association of levels of SWAP-specific IgE in serum with levels of protection in two independent experimental approaches strongly implicates a role for these antibodies in protection. To establish a clear causal relationship between IgE and resistance to reinfection will require adoptive transfer experiments of parasite-specific IgE into naive animals. We cannot exclude the possibility that other antibody isotypes, such as IgA, may also participate in protection in this model. The lack of an association with parasite-specific IgG and protection suggests that this isotype is unlikely to play a central role in protection. Schistosome antigen-induced cytokine production also failed to correlate with levels of protection. This is in contrast with our findings that SWAP-specific Th2-type cytokine responses correlate with protection in human urinary schistosomiasis (31). The weak or absent SWAP-induced cytokine production in baboons may result from a lack of chronic infection in these animals or from differences in compartmentalization of the immune response compared to humans.
The mechanism by which parasite-specific IgE contributes to protection is unclear. A logical place for parasite attrition may be schistosomules in the skin (6). Repeated infection of baboons with S. mansoni has been shown to result in development of immediate hypersensitivity reactions after skin injection of SWAP and elevated levels of parasite-specific IgE (11, 17, 18). The cercarial penetration described in these earlier studies showed immediate hypersensitivity reactions to penetrating cercariae similar to those observed in repeatedly infected baboons in the present study. Immediate hypersensitivity responses to schistosome antigens and after cercarial exposure have also been reported for humans and hypothesized to contribute to partial elimination of penetrating cercariae (22). Although subsequent studies have shown that the major site of parasite attrition in mice immunized with irradiated cercariae was during migration through the lungs and not the skin (39), this may not be the case in primates infected with schistosomiasis.
The demonstration that repeated exposure to developing larvae produces substantial levels of protection supports the hypothesis that acquired immunity to human schistosomiasis develops. With baboons, the ability to quantify exposure, treatment, and infection that would not be possible in human disease may provide further insights into the mechanisms of protective immunity to schistosomiasis in primates.
ACKNOWLEDGMENTS
This work was supported by NIH grants AI35935 and AI01202.
We are indebted to Julia Nyaundi and Michael N. Njenga for surgical expertise in collecting liver biopsies and to Simon Kiarie, Sammy Kisara, and Fred Nyundo for technical assistance.
REFERENCES
- 1.Andrew S M, Titus J A. Antibody detection and preparation. In: Coligan J E, Kruisbeek A M, Margulies D H, Shevach E M, Strober W, editors. Current protocols in immunology. Vol. 1. New York, N.Y: John Wiley & Sons; 1992. pp. 2.7.4–2.7.6. [Google Scholar]
- 2.Bliss J, Van Cleave V, Murray K, Wiencis A, Ketchum M, Maylor R, Haire T, Resmini C, Abbas A K, Wolf S F. IL-12, as an adjuvant, promotes a T helper 1 cell, but does not suppress a T helper 2 cell recall response. J Immunol. 1996;156:887–894. [PubMed] [Google Scholar]
- 3.Boulanger D, Reid G D, Sturrock R F, Wolowczuk I, Balloul J M, Grezel D, Pierce R J, Otieno M F, Guerret S, Grimaud J A, et al. Immunization of mice and baboons with the recombinant Sm28GST affects both worm viability and fecundity after experimental infection with Schistosoma mansoni. Parasite Immunol. 1991;13:473–490. doi: 10.1111/j.1365-3024.1991.tb00545.x. [DOI] [PubMed] [Google Scholar]
- 4.Butterworth, A. E. 1987. Immunity in human schistosomiasis. Acta Trop. 12(Suppl.):31–40. [PubMed]
- 5.Capron A, Dessaint J-P, Capron M, Joseph M, Pestel J. Role of anaphylactic antibodies in immunity to schistosomes. Am J Trop Med Hyg. 1980;29:849–857. doi: 10.4269/ajtmh.1980.29.849. [DOI] [PubMed] [Google Scholar]
- 6.Capron A, Dessaint J P, Capron M, Bazin H. Specific IgE antibodies in immune adherence of normal macrophages to Schistosoma mansoni schistosomules. Nature. 1975;253:474–475. doi: 10.1038/253474a0. [DOI] [PubMed] [Google Scholar]
- 7.Capron A, Dessaint J P, Capron M, Ouma J H, Butterworth A E. Immunity to schistosomes: progress toward vaccine. Science. 1987;238:1065–1072. doi: 10.1126/science.3317823. [DOI] [PubMed] [Google Scholar]
- 8.Capron M, Capron A. Immunoglobulin E and effector cells in schistosomiasis. Science. 1994;264:1876–1877. doi: 10.1126/science.8009216. [DOI] [PubMed] [Google Scholar]
- 9.Cheever A W, Kuntz R E, Myers B J, Moore J A, Huang T C. Schistosomiasis haematobia in African, hamadryas, and gelada baboons. Am J Trop Med Hyg. 1974;23:429–448. doi: 10.4269/ajtmh.1974.23.429. [DOI] [PubMed] [Google Scholar]
- 10.Damian R T, Greene N D, Fitzgerald K. Schistosomiasis mansoni in baboons. II. Acquisition of immunity to challenge infection after repeated small exposures to cercariae of Schistosoma mansoni. Am J Trop Med Hyg. 1974;23:78–80. [PubMed] [Google Scholar]
- 11.Damian R T, Greene N D, Suzuki T, Dean D A. Schistosomiasis mansoni in baboons. V. Antibodies and immediate hypersensitivity in multiply infected Papio cynocephalus. Am J Trop Med Hyg. 1981;30:836–843. [PubMed] [Google Scholar]
- 12.Dunne D W, Butterworth A E, Fulford A J, Ouma J H, Sturrock R F. Human IgE responses to Schistosoma mansoni and resistance to reinfection. Mem Inst Oswaldo Cruz. 1992;87:99–103. doi: 10.1590/s0074-02761992000800014. [DOI] [PubMed] [Google Scholar]
- 13.Farah I O, Nyindo M. Schistosoma mansoni induces in the Kenyan baboon a novel intestinal pathology that is manifestly modulated by an irradiated cercarial vaccine. J Parasitol. 1996;82:601–607. [PubMed] [Google Scholar]
- 14.Farah I O, Nyindo M, Suleman M A, Nyaundi J, Kariuki T M, Blanton R E, Elson L H, King C L. Schistosoma mansoni: development and modulation of the granuloma after single or multiple exposures in the baboon (Papio cynocephalus anubis) Exp Parasitol. 1997;86:93–101. doi: 10.1006/expr.1997.4152. [DOI] [PubMed] [Google Scholar]
- 15.Fenwick A. Baboons as reservoir hosts of Schistosoma mansoni. Trans R Soc Trop Med Hyg. 1969;63:557–567. doi: 10.1016/0035-9203(69)90172-2. [DOI] [PubMed] [Google Scholar]
- 16.Germann T, Guckes S, Bongartz M, Dlugonska H, Schmitt E, Kolbe L, Kolsch E, Podlaski F J, Gately M K, Rude E. Administration of IL-12 during ongoing immune responses fails to permanently suppress and can even enhance the synthesis of antigen-specific IgE. Int Immunol. 1995;7:1649–1657. doi: 10.1093/intimm/7.10.1649. [DOI] [PubMed] [Google Scholar]
- 17.Greene N D. Schistosoma mansoni: IgG and IgE skin-sensitizing antibodies in infected baboons. Exp Parasitol. 1974;35:219–224. doi: 10.1016/0014-4894(74)90025-3. [DOI] [PubMed] [Google Scholar]
- 18.Greene N D, Damian R T. Skin-sensitizing antibodies in Schistosoma mansoni infected baboons: evidence for the presence of two types. Proc Soc Exp Biol Med. 1972;141:291–294. doi: 10.3181/00379727-141-36761. [DOI] [PubMed] [Google Scholar]
- 19.Hagan P. Reinfection, exposure and immunity in human schistosomiasis. Parasitol Today. 1992;8:12–16. doi: 10.1016/0169-4758(92)90303-j. [DOI] [PubMed] [Google Scholar]
- 20.Hagan P, Blumenthal U J, Dunn D, Simpson A J, Wilkins H A. Human IgE, IgG4 and resistance to reinfection with Schistosoma haematobium. Nature. 1991;349:243–245. doi: 10.1038/349243a0. [DOI] [PubMed] [Google Scholar]
- 21.Harn D A, Mitsuyama M, David J R. Schistosoma mansoni. Anti-egg monoclonal antibodies protect against cercarial challenge in vivo. J Exp Med. 1984;159:1371–1387. doi: 10.1084/jem.159.5.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higashi G I, El-Asfahani A M, El-Bolkainy M N, Chu E W, Raafat M. Assessment of immediate and delayed hypersensitivity skin tests with Schistosoma mansoni adult worm antigen in a rural Egyptian population. Trop Geogr Med. 1980;32:245–250. [PubMed] [Google Scholar]
- 23.Hussain R, Hamilton R G, Kumaraswami V, Adkinson N F, Ottesen E A. IgE response in human filariasis. I. Quantitation of filaria-specific IgE. J Immunol. 1981;127:1623–1629. [PubMed] [Google Scholar]
- 24.Jankovic D, Kullberg M C, Dombrowicz D, Barbieri S, Caspar P, Wynn T A, Paul W E, Cheever A W, Kinet J P, Sher A. Fc epsilonRI-deficient mice infected with Schistosoma mansoni mount normal Th2-type responses while displaying enhanced liver pathology. J Immunol. 1997;159:1868–1875. [PubMed] [Google Scholar]
- 25.Joseph M, Capron A, Butterworth A E, Sturrock R F, Houba V. Cytotoxicity of human and baboon mononuclear phagocytes against schistosomula in vitro: induction by immune complexes containing IgE and Schistosoma mansoni antigens. Clin Exp Immunol. 1978;33:48–56. [PMC free article] [PubMed] [Google Scholar]
- 26.Kigoni E P, Elsas P P X, Lenzi H L, Dessein A J. IgE antibody and resistance to infection. II. Effect of IgE suppression on the early and late skin reaction and resistance of rats to Schistosoma mansoni infection. Eur J Immunol. 1986;16:589. doi: 10.1002/eji.1830160602. [DOI] [PubMed] [Google Scholar]
- 27.King C L, Xianli J, Malhotra I, Liu S, Mahmoud A A, Oettgen H C. Mice with a targeted deletion of the IgE gene have increased worm burdens and reduced granulomatous inflammation following primary infection with Schistosoma mansoni. J Immunol. 1997;158:294–300. [PubMed] [Google Scholar]
- 28.Koster B, Strand M. Schistosoma mansoni: immunolocalization of two different fucose-containing carbohydrate epitopes. Parasitology. 1994;108:433–446. doi: 10.1017/s0031182000075995. [DOI] [PubMed] [Google Scholar]
- 29.Lobos E. The basis of IgE responses to specific antigenic determinants in helminthiasis. Chem Immunol. 1997;66:1–25. doi: 10.1159/000058671. [DOI] [PubMed] [Google Scholar]
- 30.Mahanty S, King C L, Kumaraswami V, Regunathan J, Maya A, Jayaraman K, Abrams J S, Ottesen E A, Nutman T B. IL-4- and IL-5-secreting lymphocyte populations are preferentially stimulated by parasite-derived antigens in human tissue invasive nematode infections. J Immunol. 1993;151:3704–3711. [PubMed] [Google Scholar]
- 31.Medhat A, Shehata M, Bucci K, Mohamed S, Diab A, Badary S, Galal H, Nafeh M, King C L. Increased interleukin-4 and interleukin-5 production in response to Schistosoma haematobium adult worm antigens correlates with lack of reinfection after treatment. J Infect Dis. 1998;178:512–519. doi: 10.1086/515630. [DOI] [PubMed] [Google Scholar]
- 32.Miller J H. Papio doguera (dog face baboon), a primate reservoir host of Schistosoma mansoni in East Africa. Trans R Soc Trop Med Hyg. 1960;54:44–53. doi: 10.1016/0035-9203(60)90211-x. [DOI] [PubMed] [Google Scholar]
- 33.Mutapi F, Ndhlovu P D, Hagan P, Spicer J T, Mduluza T, Turner C M, Chandiwana S K, Woolhouse M E. Chemotherapy accelerates the development of acquired immune responses to Schistosoma haematobium infection. J Infect Dis. 1998;178:289–293. doi: 10.1086/517456. [DOI] [PubMed] [Google Scholar]
- 34.Reid G D, Sturrock R F, Harrison R A, Tarara R P. Schistosoma haematobium in the baboon (Papio anubis): assessment of protection levels against either a single mass challenge or repeated trickle challenges after vaccination with irradiated schistosomula. J Helminthol. 1995;69:139–147. doi: 10.1017/s0022149x00014024. [DOI] [PubMed] [Google Scholar]
- 35.Rempel J D, Wang M, HayGlass K T. In vivo IL-12 administration induces profound but transient commitment to T helper cell type 1-associated patterns of cytokine and antibody production. J Immunol. 1997;159:1490–1496. [PubMed] [Google Scholar]
- 36.Rihet P, Demeure C E, Bourgois A, Prata A, Dessein A J. Evidence for an association between human resistance to Schistosoma mansoni and high anti-larval IgE levels. Eur J Immunol. 1991;21:2679–2686. doi: 10.1002/eji.1830211106. [DOI] [PubMed] [Google Scholar]
- 37.Roberts M, Butterworth A E, Kimani G, Kamau T, Fulford A J, Dunne D W, Ouma J H, Sturrock R F. Immunity after treatment of human schistosomiasis: association between cellular responses and resistance to reinfection. Infect Immun. 1993;61:4984–4993. doi: 10.1128/iai.61.12.4984-4993.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sher A, Correa-Oliveira R, Hieny S, Hussain R. Mechanisms of protective immunity against Schistosoma mansoni infection in mice vaccinated with irradiated cercariae. IV. Analysis of the role of IgE antibodies and mast cells. J Immunol. 1983;131:1460–1465. [PubMed] [Google Scholar]
- 39.Smythies L E, Coulson P S, Wilson R A. Monoclonal antibody to IFN-gamma modifies pulmonary inflammatory responses and abrogates immunity to Schistosoma mansoni in mice vaccinated with attenuated cercariae. J Immunol. 1992;149:3654–3658. [PubMed] [Google Scholar]
- 40.Soisson L A, Reid G D, Farah I O, Nyindo M, Strand M. Protective immunity in baboons vaccinated with a recombinant antigen or radiation-attenuated cercariae of Schistosoma mansoni is antibody-dependent. J Immunol. 1993;151:4782–4789. [PubMed] [Google Scholar]
- 41.Sturrock R F, Butterworth A E, Houba V. Schistosoma mansoni in the baboon (Papio anubis): parasitological responses of Kenyan baboons to different exposures of a local parasite strain. Parasitology. 1976;73:239–252. doi: 10.1017/s003118200004693x. [DOI] [PubMed] [Google Scholar]
- 42.Sturrock R F, Butterworth A E, Houba V, Karamsadkar S D, Kimani R. Schistosoma mansoni in the Kenyan baboon (Papio anubis): the development and predictability of resistance to homologous challenge. Trans R Soc Trop Med Hyg. 1978;72:251–261. doi: 10.1016/0035-9203(78)90204-3. [DOI] [PubMed] [Google Scholar]
- 43.Sturrock R F, Cottrell B J, Kimani R. Observations on the ability of repeated, light exposures to Schistosoma mansoni cercariae to induce resistance to reinfection in Kenyan baboons (Papio anubis) Parasitology. 1984;88:505–514. doi: 10.1017/s0031182000054767. [DOI] [PubMed] [Google Scholar]
- 44.Sturrock R F, Cottrell B J, Lucas S, Reid G D, Seitz H M, Wilson R A. Observations on the implications of pathology induced by experimental schistosomiasis in baboons in evaluating the development of resistance to challenge infection. Parasitology. 1988;96:37–48. doi: 10.1017/s0031182000081646. [DOI] [PubMed] [Google Scholar]
- 45.Sturrock R F, James C, James E R, Webbe G. Immunization of baboons (Papio anubis) against Schistosoma haematobium with irradiated cercariae or schistosomula. Trans R Soc Trop Med Hyg. 1980;74:834–835. doi: 10.1016/0035-9203(80)90229-1. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 46.Suzuki T, Damian R T. Schistosomiasis mansoni in baboons. IV. The development of antibodies to Schistosoma mansoni adult worm, egg, and cercarial antigens during acute and chronic infections. Am J Trop Med Hyg. 1981;30:825–835. [PubMed] [Google Scholar]
- 47.Vella A T, Pearce E J. CD4+ Th2 response induced by Schistosoma mansoni eggs develops rapidly, through an early, transient, Th0-like stage. J Immunol. 1992;148:2283–2290. [PubMed] [Google Scholar]
- 48.Velupillai P, Harn D A. Oligosaccharide-specific induction of interleukin 10 production by B220+ cells from schistosome-infected mice: a mechanism for regulation of CD4+ T-cell subsets. Proc Natl Acad Sci USA. 1994;91:18–22. doi: 10.1073/pnas.91.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Verwaerde C, Auriault C, Damonneville M, Neyrinck J L, Vendeville C, Capron A. Role of serine proteases of Schistosoma mansoni in the regulation of IgE synthesis. Scand J Immunol. 1986;24:509–516. doi: 10.1111/j.1365-3083.1986.tb02165.x. [DOI] [PubMed] [Google Scholar]
- 50.Verwaerde C, Auriault C, Neyrinck J L, Capron A. Properties of serine proteases of Schistosoma mansoni schistosomula involved in the regulation of IgE synthesis. Scand J Immunol. 1988;27:17–24. doi: 10.1111/j.1365-3083.1988.tb02318.x. [DOI] [PubMed] [Google Scholar]
- 51.Webbe G, James C, Nelson G S, Smithers S R, Terry R J. Acquired resistance to Schistosoma haematobium in the baboon (Papio anubis) after cercarial exposure and adult worm transplantation. Ann Trop Med Parasitol. 1976;70:411–424. doi: 10.1080/00034983.1976.11687140. [DOI] [PubMed] [Google Scholar]
- 52.Webster M, Fallon P G, Fulford A J, Butterworth A E, Ouma J H, Kimani G, Dunne D W. Effect of praziquantel and oxamniquine treatment on human isotype responses to Schistosoma mansoni: elevated IgE to adult worm. Parasite Immunol. 1997;19:333–335. doi: 10.1046/j.1365-3024.1997.d01-211.x. [DOI] [PubMed] [Google Scholar]
- 53.Yole D S, Pemberton R, Reid G D, Wilson R A. Protective immunity to Schistosoma mansoni induced in the olive baboon Papio anubis by the irradiated cercaria vaccine. Parasitology. 1996;112:37–46. doi: 10.1017/s0031182000065057. [DOI] [PubMed] [Google Scholar]