Abstract
Structural and functional alterations to the gut microbiome, referred to as gut dysbiosis, have emerged as potential key mediators of neurodegeneration and Alzheimer disease (AD) pathogenesis through the “gut -brain” axis. Emerging data from animal and clinical studies support an important role for gut dysbiosis in mediating neuroinflammation, central and peripheral immune dysregulation, abnormal brain protein aggregation, and impaired intestinal and brain barrier permeability, leading to neuronal loss and cognitive impairment. Gut dysbiosis has also been shown to directly influence various mechanisms involved in neuronal growth and repair, synaptic plasticity, and memory and learning functions. Aging and lifestyle factors including diet, exercise, sleep, and stress influence AD risk through gut dysbiosis. Furthermore, AD is associated with characteristic gut microbial signatures which offer value as potential markers of disease severity and progression. Together, these findings suggest the presence of a complex bidirectional relationship between AD and the gut microbiome and highlight the utility of gut modulation strategies as potential preventative or therapeutic strategies in AD. We here review the current literature regarding the role of the gut-brain axis in AD pathogenesis and its potential role as a future therapeutic target in AD treatment and/or prevention.
Keywords: Gut microbiome, Alzheimer disease, Age, Lifestyle, Prevention
1. Introduction
Alzheimer’s disease (AD) is the most common cause of dementia in individuals above the age of 65 years, and results in progressive memory and cognitive impairment, behavioral changes, and functional decline (Tarawneh and Holtzman, 2012). From a neuropathological perspective, AD is characterized by the abnormal aggregation of extracellular amyloid, in the form of amyloid plaques, and intracellular hyper-phosphorylated tau protein in the form of neurofibrillary tangles, eventually leading to synaptic dysfunction and neuronal death (Price et al., 2001). As populations are aging, and in the absence of effective methods for disease prevention or treatment, AD has become a global health epidemic and a major cause of morbidity and mortality among elderly (Tarawneh and Holtzman, 2012).
Late-onset sporadic AD is a multifactorial disease that results from a complex interaction of genetic, lifestyle, and environmental factors (Tarawneh and Holtzman, 2012). Among the environmental factors implicated in AD pathogenesis, rapidly growing evidence from animal and human data suggests an important role for the gut microbiome in the onset and progression of AD pathology and supports the notion that alterations to the gut microbiome can influence central nervous system (CNS) homeostasis and disease pathogenesis through a “gut -brain axis” (Carabotti et al., 2015) (Fig. 1).
Fig. 1.

The Gut-Brain Axis. A schematic diagram demonstrating interactions between the brain and the gut via the gut-brain axis, including interactions mediated by gut secretion of toxins and metabolites, and gut modulation of the immune system leading to migration of immune cells and inflammatory markers across the blood-brain barrier. The central nervous system modulates gut motility and secretion via the enteric nervous system and the hypothalamic-pituitary axis. LPS, lipopoly-saccharide. Created with BioRender.com.
The human intestines contain approximately 1000 species and 7000 strains of bacteria which constitute the gut flora, with gram-positive or gram-negative Firmicutes (including the genus Lactobacillus, Clostridium, and Eubacterium) and gram-negative Bacteroidetes (including Bacteroides and Prevotella) being the most predominant (Huttenhower et al., 2012; Askarova et al., 2020). Dysregulation of the gut microbiome reflected by changes in the diversity and frequency of microorganism taxa and species which constitute the gut flora, also referred to as “gut dysbiosis”, has been associated with abnormal brain protein aggregation, inflammation, immune dysregulation, and impaired neuronal and synaptic activity in animal and human studies of AD (Gubert et al., 2020; Cryan et al., 2020). Furthermore, studies suggest that the presence of AD pathology is associated with characteristic changes to the gut microbiome leading to relatively disease-specific metabolic signatures which may serve as potential AD biomarkers (Vogt et al., 2017).
In this review, we will summarize the current and rapidly growing literature regarding the complex, and often bidirectional, relationship between the gut microbiome and AD, and describe various disease mechanisms and pathways by which the gut microbiome may contribute to AD pathogenesis from both animal and clinical studies. We also review effects of aging and environmental factors including diet, exercise, sleep, and stress on the gut-brain axis, and discuss potential strategies for gut modulation which may have a beneficial role in future AD prevention or treatment trials. Finally, we discuss limitations of previous studies of the gut microbiome in AD and provide directions for future research.
2. Evidence supporting the bi-directional relationship between the gut microbiome and AD
2.1. Animal studies
Data from animal studies support the notion that the gut microbiome contributes to cognitive impairment and the progression of AD pathology, including amyloid and tau aggregation, immune dysregulation, and neuroinflammation (Kowalski and Mulak, 2019; Wang, 2022). Significant alterations to the gut microbiome which may result from dietary changes, medication use, or infection have also been directly associated with synaptic dysfunction and cognitive or behavioral impairment in animal models of AD (Gareau, 2014).
Infection with Enterobacteria exacerbated AD pathology in a Drosophila AD model by promoting immune hemocyte recruitment into the brain, inflammation, and tumor necrosis factor (TNF)- and c-jun N-terminal kinase (JNK)-mediated neurodegeneration (Wu et al., 2017). Increased gut bacterial load and higher circulating levels of antimicrobial peptides targeting gram-negative bacteria have been reported in Drosophila tauopathy models, suggesting the presence of an enhanced innate immune response to gut flora (Rydbom et al., 2021). The gut microbiome also regulates the trafficking of interleukin (IL) 17-producing γδ-T cells from the gut into the meninges which triggers astrocytic release of IL-17, chemokine receptor 6 (CCR6)-mediated neuroinflammation, and synaptic dysfunction (Cipollini et al., 2019). In a study by Wang et al., intestinal dysbiosis induced by 1 month of ampicillin administration was associated with reduced hippocampal expression of the N-methyl-D-aspartic acid (NMDA) receptor, increased anxiety, and spatial memory impairment in rats, while the enrichment of the gut microbiome with Lactobacillus fermentum NS9 ameliorated these changes (Wang et al., 2015a). Infection with the pathogenic bacteria Citrobacter rodentium resulted in stress-induced memory disturbances in a C57BL/6 mouse model (Gareau et al., 2011). Additionally, in germ-free Swiss-Webster mice deprived of intestinal bacteria, spatial and working memory impairments were accompanied by reduced brain-derived neurotrophic factor (BDNF) expression which is associated with impaired synaptic plasticity (Gareau et al., 2011).
Other alterations to the gut microbiota have been shown to be beneficial in improving cognition or reducing AD pathology (Kowalski and Mulak, 2019). Treatment of APP/PS1 mice with antibiotics significantly reduced Aβ deposition and increased soluble Aβ levels even in older mice and was associated with less reactive gliosis around amyloid plaques, altered microglial morphology, and lower levels of peripheral inflammatory mediators (Minter et al., 2016, 2017). Prebiotic supplementation of APP/PS1 transgenic mice with oligosaccharides derived from Morinda officinalis maintained gut microbiota diversity, reduced Aβ pathology, and ameliorated neuronal apoptosis (Xin et al., 2018). Significant improvement in cognitive deficits was reported in specific pathogen-free Sprague-Dawley rats when their diets were enriched with Lactobacillus helveticus NS8 (Liang et al., 2015). The administration of both Lactobacillus and Bifidobacterium improved memory and learning deficits and reduced oxidative stress associated with intra-hippocampal injection of Aβ in a rat model of AD (Athari Nik Azm et al., 2018). Other studies have shown that the probiotic Bifidobacterium longum 1714 improved cognitive function in male BALB/c mice (Savignac et al., 2015) and that transplantation of fecal microbiota from wild-type to AD transgenic mice was associated with reduced amyloid and tau pathologies and improved cognition (Kim et al., 2020).
In a study by Neufeld et al., germ-free mice exhibited improved behaviors and lower anxiety associated with reduced expression of the NMDA receptor subunit, NR2B, in the central amygdala, increased BDNF, and decreased serotonin receptor 1 A (5HT1A) hippocampal expression compared to their conventionally-reared specific pathogen-free counterparts (Neufeld et al., 2011). When APP/PS1 mice are engineered to become germ-free, a decrease in Aβ pathology is observed, while colonizing germ-free APP/PS1 mice with microbiota from conventionally raised transgenic mice increases brain Aβ pathology (Harach et al., 2017). In another study, the oral administration of Bifidobacterium breve A1 strain prevented Aβ-induced cognitive impairment and attenuated behavioral deficits in an AD mouse model (Kobayashi et al., 2017). Similarly, in a rat AD model induced by intraperitoneal injection of D-galactose, treatment with Lactobacillus plantarum MTCC 1325 reduced Aβ plaque formation, restored brain acetylcholine levels, and improved cognition (Nimgampalle and Kuna, 2017). In these studies, molecular and pathological changes associated with cognitive improvement include decreased plasma levels of corticosterone and adrenocorticotropic hormone (ACTH), normalization of serotonin and norepinephrine brain expression, increased hippocampal BDNF expression, and reduced neuronal apoptosis (Askarova et al., 2020). Table 1 summarizes the main findings from animal studies examining the relationship between AD and the gut microbiome.
Table 1.
Summary of Animal Studies.
| APP/PS1 mice |
|
| 3xTg AD-mice |
|
| 5xFAD mice |
|
| AD mouse model (intra-cerebroventricular Aβ injection) |
|
| APOE−/− mice |
|
| APP NL-F and APP NL-G-F Mice |
|
| C57BL/6 Mice |
|
| Swiss Webster Germ-free mice |
|
| P301L mice |
|
| Rat Models |
|
| Drosophila models |
|
Abbreviations: APP, amyloid precursor protein; PS1; presenilin-1; Tg, transgenic; RAGE, Receptors for Advanced Glycation End-products; APP NL-F Mice, Human amyloid precursor protein (hAPP) knock-in mice which contain the Swedish and Iberian mutations; APP NL-G-F, Human APP knock-in mice which contain the Arctic mutation as third mutation; APOE, apolipoprotein E; NMDA, N-methyl-D-aspartate; SCFA, short-chain fatty acids; Aβ, amyloid-β peptide.
Evidence supporting a bi-directional relationship between the gut microbiome and AD is mostly derived from transgenic AD animal studies which examine the temporal relationship between gut microbiome structure and AD pathology in strictly controlled specific pathogen-free settings. Studies have shown that AD pathology influences the gut microbiome shifting it towards configurations that overlap with those seen in autism and inflammatory disorders (Bäuerl et al., 2018). Alterations to the gut microbiome in APP/PS1 transgenic AD mice compared to wild-type mice include increases in Bacteroidetes and Tenericutes phyla and reductions in Actinobacteria, Firmicutes, Verrucomicrobia, and Proteobacteria which are observed as early as 8 months of age (Harach et al., 2017). Alterations in gut microbiota with aging and increased gut amyloid precursor protein (APP) expression have also been reported in 5xFAD mice (Brandscheid et al., 2017). Interestingly, data from AD animal models suggest that the gut microbiome of AD mice is enriched with pro-inflammatory species (e.g. Escherichia-Shigella, Desulfovibrio, Akkermansia, and Blautia) and that gut dysbiosis is present early in life, increases with age, and often precedes the first signs of cortical Aβ deposition or microglial activation (Chen et al., 2020). Therefore, it has been postulated that AD pathology or AD-causing mutations in mouse models induce gut microbiome alterations which subsequently may contribute to further AD progression in a “forward-feedback” loop. This notion is supported by observations that gut microbial changes are often observed prior to brain amyloid aggregation, and that animals with absent intestinal flora develop less amyloid pathology than those with existing or replaced gut microbiomes (Harach et al., 2017; Chen et al., 2020; Dodiya et al., 2019, 2020). Further research is needed to clarify the extent to which AD mutations or brain AD pathology influence the gut microbiome and the mechanisms that underlie these changes.
2.2. Clinical studies
Consistent with animal data, results of clinical studies support an important role for the gut microbiome in AD pathogenesis and the presence of a gut microbiome “signature” for AD (Vogt et al., 2017). Numerous studies report the increased prevalence of bacterial lipopolysaccharide (LPS) in AD brain lysates compared to controls, including the accumulation of LPS in neocortical and hippocampal neurons and its co-localization with Aβ in amyloid plaques and perivascular Aβ aggregates (Zhan et al., 2016; Zhao et al., 2017a,b). Associations with other pathogens, including Chlamydia pneumoniae, Borrelia burgdorferi, spirochetes, herpes simplex type 1, and several others have also been reported in post-mortem AD brains (Hammond et al., 2010; Miklossy, 2016; Zhan et al., 2016). However, causal relationships and a full understanding of the mechanisms by which the gut microbiota influence brain pathology cannot be elucidated from post-mortem studies.
Conversely, recent studies in living individuals with AD have provided important insights into the role of the gut microbiome in AD pathology. Significant differences in the composition of the gut microbiomeat both the phylum and species levels-have been observed in individuals with AD compared to healthy controls in cross-sectional studies (Vogt et al., 2017; Kowalski and Mulak, 2019). In a recent study of individuals with late-onset AD and matched controls, which utilized bacterial 16 S ribosomal RNA (rRNA) gene sequencing of stool samples (Vogt et al., 2017), reductions in gut microbial diversity and significant differences in the abundance of 82 operational taxonomic units (OTUs) were observed in AD compared to controls, including differences at the phylum, family, and genus levels. Differences at the phylum level included a decreased abundance of Firmicutes and Actinobacteria, and an increased abundance of Bacteroidetes. Within Firmicutes, AD samples showed less abundance of the families Ruminococcaceae, Turicibacteraceae, Peptostreptococcaceae, Clostridiaceae, and Mogibacteriaceae, and the genera SMB53 (family Clostridiaceae), Dialister, Clostridium, Turicibacter, and cc115 (family Erysipelotrichaceae), and more abundance of the family Gemellaceae and the genera Blautia, Phascolarctobacterium, and Gemella compared to controls. Within Bacteroidetes, Bacteroidaceae and Rikenellaceae at the family level, and Bacteroides and Alistipes at the genus level were more abundant in AD. Within Actinobacteria, AD samples had lower abundance of the Bifidobacteriaceae at the family level and Bifidobacterium and Adlercreutzia at the genus level. Conversely, the genus Bilophila in the phylum Proteobacteria was more abundant in AD samples compared to controls. Furthermore, gut microbial alterations correlated with disease severity and cerebrospinal fluid (CSF) markers of amyloid (i.e., lower CSF Aβ42/Aβ40 levels) and tau (higher CSF p-tau181 levels) pathology with the strongest correlations being observed with Blautia, SMB53 and Dialister bacterial load. In this cohort, increased Bacteroides, and decreased Turicibacter and SMB53, bacterial populations also correlated with higher CSF YKL-40 levels, reflective of more severe astrocytic activation in AD (Vogt et al., 2017). Differences in intestinal populations of Bacteroides, Actinobacteria, Ruminococcus, Lachnospiraceae, and Selenomonadales phyla have also been reported in other cohorts (Zhuang et al., 2018). Consistent with these reports, a systematic meta-analysis of 11 observational and pre-interventional studies, which included 805 individuals with AD and healthy controls, found that individuals with AD dementia had lower gut microbial diversity compared to controls, more abundance of Proteobacteria, Bifidobacterium, and Phascolarcobacterium, and lower abundance of Firmicutes, Clostridiaceae, Lachnospiraceae, and Rikenellaceae compared to controls (Hung et al., 2022). Conversely, this meta-analysis suggested no significant differences in gut microbial diversity between individuals with mild cognitive impairment (MCI) and healthy controls. For several microbiota (i.e., Proteobacteria, Phascolarcobacterium, and Clostridiaceae), altered microbial abundance was found to scale with progression from normal cognition to MCI to AD dementia.
Higher intestinal levels of the pro-inflammatory Escherichia-Shigella, and lower levels of the anti-inflammatory Eubacterium rectale, have been associated with peripheral markers of inflammation, including interleukin (IL)-1β, IL-6, C-X-C motif chemokine ligand-2 [CXCL2], and NLRP3 (NOD-like Receptor [NLR] Family Pyrin Domain-Containing Protein 3) inflammasome in cognitively impaired older adults with amyloidosis compared to those without amyloidosis and healthy controls (Cattaneo et al., 2017). Other reports suggest that AD is associated with significant elevations in serum IgG antibodies targeting Fusobacterium nucleatum and Prevotella intermedia (Sparks Stein et al., 2012). Studies examining the associations of Helicobacter pylori (H. pylori) with AD have been conflicting. While previous studies suggest possible associations of H.pylori infection with AD, and higher CSF or serum H. pylori-specific IgG antibody titers with more severe cognitive impairment (Kountouras et al., 2009; Roubaud-Baudron et al., 2012), these findings were not confirmed in a recent large population-based study which showed no association between H. pylori infection and dementia risk (Fani et al., 2018).
In addition to quantitative and qualitative differences in microbial taxa, functional differences in the gut microbiome across different taxa and species have also been reported in association with AD pathology (Liu et al., 2019a). In one study which conducted functional pathway analyses of the gut microbiome using KEGG (Kyoto Encyclopedia of Genes and Genomes) and PICRUSt (Phylogenetic Investigation of Communities by Reconstruction of Unobserved States), the gut microbiome of individuals with AD was enriched with orthologs involved in LPS biosynthesis (glycan biosynthesis and metabolism) and the bacterial secretion system (membrane transport), while orthologs related to N-glycan biosynthesis, phenylalanine, tyrosine, and tryptophan biosynthesis and histidine metabolism were downregulated in AD compared to healthy controls (Liu et al., 2019a). Other studies have shown that disease-specific signatures for AD can be identified by combining clinical data with gut microbial features. The AlzBiom study combined taxonomic and functional gut microbial assessments measured by shotgun metagenomics with clinical data from 75 amyloid-positive individuals with AD dementia and 100 healthy controls. Findings from this study suggested the presence of an AD-specific signature consisting of 18 genera, 17 Gene Ontology features, and 26 KEGG ortholog features, which when combined with clinical data (i.e., age, sex, body mass index, and the apolipoprotein E4 [APOE4] genotype) discriminated AD from controls with a diagnostic accuracy of 80–92% (Laske et al., 2022).
A recent study which utilized 16 S Ribosomal RNA sequencing to examine the gut microbiome found that gut microbiome alterations were detectable in the early preclinical stages prior to the onset of cognitive impairment (e.g., increased relative abundance of Bacteroidetes and decreased abundance of Firmicutes and class Deltaproteobacteria). When the gut microbiome profile was combined with cognitive status and plasma Aβ levels, the combination of these markers differentiated preclinical AD from healthy controls with a diagnostic accuracy of 87% (Sheng et al., 2022).
Cognitively impaired individuals with AD, including those with MCI, exhibit distinctive gut metabolomic signatures compared to healthy controls. CSF levels of the gut metabolite, trimethylamine N-oxide (TMAO), are elevated in individuals with AD (Vogt et al., 2018), and correlate with CSF markers of amyloid (i.e. CSF Aβ42) and tau (i.e. p-tau181) pathology, and neurodegeneration (i.e. total tau and neuro-filament light chain). Plasma TMAO levels are also elevated in aging mice and may reflect oxidative stress and mitochondrial dysfunction associated with senescence (Li et al., 2018). Studies which examined fecal metabolomics have found significant differences in the levels of tryptophan metabolites, especially those involving indole derivatives and serotonin synthesis, and lithocholic acid in stool samples from AD compared to controls, which correlated with gut dysbiosis and cognitive impairment (Wu et al., 2021; Pappolla et al., 2021). Levels of the tryptophan metabolite, indole-3-pyruvic acid, predicted AD pathology and were progressively higher in MCI and AD dementia compared to healthy controls. Conversely, the indole derivatives, acting as the aryl hydrocarbon receptor (AhR) ligands, were reduced in the presence of AD pathology (Wu et al., 2021). In this study, levels of 5 short-chain fatty acids (formic acid, acetic acid, propanoic acid, 2-methylbutyric acid, and isovaleric acid) were found to be strongly predictive of clinical progression from MCI to AD dementia (Wu et al., 2021). Altered CSF levels of tryptophan metabolites were also reported in other AD cohorts (Kaddurah-Daouk et al., 2011). The main findings from clinical studies examining the relationship between AD and the gut microbiome are summarized in Table 2.
Table 2.
Summary of Clinical Studies.
| Post-mortem AD brains |
|
| Living AD Human Participants |
|
Abbreviations: LPS, lipopolysaccharide; TMAO, trimethylamine-N-oxide; CSF, cerebrospinal fluid; Aβ, amyloid-β peptide.
In contrast to animal studies which provide direct evidence for brain AD pathology influencing the gut microbiome, elucidating the temporal relations between AD pathology and gut microbial alterations in clinical cohorts is particularly challenging given the limited number of studies, small cohorts, unstandardized study methods in cohort characterization and microbiome analyses, and the cross-sectional study design which limits the ability to track gut microbial alterations associated with healthy aging across middle and late life, and contrast them to those associated with disease onset or progression. Therefore, while several studies have shown quantitative and qualitative differences in the gut microbiome of individuals with AD compared to controls, in the absence of longitudinal evaluations, it remains unclear whether these are the cause or result of AD pathology. Examination of gut microbial alterations early in life in individuals with dominantly inherited forms of AD (autosomal dominant AD [ADAD]; caused by autosomal dominant mutations in APP, presenilin-1 [PSEN1], or presenilin-2 [PSEN2]) represents an excellent opportunity to elucidate the effects of AD -causing mutations on gut microbiota early in life prior to the onset of AD pathology in the brain and to differentiate these from gut microbiota associated with healthy aging. Unfortunately, there is a scarcity of data regarding the gut-brain axis in ADAD cohorts, which, therefore, represents an important area for future research. Nevertheless, a few recent studies have examined the gut microbiome in individuals with Down’s syndrome (i.e., Trisomy 21), almost all of whom develop AD pathology by the fifth decade of life due to the presence of an additional copy of APP on chromosome 21. Findings from a study of Chinese children with Down’s syndrome suggested the presence of significant differences in the structure and diversity of the gut microbiome compared to controls, including a lower abundance of Acidaminococcaceae and increased modules involved in peptidases and pyrimidine metabolism (Ren et al., 2022). Importantly, gut microbiota alterations were closely associated with the severity of cognitive impairment in this cohort. Similar studies which include fluid or imaging markers of AD pathology in dominantly inherited (i.e., ADAD) or genetic forms (i.e., Trisomy 21) of AD will better elucidate the bi-directional relationship between AD-causing mutations and the gut microbiome prior to the development of significant AD pathology. Furthermore, longitudinal studies of well-characterized AD cohorts, encompassing those with early-onset inherited and late-onset sporadic forms of the disease, will provide valuable insight into the extent to which microbial alterations in cognitively normal older adults may serve as predictive markers for AD pathology and/or viable targets for potential disease modification.
Despite their anatomical continuity, studies suggest that the oral and gut microbiome profiles are distinct, being separated by the “oral-gut barrier”, and interdependently influence AD pathology (Park et al., 2021). There is growing evidence from epidemiological and experimental studies that the oral microbiome may also be associated with AD pathology and cognition (Kowalski and Mulak, 2019; Sureda et al., 2020). Periodontal disease is observed with higher frequency in patients with neurodegenerative disorders and has been associated with the severity of cognitive impairment in epidemiological studies (Ide et al., 2016). Individuals with periodontitis of 10 years were found to have a 1.7-fold increased risk of developing AD in one study (Chen et al., 2017), and those with periodontitis and gingivitis were at a higher risk of developing all-cause dementia in another study (Tzeng et al., 2016). Noble et al. found that higher serum titers of periodontal anti--Actinomyces naeslundii and anti-Eubacterium nodatum IgG were associated with a higher, and lower, risk for AD, respectively (Noble et al., 2014). Kamer et al. reported associations between periodontal disease and brain Aβ load using the positron emission tomography (PET) amyloid ligand Pittsburgh Compound B in cognitively normal older adults (Kamer et al., 2015). In a recent report, antibodies for Porphyromonas gingivalis, the most common pathogenic periodontal bacteria, were elevated in the serum of AD patients and an enzyme, gingipain, produced by P. gingivalis, was found in post-mortem AD brains (Singhrao and Olsen, 2019; Dominy et al., 2019). Other studies demonstrate differences in the prevalence of the Moraxella, Leptotrichia, and Sphaerochaeta genera in AD compared to controls (Liu et al., 2019b), and associations between oral dysbiosis and the progression of AD pathology (Bathini et al., 2020), or conditions such as diabetes mellitus (DM) and atherosclerosis which are associated with a higher risk for AD (de Groot et al., 2017; Fåk et al., 2015). While these findings suggest the presence of a potential association between oral bacteria and AD, there is limited data to support a cause-effect relationship and further research in this area is warranted.
3. Proposed mechanisms linking the gut microbiome to AD
Gut dysbiosis contributes to AD pathogenesis and cognitive impairment via several mechanisms, including immune dysregulation, neuroinflammation, disruption of the intestinal and blood-brain barriers, amyloid and tau aggregation and toxicity, and impaired synaptic plasticity, neuronal excitability, and neurogenesis. Fig. 2 represents an overview of the various mechanisms by which the gut microbiota and gut toxins or metabolites exert peripheral and central effects leading to cognitive impairment.
Fig. 2.

Potential mechanisms by which the gut microbiome contributes to AD pathogenesis. Animal and clinical studies suggest several mechanisms by which the gut microbiome contributes to the onset and progression of AD pathology, including modulation of innate and adaptive immunity, neuroinflammation and the release of cytokines and other inflammatory mediators, increased blood-brain barrier permeability, reduced integrity of the intestinal barrier leading to “leaky gut” which facilities the translocation of toxins into the circulation, increased protein aggregation including amyloid, tau, and synuclein, and direct effects on synaptic plasticity, memory, and learning processes. LPS, lipopolysaccharide; PSA, polysaccharide A; p-gp, p-glycoprotein. Created with BioRender.com.
3.1. Immune system dysregulation
Emerging evidence from genetic, histopathological, and mechanistic studies supports an important role for immune dysregulation as a central and primary substrate in AD pathogenesis (Kinney et al., 2018; Lutshumba et al., 2021). Disturbances in innate immunity including cytokine signaling, immune cell proliferation and migration, and microglial activation are observed in animal models of AD (Heneka et al., 2015). Recent findings from large-scale genetic analyses in humans suggest that over half of the AD risk loci are significantly enriched or uniquely expressed in immune cells (Wightman et al., 2020). Genetic variants in the microglial receptor, triggering receptor expressed on myeloid cells-2 (TREM2), are associated with a 2–3-fold higher risk for AD (Abduljaleel et al., 2014). The gut microbiome influences innate and adaptive immunity, including a role in innate immune system priming through the formation of gut-associated lymphoid tissue and influencing adaptive local and systemic immune responses (Galland, 2014). Alterations to the gut microbiome are associated with increased penetration of peripheral Th1 immune cells into the blood-brain-barrier (BBB), increased microglial activation, Aβ aggregation, and cognitive decline in AD mouse models (Galland, 2014). The gut microbiome may alter the peripheral immune response through the release of cytokines, complement and major histocompatibility complex (MHC) proteins, and microbial toxins or metabolites, including LPS, polysaccharide A (PSA), and butyrate (van Olst et al., 2021).
LPS is a bacterial endotoxin which is produced by several gram-negative bacteria (e.g. Bacteroidetes), and is detected in amyloid plaques and peri-vascular Aβ aggregates in human AD brains (Zhan et al., 2016). Neocortical and hippocampal LPS levels are 7 and 21-fold higher, respectively, in lysates of AD brains compared to age-matched controls (Zhao et al., 2017a) and LPS colocalizes with amyloid within plaques and surrounding blood vessels (Zhan et al., 2016). LPS induces systemic inflammation through several pathways including the activation of Toll-like receptor (TLR) 4 signaling with the subsequent release of proinflammatory cytokines (e.g., IL-1, IL-6, and tumor necrosis factor-α [TNF-α]) and the activation of T helper17 (Th17) cells which contribute to neurodegeneration via activation of the apoptotic Fas pathway in neurons (Cani et al., 2007; Tristão et al., 2017; Zhang et al., 2013; Lukiw, 2016). LPS produced by Bacteroides fragilis activates the nuclear factor kappa B (NF-κB) pathway and induces the transcription of pro-inflammatory miRNAs, which interfere with microglial functions (Lukiw, 2016). As an example, miRNA-34a inhibits the ability of microglia to phagocytose Aβ (Bhattacharjee et al., 2016). Another mechanism by which LPS contributes to brain amyloid aggregation involves disrupting Aβ flux across the BBB and reducing brain amyloid clearance into the periphery (Jaeger et al., 2009) (see section on Amyloid and Tau Aggregation). Intraperitoneal injection of LPS increased brain Aβ levels, reduced neuronal counts, and induced cognitive deficits in C57BL/6J mice (Kahn et al., 2012; Zhao et al., 2019). In another study, intraventricular LPS administration with ascorbic acid increased the immunoreactivity of intraneuronal Aβ (Hauss-Wegrzyniak and Wenk, 2002). LPS-injected mice display cognitive deficits and higher brain and peripheral levels of pro-inflammatory cytokines (i.e., TNF-α and IL-1β), higher levels of prostaglandin E2 and nitric oxide (NO), and lower levels of the anti-inflammatory cytokines IL-4 and IL-10 (Zhao et al., 2019). Treatment with the TLR-4 specific inhibitory peptide, VIPER, prevented LPS-mediated inflammation and ameliorated cognitive impairment in these models (Zhao et al., 2019).
P-glycoprotein (P-gp) is an efflux transporter which is expressed in the brain endothelium (van Assema et al., 2012) and intestinal barrier and is involved in amyloid clearance across the BBB (Wang et al., 2016a; Cirrito et al., 2005). AD is associated with reduced brain endothelial expression and impaired function of P-gp which contributes to amyloid aggregation, and with lower levels of P-gp in the intestinal epithelium compared to healthy controls and individuals with other dementias (Chiu et al., 2015; Haran et al., 2019). Several constituents of the gut microbiome, mainly gram-negative Bacteroides, alter P-gp levels with variable effects observed across different species; increased abundance of B. dorei is associated with higher P-gp levels, while lower P-gp levels are associated with a higher abundance of B. fragilis and B. vulgatus (van Olst et al., 2021). Furthermore, in a clinical study, stool samples from individuals with AD had lower p-gp levels than controls and resulted in lower p-gp levels from in vitro samples of healthy controls and individuals with other dementias (Haran et al., 2019). In addition to its role in amyloid clearance, P-gp exerts regulatory homeostatic functions which suppress the immune response to gut bacteria by exporting endocannabinoids, and balance inflammatory pathways mediated by multidrug-resistant protein 2 [MRP2]/hepoxilin A3 (Szabady et al., 2018). Therefore, gut dysbiosis may contribute to brain amyloid deposition and altered immune homeostasis through p-gp dysregulation and the loss of its physiological transport and immunomodulatory functions.
Gram-negative Bacteroides fragilis also produce the capsular carbohydrate, PSA. PSA triggers a regulatory immune response via the toll-like receptor-2 (TLR-2) which includes activation of dendritic cells and regulatory T cells, suppression of Th17 cells, and decreased production of IL-17 (Shen et al., 2012). Conversely, CD4 + stimulation by PSA was associated with increased secretion of pro-inflammatory cytokines (interferon [IFN]-γ, TNF-α, IL-6 and CXCL10), and increased surface expression of anti-inflammatory mediators (Lag3, Tim3, and PD1) in one study suggesting complex, and possibly dual, effects of PSA on inflammatory pathways (Alvarez et al., 2020).
Short-chain fatty acids (SCFA), such as butyrate, acetate, and propionate, play an important role in maintaining the structural and functional integrity of the gut microbiome, and mediating many of its immunomodulatory functions (Tan et al., 2014). Multidimensional data-driven models which utilize a systems-based approach identified over 8000 important interactions of microbial metabolites with AD pathways and ranked SFCAs among the most highly prioritized microbial metabolites associated with AD (Wang et al., 2021a). F. prausnitzii, E. rectale, and Lachnospiraceae are among the most important butyrate-producing gut bacteria and exert important regulatory effects on the peripheral immune system (Liu et al., 2018; Atarashi et al., 2013). Butyrate administration increases transforming growth factor (TGF)-β-dependent differentiation of Treg cells in cell cultures (Kespohl et al., 2017), inhibits NF-κB signaling in the intestinal epithelium (Segain et al., 2000), and is associated with higher plasma TGF-β levels and lower plasma IL-6, IL-17, and IL-23 levels (Zhang et al., 2016). Dendritic cell cultures treated with butyrate demonstrate increased IL-10 production by CD4 + cells, decreased IL-17 production, and reduced differentiation of naïve T cells into pro-inflammatory IFN-γ-producing phenotypes (Gurav et al., 2015; Kaisar et al., 2017). In LPS-treated rats, butyrate administration is associated with reduced expression of cytokine-induced neutrophil chemoattractant (CINC) 2αβ, TNF-α, and NO (Vinolo et al., 2011). Butyrate has been shown to mitigate inflammation in LPS-treated macrophages via inhibition of histone deacetylase, subsequent downregulation of IL-6, IL-12, and NO synthase 2, and G-protein receptor mediated inhibition of the NF-kB signaling pathway (Siddiqui and Cresci, 2021). While butyrate exerts predominantly anti-inflammatory effects under normal physiological conditions, interestingly-higher doses of butyrate may trigger inflammation via induction of IFN- γ and T-bet expression (Kespohl et al., 2017). SCFAs can also modulate T cell fate through G-coupled protein receptor signaling (GPR41/GPR43) and epigenetic modifications (Kim et al., 2013). Carriers of the APOE4 allele, the most significant genetic risk factor for AD, have significantly lower abundance of butyrate-producing Ruminococcacaea compared to APOE2/3 carriers (Tran et al., 2019).
Another mechanism by which the gut microbiome may influence brain pathology is through altered metabolism of bile acids. Serum and stool levels of secondary bile acids, which are produced through the deconjugation of primary bile acids by gut bacteria, are elevated in the presence of high anaerobic content of gut microbiota (Heinken et al., 2019). In a study of 1464 individuals with MCI or dementia due to AD and healthy controls, lower levels of cholic acid, and higher levels of the 7α-dehydroxylated counterpart, deoxycholic acid, were observed in AD compared to controls (MahmoudianDehkordi et al., 2019). Furthermore, higher levels of deoxycholic acid damage the tight junctions of the intestinal barrier and contribute to cognitive impairment by BBB disruption and translocation into the brain (Quinn et al., 2014; Raimondi et al., 2008; Stenman et al., 2013).
In addition to the generation of toxic metabolites, recent studies suggest that gut dysbiosis may influence peripheral immunity via amino acid secretion. High levels of phenylalanine and isoleucine (Phe/Ile) increase Th1 cells and promote neuroinflammation, while treatment with GV-971, a marine-derived oligosaccharide, reconditions the gut microbiota and reduces detrimental effects of amino acids on peripheral immunity (Wang et al., 2019). In a study by Wang et al (Wang et al., 2019), gut dysbiosis, reflected by an increased ratio of Firmicutes to Bacteroidetes, was evident at 7 months of age and coincided with an increase in the number of pro-inflammatory microglia and infiltrating peripheral Th1 cells and Aβ-mediated synaptic dysfunction in 5xFAD compared to wild-type mice. Similar increases in peripheral Th1 infiltration were observed in wild-type mice that were co-housed with, or received fecal microbiota transplantation (FMT) from, 5xFAD mice. Interestingly, higher blood and fecal levels of phenylalanine and isoleucine (Phe/Ile) were observed in 5xFAD mice compared to wild-type mice, which normalized with the oral administration of GV-971 (Wang et al., 2019). Treatment with GV-971 was also associated with lower brain Aβ burden, fewer brain Th1 cells, and less activated microglia in 5xFAD mice and improved cognitive function in APP/PS1 mice (Seo et al., 2019; Wang et al., 2019).
3.2. Leaky gut
Inflammation is associated with disruption of the intestinal epithelial barrier which facilitates the flux of bacterial constituents, endotoxins, and inflammatory cells into the circulation, a condition often referred to as “leaky gut” (Marizzoni et al., 2017). While certain gut species such as Lactobacillus plantarum, Escherichia coli Nissle, and Bifidobacterium infantis enhance the expression of tight junction proteins (Bischoff et al., 2014), others such as the Bacteroides fragilis toxin disrupt the intestinal barrier (Choi et al., 2016; König et al., 2016). Gut hyperpermeability assays have demonstrated changes in tight junction proteins such as E-cadherin, occludin, and zonula occludens (ZO-1) proteins in AD, which facilitate the translocation of bacterial endotoxins into the circulation (“endotoxemia”) (Andŕe et al., 2019). Serum samples from individuals with dementia have increased markers of gut permeability, such as serum diamine oxidase (DAO) levels, and increased inflammatory mediators including the soluble cluster of differentiation 14 (sCD14) levels compared to controls (Stadlbauer et al., 2020). Measurements of calprotectin concentrations in the stool may also offer a useful surrogate for gut inflammation (Walsham and Sherwood, 2016). Calprotectin is an inherently amyloidogenic calcium-binding protein which consists of a heterodimer of S100A8/A9 (Walsham and Sherwood, 2016) and can aggregate into oligomers and fibrils that resemble those of Aβ and induce Aβ fibrillization in vitro (Wang et al., 2014a; Zhang et al., 2012). S100A9 induces microglial activation via TLR4 and the receptor for glycation end-products (RAGE) pathways and promotes oligodendrocyte precursor cell apoptosis via activation of the NF-kB pathway (Wang et al., 2014a; Wu et al., 2018). CSF and brain calprotectin levels are increased in AD (Kowalski and Mulak, 2019), and mediate increased amyloid plaque formation in individuals with TBI (Wang et al., 2018).
3.3. Neuroinflammation, IL-17, and kynurenine pathways
Neuroinflammation is a well-documented pathological substrate of AD; increased numbers of activated microglia and reactive astrocytes are observed in human AD brains in the vicinity of amyloid plaques (Cattaneo et al., 2017; Cerovic et al., 2019). Abnormal amyloid and tau aggregation stimulate microglia, trigger the release of inflammatory cytokines and recruitment of inflammatory cells into the brain (Wang et al., 2015b). Gut dysbiosis is closely associated with activation of the NLRP3 inflammasome and higher peripheral levels of pro-inflammatory markers (Cattaneo et al., 2017). Transplantation of transgenic mice with fecal samples from individuals with AD is associated with NLRP3 induction, hippocampal microglial activation, and cognitive impairment (Shen et al., 2020). Furthermore, neuroinflammation is associated with downregulation of TREM2, leading to reduced microglial phagocytic ability of amyloid and increased amyloid aggregation (Pistollato et al., 2016; Zhao and Lukiw, 2013). Repeated exposure to bacterial toxins such as LPS may prime microglia and exacerbate microglial response to Aβ in the brain (Friedland, 2015). The secretion of meso-diaminopimelic acid (meso-DAP) from bacterial cell walls triggers the nucleotide-binding oligomerization domain-containing protein 1 (NOD1) receptor signaling and NOD1-mediated activation of bone marrow neutrophils (Clarke et al., 2010).
In AD mouse models, Aβ recruits neutrophils by increasing the affinity of the lymphocyte function association antigen-1 (LFA-1) integrin to the brain endothelium (Zenaro et al., 2015). In the brain, neutrophils increase IL-17 production which amplifies neutrophil recruitment and contributes to neurodegeneration and cognitive deficits (Cipollini et al., 2019). Blocking LFA-1 integrin or depleting neutrophils reverses cognitive deficits in mice (Zenaro et al., 2015). Increased production of reactive oxygen species due to Aβ also stimulates IL-17 secretion. IL-17 plays an important role in mediating neuroinflammation in AD and is synergistic with the pro-inflammatory effects of cytokines (Cipollini et al., 2019); CSF, serum, and hippocampal IL-17 levels are increased in AD animal models (Milovanovic et al., 2020), and their neutralization with anti-IL-17 antibodies ameliorates Aβ-mediated neuroinflammation, as evidenced by lower levels of the astrocytic markers (e.g., glial fibrillary acidic protein [GFAP], S100b, and myeloperoxidase-[MPO]), and improves cognitive functions (Cristiano et al., 2019).
Interestingly, gut microbiome alterations triggered by a high salt diet induce brain endothelial dysfunction and cognitive impairment in mice via intestinal Th17 cell polarization even in the absence of brain inflammation (Faraco et al., 2018). One proposed mechanism for this includes toxic effects of IL-17, produced by Th17 cells, on brain endothelium leading to impaired endothelial eNOS synthesis and disturbed neurovascular coupling (Faraco et al., 2018). Furthermore, high salt may have both direct effects on Th17 cell polarization as well as indirect effects mediated by gut microbiome alterations including the depletion of Lactobacillus murinus (Wilck et al., 2017). Data from clinical studies are consistent with animal studies and suggest that serum IL-17 and IL-23 levels, and the expression of the Th17 transcription factor RORγt, are higher in AD compared to controls and that Th17 cell counts correlate with CSF markers of amyloid pathology (i.e., CSF Aβ42/Aβ40) (Chen et al., 2014; Oberstein et al., 2018). Fig. 3 summarizes the main effects of IL-17 in AD pathogenesis including its relationship with the gut microbiome.
Fig. 3.
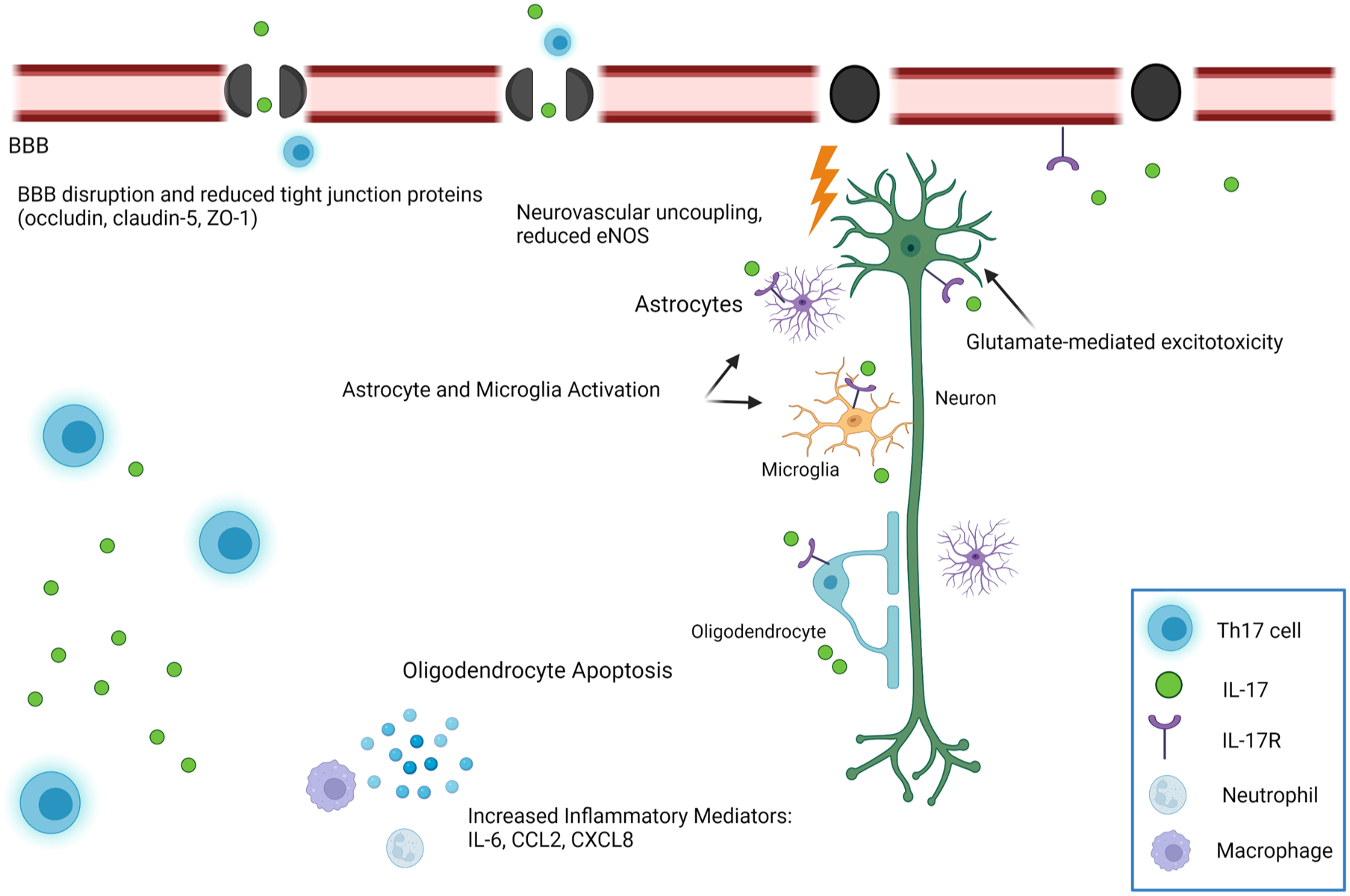
The Role of Interleukin-17 (IL-17) in AD Pathogenesis. One of the important inflammatory mediators by which gut dysbiosis contributes to AD pathogenesis is IL-17, produced by Th17 cells. IL-17 promotes neuronal and oligodendrocyte apoptosis, neuroinflammation through activation of astrocytes and microglia, neurovascular uncoupling, and increased calcium-mediated excitotoxicity, eventually leading to neuronal loss. Created with BioRender.com.
Gut dysbiosis is also associated with higher extracellular levels of the high-mobility group box 1 (HMGB1), an important non-histone nucleoprotein which has highly preserved functions in transcriptional regulation, telomere maintenance, and DNA repair (Andersson et al., 2018; Festoff et al., 2016). The extracellular exosome-mediated release of HMGB1 from the intestines into the peripheral circulation in the setting of altered gut microbiota acts as a danger-associated molecular pattern which alarms the immune system and triggers TLR-4 and NF-KB inflammatory pathways and the peripheral release of inflammatory mediators (e.g. IL-1, IL-6, and TNF-α) (Fitzgerald and Kagan, 2020). Furthermore, HMGB1 is recognized by RAGE receptor on neutrophils, monocytes, and endothelium leading to chronic low-grade inflammation and impaired BBB permeability (Liu et al., 2021; Hudson and Lippman, 2018).
Another, albeit less understood, mechanism by which the gut microbiome contributes to CNS inflammation is the dysregulation of the kynurenine pathway involved in tryptophan metabolism. Under physiological conditions, tryptophan metabolism via the kynurenine pathway results in the formation of 4 key metabolites, 3-hydroxykynurenine (3-HK), quinolinic acid (QA), kynurenic acid (KA) and picolinic acid, which play important roles in neuroplasticity (Savitz, 2020). However, in the presence of gut dysbiosis, altered ratios of these metabolites have detrimental effects leading to microglial activation, neuroinflammation, and dysregulated calcium-mediated excitotoxicity (Lugo-Huitrón et al., 2013; Guillemin, 2012). One of the key enzymes of the kynurenine pathway, indoleamine 2,3-dioxygenase 1 (IDO-1), is activated by the pro-inflammatory cytokine, IFN-γ, and co-localizes with Aβ plaques (Arora et al., 2020). The administration of Lactobacillus johnsonii to bio-breeding rats was associated with reduced endogenous IDO-1 resulting in less tryptophan breakdown, and the diversion of tryptophan towards pathways involved in serotonin synthesis (Valladares et al., 2013).
Taken together, these findings support the notion that neuro-inflammation is an important mechanism by which gut dysbiosis can influence brain pathology and immune homeostasis and has led to the proposition of a microbiome-gut-inflammasome-brain axis (Kamer et al., 2015; Shukla et al., 2021).
3.4. Blood-brain barrier disruption
The BBB, which is composed of endothelial tight junctions surrounded by pericytes and astrocytic end-foot processes, plays an important role in brain homeostasis (Sweeney et al., 2018). Disruption to the BBB, including loss of pericytes and endothelial tight junctions, is reported in even the earliest stages of AD, and is associated with increased amyloid pathology due to impaired Aβ clearance (Sweeney et al., 2018). Animal studies suggest that BBB alterations may precede Aβ and tau aggregation or neuronal loss (Szu and Obenaus, 2021). Similarly, clinical studies suggest that BBB dysfunction in the hippocampus (Montagne et al., 2015) and cortical regions is an early event in AD pathogenesis which precedes brain atrophy and cognitive decline (van de Haar et al., 2016). Toxic Aβ oligomers accelerate BBB damage by disrupting endothelial tight junctions, creating a vicious cycle that further promotes Aβ aggregation. Individuals with AD have imaging evidence of age- and disease-dependent BBB breakdown which correlates with memory loss and learning deficits and CSF markers of pericyte injury (Montagne et al., 2015).
There is growing evidence that a healthy gut microbiome is essential for BBB integrity as well as normal neuronal development (Fung et al., 2017). Gut dysbiosis is associated with increased BBB permeability in animal studies which improves after restoring gut microbial homeostasis (Braniste et al., 2014). Lower expression of the tight junction proteins, claudin-5 and occludin, and subsequent BBB disturbances have been documented in adult germ-free mice (i.e., which lack a gut microbiome), and are ameliorated with their conventionalization through fecal microbiota transplantation from pathogen-free adult mice or colonization with Bacteroides thetaiotaomicron, or Clostridium tyrobutyricum which produces butyrate (Braniste et al., 2014). Low-dose penicillin exposure in mice early in life was associated with increased hippocampal endothelial expression of the tight junction proteins, occludin and claudin-5, in one study (Leclercq et al., 2017), and treatment of senescence accelerated mice P8 (SAMP8) with probiotics was associated with increased brain expression of claudin-1, occludin, and zonula occludens-1 (ZO-1) in another study (Yang et al., 2020).
3.5. Amyloid and tau aggregation
Several pro-inflammatory bacteria such as Escherichia coli and Bacillus subtilis secrete large quantities of the amyloid peptide curli, which aids in bacterial adhesion and other bacterial surface functions (Friedland and Chapman, 2017; Hufnagel et al., 2013; Schwartz and Boles, 2013). Curli is composed of a subunit of CsgA amyloid precursor protein which shares many structural similarities to Aβ peptides, and can be recognized by the TLR2 receptor on macrophages (Rapsinski et al., 2015; Tükel et al., 2005). Activation of TLR2 by curli results in the activation of bone-marrow macrophages and T-lymphocytes and increased pro-inflammatory cytokines such as IL-6, IL-8, IL-17, and IL-22 (Rapsinski et al., 2015; Nishimori et al., 2012). The infiltration of the brain with these peripheral inflammatory mediators activates the TLR2/1 and NF-κB signaling pathways in the brain leading to inflammation, including increased production of IL-1β (Friedland, 2015). In one study, the oral administration of a curli-producing strain of E.coli in rats was associated with increased brain astrogliosis (Chen et al., 2016a). Therefore, bacterial amyloid in the gut may prime the immune system, enriching its response to endogenous brain amyloid proteins (Friedland and Chapman, 2017).
Additionally, the formation of amyloid proteins in the gut wall may facilitate their retrograde transport into the brain via the gut-brain axis (Eisele et al., 2010). While the mechanisms that control amyloid spread from the gut into the brain remain to be elucidated, transport of amyloid into the brain may be facilitated by several cell types including neurons, astrocytes, fibroblasts, and microglia (Espargaró et al., 2016). In the brain, Aβ seeding is followed by amyloid accumulation and spread to neuroanatomically connected regions and induces conformational changes of other protein molecules into the β-pleated structure, further exacerbating amyloid propagation, resembling the transcellular spread seen with prion and tau proteins (Eisele et al., 2010; Sowade and Jahn, 2017). Consistent with these findings, mice overexpressing human amyloid α-synuclein demonstrate increased brain synuclein pathology and exacerbated behavioral and motor deficits following their colonization with curli-producing E.coli (Sampson et al., 2020). Treatment of these mice with a gut-restricted amyloid inhibitor prevented curli-mediated exacerbation of synuclein aggregation and associated behavioral deficits (Sampson et al., 2020).
Interestingly, recent studies have shown that tau misfolding and aggregation may be facilitated by extracellular bacterial DNA, including B. burgdorferi, P. gingivalis, C. albicans, and E. coli (Tetz et al., 2020). The E. coli (K99 strain) and P. gingivalis are detectable in brain parenchyma and vasculature in post-mortem AD samples, including the hippocampus (Dominy et al., 2019; Zhan et al., 2016). These bacterial strains demonstrate facultative intracellular properties which create a favorable environment for interactions with the intracellular tau pathways. It has been postulated that bacterial DNA is transported to the outer membrane or released following prophage induction into the neuronal cytosol where it acts as a seed for intracellular tau aggregation (Tetz et al., 2020).
3.6. Direct effects on neuroplasticity and hippocampal learning processes
Several studies suggest direct effects of gut dysbiosis on synaptic and neuronal plasticity and modulation of hippocampal learning processes. In a study by Chu et al., antibiotic-treated mice had impaired extinction learning compared to their untreated counterparts which was attributed to reduced dendritic spine growth and remodeling and reduced activity of signal-encoding neurons in the medial prefrontal cortex (Chu et al., 2019). When gnotobiotic (i.e., germ-free) mice were colonized with diverse gut microbiota at different developmental stages, a reversal of impaired extinction learning was only observed in mice that were colonized after birth, while gnotobiotic mice colonized during weaning or adulthood did not demonstrate any cognitive benefits. Furthermore, CSF, serum, and fecal levels of 4 bacterial metabolites (phenyl sulfate, pyrocatechol sulfate, 3-[3-sulfooxyphenyl] propanoic acid, and indoxyl sulfate) were found to be differentially altered in germ-free mice compared to controls or those with restored microbiota.
Other studies provide evidence to support a role for gut dysbiosis in modulating cortical and hippocampal neuronal activity. Aβ toxicity is associated with impaired function and expression of the Na + and K + -ATPase transporters within neuronal membranes leading to impaired energy metabolism and increased oxidative stress (Ugbode et al., 2017). D-galactose administration in rats results in significant reductions in membrane-bound ATPase in the cortex and hippocampus and cognitive impairment, which are almost completely reversed with the administration of Lactobacillus plantarum MTCC1325 (Mallikarjuna et al., 2016). Taken together, these findings support the notion that gut microbiota and bacterial metabolites play an important role in learning and neuronal plasticity during the early developmental stages. Consistent with data from animal studies, results from a clinical trial of individuals with AD suggested cognitive benefits and reduced brain insulin resistance in those treated with probiotics containing Lactobacillus acidophilus, Lactobacillus casei, Bifidobacterium bifidum, and Lactobacillus fermentum (Akbari et al., 2016).
4. Age, lifestyle, and the gut microbiome
Several studies utilizing metagenomics suggest that lifestyle may influence the gut microbiome to a larger extent than genetic factors, and significantly contribute to a higher risk for AD (Hills et al., 2019; Rothschild et al., 2018). We herein review evidence supporting a link between aging or lifestyle factors (i.e., sleep, diet, stress, and exercise) and the gut microbiome, and how gut dysbiosis may mediate the effect of lifestyle factors on AD pathogenesis.
4.1. Aging
Healthy aging is associated with structural and functional changes in the gut microbiome (Salazar et al., 2017; Nagpal et al., 2018), including an increase in the number of facultative anaerobes and changes in species dominance with a relative stability of total anaerobic counts (Askarova et al., 2020; Mariat et al., 2009). One study reported lower abundance of Bifidobacterium and Lactobacillus in older compared to younger adults, and a relative predominance of the Bifidobacterium adolescentis species (Hopkins and Macfarlane, 2002). As Bifidobacterium and Lactobacillus are involved in the production of the inhibitory neurotransmitter γ-Aminobutyric acid (GABA), and as intestinal GABA levels appear to correlate with brain GABA levels, it has been postulated that the lower abundance of Bifidobacterium and Lactobacillus in older age results in impaired synaptogenesis and cognitive impairment due to altered GABA activity in the brain (Junges et al., 2018; Strandwitz, 2018). Other age-associated changes in the gut microbiome include increased prevalence of Prevotella, Eubacterium rectale, Clostridium coccoides, and Ruminococcus, proteolytic bacteria, such as Fusobacteria and Propionibacteria (Hopkins and Macfarlane, 2002; Woodmansey et al., 2004), and pro-inflammatory enterobacteria, streptococci, staphylococci, and yeast.
4.2. Sleep
The bi-directional link between midlife sleep disturbance or impaired circadian rhythms and late-life AD has been established in several epidemiological and mechanistic studies (Musiek et al., 2015; Wu et al., 2003; Uddin et al., 2020; Sabia et al., 2021). Sleep has important effects on amyloid clearance due to an increase in interstitial space which may be disturbed by even short periods of sleep restriction (Kang et al., 2009; Xie et al., 2013). Sleep disruption is a common symptom, being reported in 20–55% of individuals with AD, and may precede cognitive symptom onset by several years (Webster et al., 2020; Zhou et al., 2019). Recent studies have shown that chronic sleep fragmentation over 4 weeks increases hippocampal Aβ accumulation in mice (Duncan et al., 2022). Loss of central circadian rhythms disturbed daily oscillations of interstitial Aβ levels in the hippocampus, and targeted deletion of the core clock gene Bmal1 increased APOE expression and amyloid plaque formation (Kress et al., 2018). Data from clinical studies also support important effects of sleep disturbance on brain amyloid pathology. Normal diurnal variations in CSF Aβ42 levels in healthy adults are obliterated, and brain Aβ accumulation is increased, by even relatively short periods of sleep deprivation in humans (Shokri-Kojori et al., 2018; Ooms et al., 2014).
Sleep deprivation has also been shown to directly influence the gut microbiome (Poroyko et al., 2016; Bowers et al., 2020), providing another potential mechanism, in addition to amyloid aggregation, by which sleep disturbance may contribute to AD pathogenesis. Chronic sleep disruption shifts gut microbial structure and function in mice (Bowers et al., 2020). In humans, partial sleep deprivation is associated with an increased gut Firmicutes/Bacteroidetes ratio in healthy young adults, higher abundance of the Coriobacteriaceae and Erysipelotrichaceae, and lower abundance of Tenericutes, families (Benedict et al., 2016). Circadian rhythm disturbances have also been shown to alter gene expression within the gut microbiota, being associated with increased expression of genes involved in the synthesis and transportation of LPS, and suppression of genes involved in immune regulation (Deaver et al., 2018). Interestingly, these changes strongly resemble genetic changes in the gut microbiome observed in AD (Liu et al., 2019a). In a study which implemented meta-transcriptomic analyses of stool samples, circadian rhythm disturbance in mice was associated with higher abundance of Ruminococcus torques, and lower abundance of Lactobacillus johnsonii, which have negative and positive effects on the gut barrier integrity, respectively (Deaver et al., 2018). Reversible structural and functional changes in the gut microbiome were observed 48 h after acute sleep deprivation in rats in another study (Wang et al., 2022). Conversely, healthy sleep patterns correlated with a larger number of Verrucomicrobia and Lentisphaerae in stool samples and better cognitive performance in a clinical study of healthy older adults (Anderson et al., 2017). Despite these interesting findings, associations between sleep disturbance and gut microbiome morphology have not been replicated by other studies (Zhang et al., 2017a).
Other studies have examined the opposite direction of this relationship including the effect of the gut microbiome on sleep efficiency. In a recent clinical study, the diversity of the gut microbiome was found to be closely associated with increased sleep efficiency and total sleep time and negatively correlated with wake after sleep onset (Smith et al., 2019). An abundance of several taxa, including Lachnospiraceae, Corynebacterium, and Blautia was associated with poor sleep efficiency (Smith et al., 2019).
Given the strong associations between the gut microbiome and immune system alterations, disturbances in cytokines and other inflammatory mediators represent a potential important link between gut dysbiosis, sleep disturbance, and AD pathogenesis. It remains unclear whether the complex and multi-directional association between chronic sleep disturbance, immunity, and the gut microbiome may be influenced by other factors such as age, sex, obesity, and the metabolic syndrome. Validation of these findings in larger studies and further research in this area is warranted.
4.3. Diet
The “Western diet” consisting of a high amount of saturated fat and added sugar has been linked to a higher risk for AD in several epidemiological studies (Weisburger, 1997), and an increased risk for AD has accompanied the transition of non-Western populations to “Westernized” diets. These observations are supported by preclinical studies that demonstrate a strong association between high fat diet and dementia risk (Studzinski et al., 2009; Sanguinetti et al., 2018; Sah et al., 2017; Nam et al., 2017). The association between chronic dietary changes and a higher risk for DM, obesity, and AD is well-established and is -at least-partially mediated by an increased risk for cerebrovascular pathology. However, recent studies suggest that dietary changes may significantly alter the gut microbiome within days to weeks, thereby providing an additional mechanism by which diet may contribute to AD pathogenesis. In addition to their association with a higher incidence of vascular risk factors (e.g., DM, obesity, and the metabolic syndrome), other mechanisms by which dietary factors influence the risk for AD pathology appear to be mediated by alterations to the gut microbiome, including increased gut inflammation, oxidative stress, dysregulated NRF2 (nuclear factor erythroid 2-related factor 2) signaling, and increased neuronal apoptosis (Studzinski et al., 2009; Sanguinetti et al., 2018; Sah et al., 2017; Nam et al., 2017). A high fat diet (HFD) increased amyloid deposition in 12-month-old APP23 mice and was associated with altered brain lipid levels and lower expression of genes involved in synaptic plasticity and neuronal growth (Nam et al., 2017).
Interestingly, other studies in AD transgenic mice have shown that a HFD and genetic predisposition to AD are associated with similar gut metabolomic profiles, including higher levels of fecal ribose, lactate, ketones, trimethylamine (TMA), and TMAO, and lower levels of choline and unsaturated fatty acids (Sanguinetti et al., 2018). Gut microbiome changes in HFD-fed mice included higher cecal levels of Clostridium and Staphyloccosus species, and higher colonic abundance of Firmicutes compared to Bacteroidetes, Roseburia, Coprobacillus, and Dorea phyla, and Rikenellaceae, Lachnospiraceae, and Enterococcaceae families (Sanguinetti et al., 2018). Additionally, a HFD can lead to chronic elevation of circulating LPS levels referred to “metabolic endotoxemia” and contributes to chronic low-grade inflammation, insulin resistance, and DM (Mohammad and Thiemermann, 2021).
Higher ileal and colonic Firmicutes/Bacteroidetes ratios and lower abundance of Actinobacteria, Proteobacteria, and Verrucomicrobia have been observed in mice fed a refined high-fat or a refined low-fat diet compared to chow-diet fed mice (Dalby et al., 2017). In another study, the transition from a standard chow diet to a refined low fiber diet in mice was associated with loss of Bacteroidetes and increased Clostridia and Proteobacteria within one week, with limited additional impact of high or low dietary fat on gut microbiota composition, suggesting that fiber intake may be more closely associated with gut microbiota composition than dietary fat intake (Morrison et al., 2020).
4.4. Exercise
A sedentary lifestyle has been linked to cognitive decline in several studies, while exercise has been shown to have protective effects on brain health including a lower risk of AD (Fenesi et al., 2017). Growing evidence from animal studies suggests that physical activity slows the progression of AD pathology, including reduced amyloid and tau aggregation and inflammation, and improves lipid metabolism, neurogenesis, and cognitive function (Kim et al., 2019; Zeng et al., 2020; Rossi Daŕe et al., 2020). In one study, 12 weeks of exercise on a treadmill were associated with improved mitochondrial function, increased neurogenesis, and reduced amyloid pathology in an AD mouse model (Kim et al., 2019). Other studies have shown that physical activity is associated with reduced soluble hippocampal Aβ and TNF-α, and increased hippocampal BDNF levels (Bashiri et al., 2020; Zeng et al., 2020). A single session of physical exercise after learning improved memory consolidation in rat AD models generated by direct-hippocampal injection of Aβ (Rossi Daŕe et al., 2020). Physical exercise has also been shown to combat negative effects of a HFD on brain health including HFD-induced neuroinflammation, neuronal apoptosis, and hypothalamic microglial activation (Kim et al., 2017; Yi et al., 2012). Wu et al. demonstrated that 4 weeks of treadmill running before intraperitoneal LPS injection inhibited LPS-induced dopamine deficiency, loss of dopaminergic neurons, and motor dysfunction in male C57BL/6J mice (Wu et al., 2011).
Together, these findings support important protective roles for physical activity on brain health through several mechanisms including reduced oxidative stress, inflammation, amyloid and tau pathology, and improved neurogenesis and mitochondrial functions (Chen et al., 2016b). Recent evidence suggests that another potential mechanism by which exercise supports brain health is through modulation of the gut microbiome (Abraham et al., 2019; Fernandez et al., 2018; Mitchell et al., 2019). Physical activity has been associated with increased abundance of butyrate-producing bacteria and higher fecal butyrate levels which have homeostatic and anti-inflammatory effects and reduce LPS translocation into the bloodstream (Abraham et al., 2019; Mitchell et al., 2019). Increased diversity within the Firmicutes phyla has been consistently reported with physical activity across studies (Mitchell et al., 2019). In a study by Motiani et al (Motiani et al., 2020), sprint interval and moderate-intensity continuous training were associated with favorable changes in the gut microbiome of sedentary individuals with diabetes or pre-diabetes, including an increase in the Bacteroidetes phyla, a decrease in Firmicutes/Bacteroidetes ratio, and lower levels of systemic (e.g., TNF-α) and intestinal (e.g., LPS-binding protein) inflammatory mediators.
4.5. Stress
Stress, including environmental (e.g., noise, toxins, pollutants, or climate change), physical (e.g., sleep disturbance) or psychological (e.g., fear or anxiety) stressors, have been associated with several neurodegenerative disorders including AD (Gubert et al., 2020). Animal studies suggest that environmental stress is associated with neuroinflammation and altered expression of amyloid and tau proteins (Chong et al., 2005; Futch et al., 2017; Gubert et al., 2020; Machado et al., 2014; Ricci et al., 2012). Transgenic mice overexpressing corticotropin-releasing hormone (CRH) have increased hippocampal tau pathology, and CRH antagonism reduces both amyloid and tau aggregation in animal models (Carroll et al., 2011; Futch et al., 2017; Gubert et al., 2020).
Stress may influence the gut microbiome through activation of the hypothalamic-pituitary (HPA) axis (Misiak et al., 2020). Stress-induced HPA axis activation increases gut permeability and intestinal expression of corticotropin-releasing factor (CRF; CRH) receptor type 1 in rats (Vicario et al., 2012). Probiotics reduce HPA axis dysfunction associated with stress, and improve mood, learning, and memory functions in animal models (Misiak et al., 2020; Eutamene et al., 2007; Gareau et al., 2007). Consistent with these findings, anxiolytic effects of probiotics containing Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 due to lower cortisol levels have been observed in clinical studies (Messaoudi et al., 2011).
In a recent study, traffic-related air pollution (TRAP) was associated with gut dysbiosis including reduced microbial diversity, lower abundance of Lactobacillus and Ruminococcus flavefaciens, lower Firmicutes/Bacteroidetes ratio, and altered bile acid production at 10 months of age in a rat AD (i.e., TgF344) model (Dutta et al., 2022). In this model, TRAP was associated with increased amyloid and tau aggregation, neuronal loss, and cognitive deficits. Importantly, TRAP-mediated effects on gut dysbiosis and AD pathology appeared to be age-, sex-, and host genotype-dependent, supporting the presence of a dynamic interplay between genetics and environmental factors on the gut-brain axis.
Other studies have found that electromagnetic field exposure was associated with gut dysbiosis (i.e., lower Firmicutes/Bacteroidetes ratio) and depression-like behavior in mice (Tai et al., 2020; Luo et al., 2021). Additionally, heavy metal exposure (e.g., manganese, aluminum, and cadmium) has also been linked to gut dysbiosis in animal studies (Tinkov et al., 2021; Pineton de Chambrun et al., 2014). In one study, exposure of adult mice to cadmium for 8 weeks resulted in gut-microbiota shifts with decreased abundance of Prevotella and Lachnoclostridium and increased Escherichia coli-Shigella (Yang et al., 2021). In another study, the oral administration of benzo-[a]-pyrene for 4 weeks was associated with an increase in pro-inflammatory (e.g., Desulfovibrionaceae), and a decrease in anti-inflammatory (e.g., Lactobacillus and Akkermansia), taxa (Ribìere et al., 2016).
The relationship between the gut microbiome and the HPA axis appears to be bi-directional. Gut dysbiosis may lead to higher levels of circulating cytokines, including IL-1β, IL-6, and TNF-α, or LPS (Vakharia and Hinson, 2005), which can then penetrate the BBB and activate the HPA axis (Banks, 2005), while SCFAs may suppress HPA axis activity via gene down-regulation (van de Wouw et al., 2018). Furthermore, studies suggest an association between stress related to chronic noise exposure and increased hippocampal and prefrontal tau pathology (Manikandan et al., 2006; Cui et al., 2009, 2012a, 2012b), upregulation of enzymes involved in Aβ synthesis (i.e., APP, β- and γ-secretases) (Cui et al., 2015), and a possible role for CRF in dysregulated Aβ and tau pathologies (Gai et al., 2017; Kang et al., 2007). Interestingly, these studies suggest that chronic noise may promote AD pathology via gut dysbiosis. In a study by Cui et al., SAMP8 mice exposed to chronic noise had higher abundance of Firmicutes and lower abundance of Bacteroidetes at the phylum level, higher levels of Candidatus Jettenia and Denitratisoma at the genus level, impaired brain and intestinal endothelial tight junctions, and higher levels of peripheral inflammatory mediators compared to controls (Cui et al., 2018).
4.6. Environment, epigenetics, and the gut microbiome
With an estimated 100 trillion various microbes in the human intestine, the gut microbiome encodes 100-fold more unique genes than the human genome, making it an important component of the human epigenetic landscape. Epigenetic modifications represent an important mechanism by which the gut microbiome may mediate the effects of environmental factors (e.g., diet, toxins, air pollution, and environmental stress) on AD risk and progression. Gut microbiota and their associated metabolites have been shown to, directly and indirectly, modulate enzymatic pathways involved in epigenetic mechanisms such as histone modifications, DNA methylation, and chromatin plasticity, which contribute to AD pathogenesis and cognitive impairment (Nagu et al., 2021). For example, gut bacterial metabolites, such as SFCAs and folate, can modulate histone acetylation and DNA methylation, respectively, which in turn regulate the expression of several genes involved in the amyloid pathway (e.g., β-secretase, APP, and PSEN1) (Nagu et al., 2021; Chen et al., 2022). Furthermore, SCFAs can directly influence cognitive functions via modulation of histone deacetylase activity. In a study which conducted integrated gut-microbiome hippocampal DNA methylation analyses of APP knock-in mouse models, a positive correlation was observed between amplicon sequence variants within the Lachnospiraceae family and hippocampal APOE4 methylation (Kundu et al., 2021). Together, these findings support the presence of a dynamic cross-talk between epigenetics and the gut microbiome, in which the gut microbiome may influence the expression of AD-susceptibility genes via epigenetic mechanisms and/or epigenetic AD markers may alter intestinal physiology and influence the growth or activity of certain gut microbiota.
5. Modulating the gut microbiome for AD prevention and treatment
Modulation of the gut microbiome represents a potential strategy for AD prevention and treatment as it may reduce inflammation, oxidative stress, amyloid or tau aggregation, and help restore neurogenesis, blood-brain barrier integrity, and improve or stabilize cognitive and behavioral functions (Bonfili et al., 2021; Wang and Dykes, 2022). Potential treatment or prevention strategies of gut microbiome modulation and the mechanisms by which these may influence AD pathology are summarized in Fig. 4.
Fig. 4.

Proposed Modalities of Gut Modulation with Potential Benefits in AD Prevention or Treatment. Gut modulation through dietary modification, exercise, supplementation with probiotics, prebiotics, or the short chain fatty acid butyrate, and use of antibiotics have been investigated as potential strategies for AD prevention or treatment. This matrix summarizes the mechanisms by which each of these modalities influences AD pathogenesis. Further research is warranted to determine the safety and efficacy of other modalities, such as fecal microbiota transplantation (FMT). BBB, blood-brain barrier. Created with BioRender.com.
5.1. Dietary modification
Evidence supporting a link between dietary factors, the gut microbiome, and AD risk has generated interest in identifying brain-healthy diets. Diets rich in unsaturated fats, fruits, vegetables, and whole grains such as the Mediterranean diet are associated with improved cognition, reduced brain atrophy in regions vulnerable to AD pathology, higher plasma carotenoid levels and paraoxonase activity, higher SCFA levels, increased gut microbial diversity, and lower peripheral markers of inflammation (e.g., C-reactive protein) in several studies, supporting a positive role for the Mediterranean diet in reducing atherogenesis and supporting brain health (Blum et al., 2006; Kincaid et al., 2021; Meslier et al., 2020; Mosconi et al., 2014; Wang et al., 2021b). The Mediterranean diet has been associated with preserved cognition in older adults and with a lower risk for AD in epidemiological studies (Valls-Pedret et al., 2015; Yusufov et al., 2017). In one study of over 16,000 middle-aged and older adults who were followed over 20 years, the Mediterranean diet was associated with a 20% lower risk for dementia (Andreu-Reinón et al., 2021). Gut microbial alterations associated with the Mediterranean diet include a lower Firmicutes/Bacteroidetes ratio, and increased abundance of butyrate-producing bacteria such as F. prausnitzii and E. rectale and the butyrate-producing genus Roseburia (Ghosh et al., 2020; Nagpal et al., 2019a).
Consistent with these findings, gut microbial alterations associated with the Mediterranean diet, typically including a high intake of fiber, vitamins (e.g., B1, B9, and B6) and minerals (copper, manganese, magnesium, iron, and potassium), were associated with improved cognition and reduced frailty in another study (Ghosh et al., 2020). Similar diets, such as the Dietary Approaches to Stop Hypertension (DASH) diet, also have beneficial effects on brain health when combined with exercise (Blumenthal et al., 2019). Diets that combine elements from both the Mediterranean and DASH diets (e.g., The Mediterranean-DASH Intervention for Neurodegenerative Delay [MIND]), which is rich in fruits, vegetables, whole grains, low-fat dairy, and lean protein, may be more effective in delaying cognitive decline (Morris et al., 2015). Dietary elements rich in Vitamin D3 (e.g., dairy and fish) (Brown et al., 2003) promote the neural growth factor protein, and those rich in flavonoids (e.g., grapes, citrus, and green tea) or the polyunsaturated fatty acid, docosahexaenoic acid (e.g., fish) may reduce amyloid and tau pathology and neuroinflammation (Ayaz et al., 2019; Szczechowiak et al., 2019).
Other diets that may have protective effects on brain health include the ketogenic diet which is very low in carbohydrate and high in fat, mimicking the effects of the fasting state and promoting the production of ketones through incomplete oxidization of fatty acids (Dewsbury et al., 2021). In AD mouse models, ketones reduce oxidative stress, prevent intracellular uptake of Aβ, and improve synaptic plasticity (Yin et al., 2016). Furthermore, the ketogenic diet has been shown to alter the gut microbiome, reduce AD pathology, and improve cognition (Carranza-Naval et al., 2021). The combination of the Mediterranean and ketogenic diets is associated with increased SCFA production by gut microbiota, improved CSF markers of amyloid and tau, and better cognitive performance (Kawas et al., 2021; Nagpal et al., 2019b). Intermittent fasting has also been shown to promote hippocampal neurogenesis through activation of glycogen synthase kinase (GSK)-3β and increased BDNF, increase insulin sensitivity, reduce inflammation, and promote autophagy and protein clearance in animal studies (Baik et al., 2020; Park et al., 2020). Preliminary clinical studies also suggest protective effects of intermittent fasting on memory in older adults (Witte et al., 2009).
5.2. Probiotics
Probiotic supplementation improves gut microbial diversity, supports the integrity of the intestinal and BBB, mitigates brain amyloid accumulation, and can help reduce inflammation, and improve cognition in animal studies (Athari Nik Azm et al., 2018; Kobayashi et al., 2017). In one study, the oral administration of B. longum NK46 was effective in restoring gut dysbiosis and reducing LPS production resulting in reduced inflammation and improved cognition in AD transgenic mice (Lee et al., 2019). Other studies in AD transgenic mice have shown that probiotics containing B. lactis, L. casei, B. bifidum, and L. acidophilus suppress inflammation by inhibiting TLR4- and RIG-I-mediated NF-κB pathways (Yang et al., 2020). Probiotic supplements (containing B. bifidum and L. plantarum) combined with exercise-training reduced Aβ toxicity and improved spatial learning in a rat AD model (Shamsipour et al., 2021).
In combination with 12-weeks of memantine treatment, probiotics containing L. plantarum decreased hippocampal Aβ levels, reduced peripheral TMAO levels, and promoted neural plasticity (Wang et al., 2020a). Other studies suggest beneficial effects for probiotics containing Clostridium butyricum in reducing brain Aβ deposits and mitigating microglial activation and inflammation via increased butyrate levels (Sun et al., 2020). Butyrate production by Agathobaculum butyriciproducens SR79 was associated with lower brain Aβ deposits and improved cognition in APP/PS1 mice (Go et al., 2021).
Data from clinical trials have generally been consistent with animal studies regarding beneficial effects of probiotics on brain health. Positive effects on cognition were reported following adherence to a 12-week diet rich in probiotics including Lactobacillus acidophilus, Lactobacillus casei, Bifidobacterium bifidum, and Lactobacillus fermentum (Akbari et al., 2016), or consuming milk fermented with kefir grains in clinical trials (Ton et al., 2020). Studies in humans also support an important role for butyrate in regulating neuronal growth and synaptic differentiation (Goswami et al., 2018; Silva et al., 2020). Probiotics including B. bifidum BGN4 and B. longum BORI, or those including B. breve A1, were associated with cognitive benefits in community-dwelling older adults in 2 studies (Kim et al., 2021; Kobayashi et al., 2019). However, the effects of probiotics on cognitive function in individuals diagnosed with AD have been conflicting. While one clinical study suggested that the combination of probiotics (including L. acidophilus, B. bifidum, and B. longum) and selenium was associated with improved cognition in AD (Tamtaji et al., 2019), and another study showed significant cognitive benefits in association with 12-weeks of probiotic supplementation (mainly Lactobacillus and Bifidobacterium strains) (Akbari et al., 2016), these results were not replicated by other studies (Krüger et al., 2021).
A possible mechanism by which probiotics promote brain health is mediated by their strong anti-oxidant effects. Sirtuin-1 (SIRT-1) is a nicotinamide adenine dinucleotide (NAD)-dependent histone deacetylase which plays an important role in protecting cells from reactive oxygen species. SIRT-1 levels are reduced in human AD brains and in senescent mice resulting in increased predisposition to oxidative stress (Julien et al., 2009). Administration of a probiotic supplement containing Streptococcus thermophilus, Bifidobacterium longum, B. breve, B. infantis, Lactobacillus acidophilus, L. plantarum, L. paracasei, L. delbrueckii subsp. bulgaricus, and L. brevis was associated with significantly higher SIRT-1 expression and activity in AD transgenic mice, increased deacetylation of the SIRT-1 substrate, retinoic acid receptor-β (RARβ), and increased synthesis of ADAM-10, an APP-cleaving α-secretase involved in the non-amyloidogenic pathway, ultimately, reducing Aβ synthesis (Bonfili et al., 2018).
Results of studies are promising regarding potential protective effects of probiotics on brain health in older age. However, further research is warranted to validate these findings and provide further mechanistic insight for the effects on cognition or AD pathology and to elucidate whether such cognitive benefits extend to individuals who have evidence of AD pathology.
5.3. Prebiotics
Prebiotics are short-chain carbohydrates which can modify the composition or function of gut microbiota. Some prebiotics act as fermentation substrates for SFCA-producing microbiota such as Bifidobacteria and Lactobacilli, while others, such as fructo-oligosaccharides (FOS), have direct beneficial effects on synaptic plasticity. In a study by Sun et al., FOS supplementation was associated with upregulation of the pre-synaptic protein, synapsin-1, expression and modulation of the glucagon-like peptide (GLP-1) pathway in AD transgenic mice, leading to improved brain insulin sensitivity, reduced phosphorylation of the JNK pathway, and improved cognition (Sun et al., 2019b). Other studies have examined a similar oligosaccharide, xylo-oligosaccharide (XOS), in AD transgenic mice with post-hepatectomy cognitive dysfunction which demonstrate increased inflammation and BBB permeability and significant alterations to the gut microbiome. XOS administration reversed loss of the BBB integrity by upregulation of tight-junction proteins (e.g. ZO-1), reduced inflammation and microglial activation, restored the gut microbiome, and improved cognitive function (Han et al., 2020). Synbiotics (combinations of prebiotics and probiotics) including XOS and Lactobacillus paracasei HII01 were associated with reduced hippocampal oxidative stress and microglial activation in obese insulin-resistant rats (Chunchai et al., 2018).
Other prebiotics with potential positive effects on brain health and the gut microbiome include lactulose, dietary polyphenols (e.g., ferulic acid), and non-digestible fibers (e.g., inulin and oligofructose)(Constante et al., 2017; Lee et al., 2021). Lactulose has been shown to reduce inflammation and promote autophagy and insulin sensitivity in animal and human studies (Lee et al., 2021; Lupien-Meilleur et al., 2016). Ferulic acid (FA) is a phenolic compound which exerts strong anti-oxidant and anti-inflammatory effects and promotes neuronal stem cell proliferation through increased production of nerve growth factor (NGF) and BDNF (Nabavi et al., 2015; Lindsay, 1988; Meng et al., 2018). Large amounts of FA are produced by probiotic species such as Lactobacillus fermentum NCIMB 5221 and Bifidum animalis (Westfall et al., 2017). Pretreatment with FA was found to reduce neuroinflammation, cortical and hippocampal Aβ levels, and act as a scavenger for reactive oxygen species in AD transgenic mice (Sgarbossa et al., 2015).
It is important to note that while probiotics and prebiotics may have promising beneficial effects on cognition in animal studies, more research is needed before they can be incorporated into routine clinical practice. It will be critical to determine the extent to which, if any, they are effective in the presence of brain disease, and the optimal dose and formulation which would provide the most acceptable risk-benefit ratio. In particular, it will be important to evaluate the safety of these agents across different patient populations, including vulnerable populations (e.g., elderly, critically ill, and immunosuppressed), as some have been associated with serious side effects including sepsis, immune reactivity, and antibiotic resistance. Other limitations that will need to be addressed before these agents can be widely accepted in medical practice include the large variability in individuals’ response to these supplements and the unpredictability of their effects on gut homeostasis within and across individuals.
5.4. Antibiotics
Antibiotics have been shown to ameliorate inflammation, oxidative stress, and AD pathology in animal studies (Yulug et al., 2018). Interestingly, several animal studies suggest that these effects may be sex-dependent (Minter et al., 2016; Kaur et al., 2021). Antibiotic treatment was associated with reduced Aβ plaques and reduced microglial activity in male, but not female, APP/PS1 mice (Minter et al., 2016; Dodiya et al., 2019). Antibiotic-treated male mice have lower levels of pro-inflammatory cytokines such as IL-1β and IL-17A while the opposite effect was observed in antibiotic-treated female mice (Dodiya et al., 2019).
Results of clinical studies examining the effects of antibiotics on the gut microbiome and cognition in AD have been conflicting. High doses of D-cycloserine administered over 4 weeks (100 mg) was associated with improved cognition in individuals with AD, while lower doses (15 mg) produced no cognitive benefit (Tsai et al., 1999, 1998). Reduced rates of cognitive decline were observed after a 3-month treatment with a combination of doxycycline and rifampicin compared to placebo in another study (Loeb et al., 2004). However, these results were not replicated in a longer study which included 12-months of antibiotic treatment (Molloy et al., 2013).
5.5. Butyrate treatment
Butyrate administration or the colonization of germ-free mice with butyrate producing Clostridium tyrobutyricum is associated with beneficial effects on BBB integrity and reduced LPS-mediated inflammation and IL-1β production in mice (Braniste et al., 2014; Jiang et al., 2021). Higher butyrate levels were associated with cognitive improvement and reduced tau pathology in mice; however, their effects on Aβ aggregation appear to be stage-dependent (Fernando et al., 2020; Govindarajan et al., 2011). There is limited data regarding the effects of butyrate treatment in humans.
5.6. Fecal microbiota transplantation
There is growing evidence to suggest that FMT is safe and effective in treating gastrointestinal disease such as recurrent colitis (Khanna et al., 2017; Hazan, 2020). Due to its ability to alter the gut microbiome, FMT has also been proposed as a potential mechanism to modulate the gut-brain axis and reduce the risk of neurological disorders, including AD. Data from several animal studies suggest that FMT may effectively alter the mouse gut microbiome and influence brain pathology. In one study, transplantation of germ-free C57BL/6N humanized mice with fecal samples from an individual with AD reproduced the donor microbiota and influenced mouse behavior (Fujii et al., 2019). Other studies have shown that FMT can reduce Aβ and tau pathologies and improve cognitive deficits in AD transgenic mice through restoration of gut microbial homeostasis and increased SCFA production (Ho et al., 2018; Kim et al., 2020; Zhan et al., 2018). While these animal data are promising, FMT as a potential treatment option in humans remains controversial. More research is warranted to determine the safety and efficacy of FMT in humans, and rigorous screening methods for donor infections and recipient susceptibility will need to be incorporated into the design of clinical trials of FMT.
6. Discussion and future directions
Growing evidence supports an important role for the gut microbiome in AD pathogenesis and mediating cognitive impairment through several mechanisms including disturbance to the intestinal and blood-brain barriers, dysregulated immune response, neuroinflammation, amyloid and tau aggregation, oxidative stress, and direct effects on synaptic function, and neuronal function or survival. Importantly, animal data suggest that gut microbial alterations may be present early in life and precede AD pathology and synaptic loss, highlighting the potential value of gut modulation as a preventative or early treatment strategy in AD. Recent animal studies provide important insight into the mechanisms by which the gut-brain axis influences the onset and progression of AD pathology. While various modalities of gut modulation have shown promise in reducing AD pathology or cognitive decline in preclinical studies, more studies are needed before these findings can be translated into human studies. This is particularly important as inherent gut microbiota composition and the effects of gut microbial alterations on AD pathology may vary significantly between animals and humans (e.g., increased Firmicutes:Bacteroidetes ratio has been reported in 5xFAD mice (Wang et al., 2019) while both increased and decreased Firmicutes:Bacteroidetes ratios have been reported in clinical studies of AD) (Vogt et al., 2017; Saji et al., 2019).
Other confounding factors in the interpretation of animal data include variations in the gut microbiome of transgenic AD models due to variations in target promotors, differences in the presence or severity of other AD pathologies (e.g., amyloid or tau), or examination of AD models at different stages of disease. For example, in one study which examined gut microbiota of a tauopathy animal model (P301L), AD transgenic mice had a lower Firmicutes:Bacteroidetes ratio compared to wild-type mice (Sun et al., 2019a), while this ratio was increased in several other transgenic AD models of amyloid deposition (Bäuerl et al., 2018; Wang et al., 2019). Furthermore, other confounding factors which may limit the translation of preclinical data into human studies include differential effects of age, sex, diet, and comorbidities on the gut microbiome, which have not been adequately controlled for in all studies (Jašarevíc et al., 2016).
Most animal and clinical studies examining the role of the gut microbiome on AD pathogenesis do not adjust for sex or directly examine biological differences between males and females. Significant sex-based differences in gut microbial diversity and composition, as well as differences in immune and inflammatory responses, are evident and vary according to the female reproductive stage, with shifts towards pro-inflammatory gut microbial phenotypes in post-menopausal females (Korf et al., 2022). These differences can, at least partially, be explained by a combination of hormonal and genetic factors related to the sex chromosomes, although the mechanisms that underlie such sex differences are not yet fully understood. Sex differences in the gut microbiome are also reported in preclinical studies, in which dietary and environmental factors can be controlled. However, the specific sex-based differences in these models vary by strain, which suggests that hormonal factors or sex chromosomes alone do not fully account for these differences, and that other factors such as host genetics or differential response to environmental stimuli also play an important role. Sex-dependent differences in the effects of gut modulation on brain health offer a unique opportunity to delineate mechanistic differences in disease pathogenesis between men and women and interactions of sex with genetic and lifestyle risk factors for AD and should be integrated into future studies of the gut-brain axis.
Despite a rapidly growing number of studies examining the gut microbiome in animal AD models, data from clinical studies remain limited. So far, clinical studies have been limited by small cohorts and the scarcity of longitudinal analyses. The relatively poor reproducibility of findings across studies may be attributed to large variations in study methodology, as well as difficulty controlling for environmental factors such as diet, stress, and sleep which are known to influence gut microbial composition and metabolic activity. Genetic factors may also significantly influence the gut microbiome; therefore, validation of findings across different cohorts will require important attention to the racial and ethnic composition of study cohorts as well as their geographical locations which may confound the interpretation of these findings and their generalizability to other populations.
As environmental factors (e.g., diet, stress, and sleep) are important confounding factors in clinical or animal studies of the gut microbiome, there has been increasing interest in identifying guidelines or best practice recommendations for microbiome study design to minimize their effects on study outcomes. Recommendations include longitudinal sampling from participants and the collection of personal history (including diet, stress, sleep, and other environmental exposures) for several days prior to sampling. Longitudinal sampling from individuals accounts for intra-individual differences, which collectively reflect dietary and other short-term environmental exposures for a particular individual, and averaging these changes helps reduce intra-individual noise associated with these exposures. An average of 3–5 days of sampling has been recommended based on studies in healthy adults; however, this may vary by study design. By reducing within-person noise, longitudinal analyses improve study power and reduce the need to enroll significantly larger cohort sizes to reliably measure disease or intervention-associated outcomes (Johnson et al., 2020).
Although gut modulation represents an exciting opportunity for future research, it is worth mentioning that a few areas of investigation into the modulation of the gut-brain axis as a preventative or therapeutic strategy in AD are controversial (Goyal et al., 2021). For example, while a few studies have demonstrated positive effects of probiotics on cognition, results have been contradictory as to whether these benefits are a direct consequence of gut microbial alterations (Hanage, 2014; Hooks et al., 2018; Kristensen et al., 2016; Reid et al., 2019). This highlights the importance of improving the design of future preclinical studies to provide direct mechanistic evidence to support gut modulation as an effective strategy for disease modification, and expand the current repertoire of association studies to those that can better elucidate cause-effect relationships between gut modulation and clinical or biomarker-based outcomes of AD.
Another highly controversial topic pertains to the safety of FMT as a gut modulation strategy (Wang et al., 2016b). Despite evidence supporting its general safety as an investigational gut modulation strategy for gastrointestinal disease (e.g., recurrent Clostridium difficile infections) with strict donor screening, it is important to note that serious adverse events, including death or life-threatening infections, have been reported (Wang et al., 2016b). These appear to be mostly related to FMT use from banked stool samples where screening for multi-drug resistant organisms was not routinely performed. In 2019 and 2020, the United States Food and Drug Administration (FDA) issued safety warnings due to reports of severe illness caused by enteropathogenic Escherichia coli (EPEC) and Shigatoxin-producing E. coli associated with the use of FMT products (FDA, 2019–2020). It is also important to note that transmission of SARS-CoV-2 is a potential risk associated with FMT as the virus has been shown to remain viable in the stool even after resolution of respiratory symptoms (Xiao et al., 2020; Wang et al., 2020b). Other safety considerations pertain to the use of FMT in the treatment of chronic immune-mediated conditions, in which a pathological immune response to allogenic FMT strains is more likely to occur, and the risk of aspiration or bowel perforation during the procedure (Khoruts and Weingarden, 2014). Importantly, there is limited evidence to support effective timing and dosing regimens of FMT products and their possible interactions with antibiotic treatment or dietary modifications. Therefore, there is a need for well-designed and validated protocols for donor selection and screening to guide FMT use in clinical or research settings.
7. Conclusions
Emerging evidence from animal and clinical studies suggests a potential role for gut dysbiosis in AD pathogenesis via the gut-brain axis. Potential mechanisms by which the gut microbiome may contribute to AD pathogenesis include immune dysregulation, neuroinflammation, promoting amyloid and tau aggregation and spread, impaired BBB, as well as direct effects on synaptic plasticity and neuronal functions. Recent studies also suggest the presence of characteristic microbial signatures for AD. The effects of gut dysbiosis on brain pathology are influenced by unmodifiable factors including age, sex, and genetics as well as modifiable environmental factors including diet, exercise, environmental stress, and sleep disruption which may influence the gut-brain axis via epigenetic mechanisms. Inadequately controlling for these variable factors has limited the reproducibility of study findings across different cohorts or animal models. While addressing this may be challenging in clinical study design, it represents an important area for future mechanistic animal research.
Gut modulation offers a potential opportunity to modify AD risk; however, further research is needed to establish its safety in humans. Rigorous screening measures to ensure safety of gut microbial alterations, and a better understanding of the long-term effects of such modulation on brain and systemic health, will be needed before gut modulation may be safely implemented in clinical practice. Furthermore, there is a paucity of data regarding the effects of gut modulation on CSF or imaging markers of AD pathology, including brain atrophy and global or regional amyloid and tau aggregation. Understanding the extent to which animal data can be translated into clinical studies and the differential effects of age, sex, and metabolic risk factors on the gut-brain axis will be crucial in the design of future clinical studies.
Acknowledgements
This work was supported by the National Institute of Health Grant NIH R21-AG067755 (RT), the University of New Mexico Grand Challenges Initiative, and NIH P20-AG068077 (University of New Mexico exploratory Alzheimer Disease Research Center).
References
- Abduljaleel Z, Al-Allaf FA, Khan W, et al. , 2014. Evidence of trem2 variant associated with triple risk of Alzheimer’s disease. PLoS One 9 (3), e92648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham D, Feher J, Scuderi GL, et al. , 2019. Exercise and probiotics attenuate the development of Alzheimer’s disease in transgenic mice: role of microbiome. Exp. Gerontol 115, 122–131. [DOI] [PubMed] [Google Scholar]
- Akbari E, Asemi Z, Daneshvar Kakhaki R, et al. , 2016. Effect of probiotic supplementation on cognitive function and metabolic status in Alzheimer’s disease: a randomized, double-blind and controlled trial. Front. Aging Neurosci 8, 256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez CA, Jones MB, Hambor J, Cobb BA, 2020. Characterization of polysaccharide a response reveals interferon responsive gene signature and immunomodulatory marker expression. Front. Immunol 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JR, Carroll I, Azcarate-Peril MA, et al. , 2017. A preliminary examination of gut microbiota, sleep, and cognitive flexibility in healthy older adults. Sleep. Med 38, 104–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson U, Yang H, Harris H, 2018. High-mobility group box 1 protein (HMGB1) operates as an alarmin outside as well as inside cells. Semin. Immunol 38, 40–48. [DOI] [PubMed] [Google Scholar]
- André P, Laugerette F, Féart C, 2019. Metabolic endotoxemia: a potential underlying mechanism of the relationship between dietary fat intake and risk for cognitive impairments in humans? Nutrients 11 (8), 1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andréu-Reinon ME, Chirlaque MD, Gavrila D, et al. , 2021. Mediterranean diet and risk of dementia and Alzheimer’s disease in the EPIC-spain dementia cohort study. Nutrients 13, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora K, Green M, Prakash S, et al. , 2020. The Microbiome and Alzheimer’s Disease: Potential and Limitations of Prebiotic, Synbiotic, and Probiotic Formulations. Front Bioeng Biotechnol 14 (8), 537847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askarova S, Umbayev B, Masoud A-R, et al. , 2020. The links between the gut microbiome, aging, modern lifestyle and Alzheimer’s disease. Front Cell Infect. Microbiol 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atarashi K, Tanoue T, Oshima K, et al. , 2013. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 500 (7461), 232–236. [DOI] [PubMed] [Google Scholar]
- Athari Nik Azm S, Djazayeri A, Safa M, et al. , 2018. Lactobacilli and bifidobacteria ameliorate memory and learning deficits and oxidative stress in β-amyloid (1–42) injected rats. Appl. Physiol. Nutr. Metab. Physiol. Appl. Nutr. Et. Metab 43 (7), 718–726. [DOI] [PubMed] [Google Scholar]
- Ayaz M, Sadiq A, Junaid M, et al. , 2019. Flavonoids as prospective neuroprotectants and their therapeutic propensity in aging associated neurological disorders. Front. Aging Neurosci 11, 155–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baik S-H, Rajeev V, Fann, et al. , 2020. Intermittent fasting increases adult hippocampal neurogenesis. Brain Behav 10, e01444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks WA, 2005. Blood-brain barrier transport of cytokines: a mechanism for neuropathology. Curr. Pharm. Des 11 (8), 973–984. [DOI] [PubMed] [Google Scholar]
- Bashiri H, Enayati M, Bashiri A, Salari AA, 2020. Swimming exercise improves cognitive and behavioral disorders in male NMRI mice with sporadic Alzheimer-like disease. Physiol. Behav 223, 113003. [DOI] [PubMed] [Google Scholar]
- Bathini P, Foucras S, Dupanloup I, et al. , 2020. Classifying dementia progression using microbial profiling of saliva. Alzheimer’s. Dement 12 (1) e12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäuerl C, Collado MC, Diaz Cuevas A, Viña J, Pérez Martínez G, 2018. Shifts in gut microbiota composition in an APP/PSS1 transgenic mouse model of Alzheimer’s disease during lifespan. Lett. Appl. Microbiol 66 (6), 464–471. [DOI] [PubMed] [Google Scholar]
- Benedict C, Vogel H, Jonas W, et al. , 2016. Gut microbiota and glucometabolic alterations in response to recurrent partial sleep deprivation in normal-weight young individuals. Mol. Metab 5 (12), 1175–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharjee S, Zhao Y, Dua P, Rogaev EI, Lukiw WJ, 2016. microRNA-34a-mediated down-regulation of the microglial-enriched triggering receptor and phagocytosis-sensor TREM2 in age-related macular degeneration. PLoS One 11 (3), e0150211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff SC, Barbara G, Buurman W, et al. , 2014. Intestinal permeability – a new target for disease prevention and therapy. BMC Gastroenterol. 14 (1), 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum S, Aviram M, Ben-Amotz A, Levy Y, 2006. Effect of a Mediterranean meal on postprandial carotenoids, paraoxonase activity and C-reactive protein levels. Ann. Nutr. Metab 50 (1), 20–24. [DOI] [PubMed] [Google Scholar]
- Blumenthal JA, Smith PJ, Mabe S, et al. , 2019. Lifestyle and neurocognition in older adults with cognitive impairments: a randomized trial. Neurology 92 (3), e212–e223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfili L, Cecarini V, Berardi S, et al. , 2017. Microbiota modulation counteracts Alzheimer’s disease progression influencing neuronal proteolysis and gut hormones plasma levels. Sci. Rep 7 (1), 2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfili L, Cecarini V, Cuccioloni M, et al. , 2018. SLAB51 probiotic formulation activates SIRT1 pathway promoting antioxidant and neuroprotective effects in an AD mouse model. Mol. Neurobiol 55 (10), 7987–8000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfili L, Cecarini V, Gogoi O, et al. , 2021. Microbiota modulation as preventative and therapeutic approach in Alzheimer’s disease. FEBS J. 288 (9), 2836–2855. [DOI] [PubMed] [Google Scholar]
- Bowers SJ, Vargas F, Gonźalez A, et al. , 2020. Repeated sleep disruption in mice leads to persistent shifts in the fecal microbiome and metabolome. PLoS One 15 (2), e0229001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandscheid C, Schuck F, Reinhardt S, et al. , 2017. Altered gut microbiome composition and tryptic activity of the 5xFAD Alzheimer’s mouse model. J. Alzheimer’S. Dis.: JAD 56 (2), 775–788. [DOI] [PubMed] [Google Scholar]
- Braniste V, Al-Asmakh M, Kowal C, et al. , 2014. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med 6 (263), 263ra158–263ra158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J, Bianco JI, McGrath JJ, Eyles DW, 2003. 1,25-dihydroxyvitamin D3 induces nerve growth factor, promotes neurite outgrowth and inhibits mitosis in embryonic rat hippocampal neurons. Neurosci. Lett 343 (2), 139–143. [DOI] [PubMed] [Google Scholar]
- Cani PD, Amar J, Iglesias MA, et al. , 2007. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56 (7), 1761–1772. [DOI] [PubMed] [Google Scholar]
- Carabotti M, Scirocco A, Maselli MA, Severi C, 2015. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol 28 (2), 203–209. [PMC free article] [PubMed] [Google Scholar]
- Carranza-Naval MJ, Vargas-Soria M, Hierro-Bujalance C, et al. , 2021. Alzheimer’s disease and diabetes: role of diet, microbiota and inflammation in preclinical models. Biomolecules 11, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JC, Iba M, Bangasser DA, et al. , 2011. Chronic stress exacerbates Tau pathology, neurodegeneration, and cognitive performance through a corticotropin-releasing factor receptor-dependent mechanism in a transgenic mouse model of TauopathyJ. Neurosci. 31, 14436–14449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo A, Cattane N, Galluzzi S, et al. , 2017. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol. Aging 49, 60–68. [DOI] [PubMed] [Google Scholar]
- Cerovic M, Forloni G, Balducci C, 2019. Neuroinflammation and the gut microbiota: possible alternative therapeutic targets to counteract Alzheimer’s disease? Front Aging Neurosci. 11, 284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C-K, Wu Y-T, Chang Y-C, 2017. Association between chronic periodontitis and the risk of Alzheimer’s disease: a retrospective, population-based, matched-cohort study. Alzheimer’s Res. Ther 9 (1), 56–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Meng L, Shen L, 2022. Multiple roles of short-chain fatty acids in Alzheimer disease. Nutrition 93, 111499. [DOI] [PubMed] [Google Scholar]
- Chen JM, Jiang GX, Li QW, Zhou ZM, Cheng Q, 2014. Increased serum levels of interleukin-18, −23 and −17 in Chinese patients with Alzheimer’s disease. Dement. Geriatr. Cogn. Disord 38 (5–6), 321–329. [DOI] [PubMed] [Google Scholar]
- Chen SG, Stribinskis V, Rane MJ, et al. , 2016a. Exposure to the functional bacterial amyloid protein curli enhances alpha-synuclein aggregation in aged fischer 344 rats and caenorhabditis elegans. Sci. Rep 6 (1), 34477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W-W, Zhang X, Huang W-J, 2016b. Role of physical exercise in Alzheimer’s disease. Biomed. Rep 4 (4), 403–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Fang L, Chen S, et al. , 2020. Gut microbiome alterations precede cerebral amyloidosis and microglial pathology in a mouse model of Alzheimer’s disease. Biomed. Res Int 2020, 8456596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu C, Miller MC, Monahan R, Osgood DP, Stopa EG, Silverberg GD, 2015. P-glycoprotein expression and amyloid accumulation in human aging and Alzheimer’s disease: preliminary observations. Neurobiol. Aging 36 (9), 2475–2482. [DOI] [PubMed] [Google Scholar]
- Choi VM, Herrou J, Hecht AL, et al. , 2016. Activation of Bacteroides fragilis toxin by a novel bacterial protease contributes to anaerobic sepsis in mice. Nat Med 22 (5), 563–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Li F, Maiese K, 2005. Stress in the brain: novel cellular mechanisms of injury linked to Alzheimer’s disease. Brain Res. Rev 49 (1), 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C, Murdock MH, Jing D, et al. , 2019. The microbiota regulate neuronal function and fear extinction learning. Nature 574 (7779), 543–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chunchai T, Thunapong W, Yasom S, et al. , 2018. Decreased microglial activation through gut-brain axis by prebiotics, probiotics, or synbiotics effectively restored cognitive function in obese-insulin resistant rats. J. Neuroinflamm 15 (1), 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipollini V, Anrather J, Orzi F, Iadecola C, 2019. Th17 and cognitive impairment: possible mechanisms of action. Front. Neuroanat 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirrito JR, Deane R, Fagan AM, et al. , 2005. P-glycoprotein deficiency at the blood-brain barrier increases amyloid-beta deposition in an Alzheimer disease mouse model. J. Clin. Invest 115 (11), 3285–3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke TB, Davis KM, Lysenko ES, Zhou AY, Yu Y, Weiser JN, 2010. Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat. Med 16 (2), 228–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constante M, Fragoso G, Lupien-Meilleur J, Calvé A, Santos MM, 2017. Iron supplements modulate colon microbiota composition and potentiate the protective effects of probiotics in dextran sodium sulfate-induced colitis. Inflamm. Bowel Dis 23 (5), 753–766. [DOI] [PubMed] [Google Scholar]
- Cristiano C, Volpicelli F, Lippiello P, et al. , 2019. Neutralization of IL-17 rescues amyloid-β-induced neuroinflammation and memory impairment. Br. J. Pharm 176 (18), 3544–3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, O’Riordan KJ, Sandhu K, Peterson V, Dinan TG, 2020. The gut microbiome in neurological disorders. Lancet Neurol. 19 (2), 179–194. [DOI] [PubMed] [Google Scholar]
- Cui B, Wu M, She X, 2009. Effects of chronic noise exposure on spatial learning and memory of rats in relation to neurotransmitters and NMDAR2B alteration in the hippocampus. J. Occup. Health 51 (2), 152–158. [DOI] [PubMed] [Google Scholar]
- Cui B, Wu M, She X, Liu H, 2012a. Impulse noise exposure in rats causes cognitive deficits and changes in hippocampal neurotransmitter signaling and tau phosphorylation. Brain Res. 1427, 35–43. [DOI] [PubMed] [Google Scholar]
- Cui B, Zhu L, She X, et al. , 2012b. Chronic noise exposure causes persistence of tau hyperphosphorylation and formation of NFT tau in the rat hippocampus and prefrontal cortex. Exp. Neurol 238 (2), 122–129. [DOI] [PubMed] [Google Scholar]
- Cui B, Li K, Gai Z, et al. , 2015. Chronic noise exposure acts cumulatively to exacerbate Alzheimer’s disease-like amyloid-β pathology and neuroinflammation in the rat hippocampus. Sci. Rep 5, 12943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui B, Su D, Li W, et al. , 2018. Effects of chronic noise exposure on the microbiome-gut-brain axis in senescence-accelerated prone mice: implications for Alzheimer’s disease. J. Neuroinflamm 15 (1), 190–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalby MJ, Ross AW, Walker AW, Morgan PJ, 2017. Dietary uncoupling of gut microbiota and energy harvesting from obesity and glucose tolerance in mice. Cell Rep. 21 (6), 1521–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deaver JA, Eum SY, Toborek M, 2018. Circadian disruption changes gut microbiome taxa and functional gene composition. Front Microbiol 9, 737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewsbury LS, Lim CK, Steiner GZ, 2021. The efficacy of ketogenic therapies in the clinical management of people with neurodegenerative disease: a systematic review. Adv. Nutr 12 (4), 1571–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodiya HB, Kuntz T, Shaik SM, et al. , 2019. Sex-specific effects of microbiome perturbations on cerebral Aβ amyloidosis and microglia phenotypes. J. Exp. Med 216 (7), 1542–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodiya HB, Frith M, Sidebottom A, et al. , 2020. Synergistic depletion of gut microbial consortia, but not individual antibiotics, reduces amyloidosis in APPPS1–21 Alzheimer’s transgenic mice. Sci. Rep 10 (1), 8183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominy SS, Lynch C, Ermini F, et al. , 2019. Porphyromonas gingivalis in Alzheimer’s disease brains: evidence for disease causation and treatment with small-molecule inhibitors. Sci. Adv 5 (1) eaau3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan MJ, Guerriero LE, Kohler K, et al. , 2022. Chronic fragmentation of the daily sleep-wake rhythm increases amyloid-beta levels and neuroinflammation in the 3xTg-AD mouse model of Alzheimer’s disease. Neuroscience 481, 111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta M, Weigel KM, Patten KT, et al. , 2022. Chronic exposure to ambient traffic-related air pollution (TRAP) alters gut microbial abundance and bile acid metabolism in a transgenic rat model of Alzheimer’s disease. Toxicol. Rep 9, 432–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisele YS, Obermüller U, Heilbronner G, et al. , 2010. Peripherally applied Abeta-containing inoculates induce cerebral beta-amyloidosis. Science 330 (6006), 980–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery DC, Shoemark DK, Batstone TE, et al. , 2017. 16S rRNA next generation sequencing analysis shows bacteria in Alzheimer’s post-mortem brain. Front. Aging Neurosci 9, 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espargaró A, Busquets MA, Estelrich J, Sabate R, 2016. Key points concerning amyloid infectivity and prion-like neuronal invasion. Front. Mol. Neurosci 9, 29–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eutamene H, Lamine F, Chabo C, et al. , 2007. Synergy between Lactobacillus paracasei and its bacterial products to counteract stress-induced gut permeability and sensitivity increase in rats. J. Nutr 137 (8), 1901–1907. [DOI] [PubMed] [Google Scholar]
- Fåk F, Tremaroli V, Bergström G, Bäckhed F, 2015. Oral microbiota in patients with atherosclerosis. Atherosclerosis 243 (2), 573–578. [DOI] [PubMed] [Google Scholar]
- Fani L, Wolters FJ, Ikram MK, et al. , 2018. Helicobacter pylori and the risk of dementia: a population-based study. Alzheimers Dement 14 (10), 1377–1382. [DOI] [PubMed] [Google Scholar]
- Faraco G, Brea D, Garcia-Bonilla L, et al. , 2018. Dietary salt promotes neurovascular and cognitive dysfunction through a gut-initiated TH17 response. Nat. Neurosci 21 (2), 240–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA. Fecal Microbiota for Transplantation: Safety Communication-Risk of Serious Adverse Reactions Due to Transmission of Multi-Drug Resistant Organisms. 2019–2020; 〈https://www.fda.gov/safety/medical-product-safety-information/fecal-microbiota-transplantation-safety-communication-risk-serious-adverse-reactions-due〉.
- Fenesi B, Fang H, Kovacevic A, Oremus M, Raina P, Heisz JJ, 2017. Physical exercise moderates the relationship of apolipoprotein E (APOE) genotype and dementia risk: a population-based study. J. Alzheimer’S. Dis.: JAD 56 (1), 297–303. [DOI] [PubMed] [Google Scholar]
- Fernandez DM, Clemente JC, Giannarelli C, 2018. Physical activity, immune system, and the microbiome in cardiovascular disease. Front Physiol. 9, 763–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernando WMADB, Martins IJ, Morici M, et al. , 2020. Sodium Butyrate Reduces Brain Amyloid-β Levels and Improves Cognitive Memory Performance in an Alzheimer’s Disease Transgenic Mouse Model at an Early Disease Stage. J Alzheimers Dis 74 (1), 91–99. [DOI] [PubMed] [Google Scholar]
- Festoff BW, Sajja RK, van Dreden P, Cucullo L, 2016. HMGB1 and thrombin mediate the blood-brain barrier dysfunction acting as biomarkers of neuroinflammation and progression to neurodegeneration in Alzheimer’s disease. J. Neuroinflamm 13 (1), 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald KA, Kagan JC, 2020. Toll-like receptors and the control of immunity. Cell 180 (6), 1044–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedland RP, 2015. Mechanisms of molecular mimicry involving the microbiota in neurodegeneration. J. Alzheimer’S. Dis 45, 349–362. [DOI] [PubMed] [Google Scholar]
- Friedland RP, Chapman MR, 2017. The role of microbial amyloid in neurodegeneration. PLoS Pathog. 13 (12), e1006654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii Y, Nguyen TTT, Fujimura Y, et al. , 2019. Fecal metabolite of a gnotobiotic mouse transplanted with gut microbiota from a patient with Alzheimer’s disease. Biosci Biotechnol Biochem 83 (11), 2144–2152. [DOI] [PubMed] [Google Scholar]
- Fung TC, Olson CA, Hsiao EY, 2017. Interactions between the microbiota, immune and nervous systems in health and disease. Nat. Neurosci 20 (2), 145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futch HS, Croft CL, Truong VQ, Krause EG, Golde TE, 2017. Targeting psychologic stress signaling pathways in Alzheimer’s disease. Mol. Neurodegener 12 (1), 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gai Z, Su D, Wang Y, et al. , 2017. Effects of chronic noise on the corticotropin-releasing factor system in the rat hippocampus: relevance to Alzheimer’s disease-like tau hyperphosphorylation. Environ. Health Prev. Med 22 (1), 79–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galland L, 2014. The gut microbiome and the brain. J. Med Food 17 (12), 1261–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gareau MG, 2014. Microbiota-gut-brain axis and cognitive function. Adv. Exp. Med Biol 817, 357–371. [DOI] [PubMed] [Google Scholar]
- Gareau MG, Jury J, MacQueen G, Sherman PM, Perdue MH, 2007. Probiotic treatment of rat pups normalises corticosterone release and ameliorates colonic dysfunction induced by maternal separation. Gut 56 (11), 1522–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gareau MG, Wine E, Rodrigues DM, et al. , 2011. Bacterial infection causes stress-induced memory dysfunction in mice. Gut 60 (3), 307–317. [DOI] [PubMed] [Google Scholar]
- Ghosh TS, Rampelli S, Jeffery IB, et al. , 2020. Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: the NU-AGE 1-year dietary intervention across five European countries. Gut 69 (7), 1218–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go J, Chang DH, Ryu YK, et al. , 2021. Human gut microbiota Agathobaculum butyriciproducens improves cognitive impairment in LPS-induced and APP/PS1 mouse models of Alzheimer’s disease. Nutr. Res 86, 96–108. [DOI] [PubMed] [Google Scholar]
- Goswami C, Iwasaki Y, Yada T, 2018. Short-chain fatty acids suppress food intake by activating vagal afferent neurons. J. Nutr. Biochem 57, 130–135. [DOI] [PubMed] [Google Scholar]
- Govindarajan N, Agis-Balboa RC, Walter J, et al. , 2011. Sodium butyrate improves memory function in an Alzheimer’s disease mouse model when administered at an advanced stage of disease progression. J Alzheimers Dis 26 (1), 187–197. [DOI] [PubMed] [Google Scholar]
- Goyal D, Ali SA, Singh RK, 2021. Emerging role of gut microbiota in modulation of neuroinflammation and neurodegeneration with emphasis on Alzheimer’s disease. Prog. Neuro Psychopharmacol. Biol. Psychiatry 106, 110112. [DOI] [PubMed] [Google Scholar]
- de Groot PF, Belzer C, Aydin Ö, et al. , 2017. Distinct fecal and oral microbiota composition in human type 1 diabetes, an observational study. PLoS One 12 (12), e0188475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubert C, Kong G, Renoir T, Hannan AJ, 2020. Exercise, diet and stress as modulators of gut microbiota: implications for neurodegenerative diseases. Neurobiol. Dis 134, 104621. [DOI] [PubMed] [Google Scholar]
- Guilherme MDS, Nguyen VTT, Reinhardt C, Endres K, 2021. Impact of gut microbiome manipulation in 5xFAD mice on Alzheimer’s disease-like pathology. Microorganisms 9, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemin GJ, 2012. Quinolinic acid, the inescapable neurotoxin. FEBS J. 279 (8), 1356–1365. [DOI] [PubMed] [Google Scholar]
- Gurav A, Sivaprakasam S, Bhutia Yangzom D, Boettger T, Singh N, Ganapathy V, 2015. Slc5a8, a Na+-coupled high-affinity transporter for short-chain fatty acids, is a conditional tumour suppressor in colon that protects against colitis and colon cancer under low-fibre dietary conditions. Biochem. J 469 (2), 267–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond CJ, Hallock LR, Howanski RJ, Appelt DM, Little CS, Balin BJ, 2010. Immunohistological detection of Chlamydia pneumoniae in the Alzheimer’s disease brain. BMC Neurosci. 11, 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han D, Li Z, Liu T, et al. , 2020. Prebiotics regulation of intestinal microbiota attenuates cognitive dysfunction induced by surgery stimulation in APP/PS1 mice. Aging Dis. 11 (5), 1029–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanage WP, 2014. Microbiology: microbiome science needs a healthy dose of scepticism. Nature 512 (7514), 247–248. [DOI] [PubMed] [Google Scholar]
- Harach T, Marungruang N, Duthilleul N, et al. , 2017. Reduction of Abeta amyloid pathology in APPPS1 transgenic mice in the absence of gut microbiota. Sci. Rep 7, 41802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haran JP, Bhattarai SK, Foley SE, et al. , 2019. Alzheimer’s disease microbiome is associated with dysregulation of the anti-inflammatory P-glycoprotein pathway. mBio 10 (3) e00632–00619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauss-Wegrzyniak B, Wenk GL, 2002. Beta-amyloid deposition in the brains of rats chronically infused with thiorphan or lipopolysaccharide: the role of ascorbic acid in the vehicle. Neurosci. Lett 322 (2), 75–78. [DOI] [PubMed] [Google Scholar]
- Hazan S, 2020. Rapid improvement in Alzheimer’s disease symptoms following fecal microbiota transplantation: a case report. J. Int. Med. Res 48 (6), 300060520925930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinken A, Ravcheev DA, Baldini F, Heirendt L, Fleming RMT, Thiele I, 2019. Systematic assessment of secondary bile acid metabolism in gut microbes reveals distinct metabolic capabilities in inflammatory bowel disease. Microbiome 7 (1), 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka MT, Golenbock DT, Latz E, 2015. Innate immunity in Alzheimer’s disease. Nat. Immunol 16 (3), 229–236. [DOI] [PubMed] [Google Scholar]
- Hills RD Jr., Pontefract BA, Mishcon HR, Black CA, Sutton SC, Theberge CR, 2019. Gut microbiome: profound implications for diet and disease. Nutrients 11 (7), 1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho L, Ono K, Tsuji M, et al. , 2018. Protective roles of intestinal microbiota derived short chain fatty acids in Alzheimer’s disease-type beta-amyloid neuropathological mechanisms. Expert Rev Neurother 18 (1), 83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooks KB, Konsman JP, O’Malley MA, 2018. Microbiota-gut-brain research: a critical analysis. Behav. Brain Sci 42, e60. [DOI] [PubMed] [Google Scholar]
- Hopkins MJ, Macfarlane GT, 2002. Changes in predominant bacterial populations in human faeces with age and with Clostridium difficile infection. J. Med. Microbiol 51 (5), 448–454. [DOI] [PubMed] [Google Scholar]
- Hudson BI, Lippman ME, 2018. Targeting RAGE signaling in inflammatory disease. Annu. Rev. Med 69, 349–364. [DOI] [PubMed] [Google Scholar]
- Hufnagel DA, Tükel C, Chapman MR, 2013. Disease to dirt: the biology of microbial amyloids. PLoS Pathog. 9 (11), e1003740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung CC, Chang CC, Huang CW, Nouchi R, Cheng CH, 2022. Gut microbiota in patients with Alzheimer’s disease spectrum: a systematic review and meta-analysis. Aging 14 (1), 477–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenhower C, Gevers D, Knight R, et al. , 2012. Structure, function and diversity of the healthy human microbiome. Nature 486 (7402), 207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide M, Harris M, Stevens A, et al. , 2016. Periodontitis and cognitive decline in Alzheimer’s disease. PLoS One 11 (3) e0151081–e0151081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger LB, Dohgu S, Sultana R, et al. , 2009. Lipopolysaccharide alters the blood-brain barrier transport of amyloid beta protein: a mechanism for inflammation in the progression of Alzheimer’s disease. Brain Behav. Immun 23 (4), 507–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jašarevíc E, Morrison KE, Bale TL, 2016. Sex differences in the gut microbiomebrain axis across the lifespan. Philos. Trans. R. Soc. Lond. B Biol. Sci 371 (1688), 20150122–20150122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Li K, Li X, et al. , 2021. Sodium butyrate ameliorates the impairment of synaptic plasticity by inhibiting the neuroinflammation in 5XFAD mice. Chem Biol Interact 341, 109452. [DOI] [PubMed] [Google Scholar]
- Johnson AJ, Zheng JJ, Kang JW, Saboe A, Knights D, Zivkovic AM, 2020. A guide to diet-microbiome study design. Front. Nutr 7, 79–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julien C, Tremblay C, Emond V, et al. , 2009. Sirtuin 1 reduction parallels the accumulation of tau in Alzheimer disease. J. Neuropathol. Exp. Neurol 68 (1), 48–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junges VM, Closs VE, Nogueira GM, Gottlieb MGV, 2018. Crosstalk between gut microbiota and central nervous system: a focus on Alzheimer’s disease. Curr. Alzheimer Res 15 (13), 1179–1190. [DOI] [PubMed] [Google Scholar]
- Kaddurah-Daouk R, Rozen S, Matson W, et al. , 2011. Metabolomic changes in autopsy-confirmed Alzheimer’s disease. Alzheimers Dement 7 (3), 309–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn MS, Kranjac D, Alonzo CA, et al. , 2012. Prolonged elevation in hippocampal Aβ and cognitive deficits following repeated endotoxin exposure in the mouse. Behav. Brain Res 229 (1), 176–184. [DOI] [PubMed] [Google Scholar]
- Kaisar MM, Pelgrom LR, van der Ham AJ, et al. , 2017. Butyrate Conditions Human Dendritic Cells to Prime Type 1 Regulatory T Cells via both Histone Deacetylase Inhibition and G Protein-Coupled Receptor (109A) Signaling. Front Immunol 8, 1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamer AR, Pirraglia E, Tsui W, et al. , 2015. Periodontal disease associates with higher brain amyloid load in normal elderly. Neurobiol. Aging 36 (2), 627–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JE, Cirrito JR, Dong H, et al. , 2007. Acute stress increases interstitial fluid amyloid-beta via corticotropin-releasing factor and neuronal activity. Proc Natl Acad Sci U S A 104 (25), 10673–10678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J-E, Lim MM, Bateman RJ, et al. , 2009. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science 326, 1005–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur H, Nookala S, Singh S, Mukundan S, Nagamoto-Combs K, Combs CK, 2021. Sex-dependent effects of intestinal microbiome manipulation in a mouse model of Alzheimer’s disease. Cells 10 (9), 2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawas MI, Lockhart SN, Kim J, et al. , 2021. Modified Mediterranean ketogenic diet resolves default mode network connectivity differences between adults with normal and impaired cognition. Alzheimer’s Dement. 17, e056711. [Google Scholar]
- Kespohl M, Vachharajani N, Luu M, et al. , 2017. The microbial metabolite butyrate induces expression of Th1-associated factors in CD4+ T cells. Front. Immunol 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna S, Vazquez-Baeza Y, González A, et al. , 2017. Changes in microbial ecology after fecal microbiota transplantation for recurrent C. difficile infection affected by underlying inflammatory bowel disease. Microbiome 5 (1), 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoruts A, Weingarden AR, 2014. Emergence of fecal microbiota transplantation as an approach to repair disrupted microbial gut ecology. Immunol. Lett 162 (2 Pt A), 77–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CS, Cha L, Sim M, et al. , 2021. Probiotic supplementation improves cognitive function and mood with changes in gut microbiota in community-dwelling older adults: a randomized, double-blind, placebo-controlled, multicenter trial. J. Gerontol. A Biol. Sci. Med Sci 76 (1), 32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Cho J, Lee I, Jin Y, Kang H, 2017. Exercise attenuates high-fat diet-induced disease progression in 3xTg-AD mice. Med. Sci. Sports Exerc 49 (4), 676–686. [DOI] [PubMed] [Google Scholar]
- Kim D, Cho J, Kang H, 2019. Protective effect of exercise training against the progression of Alzheimer’s disease in 3xTg-AD mice. Behav. Brain Res 374, 112105. [DOI] [PubMed] [Google Scholar]
- Kim MH, Kang SG, Park JH, Yanagisawa M, Kim CH, 2013. Short-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice. Gastroenterology 145 (2), 396–406 e391–310. [DOI] [PubMed] [Google Scholar]
- Kim MS, Kim Y, Choi H, et al. , 2020. Transfer of a healthy microbiota reduces amyloid and tau pathology in an Alzheimer’s disease animal model. Gut 69 (2), 283–294. [DOI] [PubMed] [Google Scholar]
- Kincaid HJ, Nagpal R, Yadav H, 2021. Diet-microbiota-brain axis in Alzheimer’s disease. ANM 77 (2), 21–27 (suppl 2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney JW, Bemiller SM, Murtishaw AS, Leisgang AM, Salazar AM, Lamb BT, 2018. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimer’s. Dement 4, 575–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y, Sugahara H, Shimada K, et al. , 2017. Therapeutic potential of Bifidobacterium breve strain A1 for preventing cognitive impairment in Alzheimer’s disease. Sci. Rep 7 (1), 13510–13510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y, Kuhara T, Oki M, Xiao JZ, 2019. Effects of Bifidobacterium breve A1 on the cognitive function of older adults with memory complaints: a randomised, double-blind, placebo-controlled trial. Benef. Microbes 10 (5), 511–520. [DOI] [PubMed] [Google Scholar]
- König J, Wells J, Cani PD, et al. , 2016. Human intestinal barrier function in health and disease. Clin. Transl. Gastroenterol 7 (10), e196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korf JM, Ganesh BP, McCullough LD, 2022. Gut dysbiosis and age-related neurological diseases in females. Neurobiol. Dis 168, 105695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kountouras J, Tsolaki M, Gavalas E, et al. , 2006. Relationship between Helicobacter pylori infection and Alzheimer disease. Neurology 66 (6), 938–940. [DOI] [PubMed] [Google Scholar]
- Kountouras J, Boziki M, Gavalas E, et al. , 2009. Increased cerebrospinal fluid Helicobacter pylori antibody in Alzheimer’s disease. Int. J. Neurosci 119 (6), 765–777. [DOI] [PubMed] [Google Scholar]
- Kowalski K, Mulak A, 2019. Brain-gut-microbiota axis in Alzheimer’s disease. J. Neurogastroenterol. Motil 25 (1), 48–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kress GJ, Liao F, Dimitry J, et al. , 2018. Regulation of amyloid-β dynamics and pathology by the circadian clock. J. Exp. Med 215 (4), 1059–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen NB, Bryrup T, Allin KH, Nielsen T, Hansen TH, Pedersen O, 2016. Alterations in fecal microbiota composition by probiotic supplementation in healthy adults: a systematic review of randomized controlled trials. Genome Med. 8 (1), 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüger JF, Hillesheim E, Pereira A, Camargo CQ, Rabito EI, 2021. Probiotics for dementia: a systematic review and meta-analysis of randomized controlled trials. Nutr. Rev 79 (2), 160–170. [DOI] [PubMed] [Google Scholar]
- Kundu P, Stagaman K, Kasschau K, et al. , 2022. Fecal Implants From AppNL-G-F and AppNL-G-F/E4 Donor Mice Sufficient to Induce Behavioral Phenotypes in Germ-Free Mice. Front Behav Neurosci 8 (16), 791128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu P, Torres ERS, Stagaman K, et al. , 2021. Integrated analysis of behavioral, epigenetic, and gut microbiome analyses in AppNL-G-F, AppNL-F, and wild type mice. Sci. Rep 11 (1), 4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laske C, Müller S, Preische O, et al. , 2022. Signature of Alzheimer’s disease in intestinal microbiome: results from the AlzBiom study. Front Neurosci. 16, 792996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblhuber F, Steiner K, Schuetz B, Fuchs D, Gostner JM, 2018. Probiotic supplementation in patients with Alzheimer’s dementia - an explorative intervention study. Curr. Alzheimer Res 15 (12), 1106–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclercq S, Mian FM, Stanisz AM, et al. , 2017. Low-dose penicillin in early life induces long-term changes in murine gut microbiota, brain cytokines and behavior. Nat. Commun 8, 15062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YS, Lai DM, Huang HJ, et al. , 2021. Prebiotic Lactulose Ameliorates the Cognitive Deficit in Alzheimer’s Disease Mouse Model through Macroautophagy and Chaperone-Mediated Autophagy Pathways. J Agric Food Chem 69 (8), 2422–2437. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Lee KE, Kim JK, Kim DH, 2019. Suppression of gut dysbiosis by Bifidobacterium longum alleviates cognitive decline in 5XFAD transgenic and aged mice. Sci. Rep 9 (1), 11814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, He Y, Ma J, et al. , 2019. Mild cognitive impairment has similar alterations as Alzheimer’s disease in gut microbiota. Alzheimer’S. Dement 15 (10), 1357–1366. [DOI] [PubMed] [Google Scholar]
- Li D, Ke Y, Zhan R, et al. , 2018. Trimethylamine-N-oxide promotes brain aging and cognitive impairment in mice. Aging Cell 17 (4), e12768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang S, Wang T, Hu X, et al. , 2015. Administration of Lactobacillus helveticus NS8 improves behavioral, cognitive, and biochemical aberrations caused by chronic restraint stress. Neuroscience 310, 561–577. [DOI] [PubMed] [Google Scholar]
- Lindsay RM, 1988. Nerve growth factors (NGF, BDNF) enhance axonal regeneration but are not required for survival of adult sensory neurons. J. Neurosci.: Off. J. Soc. Neurosci 8 (7), 2394–2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Wang J, He T, et al. , 2018. Butyrate: a double-edged sword for health? Adv. Nutr 9 (1), 21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Wu L, Peng G, et al. , 2019a. Altered microbiomes distinguish Alzheimer’s disease from amnestic mild cognitive impairment and health in a Chinese cohort. Brain Behav. Immun 80, 633–643. [DOI] [PubMed] [Google Scholar]
- Liu S, Gao J, Liu K, Zhang H-L, 2021. Microbiota-gut-brain axis and Alzheimer’s disease: implications of the blood-brain barrier as an intervention target. Mech. Ageing Dev 199, 111560. [DOI] [PubMed] [Google Scholar]
- Liu XX, Jiao B, Liao XX, et al. , 2019b. Analysis of salivary microbiome in patients with Alzheimer’s disease. J. Alzheimer’s. Dis.: JAD 72 (2), 633–640. [DOI] [PubMed] [Google Scholar]
- Loeb MB, Molloy DW, Smieja M, et al. , 2004. A randomized, controlled trial of doxycycline and rifampin for patients with Alzheimer’s disease. J. Am. Geriatr. Soc 52 (3), 381–387. [DOI] [PubMed] [Google Scholar]
- Lugo-Huitrón R, Ugalde Muñiz P, Pineda B, Pedraza-Chaverrí J, Ríos C, Pérez-de la Cruz V, 2013. Quinolinic acid: an endogenous neurotoxin with multiple targets. Oxid. Med. Cell. Longev 2013, 104024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukiw WJ, 2016. Bacteroides fragilis lipopolysaccharide and inflammatory signaling in Alzheimer’s disease. Front Microbiol 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X, Huang X, Luo Z, et al. , 2021. Electromagnetic field exposure-induced depression features could be alleviated by heat acclimation based on remodeling the gut microbiota. Ecotoxicol. Environ. Saf 228, 112980. [DOI] [PubMed] [Google Scholar]
- Lupien-Meilleur J, Roy D, Lagacé L, 2016. Viability of probiotic bacteria in a maple sap beverage during refrigerated storage. LWT 74, 160–167. [Google Scholar]
- Lutshumba J, Nikolajczyk BS, Bachstetter AD, 2021. Dysregulation of systemic immunity in aging and dementia. Front. Cell Neurosci 15, 652111–652111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado A, Herrera AJ, et al. de-Pablos RM, 2014. Chronic stress as a risk factor for Alzheimer’s disease. Rev. Neurosci 25 (6), 785–804. [DOI] [PubMed] [Google Scholar]
- MahmoudianDehkordi S, Arnold M, Nho K, 2019. et al. for the Alzheimer’s Disease Neuroimaging Initiative and the Alzheimer Disease Metabolomics Consortium. Altered bile acid profile associates with cognitive impairment in Alzheimer’s disease-An emerging role for gut microbiome. Alzheimers Dement 15 (1), 76–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallikarjuna N, Praveen K, Yellamma K, 2016. Role of Lactobacillus plantarum MTCC1325 in membrane-bound transport ATPases system in Alzheimer’s disease-induced rat brain. Bioimpacts 6 (4), 203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manikandan S, Padma MK, Srikumar R, Jeya Parthasarathy N, Muthuvel A, Sheela Devi, R., 2006. Effects of chronic noise stress on spatial memory of rats in relation to neuronal dendritic alteration and free radical-imbalance in hippocampus and medial prefrontal cortex. Neurosci. Lett 399 (1–2), 17–22. [DOI] [PubMed] [Google Scholar]
- Mariat D, Firmesse O, Levenez F, et al. , 2009. The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol. 9 (1), 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marizzoni M, Provasi S, Cattaneo A, Frisoni GB, 2017. Microbiota and neurodegenerative diseases. Curr. Opin. Neurol 30 (6), 630–638. [DOI] [PubMed] [Google Scholar]
- Meng G, Meng X, Ma X, et al. , 2018. Application of ferulic acid for Alzheimer’s disease: combination of text mining and experimental validation. Front. Neuroinf 12, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meslier V, Laiola M, Roager HM, et al. , 2020. Mediterranean diet intervention in overweight and obese subjects lowers plasma cholesterol and causes changes in the gut microbiome and metabolome independently of energy intake. Gut 69 (7), 1258–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messaoudi M, Lalonde R, Violle N, et al. , 2011. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br. J. Nutr 105 (5), 755–764. [DOI] [PubMed] [Google Scholar]
- Mezö C, Dokalis N, Mossad O, et al. , 2020. Different effects of constitutive and induced microbiota modulation on microglia in a mouse model of Alzheimer’s disease. Acta Neuropathol. Commun 8 (1), 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miklossy J, 2016. Bacterial Amyloid and DNA are important constituents of senile plaques: further evidence of the spirochetal and biofilm nature of senile plaques. J. Alzheimer’s. Dis. JAD 53 (4), 1459–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minter MR, Zhang C, Leone V, et al. , 2016. Antibiotic-induced perturbations in gut microbial diversity influences neuro-inflammation and amyloidosis in a murine model of Alzheimer’s disease. Sci. Rep 6, 30028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milovanovic J, Arsenijevic A, Stojanovic B, et al. , 2020. Interleukin-17 in Chronic Inflammatory Neurological Diseases. Front Immunol 3 (11), 947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minter MR, Hinterleitner R, Meisel M, et al. , 2017. Antibiotic-induced perturbations in microbial diversity during post-natal development alters amyloid pathology in an aged APP(SWE)/PS1(ΔE9) murine model of Alzheimer’s disease. Sci. Rep 7 (1), 10411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misiak B, Łoniewski I, Marlicz W, et al. , 2020. The HPA axis dysregulation in severe mental illness: can we shift the blame to gut microbiota? Prog. Neuro Psychopharmacol. Biol. Psychiatry 102, 109951. [DOI] [PubMed] [Google Scholar]
- Mitchell CM, Davy BM, Hulver MW, Neilson AP, Bennett BJ, Davy KP, 2019. Does exercise alter gut microbial composition? A systematic review. Med. Sci. Sports Exerc 51 (1), 160–167. [DOI] [PubMed] [Google Scholar]
- Mohammad S, Thiemermann C, 2021. Role of metabolic endotoxemia in systemic inflammation and potential interventions. Front. Immunol 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molloy DW, Standish TI, Zhou Q, Guyatt G, 2013. A multicenter, blinded, randomized, factorial controlled trial of doxycycline and rifampin for treatment of Alzheimer’s disease: the DARAD trial. Int. J. Geriatr. Psychiatry 28 (5), 463–470. [DOI] [PubMed] [Google Scholar]
- Montagne A, Barnes SR, Sweeney MD, et al. , 2015. Blood-brain barrier breakdown in the aging human hippocampus. Neuron 85 (2), 296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris MC, Tangney CC, Wang Y, Sacks FM, Bennett DA, Aggarwal NT, 2015. MIND diet associated with reduced incidence of Alzheimer’s disease. Alzheimers Dement 11 (9), 1007–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison KE, Jašarevíc E, Howard CD, Bale TL, 2020. It’s the fiber, not the fat: significant effects of dietary challenge on the gut microbiome. Microbiome 8 (1), 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi L, Murray J, Tsui WH, et al. , 2014. Mediterranean diet and magnetic resonance imaging-assessed brain atrophy in cognitively normal individuals at risk for Alzheimer’s disease. J. Prev. Alzheimer’S. Dis 1 (1), 23–32. [PMC free article] [PubMed] [Google Scholar]
- Motiani KK, Collado MC, Eskelinen JJ, et al. , 2020. Exercise training modulates gut microbiota profile and improves endotoxemia. Med. Sci. Sports Exerc 52 (1), 94–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musiek ES, Xiong DD, Holtzman DM, 2015. Sleep, circadian rhythms, and the pathogenesis of Alzheimer disease. Exp. Mol. Med 47 (3), e148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabavi SF, Devi KP, Malar DS, Sureda A, Daglia M, Nabavi SM, 2015. Ferulic acid and Alzheimer’s disease: promises and pitfalls. Mini Rev. Med. Chem 15 (9), 776–788. [DOI] [PubMed] [Google Scholar]
- Nagpal R, Mainali R, Ahmadi S, et al. , 2018. Gut microbiome and aging: physiological and mechanistic insights. Nutr. Healthy Aging 4 (4), 267–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagpal R, Shively CA, Register TC, Craft S, Yadav H, 2019a. Gut microbiome-Mediterranean diet interactions in improving host health. F1000Res. 8, 699–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagpal R, Neth BJ, Wang S, Craft S, Yadav H, 2019b. Modified Mediterranean-ketogenic diet modulates gut microbiome and short-chain fatty acids in association with Alzheimer’s disease markers in subjects with mild cognitive impairment. EBioMedicine 47, 529–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagu P, Parashar A, Behl T, Mehta V, 2021. Gut microbiota composition and epigenetic molecular changes connected to the pathogenesis of Alzheimer’s disease. J. Mol. Neurosci. MN 71 (7), 1436–1455. [DOI] [PubMed] [Google Scholar]
- Nam KN, Mounier A, Wolfe CM, et al. , 2017. Effect of high fat diet on phenotype, brain transcriptome and lipidome in Alzheimer’s model mice. Sci. Rep 7 (1), 4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld KM, Kang N, Bienenstock J, Foster JA, 2011. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol. Motil 23 (3), 255–e119. [DOI] [PubMed] [Google Scholar]
- Nimgampalle M, Kuna Y, 2017. Anti-alzheimer properties of probiotic, lactobacillus plantarum MTCC 1325 in Alzheimer’s disease induced albino rats. J. Clin. Diagn. Res 11 (8), KC01–KC05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimori JH, Newman TN, Oppong GO, et al. , 2012. Microbial amyloids induce interleukin 17A (IL-17A) and IL-22 responses via Toll-like receptor 2 activation in the intestinal mucosa. Infect. Immun 80 (12), 4398–4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble JM, Scarmeas N, Celenti RS, et al. , 2014. Serum IgG antibody levels to periodontal microbiota are associated with incident Alzheimer disease. PloS One 9 (12), e114959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberstein TJ, Taha L, Spitzer P, et al. , 2018. Imbalance of circulating Th17 and regulatory T cells in Alzheimer’s disease: a case control study. Front. Immunol 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooms S, Overeem S, Besse K, Rikkert MO, Verbeek M, Claassen JA, 2014. Effect of 1 night of total sleep deprivation on cerebrospinal fluid β-amyloid 42 in healthy middle-aged men: a randomized clinical trial. JAMA Neurol. 71 (8), 971–977. [DOI] [PubMed] [Google Scholar]
- Pappolla MA, Perry G, Fang X, Zagorski M, Sambamurti K, Poeggeler B, 2021. Indoles as essential mediators in the gut-brain axis. Their role in Alzheimer’s disease. Neurobiol. Dis 156, 105403. [DOI] [PubMed] [Google Scholar]
- Park S, Zhang T, Wu X, Yi Qiu J, 2020. Ketone production by ketogenic diet and by intermittent fasting has different effects on the gut microbiota and disease progression in an Alzheimer’s disease rat model. J. Clin. Biochem. Nutr 67 (2), 188–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SY, Hwang BO, Lim M, et al. , 2021. Oral-gut microbiome axis in gastrointestinal disease and cancer. Cancers 13, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineton de Chambrun G, Body-Malapel M, Frey-Wagner I, et al. , 2014. Aluminum enhances inflammation and decreases mucosal healing in experimental colitis in mice. Mucosal Immunol. 7 (3), 589–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistollato F, Sumalla Cano S, Elio I, Masias Vergara M, Giampieri F, Battino M, 2016. Role of gut microbiota and nutrients in amyloid formation and pathogenesis of Alzheimer disease. Nutr. Rev 74 (10), 624–634. [DOI] [PubMed] [Google Scholar]
- Poole S, Singhrao SK, Chukkapalli S, et al. , 2015. Active invasion of Porphyromonas gingivalis and infection-induced complement activation in ApoE−/− mice brains. J. Alzheimer’s. Dis. JAD 43 (1), 67–80. [DOI] [PubMed] [Google Scholar]
- Poole S, Singhrao SK, Kesavalu L, et al. , 2013. Determining the presence of periodontopathic virulence factors in short-term postmortem Alzheimer’s disease brain tissue. J Alzheimers Dis 36, 665–677. [DOI] [PubMed] [Google Scholar]
- Poroyko VA, Carreras A, Khalyfa A, et al. , 2016. Chronic sleep disruption alters gut microbiota, induces systemic and adipose tissue inflammation and insulin resistance in mice. Sci. Rep 6 (1), 35405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, Ko AI, Wade MJ, Tsou SK, McKeel DW, Morris JC, 2001. Neuron number in the entorhinal cortex and CA1 in preclinical Alzheimer disease. Arch. Neurol 58 (9), 1395–1402. [DOI] [PubMed] [Google Scholar]
- Quinn M, McMillin M, Galindo C, Frampton G, Pae HY, DeMorrow S, 2014. Bile acids permeabilize the blood brain barrier after bile duct ligation in rats via Rac1-dependent mechanisms. Dig. Liver Dis. Off. J. Ital. Soc. Gastroenterol. Ital. Assoc. Study Liver 46 (6), 527–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raimondi F, Santoro P, Barone MV, et al. , 2008. Bile acids modulate tight junction structure and barrier function of Caco-2 monolayers via EGFR activation. Am. J. Physiol. Gastrointest. Liver Physiol 294 (4), G906–G913. [DOI] [PubMed] [Google Scholar]
- Rapsinski GJ, Wynosky-Dolfi MA, Oppong GO, et al. , 2015. Toll-like receptor 2 and NLRP3 cooperate to recognize a functional bacterial amyloid, curli. Infect. Immun 83 (2), 693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid G, Gadir AA, Dhir R, 2019. Probiotics: reiterating what they are and what they are not. Front Microbiol 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren S, Wang X, Qin J, et al. , 2022. Altered gut microbiota correlates with cognitive impairment in Chinese children with Down’s syndrome. Eur. Child Adolesc. Psychiatry 31 (1), 189–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribière C, Peyret P, Parisot N, et al. , 2016. Oral exposure to environmental pollutant benzo[a]pyrene impacts the intestinal epithelium and induces gut microbial shifts in murine model. Sci. Rep 6, 31027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci S, Fuso A, Ippoliti F, Businaro R, 2012. Stress-induced cytokines and neuronal dysfunction in Alzheimer’s disease. J. Alzheimer’s. Dis 28, 11–24. [DOI] [PubMed] [Google Scholar]
- Rossi Daŕe L, Garcia A, Neves B-H, Mello-Carpes PB, 2020. One physical exercise session promotes recognition learning in rats with cognitive deficits related to amyloid beta neurotoxicity. Brain Res. 1744, 146918. [DOI] [PubMed] [Google Scholar]
- Rothschild D, Weissbrod O, Barkan E, et al. , 2018. Environment dominates over host genetics in shaping human gut microbiota. Nature 555 (7695), 210–215. [DOI] [PubMed] [Google Scholar]
- Roubaud-Baudron C, Krolak-Salmon P, Quadrio I, Mégraud F, Salles N, 2012. Impact of chronic Helicobacter pylori infection on Alzheimer’s disease: preliminary results. Neurobiol. Aging 33 (5), e1011–1009, 1009. [DOI] [PubMed] [Google Scholar]
- Rydbom J, Kohl H, Hyde VR, Lohr KM, 2021. Altered gut microbial load and immune activation in a drosophila model of human tauopathy. Front. Neurosci 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabia S, Fayosse A, Dumurgier J, et al. , 2021. Association of sleep duration in middle and old age with incidence of dementia. Nat. Commun 12 (1), 2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah SK, Lee C, Jang JH, Park GH, 2017. Effect of high-fat diet on cognitive impairment in triple-transgenic mice model of Alzheimer’s disease. Biochem. Biophys. Res. Commun 493 (1), 731–736. [DOI] [PubMed] [Google Scholar]
- Saji N, Niida S, Murotani K, et al. , 2019. Analysis of the relationship between the gut microbiome and dementia: a cross-sectional study conducted in Japan. Sci. Rep 9 (1), 1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar N, Valdés-Varela L, González S, Gueimonde M, de Los Reyes-Gavilán CG, 2017. Nutrition and the gut microbiome in the elderly. Gut Microbes 8 (2), 82–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson TR, Challis C, Jain N, et al. , 2020. A gut bacterial amyloid promotes α-synuclein aggregation and motor impairment in mice. eLife 9, e53111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanguinetti E, Collado MC, Marrachelli VG, et al. , 2018. Microbiome-metabolome signatures in mice genetically prone to develop dementia, fed a normal or fatty diet. Sci. Rep 8 (1), 4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savignac HM, Tramullas M, Kiely B, Dinan TG, Cryan JF, 2015. Bifidobacteria modulate cognitive processes in an anxious mouse strain. Behav. Brain Res 287, 59–72. [DOI] [PubMed] [Google Scholar]
- Savitz J, 2020. The kynurenine pathway: a finger in every pie. Mol. Psychiatry 25 (1), 131–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz K, Boles BR, 2013. Microbial amyloids–functions and interactions within the host. Curr. Opin. Microbiol 16 (1), 93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segain JP, Raingeard de la Blétière D, Bourreille A, et al. , 2000. Butyrate inhibits inflammatory responses through NFkappaB inhibition: implications for Crohn’s disease. Gut 47 (3), 397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo DO, Boros BD, Holtzman DM, 2019. The microbiome: a target for Alzheimer disease? Cell Res 29 (10), 779–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgarbossa A, Giacomazza D, di Carlo M, 2015. Ferulic Acid: A Hope for Alzheimer’s Disease Therapy from Plants. Nutrients 7 (7), 5764–5782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamsipour S, Sharifi G, Taghian F, 2021. An 8-week administration of bifidobacterium bifidum and lactobacillus plantarum combined with exercise training alleviates neurotoxicity of Aβ and spatial learning via acetylcholine in AlzheImer RAT MODel. J. Mol. Neurosci. MN 71 (7), 1495–1505. [DOI] [PubMed] [Google Scholar]
- Shen H, Guan Q, Zhang X, et al. , 2020. New mechanism of neuroinflammation in Alzheimer’s disease: the ACTIvation of NLRP3 inflammasome mediated by gut microbiota. Prog. Neuro Psychopharmacol. Biol. Psychiatry 100, 109884. [DOI] [PubMed] [Google Scholar]
- Shen L, Liu L, Ji H-F, 2017. Alzheimer’s disease histological and behavioral manifestations in transgenic mice correlate with specific gut microbiome state. J. Alzheimer’S. Dis 56, 385–390. [DOI] [PubMed] [Google Scholar]
- Shen Y, Giardino Torchia ML, Lawson GW, Karp CL, Ashwell JD, Mazmanian SK, 2012. Outer membrane vesicles of a human commensal mediate immune regulation and disease protection. Cell Host Microbe 12 (4), 509–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng C, Yang K, He B, Du W, Cai Y, Han Y, 2022. Combination of gut microbiota and plasma amyloid-β as a potential index for identifying preclinical Alzheimer’s disease: a cross-sectional analysis from the SILCODE study. Alz Res. Ther 14 (1), 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shokri-Kojori E, Wang G-J, Wiers CE, et al. , 2018. β-Amyloid accumulation in the human brain after one night of sleep deprivation. PNAS 115 (17), 4483–4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla PK, Delotterie DF, Xiao J, et al. , 2021. Alterations in the Gut-Microbial-Inflammasome-Brain Axis in a Mouse Model of Alzheimer’s Disease. Cells 10 (4), 779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui MT, Cresci GAM, 2021. The Immunomodulatory Functions of Butyrate. J Inflamm Res 14, 6025–6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva YP, Bernardi A, Frozza RL, 2020. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front Endocrinol (Lausanne) 11, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhrao SK, Olsen I, 2019. Assessing the role of Porphyromonas gingivalis in periodontitis to determine a causative relationship with Alzheimer’s disease. J. Oral. Microbiol 11 (1), 1563405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RP, Easson C, Lyle SM, et al. , 2019. Gut microbiome diversity is associated with sleep physiology in humans. PLoS One 14 (10) e0222394-e0222394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowade RF, Jahn TR, 2017. Seed-induced acceleration of amyloid-β mediated neurotoxicity in vivo. Nat. Commun 8 (1), 512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks Stein P, Steffen MJ, Smith C, et al. , 2012. Serum antibodies to periodontal pathogens are a risk factor for Alzheimer’s disease. Alzheimer’S. Dement.: J. Alzheimer’S. Assoc 8 (3), 196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadlbauer V, Engertsberger L, Komarova I, et al. , 2020. Dysbiosis, gut barrier dysfunction and inflammation in dementia: a pilot study. BMC Geriatr. 20 (1), 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenman LK, Holma R, Eggert A, Korpela R, 2013. A novel mechanism for gut barrier dysfunction by dietary fat: epithelial disruption by hydrophobic bile acids. Am. J. Physiol. Gastrointest. Liver Physiol 304 (3), G227–G234. [DOI] [PubMed] [Google Scholar]
- Strandwitz P, 2018. Neurotransmitter modulation by the gut microbiota. Brain Res. 1693 (Pt B), 128–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studzinski CM, Li F, Bruce-Keller AJ, et al. , 2009. Effects of short-term Western diet on cerebral oxidative stress and diabetes related factors in APP × PS1 knock-in mice. J. Neurochem 108 (4), 860–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun BL, Li WW, Wang J, et al. , 2019. Gut microbiota alteration and its time course in a tauopathy mouse model. J. Alzheimer’s. Dis.: JAD 70 (2), 399–412. [DOI] [PubMed] [Google Scholar]
- Sun J, Liu S, Ling Z, Wang F, et al. , 2019b. Fructooligosaccharides Ameliorating Cognitive Deficits and Neurodegeneration in APP/PS1 Transgenic Mice through Modulating Gut Microbiota. J Agric Food Chem 67 (10), 3006–3017. [DOI] [PubMed] [Google Scholar]
- Sun J, Xu J, Yang B, Chen K, et al. , 2020. Effect of Clostridium butyricum against Microglia-Mediated Neuroinflammation in Alzheimer’s Disease via Regulating Gut Microbiota and Metabolites Butyrate. Mol. Nutr. Food Res 64, 1900636. [DOI] [PubMed] [Google Scholar]
- Sureda A, Daglia M, Argüelles Castilla S, et al. , 2020. Oral microbiota and Alzheimer’s disease: do all roads lead to Rome? Pharmacol. Res 151, 104582. [DOI] [PubMed] [Google Scholar]
- Sweeney MD, Sagare AP, Zlokovic BV, 2018. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol 14 (3), 133–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabady RL, Louissaint C, Lubben A, et al. , 2018. Intestinal P-glycoprotein exports endocannabinoids to prevent inflammation and maintain homeostasis. J. Clin. Invest 128 (9), 4044–4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczechowiak K, Diniz BS, Leszek J, 2019. Diet and Alzheimer’s dementia - nutritional approach to modulate inflammation. Pharmacol., Biochem., Behav 184, 172743. [DOI] [PubMed] [Google Scholar]
- Szu JI, Obenaus A, 2021. Cerebrovascular phenotypes in mouse models of Alzheimer’s disease. J. Cereb. Blood Flow. Metab 41 (8), 1821–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai YK, Ng C, Purnamawati K, et al. , 2020. Magnetic fields modulate metabolism and gut microbiome in correlation with Pgc-1α expression: Follow-up to an in vitro magnetic mitohormetic study. FASEB J. 34 (8), 11143–11167. [DOI] [PubMed] [Google Scholar]
- Tamtaji OR, Heidari-Soureshjani R, Mirhosseini N, et al. , 2019. Probiotic and selenium co-supplementation, and the effects on clinical, metabolic and genetic status in Alzheimer’s disease: a randomized, double-blind, controlled trial. Clin. Nutr. (Edinb., Scotl.) 38 (6), 2569–2575. [DOI] [PubMed] [Google Scholar]
- Tan J, McKenzie C, Potamitis M, Thorburn AN, Mackay CR, Macia L, 2014. Chapter three - the role of short-chain fatty acids in health and disease. In: Alt FW (Ed.), Advances in Immunology, Vol 121. Academic Press, pp. 91–119. [DOI] [PubMed] [Google Scholar]
- Tarawneh R, Holtzman DM, 2012. The clinical problem of symptomatic Alzheimer disease and mild cognitive impairment. Cold Spring Harb. Perspect. Med 2 (5), a006148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetz G, Pinho M, Pritzkow S, Mendez N, Soto C, Tetz V, 2020. Bacterial DNA promotes Tau aggregation. Sci. Rep 10 (1), 2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinkov AA, Martins AC, Avila DS, et al. , 2021. Gut microbiota as a potential player in mn-induced neurotoxicity. Biomolecules 11, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ton AMM, Campagnaro BP, Alves GA, et al. , 2020. Oxidative stress and dementia in Alzheimer’s patients: effects of synbiotic supplementation. Oxid. Med. Cell. Longev 2020, 2638703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran TTT, Corsini S, Kellingray L, et al. , 2019. APOE genotype influences the gut microbiome structure and function in humans and mice: relevance for Alzheimer’s disease pathophysiology. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol 33 (7), 8221–8231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tristão FSM, Rocha FA, Carlos D, et al. , 2017. Th17-inducing cytokines IL-6 and IL-23 are crucial for granuloma formation during experimental paracoccidioidomycosis. Front. Immunol 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai GE, Falk WE, Gunther J, 1998. A preliminary study of D-cycloserine treatment in Alzheimer’s disease. J. Neuropsychiatry Clin. Neurosci 10 (2), 224–226. [DOI] [PubMed] [Google Scholar]
- Tsai GE, Falk WE, Gunther J, Coyle JT, 1999. Improved cognition in Alzheimer’s disease with short-term D-cycloserine treatment. Am. J. Psychiatry 156 (3), 467–469. [DOI] [PubMed] [Google Scholar]
- Tükel C, Raffatellu M, Humphries AD, et al. , 2005. CsgA is a pathogen-associated molecular pattern of Salmonella enterica serotype Typhimurium that is recognized by Toll-like receptor 2. Mol Microbiol 58 (1), 289–304. [DOI] [PubMed] [Google Scholar]
- Tzeng NS, Chung CH, Yeh CB, et al. , 2016. Are chronic periodontitis and gingivitis associated with dementia? A nationwide, retrospective, matched-cohort study in Taiwan. Neuroepidemiology 47 (2), 82–93. [DOI] [PubMed] [Google Scholar]
- Uddin MS, Tewari D, Mamun AA, et al. , 2020. Circadian and sleep dysfunction in Alzheimer’s disease. Ageing Res. Rev 60, 101046. [DOI] [PubMed] [Google Scholar]
- Ugbode C, Hu Y, Whalley B, Peers C, Rattray M, Dallas ML, 2017. Astrocytic transporters in Alzheimer’s disease. Biochem. J 474 (3), 333–355. [DOI] [PubMed] [Google Scholar]
- Vakharia K, Hinson JP, 2005. Lipopolysaccharide directly stimulates cortisol secretion by human adrenal cells by a cyclooxygenase-dependent mechanism. Endocrinology 146 (3), 1398–1402. [DOI] [PubMed] [Google Scholar]
- Valladares R, Bojilova L, Potts AH, et al. , 2013. Lactobacillus johnsonii inhibits indoleamine 2,3-dioxygenase and alters tryptophan metabolite levels in BioBreeding rats. FASEB J 27 (4), 1711–1720. [DOI] [PubMed] [Google Scholar]
- Valls-Pedret C, Sala-Vila A, Serra-Mir M, et al. , 2015. Mediterranean diet and age-related cognitive decline: a randomized clinical trial. JAMA Intern. Med 175 (7), 1094–1103. [DOI] [PubMed] [Google Scholar]
- van Assema DM, Lubberink M, Bauer M, et al. , 2012. Blood-brain barrier P-glycoprotein function in Alzheimer’s disease. Brain 135 (Pt 1), 181–189. [DOI] [PubMed] [Google Scholar]
- van de Haar HJ, Burgmans S, Jansen JF, et al. , 2016. Blood-brain barrier leakage in patients with early Alzheimer disease. Radiology 281 (2), 527–535. [DOI] [PubMed] [Google Scholar]
- van de Wouw M, Boehme M, Lyte JM, et al. , 2018. Short-chain fatty acids: microbial metabolites that alleviate stress-induced brain-gut axis alterations. J. Physiol 596 (20), 4923–4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Olst L, Roks SJM, Kamermans A, et al. , 2021. Contribution of gut microbiota to immunological changes in Alzheimer’s disease. Front. Immunol 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicario M, Alonso C, Guilarte M, et al. , 2012. Chronic psychosocial stress induces reversible mitochondrial damage and corticotropin-releasing factor receptor type-1 upregulation in the rat intestine and IBS-like gut dysfunction. Psychoneuroendocrinology 37 (1), 65–77. [DOI] [PubMed] [Google Scholar]
- Vinolo MAR, Rodrigues HG, Hatanaka E, Sato FT, Sampaio SC, Curi R, 2011. Suppressive effect of short-chain fatty acids on production of proinflammatory mediators by neutrophils. J. Nutr. Biochem 22 (9), 849–855. [DOI] [PubMed] [Google Scholar]
- Vogt NM, Kerby RL, Dill-McFarland KA, et al. , 2017. Gut microbiome alterations in Alzheimer’s disease. Sci. Rep 7 (1), 13537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt NM, Romano KA, Darst BF, et al. , 2018. The gut microbiota-derived metabolite trimethylamine N-oxide is elevated in Alzheimer’s disease. Alzheimer’S. Res. Ther 10 (1), 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsham NE, Sherwood RA, 2016. Fecal calprotectin in inflammatory bowel disease. Clin. Exp. Gastroenterol 9, 21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Klechikov AG, Gharibyan AL, et al. , 2014a. The role of pro-inflammatory S100A9 in Alzheimer’s disease amyloid-neuroinflammatory cascade. Acta Neuropathol. 127 (4), 507–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Iashchishyn IA, Pansieri J, et al. , 2018. S100A9-driven amyloid-neuroinflammatory cascade in traumatic brain injury as a precursor state for Alzheimer’s disease. Sci. Rep 8 (1), 12836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DD, Nguyen LH, Li Y, et al. , 2021b. The gut microbiome modulates the protective association between a Mediterranean diet and cardiometabolic disease risk. Nat. Med 27 (2), 333–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Davis PB, Qi X, et al. , 2021a. Gut–microbiota–microglia–brain interactions in Alzheimer’s disease: knowledge-based, multi-dimensional characterization. Alz Res Ther. 13 (1), 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang QJ, Shen YE, Wang X, et al. , 2020a. Concomitant memantine and Lactobacillus plantarum treatment attenuates cognitive impairments in APP/PS1 mice. Aging 12 (1), 628–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Xu M, Wang W, et al. , 2016b. Systematic review: adverse events of fecal microbiota transplantation. PLoS One 11 (8), e0161174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Hu X, Liang S, et al. , 2015a. Lactobacillus fermentum NS9 restores the antibiotic induced physiological and psychological abnormalities in rats. Benef. Microbes 6 (5), 707–717. [DOI] [PubMed] [Google Scholar]
- Wang W, Bodles-Brakhop AM, Barger SW, 2016a. A role for P-glycoprotein in clearance of alzheimer amyloid β -peptide from the brain. Curr. Alzheimer Res 13 (6), 615–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Xu Y, Gao R, et al. , 2020b. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA 323 (18), 1843–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W-Y, Tan M-S, Yu J-T, Tan L, 2015b. Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann. Transl. Med 3 (10), 136–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Sun G, Feng T, et al. , 2019. Sodium oligomannate therapeutically remodels gut microbiota and suppresses gut bacterial amino acids-shaped neuroinflammation to inhibit Alzheimer’s disease progression. Cell Res. 29 (10), 787–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XL, Zeng J, Feng J, et al. , 2014b. Helicobacter pylori filtrate impairs spatial learning and memory in rats and increases β-amyloid by enhancing expression of presenilin-2. Front. Aging Neurosci 6, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, 2022. The role of the gut microbiota and microbial metabolites in the pathogenesis of Alzheimer’s disease. CNS Neurol. Disord. Drug Targets [DOI] [PubMed] [Google Scholar]
- Wang Y, Dykes GA, 2022. Direct modulation of the gut microbiota as a therapeutic approach for Alzheimer’s disease. CNS Neurol. Disord. Drug Targets 21 (1), 14–25. [DOI] [PubMed] [Google Scholar]
- Wang Z, Yuan K, Ji Y-B, et al. , 2022. Alterations of the gut microbiota in response to total sleep deprivation and recovery sleep in rats. Nat. Sci. Sleep 14, 121–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster L, Costafreda Gonzalez S, Stringer A, et al. , 2020. Measuring the prevalence of sleep disturbances in people with dementia living in care homes: a systematic review and meta-analysis. Sleep 43 (4) zsz251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisburger JH, 1997. Dietary fat and risk of chronic disease: mechanistic insights from experimental studies. J. Am. Diet. Assoc 97 (7 Suppl), S16–S23. [DOI] [PubMed] [Google Scholar]
- Westfall S, Lomis N, Kahouli I, Dia SY, Singh SP, Prakash S, 2017. Microbiome, probiotics and neurodegenerative diseases: deciphering the gut brain axis. Cell. Mol. life Sci.: CMLS 74 (20), 3769–3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wightman DP, Jansen IE, Savage JE, et al. , 2020. Largest GWAS (N=1,126,563) of Alzheimer’s disease implicates microglia and immune cells. medRxiv, 2020.2011.2020.20235275. [Google Scholar]
- Wilck N, Matus MG, Kearney SM, et al. , 2017. Salt-responsive gut commensal modulates T(H)17 axis and disease. Nature 551 (7682), 585–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte AV, Fobker M, Gellner R, Knecht S, Flöel A, 2009. Caloric restriction improves memory in elderly humans. Proc. Natl. Acad. Sci. USA 106 (4), 1255–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodmansey EJ, McMurdo ME, Macfarlane GT, Macfarlane S, 2004. Comparison of compositions and metabolic activities of fecal microbiotas in young adults and in antibiotic-treated and non-antibiotic-treated elderly subjects. Appl. Environ. Microbiol 70 (10), 6113–6122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Han Y, Zheng Z, et al. , 2021. Altered gut microbial metabolites in amnestic mild cognitive impairment and Alzheimer’s disease: signals in host-microbe interplay. Nutrients 13 (1), 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Xu L, Wang Y, et al. , 2018. S100A8/A9 induces microglia activation and promotes the apoptosis of oligodendrocyte precursor cells by activating the NF-κB signaling pathway. Brain Res. Bull 143, 234–245. [DOI] [PubMed] [Google Scholar]
- Wu SC, Cao ZS, Chang KM, Juang JL, 2017. Intestinal microbial dysbiosis aggravates the progression of Alzheimer’s disease in Drosophila. Nat. Commun 8 (1), 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SY, Wang TF, Yu L, et al. , 2011. Running exercise protects the substantia nigra dopaminergic neurons against inflammation-induced degeneration via the activation of BDNF signaling pathway. Brain Behav. Immun 25 (1), 135–146. [DOI] [PubMed] [Google Scholar]
- Wu YH, Feenstra MG, Zhou JN, et al. , 2003. Molecular changes underlying reduced pineal melatonin levels in Alzheimer disease: alterations in preclinical and clinical stages. J. Clin. Endocrinol. Metab 88 (12), 5898–5906. [DOI] [PubMed] [Google Scholar]
- Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H, 2020. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology 158 (6), 1831–1833 e1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie L, Kang H, Xu Q, et al. , 2013. Sleep drives metabolite clearance from the adult brain. Science 342, 373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin Y, Diling C, Jian Y, et al. , 2018. Effects of oligosaccharides from morinda officinalis on gut microbiota and metabolome of APP/PS1 transgenic mice. Front. Neurol 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Chen W, Sun Y, Liu J, Zhang W, 2021. Effects of cadmium on organ function, gut microbiota and its metabolomics profile in adolescent rats. Ecotoxicol. Environ. Saf 222, 112501. [DOI] [PubMed] [Google Scholar]
- Yang X, Yu D, Xue L, Li H, Du J, 2020. Probiotics modulate the microbiota-gut-brain axis and improve memory deficits in aged SAMP8 mice. Acta Pharm. Sin. B 10 (3), 475–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi CX, Al-Massadi O, Donelan E, et al. , 2012. Exercise protects against high-fat diet-induced hypothalamic inflammation. Physiol. Behav 106 (4), 485–490. [DOI] [PubMed] [Google Scholar]
- Yin JX, Maalouf M, Han P, et al. , 2016. Ketones block amyloid entry and improve cognition in an Alzheimer’s model. Neurobiol. Aging 39, 25–37. [DOI] [PubMed] [Google Scholar]
- Yulug B, Hanoglu L, Ozansoy M, et al. , 2018. Therapeutic role of rifampicin in Alzheimer’s disease. Psychiatry Clin. Neurosci 72 (3), 152–159. [DOI] [PubMed] [Google Scholar]
- Yusufov M, Weyandt LL, Piryatinsky I, 2017. Alzheimer’s disease and diet: a systematic review. Int. J. Neurosci 127 (2), 161–175. [DOI] [PubMed] [Google Scholar]
- Zenaro E, Pietronigro E, Bianca VD, et al. , 2015. Neutrophils promote Alzheimer’s disease–like pathology and cognitive decline via LFA-1 integrin. Nat. Med 21 (8), 880–886. [DOI] [PubMed] [Google Scholar]
- Zeng B, Zhao G, Liu HL, 2020. The differential effect of treadmill exercise intensity on hippocampal soluble Aβ and lipid metabolism in APP/PS1 mice. Neuroscience 430, 73–81. [DOI] [PubMed] [Google Scholar]
- Zhan G, Yang N, Li S, et al. , 2018. Abnormal gut microbiota composition contributes to cognitive dysfunction in SAMP8 mice. Aging 10 (6), 1257–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan X, Stamova B, Jin LW, DeCarli C, Phinney B, Sharp FR, 2016. Gram-negative bacterial molecules associate with Alzheimer disease pathology. Neurology 87 (22), 2324–2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Liu Y, Gilthorpe J, van der Maarel JR, 2012. MRP14 (S100A9) protein interacts with Alzheimer beta-amyloid peptide and induces its fibrillization. PLoS One 7 (3), e32953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Ke KF, Liu Z, Qiu YH, Peng YP, 2013. Th17 cell-mediated neuroinflammation is involved in neurodegeneration of aβ1–42-induced Alzheimer’s disease model rats. PLoS One 8 (10), e75786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Wang Y, Xiayu X, et al. , 2017b. Altered gut microbiota in a mouse model of Alzheimer’s disease. J. Alzheimer’s. Dis. JAD 60 (4), 1241–1257. [DOI] [PubMed] [Google Scholar]
- Zhang M, Zhou Q, Dorfman RG, et al. , 2016. Butyrate inhibits interleukin-17 and generates Tregs to ameliorate colorectal colitis in rats. BMC Gastroenterol. 16 (1), 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Miller RG, Gascon R, et al. , 2009. Circulating endotoxin and systemic immune activation in sporadic amyotrophic lateral sclerosis (sALS). J. Neuroimmunol 206 (1–2), 121–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SL, Bai L, Goel N, et al. , 2017a. Human and rat gut microbiome composition is maintained following sleep restriction. Proc. Natl. Acad. Sci. USA 114 (8), E1564–E1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Bi W, Xiao S, et al. , 2019. Neuroinflammation induced by lipopolysaccharide causes cognitive impairment in mice. Sci. Rep 9 (1), 5790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Cong L, Jaber V, Lukiw WJ, 2017a. Microbiome-derived lipopolysaccharide enriched in the perinuclear region of Alzheimer’s disease brain. Front. Immunol 8, 1064–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Cong L, Lukiw WJ, 2017b. Lipopolysaccharide (LPS) accumulates in neocortical neurons of Alzheimer’s disease (AD) brain and impairs transcription in human neuronal-glial primary co-cultures. Front. Aging Neurosci 9, 407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Lukiw WJ, 2013. TREM2 signaling, miRNA-34a and the extinction of phagocytosis. Front Cell Neurosci 7, 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G, Liu S, Yu X, Zhao X, Ma L, Shan P, 2019. High prevalence of sleep disorders and behavioral and psychological symptoms of dementia in late-onset Alzheimer disease: a study in Eastern China. Medicine 98 (50). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang ZQ, Shen LL, Li WW, et al. , 2018. Gut microbiota is altered in patients with Alzheimer’s disease. J. Alzheimer’s. Dis. JAD 63 (4), 1337–1346. [DOI] [PubMed] [Google Scholar]


