Abstract
Background:
Although Remdesivir has been evaluated for the treatment of coronavirus disease 2019 (COVID-19), few study has yet shown effective mortality reduction. It might be because, in almost all those studies, remdesivir therapy was started beyond 7th days from the onset of symptoms when the active viral replications have already gone.
Methods:
This study reviewed the effectiveness of early remdesivir therapy during viral phase of COVID-19 and safety of its administration at home or community care during the outbreak of COVID-19 from July to September 2021 in Myanmar. We retrospectively reviewed clinical records of 204 high risk COVID-19 patients who had received remdesivir therapy within 7 days from the onset of illness and before oxygen desaturation.
Findings:
All patients received remdesivir therapy according to standard five days course of 200 mg loading dose on day 1, followed by 100 mg daily for up to 4 additional days. Out of 204 patients, 60.75% (124/204) were aged 60 years and above with comorbidity; 21.1% (43/204) aged under 60 years with comorbidity and 18.1% (37/204) were aged more than 60 years old without comorbidity. The patients who received RDSV therapy within 1-4 days and within 5-7 days were 50.5% (103/204) and 49.5% (101/204) respectively. All patients survived to 21 days without ICU admission or mechanical ventilation. Eighty six percent of patients had no hypoxia and only five percent had moderate to severe hypoxia, requiring oxygen. Those who received RDSV therapy within 1 to 4 days from the onset of symptoms had significantly lower rate of hypoxia compared to those who received remdesivir therapy on 5 to 7 days. After RDSV therapy, increased lymphocyte count and decreased CPR were observed in 74.5% (152/204) and 52.9% (108/204) of the patients respectively. There was no report of major adverse events.
Conclusion:
Remdesivir, if given within first 4 days from the onset of symptoms, is the most effective strategy for prevention of oxygen desaturation, further progression of COVID-19 and death although it is still beneficial if given later, days 5 to 7. It is a safe drug to be prescribed in hospital at home care. It may be cost-benefit if high-risk group of patients with COVID-19 were selected for early remdesivir therapy in the community.
Keywords: ALT, COVID-19, CRP, early remdesivir therapy, hospital-at-home care, lymphocyte count, Myanmar, oxygen desaturation
Introduction
A novel coronavirus that commenced from Wuhan, China, in December 2019 has caused contagious severe acute respiratory tract infection that killed millions of people worldwide.[1]
In July 2021, a third wave caused by a highly contagious delta variant of novel SARS-CoV-2 hit Myanmar, leading to an exponential rise in the number of cases and fatalities. It made the Myanmar health care system very difficult to cope with the crisis, causing the shortage of hospital beds and the exhaustion of human resources to overcome the uprising severe cases. Consequently, many doctors had to provide care for severe and critical cases at home, with the active participation of family members in care process of family. Many desperate patients and their doctors reasonably tried to grasp every possible glimpse of hope in such a situation. These hindrances and challenges amid the most difficult situations emboldened them to try early remdesivir administration.
Remdesivir is an adenosine analogue with broad-spectrum antiviral activity, and it is a safe drug for administration in hospitalized patients approved by the United States Food Drug Administration (USFDA) on October 22, 2020.[2]
From the immunopathology aspect, COVID-19 clinical evolution is characterized by three main phases: Viral replication, pulmonary, and hyperinflammation.[3] It may be more rational to use antiviral agents like remdesivir at the early viral replication phase before pulmonary involvement and cytokine storm.[4]
Recently published most randamized controlled trials of remdesivir had not shown signifcant mortality benefits.[5,6,7,8,9] This might be due to the timing of its administration when the viral replication has already subsided. On the other hand, some case reports showed promising outcomes in high-risk patients who received early remdesivir within 48 hours of the onset of COVID-19.[10]
Besides, high mortality rate was seen in the patients with age more than 60 years, diabetes, hypertension, cardiovascular diseases (CVD), chronic respiratory diseases including asthma and chronic obstructive disease (COPD), HIV, connective tissue disorders and hence they are high-risk groups for severe COVID-19 disease.[11,12,13] It would be more rational to consider remdesivir initiation among the high-risk COVID-19 patients during viral phase.
Thus, we conducted a retrospective record review study of non-hypoxic high-risk COVID-19 patients who received early RDSV therapy in hospital-at-home care within the first 7 days from the onset of symptoms. This study aimed to determine its efficacy in preventing disease progression to oxygen desaturation (severe pneumonia) and mortality as well as the safety of remdesivir administration in primary health care setting.
Study design and data collection
This was a retrospective clinical record review study. The clinical records of confirmed COVID-19 patients who received RDSV therapy under the care of five general physicians from Yangon during the third wave of covid19 epidemic, from July to September 2021 in Myanmar were reviewed.
SARS-Cov-2 infection of the patients was confirmed by either rapid diagnostic antigen tests or PCR tests of the nasopharyngeal specimen. The records of the patients who received RDSV within seven days from the onset of the symptoms and before hypoxia; and those who had at least one risk factor for progression to severe disease were included. Risk factors for progression to severe disease were defined as those over 60 years or any age with comorbidities such as diabetes mellitus, metabolic syndrome, other non-communicable diseases, infectious diseases, and immunocompromised conditions. The records of patients who did not give informed consent for this study were excluded.
Data entry and analysis
Background demographic data (age and gender), comorbidity status, COVID-19 vaccination status, oxygen desaturation status, biochemical changes in terms of lymphocyte count, CRP, ALT and eGFR and mortality status (alive or dead) of the patients before and after RDSV administration were extracted from 204 eligible clinical records and entered into the SPSS data sheet. Changes in lymphocyte count, CRP, ALT and eGFR were computed by calculating differences between 1st time measurement before RDSV administration and the second time measurement at the end of RDSV administration; then, the results were grouped into no changes, decreased and increased from the first-time measurement. Frequency (%) and mean (SD) was applied for descriptive data analysis. Pearson X2 and Fisher’s exact test were applied to analyze oxygen desaturation changes in severity groups following RDSV administration days and to infer the relationship between background demographic data, comorbidity status, COVID-19 vaccination status, mortality status, changes of biochemical status and RDSV administration day groups. Independent sample “t” test was applied to infer association between biochemical changes (lymphocyte counts, CRP, ALT and eGFR) between two RDSV administration day groups- within 4 days and day 5 to day-7. Alpha (a = 0.05) was set for statistical significance for inferential analysis. Microsoft excels and SPSS version 20 was used for data entry and data analysis, respectively.
Ethical considerations
Informed consent for the study was taken from the patients who were the owner of eligible clinical records. Ethical approval for the study was taken from the hospital’s ethical review board where the principal investigator works.
Results
A total of 204 clinical records of high-risk Covid19 patients were included in the review. Out of 204 patients, 60.75% (124/204) had two risk factors - aged 60 years above with comorbid diseases followed by 21% (43/204) were aged under 60 years old with comorbidity and 18.1% (37/204) were aged 60 years above without comorbidity [Figure 1].
Figure 1.
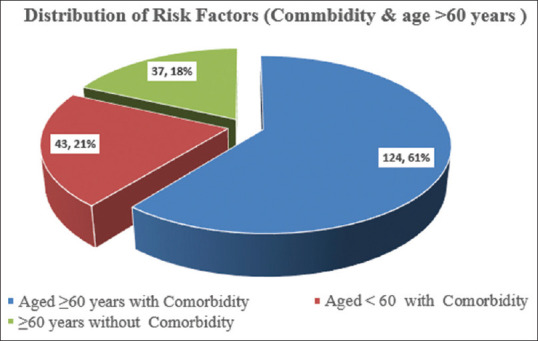
Showing having risk factors -commodity status and age more than 60 years of the COVID-19 patients under the study (n = 204)
Regarding RDSV therapy, 50.5% (103/204) were administered RDSV within day 1 to day 4 (RDSV D1-4) while 49.5% (101/204) were administrated RDSV within day 5 to day 7 (RDSV D5-7) from the onset of COVID-19 symptoms. It was noticed that there was no mortality till Day-21, end of observation. Demo-clinical background and outcomes of the patients under RDSV D1-4 were not significantly different compared with those of their counterparts, RDSV D5-7 in terms of gender, comorbidity status, and COVID-19 vaccination status [Table 1].
Table 1.
Demo-clinical background and outcomes of the Covid-19 patients in accordance with Remdesivir administration days
| Variables | RDSV administration days | Total n (%) | Significant “P” | |
|---|---|---|---|---|
|
| ||||
| Day1 - Day4 n (%) | Day 5 - Day7 n (%) | |||
| Age Group | ||||
| ≤59 years | 14 (32.6) | 29 (25.6) | 43 (21.1) | |
| ≥60 Years | 89 (55.5) | 72 (44.7) | 161 (78.9) | |
| Minimum age (Years) | 39 | 42 | 39 | |
| Maximum age (Years) | 87 | 94 | 94 | |
| Mean±SD (years) | 66±8.4 | 66.7±11.2 | 66.37±9.9 | χ2=7.009, “P”<0.05, Independent sample “t”=-0.463, “P”>0.05 |
| Gender | ||||
| Male | 45 (54.2) | 38 (45.8) | 83 (40.7) | χ2=0.777 |
| Female | 58 (47.9) | 63 (52.1) | 121 (59.3) | “P”>0.05 |
| Co-morbidity Status | ||||
| Hypertension and CVS[1]* | 16 (42.1) | 22 (57.9) | 38 (18.6) | “P”>0.05 |
| Diabetes & its complications | 53 (54.1) | 45 (45.9) | 98 (48) | |
| Chronic Respiratory disorder | 3 (37.5) | 5 (62.5) | 8 (3.9) | |
| Malignant disease | 1 (16.7) | 5 (83.3) | 6 (2.9) | |
| Connective tissue disorder and other | 7 (41.2) | 10 (58.8) | 17 (8.3) | |
| No Comorbidity | 23 (62.2) | 14 (37.8) | 37 (18.1) | |
| Covid-19 Vaccination status | ||||
| Yes | 8 (80) | 2 (20) | 10 (4.9) | “P”>0.05 |
| No | 95 (49) | 99 (51) | 194 (85.1) | |
| Mortality status | ||||
| Alive | 103 (50.5) | 101 (49.5) | 204 (100) | |
| Death | 0 (0) | 0 (0) | 0 (0) | |
| Total | 103 (50.5) | 101 (49.5) | 204 (100) | |
[1]Cardiovascular disorder including hypertension and its complications
Out of 204 patients, 78.9% (161/204) were risk age group (≥60 years old) at risk for severe COVID-19. Among them 55.5% (89/161) was RDSV D1-4 group and 44.7% (72/161) were RDSV D5-7 group. Although age group comparison of RDSV D1-4 to that of RDSV D5-7 was significantly different (p < 0.05), mean age of these two groups were not significantly different (P > 0.05). When oxygen desaturation of the patients was reviewed in accordance with days of administration of RDSV, keeping normal oxygen saturation level (≥94%) was found among the patients who received RDSV on day-1 and day-2 while severe oxygen desaturation (≤84%) was found among the patients who received RDSV on day-5 onwards [Table 2 and Figure 2]. However, this finding was not statistically significant (p > 0.05).
Table 2.
Oxygen desaturation of the covid-19 patients in accordance with Remdesivir Administration days (n=204)
| Remdesivir Administration day from COVID 19 symptoms onset | Oxygen desaturation1 of the covid-19 patients | Total n (%) | “P” | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Severe (O2 ≤84%) | Moderate (85%-89%) | Mild (90%-93%) | Normal (O2 ≥94%) | |||
| Day -1 | 0 (0) | 0 (0) | 0 (0) | 2 (100) | 2 (1) | |
| Day -2 | 0 (0) | 0 (0) | 0 (0) | 19 (100) | 19 (9.3) | >0.05 |
| Day-3 | 0 (0) | 0 (0) | 1 (1.9) | 51 (98.1) | 52 (25.5) | |
| Day-4 | 0 (0) | 0 (0) | 1 (3.3) | 29 (96.7) | 30 (14.7) | |
| Day-5 | 1 (3) | 2 (6.1) | 5 (15.2) | 25 (75.8) | 33 (16.7) | |
| Day-6 | 1 (2.7) | 4 (10.8) | 6 (16.2) | 26 (70.3) | 37 (18.1) | |
| Day-7 | 1 (3.2) | 2 (6.5) | 4 (12.9) | 24 (77.4) | 31 (15.2) | |
| Total | 3 (1.5) | 8 (3.9) | 17 (8.3) | 176 (86.3) | 204 (100) | |
Figure 2.
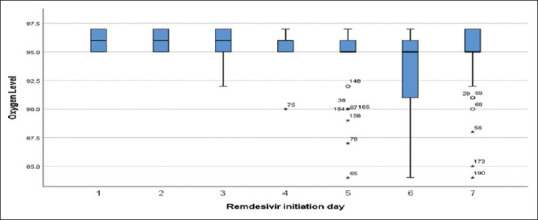
Box – Whisker plot showing oxygen desaturation of the COVID-19 patients in accordance with Remdesivir administration day
When, a cross analysis was done between oxygen desaturation in its severity groups – normal, mild, moderate and severe and two RDSV groups – RDSV D1-4 and RDSV D5-7), it was found out significantly (p < 0.05) that more normal oxygen without desaturation (SatO2 ≥ 94%) in RDSV D1-4 group than that of RDSV D5-7 (98.1% vs 74.3%).
Lymphocytes count and CRP count changes of the COVID-19 patients under study between 1st time measurement on Day-1 of RDSV and 2nd time measurement on the last day of RDSV were compared to determine bio-chemical changes of RDSV [Table 3].
Table 3.
Oxygen desaturation, Lymphocytes count and CRP changes between 1st time and 2nd time measurement in accordance with RDSV initiation days
| Outcome Variables | RDSV administration days | Total n (%) | “P” | |
|---|---|---|---|---|
|
| ||||
| Day 1-4 n (%) | Day 5-7 n (%) | |||
| Oxygen Desaturation status | ||||
| Normal (O2 ≥94%) | 101 (98.1) | 75 (74.3) | 176 (86.3) | Pearson χ2=24.76 |
| Mild (90%-93%) | 2 (1.9) | 15 (14.9) | 17 (8.3) | “P”<0.05 |
| Moderate (85%-89%) | 0 (0) | 8 (7.9) | 8 (3.9) | |
| Severe (O2 ≤84%) | 0 (0) | 3 (3) | 3 (15) | |
| Lymphocyte count Changes | ||||
| Between 1st and 2nd time | 4 (80) | 1 (20) | (2.5) | Pearson χ2=2.077 |
| No Changes (L 1 - L 2=0) | 25 (53.2) | 22 (46.8) | 47 (23) | “P”>0.05 |
| Decreased (L 1>L 2) | 74 (48.7) | 78 (51.3) | 152 (74.5) | |
| Increased (L 1<L 2) | ||||
| CRP Changes Between 1st and 2nd time | ||||
| No Changes (CRP 1- CRP 2=0) | 1 (100) | 0 (0) | 1 (0.5) | Pearson χ2=1.577 |
| Decreased (CRP 1>CRP 2) | 57 (52.8) | 51 (47.2) | 108 (52.9) | “P”>0.05 |
| Increased (Lymph 1<Lymph 2) | 45 (47.4) | 50 (52.6) | 95 (46.6) | |
| Total | 103 (50.5) | 101 (49.5) | 204 (100) | |
Out of 204 patients, 74.5% (152/204) of the patients showed increase in their lymphocyte count at 2nd time measurement while 52.9% (108/204) of the patients showed decrease in their CRP at 2nd time measurement; [Table 3]. However, lymphocyte count and CRP changes of RDSVD 1-4 was compared to those of RSDV D5-7, it was not statistically significant [Table 3 and Figure 3].
Figure 3.
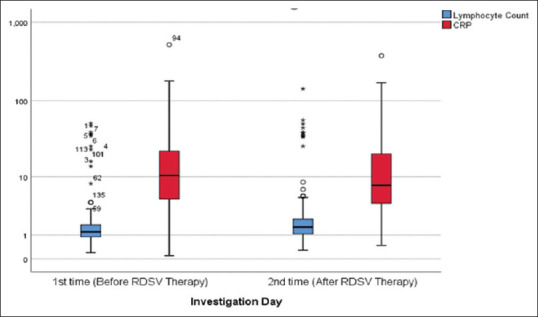
Box – Whisker plot showing Lymphocyte count and CRP changes before and after Remdesivir Therapy (n = 204)
No major adverse event was reported during drug administration and up to 21 days from initiation of therapy. However, there was no case of severe elevation (more than 5 times of upper limit of normal), requiring withholding RDSV. All cases of hepatic transaminase elevation recovered spontaneously on repeated measures. Thirty four percent of patients had mild decrease in calculated eGFR on 5th day but there was no event of clinically significant changes, requiring alteration of treatment plan. In our study, there were no report regarding anaphylactic shock, allergic reactions, and death due to side effects of RDSV.
Discussion
This is a real-world retrospective clinical record review study of the efficacy and safety of RDSV therapy at the viral replication phase, within the first 7 days from the onset of symptoms, among 204 high-risk COVID-19 patients under hospital-at-home care.
Thus, they received clinical management including diagnostic tests, therapeutic procedures, drug administration and patients’ monitoring at their homes by home visiting nurses and junior doctors under the supervision of senior physicians via teleconsultation. This finding encouraged primary care doctors to provide timely administration of RDSV to the patients under hospital at-home care in coordinated care with hospital physicians.
All patients under the review were administered locally available generic formulations of RDSV from India. Before initiation of RDSV therapy, all patients and their family members received the counselling about early RDSV therapy as a possible therapeutic option in terms of the risk of disease progression, available alternative options in a limited setting, theoretical effectiveness of RDSV therapy in the early stage of disease, based on the current available evidences.
All cases under the study received an intravenous infusion of RDSV 200 mg on the first day and 100 mg in the following four days. Any untoward effects, including heart rate and blood pressure changes, were monitored. Patients were instructed to self-monitor oxygen saturation by pulse oximeters until day 21 from the onset of symptoms. Renal and liver functions were monitored by checking ALT and eGFR on the day of RDSV initiation and repeated at the end of drug administration. CRP and lymphocyte count were tested before and after RDSV administration to check changes in inflammatory reactions. RDSV initiation days were divided into RDSV D1-4 (day 1 to 4 from symptom onset) and RDSV D5-7 (5 to 7 days from symptom onset). All these facts retrieved from the clinical records were invaluable for primary health care providers who are working in limited health care facilities to initiate RDSV therapy for their patients.
Regarding RDSV administration days and Oxygen desaturation, only 1.9% (n = 2) of the RDSV D1-4 group developed oxygen desaturation, and all of them had only mild oxygen desaturation. In contrast, 25.7% (n = 26) of the RDSV D5-7 group developed oxygen desaturation. Among them 14.9% (n = 15) had mild, 7.9% (n = 8) had moderate and 3% (n = 3) had severe degree of oxygen desaturation. Interestingly, there was no case of oxygen desaturation in the earliest initiation of remdesivir within Day 2 (48 hours) from the onset of symptoms. There was only one case of mild oxygen desaturation each in Day 3 and Day 4 RDSV, whereas more cases and more severe oxygen desaturation were found significantly if RDSV was initiated later than that. This all indicated that the effectiveness of RDSV was closely associated with its time of initiation.
Nevertheless, all our patients survived even without ICU care and ventilatory supports. It is well accepted that COVID-19 patients with comorbidities are potentially very high risk for death if they progress to oxygen desaturation and are hospitalized for complications of COVID-19.[11,12,13] However, the mortality rate of the patients under the review was surprisingly low even in the very limited health care setting. Moreover, 90% of our patients were unvaccinated. It may be due to not only keeping standard of RDSV administration, but also empowerment and active participation of the patients’ family members in the care process under closed supervision by health care provider.
Unlike other RDSV studies or guidelines, our patients received RDSV regardless of the considerations like the severity of COVID-19 symptoms, presence of hypoxia or need for hospitalization as criteria for RDSV therapy. It implies that even the highest risk patients may have good outcomes if RDSV was initiated prior to oxygen desaturation or hospitalization and while they were relatively less ill.
This study has clearly shown that the timing of the initiation of RDSV significantly influenced the rate of further oxygen desaturation and mortality. The maximal benefit with RDSV was observed if RDSV was adminstered before day 4 from the onset of the symptoms. The median duration of symptom onset before the first dose of RDSV in the randomized trials conducted by Spinner et al.[5] Goldman et al.[6] Beigel et al.[7] Wang et al.[9] were 8 days, 8 days, 9 days and 11 days, respectively. The WHO Solidarity trial, which included many patients on RDSV (n = 2743), did not mention the median duration of symptom onset to starting RDSV, and this study did not analyze the outcome based on symptom onset.[14] All these studies included hospitalized moderate to severe COVID-19 patients, and none of these studies commenced RDSV during the viremic phase, i.e., within the first five days of symptom onset when SARS-CoV2 load has reached its peak, and they have shown no significant mortality benefit.[5,6,7,9,14,15] However, the mortality benefit was found in the three retrospective studies of hospitalized COVID-19 patients by Mahendra et al., Mehta et al., and Hidalgo-Tenorio et al. when remdesivir was started within 4 days, 9 days and 5 days of the symptom onset, respectively.[16,17,18]
The article on early remdesivir therapy to prevent progression of COVID-19 in outpatients was recently published in the New England Journal of Medicine.[19] It is a randomized double-blind placebo-controlled trial showing 3-day course of remdesivir was safe and resulted in 87% lower risk of hospitalization or death than placebo. These findings support our clinical record review results for timely administration of RDSV at primary care level in management of the epidemic particularly for the resource limited situation.
Regarding changes in inflammatory markers (CRP and lymphocyte counts), majority of patients showed improvement after remdesivir. However, there were some cases who remained unchanged, or some apparently worsened on second time measure. However, we could measure only two times in each patient, day 1 of remdesivir and day 5 of remdesivir, due to limitations in resources during a crisis. If we could follow for a sufficiently long period, trend of changes might have been better visualized.
Although RDSV was a drug to be administered by intravenous route and recommended only for hospitalized patients at the time of this study, it was found to be safe to administer remdesivir at the community level if properly given by the homecare team. This study highlighted the important role of primary health care doctors in managing COVID-19 and feasibility and safety of early RDSV administration at patient’s home during early viral replication phase of covid19 infection.
Early Remdesivir treatment might be an effective preventative measure for future complications of COVID-19 pneumonia, hospitalization, ICU requirements and prevention of death. Thus, it may be a potential COVID-19 management strategy in developing countries where there are insufficiencies of healthcare resources. To be more reasonable, we should focus this therapy mainly on those high-risk group of patients as in this study. Early remdesivir initiation should be considered in high-risk patients in the future national COVID-19 control programs.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
This study was self-funded by investigators. We would like to acknowledge Dr. Aung Ko Win, Dr. Zayar Lin, Dr. Aung Myat Kyaw, Dr. Htut Myat Aung, Dr. Thawda Soe, Dr. Win Swe and Dr. Aye Thandar Win for their contribution in data collection process.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Sharma A, Farouk IA, Lal SK. COVID-19: A review on the Novel Coronavirus disease evolution, transmission, detection, control and prevention. Viruses. 2021;13:202. doi: 10.3390/v13020202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.U.S Food and Drug Administration. FDA Approves First Treatment for COVID-19. FDA. [Last accessed on2021 Nov 10]. Available from: https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-COVID-19 .
- 3.Romagnoli S, Peris A, De Gaudio AR, Geppetti P. SARS-CoV-2 and COVID-19: From the bench to the bedside. Physiol Rev. 2020;100:1455–66. doi: 10.1152/physrev.00020.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khadke S, Ahmed N, Ahmed N, Ratts R, Raju S, Gallogly M, et al. Harnessing the immune system to overcome cytokine storm and reduce viral load in COVID-19: A review of the phases of illness and therapeutic agents. Virol J. 2020;17:154. doi: 10.1186/s12985-020-01415-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spinner CD, Gottlieb RL, Criner GJ, López JRA, Cattelan AM, Viladomiu AS, et al. Effect of Remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: A randomized clinical trial. JAMA. 2020;324:1048–57. doi: 10.1001/jama.2020.16349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldman JD, Lye DCB, Hui DS, Marks KM, Bruno R, Montejano R, et al. Remdesivir for 5 or 10 days in patients with severe COVID-19. N Engl J Med. 2020;383:1827–37. doi: 10.1056/NEJMoa2015301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the treatment of COVID-19-Final report. N Engl J Med. 2020;383:1813–26. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spinner CD, Gottlieb RL, Criner GJ, Arribas López JR, Cattelan AM, Soriano Viladomiu A, et al. Effect of Remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: A randomized clinical trial. JAMA. 2020;324:1048–57. doi: 10.1001/jama.2020.16349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, et al. Remdesivir in adults with severe COVID-19: A randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569–78. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cubeddu LX, Cubeddu RJ. Early remdesivir treatment in COVID-19: Why wait another day? J Med Virol. 2021;93:4078–80. doi: 10.1002/jmv.26792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Navayi M, Fanoodi A, Salmani F, Abedi F, Shetty S, Riahi SM. Over 60 years of age as an independent prognostic factor of in-hospital mortality among COVID-19 patients: A cohort study in an Iranian high-incidence area. Public Health. 2021;200:33–8. doi: 10.1016/j.puhe.2021.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ye C, Zhong J, Cai S, Dong L, Li C, Hou X, et al. COVID-19 infection in patients with connective tissue disease: A multicity study in Hubei province, China. MedComm (2020) 2021;2:82–90. doi: 10.1002/mco2.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ejaz H, Alsrhani A, Zafar A, Javed H, Junaid K, Abdalla AE, et al. COVID-19 and comorbidities: Deleterious impact on infected patients. J Infect Public Health. 2020;13:1833–9. doi: 10.1016/j.jiph.2020.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.WHO Solidarity Trial Consortium. Repurposed antiviral drugs for COVID-19 —Interim WHO solidarity trial results. N Engl J Med. 2021;384:497–511. doi: 10.1056/NEJMoa2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cevik M, Tate M, Lloyd O, Maraolo AE, Schafers J, Ho A. SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: A systematic review and meta-analysis. Lancet Microbe. 2021;2:13–22. doi: 10.1016/S2666-5247(20)30172-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mehta RM, Bansal S, Bysani S, Kalpakam H. A shorter Symptom onset to remdesivir treatment (SORT) interval is associated with a lower mortality in moderate-to-severe COVID-19: A real-world analysis. Int J Infect Dis. 2021;106:71–7. doi: 10.1016/j.ijid.2021.02.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahendra M, Nuchin A, Kumar R, Shreedhar S, Mahesh PA. Predictors of mortality in patients with severe COVID-19 pneumonia-A retrospective study. Adv Respir Med. 2021;89:135–44. doi: 10.5603/ARM.a2021.0036. [DOI] [PubMed] [Google Scholar]
- 18.Hidalgo-Tenorio C, García-Vallecillos C, Sequera-Arquelladas S. Real-world outcomes of COVID-19 treatment with remdesivir in a Spanish hospital. Medicine (Baltimore) 2021;100:27228. doi: 10.1097/MD.0000000000027228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gottlieb RL, Vaca CE, Paredes R, Mera J, Webb BJ, Perez G, et al. Early Remdesivir to prevent progression to severe COVID-19 in outpatients. N Engl J Med. 2022;386:305–15. doi: 10.1056/NEJMoa2116846. [DOI] [PMC free article] [PubMed] [Google Scholar]


