Abstract
At the turn of the 21st century studies of the cells that resided in the adult mammalian subventricular zone (SVZ) characterized the neural stem cells (NSCs) as a subtype of astrocyte. Over the ensuing years, numerous studies have further characterized the properties of these NSCs and compared them to parenchymal astrocytes. Here we have evaluated the evidence collected to date to establish whether classifying the NSCs as astrocytes is appropriate and useful. We also performed a meta-analysis with 4 previously published datasets that used cell sorting and unbiased single-cell RNAseq to highlight the distinct gene expression profiles of adult murine NSCs and niche astrocytes. On the basis of our understanding of the properties and functions of astrocytes versus the properties and functions of NSCs, and from our comparative transcriptomic analyses we conclude that classifying the adult mammalian NSC as an astrocyte is potentially misleading. From our vantage point, it is more appropriate to refer to the cells in the adult mammalian SVZ that retain the capacity to produce new neurons and macroglia as NSCs without attaching the term “astrocyte-like.”
Keywords: Subventricular zone, stem cells, adult, mouse, human, astrocytes, review
Introduction
Whereas the concept that new neurons were produced in the adult mammalian brain was long a controversial topic, in vitro studies in the early 1990s provided strong evidence that neural stem cells (NSCs) resided within the central nervous system (CNS) and that these NSCs also existed in the human brain (Reynolds et al., 1992; Kirschenbaum et al., 1994), although the latest studies show that these adult human NSCs do not produce any new neurons (Arellano et al., 2021; Sorrells et al., 2021). Some landmark papers provided strong in vivo experimental evidence that there were, indeed, NSCs residing within the adult mouse subventricular zone (SVZ; Bystron et al., 2008; Doetsch et al., 1999a; Doetsch et al., 1999b). These studies showed that the NSCs were situated immediately adjacent to the ependymal layer lining the lateral ventricles and that they had a single apical process that penetrated the lateral ventricle. This process had a single short cilium that we now know serves as a signaling hub to enable the NSCs to respond to growth factors and cytokines that are produced by the choroid plexus, which help to maintain those NSCs in a primitive state (Liu et al., 2021). The basal process of the NSCs typically ends upon capillaries (Liu et al., 2021). Within the cytoplasm, the NSCs contained bundles of intermediate filaments comprised of glial fibrillary acidic protein (GFAP), a cytoskeletal intermediate filament protein that forms heterodimers with either vimentin or nestin. On the basis of the morphology of the cells at the light and electron microscopic level and because they expressed GFAP, Doetsch and many other colleagues classified the NSCs of the SVZ as a subtype of astrocyte (e.g. Doetsch et al., 1999b; Gonzalez-Perez and Quiñones-Hinojosa, 2012; Liu et al., 2006; Magnusson et al., 2020; Mizrak et al., 2020). Over the ensuing years, numerous studies have further characterized the properties of the NSCs and many investigators have continued to refer to them as SVZ astrocytes (e.g. Andromidas et al., 2021; Gengatharan et al., 2016; Georg Kuhn and Blomgren, 2011; Lee et al., 2018; Platel and Bordey, 2016; Schaberg et al., 2022; Urban et al., 2019). However, it is not clear that this designation is appropriate. Therefore, we have evaluated the evidence collected to date to establish whether classifying NSCs as astrocytes is appropriate and useful, for scientists newly entering this field or for non-specialists interested in NSCs.
Properties of SVZ NSCs
During CNS development, radial glial cells serve as progenitors for multiple classes of neural cells that include astrocytes, oligodendrocytes, ependymal cells, and both pyramidal and non-pyramidal neurons (Arellano et al., 2021). The term radial glial cell was introduced by Pasko Rakic (Rakic, 1988) and these proliferative cells were considered a subtype of glia in keeping with the views of classical neuroanatomists who called them “Kimzellen,” “Fetal Glia,” “Golgi Epithelial Cells,” and “Spongioblasts” (Moossy, 1982). As development proceeds, these progenitors cells become less prominent but they persist in the forebrains of adult rats and mice where their cell bodies reside in the SVZ (Breunig et al., 2011; Bystron et al., 2008; Gubert et al., 2009; Sundholm-Peters et al., 2004). While mitotic cells in the SVZ region of the adult brain had been noted near the turn of the 20th century (Allen, 1912; Schmechel and Rakic, 1979), the identities of the cells within this region were not characterized until much later (Arellano et al., 2021). With the advent of 3H-thymidine to label dividing cells (Bryans, 1959; Smart, 1961), two different cell types were originally described in the adult SVZ: cells with light nuclei and cells with dark nuclei (Rakic, 2009; Smart, 1961). Using electron microscopy, the classification scheme was extended to include three types of astrocytes (protoplasmic, fibrous, and intermediate), microglia, and mitotically active, undifferentiated subependymal cells (Blakemore, 1969). Neuropathologists often used the term “subependymal cells” for this quiescent population in the adult human brain that became active after damage to the overlaying cortex and then generated progenitors that migrated to the site of the lesion where they often differentiated into astrocytes (Haymaker and Adams, 1982).
In 1997, Fiona Doetsch, Jose Manuel Garcia-Verdugo, and Arturo Alvarez-Buylla revisited the ultrastructure of the SVZ and divided the resident cells into six cell types (excluding resident microglia). These cell types were the type B1 (presumptive NSCs) type B2 (immature astrocytes), type C (intermediate progenitors, transit amplifying cells), type A (neuroblasts), type D (tanycytes), and type E cells (ependymal cells; Doetsch et al., 1997). Then, in 1999, Fiona Doetsch and colleagues performed a series of elegant experiments that definitively identified and characterized the adult NSCs of the SVZ (Doetsch et al., 1999b). In that study, they infused the anti-mitotic drug arabinosylcytosine (Ara-C) into the ventricles, which dramatically depleted the SVZ, but the type B1 cells were resistant to this anti-mitotic drug treatment and as they divided, they eventually repopulated the SVZ. They first produced type C cells and then type A cells. Doetsch et al., (1999b) also immunostained the SVZ cells following the elimination of type A and C cells and showed that GFAP was expressed by all of the type B1 cells. They also showed that the type B1 cells were slowly dividing, “label-retaining” cells. To demonstrate that their progeny could form mature neurons they used transgenic mice where only the GFAP-expressing cells could be infected with a green fluorescence protein (GFP)-expressing replication-deficient retrovirus that allowed them to fate map the progeny of those GFAP + cells. Using this method, they showed that the GFAP + type B1 cells produced first the type C cells and subsequently the type A cells. Those type A cells then migrated to the olfactory bulb where they differentiated into post-mitotic interneurons.
Angelique Bordey's laboratory further characterized the mechanisms behind this proliferative activation of B cells in a series of experiments showing that B1 cells have projections ensheathing SVZ capillaries and can increase blood flow via calcium signaling upon entering into the S-phase (Lacar et al., 2012a, 2012b). Subsequent studies have further characterized the NSCs of the SVZ to that show that there are multiple types of NSCs in the SVZ whose gene expression differs depending upon whether they are quiescent or proliferating (Dulken et al., 2017; Mizrak et al., 2020; Pastrana et al., 2009). Furthermore, studies have shown that there is significant heterogeneity amongst that NSCs in the SVZ based on the cell types that they produce (Merkle et al., 2014). Below we discuss both the similarities and differences with astrocytes that we will discuss in the following sections.
Key Features of Astrocytes
To establish whether the SVZ NSCs are astrocytes, it is necessary to define an astrocyte. For the purpose of this article, we will limit our discussion to astrocytes of the gray matter, frequently referred to as protoplasmic or parenchymal astrocytes, as opposed to the fibrous astrocytes of white matter. Parenchymal astrocytes are a heterogeneous group of cells that perform essential homeostatic functions. They signal to each other and to the cells surrounding them and comprise part of the brain's defense mechanism (Clarke and Barres, 2013; Escartin et al., 2021; Iadecola and Nedergaard, 2007; Kettenmann and Ransom, 2013; Kimelberg and Nedergaard, 2010; Nedergaard and Verkhratsky, 2012; Oberheim et al., 2012; Parpura et al., 2012; Zhang and Barres, 2010). Astrocytes also show morphological and regional heterogeneity, possessing different properties depending upon their geographical location within the brain (Zhang and Barres, 2010). Note, however, that their morphologies are not fixed as they readily modify their shape in response to extracellular signals and especially when CNS homeostasis is disturbed (Zhang and Barres, 2010).
Mature astrocytes are quiescent cells and, thus, they do not normally participate in cellular homeostasis. Rather they provide morphological homeostasis in that they parcel the gray matter through a process termed tiling into neurovascular units, integrating astrocyte territorial domains with neuronal and vascular components (Bushong et al., 2002; Iadecola and Nedergaard, 2007; Nedergaard et al., 2003). These neurovascular units create a functional integration where the astrocytes provide molecular homeostasis (e.g. regulating ion and neurotransmitter concentrations in the CNS), metabolic homeostasis (e.g. producing lactate that they can provide neurons), and heterocellular signaling (Parpura and Verkhratsky, 2012b, 2012c; Verkhratsky and Parpura, 2015).
Astrocytes are electrically non-excitable cells as they lack high-density expression of voltage-gated channels and because they robustly express Kir 4.1, which establishes their highly negative resting potentials (Kucheryavykh et al., 2007). These potassium channels also redistribute potassium from sites of high extracellular potassium (e.g. surrounding active synapses) to areas of low extracellular potassium as the astrocytes take up the potassium that is then dissipated within an astrocyte (referred to as syphoning) or between astrocytes (referred to as buffering) through the astrocyte “syncytium” to be released at their perivascular end-feet. This scenario requires the astrocytes to be polarized whereby their end feet highly express Kir4.1. Similarly, their end feet highly express aquaporin 4 (AQP4), which is a water channel that enables the astrocytes to regulate water homeostasis in the CNS (Benfenati et al., 2011). Furthermore, their end feet almost fully cover CNS capillaries to create the perivascular “glymphatic” system that removes metabolites from the CNS parenchyma (Iliff and Nedergaard, 2013; Iliff et al., 2013). The astrocyte syncytium is maintained via gap junction coupling, predominantly by connexin 43-containing gap junctions that appear during the first two to three weeks of murine post-natal development. After that period connexin 30 is produced and then also contributes to this coupling (Kunzelmann et al., 1999; Nagy et al., 2001).
Astrocytes express a wide array of neurotransmitter receptors that are tuned to sense neighboring neuronal transmission (Parpura and Verkhratsky, 2012a; Verkhratsky et al., 2012b). Activating these receptors triggers dynamic changes in cytosolic concentrations of ions, mainly Ca2+ and Na+, which represents astrocytes’ excitability and can regulate their functions (Kirischuk et al., 2012; Parpura and Verkhratsky, 2012d; Verkhratsky et al., 2012a). Some of those diverse functions occur at the so-called tripartite synapse (Araque et al., 1999). At the tripartite synapse, astrocytic excitability leads to gliotransmission, that is, the release of gliotransmitter(s) such as adenosine triphosphate and glutamate that can, in turn, modulate synaptic transmission and plasticity of the synapses within the reach of the astrocyte's filamentous processes (Halassa et al., 2007; Navarrete et al., 2013).
Astrocytes also regulate neurotransmitter production and metabolism. They de novo synthesize glutamate owing to the astrocyte-specific mitochondrial enzyme pyruvate carboxylase. They remove glutamate, γ-aminobutyric acid (GABA), glycine, and adenosine from the perisynaptic space through the actions of specific plasmalemmal transporters. Upon internalizing neurotransmitters, astrocytes catabolize them. For example, they can use glutamine synthetase to convert glutamate to glutamine, which is then supplied to neurons as the obligate precursor for glutamate and GABA synthesis (Verkhratsky and Parpura, 2015). Astrocytes also catabolize adenosine by phosphorylating it using adenosine kinase. Notably, both glutamine synthetase and adenosine kinase are expressed almost exclusively in astrocytes. Of note, glutamate uptake occurs in astrocytes via the excitatory amino acid transporter 1 (EAAT1 in human vs. solute carrier family 1 member 3 (SLC1A3) in murine terminology) and the excitatory amino acid transporter 2 (EAAT2 in humans vs. solute carrier family 1 member 2 (SLC1A2) in mice). While EAAT1/SLC1A3 is expressed by radial glia in the fetal brain, the NSCs of the SVZ, and by immature astrocytes, EAAT2/SLC1A2 is only expressed by mature astrocytes, with post-natal day 26 in murine representing the transition point in their expression (Furuta et al., 1997). Through their actions as CNS secretory cells astrocytes not only release neuro-/glio-transmitters via multiple mechanisms but also neuromodulators, hormones, trophic, and immune factors (Malarkey and Parpura, 2008; Verkhratsky et al., 2016; Zorec et al., 2016). Indeed, the secretion of factors such as thrombospondins, cholesterol, and neuregulins is important for synaptogenesis, synaptic maturation, and myelination (Camargo et al., 2017; Christopherson et al., 2005; Eroglu and Barres, 2010; Eroglu et al., 2009; Kucukdereli et al., 2011). Astrocyte secretion also plays a role in the formation of the brain–blood barrier (Abbott et al., 2006, 2010) and in regulating parenchymal arterial blood flow (Attwell et al., 2010).
Similarity Between SVZ NSCs and Mature Astrocytes
Doetsch et al., (1999a) showed that all of the type B1 cells in the adult mouse SVZ shared microscopic features with astrocytes that included expression of GFAP and processes extended to blood vessels and, thus, referred to these cells as SVZ astrocytes. Additionally, studies have shown that type B1 cells express the glutamate transporter, SLC1A3 (Giachino et al., 2014; Liu et al., 2006). Astrocytes play a crucial role in the process of glutamate uptake from the extracellular space using two glutamate transporters, SLC1A2 and, to a lesser extent, SLC1A3. The expression of both glutamate transporters has also been found in SVZ NSCs (Table 1), as identified by immunostaining and electrophysiological recordings (Liu et al., 2006). The NSCs express functional ion channels such as K+ rectifying channels and they respond to GABA further strengthening the connection between NSCs and astrocytes.
Table 1.
Functional and Electrophysiological Similarities Between Astrocytes, SVZ Neural Stem Cells, and Radial glia.
| Astrocytes | SVZ NSCs | Radial glia | Citations |
|---|---|---|---|
| Functional gap junction coupling; express connexin 43 and exhibit dye coupling | Functional gap junction coupling; express connexin 43 and exhibit dye coupling | Functional gap junction coupling; express connexin 43 and exhibit dye coupling | (Beckervordersandforth et al., 2010; Dulken et al., 2017; Liu et al., 2006) |
| Inward/outward currents—linear current–voltage curve | Inward/outward currents—linear current–voltage curve | Voltage-dependent outward currents | (Liu et al., 2006) |
| KIR4.1/2.1 key in K+ buffering, present in SLC1A3+ cells | KIR4.1/2.1 present in SLC1A3+ cells | Unknown | (Liu et al., 2006) |
| High levels of SLC1A2, lower levels of SLC1A3 | High levels of SLC1A3 and lower levels of SLC1A2 | Express SLC1A3 only | (Beckervordersandforth et al., 2010; Furuta et al., 1997; Kugler and Schleyer, 2004; Liu et al., 2006) |
| GFAP intermediate filament protein expression varies | GFAP intermediate filament protein positive | Variable levels of GFAP intermediate filament protein depending upon species | (Doetsch et al., 1999a; Liu et al., 2006) |
| Express ALDH1L1. Catalyzes conversion of NADP+ to NADPH+ | Express ALDH1L1. Catalyzes conversion of NADP+ to NADPH+ | Express ALDH1L1. Catalyzes conversion of NADP+ to NADPH+ | (Beyer et al., 2021; Tien et al., 2012) |
Note. SVZ = subventricular zone; NSC = neural stem cell; NADP = nicotinamide-adenine-dinucleotide phosphate; NADPH = nicotinamide adenine dinucleotide phosphate; GFAP = glial fibrillary acidic protein; ALDH1L1 = aldehyde dehydrogenase 1 family member L1.
Another hallmark characteristic of astrocytes is the presence of gap junctions between the astrocytes that are involved in the cell–cell interaction and communication and as reviewed above are crucial in the proper formation of the functional syncytium of mature astrocytes. Dye-coupling methods and co-immunostaining for connexin 43 and SLC1A3 in GFAP-expressing cells indicated the presence of gap junctions and connexin 43 within SVZ NSCs (Liu et al., 2005). SVZ NSCs also express functional hemichannels (Table 1), as identified by Liu et al. (2006) using dye uptake assay and meclofenamic acid (MFA), a gap junction blocker and uncoupler. Their results showed that the fluorescent dye uptake between cells in a control environment of 0 Mg++/0 Ca++ solution (which aids the opening of connexons) was much higher than cells in the same solution with the inclusion of MFA (Liu et al., 2006). The dye reduction in the presence of MFA not only indicates the presence of hemichannels but also highlights their function, as MFA successfully inhibited intracellular communication. The functional and electrophysiological similarities between SVZ NSCs, radial glia, and astrocytes are summarized in Table 1.
Differences Between SVZ NSCs and Mature Astrocytes
Despite their functional and morphological similarities, many studies have highlighted the prominent differences between SVZ NSCs and mature parenchymal murine astrocytes in morphology as well as protein and gene expression. As Liu et al. (2006) discuss the overall morphologies of the two cell types are very different. GFAP + SVZ cells have a simple morphology in that they possess one to two main processes. Parenchymal murine astrocytes, by contrast, have a complex morphology consisting of several main processes and multiple short processes that branch extensively (Moossy, 1982). While the NSCs extend processes to blood vessels like astrocytes; those processes do not perform a key function attributed to astrocytes. Astrocytes produce transforming growth factor-β, glial-derived neurotrophic factor, angiopoietin-1, and fibroblast growth factor-2, which collaboratively induce the endothelial cells to form tight junctions and form the blood–brain barrier (Abbott et al., 2006). By contrast, studies have shown that while the NSCs in the SVZ extend a process to the blood vessel; those blood vessels are leaky (Tavazoie et al., 2008). As pointed out by others, the NSCs of the SVZ resemble the radial glia of the developing brain and the NSCs retain expression of molecules expressed by their precursors. For example, radial glia express SLC1A3 but not SLC1A2 (Shibata et al., 1997); whereas most adult astrocytes are SLC1A2+ and many SLC1A3 negative (Rothstein et al., 1994). Thus, the glutamate transporter expression of the NSCs is different from that of most astrocytes. Electrophysiologically, the NSCs have delayed rectifying K+ conductances at rest like astrocytes; however, they have a very low-level expression of the barium-sensitive inwardly rectifying potassium currents (i.e. Kir4.1) that are characteristic of mature, post-mitotic astrocytes (Bordey and Sontheimer, 1997; Liu et al., 2006). While radial glia expresses AMPA-type glutamate receptors as do subsets of astrocytes, AMPA did not induce either inward currents or increases in intracellular calcium ions from SVZ NSCs (Liu et al., 2006; Platel et al., 2010) indicating that AMPA-type glutamate receptors are not present in SVZ NSCs, highlighting another difference from mature astrocytes (Mölders et al., 2018).
NSCs and astrocytes also have marked differences in the expression of specific cell markers. These differences were uncovered by studies that combined genetic strategies and immunolabelling to identify and characterize SVZ NSCs. In a seminal study, Beckervordersandforth et al. (2010) generated a transgenic mouse expressing GFP driven by the human GFAP promoter (hGFAP:GFP mice) and stained these cells for multiple cell markers. SVZ cells co-expressing GFAP and CD133 were defined as NSCs. That strategy was used to characterize the morphology of the NSCs and to isolate NSCs by fluorescence-activated cell sorting (FACS). Transcriptomic analyses of FACS-isolated NSCs revealed that several astrocyte markers, including S100β, glutathione-S-transferase-µ, aldehyde dehydrogenase 1 family member L1 (ALDH1L1), and AQP4, were expressed in astrocytes (GFAP-expressing only) but not in the SVZ NSCs (Beckervordersandforth et al., 2010). However, a more recent study has demonstrated ALDH1L1 expression by mouse NSCs of the adult SVZ, but interestingly not the NSCs of the hippocampal subgranular zone (Beyer et al., 2021).
Along with differences in the expression of markers, there were significant differences found between SVZ NSCs and astrocytes in terms of molecular phenotype and neurogenic potential. The protein markers, nestin, and LeX/CD15 have been found to be associated with murine NSCs but not with mature astrocytes (Imura et al., 2006). Imura et al. (2006) disputed the idea that SVZ NSCs are astrocytes by highlighting the functional and morphological heterogeneity between the two cell types in terms of chemical marker expression and neurogenic potential. Despite the presence of GFAP, they found that about half of the GFAP-expressing cells from the SVZ also co-expressed nestin and, from that group, only a smaller sub-population expressed LeX/CD15. Investigating the maturation of primary astrocytes in vitro, Imura et al. (2006) found that after multiple passages, there was a significant down-regulation in the expression of nestin and LeX/CD15, accompanied by the loss of neurogenic potential and reduced ability to form multipotent neurospheres (Imura et al., 2006). Imura et al. (2006) further analyzed this relationship between GFAP-expressing NSCs and GFAP-expressing astrocytes and found that amongst the GFAP-expressing cells within primary astrocyte culture only 1% had the ability to behave as NSCs. Ultimately, Imura et al. (2006) argued that due to the evident heterogeneity of GFAP-expressing cells, particularly in phenotype, and the loss of neurogenic potential after multiple passages of primary astrocytes, the majority of GFAP-expressing cells are not multipotent NSCs. These and other comparisons between astrocytes and NSCs are summarized in Table 2.
Table 2.
Functional, Transcriptional, and Electrophysiological Differences Found Between Murine Astrocytes and SVZ NSCs.
| Physiological differences | |||
|---|---|---|---|
| Astrocytes | SVZ NSCs | Citations | |
| Bushy shape (in vivo) | Unipolar or bipolar shape | (Doetsch et al., 1999b; Fedoroff and Vernadakis, 1986) | |
| Unable to produce multipotential neurospheres in vitro | Generate multipotent neurospheres in vitro | (Imura et al., 2006) | |
| Protein and transcriptional differences | |||
| Astrocytes | SVZ NSCs | Gene/protein function | Citations |
| Lex/CD15 negative | Express Lex/CD15 | Lewis(x) is a cell-surface carbohydrate that functions as a cell–cell recognition molecule in the CNS | (Imura et al., 2006) |
| Prominin-1/CD133 negative | Express Prominin-1/CD133 | Transmembrane glycoprotein that suppresses differentiation | (Buono et al., 2012; Montana et al., 2021) |
| Nestin negative | Nestin positive | Intermediate filament required for proliferation of neural progenitors | (Imura et al., 2006) |
| High expression of S100b | No expression of S100b | Cytoplasmic EF-hand calcium-binding protein | (Beckervordersandforth et al., 2010; Hachem et al., 2007) |
| High expression of glutathione-S-transferase-µ | No expression of glutathione-S-transferase-µ | Conjugates reduced glutathione in antioxidant-responses | (Beckervordersandforth et al., 2010) |
| High expression of glutamine synthetase | No expression of glutamine synthetase | Catalyzes the synthesis of glutamine from glutamate | (Beckervordersandforth et al., 2010) |
| Subsets express AMPA/Kainate receptors (Kir4.1) | No expression of AMPA-type glutamate receptors | Inwardly rectifying potassium channel. Maintains astrocyte resting membrane potential and facilitates glutamate uptake. | (Lalo et al., 2006; Liu et al., 2006; Nwaobi et al., 2016) |
| ADK positive | ADK expressed at low levels | Regulates adenosine homeostasis in the CNS | (Beckervordersandforth et al., 2010; Dulken et al., 2017) |
| SLC7A11 (cystine-glutamate antiporter, system X(c)) positive | SLC7A11 expressed at low levels | Sodium-independent channel transports cysteine in exchange for glutamate to maintain extracellular glutamate levels | (Beckervordersandforth et al., 2010; Bender et al., 2000; Dulken et al., 2017; Sears et al., 2021) |
| CD9 (tetraspanin) high | CD9 low | Cell surface glycoprotein that binds to integrins to influence cell interactions. Regulates outgrowth of astrocytic processes | (Llorens-Bobadilla et al., 2015; Schmidt et al., 1996) |
| Low levels of nucleostemin (GNL3) | High expression of nucleostemin (especially in aNSCs) | Nucleolar GTPase required to maintain the proliferative capacity of stem cells | (Beekman et al., 2006; Dulken et al., 2017; Mizrak et al., 2019; Tsai and McKay, 2002) |
| Low expression of KIF2C | High expression of KIF2C | Kinesin-like molecular motor with microtubule depolymerizing activity | (Qin et al., 2019) |
| Low expression of TOP2A | High expression of TOP2A | DNA topoisomerase that regulates mitosis | (Qin et al., 2019) |
Note. SVZ =subventricular zone; NSC = neural stem cell; CNS = central nervous system; ADK = adenosine kinase.; GNL3 = G protein nucleolar 3; aNSCs = activated neural stem cells
Comparison of Gene Expression
Stem Cells and Resident Astrocytes in the SVZ Have Distinct Gene Expression Profiles
Many studies have investigated the transcriptomic profiles of SVZ-resident cell populations in rodents; unfortunately, to date, similar studies have not yet been performed comparing human SVZ NSCs to human protoplasmic astrocytes. Different strategies have been employed to isolate cell types and perform comparative expression analyses. The most common approaches rely on initially using fluorescently-activated cell sorting (FACS) to isolate target populations based on surface markers prior to expression analysis (Beckervordersandforth et al., 2010; Dulken et al., 2017), or on unbiased single-cell RNA sequencing (scRNAseq) followed by in silico techniques for sorting neural populations (Zywitza et al., 2018). Regardless of the approach, NSCs and resident astrocytes are consistently sorted and exhibit distinct gene expression profiles. In two studies that used FACS to isolate cell populations from mice, NSCs were identified by the dual expression of GFP driven by the hGFAP promoter and cell surface Prominin-1 (CD133), while astrocytes were selected as Prominin-1 negative and hGFAP positive (Beckervordersandforth et al., 2010; Dulken et al., 2017). Transcriptomic analysis in these sorted populations showed that even though NSCs and astrocytes have similarities in their expression profiles, there are clear expression differences that discriminate these cell types. A similar pattern is observed in studies that used scRNAseq to identify SVZ-resident populations. Unbiased clustering of sequenced cells using global gene expression consistently identified distinct clusters representing NSCs and astrocytes (Zywitza et al., 2018). This approach allows for even greater analysis depth, revealing multiple populations of NSCs and astrocytes that may represent different activation states and steps in lineage progression.
Searching for genes specifically expressed by NSCs, Qin et al. (2019) compared astrocyte and NSC gene expression profiles from two published mouse microarray studies (Beckervordersandforth et al., 2010; Nakajima-Koyama et al., 2015). Despite the differences in the origin of the samples in these two studies, Beckervordersandforth et al. (2010) used FACS-sorted cells from adult mice while Nakajima-Koyama et al. (2015) used astrocytes derived from mouse induced pluripotent stem cells (iPSCs), their bioinformatics analysis found 77 upregulated genes that overlapped. Upon further analyses of protein–protein interaction networks and tissue expression profiles they identified 24 genes that were highly expressed by the NSCs that included two key genes, KIF2C and TOP2A, that not only distinguished NSCs from astrocytes at the RNA level but also at the protein level (Qin et al., 2019).
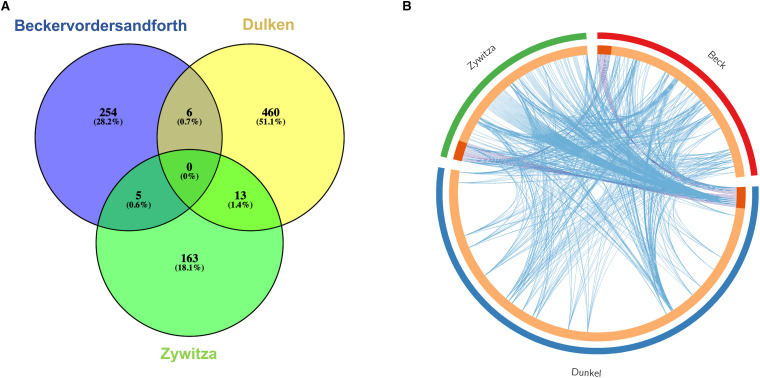
We extracted datasets from three studies that directly compared gene expression between adult murine NSCs and niche astrocytes (Beckervordersandforth et al., 2010; Dulken et al., 2017; Zywitza et al., 2018). Differentially expressed genes (DEGs) between NSCs and astrocytes were found in the three studies, albeit with some interesting differences. The number of DEGs reported varied from 181 (Zywitza et al., 2018) to 479 (Dulken et al., 2017). Interestingly, there was a very low overlap in the DEGs from these three analyses (Figure 1A). These differences in reported DEGs can be attributed to the particular strategies employed in each study. Beckervordersandforth et al. (2010) profiled NSC and astrocyte transcriptomes using microarray, a technique restricted by the number of probes in the geneset; by contrast, Dulken et al. (2017) and Zywitza et al. (2018) used single-cell sequencing, which offers improved specificity, sensitivity, and dynamic range. Moreover, Beckervordersandforth et al. (2010) and Dulken et al. (2017) employed genetically engineered mice expressing GFP driven by the GFAP promoter to FAC sort GFAP+/Prominin-1 + NSCs, while Zywitza et al. (2018) relied on in silico clustering and matching to published marker gene sets to classify NSCs and astrocytes.
Figure 1.
(A) Venn diagram for DEGs between NSC and astrocyte populations from the three studies, Beckervordersandforth et al. (2010; blue), Dulken et al. (2017; yellow) and Zywitza et al. (2018; green). Each list represents a direct comparison in gene expression between NSCs and astrocytes in the reference study. (B) Circos plot for the same gene lists as (A), representing shared genes (shaded orange and purple lines) and shared GO terms (blue lines). GO enrichment analysis performed in Metascape.
DEG = differentially expressed genes; NSC = neural stem cell; GO = gene ontology.
However, although the DEGs found in these studies were mostly unique, gene ontology (GO) enrichment analysis in these datasets revealed that these DEGs shared many of the same GO terms (Figure 1B), suggesting that these genes are part of similar signaling pathways and underlie the same cell processes. Our GO enrichment analysis highlighted important cellular processes shared by these DEGs, such as repression of the cell cycle and microtubule organization in NSCs and the silencing of neurogenesis regulatory pathways in astrocytes.
Analysis Comparing Lists of Upregulated Genes in NSCs Versus List of Upregulated Genes in Astrocytes
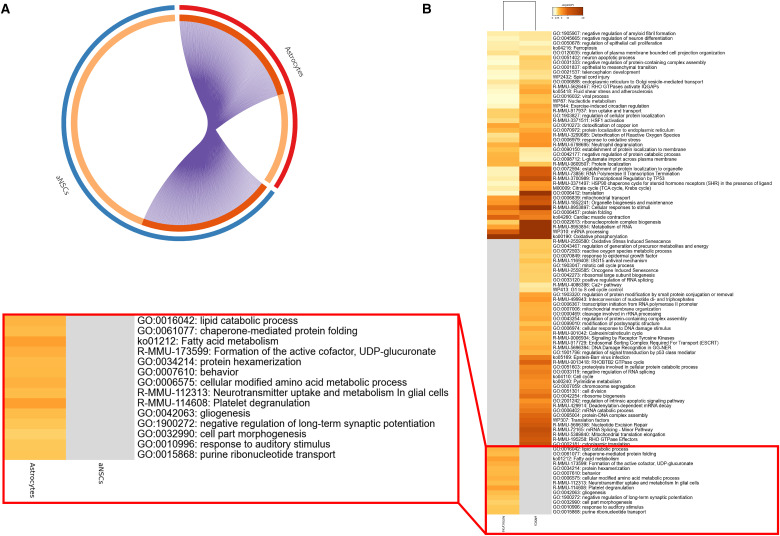
To further compare the gene expression profiles of NSCs and neighboring astrocytes, we analyzed the full lists of upregulated genes in each of these populations when compared to all sequenced cells, isolated either by FACS (Dulken et al., 2017) or scRNAq strategies (Mizrak et al., 2019; Zywitza et al., 2018). In this analysis, we performed novel comparisons between the full gene lists of NSCs versus astrocytes. As expected, the transcriptomes of NSCs and astrocytes have a high degree of overlap, sharing up to 50% of their expressed genes (Figure 2A). These similarities are well in line with the proximity of these cell types in the lineage progression toward mature astrocytes. However, as seen in the example from Dulken et al. (2017; Figure 2A), NSCs change their expression profiles during differentiation into astrocytes, silencing many genes and activating entire new gene sets, many associated with glial function. These changes can be observed in comparative GO enrichment analysis to identify common and exclusive pathways emerging from the NSC and astrocytes gene sets. Consistently, while these cells present concurring pathways that support common cell processes, both NSCs and astrocytes have exclusively enriched signaling pathways that represent the specific cell functions in each of these cell types. Among the more prominent GO terms enriched in NSCs are those related to the cell cycle, while astrocytes have a strong enrichment of pathways associated with glial function and especially neurotransmitter uptake (Figure 2B).
Figure 2.
(A) Circos plot comparing full gene lists for NSCs (blue) and astrocytes (red) from Dulken et al. (2017). Shared genes are represented by shaded orange and purple lines. (B) Heatmap representing the association of GO terms (by p-value) and clusterization of GO terms between the same NSC and astrocyte populations as in (A). Astrocyte exclusive GO terms are highlighted in red. GO enrichment analysis performed in Metascape.
NSC = neural stem cell; GO = gene ontology.
Analysis Comparing Astrocyte Markers in NSCs and Different Astrocyte Clusters
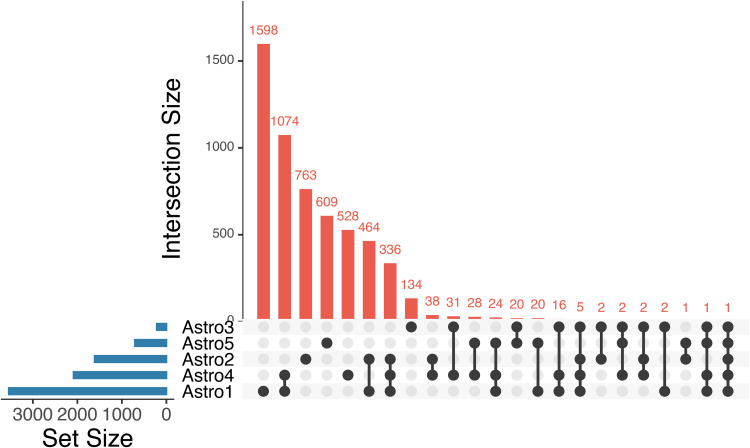
Many lineage-exclusive cell markers have been extensively validated for immunohistochemical approaches. These markers are cell-surface, cytoplasmic, or proteins that are exclusively expressed in only one or a few specific cell types. Common markers for astrocytes include GFAP (intermediate filament), S100b (Ca2+ binding protein), glutathione-S-transferase-µ (detox), glutamine synthetase (synthesis of glutamine from glutamate), EAAT1/2 (plasmalemmal glutamate transporters), ALDH1L1 (dehydrogenates aldehydes), and AQP4 (water channel). Therefore, the genes that express these proteins represent a genetic signature invaluable in the identification of astrocytes in RNA sequencing studies. Accordingly, these cell marker signatures also further support the differences between NSCs and SVZ-resident astrocytes and astrocyte progenitors. No genes for astrocyte markers were detected in the expression profiles of sorted NSCs reported by Beckervordersandforth et al. (2010) while the cells identified as astrocytes in that study had high expression of S100B, SLC1A3 (alias EAAT1/SLC1A3), ALDH1L1, and AQP4. Similarly, both the NSCs sorted by Dulken et al. (2017) and those identified in silico by Zywitza et al. (2018) had only moderate expression of glutathione-S-transferase µ1 (GSTM1) and SLC1A3 and no expression of other astrocyte markers. When compared to the astrocyte population in the respective studies all markers were strongly downregulated in NSCs. Conversely, the astrocyte population in Zywitza et al. (2018) presented enrichment of glutamate-ammonia ligase (GLUL; alias glutamine synthetase) and AQP4 when compared to NSCs. Interestingly, Mizrak et al. (2019) were able to achieve even greater resolution when clustering glial cells after scRNAseq (Mizrak et al., 2019). The authors identified not only the NSC population but also discriminated against five distinct astrocyte populations. Notably, the NSCs lacked expression of any astrocyte markers, while the five populations of astrocytes expressed unique combinations of these markers, ranging from expression of all markers, that is, Cluster4: GFAP, S100b, GSTM1, GLUL, SLC1A3, ALDH1L1, and AQP4, to only a single marker, that is, Cluster5: SLC1A3. These differences can represent either a spatial or temporal distribution of astrocytes and their progenitors in the SVZ. The expression of these astrocyte markers also correlates with the relationships between the expression profiles of the astrocyte clusters. We compared the expressed genes in each of the astrocyte clusters and found that the populations had transcriptomic signatures, with different subsets of exclusively expressed genes. Moreover, the number of exclusively expressed genes and the number of concomitant expressed genes seemed to correlate with the number of astrocyte markers found in these populations (UpSet plot from Mizrak et al., 2019). For example, astrocyte clusters 1 and 4 have the highest numbers of uniquely expressed genes and also the highest number of genes shared by two populations. These same clusters also express the highest number of specific markers among the astrocyte populations (Figure 3).
Figure 3.
Upset plot for the genes that characterize each of the five astrocyte populations identified by Mizrak et al. (2019). Blue bars represent the total number of genes in each group. Red columns represent the number of common genes defined by the corresponding relationship matrix (black connecting dots). Analysis performed in Intervene.
Human Brain Data: Delineating NCS from Astrocytes
Most non-mammalian vertebrate species such as fishes, amphibians, and reptiles produce neurons in the nervous system throughout their entire lifespan. However, this capability decreases steadily during evolution so that in birds it is much smaller, in rodents it continues only in the olfactory system and the dentate gyrus of the hippocampus, and in humans, it becomes undetectable (Arellano et al., 2022). The nomenclature for the NSCs derives from classical work on the developing human cerebrum (Bystron et al., 2008). In nonhuman primates, the production of new neurons is very small. In a recent study of the marmoset brain that used dual thymidine analogs to study the production of new neurons, the authors found that olfactory bulb and neocortical neurogenesis decreased sharply by 4 months of age. Furthermore, the vast majority of those neurons born postnatally in both the neocortex and hippocampus remained doublecortin positive. Moreover, some of them expressed Olig2 suggesting that they were not immature neurons but glial progenitors (Akter et al., 2020). While the data show that NSCs persist both periventricularly and in the hippocampus into adulthood (Kukekov et al., 1999), the production of new neurons in the adult human brain is basically extinct (Duque et al., 2022; Paredes et al., 2016; Sanai et al., 2011; Sorrells et al., 2021). It was proposed that the biological basis for this decreased capacity for producing new neurons may be essential to retain acquired complex knowledge within a stable population of neurons and their synaptic connections during the many decades of the human life span (Rakic, 1985). Thus, in the human cerebrum, the progenitors of the SVZ produce only astrocytes and oligodendrocytes, but not new neurons (Nait-Oumesmar et al., 2007).
Some reports claim that neurogenesis is occurring in adult humans using 14C to detect “new neurons.” However, this is not adequate nor sufficient evidence, since the incorporation of 14C into the DNA occurs not only at the time of cell division, but also in post-mitotic neurons attempting to repair their DNA as they are degenerating or during the process of apoptosis (Guo et al., 2011). For example, hypoxia-ischemia in the adult brain induces DNA synthesis in dying neurons in the absence of proliferation (Kuan et al., 2004). Therefore, older and dying neurons that re-enter the cell cycle can incorporate DNA replication markers used to label new neurons (Arellano et al., 2022; Duque and Spector, 2019). A recent analysis that used a single-cell transcriptome analysis could not find evidence for newly formed neurons in the hippocampus. While adult-generated neurons that expressed markers characteristic of neuroblasts, such as DCX were found in the adult hippocampus of mice, pigs, and macaques, no such neuroblasts were detected in the adult human hippocampus (Franjic et al., 2022). A thorough review of the literature shows that there is little scientifically convincing evidence for the generation and incorporation of new neurons into synaptic circuitry of the adult human brain and that cells generated in the SVZ of adult human cerebrum appear to generate exclusively oligodendrocytes or astrocytes in response to lesions and/or disorders of aging (Duque et al., 2022).
Discussion
Astrocytes are mature cell types that undergo a distinct differentiation process to obtain their complex characteristics and functionality within the CNS. Within the CNS they subserve a number of functions that include inactivating neurotransmitters, maintaining extracellular pH, regulating levels of potassium, maintaining tissue osmolarity, detoxifying byproducts of metabolism, detoxifying heavy metals, nourishing neurons, and establishing and maintaining the blood–brain barrier. They are morphologically diverse and perform region-specific functions, as well. By contrast, NSCs are, by definition, undifferentiated cells, whose function is to slowly divide to generate new cells across the lifespan. Thus, functionally these cells are quite distinct, which is evident from a review of Table 2.
As it turns out, however, even classifying a cell as an astrocyte can be complicated. A number of immunological markers have been used over the years to identify astrocytes. Astrocyte intermediate filaments are composed of GFAP and vimentin, a less cell-specific filament protein (Antanitus et al., 1975; Bignami et al., 1972; Dahl et al., 1981). Thus, a positive immunohistochemical reaction for GFAP has often been used as a major criterion for identifying astrocytes. However, GFAP expression cannot be used as the sole criterion for identifying an astrocyte. For example, in early astrocyte development, vimentin can be the major or only intermediate filament expressed (Schnitzer et al., 1981). Furthermore, some gray matter glia with the morphology and ultrastructural characteristics of astrocytes lack intermediate filaments (Herndon, 1964; Palay and Chan-Palay, 1974). Such astrocytes would be GFAP negative. Consistent with this observation, studies on GFAP protein levels and in situ hybridization for GFAP transcripts indicate that GFAP is expressed at lower levels in gray matter than in white matter (Kitamura et al., 1987). Further restraint is recommended in using GFAP as the gold standard for astrocyte identification since some cells that may wander into the brain, such as a subset of lymphocytes, can express GFAP, and GFAP also is expressed by other cells outside of the CNS, such as myoepithelial cells, osteocytes, and chondrocytes (Hainfellner et al., 2001; Neubauer et al., 1996). Consequently, molecular markers other than intermediate filaments have been used to define astrocytes. For example, S100b has long been used as a marker for astrocytes (Haan et al., 1982) and the enzymes aldolase C (Zebrin II) and glutamine synthetase are enriched in astrocytes t(Boyes et al., 1986; Cammer et al., 1989; Norenberg and Martinez-Hernandez, 1979; Staugaitis et al., 2001). However, these proteins also are not specific to astrocytes as S100b is highly expressed by Schwann cells (Barber and Lindsay, 1982) and by some oligodendrocytes (Hachem et al., 2005), glutathione-S-transferase µ is highly expressed by hepatocytes (Warholm et al., 1981) and zebrin II is expressed by Purkinje cells (Hawkes and Herrup, 1995). Thus, even with the availability of well-characterized markers, it can be difficult to posit an astrocyte.
Conclusion
Human radial glial cells have long been known to express GFAP (Levitt et al., 1981). However, the authors of this classic paper did not call these radial glial cells “astrocytes.” Rather, they understood that these radial cells, although sharing some properties with astrocytes were a distinct type of cell. Analogously, the NSCs of the adult SVZ do share several features with astrocytes; however, there are distinct differences that are functionally relevant. Perhaps we are being anti-semantic (a term introduced by Dennis Steindler when discussing this subject), but the evidence to support the conclusion that the NSCs that persist in the adult mammalian SVZ are astrocytes is, in our view, a potentially misleading classification that has been adopted without sufficient examination of the utility of adopting this terminology. From our vantage point, it is much simpler to call the cells that function as NSCs in the SVZ “NSCs” without the associated term “astrocyte.”
Acknowledgements
We thank Drs J. Arellano and A. Duque for their helpful discussions. V.P. is an Honorary Professor at the University of Rijeka, Croatia.
Footnotes
Authors Contribution: All authors had full access to all the data in the study and take responsibility for the accuracy of the data analysis. F.J.V, S.S., V.P., and S.W.L. contributed to conceptualization; F.J.V. and S.W.L. contributed to methodology; F.J.V, S.S., V.P., P.R., and S.W.L. contributed to the investigation; F.J.V, S.S., V.P., P.R., and S.W.L. contributed to formal analysis; F.J.V, S.S., V.P., P.R., and S.W.L. contributed to writing; S.W.L. contributed to supervision; F.J.V, V.P., P.R., and S.W.L. contributed to the funding.
Data Availability: The data that support the findings of this study are available from the corresponding author upon reasonable request.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by the MacBrain Resource, Kavli Institute for Neuroscience at Yale, and an NIH NIDA Merit Award DA023999 to P.R. V.P.'s work is supported by a grant from the National Institute of General Medical Sciences of the NIH (R01GM123971). S.W.L.'s work was supported by a grant from the NINDS NS116828-01.
ORCID iD: Steven W. Levison https://orcid.org/0000-0002-1264-7309
References
- Abbott N. J., Patabendige A. A., Dolman D. E., Yusof S. R., Begley D. J. (2010). Structure and function of the blood–brain barrier. Neurobiology of Disease, 37(1), 13–25. 10.1016/j.nbd.2009.07.030 [DOI] [PubMed] [Google Scholar]
- Abbott N. J., Rönnbäck L., Hansson E. (2006). Astrocyte–endothelial interactions at the blood–brain barrier. Nature Reviews Neuroscience, 7, 41–53. 10.1038/nrn1824 [DOI] [PubMed] [Google Scholar]
- Akter M., Kaneko N., Herranz-Perez V., Nakamura S., Oishi H., Garcia-Verdugo J. M., Sawamoto K. (2020). Dynamic changes in the neurogenic potential in the ventricular-subventricular zone of common marmoset during postnatal brain development. Cerebral Cortex, 30(7), 4092–4109. 10.1093/cercor/bhaa031 [DOI] [PubMed] [Google Scholar]
- Allen E. (1912). Cessation of mitosis in central nervous system of the albino rat. Journal of Comparative Neurology, 22, 547–568. [Google Scholar]
- Andromidas F., Atashpanjeh S., Myers A. J., MacKinnon B. E., Shaffer M. M., Koob A. O. (2021). The astrogenic balance in the aging brain. Current Neuropharmacology, 19(11), 1952–1965. 10.2174/1570159X19666210420095118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antanitus D. S., Choi B. H., Lapham L. W. (1975). Immunofluorescence staining of astrocytes in vitro using antiserum to glial fibrillary acidic protein. Brain Research, 89(2), 363–367. 10.1016/0006-8993(75)90729-5 [DOI] [PubMed] [Google Scholar]
- Araque A., Parpura V., Sanzgiri R. P., Haydon P. G. (1999). Tripartite synapses: Glia, the unacknowledged partner. Trends in Neurosciences, 22(5), 208–215. 10.1016/S0166-2236(98)01349-6 [DOI] [PubMed] [Google Scholar]
- Arellano J. I., Duque A., Rakic P. (2022). Comment on “impact of neurodegenerative diseases on human adult hippocampal neurogenesis”. Science, 376(6590), eabn7083. 10.1126/science.abn7083 [DOI] [PubMed] [Google Scholar]
- Arellano J. I., Morozov Y. M., Micali N., Rakic P. (2021). Radial glial cells: New views on old questions. Neurochemical Research, 46, 2512–2524. 10.1007/s11064-021-03296-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell D., Buchan A. M., Charpak S., Lauritzen M., Macvicar B. A., Newman E. A. (2010). Glial and neuronal control of brain blood flow. Nature, 468, 232–243. 10.1038/nature09613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber P. C., Lindsay R. M. (1982). Schwann cells of the olfactory nerves contain glial fibrillary acidic protein and resemble astrocytes. Neuroscience, 7(12), 3077–3090. 10.1016/0306-4522(82)90231-7 [DOI] [PubMed] [Google Scholar]
- Beckervordersandforth R., Tripathi P., Ninkovic J., Bayam E., Lepier A., Stempfhuber B., Kirchhoff F., Hirrlinger J., Haslinger A., Lie D. C., Beckers J., Yoder B., Irmler M., Gotz M. (2010). In vivo fate mapping and expression analysis reveals molecular hallmarks of prospectively isolated adult neural stem cells. Cell Stem Cell, 7(6), 744–758. 10.1016/j.stem.2010.11.017 [DOI] [PubMed] [Google Scholar]
- Beekman C., Nichane M., De Clercq S., Maetens M., Floss T., Wurst W., Bellefroid E., Marine J. C. (2006). Evolutionarily conserved role of nucleostemin: Controlling proliferation of stem/progenitor cells during early vertebrate development. Molecular and Cellular Biology, 26(24), 9291–9301. 10.1128/MCB.01183-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender A. S., Reichelt W., Norenberg M. D. (2000). Characterization of cystine uptake in cultured astrocytes. Neurochemistry International, 37(2–3), 269–276. 10.1016/S0197-0186(00)00035-8 [DOI] [PubMed] [Google Scholar]
- Benfenati V., Caprini M., Dovizio M., Mylonakou M. N., Ferroni S., Ottersen O. P., Amiry-Moghaddam M. (2011). An aquaporin-4/transient receptor potential vanilloid 4 (AQP4/TRPV4) complex is essential for cell-volume control in astrocytes. Proceedings of the National Academy of Sciences of the United States of America, 108(6), 2563–2568. 10.1073/pnas.1012867108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer F., Ludje W., Karpf J., Saher G., Beckervordersandforth R. (2021). Distribution of Aldh1L1-CreER(T2) recombination in astrocytes versus neural stem cells in the neurogenic niches of the adult mouse brain. Frontiers in Neuroscience, 15, 713077. 10.3389/fnins.2021.713077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bignami A., Eng L. F., Dahl D., Uyeda C. T. (1972). Localization of the glial fibrillary acidic protein in astrocytes by immunofluorescence. Brain Research, 43(2), 429–435. 10.1016/0006-8993(72)90398-8 [DOI] [PubMed] [Google Scholar]
- Blakemore W. F. (1969). The ultrastructure of the subependymal plate in the rat. Journal of Anatomy, 104, 423–433. [PMC free article] [PubMed] [Google Scholar]
- Bordey A., Sontheimer H. (1997). Postnatal development of ionic currents in rat hippocampal astrocytes in situ. Journal of Neurophysiology, 78(1), 461–477. 10.1152/jn.1997.78.1.461 [DOI] [PubMed] [Google Scholar]
- Boyes B. E., Kim S. U., Lee V., Sung S. C. (1986). Immunohistochemical co-localization of S-100b and the glial fibrillary acidic protein in rat brain. Neuroscience, 17(3), 857–865. 10.1016/0306-4522(86)90050-3 [DOI] [PubMed] [Google Scholar]
- Breunig J. J., Haydar T. F., Rakic P. (2011). Neural stem cells: Historical perspective and future prospects. Neuron, 70(4), 614–625. 10.1016/j.neuron.2011.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryans W. A. (1959). Mitotic activity in the brain of the adult rat. Anatomical Record, 133(1), 65–73. 10.1002/ar.1091330108 [DOI] [Google Scholar]
- Buono K. D., Vadlamuri D., Gan Q., Levison S. W. (2012). Leukemia inhibitory factor is essential for subventricular zone neural stem cell and progenitor homeostasis as revealed by a novel flow cytometric analysis. Developmental Neuroscience, 34(5), 449–462. 10.1159/000345155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushong E. A., Martone M. E., Jones Y. Z., Ellisman M. H. (2002). Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. Journal of Neuroscience, 22(1), 183–192. 10.1523/JNEUROSCI.22-01-00183.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bystron I., Blakemore C., Rakic P. (2008). Development of the human cerebral cortex: Boulder Committee revisited. Nature Reviews Neuroscience, 9, 110–122. 10.1038/nrn2252 [DOI] [PubMed] [Google Scholar]
- Camargo N., Goudriaan A., van Deijk A. F., Otte W. M., Brouwers J. F., Lodder H., Gutmann D. H., Nave K. A., Dijkhuizen R. M., Mansvelder H. D., Chrast R., Smit A. B., Verheijen M. H. G. (2017). Oligodendroglial myelination requires astrocyte-derived lipids. PLoS Biology, 15(5), e1002605. 10.1371/journal.pbio.1002605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammer W., Tansey F., Abramovitz M., Ishigaki S., Listowsky I. (1989). Differential localization of glutathione-S-transferase Yp and Yb subunits in oligodendrocytes and astrocytes of rat brain. Journal of Neurochemistry, 52(3), 876–883. 10.1111/j.1471-4159.1989.tb02536.x [DOI] [PubMed] [Google Scholar]
- Christopherson K. S., Ullian E. M., Stokes C. C., Mullowney C. E., Hell J. W., Agah A., Lawler J., Mosher D. F., Bornstein P., Barres B. A. (2005). Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell, 120(3), 421–433. 10.1016/j.cell.2004.12.020 [DOI] [PubMed] [Google Scholar]
- Clarke L. E., Barres B. A. (2013). Emerging roles of astrocytes in neural circuit development. Nature Reviews Neuroscience, 14, 311–321. 10.1038/nrn3484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl D., Bignami A., Weber K., Osborn M. (1981). Filament proteins in rat optic nerves undergoing Wallerian degeneration: Localization of vimentin, the fibroblastic 100-A filament protein, in Normal and reactive astrocytes. Experimental Neurology, 73(2), 496–506. 10.1016/0014-4886(81)90283-1 [DOI] [PubMed] [Google Scholar]
- Doetsch F., Caille I., Lim D. A., Garcia-Verdugo J. M., Alvarez-Buylla A. (1999b). Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell, 97(6), 703–716. 10.1016/S0092-8674(00)80783-7 [DOI] [PubMed] [Google Scholar]
- Doetsch F., Garcia-Verdugo J. M., Alvarez-Buylla A. (1997). Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. Journal of Neuroscience, 17(13), 5046–5061. 10.1523/JNEUROSCI.17-13-05046.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F., Garcia-Verdugo J. M., Alvarez-Buylla A. (1999a). Regeneration of a germinal layer in the adult mammalian brain. Proceedings of the National Academy of Science USA, 96(20), 11619–11624. 10.1073/pnas.96.20.11619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulken B. W., Leeman D. S., Boutet S. C., Hebestreit K., Brunet A. (2017). Single-cell transcriptomic analysis defines heterogeneity and transcriptional dynamics in the adult neural stem cell lineage. Cell Reports, 18(3), 777–790. 10.1016/j.celrep.2016.12.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque A., Arellano J. I., Rakic P. (2022). An assessment of the existence of adult neurogenesis in humans and value of its rodent models for neuropsychiatric diseases. Molecular Psychiatry, 27, 377–382. 10.1038/s41380-021-01314-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque A., Spector R. (2019). A balanced evaluation of the evidence for adult neurogenesis in humans: Implication for neuropsychiatric disorders. Brain Structure & Function, 224, 2281–2295. 10.1007/s00429-019-01917-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eroglu C., Allen N. J., Susman M. W., O’Rourke N. A., Park C. Y., Ozkan E., Chakraborty C., Mulinyawe S. B., Annis D. S., Huberman A. D., Green E. M., Lawler J., Dolmetsch R., Garcia K. C., Smith S. J., Luo Z. D., Rosenthal A., Mosher D. F., Barres B. A. (2009). Gabapentin receptor alpha2delta-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell, 139(2), 380–392. 10.1016/j.cell.2009.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eroglu C., Barres B. A. (2010). Regulation of synaptic connectivity by glia. Nature, 468, 223–231. 10.1038/nature09612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escartin C., et al. (2021). Reactive astrocyte nomenclature, definitions, and future directions. Nature Neuroscience, 24, 312–325. 10.1038/s41593-020-00783-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedoroff S., Vernadakis A. (1986). Astrocytes. Academic Press. [Google Scholar]
- Franjic D., et al. (2022). Transcriptomic taxonomy and neurogenic trajectories of adult human, macaque, and pig hippocampal and entorhinal cells. Neuron, 110(3), 452–469 e414. 10.1016/j.neuron.2021.10.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta A., Rothstein J. D., Martin L. J. (1997). Glutamate transporter protein subtypes are expressed differentially during rat CNS development. Journal of Neuroscience, 17(21), 8363–8375. 10.1523/JNEUROSCI.17-21-08363.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gengatharan A., Bammann R. R., Saghatelyan A. (2016). The role of astrocytes in the generation, migration, and integration of new neurons in the adult olfactory bulb. Frontiers in Neuroscience, 10, 149. 10.3389/fnins.2016.00149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georg Kuhn H., Blomgren K. (2011). Developmental dysregulation of adult neurogenesis. European Journal of Neuroscience, 33(6), 1115–1122. 10.1111/j.1460-9568.2011.07610.x [DOI] [PubMed] [Google Scholar]
- Giachino C., Basak O., Lugert S., Knuckles P., Obernier K., Fiorelli R., Frank S., Raineteau O., Alvarez-Buylla A., Taylor V. (2014). Molecular diversity subdivides the adult forebrain neural stem cell population. Stem Cells, 32(1), 70–84. 10.1002/stem.1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Perez O., Quiñones-Hinojosa A. (2012). Astrocytes as neural stem cells in the adult brain. Journal of Stem Cells, 7, 181–188. https://doi.org.jsc.2012.7.3.181 [PMC free article] [PubMed] [Google Scholar]
- Gubert F., Zaverucha-do-Valle C., Pimentel-Coelho P. M., Mendez-Otero R., Santiago M. F. (2009). Radial glia-like cells persist in the adult rat brain. Brain Research, 1258, 43–52. 10.1016/j.brainres.2008.12.021 [DOI] [PubMed] [Google Scholar]
- Guo J. U., Su Y., Zhong C., Ming G. L., Song H. (2011). Hydroxylation of 5-methylcytosine by TET1 promotes active DNA demethylation in the adult brain. Cell, 145(3), 423–434. 10.1016/j.cell.2011.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haan E. A., Boss B. D., Cowan W. M. (1982). Production and characterization of monoclonal antibodies against the “brain-specific” proteins 14-3-2 and S-100. Proceedings of the National Academy of Sciences of the United States of America, 79(23), 7585–7589. 10.1073/pnas.79.23.7585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachem S., Aguirre A., Vives V., Marks A., Gallo V., Legraverend C. (2005). Spatial and temporal expression of S100B in cells of oligodendrocyte lineage. Glia, 51(2), 81–97. 10.1002/glia.20184 [DOI] [PubMed] [Google Scholar]
- Hachem S., Laurenson A. S., Hugnot J. P., Legraverend C. (2007). Expression of S100B during embryonic development of the mouse cerebellum. BMC Developmental Biology, 7, 17. 10.1186/1471-213X-7-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hainfellner J. A., Voigtlander T., Strobel T., Mazal P. R., Maddalena A. S., Aguzzi A., Budka H. (2001). Fibroblasts can express glial fibrillary acidic protein (GFAP) in vivo. Journal of Neuropathology & Experimental Neurology, 60(5), 449–461. 10.1093/jnen/60.5.449 [DOI] [PubMed] [Google Scholar]
- Halassa M. M., Fellin T., Haydon P. G. (2007). The tripartite synapse: Roles for gliotransmission in health and disease. Trends in Molecular Medicine, 13(2), 54–63. 10.1016/j.molmed.2006.12.005 [DOI] [PubMed] [Google Scholar]
- Hawkes R., Herrup K. (1995). Aldolase C/zebrin II and the regionalization of the cerebellum. Journal of Molecular Neuroscience, 6, 147–158. 10.1007/BF02736761 [DOI] [PubMed] [Google Scholar]
- Haymaker W., Adams R. D. (1982). Histology and histopathology of the nervous system. Charles Thomas. [Google Scholar]
- Herndon R. M. (1964). The fine structure of the rat cerebellum. II. The stellate neurons, granule cells and glia. Journal of Cell Biology, 23(2), 277–293. 10.1083/jcb.23.2.277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C., Nedergaard M. (2007). Glial regulation of the cerebral microvasculature. Nature Neuroscience, 10, 1369–1376. 10.1038/nn2003 [DOI] [PubMed] [Google Scholar]
- Iliff J. J., Lee H., Yu M., Feng T., Logan J., Nedergaard M., Benveniste H. (2013). Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. Journal of Clinical Investigation, 123(3), 1299–1309. 10.1172/JCI67677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliff J. J., Nedergaard M. (2013). Is there a cerebral lymphatic system? Stroke, 44(6_suppl_1), S93–S95. 10.1161/STROKEAHA.112.678698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imura T., Nakano I., Kornblum H. I., Sofroniew M. V. (2006). Phenotypic and functional heterogeneity of GFAP-expressing cells in vitro: Differential expression of LeX/CD15 by GFAP-expressing multipotent neural stem cells and non-neurogenic astrocytes. Glia, 53(3), 277–293. 10.1002/glia.20281 [DOI] [PubMed] [Google Scholar]
- Kettenmann H., Ransom B. R. (eds) (2013). Neuroglia. Oxford University Press. [Google Scholar]
- Kimelberg H. K., Nedergaard M. (2010). Functions of astrocytes and their potential as therapeutic targets. Neurotherapeutics, 7, 338–353. 10.1016/j.nurt.2010.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirischuk S., Parpura V., Verkhratsky A. (2012). Sodium dynamics: Another key to astroglial excitability? Trends in Neurosciences, 35(8), 497–506. 10.1016/j.tins.2012.04.003 [DOI] [PubMed] [Google Scholar]
- Kirschenbaum B., Nedergaard M., Preuss A., Barami K., Fraser R. A., Goldman S. A. (1994). In vitro neuronal production and differentiation by precursor cells derived from the adult human forebrain. Cerebral Cortex, 4(6), 576–589. 10.1093/cercor/4.6.576 [DOI] [PubMed] [Google Scholar]
- Kitamura T., Nakanishi K., Watanabe S., Endo Y., Fujita S. (1987). GFA-protein gene expression on the astrocyte in cow and rat brains. Brain Research, 423(1–2), 189–195. 10.1016/0006-8993(87)90839-0 [DOI] [PubMed] [Google Scholar]
- Kuan C. Y., Schloemer A. J., Lu A., Burns K. A., Weng W. L., Williams M. T., Strauss K. I., Vorhees C. V., Flavell R. A., Davis R. J., Sharp F. R., Rakic P. (2004). Hypoxia-ischemia induces DNA synthesis without cell proliferation in dying neurons in adult rodent brain. Journal of Neuroscience, 24(47), 10763–10772. 10.1523/JNEUROSCI.3883-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucheryavykh Y. V., Kucheryavykh L. Y., Nichols C. G., Maldonado H. M., Baksi K., Reichenbach A., Skatchkov S. N., Eaton M. J. (2007). Downregulation of Kir4.1 inward rectifying potassium channel subunits by RNAi impairs potassium transfer and glutamate uptake by cultured cortical astrocytes. Glia, 55(3), 274–281. 10.1002/glia.20455 [DOI] [PubMed] [Google Scholar]
- Kucukdereli H., Allen N. J., Lee A. T., Feng A., Ozlu M. I., Conatser L. M., Chakraborty C., Workman G., Weaver M., Sage E. H., Barres B. A., Eroglu C. (2011). Control of excitatory CNS synaptogenesis by astrocyte-secreted proteins Hevin and SPARC. Proceedings of the National Academy of Sciences of the United States of America, 108, E440–E449. 10.1073/pnas.1104977108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugler P., Schleyer V. (2004). Developmental expression of glutamate transporters and glutamate dehydrogenase in astrocytes of the postnatal rat hippocampus. Hippocampus, 14(8), 975–985. 10.1002/hipo.20015 [DOI] [PubMed] [Google Scholar]
- Kukekov V. G., Laywell E. D., Suslov O., Davies K., Scheffler B., Thomas L. B., O'Brien T. F., Kusakabe M., Steindler D. A. (1999). Multipotent stem/progenitor cells with similar properties arise from two neurogenic regions of adult human brain. Experimental Neurology, 156(2), 333–344. 10.1006/exnr.1999.7028 [DOI] [PubMed] [Google Scholar]
- Kunzelmann P., Schroder W., Traub O., Steinhauser C., Dermietzel R., Willecke K. (1999). Late onset and increasing expression of the gap junction protein connexin30 in adult murine brain and long-term cultured astrocytes. Glia, 25(2), 111–119. [DOI] [PubMed] [Google Scholar]
- Lacar B., Herman P., Hartman N. W., Hyder F., Bordey A. (2012a). S phase entry of neural progenitor cells correlates with increased blood flow in the young subventricular zone. PLoS One, 7(2), e31960. 10.1371/journal.pone.0031960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacar B., Herman P., Platel J. C., Kubera C., Hyder F., Bordey A. (2012b). Neural progenitor cells regulate capillary blood flow in the postnatal subventricular zone. Journal of Neuroscience, 32(46), 16435–16448. 10.1523/JNEUROSCI.1457-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalo U., Pankratov Y., Kirchhoff F., North R. A., Verkhratsky A. (2006). NMDA Receptors mediate neuron-to-glia signaling in mouse cortical astrocytes. Journal of Neuroscience, 26(10), 2673–2683. 10.1523/JNEUROSCI.4689-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. H., Lee J. E., Kahng J. Y., Kim S. H., Park J. S., Yoon S. J., Um J. Y., Kim W. K., Lee J. K., Park J., Kim E. H., Lee J. H., Lee J. H., Chung W. S., Ju Y. S., Park S. H., Chang J. H., Kang S. G., Lee J. H. (2018). Human glioblastoma arises from subventricular zone cells with low-level driver mutations. Nature, 560, 243–247. 10.1038/s41586-018-0389-3 [DOI] [PubMed] [Google Scholar]
- Levitt P., Cooper M. L., Rakic P. (1981). Coexistence of neuronal and glial precursor cells in the cerebral ventricular zone of the fetal monkey: An ultrastructural immunoperoxidase analysis. Journal of Neuroscience, 1(1), 27–39. 10.1523/JNEUROSCI.01-01-00027.1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Trupiano M. X., Simon J., Guo J., Anton E. S. (2021). The essential role of primary cilia in cerebral cortical development and disorders. Current Topics in Developmental Biology, 142, 99–146. 10.1016/bs.ctdb.2020.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Bolteus A. J., Balkin D. M., Henschel O., Bordey A. (2006). GFAP-expressing cells in the postnatal subventricular zone display a unique glial phenotype intermediate between radial glia and astrocytes. Glia, 54(5), 394–410. 10.1002/glia.20392 [DOI] [PubMed] [Google Scholar]
- Liu X., Wang Q., Haydar T. F., Bordey A. (2005). Nonsynaptic GABA signaling in postnatal subventricular zone controls proliferation of GFAP-expressing progenitors. Nature Neuroscience, 8, 1179–1187. 10.1038/nn1522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorens-Bobadilla E., Zhao S., Baser A., Saiz-Castro G., Zwadlo K., Martin-Villalba A. (2015). Single-Cell transcriptomics reveals a population of dormant neural stem cells that become activated upon brain injury. Cell Stem Cell, 17(3), 329–340. 10.1016/j.stem.2015.07.002 [DOI] [PubMed] [Google Scholar]
- Magnusson J. P., Zamboni M., Santopolo G., Mold J. E., Barrientos-Somarribas M., Talavera-Lopez C., Andersson B., Frisén J. (2020). Activation of a neural stem cell transcriptional program in parenchymal astrocytes. eLife, 9, e59733. 10.7554/eLife.59733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malarkey E. B., Parpura V. (2008). Mechanisms of glutamate release from astrocytes. Neurochemistry International, 52(1–2), 142–154. 10.1016/j.neuint.2007.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkle F. T., Fuentealba L. C., Sanders T. A., Magno L., Kessaris N., Alvarez-Buylla A. (2014). Adult neural stem cells in distinct microdomains generate previously unknown interneuron types. Nature Neuroscience, 17, 207–214. 10.1038/nn.3610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizrak D., Bayin N. S., Yuan J., Liu Z., Suciu R. M., Niphakis M. J., Ngo N., Lum K. M., Cravatt B. F., Joyner A. L., Sims P. A. (2020). Single-Cell profiling and SCOPE-seq reveal lineage dynamics of adult ventricular-subventricular zone neurogenesis and NOTUM as a key regulator. Cell Reports, 31(12), 107805. 10.1016/j.celrep.2020.107805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizrak D., Levitin H. M., Delgado A. C., Crotet V., Yuan J., Chaker Z., Silva-Vargas V., Sims P. A., Doetsch F. (2019). Single-Cell analysis of regional differences in adult V-SVZ neural stem cell lineages. Cell Reports, 26(2), 394–406 e395. 10.1016/j.celrep.2018.12.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mölders A., Koch A., Menke R., Klöcker N. (2018). Heterogeneity of the astrocytic AMPA-receptor transcriptome. Glia, 66(12), 2604–2616. 10.1002/glia.23514 [DOI] [PubMed] [Google Scholar]
- Montana V., Flint D., Waagepetersen H. S., Schousboe A., Parpura V. (2021). Two metabolic fuels, glucose and lactate, differentially modulate exocytotic glutamate release from cultured astrocytes. Neurochemical Research, 46, 2551–2579. 10.1007/s11064-021-03340-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moossy J. (1982). Histology and histopathology of the nervous system. Journal of Neuropathology & Experimental Neurology, 41(5), 560–561. 10.1097/00005072-198209000-00010 [DOI] [Google Scholar]
- Nagy J. I., Li X., Rempel J., Stelmack G., Patel D., Staines W. A., Yasumura T., Rash J. E. (2001). Connexin26 in adult rodent central nervous system: Demonstration at astrocytic gap junctions and colocalization with connexin30 and connexin43. Journal of Comparative Neurology, 441(4), 302–323. 10.1002/cne.1414 [DOI] [PubMed] [Google Scholar]
- Nait-Oumesmar B., Picard-Riera N., Kerninon C., Decker L., Seilhean D., Hoglinger G. U., Hirsch E. C., Reynolds R., Baron-Van Evercooren A. (2007). Activation of the subventricular zone in multiple sclerosis: Evidence for early glial progenitors. Proceedings of the National Academy of Sciences of the United States of America, 104(11), 4694–4699. 10.1073/pnas.0606835104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima-Koyama M., Lee J., Ohta S., Yamamoto T., Nishida E. (2015). Induction of pluripotency in astrocytes through a neural stem cell-like state. Journal of Biological Chemistry, 290(52), 31173–31188. 10.1074/jbc.M115.683466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarrete M., Perea G., Maglio L., Pastor J., Garcia de Sola R., Araque A. (2013). Astrocyte calcium signal and gliotransmission in human brain tissue. Cerebral Cortex, 23(5), 1240–1246. 10.1093/cercor/bhs122 [DOI] [PubMed] [Google Scholar]
- Nedergaard M., Ransom B., Goldman S. A. (2003). New roles for astrocytes: Redefining the functional architecture of the brain. Trends in Neurosciences, 26(10), 523–530. 10.1016/j.tins.2003.08.008 [DOI] [PubMed] [Google Scholar]
- Nedergaard M., Verkhratsky A. (2012). Artifact versus reality–how astrocytes contribute to synaptic events. Glia, 60(7), 1013–1023. 10.1002/glia.22288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubauer K., Knittel T., Aurisch S., Fellmer P., Ramadori G. (1996). Glial fibrillary acidic protein—a cell type specific marker for Ito cells in vivo and in vitro. Journal of Hepatology, 24(6), 719–730. 10.1016/S0168-8278(96)80269-8 [DOI] [PubMed] [Google Scholar]
- Norenberg M. D., Martinez-Hernandez A. (1979). Fine structural localization of glutamine synthetase in astrocytes of rat brain. Brain Research, 161(2), 303–310. 10.1016/0006-8993(79)90071-4 [DOI] [PubMed] [Google Scholar]
- Nwaobi S. E., Cuddapah V. A., Patterson K. C., Randolph A. C., Olsen M. L. (2016). The role of glial-specific Kir4.1 in Normal and pathological states of the CNS. Acta Neuropathologica, 132, 1–21. 10.1007/s00401-016-1553-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberheim N. A., Goldman S. A., Nedergaard M. (2012). Heterogeneity of astrocytic form and function. In Milner R. (Ed.) Methods in molecular biology (vol. 814, pp. 23–45). Humana Press. 10.1007/978-1-61779-452-0_3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palay S. L., Chan-Palay V. (1974). Cerebellar cortex, cytology, and organization (1st ed.). Springer-Verlag. [Google Scholar]
- Paredes M. F., James D., Gil-Perotin S., Kim H., Cotter J. A., Ng C., Sandoval K., Rowitch D. H., Xu D., McQuillen P. S., Garcia-Verdugo J. M., Huang E. J., Alvarez-Buylla A. (2016). Extensive migration of young neurons into the infant human frontal lobe. Science, 354(6308), aaf7073. 10.1126/science.aaf7073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parpura V., Heneka M. T., Montana V., Oliet S. H., Schousboe A., Haydon P. G., Stout R. F., Jr., Spray D. C., Reichenbach A., Pannicke T., Pekny M., Pekna M., Zorec R., Verkhratsky A. (2012). Glial cells in (patho)physiology. Journal of Neurochemistry, 121(1), 4–27. 10.1111/j.1471-4159.2012.07664.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parpura V., Verkhratsky A. (2012a). The astrocyte excitability brief: From receptors to gliotransmission. Neurochemistry International, 61(4), 610–621. 10.1016/j.neuint.2011.12.001 [DOI] [PubMed] [Google Scholar]
- Parpura V., Verkhratsky A. (2012b). Astrocytes revisited: Concise historic outlook on glutamate homeostasis and signaling. Croatian Medical Journal, 53(6), 518–528. 10.3325/cmj.2012.53.518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parpura V., Verkhratsky A. (2012d). Homeostatic function of astrocytes: Ca2+ and Na+ signalling. Translational Neuroscience, 3(4), 334–344. 10.2478/s13380-012-0040-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parpura V., Verkhratsky A. (2012c). Neuroglia at the crossroads of homoeostasis, metabolism and signalling: Evolution of the concept. ASN Neuro, 4(4), 201–205. 10.1042/AN20120019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastrana E., Cheng L. C., Doetsch F. (2009). Simultaneous prospective purification of adult subventricular zone neural stem cells and their progeny. Proceedings of the National Academy of Sciences of the United States of America, 106(15), 6387–6392. 10.1073/pnas.0810407106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platel J. C., Bordey A. (2016). The multifaceted subventricular zone astrocyte: From a metabolic and pro-neurogenic role to acting as a neural stem cell. Neuroscience, 323, 20–28. 10.1016/j.neuroscience.2015.10.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platel J. C., Dave K. A., Gordon V., Lacar B., Rubio M. E., Bordey A. (2010). NMDA Receptors activated by subventricular zone astrocytic glutamate are critical for neuroblast survival prior to entering a synaptic network. Neuron, 65(6), 859–872. 10.1016/j.neuron.2010.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin S., Huang X., Wang D., Hu X., Yuan Y., Sun X., Tan Z., Gu Y., Cheng X., He C., Su Z. (2019). Identification of characteristic genes distinguishing neural stem cells from astrocytes. Gene, 681, 26–35. 10.1016/j.gene.2018.09.044 [DOI] [PubMed] [Google Scholar]
- Rakic P. (1985). Limits of neurogenesis in primates. Science, 227(4690), 1054–1056. 10.1126/science.3975601 [DOI] [PubMed] [Google Scholar]
- Rakic P. (1988). Specification of cerebral cortical areas. Science, 241(4862), 170–176. 10.1126/science.3291116 [DOI] [PubMed] [Google Scholar]
- Rakic P. (2009). Evolution of the neocortex: A perspective from developmental biology. Nature Reviews Neuroscience, 10, 724–735. 10.1038/nrn2719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds B. A., Tetzlaff W., Weiss S. (1992). A multipotent EGF-responsive striatal embryonic progenitor cell produces neurons and astrocytes. Journal of Neuroscience, 12(11), 4565–4574. 10.1523/JNEUROSCI.12-11-04565.1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein J. D., Martin L., Levey A. I., Dykes-Hoberg M., Jin L., Wu D., Nash N., Kuncl R. W. (1994). Localization of neuronal and glial glutamate transporters. Neuron, 13, 713–725. 10.1016/0896-6273(94)90038-8 [DOI] [PubMed] [Google Scholar]
- Sanai N., Nguyen T., Ihrie R. A., Mirzadeh Z., Tsai H. H., Wong M., Gupta N., Berger M. S., Huang E., Garcia-Verdugo J. M., Rowitch D. H., Alvarez-Buylla A. (2011). Corridors of migrating neurons in the human brain and their decline during infancy. Nature, 478, 382–386. 10.1038/nature10487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaberg E., Götz M., Faissner A. (2022). The extracellular matrix molecule tenascin-C modulates cell cycle progression and motility of adult neural stem/progenitor cells from the subependymal zone. Cellular and Molecular Life Sciences, 79, 244. 10.1007/s00018-022-04259-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmechel D. E., Rakic P. (1979). A Golgi study of radial glial cells in developing monkey telencephalon: Morphogenesis and transformation into astrocytes. Anatomy and Embryology, 156, 115–152. 10.1007/BF00300010 [DOI] [PubMed] [Google Scholar]
- Schmidt C., Kunemund V., Wintergerst E. S., Schmitz B., Schachner M. (1996). CD9 of mouse brain is implicated in neurite outgrowth and cell migration in vitro and is associated with the alpha 6/beta 1 integrin and the neural adhesion molecule L1. Journal of Neuroscience Research, 43(1), 12–31. 10.1002/jnr.490430103 [DOI] [PubMed] [Google Scholar]
- Schnitzer J., Franke W. W., Schachner M. (1981). Immunocytochemical demonstration of vimentin in astrocytes and ependymal cells of developing and adult mouse nervous system. Journal of Cell Biology, 90(2), 435–447. 10.1083/jcb.90.2.435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears S. M. S., Roberts S. H., Hewett S. J. (2021). Hyperexcitability and brain morphological differences in mice lacking the cystine/glutamate antiporter, system xc−. Journal of Neuroscience Research, 99(12), 3339–3353. 10.1002/jnr.24971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata T., Yamada K., Watanabe M., Ikenaka K., Wada K., Tanaka K., Inoue Y. (1997). Glutamate transporter GLAST is expressed in the radial glia-astrocyte lineage of developing mouse spinal cord. Journal of Neuroscience, 17(23), 9212–9219. 10.1523/JNEUROSCI.17-23-09212.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart I. H. (1961). The subependymal layer of the mouse brain and its cellular production as shown by radioautography after thymidine-3 h injection. Journal of Comparative Neurology, 116(3), 325–349. 10.1002/cne.901160306 [DOI] [Google Scholar]
- Sorrells S. F., Paredes M. F., Zhang Z., Kang G., Pastor-Alonso O., Biagiotti S., Page C. E., Sandoval K., Knox A., Connolly A., Huang E. J., Garcia-Verdugo J. M., Oldham M. C., Yang Z., Alvarez-Buylla A. (2021). Positive controls in adults and children support that very few, if any, new neurons are born in the adult human hippocampus. Journal of Neuroscience, 41(12), 2554–2565. 10.1523/JNEUROSCI.0676-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staugaitis S. M., Zerlin M., Hawkes R., Levine J. M., Goldman J. E. (2001). Aldolase C/zebrin II expression in the neonatal rat forebrain reveals cellular heterogeneity within the subventricular zone and early astrocyte differentiation. Journal of Neuroscience, 21(16), 6195–6205. 10.1523/JNEUROSCI.21-16-06195.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundholm-Peters N. L., Yang H. K., Goings G. E., Walker A. S., Szele F. G. (2004). Radial glia-like cells at the base of the lateral ventricles in adult mice. Journal of Neurocytology, 33(1), 153–164. 10.1023/B:NEUR.0000029654.70632.3a [DOI] [PubMed] [Google Scholar]
- Tavazoie M., Van der Veken L., Silva-Vargas V., Louissaint M., Colonna L., Zaidi B., Garcia-Verdugo J. M., Doetsch F. (2008). A specialized vascular niche for adult neural stem cells. Cell Stem Cell, 3(3), 279–288. 10.1016/j.stem.2008.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tien A. C., Tsai H. H., Molofsky A. V., McMahon M., Foo L. C., Kaul A., Dougherty J. D., Heintz N., Gutmann D. H., Barres B. A., Rowitch D. H. (2012). Regulated temporal-spatial astrocyte precursor cell proliferation involves BRAF signalling in mammalian spinal cord. Development, 139(14), 2477–2487. 10.1242/dev.077214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai R. Y., McKay R. D. (2002). A nucleolar mechanism controlling cell proliferation in stem cells and cancer cells. Genes & Development, 16, 2991–3003. 10.1101/gad.55671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban N., Blomfield I. M., Guillemot F. (2019). Quiescence of adult mammalian neural stem cells: A highly regulated rest. Neuron, 104(5), 834–848. 10.1016/j.neuron.2019.09.026 [DOI] [PubMed] [Google Scholar]
- Verkhratsky A., Matteoli M., Parpura V., Mothet J. P., Zorec R. (2016). Astrocytes as secretory cells of the central nervous system: Idiosyncrasies of vesicular secretion. Embo Journal, 35(3), 239–257. 10.15252/embj.201592705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A., Parpura V. (2015). Physiology of astroglia: Channels, receptors, transporters, ion signaling and gliotransmission. Colloquium Digital Library of Life Sciences: Morgan & Claypool Publishers. [Google Scholar]
- Verkhratsky A., Rodriguez J. J., Parpura V. (2012a). Calcium signalling in astroglia. Molecular and Cellular Endocrinology, 353(1–2), 45–56. 10.1016/j.mce.2011.08.039 [DOI] [PubMed] [Google Scholar]
- Verkhratsky A., Rodriguez J. J., Parpura V. (2012b). Neurotransmitters and integration in neuronal-astroglial networks. Neurochemical Research, 37, 2326–2338. 10.1007/s11064-012-0765-6 [DOI] [PubMed] [Google Scholar]
- Warholm M., Guthenberg C., Mannervik B., von Bahr C. (1981). Purification of a new glutathione S-transferase (transferase mu) from human liver having high activity with benzo(alpha)pyrene-4,5-oxide. Biochemical and Biophysical Research Communications, 98(2), 512–519. 10.1016/0006-291X(81)90870-6 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Barres B. A. (2010). Astrocyte heterogeneity: An underappreciated topic in neurobiology. Current Opinion in Neurobiology, 20(5), 588–594. 10.1016/j.conb.2010.06.005 [DOI] [PubMed] [Google Scholar]
- Zorec R., Verkhratsky A., Rodriguez J. J., Parpura V. (2016). Astrocytic vesicles and gliotransmitters: Slowness of vesicular release and synaptobrevin2-laden vesicle nanoarchitecture. Neuroscience, 323, 67–75. 10.1016/j.neuroscience.2015.02.033 [DOI] [PubMed] [Google Scholar]
- Zywitza V., Misios A., Bunatyan L., Willnow T. E., Rajewsky N. (2018). Single-Cell transcriptomics characterizes cell types in the subventricular zone and uncovers molecular defects impairing adult neurogenesis. Cell Reports, 25(9), 2457–2469 e2458. 10.1016/j.celrep.2018.11.003 [DOI] [PubMed] [Google Scholar]