Abstract
Background
The 8-Foot Up and Go (8UG) test is a widely used mobility assessment. Some dual-task mobility assessments have been developed to help detect cognitive decline.
Aims
This study developed a dual-task version of 8UG test to investigate the dual-task 8UG performance and to evaluate the ability of dual-task 8UG test in detecting cognitive decline.
Methods
A total of 101 eligible community-dwelling women aged 60–74 years were grouped into the mild cognitive impairment group (MCI, n = 49) and the non-cognitive impairment group (NCI, n = 52). The 8UG tests under single-task (ST), manual dual-task (MT), and cognitive dual-task (CT) conditions were performed respectively. The dual-task cost (DTC) and the correct response rate (CRR) were calculated to quantify the dual-task interference.
Results
Participants spent more time in performing the 8UG test under dual-task conditions. No differences were observed between NCI and MCI groups for 8UG parameters under ST and MT conditions (p > 0.05). When executing CT, significant differences were found in the number of correct answers and CRR (p < 0.05). CRR showed the strongest ability to predict MCI with a cut-off point of 0.50 (71.2% sensitivity and 61.2% specificity).
Discussion
Both manual and cognitive dual-task were found to interfere with the 8UG performance. CRR with cutoff point of 0.50 could be a potential predictor of MCI in community-dwelling older women.
Conclusions
The CRR of the cognitive dual-task 8UG test could be recommended as a potential predictor for the early detection of MCI in community-dwelling older women.
Keywords: Dual-task, 8UG, Mobility, Cognitive function, Older adult
Introduction
Cognitive impairment in older adults is a common condition and one of the major public health concerns. Age-related cognitive decline is a gradual aging process [1, 2]. Early detection and early treatment may help to delay the progression of cognitive decline [3, 4].
Mobility is found to be interrelated with cognitive function [5]. On the one hand, cognition plays an important role in normal walking [6] and physical mobility [7]. Impaired cognitive function may lead to an increased falls risk [8, 9]. On the other hand, poor mobility performance may predict cognitive decline [10]. In the aging process, the cortical gait control and cognitive function interacted with each other, and might result in motoric cognitive risk syndrome, which is related to major neurocognitive disorders [11]. The mechanism underlying their associations has not yet been completely explained. Even so, the deficit of cognitive function in attention, executive function, and working memory loss have been demonstrated to contribute to poor performance in postural control, mobility and gait [6, 12, 13].
Prior research has found that to maintain postural stability and mobility, increased recruitment of generic cognitive resources is demanded to compensate for the deficit of age-related neuromotor and sensory degeneration in older adults [14]. However, when two or more tasks are demanded to perform concurrently, a competition for common neural structures and potential trade-off of task prioritization tends to be aggravated [15]. The dual-task testing paradigm, which assesses the individuals’ mobility or balance performance while executing another cognitive or physical task concomitantly, was designed by simulating daily social activity (e.g., walking while talking on the cellphone, thinking about something else, or carrying a cup of coffee). In recent years, the dual-task testing paradigm has been shown to have important value in the early detection of cognitive decline [16], fall risk assessment [17, 18], and understanding of the interactions between cognition and motor control [19]. In addition, dual-task performance might help to explain certain relationships, for example, a mediating effect on the association between fear of falling and activities of daily living [20].
The 8-Foot Up and Go (8UG) test, which is widely applied in functional mobility assessment [21], is developed to assess the agility and dynamic balance of older adults [22]. As a modified version of the Timed Up and Go (TUG) test, the 8UG test involves the same phases as the TUG test. These phases are common functional movements of daily life, including sit to stand, walk, turn around, and turn to sit. To address the limitation of the TUG test, the distance of the 8UG test was shortened from 3 m to 2.44 m (8 feet), for the purpose of increasing the test feasibility in areas with limited space. Additionally, to signal the turn-around, the turning line of TUG was replaced by a cone placed at the turning point in the 8UG test [23]. Previous studies have indicated that compared with single-task TUG, the dual-task TUG test has a stronger ability in detecting individuals at high risk for cognitive decline [24, 25] and falls [26, 27]. Although the 8UG test is a widely used modified version of the TUG test, limited experimental evidence can support or refute whether the dual-task 8UG test has the same predictive ability for identifying older adults at high risk for falls or cognitive decline.
Community-dwelling older women exhibit a faster rate of cognitive decline and mobility decline than men [28, 29]. Comparing with young and middle-aged women, older women showed a more significant decrease in mobility performance under dual task conditions [30]. It is therefore necessary to detect early and intervene timely in these aging changes among this population. Given that physical mobility is correlated with cognitive function in older adults, dual-task versions of 8UG tests were developed with the hypothesis that dual-task 8UG performance is correlated with cognitive function. The purposes of this study were to: (1) assess the dual-task interference on 8UG performance; (2) compare single- and dual-task 8UG performance between participants with and without cognitive impairment; and (3) evaluate the ability of dual-task 8UG tests in detecting cognitive decline.
Methods
Participants
A total of 101 eligible community-dwelling older women were enrolled in this study. The inclusion criteria were: (1) community-dwelling women aged 60–74 years; (2) being able to walk independently; (3) having normal vision and hearing; and (4) being willing to and capable of providing informed consent. Participants were excluded from the study if they met the following exclusion criteria: (1) suffering from severe heart, lung, and skeletal muscle system diseases, or neurological diseases that seriously affect balance function (e.g., stroke and Parkinson disease), or mental illness (e.g., depression or take psychotropic drugs), or dementia (moderate and above); (2) using assistive devices for walking; and (3) being illiterate.
Procedures
The data collection was conducted during a single session. After signing the written informed consent, all participants underwent face-to-face interviews conducted by a trained staff member to collect the demographic and health status information including age, education, medical history (hypertension, diabetes mellitus, cardiovascular disease, hyperlipidemia), and self-reported health status (categorized as “very good”, “good”, “fair”, “poor”, and “very poor”). The Beijing version of Montreal Cognitive Assessment (MoCA-BJ) was then used to evaluate the participants’ cognitive function [31]. Body height (cm) and weight (kg) were measured according to standardized procedures [32].
The 8UG test was carried out under three conditions: 1) single-task condition (ST): 8-foot up and go (8UG) test; 2) manual dual-task condition (MT): 8UG and carrying a cup; and 3) cognitive dual-task condition (CT): 8UG and serial subtraction of 7. Two trials were performed under each condition. The order of trails for each participant was chosen randomly to avoid performance bias [33]. In addition, participants were able to undertake a rest period between each trial to reduce the possible effects of fatigue [34].
The 8UG test
The 8UG test was carried out according to the method described by Rikli and Jones [22]. A standard folding chair with 43 cm seat height was placed against the wall of a gymnasium, and a cone marker was set exactly 8 feet away from the front edge of the folding chair. Once the start signal “go” was verbally given by the assessor, the participant was instructed to get up from the chair, walk straight to the cone marker, turn around, walk back, and sit down as quickly and as safely as possible. The time taken to complete the test from the word “go” was given to the exact instance the participant sat back down on the chair and was recorded as 8UGST to the nearest 0.01 s.
In the 8UG-MT test, a standard manual task was added in the 8UG test process. In preparation for the 8UG-MT, a glass was placed on a table (70 cm high) beside the testing chair, and filled with water (300 g) [35–37]. When performing 8UG-MT test, the participants were instructed to complete the 8UG test while carrying a glass of water with their dominant hand [27, 35, 37–39]. In addition to recording the time taken to execute 8UG-MT test (recorded as 8UGMT), the weight of water spilled out during the test was calculated.
The 8UG-CT test was a subtraction task added in the 8UG test process. In the 8UG-CT test, participants performed a sequential subtraction of seven from a number randomly selected from 50 to 100 when they executing the 8UG task [33, 40]. The time spent in the 8UG-CT test (recorded as 8UGCT) was written down, and the number of correct responses in the serial subtraction task was recorded as well.
In both MT and CT dual-task 8UG tests, no prioritized task was given to simulate a real-life situation [33, 41].
Classification of cognitive impairment
Individuals’ cognitive functions were evaluated one-by-one respectively by several trained instructors in different rooms. As a widely used Chinese version of MoCA, MoCA-BJ has been shown to be able to detect mild cognitive impairments (MCI) in community-dwelling older adults [31, 42]. A cutoff was set at 26 to distinguish participants with a high risk of MCI (total score < 26) and non-cognitive impairment (NCI, at low risk of MCI, total score ≥ 26) [42].
Data analysis
With the assumption that 8UG performance might be influenced by executing two tasks concurrently, dual-task cost (DTC) was calculated to quantify the dual-task interference [43]. The DTC is determined according to the differences between single- and dual-task 8UG performances. The formulas of DTC under MT and CT were calculated as follows:
A positive value (+) of DTC represents a diminished 8UG performance (added time taken) due to dual-task, while a negative value(-) represents an enhanced performance [44]. A higher absolute value of DTC indicates a higher interference effect [45].
Additionally, in the 8UG-CT test, the response accuracy, which is also described as the correct response rate (CRR) [45–47] was calculated as follows:
The research data were initially recorded on paper forms. Double data entry was conducted in EpiData 3.1 software (EpiData Association, Odense, Denmark) to make sure the accuracy of the data. Statistical analyses of the current study were performed using SPSS 22.0 software (IBM SPSS Statistics, IBM Corporation, NY, USA).
Variables were expressed as counts (percentage) for categorical data, and as mean (standard deviation, SD) for quantitative data. Chi-square tests were used to compare categorical data between groups. Normal distribution of quantitative data was tested using the Shapiro–Wilk test. A repeated measures analysis of variance was conducted with between-factor as cognitive impairment groups (MCI, NCI), within-factor as tasks (8UGST, 8UGMT, and 8UGCT), and interactions between task and group. Independent two-sample t-tests were used to compare the difference between groups. Also, receiver operating characteristics (ROC) analysis was performed to determine the ability of the 8UG test parameters in detecting MCI. The optimal cut-off value, area under ROC curve (AUC), sensitivity, and specificity were then reported. The significance level was established as 5%.
Results
The demographic characteristics and health status of participants are summarized in Table 1. The 101 participants (65.6 ± 3.4 years) were classified into two groups based on their MoCA results. Participants with MoCA total score lower than 26 were categorized into the MCI group (n = 49, 65.3 ± 3.3 years), while others were categorized into the NCI group (n = 52, 65.9 ± 3.6 years). No significant statistical differences were found in characteristics including age, height, weight, BMI, education years, medical history, and self-reported health status between groups (p > 0.05). In general, the cognitive function performances of the MCI group were worse than that of the NCI group (p < 0.05), except for the orientation dimension.
Table 1.
Demographic characteristics and health status of participants
| Variable | Total n = 101 | NCI n = 52 | MCI n = 49 | P value |
|---|---|---|---|---|
| Age (years), mean (SD) | 65.6 (3.4) | 65.9 (3.6) | 65.3 (3.3) | 0.382 |
| Height (cm), mean (SD) | 158.5 (4.5) | 158.7 (4.6) | 158.3 (4.5) | 0.647 |
| Weight (kg), mean (SD) | 58.8 (6.7) | 59.1 (6.6) | 58.5 (6.8) | 0.674 |
| BMI (kg/m2), mean (SD) | 23.4 (2.3) | 23.4 (2.2) | 23.3 (2.4) | 0.838 |
| Education years ≤ 12, n (%) | 89 (88.1%) | 43 (82.7%) | 46 (93.9%) | 0.083 |
| No. of comorbidities | ||||
| 0 | 52 (51.5%) | 28 (53.8%) | 24 (49.0%) | 0.625 |
| 1 | 26 (25.7%) | 11 (21.2%) | 15 (30.6%) | 0.277 |
| ≥ 2 | 23 (22.8%) | 13 (25.0%) | 10 (20.4%) | 0.582 |
| Chronic disease, n (%) | ||||
| Hypertension (yes) | 23 (22.8%) | 10 (19.2%) | 13 (26.5%) | 0.382 |
| Diabetes (yes) | 6 (5.9%) | 2 (3.8%) | 4 (8.2%) | 0.620 |
| Cardiovascular disease (yes) | 10 (9.9%) | 7 (13.5%) | 3 (6.1%) | 0.368 |
| Hyperlipidemia (yes) | 5 (5.0%) | 4 (7.7%) | 1 (2.0%) | 0.396 |
| Self-reported health status, n (%) | ||||
| Very good | 21 (20.8%) | 10 (19.2%) | 11 (22.4%) | 0.474 |
| Good | 37 (36.6%) | 22 (42.3%) | 15 (30.6%) | |
| Fair | 43 (42.6%) | 20 (38.5%) | 23 (46.9%) | |
| Total score of MOCA-BJ, mean (SD) | 25.2 (3.3) | 27.8 (1.4) | 22.5 (2.5) | < 0.001* |
| Visuospatial/executive function | 3.7 (0.9) | 4.1 (0.7) | 3.3 (1.0) | < 0.001* |
| Naming | 2.8 (0.6) | 3.0 (0.2) | 2.6 (0.8) | 0.001* |
| Attention | 5.4 (0.9) | 5.8 (0.4) | 4.9 (1.0) | < 0.001* |
| Language | 1.9 (0.9) | 2.3 (0.7) | 1.4 (0.8) | < 0.001* |
| Abstraction | 1.4 (0.7) | 1.6 (0.6) | 1.1 (0.6) | < 0.001* |
| Delayed recall | 3.3 (1.5) | 4.3 (0.9) | 2.3 (1.5) | < 0.001* |
| Orientation | 5.9 (0.3) | 6.0 (0.1) | 5.9 (0.4) | 0.144 |
MOCA-BJ the Beijing Version of Montreal Cognitive Assessment, SD standard deviation; NCI refers to participants with non-cognitive impairment; MCI refers to participants with mild cognitive impairment
*Significant p < 0.05 by Student t test or chi-square test compared with NCI
Compared with the 8UG-ST test, participants spent more time in performing the 8UG test under dual-task conditions. Both MT and CT contributed to a significantly prolonged time spending in the 8UG tests compared with ST in community-dwelling older women. The descriptive statistics of 8UG time under three conditions and the results of repeated measures analysis are presented in Table 2. The time of completing the 8UG test under ST ranged from 3.90 s to 6.81 s. The 8UG test under MT and CT led to a significantly increase of time consuming in both the NCI and MCI groups. Statistically significant differences were found between tasks (p < 0.001). However, neither statistically significant differences were observed between NCI and MCI group, nor interaction effect between task and group was found (p > 0.05).
Table 2.
Descriptive and repeated measures analyses results of 8UG time under single- and dual-task conditions in Community-Dwelling older women with and without cognitive impairment
| Time | NCI | MCI | Task | ||
|---|---|---|---|---|---|
| Mean (SD) | Range | Mean (SD) | Range | ||
| 8UGST(s) | 5.35 (0.68) | 4.33–6.75 | 5.34 (0.60) | 3.90–6.81 | F = 486.606 |
| 8UGMT(s) | 7.32 (1.03) | 5.53–9.32 | 7.30 (1.04) | 5.48–9.97 | p < 0.001 |
| 8UGCT(s) | 6.76 (0.94) | 5.38–8.75 | 6.81 (0.92) | 5.19–9.26 | ηp2 = 0.909 |
8UG 8-Foot Up and Go test, 8UGST 8UG Time under Single-task, 8UGMT 8UG Time under Manual Dual-task, 8UGCT 8UG Time under Cognitive Dual-task; NCI refers to participants with non-cognitive impairment; MCI refers to participants with mild cognitive impairment
The parameters of the 8UG test performance under ST, MT and CT conditions were displayed in Table 3. In this study, all DTC values were positive (+), which suggested that both MT and CT had a negative impact on 8UG performance. The DTCMT was higher than DTCCT (p < 0.05) in both NCI and MCI groups. No statistical differences were found between NCI and MCI groups for 8UG time and DTC value under single- and dual-task conditions. When performing MT, no one spilled water out during the 8UG test. When performing CT, significant differences were observed in the cognitive task performance (the number of correct answers, p = 0.001) and cognitive dual-task interference (CRR, p = 0.001).
Table 3.
Comparison of 8UG parameters under single- and dual-task conditions in community-dwelling older women with and without cognitive impairment
| Task | Variable | NCI | MCI | P value |
|---|---|---|---|---|
| ST | 8UGST (s) | 5.35 (0.68) | 5.34 (0.60) | 0.936 |
| MT | 8UGMT (s) | 7.32 (1.03) | 7.30 (1.04) | 0.937 |
| DTCMT (%) | 37.24 (13.11) | 36.89 (12.11) | 0.890 | |
| Spilled water (%) | 0.00% (0.00%) | 0.00% (0.00%) | ||
| CT | 8UGCT (s) | 6.76 (0.94) | 6.81 (0.92) | 0.766 |
| DTCCT (%) | 27.23 (16.70) | 28.23 (15.37) | 0.755 | |
| Number of correct answers (n) | 3.77 (0.90) | 2.92 (1.41) | 0.001* | |
| CRR (n/s) | 0.57 (0.16) | 0.43 (0.21) | 0.001* |
8UG 8-Foot Up and Go test, ST Single-task, MT Manual Dual-task, CT Cognitive Dual-task, DTC Dual-task Cost, CRR Correct Response Rate; NCI refers to participants with non-cognitive impairment; MCI refers to participants with mild cognitive impairment
*Significant p < 0.05 compared with NCI
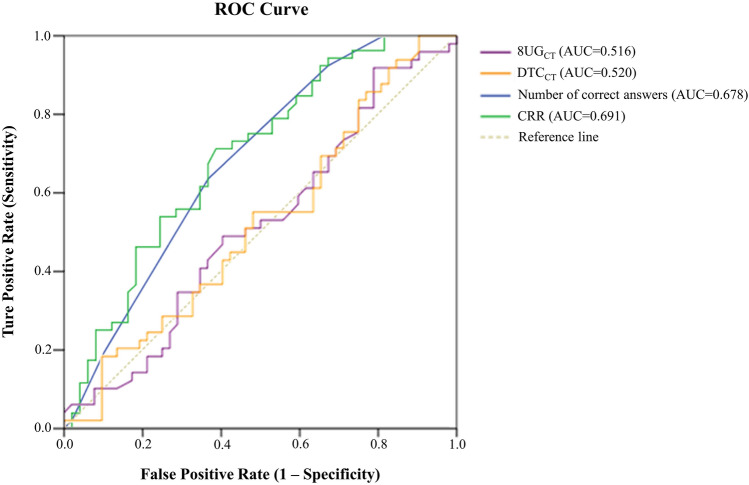
On account of that significant differences were detected in the 8UG test performance under CT in the NCI and MCI groups, an ROC analysis of the 8UG test parameters under CT condition was subsequently conducted to compare the ability to identify the participants at high risk of developing MCI. ROC curves for the four 8UG test parameters under CT to predict MCI were shown in Fig. 1. The AUC of 8UGCT, DTCCT, number of correct answers and CRR were 0.516, 0.520, 0.678 and 0.691, respectively. Numbers of correct answers and CRR showed good abilities to predict MCI in community-dwelling older women (p < 0.05).
Fig.1.
Receiver Operator Characteristic (ROC) Curves to Predict MCI for Parameters of 8UG Test under Cognitive Dule-task (CT) Condition. The predictive ability of 8UG-CT is evaluated by using ROC analysis. Area under the curve (AUC) of 8UGCT (purple curve), DTCCT (yellow curve), the number of correct answers (blue curve) and the correct response rate (CRR, green curve) is 0.516 (p = 0.786), 0.520 (p = 0.731), 0.678 (p = 0.002) and 0.691 (p = 0.001). The dotted line represents reference line
Of four parameters, CRR showed the strongest ability to predict MCI. The optimal cut-off values, AUC, sensitivity, and specificity of CRR were showed in Table 4. In order to ensure a high sensitivity (71.2%) and specificity (61.2%) for MCI prediction, the optimal cut-off point of CRR was 0.50.
Table 4.
Optimal cut-off value, AUC, sensitivity, and specificity of CRR to predict the community-dwelling older women at high risk for developing MCI
| CRR | Value |
|---|---|
| Cut-off point | 0.50 |
| AUC | 0.691 |
| Sensitivity | 71.2% |
| Specificity | 61.2% |
CRR correct response rate, AUC area under the curve
Discussion
The main findings of this study were: (1) both manual and cognitive dual-task interfered with the performance of the 8UG test, (2) no differences were observed in the 8UG test performance under single- and manual dual-task conditions among participants with and without cognitive impairment, (3) compared with NCI group, participants in MCI group performed worse in certain cognitive dual-task 8UG test parameters, including number of correct answers (cognitive task performance) and CRR (cognitive dual-task interference), and (4) CRR of cognitive dual-task 8UG test could be regarded as a potential predictor of MCI in community-dwelling older women.
In the current study, the dual-task paradigm including manual and cognitive conditions were applied to develop the 8UG test. To our knowledge, this is the first study which aimed to investigate the 8UG performance under dual-task conditions in older women with different levels of cognitive ability. Previously, an animal naming dual-task 8UG test was reported once as one of the cognitive-motor function assessments to measure the motor-cognitive performance in patients with dementia [52]. Considering that the 8UG test is a valid, reliable, and wildly used mobility assessment [48, 49], and mobility in daily life often requires performing multiple tasks simultaneously, there is substantial need for developing dual-task versions of 8UG test to detect early impairment of mobility and cognitive function. With reference to previous studies, two dual-task 8UG tests were developed in this study: manual dual-task (performing the 8UG while holding a cup filled with water concurrently) [35, 38, 50, 51], and cognitive dual-task (performing the 8UG while doing serial subtraction concurrently) [33, 52, 53].
Dual-task might elevate the central resource demand and generate potential resources competition between tasks. It is worth noting that although no task prioritization was given by the instructor, all participants in this study spent longer time in completing the 8UG test under both MT and CT than under ST. The impaired performance of the 8UG test is referred to as dual-task interference [43, 54], and could be quantified by calculating the DTC [43, 55]. In this study, the DTC values under MT and CT were positive (+) attributing to the decrement 8UG performance under dual-task conditions [44]. These findings were consistent with previous findings in dual-task TUG researches [26, 56, 57].
Task interference between motor and posture control tasks occurred often in daily life [41]. For instance, walking while carrying grocery bags or turning back while carrying a cup of coffee. A prior study has reported that participants in different cognitive level groups (NCI, MCI and Alzheimer’s disease) showed different TUG performance under both single- and dual-task conditions [51]. However, in the present study, no group differences between NCI and MCI were found in both single-task 8UG test performance and manual dual-task performance. One possible explanation might be that the participants of this study were younger and healthier, therefore most of them did not yet present severe physical and cognitive function decline.
Numerous studies have confirmed the association between mobility and cognitive function [13, 58]. The age-related mobility decline may be partly compensated by cognitive involvement. Also, the decline of cognitive function may be accelerated due to mobility impairment [58]. Based on this, dual-task performance depends on mobility, cognitive function and the interplay between cognition and motor control [59]. The cognitive dual-task testing paradigm was thus considered as a valuable tool for the early detection of mobility and cognitive decline [17, 60]. In this study, although no between-group statistical differences were found in 8UGCT and DTCCT, the MCI group tended to have a longer 8UG time and higher DTC value under CT than ST. In addition, other two parameters of 8UG-CT were observed to have significant between-group differences. These findings revealed that cognitive dual-task led to a decline in mobility performance and the decline varied according to different cognitive abilities.
To further assess the predictive ability of cognitive dual-task parameters, the ROC analysis was conducted. Among four parameters, CRR was the most informative indicator showing an AUC of 0.691 (p = 0.001). Although the AUC of CRR was less accurate, the results still implied that CRR was a valuable indicator for early detection of MCI. Previous studies showed that the sensitivity of commonly used instruments for detecting MCI was generally lower. The diagnostic accuracy across studies varied widely with different measurements and cut-points. For instance, the results by Clock Drawing Test and Mini Mental State Examination were inconsistent in detection of MCI [61]. In the current study, the sensitivity of CRR was 71.2% with a cut-off point of 0.50, indicating that CRR was a valuable indicator for early detection of MCI in large population. At the same time, CRR was a derived indicator, which was linked with both mobility and cognitive functions and their interferences. Additionally, considering that significant financial costs and resources consumptions on time, training and diagnosis for detecting MCI [62], it is necessary to conduct research on simple indicators similar to CRR in a larger population.
Studies have suggested that cognitive dual-task testing could be recommended to predict MCI [63, 64] and dementia [65, 66]. Previous studies have found that cognitive function was related to key components of mobility, including gait, turning and transitions [67]. The performance of walking straight-ahead and turning-around was affected by the complexity of secondary tasks in cognitive dual-task TUG [68]. Dual-task TUG has been suggested as an auxiliary diagnostic tool for dementia and MCI [25]. In the 8UG-CT test of this study, significant differences were found in the number of correct answers and CRR between NCI and MCI groups. Based on ROC analysis, CRR, with a cut-off point of 0.50, might be recommended as a potential predictor for the early detection of MCI.
Several limitations should be noted in this study. Firstly, this study is a cross-sectional design. Thus, it is hard to determine a causal relationship. Secondly, the use of MoCA-BJ could also be regarded as a limitation. Among five Chinese versions of MoCA, MoCA-BJ is the most popular version in the mainland China owing to the Mandarin Chinese used in its instruction [69]. Although all participants have adequate ability to understand Mandarin Chinese, language barriers between Mandarin and regional dialects are still encountered during face-to-face interviews sporadically, which might cause misunderstandings. Thirdly, only 101 community-dwelling older women aged 60 to 74 were recruited in this study. It is essential to conduct early detection among men as well, even though women are at higher risk of cognitive and mobility decline than men as we mentioned above. The main findings need to examine both women and men of a wider age bracket with larger sample size.
Future studies are required to examine: the reliability and validity of the dual-task 8UG test, the causality relationship between dual-task 8UG performance, and cognitive function with a prospective longitudinal design, as well as the dual-task 8UG performance in a broader population.
Conclusions
In conclusion, this study developed dual-task 8UG tests. The findings of the current study showed that both manual and cognitive dual-task interfered with the 8UG performance in both NCI and MCI groups, whereas only cognitive dual-task 8UG test parameters had group differences. Moreover, the CRR of cognitive dual-task 8UG test could be recommended as a potential predictor for the early detection of MCI in community-dwelling older women.
Author contributions
All authors contributed to the study conception and design. Methodology, project administration and formal analysis were performed by Jingjing Wang. Investigation and visualization were performed by Yun Xiang and Jin-Tao Hong. Supervision and validation were performed by Chunhua Zhang. The first draft of the manuscript was written by Jingjing Wang. And all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
The authors did not receive support from any organization for the submitted work.
Data availability
The datasets generated and analyzed during this study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Ethics approval
The study was approved by the Chinese Ethics Committee of Registering Clinical Trials (ChiECRCT20200388).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Budni J, Bellettini-Santos T, Mina F, Garcez ML, Zugno AI. The involvement of BDNF, NGF and GDNF in aging and Alzheimer's disease. Aging Dis. 2015;6:331–341. doi: 10.14336/AD.2015.0825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schlesinger KJ, Turner BO, Lopez BA, Miller MB, Carlson JM. Age-dependent changes in task-based modular organization of the human brain. Neuroimage. 2017;146:741–762. doi: 10.1016/j.neuroimage.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Mowszowski L, Batchelor J, Naismith SL. Early intervention for cognitive decline: can cognitive training be used as a selective prevention technique? Int Psychogeriatr. 2010;22:537–548. doi: 10.1017/S1041610209991748. [DOI] [PubMed] [Google Scholar]
- 4.Oyama A, Takeda S, Ito Y, Nakajima T, Takami Y, Takeya Y, Yamamoto K, Sugimoto K, Shimizu H, Shimamura M, Katayama T, Rakugi H, Morishita R. Novel method for rapid assessment of cognitive impairment using high-performance eye-tracking technology. Sci Rep. 2019;9:12932. doi: 10.1038/s41598-019-49275-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Montero-Odasso M, Bherer L, Studenski S, Gopaul K, Oteng-Amoako A, Woolmore-Goodwin S, Stoole P, Wells J, Doherty T, Zecevic AA, Galinsky D, Rylett RJ, Jutai J, Muir-Hunter S, Speechley M, Camicioli R. Mobility and cognition in seniors. Report from the 2008 Institute of Aging (CIHR) mobility and cognition workshop. Can Geriatr J. 2015;18:159–167. doi: 10.5770/cgj.18.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng Q, Wu M, Wu Y, Hu Y, Kwapong WR, Shi X, Fan Y, Yu X, He J, Wang Z. Weaker braking force, a new marker of worse gait stability in Alzheimer disease. Front Aging Neurosci. 2020 doi: 10.3389/fnagi.2020.554168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freiberger E, Sieber CC, Kob R. Mobility in older community-dwelling persons: a narrative review. Front Physiol. 2020;11:881. doi: 10.3389/fphys.2020.00881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ambrose AF, Paul G, Hausdorff JM. Risk factors for falls among older adults: a review of the literature. Maturitas. 2013;75:51–61. doi: 10.1016/j.maturitas.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 9.Liu Y, Chan JS, Yan JH. Neuropsychological mechanisms of falls in older adults. Front Aging Neurosci. 2014;6:64. doi: 10.3389/fnagi.2014.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tian Q, An Y, Resnick SM, Studenski S. The relative temporal sequence of decline in mobility and cognition among initially unimpaired older adults: results from the Baltimore Longitudinal Study of Aging. Age Ageing. 2017;46:445–451. doi: 10.1093/ageing/afw185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verghese J, Wang C, Lipton RB, Holtzer R. Motoric cognitive risk syndrome and the risk of dementia. J Gerontol A Biol Sci Med Sci. 2013;68:412–418. doi: 10.1093/gerona/gls191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woollacott M, Shumway-Cook A. Attention and the control of posture and gait: a review of an emerging area of research. Gait Posture. 2002;16:1–14. doi: 10.1016/s0966-6362(01)00156-4. [DOI] [PubMed] [Google Scholar]
- 13.Hsu CL, Nagamatsu LS, Davis JC, Liu-Ambrose T. Examining the relationship between specific cognitive processes and falls risk in older adults: a systematic review. Osteoporos Int. 2012;23:2409–2424. doi: 10.1007/s00198-012-1992-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boisgontier MP, Beets IA, Duysens J, Nieuwboer A, Krampe RT, Swinnen SP. Age-related differences in attentional cost associated with postural dual tasks: increased recruitment of generic cognitive resources in older adults. Neurosci Biobehav Rev. 2013;37:1824–1837. doi: 10.1016/j.neubiorev.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 15.Li KZH, Bherer L, Mirelman A, Maidan I, Hausdorff JM. Cognitive involvement in balance, gait and dual-tasking in aging: a focused review from a neuroscience of aging perspective. Front Neurol. 2018;9:913. doi: 10.3389/fneur.2018.00913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stark SL, Roe CM, Grant EA, Hollingsworth H, Benzinger TL, Fagan AM, Buckles VD, Morris JC. Preclinical Alzheimer disease and risk of falls. Neurology. 2013;81:437–443. doi: 10.1212/WNL.0b013e31829d8599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Montero-Odasso M, Verghese J, Beauchet O, Hausdorff JM. Gait and cognition: a complementary approach to understanding brain function and the risk of falling. J Am Geriatr Soc. 2012;60:2127–2136. doi: 10.1111/j.1532-5415.2012.04209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Montero-Odasso M, Speechley M. Falls in cognitively impaired older adults: implications for risk assessment and prevention. J Am Geriatr Soc. 2018;66:367–375. doi: 10.1111/jgs.15219. [DOI] [PubMed] [Google Scholar]
- 19.Borel L, Alescio-Lautier B. Posture and cognition in the elderly: interaction and contribution to the rehabilitation strategies. Neurophysiol Clin. 2014;44:95–107. doi: 10.1016/j.neucli.2013.10.129. [DOI] [PubMed] [Google Scholar]
- 20.Brustio PR, Magistro D, Zecca M, Liubicich ME, Rabaglietti E. Fear of falling and activities of daily living function: mediation effect of dual-task ability. Aging Ment Health. 2018;22:856–861. doi: 10.1080/13607863.2017.1318257. [DOI] [PubMed] [Google Scholar]
- 21.Soubra R, Chkeir A, Novella JL. A systematic review of thirty-one assessment tests to evaluate mobility in older adults. Biomed Res Int. 2019 doi: 10.1155/2019/1354362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rikli RE, Jones CJ. Development and validation of a functional fitness test for community-residing older adults. J Aging Phys Act. 1999;7:129–161. doi: 10.1123/japa.7.2.129. [DOI] [Google Scholar]
- 23.Rose DJ, Jones CJ, Lucchese N. Predicting the probability of falls in community-residing older adults using the 8-Foot Up-and-Go: a new measure of functional mobility. J Aging Phys Act. 2002;10:466–475. doi: 10.1123/japa.10.4.466. [DOI] [Google Scholar]
- 24.Cedervall Y, Stenberg AM, Ahman HB, Giedraitis V, Tinmark F, Berglund L, Halvorsen K, Ingelsson M, Rosendahl E, Aberg AC. Timed up-and-go dual-task testing in the assessment of cognitive function: a mixed methods observational study for development of the UDDGait protocol. Int J Environ Res Public Health. 2020 doi: 10.3390/ijerph17051715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahman HB, Cedervall Y, Kilander L, Giedraitis V, Berglund L, McKee KJ, Rosendahl E, Ingelsson M, Aberg AC. Dual-task tests discriminate between dementia, mild cognitive impairment, subjective cognitive impairment, and healthy controls—a cross-sectional cohort study. BMC Geriatr. 2020;20:258. doi: 10.1186/s12877-020-01645-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Asai T, Oshima K, Fukumoto Y, Yonezawa Y, Matsuo A, Misu S. Does dual-tasking provide additional value in timed "up and go" test for predicting the occurrence of falls? A longitudinal observation study by age group (young-older or old-older adults) Aging Clin Exp Res. 2021;33:77–84. doi: 10.1007/s40520-020-01510-6. [DOI] [PubMed] [Google Scholar]
- 27.Hofheinz M, Mibs M. The prognostic validity of the timed up and go test with a dual task for predicting the risk of falls in the elderly. Gerontol Geriatr Med. 2016;2:2333721416637798. doi: 10.1177/2333721416637798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nicolson PJA, Sanchez-Santos MT, Bruce J, Kirtley S, Ward L, Williamson E, Lamb SE. Risk factors for mobility decline in community-dwelling older adults: a systematic literature review. J Aging Phys Act. 2021;29:1053–1066. doi: 10.1123/japa.2020-0482. [DOI] [PubMed] [Google Scholar]
- 29.Yuan Y, Chen YP, Boyd-Kirkup J, Khaitovich P, Somel M. Accelerated aging-related transcriptome changes in the female prefrontal cortex. Aging Cell. 2012;11:894–901. doi: 10.1111/j.1474-9726.2012.00859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brustio PR, Magistro D, Rabaglietti E, Liubicich ME. Age-related differences in dual task performance: a cross-sectional study on women. Geriatr Gerontol Int. 2017;17:315–321. doi: 10.1111/ggi.12700. [DOI] [PubMed] [Google Scholar]
- 31.Yu J, Li J, Huang X. The Beijing version of the Montreal Cognitive Assessment as a brief screening tool for mild cognitive impairment: a community-based study. BMC Psychiatry. 2012;12:156. doi: 10.1186/1471-244X-12-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tian Y, Jiang C, Wang M, Cai R, Zhang Y, He Z, Wang H, Wu D, Wang F, Liu X, He Z, An P, Wang M, Tang Q, Yang Y, Zhao J, Lv S, Zhou W, Yu B, Lan J, Yang X, Zhang L, Tian H, Gu Z, Song Y, Huang T, McNaughton LR. BMI, leisure-time physical activity, and physical fitness in adults in China: results from a series of national surveys, 2000–14. Lancet Diabetes Endocrinol. 2016;4:487–497. doi: 10.1016/s2213-8587(16)00081-4. [DOI] [PubMed] [Google Scholar]
- 33.Brustio PR, Magistro D, Zecca M, Rabaglietti E, Liubicich ME. Age-related decrements in dual-task performance: comparison of different mobility and cognitive tasks. A cross sectional study. PLoS One. 2017 doi: 10.1371/journal.pone.0181698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bergamin M, Gobbo S, Zanotto T, Sieverdes JC, Alberton CL, Zaccaria M, Ermolao A. Influence of age on postural sway during different dual-task conditions. Front Aging Neurosci. 2014;6:271. doi: 10.3389/fnagi.2014.00271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lundin-Olsson L, Nyberg L, Gustafson Y. Attention, frailty, and falls: the effect of a manual task on basic mobility. J Am Geriatr Soc. 1998;46:758–761. doi: 10.1111/j.1532-5415.1998.tb03813.x. [DOI] [PubMed] [Google Scholar]
- 36.Taylor ME, Delbaere K, Mikolaizak AS, Lord SR, Close JC. Gait parameter risk factors for falls under simple and dual task conditions in cognitively impaired older people. Gait Posture. 2013;37:126–130. doi: 10.1016/j.gaitpost.2012.06.024. [DOI] [PubMed] [Google Scholar]
- 37.Chen HY, Tang PF. Contributing factors of single-and dual-task timed up & go performance in active community-dwelling middle-aged and older adults. Phys Ther. 2016;96:284–292. doi: 10.2522/ptj.20140292. [DOI] [PubMed] [Google Scholar]
- 38.Hofheinz M, Schusterschitz C. Dual task interference in estimating the risk of falls and measuring change: a comparative, psychometric study of four measurements. Clin Rehabil. 2010;24:831–842. doi: 10.1177/0269215510367993. [DOI] [PubMed] [Google Scholar]
- 39.Ansai JH, Aurichio TR, Rebelatto JR. Relationship between balance and dual task walking in the very elderly. Geriatr Gerontol Int. 2016;16:89–94. doi: 10.1111/ggi.12438. [DOI] [PubMed] [Google Scholar]
- 40.Muir-Hunter SW, Clark J, McLean S, Pedlow S, Van Hemmen A, Montero Odasso M, Overend T. Identifying balance and fall risk in community-dwelling older women: the effect of executive function on postural control. Physiother Can. 2014;66:179–186. doi: 10.3138/ptc.2013-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Granacher U, Bridenbaugh SA, Muehlbauer T, Wehrle A, Kressig RW. Age-related effects on postural control under multi-task conditions. Gerontology. 2011;57:247–255. doi: 10.1159/000322196. [DOI] [PubMed] [Google Scholar]
- 42.Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 43.Plummer P, Eskes G. Measuring treatment effects on dual-task performance: a framework for research and clinical practice. Front Hum Neurosci. 2015;9:225. doi: 10.3389/fnhum.2015.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McIsaac TL, Lamberg EM, Muratori LM. Building a framework for a dual task taxonomy. Biomed Res Int. 2015 doi: 10.1155/2015/591475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Asai T, Oshima K, Fukumoto Y, Yonezawa Y, Matsuo A, Misu S. Association of fall history with the timed up and go test score and the dual task cost: a cross-sectional study among independent community-dwelling older adults. Geriatr Gerontol Int. 2018;18:1189–1193. doi: 10.1111/ggi.13439. [DOI] [PubMed] [Google Scholar]
- 46.Yang L, He C, Pang MY. Reliability and validity of dual-task mobility assessments in people with chronic stroke. PLoS One. 2016 doi: 10.1371/journal.pone.0147833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kelly VE, Janke AA, Shumway-Cook A. Effects of instructed focus and task difficulty on concurrent walking and cognitive task performance in healthy young adults. Exp Brain Res. 2010;207:65–73. doi: 10.1007/s00221-010-2429-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hesseberg K, Bentzen H, Bergland A. Reliability of the senior fitness test in community-dwelling older people with cognitive impairment. Physiother Res Int. 2015;20:37–44. doi: 10.1002/pri.1594. [DOI] [PubMed] [Google Scholar]
- 49.Miotto JM, Chodzko-Zajko WJ, Reich JL, Supler MM. Reliability and validity of the fullerton functional fitness test: an independent replication study. J Aging Phys Act. 1999;7:339–353. doi: 10.1123/japa.7.4.339. [DOI] [Google Scholar]
- 50.Shumway-Cook A, Brauer S, Woollacott M. Predicting the probability for falls in community-dwelling older adults using the timed up & go test. Phys Ther. 2000;80:896–903. doi: 10.1093/ptj/80.9.896. [DOI] [PubMed] [Google Scholar]
- 51.Borges Sde M, Radanovic M, Forlenza OV. Functional mobility in a divided attention task in older adults with cognitive impairment. J Mot Behav. 2015;47:378–385. doi: 10.1080/00222895.2014.998331. [DOI] [PubMed] [Google Scholar]
- 52.Srygley JM, Mirelman A, Herman T, Giladi N, Hausdorff JM. When does walking alter thinking? Age and task associated findings. Brain Res. 2009;1253:92–99. doi: 10.1016/j.brainres.2008.11.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Muir SW, Speechley M, Wells J, Borrie M, Gopaul K, Montero-Odasso M. Gait assessment in mild cognitive impairment and Alzheimer's disease: the effect of dual-task challenges across the cognitive spectrum. Gait Posture. 2012;35:96–100. doi: 10.1016/j.gaitpost.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 54.Bridenbaugh SA, Kressig RW. Laboratory review: the role of gait analysis in seniors' mobility and fall prevention. Gerontology. 2011;57:256–264. doi: 10.1159/000322194. [DOI] [PubMed] [Google Scholar]
- 55.Fallahtafti F, Boron JB, Venema DM, Kim HJ, Yentes JM. Task specificity impacts dual-task interference in older adults. Aging Clin Exp Res. 2021;33:581–587. doi: 10.1007/s40520-020-01575-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gomes Gde C, Teixeira-Salmela LF, Fonseca BE, Freitas FA, Fonseca ML, Pacheco BD, Goncalves MR, Caramelli P. Age and education influence the performance of elderly women on the dual-task timed up and go test. Arq Neuropsiquiatr. 2015;73:187–193. doi: 10.1590/0004-282X20140233. [DOI] [PubMed] [Google Scholar]
- 57.Tomas-Carus P, Biehl-Printes C, Pereira C, Veiga G, Costa A, Collado-Mateo D. Dual task performance and history of falls in community-dwelling older adults. Exp Gerontol. 2019;120:35–39. doi: 10.1016/j.exger.2019.02.015. [DOI] [PubMed] [Google Scholar]
- 58.Demnitz N, Esser P, Dawes H, Valkanova V, Johansen-Berg H, Ebmeier KP, Sexton C. A systematic review and meta-analysis of cross-sectional studies examining the relationship between mobility and cognition in healthy older adults. Gait Posture. 2016;50:164–174. doi: 10.1016/j.gaitpost.2016.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bayot M, Dujardin K, Dissaux L, Tard C, Defebvre L, Bonnet CT, Allart E, Allali G, Delval A. Can dual-task paradigms predict Falls better than single task? A systematic literature review. Neurophysiol Clin. 2020;50:401–440. doi: 10.1016/j.neucli.2020.10.008. [DOI] [PubMed] [Google Scholar]
- 60.Mancioppi G, Fiorini L, Rovini E, Cavallo F. The use of Motor and Cognitive Dual-Task quantitative assessment on subjects with mild cognitive impairment: a systematic review. Mech Ageing Dev. 2021 doi: 10.1016/j.mad.2020.111393. [DOI] [PubMed] [Google Scholar]
- 61.Patnode CD, Perdue LA, Rossom RC, Rushkin MC, Redmond N, Thomas RG, Lin JS. U.S. Preventive Services Task Force Evidence Syntheses, formerly Systematic Evidence Reviews. Screening for Cognitive Impairment in Older Adults: An Evidence Update for the U.S. Preventive Services Task Force. Rockville (MD): Agency for Healthcare Research and Quality (US); 2020. eng. [PubMed]
- 62.Langa KM, Levine DA. The diagnosis and management of mild cognitive impairment: a clinical review. JAMA. 2014;312:2551–2561. doi: 10.1001/jama.2014.13806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Montero-Odasso M, Oteng-Amoako A, Speechley M, Gopaul K, Beauchet O, Annweiler C, Muir-Hunter SW. The motor signature of mild cognitive impairment: results from the gait and brain study. J Gerontol A Biol Sci Med Sci. 2014;69:1415–1421. doi: 10.1093/gerona/glu155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Klotzbier TJ, Schott N. Cognitive-motor interference during walking in older adults with probable mild cognitive impairment. Front Aging Neurosci. 2017 doi: 10.3389/fnagi.2017.00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yogev-Seligmann G, Hausdorff JM, Giladi N. The role of executive function and attention in gait. Mov Disord. 2008 doi: 10.1002/mds.21720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.de Oliveira SF, Ferreira JV, Placido J, Deslandes AC. Spatial navigation and dual-task performance in patients with Dementia that present partial dependence in instrumental activity of daily living. IBRO Rep. 2020;9:52–57. doi: 10.1016/j.ibror.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sunderaraman P, Maidan I, Kozlovski T, Apa Z, Mirelman A, Hausdorff JM, Stern Y. Differential associations between distinct components of cognitive function and mobility: implications for understanding aging, turning and dual-task walking. Front Aging Neurosci. 2019;11:166. doi: 10.3389/fnagi.2019.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Porciuncula FS, Rao AK, McIsaac TL. Aging-related decrements during specific phases of the dual-task Timed Up-and-Go test. Aging Clin Exp Res. 2016;28:121–130. doi: 10.1007/s40520-015-0372-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yu K, Zhang S, Wang Q, Wang X, Qin Y, Wang J, Li C, Wu Y, Wang W, Lin H. Development of a computerized tool for the chinese version of the montreal cognitive assessment for screening mild cognitive impairment. Int Psychogeriatr. 2014 doi: 10.1017/S1041610214002269. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed during this study are available from the corresponding author on reasonable request.