Abstract
Coronavirus disease 2019 (COVID-19) outbreak has become a global public health emergency and has led to devastating results. Mounting evidence proposes that the disease causes severe pulmonary involvement and influences different organs, leading to a critical situation named multi-organ failure. It is yet to be fully clarified how the disease becomes so deadly in some patients. However, it is proven that a condition called “cytokine storm” is involved in the deterioration of COVID-19. Although beneficial, sustained production of cytokines and overabundance of inflammatory mediators causing cytokine storm can lead to collateral vital organ damages. Furthermore, cytokine storm can cause post-COVID-19 syndrome (PCS), an important cause of morbidity after the acute phase of COVID-19. Herein, we aim to explain the possible pathophysiology mechanisms involved in COVID-19-related cytokine storm and its association with multi-organ failure and PCS. We also discuss the latest advances in finding the potential therapeutic targets to control cytokine storm wishing to answer unmet clinical demands for treatment of COVID-19.
Keywords: SARS-CoV-2, COVID-19, Multiple organ failure, Cytokine storm, Oxidative stress, Post-COVID-19 syndrome
1. Introduction
The coronavirus SARS-CoV-2, the virus responsible for the deadly Coronavirus disease 2019 (COVID-19) disease, has caused a global outbreak in pandemic proportions and killed over five million people all around the world. Clinical manifestation of coronavirus disease can range from asymptomatic presentations to severe forms, such as acute respiratory distress syndrome (ARDS) and multi-organ failure requiring intensive care unit (ICU) admission [1].
The hallmark of COVID-19 disease since its advent has been pulmonary manifestation. However, as the disease propagated all around the world, more studies have been published about its extrapulmonary effects involving the cardiovascular, gastrointestinal, urogenital, and nervous systems [2]. Multi-organ failure is the leading reason for mortality and morbidity in ICU patients. Although the exact pathogenesis of multi-organ failure in COVID-19 is still under investigation, there are two conceivable mechanisms for it [3]: 1. SARS-CoV-2 invades the host cell via angiotensin-converting enzyme 2 (ACE2) receptor. Thus, all cells that express the ACE2 receptor are vulnerable to direct virus invasion and subsequent effects of ACE2 downregulation. 2. The uncontrolled and aberrant inflammatory response to the virus, but not the virus itself, can result in unintended complications and multi-organ failure.
Accumulating evidence suggests that uncontrolled immune responses resulting from a condition called “cytokine storm” or “cytokine release syndrome” could have a disastrous effect in patients with severe COVID-19 [4]. Cytokine storm is a deleterious, fast-developing systemic inflammatory condition involving excessive circulating cytokine levels and immune-cell overactivation that can be provoked by various pathogens, autoimmune diseases, malignancies, and immunotherapies. This syndrome has been proposed to be associated with multi-organ failure, as it can give rise to a wide range of systemic presentations, including elevated levels of cytokines and growth factors, endothelial damage, vascular permeability, coagulopathy, and infiltration of immune cells into tissues [5].
To the best of our knowledge, previous studies have reviewed the role of cytokine storm in multi-organ failure in COVID-19, but they have not discussed the mechanisms of this role in details and in each organ separately. To fill this gap, here we provide a comprehensive and thorough review of cytokine storm pathophysiology mechanisms and discusses how it can lead to dysfunction and failure in each organ separately during COVID-19 and post-COVID-19 periods. We will also summarize the current data about potential therapeutic targets and exciting interventions to control cytokine storm and prevent severe complications in COVID-19 disease.
1.1. Pathophysiology of cytokine storm and SARS-CoV-2 related cytokine storm
The immune system is expected to maintain hemostasis after recognizing foreign invaders and responding properly to them. This hemostatic balance depends on sufficient cytokine secretion to combat pathogens and prevention of hyper-inflammation since the over-production of cytokines leads to severe systemic damage [6]. Indeed, cytokines play a crucial role in defense against pathogens and regulation of immune responses [7].
Although the dysregulated and excessive secretion of cytokines may cause collateral damage to vital organs, well-coordinated production of cytokines is necessary to control systemic infections [8].
Lacking a unifying, widely accepted definition for cytokine storm, Fajgenbaum and colleagues, in a recent article, proposed the following three criteria for identifying cytokine storm: high levels of circulating cytokines, uncontrolled systemic inflammatory reaction, and systemic organ damages that are merely caused by immune responses [6].
As we know, SARS-CoV-2 enters the host cell via ACE2 receptor, which is highly expressed in the lower respiratory tract, lung, heart, ileum, esophagus, kidney, and bladder. The cellular serine protease transmembrane serine protease 2 (TMPRSS2) is necessary for S protein priming and is also expressed in many tissues, such as lungs and type II alveolar cells, the kidney, liver, and the GI tract [9], [10], [11]. After viral entry, ACE2 internalization, together with the virus, results in decreased cell surface levels of the ACE2 receptor. ACE2 regulates the balance of Ang II/Ang-(1–7) levels. So, downregulation of ACE2, in turn, increases Ang-II levels and reduces Ang 1–7 levels. This condition makes a shift in the renin angiotensin system (RAS) system toward the harmful ACE/Ang II/angiotensin II receptor type 1 (AT1R) axis due to Ang II accumulation. Ang-II acts as a pro-inflammatory cytokine and induces deleterious consequences in many organs via AT1R. Hyper-activation of the Ang-II/AT1R axis mediates inflammatory responses, which leads to uncontrolled pro-inflammatory cytokine production. Ang-II/AT1R axis activates Janus kinase/signal transducer and activator of transcription (JAK-STAT) and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathways, the main signaling pathways implicated in the production of inflammatory cytokines. Over-production of pro-inflammatory cytokines causes cytokine storm, and consequently, further activation of the NF-κB pathway occurs [12], [13]. So, the entrance of the virus activates many inflammatory pathways, leading to disruptive consequences. (Fig. 1 ).
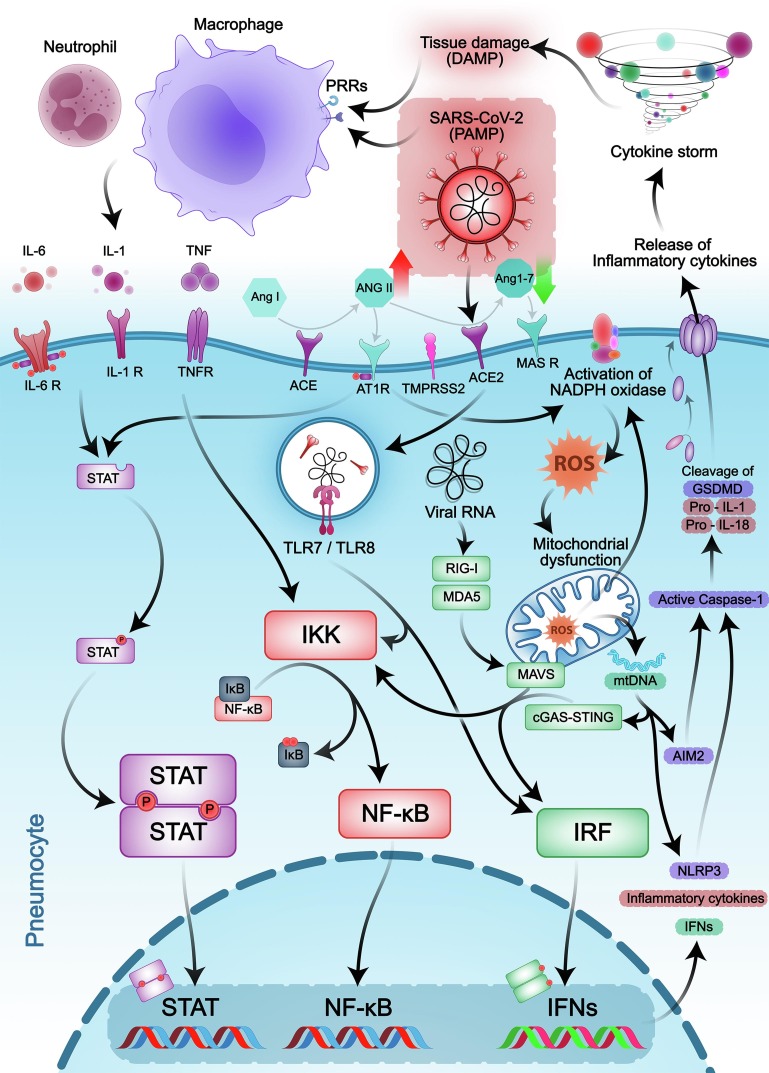
Fig. 1.
The key intracellular signaling pathways involved in COVID-19-related cytokine storm. SARS-CoV-2 enters the host cell via the ACE2 receptor resulting in downregulation of this receptor and a shift in the RAS system, which leads to elevated Ang-II levels and reduction of Ang 1–7 levels. Several pro-inflammatory cytokines produced by immune cells and Ang-II/AT1R axis utilize JAK/STAT and NF-κB intracellular signaling pathways to apply their effects. Attachment of pro-inflammatory cytokines to their cognate receptors leads to the JAKs phosphorylation, subsequent transphosphorylation and dimerization, making it possible for STATs to translocate into the nucleus and regulate specific genes expression. On the other hand, interferon regulatory factor (IRF) and NF-κB signaling pathways can be activated through RNA and DNA-sensing pathways. Mitochondrial dysfunction and reactive oxygen species (ROS) also participate in the aggravation of inflammatory responses. As a result, aberrant pro-inflammatory cytokine release causes cytokine storm and consequent multi-organ damage.
1.2. Immune system and inflammatory pathways in COVID-19
SARS-CoV-2-induced immune responses have two major phases. The first phase is based on protection against the virus via producing anti-viral components and inhibiting disease progression, mainly through activation of the dendritic cells, macrophages, and pulmonary epithelial cells. The second phase is an inflammatory state which may lead to cytokine storm and induce damage to the host [14]. It has been shown that in the early stages of COVID-19 disease, the immune responses are suppressed, which leads to more viral replication. In later stages of the disease, activation of immune responses leads to hyper-inflammatory state and cytokine storm, which cause organ damage [15]. Therefore, boosting the immune responses in the early stages and suppressing them in later stages should be considered as a potential option for the management of cytokine storm.
As mentioned, in the first phase, inflammation is crucial to make an appropriate immune response to clear the virus and inhibit the viral replication. Many important inflammatory signalings are involved in providing protective immunity against the virus. Both innate and adaptive immune responses contribute to viral elimination and protection of patients from negative consequences of the disease [14]. Here we discuss immune components and pathways contributing to immune responses against the virus.
1.2.1. Innate and adaptive immune system in COVID-19
There are complex and not fully understood interactions between various cell types, signaling pathways, and cytokines involved in cytokine storm. As the first line of defense, the innate immune system can recognize pathogens leading to the production of several cytokines [6], [16]. Activated macrophages, are implicated in various forms of the cytokine storm via producing tumor necrosis factor (TNF), interleukin-1 (IL-1), IL-6, IL-8, and IL-12. Although beneficial, the excessive production of cytokines contributes to generalized inflammation leading to multi-organ damage. Natural killer (NK) cells also provide a potent cytolytic function to diminish the viral load. Studies showed that in some types of inflammation and cytokine storm, NK cell activity is diminished, which is associated with expansion of monocytes populations which can lead to pro-inflammatory cytokine secretion and promotion of local and systemic inflammation in severe cases [17]. This condition makes it difficult to combat the pathogens and resolve the inflammation [18].
The innate immune cells express pathogen-recognition receptors (PRRs) to recognize pathogen-associated molecular patterns (PAMPs) structures on pathogens and damage-associated molecular patterns (DAMPs) released by damaged tissues. Following the binding of PRRs to PAMPs/DAMPs, PRRs activate intracellular signaling cascades to produce cytokines that activate the adaptive immune response and inhibit pathogen spreading [19].
Activation of innate immune system receptors, including the NOD-like receptors (NLRs) and the absent in Melanoma 2 (AIM)-like receptors (ALRs), leads to the inflammasome complex assembly and recruitment of pro-caspase-1. The clustering of inactive pro-caspase-1 proteins results in their self-cleavage and formation of active caspase-1. Active caspase-1 cleaves gasdermin D (GSDMD), precursor cytokines pro-IL-1β and pro-IL-18, leading to the formation of membrane pores, induction of pyroptosis, and release of biologically active IL-1β and IL-18 through these membrane pores [20], [21]. In COVID-19 patients, caspase-1 activity and higher plasma levels of IL-18 correlate with disease severity [22]. The SARS-CoV-2 virus can trigger priming and assembly of the inflammasome complex through various pathways. SARS-CoV-2 encoded N protein [23], ORF3a [24], NSP6 [25], E protein [26], and S protein [27], [28] are capable of promoting activation of inflammasomes such as NLRP3 inflammasome. Oxidization of mitochondrial DNA (mtDNA) due to mitochondrial dysfunction and subsequent ROS formation, can activate NLRP3 and AIM2 inflammasomes during SARS-CoV-2 infection [29]. Furthermore, activation of NLRP3 and AIM2 inflammasomes promotes pyroptosis of infected monocytes/macrophages and triggers systemic inflammation [30]. Activation of AIM2 in alveolar type II cells and alveolar macrophages also participates in the lung injury and increases mortality in influenza infection [31]. Thus, it may also induce inflammatory pathways in pneumocytes in COVID infection.
Upon recognition of SARS-CoV-2 by toll-like receptors (TLRs), one of the main PRRs, activation of NF-κB, interferon regulatory factor 3 and 7 (IRF3 and IRF7) transcription factors occur. Translocation of these transcription factors into the nucleus leads to priming of inflammasomes and increased expression of pro-inflammatory cytokines, chemokines, and type I interferon (IFN), which can trigger the activation of innate immune cells through a positive feedback loop. Sustained activation of innate immune response can cause ARDS [32], [33].
The adaptive immune response is carried out by B cells and T cells involved in defense against viral infections. T cells differentiate into several subtypes with different functions potentially involved in the cytokine storm. Virus-specific CD4+ T cells could differentiate into Th1 cells that secrete large amounts of IFN-γ, activate macrophages, and are crucial for cell-mediated immunity and defense against intracellular pathogens [34]. Cytokine storm might be developed due to overactivation of Th1-type inflammatory response [6].
CD8+ T cells have cytotoxic activity against infected cells, so they play a crucial role in the clearance of viruses [35]. It has been shown that SARS-CoV-2-specific CD8+ T cells and memory CD8+ T cells express high levels of activation/cycling markers in acute severe COVID-19 [36]. In most COVID-19 patients, IFN-γ+ SARS-CoV-2-specific CD8+ T cells and CD4+ T cells have been detected, representing a functional CD8+ T cell response against SARS-CoV-2 [37].
A sustained anti-viral response may cause T-cell functional exhaustion. Recent studies have reported that Tim-3 and PD-1 (exhaustion markers of T cells) expression levels on the surface of CD4+ and CD8+ T cells were significantly higher in COVID-19 patients, especially in severe conditions [38], [39], [40]. Total counts of helper T cells and cytotoxic T cells (effector T cells), and plasma cells (effector B cells which secret virus-specific neutralizing antibodies) are significantly decreased in COVID-19 [17], [32], [36], [41]. This reduction may be due to apoptosis caused by hyper-activation of innate immune responses and cytokine release [17], [32]. So, cytokine storm can cause a reduction in T cell counts. Therefore, hyper-activation or impaired function of innate and adaptive immune responses are involved in the immunopathology of COVID-19 severe stages.
1.2.2. Inflammatory cytokines and pathways in COVID-19
Inflammatory pathways, including JAK/STAT signaling pathway [42], IFN cell signaling pathway [43], NF-κB pathway [44], TLR pathway, etc. are activated in COVID-19-associated cytokine storm. Moreover, levels of IL-1, IL-6, TNF, IFN-γ, and IFN-γ-induced protein 10 (IP-10) are elevated in patients with COVID-19 and are thought to have leading roles in developing hyper-inflammatory state [45], [46]. Here, in the following sections, we provide an overview of substantial inflammatory cytokines and pathways implicated in the pathogenesis of COVID-19-associated cytokine storm.
IL-6
IL-6 is primarily released by immune cells and has a crucial role in mediating acute inflammatory responses and cytokine storm pathophysiology. Elevated levels of IL-6 may lead to multi-organ dysfunction in patients with severe inflammatory disease. IL-6 is implicated in innate and adaptive immune responses and promotes defense against pathogens, and also has a tissue repair effect. IL-6 has a particular receptor system for transmitting its signals. It can signal through the membrane-bound interleukin-6R that interacts with CD130. But the excessive and sustained release of IL-6, mainly due to exacerbated host immune response, can cause disease progression and systemic inflammation [47], [48].
IL-6 is one of the central NF-κB-driven cytokines, which can induce hyper-activation of NF-κB via activation of the JAK-STAT3 pathway in vascular inflammation. Viral induction of TLRs causes NF-κB-mediated induction of pro-inflammatory cytokines, including IL-6, TNFα, and IL-1β. TNFα or IL-1β also induces transcription of IL-6 mRNA. The binding of IL-6 to its membrane receptor IL-6R causes phosphorylation of gp130 and activates intracellular signaling via inducing activation of the JAK-STAT pathway [47], [49].
Serum IL-6 levels are significantly elevated in COVID-19 patients with complicated diseases especially among those who progressed to ARDS. There is also a relation between IL and 6 levels and mortality. Patients who died from the COVID-19 had significantly higher serum IL-6 levels [50]. Serum IL-6 levels can be used as a prognostic biomarker to detect patients with higher susceptibility to developing cytokine storm and predict the outcome of the disease [51], [52], [53]. In a study in 2020, an association between IL and 6 Levels and RNAemia in patients with severe COVID-19 was demonstrated. IL-6 level in patients with RNAemia was over 100 pg/mL, representing a higher risk of developing multiple organ damage and mortality in these patients. Excessive amounts of IL-6 expanded the vascular permeability leading to organ dysfunction and consequent increased serum SARS-CoV-2 viral RNA [54].
IL-1
Cytokines of the IL-1 family significantly promote the activity of innate immune cells and the differentiation of polarized T cells [55]. It has been shown that over-production of IL-1 β contributes to some forms of cytokine storm, presumably through activation of the NF-κB intracellular pathway. Moreover, aberrant production of IL-1β is thought to be implicated in COVID-19-related cytokine storm. SARS-COV2 virus binds to TLRs, leading to the formation of pro-IL-1β, activation of the inflammasome, and subsequently the production of IL-1 β, which mediates fever, lung fibrosis, and systemic inflammation [56], [57].
TNF
TNF is one of the potent pro-inflammatory cytokines that have a prominent role in acute viral disease and their related cytokine storm. TNF-α is produced by different immune cells, and its primary cognate receptor, TNFR1, seems to be expressed within nearly all cells of the body, which is likely responsible for TNF's multiple functions [58]. Inflammatory processes induced by TNF-α could be beneficial on a controlled level; however, on a systemic level, it can provoke fever, increased vascular permeability, coagulopathy, and even shock [59], [60], [61]. TNF-α is a prominent activator of NF-κB, the critical transcription factor in regulating a broad spectrum of biological processes, including cell proliferation, differentiation, and apoptosis [62].
In severe cases of COVID-19 disease, the levels of TNF-α and other pro-inflammatory cytokines were significantly increased [63]. In a recent study, Karki and colleagues showed that synergism of TNF-α and IFN-γ could exacerbate the COVID-19-related cytokine storm and lead to multi-organ failure as a consequence of inflammatory cell death and tissue damage [64].
JAK/STAT pathway
JAK/STAT pathway is one of the main intracellular pathways involved in cytokine storm and COVID-19 severity [42]. This pathway is initiated upon attachment of pro-inflammatory cytokines to their cognate receptors leading to the JAKs phosphorylation and then STATs transphosphorylation. The dimerization of STATs enables them to translocate into the nucleus and attach to distinct DNA sequences, consequently affecting the transcription of the immune regulatory, apoptotic, cell cycle, and differentiation-related genes [65].
Several pro-inflammatory cytokines, including IL-2, IL-4, IL-6, IL-7, IL-10, TNF-α, and IFNγ, utilize JAK/STAT pathway to apply their effects [42]. For instance, IL-6, which has been proven highly expressed in COVID-19 patients, induces JAK/STAT pathway activation and downstream inflammation-related gene expression and subsequently regulates different immune responses involved in cytokine storm [66].
Moreover, Ang II signaling can be mediated by the JAK-STAT pathway. For this purpose, AT1-R employs and phosphorylates JAK2, which in turn phosphorylates one of the STATs (based on target cells) to mediate the transcription of target genes. Considering the crucial role of the JAK/STAT pathway not only in cytokine signaling but also in Ang II signaling, targeting this pathway presents an attractive therapeutic strategy for cytokine storm [67].
NF-κB pathway
As mentioned, DAMPs and PAMPs recognized by PRRs and activated downstream signalings are implicated in anti-viral response. The NF-κB pathway is the main signaling initiated via the activation of PRRs [68]. NF-κB is a transcription factor that enhances pro-inflammatory gene expression, associated with innate and adaptive immune responses. Following activation of IKKα and IKKβ by a viral infection or pro-inflammatory cytokines, phosphorylation of IκBs occur, which leads to nuclear translocation of NF-κB. The binding of NF-κB to κB-sites in the nucleus induces transcription of pro-inflammatory genes [69], [70]. NF-κB-driven pro-inflammatory cytokines such as IL-1, IL-6, IL-8, and TNF-α can also induce activation of NF-κB through a positive feedback mechanism [71]. NF-κB is involved in promoting the polarization of macrophages into pro-inflammatory M1 phenotype due to activation of the TAK1/IKK pathway [68]. Activation of the NF-κB pathway is also involved in regulating CD4+ T-cell differentiation following TCR activation and also via regulating the cytokine production in innate immune response [72]. It has been proved that the expression of NF-κB-dependent pro-inflammatory pathways is increased in critically ill patients with COVID-19 [44]. It has also been shown that SARS-CoV-2 proteins, including M, N, and ORF7a proteins, are correlated with the severity of ARDS in COVID-19 patients via activating NF-κB function and increasing expression of pro-inflammatory cytokines such as IL-1β, IL-6, IL-8 and TNF-α [73].
So over-activation of NF-κB-dependent pro-inflammatory pathways, which can pathologically activate neutrophils and monocytes/macrophages, is responsible for excessive cytokine release and causes mortal inflammation in COVID-19 patients, leading to cytokine storm syndrome.
IFN cell signaling pathway
One of the major components in innate immune response is the IFN signaling pathway which is involved in anti-viral response through inducing transcription of IFN-stimulated genes (ISGs) [74], [75]. PRRs such as the TLRs and RIG-I-like receptors (RLRs) are involved in the production of IFNs.
TLRs are one of the main innate immune recognition receptors that recognize pathogens on the cell membrane as well as in the cytoplasm. There are 11 types of TLRs already recognized in human beings [76]; among those, specific TLRs (TLR3, TLR4, TLR7, TLR8, and TLR9) could recognize SARS-CoV-2, which leads to the activation of transcription factors such as NF‐κB, AP-1, and IRF to enhance viral elimination [49]. TLR4 is a membrane receptor of alveolar and bronchial epithelial cells that recognize PAMP as well as DAMP and has an important role in COVID-19 progression. It has been found that the accumulation of oxidized phospholipid (OxPL) could augment the levels of cytokines and promote acute lung injury (ALI) via TLR4 [49]. TLR4 is a membrane receptor of alveolar and bronchial epithelial cells that recognize PAMP as well as DAMP and has an important role in COVID-19 progression. It has been found that the accumulation of OxPL could augment the levels of cytokines and promote ALI via TLR4 [49], [77]. Since the expression of TLR4 has been reported on endothelial cells, renal tubular cells, and cardiomyocytes, it may be involved in causing ARDS and kidney and cardiac injury. Activation of endosomal TLR7 and TLR8 receptors induce pro-inflammatory cytokine production by activating MyD88/MAPK/NF-κB signaling pathways [78]. mtDNA enhances inflammatory responses via TLR9 activation. Costa et al. demonstrated that the activation of TLR9 signaling is involved in endothelial dysfunction and cardiovascular consequences in SARS-CoV-2 infection [79].
Hence, specific targeting of TLRs not completely blocking all TLRs may be beneficial in mediating the cytokine surge in COVID-19 disease and preventing ALI in those patients.
RLRs are intracellular PRRs that can sense non-self RNA (such as SARS-CoV-2 RNA) [80], [81]. RLRs such as MDA5 and RIG-I get activated in the presence of SARS-CoV-2 RNA and interact with MAVS, leading to recruitment of specific kinases and subsequent phosphorylation of inhibitor of nuclear factor kappa B (IκB), and transcription factors such as IRF3 and IRF7. Once activated, these transcription factors translocate to the nucleus and induce the transcription of genes encoding inflammatory cytokines and IFNs [33], [82], [83]. In addition to immune mechanisms that can detect viral RNA, another pathway capable of sensing cytoplasmic DNA called cyclic GMP-AMP synthase (cGAS)–stimulator of IFN genes (STING) signaling pathway, can play a critical role in immunity against the viral infection. Recent studies reported that the cGAS-STING signaling pathway could be activated by either DNA viral infections or infection by RNA viruses such as SARS-CoV-2 [84]. The cGAS-STING pathway can sense pathogenic or misallocated host DNA and subsequently activate TBK-1 and IKK, resulting in phosphorylation of IRF3 and IκBα [85]. Recent studies reported activation of the cGAS-STING pathway in SARS-CoV-2 infection upon cell-to-cell fusion and subsequent chromatin DNA leakage promoted by SARS-CoV-2 spike protein [86], [87], or upon sensing DNA of damaged mitochondria in the cytosol [88], [89]. Also, a recent study reported NF-κB-dependent production of inflammatory cytokines mediated by activation of the cGAS-STING pathway without induction of IFN signaling in epithelial cell lines infected by the SARS-CoV-2, suggesting that epithelial cells infected by the virus play a role in exacerbating the cytokine storm [90].
Once secreted, IFN binds to its receptors (IFNAR and IFNLR) and mediates its anti-viral effects via activation of the JAK-STAT pathway [75], [91]. Type I IFN production is necessary to activate the anti-viral innate immune responses during early SARS immunopathogenesis, and impaired type I IFN production leads to lower viral clearance [92]. However, overexpression of IFNs in severe COVID-19 patients has been demonstrated [43]. Delayed but high levels of type I IFN in severe COVID-19 cases with higher viral load may lead to some consequences, including 1. Accumulation of pathogenic inflammatory monocyte-macrophages (IMMs), 2. Regulating the pulmonary infiltration of monocyte-derived macrophages, 3. Induction of NFκB signaling pathway 4. Prevention of T cell proliferation 5. Secretion of inflammatory cytokines/chemokines which cause lung tissue damage [93], [94]. Therefore, it is worth mentioning that despite the role of IFNs in protecting against viral infection, they may be involved in disrupting the lung epithelial barrier [95]. Accumulating pathogenic IMMs results in pro-inflammatory cytokine expression such as IL-6 and IL-1β, which may sensitize T cells to promote apoptosis [93], [96]. So, dysregulated type I IFN is responsible for immunopathology of severe COVID-19.
In conclusion, dysregulation of immune response and subsequent uncontrolled hyper-production of pro-inflammatory cytokines can cause a fatal inflammatory state. So, it is necessary to attenuate inflammatory pathways involved in the creation of cytokine storm.
1.3. Association of cytokine storm and oxidative stress
There is an exacerbating feedback loop consisting of oxidative stress, cytokine storm, and ER stress [97]. This vicious loop between oxidative stress and cytokine storm is responsible for many lethal consequences in COVID-19 infection. SARS-CoV promotes ROS production via activation of the NF-κB pathway leading to cell apoptosis [98]. SARS-CoV-2-induced oxidative stress is mainly due to mitochondrial dysfunction [99]. Mitochondria is one of the SARS-CoV-2 targets, enhancing virus replication [100]. The localization of SARS-CoV-2 dsRNA in the host mitochondrial matrix has been proven [101]. The virus disrupts mitochondrial homeostasis through loss of mitochondrial membrane potential (ΔΨm), mitochondrial permeability transition pore (MPTP) opening, and increased ROS release, which may promote SARS-CoV-2 replication [102]. Mitochondrial ROS (mtROS) and mitochondrial DAMPs stimulate the production of additional pro-inflammatory cytokines such as IL-1β and IL-6 [103]. TNF-α, IL-6, and IFN-γ, which are the main inflammatory cytokines participating in cytokine storm creation, prevent mitochondrial oxidative phosphorylation and consequently lessen ATP production resulting in more mtROS levels [104]. Excessive production of mtROS and impaired mitophagy in platelets promote platelet dysfunction and apoptosis, resulting in activation of the coagulation cascade and promotion of thrombus formation [99].
SARS-CoV-2-induced ROS production might also be due to increased AngII levels in COVID-19 in an NADPH oxidase-dependent manner [105]. A positive feedback loop between mitochondrial and NADPH oxidase-derived ROS promotes cell and tissue degeneration as a consequence of ROS accumulation [105], [106], [107].
1.4. Cytokine storm related organ dysfunction in COVID-19
The pathophysiology of the different organ failures in COVID-19 remains to be fully elucidated. As mentioned, high levels of pro-inflammatory cytokines and chemokines play a pivotal role in local and systemic inflammation. In local inflammation, activation of inflammatory cells, endothelial damage, and parenchymal and epithelial injury occur, which leads to the extravasation of cytokines into the systemic circulation. Therefore, the systemic release of cytokines induces exaggerated inflammation that can affect remote organs and cause multi-organ failure, invading the vascular system and causing injury [108], [109], [110]. Inflammation caused by cytokine storm can disrupt homeostasis of many organs. Elevated levels of pro-inflammatory cytokines including IL-1, TNF-α, IFN-γ and IL-6 results in recruitment of various immune cells and subsequent tissue damage in COVID-19 patients [111]. Moreover, persistent and higher levels of pro-inflammatory cytokines have relationship with higher mortality rate in patients [112], [113], [114].
Over-production of IL-6 is correlated with MODS, severity of disease, and mortality. As it has been shown that IL-6 is an indicator for predicting development of organ dysfunction and mortality and is also predictor of disease severity in patients with systemic inflammation [115], [116]. IL-1β is involved in promoting inflammation in bronchoalveolar lavage fluid of patients with ARDS [117]. Pro-inflammatory cytokines, such as IL-1β, IL-8, CXCL2, and IL-6, promote and exacerbate ALI/ARDS [96], [118], [119]. Hyper-activation of NF-κB also promotes inflammatory injury to the lungs and other organs [119]. Therefore, multi-organ failure is highly associated with a pro-inflammatory state in the cytokine storm. (Fig. 2 ).
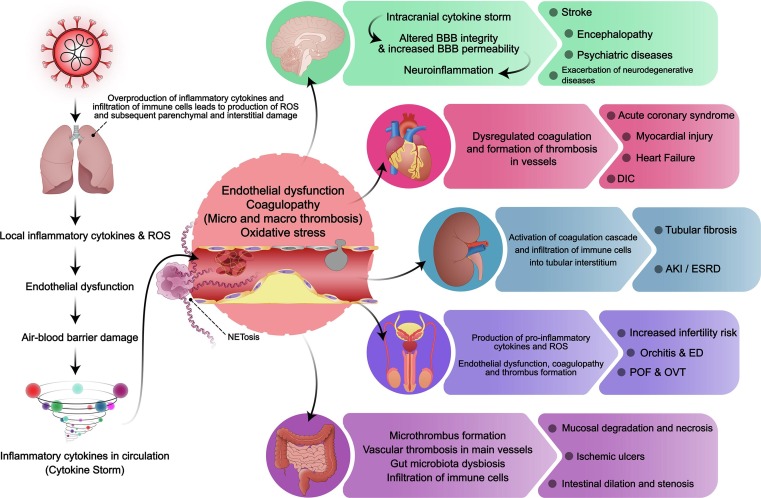
Fig. 2.
The association of SARS-CoV-2-induced cytokine storm and multi-organ failure. SARS-CoV-2 enters the body through the respiratory system and causes local inflammation and cytokine production, leading to alveolar vascular endothelial dysfunction, which subsequently leads to leakage of inflammatory cytokines to the bloodstream and the development of cytokine storm. Cytokine storm, induces endothelial dysfunction in various organs. Furthermore, ROS production and coagulopathy occur, causing thrombus formation and leading to organ damage. As for the brain, the intracranial cytokine storm arises due to a systemic cytokine storm, leading to alteration of blood–brain barrier integrity and subsequent increased permeability and development of neuroinflammation. This neuroinflammation, alongside thrombus formation, causes neurological complications such as stroke, encephalopathy, psychiatric diseases, and neurodegenerative diseases. Acute coronary syndrome, myocardial injury, heart failure and disseminated intravascular coagulopathy in the cardiovascular system, mucosal degradation and necrosis, ischemic ulcers and intestinal dilation and stenosis in the GI system and tubular fibrosis, acute kidney injury and end-stage renal disease in kidneys, are the most common complication of other organs due to systemic cytokine storm.
Hence, in addition to anti-viral therapies, anti-cytokine and immunomodulatory therapies may be effective in improving COVID-19-associated organ failure.
Here we discuss the potential mechanisms involved in cytokine storm-related organ dysfunction in COVID-19.
Respiratory system
SARS-CoV-2 mainly involves the respiratory system since the virus enters the body mainly through the pneumocytes, which highly express ACE2 receptors [120]. The most prevalent symptoms of COVID-19 patients are respiratory manifestations, including fever, dry or productive cough, dyspnea, hypoxia, respiratory failure, and ARDS [120], [121]. ARDS is a notable feature in COVID-19 and is considered the leading cause of mortality [122]. SARS-CoV-2, being a pathogen, induces the release of pro-inflammatory cytokines from local polymorpho nuclear cells (PMNs) and leukocytes, including IL-1β, IL-6, IL-8, and TNF-α [123]. These cytokines have local effects and induce endothelial cell expression of adhesion molecules, including intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VACM-1) [124]. Subsequently, other immune cells, including T-cells and PMNs, and monocytes attach to these adhesion molecules [125]. PMNs start producing ROS and more cytokines, leukotrienes, prostaglandins, and other proteases, which, together with T-cells, promote endothelial dysfunction [126]. This dysfunction is caused by cell membrane lysis, excessive permeability, cellular edema and apoptosis, and necrosis [127]. Afterward, this over-production of inflammatory mediators and immune cell infiltration producing ROS causes parenchymal and interstitial damage [108]. Subsequently, pneumocytes are injured with more infiltration, causing tissue fluid imbalance, pulmonary edema, and eventually ARDS [128], [129]. Consequently, inflammatory cytokines and activated immune cells enter the circulatory system and reach other organs, promoting multi-organ damage [6], [130].
Cardiovascular system
Myocardial injury, arrhythmia, heart failure, and acute coronary syndrome are complications following COVID-19 [131]. Acute cardiac injury and heart failure are more likely to happen in patients with cardiovascular comorbidities. Regardless of pre-existing cardiovascular disease, these cardiac complications in COVID-19 patients increase m ortality risk [132]. A multicenter study on COVID-19 patients in Italy suggested CRP levels were associated with troponin levels and cardiac injury. Patients with cardiac injury had more elevated levels of CRP, serum ferritin, IL-6, d-dimer, and NT-proBNP levels. This suggests that systemic inflammation plays an imperative role in the development of heart injury [133].
Heart failure was found to be associated with increased levels of several inflammatory cytokines, such as IL-6, IL-1β, and TNF-α. Increased levels of these cytokines are associated with the severity of heart failure. Studies have reported a strong link between pro-inflammatory cytokines (including IL-1 and IL-6) and the development of heart failure [134]. IL-1 exerts adverse inotropic effects on cardiomyocytes by impairing Ca2+ homeostasis. Cardiomyocytes produce IL-1 during chronic hypoxia [135]. IL-1β and TNF-α can cause an NF-κB-mediated upregulation of AT1R on cardiac fibroblasts after MI, causing fibrosis and subsequent cardiac dysfunction [136]. IL-6 cytokine has been shown to impair myocardial function and exert adverse inotropic effects mediated through the JAK-STAT3 pathway [137]. Studies have suggested IL-6 as an independent predictor of severity and mortality of heart failure [138], [139].
A central circulatory dysfunction in cytokine storm and multi-organ failure is dysregulated coagulation. Through excessive inflammatory cytokines in the bloodstream, tissue factor, which is an essential component in the activation of coagulation, is expressed on the surface of endothelial cells. This leads to subsequent coagulation cascade and thrombus formation in arteries and veins [140]. Moreover, there has been indirect (indicating endothelial cell dysfunction such as P-selectin) and direct evidence proving endothelial injury and endothelitis caused by COVID-19. Increased levels of IL-1β can impair lung endothelial integrity ARDS by reducing cAMP response element-binding (CREB)-mediated transcription of VE-cadherin in lung endothelial cells [141]. This endothelial cell injury leads to the release of von willebrand factor (vWF), expression of adhesion molecules, impairment of endothelial nitric oxide synthase (eNOS), and subsequent decline in NO production, which cause platelet aggregation and thrombus formation [142]. There are also considerable evidence demonstrating the occurrence of disseminated intravascular coagulopathy (DIC) in COVID-19 patients, especially in severe and critical cases [142], [143], [144]. This implies that in COVID-19-induced cytokine storm, DIC occurs through the consumption of coagulation factors due to endothelial cell injury.
Urinary system
Acute kidney injury (AKI) is prevalent in COVID-19 patients. About 28 % of hospitalized COVID-19 patients are diagnosed with AKI, and 9 % require kidney replacement therapy (KRT) [145]. COVID-19 infection causes AKI, which is considered an independent risk factor for illness progression and mortality severity. There was a higher mortality risk in COVID-19 patients with AKI compared to COVID-19 patients without AKI [146], [147], [148]. Association between respiratory failure and AKI has also been reported [149]. ARDS patients with AKI have higher IL-6 and TNF-α than those without AKI [150], [151], [152], [153]. There is also a correlation between urinary cytokines and kidney tissue injury leading to AKI [154].
An observational cohort study enrolling 223 COVID-19 patients in which 31 % of patients developed severe AKI (KDIGO stage 3) reported inflammatory markers including serum procalcitonin and white cell count were independent time-varying risk factors of severe AKI [155]. In other COVID-19 cohort studies, IL-2R, IL-6, TNF-α, CRP, and PCT were higher in the AKI group compared to the non-AKI group [156], [157].
COVID-19 induces AKI possibly via promoting tubular and endothelial dysfunction mainly caused by systemic and local inflammation, RAAS system hyper-activation, and coagulation cascade activation [158]. The lung-kidney axis is mainly impaired by the cytokine storm in COVID-19 [159].
Circulatory DAMPs and PAMPs are also involved in local inflammation in the kidney and subsequent thrombus formation and endothelial injury [158], [160], [161]. Inflammation of the vascular system causes thrombosis, microangiopathy, endothelial activation, and increased permeability in vessels [159], [162], [163].Infiltration of immune cells into tubular interstitium induces apoptosis, fibrosis, micro-vascular injury and consequent tissue damage mainly via inflammatory cytokines production [164], [165], [166].
Hyper-coagulation is another cause of AKI which can be induced by different means, including COVID-19-mediated macrophage activation, cytokine storm promotion, DAMP and PAMP activation, coagulation factors activation, and neutrophil extracellular traps (NETs) formation [160], [167]. Thrombosis can also be mediated by COVID-19-induced hypoxia. It has been shown that SARS-CoV-2 can activate platelet-mediated thrombosis via binding to platelets ACE2 [168].
Reproductive system
A few studies evaluated the presence of SARS-CoV-2 in the male and female reproductive system, representing the possible direct invasion of the virus into these organs [148], [169], [170]. However, several other studies did not detect the virus in reproductive organs by sequencing tests [171], [172], [173], [174]. This implies that reproductive organ dysfunction may result from inflammation rather than a direct invasion of SARS-CoV-2. Moreover, studies have reported decreased levels of testosterone hormone, which is vital for male reproductive function, and increased amount of inflammatory cytokines, which further suggests the impact of cytokine storm on reproductive organs [10]. The effect of inflammation and inflammatory cytokines on testis and spermatogenesis was reported [175]. In a study enrolling 43 men, the levels of IL-1β and TNF-α inversely correlated with sperm concentration [176]. The results of sperm analysis of male patients with COVID-19 showed decreased sperm count and motility compared to the results before viral infection [177], [178]. Also, the impact of oxidative stress on male and female reproductive function was evaluated and reported to play an important role in infertility in the context of COVID-19 [179], [180].
Other complications such as ischemic priapism and erectile dysfunction (ED) were also reported in male patients with COVID-19. The histologic findings reported endothelial dysfunction and blood coagulation in penile vasculature [181], [182], [183]. Furthermore, in several survey studies, some patients mentioned ED after recovery from COVID-19 [184], [185]. There were also reports of orchitis in histological findings of COVID-19 patients and impaired spermatogenesis due to increased inflammation and inflammatory cytokines [186]. An autopsy study by Wichmann et al. reported microthrombi in the prostate of male patients [38]. In female COVID-19 patients, there were also reports of primary ovarian failure (POF) and ovarian vein thrombosis (OVT) [187], [188], [189], [190], [191]. One study reported POF due to long COVID-19 [192], and another presented a case of OVT in a pregnant woman [193]. Since COVID-19-induced cytokine storm causes hyper-inflammation, endothelial dysfunction, coagulopathy and thrombus formation, considering the above evidences, it can be implied that cytokine storm also results in reproductive organs dysfunction both in men and women, which might potentially result in infertility.
Gastrointestinal and hepatobiliary system
Gastrointestinal cells, from the esophagus to the large intestine, express a considerable amount of ACE2 and TMPRSS2, which are essential for the invasion of the coronavirus to the cells. However, data has shown that esophagus mucosa expresses a small number of ACE2 receptors, so SARS-CoV2 infection of esophageal cells is less than in other GI organs [194]. Moreover, Tissue staining demonstrated lymphocyte infiltration in esophageal epithelium and infiltration of plasma cells, monocytes, and lymphocytes in lamina propria of other GI organs alongside edema of interstitium [194], [195]. Also, Laboratory studies on intestinal organoids have shown that SARS-CoV2 can infect intestinal cells [196]. The histopathological changes in the GI system due to SARS-CoV2 infection include mucosal degradation and necrosis in the GI system. Also, the submucosa's dilation and congestion of blood vessels are seen, especially in the stomach [195].
Additionally, floral dysregulation of the gut (gut dysbiosis) has been seen during COVID-19. The role of gut dysbiosis in the severity of COVID-19 and induction of cytokine storm via dysregulation of the gut-lung axis is strongly suggested in studies [197], [198]. The gut-lung axis is a bidirectional cross-talk between the lungs and GI system’s microbiota, which in physiologic states maintains and regulates the immune system [199], [200]. The disrupted GI microbial flora causes epithelial leakage and further induction of cytokine storm through different mechanisms [198], [201]. Therefore, gut dysbiosis alongside other mechanisms can cause cytokine storm and its following organ dysfunction, including the GI system.
Histological findings in GI tissue biopsies have shown evidence of vascular thrombosis in main vessels, including inferior vena cava and superior mesenteric artery, resulting in ischemia and tissue necrosis due to COVID-19 [202], [203], [204]. Additionally, in COVID-19 patients, tissue biopsies and autopsies demonstrated several digestive tract injuries, including mucosal damage and ulcers in favor of ischemia and small intestinal dilation and stenosis. Although not certain, cytokine storm is presented as a possible cause of these tissue damages [205], [206], [207].
Cytokine storm might contribute to liver and pancreas injuries as well. There have been reports of microthrombus formation in the liver in patients with critical COVID-19 undergoing cytokine storm, which subsequently leads to organ damage and liver failure [208]. Moreover, as the microthrombi form in other parts of the circulatory system, they can be displaced, resulting in emboli and ischemia in other organs such as the liver. Also, histological findings illustrated further tissue injury in the liver resulting in liver function test changes due to COVID-19-induced cytokine storm [209]. There are also reports of acute pancreatitis in severe cases of COVID-19 patients and increased cytokine levels in both severe COVID-19 and acute pancreatitis patients, implying the existence of a link between pancreatitis and cytokine storm due to COVID-19 [210].
Nervous system
Studies suggested that SARS-CoV-2 can invade the central nervous system (CNS) and peripheral nervous system (PNS) via possible mechanisms. Neuronal anterograde/retrograde viral spreading via trans-synaptic transferring is one of the possible mechanisms of SARS-CoV-2 neuroinvasion. The virus can be transferred to CNS using afferent vagus nerve and olfactory nerve leading to neuronal dysfunction and degeneration [211], [212], [213]. Another potential route for SARS-CoV-2 neuroinvasion is the hematogenous pathway via transcytosis across capillary endothelial cells, which express ACE2, and via dissemination of infected leukocytes across the blood brain barrier (BBB) and choroid plexus [213], [214]. Studies demonstrated that SARS-COV-2 alters BBB integrity and increases BBB permeability leading to neuroinflammation and neuropathology [215], [216], [217], [218]. SARS-CoV-2 S Protein induces pro-inflammatory responses in brain endothelial cells and promotes the activation of endothelial cells resulting in the upregulation of inflammatory cytokines and MMPs [217].
Direct infection of the BBB endothelial cells and cytokine/immune-mediated endothelial injuries increase the BBB permeability. Disrupted BBB and subsequent inflammatory cytokines and immune cell infiltration promote neuronal injury [219], [220].
Cytokine storm may play a vital role in the pathogenesis of COVID-19-induced neurological complications. Previous studies have reported elevated levels of cytokines such as IL-6, IL-8, IL-1, TNFα, TGF-β1, and MCP-1 in patients with different neurological complications and anti-cytokine-based therapeutics may be effective in preventing COVID-19 induced neurological complications [221], [222], [223].
1.5. Cytokine storm and post-COVID-19 syndrome
As the COVID-19 pandemia progressed, various conditions were reported, including post-COVID-19 syndrome (PCS). PCS refers to prolonged manifestations of more than three weeks after the acute phase of COVID-19 [224], [225]. Although the pathogenesis of PCS is not yet well understood, there have been reports of a sustained hyper-inflammatory state alongside microvascular thrombosis and immune dysregulation and following organ dysfunction in patients with PCS [225], [226], [227], [228]. Persistent inflammation is implicated in the deterioration of multi-organ failure recovery in the long term [229], [230]. Moreover, prolonged COVID-19 has been reported to be associated with disease severity [231].
Studies have reported the impairment of at least one major organ after recovery from the acute phase of the disease [232]. Marked increase of serum pro-inflammatory cytokine levels can cause CNS complications and impair neuronal function via penetration of the BBB. Studies have demonstrated the association between elevated IL-6 levels and post-COVID neurological consequences, pulmonary fibrosis, and other co-morbidities [233], [234], [235]. Ortelli et al. recently evaluated 12 post-COVID patients. C-reactive protein (CRP) and IL-6 serum levels were remarkably elevated in all 12 patients, representing a hyper-inflammatory state. 8 of 12 cases presented clinical neuropathological sequels [236].
A case study in 2020 has reported kawasaki disease and toxic shock syndrome among pediatrics with COVID-19 post-infection. All four children had increased pro-inflammatory cytokine levels (IL-6, IL-8, and TNF-α), representing cytokine storm. This inflammatory state could result in multi-organ failure due to impaired vascular permeability in major organs [237]. Multisystem inflammatory syndrome in children (MIS-C) is another consequence of post-COVID which can lead to inflammatory vasculopathy and myocarditis [238].
Hyper-inflammation-associated vascular impairment and coagulation abnormalities can cause post-COVID complications such as cardiac and cerebral thrombosis, pulmonary embolism, deep vein thrombosis, and also thrombosis in other organs leading to multi-organ failure [239]. A case report study reported inflammation in the main arteries of the brain and systemic circulation in a 69-year-old man Six weeks after the infection onset. Cerebral and systemic vasculitis can induce progressive brain damage and multi-organ dysfunction [240].
Post-acute-COVID-19 cardiovascular sequelae related to inflammation have been mentioned in recent studies [241], [242], [243], [244]. Persistent myocardial inflammation has been reported in 60 % of COVID-19 patients who recovered from the infection at 71 days post-infection [245]. Inflammation and cardiac biomarkers can be considered a moderate predictive value for 30-day mortality and also PCS [243].
There is a positive feedback loop between NETosis/NET formation and cytokine storm in COVID-19, which may contribute to PCS pathology. The NET formation may induce post-COVID-19 pulmonary fibrosis, long-term neurological consequences, and cancer progression and metastasis in long-term [246].
Furthermore, gut dysbiosis, which has a role in cytokine storm induction in COVID-19, may also contribute to long-COVID development [247]. Microbiome dysbiosis is associated with the development of persistent symptoms [248]. Some bacterial species are pathogenic, involved in the regulation of inflammation and positively correlate with several PCS symptoms [248], [249], [250]. In a recent study, Liu Q et al. reported that gut microbiota composition is altered in patients with COVID-19 and persisted for six months in patients with PCS [248].
In conclusion, hindering the progression of cytokine storm would be beneficial for preventing post-COVID-19 detrimental consequences.
1.6. Cytokine storm activation by different variants of SARS-CoV-2
Mutations in the spike gene of SARS-CoV-2 have led to the emergence of different variants that can evade natural or vaccine-induced immunity and jeopardize the vaccination efficacy. With the former variants of SARS-CoV-2, a prolonged hyper-inflammatory state was seen along with severe lung infection and more mortality [251], [252]. Lower disease severity caused by the Omicron variant and lower pro-inflammatory cytokine levels compared to the Delta variant suggest its lower cytokine storm induction capability. In most cases, infection with the Omicron variant provokes an acute inflammatory response which eventually solves resulting in less production of pro-inflammatory cytokines and presumably high levels of Tregs [253]. As recently reported, the Omicron infection induced significantly lower virus replication in the respiratory tracts and lungs of infected mice and also produced fewer infectious particles in human airway organoid and alveolar cells than in previous variants infections [254], [255], [256]. Moreover, the brain tissue of infected mice with the omicron variant showed a more limited amount of virus replication. Infection with the Delta variant is associated with the induction of markers of severe COVID, such as CXCL10 and CCL2. However, in the infection with the Omicron variant, the level of these markers remarkably diminishes early after infection. It has also been shown that T cells activation/exhaustion markers such as programmed cell death 1 (PD1) and cytotoxic T lymphocyte-associated protein 4 (CTLA4) were significantly fewer on pulmonary T cells of mice infected with the Omicron variant compared to other variants [256]. In conclusion, lower virus replication in the brain and lung tissues with the Omicron variant leads to a less severe disease with controlled production of pro-inflammatory cytokines and decreased amount of activated T cells in the respiratory system.
1.7. Therapeutic approaches
In addition to mechanisms discussed above, an optimal therapeutic strategy targeting inflammatory mechanisms is crucial. So here we discuss the potential treatments for this condition and focus on therapies suggested for cytokine storm in COVID-19. (Fig. 3 ).
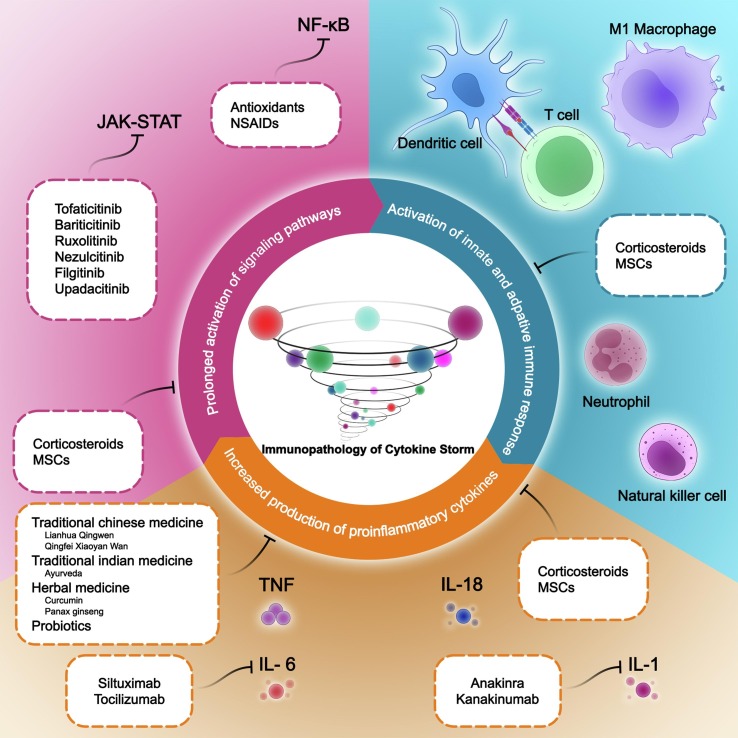
Fig. 3.
Overview of molecular and cellular mechanisms involved in SARS-CoV-2-induced cytokine storm and potential treatments. Three main causes of cytokine storm are: 1) Activation of innate and adaptive immune responses 2) increased production of pro-inflammatory cytokines 3) prolonged activation of signaling pathways. Inhibiting any of these causes can be effective in preventing the cytokine storm.
Anti-interleukin 6
Tocilizumab inhibits IL-6 action through binding to its receptors, which are both circulatory and membranous. Therefore this monoclonal antibody inhibits the downstream signaling pathways via the prevention of IL-6 binding to IL-6R [257]. On August 30, 2017, tocilizumab was approved by FDA (Food and Drug Administration) for the treatment of CAR T cell-induced cytokine release syndrome in patients ≥ two years of age. In the case of CRS in COVID-19, several types of research demonstrated the efficacy of tocilizumab in treating Cytokine storm in COVID-19 [8], [258], [259], [260]. Boregowda et al. compared the mortality rate between receiving tocilizumab and routine care in 3,641 critical COVID-19 cases. The author concluded that tocilizumab significantly reduces mortality compared to standard care [261]. By reviewing all these studies, tocilizumab is thought to be one of the main possible treatments for CRS caused by COVID-19.
Siltuximab is an antibody with binding affinity to IL-6 molecules rather than their receptors, reducing free IL-6 levels [262]. Numerous trials have assessed the effectiveness of siltuximab in CRS management, especially induced by COVID-19. The administration of this drug improved the symptoms and reduced the need for mechanical ventilation [262], [263]. However, the evidence for siltuximab's safety and efficiency in managing COVID-19-induced CRS is unsatisfactory. Therefore, as long as tocilizumab is available, siltuximab should be reserved for substitution.
Corticosteroids
Glucocorticoids are a possible treatment for COVID-19-induced CRS through their immunosuppressive and anti-inflammatory effects [264]. However, the timing and dosage of administration are essential because early administration of high-dose corticosteroids might elevate the load of SARS-CoV-2 in serum and aggravate the disease. Nevertheless, using corticosteroids in the early phases of CRS during COVID-19 reduces ARDS and inflammation [265], [266]. In a controlled open-label trial the mortality rate in the dexamethasone group was lower in patients receiving either invasive mechanical ventilation with critical conditions or non-invasive oxygenation. However, in cases with no respiratory support, dexamethasone did not significantly reduce mortality [267]. Several other studies also reported similar results for glucocorticoids in COVID-19-induced CRS [268], [269]. Nevertheless, some studies evaluated the effect of corticosteroids on CRS induced by SARS-CoV, MERS-CoV, and influenza, which showed their use results in higher mortality rates and more hospitalization periods [266], [270]. Moreover, most of the studies mentioned above suggest that corticosteroids act like a double-edged blade and should be approached with caution.
Mesenchymal stem cells
Mesenchymal stem cells (MCSs) or multi-potent MCSs are stromal cells with a self-renewal capability and the potential to be differentiated into mesodermal tissues under controlled in-vitro conditions and special media [271]. These stem cells produce anti-inflammatory products through their paracrine activity. The secretome of MSCs contains cytokines, chemokines, and other anti-inflammatory products such as Prostaglandin E2, indolamine 2,3-dioxygenase (IDO), TNF, Nitric oxide, IL-1Rα, HLA-G and IL-10 [272]. This paracrine secretion, alongside MSCs' direct cellular contact by their surface molecules, signals immune cells to promote anti-inflammatory cells, such as T-Regs, which causes immune modulation and, therefore, reduces inflammation [273]. Studies have shown that intravascular administration of MSCs will result in lung entrapment of the cells (for approximately 70 % of the total cellular population) [274]. This is considered one of the limitations of the administration of MSCs intravenously. However, it may be our best choice in the case of COVID-19-induced lung injury because stem cells will be present mainly in the lungs. In CRS situations, it could modulate the pulmonary immune system and reduce inflammatory cytokines which hampers CRS. Furthermore, it has been demonstrated that MSCs have anti-thrombotic and anti-platelet aggregation and adhesion capability [275]. Lastly, it has been demonstrated that MSCs are resistant to SARS-CoV-2 due to their diminished expression of membranous ACE2 and TMPRSS2, even in an inflammatory state. They also sustain indoleamine 2,3-dioxygenase production in contact with the virus, which implies that the immunomodulation capability of MSCs is maintained in confrontation with SARS-CoV-2 [276]. Several in vitro studies evaluated the immune-modulatory effect of MSCs on immune cells retrieved from the blood sample of COVID-19 patients. These studies have shown that MSCs diminish a broad spectrum of cytokines released by mononuclear cells, verifying that MSCs can modulate the immune system and dampen COVID-19-induced CRS [277]. There have been many clinical trials that utilized MSCs of different origins to manage COVID-19 and subsequent CRS [278]. Moreover, a pilot trial study of intravenously administered MSCs in china demonstrated the safeness and effectiveness of MSCs in managing COVID-19, especially in critically ill patients [279].
In conclusion, with these characteristics, MCSs might be a rather theoretical but powerful treatment suggestion for dampening CRS and repairing following organ dysfunction, especially in COVID-19. Moreover, not only MSCs are potential therapeutic options for CRS, but also exosomes derived from these stem cells are appropriate choices in this matter [280].
Anti-interkeukin 1
Anakinra is an IL-1α and IL-1β receptor antagonist [281], with FDA approval to treat moderate to severe rheumatoid arthritis. Several studies have assessed the efficacy of anakinra on haemophagocytic lymphohistiocytosis (HLH) or macrophage activation syndrome (MAS), syndromes of hyper-activation of the immune system, and subsequent inflammation and multiple organ damage [282], [283], [284]. The results are promising and imply that anakinra may dampen cytokine storm. Moreover, several clinical trials are assessing the effect of anakinra on COVID-19-induced cytokine storm and critical COVID-19 patients. Some studies evaluated anakinra in COVID-19 critical patients. Results were both in favor of the efficacy of anakinra [285], [286], [287], and against its effectiveness [288]. These shreds of evidence suggest that anakinra can be used effectively in CRS treatment. However, more evaluations should be considered to evaluate the effectiveness and safety of this medication.
Canakinumab is a selective IL-1β antagonist that binds to IL-1β and inhibits the molecule from attaching to its receptor, which blocks further signaling. Although experiments have reported the effectiveness of canakinumab in decreasing the necessity of mechanical ventilation in severe COVID-19 cases [289], [290], in other studies on, canakinumab did not significantly decrease the mortality rate [291]. The number of participants limits the studies in favor of canakinumab efficacy, and more clinical evidence is imperative for decision making.
JAK inhibitors
Tofacitinib is a non-selective JAK inhibitor, and Baricitinib is an anti-JAK1 and JAK2. Ruxolitinib is also an FDA-approved anti-JAK1 and JAK2 for myelofibrosis, RA, and psoriasis [292]. Upadacitinib and filgotinib are selective for JAK1 [293]. It was demonstrated that low-dose ruxolitinib and barticitinib are effective in risk reduction of mortality with no considerable adverse effect [294], [295], [296]. Also, a meta-analysis study concluded that other JAK inhibitors have benefits in the management of COVID-19 patients, including tofacitinib and nezulcitinib [297]. However, JAK inhibitors can also decrease the production of IFN-α, which is crucial for protection against virus infections, including the coronavirus [298]. Overall, the evidence for the efficacy of JAK inhibitors in COVID-19 and subsequent cytokine storm is still unsatisfying, and more complete and promising clinical trials are needed.
NF-κB inhibition
Variable medications contribute to inhibiting this protein, its stimuli, and its downstream pathways. Glucocorticoids, especially dexamethasone, are demonstrated to have an inhibitory effect on NF-κB through various mechanisms, including upregulation of I-κBα [299], [300], [301]. Also, aspirin and sodium salicylate are thought to have an anti-NF-κB activity which occurs through binding to the ATP-binding site of I-κB kinase β (IKKβ), the enzyme that causes degradation of I-κB, by competing with ATP [302], [303], [304]. Other non-steroidal anti-inflammatory drugs (NSAIDs) can also inhibit NF-κB via different mechanisms [302]. Ibuprofen, sulindac, and tepoxalin are NSAIDs that have an inhibitory effect on NF-κB [302], [305], [306]. Although these medications have a diverse anti-inflammatory mechanism of action and not just inhibition of NF-κB, the mechanism through which the clinical effects of these drugs occur cannot be distinguished.
Furthermore, ROS are another stimulus of the NF-κB pathway and a significant sign of inflammation; therefore, anti-oxidants might downregulate this pathway by decreasing oxidative stress [307], [308], [309]. N-acetylcysteine, dithiocarbamates, glutathione peroxidase, and vitamin E are anti-oxidants used in this manner [310], [311], [312], [313]. In conclusion, blocking the NF-Κb signaling pathway is a promising potential therapeutic option that may help decrease inflammation and cytokine storm. However, the clinical evidence on this matter is still inadequate.
Traditional medicine
Since there is still no definite treatment for COVID-19 and particularly COVID-19-induced cytokine storm, researchers were encouraged to assess the effects of natural products and traditional medicines, especially traditional Chinese medicine (TCM) and traditional Indian medicine. Various products of TCM have been investigated for viral infections and COVID-19, like Qingfei Xiaoyan Wan (QFXYW), Lianhua Qingwen, etc. that have immune modulatory anti-viral effects. They proved to have suppressive effects on inflammatory cytokines, especially IL-6 and TNF-α [314], [315]. Moreover, the efficacy of traditional Chinese medicine decoctions was evaluated in critically ill COVID-19 patients, which decreased mortality [316]. Moreover, various natural products derived from different plants might also have anti-inflammatory effects [317]. Curcumin and Panax ginseng are examples of these natural products which have immune-regulatory effects via increasing anti-inflammatory and decreasing pro-inflammatory cytokines [318], [319], [320].
We also have Ayurveda, the traditional Indian medicine that focuses on the maintenance of the healthiness of the body, improving the illnesses, and also enhancing the immune system [321]. The herbs in Ayurveda and Ayurvedic medicine have been proposed to have anti-viral and anti-inflammatory effects, even in the case of COVID-19, and reduce pro-inflammatory cytokines [322], [323]. These herbs also inhibit viral entry and replication and therefore are suggested for COVID-19 prophylaxis [321], [324]. Also, they are implicated to have effects on post-COVID-19 syndrome [325].
These medications and herbs of the traditional medicines have potential immune regulatory and anti-inflammatory effects, therefore, they could be used solely or in combination with other drugs to diminish cytokine storm [229], [326], [327]. Nevertheless, due to a lack of clinical assessments of the safety and efficacy of these herbs, they should be evaluated more accurately and carefully.
Probiotics
As mentioned earlier in the text, gut dysbiosis can induce cytokine storm in COVID-19. Therefore, probiotics which are live bacteria that maintain bacterial flora of the GI system may help regulate the immune system and diminish cytokine storm [328]. Several studies have assessed their efficacy and immune-regulatory effects in COVID-19 patients and also healthy individuals. They elucidated that probiotics can reduce inflammatory cytokines [329], [330]. Although the evidence is still insufficient, probiotics can possibly be used in the treatment approach of COVID-19 patients.
Therapeutic options for post-COVID-19 complications
As mentioned earlier, one major post-COVID-19 complication of the lungs is pulmonary fibrosis and subsequent pulmonary dysfunction. Corticosteroids have been shown to improve patients’ symptoms and increase their pulmonary function [331]. Also, anti-fibrotics have been utilized effectively for pulmonary fibrosis. Moreover, with the suggested role of IFN-β and IFN-γ in pulmonary vasculopathy, the application of the antagonists of these proteins might be effective in treating pulmonary fibrosis [332]. Lastly, lung transplantation is reserved for patients with end-stage conditions [333].
Other complications include venous thromboembolism (VTE) and cardiovascular complications (palpitation, chest discomfort, and myocarditis), which can be treated with anti-coagulants and renin-angiotensin-aldosterone system antagonists and β-blockers, respectively. Additionally, a thromboprophylaxis plan has been proposed to prevent thrombus formation in veins [334], [335]. Neurological complications like headaches and sleeping problems as well as psychiatric conditions (depression) may also occur. These conditions are not often critical and can be managed through routine care of these conditions in situations other than COVID-19 [336].
Side effects
It is encouraging that there are potential treatments for this critical condition in COVID-19; however, we should not neglect their adverse effects because it could change our decision-making regarding the optimum treatment approach. There have been reports of various adverse effects of tocilizumab over the past years. The most frequent ones were infections, immediate and delayed hypersensitivity reactions, liver abnormalities, cardiac disorders, and bleeding [337], [338]. Neutropenia was also commonly seen in treated patients [339], which could increase the risk of secondary infections [340]. It also induced thrombocytopenia in COVID-19 patients treated with tocilizumab [341]. Other adverse effects include interstitial lung disease [342], hepatic disorders [343], and immunogenicity to the drug [344]. Common adverse events of siltuximab are pruritus, hypertension, nausea, vomiting, fatigue, and neutropenia [345], [346]. Hyperlipidemia, cellulitis, and more severe events like urinary retention, polycythemia, leukopenia, lymphopenia, and hypersensitivity reactions were reported with the administration of siltuximab [345], [346], [347]. Increased risk of infections and cancer, hematologic effects, headache, joint pains, anaphylaxis, skin conditions, abdominal pains, and diarrhea are mentioned as adverse effects of anakinra [348], [349]. As for canakinumab, the most common effects are GI complications, including diarrhea, nausea, and gastroenteritis [350]. For some of the JAK inhibitors, studies have reported an increased risk of serious infections, headaches, and possibly increased risk of cancer. However, there is insufficient evidence to say these drugs cause cancer [87]. There is a wide range of adverse effects of corticosteroids, especially dexamethasone, which has been widely used for COVID-19. Hyperglycemia, fatty liver, insulin resistance, increased risk of diabetes, GI complications, headaches, skin acne, and dysregulated menstrual periods, are reported as their common effects [351], [352]. Lastly, it is worth mentioning that despite the efficacy of herbal medicines, they also have adverse effects that clinicians should take into account. These effects may be due to self-prescribed, bad product quality, or excessive intake [353]. It should be kept in mind that the mentioned adverse effects are not reasons for abandoning their use, but the costs and benefits of each treatment in every situation and condition must be pondered.
2. Discussion
Morbidity and mortality of COVID-19 patients are highly related to the occurrence of the “cytokine storm” induced by SARS-CoV-2, which may lead to ARDS, promoting respiratory failure and eventually causing life-threatening multi-organ failure [169]. Different variants of SARS-CoV-2 have distinct potentials to develop cytokine storm. Lower disease severity caused by the Omicron-B.1.1.529 Variant and lower pro-inflammatory cytokine levels compared to Delta variant suggest its lower cytokine storm induction capability [253], [354], [355]. As discussed earlier, the cytokine storm is a hyper-inflammatory state causing multi-organ dysfunction. Therefore, there may be a potential connection between cytokine storm and post COVID-19 complications. Additionally, evidence of organ dysfunction in prolonged COVID-19 is copious, especially cardiac and neuropsychiatric dysfunctions [333], [356], [357], [358]. Vascular dysfunction and hyper-coagulation may also implicate in post-COVID-19 multi-organ failure in response to hyper-inflammation [239]. These evidences support the possibility of cytokine storm connection with post COVID-19 complications.
There are several chronic underlying conditions that make those infected with SARS-CoV-2 more susceptible to developing severe forms of the disease. Type 2 diabetes mellitus, cardiovascular disease, hypertension, chronic kidney disease, and chronic obstructive pulmonary disease are the leading chronic conditions culprit for severe COVID-19 [132]. Furthermore, mounting evidence has been shown the significant role of metabolic syndrome in inducing inflammation leading to COVID-19 disease deterioration and its subsequent comorbidities [359]. Moreover, genetic and environmental factors may be decisive in determining the fate of the COVID-19 disease; for instance, polymorphism in genes related to inflammatory modulators makes individuals more or less vulnerable to developing cytokine storm and experiencing its collateral damages [360]. There is also a relationship between gender and COVID-19 complications. Males tend to develop more severe diseases and have higher mortality rates in comparison to females [361], [362], [363], [364], while females tend to develop prolonged COVID-19 [365]. Also, female patients tend to have lower levels of IL-6 and higher CD4+ T cells with better function [366], while male patients had higher levels of ferritin and CRP [367], [368]. Although a comprehensive review of the pathways underlying sex differences in COVID-19 is beyond the scope of this article, it is worth mentioning that the gender differences in COVID-19 might be due to sex-related hormones, different immune systems, and inflammatory responses, gender-related behaviors, and factors related to sex chromosomes.
SARS-CoV-2 enters human respiratory system cells by targeting the ACE2 receptor in type II alveolar epithelial cells. Inflammation-induced pulmonary capillary endothelium and alveolar epithelium injury are the main consequences of SARS-CoV-2 infection in the lower respiratory system, resulting in air-blood barrier damage and, consequently, entry of the virus into the bloodstream [369]. Since ACE2 is also expressed widely among body tissues such as lungs, kidneys, small intestine, heart, and other organs, cytokine storm caused by the circulating virus can promote multi-organ injury due to the wide distribution of the receptor in the body. Following viral entry through S protein-ACE2 binding, reduced membrane-bound ACE-2 expression and increased Ang-II accumulation occurs, which can lead to detrimental consequences. The imbalance of the RAAS system due to hyper-activation of the pro-inflammatory ACE/Ang-II/AT1R axis promotes inflammatory responses. This imbalance eventually enhances excessive production of pro-inflammatory cytokines such as IL-1β, IL-6, IL-8, TNF-α, and multi-organ damages [369].
Hyper-production of pro-inflammatory cytokines via activation of NF-κB and JAK/STAT pathways and generation of oxidative stress are the leading causes of systemic inflammation and cell damage. There is crosstalk between oxidative stress and cytokine storm. Increased intracellular ROS production mediates activation of inflammatory caspases and cytokines in macrophages [370]. Moreover, inflammatory cytokines induce additional intracellular and extracellular ROS production leading to mitochondrial damage and apoptosis [371]. ROS also causes endothelial dysfunction and diffuse microvascular thrombosis under the inflammatory conditions caused by the cytokine storm [3], [372], [373]. SARS-CoV-2 also develops endothelial dysfunction via upregulation of inflammatory cytokines and upstream pathways that regulate thrombosis-associated markers. This causes recruitment of immune cells, platelet activation and aggregation, and activation of coagulation cascade pathways, causing endothelial injury and thrombosis [374]. Systemic endothelium-associated or thrombotic-associated consequences can lead to multi-organ [3], [373]. Disturbed endothelial junctions from ROS and inflammatory cytokines production promote vascular leakage and transendothelial migration of immune cells [370]. Excessive recruitment of immune cells at the inflammation site is involved in tissue damage caused by the COVID-19 cytokine storm in vital organs [375] and also amplifies coagulation pathways' activation [376]. So, air-blood barrier damage caused by SARS-CoV-2-induced inflammation leads to systemic cytokine storm and consequent endothelial dysfunction, coagulopathy, and oxidative stress, which are leading causes of the multi-organ failure in COVID-19.
3. Conclusion
In addition to the harmful respiratory complications of COVID-19 disease, other organs could be affected by the SARS-CoV-2, resulting in a deadly situation called multi-organ failure. Although still under investigation, this situation could result from aberrant immune responses and the virus's direct effect on the cells expressing the ACE2 receptor. While, role of individual cytokines and immune cells in developing multi-organ failure is yet to be completely understood, it is evident that multi-organ failure is closely related to cytokine storm in COVID-19. Inflammation caused by cytokine storm can disrupt the homeostasis of organs. Thus, increasing our knowledge about the pathophysiology of COVID-19-related cytokine storm and how it can be involved in multi-organ failure may have promising results in finding potential therapeutic targets to control this situation and overcome the disastrous COVID-19 disease.
Author contributions
YN, MG, YY and AN: conceptualization. YN, MG, YY and AN: investigation. YN, MG, YY and AN: writing-original draft preparation. YN, MG, YY and AN: writing-review and editing. SMH: supervision. All authors read and agreed to the submitted version of the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
No data was used for the research described in the article.
References
- 1.Berlin D.A., Gulick R.M., Martinez F.J. Severe Covid-19. N. Engl. J. Med. 2020;383(25):2451–2460. doi: 10.1056/NEJMcp2009575. [DOI] [PubMed] [Google Scholar]
- 2.Lopes-Pacheco M., et al. Pathogenesis of Multiple Organ Injury in COVID-19 and Potential Therapeutic Strategies. Front. Physiol. 2021;12 doi: 10.3389/fphys.2021.593223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mokhtari T., et al. COVID-19 and multiorgan failure: A narrative review on potential mechanisms. J. Mol. Histol. 2020;51(6):613–628. doi: 10.1007/s10735-020-09915-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang L., et al. The signal pathways and treatment of cytokine storm in COVID-19. Signal Transduction and Targeted Therapy. 2021;6(1):255. doi: 10.1038/s41392-021-00679-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zaim S., et al. COVID-19 and Multiorgan Response. Curr. Probl. Cardiol. 2020;45(8) doi: 10.1016/j.cpcardiol.2020.100618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fajgenbaum D.C., June C.H. Cytokine Storm. N. Engl. J. Med. 2020;383(23):2255–2273. doi: 10.1056/NEJMra2026131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dembic Z. In: the Cytokines of the Immune System. Dembic Z., editor. Academic Press; Amsterdam: 2015. Chapter 1 - Introduction—Common Features About Cytokines; pp. 1–16. [Google Scholar]
- 8.Pang R., et al. Evolution of COVID-19 in patients with autoimmune rheumatic diseases. Aging (Albany NY) 2020;12(23):23427–23435. doi: 10.18632/aging.202193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou P., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoffmann M., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280. doi: 10.1016/j.cell.2020.02.052. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beyerstedt S., Casaro E.B., Rangel É.B. COVID-19: angiotensin-converting enzyme 2 (ACE2) expression and tissue susceptibility to SARS-CoV-2 infection. Eur. J. Clin. Microbiol. Infect. Dis.: Official Publ. Eur. Soc. Clin. Microbiol. 2021;40(5):905–919. doi: 10.1007/s10096-020-04138-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Devaux C.A., Rolain J.M., Raoult D. ACE2 receptor polymorphism: Susceptibility to SARS-CoV-2, hypertension, multi-organ failure, and COVID-19 disease outcome. J. Microbiol. Immunol. Infect. 2020;53(3):425–435. doi: 10.1016/j.jmii.2020.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ingraham N.E., et al. Understanding the renin-angiotensin-aldosterone-SARS-CoV axis: a comprehensive review. Eur. Respir. J. 2020;56(1) doi: 10.1183/13993003.00912-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chowdhury M.A., et al. Immune response in COVID-19: A review. J. Infect. Public Health. 2020;13(11):1619–1629. doi: 10.1016/j.jiph.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tian W., et al. Immune suppression in the early stage of COVID-19 disease. Nat. Commun. 2020;11(1):5859. doi: 10.1038/s41467-020-19706-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anaeigoudari A., et al. Severe Acute Respiratory Syndrome Coronavirus 2: The Role of the Main Components of the Innate Immune System. Inflammation. 2021;44(6):2151–2169. doi: 10.1007/s10753-021-01519-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carsetti R., et al. Different Innate and Adaptive Immune Responses to SARS-CoV-2 Infection of Asymptomatic, Mild, and Severe Cases. Front. Immunol. 2020;11(3365) doi: 10.3389/fimmu.2020.610300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perez N., et al. Impaired natural killer activity in lymphohistiocytosis syndrome. J. Pediatr. 1984;104(4):569–573. doi: 10.1016/s0022-3476(84)80549-1. [DOI] [PubMed] [Google Scholar]
- 19.Mogensen T.H. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin. Microbiol. Rev. 2009;22(2):240–273. doi: 10.1128/CMR.00046-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo H., Callaway J.B., Ting J.P. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat. Med. 2015;21(7):677–687. doi: 10.1038/nm.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vora S.M., Lieberman J., Wu H. Inflammasome activation at the crux of severe COVID-19. Nat. Rev. Immunol. 2021;21(11):694–703. doi: 10.1038/s41577-021-00588-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodrigues T.S., et al. Inflammasomes are activated in response to SARS-CoV-2 infection and are associated with COVID-19 severity in patients. J. Exp. Med. 2021;218(3) doi: 10.1084/jem.20201707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pan P., et al. SARS-CoV-2 N protein promotes NLRP3 inflammasome activation to induce hyperinflammation. Nat. Commun. 2021;12(1):1–17. doi: 10.1038/s41467-021-25015-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu H., et al. SARS-CoV-2 viroporin encoded by ORF3a triggers the NLRP3 inflammatory pathway. Virology. 2022 doi: 10.1016/j.virol.2022.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun X., et al. SARS-CoV-2 non-structural protein 6 triggers NLRP3-dependent pyroptosis by targeting ATP6AP1. Cell Death Differ. 2022:1–15. doi: 10.1038/s41418-021-00916-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng M., et al. TLR2 senses the SARS-CoV-2 envelope protein to produce inflammatory cytokines. Nat. Immunol. 2021;22(7):829–838. doi: 10.1038/s41590-021-00937-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.S.J. Theobald, et al., The SARS-CoV-2 spike protein primes inflammasome-mediated interleukin-1-beta secretion in COVID-19 patient-derived macrophages, 2020.
- 28.Theobald S.J., et al. Long-lived macrophage reprogramming drives spike protein-mediated inflammasome activation in COVID-19. EMBO Mol. Med. 2021;13(8):e14150. doi: 10.15252/emmm.202114150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaivola J., Nyman T.A., Matikainen S. Inflammasomes and SARS-CoV-2 Infection. Viruses. 2021;13(12) doi: 10.3390/v13122513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Junqueira C., et al. FcγR-mediated SARS-CoV-2 infection of monocytes activates inflammation. Nature. 2022;606(7914):576–584. doi: 10.1038/s41586-022-04702-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang H., et al. AIM2 Inflammasome Is Critical for Influenza-Induced Lung Injury and Mortality. J. Immunol. 2017;198(11):4383–4393. doi: 10.4049/jimmunol.1600714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hosseini A., et al. Innate and adaptive immune responses against coronavirus. Biomed. & Pharmacotherapy = Biomedecine & pharmacotherapie. 2020;132:110859. doi: 10.1016/j.biopha.2020.110859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schultze J.L., Aschenbrenner A.C. COVID-19 and the human innate immune system. Cell. 2021;184(7):1671–1692. doi: 10.1016/j.cell.2021.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Romagnani S. Th1/Th2 Cells. Inflamm. Bowel Dis. 1999;5(4):285–294. doi: 10.1097/00054725-199911000-00009. [DOI] [PubMed] [Google Scholar]
- 35.Sette A., Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell. 2021;184(4):861–880. doi: 10.1016/j.cell.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sekine T., et al. Robust T Cell Immunity in Convalescent Individuals with Asymptomatic or Mild COVID-19. Cell. 2020;183(1):158–168.e14. doi: 10.1016/j.cell.2020.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grifoni A., et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell. 2020;181(7):1489–1501.e15. doi: 10.1016/j.cell.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Diao B., et al. Reduction and Functional Exhaustion of T Cells in Patients With Coronavirus Disease 2019 (COVID-19) Front. Immunol. 2020;11(827) doi: 10.3389/fimmu.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zheng M., et al. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell. Mol. Immunol. 2020;17(5):533–535. doi: 10.1038/s41423-020-0402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zheng H.-Y., et al. Elevated exhaustion levels and reduced functional diversity of T cells in peripheral blood may predict severe progression in COVID-19 patients. Cell. Mol. Immunol. 2020;17(5):541–543. doi: 10.1038/s41423-020-0401-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu J., et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;55 doi: 10.1016/j.ebiom.2020.102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luo W., et al. Targeting JAK-STAT Signaling to Control Cytokine Release Syndrome in COVID-19. Trends Pharmacol. Sci. 2020;41(8):531–543. doi: 10.1016/j.tips.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lucas C., et al. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature. 2020;584(7821):463–469. doi: 10.1038/s41586-020-2588-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hadjadj J., et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369(6504):718–724. doi: 10.1126/science.abc6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu Z., et al. Clinical value of immune-inflammatory parameters to assess the severity of coronavirus disease 2019. Int. J. Infect. Dis. 2020;95:332–339. doi: 10.1016/j.ijid.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang C., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China. The Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Velazquez-Salinas L., et al. The Role of Interleukin 6 During Viral Infections. Front. Microbiol. 2019;10(1057) doi: 10.3389/fmicb.2019.01057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Narazaki M., Kishimoto T. The Two-Faced Cytokine IL-6 in Host Defense and Diseases. Int. J. Mol. Sci. 2018;19(11) doi: 10.3390/ijms19113528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.G. Magro, SARS-CoV-2 and COVID-19: Is interleukin-6 (IL-6) the ‘culprit lesion’ of ARDS onset? What is there besides Tocilizumab? SGP130Fc. Cytokine: X, 2020. 2(2): p. 100029. [DOI] [PMC free article] [PubMed]
- 50.Coomes E.A., Haghbayan H. Interleukin-6 in COVID-19: a systematic review and meta-analysis. Rev. Med. Virol. 2020;30(6):1–9. doi: 10.1002/rmv.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Han H., et al. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerging Microbes Infect. 2020;9(1):1123–1130. doi: 10.1080/22221751.2020.1770129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aykal G., et al. Could IL-6 predict the clinical severity of COVID-19? Turkish J. Biochem. 2021;46(5):499–507. [Google Scholar]
- 53.Santa Cruz A., et al. Interleukin-6 Is a Biomarker for the Development of Fatal Severe Acute Respiratory Syndrome Coronavirus 2 Pneumonia. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.613422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen X., et al. Detectable Serum Severe Acute Respiratory Syndrome Coronavirus 2 Viral Load (RNAemia) Is Closely Correlated With Drastically Elevated Interleukin 6 Level in Critically Ill Patients With Coronavirus Disease 2019. Clin. Infect. Dis. 2020;71(8):1937–1942. doi: 10.1093/cid/ciaa449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sims J.E., Smith D.E. The IL-1 family: regulators of immunity. Nat. Rev. Immunol. 2010;10(2):89–102. doi: 10.1038/nri2691. [DOI] [PubMed] [Google Scholar]
- 56.Russell B., et al. Associations between immune-suppressive and stimulating drugs and novel COVID-19-a systematic review of current evidence. Ecancermedicalscience. 2020;14:1022. doi: 10.3332/ecancer.2020.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Conti P., et al. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J. Biol. Regul. Homeost. Agents. 2020;34(2):327–331. doi: 10.23812/CONTI-E. [DOI] [PubMed] [Google Scholar]
- 58.Yang S., et al. Differential roles of TNFα-TNFR1 and TNFα-TNFR2 in the differentiation and function of CD4+Foxp3+ induced Treg cells in vitro and in vivo periphery in autoimmune diseases. Cell Death Dis. 2019;10(1):27. doi: 10.1038/s41419-018-1266-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schulte W., Bernhagen J., Bucala R. Cytokines in sepsis: potent immunoregulators and potential therapeutic targets–an updated view. Mediators Inflamm. 2013;2013:165974. doi: 10.1155/2013/165974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Okusawa S., et al. Interleukin 1 induces a shock-like state in rabbits. Synergism with tumor necrosis factor and the effect of cyclooxygenase inhibition. J. Clin. Investig. 1988;81(4):1162–1172. doi: 10.1172/JCI113431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Page M.J., Bester J., Pretorius E. The inflammatory effects of TNF-α and complement component 3 on coagulation. Sci. Rep. 2018;8(1):1812. doi: 10.1038/s41598-018-20220-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hayden M.S., Ghosh S. Regulation of NF-κB by TNF family cytokines. Semin. Immunol. 2014;26(3):253–266. doi: 10.1016/j.smim.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pedersen S.F., Ho Y.C. SARS-CoV-2: a storm is raging. J. Clin. Invest. 2020;130(5):2202–2205. doi: 10.1172/JCI137647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Karki R., et al. Synergism of TNF-α and IFN-γ Triggers Inflammatory Cell Death, Tissue Damage, and Mortality in SARS-CoV-2 Infection and Cytokine Shock Syndromes. Cell. 2021;184(1):149–168.e17. doi: 10.1016/j.cell.2020.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Goker Bagca B., Biray Avci C. The potential of JAK/STAT pathway inhibition by ruxolitinib in the treatment of COVID-19. Cytokine Growth Factor Rev. 2020;54:51–62. doi: 10.1016/j.cytogfr.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kang S., et al. Targeting Interleukin-6 Signaling in Clinic. Immunity. 2019;50(4):1007–1023. doi: 10.1016/j.immuni.2019.03.026. [DOI] [PubMed] [Google Scholar]
- 67.Seif F., et al. JAK Inhibition as a New Treatment Strategy for Patients with COVID-19. Int. Arch. Allergy Immunol. 2020;181(6):467–475. doi: 10.1159/000508247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu T., et al. NF-κB signaling in inflammation. Signal Transduction and Targeted Therapy. 2017;2(1):17023. doi: 10.1038/sigtrans.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.D'Acquisto F., May M.J., Ghosh S. Inhibition of nuclear factor kappa B (NF-B): an emerging theme in anti-inflammatory therapies. Mol. Interv. 2002;2(1):22–35. doi: 10.1124/mi.2.1.22. [DOI] [PubMed] [Google Scholar]
- 70.Hariharan A., et al. The Role and Therapeutic Potential of NF-kappa-B Pathway in Severe COVID-19 Patients. Inflammopharmacology. 2021;29(1):91–100. doi: 10.1007/s10787-020-00773-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen L.-F., Greene W.C. Shaping the nuclear action of NF-κB. Nat. Rev. Mol. Cell Biol. 2004;5(5):392–401. doi: 10.1038/nrm1368. [DOI] [PubMed] [Google Scholar]
- 72.Oh H., Ghosh S. NF-κB: roles and regulation in different CD4(+) T-cell subsets. Immunol. Rev. 2013;252(1):41–51. doi: 10.1111/imr.12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Su C.-M., Wang L., Yoo D. Activation of NF-κB and induction of proinflammatory cytokine expressions mediated by ORF7a protein of SARS-CoV-2. Sci. Rep. 2021;11(1):13464. doi: 10.1038/s41598-021-92941-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schoggins J.W., et al. A diverse range of gene products are effectors of the type I interferon antiviral response. Nature. 2011;472(7344):481–485. doi: 10.1038/nature09907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Raftery N., Stevenson N.J. Advances in anti-viral immune defence: revealing the importance of the IFN JAK/STAT pathway. Cell. Mol. Life Sci. 2017;74(14):2525–2535. doi: 10.1007/s00018-017-2520-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Moreno-Eutimio M.A., López-Macías C., Pastelin-Palacios R. Bioinformatic analysis and identification of single-stranded RNA sequences recognized by TLR7/8 in the SARS-CoV-2, SARS-CoV, and MERS-CoV genomes. Microbes Infect. 2020;22(4–5):226–229. doi: 10.1016/j.micinf.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Imai Y., et al. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell. 2008;133(2):235–249. doi: 10.1016/j.cell.2008.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Manik M., Singh R.K. Role of toll-like receptors in modulation of cytokine storm signaling in SARS-CoV-2-induced COVID-19. J. Med. Virol. 2022;94(3):869–877. doi: 10.1002/jmv.27405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Costa T.J., et al. Mitochondrial DNA and TLR9 activation contribute to SARS-CoV-2-induced endothelial cell damage. Vasc. Pharmacol. 2022;142 doi: 10.1016/j.vph.2021.106946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Onomoto K., Onoguchi K., Yoneyama M. Regulation of RIG-I-like receptor-mediated signaling: interaction between host and viral factors. Cell. Mol. Immunol. 2021;18(3):539–555. doi: 10.1038/s41423-020-00602-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Matsumiya T., Stafforini D.M. Function and regulation of retinoic acid-inducible gene-I. Critical Reviews™ in Immunology. 2010;30(6) doi: 10.1615/critrevimmunol.v30.i6.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Brisse M., Ly H. Comparative Structure and Function Analysis of the RIG-I-Like Receptors: RIG-I and MDA5. Front. Immunol. 2019;10 doi: 10.3389/fimmu.2019.01586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kim N.-E., Song Y.-J. Coordinated regulation of interferon and inflammasome signaling pathways by SARS-CoV-2 proteins. J. Microbiol. 2022:1–8. doi: 10.1007/s12275-022-1502-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rui Y., et al. Unique and complementary suppression of cGAS-STING and RNA sensing-triggered innate immune responses by SARS-CoV-2 proteins. Signal Transduction and Targeted Therapy. 2021;6(1):1–11. doi: 10.1038/s41392-021-00515-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fan Y.M., et al. Crosstalk between RNA viruses and DNA sensors: Role of the cGAS-STING signalling pathway. Rev. Med. Virol. 2022:e2343. doi: 10.1002/rmv.2343. [DOI] [PubMed] [Google Scholar]
- 86.Zhou Z., et al. Sensing of cytoplasmic chromatin by cGAS activates innate immune response in SARS-CoV-2 infection. Signal Transduction Targeted Therapy. 2021;6(1):1–13. doi: 10.1038/s41392-021-00800-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dudek P., et al. Efficacy, safety and future perspectives of JAK inhibitors in the IBD treatment. J. Clin. Med. 2021;10(23):5660. doi: 10.3390/jcm10235660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li H., Zhou F., Zhang L. STING, a critical contributor to SARS-CoV-2 immunopathology. Signal Transduction and Targeted Therapy. 2022;7(1):106. doi: 10.1038/s41392-022-00967-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Domizio J.D., et al. The cGAS–STING pathway drives type I IFN immunopathology in COVID-19. Nature. 2022;603(7899):145–151. doi: 10.1038/s41586-022-04421-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Neufeldt C.J., et al. SARS-CoV-2 infection induces a pro-inflammatory cytokine response through cGAS-STING and NF-κB. Commun. Biol. 2022;5(1):45. doi: 10.1038/s42003-021-02983-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stanifer M.L., Pervolaraki K., Boulant S. Differential Regulation of Type I and Type III Interferon Signaling. Int. J. Mol. Sci. 2019;20(6):1445. doi: 10.3390/ijms20061445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Blanco-Melo D., et al. Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19. Cell. 2020;181(5):1036–1045.e9. doi: 10.1016/j.cell.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Channappanavar R., et al. Dysregulated Type I Interferon and Inflammatory Monocyte-Macrophage Responses Cause Lethal Pneumonia in SARS-CoV-Infected Mice. Cell Host Microbe. 2016;19(2):181–193. doi: 10.1016/j.chom.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Acharya D., Liu G., Gack M.U. Dysregulation of type I interferon responses in COVID-19. Nat. Rev. Immunol. 2020;20(7):397–398. doi: 10.1038/s41577-020-0346-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Major J., et al. Type I and III interferons disrupt lung epithelial repair during recovery from viral infection. Science. 2020;369(6504):712–717. doi: 10.1126/science.abc2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Channappanavar R., Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin. Immunopathol. 2017;39(5):529–539. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Satoh T., et al. Potential Therapeutic Use of the Rosemary Diterpene Carnosic Acid for Alzheimer's Disease, Parkinson's Disease, and Long-COVID through NRF2 Activation to Counteract the NLRP3 Inflammasome. Antioxidants (Basel) 2022;11(1) doi: 10.3390/antiox11010124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lin C.-W., et al. Severe acute respiratory syndrome coronavirus 3C-like protease-induced apoptosis. FEMS Immunol. Med. Microbiol. 2006;46(3):375–380. doi: 10.1111/j.1574-695X.2006.00045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Saleh J., et al. Mitochondria and microbiota dysfunction in COVID-19 pathogenesis. Mitochondrion. 2020;54:1–7. doi: 10.1016/j.mito.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gatti P., et al. Mitochondria Targeted Viral Replication and Survival Strategies-Prospective on SARS-CoV-2. Front. Pharmacol. 2020;11 doi: 10.3389/fphar.2020.578599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ganji R., Reddy P.H. Impact of COVID-19 on Mitochondrial-Based Immunity in Aging and Age-Related Diseases. Front. Aging Neurosci. 2020;12 doi: 10.3389/fnagi.2020.614650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shang C., et al. SARS-CoV-2 Causes Mitochondrial Dysfunction and Mitophagy Impairment. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.780768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Grazioli S., Pugin J. Mitochondrial Damage-Associated Molecular Patterns: From Inflammatory Signaling to Human Diseases. Front. Immunol. 2018;9:832. doi: 10.3389/fimmu.2018.00832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li X., et al. Targeting mitochondrial reactive oxygen species as novel therapy for inflammatory diseases and cancers. J. Hematology & Oncology. 2013;6(1):19. doi: 10.1186/1756-8722-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dikalov S.I., Nazarewicz R.R. Angiotensin II-induced production of mitochondrial reactive oxygen species: potential mechanisms and relevance for cardiovascular disease. Antioxid. Redox Signal. 2013;19(10):1085–1094. doi: 10.1089/ars.2012.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dikalov S. Cross talk between mitochondria and NADPH oxidases. Free Radic. Biol. Med. 2011;51(7):1289–1301. doi: 10.1016/j.freeradbiomed.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Daiber A. Redox signaling (cross-talk) from and to mitochondria involves mitochondrial pores and reactive oxygen species. Biochim. Biophys. Acta, Mol. Cell. Biol. Lipids. 2010;1797(6–7):897–906. doi: 10.1016/j.bbabio.2010.01.032. [DOI] [PubMed] [Google Scholar]
- 108.Wang H., Ma S. The cytokine storm and factors determining the sequence and severity of organ dysfunction in multiple organ dysfunction syndrome. The Am. J. Emergency Med. 2008;26(6):711–715. doi: 10.1016/j.ajem.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 109.Aikawa N. Cytokine storm in the pathogenesis of multiple organ dysfunction syndrome associated with surgical insults. Nihon Geka Gakkai Zasshi. 1996;97(9):771–777. [PubMed] [Google Scholar]
- 110.Tisoncik J.R., et al. Into the Eye of the Cytokine Storm. Microbiol. Mol. Biol. Rev. 2012;76(1):16–32. doi: 10.1128/MMBR.05015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ragab D., et al. The COVID-19 Cytokine Storm; What We Know So Far. Front. Immunol. 2020;11(1446) doi: 10.3389/fimmu.2020.01446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jiang J.X., et al. Plasma cytokines and endotoxin levels in patients with severe injury and their relationship with organ damage. Injury. 1997;28(8):509–513. doi: 10.1016/s0020-1383(97)00057-0. [DOI] [PubMed] [Google Scholar]
- 113.Malmstrøm M.L., et al. Cytokines and Organ Failure in Acute Pancreatitis: Inflammatory Response in Acute Pancreatitis. Pancreas. 2012;41(2):271–277. doi: 10.1097/MPA.0b013e3182240552. [DOI] [PubMed] [Google Scholar]
- 114.Pinsky M.R., et al. Serum Cytokine Levels in Human Septic Shock: Relation to Multiple-System Organ Failure and Mortality. Chest. 1993;103(2):565–575. doi: 10.1378/chest.103.2.565. [DOI] [PubMed] [Google Scholar]
- 115.Frink M., et al. IL-6 predicts organ dysfunction and mortality in patients with multiple injuries. Scand. J. Trauma, Resuscitation and Emergency Med. 2009;17(1):1–7. doi: 10.1186/1757-7241-17-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Reinhart K., et al. Markers of endothelial damage in organ dysfunction and sepsis. Crit. Care Med. 2002;30(5):S302–S312. doi: 10.1097/00003246-200205001-00021. [DOI] [PubMed] [Google Scholar]
- 117.Pugin J., et al. Proinflammatory activity in bronchoalveolar lavage fluids from patients with ARDS, a prominent role for interleukin-1. Am. J. Respir. Crit. Care Med. 1996;153(6 Pt 1):1850–1856. doi: 10.1164/ajrccm.153.6.8665045. [DOI] [PubMed] [Google Scholar]
- 118.Ware L.B., Matthay M.A. The Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2000;342(18):1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 119.Fan J., Ye R.D., Malik A.B. Transcriptional mechanisms of acute lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001;281(5):L1037–L1050. doi: 10.1152/ajplung.2001.281.5.L1037. [DOI] [PubMed] [Google Scholar]
- 120.Yuki K., Fujiogi M., Koutsogiannaki S. COVID-19 pathophysiology: A review. Clin. Immunol. 2020;215 doi: 10.1016/j.clim.2020.108427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Brosnahan S.B., et al. COVID-19 and respiratory system disorders: current knowledge, future clinical and translational research questions. Arterioscler. Thromb. Vasc. Biol. 2020;40(11):2586–2597. doi: 10.1161/ATVBAHA.120.314515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.P.G. Gibson, L. Qin, S.H. Puah, COVID‐19 acute respiratory distress syndrome (ARDS): clinical features and differences from typical pre‐COVID‐19 ARDS, Med. J. Australia, 213(2) (2020) 54-56. e1. [DOI] [PMC free article] [PubMed]
- 123.Costa E., Schettino I., Schettino G. The lung in sepsis: guilty or innocent? Endocrine, Metabolic & Immune Disorders-Drug Targets (Formerly Current Drug Targets-Immune, Endocrine & Metabolic Disorders) 2006;6(2):213–216. doi: 10.2174/187153006777442413. [DOI] [PubMed] [Google Scholar]
- 124.Myers C.L., et al. Induction of ICAM-1 by TNF-alpha, IL-1 beta, and LPS in human endothelial cells after downregulation of PKC. Am. J. Physiol.-Cell Physiol. 1992;263(4):C767–C772. doi: 10.1152/ajpcell.1992.263.4.C767. [DOI] [PubMed] [Google Scholar]
- 125.Thornhill M., et al. Tumor necrosis factor combines with IL-4 or IFN-gamma to selectively enhance endothelial cell adhesiveness for T cells. The contribution of vascular cell adhesion molecule-1-dependent and-independent binding mechanisms. J. Immunol. 1991;146(2):592–598. [PubMed] [Google Scholar]
- 126.Bratt J., Palmblad J. Cytokine-induced neutrophil-mediated injury of human endothelial cells. J. Immunol. 1997;159(2):912–918. [PubMed] [Google Scholar]
- 127.Haimovitz-Friedman A., et al. Lipopolysaccharide induces disseminated endothelial apoptosis requiring ceramide generation. J. Exp. Med. 1997;186(11):1831–1841. doi: 10.1084/jem.186.11.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.C. Pelaia, et al., Lung under attack by COVID-19-induced cytokine storm: pathogenic mechanisms and therapeutic implications. Therapeutic advances in respiratory disease, 2020. 14: p. 1753466620933508. [DOI] [PMC free article] [PubMed]
- 129.C. Wang, et al., Aveolar macrophage activation and cytokine storm in the pathogenesis of severe COVID-19, 2020. [DOI] [PMC free article] [PubMed]
- 130.Montgomery A.B., et al. Causes of mortality in patients with the adult respiratory distress syndrome. Am. Rev. Respiratory Dis. 1985;132(3):485–489. doi: 10.1164/arrd.1985.132.3.485. [DOI] [PubMed] [Google Scholar]
- 131.Zhao Y.H., et al. Cardiovascular complications of SARS-CoV-2 infection (COVID-19): a systematic review and meta-analysis. Rev. Cardiovasc. Med. 2021;22(1):159–165. doi: 10.31083/j.rcm.2021.01.238. [DOI] [PubMed] [Google Scholar]
- 132.Chen T., et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368 doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Melillo F., et al. Myocardial injury in patients with SARS-CoV-2 pneumonia: Pivotal role of inflammation in COVID-19. Eur. J. Clin. Invest. 2021:e13703. doi: 10.1111/eci.13703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tassell B.W.V., et al. Targeting Interleukin-1 in Heart Disease. Circulation. 2013;128(17):1910–1923. doi: 10.1161/CIRCULATIONAHA.113.003199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kacimi R., Long C.S., Karliner J.S. Chronic Hypoxia Modulates the Interleukin-1β–Stimulated Inducible Nitric Oxide Synthase Pathway in Cardiac Myocytes. Circulation. 1997;96(6):1937–1943. doi: 10.1161/01.cir.96.6.1937. [DOI] [PubMed] [Google Scholar]
- 136.Gurantz D., et al. IL-1β and TNF-α upregulate angiotensin II type 1 (AT1) receptors on cardiac fibroblasts and are associated with increased AT1 density in the post-MI heart. J. Mol. Cell. Cardiol. 2005;38(3):505–515. doi: 10.1016/j.yjmcc.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 137.Shirazi L.F., et al. Role of Inflammation in Heart Failure. Curr. Atherosclerosis Rep. 2017;19(6):27. doi: 10.1007/s11883-017-0660-3. [DOI] [PubMed] [Google Scholar]
- 138.Deng M.C., et al. Interleukin-6 correlates with hemodynamic impairment during dobutamine administration in chronic heart failure. Int. J. Cardiol. 1996;57(2):129–134. doi: 10.1016/s0167-5273(96)02805-7. [DOI] [PubMed] [Google Scholar]
- 139.Gwechenberger M., et al. Interleukin-6 and B-type natriuretic peptide are independent predictors for worsening of heart failure in patients with progressive congestive heart failure. J. Heart Lung Transplantation. 2004;23(7):839–844. doi: 10.1016/j.healun.2003.07.023. [DOI] [PubMed] [Google Scholar]
- 140.Marshall J.C. Inflammation, coagulopathy, and the pathogenesis of multiple organ dysfunction syndrome. Crit. Care Med. 2001;29(7):S99–S106. doi: 10.1097/00003246-200107001-00032. [DOI] [PubMed] [Google Scholar]
- 141.Xiong S., et al. IL-1β suppression of VE-cadherin transcription underlies sepsis-induced inflammatory lung injury. J. Clin. Investig. 2020;130(7):3684–3698. doi: 10.1172/JCI136908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chen A.-T., et al. Coagulation Disorders and Thrombosis in COVID-19 Patients and a Possible Mechanism Involving Endothelial Cells: A Review. Aging and Disease. 2022;13(1):144. doi: 10.14336/AD.2021.0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhou X., et al. Incidence and impact of disseminated intravascular coagulation in COVID-19 a systematic review and meta-analysis. Thromb. Res. 2021;201:23–29. doi: 10.1016/j.thromres.2021.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Seitz R., Schramm W. DIC in COVID-19: Implications for Prognosis and Treatment? J. Thromb. Haemost. 2020 doi: 10.1111/jth.14878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Silver S.A., et al. The Prevalence of Acute Kidney Injury in Patients Hospitalized With COVID-19 Infection: A Systematic Review and Meta-analysis. Kidney Med. 2021;3(1):83–98.e1. doi: 10.1016/j.xkme.2020.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chan L., et al. AKI in hospitalized patients with COVID-19. J. Am. Soc. Nephrol. 2021;32(1):151–160. doi: 10.1681/ASN.2020050615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Oliveira C.B., et al. High burden of acute kidney injury in COVID-19 pandemic: systematic review and meta-analysis. J. Clin. Pathol. 2021;74(12):796–803. doi: 10.1136/jclinpath-2020-207023. [DOI] [PubMed] [Google Scholar]
- 148.Z. Li, et al., Kidney Dysfunctions of COVID-19 Patients: A Multi-Centered, Retrospective, Observational Study, SSRN Electron. J., 2020.
- 149.Hirsch J.S., et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98(1):209–218. doi: 10.1016/j.kint.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wang J., et al. Specific cytokines in the inflammatory cytokine storm of patients with COVID-19-associated acute respiratory distress syndrome and extrapulmonary multiple-organ dysfunction. Virology J. 2021;18(1):117. doi: 10.1186/s12985-021-01588-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Joseph A., et al. Acute kidney injury in patients with SARS-CoV-2 infection. Ann. Intensive Care. 2020;10(1):117. doi: 10.1186/s13613-020-00734-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Xia P., et al. Clinicopathological Features and Outcomes of Acute Kidney Injury in Critically Ill COVID-19 with Prolonged Disease Course: A Retrospective Cohort. J. Am. Soc. Nephrol. 2020;31(9):2205–2221. doi: 10.1681/ASN.2020040426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wang X., et al. Be aware of acute kidney injury in critically ill children with COVID-19. Pediatr. Nephrol. 2021;36(1):163–169. doi: 10.1007/s00467-020-04715-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gradin A., et al. Urinary cytokines correlate with acute kidney injury in critically ill COVID-19 patients. Cytokine. 2021;146 doi: 10.1016/j.cyto.2021.155589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hardenberg J.-H.-B., et al. Critical Illness and Systemic Inflammation Are Key Risk Factors of Severe Acute Kidney Injury in Patients With COVID-19. Kidney Int. Rep. 2021;6(4):905–915. doi: 10.1016/j.ekir.2021.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Molina Barragan A.M., et al. SARS-CoV-2 Renal Impairment in Critical Care: An Observational Study of 42 Cases (Kidney COVID) J. Clin. Med. 2021;10(8) doi: 10.3390/jcm10081571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wang J., et al. Identify the Risk Factors of COVID-19-Related Acute Kidney Injury: A Single-Center, Retrospective Cohort Study. Front. Med. 2020:7. doi: 10.3389/fmed.2020.00436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Legrand M., et al. Pathophysiology of COVID-19-associated acute kidney injury. Nat. Rev. Nephrol. 2021;17(11):751–764. doi: 10.1038/s41581-021-00452-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Husain-Syed F., Slutsky A.S., Ronco C. Lung-Kidney Cross-Talk in the Critically Ill Patient. Am. J. Respir. Crit. Care Med. 2016;194(4):402–414. doi: 10.1164/rccm.201602-0420CP. [DOI] [PubMed] [Google Scholar]
- 160.Delvaeye M., Conway E.M. Coagulation and innate immune responses: can we view them separately? Blood. 2009;114(12):2367–2374. doi: 10.1182/blood-2009-05-199208. [DOI] [PubMed] [Google Scholar]
- 161.Ince C. The central role of renal microcirculatory dysfunction in the pathogenesis of acute kidney injury. Nephron Clin. Practice. 2014;127(1–4):124–128. doi: 10.1159/000363203. [DOI] [PubMed] [Google Scholar]
- 162.Escher R., Breakey N., Lämmle B. Severe COVID-19 infection associated with endothelial activation. Thromb. Res. 2020;190:62. doi: 10.1016/j.thromres.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Leisman D.E., Deutschman C.S., Legrand M. Facing COVID-19 in the ICU: vascular dysfunction, thrombosis, and dysregulated inflammation. Intensive Care Med. 2020;46(6):1105–1108. doi: 10.1007/s00134-020-06059-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Singbartl K., Formeck C.L., Kellum J.A. Kidney-Immune System Crosstalk in AKI. Semin. Nephrol. 2019;39(1):96–106. doi: 10.1016/j.semnephrol.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 165.Meng X.M., Nikolic-Paterson D.J., Lan H.Y. Inflammatory processes in renal fibrosis. Nat. Rev. Nephrol. 2014;10(9):493–503. doi: 10.1038/nrneph.2014.114. [DOI] [PubMed] [Google Scholar]
- 166.Pober J.S., Sessa W.C. Inflammation and the blood microvascular system. Cold Spring Harb. Perspect. Biol. 2014;7(1) doi: 10.1101/cshperspect.a016345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Merad M., Martin J.C. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat. Rev. Immunol. 2020;20(6):355–362. doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zhang S., et al. SARS-CoV-2 binds platelet ACE2 to enhance thrombosis in COVID-19. J. Hematol. Oncology. 2020;13(1):1–22. doi: 10.1186/s13045-020-00954-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Yang X., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. The Lancet Respiratory Medicine. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Scorzolini L., et al. Comment on the potential risks of sexual and vertical transmission of COVID-19. Clin. Infect. Dis. 2020;71(16):2298. doi: 10.1093/cid/ciaa445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Qiu L., et al. SARS-CoV-2 is not detectable in the vaginal fluid of women with severe COVID-19 infection. Clin. Infect. Dis. 2020;71(15):813–817. doi: 10.1093/cid/ciaa375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Cui P., et al. Severe acute respiratory syndrome coronavirus 2 detection in the female lower genital tract. Am. J. Obstet. Gynecol. 2020;223(1):131–134. doi: 10.1016/j.ajog.2020.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Song C., et al. Absence of 2019 novel coronavirus in semen and testes of COVID-19 patients. Biol. Reprod. 2020;103(1):4–6. doi: 10.1093/biolre/ioaa050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Pan F., et al. No evidence of severe acute respiratory syndrome–coronavirus 2 in semen of males recovering from coronavirus disease 2019. Fertil. Steril. 2020;113(6):1135–1139. doi: 10.1016/j.fertnstert.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ma X., et al. Pathological and molecular examinations of postmortem testis biopsies reveal SARS-CoV-2 infection in the testis and spermatogenesis damage in COVID-19 patients. Cell. Mol. Immunol. 2021;18(2):487–489. doi: 10.1038/s41423-020-00604-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Morselli S., et al. Male reproductive system inflammation after healing from coronavirus disease 2019. Andrology. 2021 doi: 10.1111/andr.13138. [DOI] [PubMed] [Google Scholar]
- 177.Erbay G., et al. Short-term effects of COVID-19 on semen parameters: a multicenter study of 69 cases. Andrology. 2021;9(4):1060–1065. doi: 10.1111/andr.13019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Pazir Y., et al. Impaired semen parameters in patients with confirmed SARS-CoV-2 infection: A prospective cohort study. Andrologia. 2021;53(9):e14157. doi: 10.1111/and.14157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Dutta S., Majzoub A., Agarwal A. Oxidative stress and sperm function: A systematic review on evaluation and management. Arab J. Urology. 2019;17(2):87–97. doi: 10.1080/2090598X.2019.1599624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Rajak P., et al. Understanding the cross-talk between mediators of infertility and COVID-19. Reprod. Biol. 2021;21(4) doi: 10.1016/j.repbio.2021.100559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.M. Lamamri, et al., Priapism in a patient with coronavirus disease 2019 (COVID-19), Am. J. Emergency Med., 2021. 39: p. 251. e5-251. e7. [DOI] [PMC free article] [PubMed]
- 182.Kresch E., et al. COVID-19 endothelial dysfunction can cause erectile dysfunction: histopathological, immunohistochemical, and ultrastructural study of the human penis. The World J. Men's Health. 2021;39(3):466. doi: 10.5534/wjmh.210055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Alsaedi S.M., et al. Ischemic Priapism Progressing to Penile Gangrene in a Patient with COVID-19 Infection: A Case Report with Literature Review. Case Rep. Med. 2022:2022. doi: 10.1155/2022/8408216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Fang D., et al. An online questionnaire survey on the sexual life and sexual function of chinese adult men during the coronavirus disease 2019 epidemic. Sexual Med. 2021;9(1) doi: 10.1016/j.esxm.2020.100293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Sansone A., et al. “Mask up to keep it up”: Preliminary evidence of the association between erectile dysfunction and COVID-19. Andrology. 2021;9(4):1053–1059. doi: 10.1111/andr.13003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Tian Y., Zhou L.-Q. Evaluating the impact of COVID-19 on male reproduction. Reproduction. 2021;161(2):R37–R44. doi: 10.1530/REP-20-0523. [DOI] [PubMed] [Google Scholar]
- 187.Veyseh M., et al. Left gonadal vein thrombosis in a patient with COVID-19-associated coagulopathy. BMJ Case Reports CP. 2020;13(9):e236786. doi: 10.1136/bcr-2020-236786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Badrawi N., Abdulghaffar S. Ovarian vein thrombosis as a first manifestation of COVID-19 infection. Radiol. Case Rep. 2021;16(11):3491–3493. doi: 10.1016/j.radcr.2021.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Puca E., Puca E. Premature Ovarian Failure Related to SARS-CoV-2 Infection. J. Med. Cases. 2022;13(4):155–158. doi: 10.14740/jmc3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Fatimazahra M., et al. Ovarian vein thrombosis after coronavirus disease (COVID-19) mimicking acute abdomen: two case reports. J. Thromb. Thrombolysis. 2021;52(2):493–496. doi: 10.1007/s11239-021-02433-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Glanzer R., et al. Diagnostic laparoscopy and oophorectomy for ovarian vein thrombosis in a patient with COVID-19: a surgical case report and literature review. J. Surgical Case Rep. 2021;2021(9):p. rjab389. doi: 10.1093/jscr/rjab389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Madaan S., et al. Premature ovarian failure -A long COVID sequelae. Med. Sci. 2021;25:1286–1290. [Google Scholar]
- 193.Mohammadi S., et al. Ovarian vein thrombosis after coronavirus disease (COVID-19) infection in a pregnant woman: case report. J. Thromb. Thrombolysis. 2020;50(3):604–607. doi: 10.1007/s11239-020-02177-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Xiao F., et al. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158(6):1831–1833. doi: 10.1053/j.gastro.2020.02.055. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Deshmukh V., et al. Histopathological observations in COVID-19: a systematic review. J. Clin. Pathol. 2021;74(2):76–83. doi: 10.1136/jclinpath-2020-206995. [DOI] [PubMed] [Google Scholar]
- 196.Lamers M.M., et al. SARS-CoV-2 productively infects human gut enterocytes. Science. 2020;369(6499):50–54. doi: 10.1126/science.abc1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Ferreira C., Viana S.D., Reis F. Gut microbiota dysbiosis–immune hyperresponse–inflammation triad in coronavirus disease 2019 (COVID-19): impact of pharmacological and nutraceutical approaches. Microorganisms. 2020;8(10):1514. doi: 10.3390/microorganisms8101514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Vignesh R., et al. Could perturbation of gut microbiota possibly exacerbate the severity of COVID-19 via cytokine storm? Front. Immunol. 2021;11 doi: 10.3389/fimmu.2020.607734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.He Y., et al. Gut–lung axis: the microbial contributions and clinical implications. Crit. Rev. Microbiol. 2017;43(1):81–95. doi: 10.1080/1040841X.2016.1176988. [DOI] [PubMed] [Google Scholar]
- 200.Gonçalves P., Araújo J.R., Di Santo J.P. A cross-talk between microbiota-derived short-chain fatty acids and the host mucosal immune system regulates intestinal homeostasis and inflammatory bowel disease. Inflamm. Bowel Dis. 2018;24(3):558–572. doi: 10.1093/ibd/izx029. [DOI] [PubMed] [Google Scholar]
- 201.Zuo T., et al. Alterations in gut microbiota of patients with COVID-19 during time of hospitalization. Gastroenterology. 2020;159(3):944–955. doi: 10.1053/j.gastro.2020.05.048. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Keshavarz P., et al. Ischemic gastrointestinal complications of COVID-19: a systematic review on imaging presentation. Clin. Imaging. 2021;73:86–95. doi: 10.1016/j.clinimag.2020.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Norsa L., et al. Poor outcome of intestinal ischemic manifestations of COVID-19. Gastroenterology. 2020;159(4):1595–1597. doi: 10.1053/j.gastro.2020.06.041. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Cui M., et al. Vascular thrombosis and vasculitis in the gastrointestinal tract are associated with poor prognosis in patients with COVID-19. Int. J. Clin. Exp. Pathol. 2021;14(11):1069–1079. [PMC free article] [PubMed] [Google Scholar]
- 205.Wong S.H., Lui R.N., Sung J.J. Covid-19 and the digestive system. J. Gastroenterol. Hepatol. 2020;35(5):744–748. doi: 10.1111/jgh.15047. [DOI] [PubMed] [Google Scholar]
- 206.Yantiss R.K., et al. Intestinal abnormalities in patients with SARS-CoV-2 infection: histopathologic changes reflect mechanisms of disease. Am. J. Surgical Pathol. 2022;46(1):89–96. doi: 10.1097/PAS.0000000000001755. [DOI] [PubMed] [Google Scholar]
- 207.Cho M., et al. Clinical and intestinal histopathological findings in SARS-CoV-2/COVID-19 patients with hematochezia. Case Rep. Gastroenterology. 2021;15(1):408–417. doi: 10.1159/000513375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Chen R., et al. Cytokine storm: the primary determinant for the pathophysiological evolution of COVID-19 deterioration. Front. Immunol. 2021;12:1409. doi: 10.3389/fimmu.2021.589095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Garg S., et al. Unraveling the mystery of Covid-19 cytokine storm: From skin to organ systems. Dermatol. Ther. 2020;33(6):e13859. doi: 10.1111/dth.13859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.de Sá T.C., Soares C., Rocha M. Acute pancreatitis and COVID-19: A literature review. World J. Gastrointestinal Surgery. 2021;13(6):574. doi: 10.4240/wjgs.v13.i6.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Pennisi M., et al. SARS-CoV-2 and the Nervous System: From Clinical Features to Molecular Mechanisms. Int. J. Mol. Sci. 2020;21(15) doi: 10.3390/ijms21155475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Bulfamante G., et al. First ultrastructural autoptic findings of SARS -Cov-2 in olfactory pathways and brainstem. Minerva Anestesiol. 2020;86(6):678–679. doi: 10.23736/S0375-9393.20.14772-2. [DOI] [PubMed] [Google Scholar]
- 213.Reza-Zaldívar E.E., et al. Infection Mechanism of SARS-COV-2 and Its Implication on the Nervous System. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.621735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Paniz-Mondolfi A., et al. Central nervous system involvement by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) J. Med. Virol. 2020;92(7):699–702. doi: 10.1002/jmv.25915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Reynolds J.L., Mahajan S.D. SARS-COV2 Alters Blood Brain Barrier Integrity Contributing to Neuro-Inflammation. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology. 2021;16(1):4–6. doi: 10.1007/s11481-020-09975-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Zhang L., et al. SARS-CoV-2 crosses the blood–brain barrier accompanied with basement membrane disruption without tight junctions alteration. Signal Transduction and Targeted Therapy. 2021;6(1):337. doi: 10.1038/s41392-021-00719-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Buzhdygan T.P., et al. The SARS-CoV-2 spike protein alters barrier function in 2D static and 3D microfluidic in-vitro models of the human blood-brain barrier. Neurobiol. Dis. 2020;146 doi: 10.1016/j.nbd.2020.105131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.DeOre B.J., et al. SARS-CoV-2 Spike Protein Disrupts Blood-Brain Barrier Integrity via RhoA Activation. J. Neuroimmune Pharmacol. 2021;16(4):722–728. doi: 10.1007/s11481-021-10029-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Takata F., et al. Blood-Brain Barrier Dysfunction Amplifies the Development of Neuroinflammation: Understanding of Cellular Events in Brain Microvascular Endothelial Cells for Prevention and Treatment of BBB Dysfunction. Front. Cell. Neurosci. 2021;15 doi: 10.3389/fncel.2021.661838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Amruta N., et al. SARS-CoV-2 mediated neuroinflammation and the impact of COVID-19 in neurological disorders. Cytokine Growth Factor Rev. 2021;58:1–15. doi: 10.1016/j.cytogfr.2021.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Koçer A., et al. Interleukin-6 levels in tension headache patients. The Clinical journal of pain. 2010;26(8):690–693. doi: 10.1097/AJP.0b013e3181e8d9b6. [DOI] [PubMed] [Google Scholar]
- 222.Domingues R.B., et al. Increased serum levels of interleukin-8 in patients with tension-type headache. Cephalalgia. 2015;35(9):801–806. doi: 10.1177/0333102414559734. [DOI] [PubMed] [Google Scholar]
- 223.Bø S., et al. Cerebrospinal fluid cytokine levels in migraine, tension-type headache and cervicogenic headache. Cephalalgia. 2009;29(3):365–372. doi: 10.1111/j.1468-2982.2008.01727.x. [DOI] [PubMed] [Google Scholar]
- 224.van Kessel S.A., et al. Post-acute and long-COVID-19 symptoms in patients with mild diseases: a systematic review. Fam. Pract. 2022;39(1):159–167. doi: 10.1093/fampra/cmab076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Maltezou H.C., Pavli A., Tsakris A. Post-COVID syndrome: an insight on its pathogenesis. Vaccines. 2021;9(5):497. doi: 10.3390/vaccines9050497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Pierce J.D., et al. Post-COVID-19 Syndrome. Nurs. Res. 2022;71(2):164–174. doi: 10.1097/NNR.0000000000000565. [DOI] [PubMed] [Google Scholar]
- 227.Ong S.W.X., et al. Persistent Symptoms and Association With Inflammatory Cytokine Signatures in Recovered Coronavirus Disease 2019 Patients. Open Forum. Infectious Diseases. 2021;8(6) doi: 10.1093/ofid/ofab156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Durstenfeld M.S., et al. medRxiv; 2022. Inflammation during early post-acute COVID-19 is associated with reduced exercise capacity and Long COVID symptoms after 1 year. [Google Scholar]
- 229.Abou Baker D.H. Can natural products modulate cytokine storm in SARS-CoV2 patients? Biotechnol. Rep, 2022:e00749. doi: 10.1016/j.btre.2022.e00749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Castanares-Zapatero D., et al. Pathophysiology and mechanism of long COVID: a comprehensive review. Ann. Med. 2022;54(1):1473–1487. doi: 10.1080/07853890.2022.2076901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Mehandru S., Merad M. Pathological sequelae of long-haul COVID. Nat. Immunol. 2022;23(2):194–202. doi: 10.1038/s41590-021-01104-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Dennis A., et al. Multiorgan impairment in low-risk individuals with post-COVID-19 syndrome: a prospective, community-based study. BMJ open. 2021;11(3):e048391. doi: 10.1136/bmjopen-2020-048391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Alpert O., et al. Cytokine storm induced new onset depression in patients with COVID-19. A new look into the association between depression and cytokines-two case reports. Brain, Behavior, & Immunity-Health. 2020;9 doi: 10.1016/j.bbih.2020.100173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Tale S., et al. Post-COVID-19 pneumonia pulmonary fibrosis. QJM: An Int. J. Med. 2020;113(11):837–838. doi: 10.1093/qjmed/hcaa255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Sun B., et al. Characterization and Biomarker Analyses of Post-COVID-19 Complications and Neurological Manifestations. Cells. 2021;10(2):386. doi: 10.3390/cells10020386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Ortelli P., et al. Neuropsychological and neurophysiological correlates of fatigue in post-acute patients with neurological manifestations of COVID-19: Insights into a challenging symptom. J. Neurol. Sci. 2021;420 doi: 10.1016/j.jns.2020.117271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Waltuch T., et al. Features of COVID-19 post-infectious cytokine release syndrome in children presenting to the emergency department. Am. J. Emergency Med. 2020;38(10):2246.e3–2246.e6. doi: 10.1016/j.ajem.2020.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.McMurray J.C., et al. Multisystem Inflammatory Syndrome in Children (MIS-C), a Post-viral Myocarditis and Systemic Vasculitis-A Critical Review of Its Pathogenesis and Treatment. Front. Pediatr. 2020;8 doi: 10.3389/fped.2020.626182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Kumar M.A., Krishnaswamy M., Arul J.N. Post COVID-19 sequelae: venous thromboembolism complicated by lower GI bleed. BMJ Case Reports. 2021;14(1):e241059. doi: 10.1136/bcr-2020-241059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Ivanovic J., et al. Post-acute COVID-19 syndrome presented as a cerebral and systemic vasculitis: a case report. Acta Neurol. Belg. 2022;122(5):1377–1379. doi: 10.1007/s13760-022-01923-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Raman B., et al. Long COVID: post-acute sequelae of COVID-19 with a cardiovascular focus. Eur. Heart J. 2022;43(11):1157–1172. doi: 10.1093/eurheartj/ehac031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Aeschlimann F.A., et al. Myocardial involvement in children with post-COVID multisystem inflammatory syndrome: a cardiovascular magnetic resonance based multicenter international study-the CARDOVID registry. J. Cardiovasc. Magn. Reson. 2021;23(1):140. doi: 10.1186/s12968-021-00841-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Lionte C., et al. Inflammatory and Cardiac Biomarkers in Relation with Post-Acute COVID-19 and Mortality: What We Know after Successive Pandemic Waves. Diagnostics (Basel) 2022;12(6) doi: 10.3390/diagnostics12061373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Tobler D.L., et al. Long-Term Cardiovascular Effects of COVID-19: Emerging Data Relevant to the Cardiovascular Clinician. Curr. Atheroscler. Rep. 2022;24(7):563–570. doi: 10.1007/s11883-022-01032-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Puntmann V.O., et al. Outcomes of Cardiovascular Magnetic Resonance Imaging in Patients Recently Recovered From Coronavirus Disease 2019 (COVID-19) JAMA Cardiol. 2020;5(11):1265–1273. doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Zhu Y., Chen X., Liu X. NETosis and Neutrophil Extracellular Traps in COVID-19: Immunothrombosis and Beyond. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.838011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Giannos P., Prokopidis K. Gut dysbiosis and long COVID-19: Feeling gutted. J. Med. Virol. 2022 doi: 10.1002/jmv.27684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Liu Q., et al. Gut microbiota dynamics in a prospective cohort of patients with post-acute COVID-19 syndrome. Gut. 2022;71(3):544–552. doi: 10.1136/gutjnl-2021-325989. [DOI] [PubMed] [Google Scholar]
- 249.McAleer J.P., Kolls J.K. Contributions of the intestinal microbiome in lung immunity. Eur. J. Immunol. 2018;48(1):39–49. doi: 10.1002/eji.201646721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Kogut M.H., Lee A., Santin E. Microbiome and pathogen interaction with the immune system. Poult. Sci. 2020;99(4):1906–1913. doi: 10.1016/j.psj.2019.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Halfmann P.J., et al. SARS-CoV-2 Omicron virus causes attenuated disease in mice and hamsters. Nature. 2022;603(7902):687–692. doi: 10.1038/s41586-022-04441-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Alefishat E., et al. Immune response to SARS-CoV-2 variants: A focus on severity, susceptibility, and preexisting immunity. J. Infect. Public Health. 2022;15(2):277–288. doi: 10.1016/j.jiph.2022.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Elkoshi Z. SARS-CoV-2 Omicron (B.1.1.529) Variant: Corticosteroids Treatment/Respiratory Coinfection. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.856072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Shuai H., et al. Attenuated replication and pathogenicity of SARS-CoV-2 B.1.1.529 Omicron. Nature. 2022;603(7902):693–699. doi: 10.1038/s41586-022-04442-5. [DOI] [PubMed] [Google Scholar]
- 255.Meng B., et al. Altered TMPRSS2 usage by SARS-CoV-2 Omicron impacts infectivity and fusogenicity. Nature. 2022;603(7902):706–714. doi: 10.1038/s41586-022-04474-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Suryawanshi R.K., et al. Limited cross-variant immunity from SARS-CoV-2 Omicron without vaccination. Nature. 2022;607(7918):351–355. doi: 10.1038/s41586-022-04865-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Tanaka T., Narazaki M., Kishimoto T. Immunotherapeutic implications of IL-6 blockade for cytokine storm. Immunotherapy. 2016;8(8):959–970. doi: 10.2217/imt-2016-0020. [DOI] [PubMed] [Google Scholar]
- 258.Luo P., et al. Tocilizumab treatment in COVID-19: a single center experience. J. Med. Virol. 2020;92(7):814–818. doi: 10.1002/jmv.25801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Sciascia S., et al. Pilot prospective open, single-arm multicentre study on off-label use of tocilizumab in severe patients with COVID-19. Clin. Exp. Rheumatol. 2020;38(3):529–532. [PubMed] [Google Scholar]
- 260.Toniati P., et al. Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: a single center study of 100 patients in Brescia, Italy. Autoimmun. Rev. 2020 doi: 10.1016/j.autrev.2020.102568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Boregowda U., et al. Addition of Tocilizumab to the standard of care reduces mortality in severe COVID-19: A systematic review and meta-analysis. Frontiers in medicine. 2020;7 doi: 10.3389/fmed.2020.586221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Mahmoudjafari Z., et al. American Society for Blood and Marrow Transplantation Pharmacy Special Interest Group survey on chimeric antigen receptor T cell therapy administrative, logistic, and toxicity management practices in the United States. Biology of Blood and Marrow Transplantation. 2019;25(1):26–33. doi: 10.1016/j.bbmt.2018.09.024. [DOI] [PubMed] [Google Scholar]
- 263.Gritti G., et al. IL-6 signalling pathway inactivation with siltuximab in patients with COVID-19 respiratory failure: an observational cohort study. Medrxiv. 2020 [Google Scholar]
- 264.Kim J.S., et al. Immunopathogenesis and treatment of cytokine storm in COVID-19. Theranostics. 2021;11(1):316. doi: 10.7150/thno.49713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Ye Q., Wang B., Mao J. The pathogenesis and treatment of theCytokine Storm'in COVID-19. J. Infect. 2020;80(6):607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Yang Z., et al. The effect of corticosteroid treatment on patients with coronavirus infection: a systematic review and meta-analysis. J. Infect. 2020;81(1):e13–e20. doi: 10.1016/j.jinf.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Horby P., et al. Dexamethasone in Hospitalized Patients with Covid-19. N. Engl. J. Med. 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Chen R.-C., et al. Treatment of severe acute respiratory syndrome with glucosteroids: the Guangzhou experience. Chest. 2006;129(6):1441–1452. doi: 10.1378/chest.129.6.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Zhou W., et al. Potential benefits of precise corticosteroids therapy for severe 2019-nCoV pneumonia. Signal transduction and targeted therapy. 2020;5(1):1–3. doi: 10.1038/s41392-020-0127-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Russell C.D., Millar J.E., Baillie J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. The Lancet. 2020;395(10223):473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Pittenger M.F., et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 272.Zhou Y., et al. The immunomodulatory functions of mesenchymal stromal/stem cells mediated via paracrine activity. Journal of Clinical Medicine. 2019;8(7):1025. doi: 10.3390/jcm8071025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Rasmusson I. Immune modulation by mesenchymal stem cells. Exp. Cell Res. 2006;312(12):2169–2179. doi: 10.1016/j.yexcr.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 274.Assis A.C.M., et al. Time-dependent migration of systemically delivered bone marrow mesenchymal stem cells to the infarcted heart. Cell Transplant. 2010;19(2):219–230. doi: 10.3727/096368909X479677. [DOI] [PubMed] [Google Scholar]
- 275.Hashi C.K., et al. Antithrombogenic property of bone marrow mesenchymal stem cells in nanofibrous vascular grafts. Proc. Natl. Acad. Sci. 2007;104(29):11915–11920. doi: 10.1073/pnas.0704581104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Schäfer R., et al. Human mesenchymal stromal cells are resistant to SARS-CoV-2 infection under steady-state, inflammatory conditions and in the presence of SARS-CoV-2-infected cells. Stem Cell Rep. 2021;16(3):419–427. doi: 10.1016/j.stemcr.2020.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Montanucci P., et al. Microencapsulated Wharton Jelly-derived adult mesenchymal stem cells as a potential new therapeutic tool for patients with COVID-19 disease: an in vitro study. American Journal of Stem Cells. 2021;10(3):36. [PMC free article] [PubMed] [Google Scholar]
- 278.Lanzoni, G., et al., Umbilical cord mesenchymal stem cells for COVID-19 ARDS: a double blind, phase 1/2a, randomized controlled trial. 2020. [DOI] [PMC free article] [PubMed]
- 279.Leng Z., et al. Transplantation of ACE2(-) Mesenchymal Stem Cells Improves the Outcome of Patients with COVID-19 Pneumonia. Aging Dis. 2020;11(2):216–228. doi: 10.14336/AD.2020.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Taghavi-Farahabadi M., et al. Hypothesis for the management and treatment of the COVID-19-induced acute respiratory distress syndrome and lung injury using mesenchymal stem cell-derived exosomes. Med. Hypotheses. 2020;144 doi: 10.1016/j.mehy.2020.109865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Waugh J., Perry C.M. Anakinra. BioDrugs. 2005;19(3):189–202. doi: 10.2165/00063030-200519030-00005. [DOI] [PubMed] [Google Scholar]
- 282.Mehta P., et al. Silencing the cytokine storm: the use of intravenous anakinra in haemophagocytic lymphohistiocytosis or macrophage activation syndrome. The Lancet Rheumatology. 2020;2(6):e358–e367. doi: 10.1016/S2665-9913(20)30096-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Monteagudo L.A., Boothby A., Gertner E. Continuous intravenous anakinra infusion to calm the cytokine storm in macrophage activation syndrome. ACR open rheumatology. 2020;2(5):276–282. doi: 10.1002/acr2.11135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Shakoory B., et al. Interleukin-1 receptor blockade is associated with reduced mortality in sepsis patients with features of the macrophage activation syndrome: Re-analysis of a prior Phase III trial. Crit. Care Med. 2016;44(2):275. doi: 10.1097/CCM.0000000000001402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Huet T., et al. Anakinra for severe forms of COVID-19: a cohort study. Lancet Rheumatol. 2020;2(7):e393–e400. doi: 10.1016/S2665-9913(20)30164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Iglesias-Julián E., et al. High dose subcutaneous Anakinra to treat acute respiratory distress syndrome secondary to cytokine storm syndrome among severely ill COVID-19 patients. J. Autoimmun. 2020;115 doi: 10.1016/j.jaut.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Cavalli G., et al. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. The Lancet Rheumatology. 2020;2(6):e325–e331. doi: 10.1016/S2665-9913(20)30127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Declercq J., et al. Effect of anti-interleukin drugs in patients with COVID-19 and signs of cytokine release syndrome (COV-AID): a factorial, randomised, controlled trial. The Lancet Respiratory Medicine. 2021;9(12):1427–1438. doi: 10.1016/S2213-2600(21)00377-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Mastroianni, A., et al., Early use of canakinumab to prevent mechanical ventilation in select COVID-19 patients: A retrospective, observational analysis. International Journal of Immunopathology and Pharmacology, 2021. 35: p. 20587384211059675. [DOI] [PMC free article] [PubMed]
- 290.Ucciferri, C., et al., Canakinumab in a subgroup of patients with COVID-19. Lancet Rheumatol, 2020. 2(8): p. e457-ee458. [DOI] [PMC free article] [PubMed]
- 291.Caricchio R., et al. Effect of canakinumab vs placebo on survival without invasive mechanical ventilation in patients hospitalized with severe COVID-19: a randomized clinical trial. JAMA. 2021;326(3):230–239. doi: 10.1001/jama.2021.9508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Convertino I., et al. Exploring pharmacological approaches for managing cytokine storm associated with pneumonia and acute respiratory distress syndrome in COVID-19 patients. Crit. Care. 2020;24(1):1–6. doi: 10.1186/s13054-020-03020-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Reddy V., Cohen S. Jak inhibitors: What is new? Curr. Rheumatol. Rep. 2020;22(9):1–10. doi: 10.1007/s11926-020-00931-6. [DOI] [PubMed] [Google Scholar]
- 294.D’Alessio A., et al. Low-dose ruxolitinib plus steroid in severe SARS-CoV-2 pneumonia. Leukemia. 2021;35(2):635–638. doi: 10.1038/s41375-020-01087-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Chen C.-X., et al. JAK-inhibitors for coronavirus disease-2019 (COVID-19): a meta-analysis. Leukemia. 2021:1–5. doi: 10.1038/s41375-021-01266-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Wijaya I., et al. The use of Janus Kinase inhibitors in hospitalized patients with COVID-19: Systematic review and meta-analysis. Clinical epidemiology and global health. 2021 doi: 10.1016/j.cegh.2021.100755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Patoulias D., et al. Janus kinase inhibitors and major COVID-19 outcomes: time to forget the two faces of Janus! A meta-analysis of randomized controlled trials. Clin. Rheumatol. 2021;40(11):4671–4674. doi: 10.1007/s10067-021-05884-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Hu B., Huang S., Yin L. The cytokine storm and COVID-19. J. Med. Virol. 2021;93(1):250–256. doi: 10.1002/jmv.26232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Kandasamy M. NF-κB signalling as a pharmacological target in COVID-19: potential roles for IKKβ inhibitors. Naunyn-Schmiedeberg's Arch. Pharmacol. 2021;394(3):561–567. doi: 10.1007/s00210-020-02035-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Scheinman R.I., et al. Role of transcriptional activation of IκBα in mediation of immunosuppression by glucocorticoids. Science. 1995;270(5234):283–286. doi: 10.1126/science.270.5234.283. [DOI] [PubMed] [Google Scholar]
- 301.Auphan N., et al. Immunosuppression by glucocorticoids: inhibition of NF-κB activity through induction of IκB synthesis. Science. 1995;270(5234):286–290. doi: 10.1126/science.270.5234.286. [DOI] [PubMed] [Google Scholar]
- 302.D'Acquisto F., May M.J., Ghosh S. Inhibition of nuclear factor kappa B (NF-B) Mol. Interventions. 2002;2(1):22. doi: 10.1124/mi.2.1.22. [DOI] [PubMed] [Google Scholar]
- 303.Yin M.-J., Yamamoto Y., Gaynor R.B. The anti-inflammatory agents aspirin and salicylate inhibit the activity of IκB kinase-β. Nature. 1998;396(6706):77–80. doi: 10.1038/23948. [DOI] [PubMed] [Google Scholar]
- 304.Kopp E., Ghosh S. Inhibition of NF-kappa B by sodium salicylate and aspirin. Science. 1994;265(5174):956–959. doi: 10.1126/science.8052854. [DOI] [PubMed] [Google Scholar]
- 305.Scheuren N., et al. Modulation of transcription factor NF-κB by enantiomers of the nonsteroidal drug ibuprofen. Br. J. Pharmacol. 1998;123(4):645–652. doi: 10.1038/sj.bjp.0701652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Yamamoto Y., et al. Sulindac inhibits activation of the NF-κB pathway. J. Biol. Chem. 1999;274(38):27307–27314. doi: 10.1074/jbc.274.38.27307. [DOI] [PubMed] [Google Scholar]
- 307.Schoonbroodt S., Piette J. Oxidative stress interference with the nuclear factor-κB activation pathways. Biochem. Pharmacol. 2000;60(8):1075–1083. doi: 10.1016/s0006-2952(00)00371-3. [DOI] [PubMed] [Google Scholar]
- 308.Flohé L., et al. Redox regulation of NF-kappa B activation. Free Radical Biol. Med. 1997;22(6):1115–1126. doi: 10.1016/s0891-5849(96)00501-1. [DOI] [PubMed] [Google Scholar]
- 309.Blackwell T.S., et al. In vivo antioxidant treatment suppresses nuclear factor-kappa B activation and neutrophilic lung inflammation. J. Immunol. 1996;157(4):1630–1637. [PubMed] [Google Scholar]
- 310.Liu S.F., Ye X., Malik A.B. Inhibition of NF-κB activation by pyrrolidine dithiocarbamate prevents in vivo expression of proinflammatory genes. Circulation. 1999;100(12):1330–1337. doi: 10.1161/01.cir.100.12.1330. [DOI] [PubMed] [Google Scholar]
- 311.Liu S.F., Ye X., Malik A.B. Pyrrolidine dithiocarbamate prevents I-κB degradation and reduces microvascular injury induced by lipopolysaccharide in multiple organs. Mol. Pharmacol. 1999;55(4):658–667. [PubMed] [Google Scholar]
- 312.Kretz-Remy C., et al. Inhibition of I kappa B-alpha phosphorylation and degradation and subsequent NF-kappa B activation by glutathione peroxidase overexpression. J. Cell Biol. 1996;133(5):1083–1093. doi: 10.1083/jcb.133.5.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Suzuki Y.J., Packer L. Inhibition of NF-κB activation by vitamin E derivatives. Biochem. Biophys. Res. Commun. 1993;193(1):277–283. doi: 10.1006/bbrc.1993.1620. [DOI] [PubMed] [Google Scholar]
- 314.Hou J.-Y., et al. Systematic identification of the interventional mechanism of Qingfei Xiaoyan Wan (QFXYW) in treatment of the cytokine storm in acute lung injury using transcriptomics-based system pharmacological analyses. Pharm. Biol. 2022;60(1):743–754. doi: 10.1080/13880209.2022.2055090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Runfeng L., et al. Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2) Pharmacol. Res. 2020;156 doi: 10.1016/j.phrs.2020.104761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Sun Q.-G., et al. Traditional Chinese medicine decoctions significantly reduce the mortality in severe and critically ill patients with COVID-19: a retrospective cohort study. The American journal of Chinese medicine. 2021;49(05):1063–1092. doi: 10.1142/S0192415X21500518. [DOI] [PubMed] [Google Scholar]
- 317.Mardaneh, J., et al., Inhibiting NF-κB During Cytokine Storm in COVID-19: Potential Role of Natural Products as a Promising Therapeutic Approach. 2021.
- 318.Kohli K., et al. Curcumin: a natural antiinflammatory agent. Indian Journal of Pharmacology. 2005;37(3):141. [Google Scholar]
- 319.Kang S., Min H. Ginseng, the'immunity boost': the effects of Panax ginseng on immune system. J. Ginseng Res. 2012;36(4):354. doi: 10.5142/jgr.2012.36.4.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Ahn J.Y., et al. The immunomodulator ginsan induces resistance to experimental sepsis by inhibiting Toll-like receptor-mediated inflammatory signals. Eur. J. Immunol. 2006;36(1):37–45. doi: 10.1002/eji.200535138. [DOI] [PubMed] [Google Scholar]
- 321.Meenakshi S., et al. Ayurveda: The Prominence of Herbal Medicine in Containment of COVID-19. Pharmacognosy Research. 2022;14(3) [Google Scholar]
- 322.Dash, M., N. Joshi, and Y. Tripathi, Identification of therapeutic targets for controlling COVID-19 pandemic by traditional system of Ayurvedic medicines: A systematic review. 2020.
- 323.Niraj S., Varsha S. A review on scope of immuno-modulatory drugs in Ayurveda for prevention and treatment of Covid-19. Plant Science Today. 2020;7(3):417–423. [Google Scholar]
- 324.Dasgupta A., et al. Negative Interference of Ashwagandha, an Indian Ayurveda Medicine Indicated for Preventing COVID-19, in IL-6 Immunoassay. Ann. Clin. Lab. Sci. 2022;52(2):336–338. [PubMed] [Google Scholar]
- 325.Wajpeyi S.M. Approach of Ayurveda in post COVID management. International journal of health sciences. 2022;6(S2):594–605. [Google Scholar]
- 326.Bapat, V., et al., Perspectives on ‘Kamsaharitaki’: an Ayurvedic Formulation for COVID-19 related Multisystem Inflammatory Syndrome (MIS). 2022.
- 327.Ratheesh M., et al. Anti-inflammatory and anti-COVID-19 effect of a novel polyherbal formulation (Imusil) via modulating oxidative stress, inflammatory mediators and cytokine storm. Inflammopharmacology. 2022;30(1):173–184. doi: 10.1007/s10787-021-00911-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Ghattargi V., et al. Probiotics as a Potential Immuno Modulator to Combat Coronavirus Disease (covid-19) Infection. Archives of Clinical Microbiology. 2022;13(6):1–4. [Google Scholar]
- 329.Wu C., et al. The volatile and heterogeneous gut microbiota shifts of COVID-19 patients over the course of a probiotics-assisted therapy. Clinical and translational medicine. 2021;11(12) doi: 10.1002/ctm2.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Kageyama Y., et al. Lactobacillus plantarum induces innate cytokine responses that potentially provide a protective benefit against COVID19: A singlearm, doubleblind, prospective trial combined with an in vitro cytokine response assay. Exp. Therapeutic Med. 2022;23(1):1–13. doi: 10.3892/etm.2021.10942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Myall K.J., et al. Persistent post–COVID-19 interstitial lung disease. An observational study of corticosteroid treatment. Ann. Am. Thoracic Soc. 2021;18(5):799–806. doi: 10.1513/AnnalsATS.202008-1002OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.George P.M., Wells A.U., Jenkins R.G. Pulmonary fibrosis and COVID-19: the potential role for antifibrotic therapy. The Lancet Respiratory Med. 2020;8(8):807–815. doi: 10.1016/S2213-2600(20)30225-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Nalbandian A., et al. Post-acute COVID-19 syndrome. Nat. Med. 2021;27(4):601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Bai, C., et al., Updated guidance on the management of COVID-19: from an American Thoracic Society/European Respiratory Society coordinated international task force (29 July 2020). European Respiratory Review, 2020. 29(157). [DOI] [PMC free article] [PubMed]
- 335.Hendren N.S., et al. Description and proposed management of the acute COVID-19 cardiovascular syndrome. Circulation. 2020;141(23):1903–1914. doi: 10.1161/CIRCULATIONAHA.120.047349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Basu D., Chavda V.P., Mehta A.A. Therapeutics for COVID-19 and post COVID-19 complications: An update. Curr Res Pharmacol Drug Discov. 2022;3 doi: 10.1016/j.crphar.2022.100086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Rosas I.O., et al. Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. N. Engl. J. Med. 2021;384(16):1503–1516. doi: 10.1056/NEJMoa2028700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Park E.H., et al. Tocilizumab-induced anaphylaxis in patients with adult-onset Still’s disease and systemic juvenile idiopathic arthritis: a case-based review. Rheumatol. Int. 2020;40(5):791–798. doi: 10.1007/s00296-019-04456-9. [DOI] [PubMed] [Google Scholar]
- 339.Moots R.J., et al. Effect of tocilizumab on neutrophils in adult patients with rheumatoid arthritis: pooled analysis of data from phase 3 and 4 clinical trials. Rheumatology. 2017;56(4):541–549. doi: 10.1093/rheumatology/kew370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Salama C., et al. Tocilizumab in patients hospitalized with Covid-19 pneumonia. N. Engl. J. Med. 2021;384(1):20–30. doi: 10.1056/NEJMoa2030340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Roche, H.-L., A Study to Evaluate the Safety and Efficacy of Tocilizumab in Patients With Severe COVID-19 Pneumonia. 2020.
- 342.Curtis J.R., et al. Incidence and complications of interstitial lung disease in users of tocilizumab, rituximab, abatacept and anti-tumor necrosis factor α agents, a retrospective cohort study. Arthritis Research & Therapy. 2015;17(1):1–13. doi: 10.1186/s13075-015-0835-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Genovese M.C., et al. Transaminase levels and hepatic events during tocilizumab treatment: pooled analysis of long-term clinical trial safety data in rheumatoid arthritis. Arthritis & Rheumatology. 2017;69(9):1751–1761. doi: 10.1002/art.40176. [DOI] [PubMed] [Google Scholar]
- 344.Sigaux J., et al. Immunogenicity of tocilizumab in patients with rheumatoid arthritis. Joint Bone Spine. 2017;84(1):39–45. doi: 10.1016/j.jbspin.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 345.van Rhee F., et al. Long-term safety of siltuximab in patients with idiopathic multicentric Castleman disease: a prespecified, open-label, extension analysis of two trials. The Lancet Haematology. 2020;7(3):e209–e217. doi: 10.1016/S2352-3026(19)30257-1. [DOI] [PubMed] [Google Scholar]
- 346.Baracaldo-Santamaría D., et al. Immune-related adverse events of biological immunotherapies used in COVID-19. Front. Pharmacol. 2022;13:973246. doi: 10.3389/fphar.2022.973246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Sitenga J., et al. Impact of siltuximab on patient-related outcomes in multicentric Castleman’s disease. Patient Related Outcome Measures. 2018;9:35. doi: 10.2147/PROM.S140011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Bachove I., Chang C. Anakinra and related drugs targeting interleukin-1 in the treatment of cryopyrin-associated periodic syndromes. Open Access Rheumatology: Research and Reviews. 2014;6:15. doi: 10.2147/OARRR.S46017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Aysegul Ö., Taner G., Nizameddin K. A review on the toxic effects of medicines used in corona virus disease 2019 (Covid-19) treatment. DAHUDER Medical Journal. 2022;2(1):1–10. [Google Scholar]
- 350.Hossen M., et al. A review on current repurposing drugs for the treatment of COVID-19: reality and challenges. SN comprehensive clinical medicine. 2020;2(10):1777–1789. doi: 10.1007/s42399-020-00485-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Zadeh N.M., et al. Mechanism and adverse effects of COVID-19 drugs: a basic review. International Journal of Physiology, Pathophysiology and Pharmacology. 2021;13(4):102. [PMC free article] [PubMed] [Google Scholar]
- 352.Terzi F., et al. Effects of tocilizumab and dexamethasone on the downregulation of proinflammatory cytokines and upregulation of antioxidants in the lungs in oleic acid-induced ARDS. Respir. Res. 2022;23(1):1–11. doi: 10.1186/s12931-022-02172-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Sivakumar P., Gowthaman S. Characterization, potential application and limitation of medicinal herbs in COVID-19. A review. 2022 [Google Scholar]
- 354.Bentley, E., et al., SARS-CoV-2 Omicron-B.1.1.529 Variant leads to less severe disease than Pango B and Delta variants strains in a mouse model of severe COVID-19. 2021.
- 355.Diamond M., et al. The SARS-CoV-2 B.1.1.529 Omicron virus causes attenuated infection and disease in mice and hamsters. Res Sq. 2021 [Google Scholar]
- 356.Kersten J., et al. Long COVID: Distinction between organ damage and deconditioning. J. Clin. Med. 2021;10(17):3782. doi: 10.3390/jcm10173782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Wijeratne T., Crewther S. Post-COVID 19 Neurological Syndrome (PCNS); a novel syndrome with challenges for the global neurology community. J. Neurol. Sci. 2020;419 doi: 10.1016/j.jns.2020.117179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Elseidy S.A., et al. Cardiovascular complications in the Post-Acute COVID-19 syndrome (PACS) IJC Heart & Vasculature. 2022;40 doi: 10.1016/j.ijcha.2022.101012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Palmieri L., et al. Clinical Characteristics of Hospitalized Individuals Dying With COVID-19 by Age Group in Italy. J. Gerontol. A Biol. Sci. Med. Sci. 2020;75(9):1796–1800. doi: 10.1093/gerona/glaa146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Qi Y., et al. Identification of risk factors for sepsis-associated mortality by gene expression profiling analysis. Mol. Med. Rep. 2018;17(4):5350–5355. doi: 10.3892/mmr.2018.8491. [DOI] [PubMed] [Google Scholar]
- 361.Gadi N., et al. What’s sex got to do with COVID-19? Gender-based differences in the host immune response to coronaviruses. Front. Immunol. 2020:2147. doi: 10.3389/fimmu.2020.02147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Pérez-López F.R., et al. Coronavirus disease 2019 and gender-related mortality in European countries: A meta-analysis. Maturitas. 2020;141:59–62. doi: 10.1016/j.maturitas.2020.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Vahidy F.S., et al. Sex differences in susceptibility, severity, and outcomes of coronavirus disease 2019: Cross-sectional analysis from a diverse US metropolitan area. PLoS ONE. 2021;16(1):e0245556. doi: 10.1371/journal.pone.0245556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Jin J.-M., et al. Gender Differences in Patients With COVID-19: Focus on Severity and Mortality. Frontiers. Public Health. 2020;8 doi: 10.3389/fpubh.2020.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Peckham H., et al. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat. Commun. 2020;11(1):6317. doi: 10.1038/s41467-020-19741-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Conti P., Younes A. Coronavirus COV-19/SARS-CoV-2 affects women less than men: clinical response to viral infection. J. Biol. Regul. Homeost. Agents. 2020;34(2):339–343. doi: 10.23812/Editorial-Conti-3. [DOI] [PubMed] [Google Scholar]
- 367.Lau E.S., et al. Sex differences in inflammatory markers in patients hospitalized with COVID-19 infection: insights from the MGH COVID-19 patient registry. PLoS ONE. 2021;16(4):e0250774. doi: 10.1371/journal.pone.0250774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.E.P. Scully, et al., Sex and gender differences in testing, hospital admission, clinical presentation, and drivers of severe outcomes from COVID-19. in Open forum infectious diseases. 2021. Oxford University Press US. [DOI] [PMC free article] [PubMed]
- 369.Kirtipal N., et al. Understanding on the possible routes for SARS CoV-2 invasion via ACE2 in the host linked with multiple organs damage. Infection, Genetics and Evolution. 2022;99 doi: 10.1016/j.meegid.2022.105254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Mittal M., et al. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014;20(7):1126–1167. doi: 10.1089/ars.2012.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Yang D., et al. Pro-inflammatory cytokines increase reactive oxygen species through mitochondria and NADPH oxidase in cultured RPE cells. Exp. Eye Res. 2007;85(4):462–472. doi: 10.1016/j.exer.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Laforge M., et al. Tissue damage from neutrophil-induced oxidative stress in COVID-19. Nat. Rev. Immunol. 2020;20(9):515–516. doi: 10.1038/s41577-020-0407-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Ruhl L., et al. Endothelial dysfunction contributes to severe COVID-19 in combination with dysregulated lymphocyte responses and cytokine networks. Signal Transduction and Targeted Therapy. 2021;6(1):418. doi: 10.1038/s41392-021-00819-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Aid M., et al. Vascular Disease and Thrombosis in SARS-CoV-2-Infected Rhesus Macaques. Cell. 2020;183(5):1354–1366.e13. doi: 10.1016/j.cell.2020.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Ragab D., et al. The COVID-19 Cytokine Storm; What We Know So Far. Front. Immunol. 2020;11:1446. doi: 10.3389/fimmu.2020.01446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Chen R., et al. Cytokine Storm: The Primary Determinant for the Pathophysiological Evolution of COVID-19 Deterioration. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.589095. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.