Abstract
Porphyria is a challenging metabolic disease due to its heterogeneous presentation symptoms and its difficult diagnosis. Many affected individuals can complain of recurrent neuro-visceral attacks per year, some of which may be persistent and life-threatening, which is confusing if there is no established diagnosis. Although the motor manifestations, autonomic changes and seizure are highly suggestive, the diagnosis is often overlooked and needs confirmatory genetic testing. To the best of our knowledge, the acute intermittent porphyria (AIP) reported in this case, involving severe electrolyte disturbances and rapid severe weakness is a challenging neuro-metabolic case and is extremely rare worldwide. Here, we reported a case of AIP in a young girl who presented to the emergency department of Al-Araby international Hospital, Monufia, Egypt with severe abdominal pain, constipation, and headache which had started 10 days ago. It seems that the diagnosis of porphyria should be considered particularly in those patients with abdominal complaints associated with electrolyte disturbances, seizures, and severe progressive neuropathy.
Key Words: Electrolyte Imbalance, Porphyria, Acute Intermittent, Polyneuropathies
1. Introduction:
Porphyrias are a group of rare metabolic disorders presenting a wide range of clinical manifestations. As a result of the genetic mutation in the enzymatic pathway involved in heme biosynthesis, a specific subtype of acute porphyrias can arise (1). The acute porphyrias can be either autosomal dominant, such as acute intermittent porphyria (AIP), variegate porphyria, and hereditary coproporphyria, or autosomal recessive, such as delta-aminolevulinic acid (ALA) dehydratase deficiency. Enzymatic defect in the porphobilinogen deaminase (PBGD) can cause AIP; a rare autosomal dominant disease that can be easily confused with other diseases (2). Clinical presentation of AIP is rare before puberty and the diagnosis is suggested by a triad of symptoms; severe abdominal pain, quadriparesis due to peripheral nerve involvement, delirium, and depression as the most common neuropsychiatric manifestation, which dominates the clinical picture. Despite the rarity of the disease, the diagnosis may be confirmed by a decreasing level of porphobilinogen in the erythrocytes. However, although the autonomic changes and seizure are highly suggestive, the diagnosis is often overlooked and needs confirmatory genetic testing (3). Moreover, early diagnosis may considerably improve patients’ prognosis through proper management. Here we reported a case of AIP presenting to emergency department with severe electrolyte disturbances and rapid severe weakness. It seems that, the diagnosis of AIP should be considered when patients present with severe electrolyte imbalance and progressive neuropathy.
2. Case presentation:
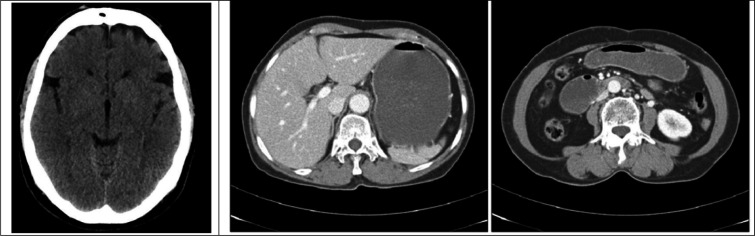
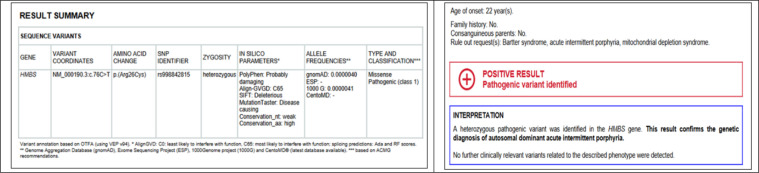
A 13-year-old girl with no history of medical or familial diseases presented to the emergency department of Al-Araby international Hospital, Monufia, Egypt with severe abdominal pain, constipation, and headache, which had started 10 days ago. Within the next few days following admission, the patient developed an attack of generalized tonic-clonic seizure associated with low-grade fever. On examination, the patient was confused in post ictal state. Otherwise, the neurological and general examination was unremarkable. Patient was hemodynamically stable. Urgent brain computed tomography (CT) scan showed brain edema (figure 1). Cerebrospinal fluid (CSF) analysis, routine blood chemistry tests, blood culture, and serum electrolyte evaluation were performed. On a clinical basis, central nervous system infection was suspected and the patient started to receive acyclovir. Due to severe abdominal pain, abdominopelvic CT scan with contrast was done, which revealed marked distention of large bowel, and no definite air-fluid level or obstructing masses (figure 1). The initial results of laboratory investigation revealed marked electrolyte disturbances; sodium level: 108 milliequivalents per liter (mEq/L), potassium level: 2.7 mEq/L, total calcium level: 4.9 mg/dl, ionized calcium level: 3.5 mg/dl, magnesium level: 0.7 mEq/L, and phosphorus level: 2.1 mEq/L (table 1). Within the next few days, the patient began to become agitated and developed generalized muscle pain despite proper correction of resistant hyponatremia with hypertonic saline 3%. In addition, the patient started to develop polyuria, and polydipsia, despite the normovolemic state, confirmed by clinical examination and central venous pressure monitoring. High suspicion to Bartter-like syndrome was raised as the renal tubular defect with increased renal loss of electrolyte was confirmed by the following laboratory investigation: urine osmolarity 277mOml/kg, serum osmolality 252 mmol/kg, serum chloride 73 mEq/L, and urinary Calcium/creatinine ratio 1.053 (table 1). Within the next few days, she developed weakness in both lower limbs, right more than left, which was rapidly progressing to involve upper limbs associated with trunk muscle affection till the patient became quadriplegic. The rest of the examination was normal. Our opinion about the case changed and we had a rising concern about genetic disease. At that time, the whole-exome sequencing was sent abroad to CENTOGENE GmbH (Rostock, Germany) for genetic analysis. Electrophysiological studies were done, which revealed evidence of purely motor axonal polyneuropathy affecting upper and lower limbs with bilateral facial axonal neuropathy (prolonged first time (F) wave latencies with reduced amplitude of compound motor action potentials). Unfortunately, the condition worsened till the patient became intubated and mechanically ventilated due to respiratory muscle involvement. The CSF examination showed cytoalbuminous dissociation, and this made the diagnosis more in favor of Guillain-Barre Syndrome (GBS). The patient received intravenous immunoglobulin (IV IG) (2gm per Kg divided over 5 days) with partial improvement. Due to the failure of multiple trials for weaning from mechanical ventilation, tracheostomy was performed. Genetic sequencing results showed a heterozygous pathogenic variant in the hydroxymethylbilane synthase (HMBS) gene, which confirmed the diagnosis of autosomal dominant AIP (figure 2). The patient received dextrose 25% and 2 doses of hemin 250 mg once daily for four days, 2 weeks apart followed by another dose after 2 months. There was a marked improvement regarding the weakness, and abdominal pain, and we managed to wean her from mechanical ventilation.
Figure 1.
left image: Brain computed tomography scan without contrast showed grey-white matter differentiation of the cerebrum and cerebellum is preserved, however there is subtly reduced density of the cerebrum relative to the cerebellum, suggesting mild/early diffuse edema. No sulcal effacement and ventricles are normal in appearance. Right and middle image: Abdominal computed tomography scan with intravenous contrast reveals marked distension of stomach and duodenum
Table 1.
The laboratory findings as well as cerebrospinal fluid and genetic analyses of the patient
| Variable | value |
|---|---|
| Complete blood count | |
| Red blood cells (cells/mcL) | 4 million |
| White blood cells (cells/mcL) | 55000 |
| Platelets count (/mcL) | 226,000 |
| Hemoglobin (gm/dl) | 13 |
| Venus blood gas analysis | |
| pH | 7.31 |
| pCO2 (mm Hg) | 42 |
| pO2(mm Hg) | 40 |
| Electrolyte | |
| Sodium (mEq/L) | 108 |
| Potassium (mEq/L) | 2.7 |
| Total calcium (mg/dl) | 4.9 |
| Ionized calcium (mg/dl) | 3.5 |
| Magnesium (mEq/L) | 0.7 |
| Phosphorus (mEq/L) | 2.1 |
| Osmolality | |
| Urine osmolality (mOml/kg) | 277 |
| Serum osmolality (mOml/kg) | 252 |
| Serum chloride (mEq/L) | 73 |
| Urinary Calcium/creatinine ratio | 1.053 |
| CSF analysis | |
| CSF appearance | Clear, colorless |
| CSF protein (mg/100 ml) | 35 |
| CSF glucose (mmol/l) | 4.2 |
| CSF chloride (mmol/l) | 125 |
| CSF lactate (mmol/l) | 2.1 |
| CSF culture and sensitivity | negative |
| Gene analysis | |
| HBMS gene | NM_000190.3:c.76C>T |
CSF: cerebrospinal fluid.
Figure 2.
Summary of genetic testing findings (left) and its interpretation (right)
3. Discussion:
Porphyria is a challenging metabolic disease due to its heterogeneous presentation symptoms and its difficult diagnosis. It is a common term incorporating many inborn metabolic diseases affecting heme biosynthesis. For each of the eight enzymes included in this pathway, there is an associated known defect. Due to these defects, an intermediate precursor is accumulated (1, 4). There are multiple classifications for porphyria but distinctly it is attributed to either the site of accumulation of the metabolite or the phenotypically dominant manifestations (5). The commonest type was AIP (3). It occurs mainly due to an autosomal dominant enzymatic defect in the third cascade of the heme synthesis pathway. Defects of the HMBS gene, which encodes PBGD enzyme, lead to the accumulation of porphyrin precursors such as ALA and porphobilinogen. These toxic metabolites accumulate in the liver and become deposited in many systems causing problems with a wide range of severity and affecting different organs. Renal failure and electrolyte imbalances are also among the possible emerging heterogeneous presentation sequelae. In the present report, we introduced a case of AIP, in which a young girl presented with a rare presentation. Due to the strong association between hormonal changes and precipitation of attacks, AIP is more prevalent in females. Our case showed severe weakness in both upper and lower limbs that progressed to involve the respiratory muscles due to severe peripheral neuropathy. This could be attributed to axonal dysfunction due to Na+/K+ pump energy dysfunction resulting from the lack of heme availability and the direct neurotoxic effect of porphyrin precursors (6). Severe resistant hyponatremia was observed in our case either due to primary salt wasting or the syndrome of inappropriate antidiuretic hormone secretion as reported in a previous study (7). The degree of axonal damage probably predicts the ultimate prognosis. However, some patients with the GBS variant remain permanently quadriparetic (8). Once a porphyria attack is diagnosed, precipitating factors or provoking agents such as dietary changes with low caloric intake, certain drugs, stressful conditions, and infections should be avoided (4, 5). Treatment for AIP includes both supportive and specific therapies. In addition, glucose supplements or parenteral administration of hematin are suggested (8). Clinical evaluation and parental targeted testing are recommended to establish whether the detected variant is inherited or de novo. Although some attacks may be persistent and life-threatening, early diagnosis may considerably improve patient prognosis through proper management.
4. Conclusion:
Porphyric neuropathy is a predominantly motor neuropathy with different clinical presentations that may mimic various neuropathies, such as GBS with diffuse axonal involvement, or lead neuropathy with specific nerve involvement, or bilateral radial neuropathy. A diagnosis of porphyria should be considered particularly in patients with abdominal complaints associated with electrolyte disturbances, seizures, and severe progressive neuropathy. Moreover, early diagnosis may considerably improve patient prognosis through proper management.
5. Declarations:
5.1. Acknowledgements
The authors would like to express their gratitude to the Department of Neurology, Faculty of Medicine, Al-Azhar University, Cairo, Egypt.
5.2. Ethics approval and consent to participate
This study was conducted in concordance with declaration of Helsinki and the participant signed a written informed consent before the submission of the case report. The institutional review board (IRB) approval was obtained from the ethical committee of Faculty of Medicine, Al-Azhar University, Cairo, Egypt.
5.3. Competing interests
The authors declare that they have no competing interests.
5.4. Funding
This study was self-funded by the authors.
5.5. Authors' contributions
All authors participated in manuscript writing and editing. All authors have read and approved the manuscript.
References
- 1.Spiritos Z, Salvador S, Mosquera D, Wilder J. Acute intermittent porphyria: current perspectives and case presentation. Ther Clin Risk Manag. 2019;15:1443–51. doi: 10.2147/TCRM.S180161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ma Y, Teng Q, Zhang Y, Zhang S. Acute intermittent porphyria: focus on possible mechanisms of acute and chronic manifestations. Intractable Rare Dis Res. 2020;9(4):187–95. doi: 10.5582/irdr.2020.03054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Phillips JD. Heme biosynthesis and the porphyrias. Mol Genet Metab. 2019;128(3):164–77. doi: 10.1016/j.ymgme.2019.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cardenas JL, Guerrero C. Acute intermittent porphyria: general aspects with focus on pain. Curr Med Res Opin. 2018;34(7):1309–15. doi: 10.1080/03007995.2018.1435521. [DOI] [PubMed] [Google Scholar]
- 5.Bustad HJ, Kallio JP, Vorland M, Fiorentino V, Sandberg S, Schmitt C, et al. Acute intermittent porphyria: An overview of therapy developments and future perspectives focusing on stabilisation of HMBS and proteostasis regulators. Int J Mol Sci. 2021;22(2):675. doi: 10.3390/ijms22020675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alqwaifly M, Bril V, Dodig D. Acute intermittent porphyria: a report of 3 cases with neuropathy. Case Rep Neurol. 2019;11(1):32–6. doi: 10.1159/000496420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hift RJ, Meissner PN. An analysis of 112 acute porphyric attacks in Cape Town, South Africa: evidence that acute intermittent porphyria and variegate porphyria differ in susceptibility and severity. Medicine. 2005;84(1):48–60. doi: 10.1097/01.md.0000152454.56435.f3. [DOI] [PubMed] [Google Scholar]
- 8.Schutte C-M, Van der Meyden CH, Van Niekerk L, Kakaza M, Van Coller R, Ueckermann V, et al. Severe porphyric neuropathy-importance of screening for porphyria in Guillain-Barre syndrome: clinical alert. S Afr Med J. 2016;106(1):44–7. doi: 10.7196/SAMJ.2016.v106i1.10118. [DOI] [PubMed] [Google Scholar]