Abstract
Interleukin-8 (IL-8) is a chemokine that belongs to the α-chemokine or CXC subfamily and is produced by a wide variety of human cells, including monocytes and polymorphonuclear cells (PMN). IL-8 is secreted in response to inflammatory stimuli, notably bacterial products such as lipopolysaccharide (LPS), but little is known about the mechanisms by which these agents mediate IL-8 induction. In this report, we show that Mycoplasma fermentans lipid-associated membrane proteins (LAMPf) induce the production of high levels of IL-8 by THP-1 (human monocyte) cells and PMN at the same extent as LPS. It was previously demonstrated that stimulation of monocytic cells with either LPS or LAMPf led to a series of common downstream signaling events, including the activation of protein tyrosine kinase and of mitogen-activated protein kinase cascades. By using PD-98059 and SB203580, two potent and selective inhibitors of MEK1 (a kinase upstream of ERK1/2) and p38, respectively, we have demonstrated that both ERK1/2 and p38 cascades play a key role in the production of IL-8 by monocytes and PMN stimulated with bacterial fractions.
Chemokines are proinflammatory cytokines that exhibit chemotactic and stimulatory activity toward blood cells (4). Interleukin-8 (IL-8) is the best-characterized member of the α-chemokine or CXC subfamily (2, 32). IL-8 acts primarily on polymorphonuclear cells (PMN) but also has potent chemotactic and stimulatory effects on T cells, basophils, or eosinophils (2). IL-8 is released by a wide variety of cell types, including monocytes/macrophages, neutrophils, T lymphocytes, fibroblasts, endothelial cells, and epithelial cells upon exposure to inflammatory stimuli such as lipopolysaccharide (LPS), interleukin-1 (IL-1), or tumor necrosis factor (TNF) (22, 23, 46). Besides its central role in inflammation process, IL-8 is involved in other biological functions such as angiogenesis (13) and hematopoiesis (8). IL-8 has been shown to play a role in the pathogenesis of various diseases, including rheumatoid arthritis, psoriasis, asthma, pancreatitis, acute respiratory distress syndrome, and sepsis (39), and its level in plasma or in inflammatory biological fluids is often correlated with the severity of the pathology and/or the outcome of the patients (19, 21).
Mycoplasma fermentans is a potent human pathogen that is suspected to be involved in rheumatoid arthritis. Unlike all the other bacteria, mycoplasmas have no cell wall, and consequently their bilipid membrane is the only structure that regulates the interaction with the external environment. One of the responses of the human macrophages to gram-negative bacterial LPS or to M. fermentans lipid-associated membrane proteins (LAMPf) is the production of proinflammatory cytokines such as IL-1β, IL-6, and TNF-α (18, 28, 29). Although the mechanisms by which LPS and LAMPf induce the secretion of proinflammatory cytokines are not completely elucidated, it has been demonstrated that signaling pathways involving protein kinases clearly participate in this processus (16, 27, 33). Whereas LPS from distinct gram-negative bacteria including Escherichia coli, Neisseria meningitidis, and Klebsiella pneumoniae have been shown to be potent inducers of IL-8 production in monocytic cells and PMN (9), the ability of M. fermentans lipoproteins to induce IL-8 secretion by these cells has not been addressed.
In this study, we have tested and compared the abilities of LAMPf and E. coli (O55:B5) LPS to induce IL-8 production by the human promyelomonocytic cell line THP-1, human monocytes/macrophages, and PMN. Furthermore, we have evaluated the role that mitogen-activated protein kinase (MAPK) pathways play in the IL-8 production induced by these bacterial products.
MATERIALS AND METHODS
Reagents.
PD-98059 and caffeic acid phenetyl ester (CAPE) were obtained from Biomol Research Laboratories (Philadelphia, Pa.) SB203580 and herbimycin A were from Calbiochem (Nottingham, United Kingdom). E. coli LPS (O55:B5) and polymyxin B were from Sigma L’Isle D’Abeauhesnes, France). MY4, an anti-human CD14 monoclonal antibody, was purchased from Coulter Diagnostics (Hialeah, Fla.).
Mycoplasma culture and LAMPf preparation.
M. fermentans PG18 was cultivated in medium containing 20% horse serum (Gibco BRL), 10% freshly prepared yeast extract, 1% glucose, and 1,000 U of penicillin G per ml. Mycoplasma cultures were incubated at 37°C and 5% CO2, then quantified as described by Rodwell and Whitcomb (31), and expressed as CFU per milliliter. LAMPf preparations were made by hydrophilic/hydrophobic fractionation using the TX-114 partitioning method as described previously (42). Protein concentrations were determined by means of micro-BCA assay (bicinchoninic acid) (Pierce, Rockford, Ill.). The endotoxin level of the preparations was <60 pg/ml, as determined by Limulus amebocyte lysate assay (Haemachem, St. Louis, Mo.).
THP-1 cell line culture and stimulation.
The human monocytic cell line THP-1 was cultured (37°C, 5% CO2) in RPMI 1640 culture medium (Gibco BRL) containing 10% fetal calf serum, 2 mM l-glutamine, and antibiotics. Cell line were tested every 2 weeks by a PCR-based detection assay for mycoplasma contamination (26). For stimulation experiments, cells were seeded at a density of 106/ml and then incubated overnight. For cytokine production, cells were stimulated with LAMPf (1 μg/ml) or LPS (1 μg/ml) for 18 h.
Isolation of human monocytes and PMN.
Fresh human blood was obtained from healthy donors (Etablissement de Transfusion Sanguine de l’Assistance Publique, Paris, France) and drawn on citrate-phosphate-dextrose. Human monocytes were selected by adherence from peripheral blood mononuclear cells (PBMC) as previously described (24). Briefly, 1:2-diluted blood in RPMI 1640 medium (Glutamax; Life Technologies, Paisley, Scotland) was layered on Ficoll-Hypaque (MSL; Eurobio, Les Ulis, France). The ratio was 2 volumes of blood to 1 volume of MSL. After centrifugation for 20 min at 15°C and 600 × g, PBMC were washed twice and then counted in 0.1% eosin.
PMN were prepared as previously described (20). Briefly, 10 volumes of blood was mixed with 2 volumes of glucose dextran (3% glucose, 3% dextran T250; Pharmacia, Uppsala, Sweden), and the leukocytes were recovered following a 40-min sedimentation at room temperature. The leukocytes were then diluted 1:2 in RPMI 1640 medium and layered on Ficoll-Hypaque. After centrifugation for 20 min at 15°C and 600 × g, the cell pellet was washed and centrifuged once for 5 min at 300 × g. Contaminating erythrocytes were lysed, and the viability of PMN was assessed by counting the cells in 0.1% eosin.
Monocytes and PMN culture and stimulation.
PBMC were adjusted to 6 × 106 cells per ml in RPMI 1640 medium supplemented with antibiotics (penicillin [100 IU/ml] and streptomycin [100 μg/ml]); 0.5-ml aliquots of PBMC suspension per well were incubated in a 5% CO2 incubator in 24-well multidish plates (Costar, Cambridge, Mass.) for 1 h at 37°C. The nonadherent cells were then discarded, and the remaining adherent cells were washed extensively; 0.5 ml of fresh medium (RPMI 1640 medium supplemented with antibiotics and 0.2% heat-inactivated normal human serum) was added, and monocytes were further cultured for 24 h at 37°C and 5% CO2. At 24 h, the adherent monocytes/macrophages were washed again and cultured for another 24-h period in 0.5 ml of fresh medium. At 48 h, cells were washed and then incubated for 24 h with stimulating agents.
PMN were adjusted to 2 × 106/ml in RPMI 1640 medium supplemented with antibiotics and 5% normal human serum. Then 0.5-ml aliquots of PMN suspension per well were incubated in 24-well multidish plates in a 5% CO2 incubator for 24 h at 37°C with stimulating agents. Dimethyl sulfoxide (DMSO) was used as the solvent control.
RNA purification and NPA.
THP-1 cells were stimulated as described above at various times, and total RNA was extracted from 107 cells by using a total RNA isolation kit from Bioprobe (Montreuil, France) according to the manufacturer’s instructions. Nuclease protection assay (NPA) for the detection of IL-8 transcript was performed with a Multi-NPA kit (Ambion, Austin, Tex.) as instructed by the manufacturer, using an IL-8 oligonucleotide probe (Clontech, Palo Alto, Calif.) and a 28S rRNA oligonucleotide probe (Ambion) for standardization. NPA gels were exposed to a PhosphorImager screen, and the signals were quantified with ImageQuant software (Molecular Dynamics, Sunnyvale, Calif.). All determinations were repeated three to four times; data are presented as means ± standard errors of the means (SEM).
Plasmids, cell transfection, activation, and assay for luciferase activity.
The NF-κB-driven and AP-1-driven (3) luciferase reporter constructs were kindly provide by O. Acuto (Institut Pasteur, Paris, France). THP-1 cells were transfected with the indicated plasmids by electroporation as described by Stacey et al. (38). Transfected cells were cultured overnight in growth medium and then either left unstimulated or stimulated with LAMPf or LPS for 6 h. Cells were then harvested, the protein concentration was determined by micro-BCA assay (Pierce), and luciferase activity was measured as described elsewhere (5). Specific luciferase activity, determined in duplicate samples by using an automated luminometer (Lumat LB 9501; EG1G Berthod, Wilbad, Germany), was determined in arbitrary units after normalization to the protein content. Luciferase fold induction was calculated as the ratio of specific luciferase activity in the stimulated cells to that in the unstimulated cells.
Cytokine ELISA.
IL-8 enzyme-linked immunosorbent assay (ELISA) was performed as previously described (21), using a monoclonal anti-human antibody obtained by J. C. Mazié (Institut Pasteur, Paris, France) and a rabbit polyclonal anti-IL-8 antibody graciously provided by N. Vita (Sanofi Recherche, Labège, France).
RESULTS
LAMPf induces IL-8 secretion by THP-1 cells, human monocytes/macrophages, and human PMN.
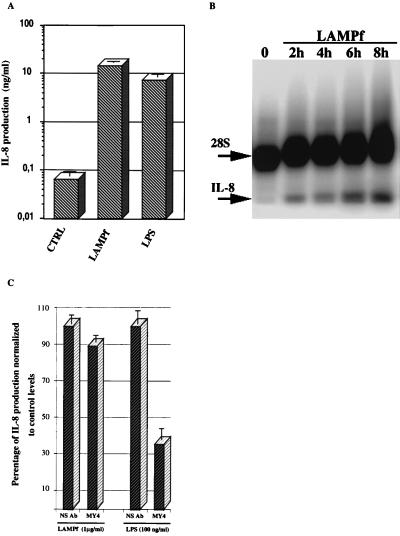
Mycoplasma membrane lipoproteins have been demonstrated to strongly stimulate the production of proinflammatory cytokines by human monocytic cells (28, 29); however, their ability to induce IL-8 secretion by these cells has not been previously addressed. As shown in Fig. 1A, Triton X-114 fractionated LAMPf (1 μg/ml) induced the release of a considerable amount of IL-8 by THP-1 cells after 18 h of stimulation. It is worth noting that the level of IL-8 in THP-1 cells stimulated with LAMPf was comparable to that induced in response to LPS (Fig. 1A). We further investigated the expression of IL-8 mRNA in THP-1 cells challenged with LAMPf by multi-NPA. As depicted in Fig. 1B, LAMPf induced the synthesis of IL-8 mRNA 2 h after stimulation, and the mRNA level increased continuously up to 8 h after stimulation. The amount of RNA in each incubation time was normalized by probing for 28S rRNA. Quantitative analysis demonstrated that at 8 h poststimulation, the IL-8 signal in LAMPf-stimulated cells, after correction by the 28S rRNA signal, was 1,033% of the IL-8 signal in control cells. Similar results were obtained when cells were stimulated with LPS (data not shown).
FIG. 1.
IL-8 production by human THP-1 cells in response to LAMPf and LPS. (A) THP-1 cells (106/ml) were not stimulated (control [CTRL]) or stimulated with either LPS (1 μg/ml) or LAMPf (1 μg/ml). IL-8 production was measured by ELISA 18 h after stimulation. Data presented are means ± SEM of three distinct assays. (B) Multi-NPA using oligonucleotide probes for IL-8 and 28S rRNA (for standardization). RNAs were prepared from untreated THP-1 cells (○) and THP-1 cells induced with LAMPf (1 μg/ml) for the indicated time and subjected to multi-NPA analysis. Multi-NPA gels were exposed to a PhosphorImager screen for 2 to 4 h; the gel shown is representative of three experiments with similar results. IL-8 and 28S rRNA oligonucleotide probes protect 24 and 35 bases of the IL-8 and 28S rRNA transcripts, respectively. The IL-8 signal in each lane was quantitatively assessed and normalized to the 28S rRNA signal. (C) Effect of anti-CD14 antibody treatments on LAMPf-induced IL-8 secretion. Human THP-1 cells were incubated with anti-human CD14 monoclonal antibody MY4 at 5 μg/ml for 1 h prior to stimulation with either LAMPf (1 μg/ml) or LPS (100 ng/ml). An irrelevant antibody (NS) was used as a control. IL-8 secretion was determined after 18 h of culture and expressed as percentage of secretion normalized to stimulated cells that received no antibody treatment. Mean values of two different experiments are shown.
Treatment of LAMPf with polymyxin B (1,000 U/ml) had no effect on the IL-8 induction mediated by LAMPf, whereas polymyxin B completely blocked LPS-induced IL-8 in the same cells (data not shown). Interestingly, the anti-CD14 monoclonal antibody MY4, which significantly reduced LPS-induced IL-8 production by THP-1 cells, had no effect on IL-8 secretion by the same cells stimulated with LAMPf (Fig. 1C).
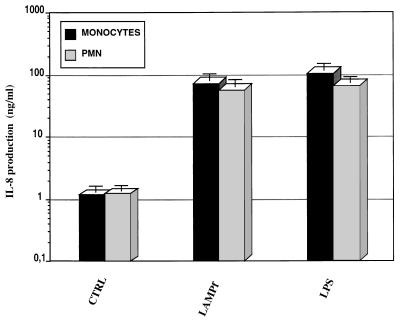
We also assessed the production of IL-8 by human monocytes/macrophages and PMN challenged with LAMPf. As shown in Fig. 2, LAMPf induced IL-8 secretion by human monocytes/macrophages after 24 h of stimulation. Culture conditions and stimulation of human monocytes/macrophages had to be adjusted in order to reduce the amount of spontaneous IL-8 release by these cells. In contrast, with the very low levels of IL-8 secreted by unstimulated THP-1 cells, a large amount of this chemokine was detected in the supernatants of 24-h-cultured human monocytes/macrophages in the absence of any stimulus. However, spontaneous IL-8 release was dramatically reduced (80% reduction) when cells were cultured for 48 h prior to stimulation and washed each 24 h (data not shown). In these conditions, LAMPf stimulation induced a 100-fold increase in IL-8 secretion in comparison to unstimulated cells (Fig. 2). Given that PMN constitute excellent producers of IL-8, we also tested the effect of mycoplasma lipoproteins on the secretion of this chemokine. LAMPf increased strongly (100-fold) the release of IL-8 by PMN (Fig. 2). Interestingly, the levels of induction of IL-8 secretion by cells stimulated with either E. coli LPS (1 μg/ml) or LAMPf were fully comparable.
FIG. 2.
IL-8 production by human monocytes and PMN stimulated with LAMPf or LPS. PBMC (6 × 106/ml) and PMN (2 × 106/ml) were unstimulated (control [CTRL]) or stimulated with either LPS (1 μg/ml) or LAMPf (1 μg/ml). IL-8 production was measured by ELISA 18 h after stimulation. Data presented are means ± SEM of four and five distinct assays for monocytes and PMN, respectively.
Tyrosine phosphorylation is required for IL-8 induction by LAMPf and LPS.
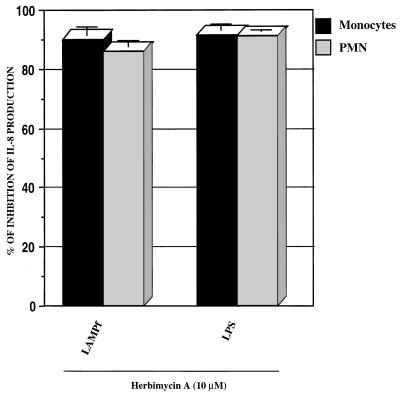
Stimulation of monocytic cells with either LAMPf or LPS results in the phosphorylation of tyrosine residues of a series of unidentified proteins. The activation of protein tyrosine kinases (PTKs) in monocytes in response to LPS or LAMPf seems to be maximal at 10 or 20 min, respectively, after stimulation (27, 28). Furthermore, tyrosine phosphorylation constitutes a crucial event in the signaling pathways leading to IL-1β, TNF-α, and IL-6 production by monocytes/macrophages stimulated with either LPS or LAMPf (12, 28). To evaluate the involvement of PTK in IL-8 production by human monocytes/macrophages and PMN stimulated with LAMPf, we used the specific PTK inhibitor herbimycin A. Prior to stimulation, human monocytes/macrophages or PMN were incubated for 1 h with herbimycin A at 10 μM (a concentration that does not affect cell viability), and IL-8 production was monitored by specific ELISA after 24 h of stimulation. As shown in Fig. 3, herbimycin A completely inhibited IL-8 production in response to LAMPf. Herbimycin A at 10 μM also completely blocked IL-8 production in response to LPS. These data clearly underscore the involvement of PTK in the signaling cascades leading to IL-8 production by monocytes/macrophages and PMN in response to both LAMPf and bacterial LPS.
FIG. 3.
Effect of PTK blockade on IL-8 production. Monocytes and PMN were incubated with herbimycin A (10 μM) for 1 h prior to LPS (1 μg/ml) or LAMPf (1 μg/ml) stimulation. DMSO (1%) was used as the solvent control. The IL-8 level was measured by ELISA 18 h after stimulation and normalized to cells that received no treatment prior to stimulation. Data presented are means ± SEM of four and three distinct experiments for monocytes and PMN, respectively.
MAPK signaling is a key event in IL-8 induction by LAMPf and LPS.
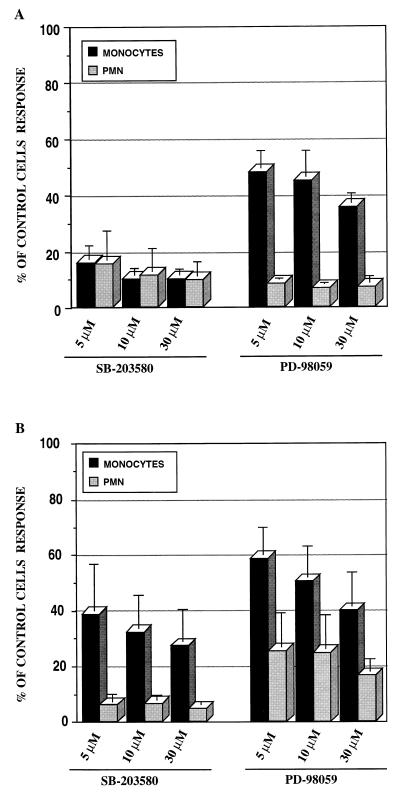
MAPKs are a group of serine/threonine-specific, proline-directed protein kinases which are activated by various extracellular stimuli. Well-characterized MAPK pathways include extracellular signal-regulated kinases 1 and 2 (ERK1/2), c-Jun NH2-terminal kinase (JNK)/stress-activated protein kinases, and p38/RK/Mpk2 (40). LPS and LAMPf are capable of activating ERK1/2 and p38 pathways in monocytes/macrophages, although with different kinetics of activation (27, 33). To determine the involvement of ERK1/2 and p38 pathways in LAMPf- and LPS-induced IL-8 production in monocytes/macrophages and PMN, we used two specific inhibitors: PD-98059, which targets the upstream effector of ERK1/2 MEK1 (1, 11); and SB203580, which inhibits p38 kinase (16).
Treatment of monocytes with SB203580 selectively inhibited the LAMPf-mediated activation of p38 without significantly affecting the stimulation of ERK1/2 or JNK (data not shown). Human monocytes/macrophages were preincubated for 1 h with various concentrations of SB203580 before stimulation. As depicted in Fig. 4A, p38 pathway inhibitor significantly reduced IL-8 production in a dose-dependent manner in response to LAMPf. Furthermore, SB203580 also inhibited IL-8 production when cells were stimulated with LPS (Fig. 4B). Similarly, treatment of PMN with SB203580 almost completely blocked IL-8 production induced by both agents. No cell toxicity was observed when cells were treated with SB203580 at the highest used concentration (30 μM). These data underscore the involvement of p38 pathway in signaling for IL-8 production in both human monocytes/macrophages and PMN.
FIG. 4.
Effect of ERK1/2 and p38 pathway-specific inhibitors on IL-8 production by human monocytes and PMN. Cells were treated for 1 h with either PD-98059 (MEK1 inhibitor) or SB203580 (p38 inhibitor) at different concentrations and then stimulated with either LAMPf (1 μg/ml) (A) or LPS (1 μg/ml) (B). DMSO (1%) was used as the solvent control. The IL-8 level was measured by ELISA 18 h after stimulation and normalized to cells that received no treatment prior to stimulation. Results are means ± SEM of four and three separate experiments for monocytes and PMN, respectively.
The MEK-1 inhibitor, PD-98059, selectively inhibited the LAMPf- or LPS-mediated activation of ERK1/2 in macrophages without significantly affecting the stimulation of p38 or JNK, and no cell toxicity was observed with this compound up to a concentration of 30 μM (data not shown). Treatment of human monocytes/macrophages with PD-98059 significantly decreased IL-8 production in a concentration-dependent manner in cells stimulated with LAMPf (Fig. 4A). In addition, PD-98059 treatment strongly reduced IL-8 production in human monocytes/macrophages challenged with LPS (Fig. 4B). Inhibition of MEK-1 in PMN resulted in a significant decrease in IL-8 production after both LAMPf and LPS challenge. As depicted in Fig. 4, PMN were found overall to be more sensitive than monocytes/macrophages to treatment with either PD-98059 or SB203580.
Altogether, these data clearly indicate the importance of MAPK pathways in the signaling leading to IL-8 induction in response to both LAMPf and E. coli LPS.
Involvement of NF-κB in IL-8 production in monocytes/macrophages and PMN.
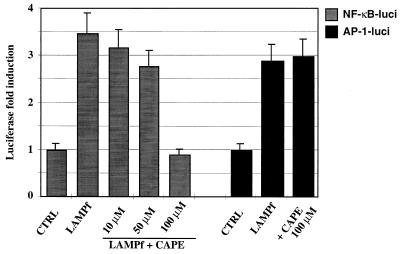
NF-κB is involved in the inflammation response (43). It is well documented that LPS induces NF-κB activation in monocytes and regulates cytokine expression (10, 43, 44). We have recently shown that LAMPf induce the activation of NF-κB in monocytic cells (29a). We therefore addressed the involvement of NF-κB in IL-8 production by LAMPf- and LPS-stimulated monocytes/macrophages and PMN. To investigate the involvement of NF-κB activation in IL-8 production induced by these bacterial stimuli, we used the recently reported specific NF-κB inhibitor CAPE (25). As shown in Fig. 5, LAMPf induced a threefold increase in luciferase activity in THP-1 cells transiently transfected with NF-κB-driven luciferase reporter plasmid. In addition, LAMPf was capable of inducing AP-1 transactivation, as determined by measuring luciferase activity in THP-1 cells transiently transfected with AP-1-driven luciferase reporter plasmid and stimulated with LAMPf (Fig. 5). Treatment of THP-1 transfected cells with CAPE (10 to 100 μM) inhibited in a concentration-dependent manner NF-κB-mediated luciferase activation induced by LAMPf (Fig. 5). No cell toxicity was observed with CAPE at 100 μM, as determined by the blue trypan uptake; at higher concentration, however, this inhibitor induced cell death (>50%). Importantly, at 100 μM CAPE completely blocked NF-κB-dependent luciferase activation but affect neither AP-1-dependent (Fig. 5) nor c-fos promoter-dependent (data not shown) luciferase activity in transfected THP-1 cells, indicating the specificity of this compound at the effective inhibitory concentration.
FIG. 5.
Effect of CAPE on NF-κB transactivation mediated by LAMPf. THP-1 cells were transiently transfected with an NF-κB- or AP-1-driven luciferase (luci) reporter plasmid. Transfected cells were stimulated with LAMPf (1 μg/ml) for 6 h prior to cell lysis. Luciferase activity was assessed in stimulated and unstimulated (control [CTRL]) cells and normalized to protein content. To assess the effect of CAPE, transfected cells were preincubated with CAPE at the indicated concentration for 1 h and then stimulated with LAMPf and analyzed for luciferase activity as indicated above. Assays were performed in duplicate, and data presented are means ± SEM of three independent experiments.
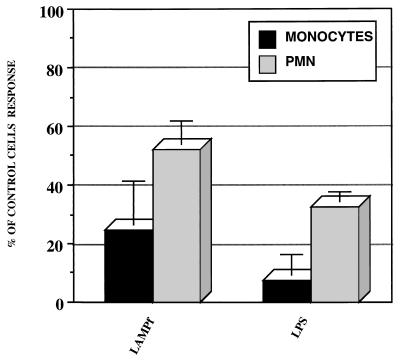
Human monocytes/macrophages or PMN were preincubated for 1 h with CAPE at 100 μM before being challenged with either LAMPf or LPS; IL-8 production was determined in cell supernatants after 24 h of stimulation and compared to that in cells preincubated with the solvent (DMSO) for 1 h prior to stimulation as a control. As shown in Fig. 6, CAPE strongly inhibited the production of IL-8 by either human monocytes/macrophages or PMN challenged with either LAMPf or LPS. These data underscore the involvement of NF-κB activation in IL-8 production by monocytes/macrophages and PMN in response to two different bacterial stimuli.
FIG. 6.
Effect of an NF-κB-specific inhibitor on IL-8 production by human monocytes and PMN. Cells were incubated with CAPE (100 μM) for 1 h prior to LPS (1 μg/ml) or LAMPf (1 μg/ml) stimulation. DMSO (1%) was used as the solvent control. IL-8 production was measured by ELISA 18 h after stimulation and normalized to cells that received no treatment prior to stimulation. Data presented are means ± SEM of four and three distinct experiments for monocytes and PMN, respectively.
DISCUSSION
Given that IL-8 can play an major role in the pathogenesis of a number of inflammatory diseases, it is important to elucidate the mechanisms by which the IL-8-inducers act on cells to activate the production of this chemokine. LPS is largely known to induce different cell types to secrete large amounts of IL-8 (2). In the present report, we have demonstrated the ability of another bacterial product, LAMPf, to induce IL-8 production by human monocytes/macrophages and PMN. The finding is helpful to more clearly understand the pathogenic mechanisms of M. fermentans, a microorganism suspected to play a role in several human inflammatory diseases, particularly rheumatoid arthritis (34, 41). This is not the first study reporting the ability of mycoplasmas to induce IL-8 secretion. M. hominis and M. salivarium recently have been shown to induce IL-8 production by human alveolar type II cells (15) and human gingival fibroblasts (37), respectively. Interestingly, the soluble protein fraction of M. salivarium was found to be the active fraction in the second study (37). This is unusual because we previously demonstrated that only Triton X-114-fractionated membrane proteins from different Mycoplasma species, including M. salivarium, were capable of inducing production of proinflammatory cytokines (IL-1β, TNF-α, and IL-6) by monocytic cells (28). Therefore, one can speculate that Mycoplasma species stimulate different cellular types by mechanisms involving distinct triggering molecular entities.
We and others have previously demonstrated that M. fermentans lipoproteins induce monocyte activation by mechanisms distinct from that of LPS (14, 28). Previous studies have shown that mycoplasma membrane lipoproteins do not stimulate monocytic cells via CD14. Unlike LPS, anti-CD14 antibodies have been shown to be inefficient in blocking the effects of mycoplasma lipoproteins on monocytic cells (14, 27). The secretion of IL-8 mediated by LAMPf seems also to be independent of CD14, given that anti-CD14 antibodies had no effect on this LAMPf activity (Fig. 1C). Although LAMPf and LPS seem to activate monocytes through distinct cellular membrane receptors, the signaling triggered by both agents converts to the activation of MAPK cascades. MAPK pathways have been demonstrated to play an important role in the control of proinflammatory cytokine induction mediated by both LPS and LAMPf (27, 33). Whereas the p38 pathway is crucial in the signaling leading to IL-1β, TNF-α, and IL-6 secretion in macrophages stimulated with LAMPf, ERK1/2 is involved in signaling for synthesis of IL-1β and TNF-α but not IL-6 (27). In the present study, we investigated the involvement of ERK1/2 and p38 pathways in IL-8 regulation in both human monocytes/macrophages and PMN stimulated with either LPS or LAMPf. Two inhibitors, PD-98059 and SB203580, that selectively inhibit ERK1/2 and p38 pathways, respectively, were used to address this issue. Data presented herein clearly show that both pathways are involved in IL-8 regulation in human monocytes/macrophages and PMN. The p38 inhibitor, SB203580, efficiently blocked IL-8 production in both cell types in response to either LAMPf or LPS, whereas slight distinct efficiency between monocytes/macrophages and PMN was observed with the ERK1/2 pathway inhibitor, PD-98059. Actually, treatment of human monocytes/macrophages with 30 μM PD-98059 inhibited about 60% of IL-8 in response to LPS or LAMPf, whereas this inhibitor at the same concentration almost completely blocked IL-8 production by PMN in response to these stimuli. These data strongly suggest that ERK1/2 is involved in IL-8 regulation in both monocytes/macrophages and PMN but to different extents. Two distinct studies have previously addressed the involvement of ERK1/2 and p38 pathways in IL-8 production by human fibroblasts. In concordance with the results presented herein, Bruder and Kovesdi recently demonstrated that the ERK1/2 pathway is involved in the expression of IL-8 in response to adenovirus (7). In contrast with our data, Ridley et al. found that IL-8 production by human fibroblast in response to IL-1 was independent of activation of the p38 pathway (30). These apparently contradictory results suggest that IL-8 regulation varies from one cell type to another and/or depends on the stimulating agent.
Both LAMPf and LPS induce the activation of NF-κB, a transcription factor known to regulate the expression of a number of cytokine genes (17, 36, 45). Brasier et al. recently demonstrated by using an agent that blocks both IκB proteolysis and NF-κB translocation that NF-κB plays an important role in the induction of IL-8 by TNF-α in A549 alveolar cells (6). In the present report, we have also shown the involvement of NF-κB in IL-8 production by monocytes/macrophages in response to LAMPf or LPS by using the recently described new NF-κB inhibitor CAPE.
The ability of some bacteria to induce IL-8 can be considered a virulence mechanism. Segal et al. (35) recently showed that the presence of a pathogenicity island in Helicobacter pylori correlates with the ability of type I strains to induce IL-8 in host cells. Therefore, type I H. pylori associated with peptic ulcer disease and gastric cancer induces IL-8 in gastric epithelial cells, whereas type II strains, more often associated with asymptomatic gastritis, do not (35). Investigation of the mechanisms involved in the induction of the host cell response to different bacteria will provide a better understanding of the pathogenesis and clinical response to these microorganisms. Although our findings remain to be tested in in vivo models, they may be informative with respect to pathologies in which excessive bacterium-induced IL-8 secretion plays a major role in the development of the disease.
ACKNOWLEDGMENTS
We thank O. Acuto (Institut Pasteur, Immunology Moleculaire, Paris, France) for the NF-κB and AP-1 reporter plasmids and J. C. Mazié (Institut Pasteur, Hybridolab, Paris, France) and N. Vita (Sanofi Recherche, Labège, France) for providing with IL-8 antibodies. We also thank I. Saint Girons for stimulating discussions as well as B. Lemercier, A. Dujeancourt, and C. Prevost for technical assistance.
C. Marie was supported by a grant from the CANAM.
REFERENCES
- 1.Alessi D R, Cuenda A, Cohen P, Dudley D T, Saltiel A R. PD-98059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- 2.Baggiolini M, Dewald B, Moser B. Interleukin-8 and related chemotactic cytokines—CXC and CC chemokines. Adv Immunol. 1994;55:97–179. [PubMed] [Google Scholar]
- 3.Baldari C T, Milia E, Di Somma M M, Baldoni F, Valitutti S, Telford J L. Distinct signaling properties identify functionally different CD4 epitopes. Eur J Immunol. 1995;25:1843–1850. doi: 10.1002/eji.1830250708. [DOI] [PubMed] [Google Scholar]
- 4.Ben-Baruch A, Michiel D F, Oppenheim J J. Signals and receptors involved in recruitment of inflammatory cells. J Biol Chem. 1995;270:11703–11706. doi: 10.1074/jbc.270.20.11703. [DOI] [PubMed] [Google Scholar]
- 5.Brasier A R. Reporter system using firefly luciferase. In: Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: Green Publishing and John Wiley & Sons; 1994. pp. 9.6.1–9.6.14. [Google Scholar]
- 6.Brasier A R, Jamaluddin M, Casola A, Duan W, Shen Q, Garofalo R P. A promoter recruitment mechanism for tumor necrosis factor-alpha-induced interleukin-8 transcription in type II pulmonary epithelial cells. Dependence on nuclear abundance of Rel A, NF-κB1, and c-Rel transcription factors. J Biol Chem. 1998;273:3551–3561. doi: 10.1074/jbc.273.6.3551. [DOI] [PubMed] [Google Scholar]
- 7.Bruder J T, Kovesdi I. Adenovirus infection stimulates the Raf/MAPK signaling pathway and induces interleukin-8 expression. J Virol. 1997;71:398–404. doi: 10.1128/jvi.71.1.398-404.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cacalano G, Lee J, Kikly K, Ryan A M, Pitts-Meek S, Hultgren B, Wood W I, Moore M W. Neutrophil and B cell expansion in mice that lack the murine IL-8 receptor homolog. Science. 1994;265:682–684. doi: 10.1126/science.8036519. [DOI] [PubMed] [Google Scholar]
- 9.Cavaillon J M, Marie C, Pitton C, Fitting C. The production of TNFα and IL-8 in whole blood assays are differently regulated aupon activation by LPS. Prog Clin Biol Res. 1995;392:433–439. [PubMed] [Google Scholar]
- 10.DeFranco A L, Hambleton J, McMahon M, Weinstein S L. Examination of the role of MAP kinase in the response of macrophages to lipopolysaccharide. Prog Clin Biol Res. 1995;392:407–420. [PubMed] [Google Scholar]
- 11.Dudley D T, Pang L, Decker S J, Bridges A J, Saltiel A R. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc Natl Acad Sci USA. 1995;92:7686–7689. doi: 10.1073/pnas.92.17.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geng Y, Zhang B, Lotz M. Protein tyrosine kinase activation is required for lipopolysaccharide induction of cytokines in human blood monocytes. J Immunol. 1993;151:6692–6700. [PubMed] [Google Scholar]
- 13.Koch A E, Polverini P J, Kunkel S L, Harlow L A, Di Pietro L A, Elner V M, Elner S G, Strieter R M. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science. 1992;258:1798–1801. doi: 10.1126/science.1281554. [DOI] [PubMed] [Google Scholar]
- 14.Kostyal D A, Butler G H, Beezhold D H. A 48-kilodalton Mycoplasma fermentans membrane protein induces cytokine secretion by human monocytes. Infect Immun. 1994;62:3793–3800. doi: 10.1128/iai.62.9.3793-3800.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kruger T, Baier J. Induction of neutrophil chemoattractant cytokines by Mycoplasma hominis in alveolar type II cells. Infect Immun. 1997;65:5131–5136. doi: 10.1128/iai.65.12.5131-5136.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee J C, Laydon J T, McDonnell P C, Gallagher T F, Kumar S, Green D, McNulty D, Blumenthal M J, Heys J R, Landvatter S W, et al. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994;372:739–746. doi: 10.1038/372739a0. [DOI] [PubMed] [Google Scholar]
- 17.Libermann T A, Baltimore D. Activation of interleukin-6 gene expression through the NF-κB transcription factor. Mol Cell Biol. 1990;10:2327–2334. doi: 10.1128/mcb.10.5.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manthey C L, Vogel S N. Interactions of lipopolysaccharide with macrophages. Immunol Ser. 1994;60:63–81. [PubMed] [Google Scholar]
- 19.Marie C, Losser M-R, Fitting C, Kermarrec N, Payen D, Cavaillon J-M. Cytokines and soluble cytokine receptors in pleural effusions from septic and nonseptic patients. Am J Respir Crit Care Med. 1997;156:1–8. doi: 10.1164/ajrccm.156.5.9702108. [DOI] [PubMed] [Google Scholar]
- 20.Marie C, Pitton C, Fitting C, Cavaillon J-M. Regulation by anti-inflammatory cytokines (IL-4, IL-10, IL-13, TGFβ) of interleukin-8 production by LPS- and/or TNFα-activated human polymorphonuclear cells. Mediators Inflamm. 1996;5:334–340. doi: 10.1155/S0962935196000488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marty C, Misset B, Tamion F, Fitting C, Carlet J, Cavaillon J M. Circulating interleukin-8 concentrations in patients with multiple organ failure of septic and nonseptic origin. Crit Care Med. 1994;22:673–679. doi: 10.1097/00003246-199404000-00025. [DOI] [PubMed] [Google Scholar]
- 22.Matsushima K, Morishita K, Yoshimura T, Lavu S, Kobayashi Y, Lew W, Appella E, Kung H F, Leonard E J, Oppenheim J J. Molecular cloning of a human monocyte-derived neutrophil chemotactic factor (MDNCF) and the induction of MDNCF mRNA by interleukin 1 and tumor necrosis factor. J Exp Med. 1988;167:1883–1893. doi: 10.1084/jem.167.6.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller M D, Krangel M S. Biology and biochemistry of the chemokines: a family of chemotactic and inflammatory cytokines. Crit Rev Immunol. 1992;12:17–46. [PubMed] [Google Scholar]
- 24.Muñoz C, Carlet J, Fitting C, Misset B, Bleriot J P, Cavaillon J M. Dysregulation of in vitro cytokine production by monocytes during sepsis. J Clin Investig. 1991;88:1747–1754. doi: 10.1172/JCI115493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Natarajan K, Singh S, Burke T, Grunberger D, Aggarwal B B. Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor NF-κB. Proc Natl Acad Sci USA. 1996;93:9090–9095. doi: 10.1073/pnas.93.17.9090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rawadi G, Lecaque D, Pirot D, Roman-Roman S. Detection and identification of mycoplasma contaminating cell cultures by polymerase chain reaction. Methods Mol Cell Biol. 1993;4:147–156. [Google Scholar]
- 27.Rawadi G, Morvan V, Lemercier B, Roman-Roman S. Activation of MAPK pathways by Mycoplasma fermentans membrane lipoproteins in murine macrophages: involvement in cytokine synthesis. J Immunol. 1998;160:1330–1339. [PubMed] [Google Scholar]
- 28.Rawadi G, Roman-Roman S. Mycoplasma membrane lipoproteins induced proinflammatory cytokines by a mechanism distinct from that of lipopolysaccharide. Infect Immun. 1996;64:637–643. doi: 10.1128/iai.64.2.637-643.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rawadi G, Roman-Roman S, Castedo M, Dutilleul V, Susin S, Marchetti P, Geuskens M, Kroemer G. Effects of Mycoplasma fermentans on the myelomonocytic lineage. Different molecular entities with cytokine-inducing and cytocidal potential. J Immunol. 1996;156:670–678. [PubMed] [Google Scholar]
- 29a.Rawadi, G., J. Garcia, B. Lemercier, and S. Roman-Roman. Signal transduction pathways involved in the activation of NF-κB, AP-1, and c-fos by Mycoplasma fermentans lipoproteins in macrophages. J. Immunol., in press. [PubMed]
- 30.Ridley S H, Sarsfield S J, Lee J C, Bigg H F, Cawston T E, Taylor D J, DeWitt D L, Saklatvala J. Actions of IL-1 are selectively controlled by p38 mitogen-activated protein kinase: regulation of prostaglandin H synthase-2, metalloproteinases, and IL-6 at different levels. J Immunol. 1997;158:3165–3173. [PubMed] [Google Scholar]
- 31.Rodwell A W, Whitcomb R F. Methods for direct and indirect measurement of mycoplasma growth. In: Razin S, Tully J H, editors. Methods in mycoplasmology. Vol. 4. New York, N.Y: Academic Press; 1983. pp. 185–196. [Google Scholar]
- 32.Rollins B J. Chemokines. Blood. 1997;90:909–928. [PubMed] [Google Scholar]
- 33.Sanghera J S, Weinstein S L, Aluwalia M, Girn J, Pelech S L. Activation of multiple proline-directed kinases by bacterial lipopolysaccharide in murine macrophages. J Immunol. 1996;156:4457–4465. [PubMed] [Google Scholar]
- 34.Schaeverbeke T, Gilroy C B, Bebear C, Dehais J, Taylor-Robinson D. Mycoplasma fermentans, but not M. penetrans, detected by PCR assays in synovium from patients with rheumatoid arthritis and other rheumatic disorders. J Clin Pathol. 1996;49:824–828. doi: 10.1136/jcp.49.10.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Segal E D, Lange C, Covacci A, Tompkins L S, Falkow S. Induction of host signal transduction pathways by Helicobacter pylori. Proc Natl Acad Sci USA. 1997;94:7595–7599. doi: 10.1073/pnas.94.14.7595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shakhov A N, Collart M A, Vassalli P, Nedospasov S A, Jongeneel C V. NF-κB-type enhancers are involved in lipopolysaccharide-mediated transcriptional activation of the tumor necrosis factor alpha gene in primary macrophages. J Exp Med. 1990;171:35–47. doi: 10.1084/jem.171.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shibata K, Hasebe A, Sasaki T, Watanabe T. Mycoplasma salivarium induces interleukin-6 and interleukin-8 in human gingival fibroblasts. FEMS Immunol Med Microbiol. 1998;19:275–283. doi: 10.1111/j.1574-695X.1997.tb01097.x. [DOI] [PubMed] [Google Scholar]
- 38.Stacey K J, Ross I L, Hume D A. Electroporation and DNA-dependent cell death in murine macrophages. Immunol Cell Biol. 1993;71:75–85. doi: 10.1038/icb.1993.8. [DOI] [PubMed] [Google Scholar]
- 39.Strieter R M, Koch A E, Antony V B, Fick R B, Jr, Standiford T J, Kunkel S L. The immunopathology of chemotactic cytokines: the role of interleukin-8 and monocyte chemoattractant protein-1. J Lab Clin Med. 1994;123:183–197. [PubMed] [Google Scholar]
- 40.Su B, Karin M. Mitogen-activated protein kinase cascades and regulation of gene expression. Curr Opin Immunol. 1996;8:402–411. doi: 10.1016/s0952-7915(96)80131-2. [DOI] [PubMed] [Google Scholar]
- 41.Taylor-Robinson D, Schaeverbeke T. Mycoplasmas in rheumatoid arthritis and other human arthritides. J Clin Pathol. 1996;49:781–782. doi: 10.1136/jcp.49.10.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wise K S, Kim M F. Major membrane surface proteins of Mycoplasma hyopneumoniae selectively modified by covalently bound lipid. J Bacteriol. 1987;169:5546–5555. doi: 10.1128/jb.169.12.5546-5555.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wulczyn F G, Krappmann D, Scheidereit C. The NK-κB/Rel and IκB gene families: mediators of immune response and inflammation. J Mol Med. 1996;74:749–769. doi: 10.1007/s001090050078. [DOI] [PubMed] [Google Scholar]
- 44.Yao J, Mackman N, Edgington T S, Fan S T. Lipopolysaccharide induction of the tumor necrosis factor-alpha promoter in human monocytic cells. Regulation by Egr-1, c-Jun, and NF-κB transcription factors. J Biol Chem. 1997;272:17795–17801. doi: 10.1074/jbc.272.28.17795. [DOI] [PubMed] [Google Scholar]
- 45.Ye K, Dinarello C A, Clark B D. Identification of the promoter region of human interleukin 1 type I receptor gene: multiple initiation sites, high G+C content, and constitutive expression. Proc Natl Acad Sci USA. 1993;90:2295–2299. doi: 10.1073/pnas.90.6.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoshimura T, Matsushima K, Oppenheim J J, Leonard E J. Neutrophil chemotactic factor produced by lipopolysaccharide (LPS)-stimulated human blood mononuclear leukocytes: partial characterization and separation from interleukin-1 (IL-1) J Immunol. 1987;139:788–793. [PubMed] [Google Scholar]