Significance
Sleep represents a window of opportunity to modulate the mnemonic fate of recent experiences. Prior work has shown that delivering auditory reminder cues can slow down overnight forgetting, and so does experimental enhancement of natural sleep rhythms. Here, we combined both methods to selectively provide auditory cues during moments of high (UP states) or low (DOWN states) neuronal excitability. We found that UP-state cueing enhanced ongoing UP states and led to significantly lower forgetting rates than DOWN-state cueing. Moreover, electrophysiological markers of memory reprocessing were more pronounced after UP-state cueing. These results illustrate the impact of delivering exogenous stimuli at optimal phases of endogenous brain rhythms and will help improve experimental approaches to strengthening memories during sleep.
Keywords: sleep, memory consolidation, targeted memory reactivation, closed-loop stimulation, slow oscillation
Abstract
Sleep constitutes a privileged state for new memories to reactivate and consolidate. Previous work has demonstrated that consolidation can be bolstered experimentally either via delivery of reminder cues (targeted memory reactivation [TMR]) or via noninvasive brain stimulation geared toward enhancing endogenous sleep rhythms. Here, we combined both approaches, controlling the timing of TMR cues with respect to ongoing slow-oscillation (SO) phases. Prior to sleep, participants learned associations between unique words and a set of repeating images (e.g., car) while hearing a prototypical image sound (e.g., engine starting). Memory performance on an immediate test vs. a test the next morning quantified overnight memory consolidation. Importantly, two image sounds were designated as TMR cues, with one cue delivered at SO UP states and the other delivered at SO DOWN states. A novel sound was used as a TMR control condition. Behavioral results revealed a significant reduction of overnight forgetting for words associated with UP-state TMR compared with words associated with DOWN-state TMR. Electrophysiological results showed that UP-state cueing led to enhancement of the ongoing UP state and was followed by greater spindle power than DOWN-state cueing. Moreover, UP-state (and not DOWN-state) cueing led to reinstatement of target image representations. Together, these results unveil the behavioral and mechanistic effects of delivering reminder cues at specific phases of endogenous sleep rhythms and mark an important step for the endeavor to experimentally modulate memories during sleep.
Memory consolidation (i.e., the stabilization and integration of newly acquired memories over time) benefits from postlearning sleep (1–4). Ignited by the finding of hippocampal “replay” in rodent sleep recordings (5–7), theoretical and computational models have highlighted the critical role of reactivation for effective systems consolidation. Specifically, reactivation of hippocampal learning representations is thought to gradually transfer memories to cortical sites for more permanent storage, and sleep—hallmarked by the absence of external distracters—constitutes a privileged state for this “hippocampal–cortical dialogue” (8–10). More recent work in humans has sought to capitalize on sleep as a window of opportunity to modulate memory consolidation experimentally. In particular, a seminal study linked learning materials to a particular environmental scent and showed that providing olfactory reminders to sleeping participants can slow down overnight forgetting (11). Known as targeted memory reactivation (TMR), a large body of work has since established the efficacy of presenting olfactory or auditory reminder cues for bolstering consolidation of recent learning experiences (reviewed in refs. 12 and 13).
What are the underlying mechanisms governing reactivation and consolidation during sleep? During non-rapid eye movement (NREM) sleep, the scalp electroencephalogram (EEG) is dominated by two cardinal signatures: slow oscillations [SOs; <1-Hz high-amplitude EEG fluctuations (14, 15)] and sleep spindles [∼12- to 16-Hz waxing and waning bursts of 0.5- to 2-s duration (16, 17)]. Both these phenomena individually as well as their co-occurrence have been implicated in memory consolidation (18–23). SOs can be thought of as global pacemakers of brain activity during sleep, toggling between intervals of neuronal excitability (UP states) and inhibition (DOWN states). Importantly, SO UP states tend to group sleep spindles (22, 24–26), which in turn, have been linked to memory reprocessing and plasticity (27–32). Given their pivotal role for orchestrating brain processes and their relatively high signal to noise ratio (i.e., detectability against the background EEG), SOs have been targeted by efforts to experimentally boost memory consolidation via noninvasive brain stimulation. One particularly promising approach has been to entrain SOs, e.g., by transcranial direct current stimulation (33) or by applying auditory clicks in a closed-loop fashion (34). In the latter case, SOs are detected algorithmically in real time, and brief bursts of noise (clicks) are presented when an SO UP state occurs. This has been shown to prolong ongoing SO trains, elicit sleep spindles coupled to SOs, and enhance behavioral expressions of memory consolidation (34–37).
In sum, both the delivery of reminder cues (TMR) and experimentally augmenting SOs have yielded promising results for the endeavor to strengthen overnight memory consolidation. This begs the question of whether the two approaches can be combined, i.e., is TMR more effective when cues are delivered at a particular phase of ongoing SOs?. Indeed, retrospective analysis of a TMR experiment suggested that the effects of memory cueing are modulated by the SO phase at which cues were presented, with the optimal presentation interval spanning the SO UP state (38). To date, only two studies have modulated consolidation by applying TMR at different SO phases. First, Shimuzi et al. (39) presented reminder cues associated with a spatial memory task during the DOWN to UP transition and found an improvement in memory performance compared with a condition without any intervention. However, the study also included a cueing phase during wakefulness embedded in an interference task, rendering it difficult to unambiguously link effects to sleep TMR. Moreover, no control condition was included in which TMR would be applied at a different SO phase. Second, Göldi et al. (40) examined the effects of TMR (linked to a vocabulary learning task) delivered at SO UP states vs. DOWN states. Results remained somewhat ambiguous, with an advantage of UP-state cued content over uncued items but no statistical difference of DOWN-state cueing compared with UP-state cueing or no cueing. Notably, none of these studies assessed whether the emergence of spindles and/or reactivation of target associations (28, 41) would vary as a function of UP- vs. DOWN-state delivery of TMR cues.
In this study, we thus used a closed-loop protocol in which we experimentally controlled the SO phase at which different mnemonic reminder cues were delivered. We hypothesized that cues delivered during SO UP states would not only lead to enhanced behavioral expressions of memory consolidation compared with cues delivered during SO DOWN states but would also entrain stronger spindle activity and more effectively trigger reactivation of target representations. This would pave the way to maximizing the efficacy of experimental/therapeutic interventions seeking to control memory processes during sleep.
Results
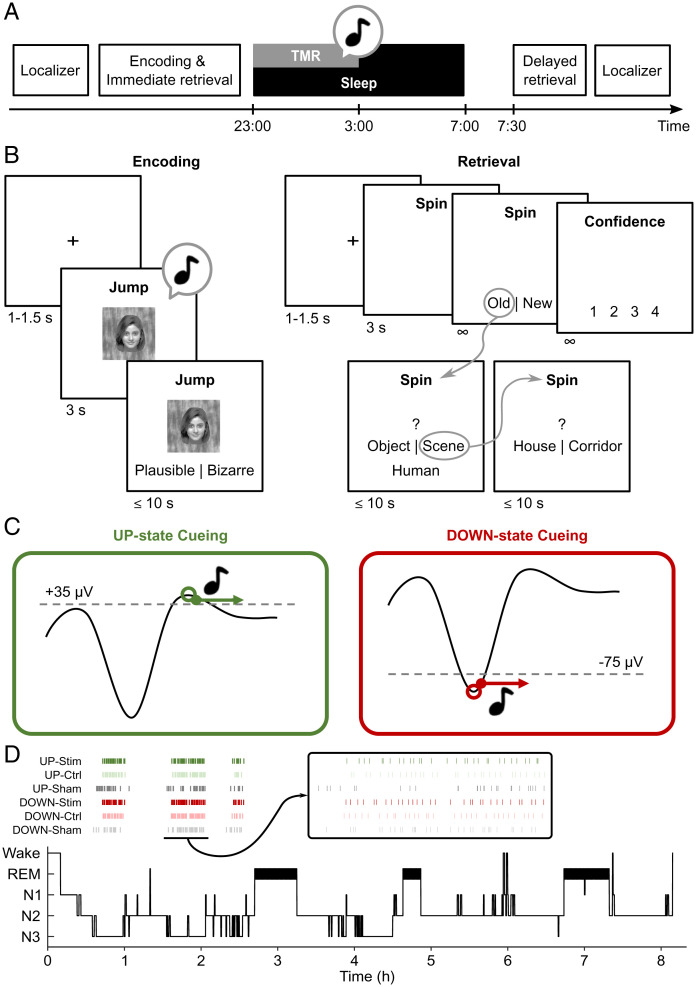
Twenty-four healthy participants took part in the overnight experiment (Fig. 1A). Prior to sleep, participants performed an episodic memory task consisting of an encoding block, a ∼5-min delay interval (psychomotor vigilance task [PVT]), and a retrieval block. During encoding, 120 trial-unique verbs were presented with one of six gray-scale images, including two objects (guitar and car), two scenes (house and corridor), and two human body parts (face and hand). Importantly, each image presentation was accompanied by a semantically related sound (e.g., the sound of a starting car engine; 500-ms duration). Participants were asked to indicate whether the mental image representing the given verb–image combination was plausible or bizarre (Fig. 1B). During retrieval, 60 (50%) of the previously presented verbs along with 30 lures were presented, and participants first made an old/new recognition judgement followed by—in case of an “old” response—recall of the associated image category. The same retrieval test was employed the next morning but using the remaining 50% of old verbs along with a new set of 30 lures.
Fig. 1.
Study design and procedures. (A) Study design. In the evening, participants performed a localizer task followed by an episodic memory task and immediate retrieval (after a 5-min distracter task). Subsequently, closed-loop controlled TMR was performed during NREM periods of the first night half. The next morning, memory was again tested (delayed retrieval) followed by another localizer task. (B) Memory task. During encoding, participants saw verb–image pairs while an image-related sound was presented. During retrieval, recognition memory of the verb and recall of the associated image were assessed. (C) The SO UP- and DOWN-state detection algorithm. Based on the 0.3- to 4-Hz band pass–filtered signal from Fz (referenced to linked mastoids), SO UP states were identified when the signal exceeded +35 µV and exhibited a local maximum (Left). DOWN-state detection was based on a negative amplitude below −75 µV and a local minimum. Upon identification of an UP or DOWN state, a sound cue was presented for 0.5 s, and the detection algorithm was paused for 8 s. (D) Hypnogram depicting the sleep architecture of an example participant. Tick marks at the top reflect time points for real (Stim), control (Ctrl), and sham UP and DOWN cues. Inset shows a magnification of the interval marked by the gray horizontal line (1,800 s).
During overnight NREM sleep, two of the six image-related sounds linked to two separate categories (e.g., objects and scenes) were presented in a TMR protocol. The remainder of the images served as a behavioral baseline condition. Critically, one of the sounds was presented during SO UP states and the other was presented during SO DOWN states as determined via a real-time closed-loop detection algorithm. To control for unspecific EEG effects induced by sounds, TMR cueing was interspersed with a nonfamiliar sound delivered during SO UP and DOWN states (Fig. 1C). Sleep architecture and subjective ratings of sleepiness are shown in Table 1.
Table 1.
Sleep architecture and subjective sleep score
| Parameter | Mean ± SEM |
|---|---|
| TST (min) | 466.5 ± 5.9 |
| Wake (%) | 3.7 ± 1.6 |
| N1 (%) | 6.2 ± 0.6 |
| N2 (%) | 50.5 ± 1.6 |
| N3 (%) | 23.1 ± 1.2 |
| REM (%) | 16.6 ± 0.9 |
| ΔSSS | −1.3 ± 0.3 |
Amount of total sleep time (TST) in minutes, relative sleep architecture, and overnight change in subjective sleepiness rating are shown. Wake indicates the relative time awake after sleep onset; ΔSSS is the difference in subjective sleep score obtained after and before sleep.
SO Phase of TMR Cues Modulates Evoked EEG Responses and Memory Consolidation.
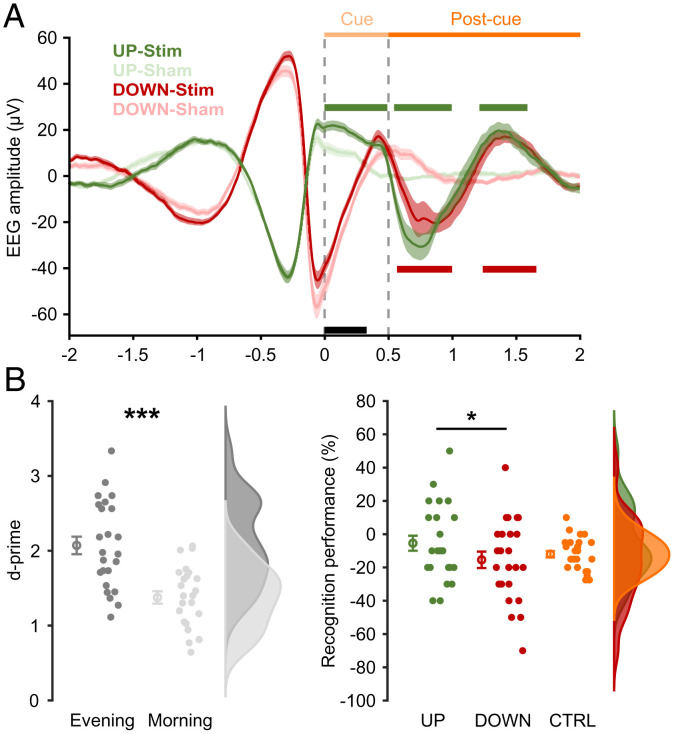
To confirm the efficacy of our closed-loop algorithm, we derived event-related potentials (ERPs) time locked to the onset of the corresponding sound cues (Fig. 2A). Importantly, to examine the electrophysiological effects of cueing relative to no cueing, we also derived sham cues by retrospectively applying the same detection algorithm on EEG segments in which no cues were delivered (Materials and Methods). Apart from the expected ERP differences before and at cue onset (targeting SO DOWN vs. UP states), this analysis yielded two key findings. First, UP-state cueing resulted in enhancement/prolongation of the UP state compared with the sham cues. Second, both UP- and DOWN-state cues elicited a second SO cycle after sound offset, with ERPs being statistically indistinguishable between the two conditions. ERPs for the novel control sounds are shown in SI Appendix, Fig. S1.
Fig. 2.
EEG and behavioral effects of UP state– vs. DOWN state–triggered TMR. (A) ERPs at Cz for the UP-Stim (green) and DOWN-Stim (red) and respective “sham” conditions (light colors). All data are shown as mean ± SEM across participants. Vertical dashed lines depict the onset and offset of TMR cues. Light and dark orange horizontal lines mark the cue and a postcue interval, respectively. The black horizontal line marks significant differences between UP- vs. DOWN-Stim conditions, the green horizontal line marks significant differences between UP-Stim vs. UP-Sham, and the red horizontal line marks significant differences between DOWN-Stim vs. DOWN-Sham (all P < 0.05, corrected; statistical analysis was performed for time of >0). Note that detection thresholds were based on data from Fz. (B) General assessment of memory performance based on old/new recognition of verbs as d prime. The rain cloud plot depicts the performance of individual participants alongside data distributions (Right). Circles with error bars show mean ± SEM across participants. Dark gray indicates presleep (evening), and light gray indicates postsleep (morning). (C) Overnight change in verb recognition associated with SO UP-state cueing (green), SO DOWN-state cueing (red), or no cueing (CTRL; orange). The same plotting conventions as in B are used. *P < 0.05; ***P < 0.001.
In terms of memory performance, we focused on old/new recognition of verbs. Note that participants were cued with image-related sounds (e.g., a car engine), each linked to a set of 20 verbs during encoding (a similar approach is used in ref. 30). Old/new recognition of verbs thus captures the efficacy of TMR cues for strengthening cue–target associations while bypassing potential response biases due to sound exposure per se (e.g., the tendency to respond with “car” due to greater familiarity with the car sound). Initial results confirmed significant overnight forgetting expressed as d prime [hit rate minus false alarms; t (23) = 6.18, P < 0.001] (Fig. 2B). Next, we directly compared forgetting rates for items associated with UP-state cues vs. items associated with DOWN-state cues. Intriguingly, UP-state cueing led to significantly lower forgetting rates than DOWN-state cueing [5 vs. 15%, t (23) = 2.28, P = 0.032 two tailed] (Fig. 2C). This result held when only including correct responses given with confidence ratings two to four, thus excluding potential guesses [t (23) = 2.18, P = 0.040]. As a point of reference, we combined all items whose associated images were not cued (“uncued” items). Forgetting rates for uncued items were 12%, which falls between forgetting rates for UP-state and DOWN-state cueing [albeit without significantly differing from either, both t (23) < 1.20, P > 0.67].
UP-State Cueing Evokes Spindle Increase.
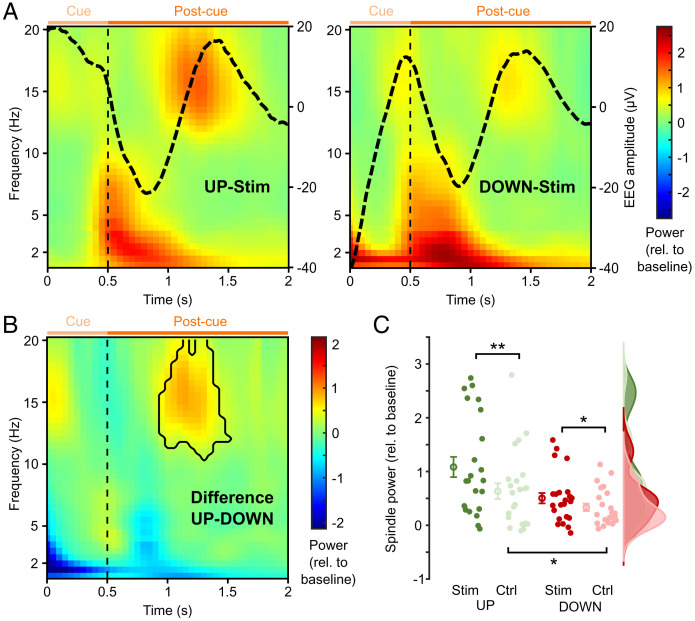
We next sought to elucidate the neurophysiological mechanisms underlying the beneficial effect of UP-state cueing. As mentioned in the introduction, recent work has linked changes in spindle power (∼12 to 16 Hz) to consolidation processes during sleep. We, therefore, first calculated time-frequency representations (TFRs) for the UP- and DOWN-state cueing conditions for frequencies between 1 and 20 Hz. As shown in Fig. 3A, the evoked SO cycle following sound offset was reflected in a <10-Hz power increase in both conditions. Critically, however, we observed an increase in spindle power coinciding with the SO DOWN to UP transition, which was significantly stronger for UP-state cueing than for DOWN-state cueing (Fig. 3B).
Fig. 3.
Greater spindle activity after SO UP-state cueing. (A) TFRs at Cz for the UP-Stim (Left) and DOWN-Stim (Right) conditions calculated time locked to the cue onset for frequencies between 1 and 20 Hz with the corresponding ERP superimposed (black lines; right y axis). The color bar indicates the relative change in power to a baseline interval from −2.5 to −2 s. Vertical dashed lines depict the offset of TMR cues. Light and dark orange horizontal lines mark the cue and postcue interval, respectively. (B) TFR showing the difference between UP-Stim and DOWN-Stim conditions as shown in A. The black contour outlines significant time-frequency clusters (P < 0.05, corrected; statistics were conducted from 10 to 20 Hz). (C) Rain cloud plot for power corresponding to the significant cluster shown in B for the UP-Stim (dark green), DOWN-Stim (dark red), and their respective control (Ctrl) conditions (novel sound; light colors). Circles with error bars show mean ± SEM across participants. Extended channel × time × frequency analyses are shown in SI Appendix, Fig. S2. *P < 0.05; **P < 0.01.
To assess whether this spindle power increase was related to processing of the reminder cue, we first compared real UP-state cues (UP-Stim) with the control sound not presented during encoding but also delivered during SO UP states (UP-Ctrl). As shown in Fig. 3C, the increase in spindle power was indeed higher for the UP-Stim than the UP-Ctrl condition [t (22) = 2.96, P = 0.007]. In the DOWN-state condition, Stim cues also elicited higher spindle power than Ctrl cues, although this difference was more moderate [t (22) = 2.11, P = 0.046], and there was a trend for a significant interaction [UP-Stim vs. UP-Ctrl > DOWN-Stim vs. DOWN-Ctrl, t (22) = 1.84, P = 0.079]. Lastly, spindle power was significantly greater in the UP-Ctrl than in the DOWN-Ctrl condition [t (22) = 2.64, P = 0.015]. These results suggest that UP-state TMR drives spindle activity by combining two effects reported previously—1) stimulating the brain at the optimal SO phase [UP > DOWN (34)] and 2) conveying mnemonic content [Stim > Ctrl (27)].
UP-State Cueing Evokes Target Category Reinstatement.
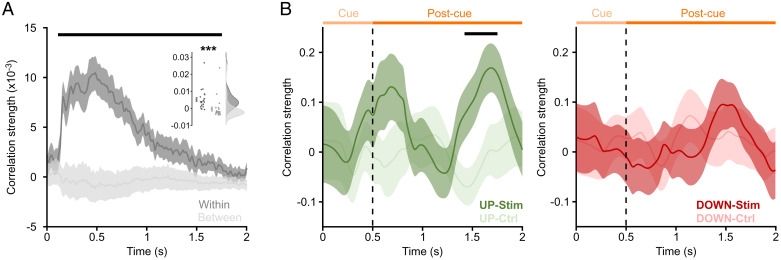
Lastly, we asked whether TMR cues delivered during UP states would be more effective in reactivating associated target representations in the sleeping brain. To this end, we capitalized on the independent localizer runs (Fig. 1A), in which multiple exemplars of the main image categories were presented (Materials and Methods has details). As a first step, we established that image categories can be reliably distinguished based on the whole-brain EEG signal. Specifically, we assessed, for each trial and each time point, the representational similarity (Pearson correlation across channels) with all other trials. Next, we compared the pooled within-category similarity (cars with other cars, houses with other houses, etc.) with the pooled between-category similarity (cars with houses, cars with faces, etc.). The difference of within- vs. between-category similarity signifies discriminability of image categories. As shown in Fig. 4A, results confirmed reliable discriminability virtually spanning the entire trial period. SI Appendix, Fig. S3 shows the time-resolved within- and between-category similarities separately for each image category.
Fig. 4.
Category reinstatement during UP-state cueing. (A) Discriminability of image categories during the wake localizer task. Dark gray indicates the pooled within-category similarity/correlation strength (car–car, face–face, etc.). Light gray indicates the pooled between-category similarity (car–face, face–house, etc.). (Inset) Rain cloud plot for within- vs. between-category similarity collapsed across the entire localizer time (0 to 2 s). ***P < 0.001. (B, Left) Reinstatement of target categories during UP-state TMR cues (dark green) and UP-state control sounds (light green). (B, Right) Reinstatement of target categories during DOWN-state TMR cues (dark red) and DOWN-state control sounds (light red). Lines show mean ± SEM across participants. Horizontal black lines denote significant differences (P < 0.05, corrected). Vertical dashed lines depict the offset of TMR cues. Light and dark orange horizontal lines mark the cue and postcue interval, respectively.
To assess category reinstatement evoked by TMR cues during sleep, we then correlated localizer data (during which only images and no sounds were presented) with the EEG data during the cueing period (during which only sounds were presented) (Materials and Methods has details). This procedure resulted in four localizer time × TMR time correlation matrices per participant—1) UP-category localizer to UP-category TMR, 2) UP-category localizer to UP-category control, 3) DOWN-category localizer to DOWN-category TMR, and 4) DOWN-category localizer to DOWN-category control. Control conditions (novel sounds delivered during UP and DOWN states) were included to provide a data-driven baseline (novel sounds should not trigger category-specific reactivation). Finally, given the sustained discriminability in the localizer data, results were collapsed across localizer time (0 to 2 s), yielding a time series of target category reinstatement during TMR/control cues (complete time × time maps are in SI Appendix, Fig. S4). As shown in Fig. 4B, UP-state TMR led to initial target reinstatement around cue offset/at the end of the first UP state, albeit without reaching statistical significance. Critically, however, a second increase in target reinstatement emerged at the end of the subsequent UP state, significantly exceeding the control condition (pcluster = 0.019). Interestingly, no evidence for target category reinstatement was observed in the DOWN-state cueing condition. Together, these results suggest that UP-state cueing successfully elicits representations of the targeted category associated with the TMR cue. It is interesting to note that this effect unfolds more strongly during the second evoked SO cycle (Discussion).
Discussion
How can we experimentally alter the mnemonic fate of our experiences? In this study, we sought to combine two recent advances in strengthening overnight memory retention (consolidation)—the delivery of reminder cues (TMR) and the modulation of endogenous electrophysiological sleep signatures via noninvasive brain stimulation. Specifically, we presented one TMR cue at SO UP states and another cue at SO DOWN states (Fig. 1). Behavioral results revealed reduced overnight forgetting of learning material linked to UP-state cues compared with learning material linked to DOWN-state cues (Fig. 2C). Physiologically, UP-state cueing enhanced ongoing UP states (Fig. 2A) and resulted in stronger spindle power during the subsequent SO cycle (Fig. 3B). Moreover, UP-state cueing elicited reinstatement of target representations, with this effect again being most pronounced during the subsequent SO cycle (Fig. 4B).
What renders SOs prone to modulating the efficacy of TMR? SOs reflect global fluctuations in cellular excitability (i.e., phases of membrane depolarization associated with synchronous firing of large neuron populations [UP states] alternating with phases of membrane hyperpolarization associated with widespread neuronal silence [DOWN states]) (14, 42, 43). Transcranial magnetic stimulation (TMS) over motor cortex is more likely to induce motor-evoked potentials (MEPs) when applied during SO UP states than during DOWN states (44). Importantly, SO-modulated neuronal excitability is not confined to neocortical regions. Rodent recordings have shown that membrane potentials in the hippocampus fluctuate according to cortical SOs (45), and human single-unit recordings have confirmed that neuronal firing rates—including those of hippocampal neurons—are elevated during SO UP states and diminished during DOWN states (46). Moreover, hippocampal ripples, strongly linked to endogenous memory reactivation (6, 9), are more prevalent at SO UP states than DOWN states (22, 24). Corroborating their relevance for memory consolidation, SO UP states have been shown to increase brain-wide coherence after learning of declarative memories (47), and enhancing SOs experimentally via transcranial electrical stimulation has been shown to reduce overnight forgetting (33). Critically, our UP-state cueing protocol enhanced ongoing UP states above and beyond endogenous levels (compared with sham stimulation) (Fig. 2A). TMR cues delivered during SO UP states may thus not only arrive at moments of elevated neuronal responsiveness, but also prolong/enhance neurophysiological conditions permissive to memory consolidation.
Apart from their role in directly modulating neuronal excitability, SOs have a strong impact on the emergence of sleep spindles (25, 26). In fact, the precision of spindle coupling to SO UP states has been shown to track memory performance across aging (22, 48, 49) and has been linked to endogenous memory reactivation during sleep (28). Interestingly, previous TMR studies have connected postcue spindle power with the processing of cue information. Specifically, spindle power was found to correlate with 1) the ability to decode cue information based on EEG activation patterns (27), 2) the number of learning stimuli paired with a particular cue (30), and 3) the behavioral benefit of TMR (50). Our results replicate the link between spindle activity and informational load (TMR sounds vs. novel control sounds) and further reveal greater spindle activity following UP-state cueing compared with DOWN-state cueing (Fig. 3 and SI Appendix, Fig. S1). This finding is consistent with the notion that cue information is more effectively processed when delivered during SO UP states than during DOWN states.
If UP-state TMR cues are more effectively processed, they should be capable of triggering reactivation of the associated target information. We thus assessed whether whole-brain EEG patterns of categorical image processing (e.g., car image) from the wake localizer task reemerge upon being cued with the corresponding TMR sounds (e.g., engine starting) during sleep. Compared with novel control sounds (where no category-specific reactivation should occur), we indeed found evidence for target reinstatement after UP-state cueing but not after DOWN-state cueing (Fig. 4). This result provides empirical evidence that TMR cues can—when delivered during optimal SO phases—elicit associated target representations derived from an independent wake session.
Examining the time course of reinstatement (Fig. 4B) reveals an intriguing cyclic pattern tracking SO UP states. This pattern is reminiscent of two previous TMR studies demonstrating that (mnemonic) processing of TMR cues tends to oscillate at ∼1 Hz (27, 51). Moreover, a recent study on motor learning found that TMR-induced reactivation of learning patterns occurs either immediately or with a delay of 1 s (52). There is of course some variability across studies in the exact effect timing with respect to the TMR onset/offset (perhaps related to the complexity of the TMR cue and/or its associated information), but the cyclic pattern strongly points to a pacemaker role of SOs in coordinating memory processes during sleep. One exciting hypothesis put forth by Lewis and Bendor (53) holds that immediate/early reactivation reflects cortical cue representations, whereas subsequent “echoes” reflect more complete reactivations mediated by hippocampal processes. In light of the relatively complex nature of our cue (sound)–target (image) associations, it is tempting to interpret the late increase in reinstatement as reflecting such hippocampal contributions. Intracranial EEG would be well suited to test whether hippocampal memory signals (e.g., ripples) indeed occur between two bouts of reactivation, with the strength of these signals perhaps predicting the fidelity of subsequent cortical reactivation.
Despite the behavioral and physiological effects of UP-state cueing observed here, processing of TMR cues is unlikely to be entirely absent when delivered at SO DOWN states, particularly as our DOWN stimulation encompassed the DOWN to UP transition (given the stimulus duration of 500 ms) (Fig. 1). Both stimulation conditions elicited an additional SO cycle compared with sham stimulation (Fig. 2A), and postcue spindle power was greater for DOWN-state TMR than for DOWN-state control sounds (Fig. 3C), although the latter effect was markedly smaller than for the corresponding UP-state conditions (UP-Stim vs. UP-Ctrl). These results are consistent with the finding that presenting clicks during DOWN states also elicited an auditory-evoked response (34) and that DOWN-state TMS also elicited MEPs, albeit diminished in strength (by ∼20%) relative to UP-state TMS (44). These data suggest that the efficacy of TMR follows a gradient rather than an all-or-none principle. This notion dovetails with a retrospective analysis of a TMR study showing that the optimal SO phase for TMR cues to elicit consolidation benefits roughly spans the entire UP- to DOWN-state transition (38). Even if there was a single optimal SO phase in theory, its identification is further complicated by factors such as unknown conduction delays between experimental apparatus and target brain regions, different durations of TMR stimuli within and across studies as well as inherent fluctuations in EEG signal to noise ratios and resulting detectability of endogenous brain rhythms. Nevertheless, by carefully controlling these factors, future closed-loop TMR studies hold great promise of improving efforts to bolster memory consolidation in laboratory settings and beyond (54).
This promising outlook notwithstanding, more work is clearly needed to establish the robustness of closed-loop TMR for different expressions of memory. For instance, while we observed the hypothesized relative benefit of UP- vs. DOWN-state cueing on consolidation, uncued items fell numerically in between these TMR conditions but did not differ statistically from either. Another recent closed-loop TMR study examined consolidation of previously learned vocabularies during a nap and found behavioral benefits of SO UP-state TMR vs. uncued items but no statistical difference to DOWN-state TMR (40). It is thus still open whether the behavioral effects of SO-based closed-loop TMR are solely mediated by UP-state cueing enhancing consolidation or whether DOWN-state cueing additionally impedes consolidation—with the latter harboring potential for therapeutic interventions. Finally, another recent closed-loop TMR study had participants learn semantically related word pairs (e.g., volcano–explosion) and delivered acoustic reminder syllables (“vol”) at SO UP or DOWN states during subsequent NREM sleep (55). No differential effects on behavioral expressions of consolidation and no differences in cue-evoked spindle power for UP- vs. DOWN-state cueing were observed in that study (reinstatement of target associations was not examined). Apart from idiosyncrasies in experimental design, the lack of behavioral effects in that study might be due to an average forgetting rate for uncued items of only 4% (as opposed to 12% in our study or 9% in ref. 40). In other words, a ceiling effect in overnight retention might have obscured the beneficial effects of UP-state TMR (56). Regarding spindles, our current study and previous TMR studies linking spindles to cue processing have used rich visual images as associated targets (27, 28, 30), which is different from the verbal/semantic associations used in Wang et al. (55). In any case, future studies will need to delineate the boundary conditions of closed-loop TMR and assess whether effects hinge on specific choices in experimental design/stimulus material.
To conclude, our study reveals that auditory reminder cues during sleep are more effective in slowing down overnight forgetting when delivered at SO UP states than at SO DOWN states. Mechanistically, this effect might be mediated by sleep spindles and reinstatement of associated target representations, both of which are more readily elicited by UP-state stimulation.
Materials and Methods
Participants.
Twenty-four participants were tested (14 females, 21.3 ± 0.5 y). Two additional participants had to be excluded: one due to irregular sleep patterns (no slow wave sleep) and one due to technical issues with the TMR procedure. All participants were healthy nonsmokers with a normal wake–sleep rhythm. Participants were asked to refrain from alcohol for at least 1 d before the experiment. On the experimental day itself, participants were instructed to wake up at 7 AM and not to consume any caffeine after 3 PM. All participants gave written consent prior to the experiment. The study was approved by the ethics committee of the University of Birmingham and conducted according to the Declaration of Helsinki.
Study Design and Procedure.
The experiment consisted of one experimental night conducted in a dedicated sleep laboratory. Participants arrived at the sleep laboratory around 9 PM and were first prepared for EEG and polysomnographic recordings. Afterward, participants performed a localizer task followed by a memory task, which was further divided into familiarization, encoding, an offline period, and immediate retrieval. The localizer task was employed to examine reinstatement during subsequent sleep. Participants then went to bed, and lights were turned off at 11 PM. TMR commenced after 5 min of stable NREM sleep for a maximal duration of 4 h and was manually halted whenever participants showed signs of arousal or transitioned into rapid eye movement (REM) sleep. After 8 h of sleep (around 7 AM), participants were woken up. Thirty minutes after waking up (mitigating sleep inertia), participants performed another memory task (delayed retrieval) as well as another localizer task and were then released from the laboratory.
Stimulus Material.
The memory task drew from a collection of 180 verbs (e.g., “jump”) and six gray-scale images from the fLoc functional localizer package (57): car, guitar, house, corridor, face, and hand. Each image was accompanied by a semantically related sound (guitar = string chord, car = engine ignition, house = squeaking door, corridor = vacuum cleaner, face = smacking kiss, hand = clapping). All sounds were 500-ms long and normalized to a comparable sound volume. During sleep, participants were presented with an additional control sound that was not presented during prior wakefulness. This sound was labeled “shooting star” and of an artificial nature, which could not be semantically related to any of the stimulus material (provided in the Open Science Framework [OSF] repository). The localizer used a total of 240 gray-scale images, including 40 images from each of the six categories. Of note, each category included the image used in the memory task, resulting in 39 other exemplars from the same category.
Memory Task.
In the evening, the memory task consisted of four phases. First, participants were familiarized with the upcoming stimulus material. To this end, participants saw a screen showing all six images. If they then pressed the key indicated below an image, all other images disappeared, and the semantically related sound was played. Participants were instructed to take their time to familiarize themselves with all six image–sound pairings.
Next, during the encoding phase, participants learned 120 verb–image associations. Each trial started with a fixation cross between 1 and 1.5 s followed by a screen displaying a verb and one of the six images below. Additionally, the semantically related sound cue was played once upon stimulus presentation. Participants had 3 s to conjure up a mental image linking verb and image. To ensure task engagement, participants were then asked to indicate within 10 s via key press whether their mental image was plausible or bizarre. Allocation of stimuli was balanced such that each image was paired with 20 randomly selected verbs. After the encoding phase, participants performed a PVT for 3 min. Finally, an immediate retrieval phase tested 50% of the encoding trials (i.e., 60 verb–image associations, with each image exemplar being the target for 10 trials). Each trial started with a fixation cross for 1 to 1.5 s, and then a verb was presented for 3 s. During this time, participants were instructed to actively remember if they have learned this verb before and if that is the case, to vividly bring back to mind the exemplar it was linked to. Memory was then tested in three steps. First, participants had unlimited time to indicate if the verb was old (seen during encoding) or from a set of 30 new verb lures (recognition memory). This was followed by a confidence rating on a four-point scale, with one for “not confident” up to four for “very confident.” If participants responded “old,” they then had 10 s to indicate whether the associated image was an object, a scene, or a human body part. They were further given the option to respond “forgotten” to discourage guessing. Finally, if a category was chosen, participants were given 10 s to choose the exact exemplar. Of note, if “object” was chosen as a category, participants could then respond with car, guitar, or forgotten—and analogously for scene (corridor, house) and human body part (face, hand). The following morning, memory was tested on the remaining 50% of the encoded material with 30 new lures during a delayed retrieval following the same procedure as in the evening.
Localizer Task.
To derive brain representations of the target image categories in an independent fashion, participants performed a continuous recognition task at the beginning and end of the experimental session (28). The 240 gray-scale images were divided into two sets of 120 images across the two localizer runs (evening and morning). Each set consisted of 20 exemplars of the six categories (guitars, cars, houses, corridors, faces, and hands). Localizer trials started with a fixation cross presented for 1.5 ± 0.1 s followed by a randomly chosen image shown on the computer screen for a minimum of 2.5 s and a maximum of 10 s. After 2 s, participants were prompted to indicate whether the image was shown for the first (new) or second (old) time. Prompting responses after 2 s ensured that trials were not contaminated by button presses. The total number of trials per localizer was 240 (2 × 120). Mean distance between successive presentations of the same image was 23.2 ± 0.3 trials. On average, accuracy across participants was 90.2 ± 1.2%.
EEG Recordings and Polysomnography.
Throughout the behavioral task and the entire night, brain activity was recorded via a Brainamp DC amplifier (Brain Products) using a 64-channel EasyCap EEG cap. These recordings were obtained with a sampling rate of 500 Hz and an online reference placed at FCz. For polysomnography, four electrodes were repurposed to record electrooculography (EOG) and electromyography (EMG). Impedance of all electrodes was kept below 5 kΩ.
SO-Locked TMR.
Closed-loop TMR phase-locked to SO UP or DOWN states was implemented with the OpenViBE Brain Computer Interface Software (58). Given the predominant origin of SOs in frontal regions (43, 59), SO detection was based on Fz referenced against linked mastoids and bandpass filtered in the slow-wave range between 0.5 and 4 Hz. To detect an SO UP state, the filtered signal had to rise, cross a threshold of +35 µV, and show a change in its slope from positive to negative, indicating a local maximum (44). Conversely, an SO DOWN state was identified whenever the falling signal crossed a threshold of −75 µV and a negative to positive change in slope polarity (i.e., a local minimum) occurred. Each time an UP or DOWN state was detected, a sound was played, and the detection/stimulation protocol paused for 8 s. Subsequent univariate analyses (ERP and TFR) focused on electrode Cz, as both SOs and sleep spindles are prevalent at that site (22, 28).
For each participant, two images (from different categories) were allocated to the UP- and DOWN-state conditions, with the combinations balanced across all participants. The semantically related sounds were thus played whenever an SO UP state or DOWN state was detected. The aforementioned novel control sound was also played during SO UP and DOWN states, resulting in a total of four cueing conditions. To control the number of stimulations in each condition, cueing was performed in a block-wise fashion, with one block consisting of the four conditions and their order being randomized after each block. On average, 78.2 ± 4.4 cues were presented for each condition across all participants. For each participant, cueing began at a volume of 20 dB SPL (sound pressure level) and was increased by 6 dB SPL after each block in a stepwise manner until a clear EEG response was evoked without any signs of arousal.
To assess the effect of cueing relative to a noncueing control condition, we retrospectively derived sham cues. These sham cues were defined as time points at which spontaneous SO UP and DOWN states met our detection criteria but at which no TMR cues were delivered (i.e., if an UP or DOWN state occurred during the stimulation refractory period of >8 s). Importantly, the event-related analysis described below was always performed on nonoverlapping 4.5-s segments.
EEG Analyses.
All data analyses were performed in MATLAB (Version 2018b) and with the Fieldtrip toolbox. Statistical analyses were performed with JASP. Due to technical difficulties, one participant had to be excluded from the EEG analyses. Data are reported as mean ± SEM.
Preprocessing included a rereferencing to linked mastoids, spline interpolation of bad channels, and visual rejection of artifacts. Furthermore, sleep stages according to standard scoring criteria (60) were determined on EEG (F3, F4, C3, C4, O1, and O2) and EOG signals band-pass filtered between 0.3 and 30 Hz, while the EMG signal was high-pass filtered at 5 Hz. Sleep stages N1, N2, N3, REM sleep, and wake were identified from lights off until waking time. The percentage of time spent in each stage was calculated as time in the respective sleep stage over total sleep time (TST).
Statistical assessment of EEG data was based on nonparametric cluster permutation tests using 1,000 permutations, a cluster threshold of P < 0.05, and a final threshold for significance of P < 0.05 (61) (all two tailed). To account for the inherent precue differences due to targeting opposing SO UP and DOWN states, the statistical evaluation was restricted to the postcue interval (i.e., time t = 0 to 2 s).
Due to the nature of our block-wise cueing procedure, trial numbers were generally balanced across conditions. However, in case of imbalances, conditions with more trials were randomly subsampled to match the condition with the fewest trials. The resulting mean ± SEM trial numbers across participants was 66.4 ± 4.3 per condition.
ERPs.
To assess the brain response evoked by TMR and control sounds, we calculated ERPs by segmenting the 0.3- to 30-Hz band pass–filtered EEG signal into 4.5-s epochs time locked to the cue onset (including 2.5 s before cue onset and 2 s after cue onset) and then for each stimulation condition, averaged across all trials.
TFRs.
To examine the spectrally resolved TMR response, we calculated TFRs time locked to the TMR cue onset using Morlet wavelets for frequencies from 1 to 20 Hz with a 0.5-Hz resolution and a time window from −2.5 to 2 s in 50-ms steps. For frequencies of ≥5 Hz, the number of cycles was set adaptively to a quarter of the corresponding frequency (or rounded up to the next integer value) but at least five cycles, resulting in time windows of ∼250 ms. For frequencies below 5 Hz (i.e., 1 to 4 Hz), cycle numbers were reduced to values ranging from two to four, reducing the window size and thereby increasing availability of artifact-free segments. Resulting TFRs were then expressed as the relative change to a baseline from −2.5 to −2 s precue onset. As TFR analyses were geared toward spindles, we restricted analyses to frequencies between 10 and 20 Hz.
Multivariate Pattern Analyses.
To unveil reinstatement of target representations during cueing, we performed a multivariate representational similarity analysis. To this end, all localizer and sleep EEG data were band-pass filtered between 0.03 and 40 Hz, down sampled to 100 Hz, and rereferenced to the common average.
First, we verified that the different image categories can be reliably differentiated based on the EEG signal. To this end, the EEG signal from the two localizers was smoothed with a 20-ms moving average and segmented into 2-s segments time locked to the onset of a visual stimulus. Before both localizers were concatenated, each localizer was separately z scored across trials. Next, we calculated—for each trial and time point—the representational similarity with all other trials based on Pearson correlations across channels. Same-trial combinations were discarded. Finally, we averaged within-category similarity (e.g., cars with cars; six within-category similarities consisting of 3,160 trials total) as well as between-category similarity (cars with guitars, cars with houses, etc.; 30 between-category similarities consisting of 6,400 trials each).
To assess localizer to TMR reinstatement, all localizer and sleep data were smoothed with a 200-ms moving average, segmented into 2-s epochs time locked to a visual stimulus or TMR cue onset, respectively, and separately z scored along the trial dimension. To examine reinstatement of the UP category following UP TMR cues, we extracted the corresponding trials from the segmented localizer and TMR data and averaged across all trials, yielding two channel × time matrices. Note that identical results were obtained when correlating the localizer average with the TMR data on a trial by trial basis and collapsing trials afterward. Next, we correlated each combination of time points from the localizer and sleep data across channels, resulting in a localizer time × TMR time correlation matrix per participant. This procedure was repeated contrasting UP localizer categories with UP control conditions, DOWN localizer categories with DOWN TMR conditions, and DOWN localizer categories with DOWN control conditions (i.e., resulting in a total of four correlation maps). Finally, we derived a one-dimensional correlation time series by averaging across localizer time (0 to 2 s), reflecting target image reinstatement relative to the TMR/control cue during sleep.
Supplementary Material
Acknowledgments
We thank Joseph Danby for assisting with the data acquisition. This work was supported by Wellcome Trust Grant 107672/Z/15/Z (to B.P.S.) and European Research Council Grant 101001121 (to B.P.S.).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2123428119/-/DCSupplemental.
Data Availability
EEG data, sound files, and analysis scripts have been deposited on the OSF (https://osf.io/2HBXZ) (62).
References
- 1.Stickgold R., Sleep-dependent memory consolidation. Nature 437, 1272–1278 (2005). [DOI] [PubMed] [Google Scholar]
- 2.Rasch B., Born J., About sleep’s role in memory. Physiol. Rev. 93, 681–766 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jenkins J. G., Dallenbach K. M., Obliviscence during sleep and waking. Am. J. Psychol. 35, 605 (1924). [Google Scholar]
- 4.Tononi G., Cirelli C., Sleep function and synaptic homeostasis. Sleep Med. Rev. 10, 49–62 (2006). [DOI] [PubMed] [Google Scholar]
- 5.Skaggs W. E., McNaughton B. L., Replay of neuronal firing sequences in rat hippocampus during sleep following spatial experience. Science 271, 1870–1873 (1996). [DOI] [PubMed] [Google Scholar]
- 6.Wilson M. A., McNaughton B. L., Reactivation of hippocampal ensemble memories during sleep. Science 265, 676–679 (1994). [DOI] [PubMed] [Google Scholar]
- 7.Ji D., Wilson M. A., Coordinated memory replay in the visual cortex and hippocampus during sleep. Nat. Neurosci. 10, 100–107 (2007). [DOI] [PubMed] [Google Scholar]
- 8.McClelland J. L., McNaughton B. L., O’Reilly R. C., Why there are complementary learning systems in the hippocampus and neocortex: Insights from the successes and failures of connectionist models of learning and memory. Psychol. Rev. 102, 419–457 (1995). [DOI] [PubMed] [Google Scholar]
- 9.Buzsáki G., The hippocampo-neocortical dialogue. Cereb. Cortex 6, 81–92 (1996). [DOI] [PubMed] [Google Scholar]
- 10.Diekelmann S., Born J., The memory function of sleep. Nat. Rev. Neurosci. 11, 114–126 (2010). [DOI] [PubMed] [Google Scholar]
- 11.Rasch B., Büchel C., Gais S., Born J., Odor cues during slow-wave sleep prompt declarative memory consolidation. Science 315, 1426–1429 (2007). [DOI] [PubMed] [Google Scholar]
- 12.Hu X., Cheng L. Y., Chiu M. H., Paller K. A., Promoting memory consolidation during sleep: A meta-analysis of targeted memory reactivation. Psychol. Bull. 146, 218–244 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paller K. A., Creery J. D., Schechtman E., Memory and sleep: How sleep cognition can change the waking mind for the better. Annu. Rev. Psychol. 72, 123–150 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steriade M., McCormick D. A., Sejnowski T. J., Thalamocortical oscillations in the sleeping and aroused brain. Science 262, 679–685 (1993). [DOI] [PubMed] [Google Scholar]
- 15.Timofeev I., Chauvette S., Sleep slow oscillation and plasticity. Curr. Opin. Neurobiol. 44, 116–126 (2017). [DOI] [PubMed] [Google Scholar]
- 16.Fernandez L. M. J., Lüthi A., Sleep spindles: Mechanisms and functions. Physiol. Rev. 100, 805–868 (2020). [DOI] [PubMed] [Google Scholar]
- 17.Loomis A. L., Harvey E. N., Hobart G., Potential rhythms of the cerebral cortex during sleep. Science 81, 597–598 (1935). [DOI] [PubMed] [Google Scholar]
- 18.Latchoumane C. V., Ngo H.-V. V., Born J., Shin H. S., Thalamic spindles promote memory formation during sleep through triple phase-locking of cortical, thalamic, and hippocampal rhythms. Neuron 95, 424–435.e6 (2017). [DOI] [PubMed] [Google Scholar]
- 19.Niethard N., Ngo H.-V. V., Ehrlich I., Born J., Cortical circuit activity underlying sleep slow oscillations and spindles. Proc. Natl. Acad. Sci. U.S.A. 115, E9220–E9229 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klinzing J. G., Niethard N., Born J., Mechanisms of systems memory consolidation during sleep. Nat. Neurosci. 22, 1598–1610 (2019). [DOI] [PubMed] [Google Scholar]
- 21.Tononi G., Cirelli C., Sleep and synaptic down-selection. Eur. J. Neurosci. 51, 413–421 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Helfrich R. F., Mander B. A., Jagust W. J., Knight R. T., Walker M. P., Old brains come uncoupled in sleep: Slow wave-spindle synchrony, brain atrophy, and forgetting. Neuron 97, 221–230.e4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Niknazar M., Krishnan G. P., Bazhenov M., Mednick S. C., Coupling of thalamocortical sleep oscillations are important for memory consolidation in humans. PLoS One 10, e0144720 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mölle M., Marshall L., Gais S., Born J., Grouping of spindle activity during slow oscillations in human non-rapid eye movement sleep. J. Neurosci. 22, 10941–10947 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mölle M., Bergmann T. O., Marshall L., Born J., Fast and slow spindles during the sleep slow oscillation: Disparate coalescence and engagement in memory processing. Sleep (Basel) 34, 1411–1421 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Staresina B. P., et al. , Hierarchical nesting of slow oscillations, spindles and ripples in the human hippocampus during sleep. Nat. Neurosci. 18, 1679–1686 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cairney S. A., Guttesen A. Á. V., El Marj N., Staresina B. P., Memory consolidation is linked to spindle-mediated information processing during sleep. Curr. Biol. 28, 948–954.e4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schreiner T., Petzka M., Staudigl T., Staresina B. P., Endogenous memory reactivation during sleep in humans is clocked by slow oscillation-spindle complexes. Nat. Commun. 12, 3112 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seibt J., et al. , Cortical dendritic activity correlates with spindle-rich oscillations during sleep in rodents. Nat. Commun. 8, 684 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schechtman E., et al. , Multiple memories can be simultaneously reactivated during sleep as effectively as a single memory. Commun. Biol. 4, 25 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sejnowski T. J., Destexhe A., Why do we sleep? Brain Res. 886, 208–223 (2000). [DOI] [PubMed] [Google Scholar]
- 32.Petzka M., Chatburn A., Charest I., Balanos G. M., Staresina B. P., Sleep spindles track cortical learning patterns for memory consolidation. Curr. Biol. 32, 2349–2356.e4 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marshall L., Helgadóttir H., Mölle M., Born J., Boosting slow oscillations during sleep potentiates memory. Nature 444, 610–613 (2006). [DOI] [PubMed] [Google Scholar]
- 34.Ngo H.-V. V., Martinetz T., Born J., Mölle M., Auditory closed-loop stimulation of the sleep slow oscillation enhances memory. Neuron 78, 545–553 (2013). [DOI] [PubMed] [Google Scholar]
- 35.Ong J. L., et al. , Effects of phase-locked acoustic stimulation during a nap on EEG spectra and declarative memory consolidation. Sleep Med. 20, 88–97 (2016). [DOI] [PubMed] [Google Scholar]
- 36.Leminen M. M., et al. , Enhanced memory consolidation via automatic sound stimulation during non-REM sleep. Sleep (Basel) 40, zsx003 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Papalambros N. A., et al. , Acoutstic enhancement of sleep slow oscillations and concomitant memory improvement in older adults. Front. Hum. Neurosci. 11, 109 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Batterink L. J., Creery J. D., Paller K. A., Phase of spontaneous slow oscillations during sleep influences memory-related processing of auditory cues. J. Neurosci. 36, 1401–1409 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shimizu R. E., et al. , Closed-loop targeted memory reactivation during sleep improves spatial navigation. Front. Hum. Neurosci. 12, 28 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Göldi M., van Poppel E. A. M., Rasch B., Schreiner T., Increased neuronal signatures of targeted memory reactivation during slow-wave up states. Sci. Rep. 9, 2715 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schönauer M., et al. , Decoding material-specific memory reprocessing during sleep in humans. Nat. Commun. 8, 15404 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Achermann P., Borbély A. A., Low-frequency (< 1 Hz) oscillations in the human sleep electroencephalogram. Neuroscience 81, 213–222 (1997). [DOI] [PubMed] [Google Scholar]
- 43.Massimini M., Huber R., Ferrarelli F., Hill S., Tononi G., The sleep slow oscillation as a traveling wave. J. Neurosci. 24, 6862–6870 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bergmann T. O., et al. , EEG-guided transcranial magnetic stimulation reveals rapid shifts in motor cortical excitability during the human sleep slow oscillation. J. Neurosci. 32, 243–253 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Isomura Y., et al. , Integration and segregation of activity in entorhinal-hippocampal subregions by neocortical slow oscillations. Neuron 52, 871–882 (2006). [DOI] [PubMed] [Google Scholar]
- 46.Nir Y., et al. , Regional slow waves and spindles in human sleep. Neuron 70, 153–169 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mölle M., Marshall L., Gais S., Born J., Learning increases human electroencephalographic coherence during subsequent slow sleep oscillations. Proc. Natl. Acad. Sci. U.S.A. 101, 13963–13968 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hahn M. A., Heib D., Schabus M., Hoedlmoser K., Helfrich R. F., Slow oscillation-spindle coupling predicts enhanced memory formation from childhood to adolescence. eLife 9, e53730 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Muehlroth B. E., et al. , Precise slow oscillation–spindle coupling promotes memory consolidation in younger and older adults. Sci. Rep. 9, 1940 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Antony J. W., et al. , Sleep spindle refractoriness segregates periods of memory reactivation. Curr. Biol. 28, 1736–1743.e4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schreiner T., Doeller C. F., Jensen O., Rasch B., Staudigl T., Theta phase-coordinated memory reactivation reoccurs in a slow-oscillatory rhythm during NREM sleep. Cell Rep. 25, 296–301 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abdellahi M. E. A., Koopman A. C. M., Treder M. S., Lewis P. A., Targeting targeted memory reactivation: Characteristics of cued reactivation in sleep. bioRxiv [Preprint] (2021). https://www.biorxiv.org/content/10.1101/2021.12.09.471945v1 (Accessed 1 April 2022). [DOI] [PubMed]
- 53.Lewis P. A., Bendor D., How targeted memory reactivation promotes the selective strengthening of memories in sleep. Curr. Biol. 29, R906–R912 (2019). [DOI] [PubMed] [Google Scholar]
- 54.Göldi M., Rasch B., Effects of targeted memory reactivation during sleep at home depend on sleep disturbances and habituation. NPJ Sci. Learn. 4, 5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang J.-Y., Heck K. L., Born J., Ngo H.-V. V, Diekelmann S., No difference between slow oscillation up- and down-state cueing for memory consolidation during sleep. J. Sleep Res., 10.1111/jsr.13562 (2022). [DOI] [PubMed] [Google Scholar]
- 56.Petzka M., Charest I., Balanos G. M., Staresina B. P., Does sleep-dependent consolidation favour weak memories? Cortex 134, 65–75 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stigliani A., Weiner K. S., Grill-Spector K., Temporal processing capacity in high-level visual cortex is domain specific. J. Neurosci. 35, 12412–12424 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Renard Y., et al. , OpenViBE: An open-source software platform to design, test and use brain-computer interfaces in real and virtual environments. Presence Teleoperators Virtual Environ. 19, 35–53 (2010). [Google Scholar]
- 59.Chauvette S., Volgushev M., Timofeev I., Origin of active states in local neocortical networks during slow sleep oscillation. Cereb. Cortex 20, 2660–2674 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Berry R. B., et al. , The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications (Version 2.2, American Academy of Sleep Medicine, Darien, IL, 2015).
- 61.Maris E., Oostenveld R., Nonparametric statistical testing of EEG- and MEG-data. J. Neurosci. Methods 164, 177–190 (2007). [DOI] [PubMed] [Google Scholar]
- 62.Ngo H.-V. V., Staresina B. P., Shaping overnight consolidation via slow-oscillation closed-loop targeted memory reactivation. Open Science Framework. https://osf.io/2HBXZ/. Deposited 7 June 2022. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
EEG data, sound files, and analysis scripts have been deposited on the OSF (https://osf.io/2HBXZ) (62).