Abstract
Objectives
Humoral vaccine responses to SARS-CoV-2 vaccines are impaired and short lasting in patients with immune-mediated inflammatory diseases (IMID) following two vaccine doses. To protect these vulnerable patients against severe COVID-19 disease, a three-dose primary vaccination strategy has been implemented in many countries. The aim of this study was to evaluate humoral response and safety of primary vaccination with three doses in patients with IMID.
Methods
Patients with IMID on immunosuppressive therapy and healthy controls receiving three-dose and two-dose primary SARS-CoV-2 vaccination, respectively, were included in this prospective observational cohort study. Anti-Spike antibodies were assessed 2–4 weeks, and 12 weeks following each dose. The main outcome was anti-Spike antibody levels 2–4 weeks following three doses in patients with IMID and two doses in controls. Additional outcomes were the antibody decline rate and adverse events.
Results
1100 patients and 303 controls were included. Following three-dose vaccination, patients achieved median (IQR) antibody levels of 5720 BAU/mL (2138–8732) compared with 4495 (1591–6639) in controls receiving two doses, p=0.27. Anti-Spike antibody levels increased with median 1932 BAU/mL (IQR 150–4978) after the third dose. The interval between the vaccine doses and vaccination with mRNA-1273 or a combination of vaccines were associated with antibody levels following the third dose. Antibody levels had a slower decline-rate following the third than the second vaccine dose, p<0.001. Adverse events were reported by 464 (47%) patients and by 196 (78%) controls. Disease flares were reported by 70 (7%) patients.
Conclusions
This study shows that additional vaccine doses to patients with IMID contribute to strong and sustained immune-responses comparable to healthy persons vaccinated twice, and supports repeated vaccination of patients with IMID.
Trial registration number
Keywords: COVID-19, vaccination, antirheumatic agents, autoimmune diseases
WHAT IS ALREADY KNOWN ON THIS TOPIC
Patients with immune-mediated inflammatory diseases (IMID) have impaired and short-lasting humoral vaccine responses to SARS-CoV-2 vaccines, and concerns have thus been raised regarding their protection against severe COVID-19 disease.
Knowledge regarding efficacy and safety of repeated vaccination in this large patient group is limited.
WHAT THIS STUDY ADDS
A third vaccine dose as primary vaccination to patients with IMID resulted in strong humoral responses comparable to healthy controls vaccinated twice, was safe and resulted in a slower decline in antibody level postvaccination.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This study supports repeated vaccination of patients with IMID to ensure a stronger and more durable humoral immune response.
Introduction
Efficient vaccines against COVID-19, as seen in the general population, are pivotal in the management of the ongoing SARS-CoV-2 pandemic.1 2 The degree of protection against COVID-19 correlates with the humoral vaccine response assessed by anti-Spike antibody levels, and patients with immune-mediated inflammatory diseases (IMIDs) on immunosuppressive therapies have demonstrated an impaired humoral response after standard two-dose vaccination.3–7 Concerns have thus been raised regarding how to optimise the protection from severe COVID-19 disease in vulnerable patients.
IMIDs comprises several prevalent chronic diseases including rheumatoid arthritis (RA), spondyloarthritis (SpA), psoriatic arthritis (PsA), ulcerative colitis (UC) and Crohn’s disease (CD). Although this is a heterogeneous group, these diseases have similar disease features and are treated with many of the same immunosuppressive medications such as tumour necrosis factor inhibitors (TNFi), non-TNFi biologics, metabolite inhibitors and targeted small molecule drugs.8 Due to a dysregulated immune system, use of immunosuppressive therapies and increased frequency of several comorbidities, these patients are vulnerable to severe outcomes of infectious diseases as well as adverse events (AEs) to vaccines including the risk of disease flare.6 9–14
Neutralising antibodies can efficiently block viral entry into host cells. Exact cut-offs have not been established, but high antibody levels are needed for efficient prevention of symptomatic disease.4 Protection against novel virus strains probably requires higher levels.15 It has become clear that the vaccine response is highly attenuated over time, with declining anti-Spike antibody levels corresponding to a reduction in protection against symptomatic disease.16–18 Thus, a need for additional vaccine doses to reactivate antibody production and keep this population protected has been argued.19–21
Two-dose primary vaccination with an additional booster dose administrated after some months is at present the most common vaccination strategy, but many countries recommend a three-dose primary vaccination regimen for immunocompromised patients. It is still not clear whether repeated vaccine delivery sufficiently can augment immunity in patients with an impaired humoral response, or whether the vaccine type plays a role. The efficacy and safety of repeated vaccinations in this large patient group remains largely unknown.22–25
The main aim of this study was to assess whether repeated vaccination by a three-dose primary vaccination strategy could raise serological responses in patients with IMIDs on immunosuppressive therapy, and also to assess the safety of repeated vaccination in relation to potential increases in disease flares and AEs. To this end, we compared the three-dose SARS-CoV-2 vaccination strategy in patients with IMID on immunosuppressive therapy with standard two-dose vaccination of healthy controls.
Methods
Participants, setting and study design
The prospective, observational Norwegian study of vaccine response to COVID-19 vaccines (Nor-vaC) is an ongoing longitudinal observational study conducted at two Norwegian IMID centres; the Division of Rheumatology at Diakonhjemmet Hospital (DH) and the Department of Gastroenterology at Akershus University Hospital (AHUS).7 Adult patients (aged ≥18 years) with RA, SpA, PsA, UC or CD who used any of the relevant immunosuppressive medications (online supplemental appendix 1) were identified by the hospital records and consecutively recruited into the study prior to the initiation of the national vaccination programme in February 2021. Healthcare workers from DH, AHUS and Oslo University Hospital (OUH) were recruited as healthy controls (online supplemental appendix 2). The study is registered at ClinialTrials.gov NCT04798625.
rmdopen-2022-002417supp001.pdf (371KB, pdf)
All participants received standard vaccines according to the national vaccination programme administered by the Norwegian Institute of Public Health with a three-dose regimen in patients and a two-dose regimen in controls. Three SARS-CoV-2 vaccine types were initially available: BNT162b2 (Pfizer), mRNA-1273 (Moderna) and ChAdOx1(Astra Zeneca). The ChAdOx1 vaccine was withdrawn from the Norwegian vaccination programme in March 2021. Patients with IMID were offered a third vaccine dose >4 weeks after the second dose. Persons subjected to a COVID-19 infection did not receive a third vaccine dose, and were not included in the main analyses of this paper.
Assessments
Patients and controls were asked to provide serum samples at a regular basis throughout the study: prior to the first vaccine dose, 2–4 weeks, and 12 weeks following each vaccine dose. For the present analyses, we included patients and healthy controls who provided blood for serological testing 2–4 weeks after the second—and third vaccine dose, respectively (online supplemental figure 1).
Assessments of immunogenicity were performed at the Department of Immunology at OUH. Antibodies to the receptor binding domain at the full-length spike protein were assessed by using an in-house bead-based method which is validated against a microneutralisation assay.26
At DH, data were collected by questionnaires created with nettskjema.no, a survey solution developed and hosted by the University of Oslo where encrypted data are sent to Services for Sensitive Data (TSD) for storage. At AHUS, data collection was handled by Viedoc, V.4 (Sweden). Demographic data were collected at baseline only, while medication use (including pausing of drugs prevaccination and/or postvaccination), patient-reported disease activity, patient-reported COVID-19 disease and other COVID-19-related questions were collected during follow-up. For healthy controls, age and gender were recorded. AEs were reported in patients and controls approximately 14 days after the first, second and third doses, respectively. The Norwegian Immunisation Registry (SYSVAK) provided information on date and type of vaccination received.27
Outcomes
The main outcome was anti-Spike antibody levels 2–4 weeks after the third vaccine dose in patients as compared with levels 2–4 weeks after the second dose in healthy controls. Additional outcomes were: Decline in anti-Spike antibody level (% per day) assessed at two time points following the second and third vaccine dose in patients, factors associated with anti-Spike antibody levels 2–4 weeks following the third vaccine dose and AEs (reported by patients after the second and third dose and in controls after the second dose).
Statistical analyses
Demographic data and AEs following each vaccine dose were summarised using descriptive statistics. The main outcome, anti-Spike antibody levels 2–4 weeks after the third vaccine dose in patients as compared with levels 2–4 weeks after the second dose in healthy controls and differences among the different vaccines were assessed by the Mann-Whitney U test. Robustness analyses were performed by matching cases and controls according to age and gender. Prevaccination and postvaccination samples in patients receiving a third dose were compared by Wilcoxon paired sampled test. The comparison of antibody level decline following the second versus the third dose was performed using a linear regression estimated via generalised estimated equations. Following each vaccination, antibody levels were assessed at two time points for each patient. The outcome in the regression was the difference between these two values, after first applying a log-transformation. The number of days between the two antibody assessments was included as a covariate both as a main effect and as an interaction with vaccination number, and the exponentiated values of their regression coefficients were used to estimate percentage daily anti-body decline. Plots supported the use of a linear model, and showed little skewness in model residuals.
Factors associated with anti-Spike antibody levels 2–4 weeks following the third dose (outcome variable) were summarised by descriptive bivariate analyses. To estimate the total effects of these factors on antibody levels we formulated a directed acyclic graph (DAG) model for the potential causal relationships between the variables. This model allowed us to determine appropriate statistical adjustments for each of the estimated total effects. All analyses were adjusted for time between vaccination and blood sampling. As plots of the residuals indicated some skewness, robustness analyses were performed using logarithmic transformation of the outcome variable. All tests were two-sided and conducted at the 0.05 significance level. All analyses were carried out using R V.4.0.3, using DAGgitty V.3.0 to guide total effect estimation.
Patient and public involvement
The research question was identified in collaboration with the user representatives in the project group who has also been involved in the planning and conduction of the study. The user representatives will also play an important role in the dissemination of the study results.
Results
General characteristics
Between, 29 July 2021 and 3 February 2022, 1100 patients (366 RA, 177 SpA, 184 PsA, 156 UC and 217 CD; median age 54 (IQR 42–64); 602 women (55%)), underwent serological testing 2–4 weeks after three vaccine doses and were included in the present analyses. Patients were compared with 303 healthy controls (median age 43 (IQR 33–55); 226 women (75%)) with serum samples available 2–4 weeks after two-dose vaccination. Disposition of patients and controls is given in online supplemental figure 1. Characteristics of patients and controls are shown in table 1. Online supplemental table 1 shows baseline characteristics of the 687 patients and 91 controls who were not included as they did not provide serum following standard vaccination. The most frequently used types of medication were tumour necrosis factor inhibitors (TNFi) (n=715) as monotherapy (n=461), or with immunosuppressive co-medication (n=254). In total 97 patients had undergone COVID-19 disease prior to the third dose and received two vaccine doses only.
Table 1.
Characteristics of patients and healthy controls
| Patients n=1100 | Healthy controls n=303 | |
| Demographics | ||
| Age, years (median, IQR) | 54.2 (42.6–64) | 43 (33–55) |
| Female, no (%) | 602 (54.7) | 226 (74.6) |
| Diseases no (%) | ||
| Rheumatoid arthritis | 366 (33) | ‧‧ |
| Spondyloarthritis | 177 (16.2) | ‧‧ |
| Psoriatic arthritis | 184 (16.8) | ‧‧ |
| Ulcerative colitis | 156 (14.1) | ‧‧ |
| Crohn’s disease | 217 (19.9) | ‧‧ |
| Medication no (%) | ||
| Tumour necrosis factor inhibitor, monotherapy* | 461 (41.9) | ‧‧ |
| Tumour necrosis factor inhibitor combination therapy† | 254 (23.1) | ‧‧ |
| Methotrexate | 220 (20) | ‧‧ |
| Vedolizumab | 46 (4.2) | ‧‧ |
| Janus kinases inhibitor | 33 (3) | ‧‧ |
| Ustekinumab, secukinumab, tocilizumab | 60 (5.5) | ‧‧ |
| Abatacept | 15 (1.4) | ‧‧ |
| Other‡ | 11 (1) | ‧‧ |
| Vaccines no (%) | ||
| BNT162b2 all doses | 596 (54.2) | 163 (53.8) |
| mRNA-1273 all doses | 186 (16.9) | 70 (23.1) |
| Combination of vaccines§ | 318 (28.9) | 70 (23.1) |
Patients received three doses, healthy controls received two doses.
*Tumour necrosis factor inhibitors: infliximab, etanercept, adalimumab, golimumab, certolizumab pegol.
†Combination therapy: Tumour necrosis factor inhibitor in combination with either methotrexate, sulfasalazine, leflunomide or azathioprine.
‡Drugs with less than 10 patients included: sulfasalazine, leflunomide, azathioprine, risankizumab, prednisolone monotherapy.
§Combination of the following vaccines: ChAdOx1, BNT162b2, mRNA-1273.
IQR, Inter quartile range.
Patients received the third vaccine dose a median of 126 (IQR 105–154) days after the second dose. Patients donated the first post-vaccination sample median 20 days (IQR 15–26) and 27 days (IQR 22–32) following the second and third dose, respectively. The postvaccination sample in controls was donated median of 23 days (IQR 17–36) following the second dose.
Humoral response to three-dose vaccination in patients
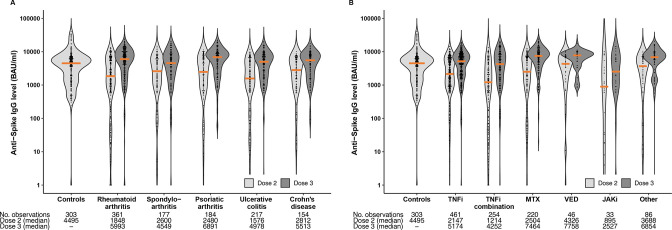
Following two vaccine doses, median anti-Spike antibody levels were significantly lower in patients (2068 BAU/mL (IQR 707–5926) compared to controls (4495 BAU/mL (IQR 1591–6639)), p<0.001 (figure 1A, B). Following the third dose, patients achieved antibody levels comparable to controls vaccinated with two doses (median 5720 BAU/ml (IQR 2138–8732), p=0.27) (table 2, figure 1A, B). In patients, anti-Spike antibody levels increased by a median of 1932 BAU/mL (IQR 150–4978) from the second to the third dose, p<0.001. Similar results were shown in a robustness analysis of 303 age- and gender-matched patients and controls (online supplemental figure 2).
Figure 1.
Anti-Spike antibody levels following three-dose vaccination in patients with IMID versus two-dose vaccination in healthy controls. (A) Anti-Spike antibody levels according to disease group, compared with healthy controls. (B) Anti-Spike antibody levels according to medication group, compared with healthy controls. Violin plot showing the probability density of the data at different values, smoothed by a kernel density estimator. Each data point is a participant, and the solid orange lines show the group median. IMID, immune-mediated inflammatory disease; JAK, Janus kinase inhibitor; MTX, methotrexate; TNFi, Tumour necrosis factor inhibitor; VED, vedolizumab.
Table 2.
Serological response following three-dose vaccination in patients
| Anti-Spike antibody level BAU/mL, median (IQR) | |
| Patients overall third dose | 5720 (2138–8732) |
| Diagnoses | |
| Rheumatoid arthritis | 5993 (2407–9855) |
| Spondyloarthritis | 4549 (1897–8358) |
| Psoriatic arthritis | 6891 (3065–9405) |
| Ulcerative colitis | 5513 (2016.5–8130) |
| Crohn’s disease | 4978 (1877–7929) |
| Medication | |
| Tumour necrosis factor inhibitor, monotherapy* | 5174 (2000–7856) |
| Tumour necrosis factor inhibitor combination therapy† | 4252 (1475–8322) |
| Methotrexate | 7464 (4239–10685) |
| Vedolizumab | 7758 (5033–9606) |
| Janus kinases inhibitor | 2527 (895–6439) |
| Tocilizumab, ustekinumab and secukinumab | 7083 (4803–9569) |
| Abatacept | 4547 (1551–6613) |
| Other‡ | 9625 (7647–10190) |
| Vaccines | |
| BNT162b2 | 4628 (1694–7719) |
| mRNA-1273 | 6610 (3764–10084) |
| Combination of vaccines§ | 7154 (2810–9797) |
| Other factors | |
| Age groups | |
| Age <30 years | 7158 (3337–9465) |
| Age 30–65 years | 5784 (2270–8701) |
| Age >65 years | 4758 (1626–8331) |
*Tumour necrosis factor inhibitors: infliximab, etanercept, adalimumab, golimumab, certolizumab pegol.
†Combination therapy: tumour necrosis factor inhibitor in combination with either methotrexate, sulfasalazine, leflunomide or azathioprine.
‡Drugs with less than 10 patients included: sulfasalazine, leflunomide, azathioprine, risankizumab, prednisolone monotherapy.
§Combination of the following vaccines: ChAdOx1, BNT162b2, mRNA-1273
BAU, binding antibody unit; IQR, Inter quartile range.
Patients receiving three doses of mRNA-1273 (6610 BAU/ml (IQR 3764–10084)) or a combination of vaccines (7154 BAU/mL (IQR 2811–9797)) had significantly higher antibody levels following the third vaccine dose than patients receiving three doses of the BNT162b2 vaccine (4628 BAU/ml (IQR 1694–7719)), p<0.001.
Patients who had previously undergone a COVID-19 infection and thus received two vaccine doses only developed comparable antibody levels to the three-dose vaccinated patients (median 5614 BAU/mL (IQR 2563–8946)).
Decline in anti-Spike antibody levels following vaccination
When comparing decline in anti-Spike antibody levels between two assessments (2–4 and 12 weeks) after the second and third doses in patients, the estimated percentage of decline per day in anti-Spike antibody levels was higher (p<0.001) following the second (2.7%) than the third vaccine dose (1.7%) (online supplemental table 2 and online supplemental figure 3).
Factors associated with anti-Spike antibody levels after the third vaccine dose
Based on the causal model (table 3, online supplemental figure 4), the following factors were found to have a positive and statistically significant effect on antibody levels after the third dose: antibody levels following the second dose, more than 3 months between the second and third vaccine dose and vaccination with mRNA-1273 or a combination of vaccines. Use of JAK inhibitors was associated with lower antibody levels, and use of methotrexate, vedolizumab and interleukin inhibitors (ustekinumab, secukinumab, tocilizumab) were associated with higher antibody levels as compared with TNFi monotherapy. Having a diagnosis of SpA, CD or UC was associated with lower antibody levels compared with RA. A robustness analysis using logarithmic transformation of the outcome variable showed similar results (online supplemental table 3).
Table 3.
Univariate associations and total effect estimates on antibody levels after third vaccine dose
| Characteristics | Beta (SE) (univariate) | P value (univariate) | Total effect (SE) | P value (total effect) |
| Age in years | −19.5 (9.2) | 0.035 | −19.5 (9.2) | 0.035 |
| Male gender | −316.3 (270.3) | 0.242 | −316.3 (270.3) | 0.242 |
| Pause in medication | 571 (359.4) | 0.112 | 383.4 (369.8) | 0.3 |
| Anti-Spike antibody level after second vaccine dose | 0.7 (0) | <0.001 | 0.6 (0) | <0.001 |
| Time between second and third dose | ||||
| Less than 3 months | (reference) | (reference) | ||
| Between 3 and 4 months | 1783.3 (421.2) | <0.001 | 1859.5 (421.2) | <0.001 |
| Between 4 and 5 months | 1753.1 (428) | <0.001 | 1963 (434.1) | <0.001 |
| More than 5 months | 2168.5 (423.7) | <0.001 | 2400.5 (430.4) | <0.001 |
| Diagnosis | ||||
| Rheumatoid arthritis | (reference) | (reference) | ||
| Spondyloarthritis | −787.7 (407.9) | 0.054 | −1150.4 (425.9) | 0.007 |
| Psoriatic arthritis | 405.4 (402.4) | 0.314 | 211.6 (408.1) | 0.604 |
| Crohn’s disease | −1084.9 (381.8) | 0.005 | −1858.4 (431) | <0.001 |
| Ulcerative colitis | −1054.7 (427.5) | 0.014 | −1732.8 (463.9) | <0.001 |
| Medication | ||||
| Tumour necrosis factor inhibitor, monotherapy* | (reference) | (reference) | ||
| Tumour necrosis factor inhibitor combination therapy† | −123.1 (339.8) | 0.717 | −463.9 (365.6) | 0.205 |
| Methotrexate | 2293.7 (356.1) | <0.001 | 2043.6 (443.5) | <0.001 |
| Vedolizumab | 1561.9 (670.3) | 0.02 | 2040.2 (694.5) | 0.003 |
| Janus kinases inhibitor | −1498.6 (784.2) | 0.056 | −1755.7 (813) | 0.031 |
| Ustekinumab, secukinumab, tocilizumab | 1919.8 (596) | 0.001 | 1962.6 (602.8) | 0.001 |
| Abatacept | −1054.8 (1137.3) | 0.354 | −1886.4 (1200.6) | 0.116 |
| Other‡ | 3335.2 (1322.8) | 0.012 | 3306.2 (1320.3) | 0.012 |
| Vaccine | ||||
| BNT162b2 | (reference) | (reference) | ||
| mRNA-1273 | 2221.6 (366.5) | <0.001 | 2212.3 (366) | <0.001 |
| Combination of vaccines§ | 1737.9 (302.9) | <0.001 | 1705.3 (303) | <0.001 |
Univariate associations with antibody level after third vaccination (BAU/mL), and estimated total effects from posited causal associations.
Total effect estimates based on posited causal model (online supplemental figure 4). Total effect of D estimated by model adjusting for M and G, denoted D|M, G; similarly: M|A, G, D; P|A, D, G, M; V|A, G; T|A, G; A|none; G|none; L|A, D, G, M. Here D=Diagnosis, M=Medication; p=Pause; V=Vaccine; T=Time between dose 2 and 3; A=Age; G=Gender; L=Antibody level after dose 2. All analyses were adjusted for time between vaccination and blood sampling.
*Tumour necrosis factor inhibitors: infliximab, etanercept, adalimumab, golimumab, certolizumab pegol.
†Combination therapy: tumour necrosis factor inhibitor in combination with either methotrexate, sulfasalazine, leflunomide or azathioprine.
‡Drugs with less than 10 patients included: sulfasalazine, leflunomide, azathioprine, risankizumab, prednisolone monotherapy.
§Combination of the following vaccines: ChAdOx1, BNT162b2, mRNA-1273.
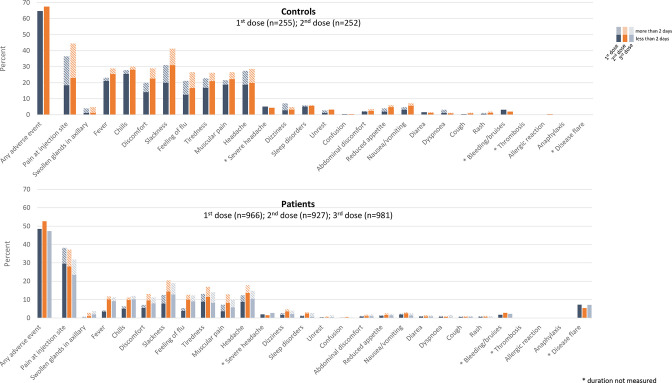
Adverse events
Any AEs were reported by 459/966 (denominator refers to the number of patients responding to the AE questionnaire) (49%), 488/927 (53%) and 464/981 (47%) of patients after first, second and third dose, respectively, and by 196/252 (78%) of healthy controls after the second dose, with a comparable safety profile (figure 2 and online supplemental table 4). After the first, second and third dose, a disease flare was reported by 70/966 (7%), 50/927 (5%) and 70/981 (7%), patients, respectively. Disease flares were mainly reported by patients with inflammatory joint diseases.
Figure 2.
Adverse events following two-dose vaccination and three-dose vaccination in controls and patients. Blue, orange and grey bars indicate adverse events reported after the first, second and third vaccine dose, respectively.
Discussion
This large observational study assessing the humoral immune response following repeated vaccination in patients with IMID on immunosuppressive therapy, demonstrated that anti-Spike antibody levels following three-dose vaccination in patients with IMID were comparable to healthy controls vaccinated twice. These findings were consistent across diagnoses and treatment groups, with no new safety issues emerging.
Prior studies of three-dose vaccination in patients with IMID which mainly have been conducted in small patient groups with an absent or minimal serological response to two-dose vaccination, suggest only moderate increases in antibody levels.22–25 Whether a third vaccine dose included in the standard vaccination programme recommended to patients with IMID on immunosuppressive therapy regardless of prior response, will increase anti-Spike antibody levels or impact antibody decay has not been fully evaluated. This study demonstrated that antibody levels in patients with IMID following three vaccine doses was comparable to levels found in healthy persons vaccinated twice. This finding is reassuring with regards to the protection of this high-risk population throughout the pandemic, and highlights the importance of repeated vaccination in this patient group. The present data indicate that the rate of antibody decline is lower following the third than the second dose, which suggests that a three-dose vaccination regimen may also increase the duration of protection against COVID-19. The effect on strength and durability of further booster vaccination in patients with IMID, with a fourth dose remains to be investigated.
Recent publications have highlighted the clinical implications of low anti-Spike antibody levels. Antibody levels correlate to neutralisation and protection against symptomatic and severe COVID-19 breakthrough infections.3 4 15 28 Virus neutralisation requires high serum antibody levels, and antigenic drift has led to the emergence of SARS-CoV-2 variants against which the vaccine induced neutralising antibody responses have a variable potency.4 15 In addition, antibody levels are proven to rapidly decay over time.17 29 Recent studies from this research group, and Simon et al have suggested that patients with IMID have a greater reduction in anti-Spike antibody level than healthy controls following two-dose standard vaccination30 31
Another notable finding is that patients receiving the mRNA-1273 vaccine for all three doses or a combination of vaccines had significantly higher antibody levels following the third vaccine dose compared with patients receiving three doses of the BNT162b2 vaccine. Prior studies have suggested that the mRNA-1273 vaccine may be more immunogenic than BNT162b2 in healthy subjects, however this is a novel finding in patients with IMID following third-dose vaccination.32
In this study, we show that time between the second and third dose was associated with response, with antibody levels being higher in patients with more than 3 months between the second and third dose. The impact on timing between the first and second dose in the healthy population has been demonstrated recently, but data on third-dose vaccination in the healthy population, or patients with IMID in particular, is currently lacking.33
These results support that additional vaccine doses are safe in an immunosuppressed population, and demonstrates that a lower proportion of patients with IMID receiving a third dose reported AEs than controls vaccinated twice, with the same range of side-effects being reported in both groups. It is thus possible that immunosuppressive medications reduce immune-mediated side effects of the SARS-CoV-2 vaccines. This result is, however, in conflict with Wieske et al who recently showed that the rate of AEs was slightly higher in patients with IMID than in healthy controls.14 This discrepancy might be due to different reporting of AEs or differences in age compositions of the control population and must be further investigated. There is limited data on the safety of SARS-CoV-2 vaccines in patients with IMID.14 34 35 Incremental AE following the second vaccine dose has been suggested in healthy individuals.1 2 However, a recent report comparing self-reported AEs following the second and third vaccine dose in patients with IMID showed no incremental risk following the third dose.14
There has been a concern that the strong immune response following mRNA SARS-CoV-2 vaccines might induce a disease flare in patients with IMID.36 The present results are reassuring, as the percentage of patients experiencing a disease flare is low, with comparable frequency after the second and third dose.
This study has many strengths including the prospective study design with regular sera assessments following each vaccine dose, a broad inclusion, well-characterised patients, a large sample size regarding both patients and controls, and including patients with a range of diagnoses and therapies.
The study has some limitations. First, we do not have data on cellular immune responses. However, a prior study from the Nor-vaC cohort showed that rituximab patients receiving a third dose obtained T-cell responses comparable to healthy controls vaccinated twice despite a lack of humoral response.22 Based on these results, it is plausible that patients on other therapies also obtain normal T-cell responses after the third dose. Second, some medication groups included a low number of patients. Third, the patients were older than the controls, raising the possibility of biased results. However, a younger patient group would be expected to show even higher postvaccination antibody levels. Further, we have corrected for age in all analyses comparing patients and controls. Sensitivity analyses matching patients and controls by age show similar results. The controls were all salaried workers and may thus be healthier than the general population. Fourth, COVID-19 infections were self-reported, and we cannot exclude that silent infections may have been missed. The current study addresses the strength and duration of humoral immunity following three vaccine doses, and do not assess the effect of three doses on the incidence of COVID-19 infections.
A third vaccine dose in patients with IMID was effective and safe and induced a humoral immune-response comparable to healthy controls vaccinated twice. This study suggests that additional vaccine doses contribute to strong and sustained immune-responses in patients on immunosuppressive therapy, and support repeated vaccination in patients with IMID.
Acknowledgments
We acknowledge the patient representatives in the study group Kristin Isabella Kirkengen Espe and Roger Thoresen for their contributions. We acknowledge all study personnel, laboratory personnel and other staff involved at the clinical departments involved and at Department of Immunology at Oslo University Hospital, particularly Synnøve Aure employed at Akershus University Hospital and May Britt Solem and Kjetil Bergsmark employed at Diakonhjemmet Hospital.
Footnotes
Twitter: @siljews, @IngridJyssum, @egeland_ingrid, @a_munthe, @Dr_EAH, @krikjo
SWS and IJ contributed equally.
JTV, SAP, KKJ and GLG contributed equally.
Contributors: SWS, IJ, JS, KKJ and GLG had full access to all the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: SWS, IJ, ATT, JS, DJW, JJ, LAM, EAH, JTV, SAP, KKJ, GLG. Acquisition, analysis and interpretation of data: SWS, IJ, ATT, JS, IEC, TTT, KHB, AC, GBK, GGr, JTV, SAP, KKJ, GLG. Drafting of the manuscript: SWS, IJ, ATT, JS, IEC, GGr, SAP, KKJ, GLG. Critical revision of the manuscript for important intellectual content: SWS, IJ, ATT, JS, IEC, TTT, KHB, SM, DJW, TKK, AC, GBK, JJ, LAM, EAH, GGr, JTV, SAP, KKJ, GLG. Statistical analyses: ATT, JS. Obtained funding: LAM, JTV, GLG. Administrative, technical or material support: JS, TTT, AC, GBK, JJ, LAM, EAH, JTV, GLG. Supervision: SWS, IJ, ATT, JS, SM, DJW, TKK, JJ, LAM, EAH, GGr, JTV, SAP, KKJ, GLG. SWS is the guarantor and acceps full responsibility for the work and/or the conduct of the study, had access to the data, and controlled the decision to publish.
Funding: Nor-vaC was an investigator-initiated study with no initial funding. During its conduct, study grants were received from The Coalition for Epidemic Preparedness Innovations (CEPI); RCN Covid (312693); a KG Jebsen Foundation (grant 19); Dr. Trygve Gythfeldt og frues forskningsfond; Karin Fossum Foundation; the Research Foundation at Diakonhjemmet Hospital; Oslo University Hospital; University of Oslo; the South-Eastern Norway Regional Health Authority.
Competing interests: TKK reports grants from AbbVie, Amgen, BMS MSD, Novartis, Pfizer, UCB, consulting fees from AbbVie, Biogen, Celltrion, Eli Lilly, Gilead, Mylan, Novartis, Pfizer, Sandoz, Sanofi, speakers bureaus Amgen, Celltrion, Egis, Evapharma, Ewopharma, Hikma, Oktal, Sandoz, Sanofi, LM reports funding from KG Jebsen foundation, support for infrastructure and biobanking from the university of Oslo and Oslo University Hospital, grants from the Coalition of Epidemic Preparedness Innovations CEPI, speakers bureaus Novartis, Cellgene, JTV reports grant from the Coalition of Epidemic Preparedness Innovations (CEPI), KKJ reports speakers bureaus from Roche and BMS, advisory board Celltrion and Norgine, GLG reports funding from The Karin Fossum foundation, Diakonhjemmet Hospital, Oslo University Hospital, Akershus University Hospital, Trygve Gydtfeldt og frues Foundation, South-East region Health authority, consulting fees AbbVie and Pfizer, speakers fees AbbVie, Pfizer, Sandoz, Orion Pharma, Novartis and UCB, advisory board Pfizer, AbbVie.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. A deidentified patient data set can be made available to researchers on reasonable request. The data will only be made available after submission of a project plan outlining the reason for the request and any proposed analyses, and will have to be approved by the Nor-vaC steering group. Project proposals can be submitted to the corresponding author. Data sharing will have to follow appropriate regulations.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The study was approved by an independent ethics committee (Regional Committees for Medical and Health Research Ethics South East, reference numbers 235424, 135924), and by appropriate institution review boards. Participants gave informed consent to participate in the study before taking part.
References
- 1. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021;384:403–16. 10.1056/NEJMoa2035389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020;383:2603–15. 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gilbert PB, Montefiori DC, McDermott AB, et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science 2022;375:43–50. 10.1126/science.abm3425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med 2021;27:1205–11. 10.1038/s41591-021-01377-8 [DOI] [PubMed] [Google Scholar]
- 5. Jena A, Mishra S, Deepak P, et al. Response to SARS-CoV-2 vaccination in immune mediated inflammatory diseases: systematic review and meta-analysis. Autoimmun Rev 2022;21:102927. 10.1016/j.autrev.2021.102927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Friedman MA, Curtis JR, Winthrop KL. Impact of disease-modifying antirheumatic drugs on vaccine immunogenicity in patients with inflammatory rheumatic and musculoskeletal diseases. Ann Rheum Dis 2021;80:1255–65. 10.1136/annrheumdis-2021-221244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Syversen SW, Jyssum I, Tveter AT, et al. Immunogenicity and safety of standard and Third-Dose SARS-CoV-2 vaccination in patients receiving immunosuppressive therapy. Arthritis Rheumatol 2022;74:1321–32. 10.1002/art.42153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schett G, McInnes IB, Neurath MF. Reframing immune-mediated inflammatory diseases through signature cytokine hubs. N Engl J Med 2021;385:628–39. 10.1056/NEJMra1909094 [DOI] [PubMed] [Google Scholar]
- 9. Listing J, Gerhold K, Zink A. The risk of infections associated with rheumatoid arthritis, with its comorbidity and treatment. Rheumatology 2013;52:53–61. 10.1093/rheumatology/kes305 [DOI] [PubMed] [Google Scholar]
- 10. Her M, Kavanaugh A. Alterations in immune function with biologic therapies for autoimmune disease. J Allergy Clin Immunol 2016;137:19–27. 10.1016/j.jaci.2015.10.023 [DOI] [PubMed] [Google Scholar]
- 11. Cordtz R, Lindhardsen J, Soussi BG, et al. Incidence and severeness of COVID-19 hospitalization in patients with inflammatory rheumatic disease: a nationwide cohort study from Denmark. Rheumatology 2021;60:SI59–67. 10.1093/rheumatology/keaa897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ungaro RC, Brenner EJ, Gearry RB, et al. Effect of IBD medications on COVID-19 outcomes: results from an international registry. Gut 2021;70:725–32. 10.1136/gutjnl-2020-322539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. D'Amico F, Rabaud C, Peyrin-Biroulet L, et al. SARS-CoV-2 vaccination in IBD: more pros than cons. Nat Rev Gastroenterol Hepatol 2021;18:211–3. 10.1038/s41575-021-00420-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wieske L, Kummer LYL, van Dam KPJ, et al. Risk factors associated with short-term adverse events after SARS-CoV-2 vaccination in patients with immune-mediated inflammatory diseases. BMC Med 2022;20:100. 10.1186/s12916-022-02310-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cromer D, Steain M, Reynaldi A, et al. Neutralising antibody titres as predictors of protection against SARS-CoV-2 variants and the impact of boosting: a meta-analysis. Lancet Microbe 2022;3:e52–61. 10.1016/S2666-5247(21)00267-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Levin EG, Lustig Y, Cohen C, et al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N Engl J Med 2021;385:e84. 10.1056/NEJMoa2114583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shrotri M, Navaratnam AMD, Nguyen V, et al. Spike-antibody waning after second dose of BNT162b2 or ChAdOx1. The Lancet 2021;398:385–7. 10.1016/S0140-6736(21)01642-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tartof SY, Slezak JM, Fischer H, et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. The Lancet 2021;398:1407–16. 10.1016/S0140-6736(21)02183-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Muik A, Lui BG, Wallisch A-K, et al. Neutralization of SARS-CoV-2 omicron by BNT162b2 mRNA vaccine-elicited human sera. Science 2022;375:678–80. 10.1126/science.abn7591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tenforde MW, Patel MM, Gaglani M, et al. Effectiveness of a Third Dose of Pfizer-BioNTech and Moderna Vaccines in Preventing COVID-19 Hospitalization Among Immunocompetent and Immunocompromised Adults - United States, August-December 2021. MMWR Morb Mortal Wkly Rep 2022;71:118–24. 10.15585/mmwr.mm7104a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Thompson MG, Natarajan K, Irving SA, et al. Effectiveness of a Third Dose of mRNA Vaccines Against COVID-19-Associated Emergency Department and Urgent Care Encounters and Hospitalizations Among Adults During Periods of Delta and Omicron Variant Predominance - VISION Network, 10 States, August 2021-January 2022. MMWR Morb Mortal Wkly Rep 2022;71:139–45. 10.15585/mmwr.mm7104e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jyssum I, Kared H, Tran TT, et al. Humoral and cellular immune responses to two and three doses of SARS-CoV-2 vaccines in rituximab-treated patients with rheumatoid arthritis: a prospective, cohort study. Lancet Rheumatol 2022;4:e177–87. 10.1016/S2665-9913(21)00394-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Simon D, Tascilar K, Fagni F, et al. Efficacy and safety of SARS-CoV-2 revaccination in non-responders with immune-mediated inflammatory disease. Ann Rheum Dis 2022;81:1023–7. 10.1136/annrheumdis-2021-221554 [DOI] [PubMed] [Google Scholar]
- 24. Schmiedeberg K, Vuilleumier N, Pagano S, et al. Efficacy and tolerability of a third dose of an mRNA anti-SARS-CoV-2 vaccine in patients with rheumatoid arthritis with absent or minimal serological response to two previous doses. Lancet Rheumatol 2022;4:e11–13. 10.1016/S2665-9913(21)00328-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wieske L, van Dam KPJ, Steenhuis M, et al. Humoral responses after second and third SARS-CoV-2 vaccination in patients with immune-mediated inflammatory disorders on immunosuppressants: a cohort study. Lancet Rheumatol 2022;4:e338–50. 10.1016/S2665-9913(22)00034-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tran TT, Vaage EB, Mehta A. Multiplexed measurement of binding- and neutralizing antibodies to SARS-CoV-2 variants in 12.000 post-vaccine sera. bioRxiv 2022:2022.2003.2026.484261. [Google Scholar]
- 27. Norwegian immunisation registry (SYSVAK). Available: https://www.fhi.no/en/hn/health-registries/norwegian-immunisation-registry-sysvak/
- 28. Bergwerk M, Gonen T, Lustig Y, et al. Covid-19 breakthrough infections in vaccinated health care workers. N Engl J Med 2021;385:1474–84. 10.1056/NEJMoa2109072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Widge AT, Rouphael NG, Jackson LA, et al. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. N Engl J Med 2021;384:80–2. 10.1056/NEJMc2032195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Simon D, Tascilar K, Fagni F, et al. Intensity and longevity of SARS-CoV-2 vaccination response in patients with immune-mediated inflammatory disease: a prospective cohort study. Lancet Rheumatol 2022;4:e614–25. 10.1016/S2665-9913(22)00191-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Christensen IE, Jyssum I, Tveter AT, et al. The persistence of anti-Spike antibodies following two SARS-CoV-2 vaccine doses in patients on immunosuppressive therapy compared to healthy controls-a prospective cohort study. BMC Med 2022;20:378. 10.1186/s12916-022-02587-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Steensels D, Pierlet N, Penders J, et al. Comparison of SARS-CoV-2 antibody response following vaccination with BNT162b2 and mRNA-1273. JAMA 2021;326:1533. 10.1001/jama.2021.15125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hall VG, Ferreira VH, Wood H, et al. Delayed-interval BNT162b2 mRNA COVID-19 vaccination enhances humoral immunity and induces robust T cell responses. Nat Immunol 2022;23:380–5. 10.1038/s41590-021-01126-6 [DOI] [PubMed] [Google Scholar]
- 34. Furer V, Eviatar T, Zisman D, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis 2021;80:1330–8. 10.1136/annrheumdis-2021-220647 [DOI] [PubMed] [Google Scholar]
- 35. Botwin GJ, Li D, Figueiredo J, et al. Adverse events after SARS-CoV-2 mRNA vaccination among patients with inflammatory bowel disease. Am J Gastroenterol 2021;116:1746–51. 10.14309/ajg.0000000000001342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vojdani A, Kharrazian D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin Immunol 2020;217:108480. 10.1016/j.clim.2020.108480 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2022-002417supp001.pdf (371KB, pdf)
Data Availability Statement
Data are available on reasonable request. A deidentified patient data set can be made available to researchers on reasonable request. The data will only be made available after submission of a project plan outlining the reason for the request and any proposed analyses, and will have to be approved by the Nor-vaC steering group. Project proposals can be submitted to the corresponding author. Data sharing will have to follow appropriate regulations.