Abstract
We reported earlier that a single gene, tsh, isolated from a strain of avian pathogenic Escherichia coli (APEC) was sufficient to confer on E. coli K-12 a hemagglutinin-positive phenotype and that the deduced sequence of the Tsh protein shared homology to the serine-type immunoglobulin A (IgA) proteases of Neisseria gonorrhoeae and Haemophilus influenzae. In this report we show that E. coli K-12 containing the recombinant tsh gene produced two proteins, a 106-kDa extracellular protein and a 33-kDa outer membrane protein, and was also able to agglutinate chicken erythrocytes. N-terminal sequence data indicated that the 106-kDa protein, designated Tshs, was derived from the N-terminal end of Tsh after the removal of a 52-amino-acid N-terminal signal peptide, while the 33-kDa protein, designated Tshβ, was derived from the C-terminal end of Tsh starting at residue N1101. The Tshs domain contains the 7-amino-acid serine protease motif that includes the active-site serine (S259), found also in the secreted domains of the IgA proteases. However, site-directed mutagenesis of S259 did not abolish the hemagglutinin activity or the extracellular secretion of Tshs indicating that host-directed proteolysis was mediating the release of Tshs. Studies with an E. coli K-12 ompT mutant strain showed that the surface protease OmpT was not needed for the secretion of Tshs. Tsh belongs to a subclass of the IgA protease family, which also includes EspC of enteropathogenic E. coli, EspP of enterohemorragic E. coli, and SepA and VirG of Shigella flexneri, which seem to involve a host endopeptidase to achieve extracellular release of their N-terminal domains. In proteolytic studies conducted in vitro, Tshs did not cleave the substrate of the IgA proteases, human IgA1 or chicken IgA, and did not show proteolytic activity in a casein-based assay. Correlation of Tsh expression and hemagglutination activity appears to be a very complex phenomenon, influenced by strain and environmental conditions. Nevertheless, for both APEC and recombinant E. coli K-12 strains containing the tsh gene, it was only the whole bacterial cells and not the cell-free supernatants that could confer hemagglutinin activity. Our results provide insights into the expression, secretion, and proteolytic features of the Tsh protein, which belongs to the growing family of gram-negative bacterial extracellular virulence factors, named autotransporters, which utilize a self-mediated mechanism to achieve export across the bacterial cell envelope.
The gram-negative bacterial cell envelope consists of the cytoplasmic and outer membranes and the periplasm, the space between the two membranes. Extracellular protein secretion in gram-negative bacteria requires the transport of a protein through the bacterial cell envelope. At least three distinct pathways are responsible for protein secretion in gram-negative bacteria (47). Most secreted proteins arrive at the cell surface via the signal peptide-dependent pathway, also known as the type II or sec-dependent, general secretory pathway (GSP) (reviewed in reference 45). In this two-step process, proteins are first translocated across the cytoplasmic membrane into the periplasm. This step is directed by the amino-terminal signal peptide of the translocated protein and is mediated by the Sec export machinery and signal peptidase I or II, which cleaves the signal peptide. The second step in the type II pathway relies on specific accessory proteins for translocation of the protein across the outer membrane. Examples of this are the secretions of the P pilus subunits (reviewed in reference 22) and the pullulanase lipoprotein of Klebsiella oxytoca (46). Secretion of the periplasmic subunits that make up the pilus macromolecular structure is completely dependent on the papC and papD gene products (54), proteins inserted in the outer membrane and present in the periplasm, respectively. Export of pullulanase across the outer membrane requires the products of at least 14 other genes (45). Furthermore, other periplasmic proteins, such as the disulfide bond isomerase DsbA-PpfA, are also involved in the export process (44).
The signal peptide-independent pathway (type I pathway) for protein secretion differs from the GSP in several ways (reviewed in reference 21). The proteins secreted via this pathway do not contain N-terminal signal peptides. The signal for secretion is instead located in the carboxy-terminal end of the protein (25). Periplasmic intermediates of proteins secreted by this pathway have never been isolated; thus, secretion occurs across both membranes simultaneously (16). The components of the secretion apparatus consist of at least three proteins: two are located in the cytoplasmic membrane, and one is located in the outer membrane, presumably forming a protein export channel through the entire cell envelope. One of the cytoplasmic membrane proteins is a member of the bacterial ABC transporter family of protein exporters (10).
The contact-dependent pathway (type III pathway) (reviewed in reference 33) was first identified in pathogenic Yersinia species expressing a number of virulence-related proteins known as Yops (7). This pathway does not involve the removal of a classical N-terminal signal peptide, even though Yop secretion signals seem to reside within the protein’s amino-terminal end. These sequences do not show any overall sequence, secondary structure, or hydrophobicity similarities (34). Yop proteins utilize a unique secretion apparatus for extracellular export, termed the Yop secretion machinery. Another peculiarity of this secretion system is the dependence on cytoplasmic chaperones, which are specific for each individual Yop protein, that presumably are needed for targeting the nascent Yop polypeptides to the secretion apparatus (58). Type III secretion systems have been also identified in several other pathogens, including Salmonella typhimurium (12), Shigella flexneri (57), and the plant pathogen Pseudomonas solanacearum (14).
A variation of the second step of the type II pathway is exemplified by the extracellular secretion of Neisseria gonorrhoeae and Haemophilus influenzae immunoglobulin A (IgA) proteases (39, 40). These proteins contain a cleavable amino-terminal signal sequence and are translocated across the cytoplasmic membrane into the periplasm by using the Sec export machinery. However, this family of proteins differs from other proteins secreted by the type II pathway in that they do not rely on accessory factors for translocation across the outer membrane. Secretion of the N. gonorrhoeae IgA protease has been extensively characterized (27, 28, 39). This protein is synthesized as a 169-kDa preproprotein that is exported across the cytoplasmic membrane. Once in the periplasm, the 45-kDa carboxy-terminal region (IgAβ) is inserted into the outer membrane, where it acts as a channel through which the amino-terminal region of the proprotein, the protease domain, is passed from the periplasm to the cell surface. This amino-terminal domain of the proprotein autoproteolytically cleaves itself at a site close to the outer membrane bound IgAβ, causing the release of the 106-kDa mature protease, whereas the IgAβ domain remains in the outer membrane. With a similar mechanism, the β-domain alone can also facilitate export to the surface of heterologous proteins fused to its amino-terminal end (27), a property that is useful for applications requiring surface display of foreign proteins and peptides (13). The IgA proteases represent the prototypes of a growing group of secreted proteins, termed autotransporters, which utilize a similar autonomous mechanism to export their amino-terminal domains across the outer membrane (4, 18, 23, 50, 53).
We recently described the isolation of a gene from an avian pathogenic E. coli (APEC) strain that is able to confer on E. coli K-12 strains a hemagglutination-positive phenotype and whose deduced amino acid sequence displays considerable homology with the serine-type IgA proteases of N. gonorrhoeae and H. influenzae (43). In this article, we report further characterization of the Tsh protein and present data on Tsh expression, secretion, and proteolytic features.
MATERIALS AND METHODS
Bacterial strains and plasmids.
APEC strain χ7122 (O78:K80:H9) is a gyrA derivative of EC1 (42). χ7141 is an ampicillin-resistant Tsh− derivative of χ7122 (43). E. coli K-12 strains CC118 [(λ−) araD139 Δ(ara leu)7697 ΔlacX74 ΔphoA20 galE galK recA1 rpsE argEamb rpsB thi] (32), DH5α [(f80dlacZΔM15) Δ(lacZYA-argF)U169 glnV44 deoR gyrA96 relA91 endA1 thi-1 hsdR17] (15), and LE392 [(λ−) lacY1 galK2 galT22 glnV44 tyrT58 metB1 hsdR574 trpR55] (36) and the E. coli B strain BL21 ΔompT gal hsdS (17) were used as recipients for plasmids. E. coli K-12 strain JM109 [(λDE3) F′ traD36 proA+ proB+ lacIq Δ(lacZ)M15/(e14−) Δ(lacZ-proAB) glnV44 gyrA96 recA relA1 endA1 thi hsdR17] was used as the host strain for T7 RNA polymerase mediated overexpression of Tsh (52). E. coli strain XL1-Blue (recA1 lac endA1 gyrA96 thi hsdR17 supE44 relA1 (F+ proAB laqIq laqZD M15 Tn10 [Tetr]) has been described (Promega). H. influenzae N187 is a nontypable and nonencapsulated strain isolated from the middle ear fluid of a child with otitis media and was a generous gift from Joseph W. St. Geme III. H. infuenzae DB117 and plasmids pGJB103 and pJS106 have been described before (18). Plasmids pYA3107 and pYA3108 contain the tsh gene subcloned in pACYC184 and pBluescript II(SK), respectively and were as described previously (43). pYA3315 and pYA3321 are derivatives of pYA3107 that contain Tn5seq1 inserted onto the tsh gene (43). For the construction of plasmid pYA3418, the tsh gene was amplified by using primers 5′-GGAATTCCGTTATGCCTGAGTAGTACTTG-3′ and 5′-CGGGATCCTTTGCTGCACAGCATCAGAATG-3′ and cloned into plasmid pWKS30 (56) at EcoRI and BamHI restriction sites under the control of the lac promoter.
Media and culture conditions.
We have shown that hemagglutination activity, the phenotype associated with tsh, is best expressed after growth of bacterial strains on colonization factor antigen (CFA) agar at 26°C for 48 h (42). Unless otherwise noted, these culture conditions were routinely used in experiments to measure Tsh expression. Phosphate-buffered agar was prepared as described previously (42). Colonies of H. influenzae N187 were used to inoculate brain heart infusion broth supplemented with hemin and NAD (51) and incubated for 16 h. L broth and L agar plates were prepared as described previously (31). Antibiotics were used at the following concentrations: ampicillin, 100 μg/ml; tetracycline, 12.5 μg/ml.
Cell fractionation.
A modification of the procedure of Achtman et al. (1) was used. Bacteria grown on a CFA agar plate at 26°C for 48 h were harvested and suspended in 2 ml of 0.85% NaCl. The titer of cells was determined spectrophotometrically, and a volume containing 4 × 109 cells was added to 10 mM Tris (pH 7.6) to a final volume of 10 ml. Cells were disrupted by eight cycles of 1-min sonication. Debris and unbroken cells were removed by centrifugation at 3,000 rpm in an SS34 rotor (Sorvall) for 20 min. The total membrane fraction was pelleted at 45,000 rpm in a TFT 65.13 rotor (Sorvall) for 1 h. The resulting supernatant contained the soluble fraction. The pellet, containing the total membranes, was resuspended in double-distilled H2O (ddH2O), extracted for 20 min at room temperature with 8 volumes of 11.1 mM Tris (pH 7.6) containing 1.67% N-lauroylsarcosine (Sarkosyl), and then pelleted at 45,000 rpm for 90 min. The supernatant, which contained the inner membrane proteins, was removed and stored at −70°C. The pellet, containing the Sarkosyl-insoluble outer membrane proteins, was suspended in 200 μl of ddH2O and frozen at −70°C. Cell supernatants were obtained by suspending bacteria from one CFA agar plate of bacteria in 2 ml of 0.85% NaCl and centrifuging this at 45,000 rpm for 4 h at 4°C. To prepare samples for resolution by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), volumes of 100 μl of soluble protein or inner membrane protein or 300-μl volumes of supernatant were precipitated with 4 volumes of cold acetone. These mixtures were incubated at −20°C for 1 h and then pelleted in a microcentrifuge for 5 min. The acetone was aspirated, and the pellet was dried in a Speed-Vac (Savant). Outer membrane protein aliquots of 10 μl were resolved without acetone precipitation.
Tsh protein purification.
We purified Tsh by exploiting the T7 promoter present upstream of tsh in pYA3108. A 35-ml standing overnight culture of JM109(λDE3)(pYA3108) in L broth with ampicillin was added to 1 liter of 37°C L broth with ampicillin in a 2-liter baffled flask and incubated at 37°C. At an optical density at 600 nm of 0.3, 0.5 mM IPTG (isopropyl-β-d-thiogalactopyranoside) was added to induce the expression of T7 RNA polymerase. After 3 h at 37°C, the cells were pelleted and washed with cold phosphate-buffered saline (PBS), pelleted again, and suspended in a final volume of 10 ml of cold PBS. Comparison of these induced cells with uninduced cells by SDS-PAGE indicated that the expression of a protein of approximately 140 kDa was induced by the addition of IPTG. The 10-ml volume of induced cells was sonicated for eight cycles of 30 s followed by another 30 s on ice. Debris and unlysed cells were removed by centrifugation at 3,000 rpm for 20 min at 4°C. A 10-ml aliquot of DNase (3 μg/ml) was added to the supernatant, which was then incubated at 37°C for 20 min. Aliquots were added to Centricon-30 microconcentrators to decrease the volume. An 8-ml volume of sonicated supernatant was reduced in this way to a volume of approximately 400 μl. The 400 μl retentate volume containing Tsh was diluted with an equal volume of 2× PBS and filtered through a 0.45-mm (pore size) membrane filter. One-half of the filtrate was loaded onto a Superose-12 FPLC column (Pharmacia Biotech, Inc.) equilibrated with PBS. The flow rate was 0.5 ml/minute, and 0.5-ml fractions were collected. Elution of protein was monitored by UV detection. Finally, aliquots of the collected fractions were resolved by SDS-PAGE. Those fractions containing Tsh were combined, acetone precipitated, and resolved by SDS-PAGE. The gel was stained with Coomassie brilliant blue R-250 dissolved in ddH2O and destained with ddH2O. The 140-kDa induced protein was cut out of the gel and electroeluted with an Elutrap (Schleicher & Schuell).
Site-directed mutagenesis.
The tsh gene was mutagenized to encode a protein with a threonine at residue 259 instead of the wild-type serine. This was accomplished by using the overlap extension procedure (19, 20). The mutagenic primers TshB (GCC GGT GAC ACC GGT TCG CCT TTA) and TshC (CCG GTG TCA CCG GCC TCG CCA TA) overlap each other by 14 nucleotides and change the serine codon AGC (nucleotides 1155 to 1157 of tsh) to the threonine codon ACC. Primers TshA (AGG AAA TGT GTT CAT AAG TCT GTC AGA) and TshC were used to PCR amplify a 714-bp fragment with Vent DNA polymerase (New England Biolabs). Primers TshC and TshD (GCC CCC TGA GAA ACC GAA TCC TTA A) were used to PCR amplify a 364-bp fragment. The template DNA for both reactions was pYA3108. The PCR products from these two reactions were gel purified to remove the primers and concentrated with Centricon-30 microconcentrators. The PCR products were combined, annealed, and extended. The primers TshA and TshD were added and used to PCR amplify a 1,065-bp fragment containing the G-to-C transversion mutation at nucleotide 1156 of tsh. This fragment was digested with AatII and NdeI. The resulting 930-bp mutagenized fragment was ligated to pYA3108 that had been digested with the same two enzymes and gel purified to remove the 928-bp fragment containing the wild-type nucleotide sequence. The resulting mixture of ligated plasmids was used to transform DH5α, and transformants were screened by DNA sequence analysis. Of the nine transformants screened, all had the desired mutation. One was chosen and designated pYA3287. The DNA sequence of the entire 928-bp PCR-derived fragment was determined and found to contain no additional mutations. The mutant protein produced by this allele was designated tsh-1156.
The tsh gene was also mutagenized to encode a Tsh protein with an alanine in the place of the Ser-259 of the putative active site. Plasmid pYA3418 was digested with enzymes NdeI and AatII, and the 0.9-kb fragment that contains the tsh region, which encodes the serine protease motif, was isolated from the gel and then ligated to pUC19 vector digested with NdeI and AatII. The resulting plasmid (designated pYA3430) was used as a template for the mutagenesis of Ser-259 by using the following long-inverse PCR approach. Two oligonucleotides with sequences GCCGGTTCGCCTTT (oligo-1) and GTCACCGGCCTCGCCATA (oligo-2) were utilized. Oligo-1 contains codon GCC in the place of AGC (nucleotides 1155 to 1157 of tsh). The 3.3-kb DNA fragment was amplified by using KlenTaq DNA polymerase. PCR products were separated on an agarose gel, and the 3.3-kb fragments were extracted, religated, and transformed into E. coli K-12 strain DH5α. The new plasmid generated by PCR was designated pYA3431. Then the 0.9-kb AatII-NdeI fragment from pYA3431 was cut, isolated from the gel, and used to replace the wild-type 0.9-kb AatII-NdeI fragment from plasmid pYA3108, generating plasmid pYA3432. The presence of codon GCC at nucleotides 1155 to 1157 of tsh in plasmid pYA3432 was confirmed by restriction enzyme digestion with enzyme BsaHI (the replacement of the codon introduced a new BsaHI site in the tsh gene) and by DNA sequencing with the primer with sequence GGACTGTCGGTAGCCTC, which hybridizes approximately 100 bases upstream of the mutagenized sequence (DNA sequencing was performed at ACGT, Inc., Northbrook, Ill.).
Antibody production and purification.
Tsh protein isolated as described above was used to subcutaneously immunize New Zealand White rabbits. The rabbits were boosted after 5 weeks, and serum was collected 4 weeks later. The antiserum was found to contain antibody that cross-reacted with a protein that migrated at the same relative molecular weight as Tsh. To absorb the antibodies that were not specific for Tsh, χ7122 was grown on phosphate-buffered agar for 48 h at 26°C. χ7122 does not agglutinate chicken erythrocytes after growth on this medium (43). χ7122 was suspended in 50 mM NaCl–33 mM Tris (pH 8.0) and sonicated as described above. Unbroken cells and debris were removed by centrifugation in an SS34 rotor (Sorvall) at 3,000 rpm for 20 min. The supernatant was dialyzed against 0.62% borate–0.952% sodium tetraborate–2.9% NaCl (pH 8.3) for 12 h at 4°C and coupled to CNBr-activated Sepharose 4B (Pharmacia Biotech, Inc.) as directed by the manufacturer. Antiserum (1 ml) was added to 0.5 ml of the coupled Sepharose and mixed end over end for 3 h at room temperature. After collection of the unabsorbed antibodies, the gel was regenerated by passing 20 ml of 0.1 M glycine (pH 2.5) through the gel, followed by 20 ml of 0.1 M glycine (pH 2.5) containing 10% Dioxane. The gel was used again after a rinsing with 20 ml of 0.1 M Tris (pH 8.0). This process was repeated until the serum had been absorbed eight times.
Determination of amino-terminal sequences.
The SDS–10% polyacrylamide gels used to resolve proteins for amino-terminal sequence analysis were allowed to polymerize for 72 h. Thioglycolate (0.1 mM) was added to the top buffer (35). To isolate the 106-kDa protein, aliquots of supernatant from CC118(pYA3107) were precipitated with cold acetone for 2 h at −20°C, then pelleted, and rinsed twice with acetone. Each aliquot was loaded into a separate well, resolved by SDS-PAGE, and transferred to 0.2-μm polyvinylidene difluoride (PVDF; Bio-Rad Laboratories, Richmond, Calif.) for 17 h at 0.15 A. The membrane was stained, destained, and rinsed 10 times for at least 5 min each time in ultrapure water (Nanopure II; Barnstead). The 106-kDa protein band was cut out of each lane and submitted for sequence analysis.
Outer membrane proteins prepared from CC118(pYA3107) were used to purify the 33-kDa outer membrane protein. CC118(pYA3107) outer membrane proteins were isolated as described above and resolved by preparative SDS-PAGE. The region containing the 33-kDa protein was cut out and electroeluted by using a Centricon-30 as described earlier (30). The proteins were further concentrated by acetone precipitation and then resolved by SDS-PAGE. The proteins were transferred to PVDF and, after being stained, destained and extensively rinsed with ultrapure water, the band corresponding to the 33-kDa outer membrane protein was cut out and submitted for sequence analysis. Proteins were sequenced on an Applied Biosystems (Foster City, Calif.) model 470A automated protein sequencer with gas-phase chemistry.
Purification of chicken IgA.
A modification of a published procedure was used (29). All manipulations were done on ice or at 4°C. A total of four 8-week-old white leghorn chickens were used. The bile contents were removed by using a syringe and needle and then diluted with the same volume of PBS. Particulate matter was removed from the bile by centrifugation in a microcentrifuge for 3 min. The supernatant was filtered through a 0.45-mm membrane. Diluted bile (3 ml) was applied to a 95- by 2.2-cm column containing Sephacryl-S300 superfine (Pharmacia Biotech, Inc.) equilibrated with PBS. After the bile had entered the column, the run was continued with 0.1 M Tris (pH 7.5), 1.0 M NaCl, and 1 mM EDTA. Fractions were collected at 5-min intervals. To determine which fractions contained IgA, 200-μl aliquots were spotted onto a nitrocellulose filter by using a Hybri-Dot manifold (Life Technologies, Inc.) and probed with affinity-purified goat anti-chicken IgA α-chain antibody (Bethyl Laboratories, Inc.). The secondary antibody used was alkaline phosphatase-conjugated affinity-purified rabbit anti-goat IgG (Sigma Chemical Company, St. Louis, Mo.). This indicated that fractions 2 through 12 contained chicken IgA. Each of these fractions was further analyzed by resolving 10-μl aliquots by SDS–10% PAGE and, after Western immunoblot transfer, the fractions were probed with anti-chicken antibody as described above. Each fraction contained a predominant band that migrated at approximately 80 kDa. Fractions 6 and 7 contained most of this protein. Aliquots of 50 μl were removed from these fractions and stored at −70°C.
Proteolysis of human IgA1 and chicken IgA.
χ7122, χ7141, CC118(pYA3107), and CC118(pACYC184) were grown for 48 h on CFA agar at 26°C. Cells were suspended in 200 μl of 0.85% NaCl and pelleted for 2 min in a microcentrifuge. H. influenzae N187 was grown and prepared as described above. Supernatant volumes of 10 μl were removed and added to either 10 μl of chicken IgA or 16.5 μl (8.25 μg) of human IgA1 (Calbiochem, Inc.). Chloramphenicol was added to a final concentration of 30 μg/ml. This mixture of bacterial supernatant and immunoglobulin was incubated at 37°C for 14 h. The entire volume was resolved by SDS–10% PAGE and transferred to nitrocellulose as described above. Proteolysis of the α-chain of each species of antibody was detected by Western immunoblot. The antibody used to detect the human IgA1 was alkaline phosphatase-conjugated affinity-purified goat anti-human IgA α-chain antibody (Kirkegaard & Perry Laboratories, Inc.). The antibody used to detect the chicken IgA was the same as that described above for purification of chicken IgA.
Hemagglutination assay.
Hemagglutination activity was measured on a 96-well round-bottom plate as follows. Bacteria grown on CFA agar plates at the temperatures indicated were harvested and suspended in 2 ml of 0.85% NaCl. When whole cells were assayed for hemagglutination activity, the suspension of cells was serially diluted in 0.85% NaCl containing methyl-α-d-mannopyranoside (Sigma) to inhibit hemagglutination by type I pili and then was added to each well of the microplate containing a suspension of chicken erythrocytes. When cell-free supernatants were assayed, the suspension of bacterial cells was first centrifuged at 36,000 rpm for 3 h at 4°C, and aliquots of the resulting supernatant were added undiluted to the wells with the erythrocytes. The reactions were incubated for 1 h on ice. Wells containing an even sheet of erythrocytes across the well were considered positive, whereas those containing a small erythrocyte pellet at the bottom of the well were considered negative. The titer is expressed as the reciprocal of the greatest dilution of bacteria that resulted in positive hemagglutination reaction.
Proteolytic assay.
Proteolytic activity in the supernatant of cultures was measured by using the EnzChek protease assay (Molecular Probes, Leiden, The Netherlands), performed according to the instructions of the manufacturer. The substrate in this assay is a casein derivative that is heavily labeled with the pH-insensitive green fluorescent BODIPY FL dye. After proteolytic hydrolysis, BODIPY FL-casein exhibits green fluorescence, which can be detected in a fluorescence microplate reader by using the appropriate excitation and emission filters. Supernatant fractions were collected from E. coli or H. influenzae cultures grown at 37°C, with or without IPTG induction as indicated, after ultracentrifugation for 1 h at 90,000 × g to remove bacterial cells and cell debris. Samples were first incubated at 37°C in the dark for 16 h in the provided digestion buffer (10 mM Tris-HCl [pH 7.8]) and in the presence of 5 μg of chloramphenicol per ml to prevent contamination and were then measured in a Microplate Fluorometer (series 7600; Cambridge Technology, Inc.) with excitation and emission filters of 485 and 530 nm, respectively. Results are presented as percentages of relative fluorescence and represent averages from three independent experiments with each experiment performed in triplicates.
General procedures.
PAGE (30) and the Western blot procedure (55) were performed as described earlier. Signal sequence cleavage site predictions were done by using the Signalp program (38) available at the Expasy Molecular Biology World Wide Web Server (http://expasy.hcuge.ch/). Carboxy-terminal sequence analysis was performed with a Teflon-blotted sample by using the Hewlett-Packard G1009A C-terminal sequencer (Argo BioAnalytica, Inc., Morris Plains, N.J.) (3).
RESULTS
Expression and secretion of Tsh in E. coli K-12 and APEC strains.
We recently described the isolation of an APEC gene, designated tsh, that endowed E. coli K-12 strains with the ability to agglutinate chicken erythrocytes (43). This gene encodes a protein that has homology to the serine-type IgA proteases of H. influenzae and N. gonorrhoeae. We wanted to determine whether Tsh is secreted in a manner similar to the serine-type IgA proteases.
pYA3107 is a pACYC184 derivative containing the gene tsh on a 10-kb DNA fragment (43). This plasmid will confer a hemagglutination positive phenotype on the E. coli K-12 strain CC118 when grown on CFA agar at 26°C. CC118(pYA3107) and CC118(pACYC184) were grown on CFA agar and subjected to cell fractionation. The deduced molecular weight of unprocessed Tsh is 148,226, and we have shown that in vitro transcription-translation assays of Tsh result in the production of a 140-kDa protein (43). However, a protein migrating at this relative molecular mass was not visible in any of the fractions from CC118(pYA3107) (Fig. 1A). Instead, two proteins were found to be expressed by CC118(pYA3107) that were not present in CC118(pACYC184) (Fig. 1A, compare lanes 4 and 5 with lanes 9 and 10). One protein with a relative molecular mass of 106 kDa was found in the supernatant, while a 33-kDa protein was found in the outer membrane fraction. The intact 140-kDa Tsh protein could not be detected in cell lysates, even when the samples were prepared in the presence of the protease inhibitor phenylmethylsulfonyl fluoride (data not shown).
FIG. 1.
Subcellular localization of two proteins expressed when the tsh gene is present in CC118. Bacterial strains were grown on CFA agar at 26°C for 48 h. Subcellular fractions were isolated as described in Materials and Methods. Aliquots were resolved by SDS–10% PAGE. (A) Gel stained with Coomassie brilliant blue. (B) Gel transferred to nitrocellulose and Western immunoblotted with absorbed anti-Tsh antibody. The two proteins produced by CC118(pYA3107) that are not produced by CC118(pACYC184) are marked by arrows. Lanes 1 to 5, CC118(pYA3107); lanes 6 to 10, CC118(pACYC184). Lanes 1 and 6, whole cells; lanes 2 and 7, soluble fractions; lanes 3 and 8, inner membrane fractions; lanes 4 and 9, outer membrane fractions; lanes 5 and 10, supernatant fractions. The molecular mass markers (in kilodaltons) are shown to the left of panel A.
We had previously isolated a series of transposon insertion mutations throughout the tsh gene (43). Two of these, tsh-84 (pYA3315) and tsh-98 (pYA3321), result in the expression of truncated Tsh proteins. Also, CC118 containing these mutant tsh alleles lose the hemagglutination-positive phenotype. Two of these insertion mutants were used here to test directly if tsh was required for the expression of the 106- and 33-kDa proteins. Supernatants and outer membrane proteins isolated from CC118 containing these mutant alleles of tsh did not contain either the 106-kDa secreted protein or the 33-kDa outer membrane protein, respectively (Fig. 2, lanes 1, 2, 4, and 5). There are two plausible explanations for this finding. Either these two proteins are derived from tsh or these two proteins are expressed by CC118 only when tsh is present.
FIG. 2.
Expression of the 106- and 33-kDa proteins by tsh mutants. CC118 containing two tsh mutations derived by transposon mutagenesis (43) and CC118 containing the wild-type tsh gene were grown on CFA agar at 26°C for 48 h. Supernatants and outer membrane proteins were isolated and resolved by SDS–10% PAGE and stained with Coomassie brilliant blue. The two proteins produced by CC118(pYA3107) that are not produced by CC118 containing the tsh mutations are marked by arrows. Lanes 1 to 3, supernatant fractions; lanes 4 to 6, outer membrane proteins. Lanes 1 and 4, CC118(pYA3315); lanes 2 and 5, CC118(pYA3321); lanes 3 and 6, CC118(pYA3107). The molecular mass markers (in kilodaltons) are shown on the left.
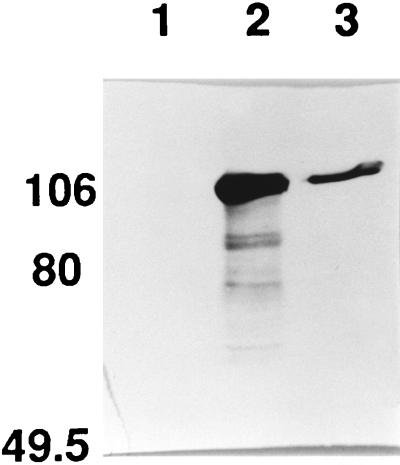
To determine if these two proteins were derived from Tsh, we used antibody produced against the full-length gene product of tsh. We overexpressed the full-length Tsh protein by using a plasmid containing tsh downstream of the T7 promoter (see Materials and Methods). Tsh was isolated and used to immunize rabbits. The antibody produced was not specific against only Tsh; it also reacted with a periplasmic or cytoplasmic protein present in E. coli K-12 that migrated at the same relative molecular weight as the full-length Tsh. This 140-kDa protein was presumably a contaminant which was eluted from the gel together with the 140-kDa Tsh protein used to raise the Tsh antiserum. Our attempts to absorb this contaminating antibody away from the anti-Tsh antibody were not completely successful. Nonetheless, in Western immunoblots, this antibody did react with the 106-kDa secreted protein produced by CC118(pYA3107) (Fig. 1B, lane 5). This observation indicates that the 106-kDa secreted protein is antigenically related to Tsh. The same antibody did not react with the 33-kDa outer membrane protein in Western immunoblots (Fig. 1B, lane 4), suggesting that the 33-kDa outer membrane protein was much less immunogenic than the secreted Tsh domain. We wanted to determine if χ7122, the wild-type strain the tsh gene was isolated from, produced the 106-kDa secreted protein. Supernatants isolated from CC118(pYA3107), χ7122, and χ7141 (the tsh-101 mutant derived from χ7122) were resolved by SDS-PAGE, transferred to nitrocellulose and probed with the anti-Tsh antibody (Fig. 3). The results show that χ7122 does produce the 106-kDa protein, and this protein is secreted. χ7141 does not produce the 106-kDa secreted protein. This indicates that the processing of Tsh we have characterized in CC118(pYA3107) is also occurring in the wild-type APEC pathogen, χ7122.
FIG. 3.
Immunoblot of supernatant fractions from χ7122, χ7141, and CC118(pYA3107). Bacterial strains were grown on CFA agar as described in Materials and Methods. Lane 1, χ7141, a tsh mutant derived from χ7122 (43); lane 2, CC118(pYA3107); lane 3, χ7122. The molecular mass markers (in kilodaltons) are shown on the left.
To further characterize the 106- and 33-kDa proteins, we determined the amino-terminal sequence of each protein. The amino-terminal sequence of the 106-kDa protein was found to be N′-()TVNNELG. This sequence matches amino acids 53 through 60 of Tsh. This amino-terminal sequence also indicates that the signal sequence cleavage site of Tsh is between amino acids 52 and 53. This cleavage site is in agreement with the site predicted by the signal sequence cleavage site prediction method Signalp (38). The amino-terminal sequence of the 33-kDa outer membrane protein was found to be N′-()LNKRMGDLRD. This sequence matches amino acids 1101 through 1111 of Tsh. The amino-terminal sequence data indicates that the 33-kDa outer membrane protein is composed of amino acids 1101 to 1377 of Tsh. The 106-kDa secreted protein begins at amino acid 53 and extends no farther than amino acid 1100. However, multiple proteolytic events may occur to result in the extracellular release of the 106-kDa secreted protein. We attempted to further characterize the 106-kDa secreted Tsh protein by determining the amino acid sequence at the carboxyl-terminal end. Unfortunately, the C-terminal sequence analysis of Tsh could not identify any sequence, indicating that the protein may be C-terminally blocked.
It has been shown (28) that when N. gonorrhoeae IgA proteins were synthesized in E. coli K-12, the processing event of the protein could be mediated by OmpT, a protease present on the cell surface of E. coli K-12 and other gram-negative bacteria (reviewed in reference 49). Likewise, the S. flexneri OmpT homologue SopA (IcsP) was shown to process the IcsA (VirG) and SepA proteins, which are both members of the IgA-protease family (9, 24). To determine if OmpT mediated the proteolytic step of the processing of Tsh, the E. coli strains BL21 and LE392 were transformed with pYA3287, pYA3108, and pBluescript II(SK). BL21 contains an ompT deletion (17), and LE392 expresses OmpT activity (17). The expression of OmpT is very low at temperatures below 32°C (49); thus, LE392 was grown at both 26 and 37°C. Tsh was processed in LE392 at both 26 and 37°C and in BL21 to result in a secreted protein migrating at 106 kDa (Fig. 4, Lanes 2, 5, and 8). This indicates that OmpT is not required for processing of Tsh, but it does not rule out the possibility that OmpT participates in the processing of Tsh.
FIG. 4.
Immunoblot of LE392 and BL21 expressing Tsh and Tsh-T259. Bacterial strains were grown on CFA agar at 26°C for 48 h or at 37°C for 24 h. Hemagglutination activity was determined as described in Materials and Methods. Supernatants were isolated, acetone precipitated and resolved by SDS–10% PAGE as described in Materials and Methods. After transfer to nitrocellulose the 106-kDa protein was detected with absorbed anti-Tsh antibody. Lanes 1 to 3, LE392 grown at 26°C; lanes 4 to 6, LE392 grown at 37°C; lanes 7 to 9, BL21 grown at 26°C. Lanes 1, 4, and 7 show strains transformed with pBluescript II(SK); lanes 2, 5, and 8 show strains transformed with pYA3108. Lanes 3, 6, and 9 show strains transformed with pYA3287. Molecular mass markers (in kilodaltons) are shown to the left of the figure. Hemagglutination titers for each strain are shown under the figure.
The serine-type IgA proteases autoproteolytically cleave themselves to cause the conversion of the preform of the enzyme to the mature secreted domain (39, 40). The serine-type IgA proteases each contain the 7-amino-acid sequence GDSGSPL (39, 40). It has been pointed out that this amino acid sequence is very similar to a 9-amino-acid motif that is highly conserved in the chymotrypsin-trypsin family of serine proteases (2). The first serine of this motif is the active site serine in this family of serine proteases. Mutagenesis of this serine to a cysteine or a threonine in the H. influenzae IgA protease results in the loss of IgA protease activity, and the mutant protein is not processed (2, 41). Tsh also has the identical 7-amino-acid motif that the other serine-type IgA proteases contain (amino acids 254 to 260). Since Tsh contains reasonable amino acid homology to the domain of the IgA proteases that contain the mature IgA protease and the homology includes the amino acid motif containing the active site serine of the IgA proteases, and since Tsh is processed in a manner similar to the IgA proteases, we investigated whether the first serine of the 7-amino-acid serine protease motif is required for the final proteolytic event that results in the extracellular release of the 106-kDa Tsh domain.
This was tested by constructing a tsh mutant that encoded a threonine in place of the putative active site serine at residue 259 of Tsh. This mutant allele, present on pYA3287, was used to transform both LE392 and BL21 and assayed for production of the 106-kDa protein and expression of hemagglutination activity. This mutation had no effect on the elaboration of the 106-kDa protein, and the molecular mass of the protein was unchanged (Fig. 4, lanes 3, 6, and 9). Both strains, grown on CFA agar for 48 h at 26°C, containing pYA3108 and pYA3287 agglutinated chicken erythrocytes; thus, the mutation did not abolish the hemagglutination activity.
However, serine and threonine have very similar structures. Since site-directed mutagenesis of the active site serine of the H. influenzae Hap protein abolished extracellular secretion only when serine was replaced with an alanine (18), we investigated if Tsh was similar to Hap in this regard. Contrary to what has been seen for Hap, mutagenesis of serine-259 to an alanine residue did not affect the processing and extracellular secretion of Tsh (data not shown).
Analysis of Tsh proteolytic activity.
The secretion similarities between Tsh and the IgA proteases prompted us to determine if Tsh could proteolytically cleave the substrate of the IgA proteases, human IgA1. Human IgA1, isolated from human plasma, was incubated with supernatants from strains grown on CFA agar. This mixture was resolved and transferred to nitrocellulose. The membrane was probed with antibody directed against the heavy chain of human IgA1. The positive control (supernatant from H. influenzae strain N187) showed a substantial decrease in the amount of heavy chain present at approximately 70 kDa and the presence of two immunoreactive bands present at approximately 36 and 39.5 kDa, thus showing the expected result of the cleavage of human IgA1 in the hinge region by the H. influenzae IgA1 protease (37). There was no loss of heavy chain at 70 kDa and no observable lower-molecular-weight bands when supernatant from strains CC118(pYA3107) and χ7122 were used (Fig. 5A). These data indicate that Tsh is not able to cleave human IgA1 under the experimental conditions used in the assay. We also investigated the ability of Tsh to cleave chicken IgA. We assayed for the cleavage of chicken IgA by Western immunoblot probed with chicken IgA-specific antibody as described above. Tsh did not cleave the heavy chain of chicken IgA that migrates at 80 kDa (Fig. 5B).
FIG. 5.
Immunoblots of human IgA1 (A) or chicken IgA (B). Bacterial strains were grown as described in Materials and Methods. Supernatants were isolated and added to aliquots of human IgA1 or chicken IgA. After 14 h of incubation at 37°C, the entire mixture was resolved by SDS–10% PAGE and then transferred to nitrocellulose. This membrane was immunoblotted with anti-human IgA1 α-chain antibody (in panel A) or anti-chicken IgA α-chain antibody (in panel B). The molecular mass markers (in kilodaltons) are shown on the right. (A) Lane 1, human IgA1 alone; lane 2, H. influenzae N187; lane 3, CC118(pACYC184); lane 4, CC118(pYA3107); lane 5, χ7141; lane 6, χ7122. (B) Lane 1, chicken IgA1 alone; lane 2, CC118(pACYC184); lane 3, CC118(pYA3107); lane 4, χ7141; lane 5, χ7122.
In the light of these findings, we decided to determine if the secreted Tsh protein displays any kind of proteolytic activity in the culture supernatant. To measure general proteolytic activity in the supernatants from Tsh− and Tsh+ cultures, we used a sensitive direct fluorescence-based assay for detecting metallo-, serine, acid, and sulfhyldryl proteases. This assay has been already used to detect proteolytic activity in the Tsh homologue PssA from a Shiga toxin-producing E. coli strain (8). Since χ7122 expressed Tsh at relatively low amounts even when grown at optimal growth conditions (Fig. 3), we used a Tsh-expressing plasmid, pYA3418, with the tsh gene under the control of the IPTG-inducible lac promoter. We collected supernatant fractions from cultures of XL1-Blue(pYA3418) and control cultures, grown in the presence of IPTG, and tested them for enzymatic activity. XL1-Blue(pYA3418) grown under these conditions secretes in the supernatant higher amounts of Tsh than does avian strain χ7122 (data not shown). As shown in Fig. 6, proteolytic activity was not detected in the supernatants from strains XL1-Blue(pYA3418) and χ7122. In contrast, proteolytic activity was detected for supernatants of H. influenzae DB117(pGJB103) secreting the H. influenzae IgA protease and H. influenzae DB117(pJS106) secreting both the H. influenzae IgA protease and the Tsh homologue Hap protein.
FIG. 6.
Casein-based assay for proteolytic activity. L broth supernatants were collected as described in Materials and Methods. Samples were incubated at 37°C for 16 h, and proteolytic activity was measured with a fluorometer. Averages from three independent experiments are shown; each assay was done in triplicate. Cultures of XL1-Blue(pWKS30) and XL1-Blue(pYA3418) were induced with 1 mM IPTG for 1 h at an optical density at 600 nm of 0.6. The samples tested are indicated by different columns as follows: A, L broth medium; B, 5 μg of trypsin; C, 5 μg of Haps; D, supernatant from culture of χ7122; E, supernatant from culture of χ7141; F, supernatant from culture of XL1-Blue(pWKS30); G, supernatant from culture of XL1-Blue(pYA3418); H, supernatant from culture of H. influenzae DB117(pJS106); F, supernatant from culture of H. influenzae DB117(pGJB103).
Environmental control of hemagglutination activity and Tsh expression in wild-type APEC.
We have shown that expression of the hemagglutination-positive phenotype of χ7122 is regulated by environmental conditions, including temperature and osmolarity (42). Hemagglutination activity is expressed maximally during growth at 26°C and decreases with increasing growth temperature. In addition, hemagglutination activity is absent after growth of χ7122 on CFA agar supplemented with 0.15 M NaCl. We wanted to determine if Tsh was expressed by χ7122 under conditions that decrease hemagglutination activity. χ7122 was grown under various conditions and supernatants were collected, resolved by SDS-PAGE, and immunoblotted with anti-Tsh antibody. While hemagglutination titers declined as the incubation temperature increased, Tsh levels expressed by χ7122 increased as the incubation temperature increased (Fig. 7, lanes 2, 3, and 4). Similarly, χ7122 grown on CFA agar supplemented with 0.15 M NaCl did not express hemagglutination activity but did express Tsh at a level similar to χ7122 grown on unsupplemented CFA agar (Fig. 7, lanes 2 and 5). In contrast, E. coli K-12 LE392 containing tsh displayed a higher level of expression of Tsh and hemagglutination activity at 26°C than at 37°C (Fig. 4, lanes 2 and 5). CC118 containing tsh expressed high hemagglutination titers after growth at both 26 and 37°C, and the level of Tsh expression after growth at these two temperatures was approximately the same (Fig. 7, lanes 6, 7, and 8). However, cell-free supernatant fractions from any tested strain grown either at 26 or at 37°C failed to produce a hemagglutination reaction (data not shown).
FIG. 7.
Immunoblot of supernatant fractions from χ7122, χ7141, and CC118(pYA3108) grown under different conditions. Bacterial strains were grown on CFA agar at 26°C for 48 h or at higher temperatures for 24 h. CFA agar was supplemented with 0.15 M NaCl as indicated. Hemagglutination activity was determined as described in Materials and Methods. Lane 1, χ7141 grown at 26°C; lane 2, χ7122, grown at 26°C; lane 3, χ7122 grown at 37°C; lane 4, χ7122 grown at 42°C; lane 5, χ7122 grown at 26°C on CFA supplemented with 0.15 M NaCl; lane 6, CC118(pYA3108) grown at 26°C, lane 7, CC118(pYA3108) grown at 37°C; lane 8, CC118(pYA3108) grown at 42°C. Molecular mass markers (in kilodaltons) are shown to the left of the figure. Hemagglutination titers for each strain are shown under the figure.
The evidence presented here shows that expression of Tsh, as measured by the expression of Tshs in the supernatant, occurs under all of the conditions we tested. Moreover, it appears that both secretion of Tshs and hemagglutination activity are influenced by strain and environmental conditions. Thus, although we have firmly established that the tsh gene alone confers on E. coli K-12 strains the ability to agglutinate chicken erythrocytes (43), the regulation of Tsh expression seems to be complex. In addition, the evidence presented here shows that in the E. coli K-12 strains LE392 and CC118, the level of expression of Tsh is correlated to the level of expression of hemagglutination activity. Thus, although in E. coli K-12 hemagglutination depends on the expression of Tsh, in the wild-type APEC strain the presence of hemagglutinin activity may be related to the expression of multiple hemagglutinins.
DISCUSSION
The APEC strain χ7122 has been shown to cause airsacculitis and colisepticemia in chickens (42). Virulence for APEC is associated with the presence of unique DNA regions in the chromosome that are absent from E. coli K-12 strains (5). We previously reported the cloning of the APEC gene tsh and exploited the ability of this gene to confer on E. coli K-12 a hemagglutination-positive phenotype (43).
The deduced amino acid sequence of the tsh gene indicated that it contained significant homology to the serine-type IgA proteases of H. influenzae and N. gonorrhoeae. The serine-type IgA proteases undergo a dramatic maturation event that results in the secretion of the 100- to 106-kDa IgA protease domain and two to three short peptides, and the remainder of the protein (approximately 45 to 56 kDa) inserted in the outer membrane (39, 40). We hypothesized that Tsh may also be proteolytically processed during maturation to result in at least two proteins. The genetic, immunological, and biochemical evidence presented here firmly establish that in E. coli K-12 the expression of the single gene tsh results in two proteins, a 106-kDa secreted protein and a 33-kDa outer membrane protein. We also demonstrate that the authentic APEC host χ7122 also secretes a 106-kDa protein that is recognized by anti-Tsh antibody. We have no way to detect small amounts of the 33-kDa protein, but we hypothesize that in APEC the 33-kDa outer membrane protein, like the 106-kDa secreted protein, is present but not expressed at high enough levels to detect without antibody.
The amino-terminal sequence of the two proteins suggests that the 106-kDa protein consists of amino acids 53 up to 1100 of Tsh, while the 33-kDa protein consists of amino acids 1101 to 1377 of Tsh. The deduced molecular weights of the 106- and 33-kDa proteins are 111,826 and 30,590, respectively, and are in good agreement with the observed relative molecular masses of the proteins. The actual carboxy-terminal amino acid of the 106-kDa protein may not be residue 1100, due to the possible occurrence of multiple proteolytic events that result in extracellular release of the protein. Carboxy-terminal amino acid sequencing of the 106-kDa protein did not provide us with any sequence, indicating that the secreted Tsh domain may be C-terminally blocked.
Since Tsh is processed in a manner similar to the serine-type IgA proteases, we have adopted a similar nomenclature for the domains of Tsh. In accordance with the terminology used to refer to the outer membrane domain of the N. gonorrhoeae serine-type IgA protease as IgAβ (27), we now refer to the 33-kDa outer membrane protein of Tsh as Tshβ. The secreted protease of the N. gonorrhoeae IgA protease is IgAp, where “p” refers to protease. Since we do not know whether the 106-kDa protein of Tsh is a protease (see below), it is designated Tshs, where “s” refers to secreted domain.
The homology between Tshs and the protease domain of the serine-type IgA proteases of H. influenzae and N. gonorrhoeae led us to assess the proteolytic properties of Tsh. Our evidence shows that the secreted 106-kDa protein derived from Tsh did not cleave human IgA1 or chicken IgA. The serine-type IgA proteases have an exceptionally limited repertoire of substrates; the proenzyme form of themselves, human IgA1, and LAMP1, an integral membrane glycoprotein of late endosomes and lysosomes. Thus, we hypothesize that Tshs is similarly limited in the substrates it can cleave and that human IgA1 and chicken IgA are not among these substrates. This is also supported by the fact that Tshs did not show proteolytic activity in a casein-based assay that has detected activity for a number of other IgA protease-like proteins, including PssA of Shiga toxin-producing E. coli (8) and Haps and IgA protease of H. influenzae (Fig. 6). Moreover, Tshs did not cleave pepsin A (data not shown), a substrate of the EspP protease of enterohemorrhagic E. coli (6). Alternatively, specific enzymatic cofactors may be required for Tshs to become proteolytically active, and these cofactors were not present in the L broth supernatants that were used for the assay.
The serine-type IgA proteases each contain the 7-amino-acid sequence GDSGSPL (39, 40). It has been pointed out that this amino acid sequence is very similar to a 9-amino-acid motif that is highly conserved in the chymotrypsin-trypsin family of serine proteases (2). The first serine of this motif is the active site serine in this family of serine proteases. Mutagenesis of this serine to a cysteine or a threonine in the H. influenzae IgA protease results in the loss of IgA protease activity, and the mutant protein is not processed (2, 41).
Tsh also has the identical 7-amino-acid motif that the other serine-type IgA proteases contain (amino acids 257 to 263). Studies by site-directed mutagenesis determined that the trypsin-like motif is not required for the extracellular secretion of Tshs or the expression of the hemagglutinin activity. Tsh was still processed when the putative active site serine at residue 259 was mutagenized to a threonine or an alanine. Thus, the extracellular release of Tshs may not depend on autoproteolysis. This is in marked contrast to the serine-type IgA protease of N. gonorrhoeae, where the extracellular release of the protease domain is due to autoproteolysis, but is in agreement with observations made previously with other members of the IgA protease family, including EspC of enteropathogenic E. coli (50), EspP of enterohemorrhagic E. coli (6), and VirG-IcsA of S. flexneri (48). The extracellular secretion for both EspC and VirG seems to depend on the presence of outer membrane associated proteases, such as OmpT and OmpT homologues found in E. coli K-12 and other gram-negative bacteria (49). However, as shown here, OmpT is not required for the processing of Tsh. As shown in Fig. 4, secretion of TshsThr-259 also occurred in the ompT strain BL21.
It is possible that the processing of Tsh may depend on the action of another outer membrane protease which has not been identified yet. The Tsh processing site Asn-Asn1101 is not a known cleavage site for the proteases of the OmpT family (49). Moreover, no asparagine-specific endopeptidases have been identified in the cell envelopes of enteric bacteria. Since the processing site Asn-Asn has been reserved in all autotransporters of the Tsh family (which includes proteins Tsh, EspP, EspC, and SepA), it is possible that the same host asparagine-specific endopeptidase may be responsible for the processing of all of these proteins. However, our data cannot rule out the possibility that multiple proteolytic events, including autoproteolysis, may occur to cause the extracellular release of Tshs.
Regulation of Tsh expression appears to be complex and dependent on strain and growth conditions. The complexity of Tsh regulation, which results in the variability of expression of the cloned tsh in different host strains, reminds one of the complexity of curli regulation (for a review on curli, see reference 22). Curli are extracellular adhesins present in E. coli and Salmonella species which, in general, are expressed optimally under conditions of low temperature and low osmolarity. However, only certain strains express curli from cloned genes, and some Salmonella spp. form curli only at high temperatures. Our results show that for E. coli K-12 strains containing tsh, the levels of Tsh secretion correlate with the levels of hemagglutinin activity. However, in the wild-type APEC strain χ7122, there is no such correlation and Tsh is secreted more efficiently at high temperatures than at low temperatures, whereas hemagglutinin activity is higher when the bacteria are grown at low temperatures. This difference may be due to (i) differences in the regulation of Tsh expression between APEC and E. coli K-12 strains and/or (ii) the presence of other hemagglutinins, in addition to Tsh, in the APEC strain.
The observed expression of Tshs at higher temperatures in χ7122 does make sense when considered within the context of the pathogenic lifestyle of APEC. We originally speculated that the expression of a hemagglutinin at a low temperature may allow the pathogen to be primed for adhering to the respiratory tract directly upon initial contact with the host mucosal surface (42). In this model, the higher temperatures prevalent within the host would lead to the expression of adhesins and other factors important in virulence and to reduced expression of the hemagglutination phenotype. After we determined that expression of one putative hemagglutinin, Tsh, led to the secretion of a molecule with the high probability of being a protease, our original hypothesis led to a paradox: if Tsh was expressed before interaction with the host, Tshs would be secreted and presumably separated from the microbe. Our results reported here show that in APEC Tshs is expressed at 42°C, the internal body temperature of the domestic fowl (11), and thus imply that Tsh may be expressed and Tshs secreted during the infectious process of APEC. The observation that increased expression of Tsh in APEC does not result in increased hemagglutination activity implies that expression of the lectin(s) responsible for the agglutination of chicken erythrocytes is not important in the pathogenesis of deeper tissues.
Finally, our results indicate that it is not the extracellular Tshs but rather a cell-associated form of Tsh that is responsible for the hemagglutinin-positive phenotype. Although, we have not been able to detect the 140-kDa Tsh protein in either APEC or recombinant E. coli K-12 strains (except when we used clones which overproduce Tsh), it is still possible that low levels of unprocessed Tsh are present on the cell surface and can mediate hemagglutination. Alternatively, the processed extracellular Tshs, which may remain associated with the bacterial surface under certain conditions, is the protein that mediates hemagglutination, but not Tshs released into the supernatant medium. The observation that in the wild-type APEC strain hemagglutination activity is higher at low temperatures, at which Tshs is secreted into the supernatant with lower efficiency, than at high temperatures, at which there is more Tshs found in the supernatant, further supports the above model for Tsh-mediated hemagglutination. Interestingly, the Tsh protein with mutagenized serine-259 (see Fig. 4) displays increased levels of hemagglutination activity compared to the wild-type Tsh protein. It will be interesting to determine whether this mutated Tsh is better associated with the bacterial surface than the wild-type Tsh. Further studies are underway in our laboratory to determine the molecular mechanism of hemagglutination as mediated by the Tsh protein.
ACKNOWLEDGMENTS
We gratefully acknowledge J. W. St. Geme III and D. R. Hendrixson for providing the H. influenzae supernatants and purified Haps, C. M. Dozois for assistance with various experiments, J. O. Hassan for assistance with collection of bile from chickens, W. S. Bollen for assistance with the construction of plasmid pYA3432, P. K. Brown for constructing plasmid pYA3418, J. Diani and D. Piachek for immunization and care of the animals used in this study, and J. Clark-Curtiss for reading the manuscript and suggestions.
This work was supported by U.S. Department of Agriculture National Research Initiative Competitive Grants Program grant 94-37204-1091 and an unrestricted grant award from Bristol-Myers Squibb.
REFERENCES
- 1.Achtman M, Mercer A, Kusecek B, Pohl A, Heuzenroeder M, Aaronson W, Sutton A, Silver R P. Six widespread bacterial clones among Escherichia coli K1 isolates. Infect Immun. 1983;39:315–335. doi: 10.1128/iai.39.1.315-335.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bachovin W W, Plaut A G, Flentke G R, Lynch M, Kettner C A. Inhibition of IgA1 proteinases from Neisseria gonorrhoeae and Hemophilus influenzae by peptide prolyl boronic acids. J Biol Chem. 1990;265:3738–3743. [PubMed] [Google Scholar]
- 3.Bailey J M, Miller C G. Current protocols in protein science, unit 15.1. New York, N.Y: Wiley; 1996. C-terminal sequence analysis. [Google Scholar]
- 4.Benjelloun-Touimi Z, Sansonetti P J, Parsot C. SepA, the major extracellular protein of Shigella flexneri: autonomous secretion and involvement in tissue invasion. Mol Microbiol. 1995;17:123–135. doi: 10.1111/j.1365-2958.1995.mmi_17010123.x. [DOI] [PubMed] [Google Scholar]
- 5.Brown P K, Curtiss R., III Unique chromosomal regions associated with virulence of an avian pathogenic Escherichia coli strain. Proc Natl Acad Sci USA. 1996;93:11149–11154. doi: 10.1073/pnas.93.20.11149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brunder W, Schmidt H, Karch H. EspP, a novel extracellular serine protease of enterohaemorrhagic Escherichia coli O157:H7 cleaves human coagulation factor V. Mol Microbiol. 1997;24:767–778. doi: 10.1046/j.1365-2958.1997.3871751.x. [DOI] [PubMed] [Google Scholar]
- 7.Cornelis G R. Yersinia pathogenicity factors. Curr Top Microbiol Immunol. 1994;192:243–263. doi: 10.1007/978-3-642-78624-2_11. [DOI] [PubMed] [Google Scholar]
- 8.Djafari S, Ebel F, Deibel C, Kramer S, Hubel M, Chakraborty T. Characterization of an exported protease from Shiga toxin-producing Escherichia coli. Mol Microbiol. 1997;25:771–784. doi: 10.1046/j.1365-2958.1997.5141874.x. [DOI] [PubMed] [Google Scholar]
- 9.Egile C, d’Hauteville H, Parsot C, Sansonetti P J. SopA, the outer membrane protease responsible for polar localization of IcsA in Shigella flexneri. Mol Microbiol. 1997;23:1063–1073. doi: 10.1046/j.1365-2958.1997.2871652.x. [DOI] [PubMed] [Google Scholar]
- 10.Fath M J, Kolter R. ABC transporters: bacterial exporters. Microbiol Rev. 1993;57:995–1017. doi: 10.1128/mr.57.4.995-1017.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freeman B M. Appendix: biochemical and physiological data. In: Freeman B M, editor. Physiology and biochemistry of the domestic fowl. Orlando, Fla: Academic Press; 1984. pp. 407–424. [Google Scholar]
- 12.Galan J E. Molecular genetic bases of Salmonella entry into host cells. Mol Microbiol. 1996;20:263–271. doi: 10.1111/j.1365-2958.1996.tb02615.x. [DOI] [PubMed] [Google Scholar]
- 13.Georgiou G, Stathopoulos C, Daugherty P S, Nayak A R, Iverson B L, Curtiss R., III Display of heterologous proteins on the surface of microorganisms: from the screening of combinatorial libraries to live recombinant vaccines. Nat Biotechnol. 1997;15:29–34. doi: 10.1038/nbt0197-29. [DOI] [PubMed] [Google Scholar]
- 14.Gough C L, Genin S, Lopes V, Boucher C A. Homology between the HrpO protein of Pseudomonas solanacearum and bacterial proteins implicated in a signal peptide-independent secretion mechanism. Mol Gen Genet. 1993;239:378–392. doi: 10.1007/BF00276936. [DOI] [PubMed] [Google Scholar]
- 15.Grant S G N, Jesse J, Bloom F R, Hanahan D. Differential plasmid rescue from transgenic mouse DNAs into Escherichia coli: methylation-restriction mutants. Proc Natl Acad Sci USA. 1990;87:4645–4649. doi: 10.1073/pnas.87.12.4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gray L, Baker K, Kenny B, Mackman N, Haigh R, Holland I B. A novel C-terminal signal sequence targets E. coli haemolysin directly to the medium. Mol Gen Genet. 1989;205:127–133. doi: 10.1242/jcs.1989.supplement_11.4. [DOI] [PubMed] [Google Scholar]
- 17.Grodberg J, Dunn J J. ompT encodes the Escherichia coli outer membrane protease that cleaves T7 RNA polymerase during purification. J Bacteriol. 1988;170:1245–1253. doi: 10.1128/jb.170.3.1245-1253.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hendrixson D R, de la Morena M L, Stathopoulos C, St. Geme J W., III Structural determinants of processing and secretion of the Haemophilus influenzae Hap protein. Mol Microbiol. 1997;26:505–518. doi: 10.1046/j.1365-2958.1997.5921965.x. [DOI] [PubMed] [Google Scholar]
- 19.Higuchi R, Krummel B, Saiki R K. A general method of in vitro preparation and specific mutagenesis of DNA fragments: study of protein and DNA interactions. Nucleic Acids Res. 1988;16:7351–7367. doi: 10.1093/nar/16.15.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ho S N, Hunt H D, Horton R M, Pullen J K, Pease L R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- 21.Holland I B, Blight M A, Kenny B. The mechanism of secretion of hemolysin and other polypeptides from Gram negative bacteria. J Bioenerg Biomembr. 1990;22:473–491. doi: 10.1007/BF00763178. [DOI] [PubMed] [Google Scholar]
- 22.Hultgren S J, Jones C H, Normark S. Bacterial adhesins and their assembly. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. Washington, D.C: ASM Press; 1996. [Google Scholar]
- 23.Jose J, Jahnig F, Meyer T F. Common structural features of IgA1 protease-like outer membrane autotransporters. Mol Microbiol. 1995;18:377–382. doi: 10.1111/j.1365-2958.1995.mmi_18020378.x. [DOI] [PubMed] [Google Scholar]
- 24.Kalpana D S, Sallustio S, Manessis A, D’Aversa T G, Goldberg M B. Disruption of IcsA, the major Shigella protease that cleaves IcsA, accelerates actin-based motility. Mol Microbiol. 1997;25:451–462. doi: 10.1046/j.1365-2958.1997.4681827.x. [DOI] [PubMed] [Google Scholar]
- 25.Kenny B, Taylor S, Holland I B. Identification of individual amino acids required for secretion within the haemolysin (HlyA) C-terminal targeting region. Mol Microbiol. 1992;6:1477–1489. doi: 10.1111/j.1365-2958.1992.tb00868.x. [DOI] [PubMed] [Google Scholar]
- 26.Klauser T, Krämer J, Otzelberger K, Pohlner J, Meyer T F. Characterization of the Neisseria Igaβ-core, the essential unit for outer membrane targeting and extracellular protein secretion. J Mol Biol. 1993;234:579–593. doi: 10.1006/jmbi.1993.1613. [DOI] [PubMed] [Google Scholar]
- 27.Klauser T, Pohler J, Meyer T F. Extracellular transport of cholera toxin B subunit using Neisseria IgA protease β-domain: conformation-dependent outer membrane translocation. EMBO J. 1990;9:1991–1999. doi: 10.1002/j.1460-2075.1990.tb08327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klauser T, Pohlner J, Meyer T F. Selective extracellular release of cholera toxin B subunit by Escherichia coli: dissection of Neisseria Igaβ-mediated outer membrane transport. EMBO J. 1992;11:2327–2335. doi: 10.1002/j.1460-2075.1992.tb05292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kobayashi K, Hirai H. Studies on subunit components of chicken polymeric immunoglobulins. J Immunol. 1980;124:1695–1704. [PubMed] [Google Scholar]
- 30.LeGendre N, Matsudaira P T. Purification of proteins and peptides by SDS-PAGE. In: Matsudaira P T, editor. A practical guide to protein and peptide purification for microsequencing. San Diego, Calif: Academic Press, Inc.; 1989. pp. 52–69. [Google Scholar]
- 31.Lennox E S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955;1:190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- 32.Manoil C, Beckwith J. TnphoA: a transposon probe for protein export signals. Proc Natl Acad Sci USA. 1985;82:8129–8133. doi: 10.1073/pnas.82.23.8129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mecsas J, Strauss E J. Molecular mechanism of bacterial virulence: type III secretion and pathogenicity islands. Emerg Infect Dis. 1996;2:271–285. doi: 10.3201/eid0204.960403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Michiels T, Cornelis G R. Secretion of hybrid proteins by the Yersinia Yop export system. J Bacteriol. 1991;173:1677–1685. doi: 10.1128/jb.173.5.1677-1685.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moos M J, Nguyen N Y, Liu T-Y. Reproducible high yield sequencing of proteins electrophoretically separated and transferred to an inert support. J Biol Chem. 1988;263:6005–6008. [PubMed] [Google Scholar]
- 36.Mulks M H, Kornfeld S J, Frangione B, Plaut A G. Relationship between the specificity of IgA proteases and serotypes in Haemophilus influenzae. J Infect Dis. 1982;146:266–274. doi: 10.1093/infdis/146.2.266. [DOI] [PubMed] [Google Scholar]
- 37.Murray N E, Brammer W J, Murray K. Lambdoid phages that simplify the recovery of in vitro recombinants. Mol Gen Genet. 1977;150:53–61. doi: 10.1007/BF02425325. [DOI] [PubMed] [Google Scholar]
- 38.Nielsen H, Engelbrecht J, Brunak S, von Heigne G. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 1997;10:1–6. doi: 10.1093/protein/10.1.1. [DOI] [PubMed] [Google Scholar]
- 39.Pohlner J, Halter R, Beyreuther K, Meyer T F. Gene structure and extracellular secretion of Neisseria gonorrhoeae IgA protease. Nature. 1987;325:458–462. doi: 10.1038/325458a0. [DOI] [PubMed] [Google Scholar]
- 40.Poulsen K, Brandt J, Hjorth J P, Thogersen H C, Kilian M. Cloning and sequencing of the immunoglobulin A1 protease gene (iga) of Haemophilus influenzae serotype b. Infect Immun. 1989;57:3097–3105. doi: 10.1128/iai.57.10.3097-3105.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poulsen K, Reinholdt J, Kilian M. A comparative genetic study of serologically distinct Haemophilus influenzae type 1 immunoglobulin A1 proteases. J Bacteriol. 1992;174:2913–2921. doi: 10.1128/jb.174.9.2913-2921.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Provence D L, Curtiss R., III Role of crl in avian pathogenic Escherichia coli: a knockout mutation of crl does not affect hemagglutination activity, fibronection binding, or curli production. Infect Immun. 1992;60:4460–4467. doi: 10.1128/iai.60.11.4460-4467.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Provence D L, Curtiss R., III Isolation and characterization of a gene involved in hemagglutination by an avian pathogenic Escherichia coli. Infect Immun. 1994;62:1369–1380. doi: 10.1128/iai.62.4.1369-1380.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pugsley A P. Translocation of a folded protein across the outer membrane in Escherichia coli. Proc Natl Acad Sci USA. 1992;89:12058–12062. doi: 10.1073/pnas.89.24.12058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pugsley A P. The complete general secretory pathway in gram-negative bacteria. Microbiol Rev. 1993;57:50–108. doi: 10.1128/mr.57.1.50-108.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pugsley A P, d’Enfert C, Reyss I, Kornacker M G. Genetics of extracellular protein secretion by gram-negative bacteria. Annu Rev Genet. 1990;24:67–90. doi: 10.1146/annurev.ge.24.120190.000435. [DOI] [PubMed] [Google Scholar]
- 47.Salmond G P, Reeves P J. Membrane traffic wardens and protein secretion in gram-negative bacteria. Trends Biochem Sci. 1993;18:7–12. doi: 10.1016/0968-0004(93)90080-7. [DOI] [PubMed] [Google Scholar]
- 48.Shere K D, Sallustio S, Manessis A, D’Aversa T G, Golberg M B. Disruption of IcsA, the major Shigella protease that cleaves IcsA, accelerates actin-based motility. Mol Microbiol. 1997;25:451–462. doi: 10.1046/j.1365-2958.1997.4681827.x. [DOI] [PubMed] [Google Scholar]
- 49.Stathopoulos C. Structural features, physiological roles, and biotechnological applications of the membrane proteases of the OmpT bacterial endopeptidase family: a micro-review. Membr Cell Biol. 1998;12:1–8. [PubMed] [Google Scholar]
- 50.Stein M, Kenny B, Stein M A, Finlay B B. Characterization of EspC, a 110-kilodalton protein secreted by enteropathogenic Escherichia coli which is homologous to members of the immunoglobulin A protease-like family of secreted proteins. J Bacteriol. 1996;178:6546–6554. doi: 10.1128/jb.178.22.6546-6554.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.St. Geme III J W, Falkow S. Haemophilus influenzae adheres to and enters cultured human epithelial cells. Infect Immun. 1990;58:4036–4044. doi: 10.1128/iai.58.12.4036-4044.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Studier F W, Moffat B A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986;189:113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- 53.Suzuki T, Lett M-C, Sasakawa C. Extracellular transport of VirG in Shigella. J Biol Chem. 1995;270:30874–30880. doi: 10.1074/jbc.270.52.30874. [DOI] [PubMed] [Google Scholar]
- 54.Thanassi D G, Saulino E T, Hultgren S J. The chaperone/usher pathway: a major terminal branch of the general secretory pathway. Curr Opin Microbiol. 1998;1:223–231. doi: 10.1016/s1369-5274(98)80015-5. [DOI] [PubMed] [Google Scholar]
- 55.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang R W, Kushner S R. Construction of versatile low-copy-number vectors for cloning, sequencing and gene expression in Escherichia coli. Gene. 1991;100:195–199. [PubMed] [Google Scholar]
- 57.Watarai M, Tobe T, Yoshikawa M, Sasakawa C. Contact of Shigella with host cells triggers release of Ipa invasins and is an essential function of invasiveness. EMBO J. 1995;14:2461–2470. doi: 10.1002/j.1460-2075.1995.tb07243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wattiau P, Woestyn S, Cornelis G R. Customised secretion chaperones in pathogenic bacteria. Mol Microbiol. 1996;20:255–262. doi: 10.1111/j.1365-2958.1996.tb02614.x. [DOI] [PubMed] [Google Scholar]