Abstract
Meal replacements and food supplements are now popular commercial weight loss and nutrition products. This review describes the efficacy, effectiveness, and therapeutic use of one such product - a soy-yoghurt-honey food formulation. The original formula of this product was created more than thirty years ago and since that time it has become well established as a food supplement supporting a healthy lifestyle. Therapeutic evidence for this product is based on numerous scientific studies and clinical trials, focusing particularly on weight management and associated metabolic risk factors and published as peer-reviewed articles. Given the availability of the product and the extent to which it has been experimentally evaluated, it is timely and important that the research is brought together under a single review to consolidate the understanding for the scientific and clinical communities. This review discusses the ingredients and the broad mechanisms of action, which are probably due to the biological properties of the three base components - soy, milk, and honey. It further summarizes and discusses the laboratory and clinical intervention studies, including the biochemical and metabolic mechanisms regarding the insulin- and lipid-lowering, anti-hypertensive, anti-inflammatory, antioxidant, and anti-microbial properties of the overall food and its base products.
Keywords: Soy-yoghurt-honey formula, Soy proteins, Biologically active peptides, Isoflavones, Weight management, Muscle mass, Prediabetes, Leptin resistance
Soy-yoghurt-honey formula; Soy proteins; Biologically active peptides; Isoflavones; Weight management; Muscle mass; Prediabetes; Leptin resistance.
1. Introduction
As part of a healthy lifestyle, functional foods can have a positive health benefit beyond their basic nutritional components and are an established feature of the food industry. Amongst the diverse range of products, a soy-yoghurt-honey formulation has been a commercially available supplement and meal replacement product for several years. This review will describe the efficacy, effectiveness, and therapeutic use of this product, particularly in the area of weight loss and metabolic health. The formulation of this product was created over thirty years ago with the initial aim to improve and activate the metabolism of patients with excess weight. The product is now established in the European and North American markets for food supplements, supported by several clinical trials and scientific studies evaluating its functionality and broad mechanism of action.
Over time, the primary objective of the product has increasingly been directed towards supporting weight loss, fat mass (FM) reduction and long-term weight stabilization. The collective findings of research and consumer use consistently demonstrate that weight management is favorably influenced by the soy-yoghurt-honey formula and that markers of metabolic risk, including type 2 diabetes (T2DM) are also improved. Thus, the nutritional product has been shown to support a healthy lifestyle and help enhance metabolic health [1, 2, 3].
The experimental findings when using this product are consistent with standard obesity treatment and prevention guidelines, including those of the German and European Obesity Societies, concerning the quality and outcomes of intervention programs [4, 5]. The main aim of weight loss strategies should not necessarily be weight loss per se, but rather an improvement in cardio-metabolic risk factors, achieved through a lifestyle that combines increased physical activity with a healthy diet which ultimately leads to an improvement in quality of life. This aim can be achieved through supervised weight management programmes, lasting at least 12 months and executed by a multi-disciplinary team including physicians, nutritionists, psychologists, exercise professionals and group behavior consultants. This strategy has to date, formed the cornerstone by which healthy modifications to diet and exercise incorporated into an individual's everyday life leads to improved body weight and health outcomes [6, 7, 8]. These lifestyle modifications are also essential in order to counterbalance the global health emergency of the current Covid-19 pandemic through preventive measures [9, 10], particularly in the elderly population [11, 12].
This review primarily centers on research that has originated from the “Rehabilitative and Preventive Sports Medicine” group at the Medical University Clinic Freiburg. In collaboration with other departments of the Medical Clinic, they have continuously researched the effect and mode of action of the soy-yogurt-honey formula. To place this review within a wider research context, a recently conducted literature search using the terms “protein-based supplements” and “protein-based meal replacement” as well as “soy protein-based supplements” and “soy protein-based meal replacement” indicated that the number of publications especially with the term “protein-based supplements” has increased substantially over the last 10 years. Restricting the search term to “soy protein”, resulted in a smaller number of publications listed, and moreover, were not necessarily focused on the topic “weight management and obesity-associated health risk”. Nevertheless, a meta-analysis of randomized controlled studies on the topic “soy products and overweight” has recently been published [1]. With this in mind, the following sections of this review will discuss the formulation of the described soy-yoghurt-honey product and how it can support a healthy lifestyle in the context of a dietary therapeutic agent.
2. Food ingredients and properties of the soy-yoghurt-honey product
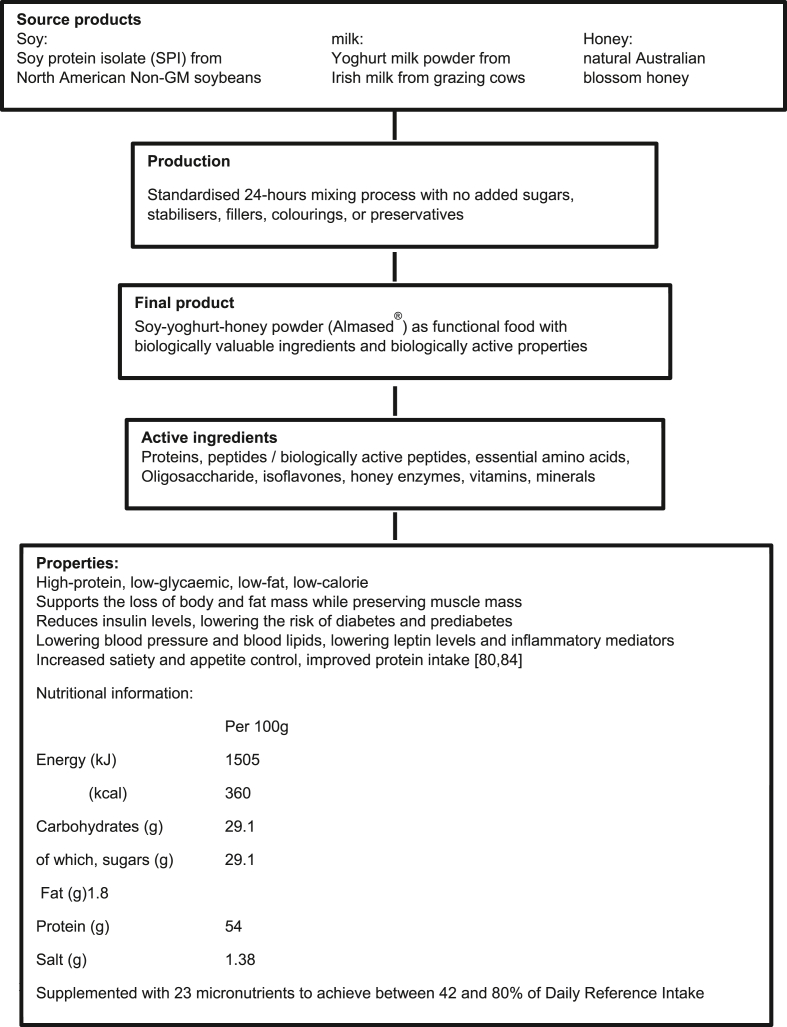
The soy-yoghurt-honey formulation presents as a dried powder which can be reconstituted with either water, skimmed cow's milk or a plant-based milk to make a drink or “shake”. It is supplemented with micronutrients and is designed to be used as a meal replacement product. Soy protein isolate from a non-genetically modified source comprises by weight the largest component of the soy-yoghurt-honey formulation. It also represents the key nutritional and biological elements [13]. The protein content of the whole product is 52.2% by weight (83% soy-protein-isolate and 17% milk protein); in addition, the product is characterized by a low glycemic index (GI = 27) and a low glycemic load (a reflection of the lactose and honey components, GL of 100 g dried powder: 7.8); a low fat (1.8 g/100 g dried powder) and low energy content (1505 kJ (360 kcal)/100 g dried powder). When reconstituted according to the manufacturer's guidelines, a single serving provides 180 kcal, together with 27 g protein which provides 60% of the energy content. Thus, the formula fulfils the condition of a high-protein, low-glycemic supplement [Figure 1]. This feature is important for many individuals, not only for weight-loss, but also for older adults (e.g., postmenopausal women or those with sarcopenia) and for healthy-weight subjects and athletes who may fail to achieve optimal daily intakes of dietary protein [12, 14, 15, 16]. In this regard, it is important to recognize that individuals who regularly consume the protein-rich low-glycemic formula not only increase their daily protein intake but also tend to prefer a protein-rich and low-glycemic diet in the long term; this is especially the case for women (see below) [17].
Figure 1.
The soy-yoghurt-honey formula – from the source product to the final product and its specific properties and nutritional information.
2.1. Proteins
In typical adult daily energy intakes, protein contributes between 10-15% of energy but in certain situations can contribute up to 20 and 30%. Any concern for potential renal tolerance under increased protein intake can be alleviated. Based on the recommendations of medical and dietetic authorities, an upper limit of 0.8 g protein/kg body weight (BW) per day is necessary only in the case of severely impaired renal function (glomerular filtration rate GFR <30 mL/min/1.73 m2) [18]. To exclude any possible negative effects on renal function in cases where an intake of the protein-rich (soy-yoghurt-honey) meal replacement is recommended, GFR has been clinically investigated. In a group of male patients [n = 10, 46 ± 13 y, 32.2 ± 3.48 kg/m2] with metabolic syndrome (MetS), following an acute consumption (a single meal) of 0.3 g protein per kg body weight, it was shown that the usual dosage of the product used in the meal replacement strategy did not have any clinically relevant effects on renal function whereas when fed at the higher level of 1.0 g protein/kg body weight, GFR was increased, being more pronounced in those with MetS. Nevertheless, it could be concluded that the amount of protein typically provided in the meal replacement therapy of the soy-yoghurt-honey preparation does not negatively affect renal function [19].
In addition to total protein content and intake, it is also important to acknowledge the protein quality; this allows for the comparability of different dietary proteins, and relates to the (essential) amino acid composition of the respective proteins and their digestibility. In nutrition practice, the amino acid score (AAS) (defined from chemical protein analysis, identifying the limiting essential amino acid), as well as the Digestible Indispensable Amino Acid Score (DIAAS) are used to describe the availability of essential and conditionally indispensable amino acids of a food [20]. Based on the quantities of essential and conditionally indispensable amino acids in the product as stated by the manufacturer (Almased Vitalkost GmbH, Bienenbüttel, Germany), a single meal (shake) reconstituted as recommended (1 g powder per kg normal body weight per meal replacement) provides the essential and conditionally indispensable amino acids in excess of the estimated adult requirements [21, 22]. If the levels and ratios of the essential and conditionally indispensable amino acids of the WHO/FAO/UNU “reference protein” [21] and the composition the soy-yoghurt-honey formula protein [23] in mg/g protein are compared, a relatively favorable composition in the amino acid pattern and hence protein quality is observed for the formula protein. If the estimated requirements [22, 23] are used, the AAS for the Almased® formula are above 1.1, if, as the limiting amino acid for the formula, the content of the sulphur-containing amino acids (methionine and (or +) cysteine is used. If the AAS for the formula protein is calculated with reference to the amino acid leucine, which is important for muscle protein synthesis and oxidation, an AAS of 1.4 is obtained. Furthermore, data presented by Bilsborough & Mann 2006 [24] indicates that the true digestibility of the proteins intrinsic to the soy-yoghurt-honey powder (in this case soy protein isolate and milk protein), is almost 100% when compared against the lumen absorption rates of equivalent loads of amino acids comprising the intrinsic proteins [24]. A large part of the formula protein is already present as peptide fragments as a result of the manufacturing process, and so it could be suggested that a correction of the AAS value based on the DIASS definition would not appear necessary. This indicates that the soy-yoghurt-honey formula contains a protein combination that is of high-quality, with high biological availability and meeting protein and amino acid requirements [25, 26, 27].
2.2. Biologically active peptides (BAPs)
Protein consumption is important not only for optimal protein synthesis but also for the uptake of biologically active peptides (BAPs), which may influence physiology, particularly in relation to obesity and its related metabolic disorders [28, 29]. These peptides are produced intra-lumenally through normal post-prandial protein digestion. Whereas most are absorbed as di-and tripeptides, larger molecular weight peptides may also be absorbed. These peptides may generate metabolic effects at physiological (microgram) quantities [26, 30, 31, 32, 33, 34, 35]. Furthermore, an excess of 1500 bioactive peptides, varying in their metabolic activity have been identified, suggesting potential biological functionality [27]. As with many peptide hormones, the chain length can range between two and twenty amino acids and in the case of the soybean peptide Lunasin, can extend up to 43 amino acids in length [36]. Generally, it is not until these peptides are cleaved from a precursor protein that they become potentially metabolically functional. Furthermore, their potential metabolic properties are dependent upon the digestibility, absorption and overall bioavailability to target tissues [27]. Milk (casein, whey) and soy proteins are considered to be sources of biologically active peptides, with other foods including fish and shellfish, egg, royal jelly, and the plant foods wheat, rice, barley and maize also being shown to be potential sources of bioactive peptides [27]. Using high-performance liquid chromatography coupled with mass spectroscopy, this soy-yoghurt-honey supplement has been shown to contain around eighty bioactive peptides [37], which may generate a variety of bioactivities with potential health benefits including blood pressure lowering, cholesterol lowering and triglyceride lowering properties, as well as anti-oxidative and even anticancer activities. Indeed, these bio-active peptides may even have anti-obesity properties [29]. Furthermore, the manufacturing process of the soy-yoghurt-honey-product and the mixing of its components may trigger proteolytic processes that not only increase the number of peptides, but also improve the digestibility as well as the metabolic activity of the final product [33, 34]. Following an exploratory approach, it has emerged that the soy-yoghurt-honey formula may be considered as having special characteristics which potentially could allow it to be designated as a “natural health product” [2, 3].
2.3. Isoflavones
Currently in a typical “Western” diet, soy protein only makes a minor dietary contribution. Nevertheless, soy-based foods have long been known as an excellent source of protein of high quality [34], coupled with a range of biologically active molecules. Some of the most studied molecules are the isoflavones, particularly in relation to cardiovascular, bone and menopausal health. The two main isoflavones are the phytoestrogens genistein and daidzein [38]. Similar in structure to the human steroidal oestrogen, these phytoestrogens bind to the nuclear oestrogen alpha and beta receptors, which in turn activate transcriptional processes and gene expression [39, 40]. Based on their molecular activities, soy isoflavones have favorable effects on fatty acid and lipoprotein metabolism and insulin sensitivity; Furthermore, conglycinin (a soy storage protein), soy saponins, and soy phospholipids appear to play a role in body weight regulation [41, 42].
As the soy-yoghurt-honey formula contains biologically available isoflavonoids at a level of approximately 1.5 mg per g dried powder, a typical serving of 50 g of the product can result in an intake of 75 mg isoflavones [23]. This level of consumption could be sufficient to raise plasma isoflavone levels (including daidzein, genistein, glycitein) to physiologically meaningful concentrations [43], detectable by Liquid Chromatography-Mass Spectroscopy analysis. There has been some concern around the association between consumption of phytoestrogens from food supplements and potential harmful effects on breast, uterine and thyroidal tissues in menopausal women. This issue has been evaluated by the European Food Safety Authority Panel on Food Additives and Nutrient Sources (EFSA ANS) [44]. They concluded that at intakes of between 35-150 mg/day from food supplements, there was no evidence for an increase in the risk of breast cancer when evaluated from cross-sectional studies, based upon either mammographic density, or on proliferation marker Ki-67 expression in interventional studies. In addition, no effect on endometrial thickness or histopathological changes in the uterus was found in response to supplementation with 150 mg/day soy isoflavones for up to 30 months. Thyroid hormone levels are also unchanged following intake of isoflavones from food supplements. In support of these conclusions, a recent systematic review was not able to find any association between isoflavone intake from food supplements and adverse effects on these target tissues. However, the Panel decided at that time that it would not be possible to determine health-based guidelines for isoflavone containing preparations, and to date, no health claims on isoflavones in foods registered in the EU Register of Nutrition and Health have been made (http://ec.europa.eu/nuhclaims/). In a study on 14 euthyroid postmenopausal women (64.2 ± 6.3 y; 24.4 ± 3.2 kg/m2) [45], we demonstrated that a daily intake of between 25 and 125 g of a soy-honey-yoghurt formula led to significant increases in the plasma concentrations of isoflavones but without concomitant changes in either thyroid or sex hormone levels. No linear increase in plasma isoflavone levels in relation to dietary isoflavone intake was observed, whereas blood isoflavone levels plateaued, particularly for genistein and daidzein, at an intake of 100 g of the soy-yoghurt-honey formula per day. If the results were interpreted as possible side effects, the formula intake had no negative impact upon either thyroid function or sex hormone levels in postmenopausal women. Furthermore, the effects on weight regulation using the soy-honey-yoghurt formula do not appear to be induced by a hyperthyroid state. However, when interpreting these findings, the sample size could be considered a limitation of the study leading to possible under powering of the statistical analyses.
In relation to cellular energy metabolism, evidence suggests that fatty acid oxidation can be increased via the actions of soy protein operating at the molecular level [46, 47]. Both insulin sensitivity and plasma lipid levels also appear to be improved by the actions of soy protein [48]. It has been proposed that these effects are brought about via the activation of peroxisome proliferator activated receptors (PPARs), affecting energy metabolism through the expression of genes involved in glucose homeostasis, lipid metabolism and fatty acid oxidation. Via this pathway, intake of isoflavone-rich soy protein in rats improved the above metabolic parameters, particularly insulin sensitivity and triglyceride concentrations. Additionally, in cell culture studies, isoflavone-rich soy extracts upregulate PPAR gene expression, suggesting a pathway by which the beneficial intake of soy protein on glucose and lipid metabolism can be mediated [51, 52]. In support of these in vitro studies, a rat feeding study of soy protein has demonstrated the upregulation of the enzymes involved in fatty acid oxidation at both mRNA and enzymatic protein levels within skeletal muscle fibres. These include carnitine palmitoyltransferase (CPT1), beta-hydroxyacyl-CoA dehydrogenase (HAD), Acyl-CoA oxidase, and medium-chain Acyl-CoA dehydrogenase [53]; this evidence provides a possible pathway by which soy protein ingestion can lead to a reduction in body fat accumulation. In addition, recently published results in human studies show that an acute intake of a soy-yoghurt-honey formula stimulates fatty acid oxidation [54].
2.4. Honey
The honey component itself is a functional food with potentially beneficial health properties, particularly in respect of its antimicrobial and antioxidant activity [55, 56]. Indeed, honey has long been used therapeutically in human diets [57]. At intakes of between 50 and 80 g per serving [58], such health enhancing properties can be observed. Even though honey is rich in sugars, its glycemic index can vary widely, depending on the botanical source of nectar and the ratio of glucose-fructose in it composition [57, 59]. A lesser-known nutritional feature is that honey also comprises of various oligosaccharides [58] as well as trace levels of proteins, enzymes, amino acids, vitamins, minerals and trace elements, aromatic compounds, and polyphenols [58, 60]. In particular, these oligosaccharide, polyphenol and flavonoid contents may explain, in part, the possible anti-diabetic, anti-obesity and anti-hyperlipidaemic properties [58, 61] and other health-related benefits of honey [62, 63]. A suggested mechanism of action for these properties could be via inhibition of the gene expression of sterol regulatory element-binding transcription Factor 1 and its target lipogenic enzyme fatty acid synthase (FAS) [61]. In addition, the gut microbiome may also be involved in the pathogenesis of insulin resistance and the dysregulation of glucose homeostasis [64, 65]. In this context, oligosaccharides (including those provided by honey) may contribute to the equilibrium of the gut microbiome through a dietary approach [66, 67], and therefore the routine substitution of sucrose with honey in the diet may beneficially modify the gut microbiome [68]. Furthermore, honey oligosaccharides may influence energy intake via specific appetite-regulating hormones [64, 69]; this could potentially be a key benefit of honey consumption in the prevention of excess weight gain [70]. As this soy-yoghurt-honey formula contains 25% by weight natural honey, a daily intake of between 50 to 75 g could be achieved when using the formula in a meal replacement approach and may be sufficient to achieve a nutritionally significant intake of oligosaccharides leading to potential health benefits.
3. Therapeutic properties of the soy-yoghurt-honey formula
The ingredients of the soy-yoghurt-honey formula are most likely responsible for the various metabolic and health effects documented across all experimental and clinical studies where the product has been evaluated. Positive effects from a regular intake can be expected on energy metabolism, (particularly fat oxidation), as well as on appetite. Furthermore, positive outcomes can be observed on serological, hormonal, immunological and body compositional variables with respect to risk of T2DM and the incidence of the MetS. In 2001, the Freiburg research group commenced the first explorative studies to identify and evaluate possible therapeutic effects following intake of the soy-yoghurt-honey formula as a foundation for further confirmatory studies [71, 72]. Since then, findings have been published in 30 peer-reviewed studies – mostly indexed in the PubMed NIH library - and available as full free texts for the scientific readership interested in these health-related lifestyle questions. These 30 studies are summarized in Table 1 and the following sections discuss their results in relation to specific areas of physiology, body composition, behavior, metabolic health and quality of life, highlighting the most important observations.
Table 1.
Summary of peer-reviewed publications to “Soy-yoghurt-honey Formula”.
| Reference | Study design | Sample size | Intervention Groups | Length of intervention | Outcomes | Main results |
|---|---|---|---|---|---|---|
| Berg et al. Ernährungs-Umschau. 2003; 50, 386–392 [72]. | Randomized controlled trial. | n = 83 with overweight and obesity. | Lifestyle education (LE-G, n = 28). Almased with physical activity (SD/PA-G, n = 27). Almased without physical activity (SD-G, n = 28). |
6 months. | Body weight, body composition, waist and hip circumferences, blood lipid profile, glucose, insulin, leptin, CRP, fibrinogen, and IL-6. | BMI, body weight, and hip circumference: ↓ more in SD/PA-G and SD-G compared with LE-G. BMI, body weight, fat mass, waist and hip circumferences, total cholesterol, HDL, LDL, and leptin: ↓ in all groups (over time). Fat-free mass: ↔. Insulin and CRP: ↓ only in SD/PA-G and in SD-G over time. IL-6: ↓ only in SD-G over time. |
| Deibert et al. International Journal of Obesity. 2004; 28, 1349–1352 [87]. | Randomized controlled trial. | n = 83 with overweight and obesity. | Lifestyle education (LE-G, n = 28). Almased with physical activity (SD/PA-G, n = 27). Almased without physical activity (SD-G, n = 28). |
24 weeks. | Body weight, body composition, metabolic and hormonal parameters. | BMI: ↓ in all groups (over time). Body weight and fat mass: ↓ more in SD/PA-G and SD-G compared with LE-G. Fat-free mass: ↔. Glycemic control and lipid profile: Improved in all groups (over tme). |
| Berg et al, Ernährungs-Umschau. 2005; 52, 310–314 [71]. | Randomized controlled trial. | n = 83 with overweight and obesity. | Lifestyle education (LE-G, n = 28). Almased with physical activity (SD/PA-G, n = 27). Almased without physical activity (SD-G, n = 28). |
1 year. | Body weight, body composition, waist and hip circumferences, blood lipid profile, glucose, insulin, leptin, CRP, fibrinogen, and IL-6. | Hip circumference: ↓ more in SD/PA-G and SD-G compared with LE-G. BMI, body weight, fat mass, waist circumference, total cholesterol, LDL, glucose, and leptin: ↓ in all groups (over time). Fat-free mass: ↔. HDL: ↑ only in LE-G and SD/PA-G over time. Insulin and CRP: ↓ only in SD/PA-G over time. IL-6: ↓ only in SD-G over time. |
| Zänker et al. Deutsche Zeitschrift für Onkologie. 2005; 37:114–121 [114]. | Prospective, open-label field study. | n = 51 with type 2 diabetes. | Almased: n = 51. | 26 weeks. | Fasting blood glucose, HbA1c, insulin, glucose tolerance test, lipid panel, and body weight. | Week 14, 20, and 26: ↓ fasting glucose and insulin compared to baseline. Week 14 and 26: ↓ HbA1c compared to baseline. Week 26: Improved insulin levels during the glucose tolerance test. Week 26: ↓ triglyceride compared to baseline. |
| Deibert et al. Nutrition Journal. 2007; 6:31 [90]. | Not specified. | n = 72 with overweight and obesity. | Premenopausal: n = 22. Postmenopausal: n = 50. Intervention in both groups (first 6 months): Almased + physical activity. |
12 months (6 months with supervision +6 months without supervision). | Body weight, fat mass, fat free mass, hip and waist circumferences, blood pressure, blood lipids, glucose, insulin, leptin, cortisol and C-reactive protein. | Body weight: ↓ in all groups (over time). Fat mass: ↓ in all groups (over time). Fat-free mass: ↓ in premenopasal women (over time). Waist and hip circumferences: ↓ in all groups (over time). Blood pressure, triglycerides, HDL-cholesterol, and glucose: Improved in postmenopausal women. Total and LDL-cholesterol: ↓ in both groups. |
| König et al. Annals of Nutrition and Metabolism. 2008; 52:74–78 [95]. | Randomized controlled trial. | n = 88 with overweight and obesity. | Fat restricted low-calorie (LCD-G, n = 29). Meal replacement (MRD-G, n = 59). |
6 weeks. | Body weight, fat mass, waist circumference, blood lipids, glucose, insulin, and leptin. | Body weight: ↓ more in MRD-G. Fat mass: ↓ more in MRD-G. Waist circumference: ↓ more in MRD-G. Triglycerides: ↓ more in MRD-G. Prevalence of metabolic syndrome: ↓ more in MRD-G. Leptin and insulin: ↓ only in MRD-G (over time). LDL-cholesterol, HDL-cholesterol, and glucose: ↓ in all groups (over time). |
| Berg et al. Deutsches Ärzteblatt International. 2008; 105 (11):197–203 [49]. | Not specified. | n = 454 with obesity. | Physical activity + nutrition counselling. Use of Almased was optional. |
12 months (7-wks start phase +17-wks weight reduction phase +6-month stabilization phase). |
Body weight, BMI, and waist cirumference. | Body weight, BMI, and waist cirumference: ↓ over time. Body weight: ↓ more with Almased. |
| Deibert et al. The Aging Male. 2011; 14 (4):273–279 [91]. | Randomized controlled trial. | n = 35 with overweight. | Resistance training + Almased (RTS-G, n = 13). Resistance training (RT-G, n = 13). Lifestyle education (LE-G, n = 9). |
12 weeks. | Body weight, waist cirumference, body composition, physical performance, lipids, glucose, fructosamines, insulin, insulin-like growth factor-1, Leptin, human growth hormone, DHEA, testosterone, CRP, Il-6. |
Body weight: ↔. Fat mass, fat-free mass, and waist circumference: ↓ in RTS-G over time. Strength: ↑ in RT-G and RTS-G over time. IGF-1 and HGH: ↑ more in RT-G + RTS-G than in LE-G. Glucose, insulin, HOMA index, and fructosamine: ↓ more in RT-G + RTS-G than in LE-G. HDL-cholesterol: ↑ more in RT-G + RTS-G than in LE-G. |
| Deibert et al. Asia Pacific Journal of Clinical Nutrition. 2011; 20 (4):527–534 [19]. | Not specified. | n = 20 (n = 10 with metabolic syndrome/n = 10 healthy controls). | Protein load of 1 g/kg body weight and 0.3 g/kg bw. | 1 day. | Glomerular filtration rate (GFR) and renal plasma flow (RPF). | GFR: ↑ more in patients compared with controls with 1 g/kg bw. GFR and RPF: ↑ in both groups with 1 g/kg bw. GFR and RPF: ↔ in patients with 0.3 g/kg bw. |
| König et al. Nutrition. 2012; 28 (1):35–39 [50]. | Randomized controlled trial. Crossover? | n = 11 with overweight and obesity + metabolic syndrome. | Almased (MR) versus isocaloric breakfast (SB). | 1 day. | Postprandial glucose, insulin, ghrelin, PYY, and substrate oxidation. | Glucose and insulin (2h after breakfast): ↓ more with Almased. AUC for glucose (4h after breakfast): ↓ more with Almased. Insulin (after lunch): ↓ more with Almased. Fat oxidation: ↑ more with Almased. Ghrelin (2h after breakfast): ↓ more with Almased. |
| Berg et al. Exercise Immunology Review. 2012; 18:128–41 [74]. | Randomized controlled trial. | n = 30 healthy young adults with a normal body weight. | Endurance training + Almased (G1, n = 15). Endurance training (G0, n = 15). |
6 weeks. | Body composition, physical performance, glucose, lactate, urea, uric acid, ammonia, cortisol, insulin, IGF-1, CK, LDH, myoglobin, hs-CRP, IL-6, IL-10, and blood cell counts. | Body weight and density: ↔. Physical performance: ↑ in both groups (over time). Lactate: ↓ in G1 (over time). Urea during exercise: ↑ more in G1. Trigliceryde and insulin 4h after field test: ↓ in G1. CK, LDH, myoglobin, blood cell count, IL-6, IL-10, and CRP: ↑ in both groups the day after field test. |
| Kempf et al. Journal of Human Nutrition and Dietetics. 2013; 27 (suppl. 2):21–27 [139]. | Proof of principle study. | n = 15 with obesity and type 2 diabetes. | Almased (PRMR, n = 15). | 1.5 year. | Insulin, HbA1c, body weight, lipid panel, creatinine, urea, and uric acid. | 12 weeks - Daily insulin dosage, HbA1c, fasting glucose, body weight, BMI, waist and hip circumferences, and triglyceride: ↓ compared to baseline. 12 weeks – HDL-Cl: ↑ compared to baseline. 1.5 year - Daily insulin dosage and body weight: ↓ compared to baseline. |
| Vitolins et al. Clinical Medicine Insights: Women's Health. 2014; 7:17–24 [94]. | Pilot study. | n = 17 E R/PR-negative breast cancer survivors. | Almased + multimodal intervention (n = 17). | 12 weeks. | Anthropometrics, biomarkers, and health-related quality of life. | Body weight, waist circumference, fat mass, total cholesterol, and triglycerides: ↓ compared to baseline. Health-related quality of life: Improved over time. |
| Koohkan et al. BMC Women's Health. 2014; 14:45 [138]. | Non-randomized controlled trial. | n = 380 women with obesity. | Lifestyle intervention (LS, n = 190. Lifestyle + Almased (LSMR, n = 190). |
12 months. | Health-related quality of life, anthropometry, clinical, physical performance, and self-reported leisure time physical activity. | All parameters improved in both groups over time. |
| König et al. Nutrition. 2014; 30 (11–12):1306–9 [92]. | Randomized controlled trial. | n = 42 with overweight and obesity. | Lifestyle (LS, n = 14). Meal replacement (MR, n = 28). |
6 weeks. | Body weight, BMI, glucose, insulin, and HOMA-IR. | Body weight and BMI: ↓ more in MR. Glucose: ↓ in both groups (over time). Insulin and HOMA-IR: ↓ more in MR. |
| Koohkan et al. Journal of Nutritional Health & Food Science. 2014; 2 (4):1–9 [73]. | Randomized controlled, crossover trial. | n = 10 healthy, normal-weight males. | Carbohydrate-rich (CH): n = 10. Fat-rich (FAT): n = 10. Protein-rich/food + Almased (P): n = 10. Water/control (CON): n = 10. |
1 morning. | Blood glucose and lactate, resting oxygen consumption, respiratory quotient, and satiety. | Blood glucose: ↑ in CH and FAT compared to CON. Blood lactate: ↑ in CH and P compared to CON. Resting oxygen consumption: ↑ in all conditions compared to CON. Respiratory quotient: ↑ in CH compared to CON. Satiety: ↑ compared to baseline and compared with the CON group in all conditions. |
| König et al. Nutrients. 2015; 7 (12):9825–9833 [100]. | Randomized trial. | n = 42 with overweight and obesity + intra-abdominal fat stores. | Lifestyle (LS): n = 22. Almased (MR): n = 20. |
24 weeks. | Body weight, body composition, metabolic blood markers, and adipokines. | Body weight: ↓ in all groups (over time). Intra-abdominal fat: ↓ in all groups (over time). Cardiometabolic risk and adipokines ↓ in all groups (over time). |
| Koohkan et al. Journal of Nutritional Health & Food Science. 2016; 4 (4):1–5 [93]. | Randomized controlled trial. | n = 83 with overweight and obesity. | Lifestyle (LS): n = 28. Almased in isolation and Almased + physical training (MR/MRPT): n = 55. |
24 weeks. | Metabolic blood markers, blood leptin, body weight, and body composition. | Lipid panel, apo B, leptin, insulin, and glucose: ↔ among groups. Body weight: ↓ in MR/MRPT group. Fat mass: ↓ in MR/MRPT group. Apo B: ↓ in MR/MRPT group. Insulin: ↓ in MR/MRPT group. HOMA Index: ↓ in MR/MRPT group. Leptin: ↓ in MR/MRPT group. |
| Kempf et al. Diabetes Care. 2017; 40 (7):863–871 [140]. | Randomized, single-blind, active-comparator controlled, intervention trial with two parallel groups. | Patients with type 2 diabetes + overweight or obesity. 12 weeks: n = 167. 26 weeks: n = 148. 52 weeks: n = 133. |
Control: n = 74 (12 weeks), n = 66 (26 weeks), n = 56 (52 weeks). TeLiPro: n = 93 (12 weeks), n = 82 (26 weeks), n = 77 (52 weeks). |
52 weeks. | HbA1c, body weight, body composition, antidiabetes medication, cardiovascular risk factors, quality of life, and eating behavior. | 12 and 52 weeks - HbA1c: ↓ more in TeLiPro. 12 weeks - Body weight, BMI, blood pressure, and 10-y cardiovascular risk: ↓ more in TeLiPro. 12 weeks - Physical health: Improved more in TeLiPro. 12 and 52 weeks - Quality of life: Improved more in TeLiPro. 12 and 52 weeks - Eating behavior: Improved more in TeLiPro. 12 and 52 weeks - Antidiabetes medication and insulin demand: ↓ more in TeLiPro. |
| Kempf et al. Nutrients. 2018; 10 (8):1022 [141]. | Randomized controlled trial. | n = 321 (12 weeks). n = 259 (52 weeks). Participants had type 2 diabetes. |
Stringent (S): n = 122 (12 weeks), n = 111 (52 weeks). Moderate (M): n = 125 (12 weeks), n = 112 (52 weeks). Control (C): n = 74 (12 weeks), n = 36 (52 weeks). |
52 weeks. | HbA1c, weight, and cardiometabolic risk factors. | 52 weeks - Triglyceride: ↓ in the S group compared to M. 52 weeks - Eating questionnaire (hunger): ↓ in the S group compared to M. 52 weeks - HbA1c: ↓ in the S group compared to C. 12 and 52 weeks - Proportion of participants who lost weight: ↑ in the S and M groups compared to C. |
| Deibert et al. World Journal of Gastroenterology. 2019; 25 (9):1116–1131 [106]. | n = 22 with non-alcoholic steatohepatitis + overweight and obesity. | Lifestyle (LC): n = 11. Almased (MR): n = 11. |
24 weeks. | Body weight, liver fat, liver enzymes, glycemic control, and lipid profile. | Max. performance (watt/kg): ↑ in the LC. Mean chain length of intrahepatic lipids: ↓ in the MR. |
|
| Röhling et al. Nutrients. 2020; 12 (7), 2022 [83]. | Randomized controlled trial - ACOORH (multicenter) | n = 141 with prediabetes and overweight or obesity. n = 123 (12 weeks), n = 105 (26 weeks), n = 93 (52 weeks). |
Lifestyle (CON): n = 35 (12 weeks), n = 31 (26 weeks), n = 28 (52 weeks). Lifestyle + Almased (INT): n = 88 (12 weeks), n = 74 (26 weeks), n = 65 (52 weeks). |
12 months. | Body weight, body composition, waist circumference, blood pressure, glucose, insulin, HbA1c, FBG, lipid panel. | 26 and 52 weeks - Conversion from prediabetes to normoglycemic: ↑ in the INT. 12 weeks - Body weight, BMI, and fat mass: ↓ more in the INT. 12 weeks - BMI, waist circumference, fat mass, and HbA1c: Improved in both groups (over time). 12 weeks - BMI, waist circumference, fat mass, fasting glucose, HbA1c, blood pressure, lipid panel: Improved in INT (over time). 52 weeks - BMI, waist circumference, and HbA1c: Improved in both groups (over time). 52 weeks - BMI, waist circumference, fat mass, HbA1c, total cholesterol, LDL cholesterol: Improved in INT (over time). |
| Röhling et al. Nutrients. 2021; 13 (2):376 [17]. | Randomized controlled trial - ACOORH (multicenter) | n = 119 with overweight or obesity. | Lifestyle (CON, n = 37). Lifestyle + Almased (INT, n = 82). |
12 months. | Dietary intake. | 12 and 52 weeks - Protein: ↑ more in the INT. 12 weeks - Fat and carbohydrate: ↓ more in the INT. |
| Halle et al. European Journal of Clinical Nutrition. 2021; 75:661–669 [84]. | Randomized controlled trial - ACOORH (multicenter) | Individuals with overweight or obesity. n = 439 (4 weeks), n = 396 (12 weeks), n = 360 (26 weeks), n = 317 (52 weeks). |
Lifestyle (CON): n = 140 (4 weeks), n = 126 (12 weeks), n = 116 (26 weeks), n = 101 (52 weeks). Lifestyle + Almased (INT): n = 299 (4 weeks), n = 270 (12 weeks), n = 244 (26 weeks), n = 216 (52 weeks). |
12 months. | Body weight, body composition, and cardiometabolic risk factors. | All time points - Body weight: ↓ more in the INT. 12 weeks - Waist circumference, fat mass, fat-free mass, total cholesterol, and LDL cholesterol: ↓ more in the INT. 52 weeks - Fat mass and fat-free mass: ↓ more in the INT. All time points - Systolic blood pressure, waist circumference, triglyceride, and fat mass: ↓ in both groups (over time). All time points - Diastolic blood pressure, LDL cholesterol, and total cholesterol: ↓ in the INT (over time). 4 weeks - Diastolic blood pressure, LDL cholesterol, and total cholesterol: ↓ in the CON (over time). 4, 12, and 26 weeks - Fasting glucose: ↓ in the INT (over time). 12, 26, and 52 weeks - Fasting glucose: ↓ in the CON (over time). |
| Kempf et al. Nutrients. 2021; 13 (5):1433 [125]. | Randomized controlled trial - ACOORH (multicenter) | n = 446 with overweight or obesity. | Lifestyle (CON, n = 145). Lifestyle + Almased (INT, n = 301). |
12 months. | Insulin and inflammatory blood markers. | Insulin and body weight: ↓ more in the INT. 26 and 52 weeks - CRP and IL-6: ↓ in the INT (over time). Participants with greater insulin reduction achieved the highest weight reduction over the course of the study. |
| Oliveira et al. The American Journal of Clinical Nutrition. 2021; 113 (2):476–487 [81]. | Randomized controlled, crossover trial. | n = 43 healthy, normal-weight adults (n = 19 females; n = 23 males). | High-protein total diet replacement (HP-TDR): n = 43. Control (CON): n = 43. |
32 h. | Energy metabolism and metabolic blood markers. | Total energy expenditure: ↑ in the HP-TDR. Protein and fat oxidation rates: ↑ in the HP-TDR. Carbohydrate oxidation rate: ↓ in the HP-TDR. Energy, fat, and carbohydrate balances: ↓ in the HP-TDR. Protein balance: ↑ in the HP-TDR. Fasting day 1 to fasting day 2 - Glycerol and triglyceride: ↓ more in the HP-TDR. Fasting day 1 to fasting day 2 - Total, LDL, and HDL cholesterol: ↑ more in the HP-TDR. Postprandial - Glucose, insulin, and glycerol: ↓ in the HP-TDR. Postprandial - Total, LDL, and HDL cholesterol: ↑ in the HP-TDR. |
| Oliveira et al. Nutrients. 2021; 13:155 [54]. | Randomized controlled, crossover trial. | n = 43 healthy, normal-weight adults (n = 19 females; n = 23 males). | High-protein meal replacement (HP-MR): n = 43. Control breakfast (CON): n = 43. |
1 morning. | Exercise energy metabolism, appetite sensations, appetite-related hormones, and metabolic blood markers. | Exercise fat oxidation: ↑ in the HP-MR. Exercise carbohydrate oxidation and RER: ↓ in the HP-MR. After exercise - Hunger: ↓ in the HP-MR. Fasting to post-exercise - Insulin, LDL cholesterol, PYY, and GLP-1: ↑ in the HP-MR. Fasting to post-exercise - Triglyceride and glycerol: ↓ in the HP-MR. |
| Röhling et al. Nutrients. 2022; 14 (7):1443 [142]. | Randomized controlled trial - ACOORH (multicenter) | n = 304 with overweight or obesity. | Lifestyle (CON, n = 101). Lifestyle + Almased (INT, n = 216). |
12 months. | Blood pressure, heart rate, and pulse wave velocity. | 26 weeks - Diastolic blood pressure, heart rate, and pulse wave velocity: ↓ more in the INT. 52 weeks - Blood pressure: ↓ more in the INT. |
| Oliveira et al. European Journal of Nutrition. 2022; 61:1849–1861 [82]. | Randomized controlled, crossover trial. | n = 43 healthy, normal-weight adults (n = 19 females; n = 23 males). | High-protein total diet replacement (HP-TDR): n = 43. Control (CON): n = 43. |
32 h. | Appetite sensations and appetite-related hormones. | In females only - 24-hour AUC for PFC: ↓ in the HP-TDR. Fasting day 1 to fasting day 2 - PYY: ↓ in the HP-TDR. Fasting day 1 to fasting day 2 - Leptin: ↑ in the HP-TDR. Postprandial - GLP-1 and PYY: ↑ in the HP-TDR. |
| Kempf et al. Nutrients. 2022; 14 (12):2537 [126]. | Randomized controlled trial - ACOORH (multicenter) | n = 427 with overweight or obesity and metabolic syndrome. | Lifestyle (CON): n = 134. Lifestyle + Almased (INT): n = 293. |
12 months. | Body weight, BMI, fat mass, fat free mass, leptin, fasting insulin, interleukin-6, and C-reactive protein. | Body weight and fat mass: ↓ compared to baseline in both groups. Leptin: ↓ in the INT group. Participants with the strongest leptin reduction achieved greatest weight reduction in both groups. Insulin: ↓ compared to baseline in both groups. |
Description of the abbreviations used.
ACOORH: The Almased Concept against Overweight and Obesity and Related Health Risk. AUC: Area under curve. BMI: Body mass index. BW (bw): Body weight. CRP: C-reactive protein. CK: Creatine kinase activity. CH: Carbohydrate rich; CON: Control group. DHEA: Dehydroepiandrosterone. FBG: Fasting blood glucose; GFR: Glomerular filtration rate. GLP-1: Glucagon-like peptide 1. HDL: High density lipoprotein cholesterol. HOMA-Index: Homeostasis model assessment index. hs: high sensitive. IGF-1: Insulin-like growth factor 1: INT: Intervention group. IL-6: Interleukin 6; IL-10: Interleukin 10. LCD-G: Low-calorie diet group. LE-G: Lifestyle education group. LDL: Low density lipoprotein cholesterol. LDH: Lactate dehydrogenase activity. LS: Lifestyle group. LSMR: Lifestyle meal replacement group. MR: Meal replacement group. MRD-G: Meal replacement diet group. MRPT: Meal replacement physical training group. PFC: Prospective food consumption. P: Protein rich. PYY: Peptide YY. RER: Respiratory exchange ratio. RPF: Renal plasma flow. RT-G: Resistance training group. RTS-G: Resistance training supplement group. SB: Standard breakfast. SD-G: Supplement diet group. SD/PA-G: Supplement diet physical activity group. TeLiPro: Telemedical Lifestyle intervention Program.
3.1. Appetite, satiety and energy metabolism
In Konig et al. 2021 [50], the postprandial responses in glycaemia and insulinaemia, the levels of satiety hormones, and substrate utilization were compared following two interventions of an isoenergetic standardized breakfast, differing in glycemic load and protein content. The test was performed in 11 overweight men (56.4 ± 4.9 y, 31.6 ± 2.6 kg/m2) with MetS and insulin resistance. The glycemic and insulinaemic responses were substantially greater following the standardized isoenergetic and higher carbohydrate breakfast compared with the formula intake. There was also a significantly less pronounced post-prandial decrease in fat oxidation following consumption of the formula meal, and this effect was also observed after consumption of a standardized lunch, suggesting a second meal effect. Ghrelin concentration was significantly lower post formula intake, and there was a trend towards higher PYY levels compared with the standardized breakfast. These post-prandial metabolic changes together with a favorable course of appetite-regulating hormones may help to explain the beneficial supplementation strategy with the high-protein, low-glycemic formula for weight management.
In Koohkan et al. 2014 [73], the choice of breakfast and its food components were examined for post-prandial energy supply and satiety. Ten healthy men (26 ± 4.4 y; 23.2 ± 0.9 kg/m2) consumed isoenergetic breakfasts but rich in either carbohydrate, fat or protein (68%, 64% and 35% of energy respectively) supplemented with a soy-yoghurt-honey formula. Plasma glucose increased significantly following the high-carbohydrate breakfast, whereas the high-protein supplemented breakfast lead to only small rises in plasma glucose. A significant increase in resting metabolic rate (VO2, up to 30%) without accompanying changes in RQ was only observed following the protein-rich supplemented breakfast. Satiety was also greatest following the high-protein breakfast. Taken together [50, 73], the results may help to explain successful weight management.
In a further RCT, a bout of moderate endurance training with (n = 15) or without (n = 15) consumption of the soy-yoghurt-honey supplement (2 × 50 g servings per day) [74] was performed to evaluate possible impacts on aerobic capacity and changes in metabolic, endocrine, and inflammatory markers induced by endurance exercise in healthy sports science students (23.6 ± 1.9 y, 22.1 ± 2.18 kg/m2, 50.9 ± 6.60 ml/kg/min VO2max). No significant changes in body composition were observed post intervention, and only marginal improvements in exercise performance and VO2max were observed across the whole group (2%, p = 0.016). However, participants in the supplemented group showed statistically significant improvement in aerobic capacity (p < 0.01) and lower lactate values (p < 0.01) post intervention in a treadmill test. In addition, the intervention group showed significantly lower differences in the exercise-induced increase in metabolic parameters (triglycerides, uric acid) and insulin in the post-exercise recovery period after an 11.5km field test. A reduction in both ammonia and uric acid production was also observed after the 11.5 km run; this is of special interest because higher levels of ammonia and uric acid are indicative of the breakdown of adenine nucleotides and the utilization of the anaerobic purine nucleotide cycle [75, 76]. In conclusion, the significant differences in triglyceride and insulin metabolism post-exercise are suggestive of a greater reliance on fatty acids for muscular energy metabolism [77, 78, 79]. These observations agree with studies which have investigated muscular energy metabolism in rats following soy-protein intake and exercise training [51].
Most recently, results of a confirmative RCT have been published by the Department of Agricultural, Food and Nutritional Science, University of Alberta whose aim was to evaluate the effect of a high-protein total diet replacement on appetite, satiety and fat oxidation [54, 80, 81, 82]. This trial compared a high-protein total diet replacement (HP-TDR, soy-yoghurt-honey formula) with a control diet (CON, typical North American) in 44 male and female healthy adults. Participants resting metabolic rate, macronutrient oxidation, metabolic blood markers and sensations of hunger and satiety were assessed inside a whole-body calorimeter for a total of 32 h. Compared with the CON diet, HP-TDR lead to a significantly higher total energy expenditure, increased rates of protein and fat oxidation and a lower rate of carbohydrate oxidation. No interaction between diet and sex was evident for any of the variables assessed [81]. These findings suggest that HP-TDR may better promote fat loss compared with a more typical isoenergetic diet. In relation to appetite sensations and the metabolic profile after breakfast and an exercise session, the HP-TR resulted in a significantly higher degree of fat oxidation, coupled with lower carbohydrate oxidation, reflected also in a significantly lower respiratory exchange ratio during exercise. Post exercise, hunger ratings were lower during the HP-TDR state. The findings confirmed that, compared to a typical North American breakfast (CON), HP-TDR resulted in higher levels of fat oxidation during the exercise session, lower levels of hunger sensation and an improved metabolic profile post exercise. These findings offer additional insights into the possible role of HP-TDR strategies during weight management.
3.2. Eating behavior and protein intake
Despite the soy-yoghurt-honey formula being declared as a low-calorie product, the energy content of a 50 g portion equates to 180 kcal. If a single portion is consumed twice a day, this may theoretically lead to an increase in body weight of about 0.35 kg per week if the soy-yoghurt-honey formula is provided as a supplement to support a healthy lifestyle. This theoretical weight gain is based on the total extra calories consumed over a week, with fat gain representing ∼75% of extra weight gain. However, this speculated weight gain is not typically observed in practice. This could be explained by factors such as a compensatory decrease in intake of other components of the diet. Also the higher protein intake from the protein-rich formula increases energy expenditure associated with its digestion, metabolism and assimilation (diet-induced thermogenesis). As an example, in an experimental RCT [33], when a healthy person consumed 2 portions of the formula per day whilst undertaking an endurance exercise training session over three months, the evaluation of diet diaries, completed before and at the end of intervention, showed that despite the supplement intake, the overall daily energy intake did not increase.
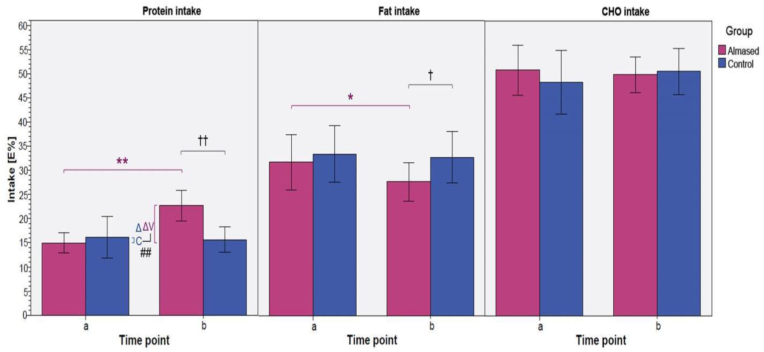
Instead, a redistribution in the intake of macronutrients in the intervention group was observed (Figure 2), with a significant increase in protein intake from 15 to 23% (of total daily energy intake), whereas the percentage of fat intake decreased significantly from 32 to 28% (p < 0.01). This observation may well be due to the already described effect of the soy-yoghurt-honey formula on satiety and appetite (see 3.1.) and that this effect also appears to occur with a normocaloric energy intake.
Figure 2.
Protein, fat, and carbohydrate intake as % of total energy intake between study groups and time points. Significances given for intra- and intergroup comparison; ∗/?p < 0.05, ∗∗/?? p < 0.01); time point a: before intervention, time point b: after intervention [33].
In addition, the results of a sub-analysis of the ACOORH (The Almased Concept against Overweight and Obesity and Related Health Risk) Study which evaluated weight loss in patients with excess weight and accompanying cardiovascular disease (CVD) risk factors, [84] indicated that the soy-yoghurt-honey formula used in a meal replacement approach, combined with a lifestyle intervention improved dietary intake by increasing the intake of protein while concurrently decreasing fat and carbohydrate intake, thus supporting successful weight loss, especially in women. This effect as a change in eating behavior was shown not only as an acute effect of the intervention, but also after a period of 12 months when the intake of the formula was no longer part of the intervention [17]. Thus, it is important to recognize that individuals who regularly consume the protein-rich low-glycemic formula not only increase their daily protein intake but also tend to prefer a protein-rich and low-glycemic diet in the long term; this is especially the case for women [12].
3.3. Body mass and body composition
Obesity, marked by an increased FM and BMI>30, has become the major global health burden. This global trend underpins the dramatic rise in the prevalence of T2DM and the incidence of the MetS, both of which drive up the risk for CVD [85]. Thus, the management of excess weight has become significant clinical and public health challenges. However, successful achievement and maintenance of weight loss by lifestyle interventions remain unsatisfactory to date [86]. As the main purpose of the low energy, low fat, low GI soy-yoghurt-honey formula is to support weight loss and weight management, almost all clinical studies conducted with the formula to date, have focused on body mass and body composition as target variables.
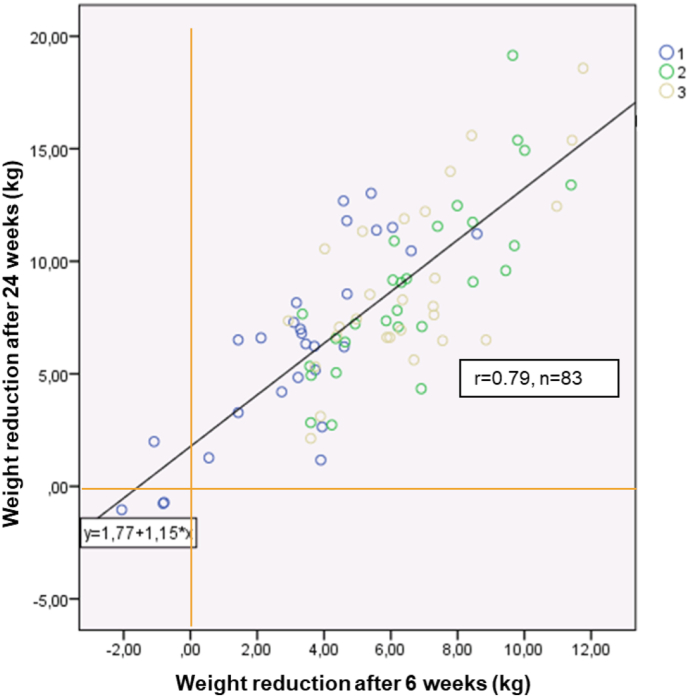
The first explorative study evaluating the mode of action of the soy-yoghurt-honey formula, demonstrated the significant positive effect of the formula on body mass and FM [72]. An additional finding was that supporting the meal replacement with an exercise program did not lead to any further significant weight loss (meta-analysis of two subgroups: control group: n = 28, meal replacement group: n = 55), the participants with the meal replacement showed significantly higher weight loss of 2.1 kg and a significantly higher decrease in FM of 2.9 kg compared with the control group following an intervention lasting 24 weeks. In addition, there was a statistically significant correlation between the weight loss after 6 weeks and the weight change achieved after 24 weeks (n = 83, r = 0.79; Figure 3). This suggests that in consultation with the patient, health and weight management professionals should consider continuing the programme even if it appears to have been unsuccessful over the initial 6 weeks of intervention.
Figure 3.
Linear regression between weight loss after 6 weeks (x) and 24 weeks (y) in a group of 83 participants of a weight management program [72]. Group 1: Lifestyle education, n = 28; Group 2: Meal replacement, n = 28; Group 3: Meal replacement and physical training, n = 27.
In addition, in this study, the loss in body mass was significantly correlated with the reduction in FM (n = 83, r = 0.883, p < 0.01), whilst there were no significant changes in fat-free mass (FFM) in all groups. This suggests that it may be possible to lose body mass without any significant and undesired reduction in FFM or muscle mass [87]. It must be emphasized that these findings were obtained using air displacement plethysmography (ADP) (Bod Pod®) – a technology more reliable for quantifying FFM changes [88] (but not skeletal muscle mass) compared with a more predictive technique such as bioelectrical impedance (BIA) [89]. From a practical perspective, however, the use of ADP in studies with high numbers of participants across different study centers is not usually feasible.
Following these earlier studies, results of further clinical studies (Table 1) have confirmed the proposal that the use of the of the soy-yoghurt-honey formula supports weight loss and the reduction of FM, while preventing or minimizing the simultaneous loss of muscle mass [49, 71, 72, 90, 91, 92, 93, 94, 95].
With improvements in diagnostic imaging techniques, it has become possible to quantify abdominal/visceral fat masses in individuals. This is important for assessing changes in body composition and differentiating between subcutaneous and intra-abdominal fat stores, as excess visceral adipose tissue is associated with adverse metabolic outcomes and is partly responsible for the synthesis of adipokines and their systemic effects regulating metabolic risk factors [96, 97, 98, 99]. Against this background, the Freiburg research group investigated the effect of the soy-yoghurt-honey meal replacement formula vs. a lifestyle modification approach on intra-abdominal fat stores using magnetic resonance imaging (MRI) in obese participants [100]. 42 overweight/obese individuals (18 men, 24 women; 49 ± 8 y, 96.3 ± 12.1 kg, 32.7 ± 2.3 kg/m2) were randomly divided in a parallel-group design. The control group (LS-G; n = 22) were provided with dietary counselling sessions and instructions on how to increase levels of physical activity. In the meal replacement group (MR-G; n = 20), meals were substituted with the soy-yoghurt-honey formula. After six months, participants in the LS-G significantly lost 8.9 ± 6.24 kg, whereas those in the MR-G lost 7.1 ± 2.33 kg (p < 0.01 for changes, with no between group differences). FFM was unchanged in either intervention group. When internal (visceral) fat was examined, MRI showed significant and comparable reductions in both groups. The greater abdominal fat loss in the LS-G was predominantly the result of a reduction in the subcutaneous site. In conclusion, both arms of the intervention led to significant reductions in body mass, total FM and abdominal fat while FFM was preserved. Nevertheless, the positive effects observed in the MR-G occurred without any further changes in lifestyle behavior, particularly leisure time physical activity [100].
By employing a new algorithm in magnetic resonance imaging (MRI), it has become possible to investigate hepatic morphology for clinical diagnostics and intervention strategies. This is important because non-alcoholic steatohepatitis (NASH) is a component of metabolic disease and is becoming one of the major forms of liver disease. Estimates of between 20 to 30% of adults in high-income countries have excess fat accumulation in the liver. This figure rises to 50% among people with, T2DM and to approximately 80% in morbidly obese individuals [101]. NASH is generally characterized by the presence of lipid in the hepatocytes together with markers of inflammation and/or liver damage. When the latter characteristic is absent, the condition is referred to as non-alcoholic fatty liver disease (NAFLD) [102]. NASH may ultimately lead on to cirrhosis and portal hypertension and potentially hepato-cellular carcinoma [103]. Weight loss via a dietary approach has been shown to lead to a reduction in hepatic fat content as well as in markers of NASH, including the enzyme alanine-amino-transferase (ALT) [104]. However, the most optimal dietary approach is still being debated. To examine this further, the Freiburg group compared a guided intensive lifestyle intervention program with a meal replacement (soy-yoghurt-honey formula) therapy in patients with NASH [106]. In this study, liver fat content, lipid composition, and markers of liver inflammation (including ALT) were analyzed following a period of weight reduction. In addition, body composition, intra-abdominal and subcutaneous adipose tissue masses, and intrahepatic lipid were evaluated. A subgroup was further evaluated to determine the proportion of saturated and unsaturated fatty acids in intrahepatic fat in relation to serum adipokines. Twenty-two participants (MR-G: n = 11; C-G: n = 11) completed the study (9 women, 13 men; 56 ± 15.0 y, 32.3 ± 3.3 kg/m2). Significant weight loss was observed in both groups, reflected in statistically significant reductions in BMI (MR-G: -2.3 ± 1.5 kg/m2; C-G: -3.0 ± 3.4 kg/m2). Intra-abdominal FM and hepatic lipid content were also substantially and comparably reduced in both groups. However, qualitative changes in liver fatty acid composition differed between groups. Marked reduction in mean fatty acid chain length was only observed in the MR-G. Overall, these findings indicated that weight management interventions may lead to reductions in both total liver fat and liver fat composition. As both weight management approaches had comparable effects with respect to weight reduction, body and liver fat content and indicators of liver inflammation, meal replacement strategies, as shown for the soy-yoghurt-honey formula, may be an option for NASH intervention, considering patient characteristics, degree of motivation, and other lifestyle factors [106, 107].
3.4. Insulin and the risk of type 2 diabetes (2TDM) and prediabetes
Numerous lifestyle interventions have demonstrated that weight loss, particularly when coupled with increases in leisure time physical activity leads to improvements in glucose metabolism, insulin sensitivity and the risk factors for cardiometabolic disease [6, 7]. This has been exemplified in two elaborate randomized controlled studies - the Diabetes Prevention Project in Finland and the Diabetes Prevention Program (DPP) in the USA [[6], [7]]. Both interventions have demonstrated that the conversion rate of prediabetes to T2DM are significantly reduced by 58% over a period of four years of weight management. In addition, the risk of developing T2DM is significantly lower in individuals whose plasma glucose levels had returned to within the normal range than in those in who maintained a pre-diabetic state. Furthermore, the risk remained significantly reduced, even if the reversion to normoglycemia was only transient [108]. Maintaining normoglycemia and accompanying risk factors is crucial for reducing the risk of progressing to CVD. Therefore, current CVD prevention guidelines endorse weight reduction and increased physical activity as lifestyle measures to reduce CVD risk [109, 110]. However, dietary change is an independent co-factor for successful weight management. These dietary changes include a protein-rich, low fat, low energy and low-glycemic diet. In this context, formula diets can be both effective and feasible approaches to obesity management and its associated risk factors [92, 111, 112, 113].
For the application of the soy-yoghurt-honey formula, several studies have confirmed the specific effects of the formula on blood glucose regulation in clinical trials after 6 and 24 weeks as well as after 26 and 52 weeks of intervention. Regular support for 26 weeks of the daily eating habit with the soy-yoghurt-honey formulation down-regulates hormonal (insulin, interleukin-6 (IL-6)) and biochemical (fasting glucose and triglyceride levels) parameters which may contribute to improvements in CVD risk, and possibly even carcinogenic risk [87, 90, 91, 92, 114].
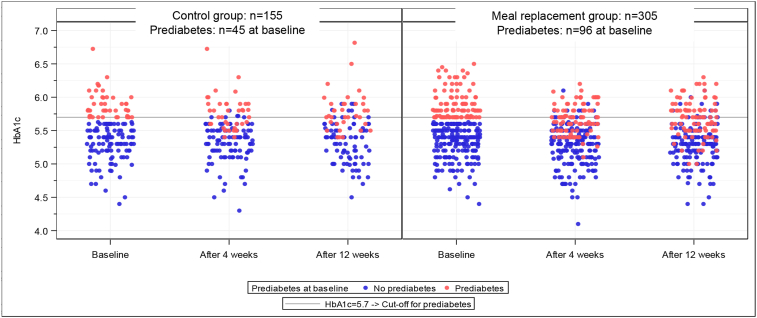
Recently published results from the ACOORH study as a 1-year-multicenter RCT for overweight and obese patients, [83, 84] in particular have demonstrated the improvements in glucose regulation could be found not only after 26 weeks, but were already present within the first weeks of intervention [115]. Commencing with comparable baseline values for glycated haemoglobin (HbA1c) in the subgroup with diagnosed prediabetes (MR-group: n = 96, HbA1c 5.90 ± 0.22%; control group: n = 45, baseline HbA1c 5.89 ± 0.21%), the results after 4 weeks of intervention (Figure 4) revealed that HbA1c decreased significantly more in the meal replacement group (MR-group -0.21 ± 0.20%) than in the lifestyle group (LS-group -0.13 ± 0.18%). These findings persisted over 12 weeks of intervention (MR-group: -0.19 ± 0.0.24% vs. LS-group -0.12 ± 0.22%).
Figure 4.
Scatterplot for HbA1c values per visit, by group and prediabetes at baseline and after 4 and 12 weeks after invention in the ACOORH study [115].
These results demonstrate the significant benefits of the formula meal replacement strategy on glucose regulation after as little as 4 weeks of intervention, such that 50% of the pre-diabetic participants were able to leave their baseline pre-diabetic state [105] and return to the normoglycemic state.
3.5. Blood pressure and blood lipids
In addition to insulin resistance and an impaired glucose regulation, excess weight (particularly in the abdominal region) is frequently accompanied with a cluster of cardiometabolic risk factors in addition to T2DM, including hypertension and atherogenic dyslipidaemia, which in their entirety are described as the MetS. MetS is linked to an increased risk of developing chronic conditions, particularly cardiovascular disease (CVD), with physical inactivity and unhealthy dietary patterns playing fundamental roles in its development [116, 117]. Thus far, treatments for hypertension and dyslipidaemia are current options for the MetS.
Considering the positive effects of the soy-yoghurt-honey formula on cardiovascular risk factors discussed already, it is understandable that MetS can also be favorably influenced, to the extent that it may no longer be detectable diagnostically in a high percentage of patients managed by the formula diet [86, 87]. This approach and outcome needs to be emphasized, because, to date, there is no contraindication against a protein-rich supplementation, even in patients with diagnosed metabolic disease [18, 19]. Together with the reductions in total body mass and FM, lifestyle intervention with additional use of the soy-yoghurt-honey formula promotes favorable changes in metabolic status and regression of systemically diagnosed components of MetS compared with more typical lifestyle changes alone. In addition, overweight patients presenting with components of MetS can also benefit substantially from the described intervention, even with only mild weight reduction [95, 100]. This reaffirms that not only a state of prediabetes, but also diagnosed MetS can be reversed by a rigorously implemented lifestyle modification [8].
Regarding the totality of the isoflavones, the defined soy proteins and biologically active peptides with likely anti-hypertensive, anti-hypercholesterolemic and anti-hypertriglyceridemic properties (see above), it is unsurprising that even after a short intervention period, e.g., 4–6 weeks, the soy-yoghurt-honey formula approach also leads to a significant decrease in triglycerides, LDL cholesterol and serum apolipoprotein-B levels. These effects could be confirmed by clinical trials using the reviewed soy-yoghurt-honey formula as part of the intervention strategy [13, 17, 80, 81, 84] and such trials should be considered, as metabolic markers of risk including elevated serum triglycerides and LDL cholesterol (such as apolipoprotein-B containing particles) should also be reduced as a result of a successful dietary and lifestyle weight management concept [118]. However, it must be recognized that changes in blood pressure as well as in the lipid profile are related to elevated baseline levels, and HDL cholesterol levels are not significantly influenced by the properties of the soy-yoghurt-honey formula.
3.6. Leptin and inflammatory mediators
Despite the adipokine leptin not being routinely measured in clinical chemistry, it must be considered not only as a key regulator of energy homeostasis and food intake but also as a risk factor for obesity and diabetes if leptin deficiency or resistance occurs [119, 120]. When leptin resistance arises in obesity (typically at the blood-brain barrier, BBB), it subsequently fails as an adipostat. As triglycerides can inhibit the transport of leptin across the BBB, it may be postulated that hypertriglyceridemia, as an obesity-related comorbidity, acts, in part to inhibit leptin transport across the BBB and invoke a starvation response [121]. Serum leptin may impact upon weight loss and maintenance of this weight loss through lifestyle changes [122, 123] and by modification of enzymes important in triglyceride metabolism [124, 125] in overweight adults. It has been shown in a retrospective study [93] that a weight loss intervention with soy-yoghurt-honey formula resulted in significant changes from baseline not only for FM but also for serum leptin levels. Despite adjustment for variations in weight loss, the participants in the formula meal-replacement group demonstrated a significantly p < 0.001 larger reduction in mean serum leptin levels post intervention (−13.9 ng/ml) than those in the control group (−9.8 ng/ml). This result may be interpreted as a positive effect on leptin resistance.
The reduction in both FM and hepatic lipid accumulation lead not only to reductions in serum leptin, but also to lower levels of inflammatory markers [106]; significant improvements have been found in levels of IL-6 and C-reactive protein (CRP), following the soy-yoghurt-honey formula intervention [125]. Obesity is known to be associated with adipose tissue inflammation [127], activation of macrophages [128], and with increased circulating plasma levels of pro-inflammatory cytokines [129]. As previously demonstrated [126] baseline levels of serum leptin were not only positively correlated with body weight, BMI, and FM, but also with the inflammatory markers IL-6 and CRP themselves.
Our most recently published observation in this area [126] is supported by findings from other studies where dietary carbohydrate restriction coupled with intermittent fasting formed part of the intervention [114]. These interventions lead not only to significant reductions in body weight in overweight participants, but also to a simultaneous reduction in the circulating concentrations of markers of inflammation [130, 131, 132, 133]. Thus, (a decreased) insulin concentration may be a regulator of immune function, as it is associated with the release of pro-inflammatory cytokines, including IL-6, from adipocytes [125, 134, 135]. In addition, these (anti-inflammatory) effects may be enhanced by isoflavones and peptides, which also exhibit a range of metabolic and molecular activities [33]. Finally, in relation to this topic, the sub-analysis of the ACOORH trial lifestyle intervention showed [126], that commencing a weight management intervention with a high-protein, low-glycemic meal replacement was more effective on body weight and leptin reduction than lifestyle intervention alone. Therefore, early, and strong leptin and insulin reduction is predictive of better long-term weight loss.
3.7. Quality of life (health related quality of life, HRQOL)
Obesity not only increases the risk of diseases such as T2DM and MetS, but it is also negatively related to HRQOL [136]. Therefore, any aims of lifestyle interventions should not necessarily only be weight loss and improvement of cardiometabolic risk factors, but also improvement of quality of life [4, 5]. Indeed, if weight losers do not feel further benefit for their HRQOL in addition to their weight reduction, it could be that the lifestyle alteration might not be optimally integrated into everyday life in the long term [137]. Meal replacement products are recommended as one effective and therapeutic approach to weight loss, particularly when introduced at the start of an intervention, a period in which the driving motivation and mood improvement is beneficial. Hence, a trial conducted by the Freiburg study group evaluated the effectiveness of a 12-month weight loss intervention strategy in obese women and in particular, to assess potential changes in HRQOL [138]. This controlled study evaluated two different approaches to a standardized 12-month weight management intervention [49]: the lifestyle program without a meal replacement (LS, n = 190) and the lifestyle program with the inclusion of the soy-yoghurt-honey meal replacement regimen (LSMR, n = 190). All participants completed the SF-36 questionnaire pre- and post-intervention – a validated tool to assess HRQOL. The LSMR sample scored lower at baseline for the HRQOL than the LS sample in six out of the eight HRQOL areas, most significant being vitality and health perception (p < 0.01). Post intervention, reductions in body weight was observed in both groups (LS: -6.6 ± 6.6 vs. LSMR: -7.6 ± 7.9 kg). However, weight loss and improvements in HRQOL scores were greater in the LSMR group (LSMR: seven of eight, LS: four of eight areas). These findings demonstrated that improvements in HRQOL can arise in middle-aged obese women who follow a standard lifestyle weight management program, and that this positive outcome can be further augmented by the inclusion of a soy protein-based meal replacement formula. Further analyses of the ACOORH study [84] also found a significant effect of meal replacement-based lifestyle interventions on scores of health-related qualities of life, comparing physically related domains against mental-emotional domains [143].
4. Critical evaluation
All research studies need to acknowledge their limitations and the extent to which the findings are credible, reproducible and generalizable, together with the quality of the study design and analyses. Overall the studies summarised in Table 1 consisted of a range of study design including explorative, retrospective as well as a number of randomized controlled trials (RCT), the latter of which is regarded as meeting the most stringent of requirements of a study design to determine whether a cause-effect relationship exists between intervention and outcome. When explorative studies were conducted, these were usually followed up with a confirmatory trial.
The RCT made up by far the majority of the studies summarized in Table 1. The ACOORH study was a multi-centred trial across Europe with a large sample size and statistically powered. Other RCTs cited were made up of varying sample sizes, some with low numbers but the majority with samples between 50-85 depending on the study design. This should be considered when interpreting the findings and caution exercised where appropriate. Nevertheless, many of the findings on weight loss, FM loss as well as changes in metabolic risk factors (such as plasma glucose, insulin and HbA1c and blood lipids) were consistent and reproducible across the individual studies and should therefore be considered as an indication of the efficacy of the soy-yoghurt-honey formula in this respect.
This is the only soy-yoghurt-honey product currently commercially available and so it is impossible to make comparisons with other studies using the same or a similar formula. However, a wide range of meal replacement products are currently available, some of which have been incorporated extensively into long term weight management interventions and in studies where T2DM prevention of reversion have been conducted. Such studies have not tended to specifically evaluate the efficacy of the meal replacement product per se, but rather the overall study design and intervention. Whilst the products used in those studies differ in the main food ingredients, formulations and energy and nutrient profiles, the interventions tend to result in similar degrees of weight loss across a similar timeframe, compared with those discussed in this review using the soy-yoghurt-honey formula. However, as has been discussed throughout this review, evidence has been presented to raise the possibility of whether specific components of the ingredients such as BAPs, isoflavones and oligosaccharides may further enhance the weight loss and metabolic outcomes of the interventions beyond the more fundamental high-protein, low energy, low GI properties of the meal replacement. To the best of our knowledge, no RCTs using other meal replacement products have undertaken a similar questioning approach.
To date the soy-yoghurt-honey formulation has been extensively evaluated in a range of physiological, metabolic and health scenarios. The product would still benefit from further rigorous testing but it can be confidently concluded that this meal replacement formulation is a safe and effective product for wider employment in weight management and health related situations.
5. Conclusions
Given the broad range of nutritional, metabolic, and cardio-protective properties of this functional food formulation, the described formula can be considered to have specific dietary properties and functions. Protein-rich formulations, especially based on plant and dairy sources could prove to be important supplements as well as meal replacements at various stages of adult life, in health and disease, for weight management and for those adults who exercise and increase physical activity.
In their entirety, the results presented underscore the efficacy of the soy-yoghurt-honey formula in weight management and the improvement of obesity associated CVD and metabolic risk factors. In addition, the regular intake of the protein-rich, low-glycemic supplement also leads to significantly improvement in eating behavior by increasing daily protein intake and also leads to improved HRQOL in physically related SF36 domains. When incorporated into a lifestyle intervention program, the soy-yoghurt-honey regimen supports a targeted healthy lifestyle and should be considered a valid therapeutic approach to successful weight management in clinical, community and personal health care settings. It can be inferred that as part of an integrated program, the observed induced lifestyle changes may be integrated into everyday life, even in the long term. The versatile soy-yoghurt-honey formula described here should continue to be experimentally evaluated as well as being made more widely available in clinical and consumer contexts for health improvement and well-being.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data will be made available on request.
Declaration of interest's statement
The authors declare the following conflict of interests: All studies and trials performed to the action and use of Almased® were financially supported by Almased-Wellness-GmbH. The funder had no influence on study design, data collection, data analysis, manuscript preparation and/or publication decisions. As scientific consultant, AB received research support for his department from the Almased-Wellness-GmbH to perform the cited Almased® studies and trials. AB and DMc have also received speaker's honoraria from Almased-Wellness-GmbH. AB was the PI of the ACOORH trial.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors would like to express their gratitude for Dr. Camila L.P. Oliveira, Department of Agricultural, Food, and Nutritional Science, University of Alberta, Canada for the production of the table summarizing the key research studies.
References
- 1.Mu Y., Kou T., Wei B., Lu X., Liu J., Tian H., Zhang W., Liu B., Li H., Cui W., Wang Q. Soy products ameliorate obesity-related anthropometric indicators in overweight or obese asian and non-menopausal women: a meta-analysis of randomized controlled trials. Nutrients. 2019;11:2790. doi: 10.3390/nu11112790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chakrabarti S., Guha S., Majumder K. Food-Derived bioactive peptides in human health: challenges and opportunities. Nutrients. 2018;10:1738. doi: 10.3390/nu10111738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Samtiya M., Aluko R.E., Dhewa T., Moreno-Rojas J.M. Potential health benefits of plant food-derived bioactive components: an overview. Foods. 2021 Apr 12;10(4):839. doi: 10.3390/foods10040839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wirth A., Wabitsch M., Hauner H. The prevention and treatment of obesity. Dtsch. Aerzteblatt Online. 2014;111:705–713. doi: 10.3238/arztebl.2014.0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yumuk V., Tsigos C., Fried M., Schindler K., Busetto L., Micic D., Toplak H. European guidelines for obesity management in adults. Obes. Facts. 2015;8:402–424. doi: 10.1159/000442721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diabetes Prevention Program Research Group. Knowler W.C., Fowler S.E., Hamman R.F., Christophi C.A., Hoffman H.J., Brenneman A.T., Brown-Friday J.O., Goldberg R., Venditti E., et al. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009;374:1677–1686. doi: 10.1016/S0140-6736(09)61457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tuomilehto J., Lindström J., Eriksson J.G., Valle T.T., Hämäläinen H., Ilanne-Parikka P., Keinänen-Kiukaanniemi S., Laakso M., Louheranta A., Rastas M., et al. Prevention of Type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N. Engl. J. Med. 2001;344:1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 8.König D., Hörmann J., Predel H.G., Berg A. A 12-month lifestyle intervention program improves body composition and reduces the prevalence of prediabetes in obese patients. Obes. Facts. 2018;11:393–399. doi: 10.1159/000492604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zupo R., Castellana F., Sardone R., Sila A., Giagulli V.A., Triggiani V., Cincione R.I., Giannelli G., De Pergola G. Preliminary trajectories in dietary behaviors during the COVID-19 pandemic: a public health call to action to face obesity. Int. J. Environ. Res. Publ. Health. 2020;17:7073. doi: 10.3390/ijerph17197073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abbas A.M., Kamel M.M. Dietary habits in adults during quarantine in the context of COVID-19 pandemic. Obes Med. 2020;19 doi: 10.1016/j.obmed.2020.100254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Artaza-Artabe I., Sáez-López P., Sánchez-Hernández N., Fernández-Gutierrez N., Malafarina V. The relationship between nutrition and frailty: effects of protein intake, nutritional supplementation, vitamin D and exercise on muscle metabolism in the elderly. A systematic review. Maturitas. 2016;93:89–99. doi: 10.1016/j.maturitas.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 12.McCarthy H.D., Berg A. Weight loss strategies and the risk of skeletal muscle mass loss. Nutrients. 2021 Jul 20;13(7):2473. doi: 10.3390/nu13072473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koohkan S., McCarthy D., Berg A. The effect of a soy-yoghurt-honey product on excess weight and related health risk factors-a review. J. Nutrition Health Food Sci. 2017;5:1–10. [Google Scholar]
- 14.Phillips S.M., Chevalier S., Leidy H.J. Protein “requirements” beyond the RDA: implications for optimizing health. Appl. Physiol. Nutr. Metabol. 2016;41:565–572. doi: 10.1139/apnm-2015-0550. [DOI] [PubMed] [Google Scholar]
- 15.Huecker M., Sarav M., Pearlman M., Laster J. Protein supplementation in sport: source, timing, and intended benefits. Curr. Nutr. Rep. 2019;8:382–396. doi: 10.1007/s13668-019-00293-1. [DOI] [PubMed] [Google Scholar]
- 16.Bopp M.J., Houston D.K., Lenchik L., Easter L., Kritchevsky S.B., Nicklas B.J. Lean mass loss is associated with low protein intake during dietary-induced weight loss in postmenopausal women. J. Am. Diet Assoc. 2008;108:1216–1220. doi: 10.1016/j.jada.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Röhling M., Stensitzky A., Oliveira C.L.P., Beck A., Braumann K.M., Halle M., Führer-Sakel D., Kempf K., McCarthy D., Predel H.G., et al. Effects of a protein-rich, low-glycemic meal replacement on changes in dietary intake and body weight following a weight-management intervention—the ACOORH trial. Nutrients. 2021;13:376. doi: 10.3390/nu13020376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bauer J., Biolo G., Cederholm T., Cesari M., Cruz-Jentoft A.J., Morley J.E., Phillips S., Sieber C., Stehle P., Teta D., et al. Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the PROT-AGE study group. J. Am. Med. Dir. Assoc. 2013;14:542–559. doi: 10.1016/j.jamda.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 19.Deibert P., Lutz L., Konig D., Zitta S., Meinitzer A., Vitolins M.Z., Becker G., Berg A. Acute effect of a soy protein-rich meal-replacement application on renal parameters in patients with the metabolic syndrome. Asia Pac. J. Clin. Nutr. 2011;20:527–534. [PubMed] [Google Scholar]
- 20.Schaafsma G. The protein digestibility–corrected amino acid score. J. Nutr. 2000;130:1865S–1867S. doi: 10.1093/jn/130.7.1865S. [DOI] [PubMed] [Google Scholar]
- 21.Joint FAO/WHO/UNU Expert Consultation on Protein and Amino Acid Requirements in Human Nutrition . Food and Agriculture Organization of the United Nations, World Health Organization & United Nations University 2007. Protein and Amino Acid Requirements in Human Nutrition: Report of a Joint FAO/WHO/UNU Expert Consultation. World Health Organization; Geneva, Switzerland: 2002. [Google Scholar]
- 22.Elmadfa I., Leitzmann C. Verlag Eugen Ulmer; Stuttgart, Germany: 2015. Ernährung des Menschen, Kap. 3.5 Proteine. 5. Auflage. [Google Scholar]
- 23.Almased Vitalkost GmbH . Personal communication; 2016. Bienenbüttel, Germany. [Google Scholar]
- 24.Bilsborough S., Mann N. A review of issues of dietary protein intake in humans. Int. J. Sport Nutr. Exerc. Metabol. 2006;16:129–152. doi: 10.1123/ijsnem.16.2.129. [DOI] [PubMed] [Google Scholar]
- 25.Souci S., Fachmann W., Kraut H. WVG; Stuttgart, Germany: 2016. Food Composition and Nutrition Tables. [Google Scholar]
- 26.Layman D.K., Anthony T.G., Rasmussen B.B., Adams S.H., Lynch C.J., Brinkworth G.D., Davis T.A. Defining meal requirements for protein to optimize metabolic roles of amino acids. Am. J. Clin. Nutr. 2015 Jun;101(6):1330S–1338S. doi: 10.3945/ajcn.114.084053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dallas D.C., Sanctuary M.R., Qu Y., Khajavi S.H., van Zandt A.E., Dyandra M., Frese S.A., Barile D., German J.B. Personalizing protein nourishment. Crit. Rev. Food Sci. Nutr. 2017 Oct 13;57(15):3313–3331. doi: 10.1080/10408398.2015.1117412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Udenigwe C.C., Aluko R.E. Food protein-derived bioactive peptides: production, processing, and potential health benefits. J. Food Sci. 2012;77:R11. doi: 10.1111/j.1750-3841.2011.02455.x. –R24. [DOI] [PubMed] [Google Scholar]
- 29.Zaloga G., Siddiqui R. Biologically active dietary peptides. Mini-Rev. Med. Chem. 2004;4:815–821. doi: 10.2174/1389557043403477. [DOI] [PubMed] [Google Scholar]
- 30.Liu C.F., Pan T.M. Vol. 14. 2011. Derived from: Soybean and Health, Prof. Hany El-Shemy (Ed.), ISBN: 978-953-307-535-8; pp. 311–328. (Beneficial Effects of Bioactive Peptides Derived from Soybean on Human Health and Their Production by Genetic Engineering). Available from: intech open science. [Google Scholar]
- 31.Mohanty D.P., Mohapatra S., Misra S., Sahu P.S. Milk derived bioactive peptides and their Impact on human health - a review. Saudi J. Biol. Sci. 2016 Sep;23(5):577–583. doi: 10.1016/j.sjbs.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Punia H., Tokas J., Malik A., Sangwan S., Baloda S., Singh N., Singh S., Bhuker A., Singh P., Yashveer S., Agarwal S., Mor V.S. Identification and detection of bioactive peptides in milk and dairy products: remarks about agro-foods. Molecules. 2020 Jul 22;25(15):3328. doi: 10.3390/molecules25153328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Röhling M., McCarthy D., Berg A. Continuous protein supplementation reduces acute exercise-induced stress markers in athletes performing marathon. Nutrients. 2021 Aug 24;13(9):2929. doi: 10.3390/nu13092929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Young V.R. Soy protein in relation to human protein and amino acid nutrition. J. Am. Diet Assoc. 1991;91:828–835. ISSN 0002-8223. [PubMed] [Google Scholar]
- 35.Singh B.P., Vij S., Hati S. Functional significance of bioactive peptides derived from soybean. Peptides. 2014;54:171–179. doi: 10.1016/j.peptides.2014.01.022. Rev Food Sci Nutr. 2017 Oct 13;57(15):3313-3331. doi: 0.1080/10408398.2015.1117412. [DOI] [PubMed] [Google Scholar]
- 36.Lule V.K., Garg S., Pophaly S.D., Hitesh, Tomar S.K. Potential health benefits of lunasin: a multifaceted soy-derived bioactive peptide. J. Food Sci. 2015;80:R485–R494. doi: 10.1111/1750-3841.12786. [DOI] [PubMed] [Google Scholar]
- 37.Proteome Factory . 2014. Berlin, Germany. Personal Communication. [Google Scholar]
- 38.Messina M.J. Legumes and soybeans: overview of their nutritional profiles and health effects. Am. J. Clin. Nutr. 1999;70:439s–450s. doi: 10.1093/ajcn/70.3.439s. [DOI] [PubMed] [Google Scholar]
- 39.Cassidy A. Potential tissue selectivity of dietary phytoestrogens and estrogens. Curr. Opin. Lipidol. 1999;10:47–52. doi: 10.1097/00041433-199902000-00009. [DOI] [PubMed] [Google Scholar]
- 40.Setchell K.D.R., Cassidy A. Dietary isoflavones: biological effects and relevance to human health. J. Nutr. 1999;129:758S. doi: 10.1093/jn/129.3.758S. 767S. [DOI] [PubMed] [Google Scholar]
- 41.Velasquez M.T., Bhathena S.J. Role of dietary soy protein in obesity. Int. J. Med. Sci. 2007:72–82. doi: 10.7150/ijms.4.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wagner J.D., Zhang L., Shadoan M.K., Kavanagh K., Chen H., Tresnasari K., Kaplan J.R., Adams M.R. Effects of soy protein and isoflavones on insulin resistance and adiponectin in male monkeys. Metabolism. 2008;57:S24–S31. doi: 10.1016/j.metabol.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Watanabe S., Yamaguchi M., Sobue T., Takahashi T., Miura T., Arai Y., Mazur W., Wähälä K., Adlercreutz H. Pharmacokinetics of soybean isoflavones in plasma, urine and feces of men after ingestion of 60 g baked soybean powder (Kinako) J. Nutr. 1998;128:1710–1715. doi: 10.1093/jn/128.10.1710. [DOI] [PubMed] [Google Scholar]
- 44.EFSA. ANS Panel Risk assessment for peri- and post-menopausal women taking food supplements containing isolated isoflavones. EFSA J. 2015;13:4241–4342. [Google Scholar]
- 45.Koohkan S., Baer M., Vitolins M., König D., Bisse E., Berg A. Poster Presentation at the 58th Symposium of the German Association of Endocrinology; Lübeck, Germany: 2015. Influence of Soy Protein Intake on Blood Isoflavone Levels, Thyroid and Sex Hormone Concentrations in Women. [Google Scholar]
- 46.Martinez-Villaluenga C., Bringe N.A., Berhow M.A., Gonzalez de Mejia E. β-Conglycinin embeds active peptides that inhibit lipid accumulation in 3T3-L1 adipocytes in vitro. J. Agric. Food Chem. 2008;56:10533–10543. doi: 10.1021/jf802216b. [DOI] [PubMed] [Google Scholar]
- 47.Moriyama T., Kishimoto K., Nagai K., Urade R., Ogawa T., Utsumi S., Maruyama N., Maebuchi M. Soybean β-conglycinin diet suppresses serum triglyceride levels in normal and genetically obese mice by induction of β-oxidation, downregulation of fatty acid synthase, and inhibition of triglyceride absorption. Biosci. Biotechnol. Biochem. 2004;68:352–359. doi: 10.1271/bbb.68.352. [DOI] [PubMed] [Google Scholar]
- 48.Torres N., Torre-Villalvazo I., Tovar A.R. Regulation of lipid metabolism by soy protein and its implication in diseases mediated by lipid disorders. J. Nutr. Biochem. 2006;17:365–373. doi: 10.1016/j.jnutbio.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 49.Berg A., Berg A., Frey I., König D., Predel H.-G. Exercise based lifestyle intervention in obese adults – results of the intervention study M.O.B.I.L.I.S. Dtsch . Aerzteblatt Online. 2008;105:197–203. doi: 10.3238/arztebl.2008.0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.König D., Muser K., Berg A., Deibert P. Fuel selection and appetite-regulating hormones after intake of a soy protein-based meal replacement. Nutrition. 2012;28:35–39. doi: 10.1016/j.nut.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 51.Mezei O., Banz W.J., Steger R.W., Peluso M.R., Winters T.A., Shay N. Soy isoflavones exert antidiabetic and hypolipidemic effects through the PPAR pathways in obese Zucker rats and murine RAW 264.7 cells. J. Nutr. 2003;133:1238–1243. doi: 10.1093/jn/133.5.1238. [DOI] [PubMed] [Google Scholar]
- 52.Ronis M.J., Chen Y., Badeaux J., Badger T.M. Dietary soy protein isolate attenuates metabolic syndrome in rats via effects on PPAR, LXR, and SREBP signaling. J. Nutr. 2009;139:1431–1438. doi: 10.3945/jn.109.107029. [DOI] [PubMed] [Google Scholar]
- 53.Morifuji M., Sanbongi C., Sugiura K. Dietary soya protein intake and exercise training have an additive effect on skeletal muscle fatty acid oxidation enzyme activities and mRNA levels in rats. Br. J. Nutr. 2006;96:469–475. [PubMed] [Google Scholar]
- 54.Oliveira C.L.P., Boulé N.G., Berg A., Sharma A.M., Elliott S.A., Siervo M., Ghosh S., Prado C.M. Consumption of a high-protein meal replacement leads to higher fat oxidation, suppression of hunger, and improved metabolic profile after an exercise session. Nutrients. 2021;13:155. doi: 10.3390/nu13010155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ramón-Sierra J.M., Villanueva M.A., Yam-Puc A., Rodríguez-Mendiola M., Arias-Castro C., Ortiz-Vázquez E. Antimicrobial and antioxidant activity of proteins isolated from Melipona beecheii honey. Food Chem. X. 2021 Dec 2;13 doi: 10.1016/j.fochx.2021.100177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Majtan J., Bucekova M., Kafantaris I., Szweda P., Hammer K., Mossialos D. Honey antibacterial activity: a neglected aspect of honey quality assurance as functional food. Trends Food Sci. Technol. 2021;118:870. Part B. [Google Scholar]
- 57.Jones R. In: Honey and Healing. Munn P., Jones R., editors. 2001. Honey and Healing through the Ages; pp. 1–4. (International Bee Research Association IBRA: Cardiff). [Google Scholar]
- 58.Bogdanov S., Jurendic T., Sieber R., Gallmann P. Honey for nutrition and health: a review. J. Am. Coll. Nutr. 2008;27:677–689. doi: 10.1080/07315724.2008.10719745. [DOI] [PubMed] [Google Scholar]
- 59.Deibert P., König D., Kloock B., Groenefeld M., Berg A. Glycemic and insulinaemic properties of some German honey varieties. Eur. J. Clin. Nutr. 2010;64:762–764. doi: 10.1038/ejcn.2009.103. [DOI] [PubMed] [Google Scholar]
- 60.Belitz H., Grosch W. Springer-Verlag; Berlin, Heidelberg, New York: 2013. Textbook of Food Chemistry. [Google Scholar]
- 61.Khairun-Nisa H., Kok-Yong C., Fairus A. The mechanism of honey in reversing metabolic syndrome. Molecules. 2021;26(4):808. doi: 10.3390/molecules26040808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Al-Waili N., Salom K., Al-Ghamdi A., Ansari M.J., Al-Waili A., Al-Waili T. Honey and cardiovascular risk factors, in normal individuals and in patients with diabetes mellitus or dyslipidemia. J. Med. Food. 2013;16:1063–1078. doi: 10.1089/jmf.2012.0285. [DOI] [PubMed] [Google Scholar]
- 63.Erejuwa O.O., Sulaiman S.A., Wahab Ab, Honey M.S. A novel antioxidant. Molecules. 2012;17:4400–4423. doi: 10.3390/molecules17044400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Erejuwa O.O., Sulaiman S.A., Wahab M.S.A. Oligosaccharides might contribute to the antidiabetic effect of honey: a review of the literature. Molecules. 2011;17:248–266. doi: 10.3390/molecules17010248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Erejuwa O.O., Sulaiman S.A., Wahab M.S.A. Honey - a novel antidiabetic agent. Int. J. Biol. Sci. 2012;8:913–934. doi: 10.7150/ijbs.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Martínez I., Lattimer J.M., Hubach K.L., Case J.A., Yang J., Weber C.G., Louk J.A., Rose D.J., Kyureghian G., Peterson D.A., et al. Gut microbiome composition is linked to whole grain-induced immunological improvements. ISME J. 2013;7:269–280. doi: 10.1038/ismej.2012.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wasielewski H., Alcock J., Aktipis A. Resource conflict and cooperation between human host and gut microbiota: implications for nutrition and health. Ann. N. Y. Acad. Sci. 2016;1372:20–28. doi: 10.1111/nyas.13118. [DOI] [PubMed] [Google Scholar]
- 68.Walsh C.J., Guinane C.M., O'Toole P.W., Cotter P.D. Beneficial modulation of the gut microbiota. FEBS Lett. 2014;588:4120–4130. doi: 10.1016/j.febslet.2014.03.035. [DOI] [PubMed] [Google Scholar]
- 69.Larson-Meyer D.E., Willis K.S., Willis L.M., Austin K.J., Hart A.M., Breton A.B., Alexander B.M. Effect of honey versus sucrose on appetite, appetite-regulating hormones, and postmeal thermogenesis. J. Am. Coll. Nutr. 2010;29:482–493. doi: 10.1080/07315724.2010.10719885. [DOI] [PubMed] [Google Scholar]
- 70.Nemoseck T.M., Carmody E.G., Furchner-Evanson A., Gleason M., Li A., Potter H., Rezende L.M., Lane K.J., Kern M. Honey promotes lower weight gain, adiposity, and triglycerides than sucrose in rats. Nutr. Res. 2011;31:55–60. doi: 10.1016/j.nutres.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 71.Berg A., Frey I., Landmann U., Deibert P., König D., Berg A., jr., Dickhuth H.H. Gewichtsreduktion durch Lebensstilintervention. Einjahresergebnisse einer klinisch kontrollierten, randomisierten Studie mit übergewichtigen Erwachsenen. Ernahrungs Umsch. 2005;53:310–314. [Google Scholar]
- 72.Berg A., Frey I., Deibert P., Landmann U., König D., Schmidt-Trucksäss A., Rücker G., Kreiter H., Dickhuth H.H. Weight reduction is feasible - preliminary results of a controlled, randomised intervention study in overweight adults. Ernahrungs- Umsch. 2003;50:386–393+374. [Google Scholar]
- 73.Koohkan S., Golsorkhi M., Schaffner D., Konig D., Deibert P., et al. Effect of different isoenergetic breakfast compositions on blood glucose regulation, energy allocation and satiety. J Nutrition Health Food Sci. 2014;2(4):1–9. [Google Scholar]
- 74.Berg A., Schaffner D., Pohlmann Y., Baumstark M.W., Deibert P., König D., Gollhofer A. A soy-based supplement alters energy metabolism but not the exercise-induced stress response. Exerc. Immunol. Rev. 2012;18:128–141. [PubMed] [Google Scholar]
- 75.Banister E., Cameron B. Exercise-induced hyperammonemia: peripheral and central effects. Int. J. Sports Med. 1990;11:S129–S142. doi: 10.1055/s-2007-1024864. [DOI] [PubMed] [Google Scholar]
- 76.Berg A., Jakob E., Lehmann M., Dickhuth H.H., Huber G., Keul J. Current aspects of modern ergometry. Pneumologie. 1990;44:2–13. [PubMed] [Google Scholar]
- 77.Achten J., Jeukendrup A.E. Optimizing fat oxidation through exercise and diet. Nutrition. 2004;20:716–727. doi: 10.1016/j.nut.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 78.Hurley B.F., Nemeth P.M., Martin W.H., Hagberg J.M., Dalsky G.P., Holloszy J.O. Muscle triglyceride utilization during exercise: effect of training. J. Appl. Physiol. 1986;60:562–567. doi: 10.1152/jappl.1986.60.2.562. [DOI] [PubMed] [Google Scholar]
- 79.Romijn J.A., Coyle E.F., Sidossis L.S., Rosenblatt J., Wolfe R.R. Substrate metabolism during different exercise intensities in endurance-trained women. J. Appl. Physiol. 2000;88:1707–1714. doi: 10.1152/jappl.2000.88.5.1707. [DOI] [PubMed] [Google Scholar]
- 80.Oliveira C.L.P., Boulé N.G., Sharma A.M., Elliott S., Siervo M., Ghosh S., Berg A., Prado C.M. Examining the effects of a high-protein total diet replacement on energy metabolism, metabolic blood markers, and appetite sensations in healthy adults: protocol for two complementary, randomized, controlled, crossover trials. Trials. 2019;20:787. doi: 10.1186/s13063-019-3950-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Oliveira C.L.P., Boulé N.G., Sharma A.M., Elliott S.A., Siervo M., Ghosh S., Berg A., Prado C.M. A high-protein total diet replacement increases energy expenditure and leads to negative fat balance in healthy, normal-weight adults. Am. J. Clin. Nutr. 2021;113:476–487. doi: 10.1093/ajcn/nqaa283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Oliveira C.L.P., Boulé N.G., Elliott S.A., Sharma A.M., Siervo M., Berg A., Ghosh S., Prado C.M. A high-protein total diet replacement alters the regulation of food intake and energy homeostasis in healthy, normal-weight adults. Eur. J. Nutr. 2022 Jun;61(4):1849–1861. doi: 10.1007/s00394-021-02747-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Röhling M., Kempf K., Banzer W., Berg A., Braumann K.-M., Tan S., Halle M., McCarthy D., Pinget M., Predel H.-G., et al. Prediabetes conversion to normoglycemia is superior adding a low-carbohydrate and energy deficit formula diet to lifestyle intervention—a 12-month subanalysis of the ACOORH trial. Nutrients. 2020;12:2022. doi: 10.3390/nu12072022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Halle M., Röhling M., Banzer W., Braumann K.M., Kempf K., McCarthy D., Schaller N., Predel H.G., Scholze J., Führer-Sakel D., et al. Meal replacement by formula diet reduces weight more than a lifestyle intervention alone in patients with overweight or obesity and accompanied cardiovascular risk factors—the ACOORH trial. Eur. J. Clin. Nutr. 2021 Apr;75(4):661–669. doi: 10.1038/s41430-020-00783-4. [DOI] [PubMed] [Google Scholar]
- 85.Field A.E., Coakley E.H., Must A., Spadano J.L., Laird N., Dietz W.H., Rimm E., Colditz G.A. Impact of overweight on the risk of developing common chronic diseases during a 10-year period. Arch. Intern. Med. 2001;161:1581–1586. doi: 10.1001/archinte.161.13.1581. [DOI] [PubMed] [Google Scholar]
- 86.Leung A.W.Y., Chan R.S.M., Sea M.M.M., Woo J. An overview of factors associated with adherence to lifestyle modification programs for weight management in adults. Int. J. Environ. Res. Publ. Health. 2017 Aug 16;14(8):922. doi: 10.3390/ijerph14080922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Deibert P., König D., Schmidt-Trucksaess A., Zaenker K.S., Frey I., Landmann U., Berg A. Weight loss without losing muscle mass in pre-obese and obese subjects induced by a high-soy-protein diet. Int. J. Obes. 2004;28:1349–1352. doi: 10.1038/sj.ijo.0802765. [DOI] [PubMed] [Google Scholar]
- 88.McCrory M.A., Gomez T.D., Bernauer E.M., Mol P.A. Evaluation of a new air displacement plethysmograph for measuring human body composition. Med. Sci. Sports Exerc. 1995;27:1686–1691. [PubMed] [Google Scholar]
- 89.Bosy-Westphal A., Schautz B., Later W., Kehayias J.J., Gallagher D., Müller M.J. What makes a BIA equation unique? Validity of eight-electrode multifrequency BIA to estimate body composition in a healthy adult population. Eur. J. Clin. Nutr. 2013;67:S14–S21. doi: 10.1038/ejcn.2012.160. [DOI] [PubMed] [Google Scholar]
- 90.Deibert P., König D., Vitolins M.Z., Landmann U., Frey I., Zahradnik H.P., Berg A. Effect of a weight loss intervention on anthropometric measures and metabolic risk factors in pre- versus postmenopausal women. Nutr. J. 2007;6:31. doi: 10.1186/1475-2891-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Deibert P., Solleder F., König D., Vitolins M.Z., Dickhuth H.-H., Gollhofer A., Berg A. Soy protein based supplementation supports metabolic effects of resistance training in previously untrained middle aged males. Aging Male. 2011;14:273–279. doi: 10.3109/13685538.2011.565091. [DOI] [PubMed] [Google Scholar]
- 92.König D., Koohkan S., Schaffner D., Deibert P., Berg A. A meal replacement regimen improves blood glucose levels in prediabetic healthy individuals with impaired fasting glucose. Nutrition. 2014;30:1306–1309. doi: 10.1016/j.nut.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 93.Koohkan S., Golesorkhi M., Vitolins M., McCarthy D., Berg A. The influence of a meal replacement formula on leptin regulation in obese adults. J. Nutrition Health Food Sci. 2016;4:1–5. [Google Scholar]
- 94.Vitolins M.Z., Milliron B.-J., Hopkins J.O., Fulmer A., Lawrence J., Melin S., Case D. Weight. Loss intervention in survivors of ER/PR-negative breast cancer. Clin. Med. Insights Women's Health. 2014 Jun 16;7:17–24. doi: 10.4137/CMWH.S13955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.König D., Deibert P., Frey I., Landmann U., Berg A. Effect of meal replacement on metabolic risk factors in overweight and obese subjects. Ann. Nutr. Metab. 2008;52:74–78. doi: 10.1159/000119416. [DOI] [PubMed] [Google Scholar]
- 96.Tchernof A., Poehlman E.T. Effects of the menopause transition on body fatness and body fat distribution. Obes. Res. 1998;6:246–254. doi: 10.1002/j.1550-8528.1998.tb00344.x. [DOI] [PubMed] [Google Scholar]
- 97.Faria A.N., Filho F.F.R., Ferreira S.R.G., Zanella M.T. Impact of visceral fat on blood pressure and insulin sensitivity in hypertensive obese women. Obes. Res. 2002;10:1203–1206. doi: 10.1038/oby.2002.164. [DOI] [PubMed] [Google Scholar]
- 98.Distefano G., Goodpaster B.H. Effects of exercise and aging on skeletal muscle. Cold Spring Harb. Perspect. Med. 2018;8:a029785. doi: 10.1101/cshperspect.a029785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Garber C.E., Blissmer B., Deschenes M.R., Franklin B.A., Lamonte M.J., Lee I.M., Nieman D.C., Swain D.P. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med. Sci. Sports Exerc. 2011;43:1334–1359. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- 100.König D., Zdzieblik D., Deibert P., Berg A., Gollhofer A., Büchert M. Internal fat and cardiometabolic risk factors following a meal-replacement regimen vs. Comprehensive lifestyle changes in obese subjects. Nutrients. 2015;7:9825–9833. doi: 10.3390/nu7125500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Younossi Z., Anstee Q.M., Marietti M., Hardy T., Henry L., Eslam M., George J., Bugianesi E. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018;15:11–20. doi: 10.1038/nrgastro.2017.109. [DOI] [PubMed] [Google Scholar]
- 102.Wong R.J., Cheung R., Ahmed A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology. 2014;59:2188–2195. doi: 10.1002/hep.26986. [DOI] [PubMed] [Google Scholar]
- 103.Church T.S., Kuk J.L., Ross R., Priest E.L., Biltoff E., Blair S.N. Association of cardiorespiratory fitness, body mass index, and waist circumference to nonalcoholic fatty liver disease. Gastroenterology. 2006;130:2023–2030. doi: 10.1053/j.gastro.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 104.Johnson N.A., Sachinwalla T., Walton D.W., Smith K., Armstrong A., Thompson M.W., George J. Aerobic exercise training reduces hepatic and visceral lipids in obese individuals without weight loss. Hepatology. 2009;50:1105–1112. doi: 10.1002/hep.23129. [DOI] [PubMed] [Google Scholar]
- 105.Tabák A.G., Herder C., Rathmann W., Brunner E.J., Kivimäki M. Prediabetes. A high-risk state for diabetes development. Lancet. 2012;379:2279–2290. doi: 10.1016/S0140-6736(12)60283-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Deibert P., Lazaro A., Schaffner D., Berg A., Koenig D., Kreisel W., Baumstark M.W., Steinmann D., Buechert M., Lange T. Comprehensive lifestyle intervention vs soy protein-based meal regimen in non-alcoholic steatohepatitis. World J. Gastroenterol. 2019;25:1116–1131. doi: 10.3748/wjg.v25.i9.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zelber-Sagi S. Nutrition and physical activity in NAFLD: an overview of the epidemiological evidence. World J. Gastroenterol. 2011;17:3377. doi: 10.3748/wjg.v17.i29.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Perreault L., Pan Q., Mather K.J., Watson K.E., Hamman R.F., Kahn S.E. Effect of regression from prediabetes to normal glucose regulation on long-term reduction in diabetes risk: results from the Diabetes Prevention Program Outcomes Study. Lancet. 2012;379:2243–2251. doi: 10.1016/S0140-6736(12)60525-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Piepoli M.F., Hoes A.W., Agewall S., Albus C., Brotons C., Catapano A.L., Cooney M.-T., Corrà U., Cosyns B., Deaton C., et al. European guidelines on cardiovascular disease prevention in clinical practice: the sixth joint task force of the European society of cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of 10 societies and by invited experts): developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR) Eur. J. Prev. Cardiol. 2016;23 doi: 10.1177/2047487316653709. [DOI] [PubMed] [Google Scholar]
- 110.Barry E., Roberts S., Oke J., Vijayaraghavan S., Normansell R., Greenhalgh T. Efficacy and effectiveness of screen and treat policies in prevention of type 2 diabetes: systematic review and meta-analysis of screening tests and interventions. BMJ. 2017;356:i6538. doi: 10.1136/bmj.i6538. [DOI] [PubMed] [Google Scholar]
- 111.Lean M.E.J., Leslie W.S., Barnes A.C., Brosnahan N., Thom G., McCombie L., Peters C., Zhyzhneuskaya S., Al-Mrabeh A., Hollingsworth K.G., et al. Durability of a primary care-led weight-management intervention for remission of type 2 diabetes: 2-year results of the DiRECT open-label, cluster-randomised trial. Lancet Diabetes Endocrinol. 2019;7:344–355. doi: 10.1016/S2213-8587(19)30068-3. [DOI] [PubMed] [Google Scholar]
- 112.Bowen M.E., Cavanaugh K.L., Wolff K., Davis D., Gregory R.P., Shintani A., Eden S., Wallston K., Elasy T., Rothman R.L. The diabetes nutrition education study randomized controlled trial: a comparative effectiveness study of approaches to nutrition in diabetes self-management education. Patient Educ. Counsel. 2016;99:1368–1376. doi: 10.1016/j.pec.2016.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Vogtschmidt Y.D., Rabe n A., Faber I., de Wilde C., Lovegrove J.A., Givens D.I., Pfeiffer A.F.H., Soedamah-Muthu S.S. Is protein the forgotten ingredient: effects of higher compared to lower protein diets on cardiometabolic risk factors. A systematic review and meta-analysis of randomised controlled trials. Atherosclerosis. 2021 Jul;328:124–135. doi: 10.1016/j.atherosclerosis.2021.05.011. [DOI] [PubMed] [Google Scholar]
- 114.Zänker K.S., Erxleben-Neis J., Gottschalk G., Schweig N. Type 2 diabetes mellitus and cancer – a nutrition-oriented intervention study. Dtsch. Z. Onkol. 2005;37:114–121. [Google Scholar]
- 115.Banzer W., Braumann K.M., Berg A., Führer-Sakel D., Halle M., Martin S., McCarthy D., Predel G.P., Scholze J., Seyller C., Toplak H. 2018. Improvement of Prediabetes Condition Applying the ACOORH Concept on Weight Control and Metabolic Regulation after 4 and 12 Weeks of Intervention – Results of a Multicenter RCT. 25th European Congress on Obesity. Vienna, Austria, May 23–26. [Google Scholar]
- 116.Scholz G.H., Hanefeld M. Metabolic vascular syndrome: new insights into a multidimensional network of risk factors and diseases. Vis. Med. 2016 Oct;32(5):319–326. doi: 10.1159/000450866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bovolini A., Garcia J., Andrade M.A., Duarte J.A. Metabolic syndrome pathophysiology and predisposing factors. Int. J. Sports Med. 2021 Mar;42(3):199–214. doi: 10.1055/a-1263-0898. [DOI] [PubMed] [Google Scholar]
- 118.Pasternak R.C. Report of the adult treatment panel III: the 2001 National Cholesterol Education Program guidelines on the detection, evaluation and treatment of elevated cholesterol in adults. Cardiol. Clin. 2003;21:393–398. doi: 10.1016/s0733-8651(03)00080-8. [DOI] [PubMed] [Google Scholar]
- 119.Zhang F., Chen Y., Heiman M., DiMarchi R. Leptin. Structure, function and biology. Vitam. Horm. 2005;71:345–372. doi: 10.1016/S0083-6729(05)71012-8. [DOI] [PubMed] [Google Scholar]
- 120.Banks W.A. The blood–brain barrier as an endocrine tissue. Nat. Rev. Endocrinol. 2019;15:444–455. doi: 10.1038/s41574-019-0213-7. [DOI] [PubMed] [Google Scholar]
- 121.Wang P., Holst C., Andersen M.R., Astrup A., Bouwman F.G., van Otterdijk S., Wodzig W.K.W.H., van Baak M.A., Larsen T.M., Jebb S.A., et al. Blood profile of proteins and steroid hormones predicts weight change after weight loss with interactions of dietary protein level and glycemic index. PLoS One. 2011;6 doi: 10.1371/journal.pone.0016773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Crujeiras A.B., Goyenechea E.b., Abete I., Lage M., Carreira M.C., Martínez J.A., Casanueva F.F. Weight regain after a diet-induced loss is predicted by higher baseline leptin and lower ghrelin plasma levels. J. Clin. Endocrinol. Metab. 2010;95:5037–5044. doi: 10.1210/jc.2009-2566. [DOI] [PubMed] [Google Scholar]
- 123.Demonty I., Lamarche B.t., Deshaies Y., Jacques H. Role of soy isoflavones in the hypotriglyceridemic effect of soy protein in the rat. J. Nutr. Biochem. 2002;13:671–677. doi: 10.1016/s0955-2863(02)00214-0. [DOI] [PubMed] [Google Scholar]
- 124.Wang Y., Jones P.J.H., Ausman L.M., Lichtenstein A.H. Soy protein reduces triglyceride levels and triglyceride fatty acid fractional synthesis rate in hypercholesterolemic subjects. Atherosclerosis. 2004;173:269–275. doi: 10.1016/j.atherosclerosis.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 125.Kempf K., Röhling R., Banzer W., Braumann K., Halle M., McCarthy D., Predel H., Schenkenberger I., Tan S., Toplak H., et al. High-protein, low-glycemic meal replacement decreases insulin and inflammation markers long-term - a 12-month subanalysis of the ACOORH trial. Nutrients. 2021;13:1433. doi: 10.3390/nu13051433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kempf K., Röhling M., Banzer W., Braumann K.M., Halle M., Schaller N., McCarthy D., Predel H.G., Schenkenberger I., Tan S., Toplak H., Martin S., Berg A., ACOORH Study Early and strong leptin reduction is predictive for long-term weight loss during high-protein, low-glycemic meal replacement-A subanalysis of the randomised-controlled ACOORH trial. Nutrients. 2022 Jun 18;14(12):2537. doi: 10.3390/nu14122537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Xu H., Barnes G.T., Yang Q., Tan G., Yang D., Chou C.J., et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Weisberg S.P., McCann D., Desai M., Rosenbaum M., Leibel R.L., Ferrante A.W., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kolb H., Mandrup-Poulsen T. An immune origin of type 2 diabetes? Diabetologia. 2005;48:1038–1050. doi: 10.1007/s00125-005-1764-9. [DOI] [PubMed] [Google Scholar]
- 130.Grassmann S., Wirsching J., Eichelmann F., Aleksandrova K. Association between peripheral adipokines and inflammation markers: a systematic review and meta-analysis. Obesity. 2017;25:1776–1785. doi: 10.1002/oby.21945. [DOI] [PubMed] [Google Scholar]
- 131.Johnson J.B., Summer W., Cutler R.G., Martin B., Hyun D.H., Dixit V.D., Pearson M., Nassar M., Telljohann R., Maudsley S., et al. Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Free Radic. Biol. Med. 2007;42:665–674. doi: 10.1016/j.freeradbiomed.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bhutani S., Klempel M.C., Berger R.A., Varady K.A. Improvements in coronary heart disease risk indicators by alternate-day fasting involve adipose tissue modulations. Obesity. 2010;18:2152–2159. doi: 10.1038/oby.2010.54. [DOI] [PubMed] [Google Scholar]
- 133.Faris M.E.A.-I.E., Kacimi S., Al-Kurd R.A.A., Fararjeh M.A., Bustanji Y.K., Mohammad M.K., Salem M.L. Intermittent fasting during Ramadan attenuates proinflammatory cytokines and immune cells in healthy subjects. Nutr. Res. 2012;32:947–955. doi: 10.1016/j.nutres.2012.06.021. [DOI] [PubMed] [Google Scholar]
- 134.Aksungar F.B., Topkaya A.E., Akyildiz M. Interleukin-6, C-reactive protein and biochemical parameters during prolonged intermittent fasting. Ann. Nutr. Metab. 2007;51:88–95. doi: 10.1159/000100954. [DOI] [PubMed] [Google Scholar]
- 135.Jang E.H., Moon J.S., Ko J.H., Ahn C.W., Lee H.H., Shin J.K., Park C.S., Kang J.H. Novel black soy peptides with antiobesity effects: activation of leptin-like signaling and AMP-activated protein kinase. Int. J. Obes. 2008;32:1161–1170. doi: 10.1038/ijo.2008.60. [DOI] [PubMed] [Google Scholar]
- 136.Wadden T.A., Phelan S. Assessment of quality of life in obese individuals. Obes. Res. 2002;10:50S. doi: 10.1038/oby.2002.190. –57S. [DOI] [PubMed] [Google Scholar]
- 137.Fontaine K.R., Bartlett S.J., Barofsky I. Health-related quality of life among obese persons seeking and not currently seeking treatment. Int. J. Eat. Disord. 2000;27:101–105. doi: 10.1002/(sici)1098-108x(200001)27:1<101::aid-eat12>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 138.Koohkan S., Schaffner D., Milliron B.J., Frey I., König D., Deibert P., Vitolins M., Berg A. The impact of a weight reduction program with and without meal-replacement on health related quality of life in middle-aged obese females. BMC Wom. Health. 2014;14:45. doi: 10.1186/1472-6874-14-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kempf K., Schloot N.C., Gärtner B., Keil R., Schadewaldt P., Martin S. Meal replacement reduces insulin requirement, HbA1c and weight long-term in type 2 diabetes patients with >100 U insulin per day. J. Hum. Nutr. Diet. 2014;27(suppl.2) doi: 10.1111/jhn.12145. First published: 02 August 2013. [DOI] [PubMed] [Google Scholar]
- 140.Kempf K., Altpeter B., Berger J., Reuß O., Fuchs M., Schneider M., Gärtner B., Niedermeier K., Martin S. Efficacy of the telemedical lifestyle intervention program TeLiPro in advanced stages of type 2 diabetes: a randomized controlled trial. Diabetes Care. 2017 Jul;40(7):863–871. doi: 10.2337/dc17-0303. [DOI] [PubMed] [Google Scholar]
- 141.Kempf K., Röhling M., Niedermeier K., Gärtner B., Martin S. Individualized meal replacement therapy improves clinically relevant long-term glycemic control in poorly controlled type 2 diabetes patients. Nutrients. 2018 Aug 4;10(8):1022. doi: 10.3390/nu10081022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Röhling M., Kempf K., Banzer W., Braumann K.M., Führer-Sakel D., Halle M., McCarthy D., Martin S., Scholze J., Toplak H., Berg A., Predel H.G., ACOORH Study Group A high-protein and low-glycemic formula diet improves blood pressure and other hemodynamic parameters in high-risk individuals. Nutrients. 2022 Mar 30;14(7):1443. doi: 10.3390/nu14071443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kempf K., Röhling M., Banzer W., Braumann M., Halle M., Schaller N., McCarthy D., Predel H.G., Schenkenberger I., Tan S., Toplak H., Martin S., Berg A., Acoorh Study. High-protein, low-glycemic meal replacement improves physical health-related quality of life in high-risk persons for metabolic syndrome—a subanalysis of the randomised-controlled ACOORH trial. Nutrients. 2022 July 30;14:3161. doi: 10.3390/nu14153161. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.