Abstract
The bacterial capsule is an important virulence determinant in animal and plant disease. Bacterial capsule and slime can be inhibited by bismuth compounds, especially when complexed with lipophilic thiol chelators. Bismuth dimercaprol (BisBAL) at 1 ppm of Bi3+ repressed Klebsiella pneumoniae capsule expression in defined medium by nearly 90%, which exposed subsurface structures. The phagocytic index for BisBAL-treated bacteria increased from <10 to 360 bacteria per 100 neutrophils in the presence of complement and anticapsular or anti-O antigen antiserum. BisBAL treatment also enhanced the reactivity of monoclonal antibodies (MAbs) specific for the O1-antigen lipopolysaccharide (LPS) or the LPS core in a dose-dependent manner as indicated by the results of enzyme-linked immunosorbent assays. When anti-O1 MAb was used, the reactivity increased significantly for fully encapsulated O1:K1 or O1:K2 cells but not for O1:K− cells. Deposition of C3b also increased significantly for BisBAL-treated O1:K1 or O1:K2 cells but not for O1:K− cells. Survival of a serum-sensitive strain was <0.1% when nonimmune human serum absorbed with O1:K1 cells was used and 107% when BisBAL-treated cells were used for absorption. Outer membrane proteins were also more accessible on the surface of K. pneumoniae after BisBAL treatment. Thus, at subinhibitory levels, BisBAL inhibited capsule expression, which promoted phagocytosis, enhanced the reactivity of specific antibodies for LPS O antigen, LPS core epitopes, or outer-membrane proteins, and enhanced complement interaction with encapsulated K. pneumoniae. By unmasking bacterial surface structures and enhancing the immune system reactivity to bacteria, bismuth thiols may prove useful as adjuncts for vaccination.
Polysaccharide capsules are produced by a broad range of bacterial species. As the outermost layer of the bacterial cell, the capsule mediates interactions with the environment. Interactions between the capsule and the host immune system often decide the outcome of infection (24). In the absence of specific immunity, the capsule confers resistance to nonspecific host defenses, such as complement-mediated phagocytosis and cell lysis (31). The capsule resists complement effects by providing a negatively charged permeability barrier that masks underlying cell surface structures (13, 17, 21). The capsule may also block phagocyte recognition of C3B or antibody deposited on the bacterial cell surface (14, 25, 26). Bacteria also release large quantities of capsular polysaccharide (CPS) complexed with endotoxin, which adds to the virulence of K. pneumoniae (7, 30) and may neutralize antibodies that would otherwise attach to and opsonize bacteria (5, 22).
A vaccine approach to the capsule (4, 6) appears impractical, since many different capsular serotypes are associated with disease. Furthermore, polysaccharides are often poorly immunogenic (24). An alternative therapeutic approach would be to inhibit the expression of CPS. Agents such as salicylate and bismuth inhibit capsule expression (8, 10), promoting the phagocytosis of encapsulated K. pneumoniae in the presence of complement and specific antibodies (12). Opsonophagocytosis was enhanced regardless of whether anti-capsular (13) or anti-lipopolysaccharide (LPS) (26) antibodies were used as opsonins. Salicylate and/or bismuth also inhibited the expression of capsule in other gram-negative bacteria, including mucoid Escherichia coli and Enterobacter spp. (11). Capsule inhibition is probably due to bypassing oxidative phosphorylation by salicylate or by inactivating redox enzymes by interaction of bismuth with thiols, both of which could reduce energy levels and curtail capsule synthesis (10). Indeed, levels of ATP in bacteria were decreased by 90% in the presence of bismuth subsalicylate (29).
Bismuth-2,3-dimercaptopropanol (BisBAL) is one of several bismuth thiol agents recently developed in our laboratory with antibacterial activity up to 1,000-fold greater than inorganic bismuth compounds (15). BisBAL is approximately 300-fold more active against a broad spectrum of bacteria than is bismuth subnitrate or bismuth subsalicylate (15). At subinhibitory concentrations, BisBAL also interferes with capsule expression in K. pneumoniae (14). The purpose of this study was to characterize the anticapsular effects of these new bismuth compounds on K. pneumoniae.
MATERIALS AND METHODS
Bacteria and media.
K. pneumoniae DL1 (O1:K1), 52145 (O1:K2), KT759 (O1:K10), KT762 (O1:K16), C3 (O1:K66), KT791 (O1:K−), and KT707 (O−:K66) were described previously (13, 32). Bacteria were subcultured on Luria-Bertani medium, nutrient agar, or sheep blood agar, with careful selection and maintenance of mucoid colonies. A defined minimal-salts liquid broth medium with high glucose and low nitrogen was prepared to promote capsule production (7, 16). Broth cultures were incubated for 18 h at 35°C with stirring at 200 rpm.
Anticapsular agents.
BisBAL was prepared by two different methods, either as a liquid or as a powder. The liquid form was prepared by combining bismuth nitrate and 2,3-dimercaptopropanol (dimercaprol, British anti-Lewisite [BAL]), both from Sigma Chemical Co., St. Louis, Mo., in propylene glycol at 5 and 2.5 mM, respectively. The powder form was prepared by precipitation of BisBAL (2:1 molar ratio in H2O) with sodium hydroxide followed by lyophilization (15). BisBAL powder or liquid was added directly to the culture media. The propylene glycol concentration in the culture media was kept at ≤0.1% by volume. The liquid preparations were used in both capsule reduction and phagocytosis experiments and the concentration is expressed in micromoles of bismuth. BisBAL powder was used in immunoassay experiments, and the concentration is expressed in micrograms per milliliter.
CPS.
The CPS concentration was determined by a chemical assay for uronic acid (2). Total CPS was measured after quantitative extraction of whole bacterial cultures with Zwittergent 3-14 (Calbiochem, La Jolla, Calif.) in citric acid (9). The CPS concentration is expressed as nanograms of uronic acid per 106 CFU. The LPS (endotoxin) concentration was measured by the Limulus amoebocyte lysate colorimetric assay (Associates of Cape Cod, Inc., Woods Hole, Mass.).
Opsonophagocytosis.
Preparation of bacteria and purification of human polymorphonuclear leukocytes (PMN) have been described previously (26). Bacteria and PMN were suspended in Hanks’ balanced salt solution (HBSS) containing 0.1% gelatin. Opsonophagocytic mixtures contained 160 μl of bacteria (2 × 107 CFU/ml), 160 μl of PMN (2 × 106 cells/ml), 40 μl of normal rabbit serum (Sigma) as a source of complement, and 40 μl of antiserum. All antisera were used undiluted except for K2 antiserum, which was diluted 1:40 in HBSS to minimize the aggregation of bacteria. The mixtures were incubated for 30 min at 37°C in a shaking water bath and then diluted to 10 ml with cold HBSS. After centrifugation at 400 × g to separate PMN and extracellular bacteria, the pelleted cells were resuspended in 1 ml of HBSS. Smears were prepared by centrifuging 125 μl onto glass slides with a Cytospin 2 centrifuge (Shandon, Pittsburgh, Pa.). Cells were stained with LeukoStat stain (Fisher Diagnostics, Orangeburg, N.Y.). A total of 100 PMN on each of duplicate slides were examined to determine the percent phagocytosis (100 × number of PMN containing bacteria/total number of PMN) and phagocytosis index (PI) (number of intracellular bacteria in 100 PMN).
Antisera.
Serotype-specific polyclonal antisera, kindly provided by R. J. Salo (13, 26), were raised in rabbits by using purified exopolysaccharides (9). Phagocytosis experiments were performed with fresh rabbit complement sera (Sigma) and either anti-K2 (diluted 1:40 to minimize agglutination) or anti-O1 antiserum.
EIAs. (i) Whole-cell immunoassays.
To assess the surface exposure of the O-antigen LPS and the LPS core and to quantitate the amount of surface CPS produced in the absence or presence of BisBAL, the whole-cell immunoassay described by Tomás et al. (32) was used. The monoclonal antibodies (MAbs) used were 2A4 (against the O-antigen LPS) and 7D2 and 12B6 (against the LPS core), all of them kindly provided by E. Mandine (18). MAb 2A4 is an immunoglobulin G3 (IgG3) antibody, MAb 7D2 is an IgG2b antibody and MAb 12B6 is an IgG3 antibody. MAb 7D2 is from the outer-core LPS, while MAb 12B6 is from the inner-core LPS (unpublished data). All MAbs were purified after two precipitations in 45% ammonium sulfate and used at a concentration of 1/500. The number of bacteria used in enzyme immunoassays (EIAs) assays was approximately 105 CFU in the exponential growth phase.
(ii) Binding of C3b to whole cells.
The interaction between whole K. pneumoniae cells and complement component C3b was quantified by an EIA (19). Briefly, bacteria that were preincubated for 5 to 20 min with 90% nonimmune human serum (NHS) were washed twice with cold phosphate-buffered saline (PBS) by microcentrifugation, incubated for 45 min at 37°C in suspension with anti-C3b (Calbiochem) (1:100 dilution in PBS plus 1% bovine serum albumin), and washed again by microcentrifugation. Then the bacteria were incubated with protein A-alkaline phosphatase (1:100 dilution in PBS) and developed with 4-nitrophenol phosphate (1 mg/ml), and the absorbance at 405 nm (A405) was recorded.
(iii) Competitive EIAs.
Purified outer membrane proteins OmpK36 (1) and OmpK17 (3) were added separately to microtiter plates. Mixtures of rabbit polyclonal antiserum against OmpK36 and OmpK17 with K. pneumoniae whole cells were incubated for 1 h, samples were centrifuged, and the supernatant was titrated for the amount of unreacted antibody. EIA development was identical to that described above. Controls without bacteria cells represented the total titer of antibody added, and the maximum A405 was recorded. Measurement of OmpK36 or OmpK17 exposure on bacteria to antibodies was expressed as the reduction of absorption at A405.
Bacterial survival in fresh nonimmune serum.
The survival of exponential-phase bacteria in NHS was measured at 37°C as previously described (32). Control measurements were made with bacteria in PBS or heat-inactivated NHS (56°C for 30 min).
Inhibition of serum bactericidal activity.
The effect of treating serum with bacterial cells in bactericidal assays was determined as previously described (19). K. pneumoniae KT707 (serum sensitive) was used as indicator.
Measurement of the anticomplement activity of whole cells.
The anticomplement activity of whole cells was measured by the method of Shafer et al. (27). The positive control consisted of sensitized sheep erythrocytes plus NHS alone, and the negative control consisted of cells without added NHS.
RESULTS
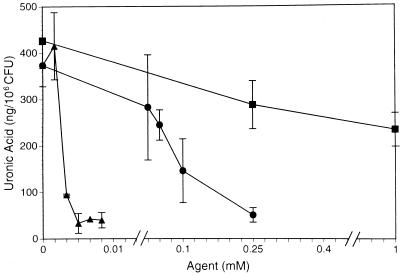
Bismuth has been shown to repress the expression of CPS in bacteria (10, 11). When it was chelated by the lipophilic thiol BAL, the CPS-inhibiting effect was far more impressive. BisBAL reduced K. pneumoniae O1:K2 CPS expression by 92% at a concentration of 5 μM or 1 ppm of Bi3+ (Fig. 1). Bismuth nitrate was orders of magnitude less effective, suppressing CPS by 85% at 250 μM. Higher concentrations of either bismuth agent proved inhibitory to cell growth. BAL alone also had an inhibitory effect on CPS expression, but this effect was not as pronounced at much higher concentrations (39% reduction at 1 mM). The solvent propylene glycol (PG) alone had no effect on CPS expression at the concentrations used (<1%). Inhibitory effects of PG on K. pneumoniae become evident at approximately 7% PG in broth medium (data not shown).
FIG. 1.
Effect of BisBAL and components on CPS expression. K. pneumoniae O1:K2 was cultured in defined medium for 18 h at 37°C with stirring at 200 rpm in the presence of different concentrations of bismuth nitrate (•), dimercaprol (■), or BisBAL (▴). CPS was extracted from whole cultures with a zwitterionic detergent in citric acid (9). Extract supernatants were ethanol precipitated and resolubilized in water. CPS was determined by measurement of its uronic acid content (2). Capsule expression is given as nanograms of uronic acid per 106 CFU. The data points represent the mean and standard deviation from at least three independent trials.
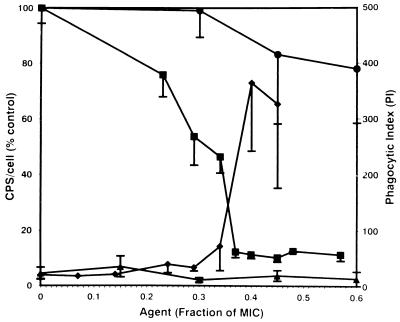
BisBAL is bacteriostatic or bactericidal to K. pneumoniae and other gram-negative bacteria at concentrations between 10 and 20 μM Bi3+ (15). At less than half the MIC, BisBAL markedly inhibited K. pneumoniae O1:K2 CPS expression (Fig. 2). At these subinhibitory concentrations, BisBAL inhibited 90% of CPS expression per viable cell. The biocide chlorhexidine showed only marginal CPS inhibition at near the MIC, causing less than a 20% reduction (Fig. 2). When biocide-treated bacteria were incubated with human PMN in the presence of complement and antiserum, the phagocytic index for BisBAL-treated cells increased from <10 to >360 (Fig. 2). In contrast, subinhibitory chlorhexidine had no effect on phagocytosis. Phagocytosis required complement and antibody as opsonins. Either polyclonal anti-K2 (diluted 1:40 to minimize agglutination) or anti-O1 antiserum was effective as opsonins.
FIG. 2.
Effect of BisBAL or chlorhexidine on CPS expression and phagocytic uptake. K. pneumoniae O1:K2 was cultured for 18 h in defined medium in the presence of subinhibitory concentrations of biocides. In phagocytic assays, bacteria were incubated at 37°C for 30 min with human PMN at a 10:1 ratio. The phagocytic index (PI; number of bacteria per 100 PMN) was determined from stained preparations by light microscopy (26). CPS was extracted with zwitterionic detergent and measured in terms of its content of uronic acid (9). Bacteria were enumerated by standard plating on agar medium. The amount of CPS per cell was expressed as a percent of the amount in the untreated control. Symbols: ■, BisBAL CPS; •, cycloheximide CPS; ⧫, BisBAL PI; ▴, cycloheximide PI. The data points represent the mean and standard deviation on at least three independent trials.
The reactivity of MAbs specific for the O1-antigen LPS or the LPS core also increased markedly with BisBAL treatment (Table 1). Whole cells of K. pneumoniae O1:K1, O1:K2, O1:K10, O1:K16, and O1:K66, but not O1:K− grown in the presence of sub-MIC BisBAL reacted to MAbs in a dose-dependent manner in EIAs. When anti-O1 MAb, for example, was used, the A405 increased from <0.1 to 0.79 for O1:K1, from 0.72 to 1.49 for O1:K2, from <0.1 to 0.83 for O1:K10, from <0.1 to 0.89 for O1:K16, and from 0.85 to 1.63 for O1:K66 but remained at 1.67 for O1:K− cells treated with 5 μg of BisBAL per ml. The results were similar when MAbs directed at the LPS core were used (Table 1). Absorption increased with increasing BisBAL concentrations from 1 to 5 μg/ml. Serotypes O1:K2 and O1:K66 showed partial reactivity with these MAbs prior to BisBAL treatment.
TABLE 1.
EIAs showing the reactivity of different MAbs against the O1-antigen LPS and the LPS core with whole cells of K. pneumoniae grown in BisBAL
| Strain | BisBAL concn (μg/ml) | Reactivity (A405) of MAb:
|
||
|---|---|---|---|---|
| 2A4 | 7D2 | 12B6 | ||
| DL1 (O1:K1) | 0 | <0.1 | <0.1 | <0.1 |
| 1 | 0.12 ± 0.03 | 0.11 ± 0.04 | 0.10 ± 0.03 | |
| 3 | 0.50 ± 0.08 | 0.33 ± 0.05 | 0.36 ± 0.09 | |
| 5 | 0.79 ± 0.12 | 0.43 ± 0.09 | 0.55 ± 0.10 | |
| 52145 (O1:K2) | 0 | 0.72 ± 0.08 | 0.52 ± 0.08 | 0.51 ± 0.07 |
| 1 | 0.98 ± 0.11 | 0.71 ± 0.10 | 0.69 ± 0.11 | |
| 3 | 1.13 ± 0.12 | 0.91 ± 0.09 | 0.81 ± 0.11 | |
| 5 | 1.49 ± 0.14 | 1.28 ± 0.13 | 1.33 ± 0.12 | |
| KT759 (O1:K10) | 0 | <0.1 | <0.1 | <0.1 |
| 1 | 0.19 ± 0.04 | 0.15 ± 0.04 | 0.15 ± 0.03 | |
| 3 | 0.59 ± 0.11 | 0.39 ± 0.09 | 0.41 ± 0.11 | |
| 5 | 0.83 ± 0.15 | 0.52 ± 0.13 | 0.58 ± 0.12 | |
| KT762 (O1:K16) | 0 | <0.1 | <0.1 | <0.1 |
| 1 | 0.22 ± 0.05 | 0.17 ± 0.04 | 0.19 ± 0.03 | |
| 3 | 0.62 ± 0.12 | 0.47 ± 0.10 | 0.52 ± 0.13 | |
| 5 | 0.89 ± 0.19 | 0.56 ± 0.15 | 0.60 ± 0.17 | |
| C3 (O1:K66) | 0 | 0.85 ± 0.12 | 0.65 ± 0.13 | 0.61 ± 0.08 |
| 1 | 1.23 ± 0.19 | 0.98 ± 0.23 | 0.92 ± 0.16 | |
| 3 | 1.45 ± 0.22 | 1.16 ± 0.19 | 1.11 ± 0.20 | |
| 5 | 1.63 ± 0.27 | 1.28 ± 0.17 | 1.25 ± 0.18 | |
| KT791 (O1:K−) | 0 | 1.67 ± 0.17 | 1.56 ± 0.15 | 1.57 ± 0.11 |
| 1 | 1.65 ± 0.16 | 1.58 ± 0.14 | 1.63 ± 0.13 | |
| 3 | 1.66 ± 0.12 | 1.57 ± 0.17 | 1.65 ± 0.14 | |
| 5 | 1.67 ± 0.14 | 1.55 ± 0.12 | 1.61 ± 0.15 | |
Data are means ± standard deviations derived from three independent experiments.
BisBAL also enhanced the interaction of complement component C3b with O1:K1, O1:K2, O1:K10, O1:K16, and O1:K66, but not with O1:K− stains (Table 2). The relative concentration of cell-bound C3b in EIAs increased from <0.1 to 0.78 for O1:K1, from 0.62 to 1.58 for O1:K2, from <0.1 to 0.83 for O1:K10, from <0.1 to 0.79 for O1:K16, and from 0.63 to 1.64 for O1:K66 but remained 1.84 for O1:K− cells treated with 5 μg of BisBAL per ml. Absorption increased with increasing BisBAL concentrations from 1 to 5 μg/ml. Again, only serotypes O1:K2 and O1:K66 showed partial interaction with C3b prior to BisBAL treatment.
TABLE 2.
Interaction of complement component C3b with K. pneumoniae whole cells grown in different concentrations of BisBAL
| Strain | BisBAL concn (μg/ml) | Relative concn (A405) of C3b (mean ± SD)a |
|---|---|---|
| DL1 (O1:K1) | 0 | <0.1 |
| 1 | 0.12 ± 0.03 | |
| 3 | 0.49 ± 0.10 | |
| 5 | 0.78 ± 0.13 | |
| 52145 (O1:K2) | 0 | 0.62 ± 0.09 |
| 1 | 0.81 ± 0.10 | |
| 3 | 1.17 ± 0.12 | |
| 5 | 1.58 ± 0.15 | |
| KT759 (O1:K10) | 0 | <0.1 |
| 1 | 0.15 ± 0.04 | |
| 3 | 0.66 ± 0.09 | |
| 5 | 0.83 ± 0.12 | |
| KT762 (O1:K16) | 0 | <0.1 |
| 1 | 0.13 ± 0.05 | |
| 3 | 0.59 ± 0.11 | |
| 5 | 0.79 ± 0.18 | |
| C3 (O1:K66) | 0 | 0.63 ± 0.08 |
| 1 | 0.99 ± 0.16 | |
| 3 | 1.35 ± 0.22 | |
| 5 | 1.64 ± 0.21 | |
| KT791 (O1:K−) | 0 | 1.84 ± 0.12 |
| 1 | 1.83 ± 0.14 | |
| 3 | 1.85 ± 0.16 | |
| 5 | 1.84 ± 0.14 |
Results (in arbitary units) from EIAs performed in triplicate at least twice. For control cells incubated in the absence of specific antibodies, the C3b concentration was always <0.1 A405 unit.
The effect of BisBAL on inhibition of the bactericidal activity of NHS by whole K. pneumoniae cells is summarized in Table 3. Untreated NHS was bactericidal (<0.1% survival) to strain KT707 (O−:K66). The bactericidal activity of NHS was not neutralized by absorption with O1:K1, O1:K10, or O1:K16 bacteria unless the cells were first treated with BisBAL. For serotypes O1:K1, O1:K10, or O1:K16, treatment with 1 μg of BisBAL per ml had only a minor effect on neutralization of bactericidal activity whereas 3 μg/ml had a marked effect. At 5 μg/ml, BisBAL was even more effective. The bactericidal activity of NHS was neutralized to a significant extent with fully encapsulated O1:K2 or O1:K66 cells, although there was a moderate increase in the survival of KT707 bacteria in NHS absorbed with BisBAL-treated cells. O1:K1, O1:K10, and O1:K16 strains exhibited anticomplement activity, as shown by the method of Shafer et al. (27), when grown in the presence of BisBAL but lacked activity without BisBAL treatment (data not shown).
TABLE 3.
Inhibition of bactericidal activity of NHS against serum-sensitive strain KT707 by whole cells of K. pneumoniae grown in BisBAL
| Strain used for NHS treatment | BisBAL concn (μg/ml) | % Survival of KT707 after incubation with treated NHSa for:
|
||
|---|---|---|---|---|
| 1 h | 2 h | 3 h | ||
| DL1 (O1:K1) | 0 | 0.6 | <0.1 | <0.1 |
| 1 | 7 | 5 | 2 | |
| 3 | 69 | 80 | 90 | |
| 5 | 85 | 97 | 105 | |
| 52145 (O1:K2) | 0 | 95 | 108 | 125 |
| 1 | 96 | 111 | 139 | |
| 3 | 100 | 121 | 178 | |
| 5 | 108 | 139 | 204 | |
| KT759 (O1:K10) | 0 | 0.5 | <0.1 | <0.1 |
| 1 | 8 | 6 | 5 | |
| 3 | 77 | 85 | 96 | |
| 5 | 90 | 101 | 111 | |
| KT762 (O1:K16) | 0 | 0.7 | <0.1 | <0.1 |
| 1 | 10 | 8 | 5 | |
| 3 | 79 | 88 | 100 | |
| 5 | 87 | 98 | 107 | |
| C3 (O1:K66) | 0 | 97 | 115 | 138 |
| 1 | 102 | 119 | 145 | |
| 3 | 104 | 123 | 150 | |
| 5 | 105 | 129 | 155 | |
| KT791 (O1:K−) | 0 | 100 | 129 | 156 |
| 1 | 118 | 142 | 174 | |
| 3 | 151 | 197 | 231 | |
| 5 | 182 | 213 | 279 | |
The bacteria were incubated at 37°C.
Outer membrane proteins were also more accessible on the surface of K. pneumoniae after BisBAL treatment. With the purified outer membrane proteins OmpK36 and OmpK17 in a competitive EIA, better penetration of specific antibodies resulted when the strains were grown in the presence of BisBAL then in its absence (Table 4). The change is more apparent for strains O1:K2 and O1:K66 than for strains O1:K1, O1:K10, and O1:K16. The outer membrane proteins of the last three strains were inaccessible to specific antibodies in the absence of BisBAL. However, they showed some accessibility to specific antibodies when grown in the presence of BisBAL (3 to 5 μg/ml) (Table 4).
TABLE 4.
Competitive EIAs with purified antigens and specific antiserum against these outer membrane proteins in competition with different K. pneumoniae whole cells grown in the presence or absence of BisBAL
| Strain | BisBAL concn (μg/ml) | Reactivity (A405) of antiserum against:
|
|
|---|---|---|---|
| OmpK36 | OmpK17 | ||
| DL1 (O1:K1) | 0 | 1.82 ± 0.11 | 1.68 ± 0.09 |
| 1 | 1.74 ± 0.14 | 1.60 ± 0.12 | |
| 3 | 1.42 ± 0.15 | 1.49 ± 0.11 | |
| 5 | 1.13 ± 0.12 | 1.05 ± 0.09 | |
| 52145 (O1:K2) | 0 | 1.68 ± 0.10 | 1.58 ± 0.11 |
| 1 | 1.01 ± 0.19 | 1.19 ± 0.17 | |
| 3 | 0.83 ± 0.15 | 0.77 ± 0.13 | |
| 5 | 0.64 ± 0.12 | 0.45 ± 0.13 | |
| KT759 (O1:K10) | 0 | 1.81 ± 0.13 | 1.69 ± 0.08 |
| 1 | 1.72 ± 0.16 | 1.62 ± 0.10 | |
| 3 | 1.46 ± 0.12 | 1.46 ± 0.11 | |
| 5 | 1.18 ± 0.11 | 1.08 ± 0.07 | |
| KT762 (O1:K16) | 0 | 1.84 ± 0.10 | 1.64 ± 0.13 |
| 1 | 1.71 ± 0.11 | 1.57 ± 0.12 | |
| 3 | 1.40 ± 0.13 | 1.38 ± 0.13 | |
| 5 | 1.07 ± 0.14 | 1.01 ± 0.11 | |
| C3 (O1:K66) | 0 | 1.62 ± 0.21 | 1.52 ± 0.19 |
| 1 | 0.91 ± 0.18 | 1.13 ± 0.20 | |
| 3 | 0.72 ± 0.16 | 0.68 ± 0.14 | |
| 5 | 0.53 ± 0.13 | 0.41 ± 0.11 | |
| None (control) | 1.83 ± 0.12 | 1.70 ± 0.10 | |
Data are means ± standard deviations derived from three independent experiments.
DISCUSSION
Bacterial capsules have the potential to interfere with complement- or antibody-mediated host defenses (17, 24, 28, 31). Antimicrobial agents that interfere with capsule and slime expression may have value in prevention and treatment of numerous infectious disease processes. Such agents may also interfere with biofilm formation, which is problematic in medicine, dentistry, and numerous industrial processes. Bismuth thiol agents have the potential to be useful in this capacity. Subinhibitory BisBAL (1 to 2 ppm of Bi3+) suppressed biofilm formation on steel by a consortium of biofilm bacteria (Pseudomonas, Bacillus, and Acidovorax spp.) for at least 19 days, while 5 ppm of chlorine was required for the same purpose (33a).
In the present study, BisBAL proved substantially more effective than bismuth nitrate in inhibiting K. pneumoniae CPS expression. Subinhibitory BisBAL promoted opsonophagocytosis, enhanced the reactivity of MAbs specific for O-antigen LPS or LPS core epitopes, enhanced C3b binding to encapsulated cells, and enhanced the penetration of specific antibodies against outer membrane proteins, including a major porin (OmpK36) and a related OmpX protein (OmpK17). BisBAL treatment of encapsulated K. pneumoniae also enhanced bacterial inhibition of the bactericidal activity of NHS. Since the O1 antigen and outer membrane proteins in O1:K2 and O1:K66 cells are already partially expressed, these cells showed disparate results compared with O1:K1, O1:K10, and O1:K16 cells, where the subcapsular antigens are completely masked (33). Even fully encapsulated O1:K2 and O1:K66 cells react partially with LPS-directed MAbs and with complement component C3b. They also can inhibit the bactericidal activity of NHS without BisBAL treatment. Clearly, strains O1:K1, O1:K10, and O1:K16 are not opsonized (i.e., they have no bound C3b) in the absence of BisBAL. Their LPS moiety is completely masked by CPS. In the presence of BisBAL, they activate complement, bind C3b, and are opsonized for phagocytosis, since the CPS is inhibited.
Some variability was encountered when determining CPS inhibition by BisBAL. Certain culture medium components has a moderating effect on these analyses. Limiting the carbon source (glucose) or the level of sulfate or iron in the defined medium reduced the threshold of anticapsular activity against bacteria by 20-fold (from 2 ppm to 100 ppb [data not shown]). Thus, bacteria starved for certain nutrients, as is common in environments outside of the laboratory, may be more sensitive to the effects of bismuth thiols. BisBAL may prove more effective in the field than can be demonstrated by standard susceptibility measurements.
Another disparity arose from comparing data obtained with liquid and powdered BisBAL. Liquid preparations worked well between 3 and 5 μM Bi3+ (0.6 to 1 ppm), whereas the powder seemed to be most active at 2 to 3 ppm Bi3+, considering that the powder is approximately two-thirds Bi3+ by weight. The difference may be accounted for by the reduced solubility of the powder form. Also, formulations in propylene glycol were nearly twice as effective as those in water, again probably due to solubility.
It is significant that CPS inhibition is occurring at subinhibitory concentrations. Most antibiotics indiscriminately kill bacteria, including members of the normal flora, thus contributing to iatrogenic sequelae (e.g., vaginal infections and diarrhea). Low levels of bismuth thiols could conceivably prevent colonization by bacteria without substantially inhibiting their growth. This alone may have quite an impact, since colonization is considered the first step in the infectious process. Also, the importance of the normal microflora comes to mind, since its indiscriminate destruction by broad-spectrum antibiotics opens the way for opportunistic infections. Countering this problem with more selective and rational approaches would avoid disrupting normal microbial communities, which are one of the first lines of defense against infection. Bismuth thiols are novel among biocides with respect to CPS inhibition. Most biocides rely on killing bacteria or inhibiting bacterial growth to be effective. BisBAL, in contrast, may be quite effective at subinhibitory levels. Bismuth thiols may prove ideal as a nondisruptive approach by containing pathogens and as a broad-spectrum device to prevent colonization by pathogenic bacteria.
Although phagocytosis of BisBAL-treated bacteria was enhanced significantly, phagocytic uptake still required the presence of a complement source and an antibody directed at the bacterial cell surface. Interaction of a variety of specific antibodies with K. pneumoniae was enhanced by subinhibitory BisBAL concentrations. Immunity to K. pneumoniae infection is presumed to require opsonic K-specific antibodies. However, a broader choice of immunogen for vaccination can be considered with the advent of bismuth thiols, since other cell surface structures become exposed when CPS is inhibited (34). Core structures in LPS may also be potential targets for immunization. Core structures are conserved across many gram-negative pathogens (23). Inhibiting CPS expression may expose complement receptors and other bacterial surface structures that exhibit more antigenicity and less antigenic variability than the K antigen. Since the vast majority of K. pneumoniae isolates are of the O1 LPS serogroup (20), O1 LPS may be the best candidate for a “subcapsular” vaccine. Other potential surface antigens are outer membrane proteins. Once vaccination has been performed, the immune response could be activated by administering bismuth thiols orally. A single oral dose of BisBAL (10 mg/kg) in mice was shown to increase the 50% lethal dose for intraperitoneal challenge with O1:K2 K. pneumoniae from 1 to 480 CFU (30a). The implications for augmenting bacterial vaccines against encapsulated pathogens are enormous. In vivo analyses are under investigation.
ACKNOWLEDGMENTS
We acknowledge grant PM97-0932 from DGICYT (Spain).
We acknowledge E. Mandine for providing the MAbs, R. J. Salo for providing rabbit antisera, and Maite Polo and Peter Wu for technical assistance.
REFERENCES
- 1.Alberti S, Rodriguez-Quinones F, Schirmer T, Rummel G, Tomás J M, Rosenbush J P, Benedi V J. A porin from Klebsiella pneumoniae: sequence homology, three-dimensional model, and complement binding. Infect Immun. 1995;63:903–910. doi: 10.1128/iai.63.3.903-910.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blumenkrantz N, Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal Biochem. 1973;54:484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- 3.Climent N, Ferrer S, Rubires X, Merino S, Tomás J M, Regué M. Molecular characterization of a 17-kDa outer membrane protein from Klebsiella pneumoniae. Res Microbiol. 1997;148:133–143. doi: 10.1016/S0923-2508(97)87644-9. [DOI] [PubMed] [Google Scholar]
- 4.Cross A S, Sadoff J C, Furer E, Cryz S J., Jr Escherichia coli and Klebsiella vaccines and immunotherapy. Infect Dis Clin North Am. 1990;4:271–282. [PubMed] [Google Scholar]
- 5.Cryz S J, Jr, Fürer E, Germanier R. Protection against fatal Klebsiella pneumoniae burn wound sepsis by passive transfer of anticapsular polysaccharide. Infect Immun. 1984;45:139–142. doi: 10.1128/iai.45.1.139-142.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cryz S J., Jr Klebsiella polysaccharide vaccines. Adv Biotechnol Processes. 1990;13:87–103. [PubMed] [Google Scholar]
- 7.Domenico P, Straus D C. Extracellular polysaccharide production by Klebsiella pneumoniae and its relation to virulence. Can J Microbiol. 1985;32:472–478. doi: 10.1139/m85-088. [DOI] [PubMed] [Google Scholar]
- 8.Domenico P, Schwartz S, Cunha B A. Reduction of capsular polysaccharide production in Klebsiella pneumoniae by sodium salicylate. Infect Immun. 1989;57:3778–3782. doi: 10.1128/iai.57.12.3778-3782.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Domenico P, Diedrich D L, Cunha B A. Quantitative extraction and purification of exopolysaccharides from Klebsiella pneumoniae. J Microbiol Methods. 1989;9:211–219. [Google Scholar]
- 10.Domenico P, Landolphi D R, Cunha B A. Reduction of capsular polysaccharide and potentiation of aminoglycoside inhibition in Gram-negative bacteria by bismuth subsalicylate. J Antimicrob Chemother. 1991;28:801–810. doi: 10.1093/jac/28.6.801. [DOI] [PubMed] [Google Scholar]
- 11.Domenico P, Marx J, Schoch P E, Cunha B A. Plasmid DNA isolation from mucoid gram-negative bacteria. J Clin Microbiol. 1992;30:2859–2863. doi: 10.1128/jcm.30.11.2859-2863.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Domenico P, Salo R J, Straus D C, Hutson J C, Cunha B A. Salicylate or bismuth salts enhance opsonophagocytosis of Klebsiella pneumoniae. Infection. 1992;20:66–72. doi: 10.1007/BF01711065. [DOI] [PubMed] [Google Scholar]
- 13.Domenico P, Salo R, Cross A S, Cunha B A. Polysaccharide capsule-mediated resistance to opsonophagocytosis in Klebsiella pneumoniae. Infect Immun. 1994;62:4495–4499. doi: 10.1128/iai.62.10.4495-4499.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Domenico P, Tomas J M, Merino S, Rubires X, Cunha B A. Bismuth-dimercaprol exposes surface components of Klebsiella pneumoniae camoflauged by the polysaccharide capsule. Ann N Y Acad Sci. 1996;797:269–270. doi: 10.1111/j.1749-6632.1996.tb52974.x. [DOI] [PubMed] [Google Scholar]
- 15.Domenico P, Salo R J, Novick S G, Schoch P E, Van Horn K, Cunha B A. Enhancement of bismuth antibacterial activity with lipophilic thiol chelators. Antimicrob Agents Chemother. 1997;41:1697–1703. doi: 10.1128/aac.41.8.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duguid J P, Wilkinson J F. The influence of culture conditions on polysaccharide production by Aerobacter aerogenes. J Gen Microbiol. 1953;9:174–189. doi: 10.1099/00221287-9-2-174. [DOI] [PubMed] [Google Scholar]
- 17.Howard C J, Glynn A A. The virulence for mice of strains of Escherichia coli related to the effects of K antigens on their resistance to phagocytosis and killing by complement. Immunology. 1971;20:767–777. [PMC free article] [PubMed] [Google Scholar]
- 18.Mandine E, Salles M F, Zalisz R, Guenounou R, Smets P. Murine monoclonal antibodies to Klebsiella pneumoniae protect against lethal endotoxemia and experimental infection with capsulated K. pneumoniae. Infect Immun. 1990;58:2828–2833. doi: 10.1128/iai.58.9.2828-2833.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Merino S, Camprubi S, Alberti S, Benedi V J, Tomás J M. Mechanisms of Klebsiella pneumoniae resistance to complement-mediated killing. Infect Immun. 1992;60:2529–2535. doi: 10.1128/iai.60.6.2529-2535.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mizuta K, Ohta M, Mori M, Hasegawa T, Nakashima I, Kato N. Virulence for mice of Klebsiella strains belonging to the O1 group: relationship to their capsular (K) types. Infect Immun. 1983;40:56–61. doi: 10.1128/iai.40.1.56-61.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moxon E R, Kroll J S. The role of bacterial polysaccharide capsules as virulence factors. Curr Top Microbiol Immunol. 1990;150:65–82. doi: 10.1007/978-3-642-74694-9_4. [DOI] [PubMed] [Google Scholar]
- 22.Pollack M. Significance of circulating capsular antigen in Klebsiella infections. Infect Immun. 1976;13:1543–1548. doi: 10.1128/iai.13.6.1543-1548.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pollack M, Chia J K S, Koles N L, et al. Specificity and cross-reactivity of monoclonal antibodies reactive with the core and lipid A regions of bacterial lipopolysaccharide. J Infect Dis. 1989;159:168–188. doi: 10.1093/infdis/159.2.168. [DOI] [PubMed] [Google Scholar]
- 24.Roberts I S, Saunders F K, Boulnois G J. Bacterial capsules and interactions with complement and phagocytes. Biochem Soc Trans. 1989;17:462–464. doi: 10.1042/bst0170462. [DOI] [PubMed] [Google Scholar]
- 25.Roberts I S. The biochemistry and genetics of capsular polysaccharide production in bacteria. Annu Rev Microbiol. 1996;50:285–315. doi: 10.1146/annurev.micro.50.1.285. [DOI] [PubMed] [Google Scholar]
- 26.Salo R J, Domenico P, Straus D C, Tomàs J M, Cunha B A. Salicylate-enhanced exposure of Klebsiella pneumoniae subcapsular components. Infection. 1995;23:371–377. doi: 10.1007/BF01713568. [DOI] [PubMed] [Google Scholar]
- 27.Shafer W M, Joiner K, Guyman L F, Cohen M S, Sparling P F. Serum sensitivity of Neisseria gonorrhoeae: the role of lipopolysaccharide. J Infect Dis. 1984;149:175–183. doi: 10.1093/infdis/149.2.175. [DOI] [PubMed] [Google Scholar]
- 28.Simoons-Smit A M, Verweij-van Vught J J, Maclaren D M. The role of K antigens as virulence factors in Klebsiella. J Med Microbiol. 1986;21:133–137. doi: 10.1099/00222615-21-2-133. [DOI] [PubMed] [Google Scholar]
- 29.Sox T E, Olsen C A. Binding and killing of bacteria by bismuth subsalicylate. Antimicrob Agents Chemother. 1989;33:2075–2082. doi: 10.1128/aac.33.12.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Straus D C. Production of an extracellular toxic complex by various strains of Klebsiella pneumoniae. Infect Immun. 1987;55:44–48. doi: 10.1128/iai.55.1.44-48.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30a.Straus, D. C. Personal communication.
- 31.Tomás J M, Benedi V J, Ciurana B, Jofre J. Role of capsule and O antigen in resistance of Klebsiella pneumoniae to serum bactericidal activity. Infect Immun. 1986;54:85–89. doi: 10.1128/iai.54.1.85-89.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tomás J M, Camprubi S, Williams P. Surface exposure of the O-antigen in Klebsiella pneumoniae O1:K1 serotype strains. Microb Pathog. 1988;5:141–147. doi: 10.1016/0882-4010(88)90016-2. [DOI] [PubMed] [Google Scholar]
- 33.Tomas J M, Camprubi S, Merino S, Davey M R, Williams P. Surface exposure of O1 serotype lipopolysaccharide in Klebsiella pneumoniae strains expressing different K antigens. Infect Immun. 1991;59:2006–2011. doi: 10.1128/iai.59.6.2006-2011.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33a.White, D. C. Personal communication.
- 34.Williams P, Lambert P A, Brown M R W, Jones R J. The role of the O and K antigens in determining the resistance of Klebsiella aerogenes to serum killing and phagocytosis. J Gen Microbiol. 1983;129:2181–2191. doi: 10.1099/00221287-129-7-2181. [DOI] [PubMed] [Google Scholar]