Abstract
Pasteurella haemolytica is an important respiratory pathogen of cattle that incites extensive infiltrates of neutrophils into the lung. In addition to the parenchymal damage caused by factors released by P. haemolytica, neutrophils contribute to the pathologic changes in the lungs. Molecules which mediate neutrophil infiltration into the lungs during P. haemolytica pneumonia are poorly characterized. To determine whether the CD18 family (β2-integrin) of leukocyte adhesion molecules mediates initial passage of neutrophils into the pulmonary bronchi and bronchioles of lungs infected with P. haemolytica, three Holstein calves homozygous for bovine leukocyte adhesion deficiency (BLAD) (CD18-deficient neutrophils), and three age- and breed-matched control calves (normal CD18 expression) were inoculated with P. haemolytica A1 via a fiberoptic bronchoscope and euthanized at 2 h postinoculation. Sections of lung were stained for neutrophils, and the intensity of neutrophilic infiltration was determined by computerized image analysis. Significantly fewer (P < 0.05) neutrophils infiltrated the lumen, epithelium, and adventitia of bronchioles and bronchi in lungs of calves with BLAD compared to normal calves, which had dense infiltrates within these sites at 2 h postinoculation. The reduced infiltration in calves with BLAD occurred despite the presence of an extremely large number of neutrophils in peripheral blood that is typical for these calves. The large number of neutrophils in the blood of calves with BLAD is probably a physiologic response that can occur without microbial colonization, since one calf with BLAD that was raised under germ-free conditions had large numbers of neutrophils in the blood that were similar to those in a calf with BLAD that was raised conventionally. Neutrophil counts in the germ-free and conventionally reared calves with BLAD were much higher than those in the three normal calves raised under germ-free conditions. The work in this study demonstrates that during the initial inflammatory response, neutrophils with normal CD18 expression pass more readily than CD18-deficient neutrophils into the walls and lumen of bronchi and bronchioles. It suggests that CD18 is needed for initial passage through the extensive extracellular matrix of the bronchi and bronchioles. This has potential importance for the development of therapies to direct or inhibit neutrophil infiltration into conducting airways rather than alveolar spaces.
Acute pulmonary infections of cattle as a result of Pasteurella haemolytica are characterized by dense infiltrates of neutrophils (21, 25). These infiltrates are associated with extensive parenchymal necrosis which is, at least in part, due to bacterial products such as leukotoxin, lipopolysaccharide, and polysaccharide and neutrophil constituents. The contribution of the neutrophils to parenchymal damage in this disease was demonstrated by work of Slocombe et al., in which calves depleted of neutrophils and inoculated with P. haemolytica had reduced parenchymal damage compared to calves with normal levels of neutrophils (21). Neutrophil constituents that potentially contribute to the tissue damage include enzymes such as elastase and acid hydrolases, as well as oxidative radicals, cytokines, and chemokines. The last two enhance the inflammatory response and incite the infiltration of additional neutrophils. The neutrophil-mediated damage is likely to be exacerbated by serum factors such as complement (24).
Little is known about the mechanisms by which adhesion molecules mediate the infiltration of neutrophils into lung alveoli, bronchi, and bronchioles during P. haemolytica pneumonia. In most tissues, passage of neutrophils into areas of bacterial infection initially involves relatively unstable interactions between selectin molecules (L-, E- and P-selectin) and their glycoconjugate receptors (7, 14, 18). Cytokines then induce the production of CD18 (β2-integrins) on neutrophils, which bind to immunoglobulin superfamily molecules such as intercellular cell adhesion molecule 1 (ICAM-1) produced by endothelial cells. Studies have shown that CD18 (β2-integrin)–ICAM-1 binding in the lungs can mediate neutrophil passage across the alveolar septa into the alveolar lumen under certain conditions (7, 18); however, a CD18-independent process of adherence is also operative in the alveoli (2, 7–9, 14).
Initial discovery of passage of CD18-deficient neutrophils into the alveoli of the lungs was seen in spontaneous cases of pneumonia in children with leukocyte adhesion deficiency type I (LAD) (3, 13) and in calves with bovine leukocyte adhesion deficiency (BLAD) (2, 7, 11, 15–17, 20). Pneumonic lungs from these calves had robust infiltrates of neutrophils in the pulmonary alveolar lumens, even though the neutrophils had impaired expression of CD18. Subsequently, we demonstrated that neutrophils in calves with BLAD (CD18-deficient neutrophils) readily infiltrate the pulmonary alveoli following intrabronchial deposition of P. haemolytica (2).
Compared with neutrophil passage in pulmonary alveoli, neutrophil infiltration into bronchi and bronchioles is less well understood and is more complicated by the extensive extracellular matrix and thick epithelium. The bronchi and bronchioles are sites vital to initial infection by P. haemolytica (14) and other pathogens, and the epithelial cells of these airways are necessary for host defense mechanisms such as mucociliary activity and the production of antimicrobial peptides (e.g., β-defensins) (22). In this study, we assess the functional contribution of CD18 to neutrophil infiltration into bronchi and bronchioles during acute P. haemolytica pneumonia. We compare the infiltration of neutrophils, in calves that produce (normal) or do not produce (CD18 deficient) functional CD18, into precise microscopic locations of the bronchioles and bronchi by image analysis. This work establishes the functional contribution of CD18 in the infiltration process of intrapulmonary airways, which are important sites of initial colonization by microbial pathogens.
MATERIALS AND METHODS
Animals and experimental design.
Three calves with impaired expression of CD18 were homozygous for BLAD as determined by a PCR-based DNA test (20) and flow cytometric analysis. Neutrophils from calves with BLAD express less than 1% of CD18, and the cells have profound functional abnormalities (11, 15–17, 20). The calves were inoculated via a fiberoptic bronchoscope with P. haemolytica (calves 4 to 6) in pyrogen-free saline according to previously described procedures outlined below (2, 5, 6). Three age-, breed-, and sex-matched control calves (calves 1 to 3) with functional neutrophils and normal expression of CD18 were similarly inoculated at the same time as each BLAD calf. Normal calves were either homozygous or heterozygous for the normal form of the CD18 allele. Both homo- and heterozygotes have normal neutrophil function and completely lack clinical features (pneumonia, enteritis, gingivitis, or tooth loss) associated with BLAD (11, 15–17, 20). The calves were housed in a heated isolated barn with cement floors.
Calves with BLAD are rare and difficult to maintain, since they are extremely susceptible to mucosal infections such as pneumonia and enteritis. All the calves in this study were closely monitored and remained healthy, lacked clinical signs of respiratory disease, and did not develop fever. Calves 4 to 6 (BLAD calves) had levels of neutrophils in blood higher than those of calves 1 to 3 (normal CD18 expression) prior to inoculation; however, this level was interpreted to be due to a physiologic response rather than being secondary to an infectious process (see gnotobiotic studies).
Briefly, all the calves were sedated with xylazine (0.1 mg/kg intravenously), and blood (5 ml in EDTA and 10 ml in serum tubes) was collected from the external jugular vein. In each calf, a Shott bronchoscope (models VFS-2 with an 1185A illuminator) was passed into the nasal orifice and to the left cranial lobe at the site of the first major bronchial bifurcation, which was identified by visual inspection as described previously (2, 5, 6). The distal tip of the bronchoscope was wedged into the cranial main bronchial bifurcation, and 10 ml of pyrogen-free saline (PFS) was delivered through a sterile plastic access port. The right tracheal bronchus was then identified, and the bronchoscope tip was passed to the cranial bronchus of the first major bifurcation, where 8 ml of 1 × 108 cells of P. haemolytica in PFS followed by 5 ml of PFS (2, 5, 6) were deposited. At 2 h postinoculation, the calves were anesthetized, bled, and euthanized by a pentobarbital overdose. The left and right cranial lung lobes were identified by incision of the trachea through the tracheal carinae into the major bronchus of the left and right cranial lobes and to the first major bronchial bifurcation. Lung tissue from the site of inoculation (10 cm in length) was collected from the dorsal, middle, and ventral regions of the inoculation site. Tissues were fixed in 10% neutral-buffered zinc-formalin for 24 h, processed routinely, embedded in paraffin, and sectioned at 4-μm thickness onto Probe On Plus slides (Fisher Scientific). The lung tissue was not fixed by intrabronchial instillation, to prevent flushing of neutrophils from the airway lumen of the bronchi and bronchioles. Peripheral blood counts were measured with an automated cell counter (Dynatech).
Microscopy, immunohistochemistry, and image analysis.
For each calf, images from at least three bronchi and six bronchioles from two tissue sections of each of the three lesion sites (dorsal, middle, and ventral) from both the right and left lung lobes were captured electronically, traced, and counted (2, 5, 6). This resulted in a minimum of 18 bronchi and 36 bronchioles from each lung lobe. The macrophages in these sections were stained by an immunohistochemical technique with an antibody to human CD68 (DAKO) which cross-reacts with bovine macrophages, to differential these cells from neutrophils (1). Neutrophils were stained by a procedure we have recently developed (19), whereby tissue sections are treated with lead thiocyanate to unmask antigens followed by a biotinylated goat anti-mouse immunoglobulin G Fab fragment. The slides were incubated with streptavidin-peroxidase (Kirkegaard & Perry) and then with diaminobezidine and counterstained with hematoxylin. The numbers of neutrophils per square micrometer were measured in histologic sections of the bronchioles and bronchi by a technique we developed previously (2, 5, 6). This technique uses a computerized image analysis system (Iowa State University Image Analysis Facility, College of Veterinary Medicine). Briefly, tissue sections for image analysis were examined with a Zeiss Axioskop fitted with NEOFLUR objectives, a 100-W light source, and a Sony DXC-3000A camera. The images were captured with VISILOG software (Noesis, St. Laurent, Quebec, Canada) on an SGI O2 workstation (Silicon Graphics, Inc., Mountain View, Calif.). Bronchi had at least one recognizable area of cartilage in the adventitia. Images were edited on a Micron workstation with Photoshop (Adobe, Mountain View, Calif.). Tracings of subcompartments of bronchi and bronchioles in edited images included outlines of airway lumens, airway epithelium, lamina propria, adventitia (bronchioles), and smooth muscle (bronchi). Neutrophils were indicated by dots. Tracings and the number of dots in edited images were measured (total area of the subcompartment and number of neutrophils within each subcompartment) with VISILOG software on the SGI O2 workstation.
Peripheral blood neutrophil counts in calves with BLAD raised under gnotobiotic (germ-free) conditions.
Calves with BLAD, including those used in this study, and human children with LAD have increased levels of neutrophils in the blood compared to reference values (11, 13, 15). The reason for this increase has been attributed to colonization by the environmental microflora and an increased incidence of mucosal infections in these animals and children due to their impaired mucosal defense. To determine if microbial colonization increases the levels of peripheral blood neutrophils in calves with BLAD, one calf with BLAD was raised under germ-free conditions (a gnotobiotic isolator) and neutrophils counts in the blood of this calf were compared to those in the blood of another calf with BLAD raised under conventional conditions (in a barn with good ventilation and straw bedding) and of three normal calves raised in gnotobiotic isolators by the same caretakers and in the same facilites. All calves (one calf with BLAD and three normal calves) raised in gnotobiotic isolators were derived aseptically by cesarean section at the U.S. Department of Agriculture Agricultural Research Service—National Animal Disease Center. The gnotobiotic isolators were monitored weekly for microbial contamination. Blood counts were determined on an automated cell counter (Dynatech).
Statistical analysis.
Leukocyte counts and the number of neutrophils per square micrometer were assessed by analysis of variance. Analysis of variance in a repeated measure and paired design was used to examine the differences in the PFS and P. haemolytica treatments and differences in the areas (lumen, epithelium, adventitia, and smooth muscle) in the calves with BLAD and the normal calves. Tukey’s Studentized range procedure was used to compensate for the multiple comparisons.
RESULTS
Microscopy, immunohistochemistry, and image analysis.
Infiltrates of neutrophils were present in the bronchiolar lumen, epithelium, and lamina propria of calves 1 to 3 (normal calves) (Fig. 1). Infiltrates of neutrophils were also present in the lumens and walls of multifocal bronchi. In the pulmonary arteries of these calves, neutrophils adhered to the endothelium and were found within the tunica intima and lamina propria. However, neutrophils were not present in the alveolar lumens. Small aggregates of neutrophils were present within dilated capillaries of the pulmonary alveoli.
FIG. 1.
Bronchus from a normal calf (normal CD18 expression). Neutrophils (arrows) are present in the lumen and lamina propria of the bronchus of a calf with normal CD18 expression. Hematoxylin and eosin stain; magnification, ×400.
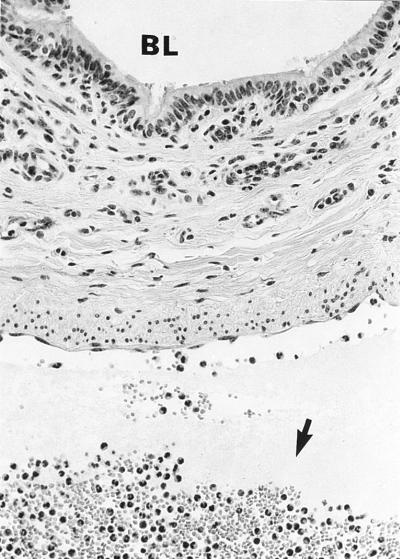
In calves 4 to 6 (BLAD calves), few neutrophils (CD18 deficient) were present in the bronchioles, bronchi, and pulmonary arteries (Fig. 2). Small aggregates of neutrophils were present in dilated capillaries of pulmonary alveolar septa, similar to those in the normal calves.
FIG. 2.
Bronchus and subjacent pulmonary artery from a calf with BLAD (CD18 deficient). The lumen of the bronchus (BL) lacks neutrophils. Few neutrophils are present within the wall of the bronchus and in the interstitium. Numerous neutrophils are present in the lumen of the subjacent pulmonary artery (arrow); however, few are adhered to the tunica intima. Hematoxylin and eosin stain; magnification, ×400.
Comparison of PFS- and P. haemolytica-inoculated lobes of normal calves and calves with BLAD 2 h postinoculation.
By analysis of tracings of electronic images, few (near zero) neutrophils were present in the bronchiolar lumen, epithelium, and adventitia of PFS-inoculated lobes of all calves (calves 1 to 3 [normal] and calves 4 to 6 [BLAD]). In contrast, P. haemolytica-inoculated lobes of all calves had significantly more neutrophils in the bronchial and bronchiolar lumens (P = 0.05) and epithelium (P = 0.04). The bronchiolar adventitia had increased numbers of neutrophils in calves 1 to 3 (normal), but this trend was not statistically significant (P = 0.13). This analysis suggests that, as expected, P. haemolytica induced infiltration of neutrophils into bronchi and bronchioles in comparison to PFS (data not shown).
Comparison of P. haemolytica-inoculated lobes of normal calves and calves with BLAD 2 h postinoculation.
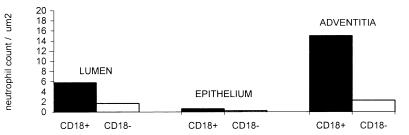
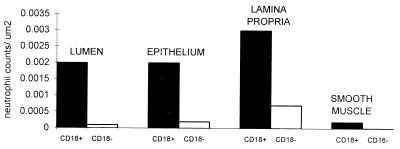
From analysis of tracings of electronic images, a significant increase (P < 0.05) in the number of neutrophils was present in all subcompartments of the bronchi and bronchioles (epithelium, adventitia, lumen, and smooth muscle of bronchi) of calves 1 to 3 (normal) compared to calves 4 to 6 (BLAD) (Figs. 3 and 4). This suggests that CD18 mediates the passage of significant numbers of neutrophils into all regions of the bronchus and bronchiole at 2 h.
FIG. 3.
Number of neutrophils within regions of bronchioles of calves with BLAD (CD18 deficient) and normal calves (normal CD18 expression). Within each of the labelled areas were significant differences between the CD18+ (β2-integrin-positive) and the CD18− neutrophil counts.
FIG. 4.
Number of neutrophils within regions of the bronchi of calves with BLAD (CD18-deficient) and normal calves (normal CD18 expression). Within each of the labelled areas there were significant differences between the CD18+ (β2-integrin-positive) and the CD18− neutrophil counts.
Peripheral blood leukocyte counts.
Total leukocyte counts were markedly elevated (P = 0.02) for calves 4 to 6 (BLAD) prior to inoculation. More than 90% of the cells were neutrophils. The counts for calves 1 to 3 (normal) were within normal ranges for young calves. The numbers of neutrophils in the blood of calf 1 (normal) and calves 4 and 6 (BLAD) decreased postinoculation; the numbers in the remaining calves increased (Table 1). There were no significant differences in blood leukocyte counts before and after challenge with P. haemolytica in any of the calves, regardless of normal or impaired CD18 expression.
TABLE 1.
Total peripheral blood leukocyte counts in calves before and after inoculation with P. haemolytica
| Time | Total leukocyte counts (leukocytes/μl) in:
|
|||||
|---|---|---|---|---|---|---|
| Calf 1 (CD18+) | Calf 2 (CD18+) | Calf 3 (CD18+) | Calf 4 (CD18−) | Calf 5 (CD18−) | Calf 6 (CD18−) | |
| Preinoculation | 14,000 | 8,300 | 6,400 | 74,500 | 50,200 | 34,300 |
| Postinoculation | 11,400 | 9,100 | 11,400 | 72,200 | 63,100 | 30,200 |
Peripheral blood neutrophil counts in calves with BLAD raised under gnotobiotic (germ-free) conditions.
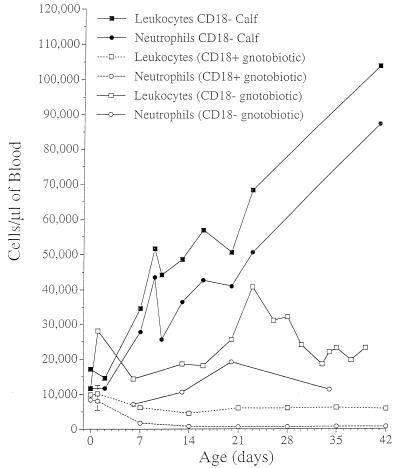
The conventionally reared calf with BLAD developed a progressive neutrophilia typical of LAD patients (Fig. 5). The gnotobiotically reared calf with BLAD developed a neutrophilia (although reduced in magnitude), indicating that the neutrophilia was due, in part, to an impaired ability of the neutrophils to exit the bloodstream or was due to extensive bone marrow myeloid hyperplasia. Gnotobiotically reared normal calves began with normal leukograms at birth, but circulating numbers fell dramatically in the absence of a normal flora (Fig. 5).
FIG. 5.
Effects of the normal flora and β2-integrin (CD18) expression on leukocytes on leukograms of gnotobiotic (germ-free) calves. The data illustrated are from a calf with BLAD (CD18 deficient) raised in a conventional environment and another calf with BLAD raised in a gnotobiotic isolator. For comparative purposes, the total leukocyte counts of three normal calves (normal CD18 expression) raised in a gnotobiotic isolator are also shown. The conventionally reared calf with BLAD has a progressive neutrophilia typical of calves with BLAD and human patients with LAD. The gnotobiotically reared calf with BLAD has a neutrophilia (although reduced in magnitude), indicating that the neutrophilia is due, in part, to entrapment of neutrophils in the bloodstream. Gnotobiotically reared normal calves begin with normal leukograms at birth, but the numbers of circulating of neutrophils fall dramatically in the absence of the normal flora.
DISCUSSION
The decreased infiltration of neutrophils into bronchi and bronchioles of calves with BLAD suggests that CD18 and its functional complementary molecule, CD11, mediate the initial passage of neutrophils following P. haemolytica inoculation into these airways. It may be that mucociliary movement or coughing transports a certain number of neutrophils from the alveolar lumen into the airway lumen of the bronchi and bronchioles. However, alveolar infiltration at 2 h postinoculation was minimal, and such retrograde movement of neutrophils through the air spaces would not account for the increased numbers of neutrophils with normal CD18 expression present in the epithelium and lamina propria of the bronchi and bronchioles of normal calves (calves 1 to 3).
The infiltration of neutrophils into bronchi and bronchioles of normal calves, in conjunction with our previous work demonstrating CD18-independent infiltration at the level of the pulmonary alveolus, suggests that CD18-mediated neutrophil infiltration can occur in certain regions of the lung (bronchi and bronchioles) during P. haemolytica infection rather than diffusely throughout the entire organ. The inability of neutrophils with impaired expression of CD18 to readily enter the bronchi and bronchioles may have clinical implications. On the one hand, neutrophil infiltration is necessary for mucosal defense and is associated with increased expression of other host defense molecules such as the β-defensin, lingual antimicrobial peptide (22). Lingual antimicrobial peptide has antimicrobial activities against many types of microorganisms, including Pseudomonas aeruginosa and Candida albicans. On the other hand, excessive neutrophil infiltration at later times postinoculation results in necrosis of the lung parenchyma, including the bronchial and bronchiolar epithelium, thus damaging the respiratory epithelial layers, which are responsible for production of defensins, surfactant, goblet cell secretions, and mucociliary clearance. Work by Slocombe et al. (21) demonstrated that calves depleted of neutrophils had greatly reduced lung lesions after inoculation with P. haemolytica compared to nondepleted calves. Identification of molecules such as CD18 that mediate initial passage into specific regions of the lung (i.e., bronchi and bronchioles) may be used therapeutically to direct or inhibit neutrophil infiltration within the lung. For example, it may prove beneficial to reduce infiltration or allow the passage of neutrophils into the bronchi and bronchioles through CD18-mediated infiltration to enhance host immunity to pathogens that colonize ciliated cells.
In this work, it was not determined which of the four β2-integrins had the greatest influence on bronchial and bronchiolar infiltration. Mac-1 (CD11a/CD18) is probably involved, since it is expressed by neutrophils at a high level and is up-regulated with activation (15, 17, 20). LFA-1 (CD11b/CD18) is not highly expressed by neutrophils, but it may play a role in adherence, since its activity can be regulated by conformational changes rather than increased expression. The β2-integrin p150,95 (CD11c/CD18) is expressed at low levels and has little functional activity for infiltration. CD11d/CD18 has not been described in the bovine. In the dog, CD11d/CD18 plays a limited, if any, role in neutrophil adherence to the vasculature (23).
The decreased infiltration of neutrophils into the bronchi and bronchioles of calves with BLAD occurred in spite of the large numbers of neutrophils present in the blood of these animals. Evidently, the increased number of neutrophils in the pulmonary microvasculature of these calves does not compensate for loss of CD18 with regard to initial infiltration. The presence of large numbers of neutrophils in the blood is common in calves with BLAD (11, 15) and children with LAD (13). The mechanism of the increase has not been elucidated; however, it is thought to be a response to ongoing mucosal infections or excessive colonization of mucosal surfaces and skin by microorganisms. Our finding that neutrophilia occurs shortly after birth, even when a calf with BLAD is kept in a germ-free environment, suggests that bacterial colonization is not essential for increased levels of neutrophils in the blood. The mechanism for the increase of CD18-deficient neutrophils in the blood was not determined but may be due to a combination of impaired transendothelial migration of neutrophils across the wall of blood vessels and enhanced bone marrow hyperplasia. Increased numbers of CD18-deficient neutrophils in calves with BLAD (11, 15) and in human LAD patients (13) are associated with increased myeloid cell hyperplasia in the bone marrow, which suggests that there is active recruitment of granulocytes at the precursor level. The granulocytic hyperplasia in the bone marrow and the subsequent neutrophilia may be exacerbated by the reduced ability of CD18-deficient neutrophils to move out of the vasculature lumen and into mucosal surfaces and the parenchyma of organs. The mechanism of impaired passage of CD18-deficient neutrophils out of the vascular lumen is supported not only by the neutrophilia but also the lack of neutrophil infiltration into ulcerated lesions of calves with BLAD and the accumulation of numerous neutrophils in the splenic red pulp in these calves (15). Reduced neutrophil egress may then increase the release of granulopoietic cytokines such as interleukin-3 or granulocyte-macrophage colony-stimulating factor by endothelial cells or other cell types associated with neutrophilic transendothelial passage.
In previous work, we found that dense infiltrates of neutrophils are present in the lumens and walls of bronchi and bronchioles in both normal calves and calves with BLAD at later times (4 and 6 h) after inoculation with P. haemolytica (2). The presence of CD18-deficient neutrophils in the lumens of bronchi and bronchioles in that study suggests that during progression of P. haemolytica pneumonia, CD18 is not essential for the total accumulation of neutrophils into these airways. Infiltration by CD18-deficient neutrophils into bronchi and bronchioles at times beyond 2 h postinoculation is probably due to two factors: (i) movement of neutrophils from the alveolar lumen into the bronchi and bronchioles during expiration and coughing, and (ii) passage of neutrophils across the bronchial and bronchiolar walls following neutrophil-mediated oxidative or enzymatic damage of capillaries and the extracellular matrix of bronchi and bronchioles.
It is known that neutrophils contribute a great deal to the pulmonary damage that occurs during P. haemolytica pneumonia, because the damage is greatly reduced in calves depleted of neutrophils prior to P. haemolytica inoculation (21). In addition, components of neutrophils such as elastase are known to contribute to the pulmonary damage in diseases such as cystic fibrosis and emphysema (4, 10, 12). The effects of elastase and other neutrophil products have not been fully characterized in P. haemolytica pneumonia; however, P. haemolytica pneumonia is characterized by extensive vascular and parenchymal damage and necrosis. Both the vascular and extracellular matrix damage may allow the passage of CD18-deficient neutrophils into the bronchi and bronchioles later in the acute inflammatory process, after the initial neutrophilic infiltration is established.
ACKNOWLEDGMENTS
We are grateful for the excellent assistance and contributions of Margie Carter, Jack Gallup, and Kim Driftmier. We thank Bruce Pesch for his technical assistance in the leukograms of the gnotobiotic calves, and we thank Jim Harp for his contribution with the leukograms.
This work was supported, in part, by the Agriculture Experiment Station, State of Iowa Healthy Livestock Initiative, and the U.S. Department of Agriculture, National Research Initiative-Competitive Grants Proposal (USDA, NRI-CGP) 97-35204-4769. Image analysis was completed at the Iowa State University Image Analysis Facility, which is supported by the Biotechnology Council.
REFERENCES
- 1.Ackermann M R, DeBey B M, Stabel T J, Gold J H, Register K B, Meehan J T. Distribution of anti-CD68 immunoreactivity in formalin-fixed, paraffin-embedded bovine tissues. Vet Pathol. 1994;31:340–348. doi: 10.1177/030098589403100307. [DOI] [PubMed] [Google Scholar]
- 2.Ackermann M R, Kehrli M E, Jr, Brogden K A. Passage of CD18− and CD18+ bovine neutrophils into pulmonary alveoli during acute pneumonia. Vet Pathol. 1996;33:639–646. doi: 10.1177/030098589603300602. [DOI] [PubMed] [Google Scholar]
- 3.Anderson D C, Schmalstieg F C, Arnaout M A, Kohl S, Tosi M F, Dana N, Buffone G J, Hughes B J, Brinkley B R, Dickey W D, Abramson J S, Springer T, Boxer L A, Hollers J M, Smith C W. Abnormalities of polymorphonuclear leukocyte function associated with a heritable deficiency of high molecular weight surface glycoprotein (GP138): common relationship to diminished cell adherence. J Clin Investig. 1984;74:536–551. doi: 10.1172/JCI111451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Birrer P, McElvaney N G, Ruderberg A, Wirz S C, Leichti-Gallati S, Kraemer R, Crystal R G. Protease-antiprotease imbalance in the lungs of children with cystic fibrosis. Am J Respir Crit Care Med. 1994;150:207–213. doi: 10.1164/ajrccm.150.1.7912987. [DOI] [PubMed] [Google Scholar]
- 5.Brogden K A, Ackermann M R, DeBey B M. Pasteurella haemolytica lipopolysaccharide associated protein induces pulmonary inflammation after bronchoscopic deposition in calves and sheep. Infect Immun. 1995;63:3595–3599. doi: 10.1128/iai.63.9.3595-3599.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brogden K A, Cutlip R C, Lehmkuhl H D. Response of sheep after localized deposition of lipopolysaccharide in the lung. Exp Lung Res. 1984;7:123–132. doi: 10.3109/01902148409069673. [DOI] [PubMed] [Google Scholar]
- 7.Burns A R, Doerschuk C M. Quantitation of L-selectin and CD18 expression on rabbit neutrophils during CD18-independent and CD18-dependent emigration in the lung. J Immunol. 1994;153:3177–3188. [PubMed] [Google Scholar]
- 8.Conlan J W, North R J. Listeria monocytogenes, but not Salmonella typhimurium, elicits a CD18-independent mechanism of neutrophil extravasation into the murine pulmonary cavity. Infect Immun. 1994;62:2702–2706. doi: 10.1128/iai.62.7.2702-2706.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doerschuk C M, Winn R K, Coxson H O, Harlan J M. CD-18 dependent and -independent mechanisms of neutrophil emigration in the pulmonary and systemic microcirculation of rabbits. J Immunol. 1990;144:2327–2333. [PubMed] [Google Scholar]
- 10.Döring G. The role of neutrophil elastase to chronic inflammation. Am J Crit Care Med. 1994;150:S114–S117. doi: 10.1164/ajrccm/150.6_Pt_2.S114. [DOI] [PubMed] [Google Scholar]
- 11.Gilbert R O, Rebhun W C, Kim C A, Kehrli M E, Jr, Shuster D E, Ackermann M R. Clinical manifestations of leukocyte adhesion deficiency in cattle: 14 cases (1977–1991) J Am Vet Med Assoc. 1993;202:445–449. [PubMed] [Google Scholar]
- 12.Hautamaki R D, Kobayashi D K, Senior R M, Shapiro S D. Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science. 1997;277:2002–2004. doi: 10.1126/science.277.5334.2002. [DOI] [PubMed] [Google Scholar]
- 13.Hawkins H K, Heffelfinger S C, Anderson D C. Leukocyte adhesion deficiency: clinical and postmortem observations. Pediatr Pathol. 1992;12:119–130. doi: 10.3109/15513819209023288. [DOI] [PubMed] [Google Scholar]
- 14.Hogg J C, Doerschuk C M. Leukocyte traffic in the lung. Annu Rev Physiol. 1995;57:97–114. doi: 10.1146/annurev.ph.57.030195.000525. [DOI] [PubMed] [Google Scholar]
- 15.Kehrli M E, Jr, Ackermann M R, Shuster D E, Van Der Maaten M, Schmalstieg F C, Anderson D C, Hughes B J. Animal model of human disease: bovine leukocyte adhesion deficiency: β2 integrin deficiency in young holstein cattle. Am J Pathol. 1992;140:1489–1492. [PMC free article] [PubMed] [Google Scholar]
- 16.Kehrli M E, Jr, Schmalstieg F C, Anderson D C, Van Der Maaten M J, Hughes B J, Ackermann M R, Wilhelmsen C L, Brown G B, Stevens M G, Whetstone C A. Molecular definition of the bovine granulocytopathy syndrome: identification of deficiecy of the Mac-1 (CD11b/CD18) glycoprotein. Am J Vet Res. 1990;51:1826–1836. [PubMed] [Google Scholar]
- 17.Kehlri M E, Shuster D E, Ackermann M R. Leukocyte adhesion deficiency among holstein cattle. Cornell Vet. 1992;82:103–109. [PubMed] [Google Scholar]
- 18.Kumasaka T, Doyle N A, Quinlan W M, Graham L, Doerschuk C. Role of CD11/CD18 in neutrophil emigration during acute and recurrent Pseudomonas aeruginosa-induced pneumonia in rabbits. Am J Pathol. 1996;148:1297–1305. [PMC free article] [PubMed] [Google Scholar]
- 19.Lee, H. Y., K. B. Register, K. Driftmier, J. M. Caverly, J. M. Gallup, and M. R. Ackermann. Detection of bovine neutrophils in formalin-fixed, paraffin-embedded lung with lead thiocyanate and anti-mouse IgG antibody. Submitted for publication.
- 20.Shuster D E, Kehrli M E, Jr, Ackermann M R, Gilbert R O. A prevalent point mutation responsible for leukocyte adhesion deficiency in holstein cattle. Proc Natl Acad Sci USA. 1992;89:9225–9229. doi: 10.1073/pnas.89.19.9225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Slocombe R F, Malark J, Ingersoll R, Derksen F J, Robinson N E. Importance of neutrophils in the pathogenesis of acute pneumonic pasteurellosis in calves. Am J Vet Res. 1985;46:2253–2258. [PubMed] [Google Scholar]
- 22.Stolzenberg E D, Anderson G M, Ackermann M R, Whitlock R H, Zasloff M A. Epithelial antibiotic induced in states of disease. Proc Natl Acad Sci USA. 1997;94:8686–8690. doi: 10.1073/pnas.94.16.8686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van der Vieren M, Le Trong H, Wood C L, Moore P F, St. John T, Staunton D E. A novel leukointegrin, αdβ2, binds preferentially to ICAM-3. Immunity. 1995;3:683–690. doi: 10.1016/1074-7613(95)90058-6. [DOI] [PubMed] [Google Scholar]
- 24.Ward P A. Role of complement in lung inflammatory injury. Am J Pathol. 1996;149:1081–1086. [PMC free article] [PubMed] [Google Scholar]
- 25.Wittum T E, Woollen N E, Perino L J, Littledike E T. Relationships among treatment for respiratory tract disease, pulmonary lesions evident at slaughter and rate of weight gain in feedlot cattle. J Am Vet Med Assoc. 1996;209:814–818. [PubMed] [Google Scholar]