Take Home Message
Androgen deprivation therapy (ADT) for advanced prostate cancer is accompanied by side effects affecting health-related quality of life (HRQL). Cotreatment with ADT and high-dose estetrol almost completely prevented the occurrence of hot flushes and improved HRQL and well being in the PCombi trial.
Keywords: Advanced prostate cancer, Androgen deprivation therapy, Estetrol, Hot flushes, Health-related quality of life, Estrogen deficiency, PCombi trial
Abstract
Background
Androgen deprivation therapy (ADT) for prostate cancer (PCa) is accompanied by side effects affecting health-related quality of life (HRQL).
Objective
To assess the effects of the fetal estrogen estetrol (E4) on symptoms related to estrogen and androgen deficiency, and on HRQL measured using the validated Functional Assessment of Cancer Therapy-Prostate (FACT-P) questionnaire.
Design, setting, and participants
This was a phase 2, double-blind, randomized, placebo-controlled study in patients with advanced PCa.
Intervention
Patients receiving ADT were randomly assigned at a 2:1 ratio to daily treatment with a high dose of E4 (HDE4; n = 41) or placebo (n = 21) for 24 wk.
Outcome measurements and statistical analysis
The primary outcome was the effect of HDE4 cotreatment on hot flushes (HFs). Secondary outcomes were the Q-Man questionnaire for evaluation of the effect on estrogen and androgen deficiency symptoms, and the FACT-P questionnaire for evaluating HRQL.
Results and limitations
At 24 wk, the number of patients experiencing HFs was significantly lower in the HDE4 group than in the placebo group (14.3% vs 60.0%; p < 0.001). HDE4 treatment was associated with lower incidence of night sweats, arthralgia, and fatigue, but more nipple tenderness and gynecomastia. At 24 wk, the mean HRQL score favored HDE4 over placebo for the FACT-P total score (122.2 ± 12.3 vs 118.7 ± 19.7) and for several other FACT subscales.
Conclusions
Daily HDE4 coadministration almost completely prevented HFs in patients with advanced PCa treated with ADT. HDE4 also had positive effects on HRQL and counteracted other estrogen deficiency symptoms caused by ADT. These data support the dual efficacy concept of ADT and HDE4 to improve HRQL and increase the antitumor effect of ADT.
Patient summary
For patients on androgen deprivation therapy for advanced prostate cancer, cotreatment with a high dose of estetrol almost completely prevents the occurrence of hot flushes and improves quality of life and well-being, but nipple sensitivity and an increase in breast size may occur.
1. Introduction
For patients diagnosed with advanced prostate cancer (PCa), androgen deprivation therapy (ADT) is the standard of care, which can be accomplished via either bilateral orchiectomy (surgical castration) or medical castration. Medical castration is achieved via continuous treatment with a gonadotrophin-releasing hormone (GnRH) analogue, especially luteinizing hormone–releasing hormone (LHRH) agonists or antagonists [1], [2], [3].
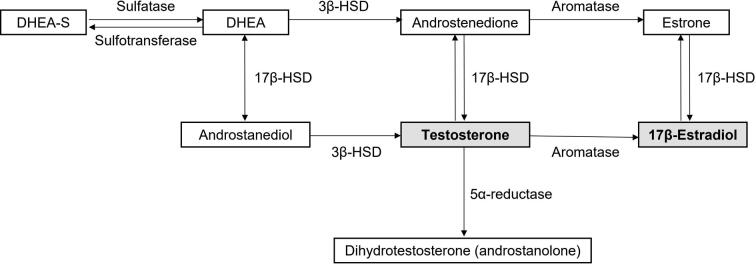
ADT effectively suppresses levels of androgens and thereby reduces disease progression and prolongs survival [4]. Since estrogens in men are derived from aromatization of androgens, lowering of androgens levels also leads to a decrease in estrogen levels (Supplementary Fig. 1) [4]. Estradiol (E2) is an important hormone for men. In fact, in men E2 circulates at concentrations exceeding those in postmenopausal women [5]. By suppressing testosterone, ADT also suppresses E2 almost completely and the resulting hypoestrogenic status causes a wide range of side effects (Table 1). Hot flushes (HFs) and sweating occur frequently, interfering with quality of life [6], and increased bone turnover predisposes to bone loss and fractures. Other symptoms of estrogen deficiency are arthralgia, sarcopenia, fatigue and loss of energy, mood changes and depression, sleep disturbances, cognition problems, and memory loss [3], [7]. Some of these symptoms are also related to suppressed testosterone levels (eg, sarcopenia, fatigue and loss of energy, sleeping problems, and apathy; Table 1), whereas other side effects of LHRH analogues are directly caused by loss of testosterone (eg, loss of libido, erection problems, change in hair pattern, and a decrease in the size of the penis and testicles; Table 1) [8].
Table 1.
Side effects of androgen deprivation therapy related to loss of testosterone (T) and estrogens (E)
| “Big four” | What you see | What is not visible | What the patient feels |
|---|---|---|---|
| Libido loss in 80–85% of patients (T) | Weight gain (E) | Bone loss and increase in fracture risk (E) | Fatigue (T & E) |
| Erection problems (T) | Gynecomastia (E) | Metabolic syndrome (E) | Sleeping problems (T & E) |
| Hot flushes and sweating (E) | Muscle atrophy (sarcopenia) (T & E) | Anemia (E) | Loss of energy (T & E) |
| Arthralgia (joint pain); most frequently occurring symptom of estrogen deficiency (E) | Decrease in size of the penis and testicles (T) | Increase in cardiovascular risk (E) | Apatheia (T & E) |
| Change in hair pattern (T) | Mood changes and depression (E) | ||
| Cognition and memory problems (E) |
Estetrol (E4) is a natural human estrogen that is synthesized exclusively by the fetal liver during pregnancy; its physiological function is unknown. E4 is considered safer than other estrogens as it has limited interaction with liver function [9], [10]. A major pharmacological difference between E4 and other natural estrogens is its high oral bioavailability of 70–80%, in comparison to approximately 5% for E2, for example [11].
Cotreatment with ADT and a high oral dose of E4 (HDE4) at a daily dose of 20–40 mg E4 could be an effective way of minimizing the side effects of ADT-induced hypoestrogenicity. In addition, HDE4 treatment has the potential to increase the antitumor activity of ADT by further suppressing levels of testosterone, prostate-specific antigen (PSA), and follicle-stimulating hormone (FSH) [12]. Currently available estrogens cause cardiovascular (CV) problems and are therefore not recommended for combination treatment with ADT [4]. However, the fetal estrogen E4 has been developed since 2001 as a natural estrogen with less of an effect on liver function and hemostasis and is therefore expected to be less harmful for the CV system [13], [14].
The purpose of the PCombi study was to evaluate HDE4 cotreatment in patients with advanced PCa who started ADT with LHRH agonists. The two primary objectives were to assess the additional suppressive effects of E4 on total and free testosterone (antitumor effects) and the on HFs. In a first publication, we reported that HDE4 treatment was reported well tolerated and no treatment-related CV adverse events (AEs) were observed [12]. Total and free testosterone, PSA, and FSH were suppressed more rapidly and profoundly, suggesting enhanced disease control with HDE4. In addition, bone turnover was substantially reduced in the E4-treated group, implying a bone-sparing effect [12]. Here we report on the effect of cotreatment with HDE4 and ADT on estrogen deficiency symptoms and health-related quality of life (HRQL).
2. Patients and methods
2.1. Study design and patients
This randomized, double-blind, placebo-controlled, phase 2, proof-of-concept study was conducted from March 2018 until May 2020 at four sites in the Netherlands (ClinicalTrials.gov NCT03361969; EudraCT 2017-003708-34). Male patients (aged ≥18 yr; body mass index 18.0–35.0 kg/m2) recently diagnosed with locally advanced or metastatic PCa qualifying for treatment with an LHRH agonist were suitable for enrollment if they had an Eastern Cooperative Oncology Group (ECOG) performance status of 0–1 [15] and life expectancy of at least 2 yr. Patients who had received any medical therapy for PCa in the last year and patients with a history of venous or arterial thromboembolic events or CV disease were excluded from participation unless they were on treatment with anticoagulants for ≥6 mo and were willing to continue anticoagulant treatment during the study. Patients with a known defect in the blood coagulation system could also not participate.
2.2. Procedures
The study comprised a 4-wk screening period, a 24-wk treatment period, and a 4-wk follow-up period. Eligible patients were randomized at baseline at a 2:1 ratio to treatment with E4 (40 mg/d) or matching placebo. The use of bicalutamide to prevent testosterone surges was allowed during the first 14 d of ADT and occurred equally in both groups. Treatment with E4 (or placebo) was initiated on the day the patient received his first injection with a depot LHRH agonist. The study medication was to be taken orally, once daily in the morning. Blinded study medication was packed per subject number according to a computer-generated randomization list that was only known to an independent biostatistician.
The study was approved by an independent ethics committee (Evaluation of Ethics in Biomedical Research, Assen, The Netherlands) and was conducted in accordance with the Declaration of Helsinki and the Good Clinical Practice guidelines of the International Council for Harmonization. All patients provided written informed consent.
2.3. Study objectives
The study had two primary objectives: (1) to assess the effect of E4 on HFs; (2) to assess the effect of E4 on suppression of total and free testosterone (reported previously [12]). The effects of HDE4 on the occurrence of symptoms related to estrogen and androgen deficiency and on HRQL were evaluated as secondary endpoints. Other study variables, reported previously [12], included various laboratory parameters (eg, endocrine parameters, PSA, bone turnover markers, and lipids) and CV and general safety.
2.4. Study assessments
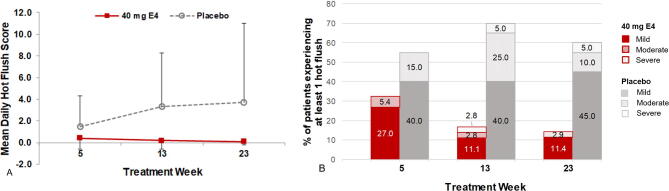
Patients were asked to record their HFs (number and severity: mild = 1; moderate = 2; severe = 3; and very severe = 4) in a daily diary over a period of 7 d during treatment weeks 5, 13, and 23. The number and percentage of patients experiencing at least one HF and the maximum reported severity by week were summarized per treatment group. Effects on HFs were also evaluated in terms of the mean daily HF score (number of HFs multiplied by their mean severity per day measured over 7 d).
To evaluate the effect on estrogen and androgen deficiency symptoms, the unvalidated Q-Man questionnaire was developed in consultation with an experienced urologist (author Y.R.; Supplementary Table 1). This questionnaire captures the presence or absence of symptoms typically related to loss of androgens and/or estrogens (Table 1) and was filled in by the patients at the clinic before treatment initiation (baseline), at treatment weeks 4, 12, and 24, and 4 wk after treatment.
To evaluate the effect on HRQL, patients were asked to complete the Functional Assessment of Cancer Therapy-Prostate (FACT-P) questionnaire [16], [17]. This is a validated questionnaire consisting of a 27-item general function status scale (FACT-G, consisting of 4 subscales: physical, social/family, emotional, and functional well-being) and a 12-item PCa-specific subscale (PCS) that assesses PCS symptoms including questions related to pain, appetite, sexual function, and urinary and bowel functions [16], [17]. The FACT-P questionnaire was completed at study visits during treatment weeks 8 and 24. Higher scores correspond to better HRQL.
2.5. Statistical analysis
No formal sample size calculation was performed for this explorative phase 2 study. The number of patients to be recruited was based on a reduction of at least 50% in the frequency of HFs, which are known to occur in 55–60% of patients treated with ADT [12]. All analyses were descriptive and explorative in nature for this proof-of-concept study. Limited statistical testing was performed. The per-protocol (PP) population, defined as all randomized patients who completed the study without major protocol deviations, was considered the primary population for the efficacy analysis. For all efficacy variables, the 24-wk visit was the primary endpoint. Baseline testing of differences in demographics and baseline disease characteristics was performed using a t test for continuous variables and a χ2 test for categorical variables. As explorative testing for efficacy, the proportion of patients experiencing at least one HF during the 7 d before the last visit was compared between the groups using Fisher’s exact test. With regard to the Q-Man questionnaire, the number and percentage of patients reporting “Yes” or “No” for the various symptoms were summarized using frequencies. Differences and similarities between the treatment groups were explored. All analyses were performed using SAS v9.4.
3. Results
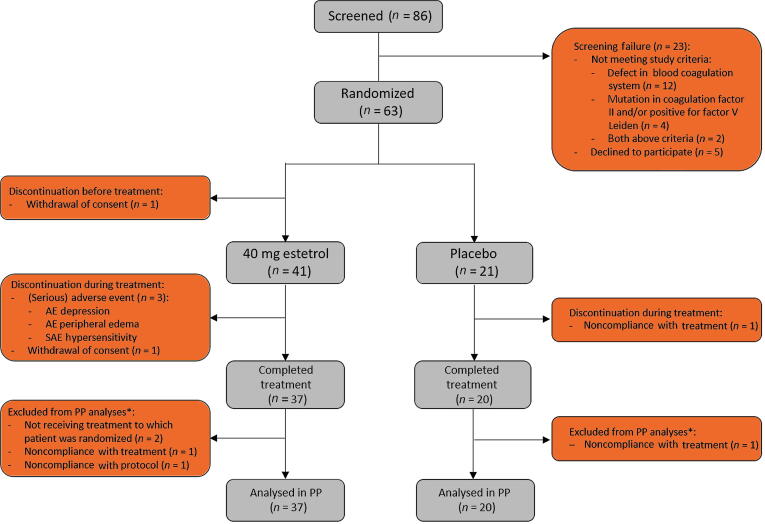
A total of 86 male patients were screened, of whom 63 were randomized to treatment. In total, 41 patients were treated with 40 mg of E4 and 21 with placebo. The PP population consisted of 57 patients; 37 on HDE4 and 20 on placebo (Fig. 1). The baseline characteristics of the study population were comparable between the groups, except for body mass index. Patients in the placebo group tended to have more advanced disease at diagnosis and screening than patients in the E4 group, as indicated by the TNM staging results (M1b–c; no difference not significant) and significantly worse ECOG performance status (Table 2). Compliance with intake of the study medication was high, with median compliance of >99% in both treatment groups. Seven CV side effects occurred during the study, two among the 21 patients on placebo (9.5%) and five among the 41 patients on HDE4 (12.2%), all considered not related to HDE4 treatment by the investigators and the independent safety officer. Table 3 summarizes the endocrine results reported earlier [12], demonstrating the highly significant and favorable endocrine antitumor effects of HDE4, even with this low number of patients, for PSA, free testosterone, and FSH, and for the bone parameters osteocalcin and CTX1. SHBG increases significantly as a parameter of estrogenicity.
Fig. 1.
CONSORT diagram. PP = per protocol. *Based on all patients who started treatment; patients not completing the study treatment could still be included in the PP analyses if all study assessments were performed (eg, end-of-study visit). This was applicable for the patients in the estetrol group, but not for the patients in the placebo group. All patients who received at least one dose of study medication were included in analyses of the safety parameters.
Table 2.
Patient demographics and baseline characteristics in the per-protocol populationa
| Parameter | 40 mg estetrol (n = 37) |
Placebo (n = 20) |
Total (n = 57) |
p valueb |
|---|---|---|---|---|
| Median age, yr (range) | 74 (59–85) | 75 (49–84) | 74 (49–85) | 0.650 |
| Mean weight, kg (standard deviation) | 82.9 (12.2) | 90.0 (14.8) | 85.4 (13.5) | 0.057 |
| Mean body mass index, kg/m2 (standard deviation) | 26.1 (3.4) | 28.2 (3.7) | 26.8 (3.6) | 0.045 |
| Time since prostate cancer diagnosis, n (%) | 0.458 | |||
| 0–3 mo | 21 (56.8) | 12 (60.0) | 33 (57.9) | |
| 3–6 mo | 3 (8.1) | 3 (15.0) | 6 (10.5) | |
| 6 mo–1 yr | 2 (5.4) | 1 (5.0) | 3 (5.3) | |
| >1 yr | 11 (29.7) | 4 (20.0) | 15 (26.3) | |
| Distant metastasis, n (%) | 0.382 | |||
| M0 | 25 (67.6) | 14 (70.0) | 39 (68.4) | |
| M1 | 7 (18.9) | 2 (10.0) | 9 (15.8) | |
| M1a | 2 (5.4) | – | 2 (3.5) | |
| M1b | 3 (8.1) | 3 (15.0) | 6 (10.5) | |
| M1c | – | 1 (5.0) | 1 (1.8) | |
| Gleason score, n (%) | 0.996 | |||
| 6 | 2 (5.4) | 1 (5.0) | 3 (5.3) | |
| 7 | 15 (40.5) | 8 (40.0) | 23 (40.4) | |
| ≥8 | 20 (54.1) | 11 (55.0) | 31 (54.4) | |
| ECOG performance status, n (%) | 0.010 | |||
| 0 | 33 (89.2) | 12 (60.0) | 45 (78.9) | |
| 1 | 4 (10.8) | 8 (40.0) | 12 (21.1) | |
| Previous prostatectomy, n (%) | 9 (24.3) | 3 (15.0) | 12 (21.1) | 0.410 |
| Radiotherapy for primary tumor, n (%) | 7 (18.9) | 2 (10.0) | 9 (15.8) | 0.378 |
| Previous hormone therapy, n (%) | – | 1 (5.0) | 1 (1.8) | 0.170 |
| Bicalutamide during baseline efficacy lab test | 16 (43.2) | 10 (50.0) | 26 (45.6) | 0.625 |
| LHRH agonist used during study, n (%) | ||||
| Leuprolide | 35 (85.4) | 19 (90.5) | (88.0) | |
| Goserelin | 6 (14.6) | 2 (9.5) | (12.0) |
ECOG = Eastern Cooperative Oncology Group; PC, prostate cancer; SD, standard deviation.
All patients were male with prostate cancer qualifying for treatment with a luteinizing hormone–releasing hormone agonist with no history of radiotherapy to bone or of chemotherapy.
The p values are for a χ2 exact test for categorical variables, or for a t test for continuous variables. Statistically significant p values are in bold font.
Table 3.
Percentage change in laboratory parameters in comparison to baseline after 24 wk of treatment for the per-protocol population [12]
| Parameter | Percentage change, % (standard deviation) |
p value | |
|---|---|---|---|
| Estetrol (n = 37) |
Placebo (n = 20) |
||
| Prostate-specific antigen | −96.4 (5.3) | −83.1 (30.2) | 0.0033 |
| Total testosterone | −97.1 (1.6) | −97.4 (1.5) | 0.2819 |
| Free testosterone | −93.2 (4.0) | −89.7 (6.9) | 0.0389 |
| Luteinizing hormone | −97.6 (1.8) | −97.6 (2.8) | 0.6805 |
| Follicle-stimulating hormone | −97.8 (1.7) | −56.7 (44.8) | <0.0001 |
| Estradiol | −81.6 (10.5) | −82.3 (14.9) | 0.7639 |
| SHBG | +185.0 (111.4) | −2.5 (15.8) | <0.0001 |
| Dehydroepiandrosterone sulfate | −26.8 (18.9) | −26.8 (18.8) | 0.5875 |
| Osteocalcin | −22.0 (19.7) | +47.6 (47.2) | <0.0001 |
| CTX1 | −24.8 (34.6) | +151.1 (109.1) | <0.0001 |
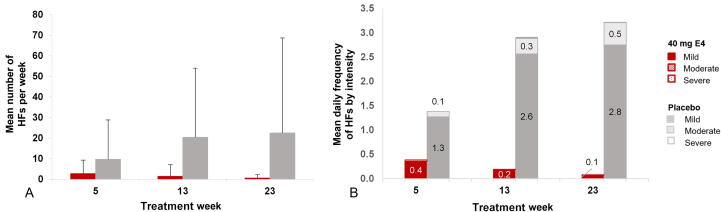
Patients treated with E4 experienced fewer and less intense HFs per week than patients on placebo at all time points during treatment (Supplementary Fig. 2). At the end of treatment, the number of patients experiencing HFs was significantly lower in the E4 group (5/35 patients, 14.3%) than in the placebo group (12/20 patients, 60.0%; Fisher’s exact test, p < 0.001; Supplementary Table 2). The mean number of HFs per week was much lower in the E4 group than in the placebo group at all time points (end of treatment: 0.6 ± 1.61 for E4 vs 22.5 ± 46.22 for placebo; Fig. 2A). In addition, patients treated with E4 suffered from less intense HFs throughout the treatment period, with only a few mild HFs reported at week 23 (mean HF daily frequency ± standard deviation: mild, 0.1 ± 0.21; moderate, 0.0 ± 0.02; severe, 0.0 ± 0.02) in comparison to the placebo group (mild, 2.8 ± 6.25; moderate, 0.5 ± 1.51; severe, 0.0 ± 0.03; Fig. 2B). The mean daily HF score in the week before visit 9 (treatment week 23) was 0.1 ± 0.3 in the E4 group and 3.7 ± 7.3 in the placebo group.
Fig. 2.
(A) Mean number of hot flushes per week and (B) mean daily frequency by intensity during treatment weeks 5, 13, and 23 with 40 mg of E4 or placebo in patients with prostate cancer treated with an LHRH agonist (per-protocol population). Severity was recorded as mild, moderate, severe, or very severe; very severe was not reported by any patient. E4 = estetrol; HF = hot flush; LHRH = luteinizing hormone–releasing hormone.
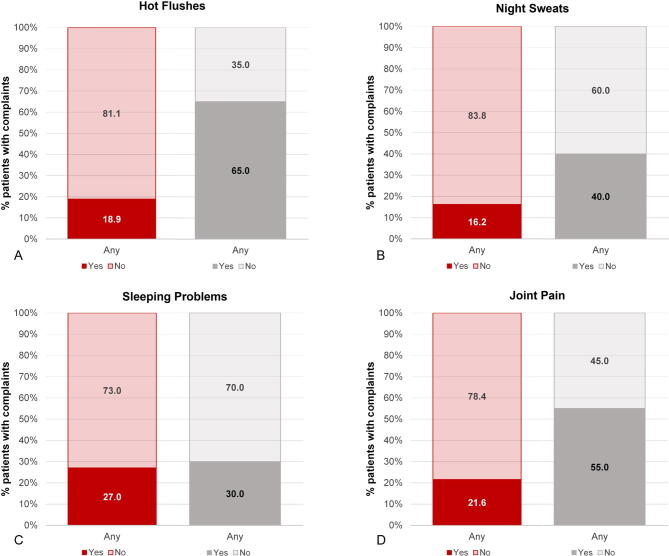
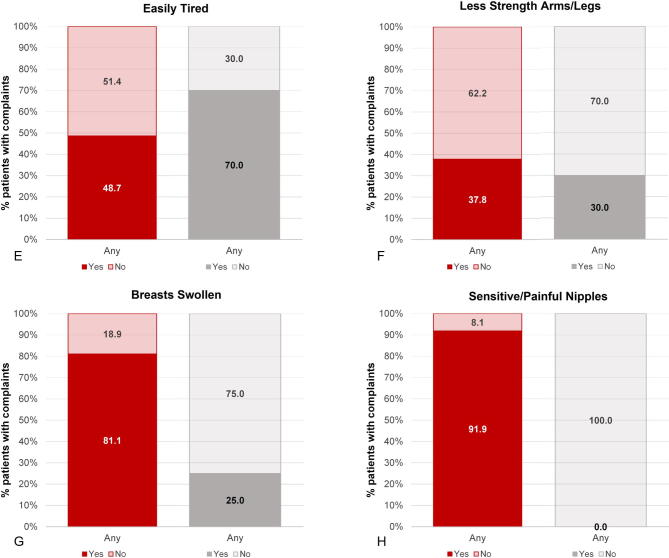
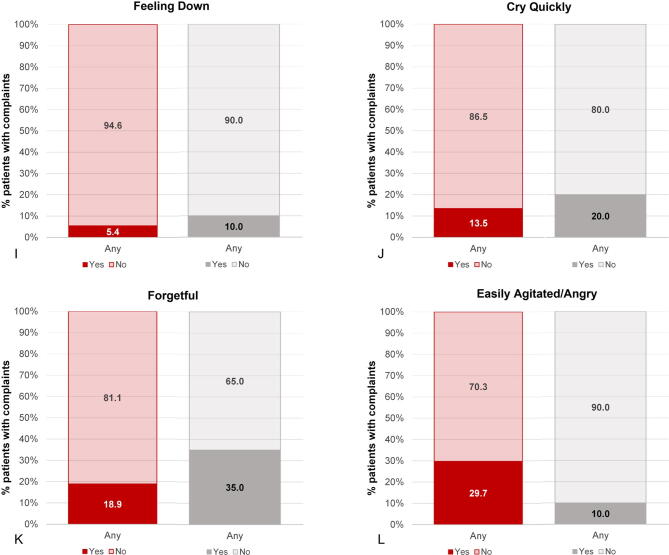
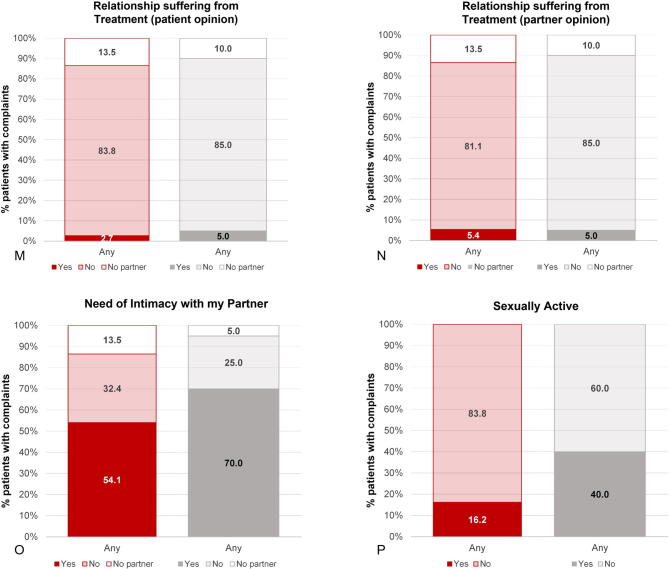
Results from the Q-Man questionnaire confirm the findings from the diary, with a much lower incidence of HFs among patients treated with E4 in comparison to patients treated with placebo (5.9% vs 55.0%). Other notable differences (>15% difference at the end of treatment) in favor of E4 were fewer complaints of night sweats (2.9% vs 30.0%), arthralgia (17.7% vs 40.0%), and fatigue (easily tired; 29.4% vs 50.0%; Table 4). More patients were sexually active in the placebo group (2.9% vs 20.0%). It should be noted that the percentage of patients who were sexually active was already lower in the E4 group at baseline (E4 24% vs placebo 45%). Complaints regarding sensitive nipples (88.2% vs 0%) and swollen breasts (82.4% vs 25.0%) were mainly reported by E4-treated patients (Table 4 and Supplementary Fig. 3). No relevant differences were observed for the other symptoms. At the follow-up visit, 4 wk after the end of treatment, most estrogen deficiency–related complaints had increased in the patients who had received E4 treatment, whereas the patients who had been treated with placebo reported no change. This was also reflected in the occurrence of HFs as an AE (ie, if the patient experienced HFs as bothersome), reported by 17.1% of patients in the E4 group versus 52.4% of patients in the placebo group during the active treatment period.
Table 4.
Patients reporting symptoms related to loss of androgens and/or estrogens as captured via the Q-Man questionnaire completed at baseline, the end of treatment (week 24), and follow-up (4 wk after treatment) by patients with prostate cancer treated with an LHRH agonist combined with 40 mg of estetrol or placebo in the per-protocol populationa
| Question | Patients, n/N (%) |
|||||
|---|---|---|---|---|---|---|
| Baseline |
End of treatmentb |
Follow-up |
||||
| Estetrol | Placebo | Estetrol | Placebo | Estetrol | Placebo | |
| I have hot flushes | 2/37 (5.4) | 0/20 (0.0) | 2/34 (5.9) | 11/20 (55.0) | 11/35 (31.4) | 10/19 (52.6) |
| I suffer from night sweats | 5/37 (13.5) | 2/20 (10.0) | 1/34 (2.9) | 6/20 (30.0) | 6/35 (17.1) | 6/19 (31.6) |
| I have sleeping problems | 7/37 (18.9) | 4/20 (20.0) | 5/34 (14.7) | 5/20 (25.0) | 10/35 (28.6) | 7/19 (36.8) |
| I have pain in my joints | 8/37 (21.6) | 3/20 (15.0) | 6/34 (17.7) | 8/20 (40.0) | 7/35 (20.0) | 6/19 (31.6) |
| I have less strength in arms and legs | NAB | NAB | 7/34 (20.6) | 6/20 (30.0) | 9/35 (25.7) | 7/19 (36.8) |
| I am tired easily | 9/37 (24.3) | 5/20 (25.0) | 10/34 (29.4) | 10/20 (50.0) | 11/35 (31.4) | 9/19 (47.4) |
| I have sensitive or painful nipples | 0/37 (0.0) | 0/20 (0.0) | 30/34 (88.2) | 0/20 (0.0) | 17/35 (48.6) | 3/19 (15.8) |
| My breasts are swollen | 0/37 (0.0) | 0/20 (0.0) | 28/34 (82.4) | 5/20 (25.0) | 27/35 (77.1) | 2/19 (10.5) |
| I am forgetful | 3/37 (8.1) | 2/20 (10.0) | 6/34 (17.7) | 4/20 (20.0) | 5/35 (14.3) | 5/19 (26.3) |
| I am easily agitated or angry | 2/37 (5.4) | 1/20 (5.0) | 3/34 (8.8) | 2/20 (10.0) | 6/35 (17.1) | 3/19 (15.8) |
| I cry quickly | 5/37 (13.5) | 1/20 (5.0) | 3/34 (8.8) | 3/20 (15.0) | 5/35 (14.3) | 4/19 (21.1) |
| I feel down | 2/37 (5.4) | 0/20 (0.0) | 0/34 (0.0) | 1/20 (5.0) | 1/35 (2.9) | 1/19 (5.3) |
| I need intimacy with my partnerc | 21/37 (56.7) | 11/20 (55.0) | 14/34 (41.2) | 11/20 (55.0) | 12/35 (34.3) | 11/19 (57.9) |
| I am sexually active | 9/37 (24.3) | 9/20 (45.0) | 1/34 (2.9) | 4/20 (20.0) | 0/35 (0.0) | 1/19 (5.3) |
| My relationship is suffering from the treatmentc | NAB | NAB | 0/34 (0.0) | 0/20 (0.0) | 0/35 (0.0) | 2/19 (10.5) |
| My partner thinks our relationship is suffering from the treatmentc | NAB | NAB | 1/34 (2.9) | 1/20 (5.0) | 1/35 (2.9) | 2/19 (10.5) |
LHRH = luteinizing hormone–releasing hormone; NAB = not asked at baseline.
The questionnaire at baseline (version A) consisted of 16 symptoms and the questionnaire used at the other time points (version B) consisted of 21 symptoms. The number of patients reporting “Yes” for the various symptoms are summarized using frequencies. Differences and similarities between the treatment groups at the end of treatment were explored. Results for questions on the ability to have an erection, an orgasm, satisfaction with sex life, less desire for sex, and less ejaculate are not included. These questions were only to be completed by patients who were sexually active. Most of the patients were not sexually active, so these questions were only completed by a few patients. Owing to the low number, the results are considered not reliable for proper interpretation.
Results in bold font indicate a difference between estetrol and placebo of >15%.
Patients without a partner did not answer these questions (estetrol, n = 5 and placebo, n = 2 at baseline and n = 3 at other visits).
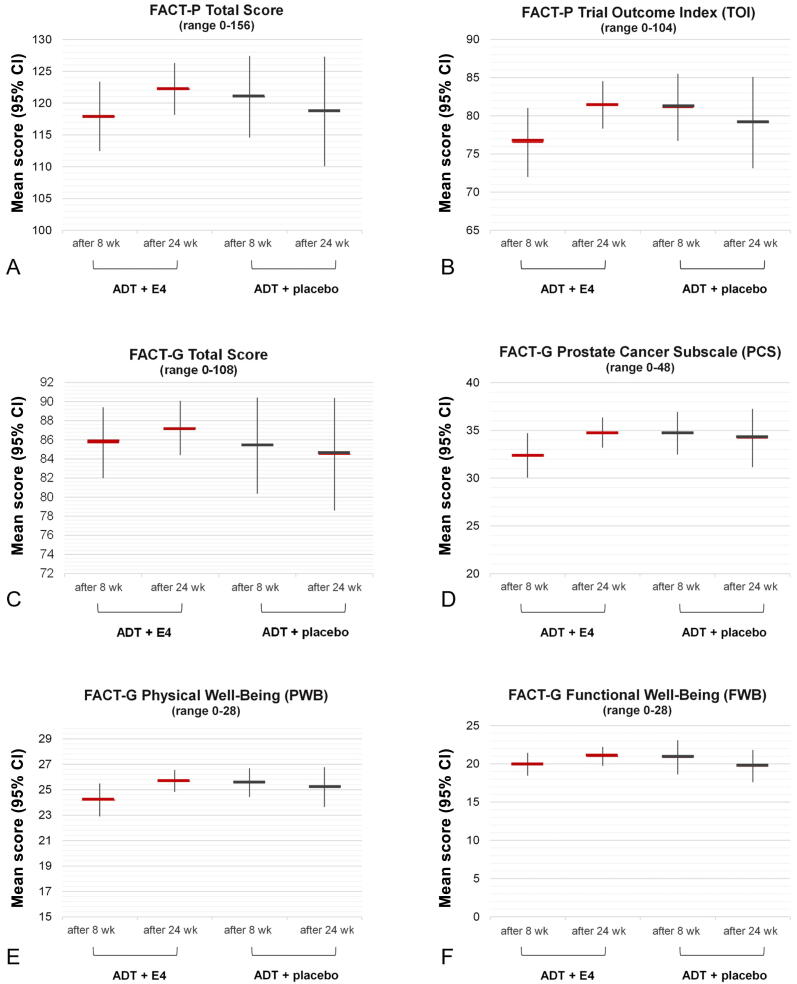
Since no baseline data for the validated FACT-P questionnaire are available, only comparisons between treatment groups are possible. Patients cotreated with ADT and E4 reported improved and better general function and PCa-related HRQL than patients on placebo (Supplementary Table 3). More specifically, in comparison to patients treated with placebo, the E4-treated patients had higher mean scores for several FACT-P (sub)scales after 24 wk of treatment: FACT-P Total, FACT-P Trial Outcome Index, FACT-G Total, FACT-P Prostate Cancer Subscale, FACT-G Physical Well-Being, and FACT-G Functional Well-Being (Fig. 3A–F). Patients in the E4 group generally reported an increase in mean scores, where patients in the placebo group often reported no change or a decrease. For the FACT Social/Family Well-Being and Emotional Well-Being subscales, there were no clear differences in mean scores between the E4 and placebo groups.
Fig. 3.
Mean scores for (A) FACT-P Total (A), (B) FACT-P Trial Outcome Index (TOI), (C) FACT-G Total, (D), FACT-G Prostate Cancer Subscale (PCS), (E) FACT-G Physical Well-Being (PWB), and (F) FACT-G Functional Well-Being (FWB) domains after 8 wk and 24 wk of treatment with 40 mg of estetrol (E4) or placebo in patients with prostate cancer treated with a luteinizing hormone–releasing hormone agonist (per-protocol population). FACT-P Total score = sum of PWB score + SWB score + EWB score + FWB score + PCS score. FACT-P TOI score = sum of PWB score + FWB score + PCS score; FACT-G Total score = sum of PWB score + SWB score + EWB score + FWB score. Higher scores correspond to better quality of life. ADT = androgen deprivation therapy; CI = confidence interval; E4 = estetrol; FACT-G = Functional Assessment of Cancer Therapy-General; FACT-P = Functional Assessment of Cancer Therapy-Prostate.
4. Discussion
Patients recently diagnosed with advanced prostate cancer who were starting ADT were cotreated with the natural fetal estrogen estetrol in the double-blind, placebo-controlled, randomized PCombi study to prevent signs and symptoms of estrogen deficiency. Daily oral coadministration of 40 mg E4 and an LHRH agonist almost completely prevented HFs and had a positive effect on HRQL. Other estrogen deficiency symptoms were also less frequent, but HDE4 was associated with a higher incidence of sensitive nipples and gynecomastia.
HFs are one of the most bothersome side effects of ADT, with incidence ranging from 50% to 80% [6], [7], [18]. HFs occur during treatment with both LHRH agonists and antagonists [19], [20], [21]. For many patients, HFs persist throughout many years of ADT use, interfering with quality of life [20], [22]. In our study, 60% of patients treated with ADT only (placebo group) suffered from HFs. By contrast, in patients cotreated with HDE4, HFs were largely prevented (86% experienced no HFs) and the intensity of any HFs that did occur was mild, as shown by data from both the diary and the Q-Man questionnaire.
Estrogen deficiency is a serious side effect of ADT; besides HFs, it is associated with subjective complaints such as night sweats, arthralgia, fatigue and loss of energy, mood changes and depression, sleep disturbances, cognition problems, and memory loss [3], [7], [8]. In our study, patients cotreated with E4 had a much lower incidence of night sweats, arthralgia, and fatigue. Nipple tenderness and gynecomastia are known estrogenic side effects in men [23], [24], [25], so the reporting of these complaints in the E4 treatment group is an expected finding, although gynecomastia was also reported in the placebo group (albeit to a lesser extent). The incidence of gynecomastia varies with ADT type and duration [26], [27]. A single irradiation session before the start of estrogen treatment may prevent nipple tenderness [28].
Some side effects of ADT are typically related to the intentional decrease in androgen levels. In our study the percentage of patients who were sexually active was low in both groups. The percentage was slightly lower in the E4 group than in the placebo group, which may be explained in part by a difference in sexual activity at baseline. More men in the E4 group complained about less strength in their arms and legs, which may be explained by the stronger suppression of testosterone on addition of E4.
Recent randomized controlled studies investigating GnRH analogues demonstrated diminished HRQL during ADT, which was also associated with the occurrence of HFs [6], [20], [22]. It could therefore be expected that treating HFs will lead to an improvement in HRQL. Indeed, in the present study, E4 had a positive effect on HRQL, with higher scores for several FACT domains reported by E4-treated patients, compared to worsening of HRQL reported by patients treated with ADT only. On the basis of on this improvement in HRQL with E4, one could speculate that patients find HFs more bothersome than nipple tenderness and breast swelling.
There are no therapies specifically approved for the treatment of HFs in patients with prostate cancer, although some agents are being used off-label (eg, cyproterone acetate) [7], [18]. Estrogens that were used in the past have been discontinued because of CV side effects [4]. Therapies currently used for HF treatment are also associated with unwanted safety risks and can often be used for single indications only [6], [7], [18], [29], [30], [31].
4.1. Study limitations
Our study has some limitations. Baseline assessment for the validated FACT-P questionnaire is missing, which hampers interpretation of results of this questionnaire and thus determination of clinically meaningful changes is not possible [17]. In addition, the new Q-Man questionnaire has not been validated yet; however, this questionnaire includes important clinical questions that are generally accepted as being related to loss of androgens and/or estrogens (Table 1) [8], [32]. Furthermore, the dose of E4 used in this study (40 mg) was higher than the E4 dose for women’s health (WH) applications such as oral contraception and menopausal hormone treatment, for which an E4 dose of 15–20 mg is typically used. The higher dose was based on an earlier dose-finding study in healthy males [33]. Therefore, we have used the terminology high-dose estetrol (HDE4) and different doses should be taken into account when comparing HDE4 data in PCa with WH applications. Finally, the current study design with its relatively low number of patients and limited treatment period of 24 wk was sufficient to obtain proof of concept. However, a further 52-wk phase 3 study in a larger population is needed to confirm the safety, HRQL improvements, effects on bone using bone mineral density measurements, and the favorable effects on secondary tumor endpoints (testosterone, PSA, FSH) of HDE4.
5. Conclusions
Daily HDE4 coadministration almost completely prevented HFs in patients with advanced PCa being treated with ADT. HDE4 also had positive effects on HRQL and counteracted other estrogen deficiency symptoms caused by ADT. These data support the dual efficacy concept of ADT cotreatment with HDE4 to improve HRQL and increase the antitumor effect of ADT.
Larger studies with longer treatment duration are needed to confirm the favorable subjective results and safety observed in our study and to evaluate long-term treatment effects on bone mass, CV safety, and antitumor effects, including progression-free survival and overall survival.
The treatment concept described in this study has been presented at various congresses and results have been presented at the 19th annual meeting of the Association of Academic European Urologists Meeting (Marseille, France; December 2021), the 32nd Annual International Prostate Cancer Update (Snowbird, UT, USA, March 2022), and at the 2022 American Association of Genitourinary Surgeons annual meeting (Palm Desert, CA, USA; April 2022).
Author contributions: Herjan J.T. Coelingh Bennink had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Coelingh Bennink, Debruyne, Zimmerman, Reisman.
Acquisition of data: Debruyne, van Moorselaar, Coelingh Bennink, Zimmerman, Krijgh, Roos, Somford, Roeleveld, de Haan, van Melick, Reisman.
Analysis and interpretation of data: All authors.
Drafting of the manuscript: Zimmerman, Coelingh Bennink, Frydenberg, van Poppel.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Zimmerman, Coelingh Bennink.
Obtaining funding: Coelingh Bennink.
Administrative, technical, or material support: Zimmerman, Krijgh, Debruyne.
Supervision: Zimmerman, Coelingh Bennink.
Other: None.
Financial disclosures: Herjan J.T. Coelingh Bennink certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Jeroen A. van Moorselaar has received grants and fees from Astellas, Ipsen, AstraZeneca, Bayer, and Janssen. Diederik M. Somford is an advisor/consultant for Astellas, Janssen, Bayer, and MSD; has received institutional research funding from Astellas, Besins, and the Dutch Cancer Society; and has performed contracted institutional research for Janssen, Eli Lilly, Astellas, Blue Earth Diagnostics, Bayer, SPL Medical, and QED Therapeutics. Frans M.J. Debruyne is a consultant for Pantarhei Oncology BV. Yvette Zimmerman is chief executive officer and a shareholder of Pantarhei Oncology. Jan Krijgh is chief medical officer of Pantarhei Oncology. Herjan J.T. Coelingh Bennink is president and a shareholder of Pantarhei Oncology, an affiliate of Pantarhei Bioscience BV. The remaining authors have nothing to disclose.
Funding/Support and role of the sponsor: Funding for the study was provided by Pantarhei Oncology. The sponsor played a role in the design and conduct of the study, and in the collection, management, and analysis of the data. Employees of the sponsor participated in interpretation of the data; in preparation, review, and approval of the manuscript; and in the decision to submit the manuscript for publication.
Acknowledgments: We thank the study staff of the clinical sites and the patients participating in the study. Carole Verhoeven (formerly at Pantarhei) and Monique Jansen are acknowledged for their participation in the set-up and organization of the study, and Ilse Christ (Ilse Pharma, Breda, The Netherlands), who performed the monitoring. Jan Egberts and Amanda Prowse (at Terminal 4 Communications, Hilversum, The Netherlands) are acknowledged for their contribution to preparation of the manuscript.
Associate Editor: Guillaume Ploussard
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.euros.2022.09.006.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary Figure 1.
Supplementary Figure 2.
Supplementary Figure 3.
Supplementary Figure 4.
Supplementary Figure 5.
Supplementary Figure 6.
References
- 1.Parker C., Castro E., Fizazi K., et al. Prostate cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31:1119–1134. doi: 10.1016/j.annonc.2020.06.011. [DOI] [PubMed] [Google Scholar]
- 2.Ng K., Smith S., Shamash J. Metastatic hormone-sensitive prostate cancer (mHSPC): advances and treatment strategies in the first-line setting. Oncol Ther. 2020;8:209–230. doi: 10.1007/s40487-020-00119-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Desai K., McManus J.M., Sharifi N. Hormonal therapy for prostate cancer. Endocr Rev. 2021;42:354–373. doi: 10.1210/endrev/bnab002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cornford P., van den Bergh R.C.N., Briers E., et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer. Part II—2020 update: treatment of relapsing and metastatic prostate cancer. Eur Urol. 2021;79:263–282. doi: 10.1016/j.eururo.2020.09.046. [DOI] [PubMed] [Google Scholar]
- 5.Hammes S.R., Levin E.R. Impact of estrogens in males and androgens in females. J Clin Invest. 2019;129:1818–1826. doi: 10.1172/JCI125755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frisk J. Managing hot flushes in men after prostate cancer—a systematic review. Maturitas. 2010;65:15–22. doi: 10.1016/j.maturitas.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 7.Guise T.A., Oefelein M.G., Eastham J.A., Cookson M.S., Higano C.S., Smith M.R. Estrogenic side effects of androgen deprivation therapy. Rev Urol. 2007;9:163–180. [PMC free article] [PubMed] [Google Scholar]
- 8.Freedland S.J., Eastham J., Shore N. Androgen deprivation therapy and estrogen deficiency induced adverse effects in the treatment of prostate cancer. Prostate Cancer Prostat Dis. 2009;12:333–338. doi: 10.1038/pcan.2009.35. [DOI] [PubMed] [Google Scholar]
- 9.Mawet M., Maillard C., Klipping C., Zimmerman Y., Foidart J.M., Coelingh Bennink H.J. Unique effects on hepatic function, lipid metabolism, bone and growth endocrine parameters of estetrol in combined oral contraceptives. Eur J Contracept Reprod Health Care. 2015;20:463–475. doi: 10.3109/13625187.2015.1068934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Douxfils J., Klipping C., Duijkers I., et al. Evaluation of the effect of a new oral contraceptive containing estetrol and drospirenone on hemostasis parameters. Contraception. 2020;102:396–402. doi: 10.1016/j.contraception.2020.08.015. [DOI] [PubMed] [Google Scholar]
- 11.Coelingh Bennink H.J., Heegaard A.M., Visser M., Holinka C.F., Christiansen C. Oral bioavailability and bone-sparing effects of estetrol in an osteoporosis model. Climacteric. 2008;11(Suppl 1):2–14. doi: 10.1080/13697130701798692. [DOI] [PubMed] [Google Scholar]
- 12.Coelingh Bennink H.J.T., Van Moorselaar J.A., Crawford E.D., et al. Estetrol cotreatment of androgen deprivation therapy in infiltrating or metastatic, castration-sensitive prostate cancer: a randomized, double-blind, phase II trial (PCombi) Eur Urol Open Sci. 2021;28:52–61. doi: 10.1016/j.euros.2021.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerard C., Arnal J.F., Jost M., et al. Profile of estetrol, a promising native estrogen for oral contraception and the relief of climacteric symptoms of menopause. Expert Rev Clin Pharmacol. 2022;15:121–137. doi: 10.1080/17512433.2022.2054413. [DOI] [PubMed] [Google Scholar]
- 14.Coelingh Bennink H.J., Holinka C.F., Diczfalusy E. Estetrol review: profile and potential clinical applications. Climacteric. 2008;11(Suppl 1):47–58. doi: 10.1080/13697130802073425. [DOI] [PubMed] [Google Scholar]
- 15.Azam F., Latif M.F., Farooq A., et al. Performance status assessment by using ECOG (Eastern Cooperative Oncology Group) score for cancer patients by oncology healthcare professionals. Case Rep Oncol. 2019;12:728–736. doi: 10.1159/000503095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Esper P., Mo F., Chodak G., Sinner M., Cella D., Pienta K.J. Measuring quality of life in men with prostate cancer using the Functional Assessment of Cancer Therapy-Prostate instrument. Urology. 1997;50:920–928. doi: 10.1016/S0090-4295(97)00459-7. [DOI] [PubMed] [Google Scholar]
- 17.Cella D., Nichol M.B., Eton D., Nelson J.B., Mulani P. Estimating clinically meaningful changes for the Functional Assessment of Cancer Therapy-Prostate: results from a clinical trial of patients with metastatic hormone-refractory prostate cancer. Value Health. 2009;12:124–129. doi: 10.1111/j.1524-4733.2008.00409.x. [DOI] [PubMed] [Google Scholar]
- 18.Shore N.D., Antonarakis E.S., Cookson M.S., et al. Optimizing the role of androgen deprivation therapy in advanced prostate cancer: challenges beyond the guidelines. Prostate. 2020;80:527–544. doi: 10.1002/pros.23967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iversen P., Karup C., van der Meulen E., Tankó L.B., Huhtaniemi I. Hot flushes in prostatic cancer patients during androgen-deprivation therapy with monthly dose of degarelix or leuprolide. Prostate Cancer Prostat Dis. 2011;14:184–190. doi: 10.1038/pcan.2011.11. [DOI] [PubMed] [Google Scholar]
- 20.Dearnaley D.P., Saltzstein D.R., Sylvester J.E., et al. The oral gonadotropin-releasing hormone receptor antagonist relugolix as neoadjuvant/adjuvant androgen deprivation therapy to external beam radiotherapy in patients with localised intermediate-risk prostate cancer: a randomised, open-label, parallel-group phase 2 trial. Eur Urol. 2020;78:184–192. doi: 10.1016/j.eururo.2020.03.001. [DOI] [PubMed] [Google Scholar]
- 21.Shore N.D., Saad F., Cookson M.S., et al. Oral relugolix for androgen-deprivation therapy in advanced prostate cancer. N Engl J Med. 2020;382:2187–2196. doi: 10.1056/NEJMoa2004325. [DOI] [PubMed] [Google Scholar]
- 22.Nishiyama T., Kanazawa S., Watanabe R., Terunuma M., Takahashi K. Influence of hot flashes on quality of life in patients with prostate cancer treated with androgen deprivation therapy. Int J Urol. 2004;11:735–741. doi: 10.1111/j.1442-2042.2004.00896.x. [DOI] [PubMed] [Google Scholar]
- 23.Langley R.E., Cafferty F.H., Alhasso A.A., et al. Cardiovascular outcomes in patients with locally advanced and metastatic prostate cancer treated with luteinising-hormone-releasing-hormone agonists or transdermal oestrogen: the randomised, phase 2 MRC PATCH trial (PR09) Lancet Oncol. 2013;14:306–316. doi: 10.1016/S1470-2045(13)70025-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gilbert D.C., Duong T., Kynaston H.G., et al. Quality-of-life outcomes from the Prostate Adenocarcinoma: TransCutaneous Hormones (PATCH) trial evaluating luteinising hormone-releasing hormone agonists versus transdermal oestradiol for androgen suppression in advanced prostate cancer. BJU Int. 2017;119:667–675. doi: 10.1111/bju.13687. [DOI] [PubMed] [Google Scholar]
- 25.Dobbs R.W., Malhotra N.R., Greenwald D.T., Wang A.Y., Prins G.S., Abern M.R. Estrogens and prostate cancer. Prostate Cancer Prostat Dis. 2019;22:185–194. doi: 10.1038/s41391-018-0081-6. [DOI] [PubMed] [Google Scholar]
- 26.Holzbeierlein J.M. Managing complications of androgen deprivation therapy for prostate cancer. Urol Clin North Am. 2006;33:181–190. doi: 10.1016/j.ucl.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 27.Harle L.K., Maggio M., Shahani S., Braga-Basaria M., Basaria S. Endocrine complications of androgen-deprivation therapy in men with prostate cancer. Clin Adv Hematol Oncol. 2006;4:687–696. [PubMed] [Google Scholar]
- 28.Cook S., Rodriguez-Antunez A. Pre-estrogen irradiation of the breast to prevent gynecomastia. Am J Roentgenol. 1973;117:662–663. doi: 10.2214/ajr.117.3.662. [DOI] [PubMed] [Google Scholar]
- 29.Hutton B., Hersi M., Cheng W., et al. Comparing interventions for management of hot flashes in patients with breast and prostate cancer: a systematic review with meta-analyses. Oncol Nurs Forum. 2020;47:E86–E. doi: 10.1188/20.ONF.E86-E106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahmadi H., Daneshmand S. Androgen deprivation therapy for prostate cancer: long-term safety and patient outcomes. Patient Relat Outcome Meas. 2014;5:63–70. doi: 10.2147/PROM.S52788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu E.Y., Getzenberg R.H., Coss C.C., et al. Selective estrogen receptor alpha agonist GTx-758 decreases testosterone with reduced side effects of androgen deprivation therapy in men with advanced prostate cancer. Eur Urol. 2015;67:334–341. doi: 10.1016/j.eururo.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 32.Allan C.A., Collins V.R., Frydenberg M., McLachlan R.I., Matthiesson K.L. Androgen deprivation therapy complications. Endocr Relat Cancer. 2014;21:T119–T129. doi: 10.1530/ERC-13-0467. [DOI] [PubMed] [Google Scholar]
- 33.Coelingh Bennink H.J.T., Zimmerman Y., Verhoeven C., et al. A dose-escalating study with the fetal estrogen estetrol in healthy men. J Clin Endocrinol Metab. 2018;103:3239–3249. doi: 10.1210/jc.2018-00147. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.