Were I still teaching upper-level biology courses, I would for sure assign the reading and discussion of Hirooka et al., “Life Cycle and Functional Genomics of the Unicellular Red Alga Galdieria for Elucidating Algal and Plant Evolution and Industrial Use” (1). It tells the engrossing tale of an unexpected discovery and the exploration of its important ramifications in both basic and applied bioscience, with a clear plot line, excellent data, and high-quality graphics.
The context of this study is a far-ranging program, in many laboratories, to develop eukaryotic unicellular algae (EUA) that can, for example, produce pharmaceuticals (2) and biofuel precursors (3) and engage in environmental remediation (4). Researchers have encountered three difficulties in pursuing these projects. First, many candidate EUAs can only grow using photosynthesis—they are obligate phototrophs, meaning that they must either be provided with expensive indoor lighting or grown in sunlit ponds. Second, ponds are difficult to maintain and algal growth is often plagued by bacterial contamination. Third, the hardiest EUA candidates prove to be endowed with robust cell walls that are refractile to mechanical or chemical disruption, meaning that their bio-products are difficult to extract; moreover, it can be challenging to introduce DNA into these cells for genetic transformation and expression of exogenous genes.
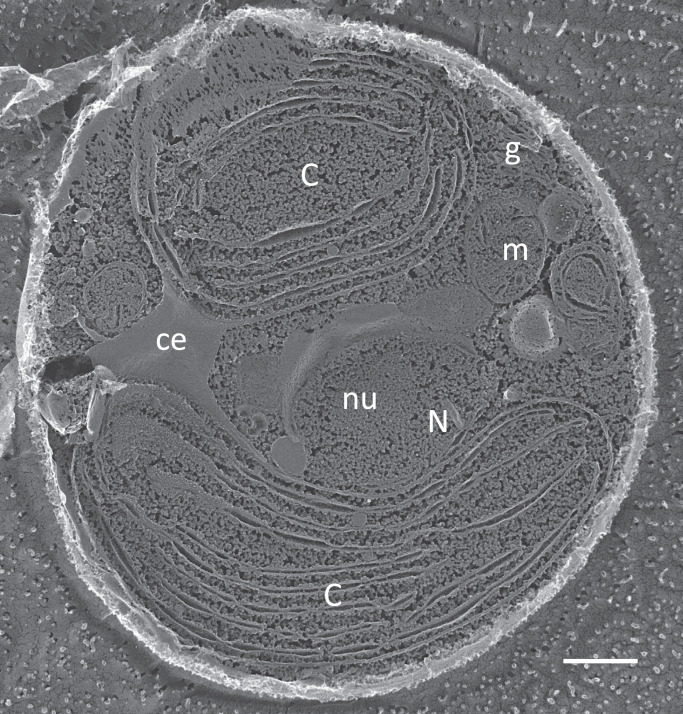
The first two difficulties are circumvented by Galdieria (Fig. 1), an EUA in the Cyanidiophyceae class of the red algal (Rhodophyta) radiation (see Fig. 1 of ref. 1). It can supplement photosynthesis with more than 50 different carbon sources, including organic materials present in wastewater, and it can grow in the dark to high densities in laboratory bioreactors when these supplements are provided (5). Its natural habitats are geothermal volcanic springs, where temperatures reach 57 °C, pH ranges between 0.5 and 3.0, and sulfuric acid is abundant, conditions that disallow the growth of most putative bacterial contaminants. However, its wall is protein-rich and rigidified by covalent cross-links, confounding breakage, and it has thus far been refractory to genetic manipulation.
Fig. 1.
Newly hatched Galdieria cell, surrounded by a dense cell wall, as visualized by the QFDEEM technique (13). C, chloroplast; ce, chloroplast envelope; g, Golgi; m, mitochondrion; N, nucleus; nu, nucleolus. Cultured cells provided by Peter Lammers, Arizona State University. (Scale bar, 500 nm.)
By contrast, Cyanidoischyson merolae cells, in the same Cyanidiophyceae class as Galdieria and fellow inhabitants of hot springs, are wall-less and have been subjected to extensive genetic manipulation, initially in the Kuroiwa laboratory (6). Importantly, C. merolae genes can be knocked out by homologous recombination—an introduced mutant gene carrying a selectable marker can replace its chromosomal homolog—a valued feature of fungal biotechnology and operant in two other EUAs (7, 8) but not found in the model EUA Chlamydomonas reinhardtii. Despite this feature, C. merolae is an obligate phototroph and hence of restricted industrial use.
While observing cells in a Galdieria culture using light microscopy, Hirooka et al. noticed two morphological types: the usual wall-encased cells and also motile cells that formed protuberant tails (see movies of ref. 1), suggesting that they might be wall-free. When one of these cells was cloned and genome-sequenced, the progeny indeed proved to be wall-less, albeit embedded in a loose polysaccharide matrix. Surprisingly, they were also found to be haploid, whereas the walled cells were known to be heterozygous diploids. Moreover, when the haploid cells were exposed to acetic acid they formed homozygous diploid cells with walls, findings that suggested the discovery of a Galdieria sexual cycle.
Pursuing this inference, the authors cloned and sequenced four additional haploid cells from the original culture and found that they were of two mating types: Type l cells (clones N1 and N3) did not form diploids when mixed together, but heterozygous diploids formed when either were mixed with Type 2 cells (clones N2, N4, and N5). This represents a demonstration of a sexual cycle in a red EUA lineage, albeit sex is widespread in the multicellular red lineages (see Fig. 1 of ref. 1).
In most sexual cycles, the gene-expression profile of haploids is different from diploids, and transcriptomic analyses showed this to be the case for Galdieria as well. As expected, genes involved in the synthesis of extracellular matrices were differentially expressed to generate the loose matrix vs. rigid cell of the two differentiated cell types. Moreover, one actin-encoding gene is transcribed in the haploids, presumably engaged in its motile behavior, while a second is transcribed in the diploids, presumably involved in the formation of actin-based cleavage furrows at cell division.
Particularly interesting are the expression patterns of TALE-class homeoprotein transcription factors that are involved in haploid/diploid transitions in other photosynthetic lineages. In the green EUA Chlamydomonas, for example, the minus mating type selectively expresses a protein in the Knox family, and the plus mating type expresses a protein in the Bell family; these form heterodimers when the mating cells fuse, and the heterodimers activate the diploid program that leads to spore formation and meiosis (9). Expanded families of KNOX and BELL genes govern the morphogenetic and sexual cycles of multicellular green land plants (10), and a KNOX gene participates in the complex sexual cycle of the multicellular red alga Pyropia (11). In Galdieria, haploid cells of a single mating type prove to express both KNOX and BELL genes whereas diploid cells express neither, and knockouts of either gene (see below) prevent self-diploidization, consistent with the possibility that Knox/Bell heterodimers participate in haploid sexual differentiation in this lineage.
Modern photosynthetic eukaryotes in the Archaeplastida belong to three evolutionary radiations (see Fig. 1 of ref. 1): the red algae (Rhodophyta), the green algae and land plants (Viridiplantae), and a small group called the Glaucophyte algae. Glaucophytes also carry KNOX and BELL homologs in their genomes (9), albeit glaucophyte sexual cycles have yet to be reported. Hence the seminal KNOX/BELL “idea” apparently arose soon after the domestication of the chloroplast and prior to the three-way branching (see Fig. 1 of ref. 1) and has gone on to undergird the vast diversity of phenotypes and life cycles found in the Archaeplastida.
With wall-less cells in hand, the authors proceeded to apply the gene-manipulation protocols developed for the wall-less C. merolae and succeeded in creating gene knockouts via homologous recombination in Galdieria, a feature that persists in the two lineages even though they last shared a common ancestor circa a billion years ago (12).
As noted earlier, the knock-out technology was used to elucidate features of the Giardia sexual cycle. It was also used to demonstrate the potential of Galdieria for industrial applications: Genes necessary to construct the core photosynthetic apparatus were knocked out, generating strains that produce the valued pigment phycocyanin without being contaminated by other light-harvesting pigments. This proof-of-concept experiment will doubtless jump-start numerous initiatives to introduce exogenous genes into Galdieria that encode proteins of industrial, biomedical, and environmental importance.
Footnotes
The author declares no competing interest.
See companion article, “Life cycle and functional genomics of the unicellular red alga Galdieria for elucidating algal and plant evolution and industrial use,” 10.1073/pnas.2210665119.
References
- 1.Hirooka S., et al. , Life cycle and functional genomics of the unicellular red alga Galdieria for elucidating algal and plant evolution and industrial use. Proc. Natl. Acad. Sci. U.S.A. 119, e2210665119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sproles A. E., et al. , Recent advancements in the genetic engineering of microalgae. Algal Res. 53, 102158 (2021). [Google Scholar]
- 3.Khan S., et al. , Production and harvesting of microalgae and an efficient operational approach to biofuel production for a sustainable environment. Fuel 311, 122543 (2022). [Google Scholar]
- 4.Henkanatte-Gedera S. M., et al. , Algal-based, single-step treatment of urban wastewaters. Bioresour. Technol. 189, 273–278 (2015). [DOI] [PubMed] [Google Scholar]
- 5.Abiusi F., et al. , Mixotrophic cultivation of Galdieria sulphuraria for C-phycocyanin and protein production. Algal Res. 61, 102603 (2022). [Google Scholar]
- 6.Fujiwara T., Ohnuma M., “Procedures for transformation and their applications in Cyanidioschyzon merolae” in Cyanidioschyzon merolae: A New Model Eukaryote for Cell and Organelle Biology, Kuroiwa T., et al., Eds. (Springer Nature, Singapore, 2017), pp. 87–103. [Google Scholar]
- 7.Kilian O., Benemann C. S., Niyogi K. K., Vick B., High-efficiency homologous recombination in the oil-producing alga Nannochloropsis sp. Proc. Natl. Acad. Sci. U.S.A. 108, 21265–21269 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lelandais G., et al. , Ostreococcus tauri is a new model green alga for studying iron metabolism in eukaryotic phytoplankton. BMC Genomics 17, 319 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee J. H., Lin H., Joo S., Goodenough U., Early sexual origins of homeoprotein heterodimerization and evolution of the plant KNOX/BELL family. Cell 133, 829–840 (2008). [DOI] [PubMed] [Google Scholar]
- 10.Joo S., et al. , Common ancestry of heterodimerizing TALE homeobox transcription factors across Metazoa and Archaeplastida. BMC Biol. 16, 136 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mikami K., Li C., Irie R., Hama Y., A unique life cycle transition in the red seaweed Pyropia yezoensis depends on apospory. Commun. Biol. 2, 299 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schönknecht G., et al. , Gene transfer from bacteria and archaea facilitated evolution of an extremophilic eukaryote. Science 339, 1207–1210 (2013). [DOI] [PubMed] [Google Scholar]
- 13.Heuser J. E., The origins and evolution of freeze-etch electron microscopy. J. Electron Microsc. (Tokyo) 60 (suppl. 1), S3–S29 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]