Abstract
Body-associated microbes were recently shown to change significantly during decomposition, undergoing an ecological succession in experimental conditions using rodent and swine models. We investigated microbial succession in soils associated with swine carcasses under experimental field conditions in summer and winter. We demonstrate that these postmortem microbial communities change in a specific, reproducible fashion, and that soil microbes represent a significant component of the postmortem microbial community, contrary to widespread belief in forensic science. However, the effects of decomposition on soil microbial communities were different in summer and winter. We suggest that the microbial ecological succession will be useful in medicolegal death investigation; however, observations in winter might not be applicable to summer, which indicates a need for a greater understanding of the seasonality of decomposition.
Keywords: postmortem microbiology, taphonomy, decomposition, bacteria, Rhabditidae
Introduction
Recent surveys of the postmortem microbiome have demonstrated the great potential of postmortem microbiology for forensic science [1–4]. These studies are exciting because they take advantage of culture-independent, high-throughput sequencing technologies to provide an accurate characterization of the incredible diversity of microbes involved in decomposition, a feat not possible a decade ago. For example, Metcalf et al. [1] characterized the bacterial, archaeal and microbial eukaryote communities associated with the decomposition of mouse (Mus musculus) carcasses on soil, in a controlled laboratory setting, and with great numbers of replicates. They [1] observed that postmortem microbial communities differ between location (skin, abdomen, soil), and that the structure of these communities changed similarly in each replicate mouse during decomposition. Furthermore, carcass breakdown triggered the proliferation of a bacterivorous nematode (Oscheius tipulae). This proliferation was probably stimulated by the flush of bacteria responding to the nutrient pulse provided by the carcass, on which the nematodes then feed. These findings are significant because they provided an accurate estimate of the time elapsed since death (postmortem interval: PMI), which is one of the most difficult challenges facing forensic pathology and medicolegal death investigation [5]. In particular, gravesoil, the soil associated with decomposing remains (as defined in Carter et al. [6]), provided highly accurate estimates of PMI.
These recent investigations [1–4] have advanced postmortem microbiology to a position where we must determine whether the observed trends are reproducible in natural, less-controlled settings in contrasting climates and seasons. These environmental parameters must be considered because temperature and moisture can significantly affect body breakdown [6] and microbial activity [7, 8]. In a field setting these variables are strongly related to seasonality yet, despite some investigations into the seasonality of carcass decomposition [4, 6, 9, 10], an analysis of postmortem microbial community structure between seasons has yet to be conducted. To advance our fundamental understanding of postmortem microbiology, we characterized gravesoil microbial communities associated with swine (Sus scrofa domesticus) decomposition in a field setting during winter and summer. We investigated (1) seasonal variation in control soils, (2) the structure of gravesoil microbiomes between summer and winter, and (3) the structure of gravesoil microbiomes within each season.
Materials and Methods
Gravesoils and control soils (soils not associated with carcasses) were collected as described in Meyer et al. [9]. Briefly, swine carcasses (~20kg) were placed on the soil surface of a tall grass prairie near Mead, Nebraska, USA, in February or June [9]. The cause of death was blunt force trauma (bolt gun) to the forehead in accordance with Institutional animal care and use standards. The experimental site is located in a cold (Dfa) climate with hot summers and no wet season [11]. Gravesoil samples were collected from underneath these carcasses to a depth of 5cm at intervals of 0, 15, 30, and 60 days postmortem. Control soils were collected from adjacent (2m) plots using the same protocol. Each sample was collected from a new location in an attempt to collect undisturbed soils. All soils were stored at −20°C until DNA extraction. DNA extraction and PCR amplification were conducted as described in Metcalf et al. [1]. 16S rRNA amplicons were sequenced using the Illumina HiSeq 2000 (100 basepair reads) and the 18S rRNA amplicons were sequenced using the Illumina MiSeq (150 basepair reads). Sequence processing and data analyses were conducted as described in Metcalf et al. [1], except that we used updated taxonomy databases (Greengenes version 13_5 for open-reference OTU picking of 16S rRNA sequences, SILVA version 111 for closed-reference OTU picking of 18S rRNA sequences). Additionally, primer and adapters were removed from the end of the 18S read, resulting in variable read lengths of approximately 120 basepairs.
For 16S sequences, taxa that were not classified in Domain Bacteria or Archaea were removed. For 18S sequences, we focused on the microbial community by filtering out taxa classified in groups Craniata, Chloroplastida, Mollusca, and Arthropoda. After these filtering steps, our 16S and 18S data sets included 6,473,512 sequence reads (mean 134,864 reads per sample) and 232,581 (mean 5,537 reads per sample), respectively. The average number of reads per sample was substantially lower for the 18S data set because of the lower depth of sequencing on the MiSeq platform and because some sample contained a high relative abundance of chloroplast, insect, and host DNA reads, which were filtered out. We rarified the 16S data set to 14,000 sequences per sample and the 18S data set to 350 sequences per sample, a level of rarefaction that standardized the sequence depth while allowing us to include most samples in analyses. Using the QIIME pipeline [12], we explored relative taxon abundances and patterns of community dissimilarity with phylogeny-based UniFrac unweighted distances. We report p-values for statistics based on unweighted UniFrac distances using a PERMANOVA statistical text (999 permutations).
Results
Temperature
Temperature differences between winter and summer were large. Mean ambient temperature during the winter trial equaled 5.3°C and ranged from −18.3°C to 25.5°C. Mean ambient temperature was ≤0°C for the initial four days postmortem. Mean soil temperature during the summer trial equaled 23.6°C and ranged from 11.7°C to 36.7°C [9].
Gross Carcass Decomposition
Significant differences were observed between the decomposition of carcasses in summer and winter [9]. In fact, carcasses during the winter trial were frozen for approximately 20 days postmortem, which resulted in little visible decomposition until 30 days postmortem. Although some maggot activity was observed in the mouth during the first few days of the winter trial, there was no other insect activity during the winter trial until approximately 25 days postmortem. In contrast, insect activity was rapid and significant during the summer trial. As a result, carcasses decomposed rapidly in the summer; a state of advanced decomposition was observed by 9 days postmortem. Carcasses in both seasons decomposed to a similar extent by 60 days postmortem, with the majority of decomposition in the winter occurring after 15 days postmortem. Conversely, the majority of decomposition during the summer trial occurred prior to 15 days postmortem [9].
Postmortem Microbial Communities
Overall, the most abundant bacterial phylum in all soils, regardless of treatment, was Verrucomicrobia. The bacterial microbial community in control soils was, however, significantly (P<0.05) different between seasons. Among the dominant (≥1%) taxa significant differences in community structure were observed as a significantly (P<0.001) greater abundance of the bacterial order Solirubrobacterales (phylum Actinobacteria) during the winter. In contrast, the structure of the eukaryotic microbiome in control soils was not significantly (P=0.538) different between seasons. Among the dominant eukaryotic taxa, however, one significant (P<0.05) difference was observed: the abundance of the protozoan Kinetoplastia (phylum Euglenozoa) was greater in the summer.
Two outliers were detected in the gravesoil samples, one in the summer and one in the winter (Fig. 1A). These samples were removed for community analysis. The bacterial communities in both summer (P<0.001) and winter (P<0.01) post-rupture gravesoils differed significantly from their respective controls, but the winter gravesoils did not change to the extent observed in the summer gravesoils (Fig. 1B, 1C). Summer gravesoils contained a significantly (P<0.001) greater relative abundance of Sphingobacterium sp. relative to control soils but carcass decomposition also reduced the relative abundance of several dominant bacterial taxa (Fig. 1A). Winter gravesoil contained a significantly (P<0.05) greater relative abundance of Psychrobacter sp. (Proteobacteria: Gammaproteobacteria) compared to control soils. As observed in the control soils, the structure of gravesoil bacterial community differed significantly (P<0.001) between seasons. In particular, the summer gravesoils were associated with a significantly (P<0.01) greater relative abundance of bacterial family Chitinophagaceae (Bacteroidetes: Sphingobacteriales) compared to winter gravesoils.
Fig. 1.
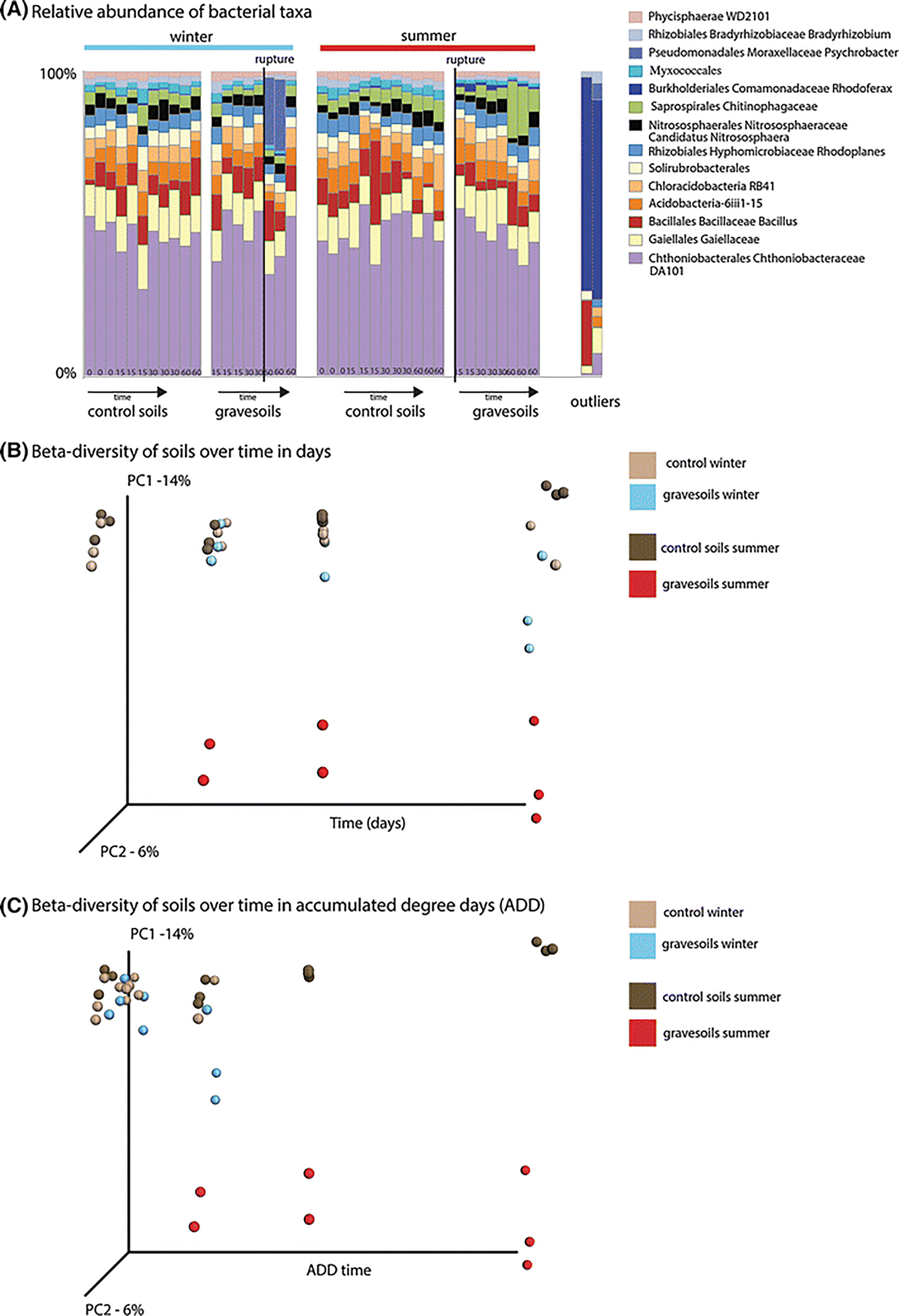
Soil bacterial community structure changes significantly during carcass decomposition. This effect is modulated by season. (A) Relative abundance of bacteria over time in gravesoil and control soil in winter (left) and summer (right) with outliers presented at the far right. (B) Principal Coordinates Analysis (PCoA) plot based on unweighted UniFrac distances showing bacterial community change associated with carcass decomposition during both summer and winter. (C) PCoA plot based on unweighted UniFrac distances displaying bacterial community change as a function of accumulated degree days, the sum of mean daily temperature during decomposition.
Post-rupture gravesoil eukaryote communities were significantly different from control soils in the summer (P<0.001) but not in the winter (P=0.868). Summer gravesoil was associated with a significantly greater relative abundance of several taxa compared to control soil including nematode Rhabditidae (P<0.001), slime mold Fonticula alba (P<0.001), amoeba Euamoebida (P<0.001), fungus Eurotiomycetes (P<0.01), and fungus Tremellomycetes (P<0.01). Post-rupture gravesoil eukaryote communities did not differ significantly between seasons (P=0.364), but when all gravesoil time points were included, we did detect a significant difference (P<0.01). Notable differences can be seen in the relative abundance of particular eukaryotic taxa in summer and winter gravesoils (Fig. 2). For example, gravesoils contained a significantly (P<0.001) greater relative abundance of rhabditid nematodes in the summer than in the winter. A high abundance of nematodes during decomposition were also observed in Metcalf et al. [1], suggesting that a nematode bloom may occur in outdoor scenarios as well as in a closed system in the laboratory.
Fig. 2.
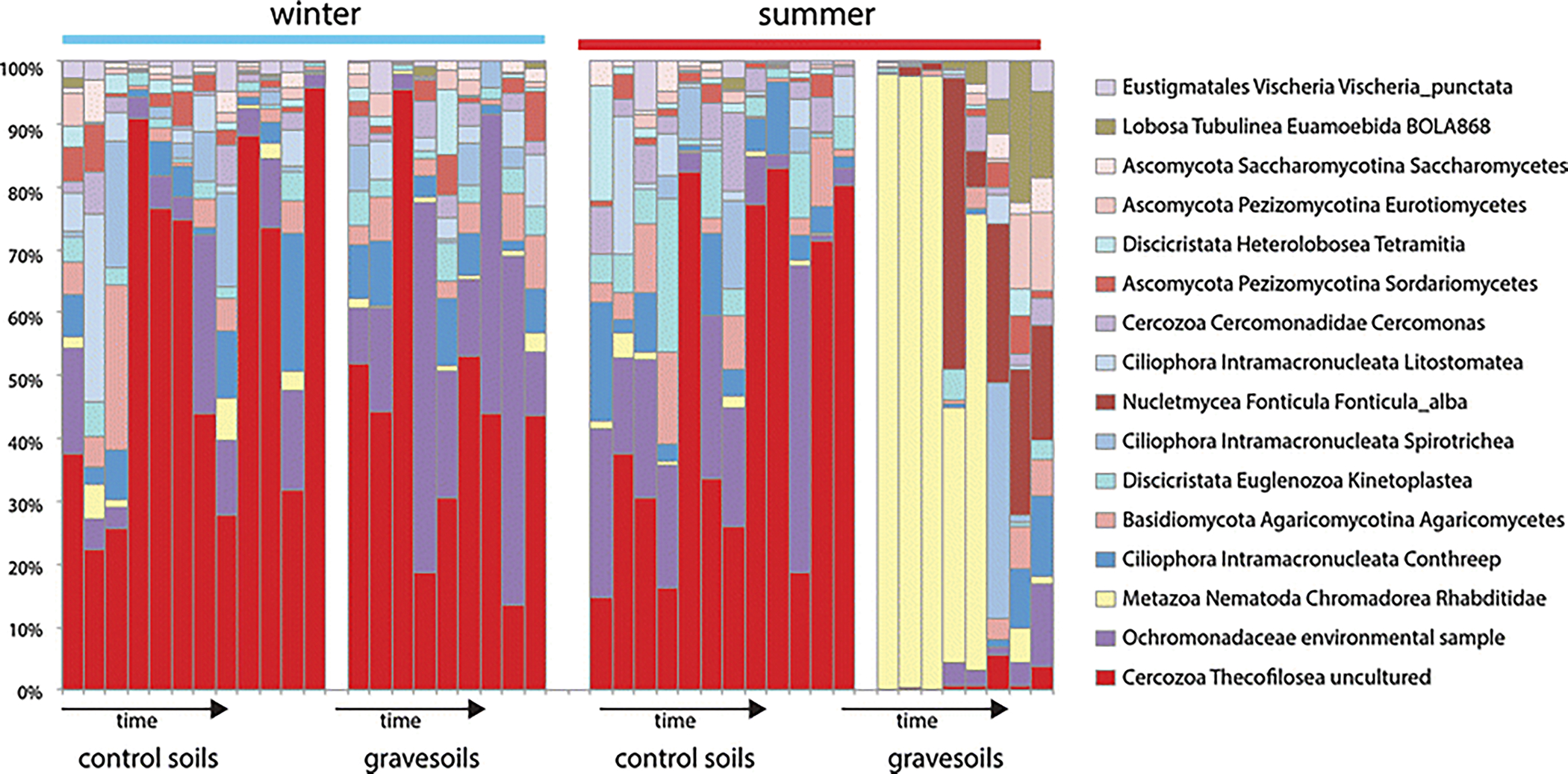
Relative abundance of the soil microbial eukaryote community during carcass decomposition in winter and summer. The structure of the summer community changes significantly during carcass decomposition with nematodes (yellow) dominating gravesoils during active decay. A significant change was not observed during the winter.
Discussion
The current data show that significant microbial community changes during decomposition are repeatable across replicates in an outdoor setting, similar to observations by Metcalf et al. [1] in a controlled laboratory setting, and Pechal et al. [2] and Hyde et al [3]. Therefore, we conclude that controlled decomposition studies using small mammalian carcasses do provide insight into the decomposition of much larger mammal carcasses, and have the potential to serve as human analogs for forensic research. The current data also demonstrate that gravesoil microbial communities are influenced significantly by season. This observation corroborates the chemical observations of Meyer et al. [9] and other investigations into the seasonality of soil microbial activity [4, 7, 8, 13, 14]. This finding is important because it indicates that investigative tools based on soil microbiology are season specific. For example, the flush of Psychrobacter might serve as an indicator of winter decomposition. In contrast, the flush of Chitinophagaceae might fill this role in the warmer months, when chitin from insects and fungi is more prevalent.
The observation that gravesoil microbial community shifts are defined by changes in the abundance of soil–dwelling communities, rather than carcass-borne communities, provides further evidence [e.g. 1, 15] that soil microbes play a significant role in the breakdown of material originating from the carcass. This observation is an important one because the opposite has widely been believed to be true in forensic science [e.g. 16]. The abundance of Verrucomicrobia in all samples is consistent with soils from a tallgrass prairie [17], which indicates the possibility of regional variation in gravesoil microbial communities. It is also necessary to recognize that although the bacterial community profile may be different among soil types and regions, the decomposer taxa that become abundant may be similar. For example, in both our study and in Metcalf et al. [1], taxa in the family Sphingobacteriaceae increased in abundance during decomposition. Additional studies will be required to understand the extent to which microbial decomposer gravesoil communities are universal.
The current results also show that changes in the bacterial community facilitate changes in the eukaryote community in the summer gravesoils. This is likely due to two primary factors. First, some members of the eukaryote community use bacterial biomass as food. This relationship can be inferred by the flush of nematodes and amoebae; these taxa are bacterivorous and probably responding to an increase in the abundance of their food supply. Nematodes have long been used as environmental indicators [18] and the current study provides additional evidence that rhabditid nematodes (Class Chromadorea) play an important role in carcass decomposition and the function of carcass decomposition islands [1].
A second explanation for the change in structure of the microbial eukaryotic community is that the bacterial community facilitates the proliferation of certain eukaryotes by changing the physical and chemical properties of gravesoil. A high level of moisture, alkalinity (8–9pH), and an abundance of anaerobic and facultatively aerobic bacteria are characteristic of early stages of carcass decomposition. This is not an environment in which fungi can compete effectively. However, the fungi proliferated as bacterial resources are depleted and moisture content and pH decreases. We observed a proliferation of Tremellomycetes (phylum Basidiomycota) in the summer and Dothideomycetes (phylum Ascomycota) in the winter, which is consistent with previous studies of the ammonia and postputrefaction fungi [19, 20].
The current study contributes substantially to our knowledge of postmortem microbiology because it describes the structure of bacterial, archaeal, and eukaryote microbiomes associated with carrion. We demonstrated that postmortem microbial communities differ through space and time and these trends are reproducible in the field and significantly influenced by season and region. We now know that these are critical factors to consider when developing microorganisms as physical evidence.
Key Points.
We show that gravesoil microbial community change is reproducible across replicates during decomposition in an outdoor setting. These results confirm and extend trends observed in controlled laboratory experiments.
In non-carcass associated control soils, season has a significant effect on the composition of bacterial communities, but not microbial eukaryotic communities.
Gravesoils associated with carcasses that have ruptured differ significantly in their bacterial soil communities from controls in the summer, but not in the winter.
Summer gravesoils contain more kinds of bacterial and eukaryotic microbial taxa than winter gravesoils.
Seasonality is likely important for determining a robust microbial clock to estimate postmortem interval.
Acknowledgements
DNA sequences have been deposited in the QIIME database as studies 1609. We thank J Huntley and the CU next-generation sequencing facility. We thank Allison Mann for assistance with sequence data processing. This work was supported in part by the National Institutes of Justice (Award 2011-DN-BX-K533) and the Howard Hughes Medical Institute. NIH-BRIC P20MD006084 supports research capacity and infrastructure at Chaminade University of Honolulu.
References
- 1.Metcalf J, Wegener-Parfrey L, Gonzalez A, Lauber CL, Knights D, Ackermann G, et al. A microbial clock provides an accurate estimate of the postmortem interval in a mouse model system. eLife. 2013;2:e01104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pechal JL, Crippen TL, Benbow ME, Tarone AM, Dowd S, Tomberlin JK. The potential use of bacterial community succession in forensics as described by high throughput metagenomic sequencing. Int J Leg Med. 2014;128:193–205. [DOI] [PubMed] [Google Scholar]
- 3.Hyde ER, Haarmann DP, Lynne AM, Bucheli SR, Pertrosino JF. The living dead: bacterial community structure of a cadaver at the onset and end of the bloat stage of decomposition. PLOS One. 2013;8:e77733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pechal JL, Crippen TL, Tarone AM, Lewis AJ, Tomberlin JK, Benbow ME. Microbial community functional change during vertebrate carrion decomposition. PLOS One. 2013;8:e79035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vass AA. The elusive universal post-mortem interval formula. Forensic Sci Int. 2011;204:34–40. [DOI] [PubMed] [Google Scholar]
- 6.Carter DO, Yellowlees D, Tibbett M. Cadaver decomposition in terrestrial ecosystems. Naturwissenschaften. 2007;94:12–24. [DOI] [PubMed] [Google Scholar]
- 7.Carter DO, Yellowlees D, Tibbett M. Temperature affects microbial decomposition of cadavers (Rattus rattus) in contrasting soils. Appl Soil Ecol. 2008;40:129–37. [Google Scholar]
- 8.Carter DO, Yellowlees D, Tibbett M. Moisture can be the dominant environmental parameter governing cadaver decomposition in soil. Forensic Sci Int. 2010;200:60–6. [DOI] [PubMed] [Google Scholar]
- 9.Meyer J, Anderson B, Carter DO. Seasonal variation of carcass decomposition and gravesoil chemistry in a cold (Dfa) climate. J Forensic Sci. 2013;58:1175–82. [DOI] [PubMed] [Google Scholar]
- 10.Benbow ME, Lewis AJ, Tomberlin JK, Pechal JL. Seasonal necrophagous insect community assembly during vertebrate carrion decomposition. J Med Entomol. 2013;50:440–50. [DOI] [PubMed] [Google Scholar]
- 11.Peel MC, Finlayson BL, McMahon TA. Updated world map of the Köppen-Geiger climate classification. Hydrol Earth Syst Sci. 2007;11:1633–44. [Google Scholar]
- 12.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. QIIME allows analysis of high-throughput community sequencing data. Nature Methods. 2010;7:335–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Putman RJ. Patterns of carbon dioxide evolution from decaying carrion. 1. Decomposition of small mammal carrion in temperate systems. Oikos. 1978;31:47–57. [Google Scholar]
- 14.Putman RJ. Flow of energy and organic matter from a carcase during decomposition. 2. Decomposition of small mammal carrion in temperate systems. Oikos. 1978;31:58–68. [Google Scholar]
- 15.Lauber CL, Metcalf JL, Keepers K, Ackermann G, Carter DO, Knight R. Vertebrate decomposition is accelerated by soil microbes. Appl Environ Microbiol. 2014;80:4920–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evans WED. The Chemistry of Death. Springfield, IL, USA: Charles C. Thomas; 1963. [Google Scholar]
- 17.Fierer N, Ladau J, Clemente JC, Leff JW, Owens SM, Pollard KS, et al. Reconstructing the microbial diversity and function of pre-agricultural tallgrass prairie soils in the United States. Science. 2013;6158:621–4. [DOI] [PubMed] [Google Scholar]
- 18.Bongers T, Ferris H. Nematode community structure as a bioindicator in environmental sampling. Trends Ecol Evol. 1999;14:224–8. [DOI] [PubMed] [Google Scholar]
- 19.Tibbett M, Carter DO. Mushrooms and taphonomy: the fungi that mark woodland graves. Mycologist. 2003;17:20–4. [Google Scholar]
- 20.Sagara N, Yamanaka T, Tibbett M. Soil fungi associated with graves and latrines: toward a forensic mycology. In: Tibbett M, Carter DO, editors. Soil analysis in forensic taphonomy: Chemical and biological effects of buried human remains. Boca Raton, FL, USA: CRC Press; 2008. pp. 67–108. [Google Scholar]


