Abstract
Background:
Cannabis legalization and use are outpacing our understanding of its long-term effects on brain and behavior, which is fundamental for effective policy and health practices. Existing studies are limited by small samples, cross-sectional measures, failure to separate long-term from recreational use, and inadequate control for other substance use. Here, we address these limitations by determining the structural brain integrity of long-term cannabis users in the Dunedin Study, a longitudinal investigation of a population-representative birth cohort followed to midlife.
Methods:
We leveraged prospective measures of cannabis, alcohol, tobacco, and other illicit drug use, in addition to structural neuroimaging in 875 Study members at age 45 to test for differences in both global and regional grey and white matter integrity between long-term cannabis users and lifelong non-users. We additionally tested for dose-response associations between continuous measures of cannabis use and brain structure, including careful adjustments for use of other substances.
Results:
Long-term cannabis users had a thinner cortex, smaller subcortical grey matter volumes, and higher machine-learning-predicted brain age than non-users. However, these differences in structural brain integrity were explained by the propensity of long-term cannabis users to engage in polysubstance use, especially with alcohol and tobacco.
Conclusions:
These findings suggest that diminished midlife structural brain integrity in long-term cannabis users reflects a broader pattern of polysubstance use, underlining the importance of understanding comorbid substance use in efforts to curb the negative effects of cannabis on brain and behavior as well as establish more effective policy and health practices.
Keywords: Cannabis, Structural MRI, Cognitive reserve, Polysubstance use, Brain age, Birth cohort
Introduction
Increasing legalization of cannabis has been accompanied by a decrease in perceived risks of use (1), despite emerging evidence of numerous adverse outcomes in long-term users, including impaired cognitive function (2-7). Neuroimaging studies of cannabis users have further revealed structural alterations in both grey and white matter supporting the very cognitive functions found to be impaired in chronic users (8-10). Most studies of structural brain integrity in cannabis users have reported on grey matter, with the most consistent differences found in cannabinoid receptor-dense regions including the hippocampus and amygdala (1,9,10). Specifically, meta-analyses have found smaller hippocampal volumes in cannabis users (11,12), with findings in other regions more mixed. Results from white matter studies are also mixed, with evidence for associations between earlier onset of use and reduced white matter microstructural integrity most consistently emerging (8,13). However, several studies have found no evidence of grey or white matter alterations in cannabis users (13-17), and additional studies have suggested that findings may be attributable to confounding influences of polysubstance use (18) or predispositional factors (19).
The absence of clear evidence for structural brain alterations in long-term cannabis users reflects, in part, limitations of existing studies that must be addressed to better understand the nature of adverse outcomes associated with long-term drug use and inform public health policy and practice. First, as with the broader clinical neuroimaging literature, heterogenous study designs and small unrepresentative samples limit power to detect associations beyond a few regions of interest or generalize to other populations (18). Additionally, existing studies face limitations common to the study of cannabis users, namely accurate usage quantification and reliance on cross-sectional retrospective usage reports (20). Reported usage levels vary widely across studies, from as little as one to >1000 lifetime uses (11,13). Further, cannabis users often engage in polysubstance use, with one population-based study demonstrating that rates of alcohol, tobacco, and other illicit drug use among cannabis users were at least two, four, and five times that of non-users, respectively (21). As such, disentangling effects of cannabis from other substances is challenging, and more research is needed to determine how the brains of cannabis users compare to those of other substance users (4). Finally, existing findings are largely based on studies of adolescents and young adults (22), some of whom used cannabis very infrequently (11), leaving open questions about the structural brain integrity of long-term cannabis users in midlife or older adulthood. As the number of older adults using cannabis reaches historical highs (23), it is increasingly important to identify how long-term use contributes to diminished cognitive reserves (24,25) and increased risk for aging-related diseases such as Alzheimer’s disease and related dementias (ADRD) (26,27).
We sought to fill these gaps by evaluating the midlife structural brain integrity of long-term cannabis users in the Dunedin Study, which offers a rich longitudinal dataset of health and behavioral measures in a population-representative birth cohort of 1,000 people followed to age 45, when neuroimaging data were first collected. To our knowledge, this neuroimaging dataset is amongst the largest of long-term cannabis users, and the only larger dataset with detailed prospective substance use measures. With this dataset, we leveraged repeated prospective cannabis use assessments over nearly 30 years to compare long-term cannabis users to lifelong non-users and to test whether associations with brain structure depend on usage level (i.e., are dose-dependent). Detailed prospective alcohol and tobacco use measures allowed us to test whether structural brain alterations in long-term cannabis users are specific to their cannabis use, or are potentially explained by the fact that they also use other substances heavily. Additionally, these measures allowed us to compare the magnitude of alterations in alcohol and tobacco users to those of cannabis users. Finally, the availability of high-quality brain structure measures at age 45 in a large number of Study members with documented drug uses histories (N=860 for grey matter; N=853 for white matter) afforded statistical power to conduct unbiased exploratory whole-brain analyses to ascertain the breadth of associations between long-term cannabis use and midlife structural brain integrity.
Specifically, we evaluated long-term cannabis users on comprehensive measures of global and regional grey matter (i.e., cortical thickness, surface area, subcortical volume) and white matter (i.e., microstructural integrity as indexed by fractional anisotropy, and volume of hyperintensities) in midlife. These MRI measures not only have demonstrated alterations in regular cannabis users (1) but also represent promising midlife biomarkers of accelerated cognitive decline and risk for ADRD (28). In addition to grey and white matter, we examined links with “brain age,” a machine-learning-based estimate derived from multiple MRI measures (29). The difference between brain age and chronological age offers an approximation of age-related deterioration in the brain using cross-sectional data (30). Individuals with the same chronological age but older brain age have accelerated cognitive decline (29), increased risk for ADRD (31), and higher mortality (32). Collectively, these design features presented a unique opportunity to establish a comprehensive portrait of midlife structural brain integrity in long-term cannabis users, which may be important in shaping their trajectories of healthy and unhealthy aging.
Methods
A brief description of the samples and measures is reported below. A full description is provided in Supplemental Methods.
Study Design and Participants
Participants were members of the Dunedin Study, a population-representative birth cohort (N=1037) born between April 1972 and March 1973 in Dunedin, New Zealand (NZ). Assessments were conducted at birth and every few years, most recently at age 45, when neuroimaging was additionally conducted in 875 Study members. The relevant ethics committees approved the Study, and all Study members provided written informed consent.
Long-Term Cannabis Users and Three Comparison Groups
At each of the six adult Study waves (ages 18, 21, 26, 32, 38, and 45), Study members self-reported the number of days (0-365) they used cannabis, the number of tobacco cigarettes smoked per day, and the number of days they used alcohol in the past year. At the four study waves from age 26-45, Study members additionally reported the number of days they used other drugs in the past year. This was used to assess past-year drug dependencies and identify long-term cannabis users and three comparison groups. Long-term cannabis users (n=82; 65% men) used cannabis at least weekly in the past year at age 45, or were dependent on cannabis at age 45, and also used at least weekly during one or more previous waves. Lifelong cannabis non-users (n=192; 41% men) never used cannabis, had no substance-use disorder diagnoses at any assessment, and never used tobacco daily. Long-term tobacco users (n=70; 40% men) smoked tobacco daily in the past year at age 45 and at one or more previous waves. Long-term alcohol users (n=56; 55% men) were at least past-year weekly drinkers at age 45 and had an alcohol dependence diagnosis at two or more waves. Long-term tobacco and alcohol users were mostly free from cannabis use at age 45 and had no history of cannabis dependence or weekly use. All comparison groups are mutually exclusive to long-term cannabis users but not each other (Figure S1).
Persistence of Cannabis Use
We created two continuous cannabis use measures. Persistence of cannabis dependence comprised Study members who (i) never used cannabis (n=248), (ii) used but were never diagnosed (n=468), and those who were diagnosed at (iii) one wave (n=78), (iv) two waves (n=34), (v) three waves (n=30), and (vi) four or more waves (n=16). Persistence of regular cannabis use (≥4 times/week) comprised Study members who (i) never used cannabis (n=248), (ii) used but never regularly (n=486), and those who used regularly at (iii) one wave (n=49), (iv) two waves (n=31), (v) three waves (n=31), or (vi) four or more waves (n=29).
Persistence of Alcohol and Tobacco Use
Continuous alcohol and tobacco dependence measures mirrored the cannabis measure. Persistence of alcohol/tobacco dependence comprised Study members who (i) never used (n=47/427), (ii) used but were never diagnosed (n=504/123), and those who were diagnosed at (iii) one wave (n=167/99), (iv) two waves (n=79/81), (v) three waves (n=45/59), or (vi) four or more waves (n=30/85).
Midlife Brain Structure
Grey matter integrity was estimated using cortical thickness (CT) and cortical surface area (SA) measures extracted from the whole brain and the 360 regions in the HCP-MPP1.0 atlas (33). Additionally, total brain volume (TBV) and grey matter volumes (GMV) for 10 subcortical structures were extracted using FreeSurfer’s "aseg" parcellation.
White matter integrity was estimated using fractional anisotropy (FA) measures averaged across the full white matter skeleton and within tract-wise regions of interest from the intersection of the skeleton and the 27 regions in the Johns Hopkins University (JHU) white matter atlas (34).
Total volume of white matter hyperintensities (WMH) was calculated with UBO Detector (35).
Finally, the brain age gap estimate (brainAGE), or difference between chronological age predicted from MRI measures of brain structure and actual chronological age, was estimated using a pre-trained publicly available algorithm (29).
Covariates
Long-term cannabis users often regularly use other licit and illicit substances (36). Thus, we included covariates for persistent use of other substances in our models. Persistent alcohol and tobacco dependence were measured as described above. Additionally, persistent illicit drug use was defined as a diagnosis of dependence on illicit drugs (other than cannabis) at two or more waves from ages 26-45 (Supplemental Methods).
Statistical Analyses
To test whether long-term cannabis users exhibit global alterations in midlife brain structure, we used t-tests comparing long-term cannabis users to each comparison group on 6 sex-adjusted global brain structure measures: average CT, total SA, TBV, average FA, WMH volume, and brainAGE. To test for dose-response associations, we used ordinary least squares (OLS) regression with each of the 2 continuous cannabis use measures. To compare the effect sizes of the associations between persistence of cannabis use and brain structure to those of persistence of alcohol and tobacco use, we used OLS regression with these respective measures. All dose-response analyses were adjusted for sex and subsequently additionally adjusted for persistent dependence on other substances (cannabis, alcohol, tobacco, and illicit drugs, as appropriate). For each model, we corrected for multiple comparisons across the 6 tests performed for each brain measure using a false discovery rate (FDR) procedure (37). We report standardized beta coefficients.
We further probed regional subdivisions of 4 of the 6 global measures to examine whether associations with global measures may be driven by localized patterns of structural differences. Specifically, we conducted all group comparisons and dose-response analyses with CT and SA in the 360 regions comprising the HCP-MPP1.0 atlas(33), GMV in the 10 subcortical volumes, and FA in the 27 tracts as described above. Given the exploratory nature of our analyses, only results with pFDR<.05 were considered statistically significant. Analyses were conducted in R version 3.6.0 (38), pre-registered (https://sites.duke.edu/moffittcaspiprojects/files/2021/07/Knodt_2020a.pdf), and checked for reproducibility by an independent analyst.
Secondary Analyses
We conducted additional pre-registered analyses comparing long-term cannabis users to midlife recreational cannabis users and cannabis quitters, as well as dose-response analyses adjusting for childhood risk factors to assess any role that antecedents to substance-misuse might have in our results (Supplemental Methods). At the request of a reviewer, we additionally repeated the global dose-response analyses for each sex separately.
Results
Cohort Characteristics
Table 1 shows substance use patterns for the 875 Study members who underwent neuroimaging at age 45, along with basic demographic information and childhood risk factors for substance misuse. While the long-term alcohol and tobacco groups were free from regular cannabis use and cannabis dependence by design, long-term cannabis users had an elevated incidence of dependence on tobacco (23.5%), alcohol (20.7%), and other illicit drugs (15.9%) at age 45.
Table 1.
Sociodemographic characteristics and substance use involvement for the full imaging cohort as well as long-term cannabis users and three comparison groups within the imaging cohort. (Childhood variables are described in the Supplemental Methods.)
| Sociodemographics | Imaging Cohort (N=875) |
Long-Term Cannabis Users (N=82) |
Lifelong Cannabis Non-Users (N=192) |
Long-term Tobacco Users (N=70) |
Long-term Alcohol Users (N=56) |
|---|---|---|---|---|---|
| Male Sex, % (N) | 50.4 (441) | 64.6 (53) | 41.1 (79) | 40.0 (28) | 55.4 (31) |
| Childhood SES, M (SD) | 3.76 (1.13) | 3.40 (1.07) | 3.92 (1.17) | 3.18 (0.96) | 3.79 (1.19) |
| Childhood Self-Control Problems, M (SD) | −0.03 (0.95) | 0.36 (1.09) | −0.19 (0.88) | 0.44 (1.18) | 0.00 (0.92) |
| Childhood IQ, M (SD) | 100.7 (13.9) | 98.6 (13.6) | 101.4 (13.8) | 92.1 (14.0) | 98.1 (11.3) |
| Family History of Substance Dependence, M (SD) | 0.15 (0.17) | 0.20 (0.20) | 0.10 (0.13) | 0.19 (0.18) | 0.14 (0.15) |
| Age 45 Depression Diagnosis, % (N) | 16.0 (140) | 26.8 (22) | 8.3 (16) | 14.3 (10) | 30.4 (17) |
| Age 45 Anxiety Disorder Diagnosis, % (N) | 18.9 (165) | 22.0 (18) | 15.6 (30) | 35.7 (25) | 28.6 (16) |
| Substance Use at Age 45 | |||||
| Cannabis Frequencya, M (SD) | 25.8 (82.7) | 255.6 (116.7) | 0 (0) | 0.11 (0.5) | 0.32 (1.2) |
| Weekly Cannabis Use, % (N) | 9.7 (85) | 98.8 (81) | 0 (0) | 0 (0) | 0 (0) |
| Regular Cannabis Useb, % (N) | 6.1 (53) | 63.4 (52) | 0 (0) | 0 (0) | 0 (0) |
| Daily Tobacco Use, % (N) | 21.3 (186) | 64.6 (53) | 0 (0) | 100 (70) | 17.9 (10) |
| Weekly Alcohol Use, % (N) | 93.1 (814) | 91.5 (75) | 91.1 (175) | 90 (63) | 100 (56) |
| Cannabis Dependence, % (N) | 2.2 (19) | 23.5 (19) | 0 (0) | 0 (0) | 0 (0) |
| Tobacco Dependence, % (N) | 11.8 (103) | 45.1 (37) | 0 (0) | 52.2 (36) | 10.7 (6) |
| Alcohol Dependence, % (N) | 11.4 (100) | 20.7 (17) | 0 (0) | 8.6 (6) | 53.6 (30) |
| Illicit Drug Dependence, % (N) | 3.2 (28) | 15.9 (13) | 0 (0) | 1.4 (1) | 1.8 (1) |
Note.
Number of days used in past year.
Regular use = 4+ days per week.
Global Brain Structure in Long-Term Cannabis Users
Group comparisons revealed that compared to lifelong cannabis non-users, Study members who were long-term cannabis users had significantly thinner average cortex (pFDR=.007) and older brainAGE (pFDR<.001) (Table 2 and Table S1). Long-term cannabis users did not differ from non-users on cortical SA, TBV, average FA, or WMH volume. Long-term cannabis users did not differ from alcohol or tobacco users on the 6 global brain structure measures.
Table 2.
A comparison of long-term cannabis users and three comparison groups on global brain measures.
| Statistical Tests of Difference Between Long-term Cannabis Users and Comparison Groups |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Brain Measure |
Long-term Cannabis Users (N=80) |
Comparison Group 1: Lifelong Cannabis Non-Users (N=187) |
Comparison Group 2: Long-term Tobacco Users (N=68) |
Comparison Group 3: Long-term Alcohol Users (N=56) |
LT vs 1 |
LT vs 2 |
LT vs 3 |
||||
| M | 95% CI | M | 95% CI | M | 95% CI | M | 95% CI | pFDR (punc) | pFDR (punc) | pFDR (punc) | |
| CT | −.180 | −.378, .019 | .216 | .074, .357 | −.335 | −.585, −.084 | −0.197 | −.466, .073 | .007 (.002) | .621 (.338) | .919 (.919) |
| SA | −.135 | −.324, .053 | .027 | −.095, .150 | −.268 | −.459, −.077 | 0.013 | −.179, .205 | .209 (.155) | .621 (.337) | .497 (.295) |
| TBV | −.145 | −.321, .032 | .073 | −.056, .202 | −.221 | −.405, −.037 | −0.009 | −.191, .174 | .126 (.063) | .621 (.559) | .497 (.309) |
| FA | −.097 | −.334, .139 | .079 | −.052, .209 | −.010 | −.222, .202 | −0.148 | −.418, .122 | .209 (.174) | .621 (.596) | .919 (.785) |
| WMH | .108 | −.128, .344 | .053 | −.080, .187 | .194 | −.046, .433 | −0.077 | −.365, .212 | .675 (.675) | .621 (.621) | .497 (.331) |
| BrainAGE | .302 | .088, .516 | −.207 | −.337, −.077 | .449 | .197, .700 | 0.116 | −.141, .373 | <.001 (<.001) | .621 (.383) | .497 (.277) |
Note. Means and statistical tests of group comparisons are adjusted for sex and standardized on the full sample (M=0, SD=1). Raw (non-standardized) means are given in Table S1. Group Ns are slightly lower than reported in the methods section and vary slightly due to missing data and varying QC exclusions for brain measures. Bolded values indicate an FDR-adjusted (across 6 measures) statistically significant difference compared with long-term cannabis users. LT=Long-term cannabis users, CT=cortical thickness, SA=surface area, TBV=total brain volume, FA=fractional anisotropy, WMH=white matter hyperintensities, BrainAGE=difference between age estimated from MRI data and actual chronological age, pFDR=FDR-adjusted p-value, punc=uncorrected p-value.
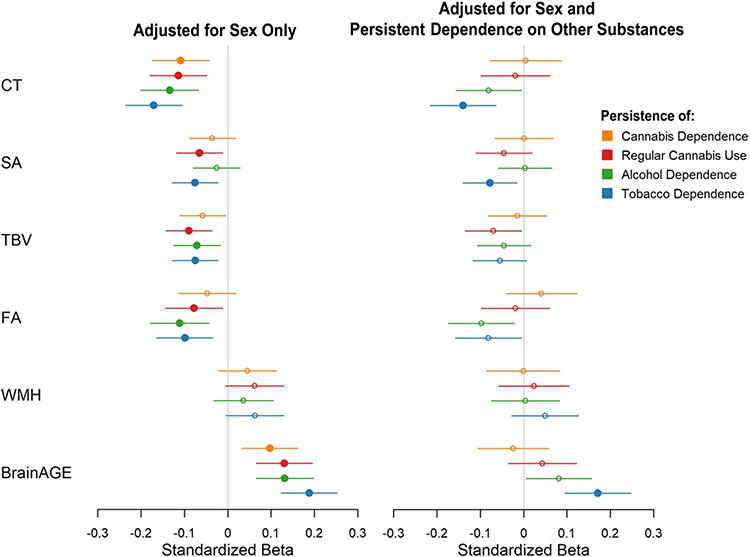
Tests of dose-response associations using the continuous measure of persistence of cannabis dependence again revealed significant associations with 2 of the 6 measures: Study members with more persistent dependence had thinner average cortex (pFDR=.007) and older brainAGE (pFDR=.010) than those with less persistent dependence (Figure 1, Table S2). Persistence of regular cannabis use showed significant associations with 5 of the 6 measures: Study members who used more persistently had thinner average cortex (pFDR=.002) and older brainAGE (pFDR=.001), along with smaller total cortical SA (pFDR=.024), smaller TBV (pFDR=.002), and lower average white matter FA (pFDR=.024) than those who used less persistently (Figure 1, Table S2). However, no associations survived adjustment for persistent dependence on other substances (all pFDR>.2).
Figure 1.
Visual representation of dose-response associations between persistence of cannabis, alcohol, and tobacco use from age 18-45 and global measures of midlife brain structure. Standardized betas are shown with adjustment for sex only, and with adjustment for sex and persistent dependence on other substances (for each of the three substances, this included persistence of dependence on the other two substances, as well as persistent dependence on other illicit drugs). Filled circles indicate p<.05 after FDR correction across 6 measures. CT=cortical thickness, SA=surface area, TBV=total brain volume, FA=fractional anisotropy, WMH=white matter hyperintensities, BrainAGE=difference between age estimated from MRI data and actual chronological age.
Global Brain Structure in Long-Term Alcohol and Tobacco Users
Persistence of alcohol dependence showed significant associations with 4 of the 6 measures: Study members with more persistent alcohol dependence had thinner average cortex (pFDR<.001), smaller TBV (pFDR=.015), lower average white matter FA (pFDR=.003), and older brainAGE (pFDR<.001) than those with less persistent dependence (Figure 1, Table S3). Again, however, no associations survived adjustment for persistent dependence on other substances (all pFDR>.07).
Persistence of tobacco dependence showed significant associations with 5 of the 6 measures: Study members with more persistent tobacco dependence had thinner average cortex (pFDR<.001), smaller total cortical SA (pFDR=.007), smaller TBV (pFDR=.007), lower average white matter FA (pFDR=.006), and older brainAGE (pFDR<.001) than those with less persistent dependence (Figure 1, Table S3). The associations between tobacco dependence and CT, SA, and brainAGE survived adjustment for other substances (all pFDR<.03).
Regional Brain Structure in Long-Term Cannabis Users
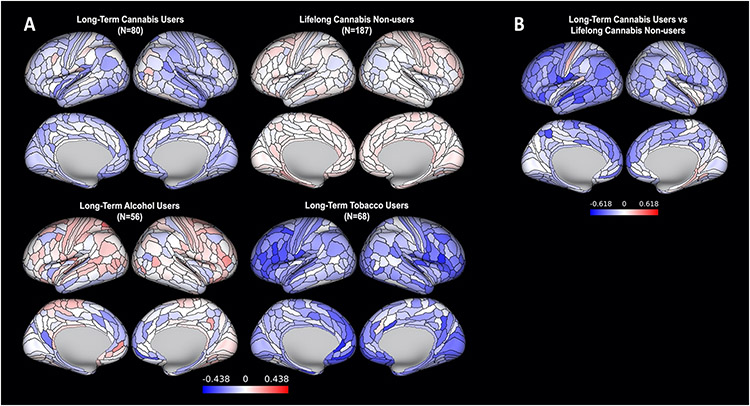
Group comparisons revealed that Study members who were long-term cannabis users had significantly thinner cortex than lifelong cannabis non-users in 34 of 360 regions (9%) (Figure 2). Long-term cannabis users did not differ from non-users in regional SA (Figure S2), subcortical GMV (Table S4), or tract-wise FA (Table S5). Long-term cannabis users did not differ from long-term alcohol or tobacco users on any regional brain structure measure.
Figure 2.
A comparison of long-term cannabis users and three comparison groups on regional cortical thickness. (A) Means for each group, adjusted for sex and standardized on the full sample (M=0, SD=1; blue regions are thinner than average and red regions are thicker than average). (B) Differences between long-term cannabis users and lifelong cannabis non-users (34 regions were significant at pFDR < .05, corrected across 360 regions). No other group comparisons revealed significant differences in regional cortical thickness. To allow for a comprehensive examination of effect sizes, maps have not been thresholded by significance.
Tests of dose-response associations revealed that Study members with more persistent cannabis dependence had thinner cortex than those with less persistent dependence in 52 of 360 regions (14%). Study members who regularly used cannabis more persistently had thinner cortex than those who used less persistently in 41 regions (11%; Dice similarity to regions associated with dependence: .67). However, no associations survived adjustment for persistent dependence on other substances (Figure 3a). Persistence of cannabis dependence and persistence of regular cannabis use were not associated with regional cortical SA. Persistence of regular cannabis use, but not persistence of cannabis dependence, was associated with smaller GMV in 4 of 10 subcortical regions: amygdala, hippocampus, thalamus, and ventral diencephalon. However, no associations survived adjustment for other substances (Figure S3). Finally, there were no associations between either continuous cannabis use measure and tract-wise FA (Figure S4).
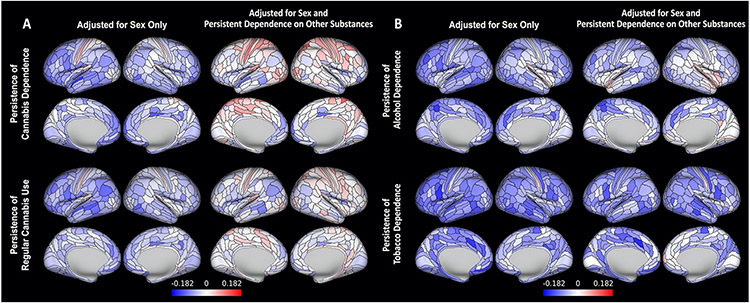
Figure 3.
Dose-response associations between persistence of (A) cannabis use and (B) alcohol and tobacco dependence from age 18-45 and regional measures of midlife cortical thickness, before and after adjustment for persistent dependence on other substances. Colors represent standardized betas, with blues reflecting negative associations with persistence of substance use. No regions had significant associations with persistence of cannabis use after adjustment for persistent use of other substances. To allow for a comprehensive examination of effect sizes, maps have not been thresholded by significance.
Regional Brain Structure in Long-Term Alcohol and Tobacco Users
Study members with more persistent alcohol dependence had thinner cortex than those with less persistent dependence in 120 regions (33%), but only two survived adjustment for persistent dependence on other substances (Figure 3b). Study members with more persistent alcohol dependence did not differ significantly from those with less persistent dependence in regional cortical SA or subcortical GMV (Figure S5); however, they had lower FA in 10 of 27 tracts (37%), including 6 that survived adjustment for other substances (Figure S6).
Study members with more persistent tobacco dependence had thinner cortex than those with less persistent dependence in 209 regions (58%), including 26 that survived adjustment for other substances (Figure 3b), along with smaller SA in 56 regions (16%), including 22 that survived adjustment for other substances (Figure S7). Study members with more persistent tobacco dependence had smaller GMV of the ventral diencephalon than those with less persistent dependence, but this association did not survive adjustment for other substances (Figure S5). These study members additionally had lower FA in 8 tracts (30%), including one that survived adjustment for other substances (Figure S6).
Secondary Analyses
We found no significant differences between long-term cannabis users and midlife recreational cannabis users or cannabis quitters (Tables S6-9 and Figure S8).
Secondary dose-response analyses revealed that associations between persistence of tobacco dependence and global CT and brainAGE survived additional adjustment for childhood risk factors (Table S10), as did associations between persistence of alcohol dependence and regional CT in 2 regions and persistence of alcohol dependence and regional FA in 6 tracts (Figures S9-10). Dose-response analyses with global brain measures separated by sex revealed patterns of associations similar to those in the full sample (Table S11).
Discussion
With increasing legalization and use of cannabis, it is important to examine the integrity of brain structures supporting cognitive functions in long-term users, particularly in midlife when cognitive reserves begin to shape aging trajectories. However, no clear picture of the structural brain integrity of long-term cannabis users, especially in midlife, has yet emerged, and questions remain regarding the role of polysubstance use in the manifestation of brain alterations. Here, we leveraged a large, population-representative birth cohort with comprehensive prospective substance use measures across five decades into midlife to address these issues. Our results advance knowledge in three ways.
First, long-term cannabis users had older brain age (i.e., higher brain age gap estimate) and thinner global and regional cortex than lifelong non-users. Moreover, tests of dose-response associations revealed that people who used cannabis more persistently had older brain age and thinner global and regional cortex than people who used cannabis less persistently or not at all, and further showed cannabis-related subcortical differences in areas demonstrating a high density of cannabinoid receptors, including the amygdala and hippocampus (10). However, no dose-response association was robust to adjustment for other substance use, and long-term cannabis users did not differ from long-term tobacco or alcohol users in any group comparison. These findings are consistent with recent large-sample studies (13,16) along with others failing to find cannabis-specific associations after careful control for alcohol use (18,39), though fewer cannabis studies have rigorously controlled for tobacco use. These findings are inconsistent with a recent study of 799 adolescents reporting associations between cannabis use and thinner prefrontal cortex even after accounting for alcohol and tobacco use (40). Another study of 89 cannabis-dependent individuals from the Human Connectome Project found grey and white matter differences in comparison with non-dependent individuals matched for alcohol use; however, this study was unable to also match for tobacco use (41). Our convergent findings across two analytic approaches for isolating cannabis effects suggest that inconsistencies reported across previous small, cross-sectional, and/or heterogeneous samples reflect, at least in part, effects of other substance use.
Importantly, our dose-response analyses were powered to detect small effects (Pearson’s r of .095), and group comparisons were powered to detect small-to-medium-sized differences (Supplemental Methods). Thus, it is unlikely that our analyses failed to identify even small alterations in long-term cannabis users. Further, we have detected cognitive deficits in long-term cannabis users in this cohort using the strategies employed here (5,6), suggesting that associations with structural brain integrity are much smaller or non-existent. For example, we recently reported on childhood-to-midlife IQ declines in Dunedin Study members that were unique to long-term cannabis users and, unlike the current structural brain associations, robust to adjustment for other substance use (6). Additionally, in this earlier study we specifically examined the hippocampus as an a priori region of interest, finding reduced GMV in long-term cannabis users. Notably, this association did not survive multiple comparison correction in the present comprehensive set of analyses. Taken together, the findings from our current and earlier studies suggest that not only could evidence pointing to unique effects of cannabis on the hippocampus be explained by the limited scope of prior analyses, but also that future work including well-powered neuroimaging studies of brain function and connectivity is needed to identify links between long-term cannabis use, cognitive impairment, and brain.
Second, we found dose-response associations between persistence of alcohol and tobacco dependence and structural brain integrity that were larger and more robust to covariate adjustment than those for cannabis use. Dose-response associations between persistence of alcohol dependence and both older brain age and thinner cortex had larger effect sizes than those for cannabis use. Persistence of alcohol dependence was additionally marginally associated with global white matter microstructural integrity, and significantly associated with regional integrity in 6 white matter tracts even after covariate adjustment. These findings are consistent with studies reporting grey matter differences in alcohol but not cannabis users (16,39,42) as well as studies consistently reporting associations between alcohol use and widespread alterations in white matter (43-45). For tobacco use, we found that continuous measures of use were more strongly associated with older brain age and thinner cortex than either cannabis or alcohol, surviving adjustments both for other substance use as well as childhood risks at the global level. Although there is evidence for associations between tobacco use and grey matter (46-49), our study represents one of few examining cannabis, alcohol, and tobacco use in the same cohort, and the dominant effect of tobacco use (rather than cannabis or alcohol) is relatively novel (16,50). This pattern is also consistent with a recent UK Biobank study reporting a slightly larger association between older brain age and tobacco use compared to alcohol use (51) (cannabis use was not assessed).
Finally, as most studies of cannabis use and the brain have been conducted in adolescents and young-adults with varying levels of use, our study shines much-needed light on the associations between substantial long-term cannabis use and brain structure in midlife, which has emerged as a critical platform in shaping how individuals experience aging in later life (52). It is reasonable to expect that effects of long-term cannabis use on the brain would be easier to detect in an older sample of persistent users, given accumulated use over many years. Indeed, we observed clear patterns of associations between long-term cannabis use and both thinner cortex and older brain age, but these were entirely explained by other substance use, suggesting there may not be mechanisms specific to the endocannabinoid system whereby cannabis use is associated with lasting alterations in structural brain integrity. Our comparatively robust finding of older midlife brain age in persistent tobacco users and, to a lesser extent, persistent alcohol users, is particularly relevant for ongoing efforts to ameliorate the impact of age-related disease, given that individuals with older brain age have increased risk for negative outcomes including accelerated cognitive decline and ADRD (29,31,32).
Our study has limitations. First, we lack structural brain integrity measures preceding initiation of cannabis use, as this cohort’s childhood predates neuroimaging technology. Future longitudinal neuroimaging studies will be essential for characterizing possible causal relationships between cannabis use and brain structure and for ruling out pre-existing alterations, as current evidence for structural brain alterations in cannabis users preceding onset of use is mixed (40,53-55). Second, cannabis use was self-reported as past-year number of days used, and a more fine-grained measure of exposure could have increased sensitivity and better captured patterns of polysubstance use. Additionally, biological assays could have helped detect under-reporting, though under-reporting for fear of admitting to illegal drug use is unlikely because Study members, interviewed repeatedly over a lifetime, have learned to trust the Study’s confidentiality guarantee (56). Third, high rates of polysubstance use prevented testing for effects specific to users of cannabis alone. However, dose-response analyses allowed for isolation of cannabis effects in more typical cannabis users through covariate control. Fourth, since all associations between cannabis use and brain structure were explained by other substance use, we do not provide an in-depth analysis on further potential confounds. Future work should seek to better understand relationships between cannabis use and other factors such as antecedents to substance use, comorbid illness, and genetic predisposition (19). Fifth, though secondary analyses revealed no differences between long-term cannabis users and cannabis quitters, an in-depth analysis of abstinence was additionally outside the scope of this work, and future work is needed to better understand the effects of cessation. Finally, our findings are based on data collected from a single New Zealand birth cohort who began using cannabis in the 1970s-80s. While Dunedin Study findings generally match findings from U.S. samples, concentrations of tetrahydrocannabinol (THC), the primary psychoactive constituent of cannabis, have risen in recent years (57). Therefore, if THC underlies associations, then cannabis-related brain differences reported here might reflect underestimates.
In summary, the detailed prospective substance use measures in our large dataset allowed for more accurate quantification of drug use than many existing studies, providing a uniquely comprehensive picture of the relative strengths of associations between cannabis, tobacco, and alcohol use with midlife structural brain integrity. This picture revealed that long-term cannabis users by midlife have widely-distributed alterations in brain structure including thinner cortex, lower subcortical GMV, and older brain age. Critically, however, all midlife structural brain alterations in long-term cannabis users were explained by their propensity to also use tobacco and alcohol, which had relatively outsized effects on brain structure. Thus, our findings collectively suggest that long-term cannabis use is not likely independently associated with midlife structural brain integrity. This emphasizes the importance of carefully accounting for polysubstance use in future studies of cannabis, brain, and behavior along with identifying convergent cellular and molecular pathways through which cannabis, alcohol, and tobacco exert effects on the brain. These findings may further help inform policy makers and health care providers as they weigh the impacts of long-term cannabis use relative to other substances, and seek to identify optimal policies for legalization and strategies for intervention, including mitigating risk for later-life ADRD.
Supplementary Material
Acknowledgements and Disclosures
This research received support from US-National Institute on Aging grants R01AG069939, R01AG032282, and R01AG049789 and UK Medical Research Council grant MR/P005918/1. We thank the Dunedin Study members, Unit research staff, and Study founder Phil Silva. The Dunedin Multidisciplinary Health and Development Research Unit is supported by the New Zealand Health Research Council (Programme Grant 16-604) and New Zealand Ministry of Business, Innovation and Employment (MBIE). The Dunedin Unit is within the Ngāi Tahu tribal area who we acknowledge as first peoples, tangata whenua (translation: people of this land).
ARK, MHM, TEM, ARH, and AC conceptualized and designed the current study. All authors were involved in data acquisition, analysis, or interpretation. ARK, MHM, TEM, ARH, and AC drafted the manuscript, and all authors provided critical revisions of the manuscript for important intellectual content. ARK and MZG conducted the statistical analyses. RP, TEM, ARH, and AC obtained funding for the study. Administrative and technical support were provided by AA, HH, DI, RP, and SR. The project was supervised by TEM, ARH, and AC.
We thank members of the Advisory Board for the Dunedin Neuroimaging Study, Dunedin Study members, unit research staff, Pacific Radiology Group staff, and Dunedin Study founder Phil Silva, Ph.D., University of Otago.
Footnotes
The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Gruber SA, Sagar KA (2017): Marijuana on the Mind? The Impact of Marijuana on Cognition, Brain Structure, and Brain Function, and Related Public Policy Implications. Policy Insights from the Behavioral and Brain Sciences. 4:104–111. [Google Scholar]
- 2.Degenhardt L, Ferrari AJ, Hall WD (2017): The Global Epidemiology and Disease Burden of Cannabis Use and Dependence. Handbook of Cannabis and Related Pathologies: Biology, Pharmacology, Diagnosis, and Treatment.89–100. [Google Scholar]
- 3.Poulton R, Robertson K, Boden J, Horwood J, Theodore R, Potiki T, et al. (2020): Patterns of recreational cannabis use in Aotearoa-New Zealand and their consequences: evidence to inform voters in the 2020 referendum. J Roy Soc New Zeal. 50:348–365. [Google Scholar]
- 4.Volkow ND, Swanson JM, Evins AE, DeLisi LE, Meier MH, Gonzalez R, et al. (2016): Effects of Cannabis Use on Human Behavior, Including Cognition, Motivation, and Psychosis: A Review. JAMA Psychiatry. 73:292–297. [DOI] [PubMed] [Google Scholar]
- 5.Meier MH, Caspi A, Ambler A, Harrington HL, Houts R, Keefe RSE, et al. (2012): Persistent cannabis users show neuropsychological decline from childhood to midlife. Proceedings of the National Academy of Sciences of the United States of America. 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meier MH, Caspi A, Knodt AR, Hall W, Ambler A, Harrington HL, et al. (2021): Long-term Cannabis Users Show Lower Cognitive Reserves and Smaller Hippocampal Volume in Midlife. American Journal of Psychiatry. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hall W (2015): What has research over the past two decades revealed about the adverse health effects of recreational cannabis use? Addiction. [DOI] [PubMed] [Google Scholar]
- 8.Sagar KA, Gruber SA (2018): Marijuana matters: reviewing the impact of marijuana on cognition, brain structure and function, & exploring policy implications and barriers to research. Int Rev Psychiatry. 30:251–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Batalla A, Bhattacharyya S, Yucel M, Fusar-Poli P, Crippa JA, Nogue S, et al. (2013): Structural and functional imaging studies in chronic cannabis users: a systematic review of adolescent and adult findings. PLoS One. 8:e55821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lorenzetti V, Solowij N, Yucel M (2016): The Role of Cannabinoids in Neuroanatomic Alterations in Cannabis Users. Biol Psychiatry. 79:e17–31. [DOI] [PubMed] [Google Scholar]
- 11.Lorenzetti V, Chye Y, Silva P, Solowij N, Roberts CA (2019): Does regular cannabis use affect neuroanatomy? An updated systematic review and meta-analysis of structural neuroimaging studies. European Archives of Psychiatry and Clinical Neuroscience. 269:59–71. [DOI] [PubMed] [Google Scholar]
- 12.Rocchetti M, Crescini A, Borgwardt S, Caverzasi E, Politi P, Atakan Z, et al. (2013): Is cannabis neurotoxic for the healthy brain? A meta-analytical review of structural brain alterations in non-psychotic users. Psychiatry and Clinical Neurosciences. 67:483–492. [DOI] [PubMed] [Google Scholar]
- 13.Orr JM, Paschall CJ, Banich MT (2016): Recreational marijuana use impacts white matter integrity and subcortical (but not cortical) morphometry. NeuroImage: Clinical. 12:47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bloomfield MAP, Hindocha C, Green SF, Wall MB, Lees R, Petrilli K, et al. (2019): The neuropsychopharmacology of cannabis: A review of human imaging studies. Pharmacology and Therapeutics. 195:132–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martín-Santos R, Fagundo AB, Crippa JA, Atakan Z, Bhattacharyya S, Allen P, et al. (2010): Neuroimaging in cannabis use: A systematic review of the literature. Psychological Medicine. 40:383–398. [DOI] [PubMed] [Google Scholar]
- 16.Mackey S, Allgaier N, Chaarani B, Spechler P, Orr C, Bunn J, et al. (2019): Mega-analysis of gray matter volume in substance dependence: General and substance-specific regional effects. American Journal of Psychiatry. 176:119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chye Y, Suo C, Lorenzetti V, Batalla A, Cousijn J, Goudriaan AE, et al. (2019): Cortical surface morphology in long-term cannabis users: A multi-site MRI study. Eur Neuropsychopharmacol. 29:257–265. [DOI] [PubMed] [Google Scholar]
- 18.Weiland BJ, Thayer RE, Depue BE, Sabbineni A, Bryan AD, Hutchison KE (2015): Daily marijuana use is not associated with brain morphometric measures in adolescents or adults. J Neurosci. 35:1505–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pagliaccio D, Barch DM, Bogdan R, Wood PK, Lynskey MT, Heath AC, et al. (2015): Shared Predisposition in the Association Between Cannabis Use and Subcortical Brain Structure. JAMA Psychiatry. 72:994–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stringer S, Minica CC, Verweij KJ, Mbarek H, Bernard M, Derringer J, et al. (2016): Genome-wide association study of lifetime cannabis use based on a large meta-analytic sample of 32 330 subjects from the International Cannabis Consortium. Transl Psychiatry. 6:e769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Degenhardt L, Hall W, Lynskey M (2001): The relationship between cannabis use and other substance use in the general population. Drug Alcohol Depend. 64:319–327. [DOI] [PubMed] [Google Scholar]
- 22.Weinstein G, Sznitman SR (2020): The implications of late-life cannabis use on brain health: A mapping review and implications for future research. Ageing Res Rev. 59:101041. [DOI] [PubMed] [Google Scholar]
- 23.Han BH, Palamar JJ (2018): Marijuana use by middle-aged and older adults in the United States, 2015-2016 (vol 191, pg 374, 2018). Drug Alcohol Depen. 192:171–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stern Y (2012): Cognitive reserve in ageing and Alzheimer's disease. Lancet Neurol. 11:1006–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whalley LJ, Deary IJ, Appleton CL, Starr JM (2004): Cognitive reserve and the neurobiology of cognitive aging. Ageing Res Rev. 3:369–382. [DOI] [PubMed] [Google Scholar]
- 26.McKetin R, Parasu P, Cherbuin N, Eramudugolla R, Anstey KJ (2016): A longitudinal examination of the relationship between cannabis use and cognitive function in mid-life adults. Drug Alcohol Depend. 169:134–140. [DOI] [PubMed] [Google Scholar]
- 27.Amen DG, Darmal B, Raji CA, Bao W, Jorandby L, Meysami S, et al. (2017): Discriminative Properties of Hippocampal Hypoperfusion in Marijuana Users Compared to Healthy Controls: Implications for Marijuana Administration in Alzheimer's Dementia. J Alzheimers Dis. 56:261–273. [DOI] [PubMed] [Google Scholar]
- 28.Elliott ML (2020): MRI-based biomarkers of accelerated aging and dementia risk in midlife: how close are we? Ageing Res Rev. 61:101075. [DOI] [PubMed] [Google Scholar]
- 29.Liem F, Varoquaux G, Kynast J, Beyer F, Kharabian Masouleh S, Huntenburg JM, et al. (2017): Predicting brain-age from multimodal imaging data captures cognitive impairment. Neuroimage. 148:179–188. [DOI] [PubMed] [Google Scholar]
- 30.Elliott ML, Belsky DW, Knodt AR, Ireland D, Melzer TR, Poulton R, et al. (2019): Brain-age in midlife is associated with accelerated biological aging and cognitive decline in a longitudinal birth cohort. Molecular Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Franke K, Ziegler G, Kloppel S, Gaser C, Alzheimer's Disease Neuroimaging I (2010): Estimating the age of healthy subjects from T1-weighted MRI scans using kernel methods: exploring the influence of various parameters. Neuroimage. 50:883–892. [DOI] [PubMed] [Google Scholar]
- 32.Cole JH, Franke K (2017): Predicting Age Using Neuroimaging: Innovative Brain Ageing Biomarkers. Trends Neurosci. 40:681–690. [DOI] [PubMed] [Google Scholar]
- 33.Glasser MF, Coalson TS, Robinson EC, Hacker CD, Harwell J, Yacoub E, et al. (2016): A multi-modal parcellation of human cerebral cortex. Nature. 536:171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mori S, Wakana S, van Zijl PCM, Nagae-Poetscher LM (2005): MRI atlas of human white matter. Elsevier Science. [Google Scholar]
- 35.Jiang J, Liu T, Zhu W, Koncz R, Liu H, Lee T, et al. (2018): UBO Detector - A cluster-based, fully automated pipeline for extracting white matter hyperintensities. Neuroimage. 174:539–549. [DOI] [PubMed] [Google Scholar]
- 36.Cohn AM, Johnson AL, Rose SW, Pearson JL, Villanti AC, Stanton C (2018): Population-level patterns and mental health and substance use correlates of alcohol, marijuana, and tobacco use and co-use in US young adults and adults: Results from the population assessment for tobacco and health. Am J Addict. 27:491–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Benjamini Y, Hochberg Y (1995): Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society: Series B (Methodological). [Google Scholar]
- 38.R Core Team (2013): R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 39.Harper J, Malone SM, Wilson S, Hunt RH, Thomas KM, Iacono WG (2021): The effects of alcohol and cannabis use on the cortical thickness of cognitive control and salience brain networks in emerging adulthood: a cotwin control study. Biological Psychiatry.1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Albaugh MD, Ottino-Gonzalez J, Sidwell A, Lepage C, Juliano A, Owens MM, et al. (2021): Association of Cannabis Use During Adolescence With Neurodevelopment. JAMA Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Manza P, Yuan K, Shokri-Kojori E, Tomasi D, Volkow ND (2020): Brain structural changes in cannabis dependence: association with MAGL. Molecular Psychiatry. 25:3256–3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thayer RE, YorkWilliams S, Karoly HC, Sabbineni A, Ewing SF, Bryan AD, et al. (2017): Structural neuroimaging correlates of alcohol and cannabis use in adolescents and adults. Addiction. 112:2144–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sutherland GT, Sheedy D, Kril JJ (2014): Neuropathology of alcoholism. Handb Clin Neurol. 125:603–615. [DOI] [PubMed] [Google Scholar]
- 44.Pfefferbaum A, Rosenbloom MJ, Chu W, Sassoon SA, Rohlfing T, Pohl KM, et al. (2014): White matter microstructural recovery with abstinence and decline with relapse in alcohol dependence interacts with normal ageing: a controlled longitudinal DTI study. Lancet Psychiatry. 1:202–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Monnig MA, Tonigan JS, Yeo RA, Thoma RJ, McCrady BS (2013): White matter volume in alcohol use disorders: a meta-analysis. Addict Biol. 18:581–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gray JC, Thompson M, Bachman C, Owens MM, Murphy M, Palmer R (2020): Associations of cigarette smoking with gray and white matter in the UK Biobank. Neuropsychopharmacology. 45:1215–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Azizian A, Monterosso J, O'Neill J, London ED (2009): Magnetic resonance imaging studies of cigarette smoking. Handb Exp Pharmacol.113–143. [DOI] [PubMed] [Google Scholar]
- 48.Karama S, Ducharme S, Corley J, Chouinard-Decorte F, Starr JM, Wardlaw JM, et al. (2015): Cigarette smoking and thinning of the brain's cortex. Mol Psychiatry. 20:778–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Elbejjani M, Auer R, Jacobs DR Jr., Haight T, Davatzikos C, Goff DC Jr. et al. (2019): Cigarette smoking and gray matter brain volumes in middle age adults: the CARDIA Brain MRI sub-study. Transl Psychiatry. 9:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gillespie NA, Neale MC, Bates TC, Eyler LT, Fennema-Notestine C, Vassileva J, et al. (2018): Testing associations between cannabis use and subcortical volumes in two large population-based samples. Addiction. 113:1661–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ning K, Zhao L, Matloff W, Sun F, Toga AW (2020): Association of relative brain age with tobacco smoking, alcohol consumption, and genetic variants. Sci Rep. 10:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moffitt TE (2020): Behavioral and Social Research to Accelerate the Geroscience Translation Agenda. Ageing Res Rev. 63:101146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cheetham A, Allen NB, Whittle S, Simmons JG, Yucel M, Lubman DI (2012): Orbitofrontal volumes in early adolescence predict initiation of cannabis use: a 4-year longitudinal and prospective study. Biol Psychiatry. 71:684–692. [DOI] [PubMed] [Google Scholar]
- 54.Wade NE, Bagot KS, Cota CI, Fotros A, Squeglia LM, Meredith LR, et al. (2019): Orbitofrontal cortex volume prospectively predicts cannabis and other substance use onset in adolescents. J Psychopharmacol. 33:1124–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jacobus J, Castro N, Squeglia LM, Meloy MJ, Brumback T, Huestis MA, et al. (2016): Adolescent cortical thickness pre- and post marijuana and alcohol initiation. Neurotoxicol Teratol. 57:20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meier MH, Hall W, Caspi A, Belsky DW, Cerda M, Harrington HL, et al. (2016): Which adolescents develop persistent substance dependence in adulthood? Using population-representative longitudinal data to inform universal risk assessment. Psychol Med. 46:877–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.ElSohly MA, Chandra S, Radwan M, Gon C, Church JC (2021): A Comprehensive Review of Cannabis Potency in the USA in the Last Decade. Biol Psychiatry Cogn Neurosci Neuroimaging. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.