Abstract
Objectives
Key challenges for a joint European Health Technology Assessment (HTA) include consolidated approaches towards the choice of adequate comparator(s), selection of endpoints that are relevant to patients with a given disease, dealing with remaining uncertainties as well as transparent and consistent management of related processes. We aimed to further crystallize related core domains within these four areas that warrant further research and scrutiny.
Methods
Building on the outcomes of a previously conducted questionnaire survey, four key areas, processes, uncertainty, comparator choice and endpoint selection, were identified. At the inaugural convention of the European Access Academy dedicated working groups were established defining and prioritizing core domains for each of the four areas. The working groups consisted of ~ 10 participants each, representing all relevant stakeholder groups (patients/ clinicians/ regulators/ HTA & payers/ academia/ industry). Story books identifying the work assignments were shared in advance. Two leads and one note taker per working group facilitated the process. All rankings were conducted on an ordinal Likert Response Scale scoring from 1 (low priority) to 7 (high priority).
Results
Identified key domains include for processes: i) address (resource-) challenge of multiple PICOs (Patient/ Intervention/ Comparator/ Outcomes), ii) time and capacity challenges, iii) integrating all involved stakeholders, iv) conflicts and aligning between different multi-national stakeholders, v) interaction with health technology developer; for uncertainty: i) early and inclusive collaboration, ii) agreement on feasibility of RCT and acceptance of uncertainty, iii) alignment on closing evidence gaps, iv) capacity gaps; for comparator choice: i) criteria for the choice of comparator in an increasingly fragmented treatment landscape, ii) reasonable number of comparators in PICOs, iii) shape Early Advice so that comparator fulfils both regulatory and HTA needs, iv) acceptability of Indirect Treatment Comparisons (ITC), v) ensure broad stakeholder involvement in comparator selection; for endpoint selection: i) approaching new endpoints; ii) patient preferences on endpoints; iii) position of HTA and other stakeholders; iv) long-term generation and secondary use of data; v) endpoint challenges in RCTs.
Conclusions
The implementation of a joint European HTA assessment is a unique opportunity for a stronger European Health Union. We identified 19 domains related to the four key areas, processes, uncertainty, comparator choice and endpoint selection that urgently need to be addressed for this regulation to become a success.
Keywords: EU HTA, Uncertainty, Comparators, Endpoints, Process, Clinical Trial Design, Patient-relevance, Access
Introduction
In December 2021 the European Regulation on Health Technology Assessment (HTA), a key pillar of the EU Pharmaceutical Strategy, was adopted by the Council and the European Parliament. Since January 2022, preparatory work has commenced including the stepwise setting up of a secretariat, a member states’ coordination group as well as respective subgroups, a stakeholders’ network, drafting implementing and delegated acts, and drafting guidance documents. The preparatory phase ends in December 2024 with a subsequent implementation phase running until January 2030. During the preparatory phase a limited number of Joint Scientific Consultations (JSC) will be offered and Joint Clinical Assessments (JCA) will be conducted in a step-wise approach. From January 2025 on, all cancer medicines and Advanced Therapy Medicinal Products (ATMPs) will be assessed according to these joint actions, and orphan medicines will follow from Jan 2028 onwards [1].
For the preparatory phase, a service contract was signed with the EUnetHTA 21 joint consortium that is led by the Dutch ‘Zorginstituut’ (ZIN) and includes a total of 13 European HTA bodies. The service contract includes a wide variety of activities building on the achievements and lessons learned from the EUnetHTA Joint Actions and supporting the stepwise implementation of the EU HTA regulation [2]. The EUnetHTA 21 work agenda covers a various deliverables including e.g., the development of methodological and process guidances and the conduct of a limited number of JSCs and JCAs until 2025 [3].
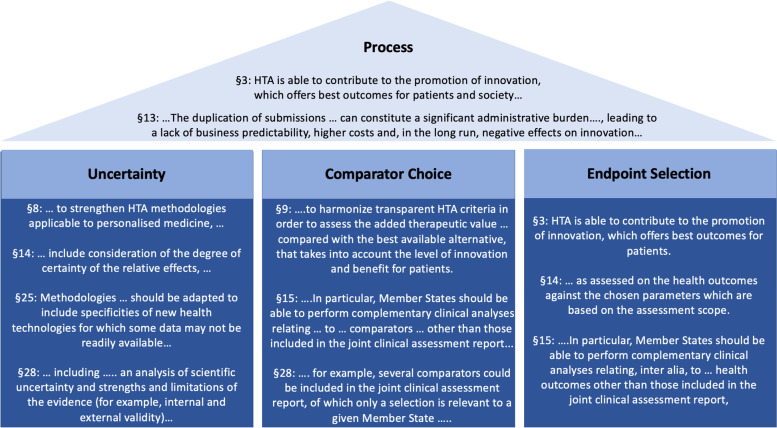
Parallel to those publicly funded activities to implement the EU HTA regulation, the ‘European Access Academy’ (EAA) was founded as a self-organized, crowd funded initiative aiming to facilitate and further support the shaping of a joint European Value Framework in order to meet the regulation’s vision ‘…to address unmet medical needs and facilitate access to innovative medicines …’ [4]. Specifically, the regulation includes extensive language guiding its implementation (Fig. 1) suggested to ensure that this regulation will strengthen the European Health Union. During the inaugural convention of the EAA a research agenda was developed highlighting key challenges areas for a joint European HTA and crystallizing related domains that warrant further research and scrutiny.
Fig. 1.
EU HTA Regulation language related to the identified challenge areas. Language derived from the preamble of the EU HTA Regulation regarding the four key areas that need to be addressed in order for the EU HTA Regulation to provide an ‘additional benefit’ compared to the status quo of many parallel independent national and subnational assessments
Methods
A total of four procedural steps were applied to determine a research agenda that focuses on achieving an additional benefit of a joint European HTA assessment over the existing national procedures. The four steps comprise i) a preparatory multi-stakeholder survey; ii) draft identification and prioritization of key domains in a working group format; iii) consolidation of the findings of the working groups; iv) final review and approval of the research agenda. An overview of the four steps is displayed in Fig. 2.
Step 1: Multi-stakeholder Survey:
Fig. 2.
The four steps in the development of the EAA’s research agenda
Prior to the inaugural convention of the EAA a multi-stakeholder survey was conducted. The semiquantitative questionnaire was developed leveraging a modified Delphi procedure and circulated across a total of n = 189 European stakeholder institutions including HTA and regulatory bodies, clinical oncology associations, patient representatives, and industry associations. Respective findings from the n = 30 responses (HTA bodies: 9; regulators: 10; patients’ and physicians’ associations: 3 each; industry: 5) were analysed and grouped into the four key challenge areas: i) processes, ii) uncertainty, iii) comparator choice, and iv) selection of endpoints that are relevant to patients. A project report was shared with the EAA faculty prior to the inaugural convention and submitted for publication [5].
Step 2: Draft Identification and Prioritization of Key Domains:
The inaugural convention of the European Access Academy was attended by 26 participants on-site and an additional 142 Unique Viewers via ZOOM during the Public Session. The EAA Working Session was set up as a hybrid meeting, allowing participation both on-site and remotely via ZOOM, and had a total of 37 participants. Building on the outcomes of the survey, four dedicated working groups were established identifying, defining, and prioritizing key domains related to the four above mentioned challenge areas. The working groups consisted of 8—10 participants each, representing a variety of involved stakeholder groups (patients/ clinicians/ regulators/ HTA & payers/ academia/ industry). The questionnaire findings as well as story books outlining the work assignments for each of the working groups were shared in advance. Two leads representing the EAA faculty and one note taker per working group facilitated the process. After the conceptual work of identifying, defining and prioritizing key domains within the working teams, results were shared across all EAA workgroups, and all participants were asked to rank relevance of the related domains from their specific point of view. Pre-generated QR codes were shared to allow for simultaneous IT based ranking using online forms generated with Microsoft Office Online. All rankings were conducted on an ordinal Likert Response Scale scoring from 1 (low priority) to 7 (high priority).
Step 3: Consolidation of the Findings of the Working Groups
In a next step findings and rankings of the EAA working groups were reviewed by the EAA secretariat (EJ, JR). Descriptive statistics were applied to the rankings derived from the EAA convention including graphical display as Box Plots (see Figs. 3, 4, 5 and 6). All analyses were conducted with Microsoft Excel Version 2019. Furthermore, a content review of convention outcomes was conducted. The descriptions of each of the work domains and related guiding questions were extracted from the workgroup notes and transferred into table format (see Tables 1, 2, 3 and 4). Any duplications were removed, and various adjustments of wording were suggested to improve clarity of the recommended key domains and related questions.
Step 4: Final Review and approval of Research Agenda
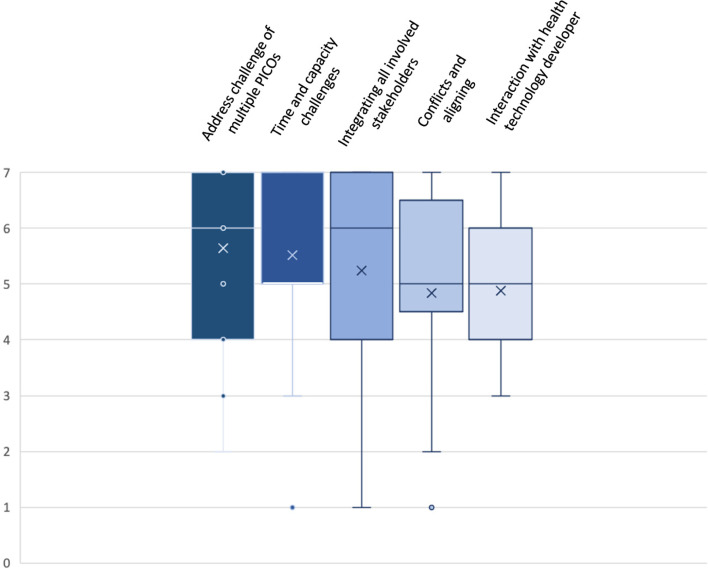
Fig. 3.
Box Plot: Ranking of Key Domains related to Challenges with Processes. Indicated are mean (x); median (bar in coloured area); interquartile range (coloured area), any individual ranks that were chosen (dots); and min/ max whiskers (dots lying outside of the whiskers are considered outliers); all rankings were conducted on an ordinal Likert Response Scale scoring from 1 (low priority) to 7 (high priority)
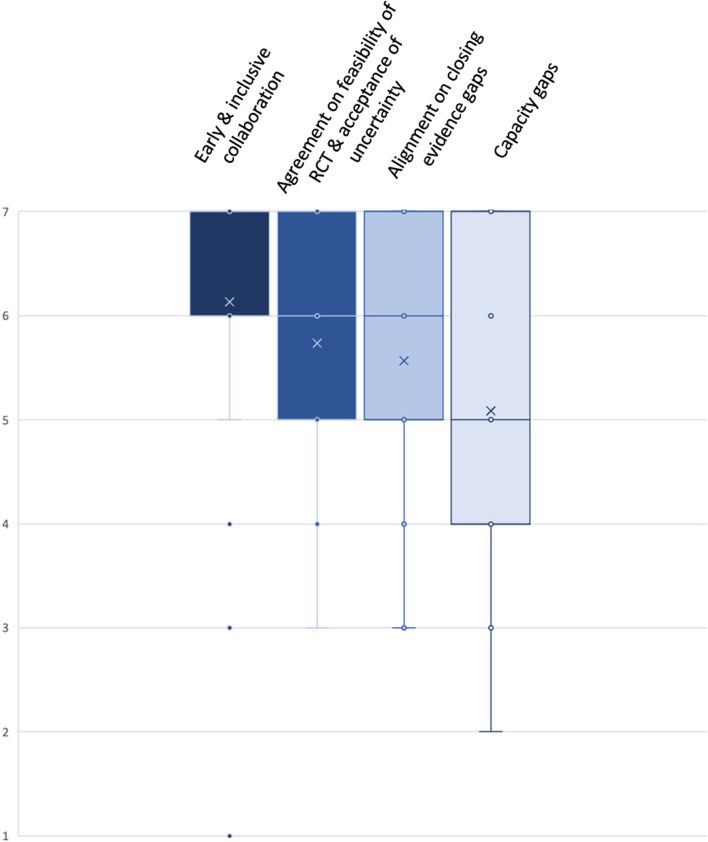
Fig. 4.
Box Plot: Ranking of Key Domains related to Challenges with Uncertainty. Indicated are mean (x); median (bar in coloured area); interquartile range (coloured area), any individual ranks that were chosen (dots); and min/ max whiskers (dots lying outside of the whiskers are considered outliers); all rankings were conducted on an ordinal Likert Response Scale scoring from 1 (low priority) to 7 (high priority)
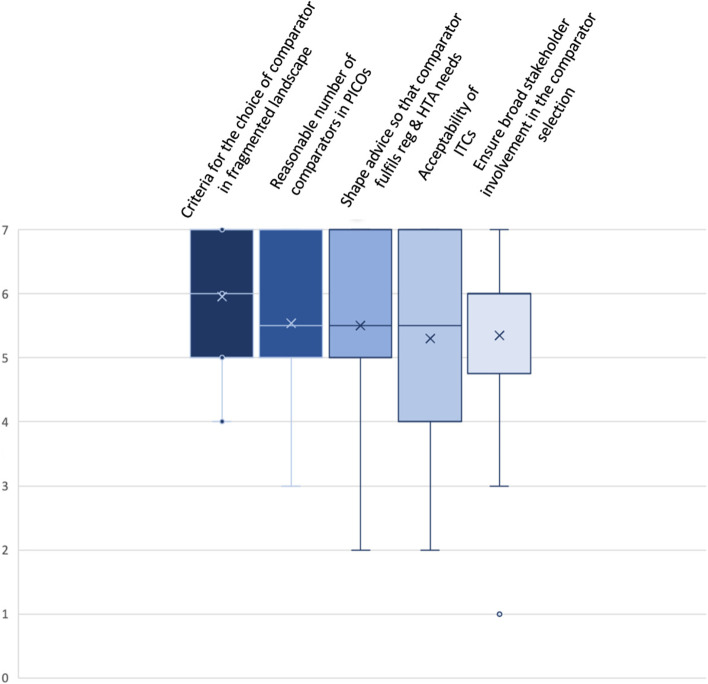
Fig. 5.
Box Plot: Ranking of Key Domains related to Challenges with Comparator Choice. Indicated are mean (x); median (bar in coloured area); interquartile range (coloured area), any individual ranks that were chosen (dots); and min/ max whiskers (dots lying outside of the whiskers are considered outliers); all rankings were conducted on an ordinal Likert Response Scale scoring from 1 (low priority) to 7 (high priority)
Fig. 6.
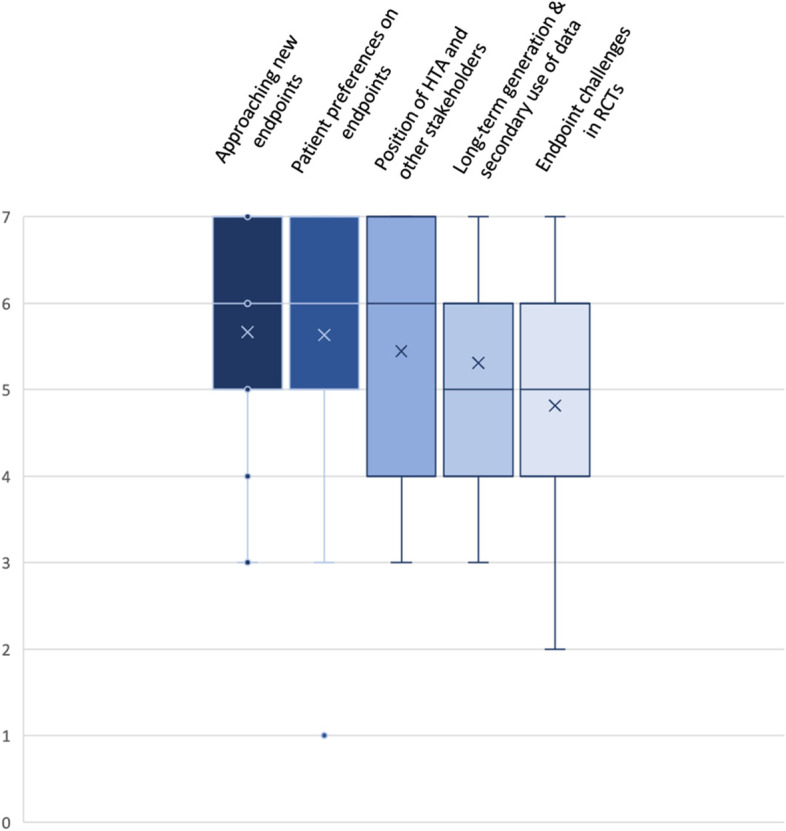
Box Plot: Ranking of Key Domains related to Challenges with Endpoint Selection. Indicated are mean (x); median (bar in coloured area); interquartile range (coloured area), any individual ranks that were chosen (dots); and min/ max whiskers (dots lying outside of the whiskers are considered outliers); all rankings were conducted on an ordinal Likert Response Scale scoring from 1 (low priority) to 7 (high priority)
Table 1.
Identified key domains to address the challenge of processes
| Process Challenges | |||||
|---|---|---|---|---|---|
| Key Domains | Address challenge of multiple PICOs | Time and capacity challenges | Integrating all involved stakeholders | Conflicts and aligning | Interaction with health technology developer |
| Description | PICO is the basis for both the advice as well as the assessment. Early discussion of PICO and agreement across regulatory and HTA stakeholders is critical to design a clinical development program | Ensure sufficient capacities to allow for early and inclusive collaboration e.g., sufficient joint early advice opportunities and to allow sufficient time for submission, generation and publication of the assessment report so national processes are not impacted | EU HTA will shape future oncology care across Europe. Early and inclusive involvement of all stakeholders (patients, HTA bodies, regulators, clinicians, industry) is key to ensure success of the regulation | Overcoming current multiplicity and/or duplication of national HTA procedures is at the heart of the EU HTA regulation. Resolution of divergences between national and EU HTA bodies and between the various other stakeholders is key | Early and inclusive collaboration between clinicians, patients, regulators, HTA bodies and the industry to ensure that the developed medicines are addressing an unmet medical need |
| Guiding Questions |
What is the rationale for different PICOs? Are there options to decrease fragmentation of PICOs? How can the challenge of a PICO changing from advice to assessment be addressed? |
What is the expected number of new oncology drug applications / year and the related joint scientific advice capabilities of EMA/ EU HTA? What can be done to increase the number of available joint scientific advice slots? |
How are the various stakeholders involved throughout a medicine’s HTA process? How will their input be used and where can it help improve the quality and usability of the assessment? How to ensure a strong clinical input? |
How to ensure national HTA bodies incorporate the JCA into their process, improving the quality of decision-making and enabling timely patient access? How to resolve or contextualise different recommendations of regulators vs HTA bodies? |
What are benefits of early and inclusive collaboration with the HTD in delivering the objectives of the Regulation? At what stages and frequency should this engagement occur? |
Table 2.
Identified key domains to address the challenge of uncertainty
| Challenges with Uncertainty | ||||
|---|---|---|---|---|
| Key Domains | Early and inclusive collaboration | Agreement on feasibility of RCT and acceptance of uncertainty | Alignment on closing evidence gaps | Capacity gaps |
| Description | Collaboration should be enhanced especially at an early stage when designing the clinical development program. Continuous involvement of regulators, HTA bodies, clinicians, patients and industry is critical | Early exploration of feasibility of RCT should be agreed by all relevant stakeholders. If an RCT is considered not feasible alternative options of generation of comparative evidence should be explored and level of acceptable uncertainty should be agreed | Early agreement on comparative evidence gaps and how to generate data to appropriately address those gaps | Ensure sufficient capacities to allow for early and inclusive collaboration e.g., sufficient joint early advice opportunities |
| Guiding Questions |
How can EU HTA take into account evolving clinical research and development paradigms in oncology to be a driver not a bottleneck for patient access to new medicines while at the same time not reducing methodological rigor? How can stakeholder involvement have meaningful impact? |
Is an RCT possible in this context? What other options for generation of comparative data exist? How can additional types of evidence and methods help increase certainty? What level of certainty can be achieved with small sample sizes e.g., in childhood cancer or in ATMPs? |
How to approach a gradually evolving evidence body with limited certainty at time of launch? What are suitable and achievable comparative evidence requirements for Real World Data and New Clinical Trial Designs? |
What is the expected number of new oncology drug applications / year and the related joint scientific advice capabilities of EMA/ EU HTA? What can be done to increase the number of available joint scientific advice slots? |
Table 3.
Identified key domains to address the challenge of comparator choice
| Challenges of Comparator Choice | |||||
|---|---|---|---|---|---|
| Key Domains | Criteria for the choice of comparator in an increasingly fragmented landscape | Reasonable number of comparators in PICOs | Shape Early Advice so that comparator fulfils both, regulatory and HTA needs | Acceptability of ITCs | Ensure broad stakeholder involvement in comparator selection |
| Description | Agreement across all involved stakeholders on the set of requirements for the appropriate comparative therapy within a clinical development program | The comparator should be the current standard of care. If multiple standards of care exist attempt should be made to limit the number of required comparisons | Agreement for early and inclusive advice covering both, regulatory and HTA needs | Agreement on applicability of ITC and methodological requirements for ITC | Ensure involvement and alignment across a wide stakeholder group incl. HTA, regulatory, clinical, patient, and industry stakeholders |
| Guiding Questions |
How to manage an ever more diverging and fast-moving comparator landscape in targeted oncology? Is the current treatment standard on-label or off-label? |
Is there a clear standard of care? How to manage different national Standards of Care and heterogeneous guideline recommendations? Is comparator selection based on best available alternative or other criteria? How should clinically interchangeable comparators be incorporated? |
How to ensure that comparator choice fulfils both, regulatory and HTA requirements? What are alternative evidence sources and methods that can be used if required comparators differ, or a comparator cannot be ethically used with a trial? |
Under what circumstances would an ITC be relevant? What are the contextual factors that can guide which ITC methods are most appropriate? |
Who should be involved in the selection of appropriate comparative therapy? What are the rationales behind their choice? |
Table 4.
Identified key domains to address the challenge of endpoint selection
| Challenges with Endpoint Selection | |||||
|---|---|---|---|---|---|
| Key Domains | Approaching new endpoints | Patient preferences on endpoints | Position of HTA and other stakeholders | Long-term generation & secondary use of data | Endpoint challenges in RCTs |
| Description | Review of applicable endpoints at the commencement of each clinical development program and early agreement across all involved stakeholders if new endpoints are required in a particular setting | The view of patients should be taken into consideration for the determination of an endpoint as ‘patient-relevant’ in HTAs | Early collaboration should make the position of all involved stakeholders transparent and lead to resolution of differing views | Agreement on the need for the generation of long-term data and secondary use of data by all involved stakeholder groups | Review of and agreement on appropriate endpoints and feasibility of RCTs to generate comparative data on these endpoints between the involved stakeholders before commencement of the clinical program |
| Guiding Questions | Are new endpoints applicable to this disease area? | How to empower patients’ voice in the determination of patient-relevance of any specific endpoint? |
Who should be involved in the definition of an endpoint? How can differing views on endpoint acceptability between stakeholders be resolved? |
How can long-term data generation and secondary use of data be facilitated? | How can we ensure that endpoints match evolving treatment paradigms in an increasingly targeted oncology environment? |
Two rounds of reviews were conducted to obtain full feed-back from the EAA faculty. In a follow up virtual meeting ~ 10 days after the EAA convention key findings and adjustments were agreed upon. Subsequently the EAA secretariat (EJ, JR) drafted the key components of the publication which was again circulated across all EAA faculty members for further review and input.
Results
A total of 19 domains warranting further research were identified, with four domains related to uncertainty, and five each to comparator choice, endpoint, and processes.
Key Domains related to Challenges with Processes:
A description of each of the domains and listing of guiding questions are displayed in Table 1. Descriptive statistics of the ranking (n = 25 responses) are presented in Fig. 3.
Identified core domains include i) address (resource-) challenge of multiple PICO (Patient/ Intervention/ Comparator/ Outcomes) schemes (mean 5.6; median 6; interquartile range (IQR) 4–7), ii) time and capacity challenges (mean 5.5; median 5; IQR 5–7), iii) integrating all involved stakeholders (mean 5.2; median 6; IQR 4–7), iv) conflicts and aligning between different multi-national stakeholders (mean 4.8; median 5; IQR 4.5–6.5), v) interactions with health technology developers (HTDs) (mean 4.9; median 5; IQR 4–6).
Process challenges were repeatedly discussed within all working groups as most of the methodological challenges include process ramifications. The challenge of multiple PICOs (identified in the scoping phase of a joint assessment), i.e., the time and resources required for manufacturers to prepare the required data and ensure availability of data for each requested PICO and the time and resources required on the side of EU HTA authorities to assess data for each PICO, was e.g., also covered in the comparator working group and was considered one of the most important hurdles for the EU HTA regulation to become a success story for Europe. Time and capacity gaps were discussed in all working groups as a major issue seriously limiting the potential of the EU HTA regulation and the ability to deliver a timely, high-quality assessment. The issue of capacity is also related to the challenge of multiple PICOs, as the higher the number of PICOs is, the more resources are needed for the assessment. Integration of all stakeholder groups in the process was considered crucial to prevent the new regulation just resulting in sophisticated technical discussions between HTA bodies and HTDs. Instead, involvement of medical societies and elaboration of relevant guidelines are considered key when e.g., determining comparative treatment regimens for a given group of patients. It was repeatedly questioned whether the outcome of the EUnetHTA 21 stakeholder network’ deliverables will allow for appropriate involvement of all relevant stakeholders, representing the EU as a whole, rather than only a few dominating countries. Consequently, ‘conflicts and aligning’ was considered a highly relevant additional domain. The evolving EU HTA system should be designed to allow for stepwise adjustments and improvements over time. Success of the implementation should be consistently tracked and reported.
Key Domains related to Challenges with Uncertainty:
A description of each of the domains and the listing of guiding questions are displayed in Table 2. Descriptive statistics (n = 23 responses) of the ranking of those domains are presented in Fig. 4.
Identified core domains include: i) early and inclusive collaboration (mean 6.1; median 7; IQR 6–7), ii) agreement on feasibility of RCT and acceptance of uncertainty (mean 5.7; median 6; IQR 5–7), iii) alignment on closing of evidence gaps (mean 5.6; median 6; IQR 5–7), and iv) capacity gaps (mean 5.1; median 5; IQR 4–7).
Initially a fifth domain was suggested, covering: ‘value and costs of closing evidence gaps’. As content of this domain was closely related to ‘alignment on closing of evidence gaps’ the decision was made to merge the two domains. A key component regarding the first domain ‘early and inclusive collaboration’ is related to the increase of predictability throughout the process and to a potential improvement of the quality of assessments. However, as ‘early and inclusive collaboration’ extends beyond predictability the name of the domain was not changed. The second domain initially only focussed on an agreement on the feasibility of an RCT. However, in situations where an RCT is not feasible, e.g., in rare diseases or due to ethical considerations, acceptance of an alternative trial design, additional types of evidence and comparison methodologies that can help reduce risk and uncertainty might be required. Thus, the adjusted wording of the second domain includes a reference to acceptance of uncertainty. Evidence gaps are related to the uncertainty in evidence/ outcomes provided and therefore constitute another important domain in this area.
Key Domains related to Challenges with Comparator Choice:
A description of each of the domains is displayed in Table 3. Descriptive statistics (n = 26 responses) of the ranking of those domains are presented in Fig. 5.
Identified core domains include i) criteria for the choice of comparator in an increasingly fragmented treatment landscape (mean 6.0; median 6; IQR 5–7), ii) reasonable number of comparators in PICOs (mean 5.5; median 6; IQR 5–7), iii) shape early advice so that comparator fulfils both, regulatory and HTA needs (mean 5.5; median 6; IQR 5–7), iv) acceptability of Indirect Treatment Comparison (ITC) (mean 5.3; median 6; IQR 4–7), v) ensure broad stakeholder involvement in comparator selection (mean 5.3; median 6; IQR 4.75–6).
An additional domain named ‘how to manage comparator in basket trials’ was removed after discussion. The challenge of comparators in basket trials is nevertheless considered very relevant and conceptually covered by the reference to the increasingly fragmented treatment landscape in the first domain. This challenge arises in particular due to advances in developing targeted treatments – not exclusively but especially in the area of oncology, where biomarker-specific approaches to tumour treatment lead to small patient numbers with different tumour types that harbour the same genetic alteration and/or molecular pattern. The challenge with multiple PICOs is covered as a main process challenge. However, to keep the focus specifically on the challenge of multiple comparators within the different PICOs identified in the scoping phase, it was decided to retain the domain within the challenges with comparator choice, as well. Lack of sufficient early advice capacities was discussed within the context of ‘shaping early advice so that comparator fulfils both, regulatory and HTA needs’. The capacity issue is also included as a major process challenge. Finally, the acceptability of ITCs is related to the challenge of comparator choice as they might be required in the context of multiple comparators, but methodological requirements should be contextualized. However, the methodological requirements for ITCs are reaching beyond comparator choice only.
Key Domains related to Challenges with Endpoint Selection:
A description of each of the domains is displayed in Table 4. Descriptive statistics (n = 26 responses) are presented in Fig. 6.
Identified core domains include i) approaching new endpoints (mean 5.7; median 6; IQR 5–7), ii) patient preferences on endpoints (mean 5.6; median 6; IQR 5–7), iii) position of HTA and other stakeholders (mean 5.4; median 6; IQR 4–7), iv) long-term generation and secondary use of data (mean 5.3; median 5; IQR 4–6), and v) endpoint challenges in RCTs (mean 4.8; median 5; IQR 4–6).
Regarding the first domain a discussion evolved whether to specify ‘definition and validation of new endpoints’. As validation did not seem appropriate in all situations relevant to HTA purposes the domain was finally named: ‘approaching new endpoints’, verbiage that includes the question whether new endpoints will be accepted by HTA bodies in a given clinical context. Although patients are also included as ‘other stakeholders’ in the third domain the determination of patient preferences with regards to endpoints was deemed particularly important and kept as a separate domain. Discussion of who should contribute to the determination of endpoints that are relevant to patients in a specific disease setting is the intent of the third domain, which had initially focussed on the role of HTA in endpoint selection only. During the discussion it became clear that early collaboration across all relevant stakeholders should be aimed for. Long-term generation of endpoint data and secondary use of data e.g., from disease registries, were originally considered two separate domains but merged into one domain as both concepts are closely related. The domain ‘endpoint challenges in RCTs’ covers the feasibility and challenges of generating comparative data on an acceptable endpoint which is relevant to patients, used in clinical practice and mature enough in the readout, in a specific disease context in an RCT. It is closely related to the concept of challenges with uncertainty, however, it received the lowest scores compared to the other domains. While the perception was that the first domain (‘approaching new endpoints’) covers some key aspects of endpoint challenges in RCTs it was still decided to retain this domain as a separate item.
Discussion
The aim of a joint European HTA assessment is stated early on in §3 of the regulation: ‘HTA is able to contribute to the promotion of innovation, which offers best outcomes for patients and society as a whole, and is an important tool for ensuring proper application and use of health technologies’ [1]. As has been repeatedly shown the challenge with the current—pre-regulation—status quo is a high level of heterogeneity of the various national and sub-national European HTA assessments resulting in different access to innovation across the EU member states and lack of predictability and a multiplicity of national HTA submissions on the side of the HTDs [6, 7]. As has been shown e.g., within the ‘SIOPE Access to Medicines Project’, ample variability in HTA decision making across Europe has an impact on availability of anticancer medicines for highly vulnerable patient groups [8]. The new EU HTA regulation is a unique opportunity to consolidate the various national HTA approaches and shape processes and methods to strengthen the European Health Union and to ensure that ‘the development of health technologies is a key driver of economic growth and innovation in the Union and is key to achieving the high level of health protection that health policies need to ensure for the benefit of all’ as stated in §1 of the regulation [1].
The presented research agenda is aimed at highlighting key challenges that warrant further research and resolution to fulfil the intentions of the regulation. While challenges were grouped into process challenges and methodological challenges (uncertainty/ comparator/ endpoints), setting up the right processes and resolving key issues in the respective domains were considered priority as they are also shaping the subsequent approach to the methodological requirements:
The challenge of multiple PICOs was considered a major hurdle for a harmonization of EU HTA efforts. Currently, the EU regulation is ambiguous as it suggests both to ‘harmonize transparent HTA criteria to assess the added therapeutic value… compared with the best available alternative’ [1, 9] and that ‘in particular member states should be able to perform complementary clinical analysis relating… to…comparators… other than those included in the joint clinical assessment report…’ [1, 15]. Where the Original Proposal for an EU HTA Regulation stated ‘Ensure the use of joint outputs in Member States’ as an Operational Objective [9] this wording is not included in the final EU HTA regulation [1]. To what extent ITCs can overcome the challenges resulting from multiple PICOs was discussed in the working group comparator choice. Therefore, ‘Acceptability of ITCs’ was selected as another domain requiring further research and resolution. Previous efforts from the EUnetHTA group to establish a European PICO [10] had aimed to implement a transparent PICO survey. However, divergence in the comparator choice remains [11], in particular due to differences in national health systems and varying levels of availability of medical treatments. This divergence will continue to make it very difficult for HTDs to shape a common clinical development program that is addressing all stakeholder needs. Therefore, it has to be considered a key objective e.g., for the developing Coordination Group and its sub-groups to not just to coordinate the assemblage of different PICOs but instead to work towards a convergence of PICO requirements in order to increase feasibility and predictability of assessment, as well as deliver a high quality report within the timeframe outlined in the regulation. Without doubt this process will cause conflicts, both between various European stakeholders (e.g., HTA bodies, regulators, medical societies, patient associations and industry) and between European and national HTA bodies. However, the success of the common EU assessment will largely depend on the capability to orchestrate this conflict in the spirit of the EU HTA Regulation. The domain ‘conflicts and alignment’ may therefore almost be considered a key performance indicator for the evolving EU HTA body.
Time and capacity challenges were considered another major limitation for a successful implementation of the new regulation. As indicated in the EUnetHTA 21 stakeholder call on March 25th, 2022, it is planned to conduct just one to two JCAs in 2023 and a very limited number of JSCs [3]. Stepwise upscaling, mutual learning and milestone tracking are important measures when implementing a new system but were not included in the scope and resources as set out in the tender specifications for the EUnetHTA 21 project by the European Commission [2]. As development of innovative oncology medicines and ATMPs is frequently based on very targeted approach with small patient numbers or where randomization is not possible, sufficient advice capacities to e.g., ensure early and inclusive collaboration across all stakeholder groups to cover methodological uncertainty challenges are critical.
Comprehensive stakeholder involvement throughout the whole process is another key topic shaping the prospects of the EU HTA regulation [12]. The stepwise establishment of a European HTA system will result in new requirements and interfaces not just for HTDs but also for patient associations, medical societies, regulators, and industry. Indeed, HTA bodies have already defined patient-relevant endpoints used for relative effectiveness assessment in Europe [13]. However, it appears important to include all relevant stakeholders in the definition of trial endpoints that are relevant to patients in a specific disease setting [14–16], as well as determination of the appropriate comparator [17]. The question remains how to approach the challenge of what level of uncertainty may be considered acceptable in disease setting e.g., what might constitute appropriate criteria, who would be involved in making a decision, etc. Such societal questions are reaching far beyond technical discussions between HTDs and HTA bodies and require involvement of a wide spectrum of stakeholders. The recently published joint EMA/ EUnetHTA work plan may be considered an important first step in the direction of comprehensive stakeholder involvement [18–20]. Collaboration with medical societies, patient representatives and industry will require similar systematic work plans to ensure societal convergence on these key value considerations.
The initial implementation phase of the EU HTA regulation is focusing on Oncology and ATMPs. This scope matches the intention of ‘Europe’s Beating Cancer plan’ [21] and reflects the high level of unmet medical need in these fields. However, research in oncology has advanced to ever more targeted interventions in ever smaller populations. Consequently, innovative oncology medicines will also require innovative methodologies to determine the additional benefit over the current standard of care in such small populations [8, 22]. Furthermore, certain rare conditions, as well as technologies such as ATMPs, might not allow for the conduct of a RCT. Agreement on alternative options to collect comparative data [23] as well as discussions on the acceptable level of uncertainty in evidence generation in a certain disease context might therefore be required [24, 25].
Conclusion
The implementation of a joint European HTA assessment is a unique opportunity for a strong European Health Union. We identified 19 domains related to the four key areas processes, uncertainty, comparator choice and endpoint selection that urgently need to be addressed for this regulation to become a success. Considering many overlapping issues and challenges, an integrated and coordinated strategy including all relevant stakeholders is needed. A continuous tracking of the regulations’ implementation milestones will be required to ensure that this HTA assessment is able to contribute to the promotion of innovation, which offers best outcomes for patients and society as a whole.
Acknowledgements
We would like to acknowledge all participants of the inaugural European Access Academy Convention in May 2022.
Abbreviations
- ATMP
Advanced Therapy Medicinal Product
- EAA
European Access Academy
- HTA
Health Technology Assessment
- HTD
Health Technology Developer
- IQR
Interquartile Range
- ITC
Indirect Treatment Comparison
- JCA
Joint Clinical Assessment
- JSC
Joint Scientific Consultation
- PICO
Patient/ Intervention/ Comparator/ Outcomes
- RCT
Randomized Controlled Trial
- SIOPE
European Society for Paediatric Oncology
- ZIN
Zorginstitut, Netherlands
Authors’ contributions
JR and EJ conceived the research project. EJ and JR drafted the preparatory survey and analyzed the survey data; reviewed the findings and rankings of the EAA working groups and drafted the manuscript. MP, OSM, FG, MT, HCB, CD, WG and PM facilitated the working group sessions during the convention and were involved in the review and analysis of the respective session outcomes. All authors reviewed and provided feedback on the manuscript. The authors read and approved the final manuscript.
Funding
This research was partly funded by an unrestricted grant from Abbvie, Astra Zeneca, Novartis, Roche and Sanofi.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
JR & EJ received an unrestricted grant by Abbvie, Astra Zeneca, Novartis, Roche and Sanofi that partially funded this research.
MP: no CoI.
OSM: no CoI.
FG: no CoI.
MT: no CoI.
HCB: no CoI.
CD: as strategic and legal consultant regularly receives honoraria for consulting from numerous health technology developers.
WG: no CoI.
PM: no CoI.
JFB received honoraria for consulting and research grants from Abbvie, Amgen, AstraZeneca, Bayer, BMS, Ferring, Gilead, GSK, IQVIA, Lilly, Novartis, Roche, Sanofi, Takeda.
TS: no CoI.
AH: employed by F. Hoffmann-La Roche.
MG: no CoI.
AC: no CoI.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.The European Parliament and the Council of the European Union. Regulation (EU) 2021/2282 of the European Parliament and of the Council of 15 December 2021 on Health Technology Assessment and amending Directive 2011/24/EU. Official Journal of the European Union L 458/1. 22.12.2021. Available from: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32021R2282&from=EN (2021). Accessed 10 June 2022.
- 2.Ted eTendering. Tender reference number CHAFEA/LUX/2020/OP/0013. Calls for tenders from the European institutions (2021). Available from: https://etendering.ted.europa.eu/cft/cft-display.html?cftId=7416 (2021) Accessed 21 June 2022.
- 3.EUnetHTA 21: Publication of Project Plans. Available from: https://www.eunethta.eu/category/eunethta-21/ (2022). Accessed 10 June 2022.
- 4.European Commission. Health Technology Assessment: Commission welcomes the adoption of new rules to improve access to innovative technologies. Brussels, 13 December 2021. Available from: https://ec.europa.eu/commission/presscorner/detail/en/IP_21_6771 (2021). Accessed 10 June 2022.
- 5.Julian E., Gianfrate F., Sola-Morales O., Mol P., Bergmann J.F., Salmonson T., Hebborn A., Grande M., Ruof J. How can a joint European Health Technology Assessment provide an ‘additional benefit’ over the current standard of national assessments? Insights generated from a multi-stakeholder survey in hematology/oncology. Health Econ Rev 2022; 12(1)30.10.1186/s13561-022-00379-7. [DOI] [PMC free article] [PubMed]
- 6.Chassagnol F, Marcelli G, Wagle J, Giuliani G, Traub D, Schaub V, Ruof J. Review of Relative effectiveness assessments (REAs) of pharmaceuticals at the European network for health technology assessment (EUnetHTA): A first step towards a consolidated European perspective on comparative effectiveness & safety? Health Policy. 2020;124:943–951. doi: 10.1016/j.healthpol.2020.06.013. [DOI] [PubMed] [Google Scholar]
- 7.Allen N, Walker S, Liberti L, Salek S. Health Technology Assessment (HTA) Case Studies: Factors Influencing Divergent HTA Reimbursement Recommendations in Australia, Canada, England, and Scotland. Value Health. 2017;20(3):320–328. doi: 10.1016/j.jval.2016.10.014. [DOI] [PubMed] [Google Scholar]
- 8.Schoot R, Otth M, Frederix G, Leufkens H, Vassal G. Market access to new anticancer medicines for children and adolescents with cancer in Europe. Eur J Cancer. 2022;165:146–153. doi: 10.1016/j.ejca.2022.01.034. [DOI] [PubMed] [Google Scholar]
- 9.The European Commission. Proposal for a Regulation of the European Parliament and of the Council on health technology assessment and amending Directive 2011/24/EU. (2018). Available from: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32021R2282&from=EN (2021). Accessed 21 June 2022.
- 10.Schreuder-Morel C, Dupree R, Willemsen A. Shaping the future of European Health Technology Assessments: Establishing a European PICO. Value Health. 2019;S798;PNS218. 10.1016/j.jval.2019.09.2118.
- 11.Kisser A, Knieriemen J, Fasan A, Eberle K, Hogger S, Werner S, Taube T, Rasch A. Towards compatibility of EUnetHTA JCA methodology and German HTA: a systematic comparison and recommendations from an industry perspective. EJHE. 2022;23:863–878. doi: 10.1007/s10198-021-01400-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dierks C. Is there a need for more patient participation in German? Analysis and outlook Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2019;62(9):1113–1119. doi: 10.1007/s00103-019-02994-y. [DOI] [PubMed] [Google Scholar]
- 13.Pavlovic M, Teljeur C, Wieseler B, Klemp M, Cleemput I, Neyt M. Endpoints for relative effectiveness assessment (REA) of pharmaceuticals. Int J Technol Assess Health Care. 2014;30(5):508–513. doi: 10.1017/S0266462314000592. [DOI] [PubMed] [Google Scholar]
- 14.Pavlovic M. Challenges for Relative Effectiveness Assessment and Early Access of Cancer Immunotherapies in Europe. Front Med (Lausanne) 2016;14(3):56. doi: 10.3389/fmed.2016.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruof J, Knoerzer D, Dünne A, Dintsios C, Staab T, Schwartz F. Analysis of endpoints used in marketing authorisations versus value assessments of oncology medicines in Germany. Health Policy. 2014;118:242–254. doi: 10.1016/j.healthpol.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 16.Pearson A, Weiner S, Adamson P, Karres D, Reaman G, Rousseau R, Blanc P, Norga K, Skolnik J, Kearns P, Scobie N, Barry E, Marshall L, Knox L, Caron H, Wariabharaj D, Pappo A, DuBois S, Gore L, Kieran M, Weigel B, Fox E, Nysom K, de Rojas T, Vassal G. ACCELERATE – Five years accelerating cancer drug development for children and adolescents. Eur J Cancer. 2022;166:145–164. doi: 10.1016/j.ejca.2022.01.033. [DOI] [PubMed] [Google Scholar]
- 17.EUnetHTA Guideline. Comparators and Comparisons. Criteria for the choice of the most appropriate comparator(s). Summary of current policies and best practice recommendations. Adapted Version (2015). Available at: https://www.eunethta.eu/wp-content/uploads/2018/03/Criteria_WP7-SG3-GL-choice_of_comparator_amend2015.pdf.
- 18.European Medicines Agency, European Network of Health Technology Assessment. EMA/188201/2022. European collaboration between regulators and health technology assessment bodies. April 11th, 2022. https://www.ema.europa.eu/en/documents/work-programme/european-collaboration-between-regulators-health-technology-assessment-bodies-joint-work-plan-2021_en.pdf (2022). Accessed 10 June 2022.
- 19.Berntgen M, Gourvil A, Pavlovic M, Goettsch W, Eichler H, Kristensen F. Improving the contribution of regulatory assessment reports to health technology assessments–a collaboration between the European Medicines Agency and the European network for Health Technology Assessment. Value Health. 2014;17(5):634–641. doi: 10.1016/j.jval.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 20.Tafuri G, Lucas I, Estevão S, Moseley J, d’Andon A, Bruehl H, Gajraj E, Garcia S, Hedberg N, Massari M, Molina A, Obach M, Osipenko L, Petavy F, Petschulies M, Pontes C, Russo P, Schiel A, Van de Casteele M, Zebedin-Brandl E, Rasi G, Vamvakas S. The impact of parallel regulatory-health technology assessment scientific advice on clinical development. Assessing the uptake of regulatory and health technology assessment recommendations. Br J Clin Pharmacol. 2018;84(5):1013–9.10.1111/bcp.13524. [DOI] [PMC free article] [PubMed]
- 21.European Commission. Communication from the Commission to the European Parliament and the Council. Europe’s Beating Cancer Plan. Brussels, 3.2.2021 COM(2021) 44 final. Available from: https://eur-lex.europa.eu/legal-content/en/TXT/?uri=COM%3A2021%3A44%3AFIN (2021) Accessed 11 March 2022.
- 22.Pavlovic M., Garnier J., Durand-Zaleski I., participants of Round Table n(o) 3 of Giens XXXI; Bilbault P., Gaudin A., Le Jeunne C., Lalaude O., Roze S., de Sahb R., Sapede C. Progression-free survival overall survival and quality of life: What is their medicoeconomic importance in oncology? Therapie. 2016 Dec;71(6):625–632. Doi: 10.1016/j.therap.2016.03.004. [DOI] [PubMed]
- 23.Wagle JA, Flacke JP, Knoerzer D, Ruof J, Merkesdal S. Intraindividual Comparisons to Determine Comparative Effectiveness: Their Relevance for GBA’s Health Technology Assessments. Value Health. 2021;24:744–752. doi: 10.1016/j.jval.2020.11.016. [DOI] [PubMed] [Google Scholar]
- 24.Djulbegovic B. Articulating and Responding to Uncertainties in Clinical Research. J Med Philosophy. 2007;32(2):79–98. doi: 10.1080/03605310701255719. [DOI] [PubMed] [Google Scholar]
- 25.Coyle D, Durand-Zaleski I, Farrington J, Garrison L, Graf von der Schulenburg J, Greiner W, Longworth L, Meunier A, Moutié A, Palmer S, Pemberton-Whiteley Z, Ratcliffe M, Shen J, Sproule D, Zhao K, Shah K. HTA methodology and value frameworks for evaluation and policy making for cell and gene therapies. Eur J Health Econ. 2020;21(9):1421–1437. doi: 10.1007/s10198-020-01212-w. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.