Abstract
Community-acquired pneumonia (CAP) is a common lower respiratory tract infection, often complicated by cardiovascular events, including cardiac arrhythmias. New-onset atrial fibrillation (newAF) has been associated with increased mortality in CAP patients, especially in those critically ill; however, limited data on the prevalence of newAF in patients with CAP are available. We aim to estimate the pooled prevalence of newAF and its impact on adverse outcomes in patients with CAP, through a systematic review and meta-analysis. MEDLINE and EMBASE were systematically searched from inception to 27 January 2022. All studies reporting the prevalence of newAF in CAP patients were included and all-cause mortality was extracted when available. The pooled prevalence of newAF, 95% Confidence Intervals (CI), and 95% Prediction Intervals (PI) were computed. The inconsistency index (I2) was calculated to measure heterogeneity. Subgroup analyses were also performed. A protocol for this study was registered on PROSPERO (CRD42022307422). Among 7,655 records retrieved, 10 studies were included, with a total of 280,589 CAP patients. Pooled prevalence of newAF in CAP patients was 7.6% (95% CI 6.4–9.0%, 95% PI 4.3–13.1%, I2 = 95%). Subgroup analyses showed no significant differences according to geographical location or study design. Patients with newAF had a higher risk of mortality among the studies included in the systematic review. NewAF is a common complication, occurring in 7.6% of CAP patients, with prediction intervals suggesting an even higher burden. CAP patients who develop newAF during hospitalization may be at higher risk of mortality in both short- and long-term follow-up.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11739-022-03135-1.
Keywords: Community-acquired pneumonia, New-onset atrial fibrillation, Prevalence, Epidemiology, Meta-analysis
Introduction
Community-acquired pneumonia (CAP) is the most common lower respiratory tract infection. It is a world-leading cause of mortality and morbidity [1], and it is often complicated by cardiovascular events [2]. CAP occurs in middle-aged and elderly patients, more likely to be at a higher risk of cardiac comorbidities. Acute infections, indeed, act as precipitating factors of acute cardiac events, such as myocardial infarction and cardiac arrhythmias [2].
The association between CAP and cardiovascular complications has been extensively studied during the last decades, and previous systematic reviews have tried to summarize the available evidence [3, 4]: a meta-analysis of observational studies showed that the incidence of overall cardiac complications were 17.7%, with the incident cardiac arrhythmias up to 4.7% [3], while a recent one [4] confirmed the high rate of overall cardiac complications, but showed a higher rate of incident cardiac arrhythmias (7.2%). However, none of these previous reports was specifically focused on atrial fibrillation.
Indeed, among cardiac arrhythmias, new-onset atrial fibrillation (newAF)—defined as a new or first detectable episode of a chaotic and irregular atrial rhythm, whether symptomatic or not [5], usually confirmed through 12-lead ECG—can be frequently observed during acute respiratory infection and has been repeatedly associated with increased mortality in CAP patients, especially in critically ill ones [6]. The pathophysiological link between newAF and infections could be explained by the systemic inflammatory response and the pro-inflammatory cytokines cascade causing both structural and electrical atrial remodeling which increases the risk for newAF [7]. However, infection type is paramount for determining the risk of newAF: pneumonia has a higher risk profile compared to other infections [8], whereas sepsis or septic shock, which are characterized by a distinct cytokine profile [9], has an even higher risk [10, 11]. Consistently, recent evidence showed that newAF is a common complication of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-associated pneumonia [12], likely due to the specific cytokines signature associated with this infection [13].
To date, there is still uncertainty about the actual prevalence of newAF in patients with CAP, and the relationship between newAF and the subsequent risk of adverse outcomes. Indeed, recent evidence has shown how newAF triggered by infections might place the patient at risk of recurrence and hospitalization for AF in the long-term [8]. These data would be of clinical relevance to implement specific preventive strategies or tailored management for patients with CAP-related newAF.
This study aims to perform a comprehensive evaluation of the prevalence and outcomes of newAF in patients with CAP, through a systematic review and meta-analysis of the current literature.
Methods
This systematic review has been conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and recommendations. A protocol for this study was registered into the international prospective register of systematic reviews (PROSPERO), N. CRD42022307422.
Search strategy
A comprehensive and systematic literature search was performed on MEDLINE and EMBASE databases, from inception to 27 January 2022. Relevant key terms were combined in the search strategy, including ‘Community-Acquired Pneumonia”, “Lower Respiratory Tract Infection”, “Pneumonia”, “Atrial Fibrillation”. The complete search strategy is detailed in Supplementary Material (Table S1). A protocol for this study was registered on PROSPERO (CRD42022307422).
Studies selection
According to PRISMA guidelines [14], all articles retrieved from the literature search were systematically and sequentially screened independently for eligibility by two co-authors (BC and FT), according to title and abstract. Each article included after the first screening phase was then evaluated according to full-text eligibility. References of the included studies were cross-checked for inclusion of other potentially relevant papers. Any disagreement was resolved by a collegial discussion with a third author (GFR).
Inclusion and exclusion criteria
The main inclusion criteria were: i) all studies reporting the number of patients with CAP who developed newAF during the hospital stay or in a time range of 30 days from hospital admission; ii) Sample size ≥ 50 patients. We excluded studies on highly selected cohorts of patients (i.e., cohorts composed only of patients with previous AF), and those that included patients with pneumonia caused by coronavirus disease 2019 (COVID-19). Finally, we excluded conference abstracts, comments, editorials, case reports, systematic reviews, and meta-analyses. In the case of two or more studies based on the same cohort of patients, we selected the study with the highest number of patients included.
Data extraction and quality assessment
Data from the studies included were independently extracted by two co-authors (BC and FT), through a standardized electronic form. We extracted data on sample size, numbers of patients with CAP, prevalence of newAF during hospital stay and data on mortality according to the newAF status. We also extracted data on sex, type of study, geographical locations, and prevalence of baseline comorbidities (i.e., hypertension, type 2 diabetes mellitus, coronary artery disease, previous cerebrovascular disease, history of atrial fibrillation, chronic obstructive pulmonary disease, intensive care unit (ICU) admission, chronic kidney disease (CKD)).
Two co-authors (BC and FT) independently evaluated the risk of bias of individual studies. For this purpose, we used a customized version of the Newcastle–Ottawa Scale (NOS) for cohort studies [15]. This scale was composed of 5 items across three domains (Selection, Comparability, Outcome), and a maximum of 5 points; each study with a NOS ≤ 3 was categorized as at high risk of bias. The scale used is reported in supplementary material, Table S2.
Patients and outcomes definition
We defined CAP according to the diagnostic method used in the original studies included (i.e., radiological, clinical, and/or microbiological). NewAF was defined as per the criteria defined in the original studies, according to 12-lead ECG diagnosis for the observational studies, or International Classification of Diseases (ICD) codes for administrative database, when reported.
Primary aim of our study was to estimate the prevalence of newAF among CAP patients. According to the criteria used in the included studies, prevalence of newAF was defined as the proportion of CAP patients with a diagnosis of newAF.
Secondary aim was to evaluate the all-cause mortality, defined as per the original studies included, among patients who developed newAF during hospitalization for CAP, compared to those without newAF.
Statistical analysis
Pooled prevalence of newAF, 95% confidence intervals (CI) and 95% prediction intervals (PI) were estimated using a generalized linear mixed model (random intercept logistic regression model). Prediction intervals represent a range of values that predicts the effect size of a new potential study, and provide a more comprehensive evaluation of the heterogeneity [16]. Inconsistency index (I2) was used to quantify the amount of the dispersion of effect size across studies that can be attributed to heterogeneity. According to pre-specified cut-offs [17], we defined low heterogeneity as an I2 of < 25%, moderate heterogeneity when I2 was between 25 and 75%, and high heterogeneity when I2 was > 75%. To investigate the potential sources of heterogeneity, we also performed subgroup analyses according to study-level characteristics (i.e., geographical location and study design). All the statistical analyses were performed using R version 4.1.2 [18], using the ‘meta’ [19], ‘metafor’ [20], and ‘dmetar’ [21] packages.
Results
A total of 7,651 studies were retrieved from the literature search (905 from MEDLINE and 6,746 from EMBASE). After duplicates removal, and sequential screening of title and abstract, we evaluated 29 full texts for eligibility, and 9 studies were included according to the inclusion and the exclusion criteria (Figure S1 in Supplementary Materials). Additionally, a cross-reference search was performed and identifies 4 studies that were assessed for eligibility, of which 1 was included. Finally, a total of 10 studies were included in the systematic review and meta-analysis with a total of 280,589 CAP patients included. A summary of the characteristics of the included studies is presented in Table 1. Briefly, 6 studies were based on prospective cohorts [22–27] of which 4 were multicentre [23–25, 27]; 2 studies were based on administrative databases [8, 28], while the other 2 were based on retrospective single-center cohorts [29, 30]. Seven studies were held in Europe [8, 22, 24, 25, 27, 28, 30]; while 3 were held in other geographical locations [23, 26, 29].
Table 1.
Characteristic of included studies
| Study | Location | Study design | Setting | NewAF Diagnosis | Age (mean) | M % | Pre-AF % | ICU % | HTN % | COPD % | T2DM % | HF % | CAD % | CVD % | CKD % | Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gundlund 2019 [8] | Dutch, Europe | Database | GH | ICD-10 | na | na | 0 | na | na | na | na | na | na | na | na | aHR; follow-up 12-month mortality |
| Mandal 2011 [28] | Scotland, Europe | Database | GH | ECG | 73* | 48 | na | 4 | na | 20 | 8 | 19 | 8 | 11 | 12 | aOR; 90-day mortality |
| Menéndez 2019 [27] | Spain, Europe | Prospective | GH | na | 70* | 64 | 11 | na | 49 | 21 | 23 | 9 | 3 | 7 | 11 | na |
| Musher 2007 [29] | Texas, Other | Retrospective | GH | ECG | na | na | na | na | na | na | na | na | na | na | na | na |
| Pieralli 2019 [22] | Italy, Europe | Prospective | MW | ECG | 75.5 | 48 | 0 | na | 56 | 30 | 25 | 22 | 17 | 14 | 15 | %; in-hospital mortality |
| Pieralli 2021 [24] | Italy Europe | Prospective | MW | ECG | 76.2 | 51 | 20 | 4 | 55 | 27 | 24 | 21 | 17 | 12 | 13 | HR; 30-day mortality |
| Rombauts 2020 [30] | Spain, Europe | Retrospective | GH | ECG | 67.1 | 64 | 15 | 13 | 34 | 30 | 22 | 10 | 10 | 8 | 9 | na |
| Ruiz 2021 [25] | Spain, Europe | Prospective | GH | ECG | 61.6 | 60 | 0 | na | 35 | 19 | 16 | 5 | 1 | 5 | 4 | %; 6-months of mortality |
| Violi 2017 [23] | Italy-Canada, Other | Prospective | MW | ECG | 73.1 | 59 | 29 | na | 65 | 33 | 36 | 29 | 38 | 12 | 20 | na |
| Seedat 1993 [26] | Africa, Other | Prospective | GH | ECG | 32.3 | na | na | 11 | na | 0 | na | 0 | 0 | na | na | na |
Legend: aHR adjusted Hazard Ratio, HR Hazard Ratio, aOR adjusted Odds Ratio, CAD Coronary artery disease, CVD CerebroVascular Disease, CKD Chronic Kidney Disease, COPD Chronic Obstructive Pulmonary Disease, ECG: Electrocardiogram, GH General Hospitalization, HF Heart Failure, HTN hypertension, ICD International Classification of Diseases, ICU Intensive Care Unit, MW Medical Ward, M Males, na not available, Pre-AF pre-existing AF, T2DM Type 2 Diabetes Mellitus, *Median °pre-existing arrhythmias
Mean age of the included patients ranged from 32.3 to 76.2 years. Male individuals accounted for 48% to 64% of patients included, and the most prevalent comorbidity was hypertension (33–65%). As for the definition of CAP, 2 studies were based on ICD-10 codes [8, 28], while in all the other studies, CAP diagnosis was based on radiological, clinical, and/or microbiological definitions. Consistently, newAF was defined on ICD-10 codes in one study [8], while in the other studies newAF diagnosis relied on direct examination of 12-leads electrocardiograms. Only in one study the definition was not reported [27]. Timing for newAF diagnosis, according to inclusion criteria, was during the hospital stay for CAP in 9 studies [8, 22–26, 28–30], and within 30 days from admission in one study [27].
Bias assessment for the prevalence of newAF in CAP patients is reported in Supplementary Materials, Table S3. Among 10 studies, 1 was determined to be at high risk of bias [29] because of a selected cohort of CAP (only pneumococcal pneumonia) and no baseline characteristics reported.
Prevalence of new-onset atrial fibrillation
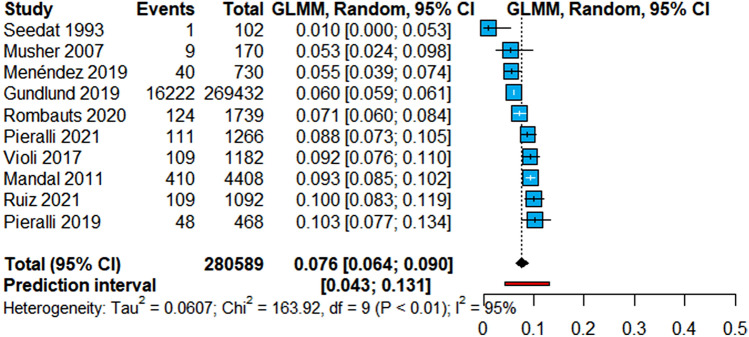
Among 10 studies, the pooled prevalence of newAF was 7.6% (95% CI 6.4–9.0%; 95% PI 4.3–13.1%) (Fig. 1). Evaluation of the heterogeneity showed a high grade of between-study variability (I2 = 95%), although 90% of studies included consistently reported a prevalence of newAF between 5.3% and 10.3%. Leave-one-out sensitivity analyses showed overall consistent results for both pooled estimate and heterogeneity (Supplementary material, Figure S2). To evaluate the potential sources of heterogeneity, we performed subgroup analyses which did not shown differences according to geographical location (Supplementary material, Figure S3), or according to the study design (Supplementary material, Figure S4).
Fig. 1.
Prevalence of newAF in CAP patients. Legend: CAP Community-acquired Pneumonia, CI Confidence Intervals, GLMM Generalized Linear Mixed Model, I2 Inconsistency Index, newAF new-onset Atrial Fibrillation
Systematic review of outcomes according to newAF
Five studies reported data about mortality according to newAF status [8, 22, 24, 25, 28]. One study reported in-hospital mortality [22], while other three reported mortality within 30 [24], 90 [28] and 180 days [25] after admission; finally, one study reported mortality within 12 months of follow-up [8]. Given the variability in the duration of follow-up, a meta-analysis was not performed, and we only report the systematic review of the studies included.
Table 2 reports a summary of the studies including data on outcomes. Overall, mortality rate was broadly higher in newAF patients than in those without. Specifically, one study reported an increased risk of 1-year mortality [8] (adjusted Hazard Ratio (aHR): 1.42, 95%CI 1.37–1.47). Consistently, other studies reported an increased mortality rate at 180 days [25] and an increased risk of 90-day mortality [28]. A non-significant increase in the in-hospital mortality rate was also observed in the study of Pieralli et al. [22] (18.8% vs 11.4%, p = 0.16). Finally, one study found no significant difference between the two groups regarding 30-day mortality [24]; however, this study included up to 20% of patients with pre-existing AF, which represents a difference compared to other studies. Moreover, in this study, newAF was defined considering both AF and atrial flutter.
Table 2.
Systematic review for outcomes of included studies
| Study | Location | Study design | Years | Total cap population | Inclusion criteria | Exclusion criteria | Follow-up | Outcomes |
|---|---|---|---|---|---|---|---|---|
| Gundlund 2019 [8] | Netherlands | Database | 1996–2016 | 269,432 | Age ≥ 18 years old, patients admitted with infection (subgroup with pneumonia) | Age > 100 years old, previous diagnosis of AF, death during hospital admission | 365 days | 12 months of mortality: aHR 1.42 (95% CI 1.37–1.47) |
| Mandal 2011 [28] | United Kingdom | Database | 2005–2007 | 4408 | Age ≥ 18 years old, diagnosis of CAP defined by ICD-10 codes | HAP, admission or transfer from a health-care facility; post-operative pneumonia; HIV | 90 days | 90-day mortality: aOR 2.39 (95% CI 1.65–2.19, p < 0.0001) |
| Pieralli 2019 [22] | Italy | Prospective | 2013–2016 | 468 | Age ≥ 18 years old, sinus rhythm confirmed by ECG on admission, no previous documented episodes of AF, diagnosis of CAP defined by clinical and radiological evidence | HAP, immunocompromised or refused or unable to give their consent | 9.5 + 5.1 days (mean, SD) | In-hospital mortality rate, newAF VS controls: 18.8% vs 11.4%, p = 0.16 |
| Ruiz 2021 [25] | Spain | Prospective | 2002–2019 | 1,092 | Hospitalized pneumococcal CAP diagnosed by clinical, radiological, and microbiological evidence | Pneumonia in the previous 3 months and pre-existing AF | 180 days | 6 months of mortality rate, newAF VS controls: 17.9% versus 2.9%, p < 0.001 |
| Pieralli 2021 [24] | Italy | Prospective | 2016–2018 | 1,266 | Age > 18 years old; diagnosis of CAP defined by clinical and radiological evidence | HAP, immunocompromised or refused or unable to give their consent | 30 days | 30-day mortality: HR 1.04 (95% CI 0.64–1.5, p = 0.99) |
Legend: aHR adjusted Hazard Ratio, aOR adjusted Odds Ratio, AF Atrial Fibrillation, newAF new-onset Atrial Fibrillation, CI Confidence Intervals, ECG Electrocardiogram, HAP Hospital-Acquired Pneumonia, HR Hazard Ratio, HIV Human Immunodeficiency Virus, ICD International Classification of Diseases, SD standard deviation
Discussion
In this systematic review and meta-analysis of 280,589 patients with CAP, we found that newAF is common among hospitalized patients with CAP, with a pooled prevalence of newAF up to 7.6%, and PIs pointing toward a potential higher prevalence, up to 13% of CAP patients. Although a high between-study variability was found among the cohorts included, 9 out of 10 studies showed an estimate of newAF prevalence between 5 and 10%, underlining the robustness of our findings and perhaps an overall low clinical relevance of the heterogeneity found. Consistently, no subgroup difference was found according to geographical location or study design. Finally, the studies that evaluated outcomes according to newAF presence found that newAF patients were at higher risk of all-cause death compared to patients who did not develop newAF during CAP.
To our knowledge, our study is the first to provide a comprehensive evaluation of the studies that investigated the relationship between CAP and newAF, and our findings deliver two key messages: first, newAF is a common cardiac arrhythmia triggered by CAP, potentially affecting up to 1 out of 13 hospitalized patients with CAP; second, patients who develop newAF are at higher risk of adverse outcomes and, specifically, mortality compared to those in stable sinus rhythm.
Several hypotheses sustain the association between CAP and newAF and may explain the prevalence observed. The hypoxia caused by CAP and due to direct lungs damage may represent the trigger for sub-clinical or manifested atrial arrhythmia [29], as suggested for other respiratory conditions associated with the presence of AF [31]. Moreover, inflammation may be a key driver of the increased risk of newAF in these patients [7], as observed also in other clinical scenarios [31]. Unsurprisingly, the onset of AF has been repeatedly described during sepsis [6, 32], a condition in which increased expression of inflammatory cytokines (including Interleukin-6 (IL-6), IL-8, and Tumor necrosis factor α) is thought to contribute to left atrial electric remodeling which in turns increases the risk of AF [7, 33, 34]. This pathophysiological hypothesis can be applied also to CAP and may explain the findings observed in our study.
Nonetheless, several reports have recently described an association between newAF and COVID-19 [35, 36]. Consistently, a recent meta-analysis [12] of hospitalized patients with COVID-19 reported a pooled prevalence of newAF (8.0%) similar to what we observed in the current study. Taken together, all these shreds of evidence reinforce the hypothesis of a close relationship between infections and newAF, and specifically for lower respiratory tract infections and CAP. Indeed, further studies are urgently needed to clarify the pathophysiological mechanisms between infections, inflammation, and the onset of newAF, with also a specific focus on the interplay between these factors and the risk of thromboembolism and other major outcomes.
Other factors may contribute to the findings observed, beyond the pathophysiological mechanisms already described. Particularly, is it possible that patients with CAP requiring hospitalization have a higher burden of cardiovascular comorbidities and risk factors, with a subsequently higher risk of developing cardiovascular complications during the in-hospital stay, including newAF [37]. This hypothesis is consistent with the observation of lower rates of cardiovascular complications in CAP outpatients [38], suggesting that newAF—beyond being directly triggered by CAP—may also represent a marker of increased clinical complexity, and may be influenced by the severity of the underlying disease [39].
Indeed, our systematic review also showed a broadly higher risk of mortality in patients that developed newAF. Although the significant variability in the definition of the outcomes (particularly regarding follow-up length) prevents us to conduct a proper meta-analysis, the evidence available from the 5 studies with available data sustains the hypothesis that patients who developed newAF during CAP would experience a higher risk of mortality during follow-up. Although further studies are clearly needed to shed light on the actual mechanisms underlying the association between newAF and worse prognosis in CAP patients, newAF may represent a marker of an overall higher clinical complexity and risk for cardiovascular complications, which may translate in worse prognosis during follow-up. It seems plausible that several factors (including older age, the higher burden of comorbidities and risk factors, and the higher severity of the CAP episode) may account for both the onset of newAF and the increased risk of mortality and worsened prognosis.
Nonetheless, mortality is only one of the adverse outcomes associated with newAF: indeed, thromboembolism and AF recurrence are, among others, events that have a higher incidence among CAP patients who develop newAF. Among the studies included in our systematic review, one showed how the risk of AF at 1-year follow-up was 25-fold higher in patients who developed newAF during infections, compared to those who maintained sinus rhythm; moreover, these patients also showed a twofold higher risk of thromboembolism [8]. Consistently, another recent analysis from a Danish-nationwide database [40] has shown how patients developing newAF during CAP, if not properly managed according to their thromboembolic risk (i.e., receiving oral anticoagulant) had a significant long-term risk of thromboembolism, when compared to patients who did not develop newAF; the risk was higher for those with high thromboembolic risk. While we still lack conclusive evidence on the rate of recurrence of AF, and on the optimal long-term management of these patients, these findings seem to suggest the need for appropriate long-term stroke prevention for patients with newAF during CAP, taking into account the risk–benefit profile for OAC prescription. This evidence supports the hypothesis that AF triggered by pneumonia should be regarded and managed consistently with its long-term thromboembolic risk. Indeed, this seems consistent with the approach introduced in the 2020 ESC guidelines for the management of AF [41]: while no-specific recommendations were produced for infection-related AF, the guidelines advocated for considering long-term OAC in those patients with post-operative AF (another form of “triggered AF”) and increased risk of stroke [42].
Taken together, our observations regarding the prevalence of newAF and the increased risk of adverse events during follow-up can be useful to shape our understanding on the epidemiology and impact of newAF in patients with CAP and, more broadly, in patients with infectious diseases. Compared to previous systematic reviews and meta-analyses, which were not specifically focused on newAF [3, 4], our study was aimed at evaluating the actual prevalence of newAF, thus providing a more detailed assessment of the actual burden of this disease in patients with CAP. Therefore, these findings are clinically relevant as they will be particularly useful to design more tailored preventive strategies in CAP patients, as well as screening programs to detect newAF in CAP patients, and tools to predict its incidence. Further studies are needed to clarify whether these approaches may provide substantial benefits in the management of CAP patients. Moreover, evidence is needed on the optimal management of newAF in patients with CAP and generally in infectious diseases and acutely ill patients (both in terms of rate control/antiarrhythmic therapy and thromboembolic risk prevention) [43, 44], and the actual need for closer follow-up to identify patients who may present recurrent episodes of AF after CAP.
Study limitations
Our study has several limitations. First, some studies also included a proportion of patients with pre-existing AF; however, these patients represented a limited proportion of the total subjects included in this study, thus being unlikely to significantly influence our estimates. Furthermore, the criteria used to define newAF in the individual studies were heterogeneous; while the vast majority defined newAF according to ECG-based diagnosis, there were insufficient data regarding duration of episodes and symptoms associated to allow us to perform additional analyses, aiming at evaluating the impact of these (and other) variables on the estimates provided. We also cannot exclude the contribution of unaccounted confounders on the strength of association between CAP and newAF, which may also contribute to the heterogeneity observed. Among these, CAP severity, and intensity of care received (whether ICU or non-ICU) were not available in most of the studies included, and did not allow us to perform subgroup analyses to explore the impact of these factors on the estimates provided. As the risk of newAF has been repeatedly shown higher in critically ill and ICU-admitted patients [45], we cannot exclude this as a potential source of bias, which may led to overestimation of the prevalence of newAF in our study. On the other side, it is conceivable that the proportion of these patients was limited in the studies included, and therefore the extent of this potential bias is likely small. However, as already been reported, the majority of the studies included were consistent in the estimate of the prevalence of newAF, reinforcing the reliability of our pooled estimate, thus making the heterogeneity observed highly unlikely to have strong clinical implications. Finally, we were unable to perform a meta-analysis on the association between newAF and mortality, given the high variability in the definition of the outcomes in the original studies; however, we have reported a systematic review of the findings of the included cohorts, which were all consistent in showing higher risk of mortality in newAF patients.
Conclusion
Among patients hospitalized with CAP, newAF represents a common complication, being found in 7.6% of patients, with prediction intervals suggesting an even higher burden. CAP patients who develop newAF during hospitalization may be at higher risk of mortality in the short- and long-term follow-up.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
BC, GFR, FT and MP contributed to the conception and the design of the work. BC and FT acquired data; BC and GFR performed the analysis. BC, GFR and MP interpreted data, wrote the first draft of the manuscript, and finalized the last version. AO, VR, RC, SB and GYHL critically revised the manuscript and gave important intellectual contributions. All authors gave final approval.
Funding
GFR and BC were supported by grants (AR11916B84DD8DCE and AR120172B872270D) issued by Sapienza – University of Rome, Rome, Italy.
Declaration
Conflict of interest
SB received research grant from MSD. GYHL has been consultant and speaker for BMS/Pfizer, Boehringer Ingelheim and Daiichi-Sankyo. No fees are directly received personally. All the disclosures happened outside the submitted work. All other authors have nothing to declare.
Footnotes
Giulio Francesco Romiti and Marco Proietti have joined as senior authors.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Bernadette Corica and Francesco Tartaglia have contributed equally.
References
- 1.Ramirez JA, Wiemken TL, Peyrani P, et al. Adults Hospitalized With Pneumonia in the United States: incidence, Epidemiology, and Mortality. Clin Infect Dis. 2017;65:1806–1812. doi: 10.1093/cid/cix647. [DOI] [PubMed] [Google Scholar]
- 2.Corrales-Medina VF, Musher DM, Shachkina S, Chirinos JA. Acute pneumonia and the cardiovascular system. Lancet. 2013;381:496–505. doi: 10.1016/S0140-6736(12)61266-5. [DOI] [PubMed] [Google Scholar]
- 3.Corrales-Medina VF, Suh KN, Rose G, et al. Cardiac complications in patients with community-acquired pneumonia: a systematic review and meta-analysis of observational studies. PLoS Med. 2011;8:e1001048. doi: 10.1371/journal.pmed.1001048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tralhão A, Póvoa P. Cardiovascular Events after Community-Acquired Pneumonia: A Global Perspective with Systematic Review and Meta-Analysis of Observational Studies. J Clin Med. 2020;9:414. doi: 10.3390/jcm9020414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.New-onset atrial fibrillation - Symptoms, diagnosis and treatment | BMJ Best Practice US. https://bestpractice.bmj.com/topics/en-us/3. Accessed 3 Oct 2022
- 6.Corica B, Romiti GF, Basili S, Proietti M. Prevalence of new-onset atrial fibrillation and associated outcomes in patients with sepsis: a systematic review and meta-analysis. J Pers Med. 2022;12:547. doi: 10.3390/jpm12040547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boos CJ. Infection and atrial fibrillation: inflammation begets AF. Eur Heart J. 2020;41:1120–1122. doi: 10.1093/eurheartj/ehz953. [DOI] [PubMed] [Google Scholar]
- 8.Gundlund A, Olesen JB, Butt JH, et al. One-year outcomes in atrial fibrillation presenting during infections: a nationwide registry-based study. Eur Heart J. 2020;41:1112–1119. doi: 10.1093/eurheartj/ehz873. [DOI] [PubMed] [Google Scholar]
- 9.Bozza FA, Salluh JI, Japiassu AM, et al. Cytokine profiles as markers of disease severity in sepsis: a multiplex analysis. Crit Care. 2007;11:R49. doi: 10.1186/cc5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu W-S, Lin C-L. Risk of incident atrial fibrillation after a prior critical illness: A retrospective cohort study. Eur J Intern Med. 2019;60:90–95. doi: 10.1016/j.ejim.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 11.Klein Klouwenberg PMC, Frencken JF, Kuipers S, et al. Incidence, predictors, and outcomes of new-onset atrial fibrillation in critically Ill patients with sepsis. A cohort study. Am J Respir Crit Care Med. 2017;195:205–211. doi: 10.1164/rccm.201603-0618OC. [DOI] [PubMed] [Google Scholar]
- 12.Romiti GF, Corica B, Lip GYH, Proietti M. Prevalence and impact of atrial fibrillation in hospitalized patients with COVID-19: a systematic review and meta-analysis. J Clin Med. 2021;10:2490. doi: 10.3390/jcm10112490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dorgham K, Quentric P, Gökkaya M, et al. Distinct cytokine profiles associated with COVID-19 severity and mortality. J Allergy Clin Immunol. 2021;147:2098–2107. doi: 10.1016/j.jaci.2021.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021 doi: 10.1136/BMJ.N71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Viswanathan M, Ansari MT, Berkman ND, et al (2008) Assessing the risk of bias of individual studies in systematic reviews of health care interventions [PubMed]
- 16.IntHout J, Ioannidis JPA, Rovers MM, Goeman JJ. Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open. 2016;6:e010247. doi: 10.1136/bmjopen-2015-010247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins JPT. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.R: The R Project for Statistical Computing. https://www.r-project.org/. Accessed 13 Apr 2022
- 19.Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22:153–160. doi: 10.1136/ebmental-2019-300117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Viechtbauer W. Conducting Meta-Analyses in R with the metafor Package. J Stat Softw. 2010 doi: 10.18637/jss.v036.i03. [DOI] [Google Scholar]
- 21.Companion R Package for the Guide Doing Meta-Analysis in R • dmetar. https://dmetar.protectlab.org/. Accessed 13 Apr 2022
- 22.Pieralli F, Biondo B, Vannucchi V, et al. Performance of the CHA 2 DS 2 -VASc score in predicting new onset atrial fibrillation during hospitalization for community-acquired pneumonia. Eur J Intern Med. 2019;62:24–28. doi: 10.1016/j.ejim.2019.01.012. [DOI] [PubMed] [Google Scholar]
- 23.Violi F, Cangemi R, Falcone M, et al. Cardiovascular complications and short-term mortality risk in community-acquired pneumonia. Clin Infect Dis. 2017;64:1486–1493. doi: 10.1093/cid/cix164. [DOI] [PubMed] [Google Scholar]
- 24.Pieralli F, Vannucchi V, Nozzoli C, et al. Acute cardiovascular events in patients with community acquired pneumonia: results from the observational prospective FADOI-ICECAP study. BMC Infect Dis. 2021;21:1–12. doi: 10.1186/s12879-021-05781-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruiz LA, Serrano L, España PP, et al. New-onset atrial fibrillation in patients with pneumococcal pneumonia. Impact of timing and duration on short- and medium-term mortality. J Infect. 2021;82:67–75. doi: 10.1016/j.jinf.2020.11.005. [DOI] [PubMed] [Google Scholar]
- 26.Seedat MA, Feldman C, Skoularigis J, et al. A study of acute community-acquired pneumonia, including details of cardiac changes. QJM. 1993;86:669–675. doi: 10.1093/qjmed/86.10.669. [DOI] [PubMed] [Google Scholar]
- 27.Menéndez R, Méndez R, Aldás I, et al. Community-acquired pneumonia patients at risk for early and long-term cardiovascular events are identified by cardiac biomarkers. Chest. 2019;156:1080–1091. doi: 10.1016/j.chest.2019.06.040. [DOI] [PubMed] [Google Scholar]
- 28.Mandal P, Chalmers JD, Choudhury G, et al. Vascular complications are associated with poor outcome in community-acquired pneumonia. QJM. 2011;104:489–495. doi: 10.1093/qjmed/hcq247. [DOI] [PubMed] [Google Scholar]
- 29.Musher DM, Rueda AM, Kaka AS, Mapara SM. The association between pneumococcal pneumonia and acute cardiac events. Clin Infect Dis. 2007;45:158–165. doi: 10.1086/518849. [DOI] [PubMed] [Google Scholar]
- 30.Rombauts A, Abelenda-Alonso G, Càmara J, et al. Host-and Pathogen-Related Factors for Acute Cardiac Events in Pneumococcal Pneumonia. Open Forum Infect Dis. 2020;7:1–8. doi: 10.1093/ofid/ofaa522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Romiti GF, Corica B, Pipitone E, et al. Prevalence, management and impact of chronic obstructive pulmonary disease in atrial fibrillation: a systematic review and meta-analysis of 4,200,000 patients. Eur Heart J. 2021;42:3541–3554C. doi: 10.1093/EURHEARTJ/EHAB453. [DOI] [PubMed] [Google Scholar]
- 32.Walkey AJ, McManus D. When rhythm changes cause the blues: new-onset atrial fibrillation during sepsis. Am J Respir Crit Care Med. 2017;195:152–153. doi: 10.1164/rccm.201608-1617ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soto-Gomez N, Anzueto A, Waterer GW, et al. Pneumonia: an arrhythmogenic disease? Am J Med. 2013;126:43–48. doi: 10.1016/j.amjmed.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Korantzopoulos P, Letsas KP, Tse G, et al. Inflammation and atrial fibrillation: A comprehensive review. J Arrhythm. 2018;34:394–401. doi: 10.1002/joa3.12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Musikantow DR, Turagam MK, Sartori S, et al. Atrial Fibrillation in Patients Hospitalized With COVID-19: Incidence, Predictors, Outcomes and Comparison to Influenza. JACC Clin Electrophysiol. 2021 doi: 10.1016/j.jacep.2021.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harrison SL, Fazio-Eynullayeva E, Lane DA, et al. Atrial fibrillation and the risk of 30-day incident thromboembolic events, and mortality in adults ≥ 50 years with COVID-19. J Arrhythm. 2021;37:231–237. doi: 10.1002/joa3.12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Han X, Chen L, Li H, et al. Prognostic factors for cardiovascular events in elderly patients with community acquired pneumonia: results from the CAP-china network. Clin Interv Aging. 2022;17:603–614. doi: 10.2147/CIA.S356925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Corrales-Medina VF, Musher DM, Wells GA, et al. Cardiac Complications in Patients With Community-Acquired Pneumonia. Circulation. 2012;125:773–781. doi: 10.1161/CIRCULATIONAHA.111.040766. [DOI] [PubMed] [Google Scholar]
- 39.Bosch NA, Massaro JM, Winter MR, et al. New-onset atrial fibrillation as a sepsis-defining organ failure. Ann Am Thorac Soc. 2019;16:1332–1334. doi: 10.1513/AnnalsATS.201902-176RL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Søgaard M, Skjøth F, Nielsen PB, et al. Thromboembolic risk in patients with pneumonia and new-onset atrial fibrillation not receiving anticoagulation therapy. JAMA Netw Open. 2022;5:e2213945–e2213945. doi: 10.1001/JAMANETWORKOPEN.2022.13945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hindricks G, Potpara T, Dagres N, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS) Eur Heart J. 2021;42:373–498. doi: 10.1093/eurheartj/ehaa612. [DOI] [PubMed] [Google Scholar]
- 42.Maisano A, Vitolo M, Imberti JF, et al. Atrial fibrillation in the setting of acute pneumonia: not a secondary arrhythmia. Rev Cardiovasc Med. 2022;23(5):176. doi: 10.31083/J.RCM2305176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Falsetti L, Proietti M, Zaccone V, et al. Impact of atrial fibrillation in critically ill patients admitted to a stepdown unit. Eur J Clin Invest. 2020 doi: 10.1111/ECI.13317. [DOI] [PubMed] [Google Scholar]
- 44.Romiti GF, Proietti M. Impact of rate control in hospitalized patients with atrial fibrillation and sepsis. Eur J Intern Med. 2021;89:126–128. doi: 10.1016/J.EJIM.2021.03.033. [DOI] [PubMed] [Google Scholar]
- 45.Sibley S, Muscedere J. New-onset atrial fibrillation in critically Ill patients. Can Respir J. 2015;22:179–182. doi: 10.1155/2015/394961. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.