Abstract
Although indigenous bacteria intimately colonize the intestinal mucosa, under normal conditions the intestinal epithelial cell is free of adherent bacteria. Nonetheless, commensal bacteria such as Escherichia coli adhere to and translocate across the intestinal epithelium in association with a number of pathologic states including hemorrhagic shock, immunosuppression, traumatic tissue injury, and lack of enteral feedings. The adhesins involved in the adherence of indigenous E. coli to the intestinal epithelium in vivo following catabolic stress are unknown. We have developed a mouse model to study the bacterial adhesins which mediate the increased intestinal adherence of E. coli after partial hepatectomy and short-term starvation. Our studies demonstrated that hepatectomy and starvation in the mouse were associated with a 7,500-fold increase in the numbers of E. coli bacteria adhering to the cecum. In addition, erythrocyte agglutination studies, as well as immunostaining of fimbrial preparations and electron micrographs of the bacteria, revealed that surface type 1 fimbriae were more abundant in the commensal E. coli harvested from the ceca of the stressed mice. These E. coli isolates adhered to a mouse colon cell line and injected cecal loops in a mannose-inhibitable manner, which suggests a role for type 1 fimbriae in the adherence of the E. coli isolates to the cecum in vivo following host catabolic stress.
In the intestinal tract, while commensal bacteria are found to intimately colonize the mucosal surface, recent reports using molecular and immunohistochemical techniques have demonstrated that under normal conditions the mammalian intestinal epithelial cell surface is free of adherent bacteria (AB) (5, 36). Nonetheless, commensal bacteria adhere to and translocate across the intestinal epithelium in association with a number of clinical situations, including hemorrhagic shock, immunosuppressive states, traumatic tissue injury, and lack of enteral feedings (4, 6, 15, 20, 28). Among the commensal bacteria, Escherichia coli in particular translocates efficiently across the intestinal epithelium in animal models and causes a large proportion of the septic episodes in critically ill patients, including patients with hepatic disease (6, 25). Moreover, a recent study has reported increased numbers of intraepithelial E. coli bacteria in intestinal biopsy specimens from patients with colorectal carcinoma (36). However, the adhesins involved in the adherence and translocation of commensal E. coli in the intestine in association with these pathologic states are unknown.
Fimbriae, also referred to as pili, are filamentous adhesins on the bacterial surface which bind to carbohydrate moieties on the surfaces of cells and can be visualized by electron microscopy (18, 22). Type 1 fimbriae bind to mannose-containing receptors and produce mannose-sensitive agglutination of erythrocytes (RBCs) as well as mannose-sensitive binding of fimbriated bacteria to other types of cells. Type 1 fimbriae are encoded by the fim gene cluster, in which the fimA gene encodes the major structural subunit, FimA, and the fimH gene encodes the adhesin, FimH (18, 22). E. coli type 1 fimbriae may be important in the pathogenesis of lower-urinary-tract infections (9, 23, 31) and in the colonization of the oropharynx in critically ill patients (10). However, the role of type 1 fimbriae in E. coli colonization of and translocation across the epithelium in the intestine remains unclear. Type 1-fimbriated strains of E. coli have been shown to bind to freshly isolated human colonic and ileal enterocytes (2). A series of investigations using streptomycin-treated mice colonized with a human fecal E. coli isolate also demonstrated that type 1 fimbriae are expressed in the intestinal tract in vivo and may be involved in the colonization of the intestinal tract by E. coli (21, 22). In addition, Cruz et al. have shown that mannose blocked the translocation of the nonpathogenic E. coli strain C25 across Caco-2 cell monolayers (9), suggesting a role for type 1 fimbriae in the translocation of bacteria across the intestinal epithelium. Nonetheless, Herias et al. reported that type 1 fimbriae did not seem to have a role in the translocation of E. coli to mesenteric lymph nodes in gnotobiotic rats (17).
We have developed a mouse model in order to study the bacterial adhesins which mediate the increased intestinal adherence of indigenous gram-negative bacteria after catabolic stress. Our studies demonstrate that partial hepatectomy, as well as short-term starvation, in the mouse was associated with increased numbers of E. coli bacteria adhering to the cecal epithelium. In addition, our investigations reveal that type 1 fimbrial expression was increased among commensal E. coli isolates harvested from the cecum after partial hepatectomy and short-term starvation, which suggests a role for type 1 fimbriae in the adherence of the E. coli isolates to the cecal mucosa in vivo.
MATERIALS AND METHODS
Bacterial adherence studies in mice following short-term starvation and partial hepatectomy.
Inbred BALB/c mice (Taconic Farms or Jackson Laboratories) were assigned to five groups. (i) Control (CONT) mice were allowed access to mouse chow and water ad libitum. (ii) Sham surgery/fed (SHAM/FED) mice were anesthetized (ketamine, 100 mg/kg of body weight; xylazine, 10 mg/kg; and atropine, 0.04 mg/kg; administered intraperitoneally), underwent a midline laparotomy and closure, and were allowed free access to mouse chow and water ad libitum. (iii) Sham surgery/starved (SHAM/STARV) mice were treated in an identical manner to the SHAM/FED group but were allowed access only to water ad libitum. (iv) Hepatectomy/fed (HEP/FED) mice were administered the standard anesthesia regimen, underwent a midline incision and a 30% surgical hepatectomy with closure, and were allowed free access to mouse chow and water ad libitum throughout the postoperative period. (v) Hepatectomy/starved (HEP/STARV) mice were treated in an identical manner to the HEP/FED group but were allowed access only to water ad libitum.
Forty-eight hours after the initiation of the study, the cecum was excised and weighed. The excised cecum then was washed extensively with phosphate-buffered saline (PBS, pH 7.3) and homogenized, and the number of CFU of adherent bacteria/gram of tissue was determined as previously described (35). Alternatively, the excised cecum was homogenized and cultured on tryptic soy agar with 5% sheep blood (TSA), eosin-methylene blue (EMB), or MacConkey agar (Difco Laboratories, Detroit, Mich.) to determine the numbers of cecal aerobic bacteria and enteric gram-negative bacteria.
Harvest of AB and LB for in vivo and in vitro studies.
Bacteria adherent to cecal tissue (AB) were cultured as described above from CONT mice (CAB) or HEP/STARV mice (HSAB). Alternatively, luminal washings were combined and centrifuged, and dilutions of the resuspended pellets (luminal bacteria [LB]) were cultured from CONT mice (CLB) or HEP/STARV mice (HSLB). Colonies identified as E. coli were inoculated into Luria broth or agar and incubated at 37°C. Aliquots of the E. coli isolates were frozen at −80°C after one passage in Luria broth.
Agglutination studies.
After initial isolation on agar, E. coli strains were passed two to three times in static Luria broth to assess type 1 fimbrial expression or on agar to assess P and S fimbrial expression (19, 32). The bacteria then were pelleted and resuspended in PBS to an optical density of 0.4 at 600 nm, and an initial 1:10 dilution was made with PBS, followed by serial 1:2 dilutions. Agglutination assays were performed as described by Friman et al. (13) with guinea pig RBCs (R3753; Sigma Chemical Co., St. Louis, Mo.), human group O RBCs (R0043; Sigma Chemical Co.), and bovine RBCs (PML Microbiologics, Wilsonville, Oreg.). Guinea pig RBC agglutinations were performed in the presence and absence of 2.5% methyl-α-d-mannopyranoside (Sigma Chemical Co.), and agglutinations of bovine RBCs were performed prior to or following treatment with 20 μg of neuraminidase (N2876; Sigma Chemical Co.)/ml. Each E. coli isolate tested was obtained from a separate mouse. Control E. coli strains constitutively expressing type 1 (HB101 pHSS22 fim+), P (HB101 pHSS22 pap+), or S fimbriae (HB101 pHSS22 sfa+) or none of these fimbriae [HB101 pHSS22 Fim− Pap− Sfa−] were kindly provided by J. Duncan, Northwestern University, Chicago, Ill. Table 1 lists the E. coli isolates used in this study.
TABLE 1.
E. coli isolates used in this study
| Strain or type | Description | Source or reference |
|---|---|---|
| CLB | Luminal E. coli harvested from the ceca of CONT mice | This study |
| CAB | Adherent E. coli harvested from the cecal mucosa of CONT mice | This study |
| HSLB | Luminal E. coli harvested from the ceca of HEP/STARV mice | This study |
| HSAB | Adherent E. coli harvested from the cecal mucosa of HEP/STARV mice | This study |
| HB101 Fim+ | E. coli strain genetically engineered to constitutively express type 1 fimbriae | 31 |
| HB101 Fim− | E. coli strain lacking type 1 fimbriae | 31 |
Immunogold labeling of electron micrographs.
Immunogold labeling studies were carried out with a polyclonal rabbit antibody which recognizes FimA, the major structural subunit of type 1 fimbriae (a gift of S. Hultgren, Washington University, Saint Louis, Mo.). All buffers were passed through a 0.22-μm-pore-size filter prior to use. Briefly, carbon-coated grids underwent glow discharge prior to incubation with approximately 106 HSAB or CLB or HB101 Fim− bacteria for 10 min. The grids then were blocked with 0.5% bovine serum albumin for 30 min. After the blocking step, the grids were incubated for 30 min with the anti-FimA antibody, which had been absorbed with HB101 Fim− bacteria for two 30-min periods to remove non-type 1 fimbrial E. coli antibodies, and then incubated for 30 min with an anti-rabbit immunoglobulin G (IgG) antibody conjugated to 5-nm-diameter gold particles (Sigma Chemical Co.). Incubations of the grids were carried out in a humidified chamber at room temperature. Between steps, the grids were washed on top of 3 separate drops of PBS for 10 min each, and then the edges of the grids were held against filter paper to remove residual fluid. All grids were stained with uranyl acetate before examination under the electron microscope.
Protein electrophoresis and immunostaining for type 1 fimbriae.
Partially purified fimbriae were prepared from 108 CLB, HSLB, HSAB, or HB101 fim+ bacteria as previously described by Eshdat et al. (12), adjusted to equivalent total protein concentrations, heated to 100°C for 5 min in concentrated hydrochloric acid to disrupt the fimbrial aggregates into monomers, brought to a neutral pH with 5.0 M sodium hydroxide, and then fractionated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis using a 15% gel. Proteins were transferred to a nitrocellulose membrane (Hybond ECL; Amersham Life Science, Arlington Heights, Ill.), immunostained with the rabbit anti-FimA antibody and detected with the ECL chemiluminescent system and horseradish peroxidase-conjugated rabbit immunoglobulin antiserum (Amersham Life Science).
Cecal injections.
E. coli recovered directly from aliquots frozen at equivalent concentrations were diluted in PBS to a concentration of approximately 2 × 108 bacteria/ml. A portion of the bacterial sample which was injected was cultured as described above to confirm the numbers of viable bacteria (in CFU per milliliter). Anesthetized mice underwent ligation of the cecum 1 mm proximal to the distal end by using a silk suture to prevent backflow of injected fluid and temporary ligation at the ileocecal junction with surgical-grade tubing to prevent injected fluid from moving into the colon. Then equal volumes (200 μl) of either PBS, CLB, HSAB, or no fluid (SHAM) were injected at the distal end of the cecum. Additionally, three of the HSAB isolates diluted in PBS with 5% mannose were injected. All animals were allowed access to water ad libitum throughout the postoperative period. Six to eight hours after injection of the samples, the ceca were harvested and the numbers of CFU of adherent bacteria per gram of tissue were determined as described above.
Cell culture system.
Young adult mouse colon (YAMC) cells (a gift from R. Whitehead, Ludwig Institute, Melbourne, Australia), epithelial cells derived from intestinal crypts of the colon of a transgenic mouse bearing a temperature-sensitive mutation of the simian virus 40 large tumor antigen gene, were grown as previously described (39). These cells proliferate continuously at the permissive temperature (33°C) in the presence of gamma interferon, but proliferation ceases at the nonpermissive temperature (39.5°C) in the absence of gamma interferon. The YAMC cells were cultured under nonpermissive conditions for 24 h prior to use in the experiments described below.
Culture of bacteria adhering to YAMC cells.
YAMC cells were trypsinized and suspended in Hank’s buffered salt solution at a concentration of 5 × 105 cells/ml. CLB or HSAB were added to the YAMC cells at a final concentration of 106 CFU of bacteria/ml in the presence or absence of 5% mannose (Sigma Chemical Co.). The bacteria and YAMC cells were incubated at 4°C for 45 min by end-over-end rotation and then centrifuged at 900 × g for 5 min. The supernatant was discarded, the cell pellet was resuspended in cold PBS, and the centrifugation step was repeated. This process was repeated one additional time to wash away nonadherent bacteria. The pellet then was resuspended in 1 ml of PBS and centrifuged at 4,000 × g for 5 min to rupture the YAMC cells. The pellet was resuspended in 0.6 ml of cold PBS, and the suspension was homogenized by using a sterile pellet pestle (Kontes Glass Company, Vineland, N.J.). Serial dilutions were cultured on MacConkey agar at 37°C for 24 h, and the numbers of CFU of E. coli per milliliter were determined by using a manual colony counter.
PCR genomic fingerprinting.
A genomic fingerprinting method using random primer PCR was modified from that described by Welsh and McClelland (38). Briefly, chromosomal DNA isolated from E. coli (Wizard Minipreps, Promega, Madison, Wis.) harvested from CONT and HEP/STARV mice was amplified by PCR using a mixture of 40 ng of DNA, 2.5 μl of 10× reaction buffer, 4 μM (each) deoxynucleoside triphosphate, 10 pmol of random 10-mer primers (GEN 1-80; Genosys Biotechnologies, The Woodlands, Tex.) and 2 U of Taq polymerase (Promega) in a total volume of 25 μl (40 cycles of 36°C for 1 min, 72°C for 2 min and 94°C for 1 min) and fractionated on a 2% agarose gel.
Statistical evaluation.
For the bacterial adherence studies in the mice, a Student two-tailed t test was used to compare the mean log10 CFU of AB per gram of tissue of the experimental groups. In addition, an analysis of variance model was fit to the logarithm (base 10) of the number of CFU of AB per gram of tissue, by using as covariates both the type of surgery (none, sham, or partial hepatectomy) and diet (starved or fed). All the mice in each of the experimental groups (78 animals total) were used in the analysis. P values and 95% confidence intervals (CI) were based on the appropriate t distribution.
For the cecal injection studies, multiple runs of each bacterial isolate-treatment (with or without mannose) combination first were averaged to yield a single value of log10 CFU of AB per gram of tissue. For the YAMC cell adherence studies, the three replicate runs for each bacterial isolate-treatment (with or without mannose) combination were averaged to yield a single value of log10 CFU of AB per milliliter. These average values then were used to compute the mean and the standard error of the mean (SEM) for each bacterial isolate type (CLB, HSLB, and HSAB) for both experiments.
RESULTS
Partial hepatectomy and short-term starvation were associated with increased numbers of E. coli bacteria adhering to the cecal epithelium.
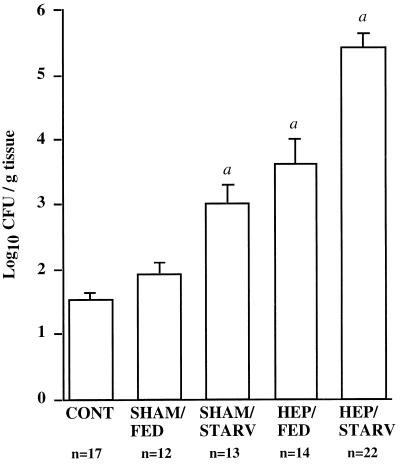
Results from the cecal culture experiments demonstrated that both short-term starvation and hepatectomy resulted in significant increases (P < 0.01) in the adherence of aerobic gram-negative bacteria to the cecum (Fig. 1). (The mean log10 CFU per gram of tissue ± standard deviation [SD] was 1.52 ± 0.10 for the CONT mice [n = 17], 3.00 ± 0.30 for the SHAM/STARV mice [n = 13], and 3.62 ± 0.39 for the HEP/FED mice [n = 14].) HEP/STARV mice had the greatest increase (P < 0.001) in the numbers of bacteria adhering to the cecum (5.40 ± 0.23 [n = 22] mean log10 CFU/g of tissue ± SD, compared with 1.52 ± 0.10 [n = 17] for the CONT mice).
FIG. 1.
Bacterial adherence to the cecal mucosa following starvation and/or partial hepatectomy compared to that in fed control and sham surgery groups. Detailed descriptions of the experimental groups are given in Materials and Methods. Values shown are the mean for each group. Error bars, SDs. α, P < 0.01 (by the Student two-tailed t test) compared with the CONT group.
Partial hepatectomy was estimated to increase the number of adherent aerobic gram-negative bacteria by 2.3 log10 CFU/g of tissue (95% CI, 1.6 to 3.0; P < 0.001) compared to no surgery. In contrast, sham surgery was associated with an increase of only 0.2 log10 CFU/g of tissue (95% CI, −0.5 to 0.9; P = 0.58), a difference that was not statistically significant. Starvation increased the average number of adherent aerobic gram-negative bacteria by an estimated 1.5 log10 CFU/g of tissue (95% CI, 1.0 to 2.0; P < 0.001). An interaction term between diet and type of surgery was not statistically significant (P = 0.19), suggesting that the two effects were additive on the log scale.
HEP/STARV mice exhibited an overgrowth of E. coli in the cecum compared to CONT mice.
Identification of the bacteria grown on selective media demonstrated that the predominant adherent organism cultured from all the experimental groups was E. coli. Numbers of total cecal bacteria (LB + AB) per gram of tissue were determined by culturing homogenized ceca from the CONT and HEP/STARV mice on TSA and EMB agar. Bacterial colonies were identified by standard microbiologic techniques. Results of a representative experiment are shown in Table 2. While the total number of cecal aerobic bacteria did not differ between CONT and HEP/STARV mice, greater numbers of enteric gram-negative bacteria were cultured from the ceca of HEP/STARV mice. E. coli and Proteus mirabilis were typically the two predominant gram-negative bacteria cultured from the mouse cecum. However, E. coli consistently predominated in HEP/STARV mouse ceca, with approximately 20-fold-greater numbers of E. coli cultured from the ceca of HEP/STARV mice compared to those from CONT mice.
TABLE 2.
Numbers of cecal aerobic bacteria in CONT and HEP/STARV micea
| Group | Log10 CFU per g of tissue (mean ± SD)
|
|||
|---|---|---|---|---|
| Total bacteria
|
E. colib | P. mirabilisb | ||
| On TSA | On EMB | |||
| CONT | 6.69 ± 0.36 | 4.30 ± 0.09 | 4.05 ± 0.13 | 3.94 ± 0.04 |
| HEP/STARV | 6.73 ± 0.14 | 5.34 ± 0.07c | 5.34 ± 0.07c | 0.00 ± 0.00c |
Homogenized ceca from CONT and HEP/STARV mice were cultured on TSA and EMB agar. Results shown are representative of multiple experiments.
Cultured on EMB agar.
P < 0.01 (by the Student two-tailed t test) compared with the corresponding CONT group.
E. coli isolated from HEP/STARV mice expressed type 1 fimbriae.
To screen the murine E. coli isolates for type 1, P, and S fimbrial expression, RBC agglutination studies were performed. As expected, the control HB101 Fim+ E. coli strain exhibited agglutination of guinea pig RBCs, which was inhibited by 2.5% mannose; the HB101 Pap+ strain exhibited agglutination of human RBCs; and the HB101 Sfa+ strain exhibited agglutination of bovine RBCs, which was abolished with neuraminidase treatment. As shown in Table 3, HSAB isolates strongly agglutinated the guinea pig RBCs in a mannose-inhibitable fashion, implicating type 1 fimbrial expression. HSAB and HSLB isolates consistently exhibited mannose-sensitive agglutination of guinea pig RBCs at higher dilutions than CLB isolates, which suggested a greater abundance of surface type 1 fimbriae among the E. coli bacteria harvested from HEP/STARV mice. Additionally, a greater proportion of HSAB (6 of 10) than CAB (0 of 4) isolates agglutinated the guinea pig RBCs at the 1:40 dilution. P and S fimbrial expression could not be demonstrated in any of the murine E. coli isolates by agglutination of human or bovine RBCs, respectively.
TABLE 3.
RBC agglutination characteristics of the murine E. coli isolates
| E. coli strain | Agglutinationa of the following RBCb at the indicated bacterial dilution:
|
|||||
|---|---|---|---|---|---|---|
| GP
|
GP + M (1:20) | Hu (1:20) | Ox (1:5) | Ox + N (1:5) | ||
| 1:20 | 1:40 | |||||
| Controls | ||||||
| HB101 Fim− | − | − | − | NT | NT | NT |
| HB101 Fim+ | + | + | − | NT | NT | NT |
| HB101 Sfa+ | NT | NT | NT | NT | + | − |
| HB101 Pap+ | NT | NT | NT | + | NT | NT |
| Murine isolates | ||||||
| HSAB | 10/10 | 6/10 | 0/10 | 0/10 | 0/10 | NT |
| HSLB | 3/5 | 0/5 | 0/5 | 0/5 | 0/5 | NT |
| CAB | 4/4 | 0/4 | 0/4 | 0/4 | 0/4 | NT |
| CLB | 0/7 | 0/7 | 0/7 | 0/7 | 0/7 | NT |
Agglutination data are given as agglutination (+), no agglutination (−), number of isolates with agglutination/number of isolates tested, or not tested (NT).
Symbols: GP, guinea pig RBCs; Hu, human RBCs; Ox, bovine RBCs; M, mannose; N, neuraminidase.
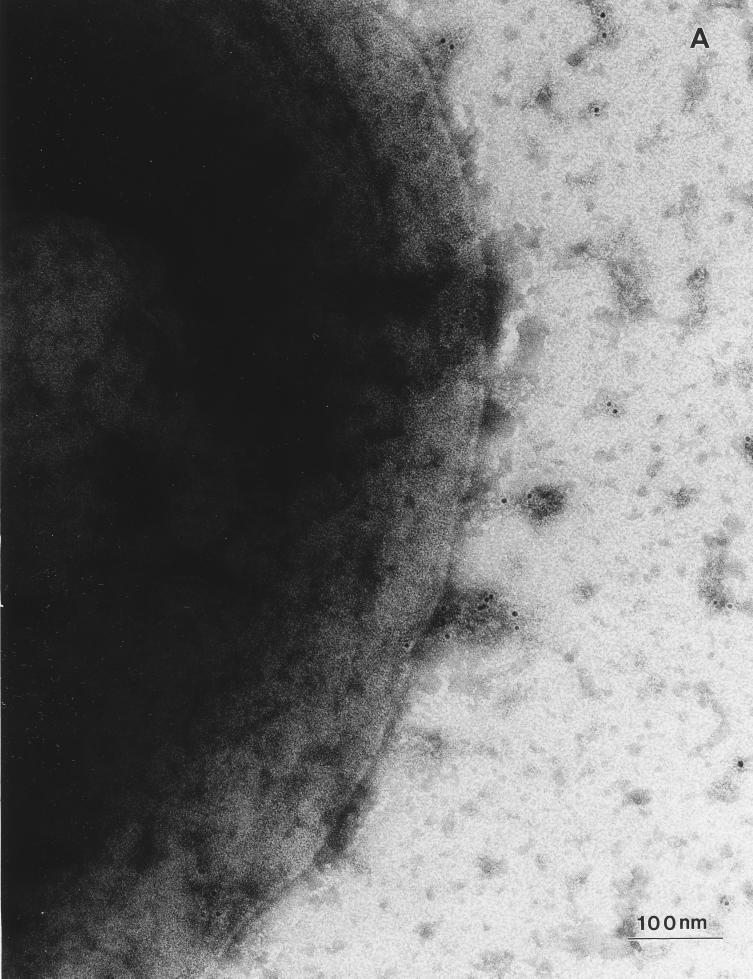
Immunogold labeling studies were carried out with two different CLB and HSAB isolates by using a polyclonal rabbit anti-FimA antibody. Examination of the immunogold-labeled bacteria by electron microscopy revealed intense fimbrial staining of every bacterium observed on the grids among the two different HSAB isolate populations (Fig. 2). In contrast, no specific staining of HB101 Fim− bacterial fimbriae was noted (data not shown). Most CLB had few or no visible fimbriae (Fig. 2), although occasionally bacteria among both CLB isolate populations exhibited fimbriae at approximately the same density as that observed among the HSAB isolates. When fimbriae were seen on CLB, they also demonstrated immunogold staining which suggested the presence of type 1 fimbriae on a portion of the CLB population. This is consistent with the observation that higher concentrations of CLB than those used in the agglutination assays described above often exhibited mannose-inhibitable agglutination of guinea pig RBCs.
FIG. 2.
Immunogold labeling of electron micrographs of CLB and HSAB with the anti-type 1 fimbrial antibody. (A) Electron micrograph of the surface of a representative CLB. (B) Electron micrograph of the surface of a representative HSAB.
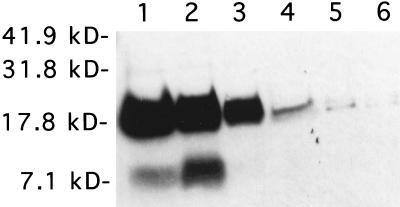
To compare the expression of type 1 fimbriae among the different murine E. coli isolates, Western blot analysis using the anti-FimA antibody was performed. Partially purified fimbriae prepared from 108 CLB, HSLB, HSAB, or HB101 Fim+ bacteria were heated in concentrated hydrochloric acid to disrupt the fimbrial aggregates into monomers, neutralized with sodium hydroxide, fractionated by SDS-polyacrylamide gel electrophoresis, and immunostained with the anti-FimA antibody. Results of a representative experiment are shown in Fig. 3. Bands consistent with type 1 fimbrial monomer were noted in fimbrial preparations from both CLB, HSLB, HSAB (Fig. 3), and HB101 Fim+ (data not shown) samples; however, HSAB fimbrial preparations consistently exhibited larger amounts of type 1 fimbriae than did CLB fimbrial preparations (Fig. 3). HSLB fimbrial preparations appeared to contain type 1 fimbriae in amounts intermediate between those of CLB and HSAB preparations (Fig. 3). The additional immunostained band of smaller molecular mass observed in the HSAB samples may be a truncated monomer.
FIG. 3.
Immunostaining with the anti-type 1 fimbrial antibody of fimbrial preparations fractionated by SDS-polyacrylamide electrophoresis. Fimbrial preparations of two representative isolates for each type of bacterium (HSAB, HSLB, and CLB) are shown. Lanes 1 and 2, HSAB isolates; lanes 3 and 4, HSLB isolates; lanes 5 and 6, CLB isolates. Molecular mass markers (in kilodaltons) are shown at the left.
HSAB adhered to the cecal epithelium to a greater extent than CLB.
To compare the adherence characteristics of the E. coli isolates, ceca were injected with either PBS, CLB, HSAB, or no fluid (sham). Six to eight hours after injection, the ceca were harvested and bacteria adhering to the cecal epithelium were cultured as described above. Two to four injection runs of each E. coli isolate were analyzed, and three different CLB isolates and five different HSAB isolates were used for these experiments. Results, expressed as mean log10 CFU per gram of tissue ± SEM, were 1.35 ± 0.32 for the sham injection and 1.97 ± 0.33 for PBS. Greater numbers of E. coli were found to be adhering to the mucosa in ceca injected with HSAB (4.68 ± 0.30 [n = 5]) than in those injected with CLB (3.75 ± 0.14 [n = 3]), despite the fact that the average number of bacteria injected was somewhat lower (3.7 × 107 CFU of HSAB/200 μl versus 5.0 × 107 CFU of CLB/200 μl). The injection of the HSAB isolates in the presence of mannose reduced the number of E. coli adhering for each of the three isolates tested (3.87 ± 0.24 [n = 3]). The mean reduction in the numbers of log10 CFU of adherent E. coli per gram of tissue with the addition of mannose was 0.42, which is relatively large compared to the SEM of 0.08.
HSAB isolates adhere to cultured murine colon cells.
YAMC cells are epithelial cells derived from intestinal crypts of the colon of a transgenic mouse bearing a temperature-sensitive mutation of the simian virus 40 large tumor antigen gene (39). Dispersed YAMC cells were incubated with two different CLB and HSLB isolates as well as with HSAB isolates in the presence or absence of 5% mannose. The YAMC cells were then washed to remove nonadherent bacteria, the YAMC cells were disrupted, and serial dilutions of the samples were cultured on selective agar to determine the mean log10 CFU of adherent bacteria per milliliter of sample. All samples run in triplicate. Results, expressed as mean log10 CFU per milliliter of sample ± SEM, were 0.00 ± 0.00 for the control (PBS). The HSAB and HSLB isolates adhered to YAMC cells in greater numbers than the CLB isolates (4.56 ± 0.17 for CLB, 5.25 ± 0.01 for HSLB, and 5.30 ± 0.08 for HSAB). The addition of mannose reduced the numbers of HSAB adhering to the YAMC cells to that of the CLB isolates (5.30 ± 0.08 for HSAB alone and 4.45 ± 0.27 for HSAB plus mannose). Both sets of CLB, HSLB, and HSAB isolates yielded similar results. These data suggest that type 1 fimbriae were involved in the adherence of the murine HSAB isolates to the YAMC cells.
No particular genotype predominated among adherent E. coli isolates.
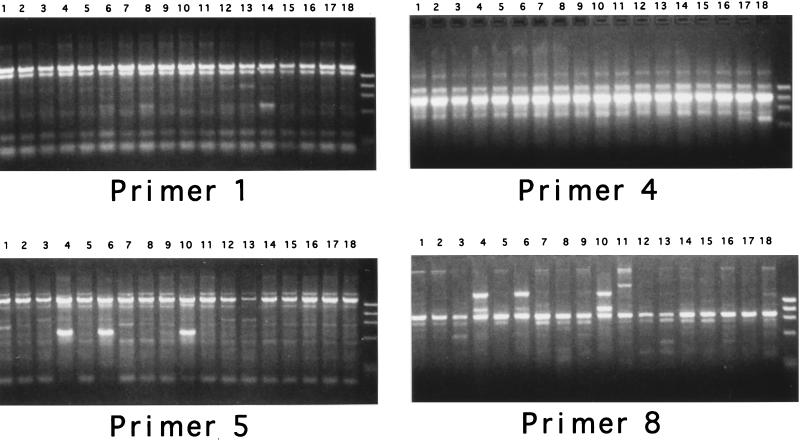
To investigate whether the genotype of the adherent E. coli isolates differed from that of the luminal E. coli isolates, 18 different murine E. coli isolates (9 HSAB, 6 CLB, and 3 HSLB) were examined by a random primer PCR genomic fingerprinting method (12). PCRs with four different random primers (Fig. 4) revealed that the murine E. coli isolates had at least 11 separate banding patterns and that three different human clinical E. coli isolates had 3 additional banding patterns (data not shown). No particular genotype predominated among the adherent E. coli isolates. Examples of LB and AB with indistinguishable banding patterns were noted, which suggested similar genotypes among at least some of the luminal and adherent E. coli isolates.
FIG. 4.
PCR genomic fingerprinting of adherent and luminal E. coli isolates harvested from CONT and HEP/STARV mice. Lanes 1 through 9, HSAB isolates; lanes 10 through 15, CLB isolates; lanes 16 through 18, HSLB isolates; rightmost lanes, DNA molecular weight standard. Results of PCRs with four different primer sets are shown.
DISCUSSION
Our data demonstrate that partial hepatectomy and short-term starvation were associated with increased numbers of aerobic gram-negative bacteria, predominantly E. coli, adhering to the cecal mucosa. Additionally, our studies revealed a greater abundance of surface type 1 fimbriae among the indigenous E. coli isolates harvested from the HEP/STARV mice and suggest a role for type 1 fimbriae in the adherence of the E. coli isolates to colonic epithelial cells.
Most of the studies examining the adherence of commensal gram-negative bacteria to the intestinal mucosa in vivo have been done in the rat model (20, 28, 35). In order to take advantage of available gene-targeted mouse lines in future studies, we developed a mouse model of catabolic stress involving partial hepatectomy, which results in increased energy demands due to tissue regeneration, and short-term starvation. Our studies also are unique in that we examined the adherence characteristics and fimbrial expression of the indigenous E. coli population in the intestinal tract as opposed to those of E. coli experimentally introduced into the mice.
Our study revealed that the numbers of E. coli increased in the cecum 20-fold following hepatectomy and short-term starvation. In contrast, the numbers of other enteric gram-negative bacteria, such as P. mirabilis did not increase in the ceca of HEP/STARV mice. The reason for the selective overgrowth of E. coli in the ceca of HEP/STARV mice is unclear. Intestinal overgrowth of bacteria, particularly Enterobacteriaceae such as E. coli, has been associated with an increased risk of bacteria translocating to the mesenteric lymph nodes (6). Consistent with this observation, bacterial cultures of feces from patients with leukemia revealed an association between the bacterial biotype present in the highest numbers in the patient’s feces with that causing subsequent septicemia in that patient (37).
While the numbers of E. coli were increased in the ceca of HEP/STARV mice, the 20-fold increase in total cecal E. coli (AB plus LB) was notably modest in comparison with the 7,500-fold increase in adherent E. coli, which suggested the contribution of other factors. Our data demonstrate that the increased expression of surface adhesins among the E. coli population in the ceca of HEP/STARV mice was likely a significant factor and that increased numbers of type 1 fimbriae appeared to play a role. RBC agglutination studies, as well as immunostaining of fimbrial preparations and electron micrographs of the bacteria, all were consistent with a spectrum of type 1 fimbrial density among the E. coli isolates harvested from the experimental mice, with CLB isolates exhibiting the least abundant and HSAB isolates exhibiting the most abundant surface type 1 fimbriae. Although the differences observed in the amount of surface type 1 fimbriae in the E. coli isolates after broth culture may have occurred by chance and may thus not reflect the in vivo state, all the HSAB isolates examined had more abundant surface type 1 fimbriae than the CLB isolates by each of the methods used. Hence, variations in the amount of surface type 1 fimbriae between CLB and HSAB isolates after growth in static broth cultures may reflect differences in the kinetics of fimbrial expression among the CLB and HSAB strains similar to those described in urinary-tract isolates of E. coli (19).
Our studies suggest that type 1 fimbriae may be involved in mediating the adherence of the indigenous E. coli to the cecal mucosa following hepatectomy and starvation. However, other factors, such as changes in intestinal transit time, alterations in mucus quality, innate immune factors, and the accessibility or quantity of fimbrial receptors on the intestinal epithelium also may be important. Although the adherence of the bacteria to the cecal epithelium in vivo may be related to adhesins other than type 1 fimbriae, virtually all the fimbriae of HSAB isolates visualized by electron microscopy reacted with the type 1 fimbrial antibody. This finding does not rule out the possibility that another unidentified factor which is coregulated with type 1 fimbriae may be important in the adherence of the HSAB isolates to the intestinal epithelium. However, mannose reduced the adherence of the HSAB isolates to injected cecal loops and to a mouse colon cell line, suggesting an involvement of type 1 fimbriae.
The expression of type 1 fimbriae involves a process known as phase variation, in which individual bacteria in a population alternate between piliated and nonpiliated states (11). Conceivably, partial hepatectomy and short-term starvation may be associated with alterations in the intestinal microenvironment which favor the piliated state in commensal E. coli. Few studies have examined the factors which influence type 1 fimbrial expression in vivo, although type 1 fimbrial phase variation has been shown to occur in animal models of experimental peritonitis and urinary-tract infection (3, 26). Type 1 fimbrial phase variation is known to be sensitive in vitro to the concentrations of certain aliphatic amino acids, such as alanine and leucine, and this regulation requires the leucine-responsive regulatory protein (14). Thus, during periods of host catabolic stress, the commensal bacteria may adhere to cells in order to gain access to nutrients within host cells. In support of this notion, Zafriri et al. have shown that adherence of gram-negative bacteria to epithelial cells allowed these bacteria to obtain nutrients from underlying cells and enhanced their toxicity due to the restricted diffusion of toxic products secreted by the bacteria (40). Thus, depleted amino acid levels in the intestinal lumen may stimulate indigenous E. coli to express type 1 fimbriae in order for the bacteria to adhere to and extract nutrients from intestinal epithelial cells. In addition, alterations in other factors, such as luminal pH or catecholamine levels, may play a role (24, 27).
Rather than a global upregulation of type 1 fimbrial expression among the indigenous E. coli population, partial hepatectomy and short-term starvation may be associated with the predominance in the cecum of a subset of E. coli strains with increased amounts of surface type 1 fimbriae. The adherent isolates possibly represent E. coli strains that were capable of upregulating type 1 fimbrial expression either to a greater extent or more quickly than the luminal isolates. In addition, FimH variants with increased mannose binding have been described; these may also account in part for the increased adherence of HSAB strains to the colonic epithelial cells (33, 34). Although a limited number of isolates were analyzed, our preliminary results using PCR genomic fingerprinting suggested the presence of multiple E. coli strains among the adherent E. coli isolates as opposed to the predominance of one or two strains. Additional studies are needed to clarify whether a subpopulation of the cecal E. coli population adheres to the mucosa in a particular HEP/STARV mouse or if the adherent strains are representative of the total cecal E. coli population.
The increased adherence of the indigenous E. coli to the cecal epithelium is likely to have pathologic consequences (4). Adherence to the intestinal epithelium is thought to be the initial step in the translocation of E. coli from the intestinal lumen to systemic sites (4, 9). Additionally, our data suggest that increased numbers of adherent commensal E. coli bacteria are associated with alterations in the electrophysiology of colonic cells (30, 35). Several studies have reported that bacterial invasion of, and conceivably bacterial adherence to, intestinal epithelial cells may stimulate secretion of proinflammatory cytokines (16, 29), which could contribute to alterations in mucosal barrier function (1, 7). Further studies are needed to define the effect of increased commensal E. coli adherence on intestinal epithelial cell function.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grant DK42086.
The technical assistance of Robert Josephs and the Electron Microscopy Facility of the Digestive Diseases Core Center at the University of Chicago are gratefully acknowledged. We also thank Phil Schumm of the Biostatistics Facility of the University of Chicago Cancer Research Center for assistance with the statistical analysis of the data.
REFERENCES
- 1.Adams R B, Planchon S M, Roche J K. IFN-γ modulation of epithelial barrier function: time course, reversability, and site of cytokine binding. J Immunol. 1993;150:2356. [PubMed] [Google Scholar]
- 2.Adlerberth I, Hanson L A, Svanborg C, Svennerholm A-M, Nordgren S, Wold A E. Adhesins of Escherichia coli associated with extra-intestinal pathogenicity confer binding to colonic epithelial cells. Microb Pathog. 1995;18:373–385. doi: 10.1006/mpat.1995.0034. [DOI] [PubMed] [Google Scholar]
- 3.Alkan M L, Wong L, Silverblatt F J. Change in degree of type 1 piliation of Escherichia coli during experimental peritonitis in the mouse. Infect Immun. 1986;54:549–554. doi: 10.1128/iai.54.2.549-554.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alverdy J C, Spitz J, Hecht G, Ghandi S. Causes and consequences of bacterial adherence to mucosal epithelia during critical illness. New Horizons. 1994;2:264–272. [PubMed] [Google Scholar]
- 5.Amann R I, Krumholz L, Stahl D A. Fluorescent-oligonucleotide probing of whole cells for determinative, phylogenetic, and environmental studies in microbiology. J Bacteriol. 1990;172:762–770. doi: 10.1128/jb.172.2.762-770.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berg R D. Bacterial translocation from the gastrointestinal tract. Trends Microbiol. 1995;3:149–54. doi: 10.1016/s0966-842x(00)88906-4. . (Review.) [DOI] [PubMed] [Google Scholar]
- 7.Colgan S P, Resnick M B, Parkos C A, Delp-Archer C, McGuirk S, Bacarra A E, Wellwe P F, Madara J L. IL-4 modulates function of a model human intestinal epithelium. J Immunol. 1994;153:2122–2129. [PubMed] [Google Scholar]
- 8.Connell I, Agace W, Klemm P, Schembri M, Marild S, Svanborg C. Type 1 fimbrial expression enhances Escherichia coli virulence for the urinary tract. Proc Natl Acad Sci USA. 1996;93:9827–9832. doi: 10.1073/pnas.93.18.9827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cruz N, Alvarez X, Specian R D, Berg R D, Deitch E A. Role of mucin, mannose, and beta-1 integrin receptors in Escherichia coli translocation across Caco-2 cell monolayers. Shock. 1994;2:121–126. doi: 10.1097/00024382-199408000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Donaldson S G, Azizi S Q, Dal Nogare A R. Characteristics of aerobic gram-negative bacteria colonizing critically ill patients. Am Rev Respir Dis. 1991;144:202–207. doi: 10.1164/ajrccm/144.1.202. [DOI] [PubMed] [Google Scholar]
- 11.Eisenstein B I. Phase variation of type 1 fimbriae in Escherichia coli is under transcriptional control. Science. 1981;214:337–339. doi: 10.1126/science.6116279. [DOI] [PubMed] [Google Scholar]
- 12.Eshdat Y, Silverblatt F J, Sharon N. Dissociation and reassembly of Escherichia coli type 1 pili. J Bacteriol. 1981;148:308–314. doi: 10.1128/jb.148.1.308-314.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friman V, Adlerberth I, Connell H, Svanborg C, Hanson L A, Wold A E. Decreased expression of mannose-specific adhesins by Escherichia coli in the colonic microfora of immunoglobulin A-deficient individuals. Infect Immun. 1996;64:2794–2798. doi: 10.1128/iai.64.7.2794-2798.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gally D L, Rucker T J, Blomfield I C. The leucine-responsive regulatory protein binds to the fim switch to control phase variation of type 1 fimbrial expression in Escherichia coli K-12. J Bacteriol. 1994;176:5665–5672. doi: 10.1128/jb.176.18.5665-5672.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gautreaux M D, Deitch E A, Berg R D. T lymphocytes in host defense against bacterial translocation from the gastrointestinal tract. Infect Immun. 1994;62:2874–2884. doi: 10.1128/iai.62.7.2874-2884.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hedlund M, Svensson M, Nilsson A, Duan R-D, Svanborg C. Role of ceramide-signaling pathway in cytokine responses to P-fimbriated Escherichia coli. J Exp Med. 1996;183:1037–1044. doi: 10.1084/jem.183.3.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herias M V, Midtvedt T, Hanson L A, Wold A E. Role of Escherichia coli P fimbriae in intestinal colonization in gnotobiotic rats. Infect Immun. 1995;63:4781–4789. doi: 10.1128/iai.63.12.4781-4789.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hultgren S J, Abraham S, Caparon M, Falk P, St. Geme III J W, Normark S. Pilus and nonpilus bacterial adhesins: assembly and function in cell recognition. Cell. 1993;73:887–901. doi: 10.1016/0092-8674(93)90269-v. . (Review.) [DOI] [PubMed] [Google Scholar]
- 19.Hultgren S J, Schwan W R, Schaeffer A J, Duncan J L. Regulation of production of type 1 pili among urinary tract isolates of Escherichia coli. Infect Immun. 1986;54:613–620. doi: 10.1128/iai.54.3.613-620.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katouli M, Nettelbladt C G, Muratov V, Ljungqvist O, Bark T, Svenberg T, Mollby R. Selective translocation of coliform bacteria adhering to the caecal epithelium of rats during catabolic stress. J Med Microbiol. 1997;46:571–578. doi: 10.1099/00222615-46-7-571. [DOI] [PubMed] [Google Scholar]
- 21.Krogfelt K A, McCormick B A, Burghoff R L, Laux D C, Cohen P S. Expression of Escherichia coli F-18 type 1 fimbriae in the streptomycin-treated mouse large intestine. Infect Immun. 1991;59:1567–1568. doi: 10.1128/iai.59.4.1567-1568.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krogfelt K A. Bacterial adhesions: genetics, biogenesis, and the role in pathogenesis of fimbrial adhesions of Escherichia coli. Rev Infect Dis. 1991;13:721–735. doi: 10.1093/clinids/13.4.721. [DOI] [PubMed] [Google Scholar]
- 23.Langermann S, Palaszynski S, Barnhart M, Auguste G, Pinkner J S, Burlein J, Barren P, Koenig S, Leath S, Jones C H, Hultgren S J. Prevention of mucosal Escherichia coli infection by FimH adhesin-based systemic vaccination. Science. 1997;276:607–611. doi: 10.1126/science.276.5312.607. [DOI] [PubMed] [Google Scholar]
- 24.Laughlin, R., and J. C. Alverdy. Unpublished data.
- 25.Le Frock, J. L., C. A. Ellis, J. B. Turchik, J. K. Zawacki, and L. Weinstein. 1975. Transient bacteremia associated with percutaneous liver biopsy. J. Infect. Dis. 131(Suppl):S104–S107. [DOI] [PubMed]
- 26.Lim J K, Gunther IV N W, Zhao H, Johnson D E, Keay S K, Mobley H L T. In vivo phase variation of Escherichia coli type 1 fimbrial genes in women with urinary tract infection. Infect Immun. 1998;66:3303–3310. doi: 10.1128/iai.66.7.3303-3310.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lyte M, Bailey M T. Neuroendocrine-bacterial interactions in a neurotoxin induced model of trauma. J Surg Res. 1997;70:195–201. doi: 10.1006/jsre.1997.5130. [DOI] [PubMed] [Google Scholar]
- 28.Nettelbladt C G, Katouli M, Volpe A, Bark T, Muratov V, Svenberg T, Mollby R, Ljungqvist O. Starvation increases the number of coliform bacteria in the caecum and induces bacterial adherence to caecal epithelium. Eur J Surg. 1997;163:135–142. [PubMed] [Google Scholar]
- 29.Parkinson J S. Signal transduction schemes of bacteria. Cell. 1993;73:857–871. doi: 10.1016/0092-8674(93)90267-t. [DOI] [PubMed] [Google Scholar]
- 30.Rocha, F., and J. C. Alverdy. Unpublished data.
- 31.Schaeffer A J, Schwan W R, Hultgren S J, Duncan J L. Relationship of type 1 pilus expression in Escherichia coli to ascending urinary tract infection of mice. Infect Immun. 1987;55:373–380. doi: 10.1128/iai.55.2.373-380.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwan W R, Seifert H S, Duncan J L. Growth conditions mediate differential transcription of fim genes involved in phase variation of type 1 pili. J Bacteriol. 1992;174:2367–2375. doi: 10.1128/jb.174.7.2367-2375.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sokurenko E V, Chesnokova V, Dykhuizen D E, Ofek I, Wu X R, Krogfelt K A, Struve C, Schembri M A, Hasty D L. Pathogenic adaptation of Escherichia coli by natural variation of the FimH adhesin. Proc Natl Acad Sci USA. 1998;95:8922–8926. doi: 10.1073/pnas.95.15.8922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sokurenko E V, Courtney H S, Maslow J, Siitonen A, Hasty D L. Quantitative differences in adhesiveness of type 1 fimbriated Escherichia coli due to structural differences in fimH genes. J Bacteriol. 1995;177:3680–3686. doi: 10.1128/jb.177.13.3680-3686.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spitz J, Hecht G, Taveras M, Aoys E, Alverdy J. The effect of dexamethasone administration on rat intestinal permeability: the role of bacterial adherence. Gastroenterology. 1994;106:35–41. doi: 10.1016/s0016-5085(94)94155-6. [DOI] [PubMed] [Google Scholar]
- 36.Swidinski A, Khilkin M, Kerjaschki D, Schreiber S, Ortner M, Weber J, Lochs H. Association between intraepithelial Escherichia coli and colorectal cancer. Gastroenterology. 1998;115:281–286. doi: 10.1016/s0016-5085(98)70194-5. [DOI] [PubMed] [Google Scholar]
- 37.Tancrede C H, Andremont A O. Bacterial translocation and gram-negative bacteremia in patients with hematological malignancies. J Infect Dis. 1985;152:99–103. doi: 10.1093/infdis/152.1.99. [DOI] [PubMed] [Google Scholar]
- 38.Welsh J, McClelland M. Fingerprinting genomes using PCR with arbitrary primers. Nucleic Acids Res. 1990;18:7213–7218. doi: 10.1093/nar/18.24.7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Whitehead R H, VanEeden P E, Noble M D, Ataliotis P, Jat P S. Establishment of conditionally immortalized epithelial cell lines from both colon and small intestine of adult H-2Kb-tsA58 transgenic mice. Proc Natl Acad Sci USA. 1993;90:587–591. doi: 10.1073/pnas.90.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zafriri D, Oron Y, Eisenstein B I, Ofek I. Growth advantage and enhanced toxicity of Escherichia coli adherent to tissue culture cells due to restricted diffusion of products secreted by the cells. J Clin Investig. 1987;79:2110–1216. doi: 10.1172/JCI112939. [DOI] [PMC free article] [PubMed] [Google Scholar]