Abstract
Background
Biological sex impacts susceptibility and presentation of cardiovascular disease, which remains the leading cause of death for both sexes. To reduce cardiovascular disease risk, statin drugs are commonly prescribed to reduce circulating cholesterol levels through inhibition of cholesterol synthesis. The effectiveness of statin therapy differs between individuals with a sex bias in the frequency of adverse effects. Limited information is available regarding the mechanisms driving sex-specific responses to hypercholesterolemia or statin treatment.
Methods
Four Core Genotypes mice (XX and XY mice with ovaries and XX and XY mice with testes) on a hypercholesteremic Apoe–/– background were fed a chow diet without or with simvastatin for 8 weeks. Plasma lipid levels were quantified and hepatic differential gene expression was evaluated with RNA-sequencing to identify the independent effects of gonadal and chromosomal sex.
Results
In a hypercholesterolemic state, gonadal sex influenced the expression levels of more than 3000 genes, and chromosomal sex impacted expression of nearly 1400 genes, which were distributed across all autosomes as well as the sex chromosomes. Gonadal sex uniquely influenced the expression of ER stress response genes, whereas chromosomal and gonadal sex influenced fatty acid metabolism gene expression in hypercholesterolemic mice. Sex-specific effects on gene regulation in response to statin treatment included a compensatory upregulation of cholesterol biosynthetic gene expression in mice with XY chromosome complement, regardless of presence of ovaries or testes.
Conclusion
Gonadal and chromosomal sex have independent effects on the hepatic transcriptome to influence different cellular pathways in a hypercholesterolemic environment. Furthermore, chromosomal sex in particular impacted the cellular response to statin treatment. An improved understanding of how gonadal and chromosomal sex influence cellular response to disease conditions and in response to drug treatment is critical to optimize disease management for all individuals.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13293-022-00474-8.
Introduction
Cardiovascular disease (CVD) remains the leading cause of death for both men and women in the United States. CVD mortality for males and females combined declined from 1980 to 2010, but has increased from 2010 to 2019 (the most recent time point for which figures are available) [1]. This is despite extensive knowledge regarding the risk factors for the development of CVDs (including hypercholesterolemia, high blood pressure, and diabetes), and the widespread use of drugs to reduce these risk factors. A fundamental factor that influences CVD susceptibility is biological sex. Men are more susceptible than women to the most common form of cardiovascular disease, coronary artery disease, through age 50 [2, 3]. However, women with coronary artery disease before age 50 have a worse prognosis than men, and following menopause, the incidence of disease in women increases and overtakes that in men at advanced ages.
Statin drugs are widely prescribed worldwide to reduce CVD risk by inhibiting hepatic cholesterol synthesis and reducing circulating cholesterol levels. Although statins are largely effective at reducing CVD risk, statin response differs among individuals. In particular, biological females are more likely to experience adverse statin effects, including myopathy and new-onset diabetes [4–6]. Elucidating the mechanisms that underlie sex-specific responses to hypercholesterolemia and statin treatment would be a valuable step toward optimizing CVD prevention and treatment for both sexes.
The liver is the central organ for cholesterol homeostasis and statin action [7]. During the postprandial period, hepatocytes take up lipids from intestinally derived lipoproteins (chylomicrons) that contain triglycerides and cholesterol esters; during the fasting state, hepatocytes take up fatty acids released from triglyceride stores in adipose tissue. The fatty acids may be oxidized, or fatty acids and cholesterol may be esterified and stored as lipid droplets. Hepatocytes package triglycerides and cholesterol esters into very low-density lipoproteins (VLDL) which are secreted and transport triglycerides and cholesterol to peripheral tissues. The hydrolysis of lipids within the core of liver-derived lipoproteins converts them to intermediate-density lipoproteins, and ultimately, low-density lipoproteins (LDL), which carry most cholesterol in the circulation and are imported into tissues throughout the body via the LDL receptor.
Hepatocytes also synthesize cholesterol and fatty acids de novo. The regulation of hepatic cholesterol biosynthesis is under complex metabolic control by multiple factors, most notably the sterol response element binding protein 2 (SREBP2) transcription factor [8]. When cellular cholesterol levels are low, SREBP2 is activated to induce expression of genes involved in cholesterol synthesis, including that of the rate-limiting enzyme, hydroxymethylglutaryl (HMG)-CoA reductase. When cholesterol accumulates, hepatocytes down-regulate SREBP2 (through the action of the liver X receptor alpha transcription factor) and reduce cholesterol synthesis. The utility of statin drugs in reduction of CVD risk stems from their action as competitive inhibitors of HMG-CoA reductase to reduce endogenous cholesterol biosynthesis [9].
Despite the long history of studies on hypercholesterolemia as a CVD risk factor, and statin drug action, little is known about the mechanistic basis for sex differences in hypercholesterolemia or statin effects. Here, we dissect sex determinants of the hepatic transcriptome in the hypercholesterolemic state and in response to statin treatment using the Four Core Genotypes (FCG) mouse model. This model independently segregates the development of gonads from the sex chromosomes to generate four sex genotypes: XX and XY mice with ovaries and XX and XY mice with testes [3, 10]. We identified specific effects of gonadal sex and chromosomal sex on plasma lipid levels and hepatic gene expression pathways in the hypercholesterolemic state. We further identified the role of biological sex components in statin lipid lowering and regulation of hepatic gene expression. These findings reveal that both gonadal and genetic sex differences contribute to the regulation of a key CVD risk factor and a widely used therapeutic intervention.
Methods
Animals
The Apoe–/–;FCG mice were maintained at UCLA for > 15 generations and were generated as previously described [11, 12]. In brief, female C57BL/6J apolipoprotein E knockout (Apoe–/–) mice (#002052, Jackson Laboratory, Bar Harbor, ME) were crossed with XY–(Sry+) Apoe–/– male mice to generate XX, XX(Sry+), XY–, and XY–(Sry+) offspring on an Apoe-deficient background. This mouse cohort is referred to as “FCG mice” throughout. At 8–10 weeks of age, mice were fed a chow diet containing 5%–6% fat calories (D1001, Research Diets, New Brunswick, NJ) or the same diet containing pharmaceutical grade simvastatin (0.1 g/kg; prepared at Research Diets under formulation D11060903i) for 8 weeks. The statin concentration was calculated to be the equivalent in mouse of an 80 mg/day dose in human. All mouse studies were conducted in accordance with UCLA Institutional Animal Research Committee (IACUC) approval under a 12-h light/dark cycle with ad libitum access to food and water. At time of tissue collection, mice were fasted for 5 h and tissues were collected and flash-frozen in liquid nitrogen, followed by storage in – 80 °C.
Plasma lipid quantitation
Total cholesterol, triglycerides and free fatty acid levels were determined in mouse plasma via enzymatic reactions and colorimetric detection [13].
RNA-sequencing
RNA from snap-frozen liver samples was isolated with TRIzol (Thermo Fisher Scientific, Waltham, MA). The RNA-seq libraries were generated as previously described [14], including poly (A) RNA selection, RNA fragmentation, oligo(dT) priming with cDNA synthesis, adaptor ligation to double-stranded DNA, strand selection and PCR amplification to produce the final libraries. Index adaptors were used to multiplex samples after quantification (Quibit) and for quality evaluation (4200 TapeStation, Agilent). Sequencing was performed on a NovaSeq 6000 sequencer at the UCLA Technology Center for Genomics & Bioinformatics. Processing of RNA-seq raw data was performed as previously described [14]. Reads were aligned to mouse genome (GRCm38.97) and read counts per gene were generated with STAR. Differential gene expression analysis was performed with DESeq2 (version 1.26, n = 3 per FCG genotype and treatment) and a P value < 0.05 demonstrating significance, unless otherwise noted [15]. Pathway analysis was performed for differentially expressed genes with > 1.25-fold change and adjusted P ≤ 0.05 (chow only comparisons) or P ≤ 0.05 (statin-treated comparisons) using Enrichr [16–18]. MA plots, PCA plot, and normalized read boxplots were generated in R. RNA-seq data have been deposited in GEO (Accession number GSE202977). For comparative analysis with additional RNA-sequencing datasets, data were downloaded from supplemental data or GEO for GSE98586 and GSE154217 [19, 20].
Quantitative real-time PCR
For gene expression quantification, RNA was reverse transcribed to cDNA with iScript reverse transcriptase (Bio-Rad, Hercules, CA) and quantified by quantitative real-time PCR (RT-PCR) with SsoAdvanced SYBR Green Supermix on Bio-Rad CFX Opux 384 Real-Time PCR Detection System. All real-time PCR data were normalized to Rplp0 (also known as 36B4) gene expression. Additional file 1: Table S1 contains all primer sequences used in this study.
Statistical analyses
Graphpad Prism 9 and RStudio were used for all statistical analyses and data representation. FCG lipid levels and gene expression were analyzed by two-way ANOVA with main factors of gonads (testes vs. ovaries) and sex chromosome complement (XX vs. XY), and assessment of an interaction between the two factors. Values for the statin-induced changes in lipid levels shown in Fig. 1B–D were calculated as the difference between the mean lipid level for each genotype without statin treatment and the lipid level of each individual mouse after statin treatment, followed by two-way ANOVA.
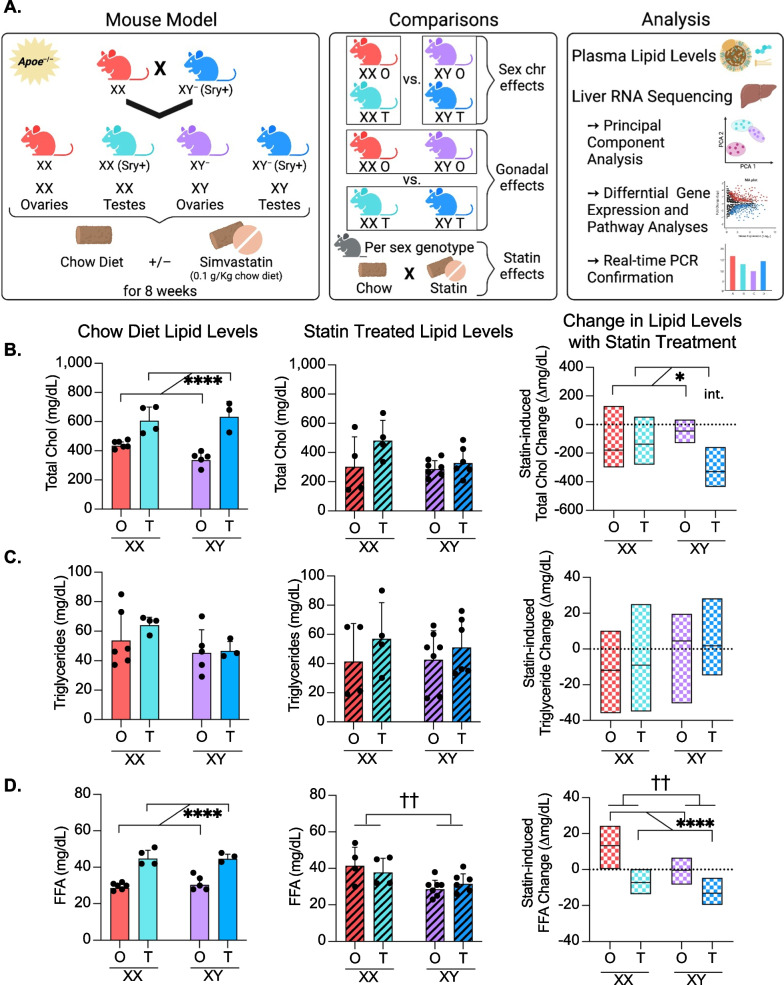
Fig. 1.
Study design using hypercholesterolemic Four Core Genotypes mice, and sex determinants of plasma lipid level response to statin. A Experimental design to assess the contribution of sex components to lipid levels and hepatic transcriptome. Left, generation of FCG mouse cohorts on hypercholesterolemic (Apoe–/–) genetic background. Center, group comparisons made throughout the study. Right, a summary of analyses performed. B Total cholesterol levels, C triglyceride levels, D and free fatty acid levels in plasma of Apoe–/– FCG mice on chow diet (left panel, N = 3–6/genotype), treated with simvastatin (center panel, N = 4–7/genotype) represented as mean ± standard deviation. The right graph in panels B–D depicts the statin-induced change in lipid levels relative to levels on chow diet (horizontal dashed line). The statin-induced lipid changes were calculated as the difference between the mean lipid level for each genotype without statin treatment and the lipid level of each individual mouse after statin treatment; box plots show the minimum, median, and maximum values for statin-induced lipid changes. All data shown in panels B–D were analyzed by two-way ANOVA for gonad and sex chromosome type, and significant differences are indicated by brackets. In two-way ANOVA analyses, two groups of mice were combined (such as XX plus XY mice with ovaries, or XX plus XY mice with testes) such that the number of mice in each group used for statistical analysis is 7–12. * indicates gonadal sex effect, † indicates chromosomal sex effect, int. indicates interaction between gonadal and chromosomal sex. Denoted significance values: *P < 0.05, ** or ††P < 0.01, ***P < 0.001, ****P < 0.0001
Results
Effects of gonadal sex on lipid levels are abolished following statin treatment
To assess the effects of sex components on hypercholesterolemia, we made use of the apolipoprotein E (apoE)-deficient (Apoe–/–) mouse model, which is widely used in the study of hypercholesterolemia and atherosclerosis. Apoe–/– mice typically develop hypercholesterolemia (300–600 mg/dL) without dietary intervention, which is associated with elevated levels of LDL and VLDL, as well as reduced high-density lipoproteins (HDL) (reviewed in [21]). We generated Apoe–/– FCG mice on a C57BL/6 background (see Methods) to evaluate the role of gonadal and chromosomal sex in response to hypercholesterolemia and statin treatment (Fig. 1A, left panel). Throughout this study, statistical analyses of the FCG mice were performed by two-way ANOVA with gonadal sex and chromosomal sex as variables. The comparison between all mice with XX chromosomes (i.e., XX mice with ovaries plus XX mice with testes) and all mice with XY chromosomes (i.e., XY mice with ovaries plus XY mice with testes) allowed the detection of sex chromosome effects (Fig. 1A, middle panel). Similarly, comparison between all mice with ovaries (XX and XY with ovaries) and those with testes (XX and XY with testes) allowed the detection of gonadal effects. Since two genotypes are combined to generate groups for two-way ANOVA, the statistical power is also augmented. The analyses that we performed include plasma lipid levels and assessment of the liver transcriptome by RNA-seq and differential gene expression with pathway analyses (Fig. 1A, right panel).
Apoe–/– FCG mice were fed chow diet with or without simvastatin for 8 weeks. Prior to statin treatment, all four FCG genotypes were hypercholesterolemic (> 240 mg/dL) due to apo E deficiency. Mice with testes had higher cholesterol levels than those with ovaries, regardless of sex chromosome type (Fig. 1B, left). After statin treatment, there were no significant sex differences in the absolute cholesterol levels (Fig. 1B, middle). However, the sex genotype influenced the statin-induced change in cholesterol levels. Reductions in cholesterol levels occurred in a gonad-dependent manner with larger reductions in mice with testes compared to mice with ovaries (Fig. 1B, right). A significant interaction between gonad type and sex chromosome complement was identified by two-way ANOVA. In contrast to cholesterol levels, triglyceride levels did not vary across sex genotypes on chow or after statin treatment (Fig. 1C).
Free fatty acid levels in hypercholesterolemic mice were influenced by gonadal type, with higher levels in mice with testes than in mice with ovaries (Fig. 1D, left). After statin treatment, the effect of gonadal sex was not evident, but rather XX mice had higher fatty acid levels than XY mice (Fig. 1D, middle). The statin-induced changes in free fatty acid levels were affected by both gonad type and sex chromosome complement (Fig. 1D, right). With statin treatment, fatty acid levels were elevated in mice with ovaries and reduced in mice with testes; XX mice had higher levels than XY mice (Fig. 1D, right). Overall, statin altered plasma cholesterol and free fatty acid levels in a sex-dependent manner with the most robust effects in mice having XX chromosomes paired with ovaries or XY chromosomes paired with testes.
Gonadal and chromosomal sex independently impact the hepatic transcriptome in hypercholesterolemic mice
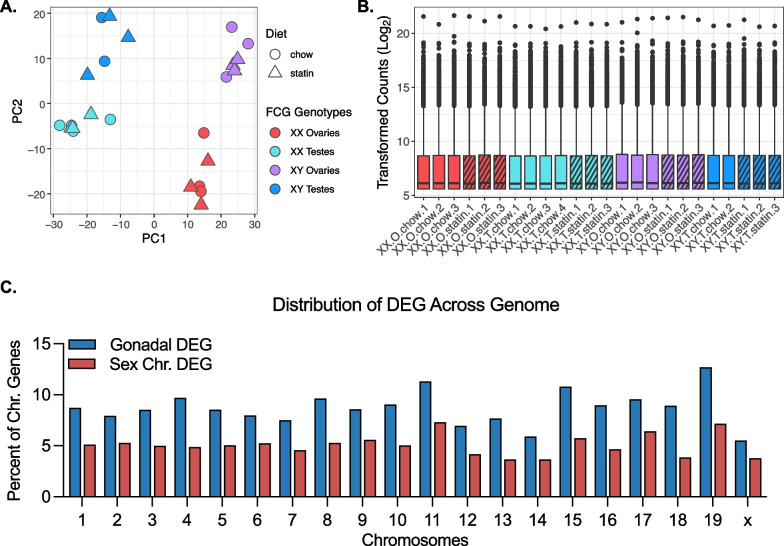
To identify genes and pathways that influence sex differences in hypercholesterolemia and statin response, we performed RNA-sequencing of liver from Apoe–/– FCG mice. To visualize the impact of gonads, sex chromosomes, and statin treatment on gene expression, we performed principal component analysis (PCA) of the RNA-seq data (Fig. 2A). The PCA generated separate clusters for gonadal type based on principal component 1, which accounted for 58% of the variation in the dataset. Within each gonadal type, the samples were separated into individual clusters for XX and XY sex chromosome complement based on principal component 2, which accounted for 23% of the variation within the dataset. Chow and statin treatments clustered together within each genotype indicating that statin treatment had a smaller impact on gene expression than sex genotype. We performed differential expression analysis with DESeq2 [15]; boxplots of the transformed counts show similar total counts across all 24 samples in the analysis (Fig. 2B).
Fig. 2.
Characterization of Apoe–/– FCG liver RNA-seq dataset. A Principal component analysis of liver RNA-seq data from Apoe–/– FCG mice fed chow diet without or with simvastatin for 8 wks. B Boxplots representing transformed RNA-seq counts across all 24 liver samples. C The genome-wide distribution of genes found to be differentially regulated by presence of ovaries vs. testes (gonadal DEG), or by presence of XX vs. XY chromosomes (Sex Chr DEG). DEG, differentially expressed genes
We assessed the influence of sex genotypes on gene expression in the hypercholesterolemic state without statin treatment. A genome-wide analysis of differential gene expression (≥ 1.25-fold difference, adj P < 0.05) identified 3223 genes with differential expression in mice with ovaries compared to testes, and 1390 genes with differential expression in XX compared to XY mice (Additional file 2: Table S2). We found that 5.9–12.7% of genes on each autosome and 5.5% of genes on the X chromosome had different expression levels in mice with ovaries compared to mice with testes (Fig. 2C, blue bars). The XX vs. XY chromosome complement conferred differential expression of 3.7–7.3% of genes/autosome, as well as 3.8% of genes on the X chromosome (Fig. 2C, red bars). The majority of Y chromosome genes are expressed at very low levels in liver and were therefore not included in this analysis. This analysis reveals that gonadal and chromosomal sex each influence expression of genes that map across all autosomes as well as on the X chromosome.
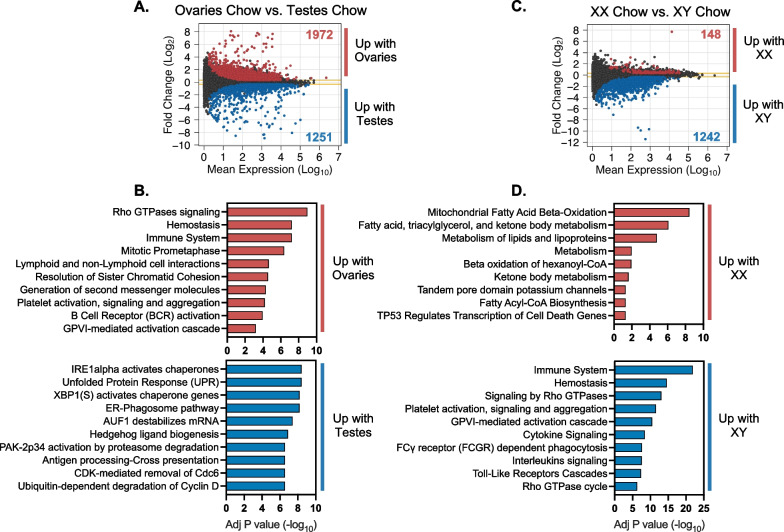
We determined the functional classification of genes that were differentially expressed based on gonadal or chromosomal sex in the liver of hypercholesterolemic mice by pathway enrichment analysis [16–18]. The 1972 DEG with elevated expression in mice with ovaries compared to testes were enriched for genes in the immune system, cell cycle, and cell signaling pathways of the 49 significant pathways identified (Fig. 3A, B, Additional file 2: Table S2, Additional file 3: Table S3). The 1251 DEG elevated in the presence of testes were enriched in functions that include the unfolded protein response (UPR) and endoplasmic reticulum (ER) stress pathways (Fig. 3A, B, Additional file 2: Table S2, Additional file 3: Table S3). These results demonstrate that distinct hepatic cellular processes are influenced at the gene expression level by presence of ovaries or testes.
Fig. 3.
Gonadal and chromosomal sex impact gene expression in Apoe–/– FCG liver. MA plot shows mean expression and fold-change of genes with differential expression in A mice with ovaries compared to mice with testes (adjusted P < 0.05, fold-change > 1.25) and B the top 10 significant cellular pathways enriched in the differentially expressed genes (adjusted P < 0.05). MA plot shows mean expression and fold-change of genes with differential expression in C XX mice compared to XY mice (adjusted P < 0.05, fold-change > 1.25) and D the top 10 significant cellular pathways enriched in the differentially expressed genes (adjusted P < 0.05)
We also assessed the functional enrichment of genes with differential expression in mice with XX (with ovaries or testes) vs. XY chromosomes (with ovaries or testes). The majority of DEG due to sex chromosome complement (1242 of 1390 genes) had elevated expression in XY compared to XX mice (Fig. 3C, Additional file 2: Table S2). The XY DEG were enriched in immune system, Rho GTPase signaling, and other cell signaling pathways (Fig. 3D, Additional file 3: Table S3). Only 148 genes were expressed at higher levels in XX compared to XY liver, but these were enriched for genes associated with fatty acid oxidation in mitochondria and other aspects of fatty acid metabolism (Fig. 3D, Additional file 3: Table S3).
Gonadally regulated genes in FCG mice extend beyond sexually determined growth hormone-regulated genes
Sex differences in hepatic gene expression have previously been attributed to growth hormone signaling, as gonad type drives sex differences in circulating levels of growth hormone through action on the pituitary. Male mice (XY testes) have low levels of circulating growth hormone with spikes at irregular frequencies while female mice (XX ovaries) have more frequent release of growth hormone leading to more consistent growth hormone-dependent signaling in the liver [22, 23]. In a study that assessed male/female differences in hepatic gene expression in CD-1 mice, 951 protein-coding genes were differentially expressed (adj P < 0.01). To identify genes that differ between the sexes due to the differential pattern of growth hormone release, growth hormone levels in male mice in that study were made to more closely resemble females by persistent growth hormone treatment for 4 days [19]. Of the original 951 genes with expression differences between males and females, the expression levels of 427 (44.9%) of these genes were altered after growth hormone treatment in male mice, suggesting that sex differences in growth hormone dynamics underlies about half of the observed male–female differences in hepatic gene expression in that model [19]. To identify growth hormone-dependent genes in our hypercholesterolemic FCG mice, we compared the 427 growth hormone-responsive genes identified by Lau-Corona et al. to the genes with differential expression in FCG mice with ovaries compared to mice with testes. Of the 427 genes, 309 genes (72.4%) were regulated by gonad type in FCG mice (adj P < 0.05) (Additional file 4: Table S4). Notably, however, our studies in hypercholesterolemic FCG mice identified an additional 2,554 genes with hepatic expression levels influenced by gonad type. 633 of the genes showing differential expression in FCG mice with ovaries compared to testes were previously found to be differentially expressed in mice with genetic deletion of the growth hormone receptor in liver (Additional file 4: Table S4) [20]. Thus, in our model, although sex differences in growth hormone may contribute to differential gene expression, additional mechanisms likely contribute to a majority of the gonad-type specific gene expression changes in our hypercholesterolemic mice.
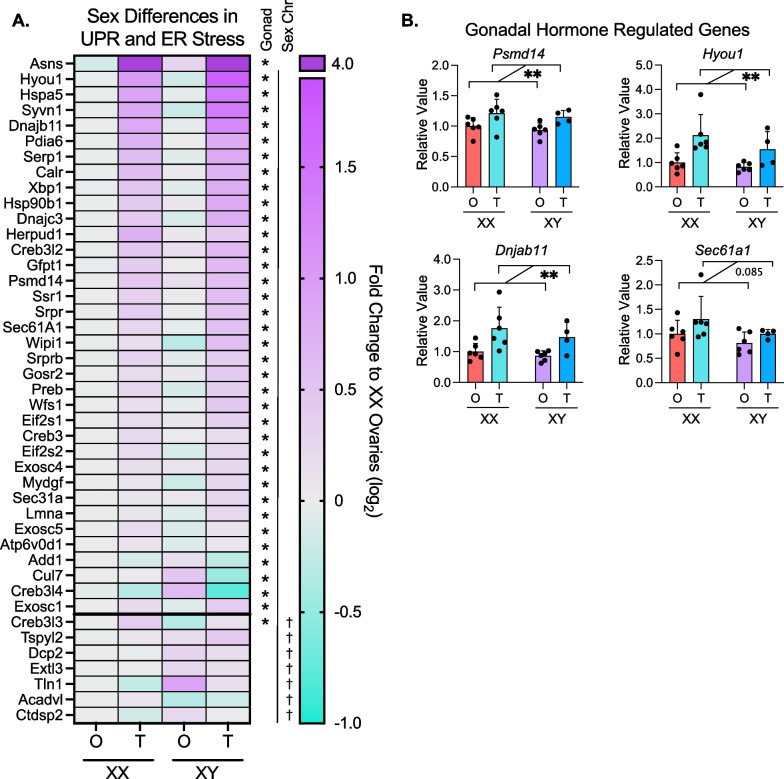
ER stress response gene expression is elevated in mice with testes
Since ER stress-related pathways were the predominant pathways upregulated in mice with testes, we further evaluated the sex determinants of expression levels for specific genes within these pathways. The ER plays key roles in protein synthesis and trafficking as well as the synthesis of lipids, including sterols, phospholipids, and triglycerides. Disruption of ER homeostasis induces stress responses that are associated with the development and progression of metabolic diseases including obesity, fatty liver, type 2 diabetes and atherosclerosis [24, 25]. All protein-encoding genes in the unfolded protein response pathway (Reactome R-HSA-381119) [26] were evaluated by two-way ANOVA on CPM values from our RNA-seq data to identify genes impacted by gonadal effects and/or sex chromosome effects. Of the 92 protein-encoding genes, 36 genes were differentially expressed in mice with testes (XX testes plus XY testes) vs. ovaries (XX ovaries plus XY ovaries), and 7 genes were influenced by sex chromosome complement (XX ovaries plus XX testes compared to XY ovaries plus XY testes) (two-way ANOVA, P < 0.05) (Fig. 4A). We confirmed the expression patterns of representative genes via real-time PCR in additional mouse liver samples beyond those used in the RNA-seq. Consistent with RNA-seq data, the expression levels of Psmd14, Hyou1, Dnjab11, and Sec61a1 were upregulated in the livers of mice with testes compared to mice with ovaries (Fig. 4B). These data suggest that gonadal sex is a key determinant of sex differences in gene expression of ER stress-associated pathways in hypercholesterolemic liver, while chromosomal sex has only a minor impact.
Fig. 4.
Genes in ER stress and UPR pathways show increased expression in mice with testes. A Heatmap displays relative hepatic expression levels of genes related to ER stress and unfolded protein response in Apoe–/– FCG genotypes (N = 3). Expression of each genotype was relative to that in XX mice with ovaries. B Real-time PCR quantification of representative ER stress genes from A (Psmd14, Hyou1, Dnjab11, and Sec61a1) in liver of Apoe–/– FCG mice. * Indicates gonadal sex effect and † indicates chromosomal sex effect. * or †P < 0.05 and **P < 0.01 by two-way ANOVA. N = 4–6. In two-way ANOVA analyses, two groups of mice were combined such that the number of mice in each group used for statistical analysis is 7–12
Fatty acid metabolism genes are independently regulated by gonadal and chromosomal sex
Our gene enrichment analyses demonstrated that gonadal and chromosomal sex each impact expression of lipid metabolism genes. In particular, XX vs. XY chromosomes influenced fatty acid metabolism and beta oxidation genes, and testes vs. ovaries influenced peroxisomal lipid metabolism (Fig. 3B, D; Additional file 3: Table S3). We further investigated the sex component regulation of genes involved in fatty acid synthesis and oxidation across the FCG genotypes. Of the 37 genes in the fatty acid synthesis pathway (Reactome R-HSA-75105.7), 21 genes were regulated by gonadal sex, sex chromosome complement, or a combination of both sex factors in livers of hypercholesterolemic mice (two-way ANOVA, *P < 0.05) (Fig. 5A). Gonadal sex influenced expression of 10 fatty acid synthesis genes. Elovl3 was strongly increased by the presence of testes, but the majority of the other 10 gonadally regulated fatty acid synthesis genes were down-regulated in mice with testes. The sex chromosome complement influenced expression of 7 fatty acid synthesis genes, with the majority upregulated in XY compared to XX mice. Four fatty acid synthesis genes showed more complex regulation, with significant effects of both gonadal and chromosomal sex.
Fig. 5.
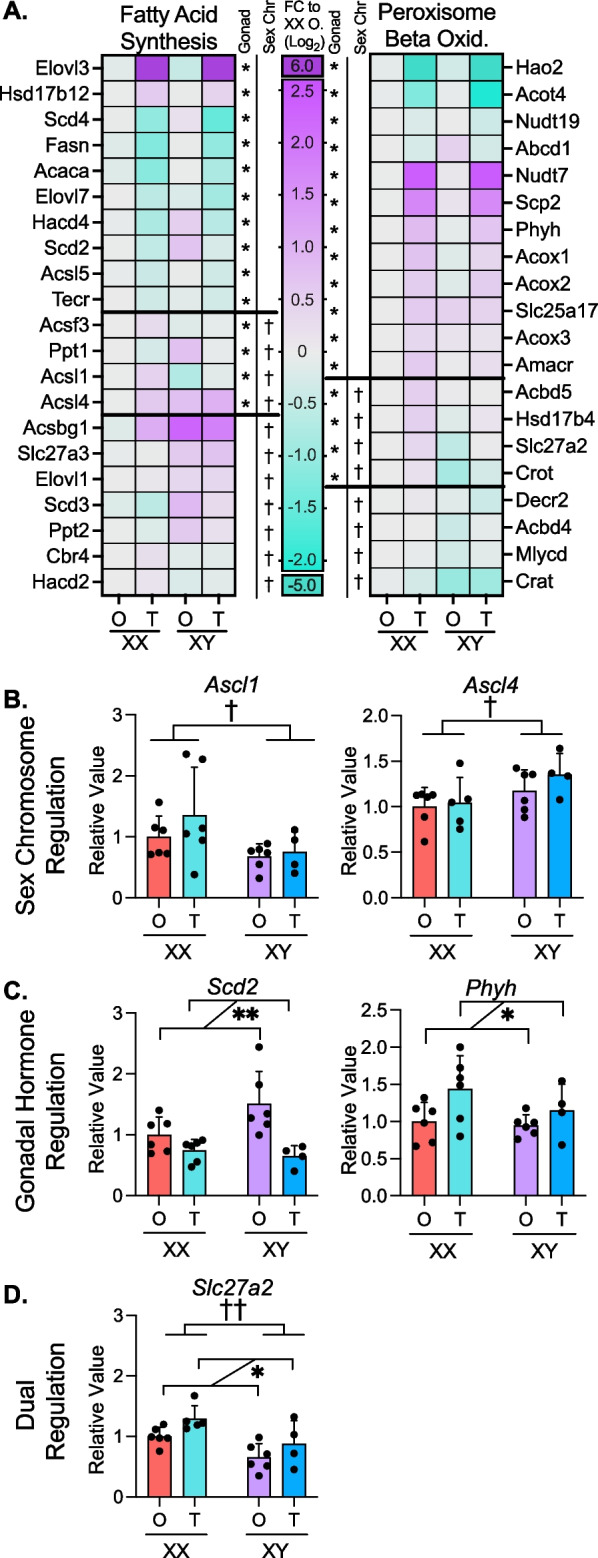
Gonadal and chromosomal sex regulate fatty acid metabolism. A Heatmaps display relative hepatic expression levels of genes related to fatty acid synthesis and peroxisomal beta oxidation in Apoe–/– FCG genotypes (N = 3). Expression of each genotype was relative to that in XX mice with ovaries. *P < 0.05 for gonadal or chromosomal sex by two-way ANOVA. Real-time PCR quantification of representative genes from A showing B chromosomal sex effects (Acsl1 and Acsl4), C gonadal sex effects (Scd2 and Phyh) and D both gonadal and chromosomal sex effects (Slc27a2). Two-way ANOVA with * donating gonadal sex effect and † denoting chromosomal sex effect. * or †P < 0.05 and ** or ††P < 0.01. N = 4–6. In two-way ANOVA analyses, two groups of mice were combined such that the number of mice in each group used for statistical analysis is 7–12
Peroxisomal fatty acid oxidation genes were also largely influenced by gonadal sex, but in a pattern distinct from the fatty acid synthesis genes. Of 29 peroxisomal lipid metabolism-related genes (Reactome R-HSA-390918.5), 12 were regulated by gonadal sex, primarily with increased expression due to testes compared to ovaries (Fig. 5A). Four of the 29 peroxisomal oxidation genes were expressed at lower levels in mice with XY compared to XX chromosomes.
We confirmed patterns of representative fatty acid metabolism genes identified by RNA-seq in a larger number of mice by real-time PCR. The acyl-CoA synthetase genes Acsl1 and Acsl4 were both regulated by sex chromosome complement, but in opposing directions: XX chromosomes promoted higher expression of Acsl1, while XY chromosomes promoted higher expression of Acsl4. (Fig. 5B). Scd2 and Phyh were oppositely regulated by gonadal sex, with ovaries promoting higher Scd2 expression, and testes enhancing Phyh expression (Fig. 5C). Slc27a2 exhibited combined gonadal and sex chromosome regulation with higher expression in XX mice compared to XY mice, but also higher expression in mice with testes compared to mice with ovaries (Fig. 5D). Overall, fatty acid metabolism exhibits complex sex-dependent regulation with fatty acid biosynthesis and peroxisomal degradation impacted in opposite directions by gonadal and chromosomal sex.
Sex-dependent transcriptional response to statin treatment is influenced by XY sex chromosome complement and testes
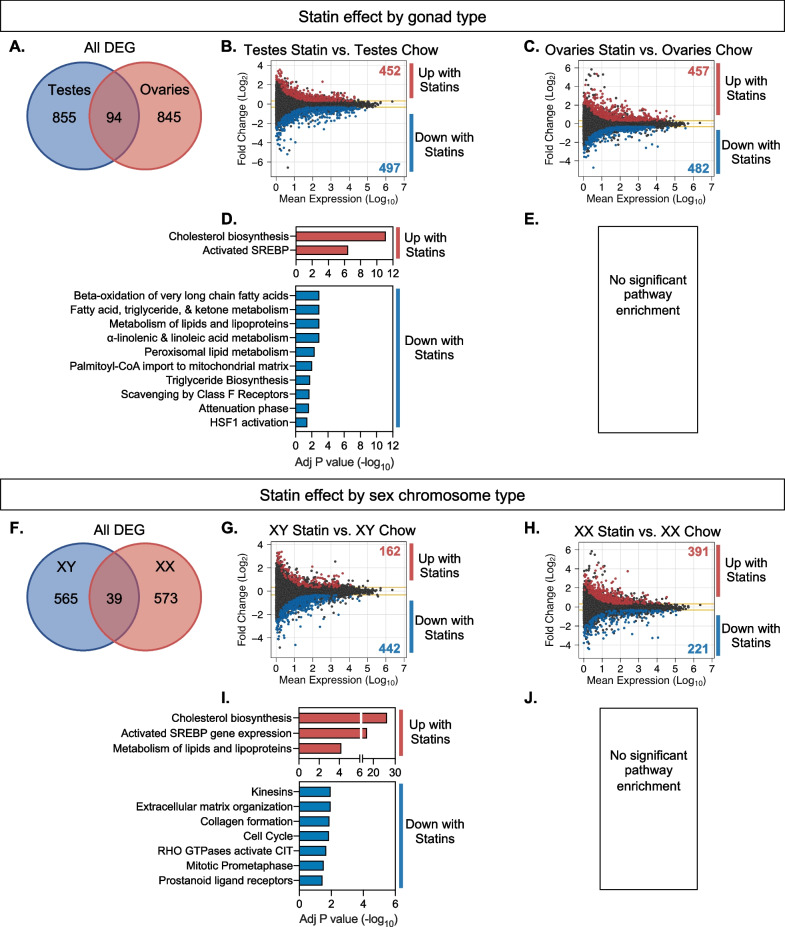
To identify sex-dependent effects of statin, Apoe–/– FCG mice received simvastatin in chow diet for 8 weeks. The role of sex components on statin-induced alterations in the hepatic transcriptome were identified by comparing statin-treated mice to chow diet controls within each genotype. Statin treatment influenced a similar number of genes in mice with testes (949 DEG between statin and chow) and ovaries (939 DEG between statin and chow) (Fig. 6A). However, only ~ 5% (94) of the genes altered by statin treatment were shared by mice with ovaries and testes, indicating distinct target genes for statin-associated regulation depending on gonadal type. We analyzed genes with up and down regulation in response to statin (Fig. 6B, C) for functional enrichment. In mice with testes, statin down-regulated genes were enriched for fatty acid metabolism pathways, and statin upregulated genes were enriched for cholesterol biosynthesis pathways (Fig. 6D). In mice with ovaries, statin-regulated genes did not show enrichment for specific biological pathways (Fig. 6E).
Fig. 6.
Statin effects on hepatic gene expression are influenced in distinct ways by gonadal and chromosomal sex. A Venn diagram shows number of genes altered by statin treatment depending on presence of ovaries or testes, and the minimal overlap between the two. MA plots display mean expression levels and fold-change of differentially expressed genes (DESeq2, > 1.25-fold altered expression, P < 0.05) in B statin-treated mice with testes compared to chow diet controls and C statin-treated mice with ovaries compared to chow diet controls. Significantly enriched cellular pathways for statin-induced gene expression changes in D mice with testes and E mice with ovaries (no enriched pathways). F Venn diagram shows number of genes altered by statin treatment depending on presence of XX or XY chromosomes, and the minimal overlap between the two. MA plots display mean expression levels and fold-change of differentially expressed genes (DESeq2) in G statin-treated XY mice compared to chow diet controls and H statin-treated XX mice compared to chow diet controls. Significantly enriched cellular pathways for statin-induced gene expression changes in I XY mice and J XX mice (no enriched pathways)
Statin-induced gene regulation was also influenced by chromosomal sex. Compared to mice fed a chow diet, statin treatment altered expression of 605 genes in XY mice and 612 genes in XX mice, but only 39 (~ 6%) of the dysregulated genes were shared between the two sex chromosome complement types (Fig. 6F). In mice with XY sex chromosomes, statin treatment upregulated 162 genes and down-regulated 442 genes. In mice with XX sex chromosomes, statin treatment upregulated 391 genes and down-regulated 221 genes (Fig. 6G,H). For XY mice, statin upregulated genes were enriched for cholesterol biosynthesis pathways, while XX mice had no significant pathway enrichment for the statin-induced DEG (Fig. 6I,J).
Statin treatment induces cholesterol biosynthesis gene expression only in mice with XY sex chromosomes or testes
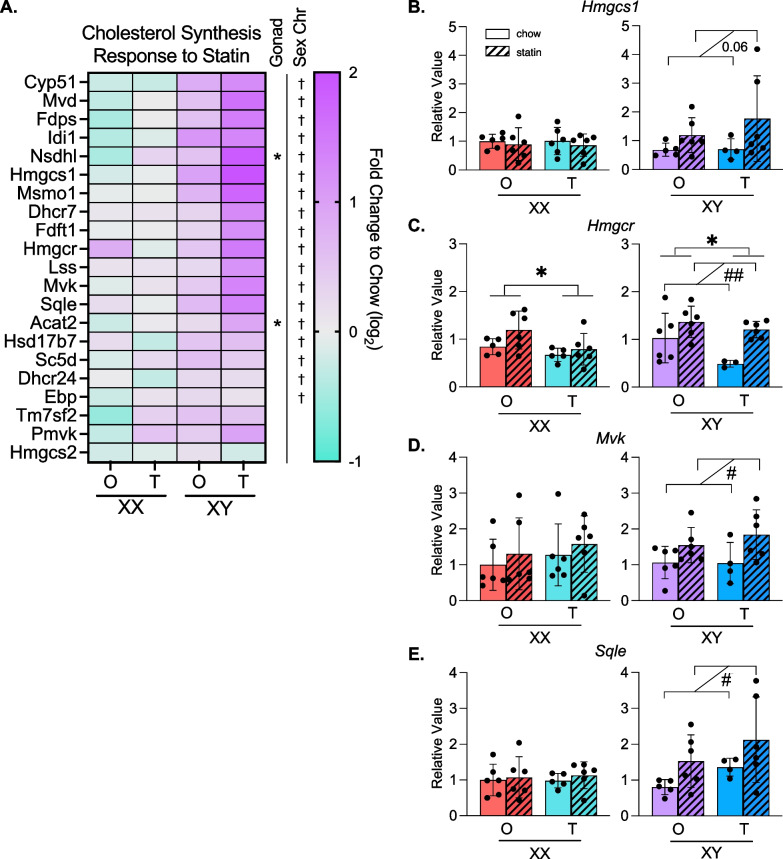
Statin drugs bind to the rate-limiting enzyme in cholesterol biosynthesis (HMG CoA reductase) to inhibit its activity. The complex feedback mechanisms that control HMGCoA reductase levels can lead to enhanced transcription of the corresponding gene, as well as other genes within the pathway, in response to statin [27, 28]. The subsequent upregulation of HMG CoA reductase protein levels may reduce the effectiveness of statin drugs. Our data above suggested that statin-induced compensatory upregulation of cholesterol synthetic gene expression is driven by testes and XY chromosome complement. We investigated further by assessing the effect of gonadal and chromosomal sex on expression of genes in the cholesterol biosynthetic pathway in response to statin. Statin upregulated 18 of 25 cholesterol biosynthetic genes in the liver of XY mice, with little or no increase in XX liver (Fig. 7A). The increased expression in XY mice was most pronounced in XY mice with testes, suggesting an additional impact from gonadal sex.
Fig. 7.
Cholesterol biosynthesis pathway gene expression is upregulated in XY mice in response to statin. A Heatmap displays relative hepatic expression levels of genes altered by sex within the cholesterol biosynthesis pathway as fold-change of statin treated compared to chow diet for each of the Four Core genotypes (N = 3 per genotype and diet). * indicates gonadal sex effect and † indicates chromosomal sex effect. * or †P < 0.05 by two-way ANOVA. B–E Real-time PCR quantification of representative genes from A illustrates the effects of gonadal and/or chromosomal sex on statin-induced alterations in gene expression in the cholesterol biosynthesis pathway. Two-way ANOVA with * denoting gonadal sex effect and # denoting effect due to statin treatment. * or #P < 0.05 and ** or ##P < 0.01. N = 4–6. In two-way ANOVA analyses, two groups of mice were combined such that the number of mice in each group used for statistical analysis is 7–12
We confirmed the sex chromosome differences in statin regulation of representative cholesterol synthetic genes by real-time PCR. Mice with XY sex chromosomes upregulated Hmgcs, Hmgcr, Mvk, and Sqle with statin treatment compared to chow diet controls, while mice with XX sex chromosomes did not (Fig. 7B–E). Hmgcr expression also showed a significant gonad effect with higher expression in mice with ovaries compared to testes, irrespective of sex chromosome type and statin treatment (Fig. 7C). Our results reveal that sex differences in statin-regulated gene expression depend on the individual gene, and specific genes may be influenced by gonadal sex, chromosomal sex, or a combination of the two.
Discussion
Differences between women and men have been characterized in plasma lipid profiles, hepatic lipid metabolism, and response to pharmaceutical approaches to reduce CVD risk [3, 4]. There is, however, limited understanding of the molecular mechanisms that underlie these sex differences. We used Apoe–/– FCG mice to investigate how gonadal and chromosomal sex independently impact hepatic gene expression in a hypercholesterolemic state and in response to statin. We focused on liver because of its central role in regulating homeostatic lipid levels and as the primary target for statin inhibition of cholesterol synthesis. Our study extends and augments previous work identifying sex differences in the hepatic transcriptome by assessing the role of sex chromosomes in hepatic gene regulation in physiological states that are relevant to human disease—hypercholesterolemia and statin treatment [29–32].
A general finding in our study is that in hypercholesterolemic mice, the presence of ovaries vs. testes leads to differential expression of ~ 6–12% of genes on each of the 19 mouse autosomes, and the presence of XX vs. XY chromosomes confers differential expression of ~ 3–6% of genes on each autosome. Several key findings related to the role of sex components and gene expression in hypercholesterolemic or statin-treated states also emerged. For example, an analysis of specific biological pathways that are influenced by sex components revealed that in the hypercholesterolemic state, gonadal sex influences the regulation of ER stress, whereas chromosomal sex and gonadal sex influence fatty acid metabolism. The upregulation of ER stress response in mice with testes may contribute to the higher circulating levels of total cholesterol and free fatty acids observed in these mice. However, while hepatic gene expression levels may impact lipoprotein synthesis, circulating lipid levels are a reflection of additional processes that include dietary lipid absorption, lipoprotein metabolism in the circulation, and lipoprotein uptake in peripheral tissues. Thus, further investigation is required to identify the processes that ultimately influence sex differences in lipoprotein levels.
Previous research has identified distinct effects of male and female gonadal hormones on growth hormone signaling to regulate sex-dependent differences in the hepatic transcriptome [19, 33]. We determined that a subset of genes found to be regulated by gonadal sex in FCG mice corresponded to known growth hormone-responsive genes, consistent with their potential regulation by gonadal hormones through the action on circulating growth hormone levels. An additional subset of the gonadally regulated genes in FCG mice corresponded to genes with sex-specific expression in the absence of growth hormone receptor, confirming that growth hormone signaling does not account for all of the gonadally regulated genes identified in our model. Furthermore, we identified an additional 2000 genes with differential hepatic expression levels in mice with ovaries vs. testes that were not described in the studies cited above. As a particular feature of the FCG model, we identified more than 1000 genes that differ in expression levels in mice with XX compared to XY chromosomes. The genes and pathways that we identified as differentially expressed in response to gonadal or chromosomal sex in hypercholesterolemic mice may be particularly relevant to differences between males and females in susceptibility to cardiometabolic diseases.
We sought to identify potential sex-dependent effects of statins on gene expression in liver, the site of statin drug metabolism. We determined that the presence of XY, but not XX, chromosomes was associated with robust upregulation of the cholesterol biosynthesis pathway genes upon statin treatment, which is known to occur through feedback regulation. These data demonstrate that the sex chromosome complement may be an important determinant of sex-dependent statin drug response. Ultimately, the complex interplay between gonadal and chromosomal sex leads to a specific gene expression environment that is expected to determine sex differences in traits related to CVD risk, including circulating plasma lipid levels and response to lipid-lowering drugs.
Significance and perspectives
Overall, our findings reveal independent effects of gonadal and chromosomal sex on the hepatic transcriptome to create sex-specific cellular phenotypes. While gonad type had the largest impact on hepatic gene expression in the conditions we assessed, we demonstrated that the sex chromosomes influence the hepatic response to statin treatment, which may have a profound impact on differential statin efficacy, effectiveness, or side effects between women and men. This may be important after middle age (when statin drugs are most commonly prescribed), as chromosomal sex effects persist after gonadal hormone levels wane. These findings join other recent data that demonstrate the integral role of sex chromosomes on cellular and whole-body physiology including mRNA and microRNA expression levels, lipoprotein metabolism, atherosclerosis, obesity, autoimmune, pulmonary, and neurological diseases [11, 14, 34–39]. Our study is the first to evaluate how gonadal and chromosomal sex impact cellular response to a drug treatment. An understanding of how sex components influence the response to disease conditions (such as hypercholesterolemia) and commonly prescribed drugs (such as statins) will lead to optimal treatment for all individuals.
Supplementary Information
Additional file 1. Table S1. PCR primers used in this study.
Additional file 2. Table S2. Differentially expressed gene group comparisons.
Additional file 3. Table S3. Pathway analyses for differentially expressed genes.
Additional file 4. Table S4. FCG gonadally-regulated genes compared to growth hormone regulated genes.
Acknowledgements
We thank Emilio Ronquillo for assistance with mouse studies and Rita Cantor for advice on statistical analyses.
Author contributions
CBW, ZWA, and KR designed the study analysis and wrote the manuscript. PZ performed mouse studies and generated RNA-seq data. CBW and ZWA analyzed transcriptome data and performed real-time PCR studies. All authors read and approved the final manuscript.
Funding
These studies were supported by funds from U.S. Public Health Service U54 DK120342 (KR), P50 GM115318 (KR), and R21 AR077782 (KR and PZ), and American Heart Association post-doctoral fellowship 20POST35100000 (CBW).
Availability of data and materials
RNA-sequencing data have been deposited in the NCBI Gene Expression Omnibus (www.ncbi.nlm.nih.gov/geo/) with accession number GSE202977. Differential gene expression enrichment analysis results are provided in Additional tables.
Declarations
Ethics approval and consent to participate
Animal studies were conducted in accordance with UCLA Institutional Animal Research Committee (IACUC) approval. No human subjects were involved.
Consent for publication
No human data are used.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, et al. Heart disease and stroke statistics-2022 update: a report from the American Heart Association. Circulation. 2022;145(8):e153–639. doi: 10.1161/CIR.0000000000001052. [DOI] [PubMed] [Google Scholar]
- 2.Regitz-Zagrosek V, Kararigas G. Mechanistic pathways of sex differences in cardiovascular disease. Physiol Rev. 2017;97(1):1–37. doi: 10.1152/physrev.00021.2015. [DOI] [PubMed] [Google Scholar]
- 3.Reue K, Wiese CB. Illuminating the mechanisms underlying sex differences in cardiovascular disease. Circ Res. 2022;130(12):1747–1762. doi: 10.1161/CIRCRESAHA.122.320259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mauvais-Jarvis F, Berthold HK, Campesi I, Carrero JJ, Dakal S, Franconi F, et al. Sex-and gender-based pharmacological response to drugs. Pharmacol Rev. 2021;73(2):730–762. doi: 10.1124/pharmrev.120.000206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goodarzi MO, Li X, Krauss RM, Rotter JI, Chen YDI. Relationship of sex to diabetes risk in statin trials. Diabetes Care. 2013;36(7):e100–e101. doi: 10.2337/dc13-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hopewell JC, Offer A, Haynes R, Bowman L, Li J, Chen F, et al. Independent risk factors for simvastatin-related myopathy and relevance to different types of muscle symptom. Eur Heart J. 2020;41(35):3336–3342. doi: 10.1093/eurheartj/ehaa574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nguyen P, Leray V, Diez M, Serisier S, Le Bloch J, Siliart B, et al. Liver lipid metabolism. J Anim Physiol Anim Nutr. 2008;92(3):272–283. doi: 10.1111/j.1439-0396.2007.00752.x. [DOI] [PubMed] [Google Scholar]
- 8.Moslehi A, Hamidi-Zad Z. Role of SREBPs in liver diseases: a mini-review. J Clin Transl Hepatol. 2018;6(3):332–338. doi: 10.14218/JCTH.2017.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Istvan ES, Deisenhofer J. Structural mechanism for statin inhibition of HMG-CoA reductase. Science. 2001;292(5519):1160–1164. doi: 10.1126/science.1059344. [DOI] [PubMed] [Google Scholar]
- 10.Mauvais-Jarvis F, Arnold AP, Reue K. A guide for the design of pre-clinical studies on sex differences in metabolism. Cell Metab. 2017;25(6):1216–1230. doi: 10.1016/j.cmet.2017.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.AlSiraj Y, Chen X, Thatcher SE, Temel RE, Cai L, Blalock E, et al. XX sex chromosome complement promotes atherosclerosis in mice. Nat Commun. 2019;10(1):2631. doi: 10.1038/s41467-019-10462-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen X, McClusky R, Chen J, Beaven SW, Tontonoz P, Arnold AP, et al. The number of X chromosomes causes sex differences in adiposity in mice. PLoS Genet. 2012;8(5):e1002709. doi: 10.1371/journal.pgen.1002709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehrabian M, Qiao JH, Hyman R, Ruddle D, Laughton C, Lusis AJ. Influence of the apoA-II gene locus on HDL levels and fatty streak development in mice. Arterioscler Thromb a J Vasc Biol. 1993;13(1):1–10. doi: 10.1161/01.ATV.13.1.1. [DOI] [PubMed] [Google Scholar]
- 14.Link JC, Wiese CB, Chen X, Avetisyan R, Ronquillo E, Ma F, et al. X chromosome dosage of histone demethylase KDM5C determines sex differences in adiposity. J Clin Invest. 2020;130(11):5688–5702. doi: 10.1172/JCI140223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuleshov MV, Jones MR, Rouillard AD, Fernandez NF, Duan Q, Wang Z, et al. Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016;44(W1):W90–W97. doi: 10.1093/nar/gkw377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen EY, Tan CM, Kou Y, Duan Q, Wang Z, Meirelles GV, et al. Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinformatics. 2013;14:128. doi: 10.1186/1471-2105-14-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xie Z, Bailey A, Kuleshov MV, Clarke DJB, Evangelista JE, Jenkins SL, et al. Gene Set knowledge discovery with enrichr. Curr Protoc. 2021;1(3):e90. doi: 10.1002/cpz1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lau-Corona D, Suvorov A, Waxman DJ. Feminization of male mouse liver by persistent growth hormone stimulation: activation of sex-biased transcriptional networks and dynamic changes in chromatin states. Mol Cell Biol. 2017;37(19):e00301. doi: 10.1128/MCB.00301-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sarmento-Cabral A, Del Rio-Moreno M, Vazquez-Borrego MC, Mahmood M, Gutierrez-Casado E, Pelke N, et al. GH directly inhibits steatosis and liver injury in a sex-dependent and IGF1-independent manner. J Endocrinol. 2021;248(1):31–44. doi: 10.1530/JOE-20-0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Emini Veseli B, Perrotta P, De Meyer GRA, Roth L, Van der Donckt C, Martinet W, et al. Animal models of atherosclerosis. Eur J Pharmacol. 2017;816:3–13. doi: 10.1016/j.ejphar.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 22.Jansson JO, Edén S, Isaksson O. Sexual dimorphism in the control of growth hormone secretion. Endocr Rev. 1985;6(2):128–150. doi: 10.1210/edrv-6-2-128. [DOI] [PubMed] [Google Scholar]
- 23.Chowen JA, Frago LM, Argente J. The regulation of GH secretion by sex steroids. Eur J Endocrinol. 2004;151(Suppl):U95–100. doi: 10.1530/eje.0.151u095. [DOI] [PubMed] [Google Scholar]
- 24.Fu S, Watkins SM, Hotamisligil GS. The role of endoplasmic reticulum in hepatic lipid homeostasis and stress signaling. Cell Metab. 2012;15(5):623–634. doi: 10.1016/j.cmet.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 25.Lemmer IL, Willemsen N, Hilal N, Bartelt A. A guide to understanding endoplasmic reticulum stress in metabolic disorders. Mol Metab. 2021;47:101169. doi: 10.1016/j.molmet.2021.101169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gillespie M, Jassal B, Stephan R, Milacic M, Rothfels K, Senff-Ribeiro A, et al. The reactome pathway knowledgebase 2022. Nucleic Acids Res. 2022;50(D1):D687–D692. doi: 10.1093/nar/gkab1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brown MS, Goldstein JL. Multivalent feedback regulation of HMG CoA reductase, a control mechanism coordinating isoprenoid synthesis and cell growth. J Lipid Res. 1980;21(5):505–517. doi: 10.1016/S0022-2275(20)42221-7. [DOI] [PubMed] [Google Scholar]
- 28.Jiang SY, Li H, Tang JJ, Wang J, Luo J, Liu B, et al. Discovery of a potent HMG-CoA reductase degrader that eliminates statin-induced reductase accumulation and lowers cholesterol. Nat Commun. 2018;9(1):5138. doi: 10.1038/s41467-018-07590-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang X, Schadt EE, Wang S, Wang H, Arnold AP, Ingram-Drake L, et al. Tissue-specific expression and regulation of sexually dimorphic genes in mice. Genome Res. 2006;16(8):995–1004. doi: 10.1101/gr.5217506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blencowe M, Chen X, Zhao Y, Itoh Y, McQuillen CN, Han Y, et al. Relative contributions of sex hormones, sex chromosomes, and gonads to sex differences in tissue gene regulation. Genome Res. 2022;32(5):807–824. doi: 10.1101/gr.275965.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldfarb CN, Karri K, Pyatkov M, Waxman DJ. Interplay between GH-regulated, sex-biased liver transcriptome and hepatic zonation revealed by single nucleus RNAseq. Endocrinology. 2022;163(7):bqac059. doi: 10.1210/endocr/bqac059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matthews BJ, Melia T, Waxman DJ. Harnessing natural variation to identify cis regulators of sex-biased gene expression in a multi-strain mouse liver model. PLoS Genet. 2021;17(11):1–48. doi: 10.1371/journal.pgen.1009588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Waxman DJ, O’Connor C. Growth hormone regulation of sex-dependent liver gene expression. Mol Endocrinol. 2006;20(11):2613–2629. doi: 10.1210/me.2006-0007. [DOI] [PubMed] [Google Scholar]
- 34.Arnold AP. Four Core Genotypes and XY* mouse models: Update on impact on SABV research. Neurosci Biobehav Rev. 2020;119:1–8. doi: 10.1016/j.neubiorev.2020.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Link JC, Hasin-Brumshtein Y, Cantor RM, Chen X, Arnold AP, Lusis AJ, et al. Diet, gonadal sex, and sex chromosome complement influence white adipose tissue miRNA expression. BMC Genomics. 2017;18(1):89. doi: 10.1186/s12864-017-3484-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Link JC, Chen X, Prien C, Borja MS, Hammerson B, Oda MN, et al. Increased high-density lipoprotein cholesterol levels in mice with XX versus XY sex chromosomes. Arterioscler Thromb Vasc Biol. 2015;35(8):1778–1786. doi: 10.1161/ATVBAHA.115.305460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Itoh Y, Golden LC, Itoh N, Matsukawa MA, Ren E, Tse V, et al. The X-linked histone demethylase Kdm6a in CD4+ T lymphocytes modulates autoimmunity. J Clin Invest. 2019;129(9):3852–3863. doi: 10.1172/JCI126250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davis EJ, Broestl L, Williams G, Garay BI, Lobach I, Devidze N, et al. A second X chromosome contributes to resilience in a mouse model of Alzheimer’s disease. Sci Transl Med. 2020;12(558):eaaz5677. doi: 10.1126/scitranslmed.aaz5677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cunningham CM, Li M, Ruffenach G, Doshi M, Aryan L, Hong J, et al. Y-chromosome gene, Uty, protects against pulmonary hypertension by reducing proinflammatory chemokines. Am J Respir Crit Care Med. 2022;206(2):186–196. doi: 10.1164/rccm.202110-2309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Table S1. PCR primers used in this study.
Additional file 2. Table S2. Differentially expressed gene group comparisons.
Additional file 3. Table S3. Pathway analyses for differentially expressed genes.
Additional file 4. Table S4. FCG gonadally-regulated genes compared to growth hormone regulated genes.
Data Availability Statement
RNA-sequencing data have been deposited in the NCBI Gene Expression Omnibus (www.ncbi.nlm.nih.gov/geo/) with accession number GSE202977. Differential gene expression enrichment analysis results are provided in Additional tables.