Abstract
A 67-years-old woman developed subacute oculomotor nerve palsy and cerebellar gait instability while receiving avelumab as immunotherapy for Merkel cell carcinoma. Brain MRI revealed oculomotor nerve T2/FLAIR hyperintensity and contrast enhancement, CSF cell number and protein concentration were slightly increased. Antibodies against intracellular and surface antigens were excluded through commercial assays, but home-made immunohistochemistry on rat brain sections showed a “neurofilament-like” pattern. Antibodies against neuronal intermediate filament (NIF-IgG) were thus tested and resulted positive in both serum and CSF, confirming the diagnosis of NIF-IgG autoimmunity. Avelumab was discontinued and treatment with steroids and intravenous immunoglobulins led to partial improvement.
Keywords: Avelumab, Immune checkpoint inhibitors, Immune-related adverse events, Neuronal intermediate filament, Paraneoplastic neurological syndromes, paraneoplastic syndrome [221], checkpoint inhibitors, immune-related adverse effects
1. Introduction
The interaction between checkpoint proteins, such as programmed cell death protein 1 (PD-1) on T cells, or its ligand PD-L1 on tumor cells, help regulate immune responses. The binding of PD-1 to PD-L1 diminishes anti-cancer T cell responses. Immune checkpoint inhibitors (ICI) are novel oncological treatments that enhance T cell responses by inhibiting PD-1 or PD-L1 (Pardoll, 2012). Immune-related adverse events (iRAE) are inflammatory or autoimmune complications of these treatments. Among iRAE, neurological autoimmune manifestations encompass central or peripheral nervous system involvement and neuromuscular disorders (Sechi et al., 2020). These events may include myositis, myasthenic syndromes, peripheral and cranial neuropathies, CNS demyelination, and encephalitis. Notably, even though known anti-neural antibodies can be detected in a discrete proportion of these patients (Sechi et al., 2020), antibodies directed against novel antigens have recently been identified in this novel population of patients with autoimmune neurological disease (McKeon et al., 2021; Zekeridou et al, 2019).
2. Case description
We present the case of a 67-year-old woman who developed a cerebellar gait instability and third cranial nerve palsy while receiving avelumab, a PD1-L inhibitor, for metastatic Merkel cell carcinoma unified by positivity in serum and CSF for a novel biomarker of neuroendocrine carcinoma-related paraneoplastic neurological autoimmunity, known as neuronal intermediate filament (NIF) IgGs.
The patient was admitted to our Neurology Unit for a 1-week history of nausea and imbalance followed by diplopia and ptosis in her left eye of subacute onset. Symptoms began 8 days after receiving the second dose of avelumab, administered for a lymph nodal metastatic relapse of cutaneous Merkel cell carcinoma. Neurological examination revealed gait ataxia and left oculomotor nerve palsy with ptosis. Brain MRI showed a T2/FLAIR hyperintensity of the left oculomotor nerve (Figure 1 a) with contrast enhancement, while no abnormalities were found in brainstem and cerebellum (Figure 1 b). CSF analysis revealed slight increased white cell count (10 mononuclear cells/ul, normal value: <5) and protein concentration (0.79 g/L, normal value: 0.15–0.45). Systemic autoimmune, endocrinological, and infectious conditions were ruled out. Although anti-acetylcholine receptor, anti-muscle-specific kinase (radioimmunoassay and cell-based assay, respectively, Euroimmun), onconeural (immunoblots, Euroimmun) and neural surface antibodies (fixed cell-based assays, Euroimmun) resulted negative, serum and CSF in-house immunohistochemistry performed at the Neuropathology and Neuroimmunology laboratory, University of Verona, Italy, as previously described, on rat cerebellum, brain, and dorsal root ganglia (Ruiz-Garcia, 2021) revealed a “neurofilament-like” pattern (Figure 1 c–e). Serum and CSF samples were thus tested at the Neuroimmunology Laboratory, Mayo Clinic (Rochester, Minnesota, USA) for antibodies to neuronal intermediate filament (NIF-IgG) with cell-based assays and resulted positive for neurofilament-light and neurofilament-heavy chain on serum, and for neurofilament-light (NF-L) and neurofilament-heavy chain and alpha internexin in CSF. The patient was diagnosed with paraneoplastic NIF-autoimmunity in association with Merkel cell carcinoma in the context of avelumab treatment.
Figure 1.
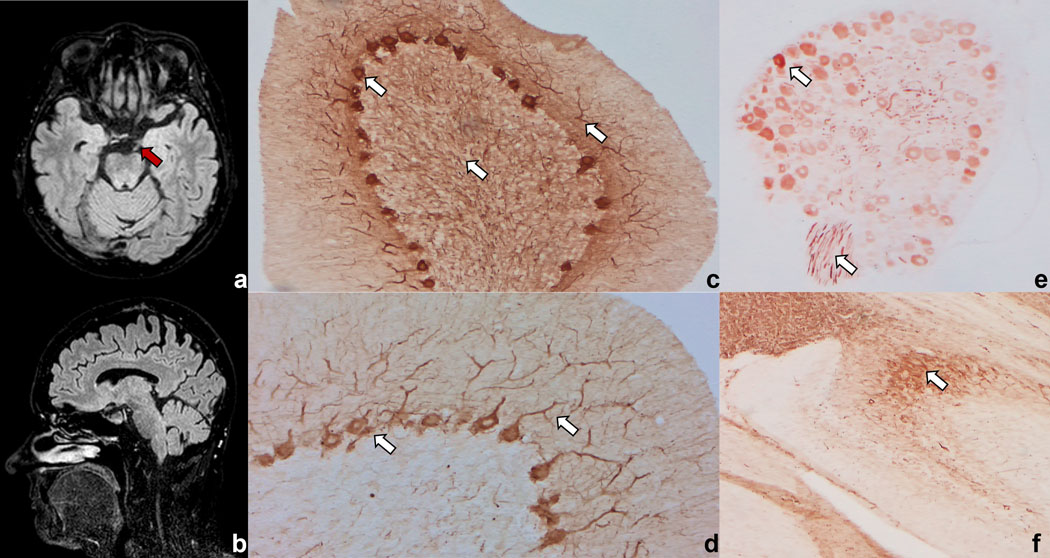
Brain MRI showing T2 hyperintensity of the left oculomotor nerve (red arrow), with contrast enhancement (not shown) (a) in absence of cerebellum or brainstem lesions (b). In-house immunohistochemistry on rat brain and cerebellum reveals a “neurofilament-like” pattern (white arrows) in the cerebellum with staining of Purkinje cells, granular, and molecular layers (c, d), in dorsal root ganglia (e), and in the hippocampus (f).
Treatment with intravenous steroids (methylprednisolone 1000 mg for 5 days) followed by slow oral tapering and intravenous immunoglobulins (0.4 g/Kg/day for 5 days) were administered because of suspicion for avelumab-related iRAE, with substantial benefit. In particular, gait imbalance rapidly improved while diplopia and ptosis slowly improved. Treatment with ICI was discontinued, and the patient received local radiotherapy for the lymph node metastasis with complete regression. At last follow-up, one year later, Merkel cell carcinoma was still in remission and neurological examination revealed only mild gait ataxia and ptosis in the left eye with inconstant vertical diplopia. Immunohistochemistry repeated on serum samples obtained at last follow-up still demonstrated a “neurofilament-like” pattern.
3. Discussion
NIF-IgG autoimmunity encompasses different clinical phenotypes including encephalopathy, ataxia/brainstem symptoms, and myeloradiculitis (McKeon et al, 2021; Basal et al, 2018). Cranial neuropathy has been reported in only a few cases with NIF-IgG, all with facial nerve involvement. Cranial neuropathy is an unusual and not predominant complication of iRAE being the facial, vestibulocochlear, and optic nerves the most frequently affected (Vogrig et al., 2021).
The NIF-IgG profile detected included positivity for all the 3 NIF subtypes tested. This profile, in particular when NF-L- IgG positivity is detected in CSF, is strongly associated with neuroendocrine-lineage carcinomas such as small cell lung cancer and Merkel cell carcinoma. So far, NIF-IgG autoimmunity has been described in 5 patients receiving ICI, three of whom developed symptoms during treatment with pembrolizumab for Merkel cell carcinoma, one treated with nivolumab plus ipilimumab for lung rhabdoid adenocarcinoma, and one under treatment with an unspecified CTLA-4 inhibitor for melanoma. All these patients presented with encephalopathy, ataxia or encephalomyelitis and had variable response to immunotherapy (Sechi et al., 2021; McKeon et al, 2021; Basal et al, 2018). In the case herein described, although the short time from avelumab initiation and the occurrence of neurological adverse event may challenge a causal association, the compatible clinical syndrome, the presence of specific autoantibodies, and the association with a peculiar neuroendocrine neoplasm support the relation between cancer, immunotherapy, and neurological symptoms.
Our patient had subacute gait instability and involvement of the oculomotor nerve that expands the spectrum of NIF-IgG autoimmunity and, in addition, includes avelumab as a potential trigger of NIF-IgG autoimmunity.
Of note, our report emphasizes the importance of searching for novel antibodies in patients experiencing neurological iRAE using home-made high sensitive technique, as tissue-based assays, since well-known antibodies generally detected by commercial single-antigen specific assays may yield negative results (Sechi et al., 2021; Wilson et al., 2018).
Funding source:
this research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Footnotes
Disclosures: The authors report no competing interests in relation to this study.
References
- Basal E, Zalewski N, Kryzer TJ, Hinson SR, Guo Y, Dubey D, Benarroch EE, Lucchinetti CF, Pittock SJ, Lennon VA, McKeon A, 2018. Paraneoplastic neuronal intermediate filament autoimmunity. Neurology 91, E1677–E1689. 10.1212/WNL.0000000000006435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeon A, Shelly S, Zivelonghi C, Basal E, Dubey D, Flanagan E, Madhavan AA, Mariotto S, Toledano M, Tracy JA, Zekeridou A, Pittock SJ, 2021. Neuronal intermediate filament IgGs in CSF: Autoimmune Axonopathy Biomarkers. Ann. Clin. Transl. Neurol 8, 425–439. 10.1002/acn3.51284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardoll DM, 2012. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 12, 252–264. 10.1038/nrc3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-García R, Muñoz-Sánchez G, Naranjo L, Guasp M, Sabater L, Saiz A, Dalmau J, Graus F, Martinez-Hernandez E, 2021. Limitations of a Commercial Assay as Diagnostic Test of Autoimmune Encephalitis. Front. Immunol 12, 1–8. 10.3389/fimmu.2021.691536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sechi E, Markovic SN, McKeon A, Dubey D, Liewluck T, Lennon VA, Lopez-Chiriboga AS, Klein CJ, Mauermann M, Pittock SJ, Flanagan EP, Zekeridou A, 2020. Neurologic autoimmunity and immune checkpoint inhibitors: Autoantibody profiles and outcomes. Neurology 95, e2442–e2452. 10.1212/WNL.0000000000010632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogrig A, Muñiz-Castrillo S, Joubert B, Picard G, Rogemond V, Skowron F, Egri M, Desestret V, Tilikete C, Psimaras D, Ducray F, Honnorat J, 2021. Cranial Nerve Disorders Associated With Immune Checkpoint Inhibitors. Neurology 96, e866–e875. 10.1212/WNL.0000000000011340 [DOI] [PubMed] [Google Scholar]
- Wilson R, Menassa DA, Davies AJ, Michael S, Hester J, Kuker W, Collins GP, Cossins J, Beeson D, Steven N, Maddison P, Rinaldi S, Jacob S, Irani SR, 2018. Seronegative antibody-mediated neurology after immune checkpoint inhibitors. Ann. Clin. Transl. Neurol 5, 640–645. 10.1002/acn3.547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zekeridou A, Kryzer T, Guo Y, Hassan A, Lennon V, Lucchinetti CF, Pittock S, McKeon A, 2019. Phosphodiesterase 10A IgG: A novel biomarker of paraneoplastic neurologic autoimmunity. Neurology 93, e815–e822. 10.1212/WNL.0000000000007971 [DOI] [PMC free article] [PubMed] [Google Scholar]


