Abstract
Conducting broad assessments of the main burden of breast cancer is the core factor for improving overdiagnosis and overtreatment of breast cancer patients as well as their survival rates. Breast cancer patients may experience neurological complications that cause devastating effects on them. Chemotherapy-induced peripheral neuropathy (CIPN) and neuropathic pain are two of the most reported complications. Objective: This study aims to review the neurological complications of breast cancer and the ways to control and treat them. Comprehensive searches were carried out about the keywords of Breast Cancer, Neurological Complications, and Breast Cancer Consequences. These keywords were searched through the most well-known databases of MEDLINE, PUBMED, Cochrane Library, Best Evidence, CancerLit, HealthSTAR, and LegalTrac. In this regard, 83 articles were chosen to be included in this study from 2010 to 2021. The identification and treatment process of neurologic syndromes are not easy. The main neurologic syndromes which the breast cancer patients face are opsoclonus myoclonus syndrome (OMS), encephalitis, sensorimotor neuropathy, retinopathy, cerebellar degeneration, and stiff-person’s syndrome. CIPN and neuropathic pain are among the most prevalent side effects which are categorized as neurological complications and mainly seen 1 year after the management of breast cancer. Aiming to minimize the burden following the treatment of breast cancer, these complications should be diagnosed and treated accurately.
Keywords: Breast cancer, complications, nervous system diseases
Introduction
One of the most prevalent tumors that could cause numerous deaths among women in the world is breast cancer. Based on the data achieved from the World Health Organization in 2021, about 12% of the annual newly detected cancer cases in the world are of breast cancer.[1] An early diagnosis of breast cancer makes the possibility of improving the survival rate to 80%. In this regard, providing screening programs for the management of breast cancer patients remains a crucial need that highlights the requirement for an intensive evaluation of the burden of breast cancer. So, having the knowledge of the management and side effects of cancer and its effect on the nervous system through metastatic disease is very important. Some of the main side effects of the management of breast cancer are neuropathic pain (NP), CIPN, immune system weakness, coagulation disorders, or paraneoplastic syndromes, which all could cause cerebrovascular disease.[2,3]
The most prevalent neurological complications following the treatment of breast cancer include NP, CIPN, cognitive impairment, stroke, and encephalopathy. CIPN and NP complications may be more disabling than the cancer itself.[4] Many chemotherapeutic agents could cause CIPN that may lead to discontinuation of treatment or/and dose reduction.[5] Despite the role of the chemotherapy regimen on the incidence of CIPN, alcohol consumption and diabetes could affect it simultaneously.[6] In more than 33% of younger breast cancer patients, chronic NP may happen after the treatment.[7]
The burden of NP and CIPN complications in women with breast cancer and their etiology, frequency, and impact on the life quality of the patients are still poorly understood. Conducting prospective studies provides a deep understanding and characterization of these complications, and makes the possibility of creating a more accurate description of the burden of breast cancer in various settings. Additionally, it could develop adequate strategies for minimizing the adverse effects of these complications during treatment.[8,9]
Following the studies carried out in this regard, it was cleared that there was no specific comprehensive study in terms of the neurological complications caused by the treatment of breast cancer.[10] Therefore, the present study aimed to assess the incidence rate of the neurological complications following breast cancer and providing suitable recommendations for the management of breast cancer patients and guidelines for future research.
Method
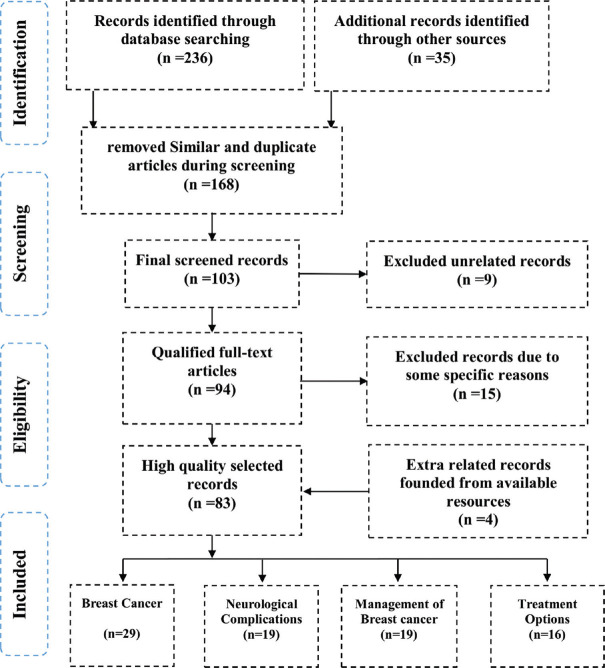
Standardized methods of Cochrane Collaboration[11] adhering to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) were performed. In this regard, all related databases of EMBASE, MEDLINE, Scopus, PubMed, PubMed Central, and Clinical Trials databases were searched. All the searched articles were in English and from 2010 to 2021. At first, 271 articles were searched based on the keywords of Breast Cancer, Epidemiology, Nervous System Diseases, and Cognition Disorders. All the searched titles were classified into four categories of highly related titles, less related titles, similar titles, and not related titles. Therefore, 168 articles were deleted due to similarity in subject and content, 15 and 9 articles were deleted due to some specific reasons, and dissimilarity in title and content, respectively. After the final screening, 83 articles were selected to be included in the main criteria of the present study and reviewed fully. The schematic process of all the steps of the method of the present study is shown in [Figure 1].
Figure 1.
The selection process of the articles in the present study is based on the PRISMA method
Etiology of breast cancer
Breast cancer is a multifactorial, heterogeneous, and complex disease. Despite the effect of some specific genetic factors such as mutations of BRCA1 and RCA2 genes in breast cancer, most breast cancer patients do not present a specific evident risk profile.[12,13] However, the mutations in the inherited high-penetrance genes are responsible for about 5–10% of all breast cancer cases.[14]
Some specific factors could result in breast cancer which include age, the personal or family history of breast cancer, hormone replacement therapy (HRT), physical inactivity, alcohol consumption, genetic predisposition, exposure to ionizing radiation, reproductive and hormonal factors, and obesity[15,16] Various factors may lead to breast cancer that are briefly mentioned in Table 1.
Table 1.
The main destructive factors of breast cancer derived in accordance with[17]
| Factors | Association | Influence | Risk threshold | Further information |
|---|---|---|---|---|
| Age at menopause and menarche[18,19] | Production of steroid hormones in the ovary | Steroid hormones affect the function and development of the breast | Late age at menopause and early age at menarche increase the risk of breast cancer | Exposure to high concentrations of endogenous estrogens for long times |
| Nulliparity[20,21] | As a result of socioeconomic development, childbirth may be delayed | Delayed childbearing and nulliparity are both associated with the increment of BC risk. | Childbearing patterns could affect the burden of BC (later age at births and fewer children) | The mother’s age at birth and spacing may influence the risk of BC |
| Breastfeeding[22] | Breastfeeding makes it possible that cells with potential DNA damage to be shed, which reduces the risk of BC | The risk of BC would be decreased by breastfeeding | Breastfeeding for at least 6 months decreases the risk of BC by 53% among mothers | Increment in the breastfeeding time decreases the risk of BC |
| Exogenous hormones[23,24] | The combination of estrogen-progestogen contraceptives; estrogen- progestogen menopausal therapy could affect BC | Long-term use of oral contraceptives and estrogen therapy increases the risk of BC | Using estrogen-progesterone therapy increases the risk of BC by 7.6%. | Utilizing HRT increases the risk of BC |
| Personal history of breast cancer[25,26] | Personal history of BC increases the risk of an invasive cancer | - | Diagnosis of tumors 1-4 years after detection of estrogen receptor-positive primary breast cancer | - |
| Family history of breast cancer[27,28,29] | In about one-third of the cases, genomic sequences and susceptibility genes cause BC | Mutation of the BRCA2 gene is responsible for the most frequent hereditary syndrome in BC | The risk of BC for women with a first-degree relative with BC is twice | Cowden syndrome increases the risk of BC |
| Ionizing radiation[30] | Gamma or X radiation are known as the main causative agents for BC in women | Exposure to radiation for medical purposes | Having too many X-rays of the body increases the risk of BC | Exposure to radiation in older women causes a lower risk of BC than that in the younger women |
| Alcohol consumption[31] | Alcoholic consumption is a carcinogenic agent for BC | The level of estrogen will be increased due to the consumption of alcohol | Consumption of >35 g of alcohol per day | - |
| Obesity[32,33] | Obesity increases the risk of BC | Increment the rate of fat could increase the risk of BC due to rising estrogen levels | Any 5-kg/m2 increase in BMI increases the risk of BC by 33%. | - |
| Physical activity[34,35] | Physical activity decreases the risk of BC | Physical activity affects the metabolism of endogenous steroid hormone and the immune system | At least 3-hour physical activity per week decreases the risk of BC by about 4-10%. | Physical inactivity increases the risk of BC by 33% |
BC: Breast Cancer
Diagnosis and staging
At first, breast cancer not only appears as a mammographic abnormality or palpable mass, but it can be detected through breast pain, breast skin change, and nipple discharge. In this regard, all the suspicious mammographic and palpable breast lesions should go under biopsy. More than two-thirds of the breast masses, particularly among young premenopausal women, are not malignant and about 15–25% of them remain in situ.[36] Abnormal cells of carcinoma in situ (CIS) are specified through the proliferation of malignant cells in the lobules or ducts of the breast without interstitial invasion of hepatocellular carcinomas (HCC). Lobular carcinoma in situ (LCIS) and ductal carcinoma in situ (DCIS) are two of the major subtypes of breast cancer. The earlier form of breast cancer is considered to be DCIS that is not invasive with a low risk of becoming invasive.[37,38]
Unlike DCIS, LCIS is microscopic and does not have any clinical and mammographic signs and is more likely to have bilateral involvement. These abnormal cells inside the milk duct are grouped in small, solid masses with a uniform, small, and round-to-oval nucleus.[39] Breast cancers can be classified by a scheme that consists of all the properties of the tumor that clarifies its life history. As per the American Joint Committee on Cancer (AJCC), TNM Classification of Malignant Tumors (TNM) is mainly based on the assumption that cancers of the same histology and anatomic site have similar patterns of extension and growth. In this classification system, T is representative of the size of the primary tumor, N is representative of the regional lymph node involvement, and M of the distant metastasis [Table 2].[40] At the time of clinical evaluation, a combination of the T, N, and M classification shows the extent of the disease.
Table 2.
Staging breast cancers based on the TNM system. Derived as per Kalli et al.[41]
| T Category | T Criteria |
|---|---|
| TX | Unable to assess primary tumors |
| T0 | The existence of a primary tumor could not be verified |
| Tis | The original place of carcinoma: LCIS, intraductal carcinoma, and/or Paget’s disease of the breast without tumor |
| T1 | The greatest dimension of the tumor is <2 cm |
| T2 | The greatest dimension of the tumor is between 2 and 5 |
| T3 | The greatest dimension of the tumor is >5 cm |
| T4 | Tumors may be of any size with the possibility of spreading to the chest |
| NX | Regional lymph nodes could not be evaluated |
| N0 | There are no metastases in the regional lymph nodes |
| N1 | Metastasis to movable ipsilateral nodes could be seen |
| N2 | Metastasis to ipsilateral nodes fixed to other structures could be seen |
| N3 | Internal mammary lymph node metastases |
| M0 | No distant metastasis could be verified |
| M1 | Distant metastases could be verified (such as metastases to ipsilateral supraclavicular lymph nodes) |
T: Tumor size (largest diameter). N: Nodal involvement (nodal status). M: Metastases
The system of stage grouping is applied for the aim of analysis and tabulation [Table 3]. The adoption of this grouping system is for ensuring that each group is more or less homogeneous for survival, and ensuring that the rate of each group is distinctive.[42]
Table 3.
Staging system of breast cancer with an adaptation of the TNM system[42]
| Stage | T | N | M |
|---|---|---|---|
| Stage 0 | Tis | N0 | M0 |
| Stage I | T11 | N0 | M0 |
| Stage IIA | T0 | N1 | M0 |
| T11 | N12 | M0 | |
| T2 | N0 | M0 | |
| Stage IIB | T2 | N1 | M0 |
| T3 | N0 | M0 | |
| Stage IIIA | T0 | N2 | M0 |
| T11 | N2 | M0 | |
| T2 | N2 | M0 | |
| T3 | N1, N2 | M0 | |
| Stage IIIA | T4 | Any N | M0 |
| Any T | N3 | M0 | |
| Stage IV | Any T | Any N | M1 |
1 T1 includes T1MIC. 2 The prognosis of the patients with pN0 is like those with pN1
Breast Cancer Metastases to the Neurocranium
Skull metastases
In patients with breast cancer, the rate of hematogenous skull metastases is higher in comparison with any other tumors.[42] As reported by Othman et al.,[43] in the early stage of the disease, in about half of the breast cancer patients, bone metastases can be detected by 99 mTc scintigraphy. Anyway, many of these lesions do not become symptomatic and it could be estimated that these lesions allow the other complications to slowly develop breast cancer. Local pain can be seen in patients with local swelling and at the time of presentation, neurologic deficits are not frequent. When the bone metastases reach a specific size and become symptomatic, they can be diagnosed. Metastases to the calvarial bones can be noted by the patients themselves as swelling.[44] When the orbit is involved, metastasis to the skull base may become symptomatic by exophthalmia or/and diplopia. In this regard, for the visualization of the bone destruction extent, a CT scan is needed. For the detection of the infiltration of the neural tissue and dura, MRI is superior to CT. Therefore, both imaging techniques are demanded preoperatively. Surgical operation should be considered in the following situations; when a massive destruction of dura and adjacent bone happen, after a neurological deficit due to solitary metastasis, and when patients are detected with painful tumors.[45,46] The purpose of surgery is for resection of the infiltrated bone in a cranioplasty operation and its replacement with bone-cement or a titan mesh. This operation is beneficial, especially in complex lesions that involve the cranial base. When metastases involved the dura, it should be resected and then replaced. The differential diagnosis of the skull includes benign tumor-like lesions (eosinophilic granuloma, hyperostosis, and fibrous dysplasia) and primary skull tumors (chordoma, chondrosarcoma, osteoma, epidermoid, and dermoid cysts).[47]
Dural metastases
Dural metastases are rare and can be found at autopsy in just about 10% of the patients with advanced systemic cancers. In their study, Chan et al.[48] introduced breast cancer as the second most common malignancy that could cause dural metastases. Dural metastases may appear as the metastatic subdural fluid collection or solid masses that assemble a chronic subdural hematoma (SDH). One of the main differential diagnoses of meningioma tumors is dural metastases and should be suspected in patients who are detected with an underlying malignant disease and a chronic SDH.[49] After removing the solid dural metastases, the dura is incised around the lesion circumferentially. Then, the tissue of the brain and the tumor are dissected from each other with cottonoids and gentle coagulation while avoiding retraction of the brain and preserving the accompanying veins. The tumor can be removed gently after circumferential dissection. Autologous transplantation or an artificial material should be used for performing dura substitute procedure. The intraoperative aspects of both systemic meningioma metastases and dural metastases are similar. However, for clarifying the diagnosis, it is essential to conduct a histological examination.[50]
Paraneoplastic syndromes
As a rare disorder following cancer, paraneoplastic neurological syndromes (PNS) affect less than 1% of breast cancer patients.[51] PNS disorders following breast cancer mainly include brainstem encephalitis, myelopathy, stiff person syndrome, sensory neuropathy, cerebellar degeneration, opsoclonus myoclonus, and encephalomyelitis.[52] The most prevalent PNS disorder following breast cancer is subacute cerebellar degeneration. Another group of rare disorders of the paraneoplastic syndromes could occur following breast cancer like retinopathy. Some systemic symptoms such as fatigue, fever, anorexia, and weight loss may also be observed.[53] The best classical onconeural antibodies that are associated with PNS and breast cancer include anti-Ma2 (anti-Ta) associated with cerebellar degeneration, brainstem encephalitis, and limbic encephalitis; a-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)-type glutamate receptors (AMPARs) associated with limbic encephalitis; Purkinje cell cytoplasmic antibody type 1 (PCA1) associated with paraneoplastic cerebellar degeneration (PCD); type II anti-neuronal nuclear antibody (ANNA-2) associated with opsoclonus myoclonus and brainstem encephalitis; and amphiphysin protein associated with sensory neuropathy, encephalomyelitis, myoclonus, myelopathy, and stiff person syndrome.[54,55]
In more than four-fifths of the cancer patients, PNS precedes the diagnosis of cancer by several months to years [183, 189]. Nearly, in all the cases, after the diagnosis of PNS, an underlying cancer could be identified within 5 months. Additionally, after 2 years, the risk of coexistent cancer will decrease and after 4 years, it is considered highly unlikely.[56] In PNS patients when there is a breast cancer, a combination of mammography and clinical examination followed by whole-body 18F-fluorodeoxyglucose-positron emission tomography/Computerized Tomography (FDG-PET/CT) (18F-FDG PET/CT) screening and breast MRI should be carried out in all the cases with negative initial screening.[52] If the initial screening is negative, repeat assessments should be performed every 6 months for at least 2 years.[54]
Because of the heterogeneity in the symptomatology and timing, and the presence of onconeural antibodies, the accurate diagnosis of the paraneoplastic syndrome is challenging. For instance, only in 60–70% of the paraneoplastic syndrome patients with breast cancer, antibodies could be found. Consequently, when the result antibodies tests is positive, it could be productive, otherwise the results of test could not be used effectively in diagnosis of paraneoplastic neurologic syndrome. Aiming to solve this challenge and the diagnosis of paraneoplastic neurological disorders, four components are defined by the international panel of neurologists[57,58]:
Existence of any trace of neurological symptoms
Existence of any signs of cancer within 4 years from the initial observation of the neurological manifestations
Exclusion of other neurological disorders
Existence of one of the following: the presence of inflammation with negative cytology in the cerebrospinal fluid (CSF) analysis, presence of a lesion in the temporal lobe in the brain MRI imaging, and seeing epileptic activity within the temporal lobe through the electroencephalogram (EEG) test.[59,60]
Neurologic complications of cancer
PNS and the central nervous system (CNS) are very susceptible to various types of cancer and their treatments. The development of the primary spinal cord and brain tumors is responsible for the most direct involvement of the nervous system. In many types of cancers, metastasis can be seen toward the CNS, and brain metastases are of the most prevalent problems caused by malignancies like breast cancer.[61] These metastases may involve the parenchyma of the spine or brain or their subarachnoid space. When PNS is involved, the spread of cancer happens through direct infiltration of the muscle, plexi, and nerve roots by the surrounding malignancies. Sometimes, the body’s nervous system may get affected by cancer in a sudden and devastating way that includes transection of the cord or compression of the epidural spinal cord caused by pathologic fractures of the bones of the spine involved by cancer; a sudden uncontrolled seizure activity caused by brain tumors that lasts for at least 5 min; and intracranial pressure (ICP) associated with the growth of the mass lesion and edema.[62,63]
PNS are the most well-known remote or indirect effects of cancer on the nervous system. The hypercoagulable state could result from cancer that causes cerebrovascular complications. It should be noted that conducting therapeutic options for the treatment of cancer could cause neurologic complications. Radiation-induced injury to the peripheral nerves, spine, and brain, and CIPN are the most prevalent complications following the treatment of cancer. Infectious complications in the nervous system ca result from the suppressant effect of cancer and its treatment on the body’s immune system.[64] Both direct and indirect complications of cancer are mentioned in Table 4.
Table 4.
Neurologic complications of cancer
| Complication | Metastases/Disorders | Description | Clinical Presentation | Diagnosis | Treatment |
|---|---|---|---|---|---|
| Direct | Brain metastases[65,66] | The most frequent direct form of involvement of the nervous system by cancer | Focal neurologic deficits, headaches, seizures | MRI and CT scan devices | Symptomatic therapy for seizures and vasogenic edema Anticonvulsants Dexamethasone Surgery and radiation therapy |
| Leptomeningeal metastases[67,68] | It is when cancer spreads to the membranes lining the spinal cord and the brain. Mostly seen in breast cancer, melanoma, and acute nonlymphocytic leukemia | Involvement of the nerve root and spinal cord Multiple cranial neuropathies Patients with weakness of limb, Pain in the involved nerve roots or bladder Bowel disturbances | Could be detected through sensory loss, weakness, and changes in deep tendon reflexes. Presence of malignant cells in the cerebrospinal fluid is a sign Techniques of polymerase chain reaction and flow cytometry | Chemotherapy Radiation Surgery Cytarabine Direct spinal fluid injection of rituximab, topotecan, etoposide, thiotepa, cytarabine, and methotrexate | |
| Spine metastases[69,70] | These types of metastases are prevalent in cancer patients Epidural spinal cord compression resulted mainly from the breast, lung, and prostate cancer It represents >95% of the spine metastases cases | Pain Involvement of the thoracic spine Mid-back pain Lower extremity bilateral weakness | Neuroimaging CT Myelography | High-dose steroid therapy (dexamethasone) Pain therapy with corticosteroids Radiation therapy Kyphoplasty or vertebroplasty for pain relief | |
| Peripheral nervous system metastases[71] | Cancer involves the nervous system directly. Metastases to the nerve roots and plexi are more common than individual nerves | Sensorimotor deficits and pain in one limb Involvement of the lower and upper plexus Injury to the entire plexus from the infiltrating neoplasm Adduction and eversion Weakness of thigh flexion Involvement of the brachial or cervical plexus Numbness or weakness Orofacial numbness | Neuroimaging with MRI Nerve conduction studies Studies with electromyography | Analgesics and steroids (pain killers) Tricyclic or other antidepressants Anticonvulsants Gabapentin and pregabalin Chemotherapy and radiation | |
| Indirect | Paraneoplastic Syndromes[72] | These syndromes are without direct infiltration Without metastases Without compression PNS or CNS structure | Insomnia, Seizures, Memory loss, Weakness, Fatigue, Autonomic dysfunction, Neuropathy | It is an autoimmune disease and provides the possibility of early cancer diagnosis | Corticosteroids Benzodiazepines Plasmapheresis IVIg Steroids Chemotherapy |
| Lambert-Eaton myasthenic syndrome[73] | It is a kind of neuromuscular syndrome | Fatigue Autonomic dysfunction Fluctuating weakness | Electromyography (EMG)/NCS | 3,4-Diaminopyridine Immunomodulatory therapy (with plasmapheresis and IgG) Long-term steroid therapy |
Neurological complications of breast cancer
In the first year following the treatment of breast cancer, nearly half of patients experience at least one neuro-oncological complication. Cognitive impairment, CIPN, and NP are among the most prevalent complications experienced by one-tenth of the patients.[74]
Both direct and indirect neurological complications include side effects of treatments, paraneoplastic syndromes, or/and vascular disorders and maybe the main source of illness among breast cancer patients.[75] Pereira et al.[76] revealed that about 50% of the women with breast cancer, who underwent treatment, experienced at least one neurooncological complication and 25% experienced two complications. In their study, the most prevalent complications were CIPN and NP.
Due to the possibility of cancer recurrence and exposure of patients to further treatments, various adverse effects of applying the treatment options for the management of cancer patients, and providing the potential of recovering the patients from some of the neurooncological complications, comprehensive evaluations of the prevalence of these complications should be carried out in long-term follow-up conditions.[76]
Paraneoplastic neuropathy
The earliest reported paraneoplastic sensorimotor neuropathy as a complication following breast cancer was reported in 1994. In their study, Chernyshkova et al.[77] stated that patients with breast cancer experienced radicular symptoms, numbness, paresthesia, muscle cramps, and neurological manifestations of the lower and upper muscle weakness. They reported that these neurological manifestations were experienced by the patients for up to 8 years before the breast cancer discovery. Breast cancer patients may present with various localized tumors in their chest and armpit lymph nodes, and stage IV tumors. After breast cancer, neurological manifestations may develop to chronic, while disability may not develop to upper levels.[77] Various studies reported that the neurological manifestations of the paraneoplastic syndrome could be relieved by conducting a plasmapheresis process.[78,79]
The rare syndrome of autoimmune paraneoplastic autonomic neuropathy in patients with breast cancer may be experienced with degenerative ataxia on the left and numbness in all extremities. In these situations, the presence of anti-neuronal antibodies can be detected through sural nerve biopsy and serum analysis.[80]
Sensorimotor polyneuropathy is a systemic process during which the nerve cells are damaged and may be observed in patients with breast cancer.[81] Criscitiello et al[82] reported that breast cancer patients who are detected with motor neuron disorders (MNDs) could be treated effectively by anastrozole, capecitabine, and docetaxel.
Anyway, the paraneoplastic neurological manifestation may be different in each breast cancer patient. For instance, some patients may experience rapidly progressive course that is mainly because of anti-Hu (ANNA-1) antibodies, while others without these antibodies will not face such a rapid progress in lower motor neuron signs, and prolonged courses. However, there is diversity in seropositivity and presentation in paraneoplastic neurological manifestation that should be considered for planning an appropriate treatment strategy.[83]
Conclusion
Cancer can affect the nervous system directly or indirectly through the side effects of drug therapy. One of the most destructive adverse effects is on the immune system that causes paraneoplastic syndromes. The application of immunosuppressive drugs for the management of cancer may affect the CNS by opportunistic infections. Neurological complications such as NP and CIPN are among the most prevalent side effects of breast cancer management during the first year after diagnosis. These complications can be present even 3 years of the diagnosis of cancer. Swift and accurate diagnosis of these complications is a critical step in decision-making about choosing an appropriate treatment option in the management process of patients with breast cancer.
One important factor that should be considered is testing the breast cancer patients for anti-neuronal antibodies due to their role in the neurologic manifestations in any neurological disorders. These antibodies could improve the accuracy of the diagnosis even up to 100%. One of the main effective mechanisms against malignancy is manipulating the immune system by conducting appropriate treatment options for improving the survival rate of the patients. In this regard, it is recommended to conduct various clinical trials to investigate the effectiveness of different therapeutic options for decreasing the possibility of neurological complications in association with breast cancer. Due to the rarity of some of these syndromes, more than two-thirds of the existing data are based on observations and just a few prospective studies are available. In this regard, treatment is often empiric and because of the diversity of the treatment options, they should be tailored for each case separately.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Joko-Fru WY, Jedy-Agba E, Korir A, Ogunbiyi OJ, Dzamalala CP, Chokunonga E, et al. The evolving epidemic of breast cancer in sub-Saharan Africa: results from the African Cancer Registry Network. Int J Cancer. 2020;147:2131–2141. doi: 10.1002/ijc.33014. [DOI] [PubMed] [Google Scholar]
- 2.Giglio P, Gilbert MR. Neurologic complications of cancer and its treatment. Curr Oncol Rep. 2010;12:50–9. doi: 10.1007/s11912-009-0071-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khasraw M, Posner JB. Neurological complications of systemic cancer. Lancet Neurol. 2010;9:1214–27. doi: 10.1016/S1474-4422(10)70220-9. [DOI] [PubMed] [Google Scholar]
- 4.Mustonen L, Vollert J, Rice ASC, Kalso E, Harno H. Sensory profiles in women with neuropathic pain after breast cancer surgery. Breast Cancer Res Treat. 2020;182:305–15. doi: 10.1007/s10549-020-05681-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miltenburg NC, Boogerd W. Chemotherapy-induced neuropathy: A comprehensive survey. Cancer Treat Rev. 2014;40:872–82. doi: 10.1016/j.ctrv.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 6.Vincenzi B, Frezza AM, Schiavon G, Spoto C, Silvestris N, Addeo R, et al. Identification of clinical predictive factors of oxaliplatin-induced chronic peripheral neuropathy in colorectal cancer patients treated with adjuvant Folfox IV. Support Care Cancer. 2013;21:1313–9. doi: 10.1007/s00520-012-1667-5. [DOI] [PubMed] [Google Scholar]
- 7.Gong Y, Tan Q, Qin Q, Wei C. Prevalence of postmastectomy pain syndrome and associated risk factors: A large single-institution cohort study. Medicine. 2020;99:19834. doi: 10.1097/MD.0000000000019834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gewandter JS, Kleckner AS, Marshall JH, Brown JS, Curtis LH, Bautista J, et al. Chemotherapy-induced peripheral neuropathy (CIPN) and its treatment: An NIH Collaboratory study of claims data. Support Care Cancer. 2020;28:2553–62. doi: 10.1007/s00520-019-05063-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colvin LA. Chemotherapy-induced peripheral neuropathy (CIPN): Where are we now? Pain. 2019;160(Suppl 1):S1–10. doi: 10.1097/j.pain.0000000000001540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang L, Shen Y, Li M, Chuang H, Ye Y, Zhao H, et al. Clinical manifestations and evidence of neurological involvement in 2019 novel coronavirus SARS-CoV-2: A systematic review and meta-analysis. J Neurol. 2020;267:2777–89. doi: 10.1007/s00415-020-09974-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. updated March 2011. The Cochrane Collaboration. 2011. Available from: http://handbook.cochrane.org .
- 12.Barth V. 1st ed. USA: Thieme; 2011. Diagnosis of Breast Diseases: Integrating the Findings of Clinical Presentation, Mammography, and Ultrasound. [Google Scholar]
- 13.Stuckey A. Breast cancer: Epidemiology and risk factors. Clin Obstet Gynecol. 2011;54:96–102. doi: 10.1097/GRF.0b013e3182080056. [DOI] [PubMed] [Google Scholar]
- 14.Palomba G, Palmieri G, Cossu A, Paliogiannis P, Cristina Sini M. Epidemiology and genetic susceptibility of breast and ovarian cancer in Sardinian population. In Breast Cancer and Breast Reconstruction. IntechOpen. 2020 doi: 10.5772/intechopen. 90517. [Google Scholar]
- 15.Colditz GA, Bohlke K. Priorities for the primary prevention of breast cancer. CA Cancer J Clin. 2014;64:186–94. doi: 10.3322/caac.21225. [DOI] [PubMed] [Google Scholar]
- 16.Lauby-Secretan B, Scoccianti C, Loomis D, Benbrahim-Tallaa L, Bouvard V, Bianchini F, et al. Breast-cancer screening–viewpoint of the IARC Working Group. N Engl J Med. 2015;372:2353–8. doi: 10.1056/NEJMsr1504363. [DOI] [PubMed] [Google Scholar]
- 17.Di Sibio A, Abriata G, Buffa R, Viniegra M, Forman D, Sierra MS. Lyon: International Agency for Research on Cancer; 2016. Etiology of breast cancer (C50) in Central and South America. In: Cancer in Central and South America. Available from: http://www-dep.iarc.fr/CSU_resources.htm . [Google Scholar]
- 18.Collaborative Group on Hormonal Factors in Breast Cancer. Menarche, menopause, and breast cancer risk: Individual participant meta-analysis, including 118 964 women with breast cancer from 117 epidemiological studies. Lancet Oncol. 2012;13:1141–51. doi: 10.1016/S1470-2045(12)70425-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang XR, Chang-Claude J, Goode EL, Couch FJ, Nevanlinna H, Milne RL, et al. Associations of breast cancer risk factors with tumor subtypes: A pooled analysis from the Breast Cancer Association Consortium studies. J Natl Cancer Inst. 2011;103:250–63. doi: 10.1093/jnci/djq526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Justo N, Wilking N, Jönsson B, Luciani S, Cazap E. A review of breast cancer care and outcomes in Latin America. Oncologist. 2013;18:248–56. doi: 10.1634/theoncologist.2012-0373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aurin J, Thorlacius H, Butt ST. Age at first childbirth and breast cancer survival: A prospective cohort study. BMC Res Notes. 2020;13:9. doi: 10.1186/s13104-019-4864-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou Y, Chen J, Li Q, Huang W, Lan H, Jiang H. Association between breastfeeding and breast cancer risk: Evidence from a meta-analysis. Breastfeed Med. 2015;10:175–82. doi: 10.1089/bfm.2014.0141. [DOI] [PubMed] [Google Scholar]
- 23.Moorman PG, Havrilesky LJ, Gierisch JM, Coeytaux RR, Lowery WJ, Peragallo Urrutia R, et al. Oral contraceptives and risk of ovarian cancer and breast cancer among high-risk women: A systematic review and meta-analysis. J Clin Oncol. 2013;31:4188–98. doi: 10.1200/JCO.2013.48.9021. [DOI] [PubMed] [Google Scholar]
- 24.Reeves GK, Pirie K, Green J, Bull D, Beral V Million Women Study Collaborators. Comparison of the effects of genetic and environmental risk factors on in situ and invasive ductal breast cancer. Int J Cancer. 2012;131:930–7. doi: 10.1002/ijc.26460. [DOI] [PubMed] [Google Scholar]
- 25.Nichols HB, Berrington de González A, Lacey JV, Jr, Rosenberg PS, Anderson WF. Declining incidence of contralateral breast cancer in the United States from 1975 to 2006. J Clin Oncol. 2011;29:1564–9. doi: 10.1200/JCO.2010.32.7395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Louro J, Roman M, Posso M, Comerma L, Vidal C, Saladié F, et al. Differences in breast cancer risk after benign breast disease by type of screening diagnosis. Breast (Edinburgh, Scotland) 2020;54:343–8. doi: 10.1016/j.breast.2020.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee E, McKean-Cowdin R, Ma H, Spicer DV, Van Den Berg D, Bernstein L, et al. Characteristics of triple-negative breast cancer in patients with a BRCA1 mutation: Results from a population-based study of young women. J Clin Oncol. 2011;29:4373–80. doi: 10.1200/JCO.2010.33.6446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weitzel JN, Clague J, Martir-Negron A, Ogaz R, Herzog J, Ricker C, et al. Prevalence and type of BRCA mutations in Hispanics undergoing genetic cancer risk assessment in the southwestern United States: A report from the Clinical Cancer Genetics Community Research Network. J Clin Oncol. 2013;31:210–6. doi: 10.1200/JCO.2011.41.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abugattas J, Llacuachaqui M, Allende YS, Velásquez AA, Velarde R, Cotrina J, et al. Prevalence of BRCA1 and BRCA2 mutations in unselected breast cancer patients from Peru. Clin Genet. 2015;88:371–5. doi: 10.1111/cge.12505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bakkach J, Pellegrino B, Elghazawy H, Novosad O, Agrawal S, Bennani Mechita M. Current overview and special considerations for second breast cancer in Hodgkin lymphoma survivors. Crit Rev Oncol Hematol. 2021;157:103175. doi: 10.1016/j.critrevonc.2020.103175. doi: 10.1016/j.critrevonc 2020.103175. [DOI] [PubMed] [Google Scholar]
- 31.Cogliano VJ, Baan R, Straif K, Grosse Y, Lauby-Secretan B, El Ghissassi F, et al. Preventable exposures associated with human cancers. J Natl Cancer Inst. 2011;103:1827–39. doi: 10.1093/jnci/djr483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arnold M, Pandeya N, Byrnes G, Renehan AG, Stevens GA, Ezzati M, et al. Global burden of cancer attributable to high body-mass index in 2012: A population-based study. Lancet Oncol. 2015;16:36–46. doi: 10.1016/S1470-2045(14)71123-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Renehan AG, Pegington M, Harvie MN, Sperrin M, Astley SM, Brentnall AR, et al. Young adulthood body mass index, adult weight gains and breast cancer risk: The PROCAS Study (United Kingdom) Br J Cancer. 2020;122:1552–61. doi: 10.1038/s41416-020-0807-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu Y, Zhang D, Kang S. Physical activity and risk of breast cancer: A meta-analysis of prospective studies. Breast Cancer Res Treat. 2013;137:869–82. doi: 10.1007/s10549-012-2396-7. [DOI] [PubMed] [Google Scholar]
- 35.Pena GG, Maia YC, Mendes MC, Furtado WR, Machado-Coelho GL, Freitas RN. Physical activity is associated with malignant and benign breast diseases in low-income Brazilian women. Nutr Cancer. 2014;66:707–15. doi: 10.1080/01635581.2013.801997. [DOI] [PubMed] [Google Scholar]
- 36.Yang H, Xu Y, Zhao Y, Yin J, Chen Z, Huang P. The role of tissue elasticity in the differential diagnosis of benign and malignant breast lesions using shear wave elastography. BMC Cancer. 2020;20:930. doi: 10.1186/s12885-020-07423-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoon JH, Kim MH, Kim EK, Moon HJ, Kwak JY, Kim MJ. Interobserver variability of ultrasound elastography: How it affects the diagnosis of breast lesions. AJR Am J Roentgenol. 2011;196:730–6. doi: 10.2214/AJR.10.4654. [DOI] [PubMed] [Google Scholar]
- 38.Lee EJ, Jung HK, Ko KH, Lee JT, Yoon JH. Diagnostic performances of shear wave elastography: Which parameter to use in differential diagnosis of solid breast masses? Eur Radiol. 2013;23:1803–11. doi: 10.1007/s00330-013-2782-5. [DOI] [PubMed] [Google Scholar]
- 39.Evans A, Whelehan P, Thomson K, McLean D, Brauer K, Purdie C, et al. Invasive breast cancer relationship between shear-wave elastographic findings and histologic prognostic factors. Radiology. 2012;263:673–7. doi: 10.1148/radiol.12111317. [DOI] [PubMed] [Google Scholar]
- 40.Hu J, Fung MW, Tsang JY, Poon IK, Chan SK, Cheung S-Y, et al. Improved prognostication for the updated AJCC breast cancer pathological prognostic staging varied in higher-stage groups. Clin Breast Cancer. 2020;20:253–61.7. doi: 10.1016/j.clbc.2020.01.011. [DOI] [PubMed] [Google Scholar]
- 41.Kalli S, Semine A, Cohen S, Naber SP, Makim SS, Bahl M. American Joint Committee on cancer's staging system for breast cancer, eighth edition: What the radiologist needs to know. Radiographics. 2018;38:1921–33. doi: 10.1148/rg.2018180056. [DOI] [PubMed] [Google Scholar]
- 42.Hortobagyi GN, Edge SB, Giuliano A. New and important changes in the TNM staging system for breast cancer. Am Soc Clin Oncol Educ Book. 2018;38:457–67. doi: 10.1200/EDBK_201313. [DOI] [PubMed] [Google Scholar]
- 43.Othman IA, Zahedi FD, Husain S. Base of skull metastatic adenocarcinoma from the breast 23 years after the primary diagnosis. Case Rep Med. 2020 doi: 10.1155/2020/2610597. 2610597. doi: 10.1155/2020/2610597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suh JH, Kotecha R, Chao ST, Ahluwalia MS, Sahgal A, Chang EL. Current approaches to the management of brain metastases. Nat Rev Clin Oncol. 2020;17:279–99. doi: 10.1038/s41571-019-0320-3. [DOI] [PubMed] [Google Scholar]
- 45.Adachi K, Maeda Y, Hayama M, Kitaguchi Y, Nojima S, Nitta K, et al. Skull base metastasis of breast cancer with oculomotor and trochlear nerve palsy. Ear Nose Throat J. 2020 doi: 10.1177/0145561320963676. 145561320963676. doi: 10.1177/0145561320963676. [DOI] [PubMed] [Google Scholar]
- 46.Pons Escoda A, Naval Baudin P, Mora P, Cos M, Gañan JH, Narváez JA, et al. Imaging of skull vault tumors in adults. Insights Imaging. 2020;11:23. doi: 10.1186/s13244-019-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kirakoya B, Kabore FA, Abubakar BM, Simpore M, Ky BD, Ouedraog A. Skull metastasis in prostate cancer: A compilation of four cases along with review of the literature. Int Arch Urol Complications. 2019;5 doi: 10.23937/2469-5742/1510064. [Google Scholar]
- 48.Chan J, Magaki S, Zhang XR, Chin C, Greenspan S, Linetsky M, et al. Intravascular carcinomatosis of the brain: A report of two cases. Brain Tumor Pathol. 2020;37:118–25. doi: 10.1007/s10014-020-00367-x. [DOI] [PubMed] [Google Scholar]
- 49.Furtner J, Oth I, Schöpf V, Nenning KH, Asenbaum U, Wöhrer A, et al. Noninvasive differentiation of meningiomas and dural metastases using intratumoral vascularity obtained by arterial spin labeling. Clin Neuroradiol. 2020;30:599–605. doi: 10.1007/s00062-019-00808-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kwon H, Kim JW, Park M, Kim JW, Kim M, Suh SH, et al. Brain metastases from lung adenocarcinoma may preferentially involve the distal middle cerebral artery territory and cerebellum. Front Oncol. 2020;10:1664. doi: 10.3389/fonc.2020.01664. doi: 10.3389/fonc2020.01664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ceyhun S, Hülya T, Erdem T, Meryem GG, Fügen VA. Determination of paraneoplastic neuropathy in newly diagnosed breast tumor patients. eNeurologicalSci. 2020;21:100265. doi: 10.1016/j.ensci.2020.100265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Graus F, Dalmau J. Paraneoplastic neurological syndromes. Curr Opin Neurol. 2012;25:795–801. doi: 10.1097/WCO.0b013e328359da15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kannoth S. Paraneoplastic neurologic syndrome: A practical approach. Ann Indian Acad Neurol. 2012;15:6–12. doi: 10.4103/0972-2327.93267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Titulaer MJ, Soffietti R, Dalmau J, Gilhus NE, Giometto B, Graus F, et al. Screening for tumours in paraneoplastic syndromes: Report of an EFNS task force. Eur J Neurol. 2011;18:19–3. doi: 10.1111/j.1468-1331.2010.03220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Psimaras D, Carpentier AF, Rossi C. PNS Euronetwork. Cerebrospinal fluid study in paraneoplastic syndromes. J Neurol Neurosurg Psychiatry. 2010;81:42–5. doi: 10.1136/jnnp.2008.159483. [DOI] [PubMed] [Google Scholar]
- 56.Hebert J, Riche B, Vogrig A, Muñiz-Castrillo S, Joubert B, Picard G, et al. Epidemiology of paraneoplastic neurologic syndromes and autoimmune encephalitides in France. Neurol Neuroimmunol Neuroinflamm. 2020;7:883. doi: 10.1212/NXI.0000000000000883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sæther SG, Schou M, Kondziella D. What is the significance of onconeural antibodies for psychiatric symptomatology?A systematic review. BMC Psychiatry. 2017;17:161. doi: 10.1186/s12888-017-1325-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huemer F, Melchardt T, Tränkenschuh W, Neureiter D, Moser G, Magnes T, et al. Anti-Hu antibody associated paraneoplastic cerebellar degeneration in head and neck cancer. BMC Cancer. 2015;15:996. doi: 10.1186/s12885-015-2020-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Said S, Cooper CJ, Reyna E, Alkhateeb H, Diaz J, Nahleh Z. Paraneoplastic limbic encephalitis, an uncommon presentation of a common cancer: Case report and discussion. Am J Case Rep. 2013;14:391–4. doi: 10.12659/AJCR.889560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fanous I, Dillon P. Paraneoplastic neurological complications of breast cancer. Exp Hematol Oncol. 2016;5:29. doi: 10.1186/s40164-016-0058-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Harrup R, White VM, Coory M, Walker R, Anazodo A, Skaczkowski G, et al. Treatment and outcomes for central nervous system tumors in Australian adolescents and young adults: A population-based national study. J Adolesc Young Adult Oncol. 2021;10:202–8. doi: 10.1089/jayao.2020.0074. [DOI] [PubMed] [Google Scholar]
- 62.Malani R. A view on the landscape of breast cancer brain metastases. CNS Oncol. 2020;9:CNS59. doi: 10.2217/cns-2020-0013. doi: 10.2217/cns-2020-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aversa C, Rossi V, Geuna E, Martinello R, Milani A, Redana S, et al. Metastatic breast cancer subtypes and central nervous system metastases. Breast (Edinburgh, Scotland) 2014;23:623–8. doi: 10.1016/j.breast.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 64.Soomro Z, Youssef M, Yust-Katz S, Jalali A, Patel AJ, Mandel J. Paraneoplastic syndromes in small cell lung cancer. J Thorac Dis. 2020;12:6253–63. doi: 10.21037/jtd.2020.03.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cacho-Díaz B, Spínola-Maroño H, Mendoza-Olivas LG, Monroy-Sosa A, Reyes-Soto G, Arrieta O. Association of neurologic manifestations and CEA levels with the diagnosis of brain metastases in lung cancer patients. Clin Transl Oncol. 2019;21:1538–42. doi: 10.1007/s12094-019-02086-y. [DOI] [PubMed] [Google Scholar]
- 66.Nayak L, Lee EQ, Wen PY. Epidemiology of brain metastases. Curr Oncol Rep. 2012;14:48–54. doi: 10.1007/s11912-011-0203-y. [DOI] [PubMed] [Google Scholar]
- 67.Pan Z, Yang G, He H, Yuan T, Li Y, Shi W, et al. Leptomeningeal metastasis from solid tumors: Clinical features and its diagnostic implication. Sci Rep. 2018;8:10445. doi: 10.1038/s41598-018-28662-w. doi: 10.1038/s41598-018-28662-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen N, Wheeler T, Coburn M, Yen AE. Leptomeningeal disease as a rare complication of primary penile urethral cancer. Case Rep Oncol Med. 2020 doi: 10.1155/2020/6349456. 6349456. doi: 10.1155/2020/6349456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huang JF, Shen J, Li X, Rengan R, Silvestris N, Wang M, et al. Incidence of patients with bone metastases at diagnosis of solid tumors in adults: A large population-based study. Ann Transl Med. 2020;8:482. doi: 10.21037/atm.2020.03.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shi D, Bai J, Chen Y, Wang X, Zhang Y, Liu H. Predicting the incidence and prognosis of bone metastatic breast cancer: A SEER-based observational study. BioMed Res Int. 2020;1068202 doi: 10.1155/2020/1068202. doi: 10.1155/2020/1068202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McCallum GA, Shiralkar J, Suciu D, Covarrubias G, Yu JS, Karathanasis E, et al. Chronic neural activity recorded within breast tumors. Sci Rep. 2020;10:14824. doi: 10.1038/s41598-020-71670-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lorusso L, Precone V, Ferrari D, Ngonga GK, Russo AG, Paolacci S, et al. Paraneoplastic neurological syndromes: Study of prevalence in a province of the Lombardy region, Italy. J Clin Med. 2020;9:3105. doi: 10.3390/jcm9103105. doi: 10.3390/jcm9103105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fukuda H, Tanaka A, Hirashima Y, Ito I. Lambert-Eaton myasthenic syndrome associated with synchronous double cancer: A combination of small cell carcinoma of the cervix and breast carcinoma. Intern Med (Tokyo, Japan) 2018;57:2409–11. doi: 10.2169/internalmedicine.0428-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. Lyon, France: International Agency for Research on Cancer; 2013. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. Available from: http://globocan.iarc.fr/ [Google Scholar]
- 75.Landolfi C. Neurologic complications of cancer and its treatment. Neurol Sci Neurosurg. 2020;1 doi: 10.47275/2692-093x-102. [Google Scholar]
- 76.Pereira S, Fontes F, Sonin T, Dias T, Fragoso M, Castro-Lopes JM, et al. Neurological complications of breast cancer: A prospective cohort study. Breast. 2015;24:582–7. doi: 10.1016/j.breast.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 77.Chernyshkova I, Estefan B, Hoque MR, Lee A. Neurologic presentation of probable seronegative paraneoplastic encephalitis in a woman with an ovarian teratoma. Cureus. 2020;12:8485. doi: 10.7759/cureus.8485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Harned ME, Salles SS, Grider JS. An introduction to trialing intrathecal baclofen in patients with hemiparetic spasticity: A description of 3 cases. Pain Physician. 2011;14:483–9. [PubMed] [Google Scholar]
- 79.Vicente-Valor MI, Garcia-Llopis P, Mejia Andujar L, Antonino de la Camara G, García del Busto N, Lopez Tinoco MJ, et al. Cannabis derivatives therapy for a seronegative stiff-person syndrome: A case report. J Clin Pharm Ther. 2013;38:71–3. doi: 10.1111/j.1365-2710.2012.01365.x. [DOI] [PubMed] [Google Scholar]
- 80.Shah S, Vazquez Do Campo R, Kumar N, McKeon A, Flanagan EP, Klein C, et al. Paraneoplastic myeloneuropathies: Clinical, oncologic, and serologic accompaniments: Clinical, oncologic, and serologic accompaniments. Neurology. 2021;96:632–9. doi: 10.1212/WNL.0000000000011218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ginsberg L. Acute and chronic neuropathies. Medicine (Abingdon) 2020;48:612–8. doi: 10.1016/j.mpmed.2020.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Criscitiello C, Esposito A, Gelao L, Fumagalli L, Locatelli M, Minchella I, et al. Immune approaches to the treatment of breast cancer, around the corner? Breast Cancer Res. 2014;16:204. doi: 10.1186/bcr3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sorensen TT, Farkas DK, Riahi EZB, Ehrenstein V, Henderson VW. Motor neuron disease and risk of cancer: A population-based cohort study in Denmark. Clin Epidemiol. 2020;12:1347–53. doi: 10.2147/CLEP.S271543. [DOI] [PMC free article] [PubMed] [Google Scholar]