Abstract
Depression has been shown to negatively impact neurocognitive functions, particularly those governed by fronto-subcortical networks, such as executive functions. Converging evidence suggests that depression-related executive dysfunction is greater at older ages, however, this has not been previously confirmed by meta-analysis. We performed a systematic review and meta-analysis, using three-level models, on peer-reviewed studies that examined depression-related differences in cognitive control in healthy community-dwelling individuals of any age. We focused on studies of cognitive control as defined by the National Institute of Mental Health (NIMH) Research Domain Criteria (RDoC) framework, which centers on goal-directed behavior, such as goal selection (updating, representations, maintenance), response selection (inhibition or suppression), and performance monitoring. In 16,806 participants aged 7 to 97 across 76 studies, both clinical depression and subthreshold depressive symptoms were associated with cognitive control deficits (Hedges’ g = −0.31). This relationship was stronger in study samples with an older mean age. Within studies with a mean age of 39 years or higher, which represents the median age in our analyses, the relationship was stronger in clinical compared to subthreshold depression and in individuals taking antidepressant medication. These findings highlight the importance of clinicians screening for cognitive control dysfunction in patients with depression, particularly in later stages of adulthood.
Keywords: Major depression, Subthreshold depression, Executive control, Executive function, Cognition, Age differences, Older adults
Major depressive disorder (MDD) is a heterogenous neuropsychiatric illness that affects individuals across the lifespan (Lupien, McEwen, Gunnar, & Heim, 2009). Depression is the second leading cause of disability in the United States and around the world, and results in significant morbidity and mortality (Collins, Patel, Joestl, March, & Insel, 2011; The US Burden of Disease Collaborators, 2018). Depressive symptoms vary across the lifespan and include changes in mood (i.e., sadness, irritability), anergia, appetite or weight changes, and changes in neurocognitive functions (Gaynes et al., 2007; McClintock et al., 2011; Westerhof & Keyes, 2010). For the latter, MDD has been found to predominantly affect neurocognitive functions governed by fronto-subcortical networks, including processing speed, attention, and executive functions (Koenig, Bhalla, & Butters, 2014; Wagner, Doering, Helmreich, Lieb, & tadic, 2012; Weisenbach et al., 2014).
Previous meta-analyses to date have repeatedly demonstrated robust associations between depression and deficits in executive control. One of the earliest meta-analyses that synthesized data from 14 studies of over 1000 participants with depression found that depression severity was significantly associated with executive dysfunction for both timed (speeded) and untimed (non-speeded) measures (McDermott & Ebmeier, 2009). Similar deficits in executive functioning have been shown to be present in patients with first-episode MDD (Lee, Hermens, Porter, & Redoblado-Hodge, 2012) as well as those who were in remission (Rock, Roiser, Riedel, & Blackwell, 2014). Executive functioning is a broad neurocognitive domain that includes multiple cognitive control functions such as planning, problem solving, set-shifting, concept formation, inhibition, and initiation (Alvarez & Emory, 2006; Miller & Wallis, 2009). Less work has examined whether the association between depression and executive functions differs across these specific cognitive control functions (McClintock, Husain, Greer, & Cullum, 2010).
Converging evidence suggests that age influences the type and severity of cognitive dysfunction, including executive deficits, in depression (Beats, Sahakian, & Levy, 1996; Lockwood, Alexopoulos, & van Gorp, 2002). Older adults with MDD consistently show greater neurocognitive deficits relative to younger cohorts with MDD (Porter, Bourke, & Gallagher, 2007). Similarly, in community samples with subthreshold symptoms, the severity of depressive symptoms has been found to be associated with poorer letter fluency performance in older but not middle-aged individuals (Dotson, Resnick, & Zonderman, 2008). Moreover, a recent meta-analysis of depressed young people between the ages of 12 and 25 found no significant depression-related deficits in executive functions including planning and organization, response inhibition, or set-shifting (Goodall et al., 2018). Together, these findings suggest that depression-related executive dysfunction is greater at older ages, however, this has not been previously confirmed by meta-analysis.
The purpose of this meta-analysis was to synthesize available data regarding the association between depression and executive functioning across the lifespan. We focused on studies of cognitive control as defined by the National Institute of Mental Health (NIMH) Research Domain Criteria (RDoC) framework (Kozak & Cuthbert, 2016; Morris & Cuthbert, 2012). Within this framework, executive functioning is codified within the cognitive control system and is centered around goal-directed behavior, including component cognitive processes such as goal selection (updating, representations, maintenance), response selection (inhibition or suppression), and performance monitoring (Paulus, 2015). We hypothesized that both clinical and subthreshold depression would be associated with poorer cognitive control performance and that age would moderate the relationship between depression and cognitive control. Based on previous evidence, we specifically expected the relationship between depression and cognitive control dysfunction to be stronger at older ages.
Methods
Literature Search
We performed a systematic review and meta-analysis using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Moher, Liberati, Tetzlaff, Altman,, & Group, 2009). In March 2018, we conducted electronic searches in PsycINFO and PubMed for studies that examined the association between clinical depression or depressive symptoms and cognitive control in the general population. Details of the search strategy for each database are reported in Table 1. Briefly, we searched for human, English language, peer-reviewed journal articles with 1) the terms depressi* or MDD or mood in the title or abstract, and 2) the terms ‘cognitive control’ or ‘executive’ in the title or abstract. Since we were interested in studies that included no major medical or psychological comorbidities, the search excluded articles with title terms dementia, Alzheimer*, MCI, “mild cognitive impairment”, Parkinson*, *stroke, HIV, cancer, diabet*, “brain injury”, TBI, “multiple sclerosis”, ADHD, and alcohol*. The searches also excluded articles with the title term postpartum or pregnan*. The asterisk provided a shorthand for including alternate endings (e.g., the search term depressi* would yield matches for the words depression, depressed, and depressive).
Table 1.
Details of the PubMed and PsycINFO literature searches in March 2018
| PubMed: |
| (depressi*[Title] OR mdd[Title] OR mood[Title]) AND (“cognitive control”[Title/Abstract] OR executive[Title/Abstract]) NOT (dementia[Title] OR Alzheimer*[Title] OR MCI[Title] OR “mild cognitive impairment”[Title] OR Parkinson*[Title] OR *stroke[Title] OR postpartum[Title] OR pregnan*[Title] OR HIV[Title] OR cancer[Title] OR diabet*[Title] OR “brain injury”[Title] OR TBI[Title] OR “multiple sclerosis”[Title] OR ADHD[Title] OR alcohol*[Title] OR therapy [Title] OR treatment[Title] OR intervention[Title]) AND Humans[Mesh] NOT “clinical trial”[Publication Type] NOT “review”[Publication Type] |
| PsycINFO: |
| (TI depressi* OR TI mdd OR TI mood) AND (TI “cognitive control” OR AB “cognitive control” OR TI executive OR AB executive)NOT (TI dementia OR TI Alzheimer* OR TI MCI OR TI “mild cognitive impairment” OR TI Parkinson* OR TI *stroke OR TI postpartum OR TI pregnan* OR TI HIV OR TI cancer OR TI diabet* OR TI “brain injury” OR TI TBI OR TI “multiple sclerosis” OR TI ADHD OR TI alcohol* OR TI therapy OR TI treatment OR TI intervention) |
| Limiters: Publication Type: Peer Reviewed Journal, English, Population Group: Human, Document Type: Journal Article, Exclude Dissertations |
| Methodology: Excluded literature review, clinical trial, meta-analysis, systematic review, treatment outcome |
Study Selection
Two reviewers screened each article to determine appropriateness for this meta-analytic study, with disagreements resolved by the first author.
Definition of Cognitive Control
We chose to conceptualize cognitive control based on the NIMH’s RDoC framework (Kozak & Cuthbert, 2016), which includes goal-directed behavior such as goal selection (updating, representations, maintenance), response selection (inhibition or suppression), and performance monitoring. Examples include measures of inhibitory control (e.g., Stroop Color-Word Test), planning (e.g., Tower of London), cognitive flexibility (e.g., Trail Making Test), and set-shifting (e.g., CANTAB Intra-Extra Dimensional Set Shift). Working memory and verbal fluency, which are considered cognitive control processes by some investigators, are not included in the RDoC classification of cognitive control. As such, studies that only examined working memory or verbal fluency tasks were not considered. For the purposes of this meta-analysis, we did not include emotion processing studies or studies in which the cognitive control task included affective stimuli.
Inclusion and Exclusion Criteria
Reviewers determined study eligibility by examining the title, abstract, and full text of each article. Only peer-reviewed journal articles presenting original research were selected, thus, we excluded reviews and meta-analyses. Studies of unipolar clinical depression, subthreshold depression, and depressive symptoms as measured by questionnaires were eligible. We selected no studies of depression with comorbid psychiatric conditions other than anxiety disorders based on the high comorbidity of depression and anxiety. Given our focus on the general population, we excluded studies conducted in inpatient settings, nursing homes, or prisons. Intervention studies were excluded unless the intervention targeted cognitive deficits, in those cases, we used only the pre-treatment data. Similarly, both longitudinal and cross-sectional studies were included, but we only considered baseline assessments. Neuroimaging studies were included if the study reported cognitive test results, which could be based on tests completed in or out of the scanner. We only selected studies with objective measures of cognitive control as the outcome (e.g., we excluded studies based solely on self-reported neurocognitive scales). Reviewers also screened out any study that, based on reviewing the full text, did not meet eligibility criteria specified in the search terms (e.g., major medical or psychological comorbidities).
Data Extraction
Two reviewers separately extracted data from each eligible article using a standardized spreadsheet. Afterward, the first author compared each dataset for discrepancies and referenced the full-text to correct any errors. Each reviewer extracted the following variables from each record: publication details, study characteristics (sample setting, study design, research type [behavioral, neuroimaging]), depression status (clinical depression or subthreshold symptoms), diagnosis method (e.g., structured interview, depressive symptom questionnaire), antidepressant medication status (on, off), age range and mean age (for the total sample as well as control and depressed groups where relevant), comorbid anxiety (yes, no), cognitive test name, and sample size (for the total sample as well as control and depressed groups where relevant). For cognitive control outcome data, reviewers extracted means and standard deviations, t values or d values for studies that compared depressed groups to controls, and correlation coefficients for studies that analyzed depressive symptoms as a continuous predictor.
Methodology quality of the included studies was assessed by the first author using the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines for Methods sections (von Elm et al., 2007). Each of the nine items in the checklist was given a score of 0 or 1 based on meeting the specified criteria of describing study design, setting, participants, variables, data sources and measurement, bias, study size, quantitative variables, and statistical methods.
Data Analysis
All effect sizes were reported using Hedges’ g (Hedges, 1981). For studies that compared depressed groups to controls, study effect sizes were calculated by subtracting the mean cognitive performance score in the depressed group from the mean cognitive performance in the healthy control group and dividing by the pooled standard deviation of the two groups. For cognitive control measures in which higher scores indicated worse performance (e.g., Trail Making Test time to completion), mean performance of the healthy group was subtracted from mean performance of the depressed group. For studies that reported correlations between depressive symptom severity and cognitive outcome, positive and negative correlation coefficients were reversed for cognitive measures in which higher scores indicated worse performance. As such, more negative effect sizes reflect lower cognitive performance among depressed individuals relative to the healthy controls, or lower cognitive performance at higher levels of depressive symptoms. Cohen’s (1988) conventions may be used for the interpretation of the size of the effect: an effect size of 0.2 is considered small, 0.5 is considered medium, and 0.8 is considered large.
Random-effects models were implemented to estimate the total association between depression and cognitive control. Random-effects models were chosen because of the high level of variation in methods among studies included in the present analysis (e.g., differences in cognitive measures, age of the sample, etc.). These models account for variability in the true effect between studies while also accounting for random error within each study.
We conducted a three-level meta-analysis, which included all data points, nesting effect sizes within studies (Cheung, 2014b) Analysis was conducted using the metaSEM package for R (Cheung, 2014a). As in traditional meta-analysis, a Q statistic tests for the degree of heterogeneity in the data; the I2 statistic is now split over levels, indicating the proportions of the total variation in the effect sizes due to heterogeneity at the different levels, which in our case are study and measures within studies. For multilevel regression models, tau2 indicates the residual heterogeneity (expressed as variance) at each of the levels, R2 indicates the proportion of estimated heterogeneity at each of the levels that is explained by the regression predictors. These models use structural equation modeling, and effect sizes are thus modeled as regression equations. Note that three-level analysis has been shown to produce unbiased estimates of effect sizes, even in the absence of information about the correlation between the different measures within each study (Moeyaert et al., 2017). An α level of 0.05 was used in all analyses.
Age and Other Moderator Analyses
A meta-regression analysis (using the maximum likelihood estimate method) was used to examine age as a possible source of between-studies heterogeneity. We also examined age as a categorical variable using subgroup meta-analyses that compare subsets of studies using Q tests. Studies were assigned to the following categories based on the age range of the sample: Child to Adolescent (age 7–17; k = 4), Adolescent (age 12–17; k = 6), Young Adult (age 18–25; k = 6), Adult (studies that did not report age ranges but had a mean age of 22–42; k = 15), Middle Aged (age 51–60; k = 2), Young to Middle Aged (age 18–65; k = 7), Young to Older Adult (age 18–85; k = 4), Middle Aged to Older Adult (age 45–85; k = 3), and Older Adult (age 60–97; k = 29). We attempted to label age groups based on commonly accepted age cutoffs throughout the lifespan (e.g., the age of 60 or 65 is generally accepted as the cutoff for older adults). The variability in the age range across studies necessitated overlap in some of the age groups for the purpose of this meta-analysis. For example, we wanted to distinguish between studies that only included adolescents (defined as age 12—17) from those that included both children under the age of 12 as well as individuals up to age 17.
Additional moderator analyses were conducted in an effort to explain significant heterogeneity in effect sizes. Subgroup meta-analyses focused on the following potential moderators: depression status (clinical depression vs. subthreshold depression), cognitive domain (inhibition, cognitive flexibility, planning, and set-shifting), antidepressant medication status (on vs. off), anxiety comorbidity (yes vs. no), and test format (paper-and-pencil vs. computerized administration). Studies that did not report information about the moderator of interest were excluded from the respective subgroup analysis.
In subgroup analyses, effect sizes are compared for two or more groups that differ on a nominal variable in order to assess whether there are significant differences in effect sizes between subgroups. A random-effects model with separate estimates of τ2 was used for subgroup analyses due to significant variability between effect sizes within groups. In studies where more than one estimate of an experimental factor was reported (e.g., a study that assessed the same cognitive domain with multiple outcome measures), an effect size was calculated for each type of estimate and treated as if it were derived from an independent study. For each of the subgroup meta-analyses, we only included groups that contained three or more studies in order to have adequate power.
Results
Description of Included Studies
As summarized in Fig. 1, the electronic database search identified 943 potentially relevant studies. After screening the title and abstract, 654 studies were excluded. Of the remaining 289 articles, 74 met all criteria. An additional two articles were added based on a review of the reference list of the 74 articles, resulting in a total of 76 articles included in the meta-analysis. Collectively, the studies included 17,051 participants who ranged in age from 7 to 97 years. Fifty-six studies compared individuals with major depression defined by structured or clinical interview to controls. The remaining studies either examined depressive symptoms as a continuous measure based on questionnaire scores, or defined depression based on some other criterion (e.g., recurrent brief depression, minor depression, dysthymia, or a cutoff on a depressive symptom questionnaire). Study characteristics are summarized in Table 2.
Fig. 1.
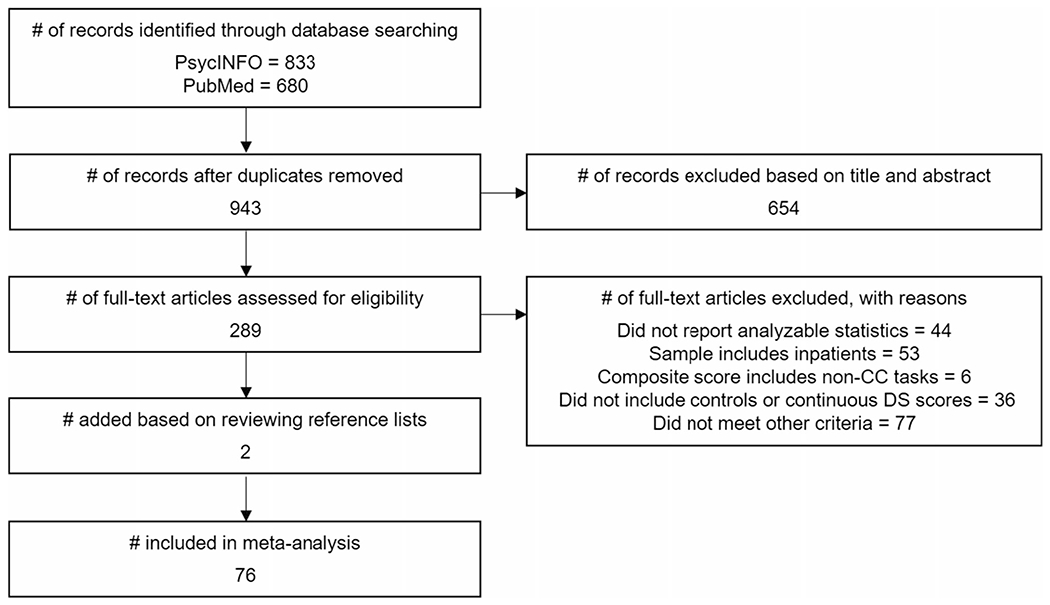
Flow diagram of study selection. CC = cognitive control, DS = depressive symptoms
Table 2.
Study Characteristics
| First Author, Year | Depression Status | Setting | N | Antidepressant Medication | Sample Age, Mean (Range) | Comorbid Anxiety |
|---|---|---|---|---|---|---|
| Aarts, 2013 | MDD | outpatient clinic | 40 | on | C = 38.9, D = 36.9 | yes |
| Aizenstein, 2009 | MDD | research center | 23 | off | C = 68.8, D = 69.1 | yes |
| Alexopoulos, 2015 | MDD | community | 83 | off | C = 72.8 (60+), D = 72.2 (60+) | yes |
| Andersson, 2010 | RBD | community | 70 | off | C = 34.2, D = 33.8 | yes |
| Baudic, 2004 | MDD | outpatient clinic | 40 | off | C = 73.6 (61–85), D = 71.8 (59–93) | NR |
| Baune, 2006 | CES-D ≥ 16 | outpatient clinic | 364 | on | C = 72.4 (65+), D = 73.7 (65+) | NR |
| Bobb, 2012 | MDD | community | 28 | off | C = 62.0 (55–85), D = 60.7 (55–85) | yes |
| Boone, 1995 | MDD, mild | community | 147 | off | C = 63.1 (45+), D = 60.5 (45+) | NR |
| MDD, moderate | community | 146 | off | C = 63.1 (45+), D = 62.2 (45+) | NR | |
| Boyle, 2010 | MDD | outpatient clinic | 378 | NR | C = 75.3 (65–95), D = 72.9 (65–88) | NR |
| minor depression | outpatient clinic | 382 | NR | C = 75.3 (65–95), D = 75.9 (65–94) | NR | |
| Bredemeier, 2016 | MDD | community | 162 | NR | 19.7 (18–26) | yes |
| Brevik, 2013 | subthreshold | community | 125 | NR | 60.0 (46–79) | no |
| Brewster, 2017 | subthreshold | community | 885 | NR | 73.5 (60+) | NR |
| Brooks, 2010 | MDD, NOS | community | 60 | on | C = 14.6 (9–17), D = 14.6 (9–17) | NR |
| Bunce, 2008 | subthreshold | community | 300 | off | 50.33 (18–85) | NR |
| Butters, 2004 | MDD | outpatient clinic | 140 | on | C = 69.9 (60+), D = 70.8 (60+) | NR |
| Channon, 1996 | BDI >11 | college | 56 | NR | C = 21.0, D = 22.3 | NR |
| Channon, 1999 | MDD | community | 22 | on | C = 41.2, D = 41.3 | NR |
| Charlton, 2014 | MDD | community | 46 | off | C = 66.3 (60–81), D = 65.7 (60–88) | no |
| Clawson, 2013 | MDD | community | 110 | on | C = 21.2, D = 22.0 | yes |
| Constant, 2005 | MDD | outpatient clinic | 46 | off | C = 48.85 (21–69), D = 47.65 (21–74) | no |
| Egger, 2008 | MDD | outpatient clinic | 34 | NR | C = 72.3 (60–84), D = 71.4 (61–86) | NR |
| Elderkin-Thompson, 2006 | MDD | community | 134 | off | C = 71.5 (60+), D = 69.2 (60+) | NR |
| minor depression | community | 103 | off | C = 71.5 (60+), D = 71.6 (60+) | NR | |
| Favre, 2008 | MDD | outpatient clinic | 63 | off | C = 13.1 (9–17), D = 12.7 (8–17) | yes |
| Franz, 2011 | subthreshold (CES-D) | Vietnam Era Twin Registry | 1237 | NR | 55.4 (51–60) | possiblea |
| Godard, 2011 | MDD | outpatient clinic | 46 | on | D = 49.5 (18–65); C = matched | yes |
| Grant, 2001 | MDD | outpatient clinic | 159 | off | C = 40.2, D = 39.0 | yes |
| Gualtieri, 2006 | MDD, medicated | outpatient clinic | 107 | off | C = 41.3, D = 38.1 | NR |
| MDD, unmedicated | outpatient clinic | 100 | off | C = 41.3, D = 43.5 | NR | |
| Halari, 2009 | MDD | outpatient clinic | 42 | off | D = 16.3 (14–17), C = matched | no |
| Halvorsen, 2012 | MDD | community and outpatient clinic | 87 | on | C = 38.1, D = 37.5 | NR |
| Han, 2016 | subthreshold (DISC-IV mother) | community | 220 | NR | 13.67 (11–16) | yes |
| Harper, 2016 | MDD | outpatient clinic | 18 | off | C = 74.1 (69–84), D = 68.3 (56–82) | NR |
| Hermens, 2010 | MDD | outpatient clinic | 37 | on | C = 22.0, D = 21.1 | NR |
| Hill, 2013 | mixed | outpatient clinic | 89 | NR | 11.0 (7–16) | yes |
| Holmes, 2008 | MDD | community | 40 | off | C = 28.8 (18–55), D = 30.6 (18–55) | yes |
| Jungwirth, 2011 | MDD, minor depression, subthreshold | community (VITA Study) | 287 | on | C = 75.8, D = 75.8) | NR |
| Katz, 2010 | MDD | outpatient clinic | 22 | on | C = 73.1, D = 73.4 | NR |
| Kaymak, 2010 | MDD | outpatient clinic | 35 | off | C = 29.3, D = 32.0 | no |
| Kikuchi, 2012 | MDD | community | 59 | on | C = 30.8 (18–65), D = 36.2 (18–65) | yes |
| Kindermann, 2000 | MDD | outpatient clinic | 45 | NR | C = 71.2 (60–82), D = 73.4 (60–82) | NR |
| Klojcnik, 2017 | subthreshold (BDI-II) | retirement community | 71 | NR | 78.9 (69–85) | NR |
| Koenig, 2015 | MDD | community | 238 | NR | C = 71.43, D = 71.23 | NR |
| Krompinger, 2011 | subthreshold (top and bottom 10% BDI-II) | college | 41 | NR | NR (undergraduates) | NR |
| Ladouceur, 2012 | MDD | outpatient clinic | 38 | off | 13.1 (7–11), D = 13.8 (7–11) | yes |
| Langanecker, 2005 | MDD | outpatient clinic | 41 | on | C = 29.5, D = 32.2 | NR |
| Lyche, 2011 | MDD | outpatient clinic | 128 | onb | C = 35.7, D = 44.2 | no |
| Maalouf, 2011 | MDD | community | 37 | on | C = 15.2, D = 15.3 | yes |
| Mackin, 2009 | MDD | unsure | 97 | off | C = 70.1 (65+), D = 70.1 (65+) | NR |
| Naismith, 2006 | MDD | outpatient clinic | 42 | on | C = 50.8 (25–69), D = 53.9 (25-77) | NR |
| Oral, 2012 | MDD | outpatient clinic | 79 | off | C = 27.2, D = 26.3 | NR |
| Pantzar, 2014 | MDD, mild | community | 2445 | off | C = 72.6, D = 78.6 | no |
| MDD, moderate-severe | community | 2438 | off | C = 72.6, D = 75.9 | no | |
| Pantzar, 2017 | MDD | community | 138 | on | C = 72.0, D = 71.8 | NR |
| Philippot, 2017 | subthreshold (GDS) | elderly homes, outpatient clinic | 43 | NR | 83.2 (75–95) | NR |
| Pizzagalli, 2006 | BDI 6 or less and 18+ | community, college | 34 | NR | 18–22 | NR |
| Porter, 2003 | MDD | outpatient clinic | 88 | off | 32.3 (18–55), 32.9 (19–61) | NR |
| Purcell, 1998 | MDD | outpatient clinic | 50 | on | C = 40.8 (18–65), D = 37.5 (18–65) | no |
| Quinn, 2012 | MDD | community | 168 | off | C = 39.22, D = 38.08 | yes |
| Rainer, 2006 | MDD, minor depression | community (VITA Study) | 280 | on | C = 75.8 (75–76), D = 75.8 (75–76) | NR |
| Rajtar-Zembaty, 2017 | MDD | NR | 187 | on | C = 66.8 (60–83), D = 68.1 (60–83) | NR |
| Rizk, 2017 | MDD | outpatient clinic | 45 | off | C = 32.8 (22–62), D = 34.7 (18–64) | yes |
| Royall, 2012 | subthreshold (GDS) | retirement community | 547 | NR | 77.9 (60–100) | NR |
| Salvat-Pujol, 2017 | MDD | outpatient clinic | 150 | on | C = 56.6, D = 57.9 | no |
| Sanders, 2006 | MDD, minor depression | outpatient clinic | 361 | NR | C = 74.7 (65–94), D = 74.6 (67–86) | NR |
| Schmid, 2013 | MDD | outpatient clinic | 60 | on | C = 26.2, D = 26.2 | yes |
| Shehab, 2016 | MDD | outpatient clinic | 48 | on | C = 14.3 (12–18), D = 14.8 (12–18) | yes |
| Sheline, 2006 | MDD | outpatient clinic | 155 | on | 68.7 | no |
| Shimada, 2014 | subthreshold depression | community | 4265 | NR | C = 71.5 (65–97), D = 73.2 (65–97) | NR |
| ≥ 6 GDS-15 | community | 3782 | NR | C = 71.5 (65–97), D = 71.1 (65–97) | NR | |
| Siegle, 2004 | MDD | NR | 51 | off | C = 31.1 (21–50), D = 40.3 (19–55) | NR |
| Tanaka, 2012 | ≥ 6 GDS-15 | NR | 35 | NR | C = 73.4 (65+), D = 72.9 (65+ | NR |
| Taylor Tavares, 2007 | MDD | outpatient clinic | 47 | off | C = 34.8 (18–59), D = 38.6 (18–59) | yes |
| Tu, 2012 | MDD | outpatient clinic | 72 | on | C = 41.8, D = 41.6 | yes |
| Uemura, 2014 | ≥ 6 GDS-15 | NR | 80 | off | C = 73.8 (65–86), D = 74.5 (68–84) | NR |
| Vergara-Lopez, 2013 | subthreshold (YSR) | community | 373 | NR | 13.1 (12–14) | NR |
| Wagner, 2015 | mixed (MDD, dysthymia, minor depression) | grade school | 486 | NR | 12.8 (12–13) | NR |
| Wingo, 2013 | subthreshold (BDI-II) | college | 77 | NR | 19.01 | NR |
| Zhang, 2007 | MDD | outpatient clinic | 39 | on | D = 68.7 (60+) C = matched | NR |
| Zhang, 2016 | MDD | outpatient clinic | 36 | on | C = 68.1, D = 70.8 | yes |
Note. MDD = major depressive disorder, C = control group, D = depressed group, RBD = recurrent brief depression, CES-D = Center for Epidemiologic Studies-Depression Scale, NR = not reported, NOS = depressive disorder not otherwise specified, BDI = Beck Depression Inventory, DISC-IV = Diagnostic Interview Schedule for Kids, Version Four, GDS = Geriatric Depression Scale, YSR = Youth Self Report
Comorbid psychological disorders were not excluded, but the authors did not specifically report anxiety disorders
On medications except for the day of testing
The average methodological quality of the included studies was 7.56 (0.80) out of 9. Most studies did not meet the criteria of explaining how the study size was determined.
Depression and Cognitive Control
A summary of the cognitive control measures in each study is provided in Table 3. Many of the studies (n = 30) included in the meta-analysis demonstrated a statistically significant relationship between depression and cognitive control, reflected in lower performance in the depressed group compared to controls or a negative correlation between depressive symptom severity and scores on cognitive control measures (Fig. 2). None of the included studies demonstrated statistically significant differences in the opposite direction. We assessed the need for a three-level model by fitting a two-level model and comparing its fit with that of the three-level model. The difference in fit was highly significant, χ2 (1) = 12.45, p < .001, with better fit for the three-level model. All data reported will therefore be from three-level models.
Table 3.
Cognitive Tests and Domains
| First Author, Year | Cognitive Test(s) | Domain |
|---|---|---|
| Aarts, 2013 | Go/No-Go (number error, number hit, speed error, speed hit) | Inhibition |
| Aizenstein, 2009 | Preparing to Overcome Prepotency (RT) | Inhibition |
| Alexopoulos, 2015 | Stroop (interference score) | Inhibition |
| TOL (total moves across 10 trials) | Planning | |
| Andersson, 2010 | D-KEFS Tower | Planning |
| Stroop (interference score) | Inhibition | |
| Baudic, 2004 | Haylings Test-B (errors) | Inhibition |
| MCST (number of categories) | Planning | |
| MCST (perseverations) | Planning | |
| Stroop (interference score) | Inhibition | |
| TMT-B (completion time) | Cognitive Flexibility | |
| Baune, 2006 | Stroop (CW – C time) | Inhibition |
| Bobb, 2012 | Stop Signal Task (RT) | Inhibition |
| Boone, 1995 | Stroop (CW) | Inhibition |
| WCST (number of categories, perseverative responses) | Planning | |
| Boyle, 2010 | TMT-B (completion time) | Cognitive Flexibility |
| Bredemeier, 2016 | Plus-Minus Task (switch cost) | Set Shifting |
| Stop Signal Task (RT) | Inhibition | |
| Brevik, 2013 | D-KEFS CW Interference Test | Inhibition |
| Brewster, 2017 | Compositea | Composite |
| Brooks, 2010 | CNS Vital Signs Stroop (complex RT, RT, simple RT) | Inhibition |
| Bunce, 2008 | Shape-color Switching Task (RT) | Set Shifting |
| Stroop (incongruent RT) | Inhibition | |
| Butters, 2004 | Stroop (interference score) | Inhibition |
| TMT-B (completion time) | Cognitive Flexibility | |
| WCST (number of errors) | Planning | |
| Channon, 1996 | WCST (nonperseverative errors, perseverative errors, trials to completion) | Planning |
| Channon, 1999 | Response Suppression Test (categorized completions, nonsensical sentences errors, total time) | Inhibition |
| Charlton, 2014 | Compositeb | Composite |
| Clawson, 2013 | Modified Flanker (incongruent preceded by congruent trial errors and RT, incongruent preceded by incongruent trial errors and RT) | Inhibition |
| Constant, 2005 | Stroop (interference score) | Inhibition |
| Egger, 2008 | TMT-B (completion time) | Cognitive Flexibility |
| Elderkin-Thompson, 2006 | WCST (number of categories) | Planning |
| Stroop (interference score) | Inhibition | |
| Favre, 2008 | Stroop (interference score) | Inhibition |
| TMT-B (completion time, errors) | Cognitive Flexibility | |
| WCST (number of categories, loss of set, perseverative errors) | Planning | |
| Franz, 2011 | D-KEFS Fluency Switching | Set Shifting |
| D-KEFS Trails Switching | Cognitive Flexibility | |
| Stroop (interference score) | Inhibition | |
| Godard, 2011 | Conditional RT Test (commission errors) | Inhibition |
| Grant, 2001 | TMT-B (completion time) | Cognitive Flexibility |
| WCST (number of categories, total errors, perseverative responses, perseverative errors, loss of set, learning to learn) | Planning | |
| Intra-Dimensional Extra-Dimensional Set Shifting (stages completed, pre-extradimensional errors, adjusted extra-dimensional shifting errors) | Set Shifting | |
| Stocking of Cambridge (problems solved in minimum moves, initial thinking time for 5, 4, 3, and 2 moves) | Planning | |
| Gualtieri, 2006 | Compositec | Composite |
| Halari, 2009 | Simon Task (Simon effect RT, errors to Simon trials) | Inhibition |
| Stop Signal Task (probability of inhibition, stop signal RT, mean RT) | Inhibition | |
| Switch Task (switch cost, errors to switch trials) | Inhibition | |
| Halvorsen, 2012 | D-KEFS CW Inhibition | Inhibition |
| D-KEFS CW Inhibition/Switching | Set Shifting | |
| Halvorsen, 2012 | D-KEFS Fluency switching | Set Shifting |
| TMT-B (completion time) | Cognitive Flexibility | |
| WCST-64 (number of categories, perseverative responses) | Planning | |
| Han, 2016 | WCST (number of trials, nonperseverative errors, perseverative errors, total errors) | Planning |
| Harper, 2016 | Stroop (interference score) | Inhibition |
| Hermens, 2010 | TMT-B (completion time) | Cognitive Flexibility |
| Hill, 2013 | TMT-B (completion time) | Cognitive Flexibility |
| WCST (percent errors) | Planning | |
| Holmes, 2008 | Stroop (accuracy, RT) | Inhibition |
| Jungwirth, 2011 | TMT-B (completion time) | Cognitive Flexibility |
| Katz, 2010 | Go/No-Go (percent hits, percent false alarm, RT) | Inhibition |
| Kaymak, 2010 | TMT-B (completion time) | Cognitive Flexibility |
| Verbal fluency-alternation | Set Shifting | |
| WCST (percent conceptual level responses, perseverative errors, total errors, trials to first category) | Planning | |
| Kikuchi, 2012 | Stroop CW (error rate, RT) | Inhibition |
| Kindermann, 2000 | Stroop (CW total) | Inhibition |
| WCST (perseverative errors, total errors, loss of set) | Planning | |
| Klojcnik, 2017 | Stroop (interference score) | Inhibition |
| Klojcnik, 2017 | TMT-B (completion time) | Cognitive Flexibility |
| Koenig, 2015 | Stroop | Inhibition |
| TMT-B (completion time) | Cognitive Flexibility | |
| WCST (total errors) | Planning | |
| Krompinger, 2011 | Stroop (incongruent accuracy, RT) | Inhibition |
| Ladouceur, 2012 | Flanker (incongruent percent errors, RT) | Inhibition |
| Langanecker, 2005 | Go/No-Go (No-Go RT easy trials, No-Go RT difficult trials, No-Go % correct inhibition easy trials, No-Go % correct inhibition difficult trials | Inhibition |
| Lyche, 2011 | ANT executive (RT) | Inhibition |
| D-KEFS CW Inhibition | Inhibition | |
| D-KEFS CW Switching | Inhibition | |
| Maalouf, 2011 | Stocking of Cambridge (number of moves) | Planning |
| Mackin, 2009 | Stroop (CW total) | Inhibition |
| TMT-B (completion time) | Cognitive Flexibility | |
| WCST-64 (total errors) | Planning | |
| Naismith, 2006 | Stroop | Inhibition |
| Naismith, 2006 | TOL | Planning |
| TMT-B (completion time) | Cognitive Flexibility | |
| WCST (total errors) | Planning | |
| Oral, 2012 | Stroop 5 (reading time) | Inhibition |
| TMT-B (completion time) | Cognitive Flexibility | |
| WCST (number of categories, percent total correct, percent error, trials to first category) | Planning | |
| Pantzar, 2014 | TMT-B (completion time) | Cognitive Flexibility |
| TMT-B (completion time) | Cognitive Flexibility | |
| Pantzar, 2017 | TMT-B (completion time) | Cognitive Flexibility |
| Philippot, 2017 | Stroop (interference errors) | Inhibition |
| TMT-B (errors) | Cognitive Flexibility | |
| Pizzagalli, 2006 | Flanker (Eriksen effect accuracy and RT) | Inhibition |
| Porter, 2003 | TOL (number of excess moves, average initial thinking time, average subsequent thinking time, perfect solution percent) | Planning |
| Purcell, 1998 | TOL (number of excess moves, initial movement time, initial thinking time, perfect solution percentage, subsequent movement time, subsequent thinking time) | Planning |
| Quinn, 2012 | Go/No-Go (commission errors, omission errors, RT, RT variability) | Inhibition |
| Rainer, 2006 | TMT-B (completion time) | Cognitive Flexibility |
| Rajtar-Zembaty, 2017 | Go/No-Go (accuracy) | Inhibition |
| TMT-B (completion time) | Cognitive Flexibility | |
| Rizk, 2017 | Stroop (interference RT) | Inhibition |
| Royall, 2012 | TMT-B (completion time) | Cognitive Flexibility |
| Salvat-Pujol, 2017 | Neuropsychological Assessment Battery Mazes | Planning |
| Stroop (CW, interference) | Inhibition | |
| TMT-B (completion time) | Cognitive Flexibility | |
| Sanders, 2006 | TMT-B (completion time) | Cognitive Flexibility |
| Schmid, 2013 | D-KEFS CW Inhibition | Inhibition |
| D-KEFS CW Inhibition Switching | Set Shifting | |
| D-KEFS Fluency Switching | Set Shifting | |
| D-KEFS Tower | Planning | |
| D-KEFS Trail Making Switching | Cognitive Flexibility | |
| Shehab, 2016 | Stockings of Cambridge (number of moves) | Planning |
| Sheline, 2006 | Composited | Composite |
| Shimada, 2014 | TMT-B (completion time) | Cognitive Flexibility |
| Siegle, 2004 | Stroop (incongruent accuracy, RT) | Inhibition |
| Tanaka, 2012 | Stroop (incongruent - congruent accuracy, RT) | Inhibition |
| Taylor Tavares, 2007 | Intra-Dimensional Extra-Dimensional Set Shifting (stages completed, total errors) | Set Shifting |
| Tu, 2012 | Color TMT-B | Cognitive Flexibility |
| WCST (number of trials, nonperseverative errors, perseverative errors) | Planning | |
| Uemura, 2014 | TMT-B (“points”) | Cognitive Flexibility |
| Vergara-Lopez, 2013 | Stop Signal Task (RT) | Inhibition |
| TOL | Planning | |
| WCST (perseverative errors) | Planning | |
| Wagner, 2015 | TEA-Ch Set Shifting (accuracy, RT) | Set Shifting |
| Wingo, 2013 | D-KEFS CW Inhibition | Set Shifting |
| D-KEFS Trail Making Number-Letter | Cognitive Flexibility | |
| Wingo, 2013 | Sequencing Stop Signal Task (RT) | Inhibition |
| Zhang, 2007 | Go/No-Go (commission errors, omission errors, RT) | Inhibition |
| WCST (“random error”, “total trial”, perseverative errors | Planning | |
| Zhang, 2016 | Go/No-Go (hit rate, RT) | Inhibition |
Note. RT = reaction time, TOL = Tower of London, D-KEFS = Delis-Kaplan Executive Function System, MCST = Modified Cart Sorting Test, TMT = Trail Making Test, CW = Color-Word, C = Color, WCST = Wisconsin Card Sorting Test, ANT = Attention Network Test
Composite comprised Stroop Color Word Test, and Trail Making Test
Composite comprised D-KEFS Category Switching (total correct), TMT-B (completion time), Stroop (interference score), Wechsler Adult Intelligence Scale, Third Edition Digits Backward (total score), and Self-ordered Pointing Task (number of errors)
Composite comprised CNS Vital Signs Stroop (RT) and Shifting Attention Test
Composite comprised phonological and semantic fluency, TMT-B, Stroop CW, Dementia Rating Scale Initiation and Perseveration score, and WCST number of categories
Fig. 2.
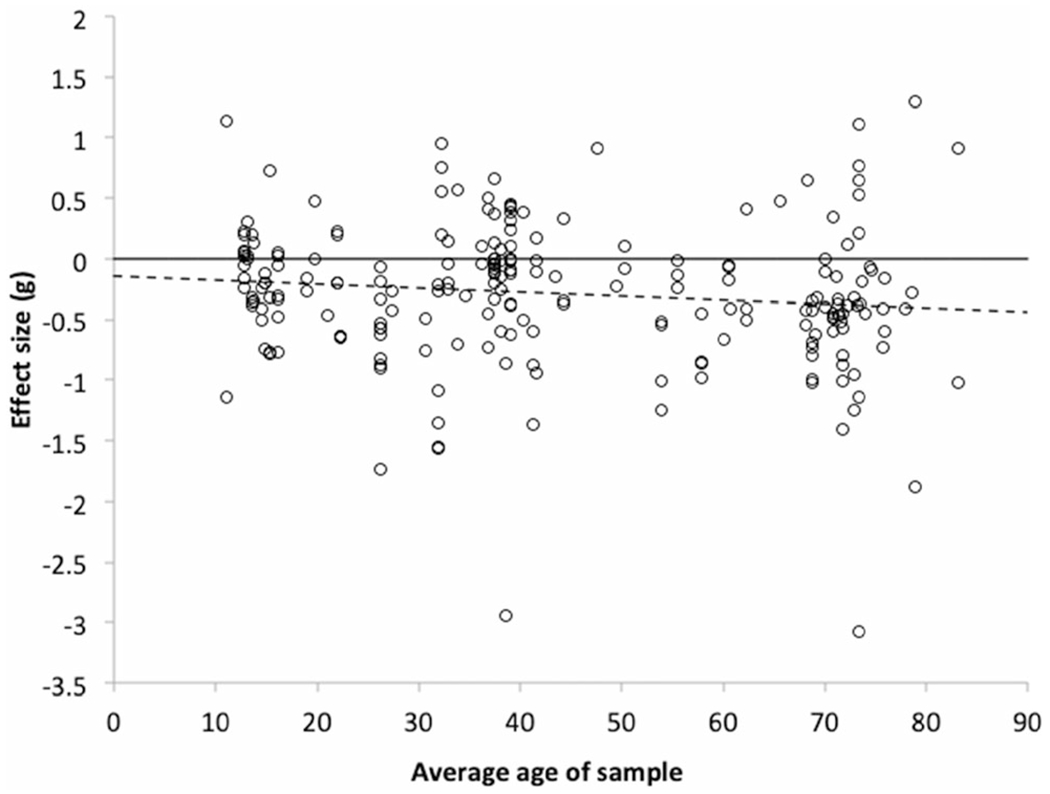
Average effect of depression on measures of cognitive control as a function of average age of sample, with the best fitting regression line as determined by three-level modeling (dashed); all available data points are represented
In the overall three-level model, the average estimated effect size was significantly different from zero (g = −0.31; 95% CIs ranging from −0.39 to −0.23; p < 0.0001). Heterogeneity was significant (Q (221) = 1307.42, p < 0.0001; tau2 at Level 2 was 0.13, p < .0001, and tau2 at Level 3 was 0.05, p = .012; I2 was .65 at Level 2 and .26 at Level 3), indicating the need for moderator analyses. Regression analysis on the funnel plot on all data points revealed no sign of asymmetry (bias <0.01; p = .75), thus providing no indication of publication bias. Note, however, that funnel plot analyses presuppose independence of data points, an assumption violated here, and so the test is only an approximation.
Age Effects
The relation between mean age and study effect size was significant, with a slope of −0.0038 (95% CI between −.0072 and −.0004, p = .027), indicating that each additional year of age added −.0038 to the effect size (see Fig. 2), R2 = .004 at Level 2 and .168 at Level 3. Heterogeneity was significant (Q (219) = 1306.17, p < 0.0001, tau2 at Level 2 was 0.13, p < .0001, and tau2 at Level 3 was 0.02, p = .024). Adding a quadratic component for age to test for non-linear effects yielded a non-significant result (slope for the quadratic component = 0.03, 95% CI = −0.06 to 0.12, p = .64; R2 = .007 at Level 2 and .168 at Level 3). Heterogeneity was significant (Q (219) = 1306.17, p < 0.0001, tau2 at Level 2 was 0.13, p < .0001, and tau2 at Level 3 was 0.04, p = .022). These p values are based on the Wald test. Because this test is based on the assumption that the sampling variances are normally distributed, an assumption likely violated here because of the small sample size, these p values are possibly inaccurate.
Limiting ourselves to age subgroupings that contained more than four studies, Hedges’ g effect size for Adolescents was −0.10 (95% CI from −0.27 to 0.06, p = .22; Q[24] = 179.82, p < 0.0001; tau2 at Level 2 was 0.07, p = .13, and tau2 at Level 3 was 0.01, p = .78; I2 = .79 at Level 2 and .11 at Level 3). For Young Adults, Hedges’ g was −.08 (95% CI from −0.31 to 0.15, p = .49; Q[14] = 57.96, p < 0.0001; tau2 at level 2 was 0.03, p = .20, and tau2 at level 3 was 0.05, p = .29; I2 = .27 at Level 2 and .48 at Level 3). For Young to Middle Aged Adults, Hedges’ g was −0.34 (95% CI from −0.77 to 0.09; Q[18] = 73.97, p < 0.0001; tau2 at Level 2 was 0.09, p = .28, and tau2 at Level 3 was 0.25, p = .17; I2 = .21 at Level 2 and .61 at Level 3). For Adults, Hedges’ g was −0.37 (95% CI from −0.52 to − 0.21, p < .0001; Q[66[= 205.32, p < 0.0001; tau2 at Level 2 was 0.07, p = .0056, and tau2 at Level 3 was 0.05, p = .10; I2 = .41 at Level 2 and .30 at Level 3). For Older Adults, Hedges’ g was −0.45 (95% CI from −0.59 to −0.31, p < .0001; Q[60]= 311.31, p < 0.0001; tau2 at Level 2 was 0.21, p = .0001, and tau2 at Level 3 was 0.00, p < .0001; I2 = .89 at Level 2 and .00 at Level 3).
Other Moderators
We performed a subgroup analysis to separately examine the association of clinical and subthreshold depression with cognitive control. For this subgroup analysis, when studies reported data for varying degrees of symptom severity among individuals diagnosed with depression (e.g., Boone et al., 1995), only data from individuals with more severe symptoms were included to minimize bias from multiple data points from a single study. Adding a term for depression status to the regression did not yield a significant effect (slope of the regression line = −0.13, 95% CI = −0.33 to 0.07, p = .20; R2 < .001 at Level 2 and .087 at Level 3). Heterogeneity was significant (Q [213] = 1115.26, p < 0.0001; tau2 at Level 2 was 0.11, p < .0001, and tau2 at Level 3 was 0.05, p = .009). Note that in our analyses of the influence of age, significant effects only appeared in the older groups. Therefore, we split our sample of studies by median average age of participant sample, only retaining studies with participants with an average age older than this median split (i.e., age 39 or over). In that analysis, depression status did yield a significant effect, increasing the average effect size from −0.16 in the subthreshold group to −0.44 in the group with participants with clinical diagnosis (slope of the regression line = −0.28, 95% CI = −0.54 to −0.03, p = .031; R2 = .010 at Level 2 and .539 at Level 3). Heterogeneity was significant at Level 2 but not Level 3 (Q[96] = 615.61, p < 0.0001; tau2 at Level 2 was 0.17, p < .0001, and tau2 at Level 3 was 0.01, p = .617).
We did not find a significant effect of antidepressant medication status (slope of the regression line = −0.08, 95% CI = −0.27 to 0.10, p = .38; R2 = .002 at Level 2 and .021 at Level 3). Heterogeneity was significant (Q [174] = 583.62, p < 0.0001; tau2 at Level 2 was 0.05, p = .0005, and tau2 at Level 3 was 0.07, p = .002). When we restricted the analysis to studies with participants with an average age older than the median split (i.e., age 39 or over), however, medication status yielded a significant effect (slope of the regression line = −0.29, 95% CI = −0.53 to −0.05, p = .016; R2 = .039 at Level 2 and .221 at Level 3). Heterogeneity was significant at Level 2, but not Level 3 (Q [71] = 291.74, p < 0.0001; tau2 at Level 2 was 0.06, p = .024, and tau2 at Level 3 was 0.05, p = .073). The average effect size was −0.19 in the studies that only assessed individuals who were not taking antidepressants, compared to −0.48 in studies that assessed individuals who were currently taking antidepressants.
We did not find significant effects for cognitive domain. We used dummy variables in the regression to test for the effects of domain, with inhibition serving as the baseline (intercept = −0.32, 95% CI = −0.43 to −0.21, p < .0001; slope for cognitive flexibility = 0.02, 95% CI = −0.15 to 0.18, p = .85; slope for planning = −0.00, 95% CI = −0.19 to 0.18, p = .97; slope for set-shifting = 0.06, 95% CI = −0.21 to 0.33, p = .65; R2 = .003 at Level 2 and .003 at Level 3). Heterogeneity was significant (Q [216] = 1280.61, p < 0.0001; tau2 at Level 2 was 0.13, p < .0001, and tau2 at Level 3 was 0.05, p = .013). Cognitive domain also failed to be a significant moderator when we restricted the analyses to studies with participants with an average age older than the median split (i.e., age 39 or over: intercept = −0.43, 95% CI = −0.58 to −0.26, p < .0001; slope for cognitive flexibility = −0.05, 95% CI = −0.30 to 0.20, p = .70; slope for planning = 0.12, 95% CI = −0.12 to 0.36, p = .34; slope for set-shifting = 0.31, 95% CI = −0.31 to 0.20, p = .32; R2 = .014 at Level 2 and .236 at Level 3). Heterogeneity was significant at Level 2 (Q [94] = 601.72, p < 0.0001; tau2 at Level 2 was 0.17, p < .0001, and tau2 at Level 3 was 0.02, p = .47).
The effect of comorbid anxiety was not significant (slope of the regression line = 0.07, 95% CI = −0.19 to 0.34, p = .59; R2 = .000 at Level 2 and .086 at Level 3). Heterogeneity was significant at Level 2 (Q [108] = 492.17, p < 0.0001; tau2 at Level 2 was 0.13, p < .0001, and tau2 at Level 3 was 0.05, p = .14). Comorbid anxiety still failed to be a significant predictor when we restricted the analyses to studies with participants with an average age older than the median split (i.e., age 39 or over: slope of the regression line = −0.02, 95% CI = −0.46 to 0.41,p = .92; R2 = .010 at Level 2 and .000 at Level 3). Heterogeneity was not significant at either Level 2 or 3 (Q [23] = 92.42, p < 0.0001; tau2 at Level 2 was 0.08, p = .14, and tau2 at Level 3 was 0.05, p = .41).
The effect of test format was not significant (slope of the regression line = −0.05, 95% CI = −0.21 to 0.10, p = .47; R2 = .004 at Level 2 and .001 at Level 3). Heterogeneity was significant (Q [221] = 1307.42, p < 0.0001; tau2 at Level 2 was 0.13, p < .0001, and tau2 at Level 3 was 0.05, p = .012). Test format was likewise not a significant predictor when we restricted the analyses to studies with participants with an average age older than the median split (slope of the regression line = −0.13, 95% CI = –−0.35 to 0.09, p = .22; R2 = .022 at Level 2 and .000 at Level 3). Heterogeneity was significant at Level 2 (Q [98] = 616.95, p < 0.0001; tau2 at Level 2 was 0.16, p < .0001, and tau2 at Level 3 was 0.02, p = −35).
Discussion
This systematic review and meta-analysis of 16,806 participants across 76 studies provides additional evidence of cognitive control deficits in community-dwelling individuals with both major and subthreshold depression, confirming previous meta-analyses of executive functioning in depression (e.g., Lee et al., 2012; McDermott & Ebmeier, 2009; Rock et al., 2014). The breadth of our literature review, which included both major and subthreshold depression as well as studies across the lifespan, allowed us to examine important moderators of the relationship between cognitive control deficits and depression that have remained unexamined in prior meta-analyses. Consistent with our hypothesis, the relationship between cognitive control deficits and depression was stronger in later stages of the lifespan. Subgroup analyses showed that effect sizes did not significantly vary based on cognitive domain (cognitive flexibility, inhibition, and planning), comorbid anxiety, or test format (computerized vs. paper-and-pencil). In studies with a mean sample age of 39 or older, the effect was stronger in studies that examined individuals with clinical depression compared to subthreshold depression, and in individuals who were taking antidepressant medication.
Regarding age differences, we found that effect sizes were larger as a function of older mean age of the study sample, and were largest in studies that included only older adults in the sample. Subsample analysis showed that depression was only significantly associated with cognitive control performance in studies that included adult, middle-aged or older adult participants, and not in those that included only children, adolescents or young adults. A recent meta-analysis in depressed youth found no depression-related differences in set-shifting and inhibition (Goodall et al., 2018), in contrast to meta-analyses of adult and older adult samples that reported significant cognitive control deficits in depressed compared to non-depressed groups (Lee et al., 2012; McDermott & Ebmeier, 2009; Rock et al., 2014).
A number of cross-sectional and longitudinal studies have shown that older age is associated with increased vulnerability to depression-related cognitive deficits and decline (Dotson et al., 2008; Dotson, Zonderman, Davatzikos, Kraut, & Resnick, 2009; Lockwood et al., 2002; Thomas et al., 2009). This vulnerability could be due at least in part to age-related changes in some of the neurobiological mechanisms related to depression, such as structural and functional changes in frontolimbic brain networks, vascular changes such as increased white matter lesions in the brain, decreased brain-derived neurotrophic factor, and increased inflammation (Naismith, Norrie, Mowszowski, & Hickie, 2012). It is possible that the cumulative effect of age-related neurobiological changes and depression-related alterations in similar mechanisms creates a “double jeopardy” for cognitive dysfunction, including cognitive control deficits. It is also possible that factors such as medical comorbidities, depression severity, and the type of tests used varied between studies of different age groups and contributed to the age difference observed in the meta-analysis. These variables were controlled for in some studies, thus the effect sizes took these factors into account. However, since not all studies did so, differences in clinical variables and study methodology might have impacted the current results.
Also important to consider is the confound between age and chronicity of depression. At later stages of the lifespan, the possibility of chronic depression or recurrent depression is higher. There is evidence that the risk for cognitive decline and dementia increases in individuals who have experienced multiple episodes of depression, even after controlling for age (Dotson, Beydoun, & Zonderman, 2010; Hasselbalch, Knorr, Hasselbalch, Gade, & Kessing, 2013). Another longitudinal study showed that chronic subthreshold depressive symptoms in adults age 50 years and older were associated with cognitive deficits over up to 26 years (Dotson et al., 2008). These deficits were more widespread than those associated with baseline depressive symptoms and concurrent symptoms (i.e., measured at the same time as the cognitive assessment). Since most studies of depression and cognitive control do not provide information about past depressive episodes or depressive symptoms, the current meta-analysis could not disentangle the impact of age versus chronicity on cognitive control. Nonetheless, given the link between executive functions and functional disability as well as poor treatment outcomes (Manning et al., 2015; Snyder, 2013), age differences in the current study highlight the importance of assessing possible cognitive control deficits in adults, and particularly older adults, with depression.
Overall, the relationship between cognitive control deficits and depression was significant in studies of both clinical depression and subthreshold depression. Depression has increasingly been recognized as a continuum that ranges from subthreshold symptoms to severe major depression (Hybels, Blazer, & Pieper, 2001; Rodriguez, Nuevo, Chatterji, & Ayuso-Mateos, 2012). There is accumulating evidence that even subthreshold symptoms are associated with multiple negative outcomes, including cognitive deficits, structural and functional brain abnormalities, functional disability, and poor health outcomes (Hybels et al., 2001; Meeks, Vahia, Lavretsky, Kulkami, & Jeste, 2011). Some studies suggest that the vulnerability to negative sequelae due to subthreshold symptoms is greater in older compared to younger adults (Dotson et al., 2008; Dotson et al., 2014; Dotson et al., 2009; Shah, Zonderman, & Waldstein, 2013). Since the studies of subthreshold depression in our meta-analysis did not include children and only three studies of adolescents were included, we cannot make a direct comparison of the relationship between cognitive control and subclinical depression in children compared to adults. However, the larger effect sizes in studies with older samples in our overall analysis suggests that depressive symptoms of any severity are a particular risk for cognitive control deficits in adulthood.
In the full sample of studies, the relationship between depression and cognitive control was not impacted by antidepressant medication status. When we restricted the analysis to studies with a mean age of 39 years or higher, which represented the median age in our meta-analysis, we found that effect sizes were significantly larger in studies that included individuals taking antidepressant medication. The finding regarding antidepressant medication does not appear to be related to depression status (clinical vs. subclinical) since nearly all of the subclinical depression studies in the meta-analysis did not report antidepressant use, and thus were excluded in the respective subsample analysis. Nonetheless, the finding might reflect the symptom severity in those studies that focused on clinical depression, and in those studies that included individuals who were taking antidepressant medication. Given the stronger relationship between depression and cognitive control in studies with older mean ages, this finding might reflect an interactive effect of age and depression severity on cognitive control. Another possible explanation could be the direct effect of antidepressant medication on cognitive control, as some studies have suggested that chronic antidepressant use can negatively impact cognitive functioning (Deakin, Rahman, Nestor, Hodges, & Sahakian, 2004; Paterniti, Dufouil, Bisserbe, & Alperovitch, 1999; Wadsworth, Moss, Simpson, & Smith, 2005). However, a recent meta-analysis found that antidepressant use had a positive, though modest effect on executive functioning, as well as divided attention, sustained attention, immediate memory, recent memory, and processing speed (Prado, Watt, & Crowe, 2018).
We selected studies for this meta-analysis based on stringent criteria that would provide a focus on unipolar depression in community-dwelling individuals who did not have significant psychological or medical comorbidities. This selection strategy allowed for greater attribution of the effect to depression rather than the impact of other disorders that have known associations with executive dysfunction or with neurobiological mechanisms underlying cognitive control. However, given the high comorbidity of depression with other disorders, the limitations in generalizability must be acknowledged. Our understanding of the relationship between depression and cognitive control will benefit from synthesis of the literature in other subgroups of depression, including vascular depression in older adults and depression in various medical populations across the lifespan. There is increasing recognition of the clinical and demographic heterogeneity within depressed individuals, and how those differences might impact depression correlates (Dotson, 2017). For example, demographic variables such as sex and race have been shown to moderate the relationship between depression and cognitive performance (Reinlieb et al., 2014; Sundermann, Katz, & Lipton, 2017). Moreover, different symptom dimensions of depression (e.g., anhedonia, sad mood, cognitive symptoms, and somatic symptoms) are differentially associated with numerous outcomes, including cognitive performance, structural and functional brain alterations, and response to treatment (Brailean et al., 2016; Fried & Nesse, 2015; McLaren et al., 2016). As more studies focus on parsing this heterogeneity, additional meta-analyses will inform our understanding of the relationship between different components of depression across the lifespan.
Publication bias may have influenced our findings. Publication bias is the result of the file-drawer phenomenon, namely, studies reporting null or negative findings are less likely to be published in peer-reviewed journals (Hopewell, Loudon, Clarke, Oxman, & Dickersin, 2009). Though the estimates of publication bias included in the present meta-analyses did not suggest the presence of such bias, its presence cannot be completely ruled out. In addition, inclusion of unpublished data and data from the gray literature (e.g., theses and dissertations) has been shown to influence meta-analytic results (Hopewell, Clarke, & Mallett, 2005). Unpublished data were not included in the present meta-analysis, as such, our estimate of the overall effect may be somewhat inflated.
Conclusion
This systematic review and meta-analysis showed a modest but significant relationship between depression and cognitive control in studies of generally healthy, community-dwelling individuals across the lifespan. Both clinical depression and subthreshold depression were associated with cognitive control deficits. This relationship was stronger in study samples with an older mean age, and within adult samples, but not child and adolescent samples. Within studies with a mean age of 39 years or higher, the relationship was stronger in clinical compared to subthreshold depression and in individuals taking antidepressant medication. The results of this study highlight the importance of clinicians screening for cognitive control dysfunction in patients with depression, particularly in later stages of adulthood.
Funding
This work was supported by funding from the National Institute of Mental Health (SMM) and the Georgia State and University Brains & Behavior graduate student fellowship (AAB and AMG).
References
- Alvarez JA, & Emory E (2006). Executive function and the frontal lobes: A meta-analytic review. Neuropsychology Review, 16(1), 17–42. [DOI] [PubMed] [Google Scholar]
- Beats BC, Sahakian BJ, & Levy R (1996). Cognitive performance in tests sensitive to frontal lobe dysfunction in the elderly depressed. Psychological Medicine, 26(3), 591–603. [DOI] [PubMed] [Google Scholar]
- Boone KB, Lesser IM, Miller BL, Wohl M, Berman N, Lee A, … Back C (1995). Cognitive functioning in older depressed outpatients: Relationship of presence and severity of depression to neuropsychological test scores. Neuropsychology, 9(3), 390–398. 10.1037/0894-4105.9.3.390. [DOI] [Google Scholar]
- Brailean A, Comijs HC, Aartsen MJ, Prince M, Prina AM, Beekman A, & Huisman M (2016). Late-life depression symptom dimensions and cognitive functioning in the longitudinal aging study Amsterdam (LASA). Journal of Affective Disorders, 201, 171–178. 10.1016/j.jad.2016.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung MW (2014a). metaSEM: An R package for meta-analysis using structural equation modeling. Frontiers in Psychology, 5, 1521. 10.3389/fpsyg.2014.01521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung MW (2014b). Modeling dependent effect sizes with three-level meta-analyses: A structural equation modeling approach. Psychological Methods, 19(2), 211–229. 10.1037/a0032968 [DOI] [PubMed] [Google Scholar]
- Cohen J (1988). Statistical power analysis for the behavioral sciences (2nd ed.). Hillsdale, N.J: L. Erlbaum Associates. [Google Scholar]
- Collins PY, Patel V, Joestl SS, March D, & Insel TR (2011). Grand challenges in global mental health. Nature, 474(7354), 27–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deakin JB, Rahman S, Nestor PJ, Hodges JR, & Sahakian BJ (2004). Paroxetine does not improve symptoms and impairs cognition in frontotemporal dementia: A double-blind randomized controlled trial. Psychopharmacology, 172(4), 400–408. 10.1007/s00213-003-1686-5 [DOI] [PubMed] [Google Scholar]
- Dotson VM (2017). Variability in cepression: What have we been missing? American Journal of Geriatric Psychiatry, 25(1), 23–24. 10.1016/j.jagp.2016.10.005 [DOI] [PubMed] [Google Scholar]
- Dotson VM, Beydoun MA, & Zondemran AB (2010). Recurrent depressive symptoms and the incidence of dementia and mild cognitive impairment. Neurology, 75(1), 27–34. 10.1212/WNL.0b013e3181e62124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotson VM, Resnick SM, & Zonderman AB (2008). Differential association of concurrent, baseline, and average depressive symptoms with cognitive decline in older adults. American Journal of Geriatric Psychiatry, 16(4), 318–330. 10.1097/JGP.0b013e3181662a9c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotson VM, Szymkowicz SM, Kirton JW, McLaren ME, Green ML, & Rohani JY (2014). Unique and interactive effect of anxiety and depressive symptoms on cognitive and brain function in young and older adults. Journal of Depression and Anxiety, Suppl, 1 10.4172/2167-1044.S1-003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotson VM, Zonderman AB, Davatzikos C, Kraut MA, & Resnick SM (2009). Frontal atrophy and attention deficits in older adults with a history of elevated depressive symptoms. Brain Imaging and Behavior, 3(4), 358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried EI, & Nesse RM (2015). Depression sum-scores don’t add up: Why analyzing specific depression symptoms is essential. BMC Medicine, 13, 72. 10.1186/s12916-015-0325-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaynes BN, Rush AJ, Trivedi MH, Wisniewski S, Balasubramani GK, Spencer DC, … Fava M (2007). Major depression symptoms in primary care and psychiatric care settings: A cross-sectional analysis. Annals of Family Medicine, 5(2), 126–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodall J, Fisher C, Hetrick S, Phillips L, Parrish EM, & Allott K (2018). Neurocognitive functioning in depressed young people: A systematic review and meta-analysis. Neuropsychology Review, 28(2), 216–231. 10.1007/s11065-018-9373-9 [DOI] [PubMed] [Google Scholar]
- Hasselbalch BJ, Knorr U, Hasselbalch SG, Gade A, & Kessing LV (2013). The cumulative load of depressive illness is associated with cognitive function in the remitted state of unipolar depressive disorder. European Psychiatry, 28(6), 349–355. 10.1016/j.eurpsy.2012.03.004 [DOI] [PubMed] [Google Scholar]
- Hedges LV (1981). Distribution theory for glass’s estimator of effect size and related estimators. Journal of Educational Statistics, 6(2), 107–128. 10.2307/1164588 [DOI] [Google Scholar]
- Hopewell S, Clarke M, & Mallett S (2005). Grey literature and systematic reviews. In Rothstein H, Sutton AJ, & Borenstein M (Eds.), Publication bias in meta-analysis : Prevention, assessment and adjustments (pp. 49–72). Chichester, England: Wiley. [Google Scholar]
- Hopewell S, Loudon K, Clarke MJ, Oxman AD, & Dickersin K (2009). Publication bias in clinical trials due to statistical significance or direction of trial results. Cochrane Database of Systematic Reviews, 1. 10.1002/14651858.MR000006.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hybels CF, Blazer DG, & Pieper CF (2001). Toward a threshold for subthreshold depression: An analysis of correlates of depression by severity of symptoms using data from an elderly community sample. Gerontologist, 41(3), 357–365. [DOI] [PubMed] [Google Scholar]
- Koenig AM, Bhalla RK, & Butters MA (2014). Cognitive functioning and late-life depression. Journal of the International Neuropsychological Society), 20(5), 461–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak MJ, & Cuthbert BN (2016). The NIMH research domain criteria initiative: Background, issues, and pragmatics. Psychophysiology, 53(3), 286–297. [DOI] [PubMed] [Google Scholar]
- Lee RSC, Hemrens DF, Porter MA, & Redoblado-Hodge MA (2012). A meta-analysis of cognitive deficits in first-episode major depressive disorder. Journal of Affective Disorders, 140(2), 113–124. 10.1016/j.jad.2011.10.023 [DOI] [PubMed] [Google Scholar]
- Lockwood KA, Alexopoulos GS, & van Gorp WG (2002). Executive dysfunction in geriatric depression. American Journal of Psychiatry, 159(7), 1119–1126. 10.1176/appi.ajp.159.7.1119 [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, & Heim C (2009). Effects of stress throughout the lifespan on the brain, behavior, and cognition. Nature Review Neuroscience, 10, 434–445. [DOI] [PubMed] [Google Scholar]
- Manning KJ, Alexopoulos GS, Banerjee S, Morimoto SS, Seirup JK, Klimstra SA, … Gunning-Dixon F (2015). Executive functioning complaints and escitalopram treatment response in late-life depression. American Journal of Geriatric Psychiatry, 23(5), 440–445. 10.1016/j.jagp.2013.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClintock SM, Husain MM, Greer TL, & Cullum CM (2010). Association between depression severity and neurocognitive function in major depressive disorder: A review and synthesis. Neuropsychology, 24(1), 9–34. 10.1037/a0017336 [DOI] [PubMed] [Google Scholar]
- McClintock SM, Husain MM, Wisniewski SR, Nierenberg AA, Stewart JW, Trivedi MH, … Rush AJ (2011). Residual depressive symptoms in depressed outpatients who respond by 50% but do not remit to antidepressant medication. Journal of Clinical Psychopharmacology, 31(2), 180–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott LM, & Ebmeier KP (2009). A nreta-analysis of depression severity and cognitive function. Journal of Affective Disorders, 119(1–3), 1–8. 10.1016/j.jad.2009.04.022 [DOI] [PubMed] [Google Scholar]
- McLaren ME, Szymkowicz SM, O’Shea A, Woods AJ, Anton SD, & Dotson VM (2016). Dimensions of depressive symptoms and cingulate volumes in older adults. Translational Psychiatry, 6, e788. 10.1038/tp.2016.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeks TW, Vahia IV, Lavretsky H, Kulkarni G, & Jeste DV (2011). A tune in “a minor” can “b major”: A review of epidemiology, illness course, and public health implications of subthreshold depression in older adults. Journal of Affective Disorders, 129(1–3), 126–142. 10.1016/j.jad.2010.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, & Wallis JD (2009). Executive function and higher-order cognition: Definition and neural substrates. In Squire LR (Ed.), Encyclopedia of Neuroscience (pp. 99–104): Academic press. [Google Scholar]
- Moeyaert M, Ugille M, Natasha Beretvas S, Ferron J, Bunuan R, & Van den Noortgate W (2017). Methods for dealing with multiple outcomes in meta-analysis: A comparison between averaging effect sizes, robust variance estimation and multilevel meta-analysis. International Journal of Social Research Methodology, 20(6), 559–572. 10.1080/13645579.2016.1252189 [DOI] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, & Group P (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Medicine, 6(7), e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris SE, & Cuthbert BN (2012). Research domain criteria: Cognitive systems, neural circuits, and dimensions of behavior. Dialogues in Clinical Neuroscience, 14(1), 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naismith SL, Norrie LM, Mowszowski L, & Hickie IB (2012). The neurobiology of depression in later-life: Clinical, neuropsychological, neuroimaging and pathophysiological features. Progress in Neurobiology, 98(1), 99–143. 10.1016/j.pneurobio.2012.05.009 [DOI] [PubMed] [Google Scholar]
- Paterniti S, Dufouil C, Bisserbe JC, & Alperovitch A (1999). Anxiety, depression, psychotropic drug use and cognitive impairment. Psychological Medicine, 29(2), 421–428. [DOI] [PubMed] [Google Scholar]
- Paulus MP (2015). Cognitive control in depression and anxiety: Out of control? Current Opinion in Behavioral Sciences, 1, 113–120. [Google Scholar]
- Porter RJ, Bourke C, & Gallagher P (2007). Neuropsychological impairment in major depression: Its nature, origin and clinical significance. Australian and New Zealand Journal of Psychiatry, 41(2), 115–128. 10.1080/00048670601109881 [DOI] [PubMed] [Google Scholar]
- Prado CE, Watt S, & Crowe SF (2018). A meta-analysis of the effects of antidepressants on cognitive functioning in depressed and non-depressed samples. Neuropsychology Review, 28(1), 32–72. 10.1007/s11065-018-9369-5 [DOI] [PubMed] [Google Scholar]
- Reinlieb ME, Persaud A, Singh D, Garcon E, Rutherford BR, Pelton GH, … Sneed JR (2014). Vascular depression: Overrepresented among African Americans? International Journal of Geriatric Psychiatry, 29(5), 470–477. 10.1002/gps.4029 [DOI] [PubMed] [Google Scholar]
- Rock PL, Roiser JP, Riedel WJ, & Blackwell AD (2014). Cognitive impairment in depression: A systematic review and meta-analysis. Psychological Medicine, 44(10), 2029–2040. 10.1017/S0033291713002535 [DOI] [PubMed] [Google Scholar]
- Rodriguez MR, Nuevo R, Chatterji S, & Ayuso-Mateos JL (2012). Definitions and factors associated with subthreshold depressive conditions: A systematic review. BMC Psychiatry, 12, 181. 10.1186/1471-244X-12-181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah MT, Zonderman AB, & Waldstein SR (2013). Sex and age differences in the relation of depressive symptoms with blood pressure. American Journal of Hypertension, 26(12), 1413–1420. 10.1093/ajh/hpt135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder HR (2013). Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: A meta-analysis and review. Psychological Bulletin, 139(1), 81–132. 10.1037/a0028727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundermann EE, Katz MJ, & Lipton RB (2017). Sex differences in the relationship between depressive symptoms and risk of amnestic mild cognitive impairment. American Journal of Geriatric Psychiatry, 25(1), 13–22. 10.1016/j.jagp.2016.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- The US Burden of Disease Collaborators. (2018). The state of US health, 1990-2016: Burden of disease, injuries, and risk factors among US states. Journal of the American Medical Association, 319(14), 1444–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas AJ, Gallagher P, Robinson LJ, Porter RJ, Young AH, Ferrier IN, & O’Brien JT (2009). A comparison of neurocognitive impairment in younger and older adults with major depression. Psychological Medicine, 39(5), 725–733. 10.1017/S0033291708004042 [DOI] [PubMed] [Google Scholar]
- von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, & Initiative S (2007). The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. Annals of Internal Medicine, 147(8), 573–577. [DOI] [PubMed] [Google Scholar]
- Wadsworth EJ, Moss SC, Simpson SA, & Smith AP (2005). SSRIs and cognitive performance in a working sample. Human Psychopharmacology, 20(8), 561–572. 10.1002/hup.725 [DOI] [PubMed] [Google Scholar]
- Wagner S, Doering B, Helmreich I, Lieb K, & Tadic A (2012). A meta-analysis of executive dysfunctions in unipolar major depressive disorder without psychotic symptoms and their changes during antidepressant treatment. Acta Psychiatrica Scandinavica, 125, 281–292. [DOI] [PubMed] [Google Scholar]
- Weisenbach SL, Kassel MT, Rao J, Weldon AL, Avery ET, Briceno EM, … Langenecker SA (2014). Differential prefrontal and subcortical circuitry engagement during encoding of semantically related words in patients with late-life depression. International Journal of Geriatric Psychiatry, 29( 11), 1104–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerhof GJ, & Keyes CLM (2010). Mental illness and mental health: The two continua model across the lifespan. Journal of Adult Development, 17(2), 110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]


