Abstract
Abstract
Atmospheric cold plasma (ACP) is a nonthermal technology that is extensively used in several industries. Within the scopes of engineering and biotechnology, some notable applications of ACP include waste management, material modification, medicine, and agriculture. Notwithstanding numerous applications, ACP still encounters a number of challenges such as diverse types of plasma generators and sizes, causing standardization challenges. This review focuses on the uses of ACP in engineering and biotechnology sectors in which the innovation can positively impact the operation process, enhance safety, and reduce cost. Additionally, its limitations are examined. Since ACP is still in its nascent stage, the review will also propose potential research opportunities that can help scientists gain more insights on the technology.
Key points
• ACP technology has been used in agriculture, medical, and bioprocessing industries.
• Chemical study on the reactive species is crucial to produce function-specific ACP.
• Different ACP devices and conditions still pose standardization problems.
Keywords: Atmospheric cold plasma, Waste management, Material modification, Medicine, Agriculture
Introduction
Plasma is the fourth state of matter along with the solid, liquid, and gas that is composed of high-energy positively charged ions, electrons, and neutrals (atoms, molecules, and radicals) (Boyd et al. 2003). During plasma generation process, the gas introduced into the system is ionized by electric current. This electrical resistivity produces significant heat which strips electrons away from the gas molecules, forming an ionized gas stream called plasma (Gomez et al. 2009). The plasma technique can be separated into two types based on the temperature of produced ions: thermal/hot and non-thermal/cold plasma (Hoffmann et al. 2013). In the thermal plasma, the plasma is produced at high pressure wherewith all the gas particles are strongly ionized which result in ions, atoms, electrons, and neutral species retaining the same high temperature range of 4000 to 20,000 K (Ruj and Ghosh 2014). Due to its high temperature and energy density, thermal plasma has a highly destructive ability and is suitable in degrading various types of waste such as organic (Huang and Tang 2007), medical (Cai and Du 2021), electronic (Rath et al. 2012), and radioactive wastes (Prado et al. 2020). In addition to waste degradation, thermal plasma can also provide rapid quenching to keep some chemical materials in the metastable stage under ambient conditions to improve their modification or synthesis. This particular advantage makes thermal plasma valuable in material processing including coating technologies, synthesis of fine powders, densification of powders, and slag metallurgy (Samal 2017). In contrast to thermal plasma, non-thermal plasma refers to the production of low-temperature particles like charged particles and radicals under atmospheric condition. Because of its low temperature property, non-thermal plasma is also called atmospheric cold plasma (ACP) (Hoffmann et al. 2013).
As previously stated, thermal plasma presents great efficacy for various waste decomposition owing to its high energy and temperature. However, a concern regarding cost-effectiveness should also be considered since the consumption of electrical energy is relatively high (Samal 2017). In contrast, ACP utilizes much lower electrical energy and can generate plasma at low or atmospheric pressure (Laroque et al. 2022). Consequently, ACP is more energy efficient for pollutant destruction compared to thermal plasma.
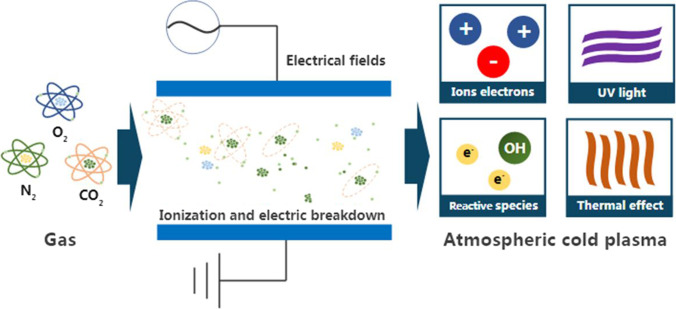
Depending on the ACP generation system and working gas (inlet gas), a variety of active species can be produced for specific applications. Figure 1 illustrates how the gas undergoes through ionization and electric breakdown to generate reactive species and other products in ACP.
Fig. 1.
The mechanism of ACP and generation of its products
Presently, many investigations on ACP focus on food modification and processing since it has an ability to inactivate pathogens and undesirable food compounds while exhibiting minimal effects on the desired attributes. Moreover, ACP devices have been improved continuously which enable them to consume less energy by 55% from 0.03–0.10 to 0.017–0.051 kWh, thereby being more economical (Niemira 2012).
Therefore, with its high effectiveness combined with low cost of operation, ACP has gained a growing interest in other fields such as waste management, material modification, medicine, and agriculture as it can contribute to safety, operation process, and cost reduction within these sectors. In spite of the progress, this highly functional technology still has slight drawbacks that may require additional studies prior to being readily employed in diverse fields. This paper explores current applications of ACP in agricultural, medical, and bioprocessing industries, in addition to proposing current limitations and future research opportunities.
ACP devices
Among the atmospheric cold plasma devices, the dielectric barrier discharge (DBD), plasma jet, gliding arc discharge (GAD), and corona discharge are the most extensively studied (Bermudez-Aguirre 2019; Stryczewska 2020). DBD is consisted of two parallel metal electrodes which are coated with dielectric materials such as quartz, polymer, or plastic. The main function of these materials is required to help regulate the consistency of output plasma (Nasiru et al. 2021). In the device, one electrode is connected to a voltage source while another is grounded. When DBD is operated, a carrier gas introduced into the parallel electrodes will be ionized by Coulombic force and disintegrated, essentially producing several reactive species. DBD cold plasma is well-recognized for the high energy of free electrons because particles would be accelerated into a gap of electric field and collided, promoting high kinetic energy and momentum (Choudhury 2017).
Plasma jet shares the similar concept to that of DBD in regard to cold plasma generation, but the schematic design differs. The needle electrode within the plasma jet is attached to the voltage source whereas the ground external electrode surrounds the needle. As the carrier gas passes between the two electrodes at a high speed, it becomes ionized, excited, and dissociated to produce various reactive species prior to being accumulated and exited through a nozzle as a beam. The beam stability is highly dependent on the type of carrier gas (Umair et al. 2021). In gliding arc discharge (GAD), air is pumped into electrode gaps which results in the formation of an arc between the electrodes (Ekezie et al. 2017). On the other hand, corona discharge employs two or more sharp pointed electrodes to generate an ionized electric field of reactive species. Generally, a point electrode with a high electric field breaks down inlet gas, creating a non-homogeneous discharge when this ACP device is in use. Nevertheless, corona discharge is inexpensive and relatively simple to operate (Ekezie et al. 2017).
Among all the devices, DBD possesses several advantages compared to other ACP generators. Firstly, DBD does not require a high area output unlike plasma jet. Moreover, compared to corona discharge, DBD is only limited by the power voltage (1 to 160 kV) whereas corona discharge depends on both the power supply and operation distance of a few millimeters. Furthermore, the ability to regulate current in DBD can help improve safety by avoiding spark which is normally present in gliding arc discharge (Nasiru et al. 2021). With these previously mentioned benefits combined with low maintenance and operational cost, DBD has become one of the most common atmospheric plasma generators to eliminate pathogens (Kilonzo-Nthenge et al. 2018; Mahnot et al. 2019; Roh et al. 2020), deactivate enzymes (Chang et al. 2021; Gu et al. 2021; Tolouie et al. 2018), and degrade pesticides (Cong et al. 2021; Liu et al. 2021a; Mousavi et al. 2017). However, microdischarges from DBD plasma still hinders its application as they cause non-uniform treatment of the sample (Choudhury 2017).
Applications of ACP in sewage disposal and waste management
ACP has been integrated in sewage disposal and wastewater management because of its antimicrobial and chemical decomposition properties. Relevant publications about waste management are summarized in Table 1.
Table 1.
Applications of ACP in sewage and waste management
| Waste and sewage disposal | |||||||
|---|---|---|---|---|---|---|---|
| Treatment target | ACP generator | Treatment | Reference | ||||
| Exposure distance (mm) | Gas | Time (min) | Input power (W) | Voltage (kV) | |||
| Municipal waste treatment | Mini glide-arc | 15 | Air | 5, 15, and 45 | - | 3.8 | (Pawłat et al. 2021) |
| Industrial waste salt treatment | DBD | - | Air and O2 | 0 ~ 25 | - | 12 ~ 16 | (Wang et al. 2021) |
| Olive mill wastewater treatment | Plasma jet | - | - | 15 and 30 | 3500 | 0.2 | (Ibrahimoglu and Yilmazoglu 2018) |
| Textile wastewater treatment | DBD | - | O2 | 45 and 90 | - | - | (Tecer et al. 2020) |
| Azo dye treatment | DBD | - | Air, N2 and O2 | 1 ~ 15 | - | 20 | (Iervolino et al. 2020) |
| Dye wastewater treatment | Plasma jet | 6 | Air, N2, Ar, and O2 | 10 | - | 3 | (Pandiyaraj et al. 2021) |
| Pesticide degradation | DBD | 37 | Air | 1 ~ 5 | - | 70 ~ 90 | (Moutiq et al. 2020) |
| Sugarcane bagasse hydrolysate | Plasma jet | 10 | Air | 25 | 200 | - | (Lin et al. 2020) |
| Pineapple peel waste | Plasma jet | 10 | Air and Ar | 60 | 80–600 | - | (Santoso et al. 2021) |
Food waste
An annual average of 1.3 billion tons of food waste reflects an inefficient utilization of global resources and hence the need to develop a more efficient management method (Tkáč et al. 2022). Food waste is defined by UN Environmental Program as any food parts that undergo through every process of the food supply chain, but still do not get consumed and are thus discarded, which are subsequently left to spoil or expire (UNEP 2021). Pawłat et al. (2021) indicated that ACP treatment can decrease a number of microorganisms from municipal waste such as vegetative bacteria and endospores, mold fungi, actinobacteria Escherichia coli, and facultative pathogens, i.e., Staphylococcus spp., Salmonella spp., Shigella spp., Enterococcus faecalis, and Clostridium perfringens. Based on the study, ACP as sterile pretreatment of municipal waste can mitigate environmental contamination during transfer and storage. Nevertheless, the caveat in regard to ACP is that microbial sterilization efficiency is highly dependent on diversity of microorganisms and plasma treatment time.
In addition to microbial inactivation, ACP may have the potential to produce value-added products from food waste by assisting in the bioconversion process. In the lignocellulosic waste pretreatment, ACP has been shown to degrade some toxic compounds that inhibit bioethanol fermentation (Nishimwe et al. 2021; Shi et al. 2017a, 2017b). Lin and colleagues (2020) successfully removed fermentation inhibitors including hydroxymethylfurfural (HMF), formic acid, and furfural from sugarcane bagasse acid hydrolysate using ACP. The detoxified hydrolysate was then used as nutrients for bioethanol production (5.24 g/L). By incorporating ACP into the pretreatment process to remove these toxic compounds, the bioethanol production industry may obtain benefits from the technology. In the similar study, Santoso et al. (2021) selected pineapple peel waste hydrolysate and exploited ACP for the detoxification process (argon based ACP under 80–200 W and air based ACP under 500–600 W) to produce bacterial cellulose. Since bacterial cellulose has extensive applications especially in the biomedical field, this study demonstrates that low-cost plant cellulose from food waste can actually be recycled to produce value-added bacterial cellulose.
Industrial wastewater
Industrial waste presents a recurring challenge in waste management not only due to a large amount of discarded materials across variety of industries, but also the complexities within the waste itself. Over 20% of water pollution comes from textile dyeing process, and a large amount of potable water is used along with more than 8000 synthetic chemicals in the process (Kant 2011). Approximately 280,000 t of highly contaminated wastewater are released during production process in textile industries worldwide; these wastewaters are highly toxic, causing enormous damage to the environment (Nippatla and Philip 2019). Nonetheless, the issue with textile wastewater is extremely difficult to solve because of its complex compositions including dyes, textile auxiliaries, and other textile chemicals (Arslan-Alaton and Alaton 2007). Nippatla and Philip (2019) attempted to use underwater parallel-multi-tube atmospheric pressure plasma to decrease textile dye dissolved in textile wastewater and discovered that almost 70% of dyes could be degraded within 10-min plasma treatment. The increased dye degradation with decreased O3, H2O2, and NO3− suggests that these active species participate in the dye decomposition. This result provides a competitive technology for conventional decomposition of textile dyes. Tecer et al. (2020) also established an ACP reactor to reduce different reactive azo dyes for at least 30 L textile wastewater. The chemical oxygen demand (COD) of ACP treated wastewater was decreased by 99%, and its color became transparent after 90-min treatment. The treated wastewater was then recycled in the new dying process, and the fabric was found to have a similar quality in terms of color, levelness, and speed performance compared to the one from osmosis water (Tecer et al. 2020).
Moreover, some chemicals can be used to assist non-thermal plasma to enhance dye removal efficiency or degradation. Iervolino and team (2020) found that an addition of hydrogen peroxide can improve the azo dye removal from 60 to 80% within 2.5 min because more hydroxyl radicals are released from hydrogen peroxide during the process. Pandiyaraj et al. (2021) also demonstrated that the addition of Cu-TiO2 nanoparticles to dye wastewater can synergistically improve the degradation effect. The photocatalytic activity of Cu-TiO2 nanoparticles leads to the formation of oxygen vacancies after plasma stimulation. Thereafter, the electrons from plasma will interact with oxidized Ti4+ to form unstable Ti3+ on the surface of Cu-TiO2 nanoparticles resulting in triggering more reactive species production. The synergistic effect of nanoparticles and plasma therefore enhances the capability of dye degradation.
Furthermore, olive mill wastewater is a byproduct of olive oil production process which is composed of organic matter, suspended solids, oil, and grease. After ACP treatment, its chemical oxygen demand (COD) and biological oxygen demand (BOD) both decreased by 94.42% and 95.37%, respectively. In addition, the dissolved oxygen also increased from 0.36 to 6.97 mg/L (Ibrahimoglu and Yilmazoglu 2018).
Additionally, industrial salt can cause a serious harm to the environmental (Li et al. 2019). When Wang et al. (2021) used ACP to treat the salt waste, they demonstrated that the physical components of ACP such as heat, light, and shock wave, as well as active species (O3, H2O2, and ∙OH) can decrease the salt content in the industrial wastewater. According to their result, the original total organic carbon (TOC) was decreased to 10 mg/kg after using the dielectric barrier discharge system, and the process did not generate additional wastewater. Although it can be observed that ACP can effectively treat various types of industrial wastewater, it should also be noted that the results generated vary which may require additional research and standardization experiments.
Agricultural wastewater
Besides industrial wastewater, agricultural wastewater is also another subject of concern due to the amount of pesticide residues. Moutiq et al. (2020) selected three types of carbamates to study the efficacy of ACP on pesticide degradation. Based on their results, 50.5%, 99.6%, and 99.3% of carbaryl, methiocarb, and aminocarb could be degraded respectively when the conditions were set at 90 kV for 5 min. While these findings suggest that ACP exhibits high potential in the wastewater treatment process, the results also confirm that the efficacy of ACP is undeniably dependent on the interactions between reactive species and initial substrates. This means that the treatment conditions should be carefully characterized to accommodate specific medium.
Applications of ACP in material modification
With an increasingly aggressive innovation in modern industrial processing, material surface modification is more essential than ever. Conventionally, material modification procedures often involve chemical processes which can be highly toxic and costly (Cheng et al. 2006). This ongoing issue urges the need to find an alternative approach. ACP can provide a non-destructive and environmental friendly method for surface modification due to its low temperature and electrical energy consumption (Hoffmann et al. 2013). As shown in Table 2, most studies have found that the use of ACP in various material alterations can achieve great performance in both metal and polymer.
Table 2.
Applications of ACP in material modification
| Materials | Plasma device | Parameters | Physicochemical change (optimization methods were chosen) | Reference | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Input power (W) | Exposure time (s) | Frequency (MHz) | ThickNess (mm) | Tensile strength (MPa) | Elastic modulus (MPa) | Elongation at break | WVP | ||||
|
CNMA- CMC/LDPE CNMA-COL/LDPE CNMA-CMC, COL/LDPE |
RF-plasma | 30 | 60 | 13.56 | + 29.97% | + 13.58% | - | - | - | (Loke et al. 2021) | |
| 1–3% SEO-CS/LDPE | - | 84 | 10 | - | + 650% | − 3% | - | − 27% | − 96.7% | (Moradi et al. 2020) | |
| Citrus/PET | Plasma jet | - | - | 30 kHz | + 150% | - | - | - | - | (Contini et al. 2014) | |
|
Nylon/PP Nylon/LDPE |
DBD | 21 kW | 180 | - | - |
Nylon/PP + 1.6% Nylon/PE-0.5% |
Nylon/PP + 1.2% Nylon/PE + 0.5% |
Nylon/PP-0.3% Nylon/PE + 0.9% |
Nylon/PP-6.25% Nylon/PE-7.7% |
(Kang et al. 2021) | |
| CMC, COL/LDPE | RF-plasma | 30 | 60 | 13.56 | - | + 7.6% | + 47.43% | - | + 114% | (Chang et al. 2021) | |
| Waterless dyeing of cotton, nylon, and polyester | Plasma jet | 300 | 180 and 300 | - | - | - | - | - | - | (Zaidy et al. 2019) | |
RF radiofrequency, WVP water vapor permeability, LDPE low-density polyethylene, DBD dielectric barrier discharge, CS chitosan, PE polyethylene, CNMA cinnamaldehyde, CMC carboxymethyl cellulose, COL collagen, SEO summer savory essential oil, PET polyethylene terephthalate, PP polypropylene
DSSC
Weerasinghe and colleagues (2021) demonstrated that argon ACP treatment can increase surface roughness of SnO2 photoanode, thereby inducing more nitrogen species incorporation and improving dye adhesion. This processing enhances the efficiency of the SnO2 applied in dye-sensitized solar cells (DSSCs) by at least five folds. Furthermore, Wante et al. (2021) used dielectric barrier discharge ACP to improve DSSC fabrication. The hydrophilicity of polyetherimide (PEI) was augmented after using air ACP system under 20.5 kV with 1 min of treatment time. Moreover, the power conversion efficiency of ACP-PEI DSSC was increased evidently by 60% compared to the untreated group.
In addition to boosting the conversion efficiency of DSSC, ACP flow could also be exploited to deposit titanium dioxide on the silica substrates as solar cell photoanode (Perraudeau et al. 2019). ACP promotes an alignment of anatase crystals microstructure on the silica scaffold which provides higher specific surface area of perovskite layer and dye, ultimately improving solar energy conversion. However, it may also cause the deposit of amorphous TiO2 particles on the silica surface which can decrease photoanode optical transmission. Hence, the extra step to remove the amorphous TiO2 particles is necessary in this method. On the other hand, different type of gas used in ACP is also an important factor in TiO2 deposition related study. Okuya et al. (2021) discovered that TiO2 layer formation of DSSC becomes more porous under an ACP treatment with the flowing mixture of nitrogen and oxygen gases. This porous structure can help decrease internal resistance within the layers and thereby enhance the conversion efficiency of DSSC. With the improved porosity, Mahmoudabadi et al. (2018) indicated that Ag/TiO2 nanocomposite can promote DSSC conversion efficiency due to the higher specific surface area of deposition and decline of band-gap energy which usually delay charge recombination. Essentially, the studies examine multiple DSSC layers with ACP processing instead of a single layer with TiO2 coating.
Omelianovych et al. (2020) also developed a CoxSn1-x/reduced graphene oxide (RGO) nanohybrids synthesized on DSSC by utilizing ACP which resulted in 5.36% of power conversion efficiency. This improved efficiency was caused by higher surface area which in turn increased stability of the alloy structure. The successful research would not only lead to a cost-effective production of dye-sensitized solar cells, but it would also provide a great potential in long-term operation. The other research teams employed ACP to synthesize Pt/Fe as photoanode in DSSC (Cao et al. 2021) or ZnO nanosheets (Zhang et al. 2021b), which show the same positive results in terms of higher catalytic activity, stability, and durability. These publications suggest that composite material photoanode through ACP synthesis presents an extremely effective technique. Moreover, another team of researchers is able to exploit properties of ACP to create a low-cost counter electrode by modifying Ni-doped TiN nanowires with graphite carbon nanofibers (CNF). According to the results, the Ni/TiN/CNF counter electrode shows high performance and stability for as long as 5 h under irradiation, highlighting its potential to replace the expensive platinum counter electrode (Liu et al. 2021b).
These studies indicate that ACP processing provides an efficient cleaning and coating method, in addition to increasing dye adhesion and improving multiple modified particles on the DSSC surface.
Polymers and films
Most studies of ACP on polymer modification are focused on celluloses (Kusano et al. 2019), proteins, and polysaccharides due to their huge applications in medical, food, and textile industry (Peran and Ercegović Ražić 2020; Shakeri 2020). ACP with He/NH3 gas mixture can be used to enhance the oxidation and wettability of cellulose nanofibers coating layer (Ferreira et al. 2019). Jannat Amani et al. (2019) modified bacterial cellulose using ACP and discovered that exposure time longer than 3 min can improve water vapor permeability of cellulose due to the removal of low-molecule cellulose fragments. Furthermore, ACP can also play an important role in modification of plastics. In polypropylene (PP), ACP creates an etching effect and causes a rough surface. The treatment results in a decrease in contact angle and an improvement of hydrophilicity caused by an increase of polar functional groups (Baniya et al. 2020). Wong et al. (2020) successfully coated gallic acid on polyethylene (PE) to create an antibacterial film. Characterization analysis revealed that the ACP-treated PE film has a rougher surface which is conducive to gallic acid coating. Furthermore, the film with 1% concentration of gallic acid or higher could effectively reduce Escherichia coli and Staphylococcus aureus bacteria from 4 to 0.5–0.6 log CFU/mL (Wong et al. 2020). Zaidy and team (2019) published the application of ACP on waterless dyeing technology and found that ACP-treated polyethylene terephthalate (PET), cotton, and nylon possess greater bonding ability to azo dye. The plausible explanation is that multiplex of energy and particles released from ACP alter the polymeric surface without changing the bulk properties of the material. Consequently, the material like PET shows an enhanced surface wettability which essentially reduces the time and temperature required for dye binding (Zaidy et al. 2019).
Similarly, carboxymethyl cellulose (CMC) and collagen (COL) can be used to coat cinnamaldehyde on low-density polyethylene (LDPE) to improve the quality of active packaging films (Loke et al. 2021). Chang et al. (2021) studied ACP-modified LDPE with different concentrations of CMC and COL coatings to gain a better understanding on the gas barrier effect. The paper reported that adding either 1% CMC or COL can inhibit the activity of polyphenol oxidase and β-1,3-glucanase, although the 1.0% COL film after plasma treatment exhibited higher effectiveness in maintaining the composition of carbon dioxide and oxygen during storage by 10–15% and 8–15%, respectively (Chang et al. 2021). When the polypropylene/LDPE film was coated with nanofibrillated cellulose and nisin using ACP, the film illustrated improved oxygen barrier performance to as low as 24.02 cc/m2·day in addition to inhibiting the growth rate of Listeria monocytogenes by up to 94% (Lu et al. 2018).
In another study, chitosan was combined with the plasma-treated zein film to enhance its application. The tensile strength and water vapor barrier property were improved by 40.67% and 46.39%, respectively. The researchers also asserted that the film’s thermal stability was increased due to the higher content of hydrogen bonds and secondary structure according to the results from ATR-FTIR (Chen et al. 2019). While the aforementioned publications highlight various advantages of ACP in a model system of active packaging, the technology is still in its infancy and should be further tested before integrating into the packaging industry.
Conclusively, these studies illustrate that the active species generated from ACP possess a surface etching ability which can be useful in cleaning and coating. In fact, one study also integrated ACP with ellipsometry to help remove human fingerprints from a surface, successfully performing contactless fingerprint detection by transferring the impurity into the gas phase (Koulouris et al. 2021). Regardless, additional research is mandatory to thoroughly evaluate its exact mechanism particularly on the physicochemical interactions between the materials’ surface and active species. In doing so, we may gain a deeper insight on its efficacy.
Applications of ACP in medicine
In medicine, ACP is a versatile technology which is commonly employed to inactivate pathogens. However, researchers has begun to discover other useful properties such as cancer therapy, wound healing, and dentistry. Some selected publications of ACP in the medical industry is shown in Table 3. Moreover, indirect plasma treatment presents a viable method to perform off-site treatments where direct plasma treatment is difficult to reach. In this sense, ACP is used to treat water or medium to produce plasma-activated water (PAW) and plasma-activated medium (PAM) (Kaushik et al. 2019).
Table 3.
Applications of ACP in the medical field
| Medical applications | |||||||
|---|---|---|---|---|---|---|---|
| Treatment target | ACP generator | Treatment | Reference | ||||
| Exposure distance (mm) | Gas | Time (min) | Input power (W) | Voltage (kV) | |||
| Drug-resistance bacteria inhibition | DBD | 2 | Air | 8 | –– | 14 | (Liao et al. 2018) |
| Fungicidal activity of A. pullulan | DBD | 30 | Ar | 30 and 60 | –– | 9.3 | (Fukuda et al. 2019) |
| Anti-cancer | Plasma jet | 6 | Ar | 5 min | –– | 17 | (Biscop et al. 2019) |
| Wound healing | Plasma jet | 15 | He + Ar | 15 ~ 90 sec | –– | 7.5 | (Lou et al. 2020) |
| Wound healing | Plasma jet | –– | Air | 90 sec | –– | 0.1 ~ 0.24 | (Wen et al. 2022) |
Microbial decontamination
The common medical applications of ACP are relevant to antimicrobial uses including anti-bacteria (Brun et al. 2018), anti-fungi (Rüntzel et al. 2019), anti-parasites (Adil et al. 2019), and anti-virus (Filipić et al. 2020). Many studies illustrate that ACP can promote faster wound healing due to its ability to deactivate pathogenic growth (Braný et al. 2020). In addition, the evaluation of PAW on bacteria indicated that the reactive species show higher inactivation efficiency on gram-negative than gram-positive bacteria (Kaushik et al. 2019).
Nosocomial infections have become a serious threat and are getting more difficult to control due to generation of drug-resistant bacteria. Liao et al. (2018) evaluated the effect of ACP for methicillin-resistant Staphylococcus aureus (MRSA). The results demonstrated that the antibiotic resistance gene-mecA expression of MRSA decreased after ACP treatment. This study highlights the potential of ACP to eliminate medical risks related to the propagation of antibiotic resistance. Aureobasidium pullulans (A. pullulans) is a known pathogen that possesses hyper-resistance to extreme conditions and can cause various human diseases (Hawkes et al. 2005). Fukuda et al. (2019) showed that A. pullulan can be efficiently killed by argon ACP treatment, but melanized A. pullulan shows high resistance to plasma. Therefore, the fungicidal effects of ACP on melanized fungi should be further investigated. As an effective sterilization tool, ACP is also expected to be applied to Covid-19 disinfecting strategy. Zhang et al. (2021a) used surface discharge ACP to treat air by deactivating the pseudovirus with the SARS-CoV-2 and the result indicated that ACP is more effective than conventional ozone in terms of virus inactivation.
Cancer therapy
Although ACP can effectively kill cancer cells in vitro using direct or indirect treatments during cancer therapy, the selection of ACP generator is necessary to avoid normal cells being harmed (Yan et al. 2018). Biscop et al. (2019) selected five cancer cell lines (A549, U87, A375, and Malme-3 M) and three normal cell lines (BEAS-2B, HA, and HEMa) as the targets in order to evaluate the effect of ACP treatment in the same type of cells. The result indicated that there is no apparent difference in sensitivity for the malignant melanoma lines (A375 and Malme-3 M) in direct ACP treatment. Moreover, in different types of cancer cell (U87 glioblastoma and A549 lung carcinoma), the experiments also demonstrated similar results which means that direct ACP treatment has no selection in regard to these cancer cell lines (Biscop et al. 2019).
In addition, since certain cultural media are optimal for cell growth, different types of PAM (DMEM, RPMI1640, BEGM, AM, AND DCBM) were analyzed. The findings are unexpected as the authors concluded that the media have little to no influence on the efficacy of ACP. Nevertheless, Malme-3 M cells presented low sensitivity compared to A375 cells when they are treated with PAM. Furthermore, the U87 cells also exhibited lower sensitivity compared to A549 cells (Biscop et al. 2019). These results illustrate the potential of indirect ACP treatment to reduce negative side effects of cancer therapy. Conclusively, the next step of indirect ACP treatment on cancer may require cell based experiments to further evaluate selected media on different cancer cells.
Recently, a study of glioblastoma in a murine brain aims to develop a micro-sized ACP for cancer therapy. Treatment conditions such as electron density and voltage were investigated, and the authors discovered that the efficacy of tumor cell death was directly proportional to the treatment dosage (Chen et al. 2017). If a micro-sized ACP is successfully developed, it may allow oncologists to improve their treatments by accessing restrictive areas such as in intracranial or any small regions within a human body.
Wound healing
ACP plumes possess antisepsis characteristics and stimulate tissue regeneration which are advantages in promoting wound healing (Bekeschus et al. 2022). Lou et al. (2020) tried to assess the effect of ACP by using a mixture gas (helium and argon). The result illustrates the enhancement of N-cadherin, cyclin D1, Ki-67, Cdk2, and p-ERK levels in human keratinocytes (HaCaT cells), which means that ACP treatment on fresh wounds can improve cell proliferation and migration (Lou et al. 2020). ACP engenders nitric oxide production and triggers endothelial cell to assemble, promoting angiogenesis and collagen synthesis (Duchesne et al. 2019). These are important features of wound healing steps. The possibility of ACP to accelerate wound closure is also confirmed in several in vivo experiments, including diabetic ulcers (He et al. 2020), bed sores (Chatraie et al. 2018), and burn wound (Duchesne et al. 2019). In addition to a direct ACP treatment, ACP-treated dressings also has the ability to promote wound healing with lower pain compared to the untreated counterparts (van Welzen et al. 2021). Furthermore, ACP can also be developed as a non-invasive pretreatment on skin to improve transdermal permeation of drug (Wen et al. 2021).
According to European Council Directive 93/42/EEC, 4 ACP devices are European Conformity (CE) certified with class-IIa for medical use: they are argon-operated glassy vacuum electrode (HF) plasma jet kINPen Med and microwave plasma torch SteriPlas, as well as air-operated DBD device PlasmaDerm FLEX and Dress and Plasma Care. These devices are specifically employed to treat pathogenic-associated skin diseases and chronic wounds (Metelmann et al. 2022).
Dentistry
In dentistry, ACP has currently been applied in several processes including instrument sterilization (Sung et al. 2013), dental caries (Kermanshah et al. 2020), root canal infection (Armand et al. 2019; Wen et al. 2022), and tooth bleaching (Pavelić et al. 2020).
Dental caries is a major oral disease caused by teeth acidification of bacteria. Conventionally, the procedure to clean the infected area involves mechanical or laser techniques. However, vibration, noise, and heat from these methods can cause discomfort to a patient which leads to the undesirable anxiety during dental treatment. Being vibration-free, ACP has the potential to be an alternative decontamination treatment to remove necrotic or non-remineralizable tissues within the infected area. The strategy could accommodate children or patients who are anxious of drilling noises (Lata et al. 2021).
In addition, ACP also has a good bleaching effect which can be applied in teeth whitening (Nam et al. 2021).
Even though it can be seen that ACP can potentially cater to numerous applications within dentistry, it should be noted that the technology still have some drawbacks. One of the limitations is the fact that this innovation may be costly to maintain due to its novelty. Despite its effectiveness, the cost that must be paid to ensure its consistent performance (Lata et al. 2021). Most importantly, studies related to ACP in dentistry may require more clinical trials as most publications are limited to in vitro experiments on agar plates or selected substrates. As dental bacteria frequently conceal themselves within tooth enamel, ACP may not always be a practical method for the operation.
Applications of ACP in agriculture
Using ACP, research confirms various positive effects on crops such as breaking dormancy (Chou et al. 2021), germination enhancement (Barba-Espín et al. 2012; Liu et al. 2018), increasing enzyme activity (Sajib et al. 2020), and promoting seedling growth (Chou et al. 2021; Ling et al. 2014).
Plant development
ACP is shown to increase the biologically active ingredient of sprouts such as Ɣ-aminobutyric acid (GABA) by 2.98 times, in addition to promoting the length and diameter of hypocotyl by 110.61% and 139.60% compared to the control group (Chou et al. 2021). A study of a plasma-activated water (PAW) irrigation system on tomato’s growth and pathogenic defense mechanism was carried out. RONS were shown to induce plant growth and endogenous defense hormones in plants. As reported by the research group, ACP can be a highly efficient method to improve plant development and immunity (Adhikari et al. 2019).
Despite the advantages previously mentioned, another research group stated that the growth of sprout is not directly proportional to plasma energy since the long treatment time can severely damage the seed surface (Chou et al. 2021). Consequently, additional studies to optimize the effect of ACP is still vital to prevent oxidative damage and seed destruction (Sajib et al. 2020).
Seed germination
Research of ACP on seed germination has been conducted on many crops including soybeans (Ling et al. 2014), mung beans (Chou et al. 2021), corn (Selcuk et al. 2008; Volin et al. 2000), rice (Khamsen et al. 2016), cotton (de Groot et al. 2018), oat (Selcuk et al. 2008), wheat (Dobrin et al. 2015; Jiang et al. 2014), and tomato (Măgureanu et al. 2018). Volin et al. (2000) published one of the earliest researches on the effect of ACP technology on seeds and proposed that suitable parameters for ACP treatment depend on the seed’s water uptake ability. Some studies also note the etching effect of plasma on the surface which improves surface hydrophilicity, thereby promoting seedling growth (Chou et al. 2021; Ling et al. 2014).
In regard to PAW, it is observed to provide two main advantages on seed promotion. First, the reactive species can inactivate microorganisms on the seed surface. In addition, nitrite and nitrate ions can serve as nutrients for plant growth. The H2O2 in PAW can also promote the early growth of pea seedlings (Barba-Espín et al. 2012). Liu et al. (2018) studied the effects of direct and indirect ACP treatment on eight different seeds and discovered that the same parameters may have different effects depending on the types of seeds. Another study reported the similar result using radishes, tomatoes, and peppers as models; a set condition may stimulate the growth of radish, but hinders that of tomato (Sivachandiran and Khacef 2017). Interestingly, although ACP could amplify the level of signal molecules like hydrogen peroxide, and nitric oxide, Sajib et al. (2020) discover that only the concentration of hydrogen peroxide in the seeds, roots, and leaves of black gram plants increases.
Based on these findings, ACP has the potential to promote biochemical substances in plants, but it is still limited by the differences in sample sources or plasma devices. Therefore, further studies are necessary to elucidate the mechanism of ACP.
Benefits and limitations
The low electrical requirement without the need of a vacuum pump makes ACP a viable operating device in many industries. In waste management, ROS generated by ACP created sterilizing effects which can be used to eliminate microorganisms (Pawłat et al. 2021), degrade pesticides (Moutiq et al. 2020), and remove textile dye from water (Iervolino et al. 2020; Pandiyaraj et al. 2021). Furthermore, while addition of hydrogen peroxide (Iervolino et al. 2020) and TiO2 (Pandiyaraj et al. 2021) showed synergistic effect of azo dye removal, it is not a mandatory procedure which makes ACP a cheaper and more eco-friendly technology compared to conventional wastewater treatments. Furthermore, ACP’s antimicrobial property is also useful in the medical field for instrument cleaning (Sung et al. 2013) and wound healing (Bekeschus et al. 2022; Duchesne et al. 2019; Lou et al. 2020), in addition to showing potential of killing cancer cells (Biscop et al. 2019).
Likewise, in material’s surface modification, ACP can be a less expensive and safer strategy than using toxic chemicals to improve functional properties on the surface of DSSC (Weerasinghe et al. 2021), polymers (Zaidy et al. 2019), and films (Ferreira et al. 2019; Jannat Amani et al. 2019). Additionally, ACP under specific conditions can accelerate seed germination (Chou et al. 2021) and plant growth (Chou et al. 2021; Ling et al. 2014). At this pace, it is highly probable that researchers may discover other novel applications of ACP.
Nevertheless, this innovation still encounters a number of limitations. According to several studies mentioned, it can be observed that the size of ACP still presents a challenge during operation (Chen et al. 2017; Feizollahi et al. 2021; Nwabor et al. 2022). Once this surmountable problem is achieved, the technology can undoubtedly be engineered to cater to various scales of processes, allowing experts to apply it in many areas. For instance, the medical and related fields may gain remarkable advantages in a micro-sized ACP, whereas waste management and engineering sectors can quickly thrive in the scale-up cold plasma system that provides a homogenous and continuous process.
Moreover, the wide variety of plasma generators may pose potential difficulty in standardization. As can be observed in the summary Tables 1, 2, and 3, treatment conditions designed by researchers may vary significantly even within the same industry. This issue stems from the availability of ACP at laboratory-scale. Hence, a variety of ACP processing in diverse fields may instigate the need to design the device to cater to specific applications. When the technology is developed to support clinical or industrial scale processing, the issue regarding standardization difficulty may eventually be reduced.
Furthermore, while many scientists have begun to study chemical interactions of ACP, additional research is still required to shed light on the chemistry of these operations. This may perhaps be the most challenging aspect within the field since the effectiveness of reactive species produced by ACP not only relies on electrode material, gas, voltage, time, and treatment distance but also depends on the sample’s constituent, surface characteristic, shape, and size. Based on this, it can be concluded that the roles of reactive species, UV, and ions are still poorly understood. Upon gaining an in-depth knowledge of its complexity, more researchers may be encouraged to implement the device into their studies that may subsequently lead to an explosive progress of this technology.
Conclusion and future outlook
Although the effects of ACP on food, microorganisms and polymeric materials are widely studied in recent years, the ongoing challenges regarding its underlying chemistry and industrial design are still unresolved. As a result, ACP would significantly benefit from additional chemical research on the roles of each reactive species. Once scientists can unravel this issue, it may subsequently give rise to design constructions of ACP that specifically cater to food treatment, medical use, or other industrial processing. After we can comprehend the mechanism this technology, it may promote process standardization and other research opportunities in which ACP can be beneficially integrated.
Author contribution
KCC, YWT, and HYH conceptualized and developed the review topic. SPL wrote the introduction and review sections including bioprocessing, medical, and material modification. YJC wrote the section on essential oil extraction. KCH prepared tables and figures. DK integrated each section and wrote the abstract, limitations, and future outlook. All authors read and approved the final manuscript.
Funding
This project was funded by the Ministry of Science and Technology, Taiwan (MOST 106–2628-E-002–009-MY3, MOST 109–2628-E-002–007-MY3).
Declarations
Ethical approval
This review article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Shin-Ping Lin and Darin Khumsupan contributed equally to the work.
Contributor Information
Yuwen Ting, Email: pywting@ntu.edu.tw.
Kuan-Chen Cheng, Email: kccheng@ntu.edu.tw.
References
- Adhikari B, Adhikari M, Ghimire B, Park G, Choi EH. Cold atmospheric plasma-activated water irrigation induces defense hormone and gene expression in tomato seedlings. Sci Rep. 2019;9(1):1–15. doi: 10.1038/s41598-019-52646-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adil BH, Al-Halbosiy MMF, Murbat HH (2019) The use of cold atmospheric plasma in pentostam enhancement as Leishmaniasis treatment in vitro. In: AIP Conference Proceedings vol 2190. AIP Publishing LLC, p 020033
- Armand A, Khani M, Asnaashari M, AliAhmadi A, Shokri B. Comparison study of root canal disinfection by cold plasma jet and photodynamic therapy. Photodiagnosis Photodyn Ther. 2019;26:327–333. doi: 10.1016/j.pdpdt.2019.04.023. [DOI] [PubMed] [Google Scholar]
- Arslan-Alaton I, Alaton I. Degradation of xenobiotics originating from the textile preparation, dyeing, and finishing industry using ozonation and advanced oxidation. Ecotoxicol Environ Saf. 2007;68(1):98–107. doi: 10.1016/j.ecoenv.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Baniya HB, Guragain RP, Baniya B, Subedi DP (2020) Cold atmospheric pressure plasma jet for the improvement of wettability of polypropylene. Int J Polym Sci 2020:3860259
- Barba-Espín G, Hernández JA, Diaz-Vivancos P. Role of H2O2 in pea seed germination. Plant Signal Behav. 2012;7(2):193–195. doi: 10.4161/psb.18881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekeschus S, Woedtke Tv, Schmidt A (2022) How does cold plasma work in medicine? Textbook Good Clin Pract Cold Plasma Ther. Springer, pp 63–86
- Bermudez-Aguirre D (2019) Advances in cold plasma applications for food safety and preservation. Academic Press, pp 198–199
- Biscop E, Lin A, Van Boxem W, Van Loenhout J, De Backer J, Deben C, Dewilde S, Smits E, Bogaerts A. The influence of cell type and culture medium on determining cancer selectivity of cold atmospheric plasma treatment. Cancers. 2019;11(9):1287. doi: 10.3390/cancers11091287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd T, Boyd T, Sanderson J. The physics of plasmas. Cambridge University Press; 2003. [Google Scholar]
- Braný D, Dvorská D, Halašová E, Škovierová H. Cold atmospheric plasma: a powerful tool for modern medicine. Int J Mol Sci. 2020;21(8):2932. doi: 10.3390/ijms21082932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brun P, Bernabè G, Marchiori C, Scarpa M, Zuin M, Cavazzana R, Zaniol B, Martines E. Antibacterial efficacy and mechanisms of action of low power atmospheric pressure cold plasma: membrane permeability, biofilm penetration and antimicrobial sensitization. J Appl Microbiol. 2018;125(2):398–408. doi: 10.1111/jam.13780. [DOI] [PubMed] [Google Scholar]
- Cai X, Du C. Thermal plasma treatment of medical waste. Plasma Chem Plasma Process. 2021;41(1):1–46. doi: 10.1007/s11090-020-10119-6. [DOI] [Google Scholar]
- Cao X, Shen Q, Zhuang Y, Zhuang G, Chen X. Atmospheric plasma reaction synthesised Pt x Fe 1–x/graphene and TiO 2 nanoparticles/graphene for efficient dye-sensitized solar cells. RSC Adv. 2021;11(12):6464–6471. doi: 10.1039/D0RA10067F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C-K, Cheng K-C, Hou C-Y, Wu Y-S, Hsieh C-W. Development of active packaging to extend the shelf life of Agaricus bisporus by using plasma technology. Polymers. 2021;13(13):2120. doi: 10.3390/polym13132120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatraie M, Torkaman G, Khani M, Salehi H, Shokri B. In vivo study of non-invasive effects of non-thermal plasma in pressure ulcer treatment. Sci Rep. 2018;8(1):1–11. doi: 10.1038/s41598-018-24049-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Dong S, Zhao S, Li S, Chen Y. Improving functional properties of zein film via compositing with chitosan and cold plasma treatment. Ind Crops Prod. 2019;129:318–326. doi: 10.1016/j.indcrop.2018.11.072. [DOI] [Google Scholar]
- Chen Z, Simonyan H, Cheng X, Gjika E, Lin L, Canady J, Sherman JH, Young C, Keidar M. A novel micro cold atmospheric plasma device for glioblastoma both in vitro and in vivo. Cancers. 2017;9(6):61. doi: 10.3390/cancers9060061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C, Liye Z, Zhan R-J. Surface modification of polymer fibre by the new atmospheric pressure cold plasma jet. Surf Coat Technol. 2006;200(24):6659–6665. doi: 10.1016/j.surfcoat.2005.09.033. [DOI] [Google Scholar]
- Chou YJ, Cheng KC, Hsu FC, Wu JSB, Ting Y. Producing high quality mung bean sprout using atmospheric cold plasma treatment: better physical appearance and higher γ-aminobutyric acid (GABA) Content. J Sci Food Agric. 2021;101(15):6463–6471. doi: 10.1002/jsfa.11317. [DOI] [PubMed] [Google Scholar]
- Choudhury AKR. Principles of textile finishing. Woodhead Publishing; 2017. [Google Scholar]
- Cong L, Huang M, Zhang J, Yan W. Effect of dielectric barrier discharge plasma on the degradation of malathion and chlorpyrifos on lettuce. J Sci Food Agric. 2021;101(2):424–432. doi: 10.1002/jsfa.10651. [DOI] [PubMed] [Google Scholar]
- Contini C, Katsikogianni MG, O’Neill F, O’Sullivan M, Boland F, Dowling D, Monahan F. Storage stability of an antioxidant active packaging coated with citrus extract following a plasma jet pretreatment. Food Bioproc Tech. 2014;7(8):2228–2240. doi: 10.1007/s11947-013-1210-9. [DOI] [Google Scholar]
- de Groot GJ, Hundt A, Murphy AB, Bange MP, Mai-Prochnow A. Cold plasma treatment for cotton seed germination improvement. Sci Rep. 2018;8(1):1–10. doi: 10.1038/s41598-018-32692-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrin D, Magureanu M, Mandache NB, Ionita M-D. The effect of non-thermal plasma treatment on wheat germination and early growth. IFSET. 2015;29:255–260. [Google Scholar]
- Duchesne C, Banzet S, Lataillade JJ, Rousseau A, Frescaline N. Cold atmospheric plasma modulates endothelial nitric oxide synthase signalling and enhances burn wound neovascularisation. J Pathol. 2019;249(3):368–380. doi: 10.1002/path.5323. [DOI] [PubMed] [Google Scholar]
- Ekezie F-GC, Sun D-W, Cheng J-H. A review on recent advances in cold plasma technology for the food industry: Current applications and future trends. Trends Food Sci Technol. 2017;69:46–58. doi: 10.1016/j.tifs.2017.08.007. [DOI] [Google Scholar]
- Feizollahi E, Misra N, Roopesh M. Factors influencing the antimicrobial efficacy of dielectric barrier discharge (DBD) atmospheric cold plasma (ACP) in food processing applications. Crit Rev Food Sci Nutr. 2021;61(4):666–689. doi: 10.1080/10408398.2020.1743967. [DOI] [PubMed] [Google Scholar]
- Ferreira DP, Cruz J, Fangueiro R (2019) Surface modification of natural fibers in polymer composites. Green composites for automotive applications. Woodhead Publishing, pp 3–41
- Filipić A, Gutierrez-Aguirre I, Primc G, Mozetič M, Dobnik D. Cold plasma, a new hope in the field of virus inactivation. Trends Biotechnol. 2020;38(11):1278–1291. doi: 10.1016/j.tibtech.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda S, Kawasaki Y, Izawa S. Ferrous chloride and ferrous sulfate improve the fungicidal efficacy of cold atmospheric argon plasma on melanized Aureobasidium pullulans. J Biosci Bioeng. 2019;128(1):28–32. doi: 10.1016/j.jbiosc.2018.12.008. [DOI] [PubMed] [Google Scholar]
- Gomez E, Rani DA, Cheeseman C, Deegan D, Wise M, Boccaccini A. Thermal plasma technology for the treatment of wastes: A critical review. J Hazard Mater. 2009;161(2–3):614–626. doi: 10.1016/j.jhazmat.2008.04.017. [DOI] [PubMed] [Google Scholar]
- Gu Y, Shi W, Liu R, Xing Y, Yu X, Jiang H. Cold plasma enzyme inactivation on dielectric properties and freshness quality in bananas. IFSET. 2021;69:102649. [Google Scholar]
- Hawkes M, Rennie R, Sand C, Vaudry W. Aureobasidium pullulans infection: fungemia in an infant and a review of human cases. Diagn Microbiol Infect Dis. 2005;51(3):209–213. doi: 10.1016/j.diagmicrobio.2004.10.007. [DOI] [PubMed] [Google Scholar]
- He R, Li Q, Shen W, Wang T, Lu H, Lu J, Lu F, Luo M, Zhang J, Gao H. The efficacy and safety of cold atmospheric plasma as a novel therapy for diabetic wound in vitro and in vivo. Int Wound J. 2020;17(3):851–863. doi: 10.1111/iwj.13341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann C, Berganza C, Zhang J. Cold Atmospheric Plasma: methods of production and application in dentistry and oncology. Med Gas Res. 2013;3(1):1–15. doi: 10.1186/2045-9912-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Tang L. Treatment of organic waste using thermal plasma pyrolysis technology. Energy Convers Manag. 2007;48(4):1331–1337. doi: 10.1016/j.enconman.2006.08.013. [DOI] [Google Scholar]
- Ibrahimoglu B, Yilmazoglu MZ. Disposal of olive mill wastewater with DC arc plasma method. J Environ Manage. 2018;217:727–734. doi: 10.1016/j.jenvman.2018.03.134. [DOI] [PubMed] [Google Scholar]
- Iervolino G, Vaiano V, Palma V. Enhanced azo dye removal in aqueous solution by H2O2 assisted non-thermal plasma technology. Environ Technol Innov. 2020;19:100969. doi: 10.1016/j.eti.2020.100969. [DOI] [Google Scholar]
- Jannat Amani H, Motamedzadegan A, Farsi M, Yousefi H. Effect of cold plasma on the physical and mechanical properties of nanopapers prepared with cellulose and chitin nanofibers. Wood Sci Technol. 2019;26(3):15–27. [Google Scholar]
- Jiang J, He X, Li L, Li J, Shao H, Xu Q, Ye R, Dong Y. Effect of cold plasma treatment on seed germination and growth of wheat. Plasma Sci Technol. 2014;16(1):54–58. doi: 10.1088/1009-0630/16/1/12. [DOI] [Google Scholar]
- Kang JH, Jeon YJ, Min SC. Effects of packaging parameters on the microbial decontamination of Korean steamed rice cakes using in-package atmospheric cold plasma treatment. Food Sci Biotechnol. 2021;30(12):1535–1542. doi: 10.1007/s10068-021-00978-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kant R (2011) Textile dyeing industry an environmental hazard
- Kaushik NK, Ghimire B, Li Y, Adhikari M, Veerana M, Kaushik N, Jha N, Adhikari B, Lee S-J, Masur K. Biological and medical applications of plasma-activated media, water and solutions. Biol Chem. 2019;400(1):39–62. doi: 10.1515/hsz-2018-0226. [DOI] [PubMed] [Google Scholar]
- Kermanshah H, Saeedi R, Ahmadi E, Ranjbar Omrani L. Efficacy of cavity liners with/without atmospheric cold helium plasma jet for dentin remineralization. Biomater Investig Dent. 2020;7(1):120–125. doi: 10.1080/26415275.2020.1803074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khamsen N, Onwimol D, Teerakawanich N, Dechanupaprittha S, Kanokbannakorn W, Hongesombut K, Srisonphan S. Rice (Oryza sativa L.) seed sterilization and germination enhancement via atmospheric hybrid nonthermal discharge plasma. ACS Appl Mater Interfaces. 2016;8(30):19268–19275. doi: 10.1021/acsami.6b04555. [DOI] [PubMed] [Google Scholar]
- Kilonzo-Nthenge A, Liu S, Yannam S, Patras A. Atmospheric cold plasma inactivation of Salmonella and Escherichia coli on the surface of golden delicious apples. Front Nutr. 2018;5(120):1–9. doi: 10.3389/fnut.2018.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koulouris N, Tasche D, Scheglov A, Mrotzek J, Gerhard C, Viöl W. Detection of atmospheric pressure plasma-induced removal of fingerprints via analysis of histograms obtained by imaging ellipsometry. J Phys Commun. 2021;5(4):045005. doi: 10.1088/2399-6528/abf3a4. [DOI] [Google Scholar]
- Kusano Y, Madsen B, Berglund L, Oksman K. Modification of cellulose nanofibre surfaces by He/NH 3 plasma at atmospheric pressure. Cellulose. 2019;26(12):7185–7194. doi: 10.1007/s10570-019-02594-8. [DOI] [Google Scholar]
- Laroque DA, Seó ST, Valencia GA, Laurindo JB, Carciofi BAM. Cold plasma in food processing: Design, mechanisms, and application. J Food Eng. 2022;312:110748. doi: 10.1016/j.jfoodeng.2021.110748. [DOI] [Google Scholar]
- Lata S, Chakravorty S, Mitra T, Pradhan PK, Mohanty S, Patel P, Jha E, Panda PK, Verma SK, Suar M (2021) Aurora Borealis in dentistry: The applications of cold plasma in biomedicine. Mater Today Bio 13:100200 [DOI] [PMC free article] [PubMed]
- Li Q, Dai S, Zheng Y, Niu D, Tang W, Jin S, Chen Y, Zhao Y, Lin S. Discussion on treatment technology and resource recovery of hazardous waste salt containing organics. Environ Eng. 2019;37(12):200–206. [Google Scholar]
- Liao X, Cullen P, Liu D, Muhammad AI, Chen S, Ye X, Wang J, Ding T. Combating Staphylococcus aureus and its methicillin resistance gene (mecA) with cold plasma. Sci Total Environ. 2018;645:1287–1295. doi: 10.1016/j.scitotenv.2018.07.190. [DOI] [PubMed] [Google Scholar]
- Lin S-P, Kuo T-C, Wang H-T, Ting Y, Hsieh C-W, Chen Y-K, Hsu H-Y, Cheng K-C. Enhanced bioethanol production using atmospheric cold plasma-assisted detoxification of sugarcane bagasse hydrolysate. Bioresour Technol. 2020;313:123704. doi: 10.1016/j.biortech.2020.123704. [DOI] [PubMed] [Google Scholar]
- Ling L, Jiafeng J, Jiangang L, Minchong S, Xin H, Hanliang S, Yuanhua D. Effects of cold plasma treatment on seed germination and seedling growth of soybean. Sci Rep. 2014;4(1):1–7. doi: 10.1038/srep05859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Honnorat B, Yang H, Arancibia J, Rajjou L, Rousseau A. Non-thermal DBD plasma array on seed germination of different plant species. J Phys D Appl Phys. 2018;52(2):025401. doi: 10.1088/1361-6463/aae771. [DOI] [Google Scholar]
- Liu H, Guo D, Feng X. Plasma degradation of pesticides on the surface of corn and evaluation of its quality changes. Sustainability. 2021;13(16):8830. doi: 10.3390/su13168830. [DOI] [Google Scholar]
- Liu R, Peng X, Han X, Mak CH, Cheng K-C, Permatasari Santoso S, Shen H-H, Ruan Q, Cao F, Yu ET, Chu PK, Hsu H-Y. Cost-effective liquid-junction solar devices with plasma-implanted Ni/TiN/CNF hierarchically structured nanofibers. J Electroanal Chem. 2021;887:115167. doi: 10.1016/j.jelechem.2021.115167. [DOI] [Google Scholar]
- Loke X-J, Chang C-K, Hou C-Y, Cheng K-C, Hsieh C-W. Plasma-treated polyethylene coated with polysaccharide and protein containing cinnamaldehyde for active packaging films and applications on tilapia (Orechromis niloticus) fillet preservation. Food Control. 2021;125:108016. doi: 10.1016/j.foodcont.2021.108016. [DOI] [Google Scholar]
- Lou B-S, Hsieh J-H, Chen C-M, Hou C-W, Wu H-Y, Chou P-Y, Lai C-H, Lee J-W. Helium/argon-generated cold atmospheric plasma facilitates cutaneous wound healing. Front Bioeng Biotechnol. 2020;8:683. doi: 10.3389/fbioe.2020.00683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P, Guo M, Xu Z, Wu M. Application of nanofibrillated cellulose on BOPP/LDPE film as oxygen barrier and antimicrobial coating based on cold plasma treatment. Coatings. 2018;8(6):207. doi: 10.3390/coatings8060207. [DOI] [Google Scholar]
- Măgureanu M, Sîrbu R, Dobrin D, Gîdea M. Stimulation of the germination and early growth of tomato seeds by non-thermal plasma. Plasma Chem Plasma Process. 2018;38(5):989–1001. doi: 10.1007/s11090-018-9916-0. [DOI] [Google Scholar]
- Mahmoudabadi ZD, Eslami E, Narimisa M. Synthesis of Ag/TiO2 nanocomposite via plasma liquid interactions: Improved performance as photoanode in dye-sensitized solar cell. J Colloid Interface Sci. 2018;529:538–546. doi: 10.1016/j.jcis.2018.06.048. [DOI] [PubMed] [Google Scholar]
- Mahnot NK, Mahanta CL, Farkas BE, Keener KM, Misra NN. Atmospheric cold plasma inactivation of Escherichia coli and Listeria monocytogenes in tender coconut water: inoculation and accelerated shelf-life studies. Food Control. 2019;106:106678. doi: 10.1016/j.foodcont.2019.06.004. [DOI] [Google Scholar]
- Metelmann H-R, von Woedtke T, Weltmann K-D, Emmert S (2022) Textbook of good clinical practice in cold plasma therapy. Springer, pp 6–9
- Moradi E, Moosavi MH, Hosseini SM, Mirmoghtadaie L, Moslehishad M, Khani MR, Jannatyha N, Shojaee-Aliabadi S. Prolonging shelf life of chicken breast fillets by using plasma-improved chitosan/low density polyethylene bilayer film containing summer savory essential oil. Int J Biol Macromol. 2020;156:321–328. doi: 10.1016/j.ijbiomac.2020.03.226. [DOI] [PubMed] [Google Scholar]
- Mousavi SM, Imani S, Dorranian D, Larijani K, Shojaee M. Effect of cold plasma on degradation of organophosphorus pesticides used on some agricultural products. J Plant Prot Res. 2017;57(1):26–35. [Google Scholar]
- Moutiq R, Pankaj S, Wan Z, Mendonca A, Keener K, Misra N. Atmospheric pressure cold plasma as a potential technology to degrade carbamate residues in water. Plasma Chem Plasma Process. 2020;40:1291–1309. doi: 10.1007/s11090-020-10093-z. [DOI] [Google Scholar]
- Nam S-H, Choi BBR, Kim G-C. The whitening effect and histological safety of nonthermal atmospheric plasma inducing tooth bleaching. Int J Environ Res Public Health. 2021;18(9):4714. doi: 10.3390/ijerph18094714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasiru MM, Frimpong EB, Muhammad U, Qian J, Mustapha AT, Yan W, Zhuang H, Zhang J. Dielectric barrier discharge cold atmospheric plasma: Influence of processing parameters on microbial inactivation in meat and meat products. CRFSFS. 2021;20(3):2626–2659. doi: 10.1111/1541-4337.12740. [DOI] [PubMed] [Google Scholar]
- Niemira BA. Cold plasma decontamination of foods. Annu Rev Food Sci Technol. 2012;3(1):125–142. doi: 10.1146/annurev-food-022811-101132. [DOI] [PubMed] [Google Scholar]
- Nippatla N, Philip L. Electrocoagulation-floatation assisted pulsed power plasma technology for the complete mineralization of potentially toxic dyes and real textile wastewater. Process Saf Environ Prot. 2019;125:143–156. doi: 10.1016/j.psep.2019.03.012. [DOI] [Google Scholar]
- Nishimwe K, Agbemafle I, Reddy MB, Keener K, Maier DE. Cytotoxicity assessment of Aflatoxin B1 after high voltage atmospheric cold plasma treatment. Toxicon. 2021;194:17–22. doi: 10.1016/j.toxicon.2021.02.008. [DOI] [PubMed] [Google Scholar]
- Nwabor OF, Onyeaka H, Miri T, Obileke K, Anumudu C, Hart A. A cold plasma technology for ensuring the microbiological safety and quality of foods. Food Eng Rev. 2022;2020:1–20. [Google Scholar]
- Okuya M, Mayumi S, Okumura R, Masuda Y, Yagi I. Porous TiO2 layer for dye-sensitized solar cell formed with non-equilibrium 2D plasma induced by dielectric barrier discharge under atmospheric pressure. Jpn J Appl Phys. 2021;60(4):045501. doi: 10.35848/1347-4065/abe79b. [DOI] [Google Scholar]
- Omelianovych O, Larina LL, Oh H-J, Park E, Dao V-D, Choi H-S. Plasma-processed CoSn/RGO nanocomposite: A low-cost and sustainable counter electrode for dye-sensitized solar cells. Sol Energy. 2020;201:819–826. doi: 10.1016/j.solener.2020.03.057. [DOI] [Google Scholar]
- Pandiyaraj KN, Vasu D, Ghobeira R, Tabaei PSE, De Geyter N, Morent R, Pichumani M, Padmanabhanan P, Deshmukh R. Dye wastewater degradation by the synergetic effect of an atmospheric pressure plasma treatment and the photocatalytic activity of plasma-functionalized Cu-TiO2 nanoparticles. J Hazard Mater. 2021;405:124264. doi: 10.1016/j.jhazmat.2020.124264. [DOI] [PubMed] [Google Scholar]
- Pavelić B, Švarc MZ, Šegović S, Bago I. Cold atmospheric plasma for bleaching endodontically treated tooth: a new clinical approach. Quintessence Int. 2020;51(5):364–371. doi: 10.3290/j.qi.a44217. [DOI] [PubMed] [Google Scholar]
- Pawłat J, Terebun P, Kwiatkowski M, Wolny-Koładka K. Possibility of Humid Municipal Wastes Hygienisation Using Gliding Arc Plasma Reactor. Water. 2021;13(2):194. doi: 10.3390/w13020194. [DOI] [Google Scholar]
- Peran J, ErcegovićRažić S. Application of atmospheric pressure plasma technology for textile surface modification. Text Res J. 2020;90(9–10):1174–1197. doi: 10.1177/0040517519883954. [DOI] [Google Scholar]
- Perraudeau A, Dublanche-Tixier C, Tristant P, Chazelas C, Vedraine S, Ratier B. Low-temperature deposition of TiO2 by atmospheric pressure PECVD towards photoanode elaboration for perovskite and solid-state dye-sensitized solar cells. EPJ Photovolt. 2019;10:5. doi: 10.1051/epjpv/2019006. [DOI] [Google Scholar]
- Prado ES, Miranda FS, de Araujo LG, Petraconi G, Baldan MR. Thermal plasma technology for radioactive waste treatment: a review. J Radioanal Nucl Chem. 2020;325(2):331–342. doi: 10.1007/s10967-020-07269-4. [DOI] [Google Scholar]
- Rath SS, Nayak P, Mukherjee PS, Chaudhury GR, Mishra B. Treatment of electronic waste to recover metal values using thermal plasma coupled with acid leaching–a response surface modeling approach. Waste Manage. 2012;32(3):575–583. doi: 10.1016/j.wasman.2011.11.001. [DOI] [PubMed] [Google Scholar]
- Roh SH, Oh YJ, Lee SY, Kang JH, Min SC. Inactivation of Escherichia coli O157:H7, Salmonella, Listeria monocytogenes, and Tulane virus in processed chicken breast via atmospheric in-package cold plasma treatment. LWT. 2020;127:109429. doi: 10.1016/j.lwt.2020.109429. [DOI] [Google Scholar]
- Ruj B, Ghosh S. Technological aspects for thermal plasma treatment of municipal solid waste—a review. Fuel Process Technol. 2014;126:298–308. doi: 10.1016/j.fuproc.2014.05.011. [DOI] [Google Scholar]
- Rüntzel CL, da Silva JR, da Silva BA, Moecke ES, Scussel VM (2019) Effect of cold plasma on black beans (Phaseolus vulgaris L.), fungi inactivation and micro-structures stability. Emir J Food Agric 31(11):864–873
- Sajib SA, Billah M, Mahmud S, Miah M, Hossain F, Omar FB, Roy NC, Hoque KMF, Talukder MR, Kabir AH. Plasma activated water: The next generation eco-friendly stimulant for enhancing plant seed germination, vigor and increased enzyme activity, a study on black gram (Vigna mungo L.) Plasma Chem Plasma Process. 2020;40(1):119–143. doi: 10.1007/s11090-019-10028-3. [DOI] [Google Scholar]
- Samal S. Thermal plasma technology: The prospective future in material processing. J Clean Prod. 2017;142:3131–3150. doi: 10.1016/j.jclepro.2016.10.154. [DOI] [Google Scholar]
- Santoso SP, Lin S-P, Wang T-Y, Ting Y, Hsieh C-W, Yu R-C, Angkawijaya AE, Soetaredjo FE, Hsu H-Y, Cheng K-C. Atmospheric cold plasma-assisted pineapple peel waste hydrolysate detoxification for the production of bacterial cellulose. Int J Biol Macromol. 2021;175:526–534. doi: 10.1016/j.ijbiomac.2021.01.169. [DOI] [PubMed] [Google Scholar]
- Selcuk M, Oksuz L, Basaran P. Decontamination of grains and legumes infected with Aspergillus spp. and Penicillum spp. by cold plasma treatment. Bioresour Technol. 2008;99(11):5104–5109. doi: 10.1016/j.biortech.2007.09.076. [DOI] [PubMed] [Google Scholar]
- Shakeri M (2020) Chapter 2: Cold plasma coating for protective textiles and clothing Advances in Functional and Protective Textiles. Woodhead publishing, pp 19–35
- Shi H, Cooper B, Stroshine RL, Ileleji KE, Keener KM. Structures of degradation products and degradation pathways of aflatoxin B1 by high-voltage atmospheric cold plasma (HVACP) treatment. J Agric Food Chem. 2017;65(30):6222–6230. doi: 10.1021/acs.jafc.7b01604. [DOI] [PubMed] [Google Scholar]
- Shi H, Ileleji K, Stroshine RL, Keener K, Jensen JL. Reduction of aflatoxin in corn by high voltage atmospheric cold plasma. Food Bioproc Tech. 2017;10(6):1042–1052. doi: 10.1007/s11947-017-1873-8. [DOI] [Google Scholar]
- Sivachandiran L, Khacef A. Enhanced seed germination and plant growth by atmospheric pressure cold air plasma: combined effect of seed and water treatment. RSC Adv. 2017;7(4):1822–1832. doi: 10.1039/C6RA24762H. [DOI] [Google Scholar]
- Stryczewska HD. Supply systems of non-thermal plasma reactors. Construction Review with Examples of Applications. Appl Sci. 2020;10(9):3242. doi: 10.3390/app10093242. [DOI] [Google Scholar]
- Sung S-J, Huh J-B, Yun M-J, Chang BMW, Jeong C-M, Jeon Y-C. Sterilization effect of atmospheric pressure non-thermal air plasma on dental instruments. J Adv Prosthodont. 2013;5(1):2–8. doi: 10.4047/jap.2013.5.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tecer LH, Gündüz A, Atav R, Soysal S, Yıldız F. Investigation of the treatment of textile wastewater with cold atmospheric plasma reactor (profoks) and reuse of recycled water in reactive dyeing process of cotton. J Nat Fibers. 2022;19(6):2382–2389. doi: 10.1080/15440478.2020.1818344. [DOI] [Google Scholar]
- Tkáč F, Košičiarová I, Horská E, Mušinská K. Socioeconomic relations of food waste in selected European countries. Economies. 2022;10(6):144. doi: 10.3390/economies10060144. [DOI] [Google Scholar]
- Tolouie H, Mohammadifar MA, Ghomi H, Yaghoubi AS, Hashemi M. The impact of atmospheric cold plasma treatment on inactivation of lipase and lipoxygenase of wheat germs. IFSET. 2018;47:346–352. [Google Scholar]
- Umair M, Jabbar S, Ayub Z, Muhammad Aadil R, Abid M, Zhang J, Liqing Z. Recent advances in plasma technology: Influence of atmospheric cold plasma on spore inactivation. Food Rev Int. 2021;1(1):1–23. doi: 10.1080/87559129.2021.1888972. [DOI] [Google Scholar]
- UNEP (2021) UNEP Food Waste Index Report 2021. Publisher
- van Welzen A, Hoch M, Wahl P, Weber F, Rode S, Tietze JK, Boeckmann L, Emmert S, Thiem A. The response and tolerability of a novel cold atmospheric plasma wound dressing for the healing of split skin graft donor sites: A controlled pilot study. Skin Pharmacol Physiol. 2021;34(6):328–336. doi: 10.1159/000517524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volin JC, Denes FS, Young RA, Park SM. Modification of seed germination performance through cold plasma chemistry technology. Crop Sci. 2000;40(6):1706–1718. doi: 10.2135/cropsci2000.4061706x. [DOI] [Google Scholar]
- Wang X, Gong Y, Qin J, Cheng J, Gong C, Jiang D. Deep removal of organic matter in glyphosate contained industrial waste salt by dielectric barrier discharge plasma. J Environ Chem Eng. 2021;9(5):106295. doi: 10.1016/j.jece.2021.106295. [DOI] [Google Scholar]
- Wante HP, Aidan J, Ezike SC (2021) Efficient dye-sensitized solar cells (DSSCs) through atmospheric pressure plasma treatment of photoanode surface. Curr Opin Green Sustain Chem 4(1):100218
- Weerasinghe J, Sen S, Kumari J, Dissanayake M, Senadeera G, Thotawatthage C, Ekanayake M, Zhou R, Cullen PJ, Sonar P. Efficiency enhancement of low-cost metal free dye sensitized solar cells via non-thermal atmospheric pressure plasma surface treatment. Sol Energy. 2021;215:367–374. doi: 10.1016/j.solener.2020.12.044. [DOI] [Google Scholar]
- Wen X, Xin Y, Hamblin MR, Jiang X. Applications of cold atmospheric plasma for transdermal drug delivery: A review. Drug Deliv Transl Res. 2021;11(3):741–747. doi: 10.1007/s13346-020-00808-2. [DOI] [PubMed] [Google Scholar]
- Wen Y, Luo Y, Wei X, Tan H, Ai R, Xiong Z, Ye L. Antibacterial effects of liquid discharge cold plasma on Enterococcus faecalis planktonic cultures and biofilms: an in vitro study of root canal treatment. J Phys D Appl Phys. 2022;55(36):365204. doi: 10.1088/1361-6463/ac7423. [DOI] [Google Scholar]
- Wong L-W, Hou C-Y, Hsieh C-C, Chang C-K, Wu Y-S, Hsieh C-W. Preparation of antimicrobial active packaging film by capacitively coupled plasma treatment. LWT. 2020;117:108612. doi: 10.1016/j.lwt.2019.108612. [DOI] [Google Scholar]
- Yan D, Xu W, Yao X, Lin L, Sherman JH, Keidar M. The cell activation phenomena in the cold atmospheric plasma cancer treatment. Sci Rep. 2018;8(1):1–10. doi: 10.1038/s41598-018-33914-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidy SS, Vacchi FI, Umbuzeiro GA, Freeman HS. Approach to waterless dyeing of textile substrates—use of atmospheric plasma. Ind Eng Chem Res. 2019;58(40):18478–18487. doi: 10.1021/acs.iecr.9b01260. [DOI] [Google Scholar]
- Zhang H, Chen M, Huang L, Guo L, Xu S, Zhang J, Xi W, Wang Z, Liu D, Kong MG. Using cold atmospheric plasma treated-air for COVID-19 disinfection in cold-chain environment. J Phys D Appl Phys. 2021;54(40):40LT01. doi: 10.1088/1361-6463/ac13f7. [DOI] [Google Scholar]
- Zhang W, Li C, Tian Z, Hou Z. Structural, photocatalytic and photoelectrochemical properties of porous ZnO nanosheets prepared by air cold plasma. Nanotechnology. 2021;32(50):505712. doi: 10.1088/1361-6528/ac2650. [DOI] [PubMed] [Google Scholar]