Abstract
Reactive arthritis is usually a self-limiting polyarthritis which develops after certain gastrointestinal or urogenital infections. Microbial antigens found in the inflamed joints are thought to play a key role in the development of this disease. It is not known how antigens of the pathogenic organisms migrate from the mucosal tissues into the joints. The data presented here show that mononuclear phagocytes which mediate the dissemination of several intracellular pathogens acquire an enhanced capacity to bind to nonstimulated vascular endothelial cells after phagocytosis of Yersinia enterocolitica O:3, one of the causative organisms of reactive arthritis. The increased binding to previously nonstimulated endothelial cells was mediated by P-selectin, whose translocation to the endothelial cell surface was induced by monocytes with intracellular Yersinia bacteria. These results suggest that mononuclear phagocytes may be responsible for the dissemination of bacterial antigens and the initiation of the joint inflammation in reactive arthritis.
Reactive arthritis is triggered by gastrointestinal or urogenital infections caused by yersiniae, salmonellae, shigellae, campylobacters or chlamydiae. Previous studies have suggested that the microbes may persist in the bodies of arthritic patients, perhaps in the mucosa-associated lymphoid tissue (13, 18). Bacterial fragments found in the inflamed joints of patients with reactive arthritis are suggested to have a role in the pathogenesis of the disease (4, 10). It is, however, unclear how these antigens eventually migrate from the primary site of the infection at mucosal surfaces to the joints. Phagocytes, especially cells of the monocyte/macrophage lineage, may participate in the process, because these cells are long-living, are highly mobile, and have been shown to be involved in persistence and dissemination of intracellular pathogens (26). Mononuclear phagocytes are also very important effector cells and contribute to both the induction and maintenance of various inflammatory conditions (5, 26). For example, in the streptococcal cell wall model of arthritis, the prevention of phagocyte accumulation inhibits the development of chronic arthritis (41).
Adhesion molecules which have been shown to be involved in mediating migration of mononuclear phagocytes into the joints include P-selectin, E-selectin, and the leukocyte integrins LFA-1, Mac-1, and VLA-4 (15, 20). In this study we investigated the possibility that phagocytosis and processing of arthritis-triggering bacteria might modify the adhesion properties of human monocytes. We found that monocytes which had processed Yersinia enterocolitica O:3 acquired the capacity to bind to nonstimulated endothelial cells via P-selectin. Induction of P-selectin expression on endothelial cells by monocytes with intracellular bacterial components may be the first event to guide microbial antigens into previously healthy joints where the microvascular bed favors the binding of mononuclear phagocytes of peripheral blood (15).
MATERIALS AND METHODS
Bacteria.
The strain of Y. enterocolitica serotype O:3 used (4147/83) was a stool isolate from a patient developing reactive arthritis as a result of infection. The strain contains a virulence-associated 72-kb plasmid (9). The presence or absence of the virulence plasmid of Yersinia bacteria was verified by autoagglutination (28). A plasmid-cured derivative of Y. enterocolitica O:3 was obtained by cultivating the bacteria on a magnesium-oxalate agar (45). As a control bacterium we used Streptococcus pyogenes (strain 8184 from the American Type Culture Collection [ATCC]). S. pyogenes was chosen as a control bacterium because it can cause postinfection joint complications which are not linked to HLA-B27. S. pyogenes does not contain lipopolysaccharide (LPS). Stock cultures were maintained at −40°C in 20% (vol/vol) glycerol-Trypticase soy broth. The Yersinia bacteria were grown in RPMI 1640 medium mimicking the extracellular conditions or in Luria-Bertani broth. The resulting bacterial cultures were suspended in saline, harvested by centrifugation (20 min, 3,000 × g), and washed three times in saline. The bacteria were killed with heat (1 h, 100°C) and stored in phosphate-buffered saline (PBS) at 4°C. S. pyogenes was grown on blood agar plates for 2 days. Enteroinvasive Escherichia coli (strain RHE-3459 from the Central Public Health Laboratory, London, United Kingdom) was grown in Luria-Bertani broth like Y. enterocolitica. The resulting bacterial cultures were harvested, killed with heat, and suspended in saline.
Preparation of LPS.
Y. enterocolitica O:3 was cultivated in nutrient broth at room temperature overnight. The LPS extraction with hot phenol-water was carried out by the method of Westphal et al. (44) as modified by Hurvell (19). After treatment with proteinase K (100 μg/ml) (Boehringer, Mannheim, Germany), RNase (100 μg/ml) (Sigma, St. Louis, Mo.), and DNase (100 μg/ml) (Boehringer), the LPS preparation was free of contaminating proteins and nucleic acids. E. coli LPS (O55:B5) was purchased from Difco Laboratories (Detroit, Mich.).
Monocyte isolation.
Monocytes from healthy blood donors (Finnish Red Cross, Turku, Finland) were isolated as described previously (45). Briefly, human peripheral blood mononuclear cells were isolated by Ficoll-Paque gradient centrifugation (Pharmacia LKB Biotechnology AB, Uppsala, Sweden), and monocytes were allowed to adhere to plastic tissue culture chambers precoated with human AB serum (Finnish Red Cross) for 1 h. Thereafter, nonadherent cells were washed off. The purity of monocyte populations was ≥95% as analyzed by using morphological characteristics and, in several samples, by also using immunofluorescence staining of the monocyte-specific CD14.
Incubation with bacteria, latex beads, or LPS.
Monocytes were allowed to phagocytose the bacteria or latex particles (Bacto latex 0.81; Difco) in RPMI medium supplemented with 10% AB serum for 1 h, and then extracellular bacteria were washed off. We used about 200 Y. enterocolitica O:3 or 20 S. pyogenes or E. coli bacteria per monocyte. Lower doses of S. pyogenes or E. coli were used because these bacteria were more toxic to monocytes and doses higher than 20 bacteria per monocyte affected the viability of the cells, especially after prolonged incubation periods. The number of heat-killed bacteria which were phagocytosed by the monocytes was studied by the indirect immunofluorescence technique. Briefly, cytocentrifuge preparations containing monocytes which had been treated with bacteria for 1 h from three individuals were stained with acridine orange (Merck, Darmstadt, Germany). The slides were studied under a fluorescence microscope, and the bacteria in five fields containing a total of 220 to 520 monocytes were counted. Sixty-seven percent of the monocytes treated with Y. enterocolitica had intracellular bacteria, and the mean number of bacteria in each of those cells was about 10. In the case of S. pyogenes, the corresponding numbers were 41% and about 20 bacteria per monocyte, and for E. coli 48% of the monocytes had intracellular bacteria and there were about 5 bacteria per cell. Over 90% of the monocytes had intracellular bacterial antigens after prolonged incubation, as shown previously (46). LPS was used at a concentration of 10 μg per 106 monocytes. LPS not bound to monocytes was washed off after 1 h. Harvesting of the monocytes was done as for cells incubated with bacteria. Control monocytes were incubated otherwise in the same way but without any exogenous stimuli. The incubation times were 1 h and 1, 2, 3, 5, or 7 days. The cells were then detached by use of 5 mM EDTA in Ca2+- and Mg2+-free Hanks’ balanced salt solution and scraping with a rubber policeman. The monocytes were >90% viable as determined by trypan blue dye exclusion.
Immunofluorescence staining and flow cytometry.
Cells for flow cytometry were stained by using a double immunofluorescence technique as described previously (45). The monoclonal antibodies (MAbs) used in this study are listed in Table 1. Analyses were performed with a FACScan flow cytometer. Monocytes were gated according to their size and granularity. This correlated well with their expression of monocyte-specific antigen CD14. Routinely, 10,000 cells were analyzed per sample. During the incubation time, the monocytes matured. Monocytes incubated with bacteria were always compared to cells which had been incubated in the same way but without any exogenous stimuli.
TABLE 1.
Antibodies used in this study
| Designation | Class | Specificity | Reference and/or source |
|---|---|---|---|
| HP2/1 | IgG1 | VLA α4 chain (CD49d) | 38; Immunotech, Marseille, France |
| SAM1 | IgG2b | VLA β5 chain (CD49e) | Immunotech |
| P5D2 | IgG1 | β1-integrin (CD29) | 43; Developmental Studies Hybridoma Bank, Iowa City, Iowa |
| TS18 | IgG1 | LFA-1 β subunit (CD18) | 39; ATCC, Rockville, Md. |
| TS22 | IgG1 | LFA-1 α subunit (CD11a) | 39; ATCC |
| PL-1 | IgG1 | PSGL-1 (CD162) | 32; R. P. McEver |
| PL-2 | IgG1 | PSGL-1 (CD162) | 32; R. P. McEver |
| WAPS12.2 | IgG1 | P-selectin (CD62P) | E. Butcher |
| P8B1 | IgG2b | VCAM-1 (CD106) | 6; Developmental Studies Hybridoma Bank |
| 5C3 | IgG1 | ICAM-1 (CD54) | 1; our laboratory |
| FD705 | IgG2b | HLA-B27 | ;34; One Lambda Inc., Canoga Park, Calif. |
| 3C10 | IgG2b | CD14 | ATCC |
| Hermes-3 | IgG2a | CD44 | 22, 23 |
| MAb 543 | IgG1 | C3b receptor (CR1; CD35) | ATCC |
| 3G6 | IgG1 | Negative control (chicken T cells) | Our laboratory |
| G10 | IgG1 | Negative control (Ly-6C) | 16; our laboratory |
Endothelial cells.
Human umbilical vein endothelial cells (HUVEC) were obtained by collagenase digestion of umbilical cord veins by the method of Jaffe et al. (21). Detached endothelial cells were cultured in RPMI 1640 medium (Gibco, Paisley, United Kingdom) supplemented with 10% heat-inactivated fetal calf serum (PAA; Labor- und Forschungsgesellschaft GmbH, Linz, Austria), 1.8 mmol of l-glutamine (Biological Industries, Kibbutz Beit Herennek, Israel), 50 μg of gentamicin (G-mycin; Orion, Espoo, Finland) per ml, and 100 μg of streptomycin and 100 U of penicillin (Biological Industries) per ml in gelatin-coated plastic cell culture flasks at 37°C in 5% CO2. The cells grew to confluence in 3 to 5 days, at which stage they were detached with trypsin-EDTA (Gibco). Second- or third-passage cells were used. For stimulation of HUVEC to express intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1), 100 U of tumor necrosis factor alpha (TNF-α) (Sigma) per ml was used. To induce the expression of VCAM-1 only, 50 U of interleukin-4 (IL-4) (a generous gift from Juha Punnonen, DNAX, Palo Alto, Calif.) per ml was added 20 h before the experiments were performed. EAhy 926 endothelial cells (8) were cultured like HUVEC and stimulated with 100 U of TNF-α per ml for 20 h.
P-selectin induction and immunostaining.
Endothelial cells (EAhy 926) were cultured on eight-well chamber slides (Nunc, Roskilde, Denmark). Isolated monocytes were incubated with Y. enterocolitica O:3, S. pyogenes, E. coli, or inert latex particles overnight. The monocytes were then detached and washed three times with Hanks’ balanced salt solution. The separate wells with confluent layers of endothelial cells were overlaid with 2 × 107 monocytes which had been incubated with bacteria or latex beads, or with control monocytes, and incubated at 37°C for 20 min. Several other amounts of monocytes per well, ranging from 1 × 106 to 2 × 107, were tested, and the more monocytes were added, the brighter was the P-selectin-specific staining. Conditioned media (200 μl/well) from different monocytes were used to stimulate the endothelial cells in separate wells to reveal the role of long-lived soluble mediators. After that, the monocytes and the conditioned media were removed by washing the chambers three times with ice-cold PBS containing 0.2% bovine serum albumin (BSA) (INC Biomedicals, Irvine, Calif.) (BSA-PBS). To study whether the P-selectin expression was induced by direct cell-cell contacts or by soluble short-lived products, the EAhy 926 endothelial cells were grown on coverslips in the lower chambers of a Transwell cell culture system (Costar, Cambridge, Mass.). Monocytes treated with Y. enterocolitica O:3 bacteria were put on grids of the upper chamber of the Transwell system or directly on the endothelial cells. The monocytes were removed after 20 min of incubation. The endothelial cells were stained with anti-P-selectin MAb (a negative control MAb) and incubated on ice at 4°C for 20 min. After three washes with BSA-PBS, fluoresceinated second-stage immunoglobulin (Ig) (Sigma Chemical Co.) was added. After 20 min of incubation, the chambers were washed again three times with BSA-PBS and fixed with 1% formaldehyde (Merck) in PBS. The slides were mounted with PBS-glycerol (1:9 [vol/vol]) which contained 1 mg of p-phenylenediamine (Sigma) per ml and studied under a Dialux 20 fluorescence microscope (Leitz, Wetzlar, Germany).
Adhesion to endothelial cells.
Endothelial cells were cultured in 96-well tissue culture plates and allowed to reach confluence. Results with HUVEC and EAhy 926 cells were comparable. In the studies of the role of β1-integrins, only HUVEC were used because only a small population of EAhy 926 cells expressed VCAM-1 after stimulation with TNF-α or IL-4. The increased expression of VCAM-1 and ICAM-1 on stimulated endothelial cells was confirmed by immunofluorescence. Monocytes incubated with Yersinia bacteria and control monocytes were labeled with 15 μg of bis-carboxyethylcarboxyfluorescein (BCECF-AM) (Lambda Fluorezenztechnologie, Graz, Austria) per ml at 37°C for 25 min. Thereafter, the monocytes were washed two times and incubated with human gamma globulin (20 μg/ml; Finnish Red Cross, Helsinki, Finland) for 20 min to block the Fc receptors and washed again two times. The monocytes were then incubated with saturating concentrations of function-blocking or negative control MAbs for 20 min. A total of 2 × 105 monocytes were added to each well in 200 μl of 10% fetal calf serum–RPMI 1640 and allowed to adhere at 37°C for 20 min. Blocking antibodies were present during the adhesion. The fluorescence of cells added to each well was measured with a Fluoroskan II fluorometer (Labsystems, Helsinki, Finland). Next, unbound cells were removed by washing the plates twice with RPMI 1640 medium, and the proportion of the fluorescence of the monocytes bound to each well was measured with the fluorometer. The percentage of monocytes binding to endothelial cells was calculated from the input fluorescence value of each well. In individual assays the percentage of monocytes binding to endothelial cells was 40% ± 15%. The results are expressed as relative binding ratios. The control binding was arbitrarily given the value 1.0.
Matrix adhesion assays.
Ninety-six-well microtiter plates were incubated with 10 μg of human plasma fibronectin (Sigma) per ml or with the chymotryptic fragments FN-120 (cell binding domain) or FN-40 (heparin II binding domain) (Calbiochem, La Jolla, Calif.) in PBS at 37°C for 2 h and then with 1% BSA–PBS at 37°C for 1 h. Next, 2 × 105 BCECF-AM-labelled monocytes treated with Yersinia bacteria and control monocytes were added to the wells and incubated at 37°C for 20 min. After that, the fluorescence of the monocytes added to each well was measured with the fluorometer, unbound cells were removed by washing the plates, and the fluorescence of the monocytes bound to each well was measured. The percentage of monocytes adherent to fibronectin was calculated from the input fluorescence value of each well. In individual assays the percentage of monocytes binding to fibronectin or the fibronectin fragments was 50% ± 22%. The results are expressed as relative binding ratios. The control binding to 1% gelatin was arbitrarily given the value 1.0.
Statistical analysis.
Statistically significant differences between monocytes incubated with Yersinia bacteria and control monocytes were determined by using a two-tailed paired Student t test (42).
RESULTS
The role of P-selectin in adhesion of monocytes incubated with Yersinia bacteria.
Numerous phagocytes containing antigens of arthritis-triggering microbes are found in inflamed joints of patients with reactive arthritis. We wanted to see whether phagocytosis and processing of one of the arthritis-triggering microbes, Y. enterocolitica O:3, would modulate the binding of mononuclear phagocytes to vascular endothelial cells. Monocytes incubated with Yersinia bacteria, but not those incubated with S. pyogenes, E. coli, LPS of E. coli, LPS of Y. enterocolitica O:3, or latex particles, showed statistically significant increases in binding to nonstimulated endothelial cells after 1 and 7 days of incubation (Fig. 1). The short duration of the adhesion experiment excluded the contributions of many adhesion molecules in mediating the increased binding. The only known molecule which can be upregulated within minutes and mediate the binding of monocytes to nonstimulated endothelial cells is P-selectin. Therefore, we analyzed whether a MAb against P-selectin blocks the binding of monocytes incubated with Yersinia bacteria to endothelial cells. The ability of a MAb against a functional epitope of P-selectin to inhibit the adhesion of monocytes incubated with Yersinia bacteria was demonstrated. Furthermore, monocytes which had processed Y. enterocolitica O:3 for 1 day in vitro also mobilized P-selectin from the intracellular storage granules to the surfaces of endothelial cells (Fig. 2). The effect was dose dependent, since the inhibition of adhesion by the MAb against P-selectin and the intensity of the staining of endothelial cells increased gradually when more monocytes incubated with Yersinia bacteria were added to the endothelial cell cultures (not shown). P-selectin upregulation was seen with both virulence plasmid-positive and -negative Y. enterocolitica O:3 bacteria, indicating that the factors responsible for the adhesion were chromosomally encoded. P-selectin upregulation was most probably mediated by short-lived soluble products. The participation of long-lived mediators was excluded by the fact that conditioned medium of the monocytes did not induce the expression of P-selectin. Direct cell-cell contacts were not necessary, because P-selectin expression on endothelial cells was induced when Yersinia-treated monocytes were incubated on grids above the endothelial cells growing in the lower wells of the Transwell cell culture system (not shown). Monocytes incubated with inert latex particles and enteroinvasive E. coli did not have the capacity to induce P-selectin-mediated adhesion (data not shown). We also studied the expression of P-selectin glycoprotein ligand-1 (PSGL-1) on monocytes incubated with Y. enterocolitica O:3 bacteria by using two different MAbs. The PSGL-1 expression on monocytes was not modified by Yersinia bacteria (Fig. 3). It is, however, possible that phagocytosis and processing of Y. enterocolitica may have affected the functionally active population of PSGL-1 molecules.
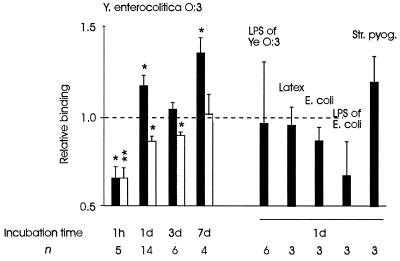
FIG. 1.
Phagocytosis and processing of Y. enterocolitica (Ye) alters the binding of monocytes to cultured endothelial cells. Results are expressed as mean relative adherence ratios ± standard errors of the means. Incubation time refers to the length of time that the monocytes were allowed to process the bacteria. Black bars and white bars indicate the binding of monocytes to nonstimulated endothelial cells and to endothelial cells which have been stimulated with TNF-α, respectively. The binding of adherent control monocytes not incubated with bacteria was given the value 1.0. An increase in relative binding from 1.0 to 1.3 means that the number of adhering monocytes increases by 30%. The increased binding by Yersinia-treated monocytes was studied with monocytes of 14 individuals in four separate experiments. ∗, P < 0.05; ∗∗, P < 0.005.
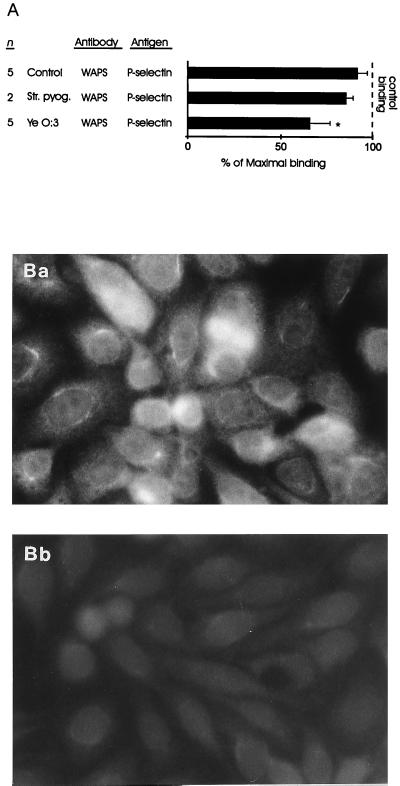
FIG. 2.
(A) MAb against P-selectin inhibits statistically significantly the binding of monocytes incubated with Yersinia bacteria (Ye), but not the adherence of S. pyogenes-treated or control monocytes, to nonstimulated endothelial cells. Endothelial cells were incubated with the inhibitory MAb against P-selectin or with the negative control (MAb 543). Thereafter, the binding of monocytes to EAhy 926 endothelial cells was analyzed. Results are expressed as mean percentages of maximal binding ± standard errors of the means, in which the number of adherent monocytes in the presence of the negative control antibody defines 100% binding. The incubation time for the monocytes after feeding of the bacteria was 1 day. The increased inhibition with the MAb against P-selectin correlated to the enhanced binding of monocytes to nonstimulated endothelial cells after 1 day of incubation (Fig. 1). ∗, P < 0.05. (B) Monocytes incubated with Yersinia bacteria induce the expression of P-selectin on cultured endothelial cells, but control monocytes do not. EAhy 926 endothelial cells were incubated with Yersinia-treated (Ba) or control (Bb) monocytes for 20 min, and then the monocytes were washed away. P-selectin molecules on adherent endothelial cells were stained without fixation by using immunofluorescence.
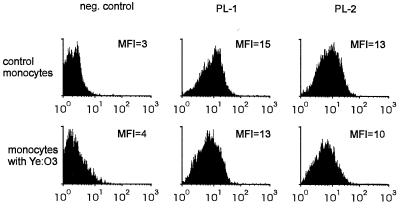
FIG. 3.
Representative experiment showing that incubation of monocytes with Yersinia bacteria does not change the expression of PSGL-1. Monocytes were treated with heat-killed Y. enterocolitica (Ye) O:3 bacteria and incubated for 1 day. The cells were stained with two different MAbs against PSGL-1 (PL-1 and PL-2) or with a negative (neg.) control MAb. MFI, mean fluorescence intensity.
Expression and function of other adhesion molecules known to contribute to the monocyte binding in arthritis.
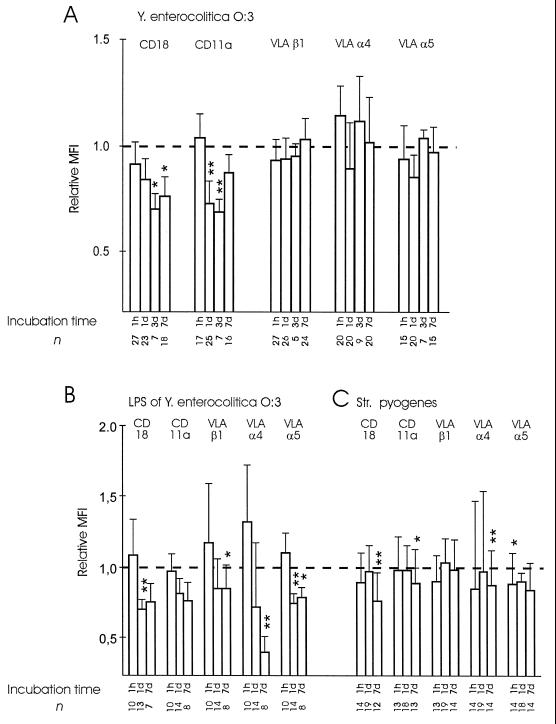
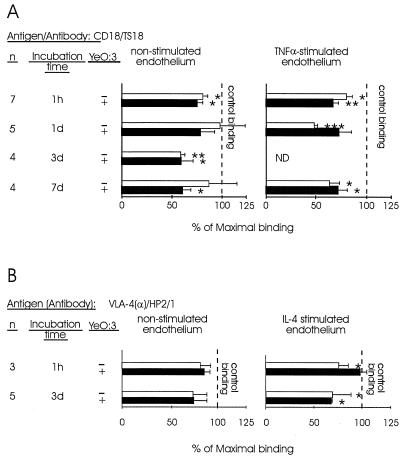
The binding of monocytes incubated with Y. enterocolitica O:3 to cytokine-activated endothelial cells was less than the binding of control monocytes for 3 days after Yersinia bacteria were phagocytosed (Fig. 1). In searching for reasons for this unexpected finding, we studied the expression of adhesion molecules which have been shown to be important in the binding of monocytes to cytokine-activated endothelial cells. We found that the expression of the CD11a molecule, which is important in mediating this binding, was significantly reduced (Fig. 4). Y. enterocolitica O:3 did not decrease the expression of β1-integrins α4 and α5 on human monocytes. The binding of monocytes to nonstimulated endothelial cells was inhibited with MAbs against CD11a and CD18. Anti-CD11a MAb gave an inhibition pattern similar to that of a MAb against CD18 (not shown). However, no significant differences between monocytes incubated with Yersinia bacteria and control monocytes in the relative contributions of these molecules to the binding were seen (Fig. 5).
FIG. 4.
Changes in the expression of adhesion molecules on monocytes incubated with Y. enterocolitica O:3, S. pyogenes, or LPS of Y. enterocolitica O:3. (A) Y. enterocolitica O:3 downregulates the expression of CD11a but not the expression of β1-integrins VLA-α4 and VLA-α5. (B) The effect of Y. enterocolitica on the expression of β2-integrins seemed to be at least partially mediated by LPS. (C) S. pyogenes was able to slightly reduce the expression of α4 and α5 integrins. Incubation time refers to the length of time that the monocytes were allowed to process the bacteria. Results are counted as net mean fluorescence intensity (MFI) values (negative control value subtracted from the experimental value) ± standard errors of the means and expressed as relative mean fluorescence intensities, in which the mean fluorescence intensity of the control monocytes was given the value 1.0. ∗, P < 0.05; ∗∗, P < 0.005.
FIG. 5.
MAbs against CD18 and VLA-4 inhibit the binding of monocytes to endothelial cells, but there is no significant difference in the relative contributions of these molecules in binding of monocytes incubated with Yersinia (Ye) bacteria and control monocytes. Monocytes were incubated with inhibitory antibodies against CD18 and VLA-α4 or with a negative control (MAb 543). Thereafter, the binding of monocytes to HUVEC or EAhy 926 cells (A) and to HUVEC (B) was analyzed. The results obtained with HUVEC and EAhy 926 cells were comparable, and therefore they were combined in panel A. Incubation time refers to the length of time that the monocytes were allowed to process the bacteria. Results after each incubation time are expressed as a mean percentage of maximal binding ± standard error of the mean, in which the number of adherent monocytes in the presence of the negative control antibody defines 100% binding. ∗, P < 0.05; ∗∗, P < 0.005; ∗∗∗, P < 0.0005.
S. pyogenes was able to reduce to some extent the expression of the α4 and α5 integrins. Differences in the capacities of Y. enterocolitica O:3 and S. pyogenes to change the expression of adhesion molecules led us to treat monocytes with isolated LPS of Y. enterocolitica O:3. We wanted to see whether LPS, which is an important component of the outer surface of Yersinia bacteria but is not present in S. pyogenes, was the cause of the observed difference. The effects of Y. enterocolitica O:3 could also be obtained with isolated LPS (Fig. 4). Interestingly, isolated LPS also effectively inhibited the expression of α4 and α5.
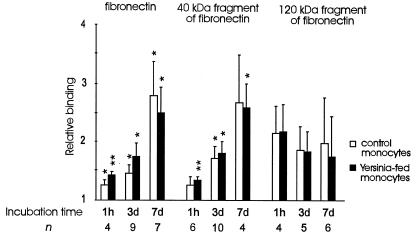
Cell-matrix interactions are important in regulating recruitment and migration of cells in tissues. We studied the binding of monocytes incubated with Yersinia bacteria to the extracellular matrix molecule fibronectin, which is a ligand for VLA-4 and VLA-5. Monocytes which had phagocytosed Yersinia bacteria bound more avidly than the control monocytes to fibronectin after 1 h of incubation (P < 0.05). This was the time of minimal endothelial cell binding (Fig. 1). Statistically, the fibronectin binding was mediated mainly by the heparin II binding domain of the molecule (Fig. 6).
FIG. 6.
Phagocytosis of Y. enterocolitica O:3 increases the binding of monocytes to fibronectin at early time points. Incubation time refers to the length of time that the monocytes were allowed to process the bacteria. Results (means ± standard errors of the means) are expressed as relative adherence ratios, in which the binding of monocytes to 1% gelatin is given the value 1.0. ∗, P < 0.05; ∗∗, P < 0.005.
Role of HLA-B27.
Thirty-two percent of the samples studied (18 of 75) were from HLA-B27-positive individuals, but they did not differ from the HLA-B27-negative ones. Therefore, the data from the HLA-B27-positive and the HLA-B27-negative samples are combined. The growth conditions of the bacteria also did not have any effect on the results (data not shown).
DISCUSSION
We observed that monocytes incubated with Yersinia bacteria acquired the capacity to bind to nonactivated vascular endothelial cells via P-selectin. The inhibition observed with a MAb against P-selectin was usually 30 to 40%. Greater inhibition would not be expected, because we used human monocytes which adhere to endothelial cells by using several different adhesion molecules. This inhibition means that one-third of the monocytes which had phagocytosed Y. enterocolitica O:3 used P-selectin in binding to previously nonstimulated endothelial cells. Moreover, in general the sum of the inhibition percentages of individual antibodies may well exceed 100% due to the multistep nature of leukocyte-endothelial cell interactions. The role of P-selectin in binding of Yersinia-treated monocytes is further strengthened by our unpublished observations showing that monocytes which have phagocytosed Y. enterocolitica O:3 but not control monocytes roll on nonstimulated endothelial cells.
The induction of P-selectin expression increased after the monocytes incubated with Yersinia bacteria had been in contact with the endothelial cells for only 20 min. This suggests that the increase of P-selectin expression could not depend on de novo protein synthesis. Rather, P-selectin was rapidly translocated to the cell surface from intracellular storage granules, the Weibel-Palade bodies, of endothelial cells (3, 29). Such a rapid P-selectin expression is induced by, for example, histamine, thrombin, and oxygen radicals (17, 31, 33). The results of this study suggest that in our system certain short-lived soluble mediators which were produced by monocytes incubated with Yersinia bacteria were responsible for the rapid mobilization of P-selectin. LPS could not have any major role, because enteroinvasive E. coli bacteria or LPS isolated from Y. enterocolitica O:3 or E. coli could not increase binding of monocytes to nonstimulated endothelial cells. Experiments clarifying the mechanisms of induction of P-selectin expression are in progress.
Numerous phagocytes with antigens of the arthritis-triggering organisms have been found in the peripheral blood of patients with reactive arthritis (12). This shows that in reactive arthritis phagocytes are able to leave the gut and enter the peripheral circulation via lymphatics or retrogradely through the vascular wall (25, 35). Other factors, like the specific properties of synovial vessels (40) and the adhesion molecules responsible for synovium-specific homing (24, 36), will contribute to the guiding of the monocytes from the mucosal areas into the joints. We observed that the change in the adhesion capacity appeared relatively soon after monocytes had phagocytosed the bacteria. This is well in line with animal models of Yersinia-induced arthritis, where antigens of the triggering microbes can be found in the joints already on the third day after the primary infection (14). In patients with reactive arthritis, bacterial antigens have been found in the first synovial fluid samples taken from the inflamed joints, which means that bacterial fragments are in the joints in large quantities already 1 week after the onset of the disease (11). A small number of phagocytes probably have entered the joints long before that.
The capacity of bacteria to induce the binding of monocytes to endothelial cells seems to be at least to some extent microbe specific, because monocytes incubated with Y. enterocolitica showed increased binding to nonstimulated endothelial cells but monocytes incubated with enteroinvasive E. coli did not. On the other hand, the expression of β2-integrins and consequently the adhesion of monocytes incubated with Yersinia bacteria to cytokine-stimulated endothelial cells expressing ICAM-1 was reduced. This suggests that different microbes can induce the binding of cells by different adhesion molecules. Previous studies have shown that S. pneumoniae can induce a CD18-independent emigration of polymorphonuclear leukocytes (PMN) into the peritoneum and lungs but that E. coli does not do this. This non-CD18-dependent mechanism of PMN emigration is augmented by the presence of macrophages (30). Mediators secreted by the macrophages in certain organs were suggested to induce PMN adherence by a CD18-independent mechanism (7), but the adhesion molecules involved in this CD18-independent pathway have not been characterized.
We monitored the usage of adhesion molecules for 1 week, and the situation may change thereafter. Most of the studies concerning involvement of adhesion molecules mediating leukocyte migration into the joints in humans have been conducted with already chronically inflamed synovium (15) and are not illustrative of the primary events. On the other hand, all molecules mediating later interactions may not participate in our experimental system. MAbs against P-selectin have been shown to block almost completely the binding of human gut-derived (37) and peripheral blood monocytes (15) to chronically inflamed synovium. This shows that the crucial role of P-selectin in mediating adhesion of monocytes to specialized synovial endothelial cells is even more important in vivo than in our experimental in vitro system. In addition to monocytes, P-selectin is also used by Th1 cells, the main T-lymphocyte subtype present in inflamed joints of patients with reactive arthritis, in binding to synovium (2, 27).
Our results show for the first time that bacteria can change the adhesion of human monocytes to vascular endothelial cells via a certain adhesion molecule, P-selectin. This may have an essential role in initiating and maintaining the arthritis and may even open new possibilities for prevention and treatment of both acute and chronic forms of joint inflammation. Further studies will reveal whether similar changes in the adhesion also operate in other diseases, such as atherosclerosis, in which the recruitment of mononuclear phagocytes has a role in the pathogenesis and a microbial etiology has been suggested.
ACKNOWLEDGMENTS
We thank Juha Punnonen, DNAX, Palo Alto, Calif., for IL-4; Eugene Butcher, Stanford University, for the MAb against P-selectin; R. P. McEver, University of Oklahoma, for the MAbs against PSGL-1; and the staff of the Labor and Delivery Unit of the University Central Hospital of Turku for providing the umbilical cords.
This work was supported by the Academy of Finland, the Technology Development Centre of Finland, the Finnish Rheumatism Research Foundation, the Sigrid Jusélius Foundation, the Maud Kuistila Foundation, the Turku University Foundation, the Finnish Medical Foundation, The European Commission Biomed 2 Programme, the Gastroenterology Research Foundation, and a Syntex Rheumatology Research Scholarship (to M. Wuorela).
REFERENCES
- 1.Airas L, Salmi M, Jalkanen S. Lymphocyte-vascular adhesion protein-2 is a novel 70-kDa molecule involved in lymphocyte adhesion to vascular endothelium. J Immunol. 1993;151:4228–4238. [PubMed] [Google Scholar]
- 2.Austrup F, Vestweber D, Borges E, Löhning M, Bräuer R, Herz U, Renz H, Hallmann R, Scheffold A, Radbruch A, Hamann A. P- and E-selectin mediate recruitment of T-helper-1 but not T-helper-2 cells into inflamed tissues. Nature. 1997;385:81–83. doi: 10.1038/385081a0. [DOI] [PubMed] [Google Scholar]
- 3.Bonfanti R, Furie B C, Furie B, Wagner D D. PADGEM (GMP140) is a component of Weibel-Palade bodies of human endothelial cells. Blood. 1989;73:1109–1112. [PubMed] [Google Scholar]
- 4.Burmester G R, Daser A, Kamradt T, Krause A, Mitchison N A, Sieper J, Wolf N. Immunology of reactive arthritides. Annu Rev Immunol. 1995;13:229–250. doi: 10.1146/annurev.iy.13.040195.001305. [DOI] [PubMed] [Google Scholar]
- 5.Burmester G R, Stuhlmuller B, Rittig M. The monocyte/macrophage system in arthritis—leopard tank or Trojan horse? Scand J Rheumatol. 1995;24:77–82. doi: 10.3109/03009749509100905. [DOI] [PubMed] [Google Scholar]
- 6.Dittel B N, McCarthy J B, Wayner E A, LeBien T W. Regulation of human B-cell precursor adhesion to bone marrow stromal cells by cytokines that exert opposing effects on the expression of vascular cell adhesion molecule-1 (VCAM-1) Blood. 1993;81:2272–2282. [PubMed] [Google Scholar]
- 7.Doerschuk C M, Winn R K, Coxson H O, Harlan J M. CD18-dependent and -independent mechanisms of neutrophil emigration in the pulmonary and systemic microcirculation of rabbits. J Immunol. 1990;144:2327–2333. [PubMed] [Google Scholar]
- 8.Edgell C-J S, McDonald C C, Graham J B. Permanent cell line expressing human factor VIII-related antigen established by hybridization. Proc Natl Acad Sci USA. 1983;80:3734–3737. doi: 10.1073/pnas.80.12.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gemski P, Lazere J R, Casey T. Plasmid associated with pathogenicity and calcium dependency of Yersinia enterocolitica. Infect Immun. 1980;27:682–685. doi: 10.1128/iai.27.2.682-685.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Granfors K. Do bacterial antigens cause reactive arthritis? Rheum Dis Clin N Am. 1992;18:37–48. [PubMed] [Google Scholar]
- 11.Granfors K, Jalkanen S, von Essen R, Lahesmaa-Rantala R, Isomäki O, Pekkola-Heino K, Merilahti-Palo R, Saario R, Isomäki H, Toivanen A. Yersinia antigens in synovial-fluid cells from patients with reactive arthritis. N Eng J Med. 1989;320:216–221. doi: 10.1056/NEJM198901263200404. [DOI] [PubMed] [Google Scholar]
- 12.Granfors K, Merilahti-Palo R, Luukkainen R, Möttönen T, Lahesmaa R, Probst P, Märker-Hermann E, Toivanen P. Yersinia antigens in peripheral blood cells from patients with Yersinia enterocolitica O:3 infection with or without reactive arthritis. Arthritis Rheum. 1998;41:855–862. doi: 10.1002/1529-0131(199805)41:5<855::AID-ART12>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 13.Granfors K, Toivanen A. IgA-anti-Yersinia antibodies in yersinia triggered reactive arthritis. Ann Rheum Dis. 1986;45:561–565. doi: 10.1136/ard.45.7.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gripenberg-Lerche C, Skurnik M, Zhang L, Söderström K-O, Toivanen P. Role of YadA in arthritogenicity of Yersinia enterocolitica serotype O:8 experimental studies with rats. Infect Immun. 1994;62:5568–5575. doi: 10.1128/iai.62.12.5568-5575.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grober J S, Brown B L, Ebling H, Athey B, Thompson C B, Fox D A, Stoolman L M. Monocyte-endothelial adhesion in chronic rheumatoid arthritis. In situ detection of selectin and integrin-dependent interactions. J Clin Invest. 1993;91:2609–2619. doi: 10.1172/JCI116500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hänninen A, Jaakkola I, Salmi M, Simell O, Jalkanen S. Ly-6C regulates endothelial adhesion and homing of CD8+ T cells by activating integrin-dependent adhesion pathways. Proc Natl Acad Sci USA. 1997;94:6898–6903. doi: 10.1073/pnas.94.13.6898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hattori R, Hamilton K K, Fugate R D, McEver R P, Sims P J. Stimulated secretion of endothelial von Willebrand factor is accompanied by rapid redistribution to the cell surface of the intracellular granule membrane protein GMP-140. J Biol Chem. 1989;264:7768–7771. [PubMed] [Google Scholar]
- 18.Hoogkamp-Korstanje J A, de Koning J, Heesemann J. Persistence of Yersinia enterocolitica in man. Infection. 1986;16:81–85. doi: 10.1007/BF01644307. [DOI] [PubMed] [Google Scholar]
- 19.Hurvell B. Serological cross-reactions between different Brucella species and Yersinia enterocolitica. Biological and chemical investigations of lipopolysaccharides from Brucella abortus and Yersinia enterocolitica type IX. Acta Pathol Microbiol Immunol Scand Sect B. 1973;81:105–112. doi: 10.1111/j.1699-0463.1973.tb02193.x. [DOI] [PubMed] [Google Scholar]
- 20.Issekutz A C, Issekutz T B. Monocyte migration to arthritis in the rat utilizes both CD11/CD18 and very late activation antigen 4 integrin mechanisms. J Exp Med. 1995;181:1197–1203. doi: 10.1084/jem.181.3.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jaffe E A, Nachman R L, Becker C G, Minick C R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973;52:2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jalkanen S, Bargatze R F, de los Toyos J, Butcher E C. Lymphocyte recognition of high endothelium: antibodies to distinct epitopes of an 85-95-kD glycoprotein antigen differentially inhibit lymphocyte binding to lymph node, mucosal, or synovial endothelial cells. J Cell Biol. 1987;105:983–990. doi: 10.1083/jcb.105.2.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jalkanen S, Jalkanen M. Lymphocyte CD44 binds the COOH-terminal heparin-binding domain of fibronectin. J Cell Biol. 1992;116:817–825. doi: 10.1083/jcb.116.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jalkanen S, Steere A C, Fox R I, Butcher E C. A distinct endothelial cell recognition system that controls lymphocyte traffic into inflamed synovium. Science. 1986;233:556–558. doi: 10.1126/science.3726548. [DOI] [PubMed] [Google Scholar]
- 25.Johnston M G, Hay J B, Movat H Z. Kinetics of prostaglandin production in various inflammatory lesions, measured in draining lymph. Am J Pathol. 1979;95:225–238. [PMC free article] [PubMed] [Google Scholar]
- 26.Kaufmann S H E. Immunity to intracellular bacteria. Annu Rev Immunol. 1993;11:129–163. doi: 10.1146/annurev.iy.11.040193.001021. [DOI] [PubMed] [Google Scholar]
- 27.Lahesmaa R, Yssel H, Batsford S, Luukkainen R, Möttönen T, Steinman L, Peltz G. Yersinia enterocolitica activates a T helper type 1-like T cell subset in reactive arthritis. J Immunol. 1992;148:3079–3085. [PubMed] [Google Scholar]
- 28.Laird W, Cavanaugh D. Correlation of autoagglutination and virulence of yersiniae. J Clin Microbiol. 1980;11:430–432. doi: 10.1128/jcm.11.4.430-432.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McEver R P, Beckstead J H, Moore K L, Marshall-Carlson L, Bainton D F. GMP-140, a platelet α-granule membrane protein, is also synthesized by vascular endothelial cells and is localized in Weibel-Palade bodies. J Clin Invest. 1989;84:92–99. doi: 10.1172/JCI114175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mileski W, Harlan J, Rice C, Winn R. Streptococcus pneumoniae-stimulated macrophages induce neutrophils to emigrate by a CD18-independent mechanism of adherence. Circ Shock. 1990;31:259–267. [PubMed] [Google Scholar]
- 31.Molenaar R, Visser W J, Verkerk A, Koster J F, Jongkind J F. Peroxidative stress and in vitro ageing of endothelial cells increases the monocyte-endothelial cell adherence in a human in vitro system. Atherosclerosis. 1989;76:193–202. doi: 10.1016/0021-9150(89)90103-2. [DOI] [PubMed] [Google Scholar]
- 32.Moore K L, Patel K D, Bruehl R E, Fugang L, Johnson D A, Lichenstein H S, Cummings R D, Bainton D F, McEver R P. P-selectin glycoprotein ligand-1 mediates rolling of human neutrophils on P-selectin. J Cell Biol. 1995;128:661–671. doi: 10.1083/jcb.128.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patel K D, Zimmerman G A, Prescott S M, McEver R P, McIntyre T M. Oxygen radicals induce human endothelial cells to express GMP-140 and bind neutrophils. J Cell Biol. 1991;112:749–759. doi: 10.1083/jcb.112.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pei R, Arjomand-Shamsai M, Deng C T, Cesbron A, Bignon J D, Lee J-H. A monospecific HLA-B27 fluorescein isothiocyanate-conjugated monoclonal antibody for rapid, simple and accurate HLA-B27 typing. Tissue Antigens. 1993;41:200–203. doi: 10.1111/j.1399-0039.1993.tb02003.x. [DOI] [PubMed] [Google Scholar]
- 35.Randolph G W, Furie M B. Mononuclear phagocytes egress from an in vitro model of the vascular wall by migrating across endothelium in the basal to apical direction: role of intracellular adhesion molecule 1 and the CD11/CD18 integrins. J Exp Med. 1996;183:451–462. doi: 10.1084/jem.183.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salmi M, Granfors K, Leirisalo-Repo M, Hämäläinen M, MacDermott R, Leino R, Havia T, Jalkanen S. Selective endothelial binding of interleukin-2-dependent human T-cell lines derived from different tissues. Proc Natl Acad Sci USA. 1992;89:11436–11440. doi: 10.1073/pnas.89.23.11436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salmi M, Rajala P, Jalkanen S. Homing of mucosal leukocytes to joints. Distinct endothelial ligands in synovium mediate leukocyte-subtype specific adhesion. J Clin Invest. 1997;99:2165–2172. doi: 10.1172/JCI119389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanchez-Madrid F, De Landazuri M O, Morago G, Cebrian M, Acevedo A, Bernabeu C. VLA-3: a novel polypeptide association within the VLA molecular complex: cell distribution and biochemical characterization. Eur J Immunol. 1986;16:1343–1349. doi: 10.1002/eji.1830161106. [DOI] [PubMed] [Google Scholar]
- 39.Sanchez-Madrid F, Krensky A M, Ware C F, Robbins E, Strominger J L, Burakoff S J, Springer T A. Three distinct antigens associated with human T-lymphocyte-mediated cytolysis: LFA-1, LFA-2, and LFA-3. Proc Natl Acad Sci USA. 1982;79:7489–7493. doi: 10.1073/pnas.79.23.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schumacher H R., Jr How micro-organisms are handled to localize to joints and within joints. Scand J Rheumatol. 1995;24:199–202. doi: 10.3109/03009749509100928. [DOI] [PubMed] [Google Scholar]
- 41.Skaleric U, Allen J B, Smith P D, Mergenhagen S E, Wahl S M. Inhibitors of reactive oxygen intermediates suppress bacterial cell wall-induced arthritis. J Immunol. 1991;147:2559–2564. [PubMed] [Google Scholar]
- 42.Swincow T D V. Statistics at square one. Plymouth, England: Latimer Trend & Company Ltd.; 1989. [Google Scholar]
- 43.Wayner E A, Gil S G, Murphy G F, Wilke M S, Carter W G. Epiligrin, a component of epithelial basement membranes, is an adhesive ligand for α3β1 positive T lymphocytes. J Cell Biol. 1993;121:1141–1152. doi: 10.1083/jcb.121.5.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Westphal O, Lüderitz O, Bister F. Über die Extraction von Bakterien mit Phenol/Wasser. Z Naturforsch. 1952;7B:148–155. [Google Scholar]
- 45.Wuorela M, Jalkanen S, Toivanen P, Granfors K. Expression of MHC class II molecules on human monocytes is regulated independently from each other after phagocytosis of bacteria. Scand J Immunol. 1996;43:39–46. doi: 10.1046/j.1365-3083.1996.d01-3.x. [DOI] [PubMed] [Google Scholar]
- 46.Wuorela M, Jalkanen S, Toivanen P, Granfors K. Yersinia lipopolysaccharide is modified by human monocytes. Infect Immun. 1993;61:5261–5270. doi: 10.1128/iai.61.12.5261-5270.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]