Abstract
Background
Public health is seriously threatened by the rise of carbapenem-resistant Enterobacterales (CRE). However, the genomic characteristics of CRE detected in pediatric patients are largely unknown. Here, we reported the genomic characteristics of a multidrug-resistant Escherichia coli strain containing the plasmid-borne blaNDM-5 and blaCTX-M-14 genes recovered from a Chinese pediatric patient.
Methods
The genome sequence of E. coli strain B379 was determined using Illumina NovaSeq 6000 and Oxford Nanopore MinION. Multiple bioinformatics tools were used to annotate the genome sequence, identify antimicrobial resistance genes and plasmid replicons and perform the in silico multilocus sequence typing (MLST) analysis. Using BacWGSTdb 2.0 server, a core genome MLST (cgMLST) comparison was made between E. coli B379 and all ST746 E. coli strains downloaded from the public database.
Results
The E. coli B379 genome sequence is comprised of six contigs totaling 5,152,502 bp, including one chromosome and five plasmids. Nineteen antimicrobial resistance genes were predicted. The blaNDM-5, which is located on a 46,161 bp IncX3 plasmid and the blaCTX-M-14 gene which is located on a 147,204 bp IncFII/IncFIA/IncFIB plasmid are two examples of these 19 genes. E. coli B379 was resistant to ampicillin, ampicillin/sulbactam, ceftriaxone, ceftazidime, imipenem, cefepime, ciprofloxacin, ertapenem, trimethoprim-sulfamethoxazole. This isolate belonged to ST746 and the closest relative was another one originating from a human material specimen in Denmark, which differed by 273 cgMLST alleles.
Conclusion
Our study reports the emergence of a multidrug-resistant E. coli strain co-carrying blaNDM-5 and blaCTX-M-14 recovered from a pediatric patient in China. These data would help us better understand the prevalence, genetic characteristics, and mechanisms of antimicrobial resistance of this recently identified multidrug-resistant bacteria in children.
Keywords: whole-genome sequencing, Escherichia coli, multidrug-resistant, bla NDM-5 , bla CTX-M-14 , pediatrics
Introduction
Escherichia coli is not only a regular component of the gut flora of people but also one of the main sources of bacterial illnesses in people. For children, the number of available antimicrobial agents is restricted; in general, cephalosporins are recommended as the first-line therapeutic option, and carbapenems are often used for the treatment of serious Gram-negative extended spectrum β-lactamase-producing bacterial infections. Nonetheless, the emergence of third-generation cephalosporin and carbapenem-resistant E. coli, has exacerbated the situation significantly. A high-priority species of E. coli was identified as carbapenem-resistant and third-generation cephalosporin-resistant in a 2007 WHO publication of antibiotic-resistant bacteria that need medical attention.1,2 Carbapenem-resistant E. coli has been identified rarely in children; nevertheless, growing occurrences globally and a more medically complicated pediatric population imply that it may emerge as a serious threat to children.3 Similarly to what has been found in adult populations, the incidence of infections caused by multidrug-resistant bacteria in children has increased over time. Carbapenem resistance has grown from 0% in 1999 and 2000 to 0.47% in 2010 and 2011 among Enterobacterales in children in the United States over the past decade.4 The nosocomial infection rate of carbapenem-resistant Enterobacterales in the Chinese pediatric population was 0.08–0.15%, with a higher rate in neonatal intensive care unit (0.63–1.93%).5
Mobile genetic elements containing genes encoding for carbapenemases, such as Klebsiella pneumoniae carbapenemase (KPC), metallo-β-lactamases and oxacillinase 48 (OXA-48), have aided the fast worldwide spread of carbapenem-resistant E. coli in recent years.6 Nearly all β-lactam antibiotics, including carbapenems, could be hydrolyzed by New Delhi metallo-β-lactamase (NDM), an Ambler class B β-lactamase.7 Numerous infections brought on by E. coli strains that produce NDM are reported to have a poor prognosis and a high death rate, particularly in individuals who are immunocompromised.8 The horizontal transfer and dissemination of antibiotic resistance genes in carbapenem-resistant E. coli strains are usually carried by conjugative plasmids.9 Despite the growing focus on carbapenem-resistant Enterobacterales in China, data on the epidemiology of these infections in children are still scarce. Here, we report the genomic features of a carbapenem-resistant E. coli from a Chinese pediatric patient that carried multiple antimicrobial resistance genes. This study provides deep insights into the characteristics and diversity of blaNDM-5 harboring E. coli and further emphasizes their role in dissemination of carbapenem resistance.
Materials and Methods
In August 2021, a pus sample from a 14-year-old hospitalized with severe appendicitis yielded the E. coli isolate B379. The identification was carried out using the GN panels (BioMérieux, France) and the VITEK 2 system (BioMérieux, France). The identified result was double-checked by a MALDI Biotyper (Bruker Daltonics, USA) and 16S rRNA gene sequencing. The VITEK 2 system (BioMérieux, France) was also used to test the antimicrobial susceptibility of E. coli B379 using the AST GN13 panels (BioMérieux, France) for the following antimicrobial agents: ampicillin, ampicillin-sulbactam, piperacillin-tazobactam, ceftriaxone, ceftazidime, cefepime, imipenem, ertapenem, aztreonam, levofloxacin, ciprofloxacin, trimethoprim-sulfamethoxazole, amikacin, gentamicin, and tobramycin. The Clinical and Laboratory Standards Institute (CLSI) 2021 standards were used to evaluate minimum inhibitory concentrations (MICs). Ethical approval of this study was obtained from the ethics committee of Children Hospital of Hangzhou (2021–71), China. Written informed consent from the patient’s parents was obtained and utilized to publish the case details. Isolation of the clinical sample was part of the routine procedure of clinical laboratory.
Using a Genomic DNA Purification Kit, the genomic DNA was extracted (QIAGEN, USA). DNA concentration and purity were assessed using a NanoDrop™ spectrophotometer (Thermo Scientific, USA). The whole-genome sequencing was carried out on two platforms: Illumina NovaSeq 6000 for the short-reads and Oxford Nanopore MinION for the long-reads. The de novo assembly of Illumina and Nanopore reads was carried out using Unicycler 0.4.8.10
The NCBI Prokaryotic Genomes Annotation Pipeline (PGAP) automatically annotated the genome sequence. IslandViewer 4,11 ISfinder,12 PHASTER 2016,13,14 CRISPRCasFinder,15 and antiSMASH 5.016 were applied to identify the genomic islands, insertion sequence (IS) elements, clustered regularly interspaced short palindromic repeat (CRISPR) sequences, prophage sequences, and secondary metabolite gene clusters, respectively, using default parameters.
ABRicate 1.0.1 was used in conjunction with ResFinder 4.1, CARD 2020, SerotypeFinder 2.0 and PlasmidFinder 2.1 to predict acquired antimicrobial resistance genes, serotype and plasmid incompatibility (Inc) groups.17–19 Virulence factors and open reading frames (ORFs) were identified by VirulenceFinder 2.020 and ORFfinder. BacWGSTdb 2.0 server was used to carry out in silico multilocus sequence typing (MLST) analysis and bacterial source tracing using a core genome MLST (cgMLST) analysis.21–23 Circular and schematic maps of the blaNDM carrying plasmid were performed by BRIG v0.9524 and SnapGene 6.1, respectively. The genetic surroundings of antibiotic resistance genes and the homologous areas shared between two plasmids were illustrated using Easyfig 2.2.5.25
Results and Discussion
The entire genome sequence of E. coli B379 is made up of six contigs with a combined length of 5,152,502 bp that have been closed and circularized. Contig 1 (4,801,759 bp) belonged to the chromosome, while other contigs belonged to different plasmids (contig 2, 152061 bp; contig 3, 147204 bp; contig 4, 46161 bp; contig 5, 2809 bp; contig 6, 2508 bp). The strain’s overall G+C content was 50.87%. Total 4643 coding sequences (CDSs), 119 RNAs (22 rRNA, 86 tRNA, and 11 ncRNA) genes were identified. At least 56 genomic islands are present in the E. coli B379 genome, as well as a number of IS elements, the majority of which belong to the IS1, IS3 and IS5 families. The genome contains a total of 11 CRISPR sequences and 15 prophage sequences. It is also possible to anticipate the existence of four putative secondary metabolite gene clusters, including the yersiniabactin, O-antigen, turnerbactin and colicin V. In addition, E. coli B379 also contained several virulence factors, including astA, gad, terC, fyuA, and irp2 carried by the chromosome; and iucABCD, iucC, iutA, sitA, and traT carried by the plasmids. According to in silico serotype prediction, E. coli B379 belongs to the nontypeable O:H44 subgroup.
Antimicrobial susceptibility tests revealed that the isolate was resistant to ampicillin/sulbactam, ceftriaxone, ampicillin, ceftazidime, cefepime, imipenem, ertapenem, ciprofloxacin, and trimethoprim–sulfamethoxazole (Table 1). The genes for resistance to β-lactams (blaTEM-1B, blaCTX-M-14 and blaNDM-5), quinolones [aac(6’)-Ib-cr], sulphonamide (sul1, sul2, sul3) and trimethoprim (dfrA1) are found in E. coli B379. The isolate has target changes in the proteins GyrA (S83L) and ParC (S80I) that confer fluoroquinolone resistance. The multidrug resistance to aminopenicillins, cephalosporins, carbapenems, quinolones, sulphonamide, and trimethoprim was well explained by these results. There are several other resistance genes, such as, aminoglycoside [two copies of aadA1, aac(6’)-Ib-cr, aac(6’)-Ib3, aadA3], macrolide [mdf(A), erm(42), erm(B)], tetracycline[tet(B)], amphenicol (floR, two copies of cmlA1), which may result resistance to tobramycin, azithromycin, chloramphenicol, and tetracycline.
Table 1.
Multidrug Resistance Profile of blaNDM-5-Carrying E. coli B379 in This Study
| Antimicrobial Agents | MICs |
|---|---|
| Ampicillin | ≥64 |
| Ampicillin-sulbactam | ≥32 |
| Piperacillin–tazobactam | 64 |
| Ceftriaxone | ≥64 |
| Ceftazidime | ≥64 |
| Cefepime | ≥64 |
| Aztreonam | <=1 |
| Imipenem | 8 |
| Ertapenem | ≥8 |
| Levofloxacin | 1 |
| Ciprofloxacin | 1 |
| Trimethoprim–sulfamethoxazole | ≥320 |
| Amikacin | 4 |
| Gentamicin | 2 |
| Tobramycin | 8 |
Abbreviation: MICs, minimal inhibitory concentrations.
The IncX3 plasmid (pB379_NDM) carrying the carbapenem resistance gene blaNDM-5 and a 46,161 bp size had a G + C content of 46.7%. Using the approach developed by Norman et al,26,27 plasmid pB379_NDM was found to include genes involved in replication (repB, copG and bis), stability (ftsH, topB, taxA, parB, hns, ykgJ, mpr, parA, trpF, umuD, taxD and dnaJ), propagation (virB1, virB2, virB3/virB4, virB5, virB6, virB8, virB9, virB10, virB11, traR, nusG, virD4, and kikA), and adaptation (tnpA-IS3000-ISAba125-IS5-blaNDM-5-blaMBL-Tn125-IS26-ISKox3-tnpR) (Figure 1). Only one resistance gene blaNDM-5, which is responsible for carbapenem resistance, was present on the IncX3 plasmid. An insertion sequence called ISAba125 that contains the genes IS5 and IS3000 exists upstream of blaNDM-5, according to analysis of the genetic environment surrounding the gene. The blaNDM-5 is located following the bleMBL, trpF, tat, catA1, and IS26 elements.
Figure 1.
Circular map of plasmid pB379_NDM. Genes at the outermost ring shown in blue, aqua, fuchsia, and orange were respectively involved in stability, propagation, replication, and application. The resistance genes were indicated in red. Hypothetical protein or other coding sequences (CDSs) are indicated in gray.
Interaction with the host or the adaptative response during horizontal transfer may cause the potential insertions or deletions of IS sequences and transposons on blaNDM-5 genetic surroundings, which could also be regarded as the variable region of the plasmid (Figure 1). pB379_NDM both had 100% query coverage and identity with multiple blaNDM-5-carrying IncX3 plasmids, according to a BLAST search. These plasmids were isolated from a variety of bacterial species, including E. coli, K. pneumoniae and P. mirabilis, indicating that this plasmid can horizontally transfer and disseminate among multiple species of Enterobacterales. It was also discovered that these plasmids were isolated from specimens of various sources, including humans, animals and vegetables, from different areas in both China and other countries, further illustrating a worldwide spread of predominant IncX3-type plasmid harboring blaNDM-5 gene.28
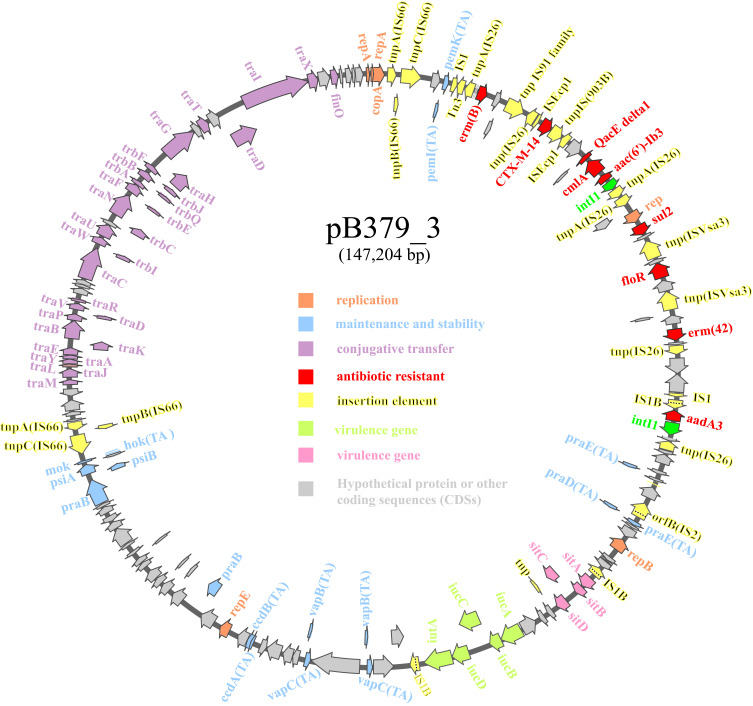
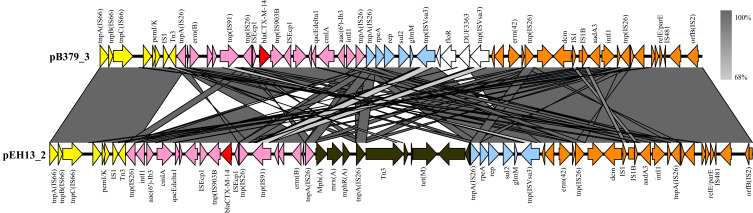
The blaCTX-M-14 gene was found on a 147,204 bp circular plasmid (pB379_3) with a G + C content of 51.69% and contained 411 predicted ORFs. This plasmid contained IncFII/IncFIA/IncFIB type of replicons. The backbone region of pB379_3 included replication genes (repA, repB and repE), maintenance and stability gene [parAB, pisAB, mok, toxin–antitoxin (TA) systems)], conjugative transfer system (tra, trb, finO genes), MDR (multidrug-resistant) region (antibiotic-resistant gene, insertion element, etc), virulence gene (iucABCD-iutA gene cluster, sitABCD gene cluster). The MDR region carried antibiotic genes including blaCTX-M-14, erm(B), erm(42), qacE, cmlA1, aac(6’)-Ib3, aadA3, sul2, floR (Figure 2). BLAST comparison indicated that pB379_3 exhibited high similarity to plasmid pEH13_2 (CP089099.1; 98% query coverage and 99.97% identity). Analysis of the MDR region of plasmid pB379_3 and pEH13_2 revealed truncated ISEcp1 exists upstream of blaCTX-M-14 and downstream of the IS903B and truncated ISEcp1, and the transposition element located on the structure with two IS26 with the opposite direction. Therefore, the blaCTX-M-14 and the associated ISEcp1-derived promoter might be able to move by the actions of IS26 elements. Another difference was that the plasmid pB379_3 carried fluoroquinolone resistance genes but the plasmid pEH13_2 carried tet(M) and mph(A) genes (Figure 3). There were several insertion sequences in the MDR region, which may result from insertions, deletions and homologous recombination event of these insertion sequences. Other antimicrobial resistance genes, ie, blaTEM-1B, two copies of aadA1, aac(6’)-Ib-cr, sul1, sul3, dfrA1, mdf(A), tet(B), cmlA1 was located on the chromosome.
Figure 2.
Schematic map of plasmid blaCTX-M-14-carrying plasmid pB379_3. Genes are denoted by arrows, and the function of genes were annotated with different colours.
Figure 3.
Linear comparison of MDR region of plasmid pB379_3 with pEH13_2. Genes are denoted by arrows. The same color denotes the same gene cluster of them. White and black represent different regions between the two plasmids. Shading indicates regions of homology (>95% nucleotide identity).
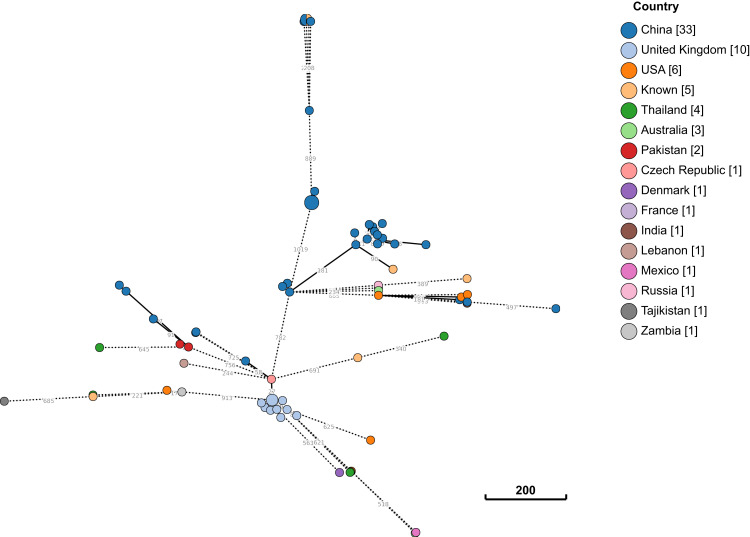
In silico MLST analysis indicated that, E. coli strain B379 belongs to sequence type (ST)746. To explore the worldwide genomic epidemiological characteristics of E. coli strains, the phylogenetic relationships between E. coli B379 and a total of 72 ST746 E. coli strains currently deposited in the NCBI GenBank database were investigated. According to cgMLST analyses, the closest relative of E. coli B379, another ST746 strain (E. coli KTE154) isolated from human feces in Denmark in 2010, differs by 273 cgMLST loci (Figure 4). This Denmark strain only carried one antimicrobial resistance genes [mdf(A)], suggesting that horizontal transmission was responsible for the acquisition of the blaCTX-M-14 and blaNDM-5 genes in E. coli B379.
Figure 4.
Phylogeny between E. coli B379 and all ST746 strains currently available in public database. The clonal relationship between two isolates is depicted by lines connecting the circles. The scale bar indicates the allelic difference between two isolates. In square brackets, the number of isolates from each nation is shown.
Currently, the majority of research focuses on the spread of carbapenemases among carbapenem-resistant Enterobacterales strains isolated from adult patients, while just a few of studies examine the spread of carbapenemases-producing strains isolated from children.29 The World Health Organization has determined that children who have carbapenem-resistant Enterobacterales strains in fecal samples are at a high risk because they are more likely to be exposed via close contact.30 A recent study investigated the carriage and molecular epidemiology of carbapenem-resistant Enterobacterales from fecal samples of outpatient children in Shanghai. They demonstrated the rate of carbapenem-resistant Enterobacterales colonization in feces collected from children was 3.6%, which was less than what had been observed in patients from community settings in other countries.31 Another study collected a total of 935 non-duplicate carbapenem-resistant Enterobacterales strains from 36 hospitals in 24 provinces across China from 2016 to 2018. They found the prevalence of blaNDM-5-carrying E. coli isolates from children was 32.9%.32 Globally, clonally different carbapenem-resistant E. coli strains (ie, ST405, ST131, ST156, and ST101) have been described; however in this work, a sporadic clone ST746 E. coli strain was discovered. This clonal lineage has been previously discovered from a wastewater treatment plant in South Korea, which also confer a high level of resistance to multiple antimicrobial agents, including carbapenems.33 To our knowledge, this is the first report of a multidrug-resistant ST746 E. coli isolate co-carrying blaNDM-5 and blaCTX-M-14 genes recovered from a pediatric patient in China.
Conclusion
Carbapenem-resistant E. coli infection remains an uncommon but serious threat in children. Our study reported the genomic characteristics of a multidrug-resistant E. coli strain carrying blaNDM-5 and blaCTX-M-14 genes recovered from a Chinese teenage patient. These data would contribute to the comprehensive understanding of the carbapenem resistance mechanisms and transmission dynamics of this bacteria among children.
Acknowledgment
This work was supported by Zhejiang Province Public Welfare Technology Application Research Project (LGF22H040011). We thank Meina Yue for providing the isolate.
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.Tacconelli E, Carrara E, Savoldi A., et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis. 2018;18(3):318–327. doi: 10.1016/S1473-3099(17)30753-3 [DOI] [PubMed] [Google Scholar]
- 2.Castanheira M, Simner PJ, Bradford PA. Extended-spectrum beta-lactamases: an update on their characteristics, epidemiology and detection. JAC Antimicrob Resist. 2021;3(3):dlab092. doi: 10.1093/jacamr/dlab092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aguilera-Alonso D, Escosa-García L, Saavedra-Lozano J, Cercenado E, Baquero-Artigao F. Carbapenem-resistant gram-negative bacterial infections in children. Antimicrob Agents Chemother. 2020;64(3). doi: 10.1128/AAC.02183-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Logan LK, Renschler JP, Gandra S, Weinstein RA, Laxminarayan R. Carbapenem-resistant Enterobacteriaceae in children, United States, 1999-2012. Emerg Infect Dis. 2015;21(11):2014–2021. doi: 10.3201/eid2111.150548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yin L, He L, Miao J, et al. Actively surveillance and appropriate patients placements’ contact isolation dramatically decreased carbapenem-resistant Enterobacteriaceae infection and colonization in pediatric patients in China. J Hosp Infect. 2020;105(3):486–494. doi: 10.1016/j.jhin.2020.03.031 [DOI] [PubMed] [Google Scholar]
- 6.Tilahun M, Kassa Y, Gedefie A, Ashagire M. Emerging carbapenem-resistant Enterobacteriaceae infection, its epidemiology and novel treatment options: a Review. Infect Drug Resist. 2021;14:4363–4374. doi: 10.2147/IDR.S337611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bahr G, Vitor-Horen L, Bethel CR, Bonomo RA, Gonzalez LJ, Vila AJ. Clinical evolution of New Delhi metallo-beta-lactamase (NDM) optimizes resistance under Zn(II) deprivation. Antimicrob Agents Chemother. 2018;62:1. doi: 10.1128/AAC.01849-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hasassri ME, Boyce TG, Norgan AP, et al. An immunocompromised child with bloodstream infection caused by two Escherichia coli strains, one harboring NDM-5 and the other harboring OXA-48-like carbapenemase. Antimicrob Agents Chemother. 2016;60(6):3270–3275. doi: 10.1128/AAC.03118-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson H, Torok ME. Extended-spectrum beta-lactamase-producing and carbapenemase-producing Enterobacteriaceae. Microb Genom. 2018;4:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wick RR, Judd LM, Gorrie CL, Holt KE. Unicycler: resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput Biol. 2017;13(6):e1005595. doi: 10.1371/journal.pcbi.1005595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bertelli C, Laird MR, Williams KP, et al. IslandViewer 4: expanded prediction of genomic islands for larger-scale datasets. Nucleic Acids Res. 2017;45(W1):W30–W35. doi: 10.1093/nar/gkx343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siguier P, Perochon J, Lestrade L, Mahillon J, Chandler M. ISfinder: the reference centre for bacterial insertion sequences. Nucleic Acids Res. 2006;34:D32–36. doi: 10.1093/nar/gkj014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou Y, Liang Y, Lynch KH, Dennis JJ, Wishart DS. PHAST: a fast phage search tool. Nucleic Acids Res. 2011;39:W347–352. doi: 10.1093/nar/gkr485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arndt D, Grant JR, Marcu A, et al. PHASTER: a better, faster version of the PHAST phage search tool. Nucleic Acids Res. 2016;44(W1):W16–21. doi: 10.1093/nar/gkw387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Couvin D, Bernheim A, Toffano-Nioche C, et al. CRISPRCasFinder, an update of CRISRFinder, includes a portable version, enhanced performance and integrates search for Cas proteins. Nucleic Acids Res. 2018;46(W1):W246–W251. doi: 10.1093/nar/gky425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blin K, Shaw S, Steinke K, et al. antiSMASH 5.0: updates to the secondary metabolite genome mining pipeline. Nucleic Acids Res. 2019;47(W1):W81–W87. doi: 10.1093/nar/gkz310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zankari E, Hasman H, Cosentino S, et al. Identification of acquired antimicrobial resistance genes. J Antimicrob Chemother. 2012;67(11):2640–2644. doi: 10.1093/jac/dks261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joensen KG, Tetzschner AM, Iguchi A, Aarestrup FM, Scheutz F. Rapid and easy in silico serotyping of Escherichia coli isolates by use of whole-genome sequencing data. J Clin Microbiol. 2015;53(8):2410–2426. doi: 10.1128/JCM.00008-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alcock BP, Raphenya AR, Lau TTY, et al. CARD 2020: antibiotic resistome surveillance with the comprehensive antibiotic resistance database. Nucleic Acids Res. 2020;48(D1):D517–D525. doi: 10.1093/nar/gkz935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joensen KG, Scheutz F, Lund O, et al. Real-time whole-genome sequencing for routine typing, surveillance, and outbreak detection of verotoxigenic Escherichia coli. J Clin Microbiol. 2014;52(5):1501–1510. doi: 10.1128/JCM.03617-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruan Z, Feng Y. BacWGSTdb, a database for genotyping and source tracking bacterial pathogens. Nucleic Acids Res. 2016;44(D1):D682–687. doi: 10.1093/nar/gkv1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruan Z, Yu Y, Feng Y. The global dissemination of bacterial infections necessitates the study of reverse genomic epidemiology. Brief Bioinform. 2020;21(2):741–750. doi: 10.1093/bib/bbz010 [DOI] [PubMed] [Google Scholar]
- 23.Feng Y, Zou S, Chen H, Yu Y, Ruan Z. BacWGSTdb 2.0: a one-stop repository for bacterial whole-genome sequence typing and source tracking. Nucleic Acids Res. 2021;49(D1):D644–D650. doi: 10.1093/nar/gkaa821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alikhan NF, Petty NK, Ben Zakour NL, Beatson SA. BLAST Ring Image Generator (BRIG): simple prokaryote genome comparisons. BMC Genomics. 2011;12:402. doi: 10.1186/1471-2164-12-402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sullivan MJ, Petty NK, Beatson SA. Easyfig: a genome comparison visualizer. Bioinformatics. 2011;27(7):1009–1010. doi: 10.1093/bioinformatics/btr039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Norman A, Hansen LH, Sorensen SJ. Conjugative plasmids: vessels of the communal gene pool. Philos Trans R Soc Lond B Biol Sci. 2009;364(1527):2275–2289. doi: 10.1098/rstb.2009.0037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tian D, Wang B, Zhang H, et al. Dissemination of the bla NDM-5 gene via IncX3-type plasmid among Enterobacteriaceae in children. mSphere. 2020;5:1. doi: 10.1128/mSphere.00699-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Köck R, Daniels-Haardt I, Becker K, et al. Carbapenem-resistant Enterobacteriaceae in wildlife, food-producing, and companion animals: a systematic review. Clin Microbiol Infect. 2018;24(12):1241–1250. doi: 10.1016/j.cmi.2018.04.004 [DOI] [PubMed] [Google Scholar]
- 29.Chiotos K, Han JH, Tamma PD. Carbapenem-resistant Enterobacteriaceae infections in children. Curr Infect Dis Rep. 2016;18(1):2. doi: 10.1007/s11908-015-0510-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwartz KL, Morris SK. Travel and the spread of drug-resistant bacteria. Curr Infect Dis Rep. 2018;20(9):29. doi: 10.1007/s11908-018-0634-9 [DOI] [PubMed] [Google Scholar]
- 31.Pan F, Tian D, Wang B, et al. Fecal carriage and molecular epidemiology of carbapenem-resistant Enterobacteriaceae from outpatient children in Shanghai. BMC Infect Dis. 2019;19(1):678. doi: 10.1186/s12879-019-4298-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Han R, Shi Q, Wu S, et al. Dissemination of carbapenemases (KPC, NDM, OXA-48, IMP, and VIM) among carbapenem-resistant Enterobacteriaceae isolated from adult and children patients in China. Front Cell Infect Microbiol. 2020;10:314. doi: 10.3389/fcimb.2020.00314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shin H, Kim Y, Han D, Hur HG. Emergence of high level carbapenem and extensively drug resistant Escherichia coli ST746 producing NDM-5 in influent of wastewater treatment plant, Seoul, South Korea. Front Microbiol. 2021;12:645411. doi: 10.3389/fmicb.2021.645411 [DOI] [PMC free article] [PubMed] [Google Scholar]