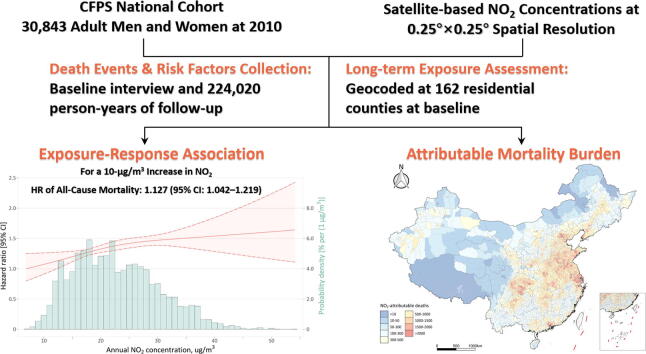
Graphical abstract
Keywords: Air pollution, Nitrogen dioxide, Adult mortality, Cohort study, Attributable deaths
Highlights
-
•
First nationwide epidemiologic evidence for long-term association between NO2 exposure and adult mortality in China.
-
•
An approximately linear NO2-mortality relationship was identified across a broad exposure range of 6.9–57.4 μg/m3.
-
•
Totally 1.65 and 1.73 million deaths were attributable to ambient NO2 in 2018 (national average 17.3 µg/m3) and 2010 (20.5 µg/m3), respectively.
Abstract
Introduction
A number of population-based studies have investigated long-term effects of nitrogen dioxide (NO2) on mortality, while great heterogeneities exist between studies. In highly populated countries in Asia, cohort evidence for NO2-mortality association was extensively sparse.
Objectives
This study aimed to quantify longitudinal association of ambient NO2 exposure with all-cause mortality in Chinese adults.
Methods
A national cohort of 30,843 adults were drawn from 25 provincial regions across mainland China, and followed up from 2010 through 2018. Participants’ exposures to ambient air pollutants were assigned according to their residential counties at baseline, through deriving monthly estimates from high-quality gridded datasets developed by machine learning methods. Cox proportional hazards models with time-varying exposures were utilized to assess the association of all-cause mortality with long-term exposure to ambient NO2. NO2-attributable deaths in China were estimated by province and county for years 2010 and 2018, with reference to the counterfactual exposure of 6.9 μg/m3 (the lowest county-level average in this cohort).
Results
We observed a total of 1662 deaths during 224020 person-years of follow-up (median 8.1 year). An approximately linear NO2-mortality relation (p = 0.273 for nonlinearity) was identified across a broad exposure range of 6.9–57.4 μg/m3. Per 10-µg/m3 increase in annual NO2 exposure was associated with an hazard ratio of 1.127 (95% confidence interval: 1.042–1.219, p = 0.003) for all-cause mortality. Risk estimates remained robust after additionally adjusting for the confounding effects of co-pollutants (i.e., PM2.5 or/and O3). In 2018, 1.65 million deaths could be attributed to ambient NO2 exposure (national average 17.3 µg/m3) in China, representing a decrease of 4.3% compared with the estimate of 1.72 million in 2010 (20.5 µg/m3).
Conclusion
This cohort study provided national evidence for elevated risk of all-cause mortality associated with long-term exposure to ambient NO2 in Chinese adults.
Introduction
Ambient nitrogen dioxide (NO2) is a common air pollutant primarily originating from fuel combustion and traffic. As a precursor to ground surface ozone, NO2 is also involved in the secondary generation of fine particles (PM2.5). In recent decades, health assessments on NO2′s independent impact raised great research interests across the globe in the context of rapid urbanization and transportation development. Systematic reviews of epidemiologic studies have well documented short-term effects of NO2 exposure on a variety of morbidity [1], [2], [3], [4], [5], [6] and mortality [2], [3], [4], [7] outcomes, including cardiovascular (e.g., stroke and ischemic heart disease) and respiratory diseases (e.g., chronic obstructive pulmonary disease and pneumonia). A recent multi-country analysis [8] from 398 cities, for instance, provided robust evidence for independent NO2-mortality associations from co-pollutants, linking a significant increase of 0.46%, 0.37%, and 0.47% in total, cardiovascular, and respiratory mortality respectively with per 10-µg/m3 rise in daily NO2 concentration. These findings highlighted the great significance and urgency for policy making to quantify the chronic effects of NO2 exposure on human life expectancy.
During past decades, longitudinal associations between NO2 exposure and mortality have been investigated in a large number of population-based cohort studies, whereas great heterogeneities were identified between regions and studies [9], [10], [11], [12]. The majority of existing studies came from the western world [9], [10] including North America and the Europe, suggesting strong evidence for NO2-induced risk of all-cause death. In highly populated countries in Asia, cohort evidence for longitudinal NO2-mortality association was extensively sparse [9], [10], thus introducing great uncertainty when performing the pooled analysis. In China, only two regional cohorts investigated chronic impacts of NO2 exposure on mortality, identifying null associations among the Hong Kong elderly [13] but a protective effect in four northeast cities [14]. These mixed findings warranted more high-quality and large-scale longitudinal investigations across mainland China, for the sake of better understanding of mortality burden due to ambient NO2 in Chinese population.
In this study, we conceived a nationwide prospective cohort of ∼ 30,000 Chinese adult men and women, utilizing population-based survey data through 2010 to 2018 from the China Family Panel Studies spanning across 126 prefecture-level cities. Our primary purpose was to quantify the association of long-term exposure to ambient NO2 with all-cause mortality in Chinese adults; A secondary purpose was to depict the spatiotemporal patterns of NO2-attributable deaths between 2010 and 2018 in China, on the basis of dose–response function derived from our cohort analysis.
Materials and methods
Study population and design
Study population in this study was drawn from the China Family Panel Studies (CFPS) [15], an ongoing national survey across 25 provincial regions in mainland China. Using a multi-stage probability strategy with stratification [16], the CFPS baseline survey during April 2010 through February 2011 totally included 33,600 adult men and women ages 16–110 years, and follow-up investigations were conducted every two years during 2012–2020. Face-to-face interviews were performed aided by computer-assisted personal interviewing technology, and participants’ data (e.g., demographic and socioeconomic characteristics, lifestyle, behavioral patterns, and health status) were thoroughly collected by well-trained investigators through standard questionnaires. Reliability and validity of CFPS interview was described in Section 1.1 of the Supplementary Material.
In purpose of investigating long-term NO2-mortality association, we conceived a longitudinal cohort design using the CFPS baseline (CFPS-2010) and four consequent waves of follow-up data (CFPS-2012, 2014, 2016, and 2018), which were publicly available at the Peking University Open Research Data Platform (https://opendata.pku.edu.cn/dataverse/CFPS/, accessed on November 30th, 2021). Death information (e.g., date and cause of death) for deceased CFPS participants were ascertained from their family members in the follow-up interviews. We only considered death events from all causes in this study, because some unknown or indeterminable causes of death were identified.
From 33,600 adult participants totally involved at CFPS baseline (Section 1.1 of the Supplementary Material), we excluded those who had no follow-up data during 2012–2018 (n = 2549) and objects whose information on death date was invalid or logically erroneous (n = 25). To avoid bias from selection of participants, we also excluded participants who died within the first year from the baseline interview [17], [18] (n = 183). A national cohort of 30,843 adult men and women were finally involved in our study, and the number of the investigated subjects in 25 survey provinces was geographically illustrated in Fig. 1.
Fig. 1.
Spatial characteristics of 8-year average NO2 concentration during 2010–2018 across China at a 0.25°× 0.25° resolution and provincial distributions of study participants (n = 30,843) included in our cohort. Islands in the South China Sea are shown in the box. NO2, nitrogen dioxide.
Ethics statement
The CFPS study involving human participants has been ethically reviewed and approved by the Peking University Biomedical Ethics Review Committee (Approval no. IRB00001052-14010), and all participants signed informed consent forms.
Exposure assessment
CFPS redacted detailed residential address from the public-access data due to privacy considerations, exposure assessments for air pollutants were thus performed at the county level as a proxy. For each CFPS participant, we identified a county-level geographic unit using uniform 6-digit administrative codes in mainland China, and linked participants in our cohort to 162 counties in 25 provincial regions [19]. Based on administrative polygons of the 162 CFPS counties, we calculated monthly concentrations during 2005–2018 for ambient air pollutants (NO2, PM2.5, and O3) through aggregating gridded estimates at spatiotemporal scales into county-level averages.
Ground surface concentrations of monthly NO2 at a 0.25°×0.25° resolution were derived from the full-coverage and high-quality datasets for near-surface air pollutants in China. Gridded datasets for NO2 (https://doi.org/10.5281/zenodo.3988349, ChinaHighNO2, accessed on November 30th, 2021) were originally generated from the OMI/Aura Tropospheric Column NO2 products based on satellite remote sensing and machine-learning method (Space-Time Extra-Trees model, STET) [20]. Estimates from ChinaHighNO2 datasets showed high consistency with nationwide ground monitors across China from 2013 to 2019, with an overall cross-validation coefficient of determination (CV-R2) of 0.72 and root mean square error (RMSE) of 9.97 µg/m3. Modelling details could be found in our previous publications [20], [21].
Monthly mean ozone concentrations at a 0.1°×0.1° resolution were estimated using a nationwide prediction model based on eXtreme Gradient Boosting (XGBoost) trees [22]. The prediction model was trained and validated using observations from 1713 sites between 2013 and 2017, and externally tested with regional monitors during 2005–2012 and nationwide measurements in 2018. Model testing results of monthly O3 estimates showed high R2 values (0.60–0.87) and low RMSEs (12.94–18.41 μg/m3) in different years [22].
Ground monthly estimates of PM2.5 concentrations at a 0.1°×0.1° resolution were extracted from a full-coverage high-resolution air pollutant dataset of Tracking Air Pollution in China (TAP, http://tapdata.org, accessed on November 30th, 2021). As the first near real-time air pollutant database in China [23], TAP PM2.5 developed by Tsinghua University is estimated based on a two-stage random forest model coupled with the synthetic minority oversampling technique and a tree-based gap-filling method [24]. This machine learning-based model exhibits good agreements with the ground monitors, with an out-of-bag CV-R2 of 0.80–0.88 and RMSE of 13.9–22.1 μg/m3 for different years.
Covariates
In light of prior cohort studies assessing NO2-mortality associations [9], [10], we considered a rich set of potential confounders, including demographic characteristics (sex, age, ethnicity, education attainment, urbanicity, marital status, and employment status), lifestyle (smoking, alcohol consumption, physical activity, and sleep duration), health status (body-mass index [BMI], chronic disease prevalence, and depressive symptom), and household characteristics (annual household income, and household air pollution from solid fuels).
Specifically, ethnicity was defined as a dichotomous variable indicating Han or minority. Education attainment was grouped into illiteracy, primary or middle school, and high school or above. Smokers and alcohol drinkers referred to former and current status of regular smoking or drinking. Current and former smokers were defined as participants who had smoked regularly within one month and a month ago, respectively, and current and former drinking status were defined as alcohol consumption at least 3 times/week (more than one cup of alcohol each time) during the last month and a month ago. Sleep duration was categorized into three groups: <6 h, 6–8 h, and more than 8 h. Physical activity was a dummy variable (1 = yes, 0 = no) indicating whether a participant was a regular physical exerciser, which was determined by asking whether she/he did sports and physical exercise over the past week. Body-mass index was calculated by dividing weight in kilogram by squared height in m2, and grouped into <18.5, 18.5–24, and 24+ kg/m3. Chronic disease prevalence was coded as 1 if a participant had one or more doctor-diagnosed chronic diseases such as hypertension, stroke, and diabetes. Depressive status was measured by the K6 screening instrument of Center for Epidemiological Studies-Depression (CES-D) scale [25], and defined as depression episode if the CES-D score is higher than a cut-off of 24. Household income was categorized into three groups of <15000 RMB, 15000–40000 RMB, and ≥40000 RMB generally based on its upper and lower quartile distribution (i.e., <P25, P25–P75, ≥P75). Detailed information about the questionnaire items for aforementioned covariates included in our analysis could be found on the CFPS website (http://www.isss.pku.edu.cn/cfps/en/documentation/questionnaires/index.htm, accessed on November 30th, 2021).
Statistical analysis
Baseline characteristics of study populations were presented as percentages for categorical variables or mean ± standard derivation (SD) for continuous variables. We assessed the long-term association between ambient NO2 exposure and all-cause death using time-varying Cox proportional hazards models, and quantified temporal and spatial changes in estimates of deaths attributable to ambient NO2 between 2010 and 2018. We did analyses with R version 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria). All tests were two-sided, and p-value <0.05 was considered to be statistically significant.
Main multivariate analysis
Cox proportional hazards models with time-varying exposures on the annual time scale, were used to quantify long-term associations of NO2 exposure with all-cause mortality. Person-years of follow-up were calculated as the intervals from the dates of baseline interviews (i.e., study enrolment) to the dates of death occurrence, loss to follow-up (the last follow-up interviews during 2012–2018), or the end of CPFS 2018, whichever came first. Motivated by prior investigations suggesting the potential for biased risk estimates using time-on-study time-scales [26], [27], [28], we adopted attained age as the time scale given the interpretability of the hazard ratio as a function of age [29]. All models were stratified by sex and age at baseline [30], which was grouped into 10-year intervals (i.e., 15–24, 25–34, 35–44, 45–54, 55–64, 65–74, and ≥75) to allow for sufficient sample sizes for assessing interactions. We assessed hazard ratios (HRs) of all-cause death and corresponding 95% confidence intervals (CIs) associated with a 10-μg/m3 increase in exposure to annual NO2 concentration, adjusting for aforementioned confounders. HR could be estimated through the formula HR = exp(β), and 95% CI could be simply calculated using exp(β ± 1.96*SE), where β is the regression coefficient and SE indicates the standard error of the estimate.
To investigate the shape of concentration–response (C-R) curve for NO2-mortality association, we input the term of annual NO2 exposure as a restricted cubic spline (RCS) with 3 knots in the multivariate-adjusted model. The number of knots for RCS smoothing was selected according to Akaike information criterion and Bayesian information criterion [31], [32] (Table S1). Nonlinearity of concentration–response curve was examined through comparing the model fit of the linear and RCS models via a likelihood-ratio test [33], [34], [35].
Subgroup and sensitivity analyses
We performed subgroup analyses stratified by sex, age, smoking and drinking status, and physical activity. In age-specific analysis, participants were divided into young adults (≤44 years old), middle-aged adults (45–59 years old), and the elderly (60+ years old) according to the World Health Organization classification. Two-sample z-tests [34], [36] were applied to identify potential effect modifications.
Several sensitivity analyses were conducted to examine the robustness of PM2.5-mortality associations estimated in our main analysis. First, to disentangle the effects of co-pollutants, we included two or three pollutants simultaneously (NO2 plus PM2.5 or/and O3) in an analysis using a consistent annual scale for time-varying exposures. We choose PM2.5 and O3 in our multi-pollutant analyses for two reasons: (1) according to existing epidemiologic evidence, the effects of long-term exposure to PM2.5 and ozone were more widely investigated and found to be associated with resident mortality; (2) at the current stage, we are unable to include other pollutants due to unavailability to high-quality gridded datasets for CO and SO2 in mainland China.
Second, we incorporated time-varying exposure for environmental temperature (e.g., annual average temperature) into our survival analysis to account for the long-term effects of weather conditions. Given emerging evidence with regard to nonlinear effects of temperature on mortality [37], we adjusted for annual average temperature through an RCS function with 3 knots [38]. Specifically, we first derived China’s daily gridded temperatures (0.1°×0.1°) during 2010–2018 from the ERA5 climate reanalysis datasets produced by the European Center for Medium-Range Weather Forecasts (https://climate.copernicus.eu/climate-reanalysis, accessed on November 30th, 2021), and then calculated county-specific average temperatures for 162 included counties to estimate participants’ time-varying exposures at annual scales.
Third, as an alternative method for selecting covariates [39], directed acyclic graph (DAG) was adopted was to determine minimal sufficient adjustment (MSA) sets for estimating the total effect of nitrogen dioxide on adult mortality (Fig. S1). Finally, our DAG-based Cox model was stratified by sex and age and adjusted for educational attainment, household income, and urbanicity. DAG in this study was created using the R package “dagitty”, with the help of online DAGitty tool (www.dagitty.net, accessed on November 30th, 2021), wherein robust MSA sets were identified by combining the evaluation of DAG-dataset consistency with the identification of valid adjustment sets for statistically equivalent DAGs [40].
Attributable deaths due to NO2 exposure
In line with the methodological strategy widely adopted in prior modelling studies [41], [42], we quantified spatiotemporal changes in NO2-attributable deaths in China between 2010 and 2018. Based on the dose–response function estimated by the RCS smoother with 3 knots, we first derived HR estimates at a range of annual NO2 concentrations with an interval of 0.1 μg/m3 through referring to a counterfactual exposure of 6.9 μg/m3 (the lowest county-average level in our cohort) [42]. For a given county (i) in China, attributable deaths (ADi) due to NO2 exposure were then calculated via multiplying total observed deaths (TDi) in a specific year (e.g., 2010 and 2018) by population attributable fraction (PAFi) estimated through the formula (HR–1)/HR × 100% [43]. Total deaths attributable to ambient NO2 in China and its provincial regions could be finally yielded by summing up county-specific AD estimates for a given year. More details for AD calculation and relevant data sources could be found in Section 1.2 of the Supplementary Material, as documented in our prior publication [38].
Results
Table 1 describes the baseline characteristics of 30,843 study participants involved in this cohort. During a total of 224020.2 person-years’ follow-up (median 8.1 year), 1662 all-cause deaths were identified from 2011 to 2018. Participants were aged 45.6 ± 16.3 years (range: 16–110 years), and 48.5% were men. 8-year mean NO2 concentrations for 162 CFPS counties varied greatly from 10.3 μg/m3 to 41.0 μg/m3, exhibiting an overall decline of 3.7 μg/m3 from 2010 (22.8 [range: 7.8–43.9] μg/m3) to 2018 (19.1 [6.2–39.2] μg/m3) in annual mean concentration (Table 2 & Fig. S2). The included study locations covered a wide span of latitudes across mainland China (Fig. 1), with a substantial temperature range of −2.0–24.2 °C in 2010 (Table 2).
Table 1.
Baseline characteristics of study participants included in the cohort.
| Variables | Entire cohort | Quartiles* of NO2 concentration, μg/m3 |
|||
|---|---|---|---|---|---|
| Q1 (10.3–17.1) |
Q2 (17.3–22.2) |
Q3 (22.4–27.0) |
Q4 (27.1–41.0) |
||
| Population | |||||
| Persons, n | 30,843 | 8096 | 7779 | 7618 | 7350 |
| Death, n | 1662 | 487 | 421 | 411 | 343 |
| Total person-years | 224020.2 | 59177.9 | 56,880 | 55921.7 | 52040.6 |
| Median year of follow-up | 8.1 | 8.1 | 8.1 | 8.1 | 8.1 |
| Demographic characteristics | |||||
| Men, % | 48.5 | 48.8 | 48.8 | 48.4 | 48.1 |
| Age (y), mean ± SD | 45.6 ± 16.3 | 45.5 ± 16.0 | 45.1 ± 16.1 | 45.7 ± 16.2 | 46.3 ± 16.8 |
| Han ethnicity, % | 91.7 | 81.8 | 92.1 | 95.1 | 98.7 |
| Educational attainment, % | |||||
| Illiteracy | 24.1 | 27.6 | 29.1 | 23.8 | 15.4 |
| Primary to middle school | 53.3 | 56.1 | 52.4 | 52.8 | 51.7 |
| High school or above | 22.5 | 16.3 | 18.4 | 23.4 | 32.9 |
| Urban, % | 43.7 | 33.5 | 33.5 | 47.2 | 62.3 |
| Married, % | 80.5 | 79.6 | 80.4 | 81.3 | 80.7 |
| Employment, % | 50.6 | 53.9 | 48.0 | 47.6 | 52.6 |
| Lifestyle factors | |||||
| Smokers, % | 36.7 | 36.2 | 39.7 | 36.1 | 35.0 |
| Alcohol drinking, % | 20.7 | 22.8 | 20.4 | 20.0 | 19.5 |
| Regular physical activity, % | 26.8 | 17.9 | 23.8 | 29.8 | 36.7 |
| Sleep duration at night, % | |||||
| <6h | 28.9 | 28.2 | 29.7 | 30.6 | 27.1 |
| 6–8 h | 63.2 | 64.8 | 61.6 | 62.0 | 64.4 |
| ≥8h | 7.9 | 7.0 | 8.7 | 7.4 | 8.5 |
| Health status | |||||
| BMI (kg/m2), mean ± SD | 21.5 ± 5.2 | 20.8 ± 5.2 | 21.2 ± 5.3 | 21.7 ± 5.4 | 22.6 ± 4.6 |
| <18.5, % | 10.9 | 14.8 | 11.5 | 9.6 | 7.3 |
| 18.5–24, % | 62.0 | 64.8 | 64.4 | 61.0 | 57.5 |
| ≥24, % | 27.1 | 20.4 | 24.1 | 29.4 | 35.2 |
| Prevalence of chronic diseases, % | 14.8 | 13.6 | 15.3 | 14.3 | 16.0 |
| Depressive symptoms, % | 3.6 | 3.6 | 4.7 | 3.7 | 2.4 |
| Household characteristics | |||||
| Annual household income, % | |||||
| <15000 RMB | 28.8 | 32.9 | 34.4 | 27.3 | 20.1 |
| 15000–40000 RMB | 42.8 | 44.1 | 43.1 | 44.5 | 39.5 |
| ≥40000 RMB | 28.3 | 23.0 | 22.5 | 28.3 | 40.5 |
| Air pollution from cooking solid fuel, % | 50.7 | 62.3 | 67.1 | 44.7 | 26.8 |
Quartiles were calculated based on average NO2 concentrations for 162 CFPS counties through 2010 to 2018. NO2, nitrogen dioxide; SD, standard deviation; BMI, body-mass index.
Table 2.
Summary statistics of environmental exposures for 162 CFPS counties between years 2010 and 2018.
| Environmental exposures | Mean | SD | Min | Percentiles |
Max | ||
|---|---|---|---|---|---|---|---|
| P25 | P50 | P75 | |||||
| Year = 2010 | |||||||
| NO2, μg/m3 | 22.8 | 7.8 | 8.9 | 16.4 | 21.6 | 27.5 | 43.9 |
| PM2.5, μg/m3 | 59.0 | 24.7 | 14.4 | 37.4 | 53.6 | 79.7 | 115.4 |
| O3, μg/m3 | 89.4 | 8.6 | 69.4 | 83.4 | 88.5 | 95.3 | 113.4 |
| Average temperature, °C | 13.5 | 5.2 | −2.0 | 9.5 | 14.4 | 16.4 | 24.2 |
| Year = 2018 | |||||||
| NO2, μg/m3 | 19.1 | 6.2 | 6.9 | 14.6 | 18.1 | 22.6 | 39.2 |
| PM2.5, μg/m3 | 38.6 | 13.3 | 14.6 | 29.2 | 35.3 | 47.5 | 77.9 |
| O3, μg/m3 | 89.8 | 8.9 | 69.3 | 83.0 | 89.7 | 95.8 | 109.7 |
| Average temperature, °C | 14.0 | 5.0 | −0.9 | 10.1 | 15.0 | 17.1 | 23.9 |
Abbreviations: SD, standard deviation; NO2, nitrogen dioxide; PM2.5, particulate matter with aerodynamic diameter ≤ 2.5 μm; O3, ozone.
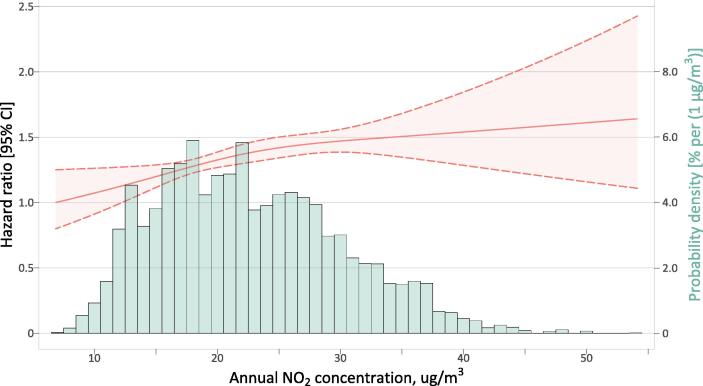
Fig. 2 depicts the concentration–response curve for long-term association between annual NO2 exposure and all-cause death risk. We identified an approximately linear NO2-mortality relation (p = 0.273 for nonlinearity) across a broad concentration range of 6.9–57.4 µg/m3, with slightly gentler slopes at high concentrations (greater than 25 µg/m3). A look-up table of concentration-response function derived from our study was available in the supplementary material (Table S2). Per 10-µg/m3 increase in annual exposure to NO2 was associated with an HR of 1.127 (95% CI: 1.042–1.219, p = 0.003). Sensitivity analyses largely supported the robustness of our findings on NO2-mortality association (Table S3). HR estimates changed little (1.144–1.169) in two-pollutant models additionally introducing PM2.5 or/and O3, and temperature-adjusted analysis gave a more comparable HR estimate of 1.121 (1.025–1.225). Our DAG-based analysis associated a slightly lower risk of 1.081 (1.007–1.159) compared to the fully adjusted model.
Fig. 2.
Concentration-response association between all-cause mortality and annual average exposure to ambient NO2 in Chinese adults. The curve is modeled using restricted cubic spline function with 3 knots. NO2, nitrogen dioxide.
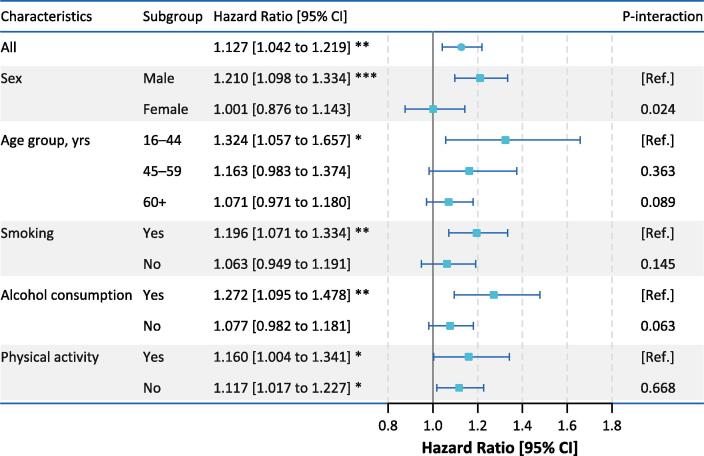
Fig. 3 summarizes estimates of subgroup analyses for NO2-mortality associations, stratified by demographic and behavioral characteristics. Only men suffered from increased risk of death (HR = 1.219 [1.101–1.340] for a 10-µg/m3 rise) associated with NO2 exposure, and we identified a significant effect modification by sex (p = 0.024). Younger participants, particularly groups aged<45 years, were at higher risks when exposed to outdoor NO2 pollution. NO2-mortality associations were observed among smokers and alcohol drinkers only, corresponding to an estimated HR of 1.200 [1.075–1.339] and 1.263 [1.087–1.468] associated with a 10-µg/m3 increase in annual NO2 exposure, both suggesting a marginal significance for effect differences between stratum. Highly similar risks of mortality were estimated for regular exercisers (1.160 [1.004–1.341]) and non-exercisers (1.117 [1.017–1.227]) related to ambient NO2 exposure.
Fig. 3.
Subgroup-specific HR estimates (with 95% CIs) of all-cause mortality associated with a 10-µg/m3 increase in annual average exposure to ambient NO2. Notes: * p < 0.05, ** p < 0.01, *** p < 0.001. HR, hazard ratio; CI, confidence interval; NO2, nitrogen dioxide.
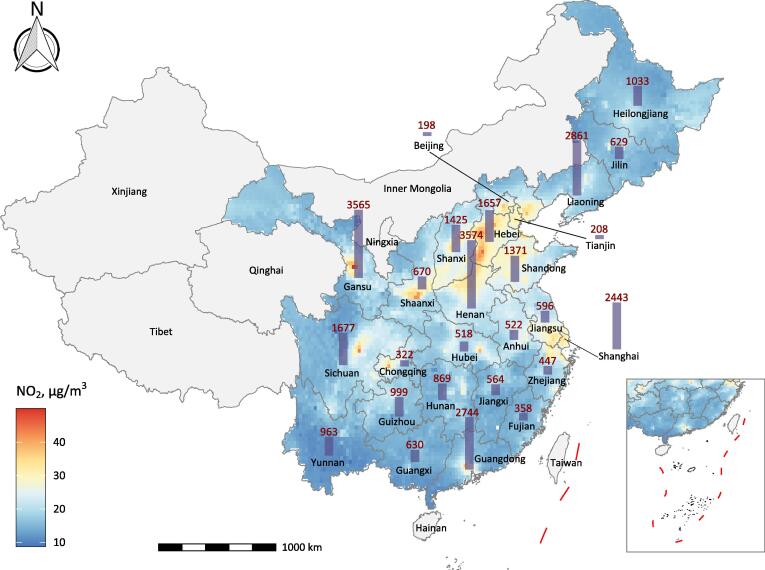
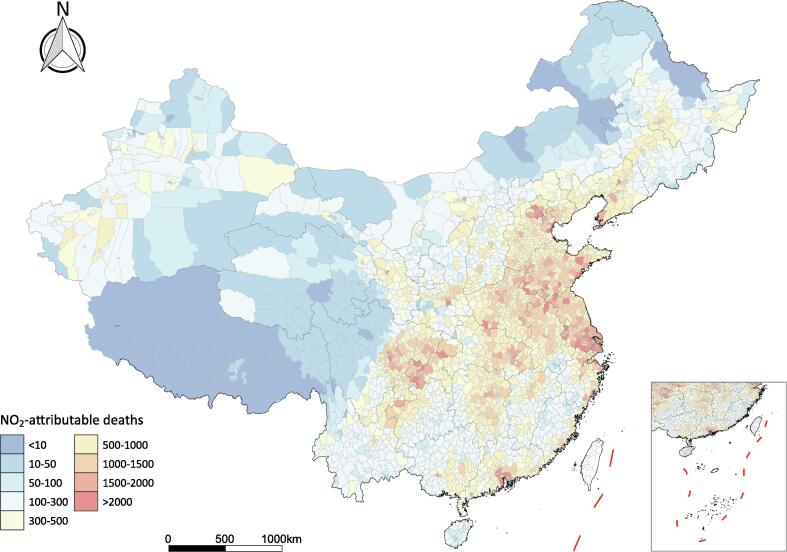
Fig. 4 & Fig. S3 illustrate county-specific estimates of NO2-attributable deaths across China for years 2018 and 2010. In 2018, a total of 1.65 million deaths could be attributed to ambient NO2 exposure (national average 17.3 µg/m3) in China, representing a decrease of 4.3% compared with the estimate of 1.72 million in 2010 (20.5 µg/m3). NO2-attributable mortality burden varied substantially between regions, and top leading regions were mainly focused in densely populated metropolitan areas including North China Plain region, Yangtze River Delta, Wuhan metropolitan region, the Sichuan Basin, and the Pearl River Delta. Great disparities across counties were also seen in temporal changes in NO2-attributable deaths in China through 2010 to 2018 (Fig. S4). The most majority of counties experienced a significant decline in NO2-related deaths during the 8-year period, wherein the reduction in NO2 concentration offset the effects of population growth. However, great increases were seen in the Pearl River Delta, Southern Jiangsu, and some top megacities such as Beijing, Shanghai, Chengdu, and Wuhan. Provincial estimates of NO2-attributable deaths for years 2010 and 2018 could be found in Table S4.
Fig. 4.
County-specific estimates of all-cause deaths attributable to ambient NO2 across China in 2018. Taiwan Province is not included for assessment and islands in the South China Sea are shown in the box. NO2, nitrogen dioxide.
Discussion
To the best of our knowledge, this is the first national perspective cohort study investigating mortality effects of long-term exposure to ambient NO2 in China. By involving 30,000+ adult participants ages 16–110 years, our study provided robust longitudinal evidence for raised death risk associated with NO2 exposure in Chinese general population. Stratified analyses revealed potential heterogeneities in NO2-mortality associations between subpopulations, suggesting significantly greater vulnerability among men.
In accordance with the up-to-date evidence synthesized from epidemiologic studies, our cohort study identified a significantly positive association between NO2 exposure and all-cause mortality. Several toxicological mechanisms may possibly interpret NO2-induced hazards. Exposure to ambient NO2 could promote a systemic vascular oxidative stress reaction [44], [45], and cause endothelial dysfunction, monocyte activation, and certain pro-atherosclerotic changes in lipoproteins, thereby initiating plaque formation, exacerbating disease, and increasing mortality [44]. For a 10-ppb rise in NO2 exposure, a recent meta-analysis of 28 cohorts estimated a pooled risk of 1.06 (95% CI: 1.04–1.08) in all-cause mortality, whereas substantial heterogeneities existed across studies (HRs ranging between 0.95 and 1.91). By enrolling a national cohort of 30,843 adults in mainland China, this study associated an excessive death risk of 27.8% (8.8–50.2%) with a 10-ppb increase in annual NO2 exposure. However, two prior Chinese investigations reported an insignificant or opposite NO2-mortality association in Hong Kong elderly [13] and northern residents [14]. Sources of huge heterogeneity between existing studies still remain not well clarified, but could possibly be related to great diversity in exposure assessment methods (e.g., fixed monitor, land use regression, and satellite-based retrievals), study demographics (e.g., age structure, locations, and NO2 exposure levels), methodological strategies (e.g., sample size, confounding adjustment, and statistical analysis) [10].
Using time series data from 398 cities in 22 low to high income countries/regions, Meng and colleagues [8] provided strong evidence for the linear associations between short-term NO2 exposure and daily total, cardiovascular, and respiratory mortality. In terms of long-term assessments, most international studies failed to investigate the dose–response curve and reported estimates by assuming a linear relation [11]. Our study did not identify significant evidence (p = 0.273) for the nonlinear effect of NO2 exposure on all-cause death at a concentration range of 6.9–57.4 µg/m3. The Dutch Environmental Longitudinal Study (DUELS) [46] reported a consistent finding (p = 0.37 for nonlinearity) in associations of NO2 with non-accidental deaths, but found a strongly superlinear relation (p < 0.005) with deaths due to circulatory diseases. Owing to limited longitudinal evidence available for C-R associations between NO2 and mortality outcomes, the state-of-art global burden of disease (GBD) studies only provided comparative estimates of deaths attributable to ambient PM2.5 and ozone, irrespective of the potential contribution from NO2 [47]. Using a counterfactual analytic framework adopted by GBD, we attributed 1.65 million all-cause deaths to NO2 exposure in China for the year 2018 only, which was higher than the latest GBD estimate (1.47 million) due to ambient PM2.5 and ozone for Chinese population [47]. Notably, even without considering the differences in analytic strategies between pollutants, the direct comparison between GBD and this study would introduce huge uncertainty. GBD assessed selected mortality causes (e.g., stroke, ischemic heart disease, and chronic obstructive pulmoriary disease [COPD]) associated with PM2.5, but only included COPD in the estimation of ozone-related deaths. NO2-mortality cohort studies focusing on C-R analyses are warranted across the world, particularly in developing countries, so as to facilitate more comprehensive GBD estimation of disease burden attributable to ambient air pollution.
Despite the substantial disparity, most counties experienced a significant decline in NO2-related deaths during 2010–2018, which was mainly due to reduction in NO2 concentration (Fig. S4) driven by the air cleaning action in mainland China [48]. However, markable increases were seen in some top megacities (e.g., Beijing and Shanghai) and major urban agglomerations (e.g., Pearl River Delta) (Fig. S5). Given China’s accelerated urbanization and rural-to-urban migration that could be foreseeable in this decade, population growth in metropolitan areas may possibly enhance NO2-attributable mortality burden. To avoid additional life lost due to ambient air pollution, more effective measurements should be implemented in these densely populated locations to achieve NO2 annual standard (10 μg/m3) recommended by the updated air quality guidelines [49], which was recently released by the World Health Organization in September 2021 [50].
Our study observed an elevated death risk associated with NO2 among adult men only, showing a significant effect modification by sex. This finding could be partially explained by great differences in intensity of work-related exposures to outdoor air pollution, as well as potential effect modification by behavioral patterns such as smoking and alcohol consumption (Fig. 3). Stronger NO2-mortality associations were also estimated for men in two European cohorts [51], while DUELS [46] reported significantly greater vulnerability among women when assessing mortality causes of respiratory disease and lung cancer. We found a tendency for higher NO2-related HR in younger age groups (16–59 years), which was in agreement with evidence of effect modification by age as highlighted in two large cohorts (over 1 million participants) in Rome [51] and Netherlands [46] for nonaccidental deaths. However, in our prior analysis of CFPS cohort [38], we identified significant PM2.5-mortality associations only in middle-aged adults (45–59 years) and the elderly (60+ years), even though risk estimates varied slightly between young (<45 years) and older groups. This inconsistency in age-specific effects of PM2.5 and NO2 might be related to greater possibility of exposure misclassification when assigning individual NO2 exposure based on a relatively coarser-resolution (0.25°×0.25°) dataset, as well as potential differences in exposure patterns to particulate and traffic-related air pollutants. Besides, the elderly may belong to older cohorts, who have experienced lower exposures to NO2 in their youth compared with newer cohorts with younger ages. Given that mortality impact of NO2 exposure may have a cumulative pattern, lower risk observed in older age groups might possibly result from cohort effect rather than age effect.
As demonstrated in our stratified analyses by behavioral factors, only ever/current smokers and alcohol drinkers were at greater death risks induced by NO2 exposure, whereas no clear evidence for modifying effects was identified in our study population. In addition, we estimated highly comparable NO2-associated hazards between those having and not having regular physical activity. Combined effects of air pollution and physical activity on mortality raised great research interest but remained not yet fully understood [52]. In several existing large cohorts of US women [52] (n = 104,990), middle-aged Danish adults [53] (n = 52,061), and Hong Kong elderly [54] (n = 66,820), no significant interactions between NO2/PM2.5 exposure and physical activity were reported in associations with total and cardiovascular mortality.
This study has several strengths. First, this cohort investigation provided the first nationwide epidemiologic evidence for long-term association between NO2 exposure and adult mortality in mainland China. Second, exposure assessment in our analysis was based on high-resolution NO2 prediction models, through taking advantages of satellite retrieved estimates and machine learning methods. This novel advance in methodology could largely reduce exposure errors as compared to the majority of prior cohort studies relying on measurements from fixed-site monitors or estimates derived from geospatial statistical methods and chemistry transport models [10]. Additionally, our analyses took into account a rich set of confounders including individual lifestyles and provided robust NO2-mortality evidence by including high-resolution PM2.5/O3 for additional adjustment.
Some limitations of our analyses should also be noted. First, participants’ NO2 exposures were assessed at the county level rather than assigned based on residential addresses, which may result in some inevitable exposure misclassifications. Second, high-quality NO2 estimates at finer spatial–temporal scales were still of wide lack globally and regionally [55], which has hampered the comparative analyses using various exposure datasets. Third, owing to data unavailability, we failed to account for residential mobility of study participants in CFPS follow-up surveys during 2012–2018. Finally, cause-specific analyses for NO2-mortality associations were not performed due to a relative high proportion of indeterminable causes of death in the CFPS database. This limitation may introduce some difficulty in direct comparison of estimates of NO2-attributable deaths between our calculation based on all-cause deaths and assessments through summing up cause-specific contributions [38].
Conclusion
In summary, this study associated elevated risk of adult mortality with long-term exposure to ambient NO2, utilizing an 8-year nationwide cohort in mainland China. Our dose–response analysis highlighted an approximately linear NO2-mortality curve, which provided a valuable opportunity to quantify mortality burden attributable to NO2 exposure in Chinese general population. Future population-based cohorts should well take advantages of high-quality exposure datasets, so as to enhance the understanding of NO2-induced health risks and promote the comprehensive assessment of regional and global disease burden due to ambient NO2.
CRediT authorship contribution statement
Yunquan Zhang: Conceptualization, Formal analysis, Methodology, Software, Validation, Visualization, Data curation, Resources, Writing – original draft, Writing – review & editing, Supervision, Funding acquisition, Project administration. Zunyan Li: Methodology, Data curation, Resources. Jing Wei: Methodology, Data curation, Resources. Yu Zhan: Methodology, Data curation, Resources. Linjiong Liu: Methodology, Data curation, Resources, Writing – review & editing. Zhiming Yang: Methodology, Data curation, Resources, Writing – review & editing. Yuanyuan Zhang: Methodology, Data curation, Resources, Writing – review & editing. Riyang Liu: Methodology, Data curation, Resources. Zongwei Ma: Methodology, Data curation, Resources.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
This study was financially supported by Youth Fund Project of Humanities and Social Sciences Research of the Ministry of Education (Grant No. 21YJCZH229). We acknowledged the China Family Panel Studies team for providing access to data involved in our cohort analysis. We appreciated the anonymous reviewers very much, whose comments and suggestions contributed a lot to improving the quality of the manuscript.
Compliance with ethics requirements
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5). The CFPS study has been ethically approved by the Peking University Biomedical Ethics Review Committee (Approval no. IRB00001052-14010), and all participants signed informed consent forms.
Footnotes
Peer review under responsibility of Cairo University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jare.2022.02.007.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Stieb D.M., Zheng C., Salama D., Berjawi R., Emode M., Hocking R., et al. Systematic review and meta-analysis of case-crossover and time-series studies of short term outdoor nitrogen dioxide exposure and ischemic heart disease morbidity. Environ Health. 2020;19(1):47. doi: 10.1186/s12940-020-00601-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang Z., Wang J., Lu W. Exposure to nitrogen dioxide and chronic obstructive pulmonary disease (COPD) in adults: A systematic review and meta-analysis. Environ Sci Pollut Res Int. 2018;25(15):15133–15145. doi: 10.1007/s11356-018-1629-7. [DOI] [PubMed] [Google Scholar]
- 3.Mills I.C., Atkinson R.W., Anderson H.R., Maynard R.L., Strachan D.P. Distinguishing the associations between daily mortality and hospital admissions and nitrogen dioxide from those of particulate matter: A systematic review and meta-analysis. BMJ Open. 2016;6(7):e010751. doi: 10.1136/bmjopen-2015-010751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shah ASV, Lee KK, McAllister DA, et al. Short term exposure to air pollution and stroke: Systematic review and meta-analysis. BMJ. 2015;350:h1295. doi: 10.1136/bmj.h1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mills IC, Atkinson RW, Kang S, et al. Quantitative systematic review of the associations between short-term exposure to nitrogen dioxide and mortality and hospital admissions. BMJ Open, 2015, 5: e006946 [DOI] [PMC free article] [PubMed]
- 6.Yee J., Cho Y.A., Yoo H.J., Yun H., Gwak H.S. Short-term exposure to air pollution and hospital admission for pneumonia: A systematic review and meta-analysis. Environ Health. 2021;20(1):6. doi: 10.1186/s12940-020-00687-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Orellano P., Reynoso J., Quaranta N., Bardach A., Ciapponi A. Short-term exposure to particulate matter (PM10 and PM2.5), nitrogen dioxide (NO2), and ozone (O3) and all-cause and cause-specific mortality: Systematic review and meta-analysis. Environ Int. 2020;142:105876. doi: 10.1016/j.envint.2020.105876. [DOI] [PubMed] [Google Scholar]
- 8.Meng X, Liu C, Chen R, et al. Short term associations of ambient nitrogen dioxide with daily total, cardiovascular, and respiratory mortality: Multilocation analysis in 398 cities. BMJ. 2021;372:n534. doi: 10.1136/bmj.n534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stieb DM, Berjawi R, Emode M, et al. Systematic review and meta-analysis of cohort studies of long term outdoor nitrogen dioxide exposure and mortality. PloS one, 2021, 16: e0246451 [DOI] [PMC free article] [PubMed]
- 10.Huang S., Li H., Wang M., Qian Y., Steenland K., Caudle W.M., et al. Long-term exposure to nitrogen dioxide and mortality: A systematic review and meta-analysis. Science Total Environ. 2021;776:145968. doi: 10.1016/j.scitotenv.2021.145968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huangfu P., Atkinson R. Long-term exposure to no2 and o3 and all-cause and respiratory mortality: A systematic review and meta-analysis. Environ Int. 2020;144:105998. doi: 10.1016/j.envint.2020.105998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Atkinson R.W., Butland B.K., Anderson H.R., Maynard R.L. Long-term concentrations of nitrogen dioxide and mortality: A meta-analysis of cohort studies. Epidemiology. 2018;29(4):460–472. doi: 10.1097/EDE.0000000000000847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang Y., Tang R., Qiu H., Lai P.-C., Wong P., Thach T.-Q., et al. Long term exposure to air pollution and mortality in an elderly cohort in Hong Kong. Environ Int. 2018;117:99–106. doi: 10.1016/j.envint.2018.04.034. [DOI] [PubMed] [Google Scholar]
- 14.Chen X.i., Zhang L.-W., Huang J.-j., Song F.-j., Zhang L.-P., Qian Z.-M., et al. Long-term exposure to urban air pollution and lung cancer mortality: A 12-year cohort study in northern china. Science Total Environ. 2016;571:855–861. doi: 10.1016/j.scitotenv.2016.07.064. [DOI] [PubMed] [Google Scholar]
- 15.Xie Y., Hu J.W. An introduction to the China Family Panel Studies (CFPS) Chin Sociol Rev. 2014;47:3–29. [Google Scholar]
- 16.Xie Y.u., Lu P. The sampling design of the China Family Panel Studies (CFPS) Chin J Sociol. 2015;1(4):471–484. doi: 10.1177/2057150X15614535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang X., Liang F., Li J., Chen J., Liu F., Huang K., et al. Associations of long-term exposure to ambient PM2.5 with mortality in chinese adults: A pooled analysis of cohorts in the China-PAR project. Environ Int. 2020;138:105589. doi: 10.1016/j.envint.2020.105589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yin P., Brauer M., Cohen A., Burnett R.T., Liu J., Liu Y., et al. Long-term fine particulate matter exposure and nonaccidental and cause-specific mortality in a large national cohort of Chinese men. Environ Health Perspect. 2017;125(11):117002. doi: 10.1289/EHP1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xue T., Zhu T., Zheng Y., Zhang Q. Declines in mental health associated with air pollution and temperature variability in China. Nat Commun. 2019;10(1):2165. doi: 10.1038/s41467-019-10196-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wei J., Li Z., Lyapustin A., Sun L., Peng Y., Xue W., et al. to 2018 in China: Spatiotemporal variations and policy implications. Remote Sens Environ. 2021;252:112136. doi: 10.1016/j.rse.2020.112136. [DOI] [Google Scholar]
- 21.Wei J., Li Z.Q., Cribb M., et al. Improved 1 km resolution PM2.5 estimates across China using enhanced space-time extremely randomized trees. Atmos Chem Phys. 2020;20:3273–3289. [Google Scholar]
- 22.Liu R., Ma Z., Liu Y., Shao Y., Zhao W., Bi J. Spatiotemporal distributions of surface ozone levels in China from 2005 to 2017: A machine learning approach. Environ Int. 2020;142:105823. doi: 10.1016/j.envint.2020.105823. [DOI] [PubMed] [Google Scholar]
- 23.Geng G, Xiao Q, Liu S, et al. Tracking air pollution in China: Near realtime PM2.5 retrievals from multiple data sources. ArXiv 2021;abs/:2103.06520.
- 24.Xiao Q., Geng G., Cheng J., Liang F., Li R., Meng X., et al. Evaluation of gap-filling approaches in satellite-based daily PM2.5 prediction models. Atmos Environ. 2021;244:117921. doi: 10.1016/j.atmosenv.2020.117921. [DOI] [Google Scholar]
- 25.Chen S., Oliva P., Zhang P. Air pollution and mental health: Evidence from China. National Bureau of Economic Research Working Paper Series. 2018;24686 [Google Scholar]
- 26.Kom E.L., Graubard B.I., Midthune D. Time-to-event analysis of longitudinal follow-up of a survey: Choice of the time-scale. Am J Epidemiol. 1997;145(1):72–80. doi: 10.1093/oxfordjournals.aje.a009034. [DOI] [PubMed] [Google Scholar]
- 27.Griffin B.A., Anderson G.L., Shih R.A., et al. Use of alternative time scales in Cox proportional hazard models: Implications for time-varying environmental exposures. Stat Med. 2012;31:3320–3327. doi: 10.1002/sim.5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cologne J., Hsu W.-L., Abbott R.D., Ohishi W., Grant E.J., Fujiwara S., et al. Proportional hazards regression in epidemiologic follow-up studies: An intuitive consideration of primary time scale. Epidemiology. 2012;23(4):565–573. doi: 10.1097/EDE.0b013e318253e418. [DOI] [PubMed] [Google Scholar]
- 29.Hvidtfeldt U.A., Sørensen M., Geels C., Ketzel M., Khan J., Tjønneland A., et al. Long-term residential exposure to PM2.5, PM10, black carbon, NO2, and ozone and mortality in a Danish cohort. Environ Int. 2019;123:265–272. doi: 10.1016/j.envint.2018.12.010. [DOI] [PubMed] [Google Scholar]
- 30.Parker J.D., Kravets N., Vaidyanathan A. Particulate Matter Air Pollution Exposure and Heart Disease Mortality Risks by Race and Ethnicity in the United States: 1997 to 2009 National Health Interview Survey With Mortality Follow-Up Through 2011. Circulation. 2018;137(16):1688–1697. doi: 10.1161/CIRCULATIONAHA.117.029376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang B., Eum K.D., Kazemiparkouhi F., et al. The impact of long-term PM2.5 exposure on specific causes of death: Exposure-response curves and effect modification among 53 million U.S. Medicare beneficiaries. Environ health. 2020;19:20. doi: 10.1186/s12940-020-00575-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liang F., Liu F., Huang K., et al. Long-term exposure to fine particulate matter and cardiovascular disease in China. J Am Coll Cardiol. 2020;75:707–717. doi: 10.1016/j.jacc.2019.12.031. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y., Wei J., Shi Y., Quan C., Ho H.C., Song Y., et al. Early-life exposure to submicron particulate air pollution in relation to asthma development in Chinese preschool children. J Allergy Clin Immunol. 2021;148(3):771–782.e12. doi: 10.1016/j.jaci.2021.02.030. [DOI] [PubMed] [Google Scholar]
- 34.Liu Y., Pan J., Fan C., et al. Short-term exposure to ambient air pollution and mortality from myocardial infarction. J Am Coll Cardiol. 2021;77:271–281. doi: 10.1016/j.jacc.2020.11.033. [DOI] [PubMed] [Google Scholar]
- 35.Beelen R., Raaschou-Nielsen O., Stafoggia M., et al. Effects of long-term exposure to air pollution on natural-cause mortality: An analysis of 22 European cohorts within the multicentre ESCAPE project. Lancet. 2014;383:785–795. doi: 10.1016/S0140-6736(13)62158-3. [DOI] [PubMed] [Google Scholar]
- 36.Di Q., Wang Y., Zanobetti A., Wang Y., Koutrakis P., Choirat C., et al. Air Pollution and Mortality in the Medicare Population. N Engl J Med. 2017;376(26):2513–2522. doi: 10.1056/NEJMoa1702747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zafeiratou S., Samoli E., Dimakopoulou K., Rodopoulou S., Analitis A., Gasparrini A., et al. A systematic review on the association between total and cardiopulmonary mortality/morbidity or cardiovascular risk factors with long-term exposure to increased or decreased ambient temperature. Science Total Environ. 2021;772:145383. doi: 10.1016/j.scitotenv.2021.145383. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Y. All-cause mortality risk and attributable deaths associated with long-term exposure to ambient PM2.5 in Chinese adults. Environ Sci Technol. 2021;55(9):6116–6127. doi: 10.1021/acs.est.0c08527. [DOI] [PubMed] [Google Scholar]
- 39.Brauer M., Brook J.R., Christidis T., et al. Mortality-Air Pollution Associations in Low-Exposure Environments (MAPLE): Phase 1. Res Rep Health Eff Inst. 2019;(203):1–87. [PMC free article] [PubMed] [Google Scholar]
- 40.Textor J., van der Zander B., Gilthorpe M.S., et al. Robust causal inference using directed acyclic graphs: The R package ‘dagitty’. Int J Epidemiol. 2017;45(6):1887–1894. doi: 10.1093/ije/dyw341. [DOI] [PubMed] [Google Scholar]
- 41.Yue H., He C., Huang Q., Yin D., Bryan B.A. Stronger policy required to substantially reduce deaths from PM2.5 pollution in China. Nat Commun. 2020;11(1) doi: 10.1038/s41467-020-15319-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burnett R., Chen H., Szyszkowicz M., et al. Global estimates of mortality associated with long-term exposure to outdoor fine particulate matter. PNAS. 2018;115:9592–9597. doi: 10.1073/pnas.1803222115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li T., Zhang Y.i., Wang J., Xu D., Yin Z., Chen H., et al. All-cause mortality risk associated with long-term exposure to ambient PM2.5 in China: A cohort study. Lancet Public Health. 2018;3(10):e470–e477. doi: 10.1016/S2468-2667(18)30144-0. [DOI] [PubMed] [Google Scholar]
- 44.Bourdrel T., Bind M.-A., Béjot Y., Morel O., Argacha J.-F. Cardiovascular effects of air pollution. Arch Cardiovasc Dis. 2017;110(11):634–642. doi: 10.1016/j.acvd.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Debprasad C., Hemanta M., Paromita B., et al. Inhibition of NO(2), PGE(2), TNF-α, and iNOS EXpression by Shorea robusta L.: An Ethnomedicine Used for Anti-Inflammatory and Analgesic Activity. Evid Based Complement Alternat Med. 2012;2012 doi: 10.1155/2012/254849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fischer P.H., Marra M., Ameling C.B., Hoek G., Beelen R., de Hoogh K., et al. Air Pollution and Mortality in Seven Million Adults: The Dutch Environmental Longitudinal Study (DUELS) Environ Health Perspect. 2015;123(7):697–704. doi: 10.1289/ehp.1408254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.GBD 2019 Risk Factors Collaborators Global burden of 87 risk factors in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1223–1249. doi: 10.1016/S0140-6736(20)30752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu Y., Di B., Luo Y., Grieneisen M.L., Zeng W., Zhang S., et al. A robust approach to deriving long-term daily surface NO2 levels across china: Correction to substantial estimation bias in back-extrapolation. Environ Int. 2021;154:106576. doi: 10.1016/j.envint.2021.106576. [DOI] [PubMed] [Google Scholar]
- 49.Hoffmann B., Boogaard H., de Nazelle A., Andersen Z.J., Abramson M., Brauer M., et al. WHO Air Quality Guidelines 2021-Aiming for Healthier Air for all: A Joint Statement by Medical, Public Health, Scientific Societies and Patient Representative Organisations. Int J Public Health. 2021;66 doi: 10.3389/ijph.2021.1604465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burki T. WHO introduces ambitious new air quality guidelines. Lancet. 2021;398(10306):1117. doi: 10.1016/S0140-6736(21)02126-7. [DOI] [PubMed] [Google Scholar]
- 51.Cesaroni G., Badaloni C., Gariazzo C., Stafoggia M., Sozzi R., Davoli M., et al. Long-term exposure to urban air pollution and mortality in a cohort of more than a million adults in Rome. Environ Health Perspect. 2013;121(3):324–331. doi: 10.1289/ehp.1205862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Elliott E.G., Laden F., James P., Rimm E.B., Rexrode K.M., Hart J.E. Interaction between Long-Term Exposure to Fine Particulate Matter and Physical Activity, and Risk of Cardiovascular Disease and Overall Mortality in U.S. Women. Environ Health Perspect. 2020;128(12):127012. doi: 10.1289/EHP7402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Andersen Z.J., de Nazelle A., Mendez M.A., Garcia-Aymerich J., Hertel O., Tjønneland A., et al. A study of the combined effects of physical activity and air pollution on mortality in elderly urban residents: the Danish Diet, Cancer, and Health Cohort. Environ Health Perspect. 2015;123(6):557–563. doi: 10.1289/ehp.1408698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sun S., Cao W., Qiu H., Ran J., Lin H., Shen C., et al. Benefits of physical activity not affected by air pollution: A prospective cohort study. Int J Epidemiol. 2020;49(1):142–152. doi: 10.1093/ije/dyz184. [DOI] [PubMed] [Google Scholar]
- 55.Di Q., Amini H., Shi L., Kloog I., Silvern R., Kelly J., et al. ssessing NO2 Concentration and Model Uncertainty with High Spatiotemporal Resolution across the Contiguous United States Using Ensemble Model Averaging. Environ Sci Technol. 2020;54(3):1372–1384. doi: 10.1021/acs.est.9b03358. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.