Graphical abstract

Keywords: Ozone, Ozone dosage, Personal Protective Equipment (PPE), Microbial inactivation, Decontamination
Abstract
Ozone – a powerful antimicrobial agent, has been extensively applied for decontamination purposes in several industries (including food, water treatment, pharmaceuticals, textiles, healthcare, and the medical sectors). The advent of the COVID-19 pandemic has led to recent developments in the deployment of different ozone-based technologies for the decontamination of surfaces, materials and indoor environments. The pandemic has also highlighted the therapeutic potential of ozone for the treatment of COVID-19 patients, with astonishing results observed. The key objective of this review is to summarize recent advances in the utilisation of ozone for decontamination applications in the above-listed industries while emphasising the impact of key parameters affecting microbial reduction efficiency and ozone stability for prolonged action. We realise that aqueous ozonation has received higher research attention, compared to the gaseous application of ozone. This can be attributed to the fact that water treatment represents one of its earliest applications. Furthermore, the application of gaseous ozone for personal protective equipment (PPE) and medical device disinfection has not received a significant number of contributions compared to other applications. This presents a challenge for which the correct application of ozonation can mitigate. In this review, a critical discussion of these challenges is presented, as well as key knowledge gaps and open research problems/opportunities.
Nomenclature
- DBP
Disinfection by-products
- FDA
Food and drug administration
- GRAS
Generally recognised as safe
- HAI
Hospital-acquired infections
- HLD
High-level disinfection
- ILD
Intermediate-level disinfection
- LLD
Low-level disinfection
- MAHT
Major autohemotherapy
- MCV
Murine coronavirus
- MiAHT
Minor autohemotherapy
- OPA
Orthophthaldehyde
- OPEX
Operating expenditure
- ORP
Oxidation-reduction potential
- OSHA
Occupational safety and health administration
- PBS
Phosphate buffered saline
- PFU
Plaque forming unit
- PEL
Permissible exposure limit
- PPE
Personal protective equipment
- RAQS
Recirculating aquacultural systems
- REL
Recommended exposure limit
- RH
Relative humidity
- RI
Rectal insufflation
- ROS
Reactive oxidative species
- SAL
Sterility assurance level
- UVC
Ultraviolet radiation – C
1. Introduction
Ozone (O3) is a triatomic gas – an allotrope of oxygen, with a distinctively pungent odour. At −112 °C, the condensation of ozone occurs yielding a dark blue liquid, with explosive properties. Although not usually noticeable at the concentrations typically produced, ozone gas also possesses a bluish colour at room temperature [1]. The physical and chemical properties of ozone are summarised in Table 1 . Ozone is significantly less stable than atmospheric oxygen [2]; this instability implies that it does not accumulate substantially and must be generated on demand via an ozone-generating system [3]. Ozone, automatically and rapidly decomposes to oxygen in both air and water, with an oxidation potential of 2.07 V. This high oxidative power and rapid decomposition make it effective against a broad spectrum of microorganisms [4]; hence its wide application for decontamination of indoor spaces, materials/surfaces, food, and water. Ozone yields faster microbial inactivation kinetics, compared to other oxidative agents used in chemical disinfectants (Table 2 ) and has been shown to be effective on notably resistant microorganisms such as Clostridium difficile [5]. It can react up to 3000 times faster with organic matter and is considered safer than chlorine, which produces harmful disinfection by-products [6]. Beyond, decontamination, ozone also possesses bleaching and deodorising properties, increasing its versatility in various industries. Nonetheless, the use of ozone for decontamination represents the most prevalent reason for its application.
Table 1.
| Property | Value |
|---|---|
| Molecular weight (g/mol) | 48 |
| Gaseous density (kg/m3) at 0 °C and 1 atm | 2.14 |
| Solid density at −195.7 °C (kg/m3) | 1728 |
| Melting point (oC) | −192.5 ± 0.4 |
| Boiling point (oC) | −111.9 ± 0.3 |
| Critical pressure (atm) | 54.6 |
| Critical temperature (oC) | −12.1 |
| Thermal conductivity at −183 °C (oC/m) | 0.000531 |
| Half-life in air (min) | 20 – 1524 |
| Half-life in water (min) | 10 – 80 |
| Dielectric constant at −183 °C | 4.74 |
| Heat of evaporation (kcal/mol) | 3.63 |
| Vapour pressure at −192.5 °C (atm) | 1.13 × 10-5 |
| Dipole moment (Debye) | 0.53 |
| Solubility (effect of temperature @ 1 atm & 6 wt% O3) | |
| Solubility in water @ 10 °C (mg/L) | 33.462 |
| Solubility in water @ 20 °C (mg/L) | 20.592 |
| Solubility in water @ 30 °C (mg/L) | 12.870 |
| Solubility (effect of pressure @ 15 °C & 6 wt% O3) | |
| Solubility in water @ 1 atm (mg/L) | 26.598 |
| Solubility in water @ 2 atm (mg/L) | 53.196 |
| Solubility in water @ 3 atm (mg/L) | 79.794 |
| Solubility (effect of ozone concentration @ 1 atm & 5 °C) | |
| Solubility in water @ 3 wt% O3 (mg/L) | 21.450 |
| Solubility in water @ 9 wt% O3 (mg/L) | 64.350 |
| Solubility in water @ 15 wt% O3 (mg/L) | 107.25 |
| Solubility ratio (@ 1 atm) | |
| Solubility ratio @ 10 °C (m3 O3/m3 H2O) | 0.390 |
| Solubility ratio @ 20 °C (m3 O3/m3 H2O) | 0.240 |
| Solubility ratio @ 30 °C (m3 O3/m3 H2O) | 0.150 |
The solubility of ozone depends on the temperature of water, pressure of water, ionic strength, presence and type of ionic salts and ozone gas concentration. Ozone dissolution in water closely follows Henry’s law; thus it is important to determine a saturation ratio. pH and organic loading are other factors that will affect the realisable solubility, relative to the theoretical maximum. Also, various half-lives have been reported by different researchers [13], [14], [15] and these tend to depend on the ambient conditions (pH, temperature, pressure, relative humidity, airflow, method of ozone dissolution and sterility of the environment).
Table 2.
| Oxidizing agent | Oxidation potential (V) |
|---|---|
| Fluorine (F2) | 3.06 |
| Hydroxyl radical (OH•) | 2.80 |
| Superoxide radical (O2•–) | 2.40 |
| Ozone (O3) | 2.07 |
| Hydrogen peroxide (H2O2) | 1.80 |
| Peracetic acid (CH3CO3H) | 1.76 |
| Hypochlorite ion (ClO–) | 1.70 |
| Perhydroxyl radical (HO2•) | 1.70 |
| Permanganate ion (MnO4–) | 1.67 |
| Chlorine dioxide (ClO2) | 1.50 |
| Hypochlorous acid (HClO) | 1.49 |
| Chlorine gas (Cl2) | 1.36 |
| Oxygen gas (O2) | 1.23 |
| Hydroperoxide ion (HO2–) | 0.88 |
Some of these radicals (for example, O2•–, OH•, HO2•) are products of ozone decomposition. They are produced to varying extents depending on the ambient humidity, (during gaseous ozonation) and water properties (during aqueous ozonation).
One of the key attributes of ozone, which makes it a widely applied antimicrobial agent, is its potency in both air and water. Moreover, very few disinfectants possess this property. Thus, industries with the flexibility of utilising either medium can apply ozone, depending on the particular attributes of the process or substrate to be decontaminated. However, the sensitivity of ozone to organic matter present in air or water implies it is more readily used up; thus reducing its concentration for the target action. Ozone generation in air is mainly carried out via ultraviolet radiation (185 nm) and corona discharge methods – the latter being more efficient (particularly for large-scale production) than the former. In water, ozone may be generated via electrolytic methods or by carefully bubbling the gas through water to enable dissolution [16], [17]. Both methods result in the generation of ozone bubbles of different sizes, which in turn determine the stability of ozone in the aqueous phase. Ozone’s poor solubility in water is also a key determinant of the attainable concentration levels in aqueous solutions and the corresponding decontamination efficiency. However, this low solubility in the aqueous phase induces the formation of ozone bubbles, which also have antimicrobial properties, particularly when they collapse on the surface of the substrate to be treated.
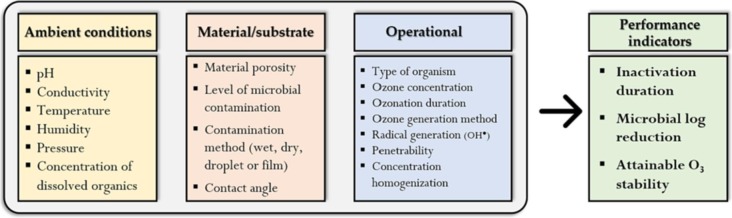
The antimicrobial performance of ozone is affected by several parameters depending on the medium of application. While temperature, pressure, and relative humidity appear to be the main ambient factors affecting gaseous ozonation, the efficiency of aqueous ozone application is affected by additional parameters such as the pH, conductivity, and organic matter composition. Besides these ambient conditions, the type and properties of the material substrate to be decontaminated (adsorbent or non-adsorbent), the nature of the microorganism, method of substrate contamination (droplets, wet, dried), method of ozone generation, exposure dosage (a product of ozone concentration and exposure duration) are other key factors affecting the efficiency of the ozonation process. Fig. 1 classifies these parameters into 3 main categories – ambient conditions, substrate/material-related properties, and operational properties. It is worth clarifying that these parameters may affect the process, by altering the stability of ozone in the medium of its application (air or water) or the attainable microbial inactivation efficiency, or both. Ozone inhalation may cause severe irritation of the respiratory tract as well as lung damage; coughing and chest tightness are characteristics of uncontrolled exposure to ozone [19]. Thus, it is important that appropriate personal protective equipment (PPE) is worn when working with ozone. Furthermore, sufficient conversion to oxygen (residual ozone concentration < OSHA worker exposure limits of 0.1 ppmv over an 8-hr shift) should be ensured, particularly during gaseous ozonation processes to prevent toxic exposure. A sequel to this review article (under development) by the authors separately explores the design and implementation considerations for small- and large-scale ozone decontamination systems.
Fig. 1.
Factors affecting the efficiency of microbial inactivation during gaseous and aqueous ozonation of materials.
Since the main context of ozone’s application discussed herein is its antimicrobial action, it becomes necessary to clearly differentiate between cleaning, disinfection, sterilisation, and decontamination – terminologies used to highlight the different levels of microbial inactivation, as reported in several studies. While cleaning refers to the removal of contamination from an item, to the extent required for further processing, disinfection is the reduction in the number of viable microorganisms on a product/material to a level specified as appropriate for its reuse [20]. It is usually recommended that cleaning be performed before disinfection and sterilisation [21]. Moreover, disinfection has been categorised in the literature into high-, intermediate-, and low-level [21], [22].
Sterilisation is the complete removal of all viable microorganisms on the product/material. Researchers have mainly adopted a concept known as the sterility assurance level (SAL), particularly because absolute sterility is hardly attainable [23]. SAL is defined as the ‘probability of a single viable micro-organism occurring on an item after sterilisation’ [24]. For example, a treated surgical instrument is considered sterile when a SAL of 10-6 is reached [25]. Decontamination is regarded as the combination of all 3 processes (cleaning, disinfection, and sterilisation) to make a reusable item safer for subsequent usage [20], [26]. In a clinical setting, disinfection may be further categorized into high-level (HLD), intermediate-level (ILD) and low-level disinfection (LLD). HLD destroys viruses, vegetative bacteria, and viruses, but not necessarily bacterial spores; ILD destroys all pathogenic vegetative bacteria, fungi and most viruses, except some non-enveloped viruses and bacterial spores; LLD eliminates most vegetative bacteria, some viruses and some fungi [22]. According to Spaulding classification [27], medical instruments that come in contact with skin are termed noncritical and require LLD or ILD. Instruments that contact mucous membranes are semi-critical and require HLD; whereas instruments that enter sterile tissue (critical), must be sterilized [28].
Ozone is capable of cleaning, disinfecting, and sterilising materials and surfaces, depending on the utilised medium and the dosage of its application. This review aims to elucidate the influence of these parameters by presenting recent advances in ozone’s utilisation in different industries. In our discussion, all ozone concentration values in parts per million or per billion are on a volume basis. Furthermore, engineering considerations required for large-scale implementation of ozone decontamination systems are critically discussed. The main knowledge gaps worthy of further investigation as well as recommendations for technology advancement are also presented.
1.1. Mechanisms of ozone’s antimicrobial action
Besides the direct action of ozone on the microorganisms of interest (direct electrophilic inactivation), microbial inactivation by ozone may also be indirect. Indirect inactivation involves the effect of the reactive oxidative species ((ROS) including OH•, HO2 •, O2 ®•, O3 ®•, HO3 ®•, H2O2, O®) that may form during spontaneous ozone decomposition in water or air [3], [29], [30], [31], [32]. It is worth pointing out the specific set of reactive oxidative species formed depend on the medium of application (air or water) and tend to be different [29], [30], [32], [33]. The hydroxyl radical (OH•), for example, is highly unstable and readily reacts with other compounds to gain the missing electron [31], [34]. It has a higher oxidation potential (2.80 V) compared to ozone itself (2.07 V). During ozone treatments, these reactive species destroy the cell membranes of the microorganism, eventually leading to their inactivation.
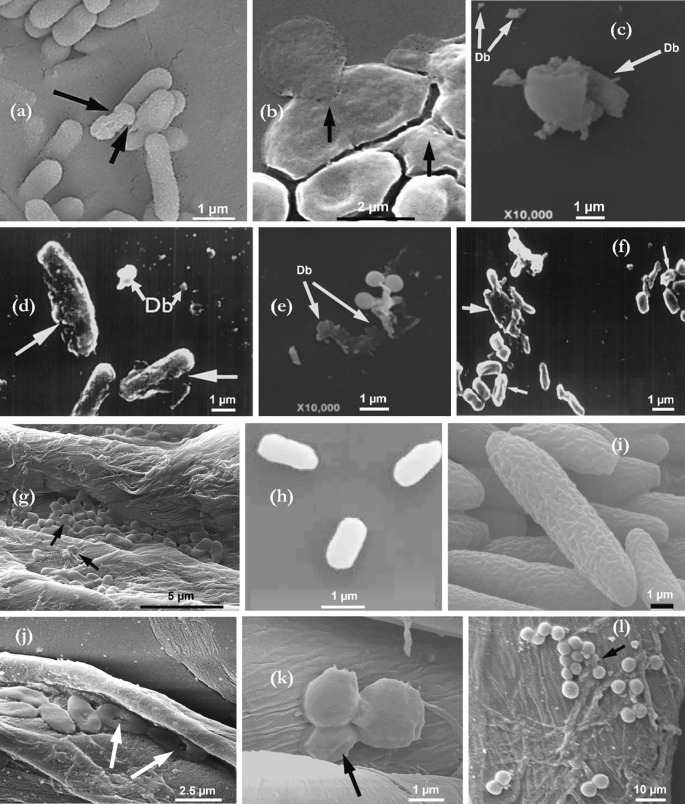
Several propositions have been made regarding ozone’s mechanism of microbial inactivation on various microorganisms (including bacteria, viruses, and fungi). We highlight some key findings from studies that present evidence of ozone’s action via electron microscopy. According to Fig. 2 (a-l) (bacterial and fungal inactivation), progressive oxidation of vital cellular compounds, from the cell surface, appears to be a common observation. Pagès et al. [43] highlighted that ozone first attacks the polyunsaturated fatty acids (cell lipid peroxidation), thereby modifying the cell membrane. This further initiates a chain reaction, which transforms these fatty acids into malondialdehyde (MDA), which eventually leads to their inactivation.
Fig. 2.
Scanning electron microscopy (SEM) bacteria and fungi, showing the cell morphological defects imposed by ozone treatment and the resulting exploded debris – Db (a) E. coli, 6 ppmv aqueous O3 for 30 s [35]; (b) E. coli, 20 ppmv gaseous O3 for 8 min [36]; (c) C. albicans, 0.8 ppm aqueous ozone [37]; (d) B. subtilis, 0.167 ppm/min aqueous O3 for 30 and 60 mins [38]; (e) S. aureus 0.8 ppm aqueous O3[37]; (f) Salmonella sp., 0.167 ppm/min aqueous O3 for 30 and 60 min [38]; (g) E. coli on textile fibres, 20 ppmv gaseous O3 for 8 min [39]; (h) B. atrophaeus, 4000 ppmv gaseous ozone for 3 h [40]; (i) F. fujikuroi, 1 L/min aqueous O3 for 30 s [41]; (j) P. aeruginosa, 10 ppmv gaseous ozone for 10 mins [42]; (k) C. albicans, 10 ppmv gaseous ozone for 10 mins [42]; (l) S. aureus, 10 ppmv gaseous ozone for 10 mins [42].
Oxygen radicals also induce cellular lysis via penetration in the cell membrane in the presence of moisture; this affects the cell’s osmotic stability and alters its metabolism [37]. Patil et al. [35] observed surface alteration (roughening) of E. coli cells on exposure to aqueous/dissolved ozone (6 ppm for 30 sec) compared to non-ozonated cells of the same bacteria (Fig. 2a). In our recent study [36], we observed similar morphological changes in E. coli cells, but additionally demonstrated the leakage of the cell constituents as a result of gaseous ozone exposure at 20 ppmv for 8 mins (Fig. 2b). dos-Santos [37] observed morphological and structural changes (via cell wall disruption), leading to the high presence of cell debris (S. aureus and C. albicans – Fig. 2c and e); aqueous ozone (0.8 ppm) was utilised in their study. Vesicle formation on the cell surface of C. albicans was observed by dos Santos – an indication of the increase in permeability of the plasma membrane. Nonetheless, the intracellular cell damage mechanisms via protein oxidation, DNA damage, and disruptions to enzymatic activities also ensue, albeit not observable by SEM [44], [45].
Growth inhibition, deformations and cell lysis were also observed in the scanning electron micrographs of Thanomsub [38] (Fig. 2d and f) for B. subtilis and Salmonella sp. after aqueous ozone treatment (0.167 ppm/min for 30 and 60 mins). The inactivation of Fusarium fujikuroi (the fungus that causes the rice bakanae disease) by aqueous ozone, also shows significant cell surface deformation via peroxidation of the membrane phospholipids (Fig. 2i) [41]. In Fig. 2g, it can be seen that the structural deformation of E. coli cells by gaseous ozone also occurs, in the presence of fibres, which may act as shields against ozone action [39]. Although the above studies consistently show structural damage of bacterial and fungal cells exposed to ozone, this was not observed in the work of Mahfoudh et al. [40] (Fig. 2h). Their study involved the exposure of bacterial spores (Bacillus atrophaeus, Bacillus pumilus, Geobacillus stearothermophilus and Deinococcus radiodurans) to dry gaseous ozone (4,000 ppmv, 3 h, relative humidity < 2 %). Thus, cell lysis (the breakdown of the cell membrane) may be bypassed during gaseous ozone inactivation of bacteria, because of ozone’s direct diffusion into the cell for the oxidative damage of the intracellular components. However, under humidified ozone application, spore swelling was evident; thus allowing for increased passage of the reactive oxidative species, and eventual collapse/rupture. Furthermore, Patil et al. [35], remarked that cell lysis was not the major inactivation mechanism of ozone on E. coli; rather, the deletion of oxidative stress-related genes was highlighted as the main reason for the increased sensitivity of E. coli cells to ozone treatment. This was a significant improvement to the general understanding of ozone’s activity, particularly in light of previous studies which suggested that the primary target of ozone’s action was the cell surface [46], [47].
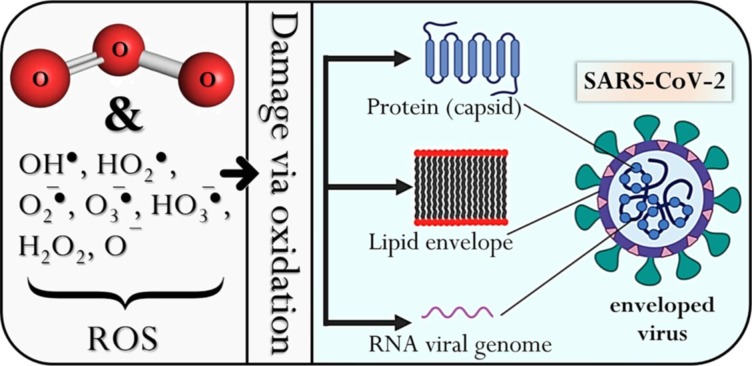
Ozone and its reactive oxidative species can attack enveloped or non-enveloped viruses at different sites (Fig. 3 ) in their structure, rendering them unable to infect a host [49]. As with bacteria and fungi, this may occur via lipid and protein peroxidation, which damages the lipid viral envelope and protein capsid [50]. This leads to further penetration by the ROS, which damages the genome capsid, and RNA, ultimately affecting its ability to reproduce. Their reaction with ROS may also yield secondary reactive species that propagate the inactivation process [48]. The destructive impact of ozone on viral nucleic acid has been highlighted as the inactivation mechanism of the poliovirus [51]. Ozone’s attack on the genome has also been demonstrated by Young et al. [52] for viral inactivation. Furthermore, since viruses are unable to repair damages induced by oxidation, they are very susceptible to ozone treatments compared to other microorganisms [53]. A recent molecular simulation study by Tizaoui [50] has also confirmed that ozone can disrupt the protein and lipid structures of the virus by attacking sulfhydryl and amino acids (especially methionine, cysteine, tryptophan, and fatty acids). The above studies provide strong evidence of ozone’s effectiveness against a wide range of microorganisms.
Fig. 3.
Oxidative action of ozone on the SARS-CoV-2 virus, showing a potential mechanism for its inactivation, via direct and indirect oxidation (adapted from Farooq and Tizaoui [48]).
1.2. How ozone’s inactivation mechanism compares to other decontamination methods
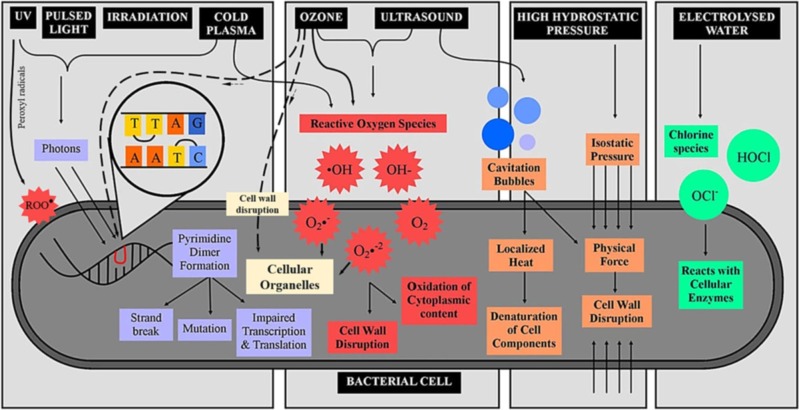
Fig. 4 further illustrates the mechanisms of ozone inactivation of a bacterial cell relative to other decontamination methods. It can be observed that the generation and transfer of reactive oxidative species is a common mechanism, and this effect is facilitated by the increase in the ambient humidity, as demonstrated by several studies [40], [54], [55]. The combined application of these methods has been shown to generally improve the efficiency of microbial inactivation relative to their independent application [56]. Of the different possible combinations, Ozone + UVC, Ozone + H2O2 and UVC + H2O2 have received considerable research attention, and are particularly useful for water and surface disinfection. Other frequently applied methods include ozone + plasma and ozone + ultrasound [21], [57], [58]. The general microbial inactivation efficiency via ozone can be increased by optimising the ambient conditions captured in Fig. 1, depending on the medium of ozone’s application. For example, low temperatures enhance ozone stability in air and water; thus reducing the spontaneous decomposition rate to oxygen [13], [32]. The use of additives (citric acid, carbonate salts, surfactants) have also been explored for the improvement of aqueous ozone stability [13], [15], [59] for prolonged microbial inactivation. Furthermore, the penetration of ozone into porous substrates can be achieved by the creation of a pressure difference between a chamber’s pressure and the entry pressure of the ozone gas feed [60]. The application of ozone nanobubbles also holds tremendous potential for improving the antimicrobial efficacy of dissolved/aqueous ozone as well as its stability [16], [42], [61].
Fig. 4.
Mechanism of ozone decontamination relative to other disinfection methods (adapted from [55]).
2. Industrial applications of ozone
2.1. Treatment of drinking water and wastewater
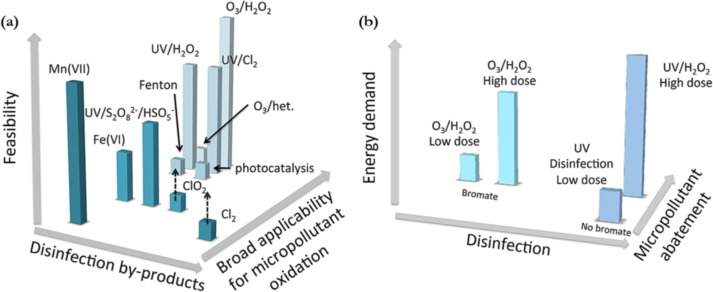
Drinking water treatment represents one of the earliest deployments of ozone in many municipalities particularly for heavy metal removal, taste and odour removal, as well as disinfection (the antimicrobial properties of ozone, are vital for this type of treatment). One of the key challenges facing the abatement of organic micropollutants during ozonation, is the formation of transformation products and/or disinfection by-products (DBP) (Fig. 5 a) – some of which have unknown toxicological consequences [62], [63]. These could even be more toxic than their parent compounds. For example, bromide-containing waters will produce bromate (a human carcinogen) during ozone treatment; its removal after formation is not viable [63], [64]. Thus, brominated by-products must be minimised as much as possible. Although the use of hybrid processes such as O3-H2O2 can significantly lower bromate formation, it was banned in France for the treatment of source waters containing pesticides [63], [65]. Incomplete mineralisation of the desired target compounds and the formation of transformation products (including: bromates, dioxane, atrazine, methyl tert-butyl ether, aldehydes, ketones, carboxylic acids) were the key reasons for this decision. Besides by-product formation, the energy requirements for these hybrid-based processes is crucial factor that determines their large-scale applicability. For example, although UV-H2O2 processes do not produce bromates, they can be up to 4 times more energy intensive than O3-H2O2 processes [65]. Thus, there exists a trade-off between target pollutant removal efficiency, by-product formation, and energy requirements when deciding on the optimal treatment route to adopt, as illustrated in (Fig. 5).
Fig. 5.
(a) Comparison of various oxidation processes for micropollutant abatement in municipal water treatment; (b) comparison of O3-H2O2 and UV-H2O2 AOPs for disinfection and micropollutant abatement [63].
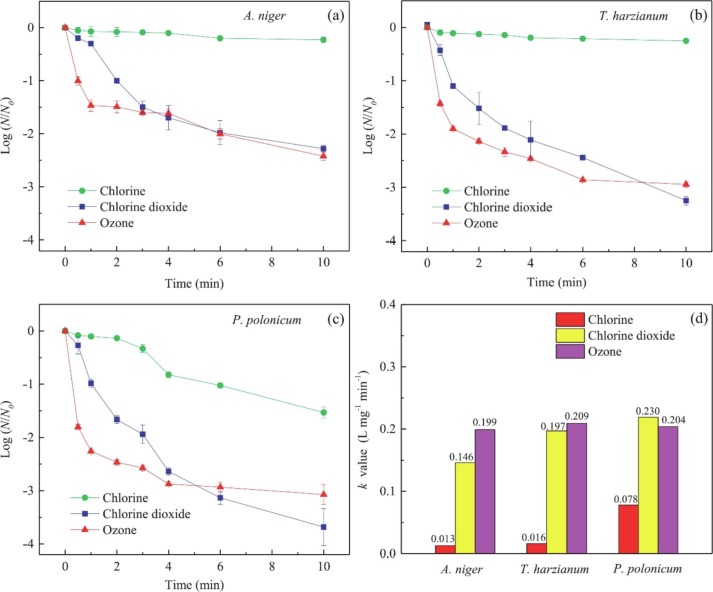
Despite the above limitations, the application of ozone has been regularly shown to outperform chlorine in most primary disinfection campaigns; for example, chlorine-resistant bacteria Bacillus cereus was more readily inactivated by ozone, for the disinfection of drinking water [67]. It has been claimed that up to 3000 times higher CT values may be required for chlorine to yield similar microbial inactivation efficiency as ozone [68]. However, the review by Collivignarelli et al. [69] illustrated approximately 10 times higher CT values for the inactivation (4-log) of viruses. Despite this disparity between both reports, already established CT values for the inactivation of several bacteria and viruses by chlorine, can be replaced with considerably lower CT values when ozone is utilised. The corresponding ozone CT value is dependent on the target organism, and it must be accurately determined for the specific application. Furthermore, the kinetics of fungal inactivation (Fig. 6 ) by ozone and chlorine was compared in the study by Wen et al. [66]. It can be observed that ozone significantly outperforms chlorine for the 3 fungal species tested (inactivation rate constants are between 2.5 and 15.5 times those of chlorine).
Fig. 6.
A comparison of the inactivation of 3 fungal spores using chlorine (2 mg/L), chlorine dioxide (2 mg/L) and ozone (2 mg/L) in 40 mM PBS; (a) Aspergillus niger; (b) Penicillium polonicum; (c) Trichoderma harzianum; (d) inactivation rate constants of the 3 disinfectants [66].
Furthermore, the concept of ozone bubble stabilisation (via the creation of ozone nanobubbles) for prolonged antimicrobial action becomes important here, particularly when considering the rapid decomposition of ozone that ensues when larger ozone bubbles are created in water [61]. The application of ozone nanobubbles enhances the gas’ solubility, with concentrations as high as 53 ppm attained at room temperature [16]. This is significantly higher than concentrations attainable with conventional ozone-contact methods [37] that generate larger micro-/macrobubbles. Ozone has also been shown to enhance biofilter performance for the removal of a wide range of contaminants during drinking water treatment [70] as well as a clogging control strategy for gas biofilters [71]. Wang et al. [70] reported that ozone below 120 mg/m3 enhanced biofilter performance during the removal of chlorobenzene. Zanacic et al. [72] emphasised the importance of estimating the ozone dosage requirements on a routine basis to capture changes in dissolved organic carbon and alkalinity during ozone-assisted biofiltration for potable water treatment. Despite these reported benefits, excessive ozone dosing may adversely affect the biofilter performance [73]. Another reason for controlling the ozone dosage administered for drinking water treatment is to ensure its complete decomposition, before it reaches the point of consumption. Usually, a very small ozone residual is maintained until disinfection is complete, at which point, all ozone disappears; a small chlorine dose is also added at this point to keep a residual in the distribution system. Additional advantages of ozonation for this application include its capability of decreasing chlorinated disinfection by-products [74], the excellent water quality aesthetics (including odour, colour, taste, turbidity) it confers post-treatment [75], and the exceptional capability to degrade a series of emerging contaminants that threaten water supplies [76], [77]. For these reasons, ozone has been more widely considered in drinking water than wastewater treatment. Moreover, many of the largest manufacturers of ozone generation equipment focus on drinking water treatment as the key/target application. The extensive implementation of ozone for drinking water treatment served as the impetus for extending ozonation to other industries, including wastewater treatment. The interested reader is referred to the following papers [16], [63], [78], which provide good overviews of ozone treatment of drinking water.
The interest in ozonation for wastewater treatment is growing, particularly for sludge reduction, the removal of recalcitrant compounds and emerging contaminants (e.g. synthetic dyes, carboxylic acids, phenolics, amoxicillin, and other pharmaceuticals) [79], [80], [81], [82], [83], [84], [85]. These tend to have a high chemical oxygen demand, a low biological oxygen demand, and a low biodegradability index and are usually resistant to biological treatments. Effective mineralisation of these compounds by ozone is important for wastewater reclamation; however, achieving full mineralisation at an industrial scale will be extremely difficult, as the ozone dosage requirements will be too high to achieve economically [76]. Thus, ozone may be applied to break these compounds into others that can be further degraded by the microorganisms in the wastewater treatment process [86], [87], [88]. The increasing reliance on ozone for wastewater treatment in recent years can be attributed to decreasing costs of ozone generation and its environmental benefits relative to chlorine [6], [79]. However, mass transfer limitations, selectivity towards certain contaminants, slow reaction rates, and relatively higher costs (capital and operating) are some drawbacks attributable to ozonation alone [31]. This has led to the prevalent application of hybrid ozonation process involving the combination of ozone, with other processes and compounds for effective water treatment. These are otherwise termed advanced oxidation processes (AOP) and involve the application of ozone with UV, sonolysis, electrocoagulation, H2O2, photocatalysts (e.g. TiO2), metal ion (Cu2+, Mn2+, Zn2+ Fe2+) homogeneous catalysts, and metal oxide heterogeneous catalysts (including activated carbon) [3], [31]. The main purpose of this hybridisation is to further induce ozone decomposition and the consequent production of hydroxyl radicals (OH•) for the oxidation of these recalcitrant compounds. A detailed explanation of the decomposition mechanisms with and without a catalyst can be found in [3], [31], [78], [79]. Furthermore, some of these AOPs (depending on the intended objective) have also been reported to be more cost-effective than the independent application of ozonation [89], [90].
Direct ozonation of raw hospital wastewater containing cyclophosphamide was utilised for effective treatment in the work of Ferre-Aracil et al. [91], with 97 % removal achieved. Furthermore, the degradation of amoxicillin (32 % mineralisation) by ozonation was demonstrated in the work of Kıdak and Doğan [57]; whereas the combination of ozone and ultrasound performed better (45 % mineralisation). Conversely, the excellent degradation of pharmaceutics in hospital water by ozone alone was demonstrated by Khan et al. [92] to be more effective than the hybrid ozone/hydrogen-peroxide treatment. Catalytic ozonation (an AOP involving the combination of ozone and granular activated carbon) was found to improve micropollutant removal in municipal wastewater effluent [93]. Besides the respective contributions by the high adsorption capacity of activated carbon, and the oxidation power of ozone (during catalytic ozonation), ozone may also be transformed into secondary oxidants such as (OH•). This facilitates the subsequent degradation of aqueous and adsorbed contaminants, eventually converting these compounds to water and carbon dioxide (mineralisation) [94]. The extensive review by Beltran et al. demonstrated the effectiveness of catalytic ozonation for the elimination of disinfection by-products in water treatment industries [95]. Laundry wastewater (from commercial textile treatment facilities) containing anionic, cationic and nonionic surfactants has also been effectively treated with ozone [3]; up to 55 % removal of dodecyl benzene sulfonate has been reported in Rivera-Utrilla et al. [96] via the application of ozonation and biodegradation, whereas, ∼95 % of nonylphenol was removed by ozonation (1 mg/L for 90 min) in the work of Wang et al. [97]. The bleaching effect of ozone on textile wastewater (containing dyes) has been demonstrated by numerous studies [98], [99], [100]; the pH appears to be a key parameter influencing the efficiency of decolourisation process. Tannery and photochemical wastewater have also been effectively treated by catalytic ozonation, as documented in [101], [102]. Ozone is also routinely applied for wastewater treatment in the pulp and paper industry, as well as for bleaching purposes [103], [104]. This discussion demonstrates the applicability of ozonation for the mineralisation of a wide range of pollutants from several chemical industries.
2.2. Aquaculture
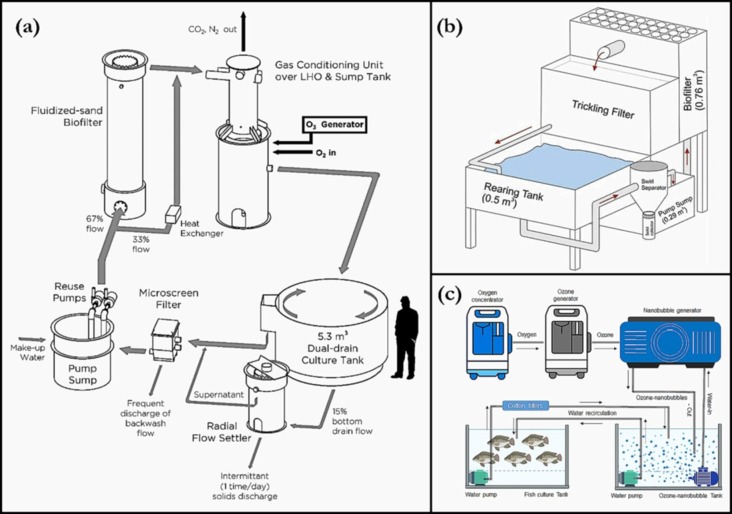
Compared to pond or cage-based aquaculture, recirculating aquacultural systems (RAQS) (Fig. 7 ) allow for more efficient water usage, with lower effluent volumes and better environmental control [105]. These systems require frequent water exchange to avoid the build-up of pathogens and parasites from food and faecal waste [106]. An economically-viable alternative is the breakdown of these organic wastes using a decontaminant such as ozone [2]. Several studies have demonstrated ozone’s capability to improve fish egg hatching rate and larva survival and reduce bacterial loading and viral particles on the fish (infection and disease rates), thereby fostering fish growth and survival in RAQS. Additionally, ozone has been used to reduce organic matter in RAQS, by promoting flocculation of organic matter [73]; thus reducing the total suspended solids (TSS), chemical oxygen demand (COD) and dissolved organic carbon (DOC); the load on the biofilters and oxygenators is also reduced via ozonation [105]. The application of ozone for shellfish depuration [107], removal of algal toxins [108], the improvement of fish taste and odour [109], and water colour elimination [110] have also been reported. More importantly, the overall enhancement of the water quality via disinfection against bacteria and viruses (including Aeromonas hydrophilia [111], Vibrio parahaemolyticus [73] and Motile Aeromonas septicaemia [112]) is well documented. However, for this to be achieved, higher ozone dosages are required to overcome the organic demand in water, for the sustenance of ozone concentration levels capable of significant bacterial and viral inactivation [105]. In addition, the detrimental effects of ozone on fish health, imply the residual ozone will have to be stripped from the water before returning the water to the culture tank. This process needs to happen separately while keeping the fish in a separate container until this disinfected water (now ozone free) is ready for the fish (Fig. 7c).
Fig. 7.
Recirculating aquacultural systems at different scales (a) large scale (adapted with permission from [119]); (b) pilot-scale (adapted from [120]); (c) laboratory scale (adapted with permission from [112]), showing the application of ozone nanobubbles for increased ozone dissolution.
Conversely, there are reports of direct ozone application to aquaculture systems yielding similar benefits to RAQS; however, it is imperative to determine a safety window or therapeutic index with regard to the applied ozone dose [2], [113]. This dose will usually be specific to the fish species, the life stage or size, the fish population in the system, and also the type of biofilter used. (Pumkaew et al. [73] demonstrated the importance of determining this ozone concentration threshold for sustained biofilter performance; in their study, this threshold was 0.3 mg/L). Direct ozone exposure as low as 1 ppbv may induce behavioural anomalies in the fish, as well as physiological changes, tissue damage (including gill damage and osmolality imbalances), and even mortality [114], [115]. Some authors do not recommend direct ozone treatment of the culture tank with ozone [105].
These lethal effects of ozone have warranted the application of proportional-integral (PI) control systems for the automatic adjustment of ozone concentrations in RAQS [115]. Another potential drawback is the production of toxic disinfection by-products such as oxidised halide species and bromates (which are carcinogenic) may cause pH depression and hamper fish health [2]. Thus, UV radiation [116] and activated carbon filtration [117] may be adopted for pre-treatment, particularly if sea water (usually high in Br) is to be utilised. These pre-treatment methods are also effective for increasing the decomposition rate of residual ozone in the treated water, before feeding back to the main culture tank of the RAQS. However, it is worth emphasising that ozone readily outperforms UV application for microbial load reduction purposes, as noted by Teitge et al. [118]. In summary, while the implications of accidental ozone overdose in RAQS can be severely detrimental, ozonation appears to be gaining acceptance, as demonstrated by the increasing number of research contributions, and full-scale utilisations worldwide.
2.3. Food
The inactivation of spoilage-causing and pathogenic microorganisms, for food contamination control and the prevention of foodborne illnesses, is paramount to the food industry. Sanitisation techniques, such as ozonation, play a huge role in achieving these objectives during production, transportation, and storage (including shelf-life extension) of fresh produce [17], [121]. Although chlorinated water has been heavily relied on for microbial load reduction, it fails to fully satisfy the decontamination and safety requirements in most food industries. For example, its reduced effectiveness against spore-forming microbes [6], [67], [122], viruses and protozoan cysts coupled with its harmful (carcinogenic) disinfection by-products such as trihalomethanes and haloacetic acids make it undesirable [1], [122]. Thus, strict regulations aimed at limiting the use of chlorine have been enforced by the European Union [123]. Conversely, ozone has non-residual properties and is the most promising disinfection technology capable of improving food safety and quality [124]. It is also listed as “Generally Recognized as Safe” (GRAS) for food matrix disinfection [125]. Ozone’s inactivation of food microorganisms depends on the type of microorganisms, the degree of attachment of the microorganisms to the food, and the composition and nature of the food surface [4]. We briefly highlight some studies on the application of gaseous and aqueous ozone for the decontamination of meat, dairy, fruit and vegetables and dry foods. Some reports on the impact of ozone on the nutritional components and sensory attributes of a variety of foods are also briefly presented.
Utilising a bespoke fresh produce ozone (<2 mg/L) and chlorine (∼100 mg/L) treatment system, Rosenblum et al. demonstrated improved inactivation of Bacillus subtilis spores on lettuce via ozonation (1.56 log reduction) compared to chlorination (1.30 log reduction); an analysis of the wastewater quality after the treatment duration of 10 – 40 mins, showed improved physicochemical and microbial quality, using ozone compared to chlorine [6]. Ozone treatment for 0.5 – 4 min was effective against 3 fungal species (Venturia inaequalis, Botrytis cinerea, and Neofabreae alba) known to attack apples; a 50 % reduction in the spore germination was observed at ozone concentrations of 0.01, 0.03, and 0.07 mg/ml, respectively [43]. Munhõs et al. [126] demonstrated the inactivation (1 log reduction) of Pseudomonas aeruginosa on skimmed and whole milk by ozone (28 mg/L for 5 min). Ozone treatment has also been recommended as a suitable alternative to pasteurisation [127], [128]. Cantalejo et al. [129] highlighted the effectiveness of a hybrid treatment procedure (0.6 ppm ozone and lyophilisation), which extended the shelf life of chicken meat fillets by up to 8 months, in comparison to lyophilisation alone; total aerobic mesophilic bacteria and lactic acid bacteria were the tested organisms. Ribero et al. demonstrated the application of ozone for the control of fungal growth (Fusarium verticillioides, Penicillium spp. and Aspergillus flavus) on maize produce and as a detoxifying agent against fumonisins [130]; ozone (13.5 mg/L for 24 h) was deemed an effective fungicidal agent with up to 86 % reduction in fumonisin B2.
As suggested by Aslam et al. [17], food decontamination by ozone for prolonged durations may affect their nutritional content; however, a necessary balance has to be maintained between food safety and the retention of nutritional aspects. Although Shynkaryk et al. [131] demonstrated that such modifications by ozone are restricted to the surface only, with no penetration into the bulk of the material observed, a 40 % reduction in the ascorbic acid (Vitamin C) contents in parsley has been reported by Karaca and Velioglu [132], after ozonation (950 ± 12 µL/L, 20 min). Despite these accounts, ozone’s interference with the protein content and quality of food has hardly been reported. With regard to sensory attributes, there have also been mixed accounts on the effect of ozone on different food; nonetheless, degradation in colour, odour, firmness, weight, and texture of fruits and vegetables, have been mitigated by ozone’s application. As demonstrated by de Souza et al. [133], gaseous (0 – 5 mg/L) and aqueous (0 – 10 mg/L) ozone treatment did not alter the weight, firmness, pH, and colour of carrots, resulting in prolonged shelf life. Conversely, the colour of chicken and duck meat was reported to be affected by gaseous ozone exposure – redness reduction associated with the oxidation of myoglobin and oxymyoglobin [134]. Ozone has also been extensively utilised for the removal of food residues from various surfaces encountered in the food processing industry, particularly because ineffective cleaning processes, may lead to the formation of biofilms on food equipment surfaces [10]. The removal of heat-denatured whey protein concentrates on stainless steel coupons was demonstrated using ozonated water (40 NL/h, 80 g/Nm3, 30 min) in the work of Jurado-Almeda et al. [135] It is worth mentioning that the application of aqueous or gaseous ozone for food processing depends on the type of food and the purpose of storage; nonetheless, the increased stability of ozone in the gaseous phases makes it somewhat preferrable. The interested reader is referred to the following critical reviews [17], [43], [124], [136], [137], [138], [139] for an expanded discussion on this subject.
2.4. Medical equipment
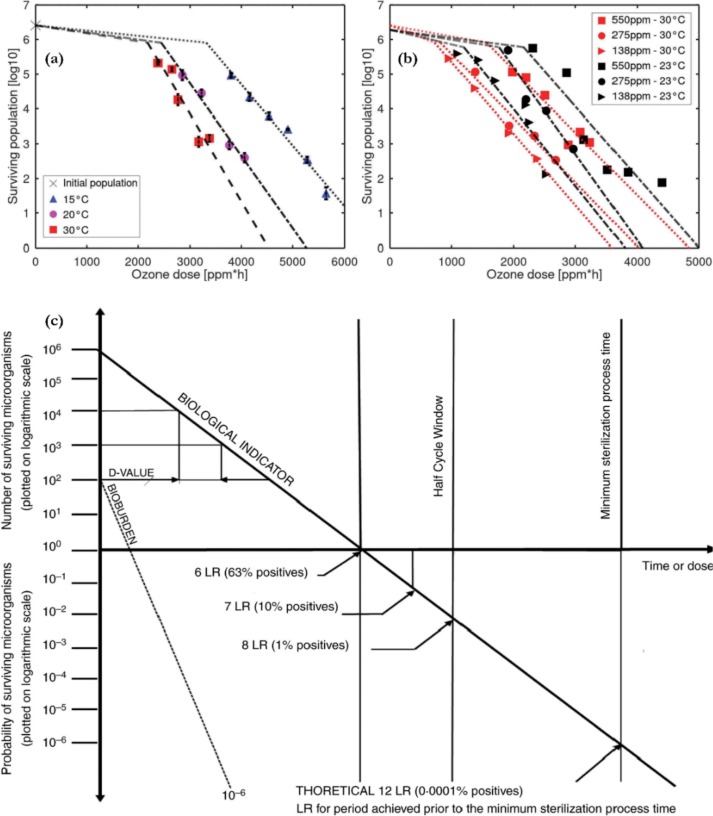
Reusable and non-reusable medical devices and surgical instruments that come in contact with a patient’s sterile tissue, mucus membranes, or skin must undergo sterilisation and disinfection before reuse or disposal to avoid infections (by pathogens) and further transmission [27], [28]. As reported by Rutala and Weber [28], there are approximately 99,000,000 inpatient and outpatient surgeries in the US each year; thus creating the need for continuous equipment sterilisation on a daily basis. Gastrointestinal endoscopes, bronchoscopes, endocavitary probes, catheters, perforators, blood pressure cuffs, dental braces, and implants are some of the equipment requiring decontamination. Furthermore, the sterilisation of PPE such as surgical facemasks, gowns, and hand gloves, may reduce waste and costs. While steam has been predominantly applied for the sterilisation of heat-tolerant medical equipment, ethylene oxide (EtO) appears to be the most implemented method for sterilising heat-sensitive materials [22]. However, it is flammable, explosive, and considered a carcinogen; thus constituting a significant safety hazard, particularly in the event of accidental exposure [22], [23], [28]. In response to this limitation, the food and drug administration (FDA) launched a sponsored innovation challenge aimed at reducing the dependence on EtO for sterilisation applications [140]. Despite the sterilisation potential of ozone, our literature search has indicated that its application for the sterilisation of medical devices has been hardly investigated. One of the few studies to address this gap was that of Thill and Spaltenstein [23]. They developed a compact ozone sterilisation system that utilises mercury-free V-UV excimer lamps (172 nm) for ozone generation (100 – 1000 ppmv). They achieved full sterilisation of a device contaminated with Geobacillus stearothermophilus spores (which is considered the most resistant microorganism to ozone [141]), based on the standards [24] for medical device sterilisation (Fig. 8 c; SAL = 10-6 or a 12 log reduction, based on extrapolation of the survivor curves). Fig. 8 illustrates the impact of temperature and ozone concentration, on microbial reduction as obtained in their work. A lag phase (during which the bacteria population remains constant) during the treatment can be noticed. This lag reduces with increased temperature and ozone concentration.
Fig. 8.
The impact of (a) temperature and (b) gaseous ozone centration on the survival population of Geobacillus stearothermophilus spores, showing a lag phase and first-order log-linear inactivation kinetics(adapted with permission from [23]); (c) an example of the relationship between a product bioburden and the biological indicator for the determination of the sterility assurance level (SAL) (adapted from [142]).
Ljungberg [143] studied the gaseous ozone disinfection of blood pressure cuffs, a drug pump, an X-ray neck collar and a CPAP bag (continuous positive airway pressure); these were originally contaminated with Geobacillus stearothermophilus spores. Applying an ozone concentration of 56 ppmv yielded a ∼ 83 % reduction (after 40 min) and ∼ 98 % reduction after 240 min. A study by Lopes et al. [21] applied a hybrid ozonation process to disinfect corrugated tubing from mechanically-ventilated tracheostomised patients. After cleaning the device, gaseous ozone (33 mg/L for 15 min) was applied, and compared with 0.2 % peracetic acid, ultrasound for 60 min, ultrasound and ozone (for 30 and 15 min, respectively, and 60 and 15 min, respectively). Ozone was deemed the most advantageous method, yielding up to 5 log microbial reduction. Similarly, the hybrid application of ozone (50–500 ppmv ozone and 3 % hydrogen peroxide) has been applied for the disinfection of healthcare spaces and surfaces contaminated with vancomycin-resistant enterococcus, Clostridioides difficile, methicillin-resistant Staphylococcus aureus, Pseudomonas aeruginosa, Bacillus subtilis, and E. coli [144]. It was shown that 80-ppmv ozone and 1 % hydrogen peroxide achieved ≥ 6 log10 reduction during 30 – 90 min exposure.
In a recent review by Rubio-Romero et al. [147], ozone was identified as a promising method for the disinfection and sterilisation of personal protective equipment (PPE), including facemasks; compared to other low-temperature sterilisation methods, which may compromise the filtration efficiency of facemasks during disinfection, gaseous ozone concentration as high as 500 ppmv has been reported to have no degradative effects on the filtration efficiency of N95 filters [148]. This retainment of filtration properties was also reported by other authors [146], [149]. In the study of Blanchard et al. [146], gaseous ozone exposure (20 ppmv for 40 min) yielded 4 log reduction of the influenza-A and respiratory syncytial virus. However, irreversible damage to the elastic bands of the facemask may occur, particularly when stretched during treatment (Fig. 9 ); stretching causes increased exposure of the polymer bonds to ozone attack, eventually leading to failures during active usage, thus leaving the wearer unprotected. It is recommended that ozone-resistant elastic materials such as silicone are utilised in the manufacturing process to increase the possible number of treatment cycles for facemasks, without compromising the functionalities of its different parts. The following studies provide an expanded discussion on ozone sterilisation of facemasks, particularly as necessitated by the COVID-19 pandemic [147], [150], [151].
Fig. 9.
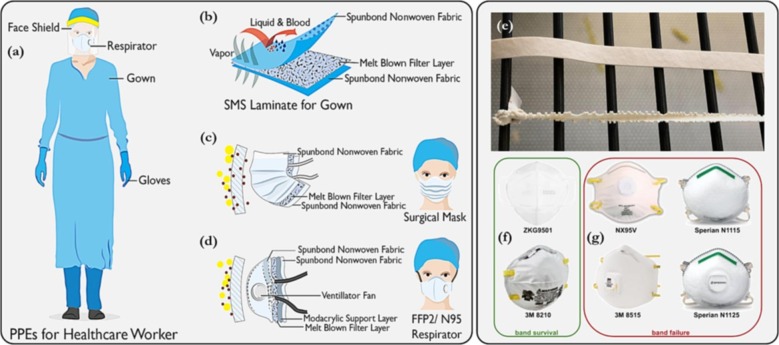
(a-d) Typical PPEs used in clinical settings showing the fabric layers that prevent the transfer of droplets to/from the host (adapted with permission from [145]); (e) Negative impact of gaseous ozone treatment on the elastic band of facemasks (strain-induced ozone damage; the top band was not stretched during ozone treatment, and displayed no damage, whereas, the lower band was stretched (×2.4), and was degraded after treatment; (f, g) elastic band compatibility of different respirators with ozone treatment. The elastic bands that survived (f), were likely made of thermoplastic elastomers or polyisoprene with polypropylene overbraid (adapted from [146]).
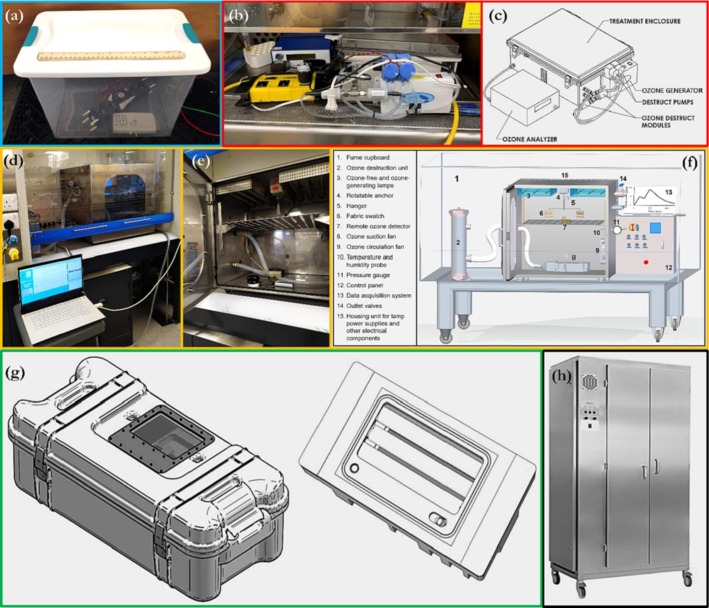
Gaseous disinfectants appear to be the preferred method for medical device sterilisation. This is because of the highly diffusive and penetrative ability of the gas compared to immersion-based disinfection in a liquid disinfectant, in which case the liquid may not be able to efficiently navigate the contaminated micro-channels of thin tubing equipment. Fig. 10 pictorially illustrates some of the gaseous ozone disinfection chambers reported in the literature; where available, schematic representations are also shown.
Fig. 10.
Small-scale gaseous ozone chambers in literature, that have been utilised for medical device sterilisation (adapted with permission from (a) [151]; (b – c) [148]; (d – f) [36]; g [23]; h [143]).
2.5. Textile processing
Ozone is applied in a variety of ways to different fibre types in the textile industry. Textile effluent/wastewater treatment, bleaching/decolourisation, disinfection, and deodorisation during laundering operations. Since the treatment of textile-related wastewater has already been highlighted in Section 2.1 of this review, we focus on the other applications of ozone, which have to do with the fabric itself. As a result of the high oxidising power of ozone, it can break down the olefin groups of the indigo dye; thus making it useful for the bleaching of denim clothing (dry bleaching) [152], [153]. Ozonation has been described as a process with reduced environmental impact because it does not require steam and water compared to conventional denim bleaching (a wet process), which generates large volumes of wastewater [152].
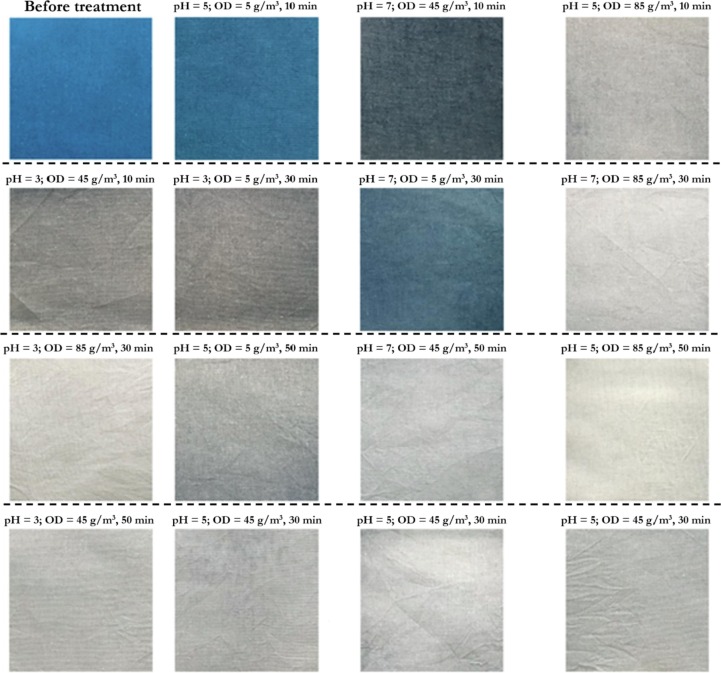
Several fabric types such as polyester, cotton, mohair, polyethylene terephthalate (PET), silk and lyocell have been successfully bleached using ozone [58], [155], [156], [157], [158]. Relative to other textile materials, it has been shown that polyethylene terephthalate (PET) degrades during fading treatments with ozone; thus it has been recommended for denim manufacturing [155]. As demonstrated by Perinçek et al. [159] ozone increases the degree of whiteness and dyeability of protein fibres. A water pick-up value (WPV) of 60 % and a pH of 7 appear to be well-accepted conditions for ozone bleaching; whereas, the time of exposure tends to be material-dependent. Hmida et al. [160] observed that ozone bleaching performance on denim textiles at WPV of 60 % is more efficient than dry ozonation. It has been shown that acidic pH of 3 during ozone treatment (85 g/m3 for 30 min) provides optimal decolourization results (Fig. 11 ) against reactive dyes on cotton fabric swatches, with less damage; however, a slight yellow colouration after treatment was observed [154]. An analysis of the physical and mechanical properties of denim fabric after ozone bleaching (10 – 100 g/m3; 5 – 30 min), revealed a significant shrinkage (∼30 %) and a profound decrease in the bagging resistance of the fabric (∼40 %) [153]; this indicates that mechanical degradation occurs with ozone treatment – ozone dosage should be carefully chosen to mitigate these effects. Furthermore, Ozone has been shown to be more effective when combined with ultrasound energy [161], [162]. This is attributable to the enhancement in ozone penetration into the fabric, produced by the ultrasonic energy. As shown in the following studies [15], [163], some stains may prove difficult for ozone action such as the oily silicone polymers found in temporary board markers, sizing agents, natural waxes and oils.
Fig. 11.
Images of the dyed fabrics before and after aqueous ozone treatment, showing ozone’s bleaching properties under the different experimental conditions; OD represents the ozone dosage (adapted from [154]).
The antimicrobial and bleaching properties of ozone are also well-suited to laundry operations. Compared to conventional high-temperature/thermal disinfection laundry cycles (71 °C), ozone laundering can be performed at low temperatures (15 °C). Cold-water wash cycles imply significant savings in energy cost and are reported to be as high as 90 % in the USA, and up to £21 k in an 800-bed commercial hotel [164]. Similar benefits have also been experienced by ACS Clothing (a prominent rental fulfilment and clothing renewal specialist in the UK). As a powerful bleaching agent and oxidiser (ozone), there is reduced consumption of other chemicals (detergents, pH stabilisers and other toxic bleaching agents); this translates to fewer rinse cycles and lower water consumption [5]. Furthermore, the reduced exposure of the fabrics to mechanical action (via fewer wash/rinse cycles and lower temperature treatment), extends the life of garments [165]. This is particularly important to the ongoing garment rental model adopted by various fashion brands in the UK. Ozone treatment is playing a key role in prolonging garments’ lives and reuse. Organic and inorganic odour-causing compounds can also be broken down by ozone; thus deodorising fabrics/garments. In addition, higher dissolved oxygen levels in the discharged laundry wastewater enhance the biodegradability of the contained pollutants; this can potentially lower sewage treatment capacities and costs [3].
The inactivation of Clostridioides difficile (commonly resistant to conventional thermal laundering) has been extensively demonstrated in the work of Cardis et al. [164] using a commercially available ozone washing machine (ECOTEX, by JLA limited). Several case studies in different healthcare establishments were presented, with excellent benefits observed (up to 7 log bacterial reduction). While Epelle et al. [15] and Eriksson [59] have demonstrated the stabilising effect of a surfactant on the ozone concentration, uncontrolled detergent addition will lead to consumption of ozone in solution, thus making it less available for fabric disinfection. Neral [166] also pointed out that the simultaneous use of ozone and surfactants may cause decreased detergency compared to the sole application of the surfactant. Thus, ozone disinfection may be left for the rinsing cycle, to improve ozone disinfection efficiency, while residual surfactant carried over from the main wash cycle will facilitate ozone’s aqueous stability/retention, alongside the low temperature. With ozone’s bleaching properties already demonstrated, its careful dosage control when washing coloured clothing, becomes paramount, compared to white-coloured clothing. Rice et al. [5] observed colour loss during ozone disinfection wash cycles; thus, the use of oxidation-resistant dyes may be further explored. They also reported that the microfibre integrity was unaffected during ozone washing [5]. However, Neral [166] reported the degradation of yarn tensile strength after prolonged exposure to ozone. This was attributed to the weak links introduced in the amorphous regions of the fibres. Although this damage was lower than that of conventional washing, it is worth considering this possibility during ozone laundering operations. It is also important to mention that this application will depend on the chemistry of the fibres, as ozone may also degrade specific constituents during the disinfection process.
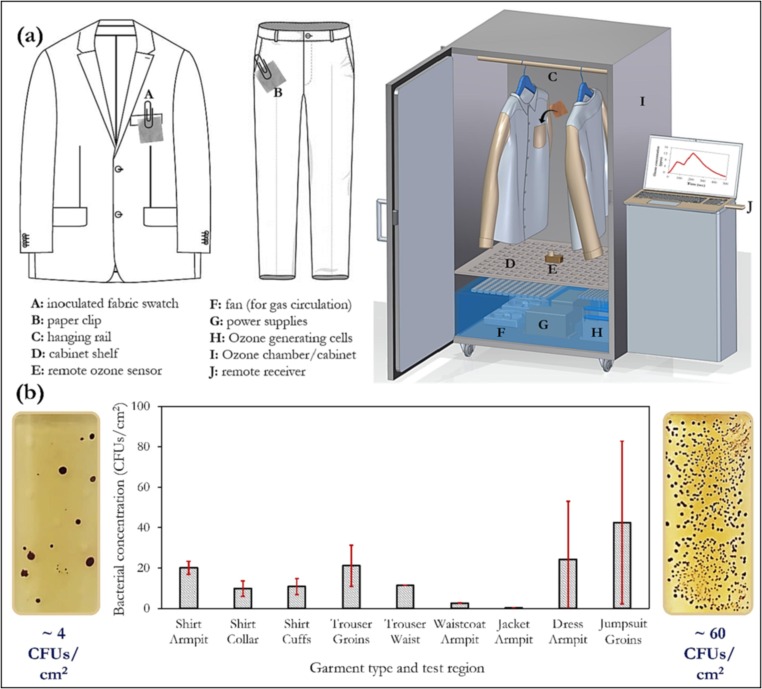
Epelle et al. demonstrated typical contamination levels (Fig. 12 ), in different regions of worn garments prone to contamination (e.g. armpit and groin areas); they examined the penetration and disinfection efficiency attainable using ozone [39]. It was discovered that the amount of ozone reaching these contamination-prone areas, may be 40 % lower than the ambient ozone concentration in a disinfection chamber. They highlighted the hanging orientation, garment packing density and the weave structure as critical parameters affecting the penetration and eventual disinfection of ozone gas. A similar study [36] by the same authors on cotton-polyester fabrics showed that an ozone dose of 80 ppmv.min (160 g.min/m3) was sufficient to fully inactivate a heavily contaminated fabric swatch (containing A. fumigatus, C. albicans, E. coli & S.aureus). Such gaseous ozonation procedures are also important for large-scale deodorisation applications. A key consideration when carrying out gaseous ozonation of textiles is the effect of the treatment on the structural integrity of the fibres (Fig. 13 ). Besides the recent heightened interest in the reuse of personal protective equipment (geared towards waste reduction), the clothing industry is also actively seeking to adopt a rental model for garments, as a means of promoting communal end-use, extending their lifespan and reducing landfill waste. The application of gaseous ozonation holds considerable potential for this purpose, while reducing the reliance of high-water consuming laundry processes for disinfection purposes. However, it should be noted that unless the fibres of these materials are made of materials not affected by strong oxidants, ozonation may not work as expected.
Fig. 12.
(a) Application of gaseous ozone for the decontamination of garments (adapted with permission from [39]), showing (b) contamination levels in different regions of used garments (adapted from [36]).
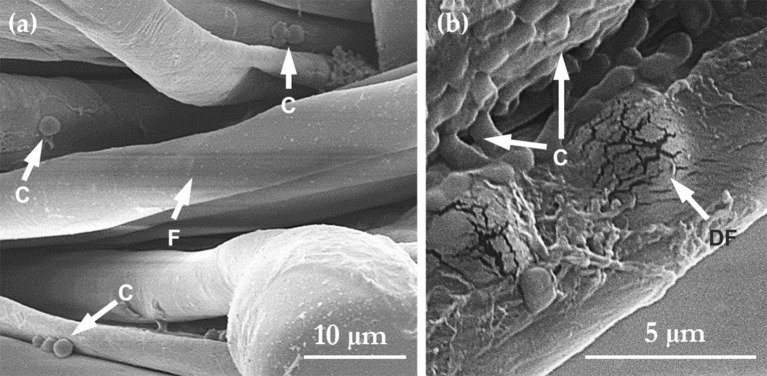
Fig. 13.
Impact of gaseous ozone treatment on the structural integrity of the cotton-polyester fibres (F); (a) shows Candida albicans cells (C) after applying an ozone doze of 100 ppmv.min; whereas (b) shows damaged fibre (DF) regions after 160 ppmv.min treatment in the presence of E.coli cells (C) (adapted with permission from [39]). Fabric swatches had undergone up to 10 previous ozone treatment cycles before this image was taken.
3. Ozone in the era of COVID-19
As the COVID-19 pandemic (officially labelled as Severe Acute Respiratory Syndrome-related Coronavirus-SARS-CoV-2) continues to ravage the world, ozone sanitisation (gaseous and aqueous) of environments, surfaces, and various objects has become paramount, in addition to other preventive and protective measures. Since viruses are unable to repair oxidative damage, ozonation is considered a good candidate for their inactivation. Although ozone has been readily applied for the inactivation of different viruses [44], [146], [167] for a very long time, this section mainly focuses on the findings of studies that utilised ozone to inactivate the novel SARS-CoV-2 virus, while briefly highlighting key studies that applied ozone to inactivate SARS-CoV-1. It is worth mentioning that the handling of SARS-CoV-2 requires biosafety level 3 facilities; thus some studies [149], [168], [169], [170] have employed biosafe substitutes/surrogates, with similar form, structure and function to the SARS-CoV-2 virus.
One of the earliest studies to demonstrate the effectiveness of ozone against the SARS-CoV-1 virus was that of Zhang et al. They reported that a concentration of 27.73 mg/L of aqueous ozone could kill the virus in 4 min [171]. A similar study by Hudson et al. using gaseous ozone showed that 20–25 ppmv of ozone (at RH > 90 %) was able to inactivate (>3 log reduction) the Murine Coronavirus (MCV) on different adsorbent and non-adsorbent surfaces within 40 min [172]. The success of ozone treatment on the SARS-CoV-1 (2002) virus, incentivised researchers in this field to investigate the action of ozone against SARS-CoV-2 (2019); also the 80 % genome sequence similarity between both viruses was a promising indication of success [173], [174].
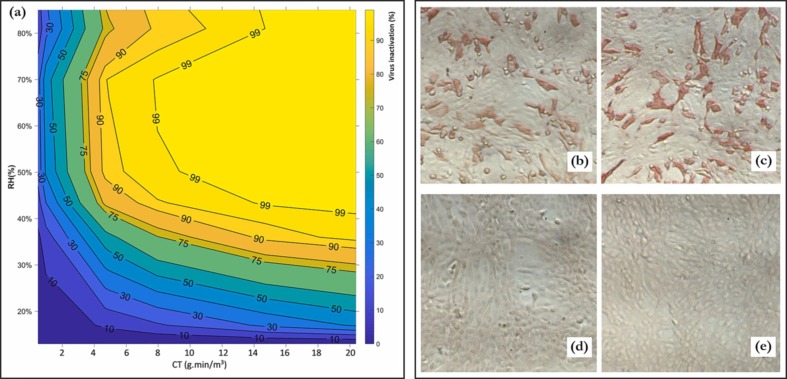
Yano et al. were the first study to demonstrate the inactivation (3 log reduction) of the SARS CoV-2 virus on stainless steel plates, utilising 6 ppmv of ozone gas for 55 mins and 60–80 % RH (from 2 × 106 PFU/mL to 1 × 106 PFU/mL) [175]. A subsequent study by Martins et al. [176] demonstrated the efficacy of ozonated water against the SARS-CoV-2 virus. 0.2 – 0.8 ppm of ozonated water yielded 2 log reduction upon 1 min of exposure. The inactivation of the SARS-COV-2 virus by aqueous and gaseous ozone was also demonstrated in the work of Tizaoui et al. [54]. The virus was suspended in liquid and also dried on several surfaces (glass, plastic (polystyrene), copper, stainless steel, and coupons of ambulance seats and floor). While the liquid treatment inactivated the virus at a rate of 0.92 ± 0.11 log reduction per ozone dose (CT value – mg.min/L), the surface treatment yielded inactivation rates between 0.01 and 0.27 log reduction per ozone dose, for the RH range of 17 % – 70 %. They realised that rigid inert surfaces gave similar inactivation results – this was also observed in the work of Hudson et al. [172]. Fig. 14 a shows the synergistic effect of ozone dosage and RH on the inactivation efficiency of the virus on a plastic surface, whereas Fig. 11b-e illustrate the effect of ozone on ozone treatment of facemasks infected with SARS-CoV-2.
Fig. 14.
(a) Combined effect of gaseous ozone dosage (CT) and RH on the inactivation of SARS-CoV-2 by ozone; these tests were carried out on a plastic (polystyrene) surface (adapted with permission from [54]). (b-c) Positive control repeats for SARS-CoV-2 virus inactivation assay; red stains indicate infected cells (VeroE6), which were stained with the virus’ nucleocapsid antibody on cotton face masks (adapted from [178]). (d-e) Effect of heat-drying (40 °C) and gaseous ozone inactivation (∼800 ppmv, 100 min) on the recovery of SARS-CoV-2 virus from cotton face masks; the samples are void of infection after treatment; d and e are repeats. [178].
Westover et al. also observed significant RNA degradation of synthetic SARS-CoV-2 RNA within 60 min of ozone exposure at 20 ppmv [177]. More recently, Wolfgruber et al. [178] showed that SARS-CoV-2 contaminated facemasks (cotton and FFP3, Fig. 14b), and glass slides can be decontaminated by 800 ppmv gaseous ozone between 10 and 60 min. A > 6 log reduction was demonstrated under these conditions. They also highlighted that the surface tension that ensues when viral droplets are placed on glass, prevented the inactivation of the virus by ozone. Additional studies highlighting the efficacy of ozone against the novel SARS-CoV-2 virus are summarised in Table 3 .
Table 3.
Ozone’s application for the inactivation of SARS-CoV-2 virus on different surfaces and in different liquid media.
| Reference & year |
Ozonation phase | Ozone conc., output, or dose (ppmv, mg/h, g.min/m3) | Time (sec, min, h) |
T (oC) |
RH (%) |
Material/substrate/media | Key result |
|---|---|---|---|---|---|---|---|
| Percivalle et al. [183] (2021) |
Gaseous | 0.5, 1, 2 ppmv | 40, 60 min | 24 °C | 55 % | Painted aluminium, non-painted aluminium FFP2 masks, glass, plastic, surgical gown, Plexiglas, stainless steel | ∼85 – 90 % viability reduction at 60 min for all surfaces at all concentrations. |
| Sallustio et al. [184] (2021) |
Gaseous | 400 mg/h 3.6 L/min |
4 min | – | – | Swabs | Negative result on real-time detection kit. |
| Westover et al. [177] (2020) |
Gaseous | 20 ppmv | 30, 60, 120, 180, 240 min | – | – | Blankets, catheters, remotes, and syringes | ∼99 % capsid RNA degradation at 240 min. |
| Volkoff et al. [185] (2021) |
Gaseous & Aqueous | 4.5 and 9 ppmv | 10 – 90 min | –* | 40 – 60 % | Polyester, stainless steel, plastic, and paper. Water treatment was also performed | 0 % virus RNA recovery from plastic surface after 4.5 and 9 ppmv O3 exposure for 60 – 90 min. |
| Inagaki et al. [186] (2021) |
Aqueous | 1, 4, 7, 10 mg/L | 5 – 20 sec | – | – | Virus stock solution prepared in MEM containing 2 % FBS, 1x NEAA, 20 mM HEPES, and 1x P/S |
Virus tier reduction rates of 81.4 %, 93.2 %, 96.6 %, and 96.6 % at 1, 4, 7, and 10 mg/L, respectively. |
| Criscuolo et al. [187] (2021) |
Gaseous | 0.2 and 4 ppmv | 30 – 120 min | –* | – | Wood, Gauze, fleece, glass and plastic | 4 ppmv Ozone exposure for 30 min yielded a 90 % reduction of viral titres for all materials. |
| Clavo et al. [188] (2020) |
Gaseous | 4 – 12 ppmv & 500 – 40,000 ppmv | 0.5, 1, 5, 10 min | 22.0 –23.7 ◦C | 53 – 65 % | PPE gowns and facemasks | No amplification of the virus after O3 exposures (at 2000 ppmv or higher). At lower concentrations (4 – 12 ppmv), inactivation significantly depends on RH. |
| Hu et al. [189] (2021) |
Aqueous | 4.5, 9, 18, 36 mg/L | 1, 5, 10 min | – | – | Virus solution inoculated into Vero E6 cells) | Full inactivation of virus stock (4 × 103 PFU/mL) in<1 min, by 36 mg/L aqueous ozonation. |
| Nicolò et al. [190] (2022) |
Gaseous | 5 g/h in a chamber of 0.033 m3 | 15, 30 min | – | – | Aerosol sampling | The virus was fully eliminated after 30 mins of ozone exposure. |
| Takeda et al. [191] (2021) |
Glycerol | 200, 500, 1000, 2000 ppm | 20 sec, 1 h, & 24 h | – | – | Virus solution in DMEM and FBS media | ≥99.91 % of the virus was inactivated by ozonated glycerol (500 ppm, 1 h) at 20 % of FBS concentration. |
| Yano et al. [175] (2020) |
Gaseous | 1, 6 ppmv | 55, 60 min | 25 °C | 60 – 80 % | Stainless steel | 6 ppmv of ozone gas for 55 mins yielded a 3-log10 reduction (PFU/mL) in the viral load. |
| Martins et al. [176] (2021) |
Aqueous | 0.2 – 0.8 ppm | 1 min | –* | – | Virus solution in DMEM media & FBS media | 2 log10 reduction in virus infectivity was observed after 0.6 ppm ozone treatment for 1 min. |
| Tizaoui et al. [54] (2022) |
Gaseous and aqueous | 0.5 – 20 g.min/m3 | 3, 5, 20 min | 26 °C | 0 – 100 % | Stainless steel, glass, copper, plastic, coupons of ambulance seat and floor; in liquid, virus cultures in DMEM media was used. | Up to 2 log reduction was observed on stainless steel and glass at 15 g.min/m3 of ozone and 81 % RH. In liquid, the rate constant of inactivation was 7 × 105 M−1s−1. |
| De Forni et al. [192] (2021) | Gaseous | 0 – 5.5 ppmv | 0 – 45 min | 21 °C ± 1 °C | 50 % ± 1 % | Virus suspensions in well plates | 3.18 ppmv of ozone inactivated > 99 % of the virus within 20 min. |
| Wolfgruber et al. [178] (2022) |
Gaseous | 800 ppmv | 10 – 60 min | 22 – 24 ◦C | 45 % | Cotton facemasks, FFP3 facemasks, glass slides | > 6 log reduction is demonstrated within 10 – 60 min of ozone exposure at 800 ppmv. |
| Murata et al. (2020) | Gaseous & Aqueous | 0.05 ppmv to 2 ppmv | 10 s – 20 h | –* | 55–80 % | Stainless steel for gaseous exposure; virus solution (with DMEM, FBS & VeroE6/ TMPRSS2 cells) mixed with ozonated water. | 1 & 2 ppm ozonated water yielded 2 & 3 log reductions in 10 s. Whereas, 0.05 & 0.1 ppmv O3 gas yielded 95 % inactivation in 10 and 20 h. |
–* means room temperature is quoted, but the exact value was not stated; – denotes missing information.
It is worth pointing out that some studies have demonstrated the ineffectiveness of ozone against certain viruses (HIV-1, HBV, EV71, MHV) [179], [180], [181], [182]. A possible reason for this observation is ozone consumption by the culture media (required by the cells that harbour the virus); thus making reducing ozone’s availability for inactivation of the virus. Further work is required to improve the general understanding of ozone’s inactivation mechanisms and the possible reasons for these failures. This is important considering the potential for mutations of the SARS-CoV-2 virus, and the efficient control and prevention of future pandemics.
Furthermore, the therapeutic applications of ozone for the treatment of SARS-CoV-2 patients have been demonstrated in several studies [193], [194], [195], [196], [197]. The administration of the ozone for the treatment of the virus can be performed via several routes, including, major autohemotherapy (MAHT), minor autohemotherapy (MiAHT), rectal insufflation (RI), ozonated saline and ozonated oils [198]. Intravenous, intramuscular, intrapleural, intra-articular, interpleural, intradiscal, infiltration and extravascular methods [199] have also been applied for the therapeutic utilisation of ozone against various illnesses such as rheumatism, Parkinson’s cancer, gangrene, osteomyelitis and several body infections [200], [201]. It has been pointed out that the human body is capable of producing ozone for protection against infectious agents [202], [203]. Despite being a powerful oxidant, ozone has been shown to possess paradoxical activity during interactions with organic molecules – this yields a pronounced antioxidant and anti-inflammatory response [203].
Ozone concentrations as high as 23 – 40 µg/mL have been successfully utilised in MAHT, MiAHT and RI treatments, regularly interspersed over days to months for a diverse range of patients [193], [194], [204], [205]. Saline treatments however tend to utilise lower concentrations (1 – 5 µg/mL) [195], [206]. This is necessary to prevent the excessive formation of H2O2 and HOCl-, which could induce irritative effects in the endothelium, and phlebitis [207]. Expedited hospital discharge, mortality rate improvement, reduced Taylor’s radiological scale, increased blood oxygen saturation as well as the reduction in the overall symptoms of COVID-19 have been reported as the benefits of ozone therapy against the virus [208], [209]. It has also been highlighted that ozone therapy provided synergistic effects with antivirals and anticoagulants conventionally applied for the treatment of the SARS-CoV-2 virus [198], [201], [210]. Only negligible side effects such as minor meteorism and bloating have been recorded in patients so far [196], [198], [205]. In summary, MAHT appears to be the most prevalent method of administering ozone for COVID-19 treatment; followed by RI and MiAHT.
4. Open research problems, opportunities & recommendations
The following points constitute the open research problems identified in this review. Furthermore, the industrial experience of the authors in the operation of industrial-scale aqueous and gaseous ozone disinfection systems has facilitated the herein provided recommendations for future exploration.
Further research contributions are needed on the utilisation of gaseous and aqueous ozone for the decontamination of medical equipment/instruments and textiles. Relative to the numerous research contributions on ozone’s application in the food industry and for drinking water treatment, those targeting these areas were fewer than expected. Notwithstanding, the use of ozone in the food industry is expected to increase in the coming years, particularly as its impact on overall food quality has been deemed minimal; thus, novel ozone contacting equipment for the treatment of various food types are required. The food industry will benefit from additional studies that further elucidate the penetrative or surface impacts of ozone treatments on a wide range of fruits and vegetables (e.g. the potential for vitamin content reduction, after ozone decontamination). In aquacultural systems, the potential for controlled ozonation to mitigate the impact of fish lice (e.g. Lepeophtheirus salmonis and Paramoeba perurans, which cause amoebic gill disease) on fish health has hardly been investigated. This could be applied in combination with conventional cage and tank systems for improved survival. If successful, the dependence on Diflubenzuron (an insecticide used to control parasites in fish farms), can be reduced.
Advancements in chemical reaction kinetics (novel computational methods) are required to facilitate accurate predictions of transformation product and by-product formation during drinking water and wastewater treatment by existing and emerging oxidation technologies. The combined application of these computational-based methods with advanced analytical techniques (e.g. for ozone nanobubble characterisation) will also facilitate the evaluation of the relative toxicities of these products; thus, ensuring the safe application of hybrid oxidation processes.
Ozone inactivation mechanisms of fungi, protozoa, and parasitic organisms require further elucidations. More research contributions are also required to determine the inherent factors instigating the antimicrobial resistance to ozone. Increased defensive enzyme (catalase) production as well as cell wall property modification (thickness, porosity and orientation) are potential cell defensive mechanisms to additionally investigate upon controlled/repeated ozone exposure. Viral inactivation kinetics via ozonation has been hardly studied in the literature, particularly the kinetics of SARS-CoV-2 inactivation (given its novelty). Although the rapid inactivation rates make it difficult to measure, such efforts will go a long way towards improving the general understanding of ozone’s antiviral action; thus facilitating improved designs of ozone contacting equipment. The viral inactivation curve proposed by Grignani et al. [211] and Tizaoui et al. [54] may constitute a good starting point for such endeavours. More insights at a molecular level are required to further substantiate the proposed mechanisms of ozone inactivation of different organisms in gaseous and aqueous conditions. The use of molecular simulations could be pursued in this regard.
There appear to be large disparities between several studies on the half-life of ozone in water (from a few minutes to some hours). This may be attributed to the method of ozone generation, and the capability of forming microbubbles or nanobubbles, which tend to be more stable than macrobubbles. Also, the half-life is affected by the presence of organic contaminants which consume ozone. Thus, reports on the half-life of ozone in water may be accompanied by details of the ozone generation method utilised, the water quality and the measured properties. Similarly, accounts of ozone’s decomposition kinetics in air should be accompanied with relevant parameters (Fig. 1). Furthermore, the impact of ozone on the fibres/microfibres of different fabric types has not been effectively studied. Similar studies to those performed to examine the integrity of facemasks bands are important for different compositions of fabric fibres. This will enable the determination of the critical ozone dosage, particularly in light of extending garment longevity.
Our review has highlighted the absence of key experimental parameters in published studies. This has hindered insightful and accurate comparisons between separate studies, particularly with regard to the efficacy of ozone treatment. Thus, it is recommended that all parameters shown in Fig. 1 be reported in subsequent studies to provide readers with a better understanding of the author(s) methodologies and to guide the interpretation of their results. Furthermore, a database of suitable/appropriate biological indicators for ascertaining sterility after ozone application will go a long way towards ensuring the validity of ozone doses claimed to yield sterility.
Studies presenting ozone’s applicability from a microbiological perspective are numerous; more engineering-based studies on ozone’s application are required (particularly for the textile, medical equipment, pulp and paper and food industries). For example, detailed techno-economic analyses are required for the large-scale application of ozone in these industries. Depending on the intended application, the determination of the specific ozone dosage requirements is vital, and its importance cannot be overemphasized. Erroneous ozone application may lead to detrimental results compared to the intended outcome. Overdosing and underdosing should be avoided if the best benefits are to be observed. As shown in Fig. 15 , we provide a broad classification of the adopted concentration ranges for different applications in the reviewed literature. Although ozone concentration should not be solely considered as the key determinant of successful ozone deployment (as exposure duration is also critical), this classification will hopefully assist experimental endeavours geared at determining the full dosage (C & t) requirements.
Fig. 15.
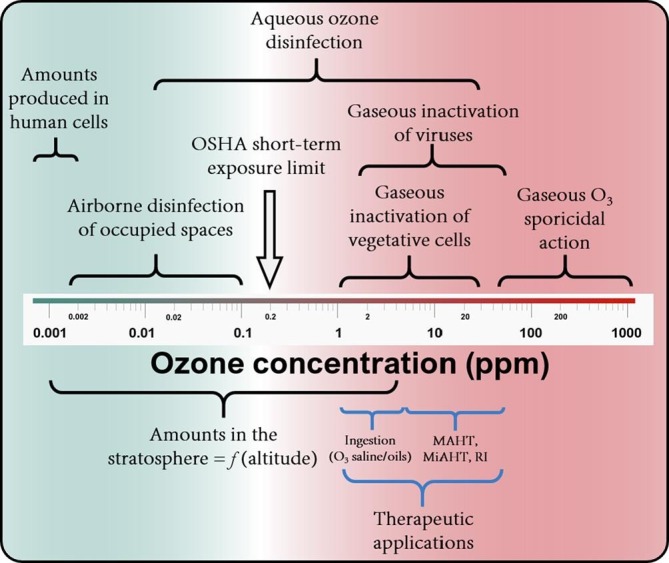
Summary of widely applied ozone concentrations for various applications at laboratory and industrial scale relative to naturally occurring ozone and Occupational Safety and Health Administration (OSHA) recommended exposure limits. It should be noted that drinking water disinfection would require rather small concentrations between 1 and 3 mg/L in a typical case; whereas wastewater disinfection (given its organic content) may require up to 10 mg/L. Higher doses than this are typically applied for industrial wastes when the oxidation of specific constituents is desired.
5. Conclusions
In response to the global need for improved decontamination techniques, this review has summarised key recent studies that demonstrate the effectiveness of ozone against a variety of microorganisms, organic and inorganic pollutants, and its applicability to a wide range of industries, including its therapeutic potential in the healthcare sector. Although several lab-scale studies outlining the impact of ozone inactivation on a variety of organisms and contaminants exist, there are few reports on the industrial deployment of this disinfection method, which expound on the practical lessons learned (particularly for the textile, medical equipment, pulp and paper and food industries). Mainly the water/wastewater treatment and aquaculture industries have been extensively studied ozonation at an industrial scale; thus, more implementation reports and insights on other industries are required. Generally, the large-scale application of ozone-related technology for disinfection is still limited by insufficient advances in the development of automated systems capable of cycle time reduction and rapid disinfection. Furthermore, the determination of the correct ozone dosage requirements (concentration × exposure time) and its controlled application during decontamination operations are essential if the benefits of this eco-friendly and cost-effective technique must be maximised.
CRediT authorship contribution statement
Emmanuel I. Epelle: Conceptualization, Methodology, Software, Data curation, Investigation, Visualization, Writing – original draft. Andrew Macfarlane: Writing – review & editing. Michael Cusack: Writing – review & editing. Anthony Burns: Writing – review & editing. Jude A. Okolie: Methodology, Writing – review & editing. William Mackay: Methodology, Writing – review & editing. Mostafa Rateb: Methodology, Writing – review & editing. Mohammed Yaseen: Conceptualization, Methodology, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors gratefully acknowledge the financial support of Innovate UK (KTP 12079), as well as ACS Clothing and the University of the West of Scotland, for providing the required software packages used in this review.
Data availability
Data will be made available on request.
References
- 1.Guzel-Seydim Z.B., Greene A.K., Seydim A.C. Use of ozone in the food industry. LWT - Food Sci. Technol. 2004;37 doi: 10.1016/j.lwt.2003.10.014. [DOI] [Google Scholar]
- 2.Powell A., Scolding J.W.S. Direct application of ozone in aquaculture systems. Rev. Aquac. 2018;10 doi: 10.1111/raq.12169. [DOI] [Google Scholar]
- 3.Joseph C.G., Farm Y.Y., Taufiq-Yap Y.H., Pang C.K., Nga J.L.H., Li Puma G. Ozonation treatment processes for the remediation of detergent wastewater: A comprehensive review. J. Environ. Chem. Eng. 2021:9. [Google Scholar]
- 4.Kim J.G., Yousef A.E., Dave S. Application of ozone for enhancing the microbiological safety and quality of foods: A review. J. Food Prot. 1999:62. doi: 10.4315/0362-028x-62.9.1071. [DOI] [PubMed] [Google Scholar]
- 5.Rice R.G., DeBrum M., Hook J., Cardis D., Tapp C. Microbiological benefits of ozone in laundering systems. Ozone Sci. Eng. 2009;31 doi: 10.1080/01919510903170419. [DOI] [Google Scholar]
- 6.Rosenblum J., Ge C., Bohrerova Z., Yousef A., Lee J. Ozonation as a clean technology for fresh produce industry and environment: Sanitizer efficiency and wastewater quality. J. Appl. Microbiol. 2012:113. doi: 10.1111/j.1365-2672.2012.05393.x. [DOI] [PubMed] [Google Scholar]
- 7.Rice R.G., Robson C.M., Miller G.W., Hill A.G. Uses of ozone in drinking water treatment. J. / Am. Water Work. Assoc. 1981;73 doi: 10.1002/j.1551-8833.1981.tb04637.x. [DOI] [Google Scholar]
- 8.T.C. Manley, S.J. Niegowski, Ozone Encyclopedia of Chemical Technology 1967.
- 9.Khadre M.A., Yousef A.E., Kim J. Microbiological aspects of ozone applications in food: a review. J. Food Sci. 2001;66:1242–1252. [Google Scholar]
- 10.Varga L., Szigeti J. Use of ozone in the dairy industry: A review. Int. J. Dairy Technol. 2016:69. [Google Scholar]
- 11.Wei C., Zhang F., Hu Y., Feng C., Wu H. Ozonation in water treatment: The generation, basic properties of ozone and its practical application. Rev. Chem. Eng. 2017;33 doi: 10.1515/revce-2016-0008. [DOI] [Google Scholar]
- 12.Oxidation Technologies LLC Fundementals of Ozone Solubility Available online: https://www.oxidationtech.com/ozone/solubility/fundementals-of-ozone-solubility.html (accessed on Jun 20, 2022).
- 13.Harding K.G., Ntimbani R., Mashwama P., Mokale R., Mothapo M., Gina N., Gina D. Decomposition of ozone in water. Chem. Technol. 2013;6–10 [Google Scholar]
- 14.Hirahara Y., Iwata K., Nakamuro K. Effect of citric acid on prolonging the half-life of dissolved ozone in water. Food Saf. 2019;7:90–94. doi: 10.14252/foodsafetyfscj.D-19-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Epelle E.I., Macfarlane A., Cusack M., Burns A., Amaeze N., Richardson K., Mackay W., Rateb M.E., Yaseen M. Stabilisation of Ozone in Water for Microbial Disinfection. Environments. 2022;9(4):1–19. doi: 10.3390/environments9040045. [DOI] [Google Scholar]
- 16.Batagoda J.H., Hewage S.D.A., Meegoda J.N. Nano-ozone bubbles for drinking water treatment. J. Environ. Eng. Sci. 2018;14 doi: 10.1680/jenes.18.00015. [DOI] [Google Scholar]
- 17.Aslam R., Alam M.S., Saeed P.A. Sanitization Potential of Ozone and Its Role in Postharvest Quality Management of Fruits and Vegetables. Food Eng. Rev. 2020:12. [Google Scholar]
- 18.Coyle E.E., Ormsbee L.E., Brion G.M. Peracetic Acid as an Alternative Disinfection Technology for Wet Weather Flows. Water Environ. Res. 2014;86 doi: 10.2175/106143014x13975035525663. [DOI] [PubMed] [Google Scholar]
- 19.UK HSE Ozone: Health hazards and control measures: Guidance Note EH38 Available online: https://www.hse.gov.uk/pubns/eh38.pdf (accessed on May 30, 2022).
- 20.Spencer W. Manual of Perioperative Care: An Essential Guide. 2013. Introduction to Decontamination and Sterilisation. [Google Scholar]
- 21.Lopes M.S., Ferreira J.R.F., da Silva K.B., de Oliveira Bacelar Simplício I., de Lima C.J., Fernandes A.B. Disinfection of corrugated tubing by ozone andultrasound in mechanically ventilated tracheostomized patients. J. Hosp. Infect. 2015;90 doi: 10.1016/j.jhin.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 22.Juwarkar C.S. Cleaning and sterilisation of anaesthetic equipment. Indian J. Anaesth. 2013:57. doi: 10.4103/0019-5049.120152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thill S.A., Spaltenstein M. Toward Efficient Low-Temperature Ozone Gas Sterilization of Medical Devices. Ozone Sci. Eng. 2020;42 doi: 10.1080/01919512.2019.1704217. [DOI] [Google Scholar]
- 24.Standardization, I.O. for ISO 11139: 2018 Sterilization of Health Care Products—Vocabulary of Terms Used in Sterilization and Related Equipment and Process Standards 2018.
- 25.EN, B.S. Sterilization of medical devices. Requirements for medical devices to be designated “STERILE”. Part 1: Requirements for terminally sterilized medical devices. 2001.
- 26.Rogers W.J. Sterilisation of Biomaterials and Medical Devices. 2012. Sterilisation techniques for polymers. [Google Scholar]
- 27.Spaulding E. Disinfection, Sterilization, and Preservation. 1968. Chemical disinfection of medical and surgical materials. [Google Scholar]
- 28.Rutala W.A., Weber D.J. Disinfection, sterilization, and antisepsis: An overview. Am. J. Infect. Control. 2019;47 doi: 10.1016/j.ajic.2019.01.018. [DOI] [PubMed] [Google Scholar]
- 29.Tomiyasu H., Fukutomi H., Gordon G. Kinetics and Mechanism of Ozone Decomposition in Basic Aqueous Solution. Inorg. Chem. 1985;24 doi: 10.1021/ic00213a018. [DOI] [Google Scholar]
- 30.Staehelin J., Buehler R.E., Hoigne J. Ozone decomposition in water studied by pulse radiolysis. 2. Hydroxyl and hydrogen tetroxide (HO4) as chain intermediates. Chem. Informationsd. 1985;16 doi: 10.1002/chin.198509020. [DOI] [Google Scholar]
- 31.Malik S.N., Ghosh P.C., Vaidya A.N., Mudliar S.N. Hybrid ozonation process for industrial wastewater treatment: Principles and applications: A review. J. Water Process Eng. 2020:35. [Google Scholar]
- 32.Batakliev T., Georgiev V., Anachkov M., Rakovsky S., Zaikov G.E. Ozone decomposition. Interdiscip. Toxicol. 2014:7. doi: 10.2478/intox-2014-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gardoni D., Vailati A., Canziani R. Decay of Ozone in Water: A Review. Ozone Sci. Eng. 2012:34. [Google Scholar]
- 34.Chapleski R.C., Zhang Y., Troya D., Morris J.R. Heterogeneous chemistry and reaction dynamics of the atmospheric oxidants, O 3, NO 3, and OH, on organic surfaces. Chem. Soc. Rev. 2016;45:3731–3746. doi: 10.1039/c5cs00375j. [DOI] [PubMed] [Google Scholar]
- 35.Patil S., Valdramidis V.P., Karatzas K.A.G., Cullen P.J., Bourke P. Assessing the microbial oxidative stress mechanism of ozone treatment through the responses of Escherichia coli mutants. J. Appl. Microbiol. 2011;111 doi: 10.1111/j.1365-2672.2011.05021.x. [DOI] [PubMed] [Google Scholar]
- 36.Epelle E.I., Macfarlane A., Cusack M., Burns A., Thissera B., Mackay W., Rateb M.E., Yaseen M. Bacterial and fungal disinfection via ozonation in air. J. Microbiol. Methods. 2022 doi: 10.1016/j.mimet.2022.106431. [DOI] [PubMed] [Google Scholar]
- 37.Santos L.M.C.dos., Silva E.S.da., Oliveira F.O., Rodrigues L.de.A.P., Neves P.R.F., Meira C.S., Moreira G.A.F., Lobato G.M., Nascimento C., Gerhardt M. Ozonized Water in Microbial Control: Analysis of the Stability, In Vitro Biocidal Potential, and Cytotoxicity. Biology (Basel) 2021;10:525. doi: 10.3390/biology10060525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thanomsub B., Anupunpisit V., Chanphetch S., Watcharachaipong T., Poonkhum R., Srisukonth C. Effects of ozone treatment on cell growth and ultrastructural changes in bacteria. J. Gen. Appl. Microbiol. 2002,:48. doi: 10.2323/jgam.48.193. [DOI] [PubMed] [Google Scholar]
- 39.Epelle E.I., Macfarlane A., Cusack M., Burns A., Amaeze N., Mackay W., Yaseen M. The Impact of Gaseous Ozone Penetration on the Disinfection Efficiency of Textile Materials. Ozone Sci. Eng. 2022:1–15. doi: 10.1080/01919512.2022.2066503. [DOI] [Google Scholar]
- 40.Mahfoudh A., Moisan M., Séguin J., Barbeau J., Kabouzi Y., Kroack D. Inactivation of vegetative and sporulated bacteria by dry gaseous ozone. Ozone Sci. Eng. 2010;32 doi: 10.1080/01919511003791971. [DOI] [Google Scholar]
- 41.Kang M.H., Pengkit A., Choi K., Jeon S.S., Choi H.W., Shin D.B., Choi E.H., Uhm H.S., Park G. Differential inactivation of fungal spores in water and on seeds by ozone and arc discharge plasma. PLoS One. 2015;10 doi: 10.1371/journal.pone.0139263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Epelle E.I., Emmerson A., Nekrasova M., Macfarlane A., Cusack M., Burns A., Mackay W., Yaseen M. Microbial Inactivation: Gaseous or Aqueous Ozonation? Ind. Eng. Chem. Res. 2022;61(27):9600–9610. doi: 10.1021/acs.iecr.2c01551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pagès M., Kleiber D., Violleau F. Ozonation of three different fungal conidia associated with apple disease: Importance of spore surface and membrane phospholipid oxidation. Food Sci. Nutr. 2020;8 doi: 10.1002/fsn3.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tseng C.C., Li C.S. Ozone for inactivation of aerosolized bacteriophages. Aerosol Sci. Technol. 2006;40 doi: 10.1080/02786820600796590. [DOI] [Google Scholar]
- 45.Ito K., Inoue S., Hiraku Y., Kawanishi S. Mechanism of site-specific DNA damage induced by ozone. Mutat. Res. - Genet. Toxicol. Environ. Mutagen. 2005;585 doi: 10.1016/j.mrgentox.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 46.Giese A.C., Christensen E. Effects of ozone on organisms. Physiol. Zool. 1954;27:101–115. [Google Scholar]
- 47.Scott D.B.M., Lesher E.C. Effect of ozone on survival and permeability of Escherichia coli. J. Bacteriol. 1963;85:567–576. doi: 10.1002/path.1700850242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Farooq S., Tizaoui C. A critical review on the inactivation of surface and airborne SARS-CoV-2 virus by ozone gas. Crit. Rev. Environ. Sci. Technol. 2022 [Google Scholar]
- 49.Murray B.K., Ohmine S., Tomer D.P., Jensen K.J., Johnson F.B., Kirsi J.J., Robison R.A., O’Neill K.L. Virion disruption by ozone-mediated reactive oxygen species. J. Virol. Methods. 2008;153 doi: 10.1016/j.jviromet.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 50.Tizaoui C. Ozone: A Potential Oxidant for COVID-19 Virus (SARS-CoV-2) Ozone Sci. Eng. 2020;42 doi: 10.1080/01919512.2020.1795614. [DOI] [Google Scholar]
- 51.Roy D., Wong P.K.Y., Engelbrecht R.S., Chian E.S.K. Mechanism of enteroviral inactivation by ozone. Appl. Environ. Microbiol. 1981;41 doi: 10.1128/aem.41.3.718-723.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Young S., Torrey J., Bachmann V., Kohn T. Relationship Between Inactivation and Genome Damage of Human Enteroviruses Upon Treatment by UV254, Free Chlorine, and Ozone. Food Environ. Virol. 2020;12 doi: 10.1007/s12560-019-09411-2. [DOI] [PubMed] [Google Scholar]
- 53.Allison K., Hook J., Cardis D., Rice R.G. Quantification of the bactericidal, fungicidal, and sporicidal efficacy of the JLA Ltd. ozone laundering system. Ozone Sci. Eng. 2009;31 doi: 10.1080/01919510903155816. [DOI] [Google Scholar]
- 54.Tizaoui C., Stanton R., Statkute E., Rubina A., Lester-Card E., Lewis A., Holliman P., Worsley D. Ozone for SARS-CoV-2 inactivation on surfaces and in liquid cell culture media. J. Hazard. Mater. 2022;428 doi: 10.1016/j.jhazmat.2022.128251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bhilwadikar T., Pounraj S., Manivannan S., Rastogi N.K., Negi P.S. Decontamination of Microorganisms and Pesticides from Fresh Fruits and Vegetables: A Comprehensive Review from Common Household Processes to Modern Techniques. Compr. Rev. Food Sci. Food Saf. 2019 doi: 10.1111/1541-4337.12453. [DOI] [PubMed] [Google Scholar]
- 56.Magbanua B., Savant G., Truax D. Combined ozone and ultraviolet inactivation of Escherichia coli. J. Environ. Sci. Heal. - Part A Toxic/Hazardous Subst. Environ. Eng. 2006;41 doi: 10.1080/10934520600620279. [DOI] [PubMed] [Google Scholar]
- 57.Kıdak R., Doğan Ş. Medium-high frequency ultrasound and ozone based advanced oxidation for amoxicillin removal in water. Ultrason. Sonochem. 2018;40 doi: 10.1016/j.ultsonch.2017.01.033. [DOI] [PubMed] [Google Scholar]
- 58.Balci N., Ömeroğullari Z., Kut D., Eren H.A. Effects Of Plasma and Ozone Treatments on Tensile and Whiteness Properties Of 100% Silk. Uludağ Univ. J. Fac. Eng. 2015;20 doi: 10.17482/uujfe.33090. [DOI] [Google Scholar]
- 59.M. Eriksson, Ozone chemistry in aqueous solution: ozone decomposition and stabilisation 2005.
- 60.J.D. Miller, Apparatus for sterilizing mail and textile articles 2011, US20110283661A1.
- 61.Seridou P., Kalogerakis N. Disinfection applications of ozone micro- And nanobubbles. Environ. Sci. Nano. 2021;8 [Google Scholar]
- 62.Beltran F.J. Ozone Reaction Kinetics for Water and. Wastewater Systems. 2003 [Google Scholar]
- 63.Von Gunten U. Oxidation Processes in Water Treatment: Are We on Track? Environ. Sci. Technol. 2018;52 doi: 10.1021/acs.est.8b00586. [DOI] [PubMed] [Google Scholar]
- 64.Lee Y., Von Gunten U. Advances in predicting organic contaminant abatement during ozonation of municipal wastewater effluent: Reaction kinetics, transformation products, and changes of biological effects. Environ. Sci. Water Res. Technol. 2016;2 [Google Scholar]
- 65.Rosenfeldt E.J., Linden K.G., Canonica S., Von Gunten U. Comparison of the efficiency of OH radical formation during ozonation and the advanced oxidation processes O3/H2O2 and UV/H2O2. Water Res. 2006;40:3695–3704. doi: 10.1016/j.watres.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 66.Wen G., Liang Z., Xu X., Cao R., Wan Q., Ji G., Lin W., Wang J., Yang J., Huang T. Inactivation of fungal spores in water using ozone: Kinetics, influencing factors and mechanisms. Water Res. 2020;185 doi: 10.1016/j.watres.2020.116218. [DOI] [PubMed] [Google Scholar]
- 67.Ding W., Jin W., Cao S., Zhou X., Wang C., Jiang Q., Huang H., Tu R., Han S.F., Wang Q. Ozone disinfection of chlorine-resistant bacteria in drinking water. Water Res. 2019;160 doi: 10.1016/j.watres.2019.05.014. [DOI] [PubMed] [Google Scholar]
- 68.Ozone Solutions Ozone vs. Chlorine for Water Disinfection Available online: https://ozonesolutions.com/blog/ozone-vs-chlorine/ (accessed on Oct 14, 2022).
- 69.Collivignarelli M.C., Abbà A., Benigna I., Sorlini S., Torretta V. Overview of the main disinfection processes for wastewater and drinking water treatment plants. Sustain. 2018;10 doi: 10.3390/su10010086. [DOI] [Google Scholar]
- 70.Wang C., Xi J.Y., Hu H.Y., Yao Y. Stimulative effects of ozone on a biofilter treating gaseous chlorobenzene. Environ. Sci. Technol. 2009;43 doi: 10.1021/es9019035. [DOI] [PubMed] [Google Scholar]
- 71.Wang Y.C., Wang C., Han M.F., Tong Z., Lin Y.T., Hu X.R., Deng J.G., Hsi H.C. Inhibiting effect of quorum quenching on biomass accumulation: A clogging control strategy in gas biofilters. Chem. Eng. J. 2022;432 doi: 10.1016/j.cej.2021.134313. [DOI] [Google Scholar]
- 72.Zanacic E., Stavrinides J., McMartin D.W. Field-analysis of potable water quality and ozone efficiency in ozone-assisted biological filtration systems for surface water treatment. Water Res. 2016;104 doi: 10.1016/j.watres.2016.08.043. [DOI] [PubMed] [Google Scholar]
- 73.Pumkaew M., Taweephitakthai T., Satanwat P., Yocawibun P., Chumtong P., Pungrasmi W., Powtongsook S. Use of ozone for Vibrio parahaemolyticus inactivation alongside nitrification biofilter treatment in shrimp-rearing recirculating aquaculture system. J. Water Process Eng. 2021;44 doi: 10.1016/j.jwpe.2021.102396. [DOI] [Google Scholar]
- 74.Méité L., Fotsing M., Barbeau B. Efficacy of Ozone to Reduce Chlorinated Disinfection By-Products in Quebec (Canada) Drinking Water Facilities. Ozone Sci. Eng. 2015;37 doi: 10.1080/01919512.2014.946592. [DOI] [Google Scholar]
- 75.Zhang T., Tao Y.Z., Yang H.W., Chen Z., Wang X.M., Xie Y.F. Study on the removal of aesthetic indicators by ozone during advanced treatment of water reuse. J. Water Process Eng. 2020;36 doi: 10.1016/j.jwpe.2020.101381. [DOI] [Google Scholar]
- 76.Lim S., Shi J.L., von Gunten U., McCurry D.L. Ozonation of organic compounds in water and wastewater: A critical review. Water Res. 2022;213 doi: 10.1016/j.watres.2022.118053. [DOI] [PubMed] [Google Scholar]
- 77.Benitez F.J., Acero J.L., Garcia-Reyes J.F., Real F.J., Roldan G., Rodriguez E., Molina-Díaz A. Determination of the reaction rate constants and decomposition mechanisms of ozone with two model emerging contaminants: DEET and nortriptyline. Ind. Eng. Chem. Res. 2013;52 doi: 10.1021/ie402916u. [DOI] [Google Scholar]
- 78.Manasfi T. Ozonation in drinking water treatment: an overview of general and practical aspects, mechanisms, kinetics, and byproduct formation. In. Comprehensive Anal. Chem. 2021;92 [Google Scholar]
- 79.Rekhate C.V., Srivastava J.K. Recent advances in ozone-based advanced oxidation processes for treatment of wastewater- A review. Chem. Eng. J. Adv. 2020;3 doi: 10.1016/j.ceja.2020.100031. [DOI] [Google Scholar]
- 80.Gupta A., Thakur I.S. Treatment of Organic Recalcitrant Contaminants in Wastewater. Biol. Wastewater Treatment Resour. Recov. 2017 [Google Scholar]
- 81.de Wilt A., van Gijn K., Verhoek T., Vergnes A., Hoek M., Rijnaarts H., Langenhoff A. Enhanced pharmaceutical removal from water in a three step bio-ozone-bio process. Water Res. 2018;138 doi: 10.1016/j.watres.2018.03.028. [DOI] [PubMed] [Google Scholar]
- 82.Szabová P., Hencelová K., Sameliaková Z., Marcová T., Staňová A.V., Grabicová K., Bodík I. Ozonation: effective way for removal of pharmaceuticals from wastewater. Monatshefte fur Chemie. 2020;151 doi: 10.1007/s00706-020-02600-x. [DOI] [Google Scholar]
- 83.Paucar N.E., Kim I., Tanaka H., Sato C. Ozone treatment process for the removal of pharmaceuticals and personal care products in wastewater. Ozone Sci. Eng. 2019;41 doi: 10.1080/01919512.2018.1482456. [DOI] [Google Scholar]
- 84.Lester Y., Mamane H., Zucker I., Avisar D. Treating wastewater from a pharmaceutical formulation facility by biological process and ozone. Water Res. 2013;47 doi: 10.1016/j.watres.2013.04.059. [DOI] [PubMed] [Google Scholar]
- 85.Dong S., Massalha N., Plewa M.J., Nguyen T.H. The impact of disinfection Ct values on cytotoxicity of agricultural wastewaters: Ozonation vs. chlorination. Water Res. 2018;144 doi: 10.1016/j.watres.2018.07.065. [DOI] [PubMed] [Google Scholar]
- 86.Wang J., Chen H. Catalytic ozonation for water and wastewater treatment: Recent advances and perspective. Sci. Total Environ. 2020:704. doi: 10.1016/j.scitotenv.2019.135249. [DOI] [PubMed] [Google Scholar]
- 87.Wu B., Majumdar R.D., Lysak D.H., Ghosh Biswas R., Tabatabaei-Anaraki M., Jenne A., You X., Soong R., Lane D., Helm P.A., et al. Towards real-time kinetic monitoring of wastewater treatment: A case study of sunlight and ozone treatment of unconcentrated wastewater using flow NMR. Chem. Eng. J. 2021;405 doi: 10.1016/j.cej.2020.126696. [DOI] [Google Scholar]
- 88.Li K., Xu L., Zhang Y., Cao A., Wang Y., Huang H., Wang J. A novel electro-catalytic membrane contactor for improving the efficiency of ozone on wastewater treatment. Appl. Catal. B Environ. 2019;249 doi: 10.1016/j.apcatb.2019.03.015. [DOI] [Google Scholar]
- 89.Cardoso J.C., Bessegato G.G., Boldrin Zanoni M.V. Efficiency comparison of ozonation, photolysis, photocatalysis and photoelectrocatalysis methods in real textile wastewater decolorization. Water Res. 2016;98 doi: 10.1016/j.watres.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 90.Mehrjouei M., Müller S., Möller D. A review on photocatalytic ozonation used for the treatment of water and wastewater. Chem. Eng. J. 2015;263 [Google Scholar]
- 91.Ferre-Aracil J., Valcárcel Y., Negreira N., de Alda M.L., Barceló D., Cardona S.C., Navarro-Laboulais J. Ozonation of hospital raw wastewaters for cytostatic compounds removal. Kinetic modelling and economic assessment of the process. Sci. Total Environ. 2016;556 doi: 10.1016/j.scitotenv.2016.02.202. [DOI] [PubMed] [Google Scholar]
- 92.Khan A.H., Khan N.A., Ahmed S., Dhingra A., Singh C.P., Khan S.U., Mohammadi A.A., Changani F., Yousefi M., Alam S., et al. Application of advanced oxidation processes followed by different treatment technologies for hospital wastewater treatment. J. Clean. Prod. 2020;269 doi: 10.1016/j.jclepro.2020.122411. [DOI] [Google Scholar]
- 93.Vatankhah H., Riley S.M., Murray C., Quiñones O., Steirer K.X., Dickenson E.R.V., Bellona C. Simultaneous ozone and granular activated carbon for advanced treatment of micropollutants in municipal wastewater effluent. Chemosphere. 2019;234 doi: 10.1016/j.chemosphere.2019.06.082. [DOI] [PubMed] [Google Scholar]
- 94.Beltrán F.J., Masa F.J., Pocostales J.P. A comparison between catalytic ozonation and activated carbon adsorption/ozone-regeneration processes for wastewater treatment. Appl. Catal. B Environ. 2009;92:393–400. [Google Scholar]
- 95.Beltrán F.J., Rey A., Gimeno O. The role of catalytic ozonation processes on the elimination of dbps and their precursors in drinking water treatment. Catalysts. 2021;11 [Google Scholar]
- 96.Rivera-Utrilla J., Bautista-Toledo M.I., Sánchez-Polo M., Méndez-Díaz J.D. Removal of surfactant dodecylbenzenesulfonate by consecutive use of ozonation and biodegradation. Eng. Life Sci. 2012;12:113–116. [Google Scholar]
- 97.Wang X.D., Lv Y., Li M.M., Liu H.Y. Removal of nonylphenol from water by ozone. Proc. Adv. Mater. Res. 2014;859 [Google Scholar]
- 98.Kasiri M.B., Modirshahla N., Mansouri H. Decolorization of organic dye solution by ozonation; Optimization with response surface methodology. Int. J. Ind. Chem. 2013;4 doi: 10.1186/2228-5547-4-3. [DOI] [Google Scholar]
- 99.Sevimli M.F., Sarikaya H.Z. Ozone treatment of textile effluents and dyes: Effect of applied ozone dose, pH and dye concentration. J. Chem. Technol. Biotechnol. 2002;77 doi: 10.1002/jctb.644. [DOI] [Google Scholar]
- 100.Bilińska L., Blus K., Gmurek M., Ledakowicz S. Coupling of electrocoagulation and ozone treatment for textile wastewater reuse. Chem. Eng. J. 2019;358 doi: 10.1016/j.cej.2018.10.093. [DOI] [Google Scholar]
- 101.Huang Y., Luo M., Xu Z., Zhang D., Li L. Catalytic ozonation of organic contaminants in petrochemical wastewater with iron-nickel foam as catalyst. Sep. Purif. Technol. 2019,:211. doi: 10.1016/j.seppur.2018.09.080. [DOI] [Google Scholar]
- 102.Kalyanaraman C., Kameswari K.S.B., Rao J.R. Studies on enhancing the biodegradation of tannins by ozonation and Fenton’s oxidation process. J. Ind. Eng. Chem. 2015;25 doi: 10.1016/j.jiec.2014.11.012. [DOI] [Google Scholar]
- 103.Hostachy J.-C., van Wyk B., Metais A. The Use of Ozone in the Pulp and Paper Industry. PaperASia. 2014;30:22–28. [Google Scholar]
- 104.XYLEM Ozone in the Pulp & Paper Industry ECONOMICAL & ECO-FRIENDLY; 2019.
- 105.Gonçalves A.A., Gagnon G.A. Ozone application in recirculating aquaculture system: An overview. Ozone Sci. Eng. 2011;33 doi: 10.1080/01919512.2011.604595. [DOI] [Google Scholar]
- 106.Sharrer M.J., Summerfelt S.T. Ozonation followed by ultraviolet irradiation provides effective bacteria inactivation in a freshwater recirculating system. Aquac. Eng. 2007;37 doi: 10.1016/j.aquaeng.2007.05.001. [DOI] [Google Scholar]
- 107.Hernández K.L., Sedas V.P., Dehaibes S.R., Valencia V.S., Mozo I.R., Herrera D.M., Primo A.F., Serrano R.U. Improved microbial safety of direct ozone-depurated shellstock eastern oysters (crassostrea virginica) by superchilled storage. Front. Microbiol. 2018;9 doi: 10.3389/fmicb.2018.02802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Figueiras Guilherme M., de Jesus Gregersen K.J., Pedersen L.F. Effects of foam fractionation and chemical disinfection on the removal of different microalgae cultures. Aquac. Res. 2020;51 doi: 10.1111/are.14663. [DOI] [Google Scholar]
- 109.Suryaningsih W., Supriyono S., Hariono B., Kurnianto M.F. Improving the Quality of Smoked Shark Meat with Ozone Water Technique. Proc. IOP Conf. Series: Earth Environ. Sci. 2020;411 [Google Scholar]
- 110.de Jesus Gregersen K.J., Pedersen L.F., Pedersen P.B., Syropoulou E., Dalsgaard J. Foam fractionation and ozonation in freshwater recirculation aquaculture systems. Aquac. Eng. 2021;95 doi: 10.1016/j.aquaeng.2021.102195. [DOI] [Google Scholar]
- 111.Dien L.T., Linh N.V., Mai T.T., Senapin S., St-Hilaire S., Rodkhum C., Dong H.T. Impacts of oxygen and ozone nanobubbles on bacteriophage in aquaculture system. Aquaculture. 2022;551 doi: 10.1016/j.aquaculture.2022.737894. [DOI] [Google Scholar]
- 112.Thanh Dien L., Linh N.V., Sangpo P., Senapin S., St-Hilaire S., Rodkhum C., Dong H.T. Ozone nanobubble treatments improve survivability of Nile tilapia (Oreochromis niloticus) challenged with a pathogenic multi-drug-resistant Aeromonas hydrophila. J. Fish Dis. 2021;44 doi: 10.1111/jfd.13451. [DOI] [PubMed] [Google Scholar]
- 113.Muller P.Y., Milton M.N. The determination and interpretation of the therapeutic index in drug development. Nat. Rev. Drug Discov. 2012;11 doi: 10.1038/nrd3801. [DOI] [PubMed] [Google Scholar]
- 114.Wedemeyer G.A., Nelson N.C., Yasutake W.T. Potentials and limits for the use of ozone as a fish disease control agent. Ozone Sci. Eng. 1979;1 doi: 10.1080/01919512.1979.10684566. [DOI] [Google Scholar]
- 115.Summerfelt S.T., Sharrer M.J., Tsukuda S.M., Gearheart M. Process requirements for achieving full-flow disinfection of recirculating water using ozonation and UV irradiation. Aquac. Eng. 2009;40 doi: 10.1016/j.aquaeng.2008.10.002. [DOI] [Google Scholar]
- 116.Petronijević M., Agbaba J., Ražić S., Molnar Jazić J., Tubić A., Watson M., Dalmacija B. Fate of bromine-containing disinfection by-products precursors during ozone and ultraviolet-based advanced oxidation processes. Int. J. Environ. Sci. Technol. 2019;16 doi: 10.1007/s13762-018-1652-8. [DOI] [Google Scholar]
- 117.Dorji P., Choi J., Kim D.I., Phuntsho S., Hong S., Shon H.K. Membrane capacitive deionisation as an alternative to the 2nd pass for seawater reverse osmosis desalination plant for bromide removal. Desalination. 2018;433 doi: 10.1016/j.desal.2018.01.020. [DOI] [Google Scholar]
- 118.Teitge F., Peppler C., Steinhagen D., Jung-Schroers V. Water disinfection by ozonation has advantages over UV irradiation in a brackish water recirculation aquaculture system for Pacific white shrimp (Litopenaeus vannamei) J. Fish Dis. 2020;43 doi: 10.1111/jfd.13238. [DOI] [PubMed] [Google Scholar]
- 119.Davidson J., Summerfelt S., Espmark Å.M.O., Mota V.C., Marancik D., Earley R.L., Snead A., Good C. Effects of ozone on post-smolt Atlantic salmon (Salmo salar) performance, health, and maturation in freshwater recirculation aquaculture systems. Aquaculture. 2021;533 doi: 10.1016/j.aquaculture.2020.736208. [DOI] [Google Scholar]
- 120.Spiliotopoulou A., Rojas-Tirado P., Chhetri R.K., Kaarsholm K.M.S., Martin R., Pedersen P.B., Pedersen L.F., Andersen H.R. Ozonation control and effects of ozone on water quality in recirculating aquaculture systems. Water Res. 2018;133 doi: 10.1016/j.watres.2018.01.032. [DOI] [PubMed] [Google Scholar]
- 121.Botondi R., Barone M., Grasso C. A review into the effectiveness of ozone technology for improving the safety and preserving the quality of fresh-cut fruits and vegetables. Foods. 2021;10:748. doi: 10.3390/foods10040748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dosti B., Guzel-Seydim Z., Greene A.K. Effectiveness of ozone, heat and chlorine for destroying common food spoilage bacteria in synthetic media and biofilms. Int. J. Dairy Technol. 2005;58 doi: 10.1111/j.1471-0307.2005.00176.x. [DOI] [Google Scholar]
- 123.Regulation, C. Council Regulation (EEC) No. 2092/91 of 24 June 1991 on organic production of agricultural products and indications referring thereto on agricultural products and foodstuffs. Off. J. L, 1991, 198, 7.
- 124.Sivaranjani S., Prasath V.A., Pandiselvam R., Kothakota A., Mousavi Khaneghah A. Recent advances in applications of ozone in the cereal industry. LWT. 2021;146 doi: 10.1016/j.lwt.2021.111412. [DOI] [Google Scholar]
- 125.Obadi M., Zhu K.X., Peng W., Sulieman A.A., Mohammed K., Zhou H.M. Effects of ozone treatment on the physicochemical and functional properties of whole grain flour. J. Cereal Sci. 2018;81 doi: 10.1016/j.jcs.2018.04.008. [DOI] [Google Scholar]
- 126.Munhõs M.C., Navarro R.S., Nunez S.C., Kozusny-Andreani D.I., Baptista A. Reduction of pseudomonas inoculated into whole milk and skin milk by ozonation. Proc. IFMBE Proc. 2019;70 [Google Scholar]
- 127.Younis F., Fayed A., Elbatawy O., Elsisi A. A comparison between Ozonation and thermal process in relation to cow’s milk attributes with emphasis on pathogens. Arab. Univ. J. Agric. Sci. 2020 doi: 10.21608/ajs.2020.20456.1131. [DOI] [Google Scholar]
- 128.J.Al. Azhar, Z.H. ALmosowy, Study the Effect of Ozone Gas in Milk Treatment on Chemical and Microbial Properties of Soft Cheese. Indian J. Ecol. 2020, 47, 1–5.
- 129.Cantalejo M.J., Zouaghi F., Pérez-Arnedo I. Combined effects of ozone and freeze-drying on the shelf-life of Broiler chicken meat. LWT. 2016;68 doi: 10.1016/j.lwt.2015.12.058. [DOI] [Google Scholar]
- 130.Ribeiro D.F., Faroni L.R.D.A., Pimentel M.A.G., Prates L.H.F., Heleno F.F., De Alencar E.R. Ozone as a Fungicidal and Detoxifying Agent to Maize Contaminated with Fumonisins. Ozone Sci. Eng. 2022;44 doi: 10.1080/01919512.2021.1924616. [DOI] [Google Scholar]
- 131.Shynkaryk M.V., Pyatkovskyy T., Mohamed H.M., Yousef A.E., Sastry S.K. Physics of fresh produce safety: Role of diffusion and tissue reaction in sanitization of leafy green vegetables with liquid and gaseous ozone-based sanitizers. J. Food Prot. 2015;78 doi: 10.4315/0362-028X.JFP-15-290. [DOI] [PubMed] [Google Scholar]
- 132.Karaca H., Velioglu Y.S. Effects of ozone treatments on microbial quality and some chemical properties of lettuce, spinach, and parsley. Postharvest Biol. Technol. 2014;88 doi: 10.1016/j.postharvbio.2013.09.003. [DOI] [Google Scholar]
- 133.L.P.de Souza, L.R.D.A. Faroni, F.F. Heleno, P.R. Cecon, T.D.C. Gonçalves, G.J.da Silva, L.H.F. Prates, Effects of ozone treatment on postharvest carrot quality. LWT - Food Sci. Technol. 2018, 90, doi:10.1016/j.lwt.2017.11.057.
- 134.Muhlisin M., Utama D.T., Lee J.H., Choi J.H., Lee S.K. Effects of gaseous ozone exposure on bacterial counts and oxidative properties in chicken and duck breast meat. Korean J. Food Sci. Anim. Resour. 2016;36 doi: 10.5851/kosfa.2016.36.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jurado-Alameda E., Altmajer-Vaz D., García-Román M., Jiménez-Pérez J.L. Study of heat-denatured whey protein removal from stainless steel surfaces in clean-in-place systems. Int. Dairy J. 2014;38 doi: 10.1016/j.idairyj.2014.01.006. [DOI] [Google Scholar]
- 136.Sarron E., Gadonna-Widehem P., Aussenac T. Ozone treatments for preserving fresh vegetables quality. A critical review. Foods. 2021:10. doi: 10.3390/foods10030605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pandiselvam R., Subhashini S., Banuu Priya E.P., Kothakota A., Ramesh S.V., Shahir S. Ozone based food preservation: a promising green technology for enhanced food safety. Ozone Sci. Eng. 2019:41. [Google Scholar]
- 138.Khanashyam A.C., Shanker M.A., Kothakota A., Mahanti N.K., Pandiselvam R. Ozone Applications in Milk and Meat Industry. Ozone Sci. Eng. 2022:44. [Google Scholar]
- 139.Brodowska A.J., Nowak A., Śmigielski K. Ozone in the food industry: Principles of ozone treatment, mechanisms of action, and applications: An overview. Crit. Rev. Food Sci. Nutr. 2018;58 doi: 10.1080/10408398.2017.1308313. [DOI] [PubMed] [Google Scholar]
- 140.FDA FDA Innovation Challenge 1: Identify New Sterilization Methods and Technologies Available online: https://www.fda.gov/medical-devices/general-hospital-devices-and-supplies/fda-innovation-challenge-1-identify-new-sterilization-methods-and-technologies (accessed on May 21, 2022).
- 141.de Souza Botelho-Almeida T., Lourenço F.R., Kikuchi I.S., Awasthi R., Dua K., Andreoli Pinto T.de J. Evaluating the Potential, Applicability, and Effectiveness of Ozone Sterilization Process for Medical Devices. J. Pharm. Innov. 2018;13 doi: 10.1007/s12247-017-9308-7. [DOI] [Google Scholar]
- 142.McEvoy B., Rowan N.J. Terminal sterilization of medical devices using vaporized hydrogen peroxide: a review of current methods and emerging opportunities. J. Appl. Microbiol. 2019;127 doi: 10.1111/jam.14412. [DOI] [PubMed] [Google Scholar]
- 143.I. Ljungberg, Evaluation of an Ozone Cabinet for Disinfecting Medical Equipment, 2018.
- 144.Zoutman D., Shannon M., Mandel A. Effectiveness of a novel ozone-based system for the rapid high-level disinfection of health care spaces and surfaces. Am. J. Infect. Control. 2011;39 doi: 10.1016/j.ajic.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 145.Karim N., Afroj S., Lloyd K., Oaten L.C., Andreeva D.V., Carr C., Farmery A.D., Kim I.D., Novoselov K.S. Sustainable personal protective clothing for healthcare applications: A review. ACS Nano. 2020;14 doi: 10.1021/acsnano.0c05537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.E.L. Blanchard, J.D. Lawrence, J.A. Noble, M. Xu, T. Joo, N.L. Ng, B.E. Schmidt, P.J. Santangelo, M.G. Finn, Enveloped Virus Inactivation on Personal Protective Equipment by Exposure to Ozone. medRxiv, 2020.
- 147.Rubio-Romero J.C., Pardo-Ferreira M.del C., Torrecilla-García J.A., Calero-Castro S. Disposable masks: Disinfection and sterilization for reuse, and non-certified manufacturing, in the face of shortages during the COVID-19 pandemic. Saf. Sci. 2020;129 doi: 10.1016/j.ssci.2020.104830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.E.P. Manning, M.D. Stephens, S. Patel, S. Dufresne, B. Silver, P. Gerbarg, Z. Gerbarg, C. Dela Cruz, L. Sharma, Disinfection of N95 Respirators with Ozone. medRxiv, 2020. [DOI] [PMC free article] [PubMed]
- 149.Lee J., Bong C., Lim W., Bae P.K., Abafogi A.T., Baek S.H., Shin Y.B., Bak M.S., Park S. Fast and Easy Disinfection of Coronavirus-Contaminated Face Masks Using Ozone Gas Produced by a Dielectric Barrier Discharge Plasma Generator. Environ. Sci. Technol. Lett. 2021;8 doi: 10.1021/acs.estlett.1c00089. [DOI] [PubMed] [Google Scholar]
- 150.Peters A., Lotfinejad N., Palomo R., Zingg W., Parneix P., Ney H., Pittet D. Decontaminating N95/FFP2 masks for reuse during the COVID-19 epidemic: a systematic review. Antimicrob. Resist. Infect. Control. 2021;10 doi: 10.1186/s13756-021-00993-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Dennis R., Cashion A., Emanuel S., Hubbard D. Ozone Gas: Scientific Justification and Practical Guidelines for Improvised Disinfection using Consumer-Grade Ozone Generators and Plastic Storage Boxes. J. Sci. Med. 2020;2 doi: 10.37714/josam.v2i1.35. [DOI] [Google Scholar]
- 152.A. Körlü, Use of Ozone in the Textile Industry. In Textile Industry and Environment; 2019.
- 153.Ben Fraj A., Jaouachi B. Effects of ozone treatment on denim garment properties. Color. Technol. 2021;137 doi: 10.1111/cote.12568. [DOI] [Google Scholar]
- 154.Powar A.S., Perwuelz A., Behary N., Hoang L., Aussenac T. Application of ozone treatment for the decolorization of the reactive-dyed fabrics in a pilot-scale process-optimization through response surface methodology. Sustain. 2020;12 doi: 10.3390/su12020471. [DOI] [Google Scholar]
- 155.He Z., Li M., Zuo D., Yi C. The effect of denim color fading ozonation on yarns. Ozone Sci. Eng. 2018;40 doi: 10.1080/01919512.2018.1435259. [DOI] [Google Scholar]
- 156.Elnagar K., Abou Elmaaty T., Raouf S. Dyeing of Polyester and Polyamide Synthetic Fabrics with Natural Dyes Using Ecofriendly Technique. J. Text. 2014;2014 doi: 10.1155/2014/363079. [DOI] [Google Scholar]
- 157.Atav R., Yurdakul A. Effect of the ozonation process on the dyeability of mohair fibres. Color. Technol. 2011;127 doi: 10.1111/j.1478-4408.2011.00293.x. [DOI] [Google Scholar]
- 158.Perincek S., Bahtiyari M.Ä., Körlü A.E., Duran K. Effect of ozone and ultrasound on the fiber properties of Angora rabbit. J. Appl. Polym. Sci. 2011;120 doi: 10.1002/app.28779. [DOI] [Google Scholar]
- 159.Perincek S., Bahtiyari M.I., Körlü A.E., Duran K. Ozone treatment of Angora rabbit fiber. J. Clean. Prod. 2008;16 doi: 10.1016/j.jclepro.2008.01.005. [DOI] [Google Scholar]
- 160.Ben Hmida S., Ladhari N. Study of Parameters Affecting Dry and Wet Ozone Bleaching of Denim Fabric. Ozone Sci. Eng. 2016;38 doi: 10.1080/01919512.2015.1113380. [DOI] [Google Scholar]
- 161.Bahtiyari M.İ., Benli H. Ozone bleaching of cotton fabrics with the aid of ultrasonic humidifier. Cellulose. 2016;23 doi: 10.1007/s10570-016-0978-y. [DOI] [Google Scholar]
- 162.Muthu S.S. Springer; 2018. Sustainable innovations in textile chemical processes. ISBN 9811084912. [Google Scholar]
- 163.Turhan Y. The Effects of Ozone Bleaching and Ozone Desizing Method on Whiteness and Water Absorption of 100% Cotton Terry Fabrics. Int. J. Mater. Sci. Appl. 2018;7 doi: 10.11648/j.ijmsa.20180703.13. [DOI] [Google Scholar]
- 164.Cardis D., Tapp C., DeBrum M., Rice R.G. Ozone in the laundry industry - Practical experiences in the United Kingdom. Ozone Sci. Eng. 2007;29 doi: 10.1080/01919510601186048. [DOI] [Google Scholar]
- 165.Rice R.G., DeBrum M., Cardis D., Tapp C. The ozone laundry handbook: A comprehensive guide for the proper application of ozone in the commercial laundry industry. Ozone Sci. Eng. 2009;31 doi: 10.1080/01919510903091318. [DOI] [Google Scholar]
- 166.Neral B. Quality of the household ozone laundering. Ind. Textila. 2018;69:304–309. [Google Scholar]
- 167.Dubuis M.E., Dumont-Leblond N., Laliberté C., Veillette M., Turgeon N., Jean J., Duchaine C. Ozone efficacy for the control of airborne viruses: Bacteriophage and norovirus models. PLoS One. 2020;15 doi: 10.1371/journal.pone.0231164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Stolle L., Nalamasu R., Rodenbeck R., Davidson K., Smarelli C., Rosko S., Wales J., LeQuang J.A.K., Pergolizzi J. Ozone: A Novel Sterilizer for Personal Protective Equipment. Cureus. 2021 doi: 10.7759/cureus.18228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Franke G., Knobling B., Brill F.H., Becker B., Klupp E.M., Belmar Campos C., Pfefferle S., Lütgehetmann M., Knobloch J.K. An automated room disinfection system using ozone is highly active against surrogates for SARS-CoV-2. J. Hosp. Infect. 2021;112 doi: 10.1016/j.jhin.2021.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Uppal T., Khazaieli A., Snijders A.M., Verma S.C. Inactivation of human coronavirus by fathhome’s dry sanitizer device: rapid and eco-friendly ozone-based disinfection of sars-cov-2. Pathogens. 2021;10 doi: 10.3390/pathogens10030339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zhang J.M., Zheng C.Y., Xiao G.F., Zhou Y.Q., Gao R. Examination of the efficacy of ozone solution disinfectant in in-activating SARS virus. Chin. J. Disinfect. 2004;1:66–70. [Google Scholar]
- 172.Hudson J.B., Sharma M., Vimalanathan S. Development of a practical method for using ozone gas as a virus decontaminating agent. Ozone Sci. Eng. 2009;31:216–223. [Google Scholar]
- 173.Muzhi Z. Ozone: A powerful weapon to combat COVID-19 outbreak. China. Org. cn. 2020;26:2002–2020. [Google Scholar]
- 174.Cristiano L. Could ozone be an effective disinfection measure against the novel coronavirus (SARS-CoV-2)? J. Prev. Med. Hyg. 2020:61. doi: 10.15167/2421-4248/jpmh2020.61.3.1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Yano H., Nakano R., Suzuki Y., Nakano A., Kasahara K., Hosoi H. Inactivation of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by gaseous ozone treatment. J. Hosp. Infect. 2020;106 doi: 10.1016/j.jhin.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Martins R.B., Castro I.A., Pontelli M., Souza J.P., Lima T.M., Melo S.R., Siqueira J.P.Z., Caetano M.H., Arruda E., de Almeida M.T.G. SARS-CoV-2 Inactivation by Ozonated Water: A Preliminary Alternative for Environmental Disinfection. Ozone Sci. Eng. 2021;43 doi: 10.1080/01919512.2020.1842998. [DOI] [Google Scholar]
- 177.Westover C., Rahmatulloev S., Danko D., Afshinnekoo E., O’Hara N.B., Ounit R., Bezdan D., Mason C.E. Ozone treatment for elimination of bacteria and SARS-CoV-2 for medical environments. BioRxiv. 2020 [Google Scholar]
- 178.Wolfgruber S., Loibner M., Puff M., Melischnig A., Zatloukal K. SARS-CoV2 neutralizing activity of ozone on porous and non-porous materials. N. Biotechnol. 2022,:66. doi: 10.1016/j.nbt.2021.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lin Y.C., Juan H.C., Cheng Y.C. Ozone exposure in the culture medium inhibits enterovirus 71 virus replication and modulates cytokine production in rhabdomyosarcoma cells. Antiviral Res. 2007;76 doi: 10.1016/j.antiviral.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 180.Guo D., Li Z., Jia B., Che X., Song T., Huang W. Comparison of the effects of formaldehyde and gaseous ozone on hbv-contaminated hospital quilts. Int. J. Clin. Exp. Med. 2015:8. [PMC free article] [PubMed] [Google Scholar]
- 181.Cía J.B., Biurrun J., Garcia B., Perez A., Kochan G., Escors D., Crespo J., Lasa I., Echarri A. Evaluation of the disinfecting capacity of ozone in emergency vehicles. medrxiv. 2020 [Google Scholar]
- 182.Mazur-Panasiuk N., Botwina P., Kutaj A., Woszczyna D., Pyrc K. Ozone treatment is insufficient to inactivate sars-cov-2 surrogate under field conditions. Antioxidants. 2021;10 doi: 10.3390/antiox10091480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Percivalle E., Clerici M., Cassaniti I., Vecchio Nepita E., Marchese P., Olivati D., Catelli C., Berri A., Baldanti F., Marone P., et al. SARS-CoV-2 viability on different surfaces after gaseous ozone treatment: a preliminary evaluation. J. Hosp. Infect. 2021;110 doi: 10.1016/j.jhin.2021.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Sallustio F., Cardinale G., Voccola S., Picerno A., Porcaro P., Gesualdo L. Ozone eliminates novel coronavirus Sars-CoV-2 in mucosal samples. New Microbes New Infect. 2021;43 doi: 10.1016/j.nmni.2021.100927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Volkoff S.J., Carlson T.J., Leik K., Smith J.J., Graves D., Dennis P., Aris T., Cuthbertson D., Holmes A., Craig K., et al. Demonstrated SARS-CoV-2 Surface Disinfection Using Ozone. Ozone Sci. Eng. 2021;43 doi: 10.1080/01919512.2020.1863770. [DOI] [Google Scholar]
- 186.Inagaki H., Saito A., Sudaryatma P.E., Sugiyama H., Okabayashi T., Fujimoto S. Rapid Inactivation of SARS-CoV-2 with Ozonated Water. Ozone Sci. Eng. 2021;43 doi: 10.1080/01919512.2021.1889318. [DOI] [Google Scholar]
- 187.Criscuolo E., Diotti R.A., Ferrarese R., Alippi C., Viscardi G., Signorelli C., Mancini N., Clementi M., Clementi N. Fast inactivation of SARS-CoV-2 by UV-C and ozone exposure on different materials. Emerg. Microbes Infect. 2021;10 doi: 10.1080/22221751.2021.1872354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Clavo B., Córdoba-Lanús E., Rodríguez-Esparragón F., Cazorla-Rivero S.E., García-Pérez O., Piñero J.E., Villar J., Blanco A., Torres-Ascensión C., Martín-Barrasa J.L., et al. Effects of ozone treatment on personal protective equipment contaminated with sars-cov-2. Antioxidants. 2020;9 doi: 10.3390/antiox9121222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hu X., Chen Z., Su Z., Deng F., Chen X., Yang Q., Li P., Chen Q., Ma J., Guan W., et al. Ozone Water Is an Effective Disinfectant for SARS-CoV-2. Virol. Sin. 2021;36 doi: 10.1007/s12250-021-00379-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Nicolò M.S., Rizzo M.G., Palermo N., Gugliandolo C., Cuzzocrea S., Guglielmino S.P.P. Evaluation of Betacoronavirus OC43 and SARS-CoV-2 Elimination by Zefero Air Sanitizer Device in a Novel Laboratory Recirculation System. Pathogens. 2022;11 doi: 10.3390/pathogens11020221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Takeda Y., Jamsransuren D., Makita Y., Kaneko A., Matsuda S., Ogawa H., Oh H. Inactivation of SARS-CoV-2 by Ozonated Glycerol. Food Environ. Virol. 2021;13 doi: 10.1007/s12560-021-09485-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.De Forni D., Poddesu B., Cugia G., Gallizia G., Licata M.La, Lisziewicz J., Chafouleas J.G., Lori F. Low ozone concentration and negative ions for rapid SARS-CoV-2 inactivation. bioRxiv. 2021:2. [Google Scholar]
- 193.Tascini C., Sermann G., Pagotto A., Sozio E., De Carlo C., Giacinta A., Sbrana F., Ripoli A., Castaldo N., Merelli M., et al. Blood ozonization in patients with mild to moderate COVID-19 pneumonia: a single centre experience. Intern. Emerg. Med. 2021;16 doi: 10.1007/s11739-020-02542-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Araimo F., Imperiale C., Tordiglione P., Ceccarelli G., Borrazzo C., Alessandri F., Santinelli L., Innocenti G. Pietro, Pinacchio C., Mauro V., et al. Ozone as adjuvant support in the treatment of COVID-19: A preliminary report of probiozovid trial. J. Med. Virol. 2021;93 doi: 10.1002/jmv.26636. [DOI] [PubMed] [Google Scholar]
- 195.Schwartz A., Martínez-Sánchez G., de Lucía A.M., Viana S.M., Constanta A.M. Complementary application of the ozonized saline solution in mild and severe patients with pneumonia COVID-19: A non-randomized pilot study. J. Pharm. Pharmacogn. Res. 2021:9. [Google Scholar]
- 196.Izzotti A., Fracchia E., Au W., Colombo M., Pfeffer U., Emionite L., Pavan S., Miotto D., Lova P., Grasselli E., et al. Prevention of covid-19 infection and related complications by ozonized oils. J. Pers. Med. 2021;11 doi: 10.3390/jpm11030226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Wu J., Tan C.S., Yu H., Wang Y., Tian Y., Shao W., Zhang Y., Zhang K., Shao H., Ni G., et al. Recovery of Four COVID-19 Patients via Ozonated Autohemotherapy. Innov. 2020;1 doi: 10.1016/j.xinn.2020.100060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ogut E., Armagan K. Evaluation of the Potential Impact of Medical Ozone Therapy on Covid-19: A Review Study. Ozone Sci. Eng. 2022:1–19. [Google Scholar]
- 199.Cattel F., Giordano S., Bertiond C., Lupia T., Corcione S., Scaldaferri M., Angelone L., De Rosa F.G. Ozone therapy in COVID-19: A narrative review. Virus Res. 2021;291 doi: 10.1016/j.virusres.2020.198207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Elvis A.M., Ekta J.S. Ozone therapy: A clinical review. J. Nat. Sci. Biol. Med. 2011:2. doi: 10.4103/0976-9668.82319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Manjunath S.N., Sakar M., Katapadi M., Geetha Balakrishna R. Recent case studies on the use of ozone to combat coronavirus: Problems and perspectives. Environ. Technol. Innov. 2021;21 doi: 10.1016/j.eti.2020.101313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Rowen R. Ozone therapy as a primary and sole treatment for acute bacterial infection: Case report. Med. Gas Res. 2018;8 doi: 10.4103/2045-9912.241078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Valdenassi L., Franzini M., Ricevuti G., Rinaldi L., Galoforo A.C., Tirelli U. Potential mechanisms by which the oxygen-ozone (O2–O3) therapy could contribute to the treatment against the coronavirus COVID-19. Eur. Rev. Med. Pharmacol. Sci. 2020:24. doi: 10.26355/eurrev_202004_20976. [DOI] [PubMed] [Google Scholar]
- 204.Shah M., Captain J., Vaidya V., Kulkarni A., Valsangkar K., Nair P.M.K., Ganu G. Safety and efficacy of ozone therapy in mild to moderate COVID-19 patients: A phase 1/11 randomized control trial (SEOT study) Int. Immunopharmacol. 2021;91 doi: 10.1016/j.intimp.2020.107301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Fernández-Cuadros M.E., Albaladejo-Florín M.J., Álava-Rabasa S., Usandizaga-Elio I., Martinez-Quintanilla Jimenez D., Peña-Lora D., Neira-Borrajo I., López-Muñoz M.J., Rodríguez-de-Cía J., Pérez-Moro O.S. Effect of rectal ozone (O3) in severe COVID-19 pneumonia: Preliminary results. SN Compr. Clin. Med. 2020;2:1328–1336. doi: 10.1007/s42399-020-00374-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Sharma A., Shah M., Lakshmi S., Sane H., Captain J., Gokulchandran N., Khubchandani P., Pradeep M.K., Gote P., Tuppekar B., et al. A pilot study for treatment of COVID-19 patients in moderate stage using intravenous administration of ozonized saline as an adjuvant treatment-registered clinical trial. Int. Immunopharmacol. 2021;96 doi: 10.1016/j.intimp.2021.107743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.V. Bocci, The Clinical Application of Ozonetherapy. OZONE, 2010.
- 208.Hernández A., Viñals M., Isidoro T., Vilás F. Potential role of oxygen–ozone therapy in treatment of COVID-19 pneumonia. Am. J. Case Rep. 2020;21 doi: 10.12659/AJCR.925849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Zheng Z., Dong M., Hu K. A preliminary evaluation on the efficacy of ozone therapy in the treatment of COVID-19. J. Med. Virol. 2020;92 doi: 10.1002/jmv.26040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Pereda R., González D., Rivero H.B., Rivero J.C., Pérez A., Lopez L.D.R., Mezquia N., Venegas R., Betancourt J.R., Domínguez R.E., et al. Therapeutic Effectiveness of Interferon Alpha 2b Treatment for COVID-19 Patient Recovery. J. Interf. Cytokine Res. 2020;40 doi: 10.1089/jir.2020.0188. [DOI] [PubMed] [Google Scholar]
- 211.Grignani E., Mansi A., Cabella R., Castellano P., Tirabasso A., Sisto R., Spagnoli M., Fabrizi G., Frigerio F., Tranfo G. Safe and Effective Use of Ozone as Air and Surface Disinfectant in the Conjuncture of Covid-19. Gases. 2020;1 doi: 10.3390/gases1010002. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.