Abstract
Background
Despite the extensive scientific evidence accumulating on the epidemiological risk factors for non-alcoholic fatty liver disease (NAFLD), evidence exploring sex- and age-related differences remains insufficient. The present cross-sectional study aims to investigate possible sex differences in the prevalence of FLI-defined NAFLD as well as in its association with common risk factors across different age groups, in a large sample of Spanish working adults.
Methods
This cross-sectional study included data from 33,216 Spanish adult workers (18–65 years) randomly selected during voluntary routine occupational medical examinations. Sociodemographic characteristics (age and social class), anthropometric (height, weight, and waist circumference) and clinical parameters (blood pressure and serum parameters) were collected. NAFLD was determined by the validated fatty liver index (FLI) with a cut-off value of ≥ 60. The presence of metabolic syndrome (MetS) was assessed according to the diagnostic criteria of the International Diabetes Federation. Cardiovascular risk was determined using the REGICOR-Framingham equation. The association between FLI-defined NAFLD and risk factors by sex and age was evaluated by multivariate logistic regression.
Results
The prevalence of FLI-defined NAFLD (FLI ≥ 60) was 19.1% overall, 27.9% (95% CI 23.3–28.5%) for men and 6.8% (95% CI 6.4–7.3%) for women and increasing across age intervals. As compared to women, men presented worse cardiometabolic and anthropometric profiles. The multivariate analysis model showed that hepatic steatosis assessed by FLI was strongly associated with age, HDL-cholesterol, social class, prediabetes, diabetes, prehypertension, hypertension, and smoking status for both men and women. The association between diabetes and hypertension with FLI-defined NAFLD was stronger in women than in men at both univariate and multivariate analyses.
Conclusions
Men presented a higher prevalence of NAFLD than women across all age intervals, as well as a worse cardiometabolic profile and a higher cardiovascular risk. Nevertheless, the association between FLI-defined NAFLD and diabetes or hypertension was significantly stronger in women than in men, possibly indicating that the presence of a dysmetabolic state might affect women more than men with regard to liver outcomes.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13293-022-00475-7.
Keywords: Fatty liver index, Non-alcoholic fatty liver disease, Cardiometabolic risk factors, Metabolic syndrome, Sex, Age
Highlights
There is a lack of evidence exploring sex- and age-related differences on the epidemiological risk factors for non-alcoholic fatty liver disease.
In our sample, hepatic steatosis assessed by FLI is more prevalent in men than women and is associated with age, lower social class and a dysmetabolic state characterized by prediabetes or diabetes, prehypertension or hypertension and low HDL-cholesterol.
Importantly, we observed that although FLI-defined NAFLD is strongly associated with cardiometabolic imbalances in both sexes, its association with diabetes and hypertension is stronger in women than in men, regardless of age.
We also observed that such association in women is evident even at stages preceding diabetes and hypertension and at younger ages.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13293-022-00475-7.
Introduction
Non-alcoholic fatty liver disease (NAFLD) is the most common and prevalent liver disease worldwide, affecting around 20–30% of adults in the western world [1]. It is characterized by lipid infiltration in the hepatocytes, not related to significant alcohol intake, which through non-alcoholic steatohepatitis can progress to liver fibrosis, cirrhosis, end-stage liver disease, and hepatocarcinoma [2, 3]. NAFLD shares pathophysiological mechanisms with the metabolic syndrome (MetS) and its components, such that they are often described as a continuum, each predicting and worsening the outcome of the other [3], and in turn significantly increasing the risk of cardiovascular (CV) disease and CV events [4–9]. In fact, patients with NAFLD besides presenting a worse cardiometabolic profile [7, 10, 11], are at significantly higher risk of dying of CV disease or non-liver cancer before suffering a liver-related death [3].
In an effort to better identify individuals at risk of chronic disease and provide effective prevention and treatment, in 2014 the National Institutes of Health requested that sex may be accounted for as a biological variable [12]. Studies on sex differences in NAFLD show that it is more prevalent in men than in women of reproductive age, and that after menopause the protective effect of estrogen fades out exposing older women to higher rates of NAFLD [13]. Sex and age differences are also observed in type 2 diabetes (T2D) [14], visceral fat [15, 16], and the MetS [17], which are major risk factors for NAFLD. What is, however, still unexplored is whether possible sex- and age-related differences exist in the association between NAFLD and its most common risk factors.
Moreover, in Spain the latest data on NAFLD prevalence, which was estimated at 25.8%, was published in 2010 and using a relatively small sample [18]. Estimating NAFLD prevalence using a large sample is crucial for obtaining updated information on the burden of the disease in the general population [19].
Although the gold standard for diagnosing presence and histopathological features of NALFD is liver biopsy, due to invasiveness, associated risks, and costs, it is often unviable as a routine procedure [7, 20, 21]. In the case of large population-based studies, the fatty liver index (FLI) is used as a valid alternative [22, 23]. The algorithm has been validated as a risk score system in the identification of NAFLD in the general population, with an area under the curve (AUC) of 0.84 [24]. FLI combines routine measurements such as triglycerides (TG), gamma-glutamyl transferase (GGT), waist circumference (WC), and body mass index (BMI) [7, 22], and has been associated with insulin resistance, MetS, and T2D [23, 25].
For all of the above, the present study aimed to assess the prevalence of FLI-defined NAFLD in a large sample of working adult population as a whole and by sex, and to evaluate the association between FLI-defined NAFLD and cardiometabolic risk factors by sex and age.
Methods
The present cross-sectional study includes a sample of 33,216 individuals (58.3% men), with available data on GGT, belonging to a larger database of 234,995 Spanish adults (18–65 years) employed in the service sector (public administration, health departments, construction, and post offices) who voluntarily underwent routine occupational health visits between January 2012 and December 2013. The study methods have been described in detail previously [26]. Upon consent, a complete medical history, including personal and family history, was recorded.
Ethical considerations
All the study protocol procedures followed the Declaration of Helsinki for research on human participants and were approved by the Balearic Ethical Committee of Clinical Research (Ref. No: CEI-IB-1887). All participants were carefully informed of the study’s purpose and procedures before providing their consent to participate.
Data collection and definition of variables
Data were collected at one single occasion for each participant during the voluntary occupational health examination. Sociodemographic characteristics were collected through a questionnaire. Social class was defined according to the Spanish Epidemiology Society classification which considers occupation, income, and education [27], and classified as “high” (executives, managers, university professionals, and intermediate occupations and employees); and “low” (manual workers). Smoking habits were recorded as “current”, “former”, or “never”.
Anthropometric measurements (height, weight, and WC) were taken according to the recommendations of the International Standards for Anthropometric Assessment (ISAK) [28] by trained examiners to minimize coefficients of variation. Body weight was measured to the nearest 0.1 kg using an electronic scale (Seca 700 scale, Hamburg). Height was measured to the nearest 0.5 cm using a stadiometer [Seca 220 (CM) Telescopic Height Rod for Column Scales, Seca GmbH, Hamburg]. WC was measured using an anthropometric tape with the participant in a standing position, midway between the last rib and the top of the iliac crest. BMI was calculated as weight (kg) divided by height (m) squared (kg/m2). Obesity, in agreement with WHO guidelines criteria, the US Preventive Services Taskforce, and the International Obesity Taskforce, was defined as a BMI ≥ 30 kg/m2 [29].
Venous blood samples were taken after a 12-h overnight fast from the antecubital vein using vacutainers without anticoagulant to obtain serum. Serum concentrations of glucose, cholesterol, HDL-cholesterol, TG, and GGT were measured in serum by standard procedures using a Beckman Coulter SYNCHRON CX® 9 PRO clinical system (La Brea, CA, USA).
Blood pressure (BP) was determined after 10 min resting in a supine position using an automatic and calibrated sphygmomanometer (OMRON M3, OMRON Healthcare Europe, Spain). BP was measured three consecutive times with a 1-min gap between each measurement, according to International Standards. The average value was calculated and used for the analyses.
Metabolic parameters and definition of MetS
The presence of MetS was defined as meeting at least three of the five criteria established in the International Diabetes Federation (IDF) consensus: (1) central obesity expressed as waist circumference: ≥ 94 cm for males and ≥ 80 cm for females (when BMI is > 30 kg/m2, central obesity can be assumed, and waist circumference is not measured); (2) TG ≥ 150 mg/dL or specific treatment; (3) HDL-cholesterol in males < 40 mg/dL and females < 50 mg/dL; (4) BP ≥ 130/85 mmHg or specific treatment; and (5) FPG > 100 mg/dL or specific treatment [30, 31].
Cardiovascular risk
Cardiovascular risk (CVR) was assessed by using the REGICOR-Framingham risk equation, adapted for the Spanish population, and validated in adults aged 35–74 years [32]. The equation uses variables associated with CVR such as age, sex, smoking habits, T2D, HDL-cholesterol, and systolic and diastolic BP, and shows the risk in percentage, such that < 5% indicates low risk, 5–9.9% indicates moderate risk, 10–14.9% high risk, and > 15% severe risk.
FLI as a surrogate measure of fatty liver
The FLI score is a non-invasive indicator for NAFLD diagnosis which has been validated against liver ultrasonography and 1H-magnetic resonance spectroscopy results [33]. FLI values range from 0 to 100, where FLI < 30 rules out steatosis with a sensitivity of 87% and a specificity of 64%, whereas FLI ≥ 60 accurately rules in hepatic steatosis with a sensitivity of 61% and a specificity of 86% [22]. FLI values between 30 and 59 indicate indeterminate risk, and fatty liver might neither be confirmed nor ruled out.
The FLI was calculated following the formula proposed by Bedogni et al. [22]:
where y = ln (TG) + 0.139 × BMI + 0.718 × ln (GGT) + 0.053 × WC − 15.745.
Here, TG is expressed as mg/dL; BMI is expressed as kg/m2; GGT is expressed as U/L; and WC is measured in cm.
Statistical analyses
Data were tested for normality distribution using the Kolmogorov–Smirnov test. Continuous variables, expressed as mean and standard deviation (± SD), were analyzed using the independent sample t-test, Pearson’s correlations, and one-way analysis of variance (ANOVA) with post hoc Bonferroni contrast method. Before performing the independent sample t-test and ANOVA, Levene’s test was run to test the assumption of homogeneity of variance. Categorical variables, expressed as counts and percentages (%), were compared by the Chi-square test (χ2) with Bonferroni post hoc method. Participants were stratified by FLI categories (FLI < 30, FLI 30–59, and FLI ≥ 60).
Bivariate logistic regression analyses were performed to calculate crude and adjusted odds ratios (ORs) and corresponding 95% confidence intervals (CI) of factors associated with FLI, while adjusting for potential confounders that showed a significant association in univariate analysis. For this analysis participants were classified into two categories: those with FLI ≥ 60 and FLI < 60. We used the Hosmer–Lemeshow test and receiver operating characteristic (ROC) curves to evaluate the goodness of fit for logistic regression models.
Statistical analysis was carried out using the Statistical Package for the Social Sciences (SPSS) version 26.0 (IBM Company, New York, NY, USA). All statistical tests were two-sided, and p values < 0.05 were considered statistically significant.
Results
General characteristics of participants
A total of 33,216 individuals, 58.3% men and 41.7% women, with a mean age of 41.3 ± 10.31 years agreed to participate in the study and were included in the current analysis. Sociodemographic characteristics, anthropometric measurements, and biochemical parameters of the sample as a whole and by sex are shown in Table 1. Most subjects (67.5%) belonged to the “low” social class, and were generally healthy (non-smokers, with a normal BMI, with normal levels of BP, and free of T2D, dyslipidemia, and MetS).
Table 1.
Anthropometric characteristics and biochemical parameters of the study population overall and in men and women
| All n = 33,216 |
Men n = 19,370 (58.32%) |
Women n = 13,846 (41.68%) |
p value* | |
|---|---|---|---|---|
| Age (years) | 41.32 (10.31) | 42.23 (9.99) | 40.05 (10.61) | < 0.001 |
| Social class | 0.079 | |||
| High | 10,788 (32.5%) | 6217 (32.1%) | 4571 (33.0%) | |
| Low | 22,428 (67.5%) | 13,153 (67.9%) | 9275 (67.0%) | |
| Smoking status | < 0.001 | |||
| Never | 18,413 (55.4%) | 10,050 (51.9%) | 8363 (60.4%) | |
| Former | 5459 (16.4%) | 3540 (18.3%) | 1919 (13.9%) | |
| Current | 9344 (28.1%) | 5780 (29.8%) | 3564 (25.7) | |
| FLI | 32.19 (26.61) | 41.92 (26.28) | 18.59 (20.39) | < 0.001 |
| FLI categories | < 0.001 | |||
| < 30 | 18,939 (57.0%) | 7979 (41.2%) | 10,960 (79.2%) | |
| 30–59 | 7923 (23.9%) | 5986 (30.9%) | 1937 (14.0%) | |
| ≥ 60 | 6345 (19.1%) | 5397 (27.9%) | 948 (6.8%) | |
| BMI (kg/m2) | 26.16 (4.82) | 26.82 (4.30) | 25.23 (5.33) | < 0.001 |
| BMI categories | < 0.001 | |||
| Normal weight | 14,849 (44.7%) | 7101 (36.7%) | 7748 (56.0%) | |
| Overweight | 12,214 (36.8%) | 8478 (43.8%) | 3736 (27.0%) | |
| Obese | 6153 (18.5%) | 3791 (19.6%) | 2362 (17.1%) | |
| WC (cm) | 83.72 (10.61) | 89.30 (8.82) | 75.91 (7.53) | < 0.001 |
| SBP (mmHg) | 121.41 (16.55) | 125.99 (15.57) | 114.99 (15.72) | < 0.001 |
| DBP (mmHg) | 74.50 (11.01) | 76.60 (10.93) | 71.58 (10.45) | < 0.001 |
| BP categories | < 0.001 | |||
| Normal | 20,630 (62.1%) | 10,119 (52.2%) | 10,511 (75.9%) | |
| Prehypertension | 4697 (14.1%) | 3441 (17.8%) | 1256 (9.1%) | |
| Hypertension | 7889 (23.8%) | 5810 (30.0%) | 2079 (15.0%) | |
| FPG (mg/dL) | 90.85 (19.27) | 92.90 (20.36) | 87.98 (17.24) | < 0.001 |
| Diabetes categories | < 0.001 | |||
| Normal | 27,015 (81.3%) | 14,941 (77.1%) | 12,074 (87.2%) | |
| Prediabetes | 4270 (12.9%) | 3036 (15.7%) | 1234 (8.9%) | |
| Diabetes | 1931 (5.8%) | 1393 (7.2%) | 538 (3.9%) | |
| GGT (IU/L) | 27.75 (28.42) | 33.78 (33.02) | 19.31 (17.02) | < 0.001 |
| AST (IU/L)a | 21.34 (11.57) | 23.77 (12.78) | 17.83 (8.41) | < 0.001 |
| ALT (IU/L)b | 25.43 (17.14) | 30.05 (18.50) | 18.96 (12.41) | < 0.001 |
| Cholesterol (mg/dL) | 195.70 (36.68) | 197.54 (37.19) | 193.14 (35.80) | < 0.001 |
| HDL-cholesterol (mg/dL) | 55.82 (11.24) | 53.71 (10.47) | 58.76 (11.61) | < 0.001 |
| TG (mg/dL) | 114.28 (73.90) | 129.21 (84.54) | 93.39 (48.55) | < 0.001 |
| Dyslipidemia | < 0.001 | |||
| No | 27,396 (82.5%) | 14,819 (76.5%) | 12,577 (90.8%) | |
| Yes | 5820 (17.5%) | 4551 (23.5%) | 1269 (9.2%) | |
| Presence of MetS | 4474 (13.5%) | 3798 (19.6%) | 676 (4.9%) | < 0.001 |
| REGICOR | < 0.001 | |||
| Low risk | 20,031 (60.3%) | 11,878 (61.3%) | 8153 (58.9%) | |
| Moderated risk | 3808 (11.5%) | 2702 (13.9%) | 1106 (8.0%) | |
| High risk | 381 (1.1%) | 234 (1.3%) | 138 (1.0%) | |
| Severe risk | 52 (0.2%) | 45 (0.2%) | 7 (0.0) | |
Data are expressed as mean (standard deviation) and as count (percentage)
FLI: fatty liver index; BMI: body mass index; BMI categories: normal weight (BMI 18.5 to 24.9 kg/m2), overweight (BMI 25 to 29.9 kg/m2), obese (BMI ≥ 30 kg/m2). WC: waist circumference; SBP: systolic blood pressure; DBP: diastolic blood pressure; BP: blood pressure; BP categories: normal BP (SBP < 130 and/or DBP < 85 mmHg), prehypertension (SBP 130 to 139 and/or DBP 85 to 89 mmHg), hypertension (SBP ≥ 140 and/or DBP ≥ 90 mmHg and/or with antihypertensive treatment); FPG, fasting plasma glucose; diabetes categories: normal (FPG < 100), prediabetes (FPG 100 to 125), Diabetes (FPG > 125 and/or antidiabetic treatment); GGT: γ-glutamyl transpeptidase; ALT: alanine aminotransferase; AST: aspartate aminotransferase; HDL: high-density lipoprotein; TG: triglycerides; dyslipidemia: no (TG < 150 and/or HDL < 40 mg/dL in men, < 46 mg/dL in women), yes (TG ≥ 150 and/or HDL ≥ 40 mg/dL in men, ≥ 46 mg/dL in women); MetS: metabolic syndrome according to the IDF; Framingham-REGICOR: low risk (< 5%), moderate risk (5–9.9%), high risk (10–14.9%), severe risk (> 15%)
*p values for comparison between men and women, obtained by independent sample t-test for continuous variables or by Chi-square for categorical variables
aAST available for n = 21,534 (men n = 12,728; women n = 8806)
bALT available for n = 33,185 (men n = 19,355; women n = 13,830)
The mean value of FLI for the whole sample was 32.19. A total of 6345 subjects (19.10%; 95% CI 18.7–19.5) presented FLI values ≥ 60, indicating an increased risk of NAFLD.
Differences across categories of FLI for the whole sample
Across FLI categories, mean BMI progressively increased from 23.37 ± 2.93 kg/m2 in the FLI < 30 category, to 32.18 ± 4.56 kg/m2 in the FLI ≥ 60 category (p < 0.001). Likewise, mean WC was higher in the FLI ≥ 60 category (96.39 ± 7.31 cm), than in the FLI < 30 (77.27 ± 7.39 cm) (p < 0.001). Mean cholesterol and TG exhibited similar behavior, being significantly lower in participants with FLI < 30 (187.86 ± 34.16 and 85.27 ± 34.73 mg/dL, respectively), compared to participants with FLI ≥ 60 (211.71 ± 38.70 and 186.87 ± 114.49 mg/dL, respectively) (p < 0.001 for both).
Characteristics of the study population by sex and across categories of FLI
Participants’ characteristics by sex are summarized in Table 1. As compared to women, men were older, and presenting higher levels of BMI, WC, systolic and diastolic BP, FPG, GGT, cholesterol and TG, and lower levels of HDL-cholesterol (all p < 0.001). As for FLI, men presented higher mean values than women (p < 0.001), as well as a higher proportion of scores of FLI ≥ 60 (p < 0.001).
General characteristics of the study population stratified by FLI categories in men and women separately are shown in Tables 2 and 3, respectively, and in Additional file 1: Fig. S1. In both men and women, those with FLI ≥ 60 were older and presented a significantly worse cardiometabolic profile.
Table 2.
Anthropometric characteristics and biochemical parameters of men subjects according to FLI categories (n = 19,362)
| Men characteristics | FLI < 30 | FLI 30–59 | FLI ≥ 60 | *p value |
|---|---|---|---|---|
| n = 7979 (41.2%) | n = 5986 (30.9%) | n = 5397 (27.9%) | ||
| Age (years) | 39.32 (10.03) | 43.72 (9.57) | 42.23 (9.99) | < 0.001a, c |
| Social class | 0.070 | |||
| High | 2586 (32.4%) | 1963 (32.8%) | 1667 (26.8%) | |
| Low | 5393 (67.6%) | 4023 (67.2%) | 3730 (69.1%) | |
| Smoking status | < 0.001 | |||
| Never | 4412 (55.3%) | 3149 (52.6%) | 2486 (46.1%) | a, b, c |
| Former | 1053 (13.2%) | 1134 (18.9%) | 1350 (25.0%) | a, b, c |
| Current | 2514 (31.5%) | 1703 (28.4%) | 1561 (28.9%) | a, c |
| BMI (kg/m2) | 23.56 (2.20) | 26.99 (2.17) | 31.44 (4.10) | < 0.001a, b, c |
| BMI categories | < 0.001 | |||
| Normal weight | 6071 (76.1%) | 940 (15.7%) | 88 (1.6%) | a, b, c |
| Overweight | 1904 (23.9%) | 4573 (76.4%) | 1905 (37.0%) | a, b, c |
| Obese | 4 (0.1%) | 473 (7.9%) | 3314 (61.4%) | a, b, c |
| WC (cm) | 82.66 (5.99) | 90.44 (6.11) | 97.85 (6.68) | < 0.001a, b, c |
| SBP (mmHg) | 121.36 (13.86) | 126.74 (14.61) | 131.99 (16.76) | < 0.001a, b, c |
| DBP (mmHg) | 72.96 (9.74) | 77.26 (10.40) | 81.25 (11.22) | < 0.001a, b, c |
| BP categories | < 0.001 | |||
| Normal | 5352 (67.1%) | 2935 (49.0%) | 1827 (33.9%) | a, b, c |
| Prehypertension | 1269 (15.9%) | 1187 (19.8%) | 983 (18.2%) | a, c |
| Hypertension | 1358 (17.0%) | 1864 (31.1%) | 2587 (47.9%) | a, b, c |
| FPG (mg/dL) | 88.82 (16.08) | 92.81 (18.33) | 99.04 (25.87) | < 0.001a, b, c |
| Diabetes categories | 6919 (86.7%) | 4570 (76.3%) | 3445 (77.1%) | < 0.001 |
| Normal | 820 (10.3%) | 1008 (16.8%) | 1207 (22.4%) | a, c |
| Prediabetes | 240 (3.0%) | 408 (6.8%) | 745 (13.8%) | a, b, c |
| Diabetes | a, b, c | |||
| GGT (IU/L) | 21.90 (11.00) | 32.92 (23.33) | 52.32 (50.77) | < 0.001a, b, c |
| AST (IU/L)d | 21.41 (9.98) | 23.77 (14.48) | 27.40 (13.72) | < 0.001a, b, c |
| ALT (IU/L)e | 21.17 (12.59) | 30.24 (17.73) | 38.57 (22.87) | < 0.001a,b,c |
| Cholesterol (mg/dL) | 185.26 (33.14) | 200.95 (35.52) | 211.88 (38.68) | < 0.001a, b, c |
| HDL-cholesterol (mg/dL) | 56.21 (10.65) | 53.29 (9.79) | 50.47 (9.97) | < 0.001a, b, c |
| TG (mg/dL) | 88.32 (34.92) | 127.27 (56.92) | 191.79 (117.73) | < 0.001a, b, c |
| Dyslipidemia | < 0.001 | |||
| No | 7457 (93.5%) | 4583 (76.6%) | 2772 (51.4%) | a, b, c |
| Yes | 522 (6.5%) | 1403 (23.4%) | 2625 (48.6%) | a, b, c |
| Presence of MetS | 52 (0.7%) | 768 (12.8%) | 2978 (55.2%) | < 0.001a, b, c |
| REGICOR | < 0.001 | |||
| Low risk | 4816 (60.4%) | 3923 (65.5%) | 3133 (58.1%) | a, c |
| Moderated risk | 476, (6.0%) | 901 (15.1%) | 1325 (24.6%) | a, b, c |
| High risk | 25 (0.3%) | 80 (1.3%) | 138 (2.6%) | a, b, c |
| Severe risk | 3 (0.0%) | 11 (0.2%) | 31 (0.6%) | a, b, c |
Data are expressed as mean (standard deviation) and as count (percentage)
FLI: fatty liver index; BMI: body mass index; BMI categories: normal weight (BMI 18.5 to 24.9 kg/m2), overweight (BMI 25 to 29.9 kg/m2), obese (BMI ≥ 30 kg/m2). WC: waist circumference; SBP: systolic blood pressure; DBP: diastolic blood pressure; BP: blood pressure; BP categories: normal BP (SBP < 130 and/or DBP < 85 mmHg), prehypertension (SBP 130 to 139 and/or DBP 85 to 89 mmHg), hypertension (SBP ≥ 140 and/or DBP ≥ 90 mmHg and/or with antihypertensive treatment); FPG: fasting plasma glucose; diabetes categories: normal (FPG < 100), prediabetes (FPG 100 to 125), diabetes (FPG > 125 and/or antidiabetic treatment); GGT: γ-glutamyl transpeptidase; ALT: alanine aminotransferase; AST: aspartate aminotransferase; HDL: high-density lipoprotein; TG: triglycerides, dyslipidemia: no (TG < 150 and/or HDL < 40 mg/dL in men, < 46 mg/dL in women), yes (TG ≥ 150 and/or HDL ≥ 40 mg/dL in men, ≥ 46 mg/dL in women); MetS: metabolic syndrome according to the IDF; Framingham-REGICOR: low risk (< 5%), moderate risk (5–9.9%), high risk (10–14.9%), severe risk (> 15%)
*p values obtained by one-way ANOVA for continuous variables or by Chi-square for categorical variables
Post hoc test by Bonferroni: asignificant difference between FLI < 30 and FLI 30–59; bsignificant difference between FLI 30–59 and FLI ≥ 60; csignificant difference between FLI < 30 and FLI ≥ 60
dAST available for n = 12,721 (FLI < 30 n = 5362; FLI 30–59 n = 3866; FLI ≥ 60 n = 3493)
eALT available for n = 19,347 (FLI < 30 n = 7973; FLI 30–59 n = 5980; FLI ≥ 60 n = 5394)
Table 3.
Anthropometric characteristics and biochemical parameters of women subjects according to FLI categories (n = 13,846)
| Women characteristics | FLI < 30 n = 10,960 (79.16%) |
FLI 30–59 n = 1937 (14%) |
FLI ≥ 60 n = 948 (6.85%) |
*p value |
|---|---|---|---|---|
| Age (years) | 39.01 (10.46) | 44.07 (10.24) | 40.05 (10.60) | < 0.001a, c |
| Social class | < 0.001 | |||
| High | 3832 (35.0%) | 507 (26.2%) | 232 (24.5%) | a, c |
| Low | 7128 (65.0%) | 1430 (73.8%) | 716 (75.5%) | a, c |
| Smoking status | < 0.001 | |||
| Never | 6572 (60.0%) | 1185 (61.2%) | 606 (63.9%) | |
| Former | 1465 (13.4%) | 307 (15.8%) | 146 (15.4%) | a |
| Current | 2923 (26.7%) | 445 (23.0%) | 196 (20.7%) | a, c |
| BMI (kg/m2) | 23.23 (3.35) | 31.05 (3.30) | 36.39 (4.75) | < 0.001a, b, c |
| BMI categories | < 0.001 | |||
| Normal weight | 7701 (70.3%) | 45 (2.3%) | 2 (0.2%) | a, b, c |
| Overweight | 3045 (27.8%) | 653 (33.7%) | 37 (3.9%) | b, c |
| Obese | 214 (2.0%) | 1,239 (64.0%) | 909 (95.9%) | a, b, c |
| WC (cm) | 73.34 (5.63) | 84.46 (5.59) | 88.09 (4.73) | < 0.001a, b, c |
| SBP (mmHg) | 112.76 (14.50) | 121.67 (16.67) | 127.09 (17.75) | < 0.001a, b, c |
| DBP (mmHg) | 70.22 (9.81) | 75.49 (10.95) | 79.22 (11.14) | < 0.001a, b, c |
| BP categories | < 0.001 | |||
| Normal | 8948 (81.6%) | 1141 (58.9%) | 421 (44.4%) | a, b, c |
| Prehypertension | 880 (8.0%) | 241 (12.4%) | 135 (14.2%) | a, b, c |
| Hypertension | 1132 (10.3%) | 555 (28.7%) | 392 (41.4%) | a, b, c |
| FPG (mg/dL) | 86.07 (13.63) | 93.01 (22.74) | 99.73 (30.05) | < 0.001a, b, c |
| Diabetes categories | < 0.001 | |||
| Normal | 9999 (91.2%) | 1485 (76.7%) | 589 (62.1%) | a, b, c |
| Prediabetes | 735 (6.7%) | 293 (15.1%) | 206 (21.7%) | a, b, c |
| Diabetes | 226 (2.1%) | 159 (8.2%) | 153 (16.1%) | a, b, c |
| GGT (UI/L) | 16.63 (9.43) | 25.70 (25.57) | 37.17 (37.20) | < 0.001a, b, c |
| AST (IU/L)d | 17.17 (7.45) | 19.64 (11.00) | 21.63 (10.72) | < 0.001a, b, c |
| ALT (IU/L)e | 17.68 (10.16) | 22.01 (16.98) | 27.50 (18.88) | < 0.001a, b, c |
| Cholesterol (mg/dL) | 189.76 (34.77) | 203.57 (35.48) | 210.77 (38.79) | < 0.001a, b, c |
| HDL-cholesterol (mg/dL) | 59.81 (11.68) | 55.57 (10.53) | 53.12 (9.93) | < 0.001a, b, c |
| TG (mg/dL) | 83.04 (34.43) | 119.89 (53.59) | 158.81 (88.95) | < 0.001 a,b,c |
| Dyslipidemia | < 0.001 | |||
| No | 10,392 (94.8%) | 1559 (80.5%) | 625 (65.9%) | a, b, c |
| Yes | 568 (5.2%) | 378 (19.5%) | 323 (34.1%) | a, b, c |
| Presence of MetS | 19 (0.2%) | 249 (12.9%) | 408 (43.0%) | < 0.001a, b, c |
| REGICOR | < 0.001 | |||
| Low risk | 6487 (59.2%) | 1177 (60.8%) | 488 (51.5%) | b, c |
| Moderated risk | 550 (5.0%) | 353 (18.2%) | 203 (21.4%) | a, b, c |
| High risk | 42 (0.4%) | 43 (2.2%) | 53 (5.6%) | a, b, c |
| Severe risk | 1 (0.0%) | 1 (0.1%) | 5 (0.5%) | b, c |
Data are expressed as mean (standard deviation) and as count (percentage)
FLI: fatty liver index; BMI: body mass index; BMI categories: normal weight (BMI 18.5 to 24.9 kg/m2), overweight (BMI 25 to 29.9 kg/m2), obese (BM I ≥ 30 kg/m2). WC: waist circumference; SBP: systolic blood pressure; DBP: diastolic blood pressure; BP: blood pressure; BP categories: normal BP (SBP < 130 and/or DBP < 85 mmHg), prehypertension (SBP 130 to 139 and/or DBP 85 to 89 mmHg), hypertension (SBP ≥ 140 and/or DBP ≥ 90 mmHg and/or with antihypertensive treatment); FPG: fasting plasma glucose; diabetes categories: normal (FPG < 100), prediabetes (FPG 100 to 125), diabetes (FPG > 125 and/or antidiabetic treatment); GGT: γ-glutamyl transpeptidase; ALT: alanine aminotransferase; AST: aspartate aminotransferase; HDL: high-density lipoprotein; TG: triglycerides, dyslipidemia: no (TG < 150 and/or HDL < 40 mg/dL in men, < 46 mg/dL in women), yes (TG ≥ 150 and/or HDL ≥ 40 mg/dL in men, ≥ 46 mg/dL in women); MetS, metabolic syndrome according to the IDF; Framingham-REGICOR: low risk (< 5%), moderate risk(5–9.9%), high risk (10–14.9%), severe risk (> 15%)
*p values obtained by one-way ANOVA for continuous variables or by Chi-square for categorical variables
Post hoc test by Bonferroni: asignificant difference between FLI < 30 and FLI 30–59; bsignificant difference between FLI 30–59 and FLI ≥ 60; csignificant difference between FLI < 30 and FLI ≥ 60
dAST available for n = 8259 (FLI < 30 n = 6939; FLI 30–59 n = 1246; FLI ≥ 60 n = 620)
eALT available for n = 13,829 (FLI < 30 n = 10,951; FLI 30–59 n = 1932; FLI ≥ 60 n = 946)
Among men, 27.9% had FLI ≥ 60, 41.2% presented FLI < 30, while 30.9% were classified as having an intermediate risk of fatty liver (FLI 30–59). Men in the highest FLI category (≥ 60) were older, and more likely to be obese, had higher WC, BP, FPG, cholesterol, and TG, and lower HDL-cholesterol as compared to men in lower categories of FLI (all p < 0.001). Accordingly, men in the highest FLI category presented a higher prevalence of hypertension, T2D, dyslipidemia, a higher risk of MetS, and a more severe CVR than the other two FLI categories (all p < 0.001). Importantly, as compared to men with FLI < 30, those with FLI 30–59 also presented a significantly higher BMI, WC, systolic and diastolic BP, GGT, FPG, and TG, and a significantly lower HDL-cholesterol (all p < 0.001). Men with FLI 30–59 also were more likely to be obese, suffer from hypertension, T2D, and dyslipidemia, and have an increased risk of MetS and CV disease than men with FLI < 30 (all p < 0.001).
Among women, 6.9% had FLI ≥ 60, 14.0% had FLI values between 30 and 59, and the majority, 79.2% had FLI < 30. Like for men, women in the FLI ≥ 60 category exhibited significantly higher levels of BMI, WC, BP, FPG, GGT, cholesterol, and TG, and lower values of HDL-cholesterol compared to the other two categories (all p < 0.001). Presence of obesity hypertension, T2D, and dyslipidemia was also more frequent in the highest FLI category than in the FLI 30–59 and FLI < 30 (all p < 0.001). Like for men, women with FLI 30–59 also presented a significantly higher BMI, WC, systolic and diastolic BP, GGT, FPG, and TG, and a significantly lower HDL-cholesterol than women with FLI < 30 (all p < 0.001); and were also more likely to be obese, suffer from hypertension, T2D, and dyslipidemia, and have an increased risk of MetS and CV disease than women with the lowest score of FLI (all p < 0.001).
Age- and sex-related differences in the prevalence of FLI-defined NAFLD
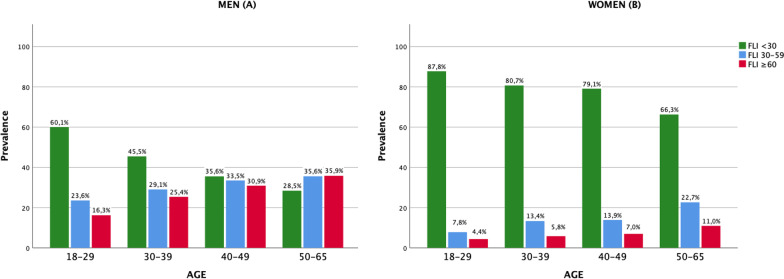
As shown in Fig. 1, FLI categories were compared across age intervals (18–29, 30–39, 40–49, 50–65 years) separately for men (A) and women (B). Moreover, prevalence of FLI categories across age intervals were compared between sexes (Additional file 2).
Fig. 1.
Prevalence of NAFLD (FLI ≥ 60) by age intervals in men (A) and women (B)
Men exhibited a significantly higher prevalence of FLI-defined NAFLD than women across all age intervals (p < 0.001). In both men and women, the percentage of cases of FLI ≥ 60 increased with age such as in the 50–65 year group the prevalence of FLI-defined NAFLD was at the highest for both men (35.9%) and women (11.0%). Interestingly, when compared, the proportion of women at low risk remained higher than the proportion of women at intermediate and high risk across all age intervals, while in men this trend was reversed as the age increased.
Association between FLI-defined NAFLD and cardiometabolic risk factors
Table 4 shows Pearson’s correlation analyses of the associations between FLI and those independent variables with are not included in the FLI equation. The association between FLI and HDL-cholesterol, systolic and diastolic BP, FPG, and REGICOR was weak–moderate and statistically significant (p > 0.001).
Table 4.
Correlation matrix between all variables for men (lower diagonal) and women (upper diagonal)
| Variables | FLI | BMI | WC | TG | HDL | GGT | SBP | DBP | FPG | REGICOR |
|---|---|---|---|---|---|---|---|---|---|---|
| FLI | 1 | –0.210** | 0.330** | 0.307** | 0.253** | 0.375** | ||||
| BMI | 1 | 0.786** | 0.280** | – | 0.146** | 0.313** | 0.297** | 0.225** | 0.326** | |
| WC | 0.740** | 1 | 0.217** | – | 0.116** | 0.224** | 0.222** | 0.156** | 0.186** | |
| TG | 0.239** | 0.183** | 1 | – | 0.193** | 0.206** | 0.192** | 0.187** | 0.346** | |
| HDL | – | – | – | – | 1 | – | – | – | – | − 0.283** |
| GGT | 0.148** | 0.105** | 0.279** | – | 1 | 0.148** | 0.105** | 0.099** | 0.147** | |
| SBP | 0.312** | 0.283** | 0.210** | 0.152** | – | 0.168** | 1 | 0.700** | 0.178** | 0.506** |
| DBP | 0.342** | 0.299** | 0.213** | 0.183** | – | 0.185** | 0.681** | 1 | 0.144** | 0.400** |
| FPG | 0.226** | 0.187** | 0.136** | 0.191** | – | 0.123** | 0.164** | 0.151** | 1 | 0.257** |
| REGICOR | 0.328** | 0.221** | 0.123** | 0.318** | – | 0.157** | 0.424** | 0.359** | 0.254** | 1 |
N = 19,830 for men (lower diagonal); N = 13,846 for women (upper diagonal)
FLI fatty liver index, BMI body mass index, WC waist circumference, TG triglycerides, HDL high-density lipoprotein cholesterol, GGT γ-glutamyl transpeptidase, SBP systolic blood pressure, DBP diastolic blood pressure, FPG fasting plasma glucose
**p < 0.001; correlations between FLI with BMI, WC, TG and GGT were not reported as such variables are included in the formula for calculating FLI
Tables 5 and 6 show factors associated with the presence of NAFLD as determined by FLI (< 60, absence and ≥ 60, presence) for men and women, respectively. At univariate analysis FLI-defined NAFLD was associated with T2D, hypertension, and age (years) in both sexes.
Table 5.
Univariate and multivariate analysis of factors associated with FLI ≥ 60 in men (n = 19,370)
| Men characteristics | OR | 95% CI | aOR | 95% CI |
|---|---|---|---|---|
| Age (years) | 1.039*** | 1.035–1.042 | 1.012*** | 1.008–1.015 |
| Social class | ||||
| Low | 1.080* | 1.010–1.156 | 1.104** | 1.026–1.189 |
| HDL-cholesterol | 0.957*** | 0.954–0.960 | 0.959*** | 0.956–0.963 |
| Diabetes categories | ||||
| Prediabetes | 2.202*** | 2.029–2.390 | 1.704*** | 1.560–1.861 |
| Diabetes | 3.878*** | 3.430–4.291 | 2.224*** | 1.966–2.516 |
| BP categories | ||||
| Prehypertension | 1.815*** | 1.659–1.985 | 1.736*** | 1.583–1.905 |
| Hypertension | 3.643*** | 3.389–3.917 | 2.859*** | 2.643–3.093 |
| Smoking | ||||
| Former smoker | 1.876*** | 1.729–2.035 | 1.442*** | 1.318–1.577 |
| Current smoker | 1.126** | 1.046–1.212 | 0.997 | 0.921–1.079 |
Qualitative explanatory variables that had more than two categories were transformed into dummy variables for inclusion in the logistic model
FLI (< 60 vs. ≥ 60)
OR: odds ratio; CI: confidence interval; aOR: adjusted odds ratio; HDL: high-density lipoprotein; diabetes categories: prediabetes (fasting plasma glucose 100 to 125), diabetes (fasting plasma glucose > 125 and/or antidiabetic treatment) BP: blood pressure; BP categories: prehypertension (systolic BP 130 to 139 and/or diastolic BP 85 to 89 mmHg), hypertension (systolic BP ≥ 140 and/or diastolic BP ≥ 90 mmHg and/or with antihypertensive treatment)
*p < 0.05, **p < 0.01, ***p < 0.001
Table 6.
Univariate and multivariate analysis of factors associated with FLI ≥ 60 in women (n = 13,846)
| Women characteristics | OR | 95% CI | aOR | 95% CI |
|---|---|---|---|---|
| Age (years) | 1.037*** | 1.030–1.043 | 0.997 | 0.990–1.005 |
| Social class | ||||
| Low | 1.565*** | 1.343–1.822 | 1.203* | 1.023–1.414 |
| HDL-cholesterol | 0.949*** | 0.942–0.955 | 0.954*** | 0.947–0.961 |
| Diabetes categories | ||||
| Prediabetes | 3.907*** | 3.293–4.636 | 2.712*** | 2.255–3.262 |
| Diabetes | 7.749*** | 6.314–9.510 | 4.029*** | 3.201–5.070 |
| BP categories | ||||
| Prehypertension | 2.886*** | 2.355–3.537 | 2.545*** | 2.057–3.149 |
| Hypertension | 5.569*** | 4.808–6.450 | 4.010*** | 3.383–4.753 |
| Smoking | ||||
| Former smoker | 1.054 | 0.873–1.272 | 0.922 | 0.754–1.129 |
| Current smoker | 0.745*** | 0.631–0.879 | 0.704*** | 0.592–0.839 |
Qualitative explanatory variables that had more than two categories were transformed into dummy variables for inclusion in the logistic model
FLI (< 60 vs. ≥ 60)
OR: odds ratio; CI: confidence interval; aOR: adjusted odds ratio; HDL: high-density lipoprotein; diabetes categories: prediabetes (fasting plasma glucose 100 to 125), diabetes (fasting plasma glucose > 125 and/or antidiabetic treatment) BP: blood pressure; BP categories: prehypertension (systolic BP 130 to 139 and/or diastolic BP 85 to 89 mmHg), hypertension (systolic BP ≥ 140 and/or diastolic BP ≥ 90 mmHg and/or with antihypertensive treatment)
*p < 0.05, **p < 0.01, ***p < 0.001
In men, the multivariate analysis model showed that age, low social class, prediabetes, T2D, prehypertension, hypertension, and formerly smoking remained independently associated with FLI-defined NAFLD, while no association was found between FLI ≥ 60 and currently smoking. On the other hand, HDL-cholesterol was inversely associated with FLI ≥ 60. In women, low social class, prediabetes, T2D, prehypertension, and hypertension, remained associated with FLI-defined NAFLD, while for age and formerly smoking no association was found. HDL-cholesterol and currently smoking were inversely associated with FLI ≥ 60.
A ROC analysis showed that the model including age, sex, social class, HDL-cholesterol, T2D, blood pressure, and smoking had very high discriminative capacity for fatty liver disease both in men (AUC = 0.719, 95% CI 0.711 to 0.727) and women (AUC = 0.776, 95% CI 0.760 to 0.792).
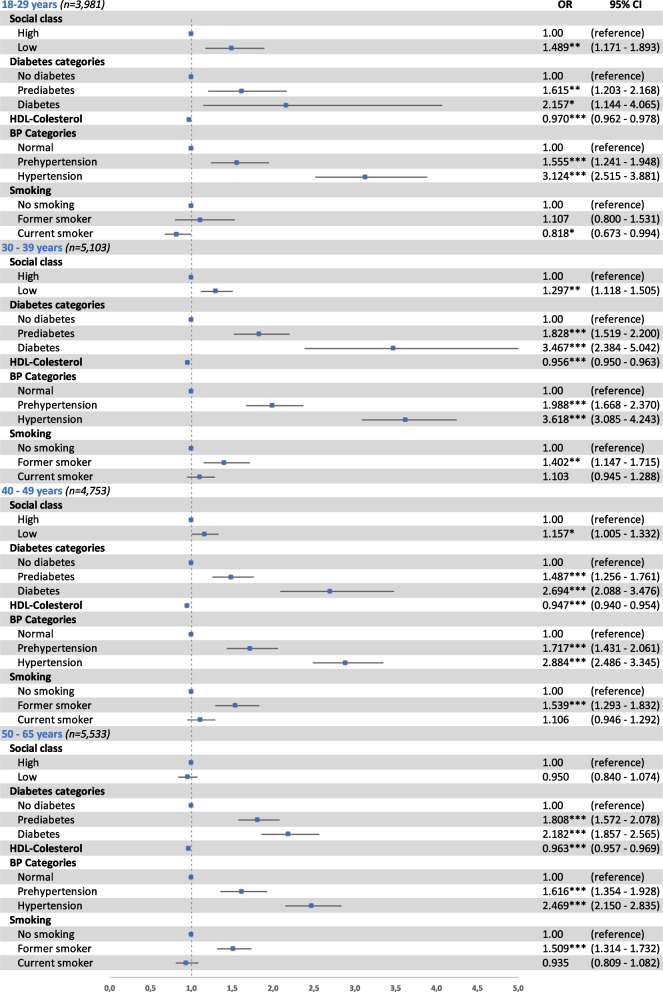
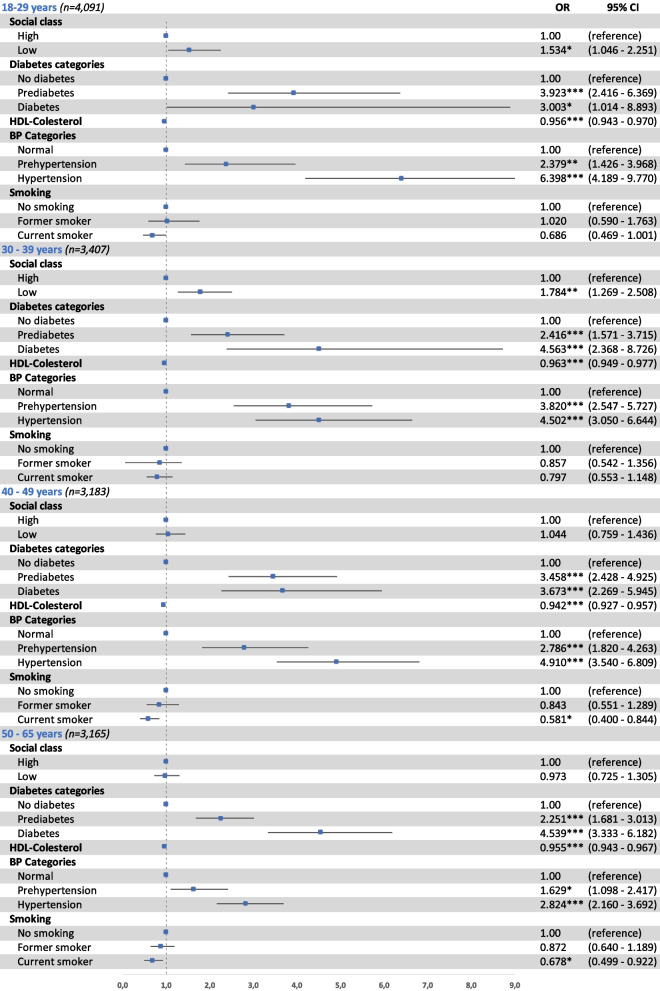
Figures 2 and 3 show risk factors associated with FLI-defined NAFLD determined by FLI ≥ 60 across different age groups (18–29, 30–39, 40–49, and 50–65 years) for men and women, respectively. Independently of age, subjects with prediabetes, T2D, prehypertension, or hypertension had a significantly higher risk of FLI-defined NAFLD, and among women most of these associations were stronger than for men. For instance, the OR for T2D in males aged 50–65 years was 2.182 (95% CI 1.857 to 2.565) and 4.539 (95% CI 3.333 to 6.128) in women of the same age. Furthermore, low social class was a significant risk factor among young and middle-aged adults only (18–49 years in men and 18–39 years in women). Contrarily, HDL-cholesterol was a protector at any age independently of sex, and currently smoking was also inversely related to FLI-defined NAFLD in women of 40–65 years only.
Fig. 2.
Factors associated with FLI ≥ 60† in men by age groups. Odds ratios (OR) and 95% confidence interval (95% CI) of the association between FLI ≥ 60† and social class (high; low), categories of diabetes (no diabetes; prediabetes; diabetes), HDL-cholesterol (mg/dL), categories of blood pressure (BP) (normal; prehypertension; hypertension), and smoking (no smoking, former smoker, current smoker), in men by age groups. Binary logistic regression adjusted by age. *p < 0.05, **p < 0.01, ***p < 0.001. †FLI (< 60 vs. ≥ 60). FLI: fatty liver index; diabetes categories: normal (fasting plasma glucose < 100), prediabetes (fasting plasma glucose 100 to 125), diabetes (fasting plasma glucose > 125 and/or antidiabetic treatment); BP: blood pressure; BP categories: normal BP (systolic BP < 130 and/or diastolic BP < 85 mmHg), prehypertension (systolic BP 130 to 139 and/or diastolic BP 85 to 89 mmHg), hypertension (systolic BP ≥ 140 and/or diastolic BP ≥ 90 mmHg and/or with antihypertensive treatment)
Fig. 3.
Factors associated with FLI ≥ 60† in women by age groups. Odds ratios (OR) and 95% confidence interval (95% CI) of the association between FLI ≥ 60† and social class (high; low), categories of diabetes (no diabetes; prediabetes; diabetes), HDL-cholesterol (mg/dL), categories of blood pressure (BP) (normal; prehypertension; hypertension), and smoking (no smoking, former smoker, current smoker), in men by age groups. Binary logistic regression adjusted by age. *p < 0.05, **p < 0.01, ***p < 0.001. †FLI (< 60 vs. ≥ 60). FLI: fatty liver index; diabetes categories: normal (fasting plasma glucose < 100), prediabetes (fasting plasma glucose 100 to 125), diabetes (fasting plasma glucose > 125 and/or antidiabetic treatment); BP: blood pressure; BP categories: normal BP (systolic BP < 130 and/or diastolic BP < 85 mmHg), prehypertension (systolic BP 130 to 139 and/or diastolic BP 85 to 89 mmHg), hypertension (systolic BP ≥ 140 and/or diastolic BP ≥ 90 mmHg and/or with antihypertensive treatment)
Discussion
The present cross-sectional study showed that 19.1% of our population of Spanish workers presented a FLI-defined NAFLD (FLI ≥ 60), and that it was significantly more prevalent in men (27.9%) than in women (6.8%). Subjects with high FLI values were generally more obese, presented worse cardiometabolic and anthropometric profiles, and had a higher CVR. FLI-defined NAFLD was associated with age (in men only), HDL-cholesterol, social class, smoking status, prediabetes, T2D, prehypertension and hypertension. In both sexes, T2D and hypertension were strongly associated with FLI-defined NAFLD than prediabetes and hypertension, however such associations were stronger in women than men.
Prevalence of FLI-defined NAFLD
The prevalence of NAFLD by FLI in this study is lower than the worldwide prevalence estimated at 24% [8], but in agreement with the percentages of between 20 and 30% reported for Western countries [34–37]. Specific to Spain, according to the most recent cross-sectional population study published in 2010, which included men and women between 15 and 85 years of age, the prevalence of NAFLD diagnosed by abdominal echography was 25.8% [18]. Such difference in the percentage of prevalence could be explained by the broader age range of the study population. Age strongly correlates with a variety of major cardiometabolic risk factors which in turn might contribute to the development of NAFLD [3, 38], and in the same study the highest prevalence was among those above 60 years (35.6%).
Recent population-based studies also indicate that NAFLD is more prevalent in men than in women [13], at least up the age of menopause, after which such differences tend to level out [39]. Specific to age, this phenomenon has been previously described as an inverted U-shaped curve, where prevalence of NAFLD in men increases during adulthood, and declines after the age of 50–60 years, while in women it rises during menopausal age, to decline after the age of 70 years [40, 41]. The later rise of NAFLD in women may be influenced by hormonal changes [42, 43], increased insulin resistance and visceral fat occurring during menopause, predisposing them to NAFLD and CV disease [44]. Moreover, the reported decreased ratios of NAFLD in older people could possibly reflect the decreased survival in those with NAFLD [42, 45]. Our sample included adult subjects up to 65 years, thus our results simply showed a linear association between FLI-defined NAFLD and age in both sexes, however the prevalence in men was consistently higher than in women across all ages.
FLI-defined NAFLD and associated comorbidities
Several studies reported a high burden of cardiometabolic comorbidities associated with NAFLD [46, 47]. Obesity, and more specifically visceral fat, are particularly important in the pathogenesis of NAFLD [8, 18, 48–51]. Visceral fat is a recognized major endocrine organ secreting cytokine and adipokines responsible for increased liver fat accumulation as an effect of insulin resistance [52, 53]. Moreover, in recent years it has been observed that excess visceral adipose tissue can affect lean individuals as much as obese, such that individuals with an apparent healthy weight can manifest MetS [54]. In our study, we observed that even at lower BMI levels, men were more at risk of NAFLD than women, which could be explained by the possible difference in body fat distribution between sexes [15, 16]. Although women present higher percentages of total body fat, it is mainly distributed around the hips and thighs, while the tendency for men is to accumulate it around the waist, making men more possibly prone to fatty liver infiltrations even at lean BMI levels [55].
NAFLD significantly increases the risk of prediabetes and its progression to T2D [11, 56]; moreover, once T2D and NAFLD are both present, it has been observed that the former becomes an independent predictor of the progression of NAFLD to advanced fibrosis [57, 58] and that patients with T2D have more severe NAFLD and a higher prevalence of NASH and fibrosis as compared to those without [59]. As expected, in our study, prediabetes and T2D had a significantly higher prevalence in the FLI ≥ 60 category compared to the other two and were strongly associated with FLI-defined NAFLD in both men and women. When comparing sexes, although if the percentage of diabetic men was higher than that of women, the strength of the association between prediabetes and T2D with FLI-defined NAFLD was almost doubled in women as compared to men. Across age groups, in men, T2D was most strongly associated with FLI-defined NAFLD in the 30–39 year category, whereas in women, except for the youngest age group, the strength of the association was similar across all ages and comparable or even higher to that of men of 30–39 years. To the best of our knowledge, besides the few studies examining menopause or age-specific sex differences in NAFLD prevalence, which suggest that estrogen protects from NAFLD [40, 41], there is no evidence available which specifically explores possible sex differences in the association between prediabetes or T2D with NAFLD. What has been observed is that in patients with biopsy proven or imaging assessed NAFLD those who were females and type 2 diabetics were more likely to present NASH and elevated liver enzymes [60], however the median age of the sample was 50 years and thus older than our current population. In any case, when it comes to diabetic women, the protective effect of the female sex is attenuated and, across all ages, women are at higher risk than men of diabetic complications and associated comorbidities [14]. For what we could observe, in the present study, the female sex seemed to protect against FLI-defined NAFLD only in the absence of a dysmetabolic state, which, when present, leave women exposed to suffer from liver fat accumulation.
Hypertension is another risk factor associated with NAFLD. As in the case of T2D, also NAFLD and hypertension have a bidirectional relationship, where NAFLD may influence the development of hypertension and hypertension can lead to more severe liver disease [53], and greatly contribute to the increased risk of stroke and ischemic heart disease in the NAFLD population [61]. Our study showed that the prevalence of prehypertension and hypertension increased across FLI categories for both men and women; moreover, for both sexes, prehypertension and hypertension were independently associated with FLI ≥ 60, although the association was stronger in women than men for each age group. Like for prediabetes and T2D there is a lack of evidence available on the possible sex differences in the association between prehypertension or hypertension with NAFLD; nevertheless, as mentioned above, the presence of a dysmetabolic state might affect women more than men with regard to liver outcomes.
Growing evidence suggests that socio-economic status is inversely related to the prevalence of NAFLD [62–65], and that lower incomes and education levels are associated with worse liver outcomes [66]. Social status has a significant influence in predicting NAFLD, and during routine clinical practice, those who belong to lower social classes should be accurately screened for a possible higher risk [66]. The social class effect on health and disease has been observed to possibly start from early adolescence and diminish with increasing age [67]. Accordingly, we observed a significant association between low social class and FLI ≥ 60 only up to the age of 49 years for men and 39 years for women, after which it becames non-significant, independently of sex.
The role of smoking status in NAFLD development remains unclear because of conflicting results. Some studies showed that smoking was an independent risk factor for incident NAFLD [68, 69], however others found no association [70, 71]. According to our results, smoking is not associated with FLI-defined NAFLD but rather with its absence; however, it could be argued that it is not smoking per se to be a protective factor, but its anorexigenic effect [72].
Study limitations
Our study also has a few limitations to be considered. First, the cross-sectional design limits inferences on causality. Second, NAFLD was assessed by FLI; in a clinical setting, the gold standard for diagnosing the presence of NAFLD is by liver biopsy. However due to invasiveness, associated risks, and costs, it is unviable in large population-based studies; moreover it is advised only in cases of severe liver disease [7, 20, 21]. Alternatively, imaging tests of the liver are widely used [24], however, these procedures may be costly, time-consuming, and generally unpractical. The FLI equation is a simple alternative when performing diagnostic imaging is not feasible and has been shown to have high predictive accuracy in identifying people at risk of NAFLD in different large epidemiological studies [24]. Third, a selection bias and, more specifically, the one described as the “healthy workers effect” should also be considered [73]. The bias, if present, could lead to the underestimation of the prevalence rates; nevertheless the study is providing information on a working population.
Study strengths
The main strength of our study is the large sample size, which allowed us to analyze and compare different age and sex groups in detail. Furthermore, we included subjects belonging to multiple occupations, indicating that our sample may be considered representative of the Spanish workforce.
Conclusions
Our results indicate that FLI-defined NAFLD is more prevalent in men than women, and it is associated with age, lower social class, and a dysmetabolic state characterized by prediabetes or diabetes, prehypertension or hypertension, and low HDL-cholesterol. Most importantly, although FLI-defined NAFLD affects men more than women, when a dysmetabolic state is present, women also become exposed to liver fat infiltrations independently of age.
Previous observations show that diabetic women are at higher risk of diabetes-related complications and associated comorbidities than men, and that at the time of menopause they might also present more advances stages of NAFLD. Our study further suggests that worse liver outcomes can be experienced at younger ages and at stages in which diabetes and hypertension are not yet fully manifested.
The understanding of sex and age differences in risk factors for NAFLD could help clinicians correctly implement more personalized strategies for the prevention of this condition. Most risk factors associated with NAFLD are modifiable, and sex- and age-specific lifestyle interventions, if timely implemented when cardiometabolic imbalances are still subclinical, could prevent the onset of NAFLD and its progression.
Supplementary Information
Additional file 1: Figure S1. BMI (kg/m2), waist circumference (cm) and fasting plasma glucose (mg/dL), total cholesterol (mg/dL), HDL-cholesterol (mg/dL) and triglycerides (mg/dL) for men and women by categories of FLI.
Additional file 2: Table S1. Differences between men and women in the prevalence of categories of FLI by age intervals.
Acknowledgements
The authors are grateful to the field staff and participants of this study.
Author contributions
Conceptualization, SF, MB-V, CB-C, and AY; methodology, SF, MA, and MB-V; formal analysis, SF, MA, MB-V and AY; investigation, AL-G; data curation, SF, CB-C, MA, and AL-G; writing—original draft preparation, SF, CB-C, and MA; writing—review and editing, MB-V and AY. All authors read and approved the final manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The protocol was approved by the Balearic Ethical Committee of Clinical Research (Ref. No: CEI-IB-1887). All participants provided written informed consent prior to enrollment in the study.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sergio Fresneda, Email: s.fresneda@uib.es.
Manuela Abbate, Email: manuela.abbate@uib.es.
Carla Busquets-Cortés, Email: c.busquets@eua.edu.es.
Arturo López-González, Email: a.lopez@eua.edu.es.
Pilar Fuster-Parra, Email: pilar.fuster@uib.es.
Miquel Bennasar-Veny, Email: miquel.bennasar@uib.es.
Aina M. Yáñez, Email: aina.yanez@uib.es
References
- 1.Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15(1):11–20. doi: 10.1038/nrgastro.2017.109. [DOI] [PubMed] [Google Scholar]
- 2.Brown GT, Kleiner DE. Histopathology of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Metabolism. 2016;65(8):1080–1086. doi: 10.1016/j.metabol.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilman HR, Kelly M, Garratt S, Matthews PM, Milanesi M, Herlihy A, et al. Characterisation of liver fat in the UK Biobank cohort. PLoS ONE. 2017;12(2):e0172921. doi: 10.1371/journal.pone.0172921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Targher G, Byrne CD, Lonardo A, Zoppini G, Barbui C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: a meta-analysis. J Hepatol. 2016;65(3):589–600. doi: 10.1016/j.jhep.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 5.Nishi T, Babazono A, Maeda T, Imatoh T, Une H. Evaluation of the fatty liver index as a predictor for the development of diabetes among insurance beneficiaries with prediabetes. J Diabetes Investig. 2015;6(3):309–316. doi: 10.1111/jdi.12290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Forlani G, Giorda C, Manti R, Mazzella N, De Cosmo S, Rossi MC, et al. The burden of NAFLD and its characteristics in a nationwide population with type 2 diabetes. J Diabetes Res. 2016;2016:2931985. doi: 10.1155/2016/2931985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Franch-Nadal J, Caballeria L, Mata-Cases M, Mauricio D, Giraldez-Garcia C, Mancera J, et al. Fatty liver index is a predictor of incident diabetes in patients with prediabetes: the PREDAPS study. PLoS ONE. 2018;13(6):e0198327. doi: 10.1371/journal.pone.0198327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 9.Lorbeer R, Bayerl C, Auweter S, Rospleszcz S, Lieb W, Meisinger C, et al. Association between MRI-derived hepatic fat fraction and blood pressure in participants without history of cardiovascular disease. J Hypertens. 2017;35(4):737–744. doi: 10.1097/HJH.0000000000001245. [DOI] [PubMed] [Google Scholar]
- 10.Soderberg C, Stal P, Askling J, Glaumann H, Lindberg G, Marmur J, et al. Decreased survival of subjects with elevated liver function tests during a 28-year follow-up. Hepatology. 2010;51(2):595–602. doi: 10.1002/hep.23314. [DOI] [PubMed] [Google Scholar]
- 11.Ekstedt M, Franzen LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44(4):865–873. doi: 10.1002/hep.21327. [DOI] [PubMed] [Google Scholar]
- 12.Clayton JA. Studying both sexes: a guiding principle for biomedicine. FASEB J. 2016;30(2):519–524. doi: 10.1096/fj.15-279554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lonardo A, Nascimbeni F, Ballestri S, Fairweather D, Win S, Than TA, et al. Sex differences in nonalcoholic fatty liver disease: state of the art and identification of research gaps. Hepatology. 2019;70(4):1457–1469. doi: 10.1002/hep.30626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kautzky-Willer A, Harreiter J, Pacini G. Sex and gender differences in risk, pathophysiology and complications of type 2 diabetes mellitus. Endocr Rev. 2016;37(3):278–316. doi: 10.1210/er.2015-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Janssen I, Heymsfield SB, Allison DB, Kotler DP, Ross R. Body mass index and waist circumference independently contribute to the prediction of nonabdominal, abdominal subcutaneous, and visceral fat. Am J Clin Nutr. 2002;75(4):683–688. doi: 10.1093/ajcn/75.4.683. [DOI] [PubMed] [Google Scholar]
- 16.Ballestri S, Nascimbeni F, Baldelli E, Marrazzo A, Romagnoli D, Lonardo A. NAFLD as a sexual dimorphic disease: role of gender and reproductive status in the development and progression of nonalcoholic fatty liver disease and inherent cardiovascular risk. Adv Ther. 2017;34(6):1291–1326. doi: 10.1007/s12325-017-0556-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tramunt B, Smati S, Grandgeorge N, Lenfant F, Arnal JF, Montagner A, et al. Sex differences in metabolic regulation and diabetes susceptibility. Diabetologia. 2020;63(3):453–461. doi: 10.1007/s00125-019-05040-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caballeria L, Pera G, Auladell MA, Toran P, Munoz L, Miranda D, et al. Prevalence and factors associated with the presence of nonalcoholic fatty liver disease in an adult population in Spain. Eur J Gastroenterol Hepatol. 2010;22(1):24–32. doi: 10.1097/MEG.0b013e32832fcdf0. [DOI] [PubMed] [Google Scholar]
- 19.Riazi K, Azhari H, Charette JH, Underwood FE, King JA, Afshar EE, et al. The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2022;7(9):851–861. doi: 10.1016/S2468-1253(22)00165-0. [DOI] [PubMed] [Google Scholar]
- 20.Fazel Y, Koenig AB, Sayiner M, Goodman ZD, Younossi ZM. Epidemiology and natural history of non-alcoholic fatty liver disease. Metabolism. 2016;65(8):1017–1025. doi: 10.1016/j.metabol.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 21.Nascimbeni F, Pais R, Bellentani S, Day CP, Ratziu V, Loria P, et al. From NAFLD in clinical practice to answers from guidelines. J Hepatol. 2013;59(4):859–871. doi: 10.1016/j.jhep.2013.05.044. [DOI] [PubMed] [Google Scholar]
- 22.Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, et al. The fatty liver index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:33. doi: 10.1186/1471-230X-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calori G, Lattuada G, Ragogna F, Garancini MP, Crosignani P, Villa M, et al. Fatty liver index and mortality: the Cremona study in the 15th year of follow-up. Hepatology. 2011;54(1):145–152. doi: 10.1002/hep.24356. [DOI] [PubMed] [Google Scholar]
- 24.Papagianni M, Sofogianni A, Tziomalos K. Non-invasive methods for the diagnosis of nonalcoholic fatty liver disease. World J Hepatol. 2015;7(4):638–648. doi: 10.4254/wjh.v7.i4.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jager S, Jacobs S, Kroger J, Stefan N, Fritsche A, Weikert C, et al. Association between the fatty liver index and risk of type 2 diabetes in the EPIC-Potsdam study. PLoS ONE. 2015;10(4):e0124749. doi: 10.1371/journal.pone.0124749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bennasar-Veny M, Fresneda S, Lopez-Gonzalez A, Busquets-Cortes C, Aguilo A, Yanez AM. Lifestyle and progression to type 2 diabetes in a cohort of workers with prediabetes. Nutrients. 2020;12(5):1538. doi: 10.3390/nu12051538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Domingo-Salvany A, Bacigalupe A, Carrasco JM, Espelt A, Ferrando J, Borrell C, et al. Proposals for social class classification based on the Spanish national classification of occupations 2011 using neo-Weberian and neo-Marxist approaches. Gac Sanit. 2013;27(3):263–272. doi: 10.1016/j.gaceta.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 28.Stewart A, Marfell-Jones M, Olds T, De Ridder H. International standards for anthropometric assessment; 2011.
- 29.Obesity WHOCo, World Health O. Obesity: preventing and managing the global epidemic: report of a WHO consultation. Geneva: World Health Organization; 2000. [PubMed]
- 30.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112(17):2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 31.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 32.Marrugat JSP, D’Agostino R, Sullivan L, Ordovas J, et al. Coronary risk estimation in Spain using a calibrated Framingham function. Rev Esp Cardiol. 2003;56:253–261. doi: 10.1016/s0300-8932(03)76861-4. [DOI] [PubMed] [Google Scholar]
- 33.Koehler EM, Schouten JN, Hansen BE, Hofman A, Stricker BH, Janssen HL. External validation of the fatty liver index for identifying nonalcoholic fatty liver disease in a population-based study. Clin Gastroenterol Hepatol. 2013;11(9):1201–1204. doi: 10.1016/j.cgh.2012.12.031. [DOI] [PubMed] [Google Scholar]
- 34.Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD single topic conference. Hepatology. 2003;37(5):1202–1219. doi: 10.1053/jhep.2003.50193. [DOI] [PubMed] [Google Scholar]
- 35.Bedogni G, Bellentani S. Fatty liver: how frequent is it and why? Ann Hepatol. 2004;3(2):63–65. [PubMed] [Google Scholar]
- 36.Bellentani S, Saccoccio G, Masutti F, Croce LS, Brandi G, Sasso F, et al. Prevalence of and risk factors for hepatic steatosis in northern Italy. Ann Intern Med. 2000;132(2):112–117. doi: 10.7326/0003-4819-132-2-200001180-00004. [DOI] [PubMed] [Google Scholar]
- 37.Bedogni G, Miglioli L, Masutti F, Tiribelli C, Marchesini G, Bellentani S. Prevalence of and risk factors for nonalcoholic fatty liver disease: the Dionysos nutrition and liver study. Hepatology. 2005;42(1):44–52. doi: 10.1002/hep.20734. [DOI] [PubMed] [Google Scholar]
- 38.Kim IH, Kisseleva T, Brenner DA. Aging and liver disease. Curr Opin Gastroenterol. 2015;31(3):184–191. doi: 10.1097/MOG.0000000000000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Z, Xu M, Hu Z, Hultstrom M, Lai E. Sex-specific prevalence of fatty liver disease and associated metabolic factors in Wuhan, south central China. Eur J Gastroenterol Hepatol. 2014;26(9):1015–1021. doi: 10.1097/MEG.0000000000000151. [DOI] [PubMed] [Google Scholar]
- 40.Fan JG, Zhu J, Li XJ, Chen L, Li L, Dai F, et al. Prevalence of and risk factors for fatty liver in a general population of Shanghai, China. J Hepatol. 2005;43(3):508–514. doi: 10.1016/j.jhep.2005.02.042. [DOI] [PubMed] [Google Scholar]
- 41.Eguchi Y, Hyogo H, Ono M, Mizuta T, Ono N, Fujimoto K, et al. Prevalence and associated metabolic factors of nonalcoholic fatty liver disease in the general population from 2009 to 2010 in Japan: a multicenter large retrospective study. J Gastroenterol. 2012;47(5):586–595. doi: 10.1007/s00535-012-0533-z. [DOI] [PubMed] [Google Scholar]
- 42.Bertolotti M, Lonardo A, Mussi C, Baldelli E, Pellegrini E, Ballestri S, et al. Nonalcoholic fatty liver disease and aging: epidemiology to management. World J Gastroenterol. 2014;20(39):14185–14204. doi: 10.3748/wjg.v20.i39.14185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Puig J, Blasco G, Daunis IEJ, Loshuertos E, Codina J, Cuba V, et al. Nonalcoholic fatty liver disease and age are strong indicators for atherosclerosis in morbid obesity. Clin Endocrinol (Oxf) 2015;83(2):180–186. doi: 10.1111/cen.12698. [DOI] [PubMed] [Google Scholar]
- 44.Lee C, Kim J, Jung Y. Potential therapeutic application of estrogen in gender disparity of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Cells. 2019;8(10):1259. doi: 10.3390/cells8101259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ballestri S, Lonardo A, Bonapace S, Byrne CD, Loria P, Targher G. Risk of cardiovascular, cardiac and arrhythmic complications in patients with non-alcoholic fatty liver disease. World J Gastroenterol. 2014;20(7):1724–1745. doi: 10.3748/wjg.v20.i7.1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee JY, Kim KM, Lee SG, Yu E, Lim YS, Lee HC, et al. Prevalence and risk factors of non-alcoholic fatty liver disease in potential living liver donors in Korea: a review of 589 consecutive liver biopsies in a single center. J Hepatol. 2007;47(2):239–244. doi: 10.1016/j.jhep.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 47.Chung GE, Yim JY, Kim D, Lim SH, Yang JI, Kim YS, et al. The influence of metabolic factors for nonalcoholic fatty liver disease in women. Biomed Res Int. 2015;2015:131528. doi: 10.1155/2015/131528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Caballeria J, Caballeria L. Nonalcoholic steatohepatitis and diabetes. Endocrinol Nutr. 2016;63(8):377–379. doi: 10.1016/j.endonu.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 49.Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol. 2013;10(6):330–344. doi: 10.1038/nrgastro.2013.41. [DOI] [PubMed] [Google Scholar]
- 50.Balkau B, Lange C, Vol S, Fumeron F, Bonnet F. Nine-year incident diabetes is predicted by fatty liver indices: the French DESIR study. BMC Gastroenterol. 2010;10:56. doi: 10.1186/1471-230X-10-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fung J, Lee CK, Chan M, Seto WK, Lai CL, Yuen MF, et al. High prevalence of non-alcoholic fatty liver disease in the Chinese—results from the Hong Kong liver health census. Liver Int. 2015;35(2):542–549. doi: 10.1111/liv.12619. [DOI] [PubMed] [Google Scholar]
- 52.Saponaro C, Gaggini M, Gastaldelli A. Nonalcoholic fatty liver disease and type 2 diabetes: common pathophysiologic mechanisms. Curr Diabetes Rep. 2015;15(6):607. doi: 10.1007/s11892-015-0607-4. [DOI] [PubMed] [Google Scholar]
- 53.Lonardo A, Nascimbeni F, Mantovani A, Targher G. Hypertension, diabetes, atherosclerosis and NASH: cause or consequence? J Hepatol. 2018;68(2):335–352. doi: 10.1016/j.jhep.2017.09.021. [DOI] [PubMed] [Google Scholar]
- 54.Ding C, Chan Z, Magkos F. Lean, but not healthy: the ‘metabolically obese, normal-weight’ phenotype. Curr Opin Clin Nutr Metab Care. 2016;19(6):408–417. doi: 10.1097/MCO.0000000000000317. [DOI] [PubMed] [Google Scholar]
- 55.Karastergiou K, Smith SR, Greenberg AS, Fried SK. Sex differences in human adipose tissues—the biology of pear shape. Biol Sex Differ. 2012;3(1):13. doi: 10.1186/2042-6410-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Busquets-Cortes C, Bennasar-Veny M, Lopez-Gonzalez AA, Fresneda S, Aguilo A, Yanez A. Fatty liver index and progression to type 2 diabetes: a 5-year longitudinal study in Spanish workers with pre-diabetes. BMJ Open. 2021;11(8):e045498. doi: 10.1136/bmjopen-2020-045498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pais R, Charlotte F, Fedchuk L, Bedossa P, Lebray P, Poynard T, et al. A systematic review of follow-up biopsies reveals disease progression in patients with non-alcoholic fatty liver. J Hepatol. 2013;59(3):550–556. doi: 10.1016/j.jhep.2013.04.027. [DOI] [PubMed] [Google Scholar]
- 58.Fracanzani AL, Valenti L, Bugianesi E, Andreoletti M, Colli A, Vanni E, et al. Risk of severe liver disease in nonalcoholic fatty liver disease with normal aminotransferase levels: a role for insulin resistance and diabetes. Hepatology. 2008;48(3):792–798. doi: 10.1002/hep.22429. [DOI] [PubMed] [Google Scholar]
- 59.Goh GB, Pagadala MR, Dasarathy J, Unalp-Arida A, Sargent R, Hawkins C, et al. Clinical spectrum of non-alcoholic fatty liver disease in diabetic and non-diabetic patients. BBA Clin. 2015;3:141–145. doi: 10.1016/j.bbacli.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aller de la Fuente R, Mora Cuadrado N, Tafur C, Lopez Gomez JJ, Gomez de la Cuesta S, Garcia Sanchez MC, et al. Histopathological differences in patients with biopsy-proven non-alcoholic fatty liver disease with and without type 2 diabetes. Endocrinol Diabetes Nutr. 2018;65(6):354–360. doi: 10.1016/j.endinu.2017.12.011. [DOI] [PubMed] [Google Scholar]
- 61.Tana C, Ballestri S, Ricci F, Di Vincenzo A, Ticinesi A, Gallina S, et al. Cardiovascular risk in non-alcoholic fatty liver disease: mechanisms and therapeutic implications. Int J Environ Res Public Health. 2019;16(17):3104. doi: 10.3390/ijerph16173104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dai W, Ye L, Liu A, Wen SW, Deng J, Wu X, et al. Prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus: a meta-analysis. Medicine (Baltimore) 2017;96(39):e8179. doi: 10.1097/MD.0000000000008179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hu W, Liu Z, Hao HR, Yu WN, Wang XQ, Shao XJ, et al. Correlation between income and non-alcoholic fatty liver disease in a Chinese population. Ann Endocrinol (Paris) 2020;81(6):561–566. doi: 10.1016/j.ando.2020.07.1109. [DOI] [PubMed] [Google Scholar]
- 64.Orkin S, Brokamp C, Yodoshi T, Trout AT, Liu C, Meryum S, et al. Community socioeconomic deprivation and nonalcoholic fatty liver disease severity. J Pediatr Gastroenterol Nutr. 2020;70(3):364–370. doi: 10.1097/MPG.0000000000002527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cho J, Lee I, Park DH, Kwak HB, Min K. Relationships between socioeconomic status, handgrip strength, and non-alcoholic fatty liver disease in middle-aged Adults. Int J Environ Res Public Health. 2021;18(4):1892. doi: 10.3390/ijerph18041892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Giammarino AM, Qiu H, Bulsara K, Khan S, Jiang Y, Da BL, et al. Community socioeconomic deprivation predicts nonalcoholic steatohepatitis. Hepatol Commun. 2022;6(3):550–560. doi: 10.1002/hep4.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang YC, Schorpp K, Boen C, Johnson M, Harris KM. Socioeconomic status and biological risks for health and illness across the life course. J Gerontol B Psychol Sci Soc Sci. 2020;75(3):613–624. doi: 10.1093/geronb/gby108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jung HS, Chang Y, Kwon MJ, Sung E, Yun KE, Cho YK, et al. Smoking and the risk of non-alcoholic fatty liver disease: a cohort study. Am J Gastroenterol. 2019;114(3):453–463. doi: 10.1038/s41395-018-0283-5. [DOI] [PubMed] [Google Scholar]
- 69.Hamabe A, Uto H, Imamura Y, Kusano K, Mawatari S, Kumagai K, et al. Impact of cigarette smoking on onset of nonalcoholic fatty liver disease over a 10-year period. J Gastroenterol. 2011;46(6):769–778. doi: 10.1007/s00535-011-0376-z. [DOI] [PubMed] [Google Scholar]
- 70.Chavez-Tapia NC, Lizardi-Cervera J, Perez-Bautista O, Ramos-Ostos MH, Uribe M. Smoking is not associated with nonalcoholic fatty liver disease. World J Gastroenterol. 2006;12(32):5196–5200. doi: 10.3748/wjg.v12.i32.5196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shen H, Peng JL, Tayarachakul S, Liangpunsakul S. Association between serum cotinine level and prevalence of non-alcoholic fatty liver disease: a cross-sectional study from the third national health and nutrition examination survey. J Investig Med. 2017;65(1):43–48. doi: 10.1136/jim-2016-000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stojakovic A, Espinosa EP, Farhad OT, Lutfy K. Effects of nicotine on homeostatic and hedonic components of food intake. J Endocrinol. 2017;235(1):R13–R31. doi: 10.1530/JOE-17-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chowdhury R, Shah D, Payal AR. Healthy worker effect phenomenon: revisited with emphasis on statistical methods—a review. Indian J Occup Environ Med. 2017;21(1):2–8. doi: 10.4103/ijoem.IJOEM_53_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. BMI (kg/m2), waist circumference (cm) and fasting plasma glucose (mg/dL), total cholesterol (mg/dL), HDL-cholesterol (mg/dL) and triglycerides (mg/dL) for men and women by categories of FLI.
Additional file 2: Table S1. Differences between men and women in the prevalence of categories of FLI by age intervals.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.