Abstract
Pathogenic Yersinia spp. utilize a plasmid-encoded type III secretion system to deliver a set of Yop effector proteins into eukaryotic cells. Previous studies have shown that the effector YopJ is required for Yersinia to cause downregulation of the mitogen-activated protein (MAP) kinases c-Jun N-terminal kinase (JNK), p38, and extracellular signal-regulated kinase (ERK) 1 and 2 in infected macrophages. Here we demonstrate that YopJ is sufficient to cause downregulation of multiple MAP kinases in eukaryotic cells. Cellular fractionation experiments confirmed that YopJ is delivered into the cytoplasmic fraction of macrophages by the type III system. Production of YopJ in COS-1 cells by transfection significantly reduced (5- to 10-fold) activation of JNK, p38, and ERK in response to several different stimuli, including serum and tumor necrosis factor alpha. JNK activation mediated by RacV12, an activated mutant of Rac1, was also blocked by YopJ in COS-1 cells, indicating that YopJ acts downstream of this small GTPase to downregulate MAP kinase signaling. Analysis of transfected COS-1 cells by immunofluorescence microscopy revealed that YopJ is recruited from the cytoplasmic compartment to the cell periphery in response to stimuli (e.g., serum) that induce membrane ruffling. These data indicate that YopJ functions as a “MAP kinase toxin” to selectively block nuclear responses that are triggered by Yersinia-host cell interaction.
Three bacterial spp. in the genus Yersinia cause human disease. Yersinia pestis is the agent of bubonic plague (9). Yersinia enterocolitica and Yersinia pseudotuberculosis cause diarrhea, mesenteric lymphadenitis, and septicemia (8, 10). All three Yersinia spp. carry a 70-kb plasmid, referred to as pYV (plasmid of Yersinia virulence), that is required for replication in lymphoid tissues. pYV encodes a type III secretion pathway that allows extracellular Yersinia to deliver a set of virulence proteins into the cytoplasmic compartments of host eukaryotic cells (reviewed in references 11, 15, and 40). The type III pathway is comprised of three basic components: a ysc-encoded secretion system that delivers proteins to the bacterial surface; a translocation apparatus represented minimally by LcrV, YopB, and YopD; and a set of effector proteins (YopO [YpkA], YopH, YopM, YopJ [YopP], and YopE) (11, 15, 40). The translocated effectors paralyze and kill host cells and block their ability to produce cytokines (11, 15, 24, 26, 29, 31, 32, 40).
Wild-type strains of Yersinia inhibit production of the proinflammatory cytokine tumor necrosis factor alpha (TNF-α) in vivo (27) and in cultured macrophages (2, 5, 29, 34). Wild-type strains of Yersinia also downregulate the mitogen-activated protein (MAP) kinases c-Jun N-terminal kinase (JNK), p38, and extracellular signal-regulated kinase (ERK) in macrophages (5, 29, 34). As MAP kinases play an important role in the regulation of proinflammatory cytokine biosynthesis (20, 22, 25, 30, 41, 45), this is likely to represent the mechanism used by these bacteria to inhibit cytokine production (5, 29, 34).
The effector YopJ is required for Yersinia to inhibit TNF-α production and cause downregulation of MAP kinases in macrophages (5, 29). The ability of Yersinia to kill macrophages by apoptosis is also dependent upon YopJ (24, 26). YopJ is a 30-kDa protein of unknown activity (39). The translocation factor YopB is required for suppression of TNF-α production (5, 29), indicating that YopJ (and possibly other essential effectors) must be delivered into cells for this repressive activity to be expressed. Consistent with the idea that YopJ is translocated into host cells, a cyclase reporter protein linked to the first 99 residues of YopJ can be internalized into macrophages at low but detectable levels in a YopB-dependent manner (36). Furthermore, AvrA, a YopJ-related protein found in Salmonella, is translocated into host cells via the invasion-associated type III pathway (17).
Although the above data indicate that YopJ is required for Yersinia to cause downregulation of MAP kinases, it has not been demonstrated that it is sufficient for this activity. It is possible that another type III effector protein, in addition to YopJ, is required for this activity. A second complication is that certain activities attributed to YopJ in macrophages may result from a general decrease in cell viability due to apoptosis. In order to avoid the effects of YopJ-mediated cell killing in our studies, we analyzed MAP kinase downregulation in macrophages prior to the first detectable signs of apoptosis. The results of infection experiments indicated that full-length YopJ is translocated into host cells in a YopB-dependent manner and that it is the only effector encoded by pYV that is required to cause downregulation of MAP kinases. In addition, we demonstrated by transfection of fibroblasts that production of YopJ in eukaryotic cells is sufficient to cause downregulation of JNK, p38, and ERK kinases.
MATERIALS AND METHODS
Reagents.
The following oligonucleotides were obtained from Life Technologies: J3 (5′-GGGAATTCATATGATCGGACCAATATCACAAATAAAT-3′) and J9 (5′-AATAAGGATAAATAAATGGAAAGGAATTATCAGTTTCGGTA-3′). FuGENE 6 transfection reagent was purchased from Boehringer Mannheim. Sorbitol and phorbol 12-myristate 13-acetate (PMA) were purchased from Sigma. TNF-α was purchased from Calbiochem.
Antibodies and immunoblotting conditions.
Phosphospecific antibodies to p38, JNK, and ERK1 and -2 and standard antibodies to JNK and ERK1 and -2 were purchased from New England Biolabs. The standard anti-p38 antibody (Santa Cruz Biotechnology, Inc.) was a gift from J. Galán (State University of New York [SUNY], Stony Brook). The T7 · Tag antibody (Novagen) was a gift from D. Bar-Sagi (SUNY, Stony Brook). Immunoblotting with commercial primary antibodies was done as suggested by the suppliers. The mouse monoclonal antibody M45 (28), which recognizes the 12-amino acid epitope SRDRLPPFETET, was provided as a hybridoma supernatant by P. Hearing (SUNY, Stony Brook). Immunoblotting with the M45 hybridoma supernatant was performed at a dilution of 1:500. Anti-mouse immunoglobulin G and anti-rabbit immunoglobulin G, each conjugated to horseradish peroxidase (Sigma) were used at a dilution of 1:20,000 or 1:2,000, respectively. Immunoblots were developed with enhanced chemiluminescence reagents as recommended by the supplier (New England Nuclear). Films exposed in the linear range were scanned with an LKB laser densitometer to quantitate relative levels of activated MAP kinases.
Bacterial strains and growth conditions.
The Y. pseudotuberculosis strains used in this study are shown in Table 1. Bacteria were routinely cultivated at 26°C in Luria-Bertani broth or on Luria-Bertani agar plates containing appropriate antibiotics. For infection assays, overnight cultures of bacteria were diluted into Luria broth containing 20 mM sodium oxalate and 20 mM MgCl2 to an optical density at 600 nm of 0.1. Bacteria were grown with shaking at 26°C for 1 h and then shifted to 37°C for 2 to 3 h to induce maximal Yop expression. Where indicated, IPTG (isopropyl-β-d-thiogalactopyranoside) was added (100 μM final concentration) to the growth media at the time of the temperature shift to 37°C in order to induce expression of yopJM45. Bacteria were pelleted by microcentrifugation, resuspended to an optical density at 600 nm of 1.0 in Hanks balanced salt solution, and used to infect macrophages at a multiplicity of infection of 100.
TABLE 1.
Strains and plasmids
| Strain or plasmid | Relevant characteristics | Source or reference |
|---|---|---|
| Y. pseudotuber-culosis | ||
| YP126 | Wild type, YPIII(pYV+) | 6 |
| YP71 | YP126 ysc::Tn5 (secretion mutant) | 7 |
| YP17 | YP126 yopE::kan yopH::cam | 3 |
| YP18 | YP126 ΔyopB (deficient in YopB) | 29 |
| YP19 | YP17 ΔyopB (deficient in Yops E, H, B) | This work |
| YP22 | YP17 ΔyopK (deficient in Yops E, H, K) | 29 |
| YP23 | YP17 ΔyopM (deficient in Yops E, H, M) | 29 |
| YP24 | YP17 ΔyopO (deficient in Yops E, H, O) | 29 |
| YP25 | YP17 yopO::pLP8 (deficient in Yops E, H, J) | 29 |
| YP26 | YP126 ΔyopJ (deficient in YopJ) | 29 |
| YP27 | YP17 ΔyopJ (deficient in Yops E, H, J) | This work |
| YP29 | YP19 ΔyopJ (deficient in Yops E, H, B, J) | This work |
| IP2666 | Plasmid cured | 4 |
| IP27 | IP2666 transformed with virulence plasmid from YP27 (deficient in Yops E, H, J) | This work |
| IP29 | IP2666 transformed with virulence plasmid from YP29 (deficient in Yops E, H, B, J) | This work |
| Plasmids | ||
| pMMB67HE | Expression vector | 14 |
| pYOPHM45 | pMMB67HE yopHM45 | This work |
| pGEX-2T | GST fusion vector | 38 |
| pLP15 | pGEX-2T yopHM45 | This work |
| pLP16 | pGEX-2T yopJM45 | This work |
| pLP17 | pMMB67HE yopJM45 | This work |
| pSFFV | pCMV3Rluc with SFFV promoter | 15a |
| pLP20 | pSFFV yopJM45 | This work |
| pCGT | pCGN with T7 epitope tag | 42 |
| pCGT-RacV12 | pCGT containing RacV12 | 42 |
Construction of yop mutants.
Mutants were constructed by allelic recombination as described previously (29). YP19 (yopEHB) was derived from YP17 (yopEH) by deleting nucleotides 496 to 774 of yopB with the suicide plasmid pJB4 (29). YP27 (yopEHJ) and YP29 (yopEHJB) were derived from YP17 and YP19, respectively, by deleting nucleotides 1 to 795 of yopJ with the suicide plasmid pLP13 (29). Virulence plasmid DNA from YP27 and YP29 was purified and electroporated into the plasmid-cured strain IP2666, resulting in IP27 and IP29, respectively.
Construction of plasmids.
Plasmids are listed in Table 1. A DNA fragment containing yopH fused to a carboxy-terminal M45 epitope (yopHM45) was inserted into the multicloning site of pGEX-2T, resulting in pLP15. In pLP15, yopHM45 is flanked by a 5′ NdeI and 3′ EcoRI site, and an XmaI site is present at the point of fusion between the yopH and M45 sequences. pLP16 was constructed by replacing the yopH sequence in pLP15 with an NdeI-XmaI fragment containing the yopJ coding region. The yopJ coding region was obtained by PCR amplification, by using primers J3 and J9, followed by digestion with NdeI and XmaI. The structure of the yopJM45 gene in pLP16 was verified by sequencing. To construct pYOPHM45, the yopHM45 gene was inserted into the multicloning site of pMMB67HE (14), placing it under the control of the ptac promoter. pLP17 was constructed by replacing the yopJM45 sequence in pYOPHM45 with an NdeI-EcoRI fragment containing the yopJM45 coding region. pSFFV (15a) was derived from pCMV3Rluc (12) by replacing the cytomegalovirus promoter with the spleen focus-forming virus (SFFV) promoter (provided by Michel Nussenswieg). The yopJM45 gene was inserted between the ApaI and NotI sites of pSFFV, yielding pLP20.
Analysis of MAP kinase downregulation in macrophages.
J774A.1 murine macrophage-like cells were grown as described previously (29). Twenty-four hours prior to infection, 2 × 106 macrophages in 3 ml of Dulbecco modified Eagle medium containing 10% fetal bovine serum (DMEM-10% FBS) (GIBCO BRL) were seeded into 60-mm-diameter tissue culture dishes. Fifteen minutes prior to infection, the macrophages were overlaid with 3 ml of fresh DMEM-10%FBS. A total of 2 × 108 bacteria grown as described above were then added to each well. After 15 or 45 min, the cells were washed once with ice-cold phosphate-buffered saline (PBS) containing 1 mM Na3VO4 and 10 mM NaF. Washed cells were lysed for 15 min on ice with 0.5 ml of modified radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris [pH 8.0], 150 mM NaCl, 1% Nonidet P-40, 0.5% deoxycholate, 0.1% sodium dodecyl sulfate [SDS], 1 mM Na3VO4, 10 mM NaF). Lysates were clarified by centrifugation for 15 min at 4°C at 12,000 × g. Protein concentrations were determined by using the Bio-Rad protein assay. Samples containing approximately 20 μg of protein were boiled in Laemmli sample buffer (1× Laemmli sample buffer containing 10 mM dithiothreitol), separated on SDS–10% polyacrylamide gels, and transferred to nitrocellulose membranes. Membranes were probed with phosphospecific antibodies or standard antibodies according to the manufacturers’ instructions.
Detergent solubility assay.
Macrophages were infected with bacteria as described above. Where indicated, the tissue culture medium was supplemented with IPTG (100 μM final concentration) to induce expression of yopJM45. Two hours postinfection, the dishes were washed three times in ice-cold PBS containing 1 mM Na3VO4 and 10 mM NaF. To each dish 0.5 ml of cold 1% Triton X-100 buffer (10 mM Tris [pH 7.6], 150 mM NaCl, 5 mM EDTA, 10% glycerol, 1% Triton X-100, 1 mM Na3VO4, 10 mM NaF, 200 μM AEBSF [aminoethylbenzenesulfonyl fluoride], 20 μM leupeptin, and 1 μM pepstatin) was added, and the dishes were incubated for 15 min on ice with occasional rocking. Infected cells were scraped with the lysis buffer into microcentrifuge tubes and centrifuged 10 min at 12,000 × g at 4°C. Supernatants (soluble fractions) were transferred to new tubes, and protein concentrations were determined by using the Bio-Rad protein assay. Soluble fractions were diluted to 0.8 μg per μl in Laemmli sample buffer and boiled. Pellets (insoluble fractions) were carefully washed in lysis buffer, resuspended in 100 μl of Laemmli sample buffer, and boiled. A volume of 25 μl of each soluble fraction (approximately 2% of the total) and 10 μl of each insoluble fraction (10% of the total) were separated on SDS–10% polyacrylamide gels and transferred to a nitrocellulose membrane. Immunoblots were developed with the M45 antibody. In mock infection experiments, 2 × 108 bacteria were inoculated into 60-mm-diameter dishes containing 3 ml of DMEM-10%FBS. Incubations, extractions, and immunoblots were carried out exactly as described above, with the exception that 10 μl of each soluble protein sample (approximately 2% of the total) was analyzed.
Analysis of MAP kinase downregulation in COS-1 cells.
COS-1 cells were passaged in DMEM containing 5% FBS (DMEM-5%FBS). Twenty-four hours prior to transfection, 105 COS-1 cells in 2 ml of DMEM-5%FBS were seeded into the wells of a 6-well dish. COS-1 cells were transfected with 1 μg of pSFFV or pLP20 mixed with 3 μl of the FuGENE 6 transfection reagent according to the manufacturer’s instructions. Twenty-four hours after transfection, the medium was replaced with serum-free DMEM. After 16 h, the medium overlaying the transfected cells was removed, and the cells were incubated for 30 min with DMEM alone or with DMEM supplemented with 10% FBS, 0.4 M sorbitol, 50 ng of TNF-α per ml, or 100 ng of PMA per ml. COS-1 cells were washed with ice-cold PBS containing 1 mM Na3VO4 and 10 mM NaF and lysed in 200 μl of modified RIPA buffer, as described above. Protein concentrations of soluble lysates were determined by using the Bio-Rad protein assay. Samples of the lysates containing approximately 15 μg of protein were separated on SDS–10% polyacrylamide gels and transferred to nitrocellulose membranes. Membranes were developed with either the M45 antibody, the phosphospecific MAP kinase antibodies, or the standard MAP kinase antibodies.
For cotransfection experiments, COS-1 cells were prepared and transfected as described above, with the exception that 1 μg of each indicated plasmid and 6 μl of the FuGENE 6 transfection reagent were used. Twenty-four hours after transfection, the medium overlaying the transfected COS-1 cells was replaced with serum-free DMEM. Twelve hours later, the transfected cells were washed and lysed in 200 μl of modified RIPA buffer, as described above. Samples of the lysates containing approximately 20 μg of protein were separated on SDS–10% polyacrylamide gels and transferred to nitrocellulose membranes. Membranes were developed with either the M45 antibody, T7 · Tag antibody, the phosphospecific MAP kinase antibodies, or the standard MAP kinase antibodies.
Immunofluorescence microscopy.
Twenty-four hours prior to transfection, 104 COS-1 cells in 1 ml of DMEM-5%FBS were seeded into the wells of a 24-well dish containing glass coverslips. COS-1 cells were transfected with 0.5 μg of pLP20 and 1.5 μl of FuGENE 6 transfection reagent according to the manufacturer’s instructions. Cells were serum starved and stimulated as described above. Thirty minutes after stimulation, the transfected cells were processed for immunofluorescence as described previously (3) by using the M45 antibody (1:100 dilution) as the primary antibody and anti-mouse fluorescein isothiocyanate conjugate (1:500 dilution) as the secondary antibody. Images were captured with a confocal laser microscope as described previously (3).
RESULTS
YopJ is required for downregulation of ERK at early infection times.
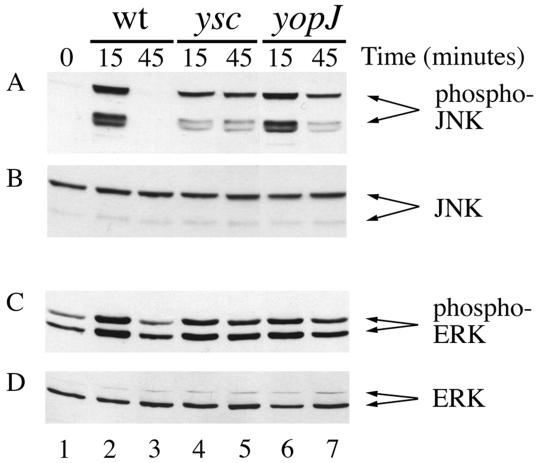
Previous results indicated that YopJ is required for Y. pseudotuberculosis to cause downregulation of JNK and p38 in macrophages (29). In addition, Boland and Cornelis (5) found that YopJ is required for inhibition of ERK activity in macrophages infected with Y. enterocolitica. However, ERK kinase activity was measured 3.5 h postinfection (5), at which time up to 80% of infected macrophages may be killed in a YopJ-dependent fashion (24, 26, 35). The results of a time course infection experiment using macrophages indicated that JNK is completely downregulated by Y. pseudotuberculosis within 45 min of infection (data not shown), which is prior to the first detectable signs of apoptosis (24, 26, 35). We analyzed ERK activation at 15 and 45 min postinfection in order to rule out an indirect effect of macrophage death on ERK kinase activity. J774A.1 macrophages were infected with a wild-type serogroup III strain (YP126), an isogenic yopJ mutant (YP26), or an isogenic ysc mutant (YP71) defective in the type III pathway (Table 1). Within 15 or 45 min of infection, the macrophages were lysed and samples of the lysates were analyzed by immunoblotting with an antibody that specifically recognizes the activated form of the MAP kinase (phosphospecific antibody). Initially, the blots were developed with a phosphospecific JNK antibody as a control. As shown in Fig. 1A, little or no activated JNK was detected in uninfected macrophages (lane 1). The two major isoforms of JNK (p54 JNK and p46 JNK) were activated at 15 min postinfection when macrophages were infected with either the wild-type strain, the ysc mutant, or the yopJ mutant (Fig. 1A, lanes 2, 4, and 6, respectively). At 45 min postinfection, JNK was downregulated in macrophages infected with the wild-type strain (Fig. 1A, lane 3), but it remained activated in macrophages infected with the ysc or yopJ mutants (lanes 5 and 7, respectively). A control immunoblot probed with a standard JNK antibody demonstrated equal loading of JNK in each lane (Fig. 1B). Thus, as shown previously, YopJ is required for Y. pseudotuberculosis to downregulate JNK at 45 min postinfection (29). Identical results have been reported for p38 (29).
FIG. 1.
YopJ is required for Y. pseudotuberculosis to cause downregulation of JNK and ERK in macrophages. Macrophages were left uninfected (lane 1) or were infected with YP126 (wt) (lanes 2 and 3), the secretion mutant YP71 (ysc) (lanes 4 and 5), or YP26 (yopJ) (lanes 6 and 7), as described in Materials and Methods. Macrophages were lysed at the indicated times, and samples of the lysates were analyzed by immunoblotting with a phosphospecific JNK antibody (A), a standard JNK antibody (B), a phosphospecific ERK antibody (C), or a standard ERK antibody (D).
Duplicate samples of the same lysates analyzed above were then analyzed by immunoblotting with a phosphospecific ERK antibody (Fig. 1C) or a standard ERK antibody (Fig. 1D). The two isoforms of ERK (p44 ERK1 and p42 ERK2) were activated at a low level in uninfected macrophages (Fig. 1C, lane 1). ERK activation increased significantly above this baseline level after a 15-min infection with the wild type, the ysc mutant, or the yopJ mutant strains (Fig. 1C, lanes 2, 4, and 6, respectively). ERK activation returned to baseline levels after a 45-min infection with the wild-type strain, but it remained elevated in macrophages infected with the ysc or yopJ mutants (Fig. 1C, lanes 5 and 7, respectively). Relative levels of active p42 ERK2 in uninfected cells or in cells infected with the different strains for 45 min were quantified by laser densitometry. The level of activated ERK2 at 45 min was approximately fourfold higher in macrophages infected with the ysc or yopJ mutants than those infected with the wild type. These results indicate that YopJ is required for Y. pseudotuberculosis to downregulate ERK in macrophages after 45 min of infection. One interesting difference between JNK and ERK with respect to their downregulation by wild-type Yersinia was that the level of active ERK at 45 min postinfection never dropped below the baseline level in uninfected cells, while the level of active JNK at 45 min was consistently lower than the baseline level (Fig. 1; data not shown). This may reflect a difference in the sensitivity of ERK and JNK kinase cascades to inhibition by YopJ (see Discussion).
To determine if other known effectors encoded by pYV were required for downregulation of MAP kinases, we infected macrophages with a series of isogenic mutants defective in multiple yop effectors (yopO, yopH, yopM, or yopE) (11, 40) or the translocation regulator yopK (19) (Table 1). Downregulation of MAP kinases was examined at 15 and 45 min postinfection by immunoblotting with the phosphospecific JNK or standard JNK antibody. In agreement with Boland and Cornelis (5), we found that yopO, yopH, yopM, or yopE and yopK were dispensable for downregulation of MAP kinases in macrophages (data not shown). These results pointed to the possibility that YopJ is sufficient for this inhibitory activity.
YopJ is delivered into the cytoplasmic fraction of macrophages in a YopB-dependent process.
A fusion protein consisting of the first 99 residues of YopJ linked to a cyclase reporter protein can be translocated into the cytosol of macrophages in a YopB-dependent manner (36). To extend these results to the native YopJ protein, we attempted to detect intracellular delivery of YopJ into infected host cells by using immunofluorescence microscopy. For this experiment, we fused the M45 epitope onto the 3′ end of the YopJ open reading frame (see Materials and Methods). The yopJM45 gene was inserted into a multicopy expression vector under the control of the ptac promoter, and the resulting plasmid was introduced into a Y. pseudotuberculosis yopEHJ mutant (IP27) (Table 1). IP27 is derived from the serogroup III strain IP2666. We have found that this strain is able to translocate higher levels of Yop proteins into host cells than the YPIII strain (data not shown). As a positive control, we also fused the M45 epitope onto the carboxy-terminal end of YopH and expressed this protein in the same genetic background. Macrophages or HeLa cells were infected for 2 h with these strains, stained with the M45 antibody, and examined by immunofluorescence microscopy. Although YopHM45 could be readily detected in the cytoplasmic compartments of the infected cells, YopJM45 could not be detected inside host cells in the presence or absence of IPTG induction (data not shown). The YopJM45 protein had wild-type activity, as determined by its ability to complement a yopJ mutant in a TNF-α suppression assay (data not shown). From these results, we concluded that YopJ is translocated at levels that are too low to allow detection by standard immunofluorescence microscopic techniques.
As a second approach, we used a detergent solubility assay to test for the translocation of YopJ. This assay relies on the fact that Yersinia bacteria are resistant to lysis with 1% Triton X-100. Therefore, the appearance of a Yop protein in the soluble fraction of infected macrophages is indicative of its presentation either at the bacterial surface or of its delivery into the cytoplasmic compartment of the host cell. To discriminate between surface and cytoplasmic localization, parallel infections were carried out with a strain defective in the translocation factor YopB. We initially analyzed the translocation of YopHM45 by this assay. Macrophages infected with either the yopEHJ mutant or the yopEHJB mutant were lysed in 1% Triton X-100 and separated into soluble or insoluble fractions by centrifugation. Samples of the soluble and insoluble fractions were analyzed by immunoblotting with the M45 antibody. YopHM45 was detected in both the soluble and insoluble cell fractions when macrophages were infected with the yopEHJ mutant (Fig. 2, lane 5). In contrast, YopHM45 was not detected in the soluble fraction of macrophages infected with the yopEHJB mutant (Fig. 2, lane 6). Similar results were obtained when the translocation of YopJM45 was monitored by this assay, although a small amount of YopJM45 was detected in the soluble fraction of cells infected with the yopEHJB mutant (Fig. 2, lanes 2 and 4). When mock infections were carried out in the absence of macrophages and extracted as before, YopJM45 was not detected in the detergent-soluble fractions (data not shown). This result indicated that the YopJM45 detected in the soluble fractions of infected macrophages corresponded either to translocated protein or to protein exposed on the bacterial surface in response to host cell contact. The small amount of YopJM45 detected in the soluble fraction of cells infected with the yopEHJB mutant may therefore have resulted from the accumulation of YopJM45 at the bacterial surface in the absence of a functional translocation apparatus, or alternatively, it is possible that a small fraction of YopJ is translocated in a YopB-independent manner. We concluded that the efficient translocation of YopJ into macrophages is dependent upon YopB.
FIG. 2.
Analysis of YopJ translocation by Triton X-100 solubility. Macrophages were infected with IP27 (yopE/H/J) or IP29 (yopE/H/J/B) containing pMMB67HE (vector), pYOPHM45 (pyopHM45), or pLP17 (pyopJM45) in trans. The results shown in lanes 1 to 4 were for infections carried out in the presence of 100 μM IPTG to induce expression of yopJM45. Two hours postinfection, macrophages were lysed in 1% Triton X-100. Lysates were separated into soluble and insoluble fractions by centrifugation. Samples of the soluble protein (20 μg or approximately 2.5% of the total) and insoluble protein (10% of the total) were analyzed by immunoblotting with the M45 antibody.
YopJ is sufficient to cause downregulation of JNK, p38, and ERK in eukaryotic cells.
To determine if YopJ is sufficient to cause downregulation of JNK, p38, and ERK in host cells, we inserted yopJM45 into a eukaryotic expression vector under the control of the SFFV promoter and introduced the resulting plasmid (pLP20) into cultured mammalian cells by a highly efficient transfection procedure (see Materials and Methods). Since macrophage cell lines are difficult to transfect, we initially introduced pLP20 into the highly transfectable kidney cell line COS-1. Thirty hours posttransfection the cells were examined by immunofluorescence microscopy after staining with the M45 antibody. Approximately 90% of the cells transfected with pLP20 were stained brightly with the M45 antibody (see Fig. 6), while no specific staining was observed in cells transfected with the empty vector (data not shown). To determine if expression of YopJM45 in COS-1 cells induced apoptosis, transfected cells were simultaneously stained with the M45 antibody and DAPI (4′,6-diamidino-2-phenylindole) to label the nuclei. We observed no fragmentation of nuclei (an indicator of apoptosis) in cells producing YopJM45 (data not shown). To determine if YopJ is sufficient to cause downregulation of MAP kinases in COS-1 cells, we treated transfected cells with serum, sorbitol, TNF-α, or PMA. These stimuli have been shown to activate JNK, p38, and ERK in various cell types (20, 25, 30, 45). Lipopolysaccharide, which is a potent activator of MAP kinases in macrophages (41), was not used as a stimulus in this application, since lipopolysaccharide treatment of COS-1 cells does not cause significant activation of MAP kinases (15a). Serum-starved COS-1 cells transfected with the empty vector or pLP20 were stimulated with these agonists for 30 min and then lysed. Lysates were first analyzed by immunoblotting with the M45 antibody to verify the equal expression of YopJM45 under all conditions (Fig. 3A). Lysates were then analyzed by immunoblotting with the phosphospecific or standard JNK antibody (Fig. 3B and C, respectively). Unstimulated COS-1 cells transfected with vector alone contained low levels of active JNK (Fig. 3B, lane 1). The baseline level of JNK activation was reduced approximately 10-fold in cells transfected with pLP20 (Fig. 3B, lane 2). JNK activation increased significantly upon the exposure of vector-transfected cells to serum, sorbitol, TNF-α, or PMA (Fig. 3B, lanes 3, 5, 7, and 9). JNK activation in response to serum, sorbitol, TNF-α, or PMA was approximately 10-fold lower in cells transfected with pLP20 (Fig. 3B, lanes 4, 6, 8, and 10). Relative levels of active p54 JNK in cells treated with serum or TNF-α were quantitated by densitometry and are shown in Fig. 4A.
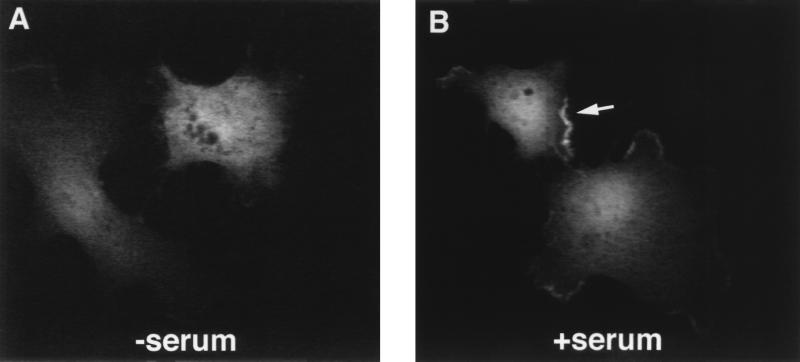
FIG. 6.
YopJ localizes to the periphery of serum-stimulated COS-1 cells. COS-1 cells plated on glass coverslips were transfected with pLP20 and then serum starved for 16 h. Cells were exposed to media lacking serum (A) or to media containing 10% serum (B). After 30 min, cells were fixed, made permeable, stained with the M45 antibody, and examined by confocal microscopy, as described in Materials and Methods.
FIG. 3.
YopJ is sufficient to cause downregulation of MAP kinases in eukaryotic cells. COS-1 cells were transfected with the empty vector pSFFV (V) or with pLP20 (J), as described in Materials and Methods. Twenty-four hours posttransfection, the cells were deprived of serum. After 16 h of serum starvation, the cells were exposed to fresh medium alone (−) or medium supplemented with 10% serum, 0.4 M sorbitol, 50ng of TNF-α per ml, or 100ng of PMA per ml for 30 min. Cells were lysed, and samples of the lysates were analyzed by immunoblotting with an antibody specific for the M45 epitope (A), a phosphospecific JNK antibody (B), a standard JNK antibody (C), a phosphospecific p38 antibody (D), a standard p38 antibody (E), a phosphospecific ERK antibody (F), or a standard ERK antibody (G). The identity of the protein band migrating below the position of p46 JNK in panel B is unknown, although it may represent an additional JNK-related kinase expressed in COS-1 cells.
FIG. 4.
Relative levels of activated JNK and ERK in COS-1 cells stimulated with serum or TNF-α. (A) Relative levels of activated p54 JNK. (B) Relative levels of activated p42 ERK2. Levels of activated MAP kinases, as shown in Fig. 3, were normalized to total MAP kinase levels by laser densitometry. Fold activation was calculated relative to the value for unstimulated vector-transfected cells. V, pSFFV; J, pLP20.
Similar results were obtained when samples of the same lysates were analyzed by immunoblotting with the phosphospecific p38 antibody (Fig. 3D). A less dramatic effect of YopJM45 expression on MAP kinase activation was observed when the lysates were analyzed with the phosphospecific ERK antibody (Fig. 3F). Relative levels of activated ERK in unstimulated cells and cells treated with serum or TNF-α are plotted in Fig. 4B. There was a fivefold decrease in the level of active ERK in cells transfected with pLP20 compared to cells transfected with the empty vector (Fig. 3F and 4B). These results indicated that YopJ is sufficient to cause downregulation of the JNK, p38, and ERK kinases in host cells exposed to several different activators of MAP kinase pathways.
YopJ acts downstream of Rac activation to downregulate JNK.
The small GTPase Rac1 acts as an upstream activator of the JNK kinase cascade (20, 25, 30, 45). To determine if YopJ acts at a level above or below that of Rac to downregulate JNK activity, we introduced a constitutively activated mutant of Rac1 (RacV12) into COS-1 cells by transfection. Production of RacV12 was confirmed by immunoblotting with an antibody specific for the T7 epitope fused to RacV12 (Fig. 5B, lanes 3 and 4). Figure 5C shows that production of RacV12 in serum-starved COS-1 cells caused a significant increase in JNK activation (compare lanes 1 and 3). In contrast, activated JNK was not detected when YopJM45 was produced together with RacV12 in COS-1 cells (Fig. 5C, lane 4). These results indicate that YopJ acts at a step below Rac activation to cause downregulation of the JNK kinase cascade.
FIG. 5.
YopJ acts below the level of Rac activation to downregulate JNK. COS-1 cells were cotransfected with the indicated plasmids and serum starved, as described in Materials and Methods. Cells were lysed, and samples of the lysates were analyzed by immunoblotting with an antibody specific for the M45 epitope fused to YopJ (A), the T7 epitope fused to RacV12 (B), a phosphospecific JNK antibody (C), or a standard JNK antibody (D).
YopJ concentrates at the periphery of host cells in response to stimuli that induce membrane ruffling.
To examine the localization of YopJ within host cells, COS-1 cells were transfected with pLP20 and serum starved as before. The transfected cells were processed for immunofluorescence microscopy by using the M45 antibody and anti-mouse fluorescein isothiocyanate antibody as the secondary antibody. The cells were then examined by confocal microscopy. Figure 6A shows a single representative image of a section through two serum-starved transfected cells. Under these conditions, YopJM45 localized throughout cytoplasmic compartments of the transfected cells. When the transfected cells were stimulated with serum for 30 min and then analyzed as before, strong staining was detected at the cell periphery (Fig. 6B). No peripheral staining was seen in vector-transfected cells following exposure to serum (data not shown). A similar peripheral staining of YopJM45 at the cell boundary was observed when cells were treated with PMA for 30 min, while no such staining was seen in cells stimulated with sorbitol or TNF-α (data not shown). As serum and PMA are known to stimulate membrane ruffling as well as MAP kinase activation (for a review see reference 16), the peripheral staining observed in the presence of these agonists may reflect the recruitment of YopJ to membrane ruffles.
DISCUSSION
The goal of this study was to determine if Yersinia YopJ is sufficient to cause downregulation of MAP kinases in host cells. Initially, we asked if other known Yops encoded by pYV are required for Y. pseudotuberculosis to downregulate MAP kinases in macrophages. We analyzed strains defective in multiple yop genes and found that the effectors YopH, YopE, YopM, and YopO and the translocation regulator YopK were dispensable for this activity, while YopJ was required. These results are consistent with those of Boland and Cornelis (5), who showed that YopH, YopE, YopM, YopO, and YopK were dispensable for Y. enterocolitica to inhibit activation of p38 and ERK in macrophages. An important difference between our study and that of Boland and Cornelis is that we analyzed MAP kinase downregulation at 45 min postinfection, prior to the first detectable signs of apoptosis, while they measured MAP kinase activation at 3.5 h, at which time a significant fraction of macrophages are dead. Thus, our data rule out the possibility that YopJ-mediated inhibition of ERK kinase in macrophages by Yersinia is an indirect effect of cell death.
We next addressed the issue of whether full-length YopJ is translocated into host cells via the type III pathway. Sarker et al. (36) have shown that the first 99 residues of YopJ can direct the translocation of a cyclase fusion protein into macrophages in a YopB-dependent manner, although at levels approximately fourfold lower than a YopE-cyclase fusion protein. We were unable to detect the intracellular delivery of native YopJ into infected host cells by standard immunofluorescence microscopy techniques, apparently because of the low level at which this protein is translocated. Using a detergent solubility assay that is sensitive and discriminates between bacteria-associated and translocated Yops, we obtained results consistent with the idea that full-length YopJ is translocated into macrophages in a YopB-dependent process. These results are consistent with the demonstration that YopB is required for Yersinia to inhibit TNF-α production (5, 29).
As all available evidence indicated that YopJ is translocated into host cells via the plasmid-encoded type III pathway, we next addressed the question of whether YopJ is sufficient to cause downregulation of MAP kinases. For this purpose, the yopJ gene was inserted into a eukaryotic expression vector and transfected into COS-1 cells. Using a highly efficient transfection protocol we were able to obtain transfection efficiencies greater than 90%. Expression of YopJ in COS-1 cells did not appear to induce apoptosis based on DAPI staining of nuclei. This was expected since epithelial cell lines do not undergo apoptosis in response to infection with Yersinia unless they are simultaneously exposed to TNF-α (33). We used immunoblotting to monitor the levels of active JNK, p38, and ERK in transfected COS-1 cells in the presence or absence of several different activators of MAP kinase pathways. Expression of YopJ in COS-1 cells caused a 10-fold reduction in the baseline level of active JNK or p38 in these cells, as well as a 10-fold reduction in JNK or p38 activation following stimulation. Similar results were obtained in the case of ERK, although the magnitude of YopJ-mediated kinase downregulation appeared to be lower, approximately fivefold. Upon stimulation of transfected COS-1 cells with serum or PMA, which are known to induce membrane ruffling as well as activate MAP kinases, we observed a redistribution of YopJ from the cytoplasmic compartment to the cell periphery. This may indicate that YopJ interacts with a component of MAP kinase signaling cascades that is recruited to membrane ruffles. Expression of YopJ did not appear to interfere with the formation of membrane ruffles in COS-1 cells, based on preliminary studies in which serum-stimulated cells transfected with pLP20 were simultaneously stained with the M45 antibody and phalloidin to label F-actin-rich membrane ruffles (data not shown). These results show that YopJ is sufficient to cause downregulation of ERK, p38, and JNK kinases in eukaryotic cells. Furthermore, our data suggest that YopJ selectively blocks the nuclear responses and not the cytoskeletal rearrangements that can accompany activation of MAP kinase cascades.
One major issue that remains to be addressed is the mechanism by which YopJ causes downregulation of MAP kinases. It is generally thought that the ERK kinase signaling cascade is distinct and separate from the signaling pathway leading to JNK or p38 (20, 25, 30, 45). Stimulation of ERK proceeds via the sequential activation of Ras, Raf-1, and MEK1. Stimulation of JNK and p38 proceeds by the sequential activation of Rac and/or Cdc42hs, PAK, a MAP kinase kinase kinase (MEKK), and a MAP kinase kinase (MKK). Thus, there appears to be no common upstream signaling intermediate that is required for activation of ERK, p38, and JNK kinases. As a consequence, YopJ may need to interact with multiple, possibly functionally related targets to cause downregulation of all three kinases. As an initial step toward defining the level at which YopJ acts to downregulate MAP kinase cascades, we introduced the constitutively activated RacV12 protein into COS-1 cells. We found that YopJ was able to downregulate JNK activation mediated by RacV12. This result argues that YopJ acts below the level of GTPase activation to block MAP kinase signaling pathways. Ruckdeschel et al. (34) have reported that wild-type Y. enterocolitica impairs activation of Raf-1. If this repressive effect is in fact due to YopJ, it would suggest that downregulation occurs at or above the level of a MAP kinase kinase kinase. Recently, Frost et al. (13) presented evidence for a “cross-cascade” interaction between the ERK and JNK signaling pathways. This appears to involve phosphorylation of MEK1 by PAK on serine 298, a site important for binding of Raf-1 to MEK1 (13). This cross-cascade interaction is not required for activation of ERK, but it can increase the duration and amplitude of the response (13). It is possible that YopJ acts at a step in the JNK and p38 signaling pathways that is at or above the point at which this cross-cascade regulation occurs. According to this model, YopJ would be expected to reduce, but not completely block, activation of the ERK kinase. We have observed that ERK activation is less sensitive to YopJ downregulation than JNK and p38, a finding which is consistent with this model.
Several groups have reported that Yersinia induces apoptosis in macrophages (24, 26, 35) and that this cell death requires YopJ (24, 26). In addition, Ruckdeschel et al. (33) have shown that Y. enterocolitica impairs activation of the transcription factor NF-κB in macrophages. NF-κB plays an essential role in preventing apoptosis in response to treatment of eukaryotic cells with several different stimuli, including TNF-α (1, 43, 44). Thus, Yersinia may induce apoptosis in macrophages by antagonizing NF-κB rather than by activating an apoptotic program. While this paper was in preparation, Schesser et al. reported that YopJ is required for Y. pseudotuberculosis to inhibit activation of NF-κB in HeLa cells (37). In addition, they identified a region in YopJ that shows similarity to a segment of eukaryotic Src homology 2 domains (37). These results suggest that YopJ directly downregulates the signaling pathway leading to activation of NF-κB. How this occurs is at present unclear, although recent studies have suggested that MEKK is involved in the pathway leading to NF-κB activation (18, 21, 23). Therefore, it is possible that all of the biological activities attributed to YopJ are a direct consequence of its ability to inhibit upstream components of MAP kinase cascades.
ACKNOWLEDGMENTS
We thank J. Galán, D. Black, and L. Montagna for comments on the manuscript and D. Bar-Sagi and P. Hearing for providing reagents.
This research was supported by a grant from the National Institutes of Health (AI35175). L.E.P. was supported by a National Institutes of Health training grant (2T32CAO9176). A.R.P. was supported by the Conselho Nacional Para O Desenvolvimento Cientifico E Technológico, Brazil. J.B.B. is a PEW scholar in the biomedical sciences.
REFERENCES
- 1.Beg A A, Baltimore D. An essential role for NF-κB in preventing TNFα-induced cell death. Science. 1996;274:782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- 2.Beuscher H U, Rödel F, Forsberg A, Röllinghoff M. Bacterial evasion of host immune defense: Yersinia enterocolitica encodes a suppressor for tumor necrosis factor alpha expression. Infect Immun. 1995;63:1270–1277. doi: 10.1128/iai.63.4.1270-1277.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Black D S, Bliska J B. Identification of p130Cas as a substrate of Yersinia YopH (Yop51), a bacterial protein tyrosine phosphatase that translocates into mammalian cells and targets focal adhesions. EMBO J. 1997;16:2730–2744. doi: 10.1093/emboj/16.10.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bliska J B, Guan K, Dixon J E, Falkow S. Tyrosine phosphate hydrolysis of host proteins by an essential Yersinia virulence determinant. Proc Natl Acad Sci USA. 1991;88:1187–1191. doi: 10.1073/pnas.88.4.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boland A, Cornelis G R. Role of YopP in suppression of tumor necrosis factor alpha release by macrophages during Yersinia infection. Infect Immun. 1998;66:1878–1884. doi: 10.1128/iai.66.5.1878-1884.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bölin I, Norlander L, Wolf-Watz H. Temperature-inducible outer membrane protein of Yersinia pseudotuberculosis and Yersinia enterocolitica is associated with the virulence plasmid. Infect Immun. 1982;37:506–512. doi: 10.1128/iai.37.2.506-512.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bölin I, Wolf-Watz H. Molecular cloning of the temperature-inducible outer membrane protein 1 of Yersinia pseudotuberculosis. Infect Immun. 1984;43:72–78. doi: 10.1128/iai.43.1.72-78.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bottone E J. Yersinia enterocolitica: the charisma continues. Clin Microbiol Rev. 1997;10:257–276. doi: 10.1128/cmr.10.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brubaker R R. Factors promoting acute and chronic diseases caused by yersiniae. Clin Microbiol Rev. 1991;4:309–324. doi: 10.1128/cmr.4.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cornelis G, Laroche Y, Balligand G, Sory M P, Wauters G. Yersinia enterocolitica, a primary model for bacterial invasiveness. Rev Infect Dis. 1987;9:64–87. doi: 10.1093/clinids/9.1.64. [DOI] [PubMed] [Google Scholar]
- 11.Cornelis G R, Wolf-Watz H. The Yersinia Yop virulon: a bacterial system for subverting eukaryotic cells. Mol Microbiol. 1997;23:861–867. doi: 10.1046/j.1365-2958.1997.2731623.x. [DOI] [PubMed] [Google Scholar]
- 12.Cox D, Chang P, Zhang Q, Reddy P G, Bokoch G M, Greenberg S. Requirements for both Rac1 and Cdc42 in membrane ruffling and phagocytosis in leukocytes. J Exp Med. 1997;186:1487–1494. doi: 10.1084/jem.186.9.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frost J A, Steen H, Shapiro P, Lewis T, Ahn N, Shaw P E, Cobb M H. Cross-cascade activation of ERKs and ternary complex factors by Rho family members. EMBO J. 1997;16:6426–6438. doi: 10.1093/emboj/16.21.6426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furste J P, Pansegrau W, Frank R, Blocker H, Scholz P, Bagdasarian M, Lanka E. Molecular cloning of the plasmid RP4 primase region in a multi-host range tacP expression vector. Gene. 1986;48:119–131. doi: 10.1016/0378-1119(86)90358-6. [DOI] [PubMed] [Google Scholar]
- 15.Galán J E, Bliska J B. Cross-talk between bacterial pathogens and their host cells. Annu Rev Cell Dev Biol. 1996;12:221–255. doi: 10.1146/annurev.cellbio.12.1.221. [DOI] [PubMed] [Google Scholar]
- 15a.Greenberg, S. Unpublished data.
- 16.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 17.Hardt W-D, Galán J E. A secreted Salmonella protein with homology to an avirulence determinant of plant pathogenic bacteria. Proc Natl Acad Sci USA. 1997;94:9887–9892. doi: 10.1073/pnas.94.18.9887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirano M, Osada S, Aoki T, Hirai S, Hosaka M, Inoue J, Ohno S. MEK kinase is involved in tumor necrosis factor α-induced NF-κB activation and degradation of IκB-α. J Biol Chem. 1996;271:13234–13238. doi: 10.1074/jbc.271.22.13234. [DOI] [PubMed] [Google Scholar]
- 19.Holmstrom A, Pettersson J, Rosqvist R, Hakansson S, Tafazoli F, Fallman M, Magnusson K-E, Wolf-Watz H, Forsberg A. YopK of Yersinia pseudotuberculosis controls translocation of Yop effectors across the eukaryotic cell membrane. Mol Microbiol. 1997;24:73–91. doi: 10.1046/j.1365-2958.1997.3211681.x. [DOI] [PubMed] [Google Scholar]
- 20.Kyriakis J M, Avruch J. Protein kinase cascades activated by stress and inflammatory cytokines. Bioessays. 1996;18:567–577. doi: 10.1002/bies.950180708. [DOI] [PubMed] [Google Scholar]
- 21.Lee F S, Hagler J, Chen Z J, Maniatis T. Activation of the IκBα kinase complex by MEKK1, a kinase of the JNK pathway. Cell. 1997;88:213–222. doi: 10.1016/s0092-8674(00)81842-5. [DOI] [PubMed] [Google Scholar]
- 22.Lee J C, Young P R. Role of CSB/p38/RK stress response kinase in LPS and cytokine signaling mechanisms. J Leukoc Biol. 1996;59:152–157. doi: 10.1002/jlb.59.2.152. [DOI] [PubMed] [Google Scholar]
- 23.Meyer C F, Wang X, Chang C, Templeton D, Tan T-H. Interaction between c-Rel and the mitogen-activated protein kinase kinase 1 signaling cascade in mediating κB enhancer activation. J Biol Chem. 1996;271:8971–8976. doi: 10.1074/jbc.271.15.8971. [DOI] [PubMed] [Google Scholar]
- 24.Mills S D, Boland A, Sory M-P, van der Smissen P, Kerbourch C, Finlay B B, Cornelis G R. Yersinia enterocolitica induces apoptosis in macrophages by a process requiring functional type III secretion and translocation mechanisms and involving YopP, presumably acting as an effector protein. Proc Natl Acad Sci USA. 1997;94:12638–12643. doi: 10.1073/pnas.94.23.12638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Minden A, Karin M. Regulation and function of the JNK subgroup of MAP kinases. Biochim Biophys Acta. 1997;1333:F85–F104. doi: 10.1016/s0304-419x(97)00018-8. [DOI] [PubMed] [Google Scholar]
- 26.Monack D M, Mecsas J, Ghori N, Falkow S. Yersinia signals macrophages to undergo apoptosis and YopJ is necessary for this cell death. Proc Natl Acad Sci USA. 1997;94:10385–10390. doi: 10.1073/pnas.94.19.10385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakajima R, Brubaker R R. Association between virulence of Yersinia pestis and suppression of gamma interferon and tumor necrosis factor alpha. Infect Immun. 1993;61:23–31. doi: 10.1128/iai.61.1.23-31.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Obert S, O’Connor R J, Schmid S, Hearing P. The adenovirus E4-6/7 protein transactivates the E2 promoter by inducing dimerization of a heteromeric E2F complex. Mol Cell Biol. 1994;14:1333–1346. doi: 10.1128/mcb.14.2.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Palmer L E, Hobbie S, Galan J E, Bliska J B. YopJ of Yersinia pseudotuberculosis is required for the inhibition of macrophage TNFα production and the downregulation of the MAP kinases p38 and JNK. Mol Microbiol. 1998;27:953–965. doi: 10.1046/j.1365-2958.1998.00740.x. [DOI] [PubMed] [Google Scholar]
- 30.Robinson M J, Cobb M H. Mitogen-activated protein kinase cascades. Curr Opin Cell Biol. 1997;9:180–186. doi: 10.1016/s0955-0674(97)80061-0. [DOI] [PubMed] [Google Scholar]
- 31.Rosqvist R, Bolin I, Wolf-Watz H. Inhibition of phagocytosis in Yersinia pseudotuberculosis: a virulence plasmid-encoded ability involving the Yop2b protein. Infect Immun. 1988;56:2139–2143. doi: 10.1128/iai.56.8.2139-2143.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosqvist R, Forsberg A, Rimpilainen M, Bergman T, Wolf-Watz H. The cytotoxic protein YopE of Yersinia obstructs the primary host defence. Mol Microbiol. 1990;4:657–667. doi: 10.1111/j.1365-2958.1990.tb00635.x. [DOI] [PubMed] [Google Scholar]
- 33.Ruckdeschel K, Harb S, Roggenkamp A, Hornef M, Zumbihl R, Kohler S, Heesemann J, Rouot B. Yersinia enterocolitica impairs activation of transcription factor NF-κB: involvement in the induction of programmed cell death and in the suppression of the macrophage tumor necrosis factor α production. J Exp Med. 1998;187:1069–1079. doi: 10.1084/jem.187.7.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ruckdeschel K, Machold J, Roggenkamp A, Schubert S, Pierre J, Zumbihl R, Liautard J-P, Heesemann J, Rouot B. Yersinia enterocolitica promotes deactivation of macrophage mitogen-activated protein kinases extracellular signal-regulated kinase-1/2, p38, and c-Jun NH2-terminal kinase. J Biol Chem. 1997;272:15920–15927. doi: 10.1074/jbc.272.25.15920. [DOI] [PubMed] [Google Scholar]
- 35.Ruckdeschel K, Roggenkamp A, Lafont V, Mangeat P, Heesemann J, Rouot B. Interaction of Yersinia enterocolitica with macrophages leads to macrophage cell death through apoptosis. Infect Immun. 1997;65:4813–4821. doi: 10.1128/iai.65.11.4813-4821.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sarker M, Sory M-P, Boyd A P, Iriarte M, Cornelis G R. LcrG is required for efficient translocation of Yersinia Yop effector proteins into eukaryotic cells. Infect Immun. 1998;66:2976–2979. doi: 10.1128/iai.66.6.2976-2979.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schesser K, Spiik A-K, Dukuzumuremyi J-M, Neurath M F, Pettersson S, Wolf-Watz H. The yopJ locus is required for Yersinia-mediated inhibition of NF-κB activation and cytokine expression: YopJ contains a eukaryotic SH2-like domain that is required for its repressive activity. Mol Microbiol. 1998;28:1067–1080. doi: 10.1046/j.1365-2958.1998.00851.x. [DOI] [PubMed] [Google Scholar]
- 38.Smith D B, Johnson K S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988;67:31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- 39.Straley S C, Bowmer W S. Virulence genes regulated at the transcriptional level by Ca2+ in Yersinia pestis include structural genes for outer membrane proteins. Infect Immun. 1986;51:445–454. doi: 10.1128/iai.51.2.445-454.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Straley S C, Skrzypek E, Plano G V, Bliska J B. Yops of Yersinia spp. pathogenic for humans. Infect Immun. 1993;61:3105–3110. doi: 10.1128/iai.61.8.3105-3110.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ulevitch R J, Tobias P S. Receptor-dependent mechanisms of cell stimulation by bacterial endotoxin. Annu Rev Immunol. 1995;13:437–457. doi: 10.1146/annurev.iy.13.040195.002253. [DOI] [PubMed] [Google Scholar]
- 42.Van Aelst L, Joneson T, Bar-Sagi D. Identification of a novel Rac1-interacting protein involved in membrane ruffling. EMBO J. 1996;15:3778–3786. [PMC free article] [PubMed] [Google Scholar]
- 43.Van Antwerp D J, Martin S J, Kafri T, Green D R, Verma I M. Suppression of TNFα-induced apoptosis by NF-κB. Science. 1996;274:787–789. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- 44.Wang C-U, Mayo M W, Baldwin A S., Jr TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-κB. Science. 1996;274:784–787. doi: 10.1126/science.274.5288.784. [DOI] [PubMed] [Google Scholar]
- 45.Witmarsh A J, Davis R J. Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. J Mol Med. 1996;74:589–607. doi: 10.1007/s001090050063. [DOI] [PubMed] [Google Scholar]