Abstract
Moraxella catarrhalis-induced otitis media continues to be a significant cause of infection in young children, prompting increased efforts at identifying effective vaccine antigens. We have previously demonstrated that M. catarrhalis expresses specific outer membrane proteins (OMPs) in response to iron limitation and that this organism can utilize transferrin and lactoferrin for in vitro growth. One of these proteins, which binds human transferrin, is OMP B1. As the human host presents a naturally iron-limited environment, proteins, like OMP B1, which are expressed in response to this nutritional stress are potential vaccine antigens. In this study, we have developed monoclonal antibody (MAb) 11C6, which reacts to a surface-exposed epitope of OMP B1 expressed by M. catarrhalis 7169. This antibody was used to clone ompB1, and sequence analysis suggested that OMP B1 is the M. catarrhalis homologue to the transferrin binding protein B described for pathogenic Neisseriaceae, Haemophilus influenzae, Actinobacillus pleuropneumoniae, and M. catarrhalis. Expression of recombinant OMP B1 on the surface of Escherichia coli confers transferrin binding activity, confirming that this protein is likely involved in iron acquisition. In addition, ompB1 was used to construct an isogenic mutant in M. catarrhalis 7169. This mutant, termed 7169b12, was used as the control in bactericidal assays designed to determine if OMP B1 elicits protective antibodies. In the presence of MAb 11C6 and human complement, wild-type 7169 demonstrated a 99% decline in viability, whereas the ompB1 isogenic mutant was resistant to this bactericidal activity. Further analysis with MAb 11C6 revealed the presence of this OMP B1 epitope on 31% of the clinical isolates tested. These data suggest that OMP B1 is a potential vaccine antigen against M. catarrhalis infections.
Moraxella catarrhalis is a gram-negative diplococcus which has emerged as an important human pathogen over the past decade. This bacterium is the third leading cause of otitis media in young children, resulting in approximately 15 to 20% of all cases reported (13, 26). This infection rate is significant, as it is estimated that 70 to 80% of all children will have had at least a single episode of middle ear disease by the age of three years (13, 26). Many of these young patients will experience recurrent otitis media, resulting in substantial morbidity and possible developmental and learning problems as these children reach school age (36). Recent data from various centers throughout the United States show that M. catarrhalis is responsible for over three million episodes of otitis media annually, resulting in a substantial financial burden on the health care system at present (26). M. catarrhalis has also been shown to be an important pathogen in adults in certain settings. This organism causes lower respiratory tract infection in adults with chronic bronchitis and chronic obstructive pulmonary disease (COPD), often leading to acute exacerbations of COPD (5, 15, 28).
Given these statistics, it is obvious that an effective vaccine, designed to prevent M. catarrhalis infections, would result in a substantial decline in the estimated two billion dollars spent annually on treatments involving otitis media infections (4). The rapid identification of specific vaccine candidates becomes even more important based on recent reports which show that nearly 90% of the M. catarrhalis clinical isolates are now β-lactamase positive (13).
In general, there are multiple characteristics which are essential for a good vaccine candidate against bacterial infections. The specific component(s) in question should contain surface-exposed epitopes that are conserved among all, or many, of the strains of a given species. In addition, an effective vaccine antigen must be expressed in vivo and should elicit a protective or neutralizing immune response in the susceptible population.
In previous studies, several outer membrane proteins (OMPs) of M. catarrhalis have been investigated as potential vaccine antigens. Helminen et al. demonstrated that antibodies directed to CopB, a major iron-repressible OMP, enhanced pulmonary clearance of M. catarrhalis in a mouse model (17). It has also been shown that antibodies to the high-molecular-weight proteins UspA1 and UspA2 elicit biologic activity against M. catarrhalis in the same model (16). In addition, in vitro studies have demonstrated that antibodies directed to the highly conserved OMP CD exhibit complement-mediated bactericidal activity in vitro (38).
In addition to the proteins mentioned above, we have previously reported the identification and isolation of an iron-regulated protein, termed OMP B1, from M. catarrhalis which has several characteristics of a good vaccine antigen. This protein is conserved in the outer membrane of all M. catarrhalis strains evaluated (7). Our studies suggest that expression of OMP B1 is increased in response to iron limitation, a condition which naturally exists in the human body (6, 7, 12, 14, 21). In addition, we have demonstrated that OMP B1 binds human transferrin in vitro, similarly to the transferrin receptor TbpB described for other pathogenic bacteria, such as Neisseria meningitidis, Neisseria gonorrhoeae, Haemophilus influenzae, Actinobacillus pleuropneumoniae, and more recently, other M. catarrhalis strains (7, 9, 10, 18–20, 22, 23, 27, 29, 30, 32–34). In the absence of siderophore production, receptors for iron carrier proteins, such as human transferrin, are probably expressed in vivo and represent an important mechanism for survival of these pathogens in the host. This has prompted recent studies to evaluate bacterial transferrin receptors of the pathogenic Neisseriaceae, and TbpB in particular, as potential vaccine antigens (1–3). Investigators have shown that antibodies to the meningococcal TbpB, elicited in animals and in humans, exhibit bactericidal activity (1, 2). Myers et al. have shown that polyclonal antibodies to the TbpB of M. catarrhalis are biologically active (27). This latter data is particularly relevant to our current study because OMP B1 is homologous to the M. catarrhalis TbpB recently described (27). In addition, we have also demonstrated that children recovering from M. catarrhalis-induced otitis media have immunoglobulin G (IgG) antibodies to OMP B1 in their convalescent-phase sera (7). These data, taken together, suggest that OMP B1 is an attractive vaccine candidate.
In this study, we have extended our evaluation of OMP B1 as a potential vaccine antigen against M. catarrhalis infections. We have developed monoclonal antibody (MAb) 11C6 to OMP B1 and characterized this antibody for surface reactivity, conservation, and biologic activity. The gene which codes for OMP B1 has been cloned, and an isogenic mutant, deficient in OMP B1 expression, has been constructed in M. catarrhalis 7169. In addition, we have presented bactericidal studies comparing the isogenic mutant to the wild-type strain, which have detected a potentially protective epitope of OMP B1 as defined by the specific MAb 11C6.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
M. catarrhalis 7169 is a low-passage clinical isolate obtained by tympanocentesis of the middle ear of a child with otitis media. This strain was kindly provided by Howard Faden (Children’s Hospital, Buffalo, N.Y.). The completely defined growth media (CDM) and the culturing conditions for M. catarrhalis were described previously (8, 25, 37). The antibiotic-resistant isogenic mutant was cultured in the presence of 20 μg of kanamycin per ml. Escherichia coli strains XL1-Blue and BM25.8 (Clonetech, Palo Alto, Calif.) were cultured at 37°C on Luria-Bertani (LB) agar plates or in LB broth in the presence of the appropriate antibiotic (100 μg of ampicillin per ml or 20 μg of kanamycin per ml).
Chemicals.
Biotinylated human transferrin (bTf), holo-human transferrin (hTf) and CNBr-activated Sepharose 4B were purchased from Sigma Chemical Co., St. Louis, Mo. The restriction endonucleases, T4 ligase, DNA polymerase 1 and molecular biology reagents were purchased from New England Biolabs, Inc., Beverly, Mass.
MAb.
MAbs against OMP B1 were developed by injecting BALB/c mice with viable, iron-stressed bacteria of M. catarrhalis 7169 by a previously described method (7).
OMPs, SDS-PAGE, and Western blot analyses.
OMPs were prepared by extraction with Zwittergent as previously described (7, 8). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot assays were performed by using our standard methods (7, 8).
Flow cytometry.
MAb 11C6 was evaluated by flow cytometry by a modification of a previously described method for M. catarrhalis (35). Briefly, bacteria were grown to mid-logarithmic phase in completely defined growth media as described (8, 25, 37). Two hundred microliters of culture was suspended in 800 μl of affinity- purified MAb and incubated for 1 h at 37°C. The bacteria were collected, suspended in fluorescein-labeled goat anti-mouse IgG or IgM (Kirkegaard and Perry Laboratories, Gaithersburg, Md.) and incubated for 30 min at 37°C. Phosphate-buffered saline was added, and the bacteria were subjected to flow cytometry with a FACScan (Becton Dickinson, Bedford, Mass.). A total of 20,000 CFU were counted in a gated region of single cells. Data were obtained by using an instrument status of logarithmic mode for forward scatter, side scatter, and fluorescence. MAb 7B3 was used as the negative control, and MAb 4G5, specific for a surface-exposed M. catarrhalis lipooligosaccharide epitope, served as the positive control.
Cloning and sequencing of ompB1.
M. catarrhalis genomic DNA was isolated by standard methods as previously described (31). Chromosomal M. catarrhalis 7169 DNA was partially digested with ApoI, ligated into λTriplEx arms (Clonetech, Palo Alto, Calif.) and packaged with Gigapack III Gold Packaging Extract (Stratagene, La Jolla, Calif.). The amplified library was plated on Escherichia coli XL1-Blue, and the recombinant clones were immunoscreened by probing nitrocellulose plaque lifts with MAb 11C6. Reactive plaques were purified, and recombinant pTriplEx plasmids were released from the λTriplEx clones via Cre-lox-mediated recombination upon transduction into E. coli BM25.8, per the manufacturer’s instructions. The plasmid p2DA was purified (Qiagen, Santa Clarita, Calif.), and the insert sequence (3.9 kb) was obtained via automated DNA sequencing (Sequencing Facility, State University of New York at Buffalo, Buffalo, N.Y.). Sequence analysis of the cloned gene was performed using MacVector 6.0 and the Wisconsin Sequence Analysis Packages (Genetics Computer Group, Madison, Wis.).
Insertional mutagenesis.
p2DA was restriction digested with SphI, to remove a 1.7-kb DNA fragment located 5′ of ompB1, and religated to form the subclone pB1a. pB1a was digested with HindIII to remove a 1,509-bp internal fragment of ompB1. The resulting subclones were screened with MAb 11C6, and a negative subclone, pdelB1, was identified. pdelB1 was redigested with HindIII and ligated to an EcoRI-HindIII fragment of pUC18K containing the aphA-3 nonpolar cassette (24). The resultant kanamycin-resistant plasmid, pdelB1-kan, was linearized by restriction digestion with BglII and NotI and electroporated into M. catarrhalis 7169 by a previously described method (17). Kanamycin-resistant M. catarrhalis 7169 colonies were screened for loss of reactivity to MAb 11C6, and one of these, 7169b12, was selected for further analysis.
PCR amplification.
Chromosomal DNA, isolated from strains 7169 and 7169b12, was subjected to PCR amplification by using oligonucleotide primers based on the sequences 5′-CGTCTTATTAACCGCTTGTGG-3′ (sense) and 5′-TCGACCGCTTTCAGTGTTC-3′ (antisense), which are located within ompB1 and flank the predicted insertional region of the aphA-3 cassette. PCR amplification was performed for 30 cycles, with an annealing temperature of 55°C. Each reaction mixture, containing a single amplified fragment as demonstrated by agarose gel analysis, was cleaned by using the QIAquick PCR Purification Kit (Qiagen) and sequenced as described above.
Bactericidal assay.
M. catarrhalis cells were cultured in iron-deficient CDM to induce maximum expression of OMP B1. Iron-stressed bacteria (A600 of 0.2) were diluted 100-fold in Gey’s balanced salt solution (GBSS). One hundred microliters of this bacterial stock suspension was added to each experimental tube. Pooled normal human serum (NHS), prepared by standard methods and stored at −80°C, was used as a source of complement. Heat-inactivated NHS (56°C for 30 min) was used as the complement-depleted control. Bactericidal activity was measured under the following experimental conditions: (i) bacteria with 15% NHS, (ii) bacteria with 15% NHS and 33 μg of purified MAb 11C6, and (iii) bacteria with 45% heat-inactivated NHS and 33 μg of purified MAb 11C6. The final reaction volume was adjusted to 1 ml with GBSS. The tubes were incubated in a 37°C water bath with constant agitation of 80 rpm. At 0, 60, 120, and 240 min, 100-μl aliquots were removed from each tube, serially diluted, and plated in triplicate. CFU were counted after overnight incubation. This assay was repeated three times.
Nucleotide sequence accession number.
The sequence for M. catarrhalis 7169 ompB1 was deposited with GenBank under the accession no. AF105251.
RESULTS
Development and analysis of MAb 11C6.
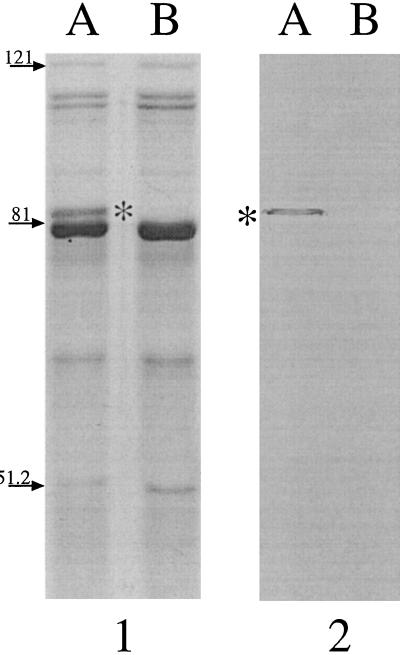
We have previously described MAb 7B3, which reacts to a conserved epitope of OMP B1 expressed by all strains of M. catarrhalis tested to date (7). However, the epitope which reacts with MAb 7B3 was not detected on the surface of the bacteria, and this antibody did not elicit biologic activity against M. catarrhalis (data not shown). A subsequent fusion was performed resulting in the development of MAb 11C6. Figure 1 shows an SDS-PAGE gel (panel 1) and the corresponding Western blot probed with MAb 11C6 (panel 2). In panel 1, OMPs isolated from iron-replete M. catarrhalis 7169 (lane A) are shown with those isolated from iron-stressed organisms (lane B). It is evident from this gel that there are multiple OMPs which have apparently increased in expression level in response to iron stress (lane B). One of these proteins is the previously described OMP B1, with an apparent molecular mass of 82 kDa (7). Panel 2 demonstrates that MAb 11C6 reacts with OMP B1 (asterisk), which was detected in the OMP profile of iron-stressed bacteria (lane B), but not in the outer membranes of organisms grown in the presence of iron (lane A). The OMP B1 epitope recognized by this antibody was also detected in flow cytometry by using viable, iron-stressed bacteria (data not shown). This reactivity confirms that OMP B1 contains epitopes that are expressed on the surface of the bacteria, an essential characteristic of a good vaccine antigen.
FIG. 1.
An SDS-PAGE gel (7.5% polycrylamide) (panel 1) showing the OMP profile from M. catarrhalis 7169 grown under iron-replete (lane A) and iron-deficient (lane B) conditions. Panel 2 is the corresponding Western blot probed with MAb 11C6, which detects the presence of OMP B1, with an approximate molecular mass of 82 kDa (asterisk), in outer membranes of cells grown only under iron limitation (lane B). Molecular mass standards (left) are expressed in kilodaltons.
Cloning of ompB1.
To further characterize OMP B1, the gene which codes for this protein was cloned by probing a genomic library of M. catarrhalis 7169 with MAb 11C6. The details of the construction of this library are presented in Materials and Methods. Several positive clones were identified, and one (p2Da), which contained a 3.9-kb DNA insert, was chosen for further evaluation. Sequence analysis of p2Da identified an open reading frame of 2,133 bp which encodes the intact ompB1. Plasmid pB1a, containing ompB1 flanked by 61 bp of 5′ sequence and 170 bp of 3′ sequence, was subcloned from p2DA and used for further analysis (see the description of construction of the OMP B1 mutant in Materials and Methods). Comparison of the amino acid sequence of OMP B1 to sequences deposited in databanks reveals identity with transferrin binding protein B of the pathogenic Neisseriaceae (34 to 40% identity), H. influenzae (33 to 36% identity), and A. pleuropneumoniae (35 to 39% identity). Subsequent comparison of ompB1 to the M. catarrhalis tbpB sequences recently reported by Myers et al. (27) revealed 50 to 71% identity and 61 to 77% similarity between OMP B1 and these six TbpBs at the amino acid level (data not shown).
Expression of ompB1 in E. coli was achieved by transformation with either p2DA or pB1a. Figure 2 shows a Western blot assay probed with MAb 11C6. This blot shows that MAb 11C6 reacts to recombinant OMP B1 (rOMP B1) (lane C), which has an apparent molecular mass of 82 kDa, in a manner indistinguishable from its reaction to native OMP B1 produced by M. catarrhalis 7169 (lane A). In addition, a portion of the total membranes from E. coli BM25.8 expressing rOMP B1 was subjected to affinity chromatography on a matrix containing human holotransferrin. The resulting binding fraction, containing a single protein, was analyzed (Fig. 2, lane D). The reactivity detected by MAb 11C6 confirmed that rOMP B1 retained the ability to bind human transferrin.
FIG. 2.
A Western blot, of an SDS-PAGE gel (7.5% polyacrylamide), probed with MAb 11C6. Total membrane proteins from iron-stressed M. catarrhalis 7169 (lane A), from wild-type E. coli BM25.8 (lane B), and from transformed E. coli BM25.8 expressing rOMP B1 (lane C) were analyzed. Lane D contains the binding fraction from a holotransferrin affinity matrix that was incubated with total membranes of BM25.8 expressing rOMP B1, demonstrating that the recombinant protein retains transferrin binding activity. Molecular mass standards (left) are in kilodaltons.
Figure 3 is a composite showing colony lift assays probed with either MAb 11C6 (panels A and B) or biotinylated human holotransferrin (panels C and D). MAb 11C6 detects the expression of rOMP B1 on the surface of the transformed E. coli (panel A) but not on that of the wild-type control (panel B). Furthermore, in contrast to the wild type (panel D) E. coli expressing rOMP B1 bound biotinylated human holotransferrin at the bacterial cell surface (panel C). These data show that OMP B1 was expressed, processed, and assembled on the surface of the transformed E. coli, confirming that OMP B1 is a receptor for human transferrin.
FIG. 3.
A composite of colony lift assays of E. coli expressing OMP B1 (A and C) and wild-type E. coli (B and D). The blots shown in panels A and B were probed with MAb 11C6 and demonstrate that rOMP B1 is expressed on the cell surface (A). The blots shown in panels C and D were probed with biotinylated human holotransferrin and demonstrate that rOMP B1 retains transferrin binding activity.
Construction and characterization of the ompB1 isogenic mutant.
An isogenic mutant was constructed with M. catarrhalis 7169 by using the kanamycin resistance determinant from pUC18K for cassette mutagenesis of the ompB1 coding region (24). This mutant was developed to serve as a control in studies designed to evaluate the significance of antibodies directed to OMP B1. However, this mutant has also provided an important tool for comparative growth studies designed to characterize the role of OMP B1 in iron acquisition from human transferrin (unpublished data). A 1,509-bp internal fragment of ompB1 was replaced with a fragment of pUC18K that contains the aphA-3 nonpolar cassette, resulting in pdelB1-kan (24). pdelB1-kan contains an ATG codon placed 3′ of the resistance gene and in frame with the TAA codon of ompB1 and therefore is nonpolar. The plasmid pdelB1-kan was linearized by digestion with BglII and NotI and electroporated into M. catarrhalis 7169. All of the resulting kanamycin-resistant clones were unreactive by immunoscreening with MAb 11C6.
Chromosomal DNA was isolated from four of these negative clones, and a portion of the disrupted ompB1 containing aphA-3 was amplified via PCR. Subsequent DNA sequence analysis of these PCR-amplified fragments confirmed that the kanamycin resistance cassette was inserted into ompB1 of M. catarrhalis 7169 at the predicted location (data not shown). One of the ompB1 isogenic mutants, 7169b12, was selected for further analysis.
To confirm the loss of OMP B1 expression, OMPs from wild-type 7169 and the isogenic mutant 7169b12 were prepared from cells grown under iron-limiting conditions and analyzed. Figure 4 shows an SDS-PAGE gel (panel 1) demonstrating that OMP B1 is expressed only in the wild-type strain (lane A). In contrast, the 82-kDa OMP B1 protein is absent in the OMPs from the mutant 7169b12 (lane B). Further comparison of the OMP profiles did not reveal any other difference between these two strains. Lane A of panel 2 shows that MAb 11C6 reacts to the 82-kDa OMP B1 present in the 7169 OMPs but does not recognize this protein in the OMPs isolated from 7169b12 (lane B), confirming the absence of OMP B1 from the isogenic mutant 7169b12.
FIG. 4.
An SDS–7% PAGE gel (panel 1) showing iron-stressed OMPs of wild-type M. catarrhalis 7169 (lane A) and those isolated from the isogenic mutant 7169b12. Panel 2 shows a Western blot probed with MAb 11C6, corresponding to the gel shown in panel 1 and confirming the loss of OMP B1 expression (asterisk) in the isogenic mutant 7169b12 (lane B). Molecular mass standards (left) are expressed in kilodaltons.
Bactericidal activity of monoclonal antibody 11C6.
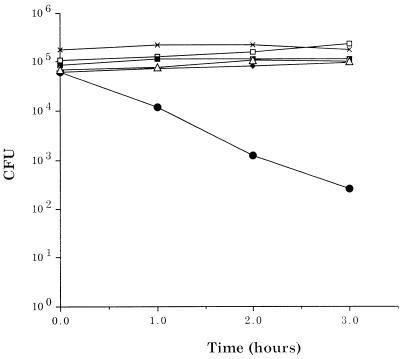
In order to investigate whether OMP B1 elicits a functional antibody response, we analyzed the ability of affinity-purified MAb 11C6 to activate complement and promote bactericidal activity against M. catarrhalis 7169. Figure 5 shows the data from a representative bactericidal assay obtained by using pooled NHS as the complement source and MAb 11C6 as the specific antibody. In the presence of MAb 11C6 and 15% NHS, wild-type 7169 demonstrated a substantial decline in viability. After 1 h, the percent viability had decreased by approximately 80%. This percentage continued to decline, resulting in a 2.5 log decrease in the number of viable bacteria at the final time point, which corresponds to a 99.9% rate of kill. The ompB1 isogenic mutant 7169b12, in contrast, was resistant to this complement-mediated bactericidal activity (Fig. 5). In addition, the viability of the strains was not affected by exposure to heat-inactivated NHS in the presence of MAb 11C6 or to 15% NHS alone. These data demonstrate that OMP B1, and specifically the epitope defined by MAb 11C6, elicits biologically protective antibodies.
FIG. 5.
A bactericidal assay comparing the sensitivities of wild-type M. catarrhalis 7169 and the ompB1 isogenic mutant 7169b12 to MAb 11C6. In the presence of MAb 11C6 and 15% NHS, the wild type (•) exhibited a 99% decline in viability. In contrast, the viability of 7169b12 (■) was unaffected under the same conditions. As controls, the mutant (×) and the wild type (⧫) were incubated with 15% NHS and the mutant (▵) and the wild type (□) were incubated with 45% heated-inactivated NHS plus MAb 11C6.
Conservation of the OMP B1 epitope recognized by MAb 11C6.
To further investigate the conservation of the OMP B1 epitope that reacts with MAb 11C6, 16 clinical isolates of M. catarrhalis were analyzed by Western blot assay and flow cytometry and their reactivities detected by MAb 7B3 were compared. Table 1 summarizes the results of these studies. These data show that MAb 11C6 reacts to a surface-exposed epitope conserved on 31% of the isolates tested. It should be noted that strains were isolated from both children and adults from various geographic locations. This table also shows that MAb 7B3 reacts to a highly conserved epitope that is not expressed on the surface of the bacteria. This demonstrates that OMP B1 contains conserved and variable epitopes, which is consistent with the data characterizing the TbpB of other pathogenic bacteria.
TABLE 1.
MAb analysis of OMP B1 epitopes
| M. catarrhalis isolate | MAb 7B3
|
MAb 11C6
|
Isolation sitea | Geographic location | ||
|---|---|---|---|---|---|---|
| Wbb | Fcc | Wb | Fc | |||
| sk633 | + | − | + | + | BAL | Buffalo, N.Y. |
| 7169 | + | − | + | + | ME | Buffalo, N.Y. |
| 3584 | + | − | − | − | ME | Buffalo, N.Y. |
| 555 | + | − | − | − | SP | Buffalo, N.Y. |
| 9485 | + | − | + | + | ME | Buffalo, N.Y. |
| 5190 | + | − | + | + | ME | Birmingham, England |
| Pw640 | + | − | − | − | BAL | Birmingham, England |
| 524DH | + | − | − | − | SP | Birmingham, England |
| 481DH | + | − | − | − | SP | Birmingham, England |
| Af218 | + | − | − | − | BAL | Birmingham, England |
| M7 | + | − | − | − | SP | Houston, Tex. |
| M10 | + | − | + | + | SP | Houston, Tex. |
| 035E | + | − | − | − | ME | Houston, Tex. |
| Tal3 | + | − | − | − | SN | Philadelphia, Pa. |
| Tal1 | + | − | − | − | SP | Philadelphia, Pa. |
| MC56 | + | − | − | − | SP | Mt. Home, Tenn. |
BAL, bronchoaveolar lavage; ME, middle ear; SP, sputum; SN, sinus.
Wb, Western blot reactivity.
Fc, flow cytometry reactivity.
DISCUSSION
In this report, we describe studies which suggest that the iron-regulated protein OMP B1, expressed by all strains of M. catarrhalis, is a potential vaccine candidate. We have developed MAb 11C6 which reacts to a surface-exposed epitope of OMP B1. This antibody was used to clone ompB1 from M. catarrhalis 7169. Comparative sequence analysis shows that OMP B1 has homology with the TbpB described for various other pathogenic bacteria, including M. catarrhalis (27). Recombinant OMP B1 was expressed in E. coli, and this construct exhibited human transferrin binding activity at the bacterial cell surface, demonstrating that OMP B1 is a transferrin receptor. An isogenic mutant, defective in OMP B1 expression, was constructed in M. catarrhalis 7169. This mutant, termed 7169b12, was included as an important control in the bactericidal studies analyzing MAb 11C6. In addition, this isogenic mutant is an important tool for defining the role of OMP B1 in the acquisition of iron from human transferrin (unpublished data). We performed bactericidal assays, which have demonstrated that MAb 11C6 elicits complement-mediated bactericidal activity against M. catarrhalis 7169. Finally, we have also used MAbs to demonstrate that OMP B1 expresses both conserved and variable epitopes among various clinical isolates of M. catarrhalis.
Our initial sequence analysis of ompB1 showed homology with the tbpB genes of N. meningitidis, N. gonorrhoeae, H. influenzae, and A. pleuropneumoniae (9, 10, 18–20, 22, 29, 30, 32–34). Subsequently, a recent study by Myers et al. described the cloning and sequencing of the genes which code for transferrin binding proteins from various M. catarrhalis strains (27). A comparative sequence analysis of ompB1 and these M. catarrhalis transferrin binding proteins reveals that OMP B1 has 50 to 71% identity and 61 to 77% similarity to TbpB at the amino acid level (data not shown). Perhaps the most significant evidence we have presented, which confirms that OMP B1 is a transferrin receptor, involves the expression of rOMP B1 in E. coli. Our data demonstrate that rOMP B1 was expressed on the E. coli membrane, and we have also correlated this expression with binding to human holotransferrin on the bacterial surface. This is an important result because this data supports the conclusion that OMP B1 was processed, expressed, and inserted into the E. coli membrane as the mature, functioning lipoprotein. This data differs from the studies presented by Myers et al., who were unable to achieve surface expression of TbpA or TbpB in E. coli (27). The explanation for this difference is unclear; however, analysis of our constructs indicates that ompB1 is in the opposite orientation of the lacZ promoter contained within the cloning vector, suggesting that rOMP B1 expression is under the control of an M. catarrhalis promoter.
It was important to confirm that OMP B1 is a functional M. catarrhalis receptor for human transferrin because the characteristics of bacterial transferrin receptors are consistent with good vaccine antigens. Based on these characteristics, these receptors have been evaluated as potential components in vaccines in other human pathogens. It has been shown that TbpA and TbpB of N. meningitidis elicit both strain-specific and cross-reactive antibodies in sera from humans and animals (1, 2). Further analysis has shown that antibodies to meningococcal transferrin binding proteins are capable of killing N. meningitidis in the presence of complement (1, 2). The data recently presented by Myers et al. also shows that rTbpB from M. catarrhalis elicits bactericidal antibodies in guinea pigs (27). These studies suggest that transferrin receptors warrant further evaluation as vaccine antigens.
One of the important strengths of our studies evaluating the vaccine potential of OMP B1 involves the utilization of affinity- purified MAb 11C6 in our bactericidal assays. Although the work by Myers et al. suggests that the bactericidal activity of their polyclonal antisera is directed to TbpB, the possibility of undetected contaminating antibodies to other bacterial proteins cannot be excluded (27). This is not the case when MAbs are employed in these assays. In addition, the inclusion of the ompB1 isogenic mutant provides the ideal negative control for our studies. By measuring the biologic effect of MAb 11C6 against the isogenic mutant and the wild type, we have unequivocally demonstrated that complement-mediated bactericidal activity is the direct result of antibody binding to a specific epitope of OMP B1.
Although the epitope defined by MAb 11C6 was detected on only 31% of the isolates evaluated, this epitope elicits potentially protective antibodies. In addition, MAb 11C6 reacts to a single OMP B1 epitope which exists on a complex protein containing multiple antigenic determinants. We are currently extending our MAb studies to define other OMP B1 epitopes, from various clinical isolates, that are potentially protective. Our overall goal is to identify a series of OMP B1 antigenic determinants which collectively will elicit antibodies that react to a majority of M. catarrhalis clinical isolates. Once we have identified such an important group of determinants, we will perform a detailed analysis of the human immune response to these OMP B1 epitopes. These studies are designed to determine which specific OMP B1 epitopes warrant further consideration in a multicomponent vaccine against M. catarrhalis infections.
Until recently, it was difficult to confirm that transferrin receptors were expressed in vivo and important for disease. However, a recent study by Cornelissen et al. was the first study to directly link gonococcal transferrin receptors to in vivo pathogenesis in humans, the natural host (11). These investigators demonstrated that a mutant defective in expression of TbpA and TbpB was incapable of causing gonococcal infection in male volunteers, confirming that these proteins must be expressed in vivo (11). Unfortunately, it is difficult to study M. catarrhalis in vivo, because this organism is a strictly human pathogen, like the pathogenic Neisseriaceae. In addition, there is currently no human model available with which to begin to assess the importance of OMP B1 to M. catarrhalis infections. While the homology of OMP B1 to the gonococcal TbpB, together with the results of the human studies reported by Cornelissen et al., implies that OMP B1 may be expressed in vivo, more studies are needed to confirm this hypothesis. To date, the most compelling evidence to suggest that OMP B1 is expressed during natural infection is our previous data which showed that both adults and children have cross-reactive antibodies to OMP B1 in their convalescent-phase sera (7, 8). Despite the antigenic heterogeneity which exists for OMP B1, this protein is highly conserved, contains surface-exposed epitopes, elicits biologically active antibodies, and stimulates an immune response in humans. These characteristics strongly suggest that OMP B1 warrants further evaluation as a potential vaccine antigen against M. catarrhalis infections.
REFERENCES
- 1.Ala’Aldeen D A. Transferrin receptors of Neisseria meningitidis: promising candidates for a broadly cross-protective vaccine. J Med Microbiol. 1996;44:237–243. doi: 10.1099/00222615-44-4-237. [DOI] [PubMed] [Google Scholar]
- 2.Ala’Aldeen D A, Borriello S P. The meningococcal transferrin-binding proteins 1 and 2 are both surface exposed and generate bactericidal antibodies capable of killing homologous and heterologous strains. Vaccine. 1996;14:49–53. doi: 10.1016/0264-410x(95)00136-o. [DOI] [PubMed] [Google Scholar]
- 3.Ala’Aldeen D A, Stevenson P, Griffiths E, Gorringe A R, Irons L I, Robinson A, Hyde S, Borriello S P. Immune responses in humans and animals to meningococcal transferrin-binding proteins: implications for vaccine design. Infect Immun. 1994;62:2984–2990. doi: 10.1128/iai.62.7.2984-2990.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bluestone C D. Modern management of otitis media. Pediatr Clin N Am. 1989;36:1371–1387. doi: 10.1016/s0031-3955(16)36794-3. [DOI] [PubMed] [Google Scholar]
- 5.Boyle F M, Georghiou P R, Tilse M H, McCormack J G. Branhamella (Moraxella) catarrhalis: pathogenic significance in respiratory infections. Med J Aust. 1991;154:592–596. doi: 10.5694/j.1326-5377.1991.tb121219.x. [DOI] [PubMed] [Google Scholar]
- 6.Bullen J J. The significance of iron in infection. Rev Infect Dis. 1981;3:1127–1138. doi: 10.1093/clinids/3.6.1127. [DOI] [PubMed] [Google Scholar]
- 7.Campagnari A A, Ducey T F, Rebmann C A. Outer membrane protein B1, an iron-repressible protein conserved in the outer membrane of Moraxella (Branhamella) catarrhalis, binds human transferrin. Infect Immun. 1996;64:3920–3924. doi: 10.1128/iai.64.9.3920-3924.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campagnari A A, Shanks K L, Dyer D W. Growth of Moraxella catarrhalis with human transferrin and lactoferrin: expression of iron-repressible proteins without siderophore production. Infect Immun. 1994;62:4909–4914. doi: 10.1128/iai.62.11.4909-4914.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cornelissen C N, Anderson J E, Sparling P F. Characterization of the diversity and the transferrin-binding domain of gonococcal transferrin-binding protein 2. Infect Immun. 1997;65:822–828. doi: 10.1128/iai.65.2.822-828.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cornelissen C N, Biswas G D, Tsai J, Paruchuri D K, Thompson S A, Sparling P F. Gonococcal transferrin-binding protein 1 is required for transferrin utilization and is homologous to TonB-dependent outer membrane receptors. J Bacteriol. 1992;174:5788–5797. doi: 10.1128/jb.174.18.5788-5797.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cornelissen C N, Kelley M, Hobbs M M, Anderson J E, Cannon J G, Cohen M S, Sparling P F. The transferrin receptor expressed by gonococcal strain FA1090 is required for the experimental infection of human male volunteers. Mol Microbiol. 1998;27:611–616. doi: 10.1046/j.1365-2958.1998.00710.x. [DOI] [PubMed] [Google Scholar]
- 12.Cornelissen C N, Sparling P F. Iron piracy: acquisition of transferrin-bound iron by bacterial pathogens. Mol Microbiol. 1994;14:843–850. doi: 10.1111/j.1365-2958.1994.tb01320.x. [DOI] [PubMed] [Google Scholar]
- 13.Enright M C, McKenzie H. Moraxella (Branhamella) catarrhalis—clinical and molecular aspects of a rediscovered pathogen. J Med Microbiol. 1997;46:360–371. doi: 10.1099/00222615-46-5-360. [DOI] [PubMed] [Google Scholar]
- 14.Finkelstein, R. A., C. V. Sciortino, and M. A. McIntosh. 1983. Role of iron in microbe-host interactions. Rev. Infect. Dis. 5(Suppl. 4):S759–S777. [DOI] [PubMed]
- 15.Hager H, Verghese A, Alvarez S, Berk S L. Branhamella catarrhalis respiratory infections. Rev Infect Dis. 1987;9:1140–1149. doi: 10.1093/clinids/9.6.1140. [DOI] [PubMed] [Google Scholar]
- 16.Helminen M E, Maciver I, Latimer J L, Klesney-Tait J, Cope L D, Paris M, McCracken G H, Jr, Hansen E J. A large, antigenically conserved protein on the surface of Moraxella. J Infect Dis. 1994;170:867–872. doi: 10.1093/infdis/170.4.867. [DOI] [PubMed] [Google Scholar]
- 17.Helminen M E, Maciver I, Paris M, Latimer J L, Lumbley S L, Cope L D, McCracken G H, Jr, Hansen E J. A mutation affecting expression of a major outer membrane protein of Moraxella catarrhalis alters serum resistance and survival in vivo. J Infect Dis. 1993;168:1194–1201. doi: 10.1093/infdis/168.5.1194. [DOI] [PubMed] [Google Scholar]
- 18.Holland J, Towner K J, Williams P. Isolation and characterisation of Haemophilus influenzae type b mutants defective in transferrin-binding and iron assimilation. FEMS Microbiol Lett. 1991;61:283–287. doi: 10.1016/0378-1097(91)90566-s. [DOI] [PubMed] [Google Scholar]
- 19.Irwin S W, Averil N, Cheng C Y, Schryvers A B. Preparation and analysis of isogenic mutants in the transferrin receptor protein genes, tbpA and tbpB, from Neisseria meningitidis. Mol Microbiol. 1993;8:1125–1133. doi: 10.1111/j.1365-2958.1993.tb01657.x. [DOI] [PubMed] [Google Scholar]
- 20.Legrain M, Mazarin V, Irwin S W, Bouchon B, Quentin-Millet M J, Jacobs E, Schryvers A B. Cloning and characterization of Neisseria meningitidis genes encoding the transferrin-binding proteins Tbp1 and Tbp2. Gene. 1993;130:73–80. doi: 10.1016/0378-1119(93)90348-7. [DOI] [PubMed] [Google Scholar]
- 21.Litwin C M, Calderwood S B. Role of iron regulation of virulence genes. Clin Microbiol Rev. 1993;6:137–149. doi: 10.1128/cmr.6.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loosmore S M, Yang Y P, Coleman D C, Shortreed J M, England D M, Harkness R E, Chong P S, Klein M H. Cloning and expression of the Haemophilus influenzae transferrin receptor genes. Mol Microbiol. 1996;19:575–586. doi: 10.1046/j.1365-2958.1996.406943.x. [DOI] [PubMed] [Google Scholar]
- 23.Mathers K E, Goldblatt D, Aebi C, Yu R, Schryvers A B, Hansen E J. Characterisation of an outer membrane protein of Moraxella. FEMS Immunol Med Microbiol. 1997;19:231–236. doi: 10.1111/j.1574-695X.1997.tb01092.x. [DOI] [PubMed] [Google Scholar]
- 24.Menard R, Sansonetti P J, Parsot C. Nonpolar mutagenesis of ipa genes defines IpaB, IpaC, and IpaD as effectors of Shigella flexneri entry into epithelial cells. J Bacteriol. 1993;175:5899–5906. doi: 10.1128/jb.175.18.5899-5906.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morse S A, Bartenstein L. Purine metabolism in Neisseria gonorrhoeae; the requirement for hypoxanthine. Can J Microbiol. 1980;26:12–20. doi: 10.1139/m80-003. [DOI] [PubMed] [Google Scholar]
- 26.Murphy T F. Branhamella catarrhalis: epidemiology, surface antigenic structure, and immune response. Microbiol Rev. 1996;60:267–279. doi: 10.1128/mr.60.2.267-279.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Myers L E, Yang Y-P, Du R-P, Wang Q, Harkness R E, Schryvers A B, Klein M H, Loosmore S M. The transferrin binding protein B of Moraxella catarrhalis elicits bactericidal antibodies and is a potential vaccine antigen. Infect Immun. 1998;66:4183–4192. doi: 10.1128/iai.66.9.4183-4192.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nicotra B, Rivera M, Luman J I, Wallace R J., Jr Branhamella catarrhalis as a lower respiratory tract pathogen in patients with chronic lung disease. Arch Intern Med. 1986;146:890–893. [PubMed] [Google Scholar]
- 29.Pettersson A, Klarenbeek V, van Deurzen J, Poolman J T, Tommassen J. Molecular characterization of the structural gene for the lactoferrin receptor of the meningococcal strain H44/76. Microb Pathog. 1994;17:395–408. doi: 10.1006/mpat.1994.1085. [DOI] [PubMed] [Google Scholar]
- 30.Pettersson A, van der Ley P, Poolman J T, Tommassen J. Molecular characterization of the 98-kilodalton iron-regulated outer membrane protein of Neisseria meningitidis. Infect Immun. 1993;61:4724–4733. doi: 10.1128/iai.61.11.4724-4733.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Russo T A, Guenther J E, Wenderoth S, Frannk M M. Generation of isogenic K54 capsule-deficient Escherichia coli strains through TnphoA-mediated gene disruption. Mol Microbiol. 1993;9:357–364. doi: 10.1111/j.1365-2958.1993.tb01696.x. [DOI] [PubMed] [Google Scholar]
- 32.Schryvers A B. Identification of the transferrin- and lactoferrin-binding proteins in Haemophilus influenzae. J Med Microbiol. 1989;29:121–130. doi: 10.1099/00222615-29-2-121. [DOI] [PubMed] [Google Scholar]
- 33.Schryvers, A. B., and S. Gray-Owen. 1992. Iron acquisition in Haemophilus influenzae: receptors for human transferrin. J. Infect. Dis. 165(Suppl. 1):S103–S104. [DOI] [PubMed]
- 34.Schryvers A B, Lee B C. Comparative analysis of the transferrin and lactoferrin binding proteins in the family Neisseriaceae. Can J Microbiol. 1989;35:409–415. doi: 10.1139/m89-063. [DOI] [PubMed] [Google Scholar]
- 35.Sethi S, Surface J M, Murphy T F. Antigenic heterogeneity and molecular analysis of CopB of Moraxella (Branhamella) catarrhalis. Infect Immun. 1997;65:3666–3671. doi: 10.1128/iai.65.9.3666-3671.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stool S E, Field M J. The impact of otitis media. Pediatr Infect Dis J. 1989;8:S11–S14. [PubMed] [Google Scholar]
- 37.West S E H, Sparling P F. The response of Neisseria gonorrhoeae to iron limitation: alterations in expression of membrane proteins without apparent siderophore production. Infect Immun. 1985;47:388–394. doi: 10.1128/iai.47.2.388-394.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang Y P, Myers L E, McGuinness U, Chong P, Kwok Y, Klein M H, Harkness R E. The major outer membrane protein, CD, extracted from Moraxella. FEMS Immunol Med Microbiol. 1997;17:187–199. doi: 10.1111/j.1574-695X.1997.tb01012.x. [DOI] [PubMed] [Google Scholar]