This decision analytical model study quantifies the estimated US population-level impact of reducing heart failure events with sodium-glucose cotransporter-2 inhibitors in left ventricular ejection fraction more than 40%.
Key Points
Question
What is the population-level impact in the US of comprehensive implementation of sodium-glucose cotransporter-2 (SGLT-2) inhibitors in patients with heart failure (HF) and a left ventricular ejection fraction (LVEF) more than 40%?
Findings
In this decision analytical model study of 4 794 524 adults with HF in the US, a total of 2 619 248 patients with LVEF more than 40% were newly eligible for SGLT-2 inhibitor therapy. With the optimal implementation of SGLT-2 inhibitors over 3 years, an estimated 630 000 worsening HF events were projected to be prevented/postponed across the LVEF spectrum, of which approximately 230 000 to 280 000 would be prevented/postponed in HF with LVEF more than 40%.
Meaning
Optimal implementation of SGLT-2 inhibitors to approximately 4.8 million patients with HF in the US is estimated to potentially cause a reduction of about 250 000 worsening HF events in patients with LVEF more than 40% over 3 years, contributing to possible prevention/postponement of total approximately 630 000 events across all the LVEF spectrum.
Abstract
Importance
The expansion of sodium-glucose cotransporter-2 (SGLT-2) inhibitor use in patients with heart failure (HF) and left ventricular ejection fraction (LVEF) more than 40% following the EMPEROR-Preserved (Empagliflozin Outcome Trial in Patients with Chronic Heart Failure with Preserved Ejection Fraction) and the DELIVER (Dapagliflozin Evaluation to Improve the Lives of Patients with Preserved Ejection Fraction Heart Failure) trials have major implications in the US.
Objective
To quantify the estimated US population-level impact of reducing worsening HF events with SGLT-2 inhibitors in individuals with LVEF more than 40%.
Design, Setting, and Participants
This decision analytical model study used self-reported HF data from the National Health and Nutritional Examination Survey from 2015 to 2018, which was weighted across the entire US population and subsequently mapped onto newly eligible LVEF distributions from the Get With The Guidelines–Heart Failure registry. All patients older than 18 years with HF from the National Health and Nutritional Examination Survey were grouped into the following categories: all LVEF and LVEF more than 40%. Numbers needed to treat estimations over 3 years were obtained for outcome measures from the EMPEROR-Reduced (Empagliflozin Outcome Trial in Patients with Chronic Heart Failure with Reduced Ejection Fraction), EMPEROR-Preserved, DAPA-HF (Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure), and DELIVER trials.
Main Outcomes and Measures
Worsening HF events (unplanned HF hospitalizations, urgent HF visits requiring intravenous therapy, or cardiovascular death).
Results
A projected 4 794 524 (95% CI, 3 997 363-5 591 684) adults (57% male; 67% White; mean age, 66 years) with HF would be eligible for SGLT-2 inhibitors. Of this total population, 2 619 248 (95% CI, 2 183 759-3 054 737) would be estimated as newly eligible with LVEF more than 40%. Based on estimates from the EMPEROR-Reduced/EMPEROR-Preserved and DAPA-HF/DELIVER trials, a projected 624 247 (95% CI, 520 457-728 037) to 627 124 (95% CI, 522 855-731 392) worsening HF events could be prevented across the LVEF spectrum with SGLT-2 inhibitors over 3 years, of which 232 589 (95% CI, 193 918-271 260) to 282 879 (95% CI, 235 846-329 912) events could be prevented in individuals with LVEF more than 40%. Moreover, an estimated 468 904 (95% CI, 390 942-546 867) to 499 110 (95% CI, 416 125-582 094) total HF hospitalizations could be prevented across the LVEF spectrum, of which 172 870 (95% CI, 144 128-201 613) to 231 018 (95% CI, 192 608-269 428) could be prevented in individuals with LVEF more than 40%.
Conclusions and Relevance
In addition to the proven benefit in HF with LVEF of 40% and less, optimal implementation of SGLT-2 inhibitor therapy for HF with LVEF more than 40% can potentially prevent/postpone an additional approximately 250 000 worsening HF events over 3 years in the US.
Introduction
Sodium-glucose cotransporter-2 (SGLT-2) inhibitors were initially found to decrease heart failure (HF) hospitalizations and cardiovascular (CV) deaths in the EMPA-REG OUTCOME (Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients) trial1 that was specifically designed to assess efficacy and safety of empagliflozin in patients with type 2 diabetes. Subsequently, in DAPA-HF (Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure)2 and EMPEROR-Reduced (Empagliflozin Outcome Trial in Patients with Chronic Heart Failure with Reduced Ejection Fraction),3 SGLT-2 inhibitors were found to reduce CV death and HF hospitalizations in patients with HF with reduced ejection fraction, irrespective of diabetes status. More recently, the EMPEROR-Preserved (Empagliflozin Outcome Trial in Patients with Chronic Heart Failure with Preserved Ejection Fraction) trial4 reported a significant reduction in the composite outcome of CV death or HF hospitalization with empagliflozin in patients with HF and a left ventricular ejection fraction (LVEF) more than 40%, ie, with mildly reduced or preserved ejection fraction. Similar findings were reported in the DELIVER (Dapagliflozin Evaluation to Improve the Lives of Patients with Preserved Ejection Fraction Heart Failure) trial,5 which reported a significant reduction in the composite outcome of HF hospitalizations, urgent HF visits, or CV deaths.
We sought to estimate the potential US population-level implications of SGLT-2 inhibitor therapy in HF by performing a decision analytical model study of all participants in the EMPEROR-Reduced, EMPEROR-Preserved, DAPA-HF, and DELIVER trials. We also aim to assess the benefit of the expansion of SGLT-2 inhibitors therapy to patients with HF with LVEF more than 40% by the US Food and Drug Administration in the rates of worsening HF events, CV deaths, and adverse drug events.
Methods
National Health and Nutritional Examination Survey
The National Health and Nutritional Examination Survey (NHANES),6 which is a biannually conducted survey designed to project national prevalence estimates of diseases in the US, was used to estimate the weighted prevalence of patients with HF in the US. Estimates of self-reported HF from 2015 to 2018 (n = 19 255) were obtained, as performed by Vaduganathan et al.7 The following criteria were applied to the initial cohort to determine a clinically actionable population of interest: patients 18 years and older with a diagnosis of HF irrespective of LVEF were included, and patients with an estimated glomerular filtration rate (<20 mL/min/1.73 m2) and systolic blood pressure less than 100 mm Hg were excluded. Blood pressure measurements were obtained by physical examination and estimated glomerular filtration rate was determined based on creatinine measurements. In a sensitivity analysis, the following exclusions were further applied: patients undergoing inotropic support or hospice/comfort care, ventricular assist device or heart transplant recipients, type 1 diabetes, and patients with New York Heart Association functional class 1 symptoms.
Get With The Guidelines–Heart Failure Registry
Data were obtained from the American Heart Association Get With The Guidelines–Heart Failure (GWTG-HF) registry8 to identify a distribution of LVEF among patients 18 years and older hospitalized between January 1, 2014, and September 30, 2019, at 529 US hospitals. Population-level estimates were ascertained for the following groups: (1) all patients with HF irrespective of LVEF in the EMPEROR-Reduced, EMPEROR-Preserved, DAPA-HF, and DELIVER trials and (2) HF with LVEF more than 40%, including all patients in the EMPEROR-Preserved and DELIVER trials.
End Point Assessment
The EMPEROR trials primarily assessed the composite end point of CV death or HF hospitalization. The DAPA-HF and DELIVER trials primarily assessed the composite end point of worsening HF event (including unplanned HF hospitalizations or urgent visits requiring intravenous therapy) or CV death. Both trials also studied the occurrence of total (first and recurrent) HF hospitalizations, which were included in this analysis. We designated the following end points for assessment of population-level impact, standardized over 1 and 3 years: (1) total (first and recurrent) HF hospitalizations, (2) composite of total (first and recurrent) HF hospitalizations and CV deaths, and (3) composite of worsening HF event (expanded composite inclusive of urgent HF visits and total HF hospitalizations) or CV death.7 Urgent HF visits were urgent or emergency visits for HF that required intravenous therapy.
Adverse Events
The following adverse events of interest were also assessed and standardized at 1 and 3 years: (1) symptomatic hypotension (investigator-defined events, no blood pressure cutoff as mentioned in the EMPEROR-Preserved trial) and (2) genital infections. To avoid heterogeneity in reporting projected population effect, adverse event assessment was performed only from the EMPEROR-Reduced and EMPEROR-Preserved trials and not from the DAPA-HF and DELIVER trials since the latter studies only collected data on serious adverse events and adverse events for genital infections that led to treatment discontinuation.
Numbers Needed to Treat for Recurrent Events
Incidence rates were calculated for all efficacy end points of interest from the EMPEROR-Reduced, EMPEROR-Preserved, DAPA-HF, and DELIVER trials. Numbers needed to treat (NNT) were calculated for each CV end point per patient-year observed using data at 1 and 3 years after randomization. NNT is usually interpreted as the number of patients who need to be treated to prevent 1 patient from experiencing an adverse event. In this study, we reported an event-based NNT reflecting the number of patients who need to be treated for 3 years to prevent 1 event for all end points of interest. NNT was calculated by using the inverse of the estimated rate difference (calculated as events per 100 patient-years). For comparison, NNTs were also calculated using data up to 1 year after randomization and NNTs reflecting the number of patients that need to be treated for 1 year (or the number of patient-years of treatment) to prevent 1 event were also calculated.
A secondary analysis was performed using efficacy end point data from the EMPEROR-Reduced and EMPEROR-Preserved trials where NNT was calculated using the inverse of the estimated rate difference of events between the treatment and control groups using a negative binomial model assuming a constant event rate (offset of log[time at risk]). We calculated the confidence interval (CI) for the rate difference using a negative binomial model between the treatment and control groups. The inverse of the CI was then used to transform it into the CI for NNT. The results of this analysis are included in the eTable 1 in the Supplement.
Numbers Needed to Harm for Adverse Events
Event-based numbers needed to harm (NNH) were calculated for the first events of symptomatic hypotension and genital infections calculating expected event rates assuming an exponential distribution and following similar principles as above (using data up to 1 and 3 years) from the EMPEROR-Reduced and EMPEROR-Preserved trials. NNH is interpreted as the number of patients who needed to be treated for 1 or 3 years for 1 patient to experience an adverse event. The calculations for NNH were like those made for NNT except focused on the first event rather than recurrent events. For NNT, calculations followed this intention-to-treat analysis (as the primary trial analysis) and NNH calculations used data up to 7 days after treatment discontinuation in line with safety analyses of the trials.
Results
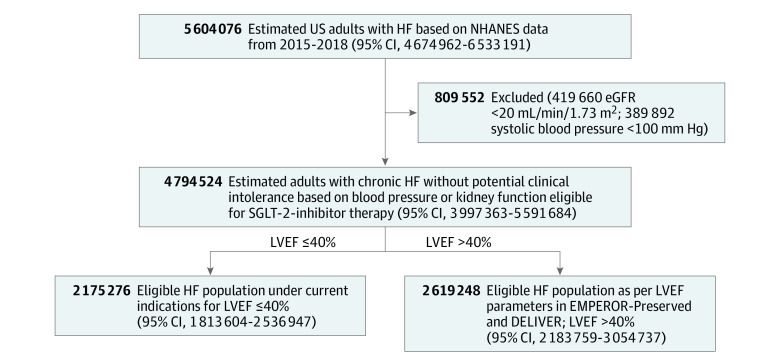
The prevalence of self-reported HF among US adults was estimated to be 5 604 076 (95% CI, 4 674 944-6 533 208). After excluding those with systolic blood pressure of less than 100 mm Hg or estimated glomerular filtration rate less than 20 mL/min/1.73 m2, 4 794 524 (95% CI, 3 997 363-5 591 684) patients with HF were identified (Figure 1). The mean age was 66.4 (95% CI, 64.8-68.0) years. The total HF population was predominantly male (56.5%) and non-Hispanic White (67.3%). The HF population also included Asian (2.3%), Black (16.1%), other Hispanic (6.4%), Mexican American (4.1%), and individuals of other race and ethnicity (3.8%). Other demographic and clinical characteristics of individuals with HF in NHANES are described in the eTable 2 in the Supplement.
Figure 1. Total Heart Failure (HF) Population Estimated From the National Health and Nutritional Examination Survey (NHANES) That Would be Eligible for Sodium-Glucose Cotransporter-2 (SGLT-2) Inhibitor Therapy.
DELIVER indicates Dapagliflozin Evaluation to Improve the Lives of Patients with Preserved Ejection Fraction Heart Failure; eGFR, estimated glomerular filtration rate; EMPEROR-Preserved, Empagliflozin Outcome Trial in Patients with Chronic Heart Failure with Preserved Ejection Fraction; LVEF, left ventricular ejection fraction.
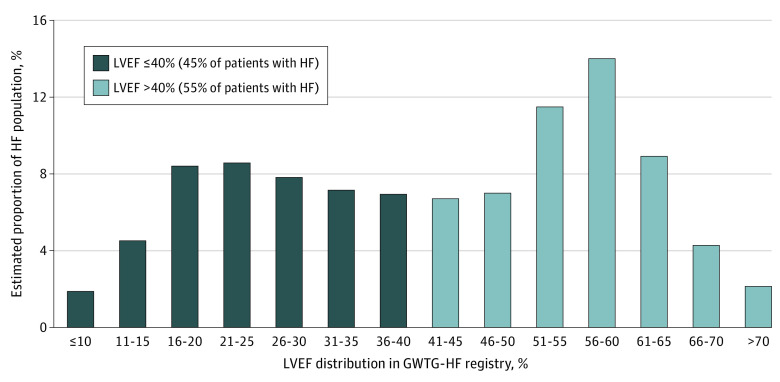
Among 586 580 patients hospitalized for HF in the GWTG-HF registry, 27 060 (4.6%) did not have available data for LVEF. Among those with available data (n = 559 520), relative proportions of patients based on LVEF subgroups, 40% or less and more than 40%, are illustrated in Figure 2. Overall, 45.3% of patients with HF with LVEF 40% or less, an estimated 2 175 276 individuals, would have already met the LVEF criteria of the prior indication for SGLT-2 inhibitors for HF with reduced ejection fraction. Expanded treatment eligibility after the EMPEROR-Preserved and DELIVER trials, which included patients with HF with LVEF more than 40%, leads to an additional 2 619 248 (95% CI, 2 183 759-3 054 737) individuals who are eligible to receive SGLT-2 inhibitors.
Figure 2. Proportion of Patients With Heart Failure (HF) Stratified According to Left Ventricular Ejection Fraction (LVEF) Subgroups in Get With The Guidelines–Heart Failure Registry (GWTG-HF).
Population-Level Treatment Effect Sizes Irrespective of Left Ventricular Ejection Fraction
Among 9718 trial participants in the EMPEROR-Reduced and EMPEROR-Preserved trials, the 3-year NNT for total HF hospitalizations, the composite of HF hospitalizations and CV deaths, and worsening HF event or CV death were 10, 9, and 8, respectively (Table 1). The findings were similar among 8007 participants in the DAPA-HF and DELIVER trials where the 3-year NNT for total HF hospitalizations, the composite of HF hospitalizations and CV deaths, and worsening HF event or CV death were 11, 9, and 8, respectively (Table 1). Based on these estimates, optimal implementation of SGLT-2 inhibitors across the LVEF spectrum is projected to prevent/postpone 499 110 (95% CI, 416 125-582 094) to 468 904 (95% CI, 390 942-546 867) total HF hospitalizations, 562 397 (95% CI, 468 890-655 905) to 591 165 (95% CI, 492 875-689 455) total HF hospitalizations and CV deaths, and 624 247 (95% CI, 520 457-728 037) to 627 124 (95% CI, 522 855-731 392) worsening HF events or CV deaths over a 3-year period (Table 2 and Figure 3).
Table 1. Treatment Effect Sizes and Number Needed to Treat With SGLT-2 Inhibitors in the EMPEROR-Reduced, EMPEROR-Preserved, DAPA-HF, and DELIVER Trials.
| End point | Overall rate (per 100 patient-years) | Overall rate measure (95% CI) | Rate difference, 1-y outcome | Event rate (per 100 patient-years) in the first 3 y | Rate difference, 3-y outcome | Number needed to treat | |||
|---|---|---|---|---|---|---|---|---|---|
| Placebo | SGLT-2 inhibitor | Placebo | SGLT-2 inhibitor | 1-y Outcome | 3-y Outcome | ||||
| EMPEROR-Reduced and EMPEROR-Preserved trials, all LVEFs | |||||||||
| Total HF hospitalizations | 12.49 | 9.07 | 0.72 (0.63-0.83) | 5.13 | 12.56 | 9.09 | 10.41 | 20 | 10 |
| Total HF hospitalizations and CV deaths | 17.58 | 13.70 | 0.77 (0.69-0.87) | 5.41 | 17.64 | 13.73 | 11.73 | 19 | 9 |
| Worsening HF eventa or CV death | 19.17 | 14.84 | 0.76 (0.68-0.86) | 6.22 | 19.22 | 14.88 | 13.02 | 17 | 8 |
| DAPA-HF and DELIVER trials, all LVEFs | |||||||||
| Total HF hospitalizations | 11.44 | 8.20 | 0.72 (0.63-0.82) | 4.78 | 11.47 | 8.21 | 9.78 | 21 | 11 |
| Total HF hospitalizations and CV deaths | 17.34 | 13.28 | 0.76 (0.69-0.85) | 5.50 | 17.40 | 13.29 | 12.33 | 19 | 9 |
| Worsening HF event or CV death | 17.78 | 13.46 | 0.76 (0.68-0.84) | 6.15 | 17.83 | 13.47 | 13.08 | 17 | 8 |
| EMPEROR-Preserved trial, LVEF >40% | |||||||||
| Total HF hospitalizations | 8.60 | 6.46 | 0.73 (0.60-0.89) | 3.74 | 8.64 | 6.44 | 6.60 | 27 | 16 |
| Total HF hospitalizations and CV deaths | 12.47 | 9.93 | 0.78 (0.66-0.93) | 3.75 | 12.48 | 9.90 | 7.74 | 27 | 13 |
| Worsening HF event or CV death | 13.79 | 10.83 | 0.77 (0.65-0.91) | 4.14 | 13.78 | 10.82 | 8.88 | 25 | 12 |
| DELIVER trial, LVEF >40% | |||||||||
| Total HF hospitalizations | 10.25 | 7.35 | 0.72 (0.60-0.85) | 5.31 | 10.30 | 7.36 | 8.82 | 19 | 12 |
| Total HF hospitalizations and CV deaths | 14.04 | 10.69 | 0.76 (0.66-0.88) | 5.50 | 14.11 | 10.73 | 10.14 | 19 | 10 |
| Worsening HF event or CV death | 15.33 | 11.79 | 0.77 (0.67-0.89) | 6.40 | 15.39 | 11.79 | 10.80 | 16 | 10 |
Abbreviations: CV, cardiovascular; DAPA-HF, Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure; DELIVER, Dapagliflozin Evaluation To Improve The Lives Of Patients With Preserved Ejection Fraction Heart Failure; EMPEROR-Preserved, Empagliflozin Outcome Trial In Patients With Chronic Heart Failure With Preserved Ejection Fraction; EMPEROR-Reduced, Empagliflozin Outcome Trial In Patients With Chronic Heart Failure With Reduced Ejection Fraction; HF, heart failure; LVEF, left ventricular ejection fraction; SGLT-2, sodium-glucose cotransporter-2.
Worsening HF events include HF hospitalizations and urgent visits for HF requiring intravenous therapy.
Table 2. Estimated Events Prevented/Postponed or Associated With SGLT-2 Inhibitor Therapy Among Newly Eligible Patients Treated for 3 Years.
| LVEF range | No. (95% CI) | |||||
|---|---|---|---|---|---|---|
| Potentially newly eligible candidates | Estimated events prevented/postponed by the implementation of SGLT-2 inhibitors among newly eligible patients for 3 ya | Estimated No. of patients who experience an event caused by the implementation of SGLT-2 inhibitors among newly eligible patients for 3 ya | ||||
| Total HF hospitalizations | Total HF hospitalizations and CV deaths | Worsening HF eventb or CV death | Symptomatic hypotension | Genital infections | ||
| EMPEROR-Reduced and EMPEROR-Preserved trials | ||||||
| All LVEFs | 4 794 524 (3 997 363-5 591 684) | 499 110 (416 125-582 094) | 562 397 (468 890-655 905) | 624 247 (520 457-728 037) | 77 672 (64 758-90 586) | 119 384 (99 535-139 233) |
| LVEF >40% | 2 619 248 (2 183 759-3 054 737) | 172 870 (144 128-201 613) | 202 730 (169 023-236 437) | 232 589 (193 918-271 260) | 56 578 (47 170-65 985) | 64 434 (53 721-75 147) |
| DAPA-HF and DELIVER trials | ||||||
| All LVEFs | 4 794 524 (3 997 363-5 591 684) | 468 904 (390 942-546 867) | 591 165 (492 875-689 455) | 627 124 (522 855-731 392) | NA | NA |
| LVEF >40% | 2 619 248 (2 183 759-3 054 737) | 231 018 (192 608-269 428) | 265 592 (221 433-309 750) | 282 879 (235 846-329 912) | NA | NA |
Abbreviations: CV, cardiovascular; DAPA-HF, Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure; DELIVER, Dapagliflozin Evaluation To Improve The Lives Of Patients With Preserved Ejection Fraction Heart Failure; EMPEROR-Preserved, Empagliflozin Outcome Trial in Patients with Chronic Heart Failure with Preserved Ejection Fraction; EMPEROR-Reduced, Empagliflozin Outcome Trial In Patients With Chronic Heart Failure With Reduced Ejection Fraction; HF, heart failure; LVEF, left ventricular ejection fraction; NA, not applicable; SGLT-2, sodium-glucose cotransporter-2.
Using 95% CI of the potentially newly eligible candidates and the estimates of differences in event rates.
Worsening HF events include HF hospitalizations and urgent visits for HF requiring intravenous therapy.
Figure 3. Heart Failure (HF) Events Prevented Using Event Rates From Trials .
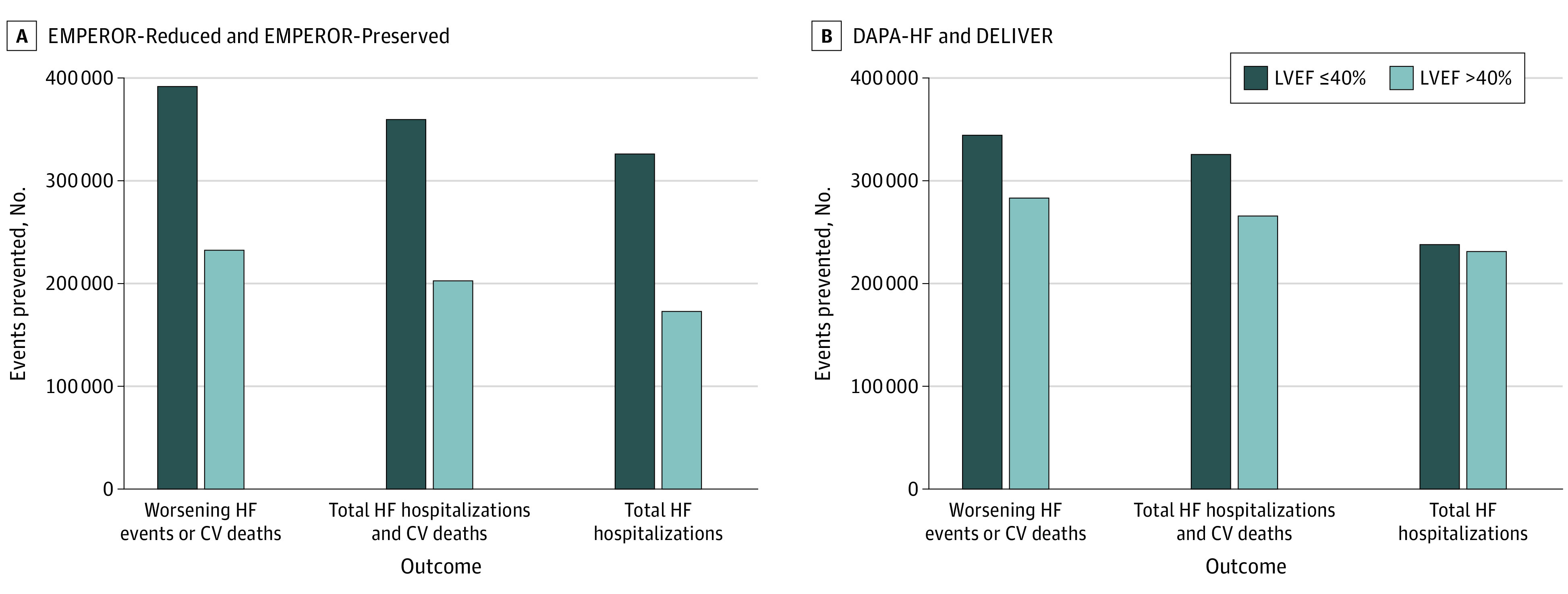
CV indicates cardiovascular; DAPA-HF, Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure; DELIVER, Dapagliflozin Evaluation to Improve the Lives of Patients with Preserved Ejection Fraction Heart Failure; EMPEROR-Preserved, Empagliflozin Outcome Trial in Patients with Chronic Heart Failure with Preserved Ejection Fraction; EMPEROR-Reduced, Empagliflozin Outcome Trial in Patients with Chronic Heart Failure with Reduced Ejection Fraction; LVEF, left ventricular ejection fraction.
The 3-year NNH for symptomatic hypotension and genital infections was 70 and 42, respectively. Optimal implementation of SGLT-2 inhibitor therapy could cause a projected 77 672 (95% CI, 64 758-90 586) episodes of symptomatic hypotension and 119 384 (95% CI, 99 535-139 233) genital infections over a 3-year period (Table 2).
Population-Level Treatment Effect Sizes in LVEF >40%
Among 5988 participants in the EMPEROR-Preserved trial with LVEF more than 40%, the 3-year NNT for total HF hospitalizations, the composite of HF hospitalizations and CV deaths, and worsening HF event or CV death were 16, 13, and 12, respectively (Table 1). Among 6263 participants in the DELIVER trial, the 3-year NNTs for total HF hospitalizations, the composite of HF hospitalizations and CV deaths, and worsening HF event or CV death were 12, 10, and 10, respectively (Table 1). Based on these estimates, optimal implementation of SGLT-2 inhibitors in HF with LVEF more than 40% is projected to prevent/postpone 172 870 (95% CI, 144 128-201 613) to 231 018 (95% CI, 192 608-269 428) total HF hospitalizations, 202 730 (95% CI, 169 023-236 437) to 265 592 (95% CI, 221 433-309 750) total HF hospitalizations and CV deaths, and 232 589 (95% CI, 193 918-271 260) to 282 879 (95% CI, 235 846-329 912) worsening HF events or CV deaths over a 3-year period (Table 2 and Figure 3).
The 3-year NNH for symptomatic hypotension and genital infections was 52 and 42, respectively (eTable 3 in the Supplement). Optimal implementation of SGLT-2 inhibitor therapy could cause a projected 56 578 (95% CI, 47 170-65 985) patients to experience a symptomatic hypotension event and 64 434 (95% CI, 53 721-75 147) patients to experience a genital infection over a 3-year period (Table 2).
Sensitivity Analysis of Conservative Estimate of Potentially Eligible Treatment Candidates
In a sensitivity analysis, those with New York Heart Association class I functional status (239 726 [5%]), those receiving hospice care or comfort measures only (191 781 [4%]), inotropic agents, ventricular assist devices, urgent transplant (47 945 [1%]), and those with type 1 diabetes (960 [0.02%]) were additionally excluded. The potential number of adults projected to be eligible ranged from 4 314 112 (95% CI, 3 596 827-5 031 397) for all LVEFs to 2 356 798 (95% CI, 1 964 946-2 748 652) for individuals with LVEF more than 40%. Optimal implementation of SGLT-2 inhibitors in this more selected HF population would be expected to avert up to 561 697 (95% CI, 468 306-655 088) to 564 285 (95% CI, 470 465-658 107) worsening HF events or CV deaths across all LVEFs and 209 284 (95% CI, 174 487-244 080) to 254 534 (95% CI, 212 214-296 854) worsening HF events or CV deaths for HF with LVEF more than 40%. Optimal implementation of SGLT-2 inhibitors would be expected to cause up to 107 422 (all LVEF) and 57 978 (LVEF >40%) patients to experience a genital infection. Moreover, it will also be expected to cause 69 889 (all LVEF) and 49 769 (LVEF >40%) patients to experience a symptomatic hypotension event over 3 years.
Discussion
The indication for SGLT-2 inhibitors has expanded to patients with HF across the complete LVEF spectrum after the EMPEROR-Preserved trial found a significant reduction in HF events with SGLT-2 inhibitors in HF with LVEF more than 40%. The DELIVER study further confirmed these findings and extended similar benefits in patients regardless of LVEF in those enrolled in the hospital or recently hospitalized and in those with HF with improved LVEF (those with prior LVEF ≤40%).5 The current study evaluated the projected benefit of the maximal implementation of both SGLT-2 inhibitors based on the current body of evidence and guideline recommendations. An estimation of approximately 630 000 total worsening HF events or CV deaths are projected to be prevented/postponed over 3 years of SGLT-2 inhibitor therapy across the LVEF spectrum. This includes an estimated prevention/postponement of approximately 230 000 to 280 000 worsening HF events or CV deaths in HF with LVEF more than 40%.
The NNTs for 3 years of treatment for all efficacy end points were similar across the LVEF spectrum included in this study and ranged from 9 to 13 for the composite end point of total HF hospitalizations and CV deaths and 10 to 16 for total HF hospitalizations. The NNTs for LVEF more than 40% were numerically higher overall compared with those for all LVEFs for all efficacy end points. Worsening HF events included urgent HF visits and total (first and recurrent) hospitalizations and were included within an expanded composite end point of worsening HF event or CV death. This was deemed an important outcome for this study as urgent HF visits also represent an indicator of HF morbidity, quality of life, and a measure of HF-associated health care costs. According to a retrospective evaluation of linked US claims data and electronic health records from 2012 to 2018,9 an estimated 16% of patients with HF with preserved ejection fraction underwent HF hospitalization, while the rate of urgent outpatient HF visits was similar (approximately 12%). More alarmingly, 27% of patients with an urgent outpatient HF visit were admitted to the hospital for HF within 30 days. This contributes to a mean monthly direct health care cost to be approximately $9000 (slightly lower for HF with preserved ejection fraction, approximately $7400).
The projected decrease in HF-associated morbidity that could be achieved with optimal implementation of SGLT-2 inhibitors could substantially reduce the economic burden of HF events on the US health care system. According to a contemporary systematic review of all HF hospitalizations in the US from 2014 to 2020,10 the mean cost of an HF hospitalization in the US is approximately $13 000 to $14 000. Based on these estimates and total HF hospitalizations projected to be prevented/postponed in this study, maximal implementation of SGLT-2 inhibitors would be expected to reduce the costs of HF hospitalizations by close to $7 billion across the LVEF spectrum and $3 billion in all individuals with HF with LVEF more than 40%. These estimates are optimistic in that they do not account for the cost of SGLT-2 inhibitors given the obvious difficulty in ensuring maximal indicated use of SGLT-2 inhibitors in HF; however, even a more conservative implementation estimate will still significantly bring down costs of HF hospitalizations.
A potential barrier to the use of SGLT-2 inhibitors across populations is the perceived notion of adverse reactions associated with the drug among patients and clinicians alike. In this analysis, we included 2 adverse events reported in the EMPEROR trials that were slightly more prevalent in the SGLT-2 inhibitor group: symptomatic hypotension and genital infections. The NNH for both was high across all LVEF subgroups (52 to 70 for symptomatic hypotension and 39 to 42 for genital infections), leading to a maximum of projected 77 672 patients to experience a symptomatic hypotension event and projected 119 384 patients to experience a genital infection across 3 years. The rate difference for genital infections was higher with SGLT-2 inhibitors vs placebo compared with that for symptomatic hypotension; however, uncomplicated infections are not an absolute contraindication to SGLT-2 inhibitors and the risk of complicated genital infections is quite low at less than 3% in the EMPEROR-Preserved trial.
This analysis addresses the optimal implementation of SGLT-2 inhibitors across the US, which is limited by several factors, including the high retail cost of SGLT-2 inhibitors, estimated to be approximately $500 per month. An analysis from the Medical Expenditure Panel Survey11 from 2014 to 2018 revealed that out-of-pocket costs for SGLT-2 inhibitors were about $140 per month for uninsured patients, while those who were insured by Medicare were still required to pay about $50 per month out of pocket in medication costs. Moreover, most of these patients are older with multiple chronic conditions and are already taking multiple medications, and the addition of this relatively expensive medication, even among insured patients, can be a major deterrent to populationwide SGLT-2 inhibitor uptake. A recent analysis by Vaduganathan et al12 suggested that cardiologists comprise only 5% of total prescribing physicians for SGLT-2 inhibitors, with the majority citing insufficient knowledge about the medication and perception of SGLT-2 inhibitors as drugs for diabetes as reasons for not prescribing them. Moreover, another contemporary analysis reported the uptake of SGLT-2 inhibitors in patients with type 2 diabetes and CV disease in less than 2% of the eligible population.13 Several patient and clinician-related barriers exist to optimal implementation of SGLT-2 inhibitors, which if alleviated can lead to a profound reduction in HF events as discussed in this study.
Limitations
The results of this study are subject to certain limitations. First, the projected estimates assume that patients will comply with therapy for 3 years, not accounting for adherence patterns, medication costs, and potential perceived or true adverse reactions causing drug discontinuation. Second, the projected HF population from the NHANES registry is a weighted mean of self-reported HF data in the US and hence may not be a true estimate of HF prevalence. Third, the LVEF distribution obtained from the GWTG-HF registry might not be a true assessment of LVEF distribution across the US population as the GWTG-HF registry derives its data only from patients hospitalized for HF rather than the overall HF population. However, as discussed in a previous similar study by Vaduganathan et al,7 the LVEF measured during hospitalizations is usually the most recent, objective assessment of ventricular function, and hence provides a reliable estimate of LVEF distribution in the overall HF population. Fourth, there was heterogeneity in safety end point definition in DAPA-HF and DELIVER trials as data were only collected for serious adverse events or adverse events that led to treatment discontinuation, and hence safety event rates from these 2 trials were not included. Lastly, urgent HF visits may have led to subsequent HF hospitalization and were counted as 2 separate events in the EMPEROR-Reduced and EMPEROR-Preserved trials. This may have led to double counting of worsening HF events and overestimation of the treatment effect.
Conclusions
SGLT-2 inhibitors have been expanded to a further 2.6 million patients with HF in the US with mildly reduced and preserved LVEF based on findings from the EMPEROR-Preserved and DELIVER trials. In addition to clinical events prevented among patients with LVEF 40% or lower, optimal implementation is estimated to maximally prevent/postpone a further approximately 250 000 worsening HF events or CV deaths in LVEF more than 40% leading to projected prevention/postponement of approximately 630 000 events across the LVEF spectrum over 3 years, with a low adverse event rate. To mirror this effect in routine clinical practice, efforts are needed to advocate for initiating SGLT-2 inhibitors in HF among hospital systems, clinicians, and patients alike given the magnitude of the morbidity benefits and potential reduction in HF-related health care costs.
eTable 1. Treatment effects and number needed to treat with SGLT-2 inhibitors in the EMPEROR-Reduced and EMPEROR-Preserved trials using negative binomial analysis
eTable 2. Characteristics of NHANES participants with heart failure without potential clinical intolerance based on blood pressure or kidney function
eTable 3. Adverse event rates and numbers needed to harm with SGLT-2 inhibitors in the EMPEROR-Reduced and EMPEROR-Preserved trials
References
- 1.Zinman B, Wanner C, Lachin JM, et al. ; EMPA-REG OUTCOME Investigators . Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117-2128. doi: 10.1056/NEJMoa1504720 [DOI] [PubMed] [Google Scholar]
- 2.McMurray JJV, Solomon SD, Inzucchi SE, et al. ; DAPA-HF Trial Committees and Investigators . Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995-2008. doi: 10.1056/NEJMoa1911303 [DOI] [PubMed] [Google Scholar]
- 3.Packer M, Anker SD, Butler J, et al. ; EMPEROR-Reduced Trial Investigators . Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383(15):1413-1424. doi: 10.1056/NEJMoa2022190 [DOI] [PubMed] [Google Scholar]
- 4.Anker SD, Butler J, Filippatos G, et al. ; EMPEROR-Preserved Trial Investigators . Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385(16):1451-1461. doi: 10.1056/NEJMoa2107038 [DOI] [PubMed] [Google Scholar]
- 5.Solomon SD, McMurray JJV, Claggett B, et al. ; DELIVER Trial Committees and Investigators . Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med. 2022;387(12):1089-1098. doi: 10.1056/NEJMoa2206286 [DOI] [PubMed] [Google Scholar]
- 6.National Health and Nutrition Examination Survey, 2015-2018: sample design and estimation procedures: data evaluation and methods research. National Center for Health Studies. Published April 2020. Accessed October 18, 2022. https://www.cdc.gov/nchs/data/series/sr_02/sr02-184-508.pdf
- 7.Vaduganathan M, Claggett BL, Greene SJ, et al. Potential implications of expanded US Food and Drug Administration labeling for sacubitril/valsartan in the US. JAMA Cardiol. 2021;6(12):1415-1423. doi: 10.1001/jamacardio.2021.3651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pandey A, Patel KV, Liang L, et al. Association of hospital performance based on 30-day risk-standardized mortality rate with long-term survival after heart failure hospitalization: an analysis of the Get With The Guidelines-Heart Failure Registry. JAMA Cardiol. 2018;3(6):489-497. doi: 10.1001/jamacardio.2018.0579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lam CSP, Wood R, Vaduganathan M, et al. Contemporary economic burden in a real-world heart failure population with commercial and Medicare supplemental plans. Clin Cardiol. 2021;44(5):646-655. doi: 10.1002/clc.23585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Urbich M, Globe G, Pantiri K, et al. A systematic review of medical costs associated with heart failure in the USA (2014-2020). Pharmacoeconomics. 2020;38(11):1219-1236. doi: 10.1007/s40273-020-00952-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aggarwal R, Vaduganathan M, Chiu N, Bhatt DL. Out-of-pocket costs for SGLT-2 (sodium-glucose transport protein-2) inhibitors in the United States. Circ Heart Fail. 2022;15(3):e009099. doi: 10.1161/CIRCHEARTFAILURE.121.009099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vaduganathan M, Sathiyakumar V, Singh A, et al. Prescriber patterns of SGLT2i after expansions of U.S. Food and Drug Administration labeling. J Am Coll Cardiol. 2018;72(25):3370-3372. doi: 10.1016/j.jacc.2018.08.2202 [DOI] [PubMed] [Google Scholar]
- 13.Hamid A, Vaduganathan M, Oshunbade AA, et al. Antihyperglycemic therapies with expansions of US Food and Drug Administration indications to reduce cardiovascular events: prescribing patterns within an Academic medical center. J Cardiovasc Pharmacol. 2020;76(3):313-320. doi: 10.1097/FJC.0000000000000864 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Treatment effects and number needed to treat with SGLT-2 inhibitors in the EMPEROR-Reduced and EMPEROR-Preserved trials using negative binomial analysis
eTable 2. Characteristics of NHANES participants with heart failure without potential clinical intolerance based on blood pressure or kidney function
eTable 3. Adverse event rates and numbers needed to harm with SGLT-2 inhibitors in the EMPEROR-Reduced and EMPEROR-Preserved trials