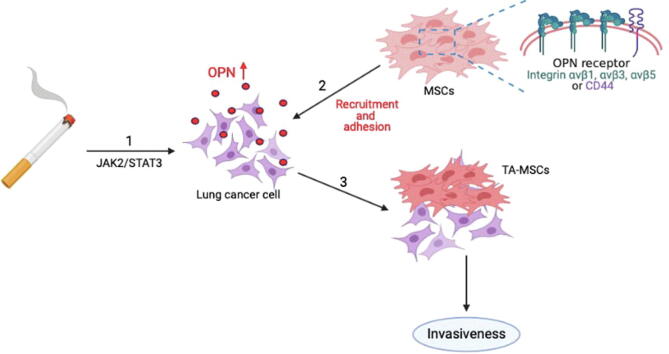
Graphical abstract
Keywords: Lung cancer, Benzo[α]pyrene, Cigarette smoke extract, Mesenchymal stem cell, Osteopontin
Highlights
-
•
Cigarette smoke-induced increases in OPN expression in lung cancer cells.
-
•
Cigarette smoke-induced increases in OPN expression recruit mesenchymal stem cells to the lung cancer cells.
-
•
The JAK2/STAT3 axis is required for OPN expression and the recruitment of MSCs to lung cancer cells.
-
•
OPN receptors are critical for MSC homing to lung cancer cells.
-
•
Tumor-associated MSCs facilitate invasiveness of lung cancer cells.
Abstract
Introduction
Cigarette smoking is the main risk factor for lung cancer. MSCs in the TME promoting tumor angiogenesis, growth, and metastasis. SIBLING proteins enable cancer cells to extend, invade and metastasize.
Objectives
Cigarette smoke promotes the progression and metastasis of lung cancer, although how this occurs is poorly understood. We evaluated the impact of whether cigarette smoking motivates SIBLING protein expression and is involved in MSC-mediated lung tumor metastasis.
Methods
We investigated the expression of OPN in the Gene Expression Omnibus (GEO) databases and confirmed the results by immunohistochemistry (IHC), qPCR and Western blotting (WB) of lung cancer cells and tissues. The effect of OPN on the recruitment and adhesion of mesenchymal stem cells (MSCs) to lung cancer cells and lung cancers metastasis was investigated by Transwell, adhesion assays. A series of in vitro and in vivo experiments were conducted to demonstrate the mechanisms by which OPN modulates recruitment and adhesion of MSCs to lung cancer cells and lung cancer metastasis.
Results
Cigarette smoke extract (CSE) and benzo[α]pyrene (B[α]P) increased levels of OPN expression and facilitated the recruitment and adhesion of MSCs to lung cancer cells via JAK2/STAT3 signaling. We also observed that OPN promotes tumor-associated MSC (TA-MSC) formation through the OPN receptor (integrins αvβ1, αvβ3, αvβ5 or CD44), inducing lung cancer cell migration and invasion. In an orthotopic mouse model of lung cancer, increases in OPN expression promoted by cigarette smoke upregulated MSC recruitment and facilitated lung cancer metastasis. Knockdown of OPN expression inhibited cigarette smoke-induced lung cancer metastasis in vivo.
Conclusion
Cigarette smoke increases OPN expression through the JAK2/STAT3 signaling pathway to attract MSC cell recruitment and promote lung cancer metastasis. Our findings offer important insights into how lung cancer metastasis develops in smokers.
Introduction
Cigarette smoking, the main risk factor for lung cancer, contains at least 93 carcinogens that are harmful to human health, including nicotine, nitrosamines, and benzene [1]. On average, the risk of developing lung cancer is increased 20-fold among lifetime smokers compared with nonsmokers [2]. Importantly, lung cancer patients who continue to smoke tobacco reduce the effectiveness of their treatments such as chemotherapy, increase their risk for recurrence and decrease their overall survival and quality of life [3]. Improving our understanding as to how cigarette smoking worsens mortality in lung cancer is expected to improve therapeutic strategies for ever-smokers with lung cancer.
Advances in cancer research have witnessed treatment benefits extending from the tumor site to the tumor microenvironment (TME), involving stromal cells, mesenchymal stem cells (MSCs), immune cells and blood vessels [4]. Cancer cells have been termed the “heart” of the TME, harnessing nonmalignant cells to work for the benefit of cancer cells, leading to tumor progression and distant metastasis [5]. MSCs have the ability to self-renew and differentiate into myocytes, osteoblasts, chondrocytes and adipocytes [6]. Critically, MSCs confer pro-oncogenic effects in many different types of tumors, helping cells in the TME to avoid immune-mediated attacks and promoting tumor angiogenesis, growth, and eventual metastasis [7], [8]. Targeting this crosstalk between cancer cells and MSCs in the TME could help to improve the outcomes of cancer treatment.
The family of glycophosphoproteins consisting of osteopontin (OPN), dentin matrix protein 1 (DMP1), bone sialoprotein (BSP), matrix extracellular phosphoglycoprotein (MEPE) and dentin sialophosphoprotein (DSPP) are small integrin-binding ligand N-linked glycoproteins (SIBLINGs) [9] that were once thought to be present only in mineralized tissues, such as bones and teeth [10]. Now, numerous studies suggest that SIBLING proteins enable cancer cells to expand, invade and metastasize [9]. For instance, osteoinductive carcinoma expresses all five SIBLING members, which is believed to be the reason for the ability of this cancer to metastasize to bone [11], [12]. OPN, one of the most closely studied SIBLING proteins, directly affects many different functions of cells and tissues, such as assisting with cellular survival by inhibiting apoptotic signaling of melanocytes [13] and, in particular, OPN promotes cancer cell migration, invasion and angiogenesis [14], [15]. OPN is over-expressed in various human cancers and is upregulated in the blood of patients with metastatic cancers [16]. The OPN protein is therefore considered to be a potential prognostic marker and an appropriate therapeutic target.
Our research sought to determine whether cigarette smoking stimulates SIBLING protein expression and is involved in MSC-mediated lung tumor metastasis. Our analysis of records from the Gene Expression Omnibus (GEO) database revealed upregulated OPN expression in lung cancer patients who were cigarette smokers compared with nonsmokers; no other SIBLING proteins were affected by cigarette smoking status. Our in vitro and in vivo evidence showed that long-term exposure (for 30 weeks) to CSE and its carcinogen polycyclic aromatic hydrocarbon benzo[a]pyrene (B[α]P) elevates OPN secretion from lung cancer cells through the JAK2/STAT3 signaling pathway. We observed that high levels of OPN in the lung TME stimulated MSCs to infiltrate and adhere to cancer cells, then promote tumor growth. Our findings emphasize the importance of further investigations into strategies that can silence OPN and sabotage the crosstalk between cancer cells and MSCs in smokers with lung cancer.
Materials and Methods
Configuration of cigarette smoke extract (CSE) medium
Twenty-five commercially available cigarettes (Taiwan Tobacco & Liquor Corporation, Taipei, Taiwan) were used in this study, each containing 0.8 mg of nicotine and 10 mg of tar, to prepare 250 mL of culture medium. After adjusting the pH value of the culture medium to 7.4, the mixture was filtrated using a 0.22 μm filter to remove all large particles. The CSE stock medium was 100% (1 cigarette per mL of medium) and was diluted with complete medium, then kept frozen at –20 °C until use [17].
Analysis of the GEO dataset
Lung cancer tissue samples were collected from the GEO dataset (GSE31210) involving 123 ever-smokers and 123 nonsmokers and analyzed for SIBLING gene expression.
Gene set enrichment analysis
Gene set enrichment analysis (GSEA) methodology was downloaded (http://www.broadinstitute.org/gsea/index.jsp) to determine which signaling pathway is involved in promoting the secretion of OPN when lung cancer cells are exposed to cigarette smoke. The 246 lung cancer samples in GEO database GSE31210 were divided into ever-smokers and non-smokers. One thousand gene sampling permutations were examined for statistical significance to ensure the credibility of the results. The p-values were normalized to < 0.05 and the false discovery rate (FDR) was < 25%.
Cell culture
Human A549 cells were exposed to 1% CSE or 1 μΜ B[α]P for 1 week (short-term; A549-S stable cells) or for 30 weeks (long-term; A549-L stable cells). The A549-S cell line was generated in our laboratory; the A549-L cell line and CSE were kindly provided by Dr. Wei-Chien Huang (China Medical University, Taiwan). Both cell lines were cultured in RPMI 1640 medium (Gibco, USA). MSC cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) medium (Gibco, USA). Streptomycin (100 μg/mL), penicillin (100 U/mL), N-(2-hydroxyethyl)piperazine-N’-(2-ethanesulfonic acid) (HEPES) (20 mM), glutamine (2 mM) and 10% fetal bovine serum (FBS; Gibco, USA) were added to the cell line medium. The cells were maintained at 37 °C in an atmosphere of humidified air with 5% CO2 [17].
Transwell migration assay
Cell migration assays were performed using Transwell® inserts (8-μm pore size; Costar®, NY, USA) in 24-well dishes. After seeding MSCs or A549-L cells (1 × 104 in 200 µL of serum-free medium) into the upper chamber of the Transwell assay, 30% of the resulting conditioned medium (CM) was placed in the lower chamber. After 18 h, migratory cells were stained with crystal violet and manually counted under a microscope.
Adhesion assay
A549-L cells were cultured in RPMI medium on 24-well plates. MSC activity was quantified with 10 µM of the fluorescent probe, 2′,7′-bis(2-carboxyethyl)-5(6)-carboxyfluorescein (BCECF) (Catalog No. 14562, Sigma-Aldrich, St. Louis, MO, USA) in 10% culture medium for 1 h. MSCs were centrifuged at 1,200 revolutions per minute (rpm) for 10 min, then the culture medium was replaced with fresh medium containing 1% FBS. MSCs (104/well) were counted under the microscope and added to 24-well plates coated with A549-L cells for 1 h. Unattached MSC cells were removed by washing the A549-L cells once with PBS, before fixing with 3.7% formalin and photographing them.
Establishment of OPN knockdown A549-L cells
To establish OPN knockdown A549-L cells, a lentiviral vector capable of expressing OPN-specific short hairpin RNA (shRNA) was purchased from the National RNAi Core Facility Platform (Taipei, Taiwan). A lentivirus was prepared according to a standard protocol [18]. For infection, A549-L cells were seeded in a 6-well dish and the lentivirus was added to the medium (multiplicity of infection = 10). After 24 h, the culture medium was changed and then at 48 h, 2 μg/mL of puromycin was added to select for OPN shRNA-expressing cells.
Orthotopic animal model and bioluminescence imaging
Following an established protocol, 6- to 8-week-old male SCID mice were anesthetized with isoflurane inhalation, then inoculated with 5 × 106 A549-Luc cells suspended in 100 μL Matrigel into the upper margin of the sixth intercostal rib on the left side [19], [20]. Pleural metastasis of lung carcinoma was monitored by bioluminescence imaging with an in vivo imaging system (IVIS) (Xenogen, UK). The mice were sacrificed after 5 weeks and their dissected lungs underwent immunohistochemical (IHC) staining. All mice were handled in accordance with the Animal Care and Use Guidelines of China Medical University, Taichung, Taiwan, under a protocol approved by the Institutional Animal Care and Use Committee (No. CMU IACUC-2019–134).
Statistical analysis
All values are expressed as the mean ± standard deviation (SD). Statistical differences between the experimental groups were assessed for significance using the Student’s t-test. Statistical comparisons of more than two groups were performed using one-way analysis of variance (ANOVA) with Bonferroni’s post hoc test. Between-group differences were considered to be significant if the p value was<0.05.
Ethics statement
All experiments involving animals were conducted according to the ethical policies and procedures issued by the Institutional Animal Care and Use Committee of China Medical University, which approved our experiments (Approval no. CMU IACUC-2019–134).
Results
Cigarette smoking increases OPN expression in lung cancer patients
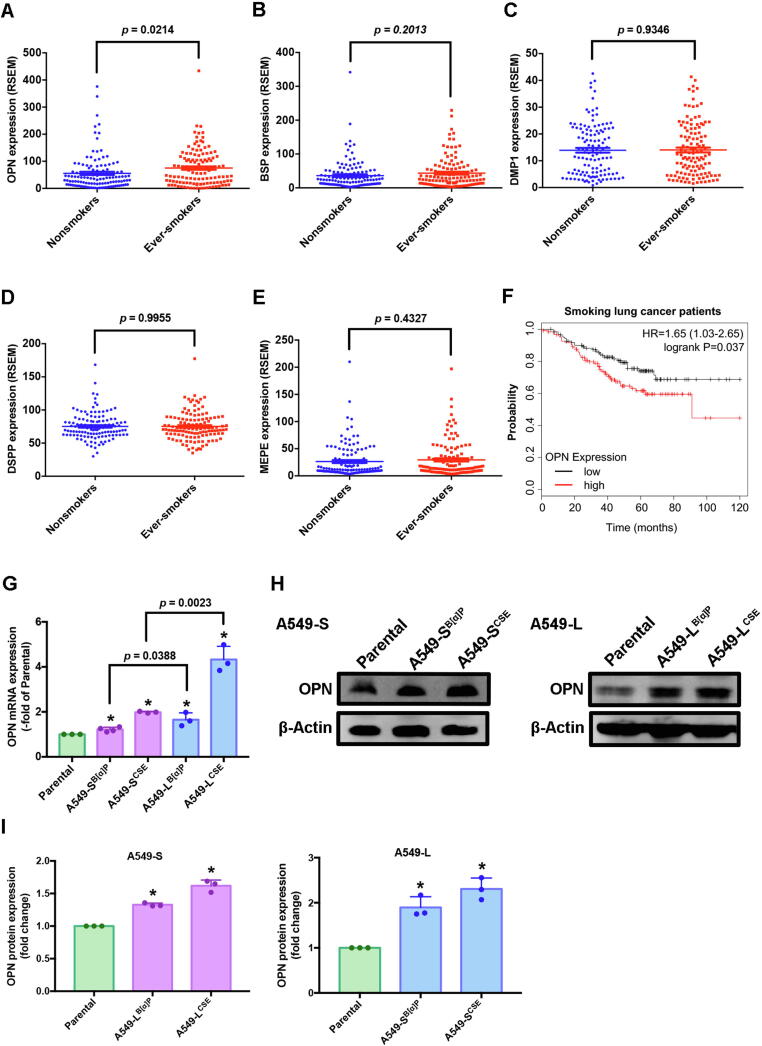
The expression of SIBLING family members is important in the process of malignant transformation, invasion and metastasis in several different types of cancers [9]. However, it is uncertain as to whether or not SIBLING family members worsen cigarette smoke-induced lung cancers. Our analysis of 246 lung cancer samples from the GEO database for SIBLING gene expression included 123 samples from ever-smokers and 123 samples from nonsmokers. OPN levels in the ever-smokers were significantly higher than those in the nonsmokers (Fig. 1A, p = 0.0214) and higher than all other SIBLING measurements; no other significant between-group differences were observed (Fig. 1B-E). In Kaplan-Meier analysis, progressively higher OPN expression among the ever-smokers was associated with correspondingly lower overall survival rates (hazard ratio 1.65; 95% confidence interval, 1.03 to 2.65; logrank p = 0.037) (Fig. 1F). Thus, OPN overexpression among lung cancer patients who were ever-smokers positively correlated with a poor clinical prognosis.
Fig. 1.
OPN levels were higher in ever-smokers than in nonsmokers in patients with lung cancer. (A-E) Lung cancer tissue samples from ever-smokers and nonsmokers in the Gene Expression Omnibus (GEO) GSE31210 dataset were analyzed for SIBLING expression. (F) Kaplan-Meier analysis determined levels of OPN expression and overall survival rates of patients with lung cancer. (G-I) The effects of CSE and B[α]P on OPN expression in A549-S and A549-L cells were examined by quantitative real-time PCR and Western blot. Densitometric analysis of protein expression normalized to β-actin. All values are expressed as the mean ± SD of three independent samples. *p < 0.05 compared with parental expression.
To validate whether cigarette smoke increases levels of OPN protein in lung cancer cells, we exposed A549 cells (Parental) to 1% CSE or 1μΜ B[α]P as either short-term (A549-SB[α]P and A549-SCSE) or long-term exposure (A549-LB[α]P and A549-LCSE) (hereafter referred as to A549-S and A549-L, respectively), to mimic cigarette smoking behavior. Levels of OPN mRNA and protein expression were significantly increased in A549-S and A549-L cells compared with levels in parental cells (Fig. 1G-I). We selected A549-L cells for follow-up experiments, as the levels of significance in comparison with parental cells were greater than those with A549-S cells.
Cigarette smoke-induced increases in OPN expression recruits mesenchymal stem cells to the lung cancer cells
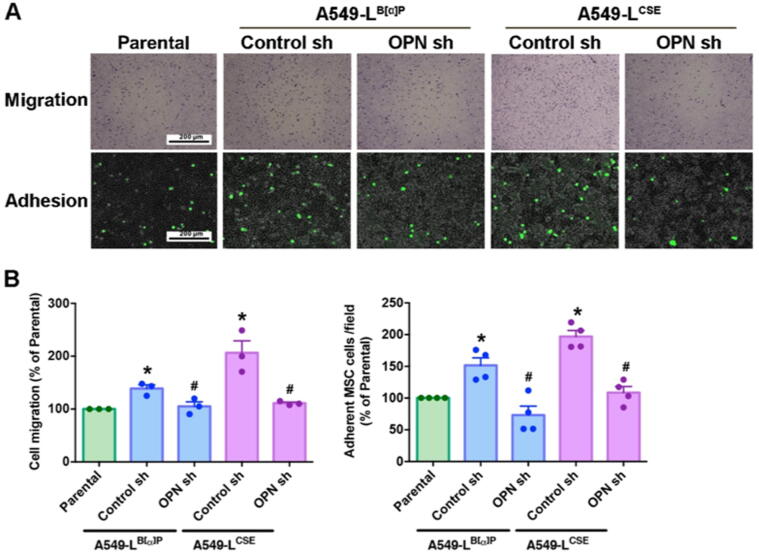
Multiple signals generated by cancer cells recruit MSCs to the TME, where the MSCs adhere to the cancer cells and promote tumor progression and metastasis [21]. To investigate whether the amount of OPN secreted from tumor cells affects the recruitment and attachment of MSCs, we collected CM from A549-L cells, MSCs were added to the upper chamber and A549-L CM to the lower chamber of the Transwell migration assay for 18 h. We found that A549-L CM attracted MSCs and that OPN shRNA inhibited the ability of A549-L CM to recruit MSCs (Fig. 2A). In the adhesion experiment, we found that A549-L cells increased MSCs attachment ability and that this effect was inhibited when the cancer cells were treated with OPN shRNA (Fig. 2B). Clearly, OPN secreted from lung cancer cells affects MSC recruitment and adhesion.
Fig. 2.
OPN attracts MSCs to lung cancer cells. (A) A549-L cells were incubated with OPN shRNA (1.5 ug/mL) for 24 h. The Transwell assay determined MSC recruitment in the CM. An adhesion assay examined levels of MSC adhesion in A549-L cells. (B) Quantification chart of the migration and adhesion assay data. All results are expressed as the mean ± SD of three independent samples. *p < 0.05 compared with the parental group; #p < 0.05 compared with 1 μM B[α]P or 1% CSE treatment.
The JAK2/STAT3 axis is required for OPN expression and the recruitment of MSCs to lung cancer cells
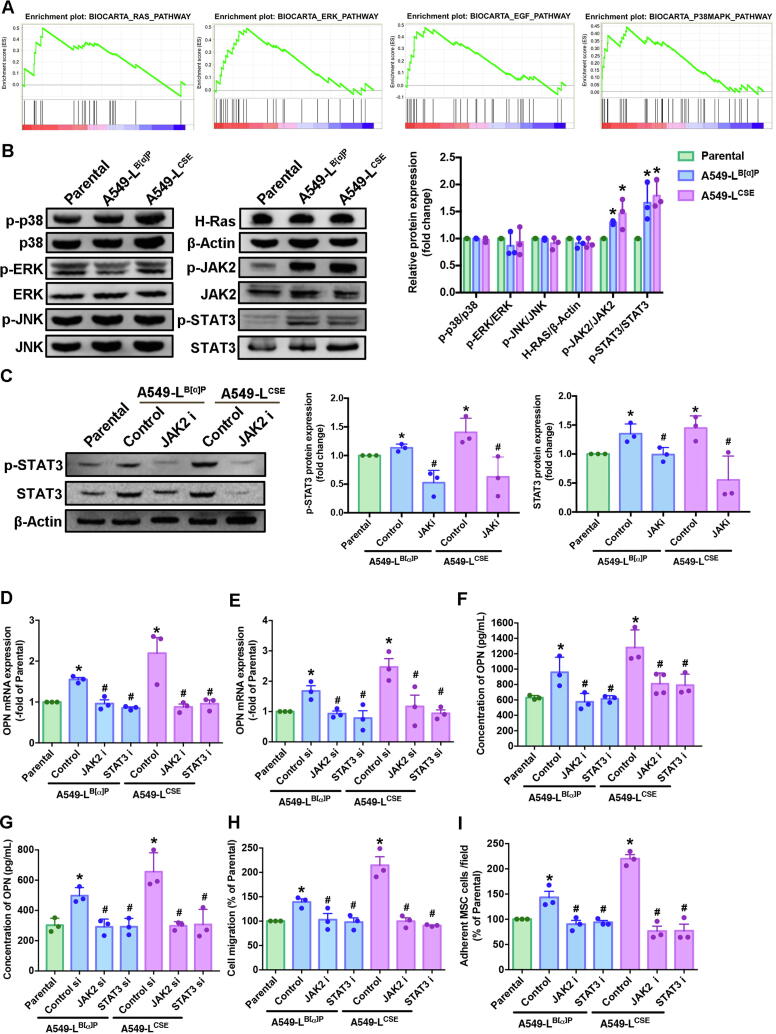
Cigarette smoke is linked with genetic abnormalities and tumor promotion by inducing the abnormal regulation of several signaling transduction pathways [22], [23]. We sought to determine which signaling pathway is involved in promoting the secretion of OPN when lung cancer cells are exposed to cigarette smoke. Performing GSEA upon the BioCarta records in the GEO database revealed 12 gene sets between smokers and nonsmokers in lung cancer patients (Fig. 3A, Supplementary Fig. 1). In our experiments, A549-L cells increased the phosphorylation of JAK2 and STAT3 protein expression; no marked effects were observed with the other signaling pathway proteins (Fig. 3B). We then treated the A549-L cells with the JAK2 inhibitor for 24 h, to determine whether phosphorylation of STAT3 occurs upstream or downstream in the JAK2/STAT3 signaling pathway. We found that STAT3 is a downstream protein of JAK2 (Fig. 3C).
Fig. 3.
CSE and B[α]P increased levels of JAK2/STAT3 pathway expression and induced increases OPN expression and MSC recruitment in human lung cancer cells. (A) A Gene Set Enrichment Analysis (GSEA)-based pathway analysis included RAS, ERK, EGF and p38 MAPK gene sets associated with lung cancer in smokers and nonsmokers obtained from the BioCarta database. (B) Western blot analysis examined levels of MAPK (ERK, JNK, p38), H-Ras, β-actin, JAK2 and STAT3 phosphorylation in A549-L cells. The graphs demonstrate densitometric analysis of p-p38/p38, p-ERK/ERK, p-JNK/JNK, H-Ras/β-actin, p-JAK2/JAK2 and p-STAT3/STAT3. (C) A549-L cells were incubated with a JAK2 inhibitor for 24 h. Western blot examined levels of STAT3 phosphorylation. A549-L cells were incubated with JAK2/STAT3 inhibitors or JAK2/STAT3 siRNAs for 24 h. Densitometric analysis of protein expression was normalized to β-actin. (D-G) Levels of OPN expression were examined by qRT-PCR and ELISA assays. (H) The Transwell assay examined MSC recruitment in CM collected from A549-L cells after 24 h of incubation. (I) The adhesion assay examined levels of MSC adhesion with A549-L cells after 24 h of incubation. All results are expressed as the mean ± SD of three independent samples. *p < 0.05 compared with levels of the parental group; #p < 0.05 compared with 1 μM B[α]P or 1% CSE treatment.
Next, we sought to determine whether JAK2/STAT3 signaling affects OPN expression, by treating A549-L cells with JAK2 and STAT3 inhibitors and their respective short-interfering RNAs (siRNAs) and control were purchased from Dharmacon (Lafayette, CO, USA) for 24 h. According to qPCR and ELISA results, CSE and B[α]P exposure increased OPN expression, while treatment with JAK2 and STAT3 inhibitors and their respective siRNAs inhibited this phenomenon (Fig. 3D-G). Most importantly, blocking OPN expression with JAK2 and STAT3 inhibitors attenuated OPN-induced migration and adhesion of MSCs to lung cancer cells (Fig. 3H-I). These results reveal that cigarette smoke increases OPN expression in lung cancer cells through JAK2/STAT3 signaling and thus facilitates MSC homing to lung cancer cells.
OPN receptors are critical for MSC homing to lung cancer cells
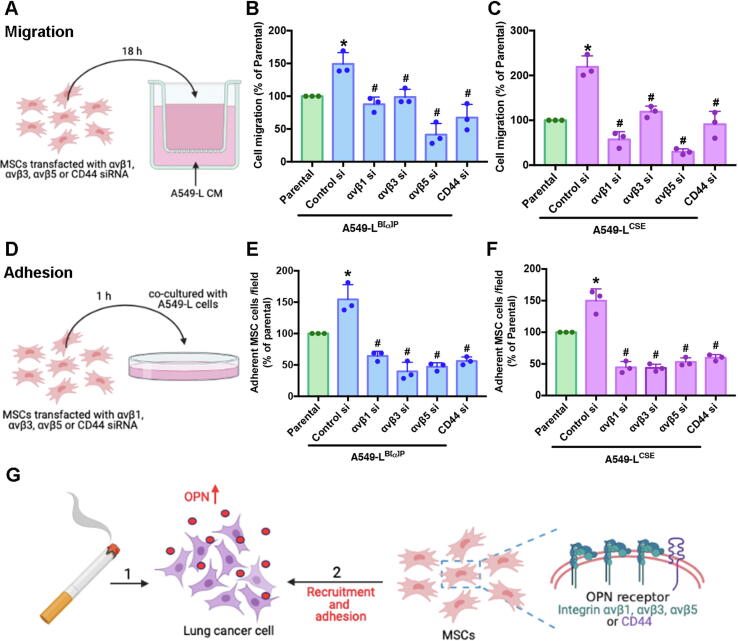
Specific OPN receptors containing αvβ1, αvβ3, αvβ5 and CD44 have been discovered on the MSC membrane [24]. To examine whether cigarette smoke-triggered OPN expression affects the homing of MSCs to tumor sites and whether their adherence to lung cancer cells is affected by the integrins and CD44 on the MSC membranes, we collected the CM from A549-L cells and transfected MSCs with αvβ1, αvβ3, αvβ5, or CD44 siRNAs for 24 h. The MSCs were then incubated with A549-L CM for Transwell migration analysis (Fig. 4A). We found that the αvβ1, αvβ3, αvβ5 and CD44 siRNAs inhibited A549-L CM-mediated recruitment of MSCs (Fig. 4B-C). Next, A549-L cells were seeded into 24-well plates and co-cultivated with the MSCs for 1 h (Fig. 4D). We found that αvβ1, αvβ3, αvβ5 and CD44 siRNAs inhibited the attachment of MSCs to A549-L cells (Fig. 4E-F). These results suggest that cigarette smoke induced increases in OPN expression and binding with OPN receptors on MSC cell membranes, enabling MSCs to infiltrate and attach to lung cancer cells (Fig. 4G).
Fig. 4.
The OPN receptor on the MSC membrane plays a vital role in MSC recruitment and adhesion to lung cancer cells. (A-C) CM was collected from A549-L cells. MSCs were treated with αvβ1, αvβ3, αvβ5, or CD44 siRNAs for 24 h. The CM was placed into the lower chamber of a Transwell assay and MSCs were placed into the upper chamber. The Transwell assay examined MSC recruitment. (D-F) MSCs were treated with αvβ1, αvβ3, αvβ5, or CD44 siRNAs for 24 h. A549-L cells were seeded into 24-well plates and co– cultivated with the MSCs. An adhesion assay examined levels of adhesion with lung cancer cells. (G) The model shows how cigarette smoke induces increases in OPN expression in lung cancer cells and OPN binding with OPN receptors on MSC cell membranes stimulates the recruitment and adhesion of MSCs to lung cancer cells. All results are expressed as the mean ± SD of three independent samples. *p < 0.05 compared with the parental group; #p < 0.05 compared with 1 μM B[α]P or 1% CSE treatment.
Tumor-associated MSCs facilitate invasiveness of lung cancer cells.
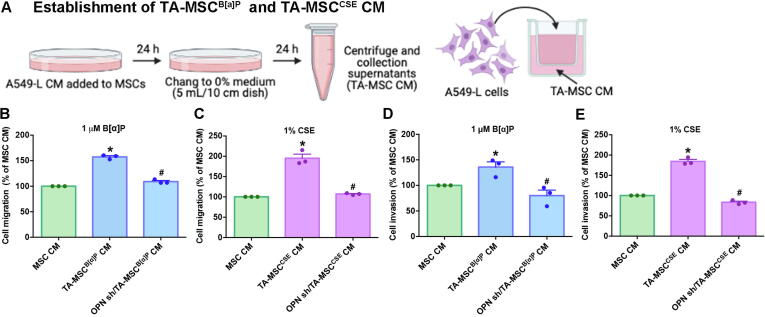
Evidence suggests that MSCs home to tumor sites, where the communication between tumor and MSCs contributes to the formation of tumor-associated MSCs (TA-MSCs), enabling TA-MSCs to facilitate tumor progression and metastasis [25], [26]. Although we identified that CSE-induced increases in OPN expression enables MSC homing to lung cancer cells, it was unclear as to whether OPN involvement in the formation of TA-MSCs promotes invasiveness of lung cancer cells. CM collected from parental and A549-L cells, as well as from A549-L cells transfected with OPN shRNA, was cultured with MSCs for 24 h to promote TA-MSC formation. The resulting TA-MSC CM was collected and used in Transwell migration and invasion experiments involving A549-L cells (Fig. 5A). TA-MSC CM increased the migratory and invasive abilities of A549-L cells, which were significantly reduced when MSCs were cultured with CM from A549-L cells transfected with OPN shRNA (Fig. 5B-E).
Fig. 5.
OPN stimulated TA-MSC-induced promotion of lung cancer cell migration and invasion. (A) A549-L cells were transfected with or without OPN shRNA for 24 h, prior to collection of CM. The resulting CM was used to treat MSCs for another 24 h, then the medium was collected as TA-MSC CM and used to detect cancer cell migration and invasion. (B-E) The migratory and invasive abilities of lung cancer cells were examined using the Transwell assay. All results are expressed as the mean ± SD of three independent samples. *p < 0.05 compared with controls (MSC CM); #p < 0.05 compared with 1 μM B[α]P or 1% CSE treatment.
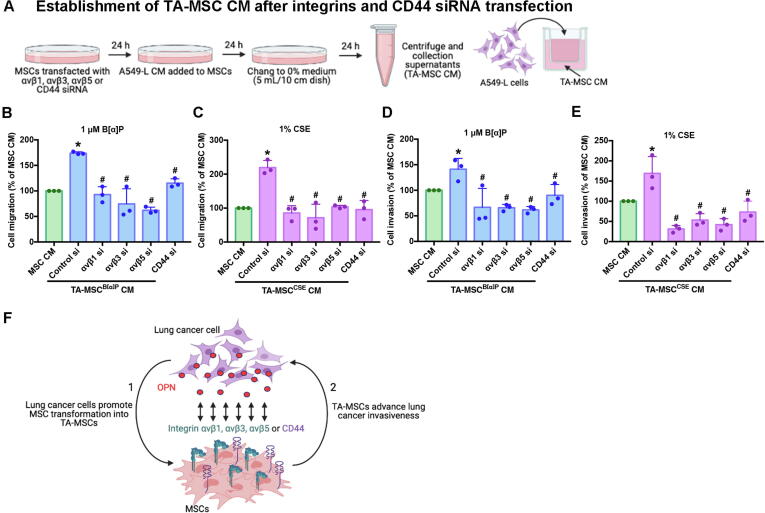
Next, we transfected MSCs with integrins and CD44 siRNAs for 24 h, then cultured the MSCs with A549-L CM, to promote the formation of TA-MSCs (Fig. 6A). The resulting TA-MSC CM was incubated with A549-L cells for 18 h in the Transwell migration and invasion assay (Fig. 6A). Integrin and CD44 siRNAs inhibited TA-MSC-mediated lung cancer cell migratory and invasive activities (Fig. 6B-E). It appears that OPN promotes TA-MSC formation via integrins and CD44, then subsequently encourages lung cancer cell migratory and invasive activities (Fig. 6F).
Fig. 6.
OPN receptors on the MSC cell membrane affect OPN-mediated TA-MSC formation. (A) Pretreatment of MSCs with αvβ1, αvβ3, αvβ5, or CD44 siRNAs for 24 h was followed by A549-L CM incubation for another 24 h. The resulting TA-MSC CM was collected and used to quantify cancer cell migration and invasion. (B and C) The Transwell assay examined levels of lung cancer cell migration. (D and E) The invasion assay examined levels of lung cancer cell invasion. (F) The model shows that lung cancer cells secrete OPN, which promotes the transformation of MSC cells into TA-MSCs via integrins and CD44, stimulating the invasiveness of lung cancer cells. All results are expressed as the mean ± SD of three independent samples. *p < 0.05 compared with controls; #p < 0.05 compared with 1 μM B[α]P or 1% CSE treatment.
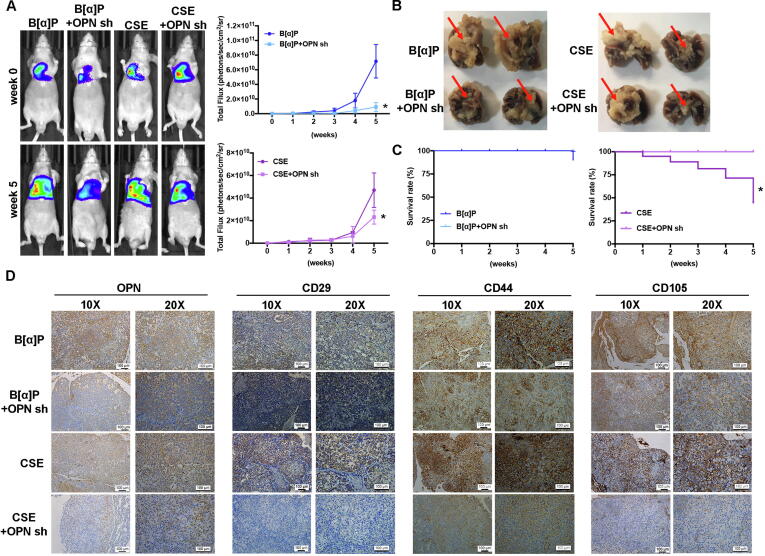
Knockdown of OPN expression inhibits cigarette smoke-triggered lung cancer metastasis in the orthotopic model
When we examined the in vivo role of OPN in lung cancer cells treated with CSE or B[α]P, we found that A549-L cells expressing OPN shRNA exhibited less tumor growth and didn’t metastasis from the right lung to the left lung (Fig. 7A-B). Furthermore, OPN shRNA lowered overall survival of mice in the CSE group (Fig. 7C). When we analyzed the expression levels of OPN and MSC cell markers (CD29, CD44 and CD105) in tumor specimens, we discovered that OPN shRNA downregulates OPN and CD29, CD44 and CD105 expression (Fig. 7D). Evidently, increases in lung cancer cell OPN expression promoted by cigarette smoke upregulate MSC recruitment and facilitate lung cancer metastasis, which suggests that targeting OPN may be beneficial in smokers with lung cancer.
Fig. 7.
Knockdown OPN expression inhibits MSC recruitment and lung metastasis in vivo. (A) A549-L-Luc cells were injected into the right lungs of nude mice and tumor growth was monitored by bioluminescence imaging at baseline and on Day 35. (B) On Day 35, the mice were sacrificed, and the lungs were excised and photographed. (C) Survival rates over 35 days of mice in the control (B[α]P or CSE) and OPN shRNA groups. (D) Lung specimens from sacrificed mice were stained with OPN and MSC markers (CD29, CD44 and CD105).
Discussion
Survival rates are lower and more tumor recurrences are detected among patients with lung cancer who smoke compared with nonsmokers [27]. Cigarette smoke plays a role in lung cancer progression and metastasis [28]. The recruitment of MSCs by cancer cells promotes cancer metastasis by altering the TME, remodeling the tumor site and enabling tumor cells to proliferate and enter the circulation [8], [29]. We found that the JAK2/STAT3 signaling pathway stimulates cigarette smoke to increase levels of OPN expression in lung cancer cells. We also observed that MSC recruitment by lung cancer cells is regulated by the OPN receptors (integrins αvβ1, αvβ3, αvβ5 and CD44) and that this process promotes lung cancer metastasis. Our findings indicate that targeting OPN may be a worthwhile therapeutic strategy in the management of lung cancer.
The SIBLING family is important for the progression of lung cancer and its metastasis [9]. Our analysis of SIBLING gene variation in the GEO database records identified abnormal upregulation of OPN in patients with lung cancer who were smokers compared with patients who were nonsmokers; no other significant between-group differences were found for other SIBLING family members. Our in vitro and in vivo findings demonstrate that upregulated OPN levels due to cigarette smoke exposure play a critical role in the worsening of lung cancer. Similarly, previous study have shown that cigarette smoke increases OPN expression in pancreatic ductal adenocarcinoma cells and thereby promotes tumor progression [30]. Previous research has attributed 17 different cancers to cigarette smoking, including liver, oral and pancreatic cancer [31]. Whether cigarette smoking increases OPN expression in other types of cancer cells besides lung cancer remains to be clarified.
Our GSEA analysis of GEO records found higher levels of JAK2/STAT3 signaling in patients with lung cancer who smoked versus those who did not. Much evidence highlights the importance of the JAK2/STAT3 signaling pathway in lung cancer pathogenesis [32], [33], [34]. For instance, the oncogene zinc finger protein multitype 2 antisense RNA 1 (ZFPM2-AS1) acts through the JAK, STAT and AKT pathways to promote the proliferation and migration of lung cancer cells [32], while the prostate androgen-regulated transcript 1 (PART1) gene targets microRNA (miR)‐635 and activates JAK/STAT signaling to promote lung cancer progression [33]. The ataxia telangiectasia mutated (ATM) protein kinase regulates tumor programmed death ligand 1 (PD-L1) expression by activating JAK/STAT3 signaling in cisplatin-resistant lung cancer cells, while the inhibition of ATM suppresses JAK/STAT3 signaling, PD-L1 expression and tumor metastasis in a xenograft mouse model [34]. Our findings revealed that cigarette smoke increases the phosphorylation of JAK2/STAT3 in lung cancer cells, and that pharmacologic and genetic inhibition of the JAK2/STAT3 pathway inhibits cigarette smoke-induced OPN secretion and MSC recruitment by lung cancer cells. Our study data are in agreement with previous research suggesting that the JAK2/STAT3 pathway is important in the progression and metastasis of lung cancer [32], [33], [34].
Previous research has described distinct TME alterations induced by tobacco smoking that contribute to the development and progression of lung tumors [35], [36]. The TME includes lymphatic circulation systems, fibroblasts, macrophages, mast cells and, in particular, MSCs [37], which enhance tumor cell proliferation and facilitate tumor cell growth [38]. The formation of TA-MSCs promotes tumor progression and metastasis [25], [26], [39], [40]. For instance, when prostate cancer cells recruit C-X-C motif chemokine receptor 6-positive (CXCR6+) MSCs, the CSCR6+ MSCs transform into cancer-associated fibroblasts (CAFs) that promote prostate cancer metastasis [41]. Similarly, triple-negative breast cancer (TNBC) cells recruit platelet-derived growth factor receptor beta-positive (PDGFRβ+) MSCs that transform into CAFs and promote TNBC metastasis [42]. Our study data confirm this phenomenon, as we observed cigarette smoking-induced increases in OPN expression that promoted recruitment of integrin+ and CD44+ MSCs to lung cancer cells. Moreover, the OPN receptor integrins (αvβ1, αvβ3 and αvβ5) and CD44 facilitate the attachment of MSCs to lung cancer cells and subsequent transformation into TA-MSCs, leading to lung cancer metastasis. In our in vivo experiments, IHC staining revealed increases in levels of OPN and MSC markers CD29, CD44 and CD105 in lung cancer tissue, exhibiting cigarette smoke-induced increases in lung cancer OPN expression and MSC recruitment to tumor sites. Recruitment of MSCs by tumors was inhibited by knockdown of OPN expression in lung cancer cells, while blocking the expression of the OPN receptor integrins and CD44 decreased MSC expression, all of which inhibited lung cancer metastasis. This suggests that cigarette smoke-induced promotion of OPN expression in lung cancer cells recruits MSCs to tumors and promotes tumor growth and metastasis via the OPN receptors.
Conclusion
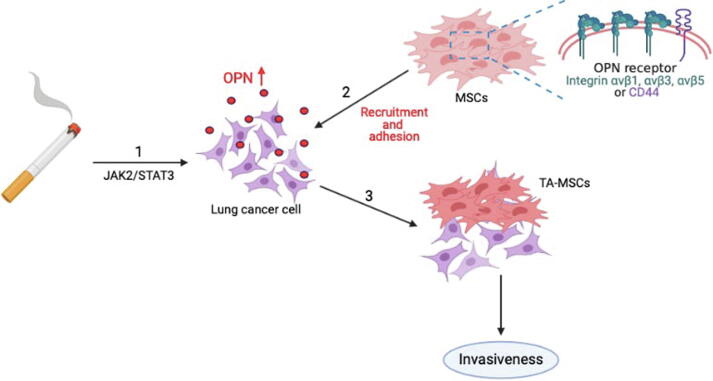
In conclusion, our study results reveal that cigarette smoke facilitates lung cancer metastasis in the following ways: (1) long-term CSE and B[α]P exposure can increase the expression of OPN in lung cancer cells through the JAK2/STAT3 signaling pathway; (2) as the levels of OPN expression increase in the lung TME, integrin- and CD44-positive MSC cells will be attracted to the lung TME and adhere to lung cancer cells; and (3) OPN induces the transformation of MSCs to TA-MSCs, which facilitates the metastasis of lung cancer cells (Fig. 8).
Fig. 8.
A schematic diagram showing how cigarette smoke increases OPN expression and stimulates the recruitment of MSCs to lung cancer cells. Our model illustrates the potential mechanisms that facilitate cigarette smoke-induced promotion of OPN expression and MSC recruitment by lung cancer cells. Arrow 1 refers to the effects of long-term CSE and B[α]P exposure, with subsequent increases in OPN expression in lung cancer cells via the JAK2/STAT3 signaling pathway. Arrow 2 refers to the process whereby integrin-positive and CD44-positive MSC cells are recruited to the lung TME and adhere to lung cancer cells. Arrow 3 refers to the events that occur when OPN induces MSC formation to TA-MSCs, which stimulates the invasiveness of lung cancer cells in vitro and lung tumor metastasis in vivo (3).
CRediT authorship contribution statement
Ya-Jing Jiang: Data curation, Formal analysis, Methodology, Software, Writing – original draft. Chia-Chia Chao: Data curation, Formal analysis, Investigation, Software, Writing – original draft. An-Chen Chang: Conceptualization, Project administration, Resources, Software. Po-Chun Chen: Methodology, Project administration, Software. Fang-Ju Cheng: Formal analysis, Methodology. Ju-Fang Liu: Investigation. Po-I Liu: Resources. Chang-Lun Huang: Resources. Jeng-Hung Guo: Resources. Wei-Chien Huang: Supervision, Writing – review & editing. Chih-Hsin Tang: Conceptualization, Supervision, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We would like to thank Iona J. MacDonald from China Medical University, Taichung, Taiwan, for her English language revision of this paper.
Funding
This study was supported by grants from Ministry of Science and Technology of Taiwan (MOST 109-2320-B-341-002-;MOST 110-2320-B-039 -022 -MY3), China Medical University Hospital (DMR-110-091;DMR-111-164), Asia University Hospital (10951002) and Changhua Christian Hospital (-108-CCH-IRP-028)
Footnotes
Peer review under responsibility of Cairo University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jare.2021.12.011.
Contributor Information
Wei-Chien Huang, Email: whuang@mail.cmu.edu.tw.
Chih-Hsin Tang, Email: chtang@mail.cmu.edu.tw.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Kelley D.E., Boynton M.H., Noar S.M., Morgan J.C., Mendel J.R., Ribisl K.M., et al. Effective Message Elements for Disclosures About Chemicals in Cigarette Smoke. Nicotine Tob Res. 2018;20(9):1047–1054. doi: 10.1093/ntr/ntx109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alberg A.J., Samet J.M. Epidemiology of lung cancer. Chest. 2003;123(1):21S–49S. doi: 10.1378/chest.123.1_suppl.21s. [DOI] [PubMed] [Google Scholar]
- 3.Daniel M., Keefe F.J., Lyna P., Peterson B., Garst J., Kelley M., et al. Persistent smoking after a diagnosis of lung cancer is associated with higher reported pain levels. J Pain. 2009;10(3):323–328. doi: 10.1016/j.jpain.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arneth B. Tumor Microenvironment. Medicina (Kaunas) 2019;56(1):15. doi: 10.3390/medicina56010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baghban R., Roshangar L., Jahanban-Esfahlan R., Seidi K., Ebrahimi-Kalan A., Jaymand M., et al. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun Signal. 2020;18(1) doi: 10.1186/s12964-020-0530-410.21203/rs.3.rs-37680/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ullah I., Subbarao R.B., Rho G.J. Human mesenchymal stem cells - current trends and future prospective. Biosci Rep. 2015;35(2) doi: 10.1042/BSR20150025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han Z., Tian Z., Lv G., Zhang L., Jiang G., Sun K., et al. Immunosuppressive effect of bone marrow-derived mesenchymal stem cells in inflammatory microenvironment favours the growth of B16 melanoma cells. J Cell Mol Med. 2011;15(11):2343–2352. doi: 10.1111/j.1582-4934.2010.01215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karnoub A.E., Dash A.B., Vo A.P., Sullivan A., Brooks M.W., Bell G.W., et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449(7162):557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 9.Bellahcène A., Castronovo V., Ogbureke K.U.E., Fisher L.W., Fedarko N.S. Small integrin-binding ligand N-linked glycoproteins (SIBLINGs): multifunctional proteins in cancer. Nat Rev Cancer. 2008;8(3):212–226. doi: 10.1038/nrc2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fisher L.W., Jain A., Tayback M., Fedarko N.S. Small integrin binding ligand N-linked glycoprotein gene family expression in different cancers. Clin Cancer Res. 2004;10(24):8501–8511. doi: 10.1158/1078-0432.CCR-04-1072. [DOI] [PubMed] [Google Scholar]
- 11.Kruger T.E., Miller A.H., Godwin A.K., Wang J. Bone sialoprotein and osteopontin in bone metastasis of osteotropic cancers. Crit Rev Oncol Hematol. 2014;89(2):330–341. doi: 10.1016/j.critrevonc.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang L.i., Hou X., Lu S., Rao H., Hou J., Luo R., et al. Predictive significance of bone sialoprotein and osteopontin for bone metastases in resected Chinese non-small-cell lung cancer patients: a large cohort retrospective study. Lung Cancer. 2010;67(1):114–119. doi: 10.1016/j.lungcan.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 13.Geissinger E., Weisser C., Fischer P., Schartl M., Wellbrock C. Autocrine stimulation by osteopontin contributes to antiapoptotic signalling of melanocytes in dermal collagen. Cancer Res. 2002;62(16):4820–4828. [PubMed] [Google Scholar]
- 14.Tuck A.B., Arsenault D.M., O'Malley F.P., Hota C., Ling M.C., Wilson S.M., et al. Osteopontin induces increased invasiveness and plasminogen activator expression of human mammary epithelial cells. Oncogene. 1999;18(29):4237–4246. doi: 10.1038/sj.onc.1202799. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi F., Akutagawa S., Fukumoto H., Tsukiyama S., Ohe Y., Takahashi K., et al. Osteopontin induces angiogenesis of murine neuroblastoma cells in mice. Int J Cancer. 2002;98(5):707–712. doi: 10.1002/ijc.10261. [DOI] [PubMed] [Google Scholar]
- 16.Furger K.A., Menon R.K., Tuck A.B., Bramwell V.H., Chambers A.F. The functional and clinical roles of osteopontin in cancer and metastasis. Curr Mol Med. 2001;1(5):621–632. doi: 10.2174/1566524013363339. [DOI] [PubMed] [Google Scholar]
- 17.Cheng F.-J., Chen C.-H., Tsai W.-C., Wang B.-W., Yu M.-C., Hsia T.-C., et al. Cigarette smoke-induced LKB1/AMPK pathway deficiency reduces EGFR TKI sensitivity in NSCLC. Oncogene. 2021;40(6):1162–1175. doi: 10.1038/s41388-020-01597-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen J.-C., Huang C., Lee I.-N., Wu Y.-P., Tang C.-H. Amphiregulin enhances cell migration and resistance to doxorubicin in chondrosarcoma cells through the MAPK pathway. Mol Carcinog. 2018;57(12):1816–1824. doi: 10.1002/mc.22899. [DOI] [PubMed] [Google Scholar]
- 19.Chao C.C., Chen P.C., Chiou P.C., Hsu C.J., Liu P.I., Yang Y.C., et al. Melatonin suppresses lung cancer metastasis by inhibition of epithelial-mesenchymal transition through targeting to Twist. Clin Sci (Lond) 2019;133(5):709–722. doi: 10.1042/CS20180945. [DOI] [PubMed] [Google Scholar]
- 20.Matsumoto S., Tanaka F., Sato K., Kimura S., Maekawa T., Hasegawa S., et al. Monitoring with a non-invasive bioluminescent in vivo imaging system of pleural metastasis of lung carcinoma. Lung Cancer. 2009;66(1):75–79. doi: 10.1016/j.lungcan.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 21.Hill B.S., Pelagalli A., Passaro N., Zannetti A. Tumor-educated mesenchymal stem cells promote pro-metastatic phenotype. Oncotarget. 2017;8(42):73296–73311. doi: 10.18632/oncotarget.20265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones P.A., Baylin S.B. The epigenomics of cancer. Cell. 2007;128(4):683–692. doi: 10.1016/j.cell.2007.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Macaluso M., Paggi M.G., Giordano A. Genetic and epigenetic alterations as hallmarks of the intricate road to cancer. Oncogene. 2003;22(42):6472–6478. doi: 10.1038/sj.onc.1206955. [DOI] [PubMed] [Google Scholar]
- 24.Chen Q., Shou P., Zhang L., Xu C., Zheng C., Han Y., et al. An osteopontin-integrin interaction plays a critical role in directing adipogenesis and osteogenesis by mesenchymal stem cells. Stem Cells. 2014;32(2):327–337. doi: 10.1002/stem.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi Y., Du L., Lin L., Wang Y. Tumour-associated mesenchymal stem/stromal cells: emerging therapeutic targets. Nat Rev Drug Discov. 2017;16(1):35–52. doi: 10.1038/nrd.2016.193. [DOI] [PubMed] [Google Scholar]
- 26.Li P., Gong Z., Shultz L.D., Ren G. Mesenchymal stem cells: From regeneration to cancer. Pharmacol Ther. 2019;200:42–54. doi: 10.1016/j.pharmthera.2019.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang S., Liu T., Liang G. The benefits of smoking cessation on survival in cancer patients by integrative analysis of multi-omics data. Mol Oncol. 2020;14(9):2069–2080. doi: 10.1002/1878-0261.12755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Di Vincenzo S., Sangiorgi C., Ferraro M., Buscetta M., Cipollina C., Pace E. Cigarette smoke extract reduces FOXO3a promoting tumor progression and cell migration in lung cancer. Toxicology. 2021;454 doi: 10.1016/j.tox.2021.152751. [DOI] [PubMed] [Google Scholar]
- 29.Sai B., Dai Y., Fan S., Wang F., Wang L., Li Z., et al. Cancer-educated mesenchymal stem cells promote the survival of cancer cells at primary and distant metastatic sites via the expansion of bone marrow-derived-PMN-MDSCs. Cell Death Dis. 2019;10(12) doi: 10.1038/s41419-019-2149-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chipitsyna G., Gong Q., Anandanadesan R., Alnajar A., Batra S.K., Wittel U.A., et al. Induction of osteopontin expression by nicotine and cigarette smoke in the pancreas and pancreatic ductal adenocarcinoma cells. Int J Cancer. 2009;125(2):276–285. doi: 10.1002/ijc.24388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alexandrov L.B., Ju Y.S., Haase K., Van Loo P., Martincorena I., Nik-Zainal S., et al. Mutational signatures associated with tobacco smoking in human cancer. Science. 2016;354(6312):618–622. doi: 10.1126/science.aag0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang X., Tang J., Zhao J., Lou B., Li L. ZFPM2-AS1 promotes the proliferation, migration, and invasion of human non-small cell lung cancer cells involving the JAK-STAT and AKT pathways. PeerJ. 2020;8:e10225. doi: 10.7717/peerj.10225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu D., Yu Y., Wang W., Wu K., Liu D., Yang Y., et al. Long noncoding RNA PART1 promotes progression of non-small cell lung cancer cells via JAK-STAT signaling pathway. Cancer Med. 2019;8(13):6064–6081. doi: 10.1002/cam4.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shen M., Xu Z., Xu W., Jiang K., Zhang F., Ding Q., et al. Inhibition of ATM reverses EMT and decreases metastatic potential of cisplatin-resistant lung cancer cells through JAK/STAT3/PD-L1 pathway. J Exp Clin Cancer Res. 2019;38(1) doi: 10.1186/s13046-019-1161-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Desrichard A., Kuo F., Chowell D., Lee K.W., Riaz N., Wong R.J., et al. Tobacco Smoking-Associated Alterations in the Immune Microenvironment of Squamous Cell Carcinomas. J Natl Cancer Inst. 2018;110(12):1386–1392. doi: 10.1093/jnci/djy060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li X., Li J., Wu P., Zhou L., Lu B., Ying K., et al. Smoker and non-smoker lung adenocarcinoma is characterized by distinct tumor immune microenvironments. Oncoimmunology. 2018;7(10):e1494677. doi: 10.1080/2162402X.2018.1494677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Joyce J.A., Pollard J.W. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9(4):239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suzuki K., Sun R., Origuchi M., Kanehira M., Takahata T., Itoh J., et al. Mesenchymal stromal cells promote tumor growth through the enhancement of neovascularization. Mol Med. 2011;17(7-8):579–587. doi: 10.2119/molmed.2010.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reagan M.R., Kaplan D.L. Concise review: Mesenchymal stem cell tumor-homing: detection methods in disease model systems. Stem Cells. 2011;29(6):920–927. doi: 10.1002/stem.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xie C., Yang Z., Suo Y., Chen Q., Wei D., Weng X., et al. Systemically Infused Mesenchymal Stem Cells Show Different Homing Profiles in Healthy and Tumor Mouse Models. Stem Cells Transl Med. 2017;6(4):1120–1131. doi: 10.1002/sctm.16-0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jung Y., Kim J.K., Shiozawa Y., Wang J., Mishra A., Joseph J., et al. Recruitment of mesenchymal stem cells into prostate tumours promotes metastasis. Nat Commun. 2013;4:1795. doi: 10.1038/ncomms2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Camorani S., Hill B.S., Fontanella R., Greco A., Gramanzini M., Auletta L., et al. Inhibition of Bone Marrow-Derived Mesenchymal Stem Cells Homing Towards Triple-Negative Breast Cancer Microenvironment Using an Anti-PDGFRbeta Aptamer. Theranostics. 2017;7(14):3595–3607. doi: 10.7150/thno.18974. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.