Abstract
Purpose
Laparoscopic sleeve gastrectomy (SG) is the most popular bariatric surgery. Nonetheless, only a few studies have reported its long-term outcomes. This study aimed to evaluate changes in weight and body mass index (BMI) parameters, resolution of comorbidities, and frequency of re-operations in a follow-up period of at least 10 years.
Materials and Methods
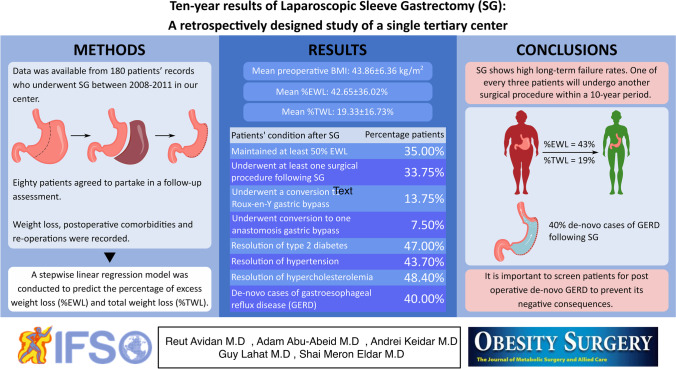
Data was available from 180 patients’ records who underwent SG between 2008 and 2011 in the Tel Aviv Sourasky Medical Center. Eighty patients agreed to partake in a follow-up assessment that was completed via a phone call questionnaire and hospital computed registry.
Results
Patients’ mean preoperative BMI was 43.86 ± 6.36 kg/m2 which was significantly higher when compared to mean nadir BMI and last follow-up BMI (29.44 ± 7.12 and 36.34 ± 9.7; p < 0.001). Mean percentage of excess weight loss (%EWL) at 10 years was 42.65 ± 36.02% and mean percentage of total weight loss was 19.33 ± 16.73%. Twenty-eight patients (35%) maintained at least 50% EWL. Twenty-seven (33.75%) patients underwent at least one surgical procedure following SG. Eleven patients (13.75%) underwent a conversion to Roux-en-Y gastric bypass and six patients (7.5%) underwent conversion to one anastomosis gastric bypass. Resolution of type 2 diabetes (T2D), hypertension, and hypercholesterolemia occurred in 47%, 43.7%, and 48.4%, respectively. De-novo cases of gastroesophageal reflux (GERD) were present in 40%.
Conclusion
SG shows high long-term failure rates. One of every three patients will undergo another surgical procedure within a 10-year period.
Graphical Abstract
Keywords: Sleeve gastrectomy, Bariatric, Roux-en-Y gastric bypass, Body weight, Ten years
Introduction
Obesity, defined as a body mass index (BMI) of ≥ 30 kg/m2, has become a worldwide epidemic. According to the World Health Organization (WHO) [1], the prevalence of obesity nearly tripled between 1975 and 2016, and about 13% of the global adult population is now obese. By contributing towards multiple cardiovascular and metabolic comorbidities, obesity was responsible for 4.7 million premature deaths in 2017 [2], and the yearly figure continues to rise.
Currently, metabolic and bariatric surgery (MBS) is the most effective intervention for inducing weight loss in patients with obesity, mainly through gastric volume restriction and malabsorption [3]. Laparoscopic vertical sleeve gastrectomy (SG) is the most commonly performed MBS worldwide and has grown in popularity among patients and surgeons due to its relative technical ease and high safety profile. In fact, it accounted for nearly 50% of all MBS performed in the USA between 2014 and 2018 [4]. However, most of the available evidence for its efficacy is based on relatively short-term follow-up (up to 5 years) [5–7]. Few large cohort studies have reported the long-term effects on weight loss and comorbidities [8–11]. Since obesity is a chronic disease, it is more meaningful to understand long-term rather than short-term outcomes.
Beyond the success of SG in lowering BMI, the surgery has also been shown to significantly reduce the frequency or severity of concomitant diseases associated with obesity. These include type 2 diabetes (T2D), hypertension (HTN), and hypercholesterolemia. In cases of insufficient weight loss, there are various further surgical options available including a secondary sleeve gastrectomy, conversion to Roux-en-Y gastric bypass (RYGB), conversion to one anastomosis gastric bypass (OAGB), or gastric banding. As with all surgical procedures, appropriate patient selection is important.
Our study aimed to examine the effects of SG over a long-term period of at least 10 years. Specifically, the effects of the procedure regarding weight loss, BMI, and obesity-related co-morbidities, such as T2D, HTN, hypercholesterolemia, and gastroesophageal reflux disease (GERD). Post-SG re-operations were documented with an emphasis on conversion to RYGB or OAGB.
Materials and Methods
This was a single-center retrospective study of adult patients who underwent SG surgery at a single tertiary medical center (Tel Aviv Sourasky Medical Center), between 2008 and 2011. Patients were included in the study if they had follow-up data for a minimum of 10 years. All patients underwent standard preoperative workup, including a complete history and physical examination, routine laboratory tests, chest x-ray, EKG, abdominal ultrasound, esophagogastroduodenoscopy or upper GI barium swallow, nutritional and psychiatric evaluation, and additional examination and/or consultations when necessary.
Data from medical databases at our institution were retrospectively analyzed. Baseline age, gender, height, weight, BMI, and obesity-related co-morbidities were recorded. Data was available from 180 individual records who underwent SG surgery in our bariatric center, of which 80 patients agreed to partake in a follow-up assessment at 10 years after the procedure. Follow-up was completed via telephone call questionnaire and hospital computed registry. Phone contact rather than outpatient appointments was preferable at that time due to social distancing restrictions imposed during the second wave of COVID-19. Weight loss, postoperative comorbidities, and re-operations were recorded. Weight loss was expressed as BMI change, percentage of excess weight loss (%EWL), and percentage total weight loss (%TWL). The ideal body weight was calculated as weight × 22/BMI. The %EWL was calculated as (preoperative weight − current weight)/(preoperative weight − ideal body weight) × 100. The %TWL was calculated as (current weight–preoperative weight)/(preoperative weight) × 100. The goal for good weight loss outcome rates for SG was defined by a %EWL of at least 50%. The measured comorbidities included T2D, HTN, hypercholesterolemia, and GERD. GERD symptoms and proton pump inhibitor (PPI) intake were assessed both pre- and postoperatively.
Definition of Obesity-Related Diseases and Their Resolution
The measured comorbidities included T2D, HTN, hypercholesterolemia, and GERD. T2D was diagnosed according to the American Diabetes Association guidelines [12], as fasting plasma glucose ≥ 126 mg/dl and/or Hba1c ≥ 6.5%, and its remission was defined as Hba1c < 6.5% off antidiabetic medications. Hypertension was defined as a systolic blood pressure of > 140 mmHg or a diastolic blood pressure of > 90 mmHg. Remission was defined as a blood pressure < 140/90 mmHg off antihypertensive medications [13]. Hypercholesterolemia was defined as a blood total cholesterol level of ≥ 200 mg/dL, resolution of it was defined as total cholesterol < 200 mg/dL off medications. GERD symptoms and proton pump inhibitor (PPI) intake were assessed both pre- and postoperatively. GERD resolution was defined as symptomatic relief without PPI treatment. De-novo GERD was defined as the new onset of postoperative development of heat burn and regurgitation in patients who did not have symptomatic GERD previously [14].
Surgical Technique
All SG operations were performed by two surgeons who used the standardized laparoscopic technique. The greater curvature of the stomach was mobilized and dissected starting 4 cm from the pylorus. A Bougie sized 32–40 Fr was inserted along the lesser curvature. The stomach was vertically transected using linear staplers. No stapler line reinforcement or over suturing was used. A drain was placed selectively. Patients were allowed clear liquids on postoperative (POD) and discharged on POD 3.
Postoperative Follow-up
Following discharge, all patients are scheduled for an appointment to the bariatric outpatient clinic 2 weeks following discharge. Following the first visit, patients are routinely invited for visits at 1 month, 3 months, 6 months, 12 months, and then annually. If patients require further evaluation, these intervals may shorten. At all visits, patients are evaluated for weight loss, obesity–related co-morbidities, and early and late complications.
Statistical Analysis
Continuous variables are expressed as means ± standard deviations. Categorical variables are expressed as counts or percentages. Comparisons between groups were made using paired t tests. A stepwise linear regression model was conducted to predict the %EWL at a minimum 10-year follow-up. Age and sex were entered at the first step, preoperative occurrences of comorbidities (T2D, HTN, hypercholesterolemia, GERD) were entered as the second step, and preoperative BMI was entered as the third and final step. All analyses were performed with SPSS software version 25 (SPSS, Inc., Chicago, IL, USA).
Results
There were 180 patients who underwent SG underwent during the study’s timeframe. During the follow up, there was no mortality within the cohort. Eighty patients out of 180 patients consented to participate in our study (44.4%). Of these, 56 (70%) were women. The participants had a mean age of 41.3 ± 13.9 years at the time of surgery. The median follow-up time was 115 months (range, 10–11 years).
Paired t-tests revealed that participants maintained a significant long-term weight loss, as measured by the change in preoperative versus postoperative weight at ≥ 10 years after the SG procedure: 120.11 ± 22.03 versus 98.41 ± 26.24 kg, p < 0.001. Similarly, the mean BMI was significantly reduced at follow-up: 43.21 ± 8.01 kg/m2 preoperatively versus 36.34 ± 9.77 kg/m2 at ≥ 10 years postoperatively; p < 0.0001. Significant weight and BMI reductions were also shown when compared to the participants’ minimum (nadir) weight and minimum (nadir) BMI (Table 1).
Table 1.
Patients’ weight and BMI parameters pre- and post-SG procedure*
| Preoperative weight (mean ± SD, kg) | 120.11 ± 22.03 |
| Preoperative BMI (mean ± SD, kg/m2) | 43.21 ± 8.01 |
| Postoperative minimum weight (mean ± SD, kg) | 81.23 ± 19.61 |
| Postoperative minimum BMI (mean ± SD, kg/m2) | 29.44 ± 7.12 |
| ≥ 10 years postoperative weight (mean ± SD, kg) | 98.41 ± 26.24 |
| ≥ 10 years postoperative BMI (mean ± SD, kg/m2) | 36.34 ± 9.77 |
| ≥ 10 years %EWL (mean ± SD) | 42.65 ± 36.02 |
| ≥ 10 years %EWL in non-converted patients (n = 62) | 42.49 ± 35.9 |
| ≥ 10 years %EWL in converted patients (n = 18) | 42.93 ± 35.1 |
| ≥ 10 years %EWL in converted vs non-converted patients | p = 0.96 |
| ≥ 10 years %TWL (mean ± SD) | 19.33 ± 16.73 |
| ≥ 10 years %TWL in non-converted patients (n = 62) | 19.47 ± 17.5 |
| ≥ 10 years %TWL in converted patients (n = 18) | 17.97 ± 13.02 |
| ≥ 10 years %TWL in converted vs non-converted patients | p = 0.72 |
BMI, body mass index; SG, sleeve gastrectomy; SD, standard deviation; EWL, excess weight loss; TWL, total weight loss
*Values correspond to entire cohort (n = 80) unless differently stated
Mean %EWL at ≥ 10 years was 42.65 ± 36.02%. The mean %TWL was 19.33 ± 16.73%. We compared the %EWL and %TWL for patients who during the follow-up period underwent revisional surgery to patients who did not and found no significant difference between groups (42.93 ± 35.1 vs 42.49 ± 35.9; p = 0.96, and 17.97 ± 13.02 vs 19.47 ± 17.5; p = 0.73, respectively). SG showed satisfactory weight loss outcomes (%EWL ≥ 50) in only 35% (n = 28) of the study’s cohort. Among the population, 50% had BMI ≥ 35 at ≥ 10 years postoperatively. In addition, there were 13 participants (16.25%) who had a BMI ≥ 50 preoperatively. In this subgroup, there was a mean weight regain from nadir of 23.69 ± 25.16 kg at 10 years following SG, but the long-term success rate (%EWL ≥ 50) was fairly good at 61.5% (8 out of 13 participants).
Pre-operative rates of obesity-related comorbidities baseline status, resolution, and de-novo occurrence are depicted in Table 2. T2D, HTN, and hypercholesterolemia resolved in 47%, 43.7%, and 48.4%. De-novo GERD occurred in 40% of patients (n = 30).
Table 2.
Rates of pre- and post-SG comorbidities, including resolution and de novo cases
| Baseline | Resolution | De Novo | |
|---|---|---|---|
| 23.8% (19) | 47% (9) | 8.9% (7) | |
| 40.5% (32) | 43.7% (14) | 11.4% (9) | |
| 38.8% (31) | 48.4 (15) | 8.8% (7) | |
| 16% (12) | 50% (6) | 40% (30) |
SG, sleeve gastrectomy; T2D, type 2 diabetes; GERD, gastroesophageal reflux disease
Twenty-seven (33.75%) patients underwent at least one surgical procedure following SG. Eleven patients (13.75%) underwent conversion to RYGB due to weight regain, five of them also suffered from de-novo GERD. Six patients (7.5%) underwent conversion to OAGB due to weight regain. Two patients underwent placement of an adjustable gastric band due to weight regain. The mean time between SG conduction to revisional surgery for weight regain was 6.02 ± 3.96 years. Other procedures performed included cholecystectomy (n = 6), trocar-site hernia repair (n = 3), and diaphragmatic hernia repair (n = 1).
Discussion
This study is one of only a handful of studies reporting long-term outcomes of over 10 years following SG. Our main findings at ≥ 10 years post-surgery were a significant reduction in patients’ weight and BMI with a mean excess weight loss of 42.65%, but only 35% achieved a %EWL ≥ 50, and only 50% reached the 10-year point with a BMI < 35 kg/m2. We found that 40–50% of our patients with preoperative obesity-related comorbidities had resolution of their condition. The exception to this was with GERD where we found a relatively high rate of 40% of de-novo cases following SG. Finally, more than a fifth of our patients required a conversion to an additional MBS due to weight regain.
There have been very few recent studies looking at the long-term effects of SG surgery, a major reason for carrying out this study. Table 3 summarizes most of the recently relevant published studies evaluating 10-year outcomes of SG. One of the most rigorous studies was carried out by Felsenreich et al. [15] in 2016 on a smaller patient sample of 53 patients who underwent SG. At 10 years, they reported a mean %EWL of 53 ± 25% in 32 of their 53 patients, compared to our total mean of 42.65 ± 36.02%. We could consider their results to be therefore similar to ours despite some noticeable differences. Their patients had a higher percentage of women (80% compared to our 70%), a higher mean preoperative weight and BMI of 137.1 ± 28.5 kg and 48.9 ± 9.4 kg/m2, respectively, a higher number of patients with BMI ≥ 50 (36% compared to our 16%), and an earlier and higher rate of conversion to Roux-en-Y gastric bypass (36% compared to our 21%) at baseline between the two patient cohorts. Conversely, our findings did not reach the success of another study examining 10-year outcomes following SG [16], which reported a mean %TWL of 30.9 ± 12.4 (compared to our 19.33 ± 16.73%) and a mean %EWL of 52.5 ± 21.1 (compared to our 42.65 ± 36.02). One reason for their greater success may be due to them making a specific effort to refer their patients for regular nutritional and psychological counselling throughout the follow-up period. These interventions have been reported to promote lifestyle modifications and increase weight loss outcomes [17]. We showed that 28 patients (35%) reached the satisfactory goal of %EWL ≥ 50. Hauters et al. [18] reported that 41% of patients reached this goal at 10 years after SG. This was further supported by an additional retrospective study reporting 48.6% of patients reaching EWL > 50 [19]. We agree with conclusions drawn in these studies that SG may be of benefit for selected patients.
Table 3.
Series of Sleeve Gastrectomy studies with at least 10-year FU
| Author, year | Number of patients | Study design | Mean EWL (SD) | T2D resolution | HTN resolution | De-novo GERD | Revisional surgery during FU |
|---|---|---|---|---|---|---|---|
| Felsenreich, 2016 [15] | 53 | Retrospective | 53.5% (26.6) | 1.9%** | 23.5%** | N/A | 36% |
| Castagneto Gissey, 2018 [16] | 114 | Retrospective | 52.5% (21.1) | 64.7% | 44.2% | 42.9% | 44% |
| Hauters, 2021 [18] | 34 | Retrospective | 42% (37) | 12% | 17% | 41% | 18% |
| Musella, 2019 [19] | 76 | Retrospective | 50.1% (30.5) | 0% | 51.4% | 25.7% | 15.8% |
| Kraljević, 2021 [20] | 215 | Retrospective | 53.6% (24.6)* | 61% | 60.5% | 32.4% | 19.2% |
| Chang, 2018 [21] | 65 | Retrospective | 70.5% (27.8) | 39.6% | 78.4% | 58.4% | 21.5% |
| Arman, 2016 [24] | 65 | Retrospective | 67.4% | N/A | 28.6%** | 21.4% | 31.7% |
FU, follow-up; T2D, type 2 diabetes; HTN, hypertension; EWL, excess weight loss; GERD, gastroesophageal reflux disease; N/A, not available
*Corresponds to primary sleeve gastrectomy only
**Includes patients with resolution and/or improvement of T2D/HTN
Patients with BMI ≥ 50 exhibited greater weight loss rate of 61.5% compared to the 35% for the whole cohort, as defined by %EWL ≥ 50. Interestingly, baseline comorbidities did not predict long-term weight loss. In the majority of our sample, patients with baseline comorbidities showed complete remission of their diseases during the 10-year follow-up. In a retrospective study by Kraljevic et al. [20], T2D, HTN, and hyperlipidemia were reported to resolve 10 years after SG in 61%, 60.5%, and 54.8%, respectively. Similarly, Chang et al. [21] reported 39.6% remission of T2D, 78.4% for HTN, and 51.3% for dyslipidemia. On the contrary, Musella et al. [19] showed in their cohort showed 0% remission of T2D and 51.4% remission of HTN. Our results showed 47% remission for T2D, 43.7% remission for HTN, and 48.4% remission for hypercholesterolemia. The variations of reports in the literature are possibly due to different sample sizes, demographics and variable loss to follow-up rates (50–60%) [18–21]. It is also important to note that during the last decade, we have developed a trend referring patients with higher BMI and poorly controlled obesity related co-morbidities to other procedures (OAGB, RYGB) as they have shown to have satisfactory outcomes [22, 23].
De-novo cases of symptomatic GERD after SG were fairly high at 40% (n = 30) in our study and was a common indication for conversion to RYGB. All patients with de novo GERD were scheduled for an appointment in the MBS clinic in our center following the telephone interview. In total, five patients required conversion to RYGB with complete resolution of symptoms. Despite the high number of de-novo GERD (40%), most patients were treated conservatively and did not require revision to RYGB. This was shown in several other studies evaluating results of SG at 10 years reporting de-novo GERD rate of 25.7–58.4% and a conversion rate of 2.6–20.4% due to de-novo GERD [18–21]. It is of major importance to follow-up, diagnose, monitor, and treat chronic post-SG de novo GERD as they may advance to Barret’s esophagus and increase cancer risk.
In our cohort, 11 patients (13.75%) underwent conversion to RYGB, and 6 patients (7.5%) underwent conversion to OAGB with a mean interval time of 6.02 ± 3.96 years. Arman et al. [24] reported a conversion rate of 31.7% at 11.7 years after SG, mostly due to weight regain/insufficient weight loss, similar to other study. Some of the earlier conversions to gastric bypass may represent our learning curve with the sleeve gastrectomy procedure as it was the early era of SG in our institution, similarly, Chang et al. [21] reported that major complication rate was higher in the first years of their experience with SG. It should be emphasized that when the indication for SG revision is for weight regain/insufficient weight loss, we prefer the OAGB as it is a more aggressive malabsorptive procedure, but when the main indication for revision is GERD, we definitely prefer the RYGB.
Long-term results of SG have been compared to other MBS. The SLEEVEPASS trial was recently published and compared SG to RYGB [25]. Interestingly, it was shown that RYGB was associated with a significantly higher %EWL (50.7% vs 43.5%), and HTN remission (24% vs 8%); however, no significant difference was found in remission of T2D, dyslipidemia, and obstructive sleep apnea. In addition, esophagitis prevalence was significantly higher after SG (31% vs 7%) but no difference was seen in the prevalence of Barret’s esophagus. Musella et al. [19] retrospectively analyzed patients undergoing SG vs gastric band at 10 years and did not show any advantage for SG over the gastric band in terms of weight loss and remission of co-morbidities except for postoperative GERD which was significantly higher in the SG group (25.7% vs 6.5%). We think that additional comparative studies are required to further clarify long term outcomes of SG compared to other MBS.
There are several limitations to our study. The first limitation relates to its retrospective design, with no comparative group. Follow-up rate was 44.4% (80 out of 180 patients). This could be due to the difficulties patients face when seeking funding of nutritional support, psychologic support, and routine ambulatory visits. We can also relate that to COVID-19 restrictions which took place when performing this study. Nevertheless, the loss to follow-up is a known phenomenon after MBS and it increases over the years. We also could not include postoperative endoscopic findings of patients with de-novo GERD which could aid us in evaluating esophagitis grade.
Despite that, the cohort size is relatively large compared to several studies We also acknowledge the single-center characteristic of the study. Finally, this study looked into the long-term outcomes of SG.
Conclusions
Our study confirms that SG is an effective bariatric procedure for patients with obesity in terms of weight loss and resolution of comorbidities. Due to the current scarcity of long-term studies, our findings are able to further support that SG can provide patients with a sustained weight loss a decade later. Yet only 35% maintain a %EWL of more than 50%. It is important to screen patients for postoperative de-novo GERD that may occur in a considerable number of patients to prevent its negative consequences.
Declarations
Ethical approval
All procedures performed in this study were in accordance with ethical standards of the institutional and/or national health research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Consent to Participate
Informed consent was obtained from all individuals included in this study.
Conflict of Interest
The authors declare no competing interests.
Footnotes
Key Points
• SG resulted in a mean excess weight loss of 43% within a 10-year follow-up.
• Sixty-five percent of patients showed did not reach EWL ≥ 50%.
• SG caused obesity-related comorbidities to resolve in nearly 50% of patients.
• Forty percent of patients developed de-novo GERD following SG.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO (2021) – Fact sheet – obesity and overweight. Updated June 2021. [Electronic resource]. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight
- 2.Hannah Ritchie and Max Roser. Obesity. Published online at OurWorldInData.org. 2017. Retrieved from: https://ourworldindata.org/obesity.
- 3.Cavin JB, Bado A, Le Gall M. Intestinal adaptations after bariatric surgery: consequences on glucose homeostasis. Trends Endocrinol Metab. 2017;28(5):354–364. doi: 10.1016/j.tem.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Welbourn R, Hollyman M, Kinsman R, Dixon J, Liem R, Ottosson J, Ramos A, Våge V, Al-Sabah S, Brown W, Cohen R, Walton P, Himpens J. Bariatric Surgery Worldwide: Baseline Demographic Description and One-Year Outcomes from the Fourth IFSO Global Registry Report 2018. Obes Surg. 2019;29(3):782–795. doi: 10.1007/s11695-018-3593-1. [DOI] [PubMed] [Google Scholar]
- 5.Baltasar A, Serra C, Pérez N, Bou R, Bengochea M, Ferri L. Laparoscopic sleeve gastrectomy: a multi-purpose bariatric operation. Obes Surg. 2005;15(8):1124–1128. doi: 10.1381/0960892055002248. [DOI] [PubMed] [Google Scholar]
- 6.Himpens J, Dapri G, Cadière GB. A prospective randomized study between laparoscopic gastric banding and laparoscopic isolated sleeve gastrectomy: results after 1 and 3 years. Obes Surg. 2006;16(11):1450–1456. doi: 10.1381/096089206778869933. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen NT, Slone JA, Nguyen XMT, Hartman JS, Hoyt DB. A prospective randomized trial of laparoscopic gastric bypass versus laparoscopic adjustable gastric banding for the treatment of morbid obesity: outcomes, quality of life, and costs. Ann Surg. 2009;250(4):631–641. doi: 10.1097/SLA.0b013e3181b92480. [DOI] [PubMed] [Google Scholar]
- 8.Bohdjalian A, Langer FB, Shakeri-Leidenmühler S, Gfrerer L, Ludvik B, Zacherl J, Prager G. Sleeve gastrectomy as sole and definitive bariatric procedure: 5-year results for weight loss and ghrelin. Obes Surg. 2010;20(5):535–540. doi: 10.1007/s11695-009-0066-6. [DOI] [PubMed] [Google Scholar]
- 9.Lemanu DP, Singh PP, Rahman H, Hill AG, Babor R, MacCormick AD. Five-year results after laparoscopic sleeve gastrectomy: a prospective study. Surg Obesity Related Dis. 2015;11(3):518–524. doi: 10.1016/j.soard.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 10.Sieber P, Gass M, Kern B, Peters T, Slawik M, Peterli R. Five-year results of laparoscopic sleeve gastrectomy. Surg Obesity Related Dis. 2014;10(2):243–249. doi: 10.1016/j.soard.2013.06.024. [DOI] [PubMed] [Google Scholar]
- 11.Hoyuela C. Five-year outcomes of laparoscopic sleeve gastrectomy as a primary procedure for morbid obesity: a prospective study. World J Gastrointest Surg. 2017;9(4):109. doi: 10.4240/wjgs.v9.i4.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(Suppl 1):S62–9. 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed]
- 13.National Institute for Health and Clinical excellence. (2019). Hypertension in adults: diagnosis and management (NICE guideline [NG136]). https://www.nice.org.uk/guidance/ng136/chapter/recommendations.
- 14.Gyawali CP, Kahrilas PJ, Savarino E, Zerbib F, Mion F, Smout AJPM, Vaezi M, Sifrim D, Fox MR, Vela MF, Tutuian R, Tack J, Bredenoord AJ, Pandolfino J, Roman S. Modern diagnosis of GERD: the Lyon Consensus. Gut. 2018;67(7):1351–1362. doi: 10.1136/gutjnl-2017-314722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Felsenreich DM, Langer FB, Kefurt R, Panhofer P, Schermann M, Beckerhinn P, Sperker C, Prager G. Weight loss, weight regain, and conversions to Roux-en-Y gastric bypass: 10-year results of laparoscopic sleeve gastrectomy. Surg Obesity related Diseas: official J American Soc Bariat Surg. 2016;12(9):1655–1662. doi: 10.1016/j.soard.2016.02.021. [DOI] [PubMed] [Google Scholar]
- 16.Castagneto Gissey L, Casella Mariolo JR, Genco A, Troisi A, Basso N, Casella G. 10-year follow-up after laparoscopic sleeve gastrectomy: Outcomes in a monocentric series. SurG Obesity Related diseas: official J American Soc Bariat Surg. 2018;14(10):1480–1487. doi: 10.1016/j.soard.2018.06.021. [DOI] [PubMed] [Google Scholar]
- 17.Casella G, Soricelli E, Giannotti D, Collalti M, Maselli R, Genco A, Redler A, Basso N. Long-term results after laparoscopic sleeve gastrectomy in a large monocentric series. Surg Obes Relat Dis. 2016;12(4):757–762. doi: 10.1016/j.soard.2015.09.028. [DOI] [PubMed] [Google Scholar]
- 18.Hauters P, Dubart JW, Desmet J, Degolla R, Roumain M, Malvaux P. Ten-year outcomes after primary vertical sleeve gastrectomy for morbid obesity: a monocentric cohort study. Surg Endosc. 2021;35(12):6466–6471. doi: 10.1007/s00464-020-08137-8. [DOI] [PubMed] [Google Scholar]
- 19.Musella M, Berardi G, Velotti N, Schiavone V, Vitiello A. Ten-year results of laparoscopic sleeve gastrectomy: retrospective matched comparison with laparoscopic adjustable gastric banding-is there a significant difference in long term? Obes Surg. 2021;31(12):5267–5274. doi: 10.1007/s11695-021-05735-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kraljević M, Cordasco V, Schneider R, Peters T, Slawik M, Wölnerhanssen B, Peterli R. Long-term effects of laparoscopic sleeve gastrectomy: what are the results beyond 10 years? Obes Surg. 2021;31(8):3427–3433. doi: 10.1007/s11695-021-05437-3. [DOI] [PubMed] [Google Scholar]
- 21.Chang DM, Lee WJ, Chen JC, Ser KH, Tsai PL, Lee YC. Thirteen-Year Experience of Laparoscopic Sleeve Gastrectomy: Surgical Risk, Weight Loss, and Revision Procedures. Obes Surg. 2018;28(10):2991–2997. doi: 10.1007/s11695-018-3344-3. [DOI] [PubMed] [Google Scholar]
- 22.Abu-Abeid A, Goren O, Abu-Abeid S, Dayan D. One Anastomosis Gastric Bypass for Revision of Restrictive Procedures: Mid-Term Outcomes and Analysis of Possible Outcome Predictors. Obes Surg. 2022;32(10):3264–3271. doi: 10.1007/s11695-022-06235-1. [DOI] [PubMed] [Google Scholar]
- 23.Angrisani L, Ferraro L, Santonicola A, Palma R, Formisano G, Iovino P. Long-term results of laparoscopic Roux-en-Y gastric bypass for morbid obesity: 105 patients with minimum follow-up of 15 years. Surg Obes Relat Dis. 2021;17(4):727–736. doi: 10.1016/j.soard.2020.11.028. [DOI] [PubMed] [Google Scholar]
- 24.Arman GA, Himpens J, Dhaenens J, Ballet T, Vilallonga R, Leman G. Long-term (11+years) outcomes in weight, patient satisfaction, comorbidities, and gastroesophageal reflux treatment after laparoscopic sleeve gastrectomy. Surg Obes Relat Dis. 2016;12(10):1778–1786. doi: 10.1016/j.soard.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 25.Salminen P, Grönroos S, Helmiö M, Hurme S, Juuti A, Juusela R, Peromaa-Haavisto P, Leivonen M, Nuutila P, Ovaska J. Effect of laparoscopic sleeve gastrectomy vs roux-en-y gastric bypass on weight loss, comorbidities, and reflux at 10 years in adult patients with obesity: the SLEEVEPASS randomized clinical trial. JAMA Surg. 2022;157(8):656–666. doi: 10.1001/jamasurg.2022.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]