Abstract
A new α-Tetralone based chalcone compound, (2E)-2-(4-ethoxybenzylidene)-3,4-dihydro-2H-naphthalen-1-one (EBDN) has been synthesized by Claisen–Schmidt condensation reaction of α-Tetralone (1) with 4-Ethoxybenzaldehyde (2) in basic medium. Then it was allowed to grow through slow evaporation solution growth technique. The molecular structure of grown EBDN has been systematically characterized by SCXRD, FT-IR, 1H NMR and 13C NMR spectroscopic studies. The micro-hardness, thermal (TGA & DTA) and photoluminence studies of the synthesized EBDN were also examined. The EBDN was screened for its anti-inflammatory, antidiabetic and anti-oxidant activity. It has shown admirable anti-inflammatory and antidiabetic activity. Protein-Ligand interactions of EBDN with SARS-CoV-2 main protease (PDB code: 6yb7) also performed.
Keywords: Claisen-Schmidt condensation, Chalcone, Crystal growth, Crystal structure, Biological activity, SARS-CoV-2
Grapghical abstract
1. Introduction
Chalcones ((E)-1,3-diphenylprop-2-ene-1-ones, Fig. 1 ) are one of the most overbearing classes and a modest chemical scaffolding of numerous naturally occurring compounds across the plant kingdom (vegetables, spices, fruits, teas and etc.,) [1], [2], [3], [4], [5], [6]. Chalcones belong to the family of flavonoids and they are also known as chalconoids. Which exhibits geometrical isomers cis and trans, and the thermodynamically more stable one is the trans isomer (Fig. 1).
Fig. 1.
Structure representation of chalcone and two clinically approved chalcone moiety containing drugs.
Chalcones are organic compounds possessing acceptor and donor groups, which enhance their importance in various fields. The chalcone compounds have attained significance because of their excellent NLO properties [7]. The NLO response was found to increase as the donor strength of the molecules increased.
The chalcone family has drawn a lot of attention not only from synthetic and biosynthetic perspectives, but also from pharmacological applications because they have revealed a lot of biological actions that include the treatment of medical disorders like microbial and viral infections [8], [9], [10], [11], cancer [12], [13], [14], [15], cardiovascular diseases and their risk factors [16], [17], [18], [19], anti-inflammatory [20] and antioxidant activities [21,22]. The maximum reactive moiety is due to the, [23] α,β-unsaturated carbonyl bridge, which is related to chalcone biological actions [24]. Some chalcone-moiety containing compounds have additionally been permitted for clinical use. For example, metochalcone ((E)-1-(2,4-dimethoxyphenyl)-3-(4-methoxyphenyl) prop-2-en-1-one) was approved as a choleretic while sofalcone was approved as an antiulcer and mucoprotective drug (Fig. 1) [25,26].
1-Tetralones are an essential elegance of compounds, and their numerous derivatives have proven important biological applications, including antimicrobial activity [27], anti-tumor activity [28], and neurological disorders. [29,30,43] Several (E)-2-(4′-substitutedbenzylidene)-1-tetralones (Fig. 2 ) have been synthesized, characterized by using spectroscopic tools and also performed their biological applications [31], [32], [33], [34], [35], [36], [37], [38].
Fig. 2.

Structure representation of 1-Tetralone based chalcones.
Since it's unveiling at the end of December 2019, SARS-CoV-2 has remained to spread chop-chop throughout the world. Despite the fact that the number of deaths caused by COVID-19 is rapidly increasing, an effective drug treatment other than vaccines has yet to be developed.The existing literature comprises many investigations on SARS-CoV-2 main protease and its potential inhibitors [39], [40], [41], [42], [43], [44], [45].
In this present investigation, a new α-Tetralone based chalcone compound, (2E)-2-(4-ethoxybenzylidene)-3,4-dihydro-2H-naphthalen-1-one (EBDN) has been synthesized and grown through a slow evaporation solution growth technique. The molecular structure of grown EBDN has been characterized by SCXRD, FT-IR, 1H NMR and 13C NMR spectroscopic studies. The micro-hardness, thermal (TGA & DTA) and photoluminence studies were also examined. The EBDN was screened for its anti-inflammatory, antidiabetic and anti-oxidant activity. Protein-Ligand interactions of EBDN with SARS-CoV-2 main protease also performed.
2. Experimental methods
2.1. Materials
α-tetralone (97%, Sigma Aldrich), p-Ethoxy benzaldehyde (99%, Sigma Aldrich), Absolute alcohol and sodium hydroxide (Spectrochem) were purchased from commercially available and were used.
2.2. Methods
2.2.1. Synthesis of (2E)-2-(4-ethoxybenzylidene)-3,4-dihydro-2H-naphthalen-1-one
In a 250 mL round-bottomed flask, α-tetralone (0.05 mol, 7.3 mL) and p-ethoxy benzaldehyde (0.05 mol, 7.0 mL) were taken to which 100 mL of absolute alcohol was added and stirred well for complete dissolution. After a span of 10 min, about 10 mL of 10% sodium hydroxide solution was added and the solution was stirred for about 1 hour at room temperature. After 12 h, ice cold water was added to precipitate the obtained light yellow color product which was filtered, washed with double distilled water and dried (Scheme 1 : Yield: 85%, 11.8297 g).
Scheme 1.
Synthesis of (2E)-2-(4-ethoxybenzylidene)-3,4-dihydro-2H-naphthalen-1-one.
EBDN: Light yellow single crystal. m.p.: 141–142 °C. 1H NMR (δ in ppm, CDCl3, 400 MHz): 8.13, 8.10 (d, 1H, J = 12 Hz), 7.84 (s, 1H), 7.49, 7.47 (d, 1H, J = 8 Hz), 7.45–7.40 (dd, 2H, J = 20 Hz), 7.37–7.33 (m, 1H, J = 16 Hz), 7.25, 7.23 (d, 1H, J = 8 Hz), 6.94, 6.92 (d, 2H, J = 8 Hz), 4.10–4.05 (q, 2H, J = 20 Hz), 3.15–3.13 (t, 2H, J = 8 Hz), 2.96–2.93 (t, 2H, J = 12 Hz), 1.45–1.41 (t, 3H, J = 16 Hz). 13C NMR (δ in ppm, CDCl3, 400 MHz): 188.07 (C=O), 159.59 (C15), 143.13 (C5), 136.97 (C11), 133.97 (C6 & C9), 133.62 (C2), 131.97 (C13 & C17), 128.45 (C1), 128.35 (C4), 128.27 (C12), 127.16 (C3), 114.67 (C14 & C16), 63.77 (C18), 29.01 (C8), 27.43 (C7), 14.98 (C19).
2.2.2. Crystal growth (slow evaporation solution method)
The slow evaporation solution method [38] was adopted to crystallize the formed chalcone. About one gram of the synthesized EBDN was taken in a 100 mL beaker to which 50 mL of acetone was added and then stirred at room temperature for about half-an-hour. Then it was filtered and the filtrate was collected in a 100 mL beaker. It was allowed to undergo slow evaporation at room temperature. When all the solvent was evaporated, the crystals were harvested and dried for crystallographic study (Fig. 3 ).
Fig. 3.
Photograph of the Grown Crystal of EBDN.
2.2.3. Characterization techniques
2.2.3.1. Single crystal X-Ray diffraction studies
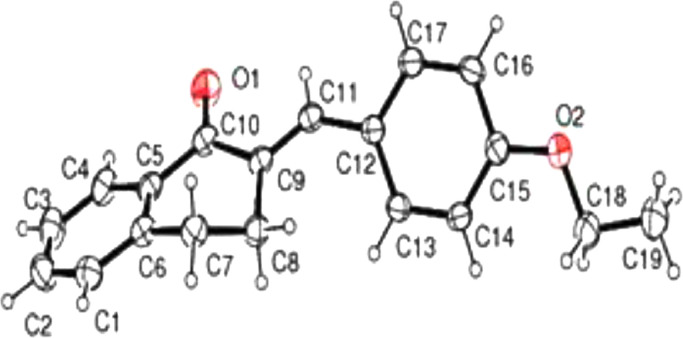
X-ray diffraction data for the crystalline EBDN was collected by employing a Bruker APEX DUO CCD diffractometer with a graphite monochromatic Mo-Kα radiation at a detector distance of 5 cm with APEX2 software [46]. The collected data was reduced using the SAINT program and the empirical absorption corrections were performed with the SADABS program. The molecular structure of the (2E)-2-(4-ethoxybenzylidene)-3,4-dihydro-2H-naphthalen-1-one (EBDN) crystalline was solved by direct methods and was refined using a full-matrix least-squares method on F2 using the SHELXTL program (Fig. 4 ) [46].
Fig. 4.
ORTEP diagram of crystalline EBDN.
2.2.3.2. FT-IR spectral analysis
The FT-IR spectrum of the grown crystal EBDN was recorded in the wave number region from 400 to 4000 cm−1 using a Perkin Elmer FT-IR spectrometer by KBr pellet technique.
2.2.3.3. NMR spectral analysis
1H NMR and 13C NMR spectrum of the synthesized grown crystal EBDN was recorded by using have been Bruker AV 400 NMR spectrometer (400 MHz) in CDCl3.
2.2.4. Biological evaluation
2.2.4.1. Anti-inflammatory analysis (BSA denaturation technique)
The grown chalcone compound EBDN and standard diclofenac sodium were screened for anti-inflammatory activity in five different concentrations (10, 25, 50, 125 & 250 μg/mL) by using the inhibition of albumin denaturation technique [47] with minor modification. The standard drug and compound were dissolved in a minimum quantity of Dimethyl formamide (DMF) and diluted with phosphate buffer (0.2 M, PH 7.4). The final concentration of DMF in all solutions was less than 2.5%. Test Solution (4 mL) containing different concentrations of the drug was mixed with 1 mL of 1 mM Bovine serum albumin solution in phosphate buffer and incubated at 37 °C in an incubator for 15 min. Denaturation was induced by keeping the reaction mixture at 70 °C in a water bath for 30 min. After cooling, the turbidity was measured at 660 nm. The percentage of inhibition of denaturation was calculated from control where no drug was added. The percentage inhibition of denaturation was calculated by using the following formula.
At: Absorbance of test
Ac: Absorbance of control
2.2.4.2. Anti-diabetic analysis (α-amylase inhibition technique)
The antidiabetic activity of the grown chalcone compound EBDN and standard Acarbose in five different concentrations (10, 25, 50, 125 & 250 μg/mL) were performed using α-amylase inhibition method [47]. Briefly, Amylase (0.5 mg/mL) was incubated with and without extract and standard for 10 min at 25 °C. This experiment was performed in 20 mM sodium phosphate buffer (pH 6.9). After pre incubation, the 1% starch solution (1 mL) was added and the reaction mixture was incubated for 30 min at 25 °C. In order to stop the enzymatic reaction, DNSA reagent (1 mL) was added as the color reagent and then incubated in a boiling water bath for15 min. After cooling down to the room temperature, the absorbance measured at 540 nm using a spectrophotometer instrument. The measured absorbance was compared with that of the control experiment. The percentage inhibition was calculated from the given formula.
At: Absorbance of test
Ac: Absorbance of control
2.2.4.3. Total antioxidant activity (phosphomolybdenum method)
Total antioxidant activity of the grown chalcone compound EBDN and standard Vitamin C in five different concentrations (10, 25, 50, 125 & 250 μg/mL) were observed using phosphomolybdenum method. About 3 mL of antioxidant reagent (0.6 M H2SO4, 28 mM Na3PO4 and 4 mM ammonium molybdate) were added to the test samples with various concentrations. The test mixture to accomplish proper diffusion with phosphomolybdenum reagent was incubated at 95 °C for 90 min in a water bath. The total antioxidant activity of extracts and vitamin C standard drug were measured and determined their absorbance at 695 nm using a spectrophotometer. The total antioxidant activities were calculated using the given formula.
At: Absorbance of test
Ac: Absorbance of control
2.2.5. Protein-ligand interactions
The 3D structure of the SARS-Cov-2 main protease enzyme (6yb7.pdb) was downloaded from the protein data bank [48]. The molecular docking poses are visualization using discovery studio. Molecular docking was performed using the Auto-Dock Tool (1.5.6). The 3D structure of EBDN was optimized using Gaussian 09 W [49]. All avoidable water and ligand were removed from the enzyme and polar hydrogen was added to the enzyme. The target was generated as a PDBQT format. PDBQT file of the ligands also generated and performed. The grid box size is fixed as 60 × 60 × 60 points in X, Y, and Z directions. Similarly, the grid space is fixed as 0.375 Å and ten runs were created by using Lamarckian genetic algorithm searches. The default docking parameters were fixed and performed.
3. Results and discussion
3.1. Synthesis and crystal growth of EBDN
The title chalcone compound (2E)-2-(4-ethoxybenzylidene)-3,4-dihydro-2H-naphthalen-1-one (EBDN) was successfully synthesized by the simple Claisen-Schmidt condensation reaction of α-Tetralone with 4-ethoxybenzaldehyde in a basic medium (Scheme 1). 85% of EBDN were obtained. Then it was grown through a slow evaporation solution growth technique. The single crystal of grown EBDN was obtained.
3.2. Characterization of EBDN
The molecular structure of the title chalcone compound (2E)-2-(4-ethoxybenzylidene)-3,4-dihydro-2H-naphthalen-1-one (EBDN) was successfully confirmed and characterized by SCXRD, FT-IR and NMR spectral analysis.
3.2.1. Single crystal X-ray diffraction study of EBDN
The title chalcone compound, (2E)-2-(4-ethoxybenzylidene)-3,4-dihydro-2H-naphthalen-1-one (EBDN) crystallizes in a monoclinic crystal system with P21/n space group with the unit cell parameters shown in Table 1 . The crystal packing of the compound viewed along the ‘b’ axis is presented in Fig. 5 .
Table 1.
Crystal data and structure refinement for the EBDN crystal.
| CCDC No. | 1954163 | |
|---|---|---|
| Identification code | EBDN | |
| Empirical formula | C19 H18 O2 | |
| Formula weight | 278.33 | |
| Temperature | 296(2) K | |
| Wavelength | 0.71073 Å | |
| Crystal system | Monoclinic | |
| Space group | P21 | |
| Unit cell dimensions | a = 6.9532(6) Å | α = 90° |
| b = 14.8211(14) Å | β = 101.969(3)° | |
| c = 14.5529(13) Å | γ = 90° | |
| Volume | 1467.1(2) Å3 | |
| Z | 4 | |
| Density (calculated) | 1.260 mg/mm3 | |
| Absorption coefficient | 0.080 mm−1 | |
| F (000) | 592 | |
| Crystal size | 0.200 × 0.150 × 0.150 mm3 | |
| Theta range for data collection | 2.749 to 25.133° | |
| Index ranges | −7<=h<=8, −17<=k<=17, −17<=l<=17 | |
| Reflections collected | 23,021 | |
Fig. 5.
Crystal packing of Crystalline EBDN.
The molecule exists in an E configuration with respect to the C9 C11 double bond and the dihedral angle existing between the mean plane of the two phenyl rings is 34.4°. The bond lengths and angles are within the normal ranges and comparable with the previously reported structures of chalcone [38]. Table 2 provides experimental values of the bond lengths and bond angles of the synthesised compound molecular structure.
Table 2.
The geometrical parameters of the grown EBDN.
| Bond length (Å) |
Bond angles(˚) |
Dihedral angles [˚] |
|||
|---|---|---|---|---|---|
| Atoms | Bond length | Atoms | Bond angle | Atoms | Dihedral angle |
| C(1)-C(6) | 1.384(6) | C(6)-C(1)-C(2) | 121.0(4) | C(6)-C(1)-C(2)-C(3) | −0.9(7) |
| C(1)-C(2) | 1.389(5) | C(6)-C(1)-H(1) | 119.5 | C(1)-C(2)-C(3)-C(4) | 0.2(7) |
| C(1)-H(1) | 0.93 | C(2)-C(1)-H(1) | 119.5 | C(2)-C(3)-C(4)-C(5) | 0.3(7) |
| C(2)-C(3) | 1.372(6) | C(3)-C(2)-C(1) | 120.1(4) | C(3)-C(4)-C(5)-C(6) | −0.2(6) |
| C(2)-H(2) | 0.93 | C(3)-C(2)-H(2) | 119.9 | C(3)-C(4)-C(5)-C(10) | −179.1(4) |
| C(3)-C(4) | 1.372(6) | C(1)-C(2)-H(2) | 119.9 | C(2)-C(1)-C(6)-C(5) | 1.0(6) |
| C(3)-H(3) | 0.93 | C(2)-C(3)-C(4) | 119.7(4) | C(2)-C(1)-C(6)-C(7) | 180.0(4) |
| C(4)-C(5) | 1.392(5) | C(2)-C(3)-H(3) | 120.1 | C(4)-C(5)-C(6)-C(1) | −0.4(6) |
| C(4)-H(4) | 0.93 | C(4)-C(3)-H(3) | 120.1 | C(10)-C(5)-C(6)-C(1) | 178.4(4) |
| C(5)-C(6) | 1.388(5) | C(3)-C(4)-C(5) | 120.3(4) | C(4)-C(5)-C(6)-C(7) | −179.5(4) |
| C(5)-C(10) | 1.485(5) | C(3)-C(4)-H(4) | 119.8 | C(10)-C(5)-C(6)-C(7) | −0.6(6) |
| C(6)-C(7) | 1.493(5) | C(5)-C(4)-H(4) | 119.8 | C(1)-C(6)-C(7)-C(8) | 144.6(4) |
| C(7)-C(8) | 1.521(6) | C(6)-C(5)-C(4) | 120.5(4) | C(5)-C(6)-C(7)-C(8) | −36.4(5) |
| C(7)-H(7A) | 0.97 | C(6)-C(5)-C(10) | 120.3(3) | C(6)-C(7)-C(8)-C(9) | 54.7(5) |
| C(7)-H(7B) | 0.97 | C(4)-C(5)-C(10) | 119.1(4) | C(7)-C(8)-C(9)-C(11) | 142.5(4) |
| C(8)-C(9) | 1.500(5) | C(1)-C(6)-C(5) | 118.2(4) | C(7)-C(8)-C(9)-C(10) | −38.4(5) |
| C(8)-H(8A) | 0.97 | C(1)-C(6)-C(7) | 121.9(4) | C(6)-C(5)-C(10)-O(1) | −162.8(4) |
| C(8)-H(8B) | 0.97 | C(5)-C(6)-C(7) | 119.8(4) | C(4)-C(5)-C(10)-O(1) | 16.1(6) |
| C(9)-C(11) | 1.334(5) | C(6)-C(7)-C(8) | 110.9(4) | C(6)-C(5)-C(10)-C(9) | 18.8(6) |
| C(9)-C(10) | 1.490(5) | C(6)-C(7)-H(7A) | 109.4 | C(4)-C(5)-C(10)-C(9) | −162.3(4) |
| C(10)-O(1) | 1.223(4) | C(8)-C(7)-H(7A) | 109.4 | C(11)-C(9)-C(10)-O(1) | 2.9(6) |
| C(11)-C(12) | 1.459(5) | C(6)-C(7)-H(7B) | 109.4 | C(8)-C(9)-C(10)-O(1) | −176.2(4) |
| C(11)-H(11) | 0.93 | C(8)-C(7)-H(7B) | 109.4 | C(11)-C(9)-C(10)-C(5) | −178.6(4) |
| C(12)-C(13) | 1.390(5) | H(7A)-C(7)-H(7B) | 108 | C(8)-C(9)-C(10)-C(5) | 2.2(5) |
| C(12)-C(17) | 1.394(5) | C(9)-C(8)-C(7) | 111.3(3) | C(10)-C(9)-C(11)-C(12) | −172.6(3) |
| C(13)-C(14) | 1.380(5) | C(9)-C(8)-H(8A) | 109.4 | C(8)-C(9)-C(11)-C(12) | 6.5(7) |
| C(13)-H(13) | 0.93 | C(7)-C(8)-H(8A) | 109.4 | C(9)-C(11)-C(12)-C(13) | 34.4(6) |
| C(14)-C(15) | 1.376(5) | C(9)-C(8)-H(8B) | 109.4 | C(9)-C(11)-C(12)-C(17) | −150.1(5) |
| C(14)-H(14) | 0.93 | C(7)-C(8)-H(8B) | 109.4 | C(17)-C(12)-C(13)-C(14) | 0.4(6) |
| C(15)-O(2) | 1.365(4) | H(8A)-C(8)-H(8B) | 108 | C(11)-C(12)-C(13)-C(14) | 176.0(4) |
| C(15)-C(16) | 1.385(5) | C(11)-C(9)-C(10) | 117.9(3) | C(12)-C(13)-C(14)-C(15) | −0.3(6) |
| C(16)-C(17) | 1.368(5) | C(11)-C(9)-C(8) | 124.8(4) | C(13)-C(14)-C(15)-O(2) | −179.0(4) |
| C(16)-H(16) | 0.93 | C(10)-C(9)-C(8) | 117.3(3) | C(13)-C(14)-C(15)-C(16) | 0.8(6) |
| C(17)-H(17) | 0.93 | O(1)-C(10)-C(5) | 121.0(3) | O(2)-C(15)-C(16)-C(17) | 178.4(4) |
| C(18)-O(2) | 1.421(5) | O(1)-C(10)-C(9) | 121.8(3) | C(14)-C(15)-C(16)-C(17) | −1.4(6) |
| C(18)-C(19) | 1.492(5) | C(5)-C(10)-C(9) | 117.2(3) | C(15)-C(16)-C(17)-C(12) | 1.6(6) |
| C(18)-H(18A) | 0.97 | C(9)-C(11)-C(12) | 129.5(3) | C(13)-C(12)-C(17)-C(16) | −1.0(6) |
| C(18)-H(18B) | 0.97 | C(9)-C(11)-H(11) | 115.2 | C(11)-C(12)-C(17)-C(16) | −176.8(4) |
| C(19)-H(19A) | 0.96 | C(12)-C(11)-H(11) | 115.2 | C(14)-C(15)-O(2)-C(18) | −0.8(5) |
| C(19)-H(19B) | 0.96 | C(13)-C(12)-C(17) | 117.0(4) | C(16)-C(15)-O(2)-C(18) | 179.4(4) |
| C(19)-H(19C) | 0.96 | C(13)-C(12)-C(11) | 123.4(4) | C(19)-C(18)-O(2)-C(15) | −176.7(4) |
| C(20)-C(25) | 1.380(6) | C(17)-C(12)-C(11) | 119.5(4) | C(25)-C(20)-C(21)-C(22) | −0.3(7) |
| C(20)-C(21) | 1.384(6) | C(14)-C(13)-C(12) | 121.6(4) | C(20)-C(21)-C(22)-C(23) | 1.8(7) |
| C(20)-H(20) | 0.93 | C(14)-C(13)-H(13) | 119.2 | C(21)-C(22)-C(23)-C(24) | −2.1(7) |
| C(21)-C(22) | 1.385(6) | C(12)-C(13)-H(13) | 119.2 | C(22)-C(23)-C(24)-C(25) | 0.8(6) |
| C(21)-H(21) | 0.93 | C(15)-C(14)-C(13) | 120.2(4) | C(22)-C(23)-C(24)-C(29) | −179.5(4) |
| C(22)-C(23) | 1.375(5) | C(15)-C(14)-H(14) | 119.9 | C(21)-C(20)-C(25)-C(24) | −0.9(6) |
| C(22)-H(22) | 0.93 | C(13)-C(14)-H(14) | 119.9 | C(21)-C(20)-C(25)-C(26) | 179.7(4) |
| C(23)-C(24) | 1.389(5) | O(2)-C(15)-C(14) | 124.7(4) | C(23)-C(24)-C(25)-C(20) | 0.7(6) |
| C(23)-H(23) | 0.93 | O(2)-C(15)-C(16) | 116.1(4) | C(29)-C(24)-C(25)-C(20) | −179.0(4) |
| C(24)-C(25) | 1.398(5) | C(14)-C(15)-C(16) | 119.3(4) | C(23)-C(24)-C(25)-C(26) | −180.0(4) |
| C(24)-C(29) | 1.486(5) | C(17)-C(16)-C(15) | 120.2(4) | C(29)-C(24)-C(25)-C(26) | 0.4(6) |
| C(25)-C(26) | 1.505(5) | C(17)-C(16)-H(16) | 119.9 | C(20)-C(25)-C(26)-C(27) | −144.2(4) |
| C(26)-C(27) | 1.519(5) | C(15)-C(16)-H(16) | 119.9 | C(24)-C(25)-C(26)-C(27) | 36.5(5) |
| C(26)-H(26A) | 0.97 | C(16)-C(17)-C(12) | 121.8(4) | C(25)-C(26)-C(27)-C(28) | −55.4(4) |
| C(26)-H(26B) | 0.97 | C(16)-C(17)-H(17) | 119.1 | C(26)-C(27)-C(28)-C(30) | −139.3(4) |
| C(27)-C(28) | 1.505(5) | C(12)-C(17)-H(17) | 119.1 | C(26)-C(27)-C(28)-C(29) | 39.5(5) |
| C(27)-H(27A) | 0.97 | O(2)-C(18)-C(19) | 108.3(4) | C(23)-C(24)-C(29)-O(3) | −14.3(6) |
| C(27)-H(27B) | 0.97 | O(2)-C(18)-H(18A) | 110 | C(25)-C(24)-C(29)-O(3) | 165.3(4) |
| C(28)-C(30) | 1.327(5) | C(19)-C(18)-H(18A) | 110 | C(23)-C(24)-C(29)-C(28) | 162.5(3) |
| C(28)-C(29) | 1.489(5) | O(2)-C(18)-H(18B) | 110 | C(25)-C(24)-C(29)-C(28) | −17.8(6) |
| C(29)-O(3) | 1.224(4) | C(19)-C(18)-H(18B) | 110 | C(30)-C(28)-C(29)-O(3) | −7.9(6) |
| C(30)-C(31) | 1.461(5) | H(18A)-C(18)-H(18B) | 108.4 | C(27)-C(28)-C(29)-O(3) | 173.3(4) |
| C(30)-H(30) | 0.93 | C(18)-C(19)-H(19A) | 109.5 | C(30)-C(28)-C(29)-C(24) | 175.3(4) |
| C(31)-C(32) | 1.388(5) | C(18)-C(19)-H(19B) | 109.5 | C(27)-C(28)-C(29)-C(24) | −3.5(5) |
| C(31)-C(36) | 1.396(5) | H(19A)-C(19)-H(19B) | 109.5 | C(29)-C(28)-C(30)-C(31) | 174.4(4) |
| C(32)-C(33) | 1.373(5) | C(18)-C(19)-H(19C) | 109.5 | C(27)-C(28)-C(30)-C(31) | −6.8(7) |
| C(32)-H(32) | 0.93 | H(19A)-C(19)-H(19C) | 109.5 | C(28)-C(30)-C(31)-C(32) | 148.8(5) |
| C(33)-C(34) | 1.383(5) | H(19B)-C(19)-H(19C) | 109.5 | C(28)-C(30)-C(31)-C(36) | −35.8(6) |
| C(33)-H(33) | 0.93 | C(15)-O(2)-C(18) | 118.1(3) | C(36)-C(31)-C(32)-C(33) | 1.5(6) |
| C(34)-O(4) | 1.369(4) | C(25)-C(20)-C(21) | 121.1(5) | C(30)-C(31)-C(32)-C(33) | 177.3(4) |
| C(34)-C(35) | 1.385(5) | C(25)-C(20)-H(20) | 119.5 | C(31)-C(32)-C(33)-C(34) | −2.1(6) |
| C(35)-C(36) | 1.380(5) | C(21)-C(20)-H(20) | 119.5 | C(32)-C(33)-C(34)-O(4) | −178.3(4) |
| C(35)-H(35) | 0.93 | C(20)-C(21)-C(22) | 119.8(4) | C(32)-C(33)-C(34)-C(35) | 1.3(6) |
| C(36)-H(36) | 0.93 | C(20)-C(21)-H(21) | 120.1 | O(4)-C(34)-C(35)-C(36) | 179.4(4) |
| C(37)-O(4) | 1.425(5) | C(22)-C(21)-H(21) | 120.1 | C(33)-C(34)-C(35)-C(36) | −0.1(6) |
| C(37)-C(38) | 1.504(6) | C(23)-C(22)-C(21) | 119.6(4) | C(34)-C(35)-C(36)-C(31) | −0.4(6) |
| C(37)-H(37A) | 0.97 | C(23)-C(22)-H(22) | 120.2 | C(32)-C(31)-C(36)-C(35) | −0.3(6) |
| C(37)-H(37B) | 0.97 | C(21)-C(22)-H(22) | 120.2 | C(30)-C(31)-C(36)-C(35) | −175.9(4) |
| C(38)-H(38A) | 0.96 | C(22)-C(23)-C(24) | 120.8(4) | C(33)-C(34)-O(4)-C(37) | −176.5(4) |
| C(38)-H(38B) | 0.96 | C(22)-C(23)-H(23) | 119.6 | C(35)-C(34)-O(4)-C(37) | 3.9(6) |
| C(38)-H(38C) | 0.96 | C(24)-C(23)-H(23) | 119.6 | C(38)-C(37)-O(4)-C(34) | 171.6(4) |
| C(23)-C(24)-C(25) | 119.7(4) | ||||
| C(23)-C(24)-C(29) | 120.2(4) | ||||
| C(25)-C(24)-C(29) | 120.1(3) | ||||
| C(20)-C(25)-C(24) | 118.9(4) | ||||
| C(20)-C(25)-C(26) | 122.1(4) | ||||
| C(24)-C(25)-C(26) | 119.0(4) | ||||
| C(25)-C(26)-C(27) | 111.6(4) | ||||
| C(25)-C(26)-H(26A) | 109.3 | ||||
| C(27)-C(26)-H(26A) | 109.3 | ||||
| C(25)-C(26)-H(26B) | 109.3 | ||||
| C(27)-C(26)-H(26B) | 109.3 | ||||
| H(26A)-C(26)-H(26B) | 108 | ||||
| C(28)-C(27)-C(26) | 111.0(3) | ||||
| C(28)-C(27)-H(27A) | 109.4 | ||||
| C(26)-C(27)-H(27A) | 109.4 | ||||
| C(28)-C(27)-H(27B) | 109.4 | ||||
| C(26)-C(27)-H(27B) | 109.4 | ||||
| H(27A)-C(27)-H(27B) | 108 | ||||
| C(30)-C(28)-C(29) | 117.5(3) | ||||
| C(30)-C(28)-C(27) | 125.8(3) | ||||
| C(29)-C(28)-C(27) | 116.6(3) | ||||
| O(3)-C(29)-C(24) | 119.6(3) | ||||
| O(3)-C(29)-C(28) | 122.0(4) | ||||
| C(24)-C(29)-C(28) | 118.3(3) | ||||
| C(28)-C(30)-C(31) | 129.6(3) | ||||
| C(28)-C(30)-H(30) | 115.2 | ||||
| C(31)-C(30)-H(30) | 115.2 | ||||
| C(32)-C(31)-C(36) | 116.8(4) | ||||
| C(32)-C(31)-C(30) | 119.6(3) | ||||
| C(36)-C(31)-C(30) | 123.4(3) | ||||
| C(33)-C(32)-C(31) | 122.3(4) | ||||
| C(33)-C(32)-H(32) | 118.8 | ||||
| C(31)-C(32)-H(32) | 118.8 | ||||
| C(32)-C(33)-C(34) | 119.7(4) | ||||
| C(32)-C(33)-H(33) | 120.1 | ||||
| C(34)-C(33)-H(33) | 120.1 | ||||
| O(4)-C(34)-C(33) | 115.6(4) | ||||
| O(4)-C(34)-C(35) | 124.8(4) | ||||
| C(33)-C(34)-C(35) | 119.7(4) | ||||
| C(36)-C(35)-C(34) | 119.7(4) | ||||
| C(36)-C(35)-H(35) | 120.2 | ||||
| C(34)-C(35)-H(35) | 120.2 | ||||
| C(35)-C(36)-C(31) | 121.8(4) | ||||
| C(35)-C(36)-H(36) | 119.1 | ||||
| C(31)-C(36)-H(36) | 119.1 | ||||
| O(4)-C(37)-C(38) | 106.9(4) | ||||
| O(4)-C(37)-H(37A) | 110.3 | ||||
| C(38)-C(37)-H(37A) | 110.3 | ||||
| O(4)-C(37)-H(37B) | 110.3 | ||||
| C(38)-C(37)-H(37B) | 110.3 | ||||
| H(37A)-C(37)-H(37B) | 108.6 | ||||
| C(37)-C(38)-H(38A) | 109.5 | ||||
| C(37)-C(38)-H(38B) | 109.5 | ||||
| H(38A)-C(38)-H(38B) | 109.5 | ||||
| C(37)-C(38)-H(38C) | 109.5 | ||||
| H(38A)-C(38)-H(38C) | 109.5 | ||||
| H(38B)-C(38)-H(38C) | 109.5 | ||||
| C(34)-O(4)-C(37) | 117.9(3) | ||||
3.2.2. FTIR spectral charatcerization
The FT-IR spectrum and vibrational frequencies of grown EBDN are shown in Fig. 6 and Table 3 . For the FT-IR spectrum of EBDN, the aromatic and aliphatic C—H stretching vibrations are observed at 3068 and 2960 cm−1 respectively [38,50]. The strong peak was observed at 1675 cm−1 which is due to the C=O group of the tetralone moiety [38]. A very strong peak at 1599 cm−1 is shown which is attributed to the C=C and C=O groups. The C=C group has also shown a very strong peak at 1508 cm−1. The C—O group is shown a very strong peak at 1253 cm−1. The C—H vibrations were observed at 977, 838, 728 and 541 cm−1 [38].
Fig. 6.
FT-IR spectrum of grown EBDN.
Table 3.
FT-IR data and functional group confirmation of grown EBDN crystal.
| S. No. | Vibrational frequency (ν, cm−1) | Vibrational assignment(s) |
|---|---|---|
| 1 | 3068, vw | C-H |
| 2 | 2960, vs | C—H |
| 3 | 1675, s | C=O |
| 4 | 1599, vs | C=C/C=O |
| 5 | 1508, vs | C=C |
| 6 | 1253,vs | C-O |
| 7 | 977, s | C—H |
| 8 | 838, s | C-H |
| 9 | 728, s | C—H |
| 10 | 541, s | C-H |
3.2.3. NMR spectral charatcerization
The 1H NMR and 13C NMR spectra of the title compound EBDN was recorded in CDCl3 at 400 MHz and its neat spectrum is shown in Figs. 7 and 8 .
Fig. 7.
1H NMR and 13C NMR spectral data representation of the grown EBDN.
Fig. 8.
1H NMR spectrum of the grown EBDN.
3.2.3.1. 1H NMR spectral charatcerization
The 1H NMR spectrum of the title compound, EBDN, is shown.A triplet peaks at 1.41–1.45 ppm and a quartet peaks at 4.10–4.05 ppm are attributed to the methyl and methylene protons of the ethoxy group present in the 4-ethoxybenzylidene moiety respectively [51]. For the 4-ethoxybenzylidene moiety, two aromatic protons (C13H & C17H) are shown multiplet peaks at 7.40–7.45 ppm and another two aromatic protons (C14H & C16H) are shown doublet peaks at 6.94, 6.92 ppm (Fig. 7 & 8) [51]. The four aromatic protons of the α-Tetralone moiety are shown at four different peaks. The C1H proton has mulplet peaks at 7.33–7.37 ppm, the C2H proton has doublet peaks at 7.47, 7.49 ppm, the C3H proton has doublet peaks at 7.23, 7.25 ppm, and the C4H proton has doublet peaks at 8.10, 8.12 ppm [38]. Two triplet peaks are shown in the 1H NMR spectrum of the EBDN, which are attributed to two methylene protons (C7H2 & C8H2) of the α-Tetralone moiety. And finally, a characteristic singlet peak is shown at 7.84 ppm, which is assigned to methine (Ar-CH C) proton (Fig. 7 & 8) [38].
3.2.3.2. 13C NMR spectral charatcerization
In the 13C NMR spectrum of the title compound EBDN, a peak is shown at 188.07 ppm, which is attributed to the carbonyl (C=O) group (Fig. 7 & 9 ). In the 4-ethoxybenzylidene moiety, the methyl carbon (C19) is shown a peak at 14.98 ppm and methylene carbon (C18) is shown a peak at 63.77 ppm [51]. Peaks for the four equivalent carbons are shown at 128.27 ppm (C12), 131.97 ppm (C13 & C17), 114.97 ppm (C14 & C16), and 159.59 ppm (C15). A peak at 136.97 ppm is shown for newly formed methine carbon (C11).Two distinct peaks are shown for the two methylene carbons found in the α -tetralone moiety (Fig. 7 & 9). One is observed at 27.43 ppm (C7) and another one is 29.01 ppm (C8). Both C6 and C9 are shown a peak at 133.97 ppm. Peaks are shown for the five aromatic carbons at 128.45 for C1, 133.62 for C2, 127.16 for C3, 128.35 for C4 and 143.13 (C5) [38,50,51].
Fig. 9.
13C NMR spectra spectrum of the grown EBDN.
3.2.4. Thermal studies
Thermal gravimetric (TG) and Differential thermal analysis (DTA) were performed on the grown crystal in the nitrogen atmosphere. The studies were embedded in the temperature range of 30–450 °C with a slow heating rate of 20 °C/min. Initially, 2.078 mg of the sample weighing was taken for the measurement, and the thermogram is illustrated in Fig. 10 . Because there is no weight loss in the TG curve at temperatures up to 238 °C, it is clear that the crystal is thermally stable. The small exothermic peak exists at 141 °C (The melting point of the EBDN is 141–142 °C) in the DTA curve (Fig. 10) confirms the melting point of the crystal. The weight loss begins gradually above 238 °C and becomes almost steep until it reaches 365 °C, when 95% of the material decomposes into a gaseous state. The two endothermic peaks in the DTA curve at 335 °C and 352 °C correspond to energy absorbed by the material to decompose in the temperature range of 238 °C-365 °C. There is no weight loss observed above 365 °C.
Fig. 10.
TG and DTA curve of grown EBDN.
3.2.5. Photoluminescence studies
The FP-6500 Spectro-fluorometer was used to analyze the photoluminescence (PL) of the grown crystal at an optical excitation wavelength of 375 nm, and spectra were recorded in the range 350–700 nm. The recorded PL spectrum is shown in Fig. 11 . Strong emission in the blue range is observed with two peaks at 452 nm and 464 nm for this excitation. The luminescence property of the chalcone derivatives can be used effectively in the field of design of optoelectronic devices and laser applications.
Fig. 11.
Photoluminescence graph of grown EBDN.
3.2.6. Mechanical studies
The structure and composition of the crystalline solids are inviolably related to the mechanical hardness. The EBDN crystal's microhardness was measured using a Leitz-Wetzlar Vickers' microhardness tester equipped with a diamond pyramidal indenter and attached to an optical microscope.Microhardness studies were performed on EBDN crystal for loads in the range of 10–60 g. From Fig. 12 , it is observed that Vicker's hardness (Hv) number increases initially with load up to 50 g and cracks were observed beyond 50 g. This type of load variation of hardness is termed as the reverse indentation size effect. At low loads, the indenter penetrates only the top surface layers, generating dislocations, which results in the increase of hardness in this region. The load independence to hardness at higher loads can be attributed to the mutual interaction or rearrangement of dislocations. The relation between load and the size of indentation can be correlated using Meyer's law, P = k1dn, where k1 is a constant and ‘n’ is the Meyer's index. The slope of log P versus log d gives the work hardening coefficient (n) and it is found to be 1.36, which indicates that EBDN crystal belongs to the hard material category (Fig. 13 ).
Fig. 12.
Plot of Vickers’ hardness (Hv) against load (P) of EBDN crystal.
Fig. 13.
Plot of log d vs log P of EBDN crystal.
3.2.7. Biological evaluation
The grown EBDN was screened for its anti-inflammatory (see Section 3.2.7.1.), anti-diabetic (see Section 3.2.7.2.) and antioxidant (see Section 3.2.7.3.) activities and percentage of inhibition are shown in Fig. 14, Fig. 15, Fig. 16 and its results are summarized in Table 4 .
Fig. 14.
Anti-inflammatory activity of EBDN.
Fig. 15.
Anti-diabetic activity of grown EBDN.
Fig. 16.
Anti-oxidant activity of grown EBDN.
Table 4.
Anti-inflammatory, anti-diabetic and anti-oxidant activity of grown EBDN.
| Compound | Percentage of inhibition (%) |
IC50 (µg/mL) | ||||
|---|---|---|---|---|---|---|
| Concentration (µg/mL) | ||||||
| 10 | 25 | 50 | 125 | 250 | ||
| Anti-inflammatory activity | ||||||
| EBDN | 17.62 | 28.79 | 61.43 | 81.19 | 98.71 | 133.95 |
| Diclofenac sodium* | 14.82 | 25.63 | 59.44 | 78.59 | 96.22 | 150.66 |
| Anti-diabetic activity | ||||||
| EBDN | 9.89 | 20.74 | 48.73 | 67.42 | 77.31 | 221.87 |
| Acarbose* | 13.37 | 24.15 | 53.67 | 73.48 | 85.32 | 181.95 |
| Anti-oxidant activity | ||||||
| EBDN | 3.61 | 5.80 | 8.37 | 11.41 | 17.22 | 1736.14 |
| Vitamin C* | 12.42 | 20.36 | 51.62 | 70.28 | 83.44 | 198.96 |
Standard drug.
3.2.7.1. Anti-inflammatory activity of grown EBDN
The grown EBDN was screened for its anti-inflammatory activity in five different concentrations (10, 25, 50, 125 & 250 μg/mL) and the percentage of inhibition (%) is summarized in Table 4 and shown in Fig. 14. Diclofenac sodium was used as a standard drug for this present work. The 10 µg/mL concentration of grown EBDN was shown 17.62% of inhibition of anti-inflammatory activity. The standard drug was shown 14.82% of inhibition. The grown EBDN and standard drug were shown 28.79 and 25.63%, 61.43 and 59.44%, 81.19 and 78.59% and, 98.71 and 96.22% in the concentration of 25, 50, 125 and 250 µg/mL respectively. When compared to the standard drug (IC50 value 150.66 µg/mL), the percentage of inhibition of grown EBDN (IC50 value 133.95 µg/mL) was excellent, and its results were strongly cleared as an admirable anti-inflammatory agent.
3.2.7.2. Antidiabetic activity of grown EBDN
The grown EBDN was screened for its anti-diabetic activity in five different concentrations (10, 25, 50, 125 & 250 μg/mL) and the percentage of inhibition (%) is summarized in Table 4 and shown in Fig. 15. Acarbose was used as a standard drug for this present work. The anti-diabetic activity of grown EBDN was inhibited by 9.89% at 10 g/mL concentration. The standard drug was shown 13.37% of inhibition. The grown EBDN and standard drug were shown 20.74 and 24.15%, 48.73 and 53.67%, 67.42 and 73.48% and, 77.31 and 85.32% in the concentration of 25, 50, 125 and 250 µg/mL respectively. The percentage of inhibition of grown EBDN (IC50 value 221.87 µg/mL) was very close to that of the standard drug (IC50 value 181.95 µg/mL), and its results were strongly supported as an excellent anti-inflammatory agent.
3.2.7.3. Antioxidant activity of grown EBDN
The grown EBDN was screened for its anti-oxidant activity in five different concentrations (10, 25, 50, 125 & 250 μg/mL) and the percentage of inhibition (%) is summarized in Table 4 and shown in Fig. 16. Vitamin C was used as a standard drug for this present work. The 10 µg/mL concentration of grown EBDN was shown 3.61% of inhibition of anti-oxidant activity. The standard drug was shown 12.42% of inhibition. The grown EBDN and standard drug were shown 5.80 and 20.36%, 8.37 and 51.62%, 11.41 and 70.28% and, 17.22 and 83.44% in the concentration of 25, 50, 125 and 250 µg/mL respectively. The% of inhibition of grown EBDN (IC50 value 1736.14 µg/mL) was moderate activity compared to the standard drug (IC50 value 198.96 µg/mL) and its results indicate the compound EBDN should be further modified structurally.
3.2.7.4. Protein-ligand binding interaction of EBDN with 6yb7
The binding energy, number of hydrogen bond interactions, inhibition constant and interacting amino acid residues were summarized in Table 5 . The binding energy of the synthesized chalcone compound EBDN is −7.25 Kcal/mol [39], [40], [41], [42], [43], [44], [45]. The EBDN has shown strong four hydrogen bond (H-bond) interaction with targeted SARS-CoV-2 main protease (6yb7). The first H-bond interaction is shown with the amino acid residue of THR26, which is due to the H-A distance of 3.03 Å (Table 5, Fig. 17 ). The second H-bond interaction is shown with CYS145 and the H-A distance is 3.68 Å (Table 5, Fig. 17). Both third and fourth H-bond interactions were shown with the GLU166 amino acid residue of the SARS CoV-2 main protease and the H-A distances are 3.65 and 3.04 Å (Table 5, Fig. 17) respectively. Among them, four H-bond interactions, H-A distance of two interactions are shown a strong binding with SARS CoV-2 main protease, which is due to THR26 (3.03 Å) and GLU166 (3.04 Å) [43].
Table 5.
Protein-ligand binding interactions of EBDN with 6yb7.
| Compound | Binding energy (Kcal/mol) | Number of hydrogen bonds | Inhibition constant (µM) | Interacting residues (H-A distance) |
|---|---|---|---|---|
| EBDN | −7.25 | 4 | 4.85 | THR26 (3.03 Å), CYS145 (3.68 Å), GLU166 (3.65 Å), GLU166 (3.04 Å) |
Fig. 17.
Protein-Ligand binding interaction of EBDN in the active site pocket of 6yb7.
4. Conclusion
To summarize, we have presented in this paper, the synthesis and crystal growth (2E)-2-(4-ethoxybenzylidene)-3,4-dihydro-2H-naphthalen-1-one single crystal (EBDN) in excellent yield under very mild conditions. It has been fully characterized by cyrstallographic and spectral analysis. The synthesized EBDN was successfully screened for its anti-inflammatory, anti-diabetic and anti-oxidant activities. Its results have also been shown as an excellent anti-inflammatory as well as anti-diabetic agents and moderate anti-oxidant activity. The EBDN's binding interactions and binding affinity with SARS-CoV-2 are also investigated, and the results show a very good binding with the targeted main protease (6yb7).
CRediT authorship contribution statement
N. Afsar: Conceptualization, Data curation, Formal analysis, Investigation, Validation, Writing – original draft. D. Reuben Jonathan: Conceptualization, Formal analysis, Investigation, Visualization. B.K. Revathi: Methodology, Software. Dhurairaj Satheesh: Data curation, Formal analysis, Validation, Visualization. S. Manivannan: Conceptualization, Supervision, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Zhou B., Xing C. Diverse molecular targets for chalcones with varied bioactivities. Med. Chem. 2015;5:388–404. doi: 10.4172/2161-0444.1000291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Batovska D.I., Todorova I.T. Trends in utilization of the pharmacological potential of chalcones. Curr. Clin. Pharmacol. 2010;5:1–29. doi: 10.2174/157488410790410579. [DOI] [PubMed] [Google Scholar]
- 3.Sahu N.K., Balbhadra S.S., Choudhary J., Kohli D.V. Exploring pharmacological significance of chalcone scaffold: a review. Curr. Med. Chem. 2012;19:209–225. doi: 10.2174/092986712803414132. [DOI] [PubMed] [Google Scholar]
- 4.Singh P., Anand A., Kumar V. Recent developments in biological activities of chalcones: a mini review. Eur. J. Med. Chem. 2014;85:758–777. doi: 10.1016/j.ejmech.2014.08.033. [DOI] [PubMed] [Google Scholar]
- 5.Karthikeyan C., Moorthy N.S., Ramasamy S., Vanam U., Manivannan E., Karunagaran D., Trivedi P. Advances in chalconeswith anticancer activities. Recent Pat. Anti-Cancer Drug Discov. 2015;10:97–115. doi: 10.2174/1574892809666140819153902. [DOI] [PubMed] [Google Scholar]
- 6.Sebti S., Solhy A., Smahi A., Kossir A., Oumimoun H. Dramatic activity enhancement of natural phosphate catalyst by lithium nitrate. An efficient synthesis of chalcones. Catal. Commun. 2002;3:335–339. [Google Scholar]
- 7.Arshad M.N., Al-Dies A.-A.M., Asiri A.M., Khalid M., Birinji A.S., Al-Amry K.A., Ataualpa A., Braga C. J. Mol. Struct. 2017;1141:142–156. [Google Scholar]
- 8.Mascarello A., Chiaradia L.D., Vernal J., Villarino A., Guido R.V., Perizzolo P., Poirier V., Wong D., Martins P.G., Nunes R.J., Yunes R.A., Andricopulo A.D., Av-Gay Y., Terenzi H. Inhibition of Mycobacterium tuberculosis tyrosine phosphatase PtpA by synthetic chalcones: kinetics, molecular modeling, toxicity and effect on growth. Bioorg. Med. Chem. 2010;18(11):3783–3789. doi: 10.1016/j.bmc.2010.04.051. [DOI] [PubMed] [Google Scholar]
- 9.Tomar V., Bhattacharjee G., Kamaluddin, Rajakumar S., Srivastava K., Puri S.K. Synthesis of new chalcone derivatives containing acridinyl moiety with potential antimalarial activity. Eur. J.Med. Chem. 2010;45(2):745–751. doi: 10.1016/j.ejmech.2009.11.022. [DOI] [PubMed] [Google Scholar]
- 10.Rizvi S.U.F., Siddiqui H.L., Johns M., Detorio M., Schinazi R.F. Anti-HIV-1 and cytotoxicity studies of piperidyl-thienyl chalcones and their 2-pyrazoline derivatives. Med. Chem. Res. 2012;21(21):3741–3749. [Google Scholar]
- 11.Abdullah M.I., Mahmood A., Madni M., Masood S., Kashif M. Synthesis, characterization, theoretical, anti-bacterial and molecular docking studies of quinoline based chalcones as a DNA gyrase inhibitor. Bioorg. Chem. 2014;54:31–37. doi: 10.1016/j.bioorg.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 12.Mahapatra D.K., Bharti S.K., Asati V. Anti-cancer chalcones: structural and molecular target perspectives. Eur. J. Med. Chem. 2015;98:69–114. doi: 10.1016/j.ejmech.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Wang L., Chen G., Lu X., Wang S., Han S., Li Y., Ping G., Jiang X., Li H., Yang J., Wu C. Novel chalcone derivatives as hypoxiainducible factor (HIF)-1 inhibitor: synthesis, anti-invasive and antiangiogenic properties. Eur. J. Med. Chem. 2015;89:88–97. doi: 10.1016/j.ejmech.2014.10.036. [DOI] [PubMed] [Google Scholar]
- 14.Winter E., Locatelli C., Di Pietro A., Creczynski-Pasa T.B. Recent trends of chalcones potentialities as antiproliferative and antiresistance agents. Anti-Cancer Agents Med. Chem. 2015;15(5):592–604. doi: 10.2174/1871520615666150101130800. [DOI] [PubMed] [Google Scholar]
- 15.Qin H.-L., Shang Z.-P., Jantan I., Tan O.U., Hussain M.A., Sher M., Bukhari S.N.A. Molecular docking studies and biological evaluation of chalcone based pyrazolines as tyrosinase inhibitors and potential anticancer agents. RSC Adv. 2015;5:46330–46338. [Google Scholar]
- 16.Birari R.B., Gupta S., Mohan C.G., Bhutani K.K. Antiobesity and lipid lowering effects of glycyrrhizachalcones: experimental and computational studies. Phytomedicine. 2011;18(8–9):795–801. doi: 10.1016/j.phymed.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 17.Kantevari S., Addla D., Bagul P.K., Sridhar B., Banerjee S.K. Synthesis and evaluation of novel 2-butyl-4-chloro-1-methylimidazole embedded chalcones and pyrazoles as angiotensin converting enzyme (ACE) inhibitors. Bioorg. Med. Chem. 2011;19(16):4772–4781. doi: 10.1016/j.bmc.2011.06.085. [DOI] [PubMed] [Google Scholar]
- 18.Sashidhara K.V., Palnati G.R., Sonkar R., Avula S.R., Awasthi C., Bhatia G. Coumarinchalcone fibrates: a new structural class of lipid lowering agents. Eur. J. Med. Chem. 2013;64:422–431. doi: 10.1016/j.ejmech.2013.04.026. [DOI] [PubMed] [Google Scholar]
- 19.Mahapatra D.K., Asati V., Bharti S.K. Chalcones and their role in management of diabetes mellitus: structural and pharmacological perspectives. Eur. J. Med. Chem. 2015;92:839–865. doi: 10.1016/j.ejmech.2015.01.051. [DOI] [PubMed] [Google Scholar]
- 20.Gomez-Rivera A., Aguilar-Mariscal H., Romero-Ceronio N., Roa-de la Fuente L.F., Lobato-Garcia C.E. Synthesis and anti-inflammatory activity of three nitro chalcones. Bioorg. Med. Chem. Lett. 2013;23:5519–5522. doi: 10.1016/j.bmcl.2013.08.061. [DOI] [PubMed] [Google Scholar]
- 21.Aoki N., Muko M., Ohta E., Ohta S. C-Geranylated chalcones from the stems of Angelica keiskei with superoxide-scavenging activity. J. Nat. Prod. 2008;71(7):1308–1310. doi: 10.1021/np800187f. [DOI] [PubMed] [Google Scholar]
- 22.Bukhari S.N., Tajuddin Y., Benedict V.J., Lam K.W., Jantan I., Jalil J., Jasamai M. Synthesis and evaluation of chalcone derivatives as inhibitors of neutrophils’ chemotaxis, phagocytosis and production of reactive oxygen species. Chem. Biol. Drug Des. 2014;83(2):198–206. doi: 10.1111/cbdd.12226. [DOI] [PubMed] [Google Scholar]
- 23.Bukhari S.N., Zhang X., Jantan I., Zhu H.L., Amjad M.W., Masand V.H. Synthesis, molecular modeling, and biological evaluation of novel 1, 3-diphenyl-2-propen-1-one based pyrazolines as anti-inflammatory agents. Chem. Biol. Drug Des. 2015;85(6):729–742. doi: 10.1111/cbdd.12457. [DOI] [PubMed] [Google Scholar]
- 24.Dimmock J.R., Elias D.W., Beazely M.A., Kandepu N.M. Bioactivities of chalcones. Curr. Med. Chem. 1999;6(12):1125–1149. [PubMed] [Google Scholar]
- 25.Sahu N.K., Balbhadra S.S., Choudhary J., Kohli D.V. Exploring pharmacological significance of chalcone scaffold: a review. Curr. Med. Chem. 2012;19(2):209–225. doi: 10.2174/092986712803414132. [DOI] [PubMed] [Google Scholar]
- 26.Batovska D.I., Todorova I.T. Trends in utilization of the pharmacological potential of chalcones. Curr. Clin. Pharmacol. 2010;5:1–29. doi: 10.2174/157488410790410579. [DOI] [PubMed] [Google Scholar]
- 27.Arora S., Pareek A., Agrawal N., Nagori B. Synthesis and antimicrobial activity of indazoline tetralone. Int. J. Res. Pharm. Chem. 2013;3:797–802. [Google Scholar]
- 28.Shih H., Deng L., Carrera C.J., Adachi S., Cottam H.B., Carson D.A. Rational design, synthesis and structure–activity relationships of antitumor (E)-2-benzylidene-1-tetralones and (E)-2-benzylidene-1-indanones. Bioorg. Med. Chem. Lett. 2000;10:487–490. doi: 10.1016/s0960-894x(00)00032-9. [DOI] [PubMed] [Google Scholar]
- 29.Legoabe L.J., Van der Walt M.M., Terre'Blanche G. Evaluation of 2-benzylidene-1-tetralone derivatives as antagonists of A1 and A2A adenosine receptors. Chem. Biol. Drug. Des. 2018;91:234–244. doi: 10.1111/cbdd.13074. [DOI] [PubMed] [Google Scholar]
- 30.Janse van Rensburg H.D., Terre'Blanche G., van der Walt M.M., Legoabe L.J. 5-Substituted 2-benzylidene-1-tetralone analogues as A 1 and/or A 2A antagonists for the potential treatment of neurological conditions. Bioorganic Chem. 2017;74:251–259. doi: 10.1016/j.bioorg.2017.08.013. [DOI] [PubMed] [Google Scholar]
- 31.Shih H., Deng L., Carrera C.J., Adachi S., Cottam H.B., Carson D.A. Rational design, synthesis and structure–activity relationships of antitumor (E)-2-benzylidene-1-tetralones and (E)-2-benzylidene-1-indanones. Bioorg. Med. Chem. Lett. 2000;10:487–490. doi: 10.1016/s0960-894x(00)00032-9. [DOI] [PubMed] [Google Scholar]
- 32.Legoabe L.J., Van der Walt M.M., Terre'Blanche G. Evaluation of 2-benzylidene-1-tetralone derivatives as antagonists of A1 and A2A adenosine receptors. Chem. Biol. Drug. Des. 2018;91:234–244. doi: 10.1111/cbdd.13074. [DOI] [PubMed] [Google Scholar]
- 33.Janse van Rensburg H.D., Terre'Blanche G., van der Walt M.M., Legoabe L.J. 5-Substituted 2-benzylidene-1-tetralone analogues as A 1 and/or A 2A antagonists for the potential treatment of neurological conditions. Bioorganic Chem. 2017;74:251–259. doi: 10.1016/j.bioorg.2017.08.013. [DOI] [PubMed] [Google Scholar]
- 34.Kamakshi R., Swarna Latha S., Reddy B.S.R. An efficient synthesis of bioactive fluorescent benzylidine tetralones. Indian J. Chem. Sect. B Org. Chem. Incl. Med. Chem. 2010;49:944–947. [Google Scholar]
- 35.Perje´si P., Linnanto J., Kolehmainen E., O˝sz E., Virtanen E. E-2-Benzylidenebenzocycloalkanones. IV. Studies on transmission of substituent effects on 13C NMR chemical shifts of E-2-(X-benzylidene)-1-tetralones, and -benzosuberones. Comparison with the 13C NMR data of chalcones and E-2-(X-benzylidene)-1-indanones. J. Mol. Struct. 2005;740:81–89. [Google Scholar]
- 36.Štefanišinová M., Tomečková V., Kožurková M., Ostró A., Mareková M. Study of DNA interactions with cyclic chalcone derivatives by spectroscopic techniques. Spectrochim. Acta Part A. 2011;81(1):666–671. doi: 10.1016/j.saa.2011.06.071. [DOI] [PubMed] [Google Scholar]
- 37.Tomečková V., Guzy J., Kušnír J., Fodor K., Mareková M., Chavková Z., Perjési P. Comparison of the effects of selected chalcones, dihydrochalcones and some cyclic flavonoids on mitochondrial outer membrane determined by fluorescence spectroscopy. J. Biochem. Biophys. Methods. 2006;69(1–2):143–150. doi: 10.1016/j.jbbm.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 38.Katila P., Shrestha A., Shrestha Ritina Shrestha A., Park P.-H., SeokLee E. Introduction of amino moiety enhances the inhibitory potency of 1-tetralone chalcone derivatives against LPS-stimulated reactive oxygen species production in RAW 264.7 macrophages. Bioorg. Chem. 2019;87:495–505. doi: 10.1016/j.bioorg.2019.03.055. [DOI] [PubMed] [Google Scholar]
- 39.Rameshkumar M.R., Indu P., Arunagirinathan N., Venkatadri B., El-Serehy H.A., Ahmad A. Computational selection of flavonoid compounds as inhibitors against SARS-CoV-2 main protease, RNA-dependent RNA polymerase and spike proteins: a molecular docking study. Saudi J. Biol. Sci. 2021;28:448–458. doi: 10.1016/j.sjbs.2020.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Erdogan T. DFT, molecular docking and molecular dynamics simulation studies on some newly introduced natural products for their potential use against SARS-CoV-2. J. Mol. Struct. 2021;1242 doi: 10.1016/j.molstruc.2021.130733. Available online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mohapatra R.K., Perekhoda L., Azam M., Suleiman M., Sarangi A.K., Semenets A., et al. Computational investigations of three main drugs and its comparison with synthesized compounds as potent inhibitors of SARS-CoV-2 main protease (M(pro)): DFT, QSAR, molecular docking, and in silico toxicity analysis. J. King Saud. Univ. Sci. 2020;33(2) doi: 10.1016/j.jksus.2020.101315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ercan S., Çınar E. A molecular docking study of potential inhibitors and repurposed drugs against SARS-CoV-2 main protease enzyme. J. Indian Chem. Soc. 2021;98 [Google Scholar]
- 43.Satheesh D., Rajendran A., Chithra K. Protein-ligand binding interactions of imidazolium salts with SARS CoV-2. Heliyon. 2020;6:e05544. doi: 10.1016/j.heliyon.2020.e05544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vijayakumar B.G., Ramesh D., Joji A., Prakasan J.J., Kannan T. In silico pharmacokinetic and molecular docking studies of natural flavonoids and synthetic indole chalcones against essential proteins of SARS-CoV-2. Eur. J. Pharmacol. 2020;886 doi: 10.1016/j.ejphar.2020.173448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Syahri J., Nurohmah B.A., Yuanita E. Effectivity of Remdesivir and some compounds as therapeutic potential drugs for anti-SARS-CoV-2: in silico study. Jordan J. Pharm. Sci. 2021;14:1. [Google Scholar]
- 46.Renuka V., Revathi B.K., Reuben Jonathan D., Priya M.K., Samuel Asirvatham P. Synthesis, growth and characterization of a new NLO active chalcone derivative -4-chloro-N-{3-[(2E)-3-(methoxyphenyl)prop-2-Enoyl]phenyl}benzamide monohydrate. J. Mol. Struct. 2021;1176:838–846. [Google Scholar]
- 47.D. Satheesh, R. Roshini, S. Jeevitha, K. Chithra, S. Vasanth Kumar, P. Sellam, Anti-inflammatory and anti-diabetic activity of 8-hydroxyquinolinium 3,5-dinitrobenzoate. 33, 2021, 100726.
- 48.C.D. Owen, P. Lukacik, C.M. Strain-Damerell, A. Douangamath, A.J. Powell, D. Fearon, J. Brandao-Neto, A.D. Crawshaw, D. Aragao, M. Williams, R. Flaig, D.R. Hall, K.E. McAuley, M. Mazzorana, D.I. Stuart, F. von Delft, M.A. Walsh SARS-CoV-2 main protease with unliganded active site (2019-nCoV, coronavirus disease 2020, COVID-19). DOI: 10.2210/pdb6YB7/pdb.
- 49.Frisch M.J., Trucks G.W., Schlegel H.B., Scuseria G.E., Robb M.A., Cheeseman J.R., Scalmani G., Barone V., Petersson G.A., Nakatsuji H., Li X., Caricato M., Marenich A., Bloino J., Janesko B.G., Gomperts R., Mennucci B., Hratchian H.P., Ortiz J.V., Izmaylov A.F., Sonnenberg J.L., Williams-Young D., Ding F., Lipparini F., Egidi F., Goings J., Peng B., Petrone A., Henderson T., Ranasinghe D., Zakrzewski V.G., Gao J., Rega N., Zheng G., Liang W., Hada M., Ehara M., Toyota K., Fukuda R., Hasegawa J., Ishida M., Nakajima T., Honda Y., Kitao O., Nakai H., Vreven T., Throssell K., Montgomery J.A., Jr., Peralta J.E., Ogliaro F., Bearpark M., Heyd J.J., Brothers E., Kudin K.N., Staroverov V.N., Keith T., Kobayashi R., Normand J., Raghavachari K., Rendell A., Burant J.C., Iyengar S.S., Tomasi J., Cossi M., Millam J.M., Klene M., Adamo C., Cammi R., Ochterski J.W., Martin R.L., Morokuma K., Farkas O., Foresman J.B., Fox D.J. Gaussian, Inc.; Wallingford CT: 2016. Gaussian 09, Revision A.02. [Google Scholar]
- 50.Chithra K., Jayanthi K., Satheesh D. Acetophenone based Mannich bases: synthesis, characterization and their antimicrobial activity. World J. Pharm. Chem. 2017;6(2):1439–1449. [Google Scholar]
- 51.Kannan S., Gomathi A., Chithra K., Satheesh D. Binuclear copper (II) complexes of salphen-type tetra-imine Schiff's bases derived from 3,3′-diaminobenzidine with 3-allylsalicylaldehyde and 3-ethoxysalicylaldehyde: synthesis, characterization and their antimicrobial activity. Infokara Res. 2020;9(10):236–254. [Google Scholar]