Abstract
In contrast to immunocompetent controls, interleukin-10 (IL-10) knockout (KO) mice eliminated an experimental intravenous inoculation with Candida albicans from their kidneys. Improved clearance of C. albicans from the kidneys of IL-10 KO mice was evident at 24 h after intravenous challenge with the fungus. Conversely, mice with a deletion of the IL-4 cytokine gene were more susceptible to systemic candidiasis than were immunocompetent controls. The hyperresistance of IL-10 KO mice to acute systemic candidiasis did not seem to correlate with nitric oxide-mediated immunity, but rather, it appeared to be associated with more efficient effector function of innate cells, possibly neutrophils. In support of the latter hypothesis, we observed that neutrophils from IL-10 KO mice were more efficient at killing C. albicans blastoconidia and hyphae than were neutrophils from immunocompetent control mice. Neither IL-10 KO nor IL-4 KO mice that were monoassociated with C. albicans for 4 weeks showed any histologic evidence of systemic candidiasis of endogenous origin. In contrast to systemic candidiasis, we observed no significant (P < 0.05) differences in susceptibility among IL-10 KO, IL-4 KO, and wild-type (immunocompetent) mice to orogastric candidiasis. Our results suggest that IL-10 exerts a negative effect on the early, innate response to acute systemic candidiasis; however, in comparison to immunocompetent control (wild-type) mice, neither IL-10 nor IL-4 deficiency enhanced susceptibility to orogastric candidiasis.
Immunodeficient individuals frequently suffer from infections caused by the opportunistic fungus Candida albicans (6, 10, 18). Of the many immunodeficient and immunosuppressive factors that predispose humans to candidiasis, defects in T cells have been well recognized over time (1). The high incidence of oral thrush in human immunodeficiency virus-infected individuals highlights the importance of CD4-positive T cells in resistance to oral candidiasis (15). More recently, it has become apparent that CD4-positive T cells are not only important for resistance to mucosal candidiasis but are also involved in immunity to vaginal and chronic mucocutaneous C. albicans infections and, under certain circumstances, to systemic candidiasis, as well (3, 8, 9, 16, 26). According to the cytokine profile, the T-cell responses to C. albicans antigens can be categorized as T-helper 1 (Th1; predominance of gamma interferon and interleukin-2 [IL-2]) or Th2 (predominance of IL-4 and IL-10). Whereas the former phenotype has been associated with resistance, the latter has been, in general, associated with susceptibility to mucocutaneous, systemic, and mucosal candidiasis (8, 14, 16, 23).
Much of the work on the paradigm of the Th response to C. albicans has been performed with hosts with intact antibody- and/or cell-mediated immune responses. Macrophages are central to the Th response paradigm. By virtue of their IL-12- and IL-10-producing capacity, macrophages not only promote the development of Th1 and Th2 responses, but some of their anti-Candida effector functions are also targets for the regulatory effects of gamma interferon, IL-4, and IL-10 (25–28). Recently, it has been suggested that neutrophils, due to their capacity to synthesize IL-12 and IL-10, can also modulate the development of a subsequent acquired immune response to C. albicans (22). It is not known, however, whether cytokines that define the different Th subsets have any effect on the acute-phase response to C. albicans and on neutrophils, the leukocytes which constitute the main cellular component of innate immunity to this dimorphic fungus (11).
In this study, we assessed the effect of Th2 cytokines on murine susceptibility to acute systemic candidiasis (intravenous [i.v.]) and to mucosal and systemic candidiasis of endogenous origin by colonizing the alimentary tracts of germfree IL-4 and IL-10 knockout (KO) mice with C. albicans.
MATERIALS AND METHODS
Microorganisms.
C. albicans B311 (type A) was maintained by monthly transfer on Sabouraud dextrose agar (SDA; Difco, Detroit, Mich.) slants. Before investigation, the yeast cells were cultured for 24 h at 37°C on SDA, washed from the slants, and adjusted to the desired concentration in phosphate-buffered saline (PBS) or RPMI+ medium (RPMI supplemented with 10% heat-inactivated fetal calf serum, 200 mM l-glutamine, Penstrep [100 U/ml, 10 mg/ml], 15 mM HEPES buffer, and 1% [vol/vol] minimum essential medium; all of these reagents were purchased from Sigma Chemical Co., St. Louis, Mo.).
Mice.
Germfree male and female C57BL/6xL129 IL-10 KO mice, C57BL/6xL129 IL-4 KO mice, and C57BL/6xL129 immunocompetent controls between 6 and 9 weeks of age were used in these studies. The IL-4 KO mice were purchased from Jackson Laboratories (Bar Harbor, Maine), and the IL-10 KO mice were supplied by Donna Rennick (DNAX Research Institute for Molecular Biology, Palo Alto, Calif.). Pups in the germfree state were derived by cesarean section and raised to adulthood on germfree BALB/c mothers. A breeding colony of IL-4 and IL-10 KO germfree mice was maintained at the University of Wisconsin Gnotobiotic Research Laboratory, Madison. The gnotobiotic state of the mice was assessed as previously described (13).
Genotype determination.
The genotypes of the mice used in this study were determined by using a PCR assay on genomic DNA. The DNA samples were isolated from 0.5-cm sections of tails by using the Qiamp DNA isolation system in accordance with the manufacturer’s instructions (Qiagen, Inc., Santa Clarita, Calif.). The genotyping PCR procedures and primer sequences were provided by Donna Rennick at the DNAX Research Institute for Molecular Biology. For genotyping of IL-10 KO mice, a 5-μl aliquot of genomic DNA was added to 45 μl of a PCR mixture consisting of 33.5 μl of H2O; 5 μl of 5× Taq polymerase reaction buffer (Fisher Scientific, Itasca, Ill.); 5 μl of MgCl2 (1.5 mM); 2 μl of a mixture of 5 mM dGTP, dTTP, dATP, and dCTP; 0.4 μl of oligonucleotide P1.4; 0.2 μl of oligonucleotide P2.2; and 0.2 μl of oligonucleotide Pneo (each of the three oligonucleotide primers were used at 50 optical density U/ml); and 0.25 μl of Taq DNA polymerase (5 U/μl). The oligonucleotide primers were P1.4 (5′ GCCTTCAGTATAAAAGGGGGACC 3′), P2.2 (5′ GTGGGTGCAGTTATTGTCTTCCCG 3′), and Pneo (5′ CCTGCGTGCAATCCATCTTG 3′). The PCR mixtures were preincubated at 92°C for 2 min, and then 30 cycles were run at 94°C for 30 s, 55°C for 30 s, and 72°C for 1 min. The PCR was followed by incubation at 72°C for 10 min.
The genotyping procedure for IL-4 KO mice used similar parameters, except that the four oligonucleotide primers were IMR077 (5′ GCACAGAGCTATTGATTGGGTC 3′), IMRO78 (5′ GCTGTGAGGACGTTTGGC 3′), IMRO79 (5′ TCAGGACATAGCGTTGGC 3′), and Neo-75 (5′ TCGAATTCGCCAATGACACGCT 3′).
The PCR products were separated by electrophoresis on a 2% agarose gel and stained with ethidium bromide. The wild-type IL-10 allele yielded a DNA band with an apparent molecular size of 200 bp, and the neomycin insert band in DNA from IL-10 KO mice had an apparent molecular size of 358 bp. The wild-type IL-4 band was 444 bp, and the neomycin insert band in DNA from IL-4 KO mice was 300 bp. Molecular weights were compared with a HaeIII digest of ΦX-174 DNA (Promega, Madison, Wis.).
Resistance to systemic infection.
Specific-pathogen-free and C. albicans-monoassociated IL-4 and IL-10 KO mice and immunocompetent controls received i.v. injections of 104 CFU of C. albicans as described elsewhere (29). At 1, 3, 7, and 14 days after challenge, three mice were sacrificed per group and the numbers of C. albicans CFU present in the spleen, liver, kidney, and brain were quantified after serial dilutions were plated on SDA plates. Data from two experiments are reported as CFU per gram (dry weight) of tissue. To determine the contribution of nitric oxide (NO) to resistance to systemic candidiasis, some mice were inoculated intraperitoneally with the selective inducible NO inhibitor aminoguanidine (Sigma) at 250 mg/kg/day as previously described (28). Aminoguanidine (250 μM) effectively inhibited the nitrite-producing capacity of IFN-γ–lipopolysaccharide-activated murine macrophages (data not shown).
Neutrophil isolation.
Isolation of neutrophils was performed as described by Jones-Carson et al. (12). Briefly, peritoneal exudate cells were harvested by lavage of the peritoneal cavity with sterile, prewarmed Dulbecco’s PBS supplemented with 5% bovine serum albumin (Sigma) 5 to 7 h after the mice were injected with 107 heat-killed C. albicans blastoconidia. The peritoneal exudate cells were centrifuged at 200× g for 10 min at room temperature, resuspended in prewarmed RPMI+ medium, and counted in a hemacytometer. The cell viability was >95% as determined by trypan blue exclusion. The percentage of neutrophils was determined by Wright staining of the cytocentrifuged preparations.
Candidacidal activity of neutrophils.
Killing of C. albicans blastoconidia and hyphae was assessed as previously described (12). Briefly, neutrophils were incubated with live C. albicans yeast cells at an effector-to-target cell ratio of 10:1 at 37°C in a 5% CO2 incubator. At 2 h of incubation, the neutrophils were lysed with 0.1% Triton X in sterile, double-distilled water, and the content of each well was serially diluted and plated onto SDA. The number of CFU was scored after overnight culture at 37°C. For the neutrophil killing of germinated C. albicans, the yeast cells (103 CFU/well) were germinated on gelatin-coated plates at 37°C for 2 h in RPMI+ medium. Neutrophils (effector-to-target cell ratio of 750:1) were added to the wells when >98% of the C. albicans had germinated, and the culture was incubated for an additional 2 h at 37°C in a 5% CO2 incubator. Germ tubes were removed by adding 10 μl of tissue culture grade trypsin-EDTA (10× solution; Sigma) and incubating the mixture for another 30 min at 37°C to allow for enzymatic digestion of the gelatin. Triton X (0.5% final concentration) was then added to the wells, and the plates were incubated for another 5 min on ice. The C. albicans hyphae were removed from the wells, serially diluted, and plated on SDA as described for the blastoconidia to count viable cells. The candidacidal activity was calculated as [(CFU in control well CFU in test well)/CFU in control well] × 100.
Histopathology.
The IL-10 KO, IL-4 KO, and immunocompetent control C57BL/6xL129 mice were removed from the gnotobiotic isolator at 4 weeks after oral inoculation with C. albicans (13). The tongue, palate, esophagus, stomach, large and small intestines, spleen, vagina, mesenteric lymph nodes, liver, thymus, lungs, and sternum were aseptically collected and fixed in 10% formaldehyde buffered in PBS. The tissues were processed in graded (100, 95, 80, and 70%) alcohol and xylene solutions and embedded in paraffin. Sections of tissues were cut (5 μm), placed on slides, and stained with hematoxylin and eosin and Grocott’s methenamine silver stain for fungi. Tissue sections were scored (blinded, by a pathologist) as follows: 0, no yeast or hyphae observed in a high-power field (HPF; (×40) of tissues; 1, partial yeast cells and hyphae (1 to 10 organisms/HPF (×40); 2, numerous yeast cells and hyphae (10 to 50 organisms/HPF); 3, abundant but not confluent yeast cells and hyphae (50 to 100 organisms/HPF); 4, confluent hyphal invasion of the tissues (>100 organisms/HPF); 5, confluent infection of the tissues with yeast cells and hyphae and penetration of hyphae into viable epithelial tissues.
Reactive oxygen intermediate production.
Reactive oxygen intermediates synthesized by C. albicans-elicited neutrophils was estimated by recording the oxidation of luminol (5-amino-2,3-dihydro-1,4-phthalazinedione; Sigma) with a Los Alamos Diagnostics model 633 chemiluminometer as described previously (27). The neutrophils were incubated with live C. albicans hyphae at an effector-to-target cell ratio of 1:1 in a cuvette containing 100 μM luminol, and chemiluminescence was recorded in a computer interface. The number of neutrophils used for these experiments was estimated from Wright-stained peritoneal exudate cells as described above.
Statistical analysis.
Student’s t test was performed with SigmaStat software (SPSS Corporation, Chicago, Ill.) to determine statistically significant differences between groups. P < 0.05 was considered significant. Fisher’s (two-tailed) exact test was used to assess the significance of the histopathology data.
RESULTS
Resistance of IL-10 KO mice to acute systemic candidiasis.
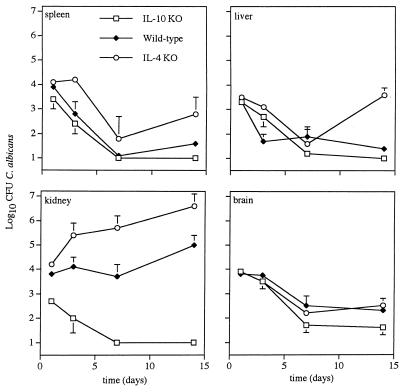
IL-10 KO mice and immunocompetent controls were similar in resistance to splenic, hepatic, and cerebral candidiasis; however, the IL-10 KO (−/−) mice showed enhanced resistance (P < 0.05) to renal candidiasis compared to their immunocompetent littermates (+/− and +/+) (Fig. 1). The resistance of IL-10 KO mice to renal candidiasis was evident on day 1 after i.v. inoculation with C. albicans when, compared to control mice, a 1-log decrease in the number of C. albicans CFU recovered from the kidneys was observed. By day 7, the IL-10 KO mice had sterile (no C. albicans) kidneys, whereas the fungal burden in their immunocompetent controls was around 4 logs/g (dry weight) of renal tissue.
FIG. 1.
Disparate resistance of IL-4 and IL-10 KO mice to acute systemic candidiasis. Immunocompetent control, IL-4 KO, and IL-10 KO mice were given an i.v. challenge with C. albicans, and their susceptibility to acute systemic candidiasis was assessed as described in Materials and Methods. The data represent the mean ± the standard error of the mean from at least six mice per time point.
In contrast to IL-10 KO mice, mice genetically deficient in IL-4 were more susceptible (P < 0.05) to acute systemic candidiasis than were immunocompetent controls (Fig. 1); the fungal burden in the spleens, livers, and kidneys of IL-4 KO mice was significantly greater than in C57BL/6xL129 controls given an i.v. challenge with the same number of yeast cells. The increased susceptibility of IL-4 KO mice to renal candidiasis was evident at early time points (i.e., day 3) after i.v. inoculation. No differences in cerebral candidiasis were observed between IL-4 KO mice and immunocompetent controls.
Neutrophilic responses to C. albicans.
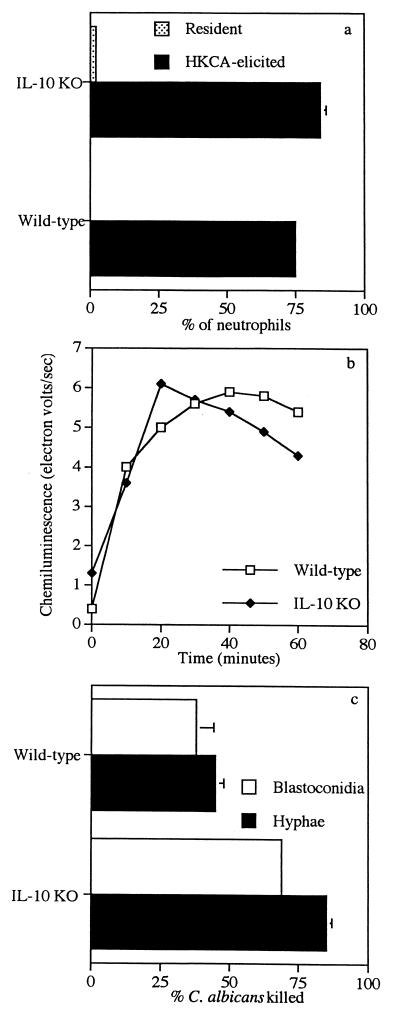
The neutrophilic responses of IL-10 KO mice and the immunocompetent controls to C. albicans inoculated intraperitoneally can be seen in Fig. 2. Very few resident neutrophils were present in the peritoneal cavities of specific-pathogen-free, immunocompetent and IL-10 KO mice (Fig. 2a). At 5 to 7 h after intraperitoneal inoculation of IL-10 KO mice with heat-killed C. albicans, the number of neutrophils present in their peritoneal cavities was not significantly different (P > 0.05) from that of the immunocompetent controls. Neutrophils at this time point made up approximately 75 to 80% of the peritoneal exudate cells. Heat-killed C. albicans-elicited peritoneal neutrophils from immunocompetent and IL-10 KO mice had a luminol-dependent chemiluminescence in response to C. albicans hyphae similar to that of neutrophils from immunocompetent controls (Fig. 2b). In contrast, neutrophils elicited from IL-10 KO mice with heat-killed C. albicans had a greater capacity (P < 0.05) to kill C. albicans blastoconidia and hyphae than did neutrophils elicited from immunocompetent controls (Fig. 2c).
FIG. 2.
Neutrophilic responses to C. albicans. Percentages of resident and elicited neutrophils harvested from the peritoneal cavities of IL-10 KO and control C57BL/6xL129 mice (a) and luminol-dependent chemiluminescence (b) and candidacidal activity (c) of heat-killed C. albicans were quantified as described in Materials and Methods.
Effect of aminoguanidine on the susceptibility of IL-10 KO mice to acute systemic candidiasis.
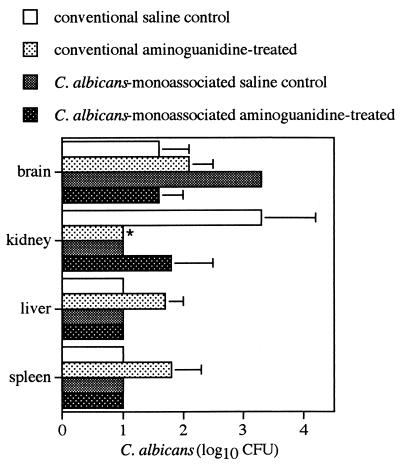
Aminoguanidine, an inhibitor with high specificity for inducible NO synthase (7), did not increase the susceptibility of IL-10 KO mice to acute cerebral, hepatic, or splenic candidiasis (Fig. 3). On the contrary, aminoguanidine treatment increased the resistance of these IL-10 KO mice to renal candidiasis. When conventional and C. albicans-monoassociated (saline- or aminoguanidine-treated) IL-10 KO mice were compared for the ability to clear C. albicans from the internal organs after i.v. inoculation with 104 viable C. albicans cells, no significant differences (P < 0.05) were observed.
FIG. 3.
Role of NO in the resistance of IL-10 KO mice to systemic candidiasis. Conventional and C. albicans-monoassociated IL-10 KO mice were given an i.v. inoculation of 104 C. albicans CFU and inoculated intraperitoneally with the inducible NO inhibitor aminoguanidine at 250 mg/kg/day. The numbers of CFU were quantified at day 14 after the i.v. challenge. ∗, P < 0.05.
Susceptibility of IL-10 and IL-4 KO mice to orogastric candidiasis.
Table 1 shows that gnotobiotic IL-10 KO mice had lingual and gastric candidiasis at 4 weeks after oral colonization with C. albicans. The IL-4 KO mice manifested gastric but not lingual candidiasis at 4 weeks after oral colonization, whereas immunocompetent controls had lingual but not gastric candidiasis at this time point. Neither IL-10 KO nor IL-4 KO mice, colonized for 4 weeks with a pure culture of C. albicans, showed any histologic evidence (Grocott’s stain) of systemic candidiasis of endogenous origin (data not shown). Histopathology scores, a measure of the severity of the orogastric candidiasis in these mice, ranged between 2 and 3 (over a range of 0 to 5; see Materials and Methods) for the infected mice. At the 4-week time point, no significant differences (P < 0.05) in lingual or gastric candidiasis were evident (by Fisher’s exact test) among the three strains of mice.
TABLE 1.
Gastric and lingual candidiasis in IL-10 KO, IL-4 KO, and wild-type mice
| Genotype | No. of mice infected/no. assayed (% infected)a
|
|
|---|---|---|
| Tongue | Stomach | |
| IL-10 KO | 7/15 (46) | 5/15 (33) |
| IL-4 KO | 0/6 (0) | 2/6 (33) |
| Control (+/+) | 4/6 (66) | 0/6 (0) |
All mice were colonized for 4 weeks with C. albicans. There were no significant differences (P < 0.05) by Fisher’s (two-tailed) exact test.
DISCUSSION
In candidiasis, a dominant Th2 phenotype has been correlated with increased susceptibility of immune humans and mice to systemic and mucosal manifestations of the disease (19, 26). In the present report, we show that IL-10 may predispose naive mice to acute systemic candidiasis; however, the susceptibility of naive mice to acute systemic candidiasis does not completely segregate with a Th2 response because mice deficient in IL-4 (a cytokine that, together with IL-10, defines the Th2 subset) were more susceptible to systemic challenge with C. albicans than were the immunocompetent controls. Our observations with Candida-naive mice are in sharp contrast to others which correlated the presence of IL-4 in immune mice with a worsening of systemic candidiasis (21, 24, 26). The view that IL-4 is important in resistance to primary systemic candidiasis is in accord with a more recent study which described a key role for this cytokine in promoting the development of a protective Th1 response in later phases of acute systemic candidiasis (20). Our data suggest that IL-4 plays an important role in resistance to early and late stages of systemic C. albicans infections as suggested by the increased susceptibility of IL-4 KO mice to splenic and renal candidiasis which was evident 3 days after experimental induction of fungemia by i.v. challenge.
A number of investigations have associated the detrimental effects of IL-10 on resistance to systemic candidiasis with decreased NO-mediated immunity (25, 26). The hypersusceptibility of DBA/2 mice, which are congenitally deficient in the C5 component of complement, to experimentally induced systemic C. albicans infections has been correlated with poor NO-mediated candidacidal activity of their macrophages (25). The poor macrophage candidacidal activity, low macrophage NO synthesis, and hypersusceptibility of immune DBA/2 mice to experimentally induced systemic candidiasis were reversed by treatment with anti-IL-10 neutralizing antibodies (25, 26). In our model, however, the beneficial effect that genetic abrogation of IL-10 had on resistance to systemic candidiasis does not seem to correlate with more-effective utilization of NO-mediated immunity. In favor of the latter hypothesis, we observed that aminoguanidine, a selective inhibitor of inducible NO synthase (7), did not interfere with the resistance of C. albicans-monoassociated or specific-pathogen-free IL-10 KO mice to an acute i.v. challenge with the fungus.
The disparate effect of IL-10 on the development of an NO-dominated immune response to a systemic infection by C. albicans may be explained by different experimental conditions. Whereas Romani and collaborators (25, 26) tested the contribution of IL-10 to the resistance of immune mice to systemic candidiasis, we tested its role in the resistance of Candida-naive mice. Other possibilities that could account for the disparate roles that NO seems to play in these two IL-10-deficient models include the different mouse strains used and the different approaches used to deplete IL-10 production. Genetic disruption of the IL-10 gene is likely to be a more efficient way to abrogate IL-10 synthesis from the beginning of the experimental challenge than immunological cytokine neutralization. Abrogation of IL-10 synthesis by genetic procedures may allow for the derepression of early innate effector functions, which, in turn, could block the subsequent development of NO-mediated immunity in IL-10 KO mice subjected to i.v. infection with C. albicans.
Recent observations suggest that, through their potential to synthesize IL-12 or IL-10, neutrophils can direct the host’s acquired immune response to C. albicans toward a curing Th1 or a disease-promoting Th2 response, respectively (12). According to the latter model, it would be expected that an IL-12-dominated neutrophilic response in IL-10 KO mice would result in the establishment of a protective Th1 immune response; however, the early resistance of IL-10 KO mice to acute systemic candidiasis is more in agreement with the hypothesis of direct IL-10 action on the effector mechanisms responsible for clearing C. albicans from the kidney. Accordingly, we observed that neutrophils from IL-10 KO mice killed more C. albicans blastoconidia and hyphae than did neutrophils from immunocompetent controls. The observation that neutralization of IL-10, although effective in immunocompetent mice, did not enhance the resistance of highly susceptible granulocytopenic mice to acute systemic candidiasis (22) is in agreement with our hypothesis that IL-10 affects a neutrophil effector function(s).
Others have previously documented that IL-10 down-regulates neutrophil oxidative metabolism, phagocytosis, and bactericidal activity (4, 17). In our system, the reduced candidacidal activity exhibited by neutrophils from immunocompetent mice seems to be due to IL-10-mediated inhibition of an oxygen-independent killing mechanism(s). In support of the latter, neutrophils from immunocompetent and IL-10 KO mice generated a similar oxidative burst (as measured by luminoldependent chemiluminescence) in response to C. albicans hyphae. Thus, our data suggest that elimination of IL-10 enhances a neutrophil effector function(s) at early stages of experimentally induced acute systemic candidiasis and results in enhanced resolution of the systemic infection.
Our histology data, which showed no significant differences (P < 0.05) in orogastric candidiasis among the three strains of mice at 4 weeks after oral challenge, suggest that neither IL-10 nor IL-4 is required for resistance to orogastric candidiasis. Our data contrast with other observations which noted that (i) treatments with an IL-4 antagonist (anti-IL-4 receptor) potentiated a Th1-type response and decreased the number of CFU recovered from the stomachs of mice after an intragastric challenge with the fungus (5) and (ii) C. albicans infection in the gastric mucosae of hypersusceptible DBA/2 mice was accompanied by decreased IL-4 expression in Peyer’s patches (2). Further experimentation is needed to clarify the role of IL-4 in resistance to mucosal candidiasis.
ACKNOWLEDGMENTS
We thank Donna Brackett for her secretarial assistance and JoAnne Croft, Barbara Reese, and June Sowatzke, University of Wisconsin Gnotobiote Laboratory, Madison, for providing the gnotobiotic mice used in this study.
REFERENCES
- 1.Ashman R B, Papadimitriu J M. Production and function of cytokines in natural and acquired immunity to Candida albicans infection. Microbiol Rev. 1995;59:646–672. doi: 10.1128/mr.59.4.646-672.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bistoni F, Cenci E, Mencacci A, Schiaffella E, Mosci P, Puccetti P, Romani L. Mucosal and systemic T helper cell function after intragastric colonization of adult mice with Candida albicans. J Infect Dis. 1993;168:1449–1457. doi: 10.1093/infdis/168.6.1449. [DOI] [PubMed] [Google Scholar]
- 3.Burford-Mason A P, Matthews R C, Williams J R. Transient abrogation of immunosuppression in a patients with chronic mucocutaneous candidiasis following vaccination with Candida albicans. J Infect. 1987;14:147–157. doi: 10.1016/s0163-4453(87)91977-3. [DOI] [PubMed] [Google Scholar]
- 4.Capsoni F, Minonzio F, Ongari A M, Carbonelli V, Galli A, Zanussi C. Interleukin-10 down-regulates oxidative metabolism and antibody-dependent cellular cytotoxicity of human neutrophils. Scand J Immunol. 1997;45:269–275. doi: 10.1046/j.1365-3083.1997.d01-393.x. [DOI] [PubMed] [Google Scholar]
- 5.Cenci E, Mencacci A, Del Sero G, Bistoni F, Romani L. Induction of protective Th1 responses to Candida albicans by antifungal therapy alone or in combination with an interleukin-4 antagonist. J Infect Dis. 1997;176:217–226. doi: 10.1086/514027. [DOI] [PubMed] [Google Scholar]
- 6.Clift R A. Candidiasis in the transplant patient. Am J Med. 1984;77:34–38. [PubMed] [Google Scholar]
- 7.Corbett J A, Tilton R G, Chang K, Hasan K S, Ido Y, Wang J L, Sweetland M A, Lancaster J R, Williamson J R, McDaniel M L. Aminoguanidine, a novel inhibitor of nitric oxide formation, prevents diabetic vascular dysfunction. Diabetes. 1992;41:552–556. doi: 10.2337/diab.41.4.552. [DOI] [PubMed] [Google Scholar]
- 8.Fidel P L, Lynch M E, Sobel J D. Candida-specific Th1-type responsiveness in mice with experimental vaginal candidiasis. Infect Immun. 1993;61:4202–4207. doi: 10.1128/iai.61.10.4202-4207.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fidel P L, Lynch M E, Sobel J D. Circulating CD4 and CD8 T cells have little impact on host defense against experimental vaginal candidiasis. Infect Immun. 1995;63:2403–2408. doi: 10.1128/iai.63.7.2403-2408.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hostetter M K. Handicaps to host defense. Effects of hyperglycemia on C3 and Candida albicans. Diabetes. 1990;39:271–275. doi: 10.2337/diab.39.3.271. [DOI] [PubMed] [Google Scholar]
- 11.Jensen J, Warner T, Balish E. Resistance of SCID mice to C. albicans administered intravenously or colonizing the gut: role of polymorphonuclear leukocytes and macrophages. J Infect Dis. 1993;167:912–919. doi: 10.1093/infdis/167.4.912. [DOI] [PubMed] [Google Scholar]
- 12.Jones-Carson J, Vazquez-Torres A, Balish E. Defective killing of Candida albicans hyphae by neutrophils from beige mice. J Infect Dis. 1995;171:1664–1667. doi: 10.1093/infdis/171.6.1664. [DOI] [PubMed] [Google Scholar]
- 13.Jones-Carson J, Vazquez-Torres A, Balish E. B cell-independent selection of memory T cells after mucosal immunization with Candida albicans. J Immunol. 1997;158:4328–4335. [PubMed] [Google Scholar]
- 14.Kaposzta R, Tree P, Marodi L, Gordon S. Characteristics of invasive candidiasis in gamma interferon- and interleukin-4-deficient mice: role of macrophages in host defense against Candida albicans. Infect Immun. 1998;66:1708–1717. doi: 10.1128/iai.66.4.1708-1717.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klein R S, Harris C A, Small C B, Moll B, Lesser M, Friedland G H. Oral candidiasis in high-risk patients as the initial manifestation of the acquired immunodeficiency syndrome. N Engl J Med. 1984;311:354–358. doi: 10.1056/NEJM198408093110602. [DOI] [PubMed] [Google Scholar]
- 16.Kobrynski L J, Tanimune L, Kilpatrick L, Campbell D E, Douglas S D. Production of T-helper cell subsets and cytokines by lymphocytes from patients with chronic mucocutaneous candidiasis. Clin Diagn Lab Immunol. 1996;3:740–745. doi: 10.1128/cdli.3.6.740-745.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laichalk L L, Danforth J M, Standiford T J. Interleukin-10 inhibits neutrophil phagocytic and bactericidal activity. FEMS Immunol Med Microbiol. 1996;15:181–187. doi: 10.1111/j.1574-695X.1996.tb00084.x. [DOI] [PubMed] [Google Scholar]
- 18.Maksymiuk A W, Thongpraser S, Hopfer R, Luna M, Fainstein V, Bodey G P. Systemic candidiasis in cancer patients. Am J Med. 1984;77:20–27. [PubMed] [Google Scholar]
- 19.Mencacci A, Cenci E, Boelaert J R, Bucci P, Mosci P F, d’Ostiani C, Bistoni F, Romani L. Iron overload alters innate and T helper cell responses to Candida albicans in mice. J Infect Dis. 1997;175:1467–1476. doi: 10.1086/516481. [DOI] [PubMed] [Google Scholar]
- 20.Mencacci A, Del Sero G, Cenci E, d’Ostiani C F, Bacci A, Montagnoli C, Kopf M, Romani L. Endogenous interleukin 4 is required for development of protective CD4+ T helper type 1 cell responses to Candida albicans. J Exp Med. 1998;187:307–317. doi: 10.1084/jem.187.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Puccetti P, Mencacci A, Cenci E, Spaccapelo R, Mosci P, Enssle K-H, Romani L, Bistoni F. Cure of murine candidiasis by recombinant soluble interleukin-4 receptor. J Infect Dis. 1994;169:1325–1331. doi: 10.1093/infdis/169.6.1325. [DOI] [PubMed] [Google Scholar]
- 22.Romani L, Mencacci A, Cenci E, Del Sero G, Bistoni F, Puccetti P. An immunoregulatory role for neutrophils in CD4+ T helper subset selection in mice with candidiasis. J Immunol. 1997;158:2356–2362. [PubMed] [Google Scholar]
- 23.Romani L, Mencacci A, Cenci E, Spaccapelo R, Mosci P, Puccetti P, Bistoni F. CD4+ subset expression in murine candidiasis. Th responses correlate directly with genetically determined susceptibility of vaccine-induced resistance. J Immunol. 1993;150:925–931. [PubMed] [Google Scholar]
- 24.Romani L, Mencacci A, Grohmann U, Mocci S, Mosci P, Puccetti P, Bistoni F. Neutralizing antibody to interleukin-4 induces systemic protection and T helper type 1-associated immunity in murine candidiasis. J Exp Med. 1992;176:19–25. doi: 10.1084/jem.176.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romani L, Puccetti P, Mencacci A, Cenci E, Spaccapelo R, Tonnetti L, Grohmann U, Bistoni F. Neutralization of IL-10 up-regulates nitric oxide production and protects susceptible mice from challenge with Candida albicans. J Immunol. 1994;152:3514–3521. [PubMed] [Google Scholar]
- 26.Tonnetti L, Spacccapelo R, Cenci E, Mencacci A, Puccetti P, Coffman R L, Bistoni F, Romani L. Interleukin-4 and -10 exacerbate candidiasis in mice. Eur J Immunol. 1995;25:1559–1565. doi: 10.1002/eji.1830250614. [DOI] [PubMed] [Google Scholar]
- 27.Vazquez-Torres A, Jones-Carson J, Balish E. Peroxynitrite contributes to the candidacidal activity of nitric oxide-producing macrophages. Infect Immun. 1996;64:3127–3133. doi: 10.1128/iai.64.8.3127-3133.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vazquez-Torres A, Jones-Carson J, Warner T, Balish E. Nitric oxide enhances resistance of SCID mice to mucosal candidiasis. J Infect Dis. 1995;172:192–198. doi: 10.1093/infdis/172.1.192. [DOI] [PubMed] [Google Scholar]
- 29.Wagner R D, Vazquez-Torres A, Jones-Carson J, Warner T, Balish E. B cell knockout mice are resistant to mucosal and systemic candidiasis of endogenous origin, but susceptible to experimental systemic candidiasis. J Infect Dis. 1997;174:589–597. doi: 10.1093/infdis/174.3.589. [DOI] [PubMed] [Google Scholar]