Abstract
Guillain–Barré syndrome (GBS) is an adverse event of special interest (AESI) for surveillance systems monitoring adverse events following immunisation (AEFI) with COVID-19 vaccines. Emerging data support a temporal association between GBS and adenovirus-vector COVID-19 vaccines. We present a case series of GBS reports submitted between February and November 2021 to our enhanced spontaneous surveillance system (SAEFVIC) in Victoria, Australia, following vaccination with either the adenovirus-vector vaccine Vaxzevria ChadOx1-S (AstraZeneca) or an mRNA vaccine (Comirnaty BNT162b2 [Pfizer-BioNTech] or Spikevax mRNA-1273 [Moderna]). For each report, Brighton Collaboration case definitions were used to describe diagnostic certainty. Severity was graded using the GBS Disability Score. The observed incidence of GBS following immunisation against COVID-19 was compared to expected background ICD10-AM G61.0 coded hospitalisations. There were 41 total cases of GBS reported to SAEFVIC following Vaxzevria (n = 38), Comirnaty (n = 3), or Spikevax (n = 0) vaccines. The observed GBS incidence rate exceeded the expected background rate for Vaxzevria only, with 1.85 reports per 100,000 doses following dose 1, higher than the expected rate of 0.39 hospital admissions per 100,000 adults within 42 days of vaccination. Of 38 GBS reports following Vaxzevria, the median age at vaccination was 66 years and median onset of symptoms was 14 days following immunisation. There was one death. Four cases initially categorised as GBS were later reclassified as acute-onset chronic inflammatory demyelinating polyneuropathy. Fatigue was the predominant persisting symptom reported at follow up. Additional global studies are required to characterise risk factors, clinical variability, and to provide precision and generalizability regarding AEFI risks such as GBS associated with different vaccine platforms, which will help inform communication of the potential benefits and risks of COVID19 vaccination.
Keywords: Guillain–Barré syndrome, Vaccine, Vaccination, COVID-19, SARS-CoV-2
1. Introduction
From the beginning of the global COVID-19 vaccination campaign, Guillain–Barré syndrome (GBS) was identified as an Adverse Event of Special Interest (AESI) for surveillance systems monitoring Adverse Events Following Immunization (AEFI), considering its ‘proven or theoretical association with immunization in general,’ best described in association with influenza vaccines [1], [2]. It has since also become clearer that GBS is a rare complication of SARS-CoV-2 infection itself, adding biological plausibility to the rationale for considering GBS as an AESI [3]. In addition to steadily accumulating case reports there is now higher quality evidence regarding diverse Serious Acute Neurological Events (SANE) following immunization against COVID-19, with emerging data suggesting a temporal association between GBS and adenovirus-vector vaccines including Vaxzevria ChadOx1-S (AstraZeneca) and Janssen Ad26.COV2.S products[3]. However, interpretation of these data and attribution of a causal relationship has been challenging against the confounding backdrop of uncontrolled SARS-CoV-2 transmission, including potential subclinical infection, and associated stresses from COVID-19 in many countries across all aspects of public health, including surveillance systems.
The Australian state of Victoria is well placed to help address this question, combining a relatively successful public health effort to suppress SARS-CoV-2 infections through 2021[4], an established AEFI surveillance system[5], and a COVID-19 vaccination program delivering adenovirus-vector (Vaxzevria) and mRNA vaccines (predominantly Comirnaty BNT162b2 [Pfizer-BioNTech]) in parallel. Public health measures instituted from 2020 in Victoria also saw the near-elimination through 2021 of influenza and other non-COVID-19 respiratory viral diseases thought to contribute to the pathogenesis of GBS under normal conditions, enabling an even clearer focus on any temporal relationship between COVID-19 vaccine administration and GBS[4], [6]. Here, using a similar approach to our recent publication focussed on post-vaccine immune thrombocytopenia[7], we present a case series of GBS reports to our enhanced spontaneous surveillance system (SAEFVIC), following COVID-19 vaccination in Victoria with Vaxzevria and Comirnaty, from the launch of the local COVID-19 vaccination program in February until November 2021, compare these cases to expected background rates, and describe medium-term impacts using validated patient-reported outcome measures.
2. Methods
SAEFVIC comprises an enhanced passive (spontaneous) and active surveillance system integrated with clinical services, serving the Australian state of Victoria (population 6.6. million)[7]. AEFI are spontaneously reported to SAEFVIC by vaccine recipients, carers, and health-care providers, and follow-up arranged as necessary. Any serious AEFI identified via active surveillance are reviewed and added to our centralised SAEFVIC database. To support the COVID-19 vaccination program, the Victorian Specialist Immunisation Services (VicSIS) network of specialist clinics was established to coordinate follow-up clinical assessment and ongoing care and follow-up[8]. SAEFVIC forwards all AEFI reports to the Australian Therapeutic Goods Administration (TGA), responsible for pharmacovigilance and national collation of spontaneous adverse event reports.
Reports of possible GBS were identified using free text word search of reports submitted to SAEFVIC following COVID-19 vaccine doses administered between 22 February 2021 (launch of the Australian national program) and 30 September 2021. To capture a 42-day risk period after vaccine administration, GBS reports received with symptom onset until 12 November were considered. Data recorded for each patient included age, sex, interval between vaccination and symptom onset, neurologic history and examination, investigation results, treatment, vaccine brand and dose and details of subsequent COVID-19 vaccines if applicable. Additional information was collected (where possible), from vaccinees, carers, treating clinicians, and hospital sites. A specialist neurologist with experience in neuromuscular disease and neurophysiology reviewed the clinical data, nerve conduction studies, cerebrospinal fluid, and neuroimaging results for each case to determine a level of diagnostic certainty according to Brighton Collaboration case definitions and to grade severity using the GBS Disability Score (GBSDS)[9], [10].
To explore medium-term outcomes, repeated attempts were made to follow up each vaccine recipient with confirmed GBS to complete validated patient-reported outcome measures for immune-mediated neuropathies[11], [12]. Cases were excluded from follow up if detailed evaluation revealed a clear aetiology other than vaccination as the cause of GBS (e.g., Campylobacter jejuni infection), or a clear diagnosis other than GBS as the cause for the reported symptoms (e.g., spinal cord lesion). Eligible cases were contacted three times before being deemed as lost to follow up. Outcome measures used were the Fatigue Severity Score (FSS), Rasch-built Overall Disability Scale (R-ODS), a quality of life rating from 0 (poor) to 10 (excellent), and a rating of their health from 0 (poor) to 10 (excellent). The relationship between GBSDS and outcome measures was investigated.
2.1. Statistical analysis
Data were analysed using Microsoft PowerBI (version 2.92.943.0) and reporting rates and 95 % Poisson confidence intervals calculated in RStudio (version 2022.07.1) by population demographics including sex,and indigenous status to identify specific at-risk groups[13], [14]. Reporting rates were calculated using doses administered as per the Australian Immunisation Register (AIR). Similar to previous SAEVFIC studies[15], [16], expected background rates were estimated from ICD10-AM G61.0 coded separations recorded in the state-wide hospital Victorian Admissions Episode Dataset (VAED) during 20157–2019 (with a 12-month washout period applied), calculated as annual average per 100,000 population in adults. The annual rate was divided by 8.69 to adjust for a 42-day risk window period[15].
2.2. Ethics approval
Ethics approval was not required for this study as the case review and follow-up was undertaken as part of routine public health AEFI management. Data were recorded in the registered SAEFVIC database for clinical management.
3. Results
3.1. Initial case review
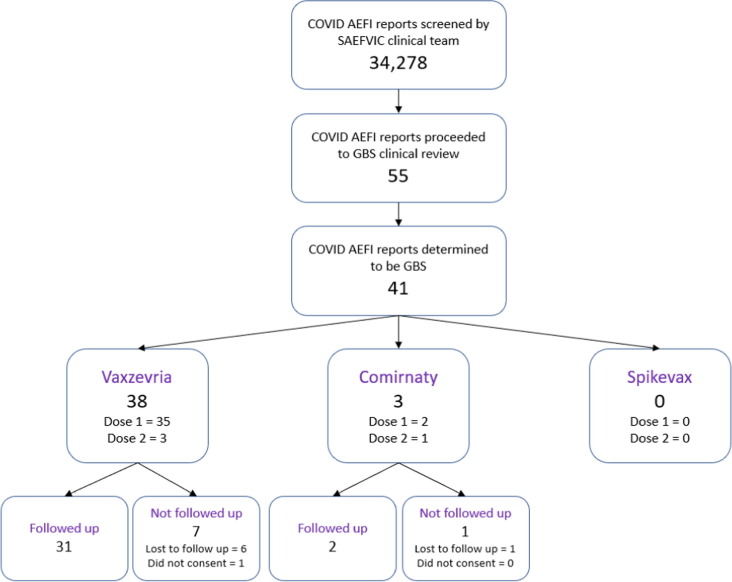
Fifty-five possible reports were identified from the SAEFVIC database following 3,749,291 doses of Vaxzevria. All possible cases were reviewed by a specialist neurologist, with 41 included in the final analysis (Fig. 1 ). Fourteen patients were considered to have an alternate diagnosis – including transverse myelitis, sensory axonal neuropathies, Bell’s palsy, and post-vaccine sensory symptoms attributed to anxiety. The 41 cases were in adults (range 28–85 years), both male and female (19 male, 22 female) and with the majority following Vaxzevria vaccination (n = 38, 92.7 %). None of the 41 cases self-identified as being Indigenous Australian. For Vaxzevria, 35 cases were reported following dose 1, with an reporting rate of 1.85 per 100,000 doses (95 % CI 1.28, 2.56), exceeding the expected background rate of 0.39 presentations per 100,000 adult population within 42 days of vaccination.
Fig. 1.
Inclusion/exclusion flowchart (February- September 2021). AEFI: adverse event following immunization; GBS: Guillain-Barré syndrome; SAEFVIC: Surveillance of Adverse Events Following Vaccination In the Community.
Of the 38 reports following Vaxzevria administration, in 36 (94.7 %) the time from COVID-19 vaccination to symptom onset was between 10 and 28 days, with a median of 14 days. In the other 2 cases, there was an intervening seasonal influenza vaccine administered at a relevant time point between COVID-19 vaccine and onset of symptoms. Twenty-seven of these cases (71.5 %) were considered definite (level 1) or probable (level 2), according to Brighton Collaboration criteria and eleven (28.9 %) as possible/level 3–4 (Table 1 ). Half of the cases (n = 19, 50.0 %) were at least partly ambulant (GBSDS 1, 2, or 3), with 16 (42.1 %) individuals bedridden or chairbound (GBSDS 4) and two (5 %) requiring assisted ventilation at the peak of their illness (GBSDS 5). A female over 70 years died from complications related to GBS (GBSDS 6). Clinical GBS phenotypes included: typical sensorimotor GBS (n = 26), 3 with unilateral facial weakness and 3 electrophysiologically subtyped as acute motor axonal neuropathy (AMAN); acute sensory ataxic neuropathy (n = 1); bifacial weakness with paraesthesias (BFP, n = 2); small fibre painful neuropathy (n = 1); and acute paraparetic GBS (n = 4). Intravenous immunoglobulin was administered in all but 4 mild cases (GBS disability score 1 or 2).
Table 1.
Characteristics of reported cases of Guillain-Barré Syndrome following COVID-19 vaccination.
| Vaxzevria | Comirnaty | Total* | |
|---|---|---|---|
| Total doses | 3,749,291 | 6,502,290 | 10,613,528 |
| Case count (Reporting rate per 100,000 [95 % CI]) | |||
| Observed Total | 38 (1.01 [0.72,1.40]) | 3 (0.05 [0.01,0.14]) | 41 (0.39 [0.28,0.53]) |
| Dose 1 | 35 (1.85 [1.28,2.56]) | 2 (0.06 [0.01,0.22]) | 37 (0.69 [0.49,0.95]) |
| Dose 2 | 3 (0.16 [0.03,0.48]) | 1 (0.03 [0.00,0.18]) | 4(0.08 [0.02,0.20]) |
| Expected | 20 | 25 | 45 |
| Demographics case count (Reporting rate per 100,000 [ 95 % CI]) | |||
| Age range (median) | 36 to 85 (66) | 28 to 43 (34) | 28 to 85 (65) |
| Sex | |||
| Male | 18(0.97 [0.57,1.53]) | 1(0.03[0.00,0.18]) | 19 (0.37[0.22,0.57]) |
| Female | 20(1.07[0.65,1.65]) | 2(0.06[0.01,0.22]) | 22(0.41[0.26,0.62]) |
| Indigenous status | |||
| Indigenous | 0 | 0 | 0 |
| Non– Indigenous | 38(0.83[0.57,1.18] | 3(0.05[0.01,0.14] | 41 (0.32[0.22,0.45] |
| Clinical information | |||
| Days to symptom onset (median) | 4 to 70 (14) | 5 to 26 (14) | 3 to 70 (14) |
| Brighton Collaboration Level | |||
| Brighton Collaboration 1–2 | 27 | 1 | 28 |
| Brighton Collaboration 3–4 | 11 | 2 | 13 |
| Disability score | |||
| DS 1 | 5 | 0 | 5 |
| DS 2 | 7 | 1 | 8 |
| DS 3 | 7 | 1 | 8 |
| DS 4 | 16 | 1 | 17 |
| DS 5 | 2 | 0 | 2 |
| DS 6 | 1 | 0 | 1 |
| GBS Phenotype | |||
| Typical GBS | 26 | 1 | 27 |
| MFS | 0 | 1 | 1 |
| Acute sensory ataxic neuropathy | 1 | 0 | 1 |
| Small fibre painful neuropathy | 1 | 0 | 1 |
| BFP | 2 | 1 | 3 |
| Paraparetic GBS | 4 | 0 | 4 |
| Acute onset CIDP | 4 | 0 | 4 |
BFP: bifacial weakness with paresthesias; CIDP: chronic inflammatory demyelinating polyradiculoneuropathy; GBS: Guillain-Barré syndrome; MFS: Miller Fisher Syndrome.
Total vaccine numbers and rates include Moderna Spikevax doses given for which there were 0 GBS cases.
Four cases were subsequently reclassified months later, as acute-onset chronic inflammatory demyelinating polyneuropathy (ACIDP), as defined by either 3 or more treatment-related fluctuations or progression 4 weeks after onset of symptoms. One case was initially diagnosed with the bifacial weakness with paraesthesias (BFP) variant of GBS after dose one Vaxzevria, had incomplete recovery after initial intravenous immune globulin (IVIG) treatment, and a progressive course characterised by limb weakness requiring retreatment with IVIG. The case was later reclassified as CIDP after further clinical deterioration and nerve conduction studies demonstrated a severe sensorimotor demyelinating polyneuropathy shortly after a second COVID-19 vaccine dose with Comirnaty. This case was considered post Vaxzevria as symptom onset occurred prior to Comirnaty administration. This was the only AEFI reported amongst the 21 cases who had a subsequent dose of a COVID-19 vaccine during the time period covered by this investigation, including 20 individuals administered 1 or more doses of Comirnaty and a single individual who received a further dose of Vaxzevria.
3.2. Follow up
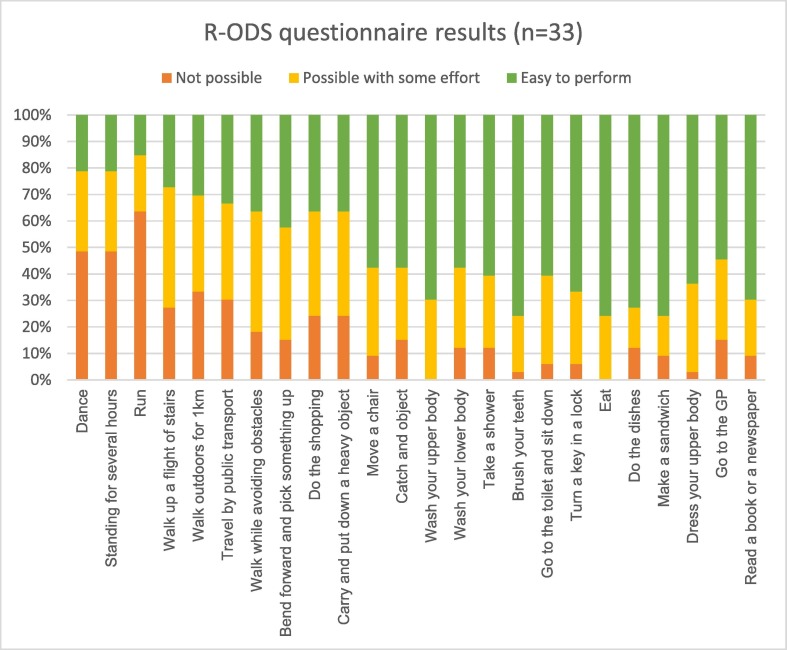
Thirty-three cases (Vaxzevria n = 31, Comirnaty n = 2) across a range of age and disability score levels provided follow up data (mean 252 days after vaccination [range 161 to 342]). 1 case did not consent to follow up, and 7 cases were lost to follow up. All but one of the cases reported ongoing challenges in at least one daily activity. The most frequently reported challenges in the R-ODS questionnaire involved lower limb function, while most cases were able to resume activities involving the upper limb (Fig. 2 ). The median R-ODS score is 35 (range 3 to 48).
Fig. 2.
R-ODS results for GBS cases (n = 33) at months follow up.
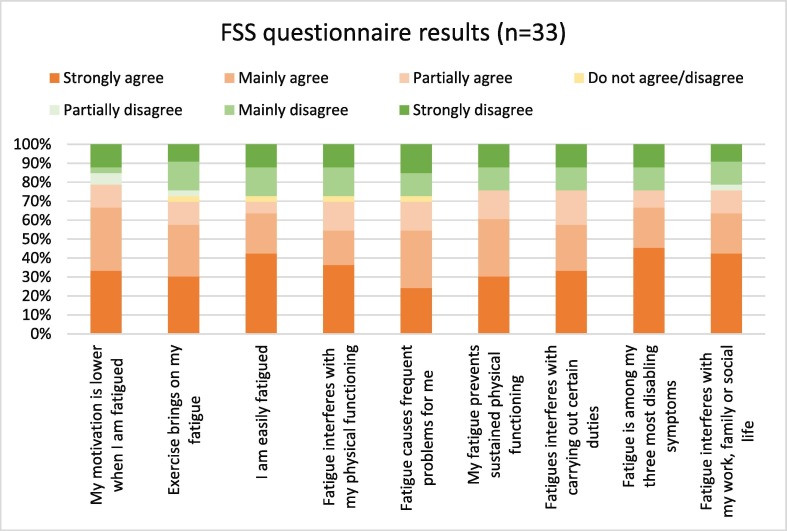
Twenty-eight (75.7 %) cases strongly or mainly agreed to experiencing negative impacts associated with fatigue in their follow up questionnaire. 67.6 % of cases strongly or mainly agreed that fatigue was one of their three most disabling symptoms and 64.7 % of cases strongly or mainly agreed that fatigue interfered with their work, family or social life (Fig. 3 ). The median FSS score was 55 (range 9 to 63). The median quality of life score self-reported by cases (scale from 0 worst to 10 best) was 7 (range 1 to 10). Similarly, when asked to comment on their health on the day of the questionnaire using the same scale, the median score was 7 (range 1 to 9). Follow-up scores for all indices were poorest for individuals with GBSDS 3 and 4 during their initial admission.
Fig. 3.
Fatigue severity score results for GBS cases (n = 33) at months follow up.
4. Discussion
Through 2021, when adenovirus-vector and mRNA COVID-19 vaccines were both deployed, the number of cases of GBS reported to SAEFVIC following immunisation with the adenovirus-vector AZ COVID-19 vaccine Vaxzevria exceeded expected background rates. Similar to vaccine induced thrombotic thrombocytopenia (VITT) the majority were following the 1st vaccine dose[16]. As in our smaller preliminary study, no such temporal association was observed for the mRNA vaccines[17]. A number of different GBS variants were observed, and in 4 individuals, a relapsing-remitting pattern following the reported episode led to a diagnosis of acute-onset chronic inflammatory demyelinating polyneuropathy (ACIDP). Most epidemiologic studies suggest that men are 1.5 times more likely to be affected than women[17] in contrast to our post-vaccination series with a preponderance of females (F:M = 1.16:1). For the first time in the scientific literature to date, we have highlighted the lasting and relatively severe impact of COVID-19 vaccine-related GBS.
Although a clear causal mechanism for GBS (and other neurological complications) following immunization against COVID-19 has not been established, this study adds to accumulating data showing a temporal association. In a large linked dataset analysis utilising the self-controlled case series methodology, spanning December 1, 2020 to May 31, 2021 and capturing more than 20 million Vaxzevria and 12 million Comirnaty first doses delivered in England, Patone and colleagues noted an increased risk of GBS in the 28 days following vaccination after Vaxzevria with an incidence rate ratio (IRR) of 2.04 (95 % confidence interval [CI]: 1.60–2.60) but not after Comirnaty (IRR, 0.86; 95 % CI: 0.54–1.36). The Vaxzevria signal was replicated in a smaller independent Scottish cohort (IRR, 2.32; 95 % CI: 1.08–5.02)3. By comparison, in the larger English cohort, the IRR for GBS in the 28 days after a positive SARS-CoV-2 test was 5.25 (95 % CI: 3.00–9.18).
A strength of the Patone et al. paper is that it utilises the self-controlled case series methodology[18] to describe the attributable risk, or excess of cases of GBS in the 1–28 days following the 1st dose of the Vaxzevria, AstraZeneca COVID-19 vaccine, as well as following a documented SARS Cov2 infection. Like our study, Patone and colleagues identified no increase in risk following the mRNA vaccine, Comirnaty (Pfizer COVID-19 vaccine).
A primary care linked-database study, utilising UK and Spanish records, led by Li and colleagues had different findings, with no increase in GBS attributable to Vaxzevria detected[19]. An associated editorial by Pottegard et al. highlighted some of the limitations of the Li et al study compared to Patone[20]. In particular, the smaller sample size, and reliance upon capture of GBS diagnoses in primary care systems when most cases are expected to be admitted to hospitals were noted.
GBS diagnostic certainty requires additional investigations as per the Brighton criteria, so a hospitalisation dataset will be more robust, noting they only had 11 cases of GBS identified in the vaccine window. The Li study also utilised a shorter window post vaccine (21 days, compared with 28 days) post the 1st vaccine dose. Most epidemiological vaccine safety studies reviewing auto-immune phenomenon like GBS, use a 42-day window[1] so additional sensitivity analysis may help address some of the limitations of these studies.
An significant excess of observed GBS cases over the expected rate following another adenovirus vector COVID-19 vaccine, the Ad.26.COV2.S vaccine (Janssen) has also been described in the US Vaccine Safety Datalink[21]. This has led to a preferential recommendation by the US Advisory Committee on Immunisation Practice in December 2021 for mRNA COVID-19 vaccines over the Janssen COVID-19 vaccine for all persons over 18 years of age[22].
The clinical GBS phenotypes identified in our study are consistent with other reports. Similar to our study, there have been several reported cases of ACIDP following COVID-19 vaccination, diverging from the monophasic trajectory of GBS after an initial acute episode indistinguishable from GBS[18], [19], [20]. Differentiation of ACIDP from GBS may only be possible retrospectively, in the setting of longitudinal follow-up. The 4 individuals with ACIDP in our study included the single individual who had a further AEFI reported after a second dose of COVID-19 vaccine.
We recorded 1 death due to GBS. In total, the TGA has identified 11 reports across Australia where the cause of death was linked to COVID-19 vaccination, including 1 additional case of GBS (plus 8 VITT, 1 immune thrombocytopenia), all following the first dose of Vaxzevria[23]. Understanding the pathophysiology and mechanisms behind these serious adverse events raise important questions for the long-term sustainability of adenovirus-vector vaccines in the future, which have saved many more lives from COVID-19 disease. The fatal GBS case in our study was among 19 with an acute GBSDS score of 4 or greater (i.e. at least bedridden or chairbound). Three-quarters of all the GBS cases had ongoing negative impacts 3 months after their initial presentation.
Some limitations of the study include case ascertainment, as it possible GBS cases temporally associated with a COVID-19 vaccine were not identified via active or spontaneous AEFI reporting. While cases were screened for SARS CoV2 infection, not all cases had a detailed review excluding all possible infectious causes, noting the COVID-19 public health in Victoria, reduced the number of cases of circulating viruses, including influenza. Key strengths of this analysis are the longitudinal follow-up, critical to the ACIDP finding, and the stringent approach to GBS diagnosis, with specialist review of clinical details and results of electrophysiology, radiology, cerebrospinal fluid, and other laboratory investigations for every case. These cases occurred during a time period of very low local incidence of concurrent non-vaccine causes of GBS, owing to wide-ranging public health interventions, which would otherwise be expected to confound these findings. This has produced a case series of high diagnostic specificity from a large dataset of SAEFVIC reports collected by passive and active surveillance mechanisms. In contrast, the historical background rates are generated from comprehensive coding databases without specialist diagnostic confirmation of individual cases. Added to the fact that some GBS cases following COVID-19 immunization were likely to have gone unreported, this intrinsic limitation actually strengthens the key finding of this study.
Brighton Collaboration case definitions are intended to increase comparability of AEFI data across surveillance systems or research studies for retrospective analyses, and through standardized prospective data collection in a variety of geographic settings[24]. Our case series highlights a deficiency in these criteria for capturing GBS variants, for which limb weakness and areflexia are not cardinal features. These regional GBS variants are clearly accepted to be within the spectrum of GBS, yet most are only classified as Level 4, and were infrequent in previous validation efforts[25].
Noting the importance of adenovirus-vector vaccines in the continuing global COVID-19 vaccination program (especially the AstraZeneca product Vaxzevria) and continuing development of adenovirus-vector vaccines against Ebola, Lassa fever, rabies, Middle East Respiratory Syndrome (MERS), Zika, malaria, and tuberculosis, the authors believe there is an urgent need to support research into the role the adenoviral vector may play in these AESI and whether any predictive biomarkers may help to minimise the risk of an adverse events in the future (e.g. PF4 and VITT[26]). Global collaborative studies are required to characterise risk factors, clinical variability, and to provide precision and generalizability regarding AEFI risks associated with different vaccine platforms[27], [28] to inform understanding and communication of the potential benefits and risks of vaccination.
Funding.
This research was not supported by any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
Victorian Department of Health – Sally Gordon, Sam Axford, Anita Ona, Anna Power, Thiruverni Ananthanathan, Ngaree Blow, Elise Virah Sawmy, Eleanor Duckworth, Michelle Wolthuizen, Naveen Tenneti, Naomi Bromley.
VicSIS clinic sites - Michelle Giles (Alfred Health), Sarah Bullen (Alfred Health), Danielle Kennedy (Alfred Health), Kayleigh Malone (Alfred Health), Ciara Burke (Alfred Health), Brian Price (Alfred Health), Joseph de Luca (Austin Health), Jason Trubiano (Austin Health), Kerryn McInnes (Austin Health), Jamie Rotin (Austin Health), Callum Maggs (Barwon Health), Elyse Stevens (Barwon Health), Julie Carlilse (Barwon Health), Loretta Mithen (Barwon Health), Katrina Bellamy (Barwon Health), Caroline Poynder (Barwon Health), Susan Cirillo (Barwon Health), Katherine Gibney (Melbourne Health), Charlotte Slade (Melbourne Health), Elise Wang (Melbourne Health), Tony Korman (Monash Health), Sara Barnes (Monash Health), Karen Bellamy (Monash Health), Jo Hickman (Monash Health), Elizabeth Leahy (Monash Health), Sara Pitts (Monash Health), Craig Aboltins (Northern Health), Jenna Paterson (Northern Health), Hayley Gray (Northern Health), Jade Mertens (Northern Health), Lumnise Gashi (Northern Health), Ben The (Peter MacCallum Cancer Centre), Cindy Yuen (Peter MacCallum Cancer Centre), Marion Kainer (Western Health), Katherine Langan (Western Health), Claire Sanguinetti (Western Health), Kayleen Kraal (Western Health).
SAEFVIC – Emma Roney, Daryl Cheng, Priya Shenton, Mel Addison, Louise Dempsey, Veronica Abruzzo, Georgie Lewis, Bianca Penak, Laura Voss, Jaimee Craft, Victoria Scott, Lois Tham, Gerardo Luis Dimaguila, Li-yin Goh.
Neurologists involved in patient management, plus medical, nursing and administrative staff that support the VicSIS clinics.
All other hospital sites and clinicians involved in providing information on cases.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2022.10.084.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- 1.Crawford N.W., et al. Guillain-Barre syndrome following pandemic (H1N1) 2009 influenza A immunisation in Victoria: a self-controlled case series. Med J Aust. 2012;197:574–578. doi: 10.5694/mja12.10534. [DOI] [PubMed] [Google Scholar]
- 2.Priority list of adverse events of special interest: COVID-19. 2020. (Accessed May 23, 2022, at https://brightoncollaboration.us/priority-list-aesi-covid/.).
- 3.Patone M., et al. Neurological complications after first dose of COVID-19 vaccines and SARS-CoV-2 infection. Nat Med. 2021 doi: 10.1038/s41591-021-01556-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Population-based analysis of the epidemiological features of COVID-19 epidemics in Victoria, Australia, January 2020 - March 2021, and their suppression through comprehensive control strategies. Lancet Reg Health West Pac 2021;17:100297. [DOI] [PMC free article] [PubMed]
- 5.Clothier H.J., et al. Evaluation of 'SAEFVIC', A Pharmacovigilance Surveillance Scheme for the Spontaneous Reporting of Adverse Events Following Immunisation in Victoria. Australia Drug Saf. 2017;40:483–495. doi: 10.1007/s40264-017-0520-7. [DOI] [PubMed] [Google Scholar]
- 6.Todd I.M.F., et al. Changes in infection-related hospitalizations in children following pandemic restrictions: an interrupted time-series analysis of total population data. Int J Epidemiol. 2021;50:1435–1443. doi: 10.1093/ije/dyab101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clothier H.J., et al. Surveillance of adverse events following immunisation: the model of SAEFVIC. Victoria Commun Dis Intell Q Rep. 2011;35:294–298. doi: 10.33321/cdi.2011.35.28. [DOI] [PubMed] [Google Scholar]
- 8.Gordon S.F., et al. Victorian Specialist Immunisation Services (VicSIS) - bolstering adult clinics for COVID-19 vaccines. Hum Vaccin Immunother. 2022:1–6. doi: 10.1080/21645515.2022.2052701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Koningsveld R., et al. A clinical prognostic scoring system for Guillain-Barre syndrome. Lancet Neurol. 2007;6:589–594. doi: 10.1016/S1474-4422(07)70130-8. [DOI] [PubMed] [Google Scholar]
- 10.Sejvar J.J., et al. Guillain-Barré syndrome and Fisher syndrome: case definitions and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine. 2011;29:599–612. doi: 10.1016/j.vaccine.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 11.van Nes S.I., et al. Improving fatigue assessment in immune-mediated neuropathies: the modified Rasch-built fatigue severity scale. J Peripher Nerv Syst. 2009;14:268–278. doi: 10.1111/j.1529-8027.2009.00238.x. [DOI] [PubMed] [Google Scholar]
- 12.van Nes S.I., et al. Rasch-built Overall Disability Scale (R-ODS) for immune-mediated peripheral neuropathies. Neurology. 2011;76:337–345. doi: 10.1212/WNL.0b013e318208824b. [DOI] [PubMed] [Google Scholar]
- 13.Gold M., et al. Use of the Australian Childhood Immunisation Register for vaccine safety data linkage. Vaccine. 2010;28:4308–4311. doi: 10.1016/j.vaccine.2010.04.021. [DOI] [PubMed] [Google Scholar]
- 14.Tuckerman J., et al. COVID-19 and changes in the National Immunisation Program: a unique opportunity to optimise the Australian Immunisation Register (AIR) Med J Aust. 2021;214(247–9):e1. doi: 10.5694/mja2.50971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clothier H.J., et al. Human papillomavirus vaccine in boys: background rates of potential adverse events. Med J Aust. 2013;198:554–558. doi: 10.5694/mja12.11751. [DOI] [PubMed] [Google Scholar]
- 16.Gordon S.F., et al. Immune thrombocytopenia following immunisation with Vaxzevria ChadOx1-S (AstraZeneca) vaccine, Victoria. Australia Vaccine. 2021;39:7052–7057. doi: 10.1016/j.vaccine.2021.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bogliun G., et al. Incidence and clinical features of acute inflammatory polyradiculoneuropathy in Lombardy, Italy, 1996. Acta Neurol Scand. 2004;110:100–106. doi: 10.1111/j.1600-0404.2004.00272.x. [DOI] [PubMed] [Google Scholar]
- 18.Hawken S., et al. The self-controlled case series method for evaluating safety of vaccines. Med J Aust. 2012;197:578–579. doi: 10.5694/mja12.11087. [DOI] [PubMed] [Google Scholar]
- 19.Li X., et al. Association between covid-19 vaccination, SARS-CoV-2 infection, and risk of immune mediated neurological events: population based cohort and self-controlled case series analysis. BMJ. 2022;376:e068373. doi: 10.1136/bmj-2021-068373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pottegard A., et al. The neurological safety of covid-19 vaccines. BMJ. 2022;376 doi: 10.1136/bmj.o522. [DOI] [PubMed] [Google Scholar]
- 21.Hanson K.E., et al. Incidence of Guillain-Barre Syndrome After COVID-19 Vaccination in the Vaccine Safety Datalink. JAMA Netw Open. 2022;5:e228879. doi: 10.1001/jamanetworkopen.2022.8879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oliver S.E., et al. Use of the Janssen (Johnson & Johnson) COVID-19 Vaccine: Updated Interim Recommendations from the Advisory Committee on Immunization Practices - United States, December 2021. MMWR Morb Mortal Wkly Rep. 2022;71:90–95. doi: 10.15585/mmwr.mm7103a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.COVID-19 vaccine weekly safety report - 05-05-2022. 2022. (Accessed May 23, 2022, at https://www.tga.gov.au/periodic/covid-19-vaccine-weekly-safety-report-05-05-2022.).
- 24.Kohl K.S., et al. Applicability, reliability, sensitivity, and specificity of six Brighton Collaboration standardized case definitions for adverse events following immunization. Vaccine. 2008;26:6349–6360. doi: 10.1016/j.vaccine.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 25.Fokke C., et al. Diagnosis of Guillain-Barre syndrome and validation of Brighton criteria. Brain. 2014;137:33–43. doi: 10.1093/brain/awt285. [DOI] [PubMed] [Google Scholar]
- 26.Greinacher A., et al. Insights in ChAdOx1 nCoV-19 vaccine-induced immune thrombotic thrombocytopenia. Blood. 2021;138:2256–2268. doi: 10.1182/blood.2021013231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petousis-Harris H., et al. Progress Toward a Global Vaccine Data Network. Pediatr Infect Dis J. 2020;39:1023–1025. doi: 10.1097/INF.0000000000002785. [DOI] [PubMed] [Google Scholar]
- 28.International Network of Special Immunization Services. Canadian Center for Vaccinology, 2022. (Accessed May 23, 2022, at https://insisvaccine.org.).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.