Take Home Message
We examined perioperative and oncologic outcomes among patients with inferior vena cava tumor thrombus extension, arising from clear cell versus non–clear cell renal cell carcinoma, undergoing surgical resection. We illustrate that both histological groups displayed similar morbidity and survival outcomes over several years.
Keywords: Renal cell carcinoma, Non–clear cell, Histology, Thrombus, Inferior vena cava thrombectomy, Outcomes
Abstract
Background
Renal cell carcinoma (RCC) can exhibit a unique vascular tropism that enables tumor thrombus extension into the inferior vena cava (IVC). While most RCC subtypes that form tumor thrombi are of clear cell (cc) histology, non–clear cell (ncc) subtypes can also exhibit this unique growth pattern.
Objective
To characterize clinicopathologic differences and survival outcomes among patients with IVC tumor thrombus arising from ccRCC versus nccRCC.
Design, setting, and participants
Patients diagnosed with IVC tumor thrombus secondary to RCC in our institutional experience from 2003 to 2021 were identified.
Outcome measurements and statistical analysis
Clinicopathologic characteristics were compared by histology. Perioperative and oncologic outcomes including recurrence-free (RFS), overall (OS), and cancer-specific (CSS) survival were assessed using multivariable Cox regression analyses.
Results and limitations
The analyzed cohort included 103 patients (82 ccRCC and 21 nccRCC). There were no significant differences in baseline demographic parameters. Patients with nccRCC were more likely to have regional lymph node involvement (42.9% vs 20.7%, p = 0.037). No differences in perioperative outcomes, IVC resection, or IVC reconstruction were observed between groups. The median follow-up time was 30 mo. The median RFS was 30 (nccRCC) versus 53 (ccRCC) mo (p = 0.1). There was no significant difference in OS or CSS. This study was limited by its small sample size.
Conclusions
Patients with IVC tumor thrombus arising from ccRCC and nccRCC exhibit similar perioperative and oncologic outcomes. While surgical appropriateness was not impacted by histologic subtype, multimodal strategies are needed to improve outcomes for patients with tumor thrombus.
Patient summary
Renal cell carcinoma (RCC) can uniquely invade vasculature and form a tumor thrombus. This study examined the difference in outcomes of patients with tumor thrombus based on RCC subtype (clear cell vs non–clear cell). We found that patients exhibited similar surgical and survival outcomes regardless of RCC type.
1. Introduction
Renal cancer is among the ten most common malignancies in the USA, with an estimated incidence of 76 080 and mortality of 13 780 cases in 2021 [1]. In up to 10% of patients, renal cell carcinoma (RCC) can exhibit a unique biological tropism for vascular extension into the renal vein and inferior vena cava (IVC) [2]. Extension into the right atrium has been reported in up to 1% of RCC cases [3]. Similar to lymph node involvement and extent of metastasis, tumor thrombus extension has been shown to play a vital role in overall prognosis [3]. The median survival in patients with tumor thrombus managed expectantly is poor at 5 mo, with 1-yr disease-specific survival of under 30% [4], [5].
Clear cell RCC (ccRCC) is the most common subtype of RCC, comprising 70–80% of all RCCs [6]. Histologic studies have described intravenous extension with subsequent secondary sinus fat invasion as the first step toward forming a tumor thrombus and, with time, metastasizing [7]. Although most studies involving vascular extension focus on ccRCC, this phenomenon is not unique to ccRCC. Few studies have reported tumor thrombus extension in papillary RCC (pRCC) and have associated it with worse cancer-specific outcomes compared with ccRCC [8], [9]. Recent efforts have uncovered molecular underpinnings of IVC tumor thrombus arising from RCC, with inclusion of a small subset of patients with non–clear cell (nccRCC) histology as well [10]. Additional studies are needed to further characterize tumor thrombus arising from nccRCC histologic subtypes. Given the paucity of data for managing nccRCC with IVC tumor thrombus, we herein sought to investigate the differences in clinicopathologic characteristics, perioperative outcomes, and oncologic outcomes among patients with IVC tumor thrombus arising from ccRCC versus nccRCC managed surgically.
2. Patients and methods
2.1. Patient population and data collection
An institutional review board–approved renal mass registry was queried to identify all patients with RCC who underwent radical nephrectomy at our institution between 2003 and 2021. The registry is maintained prospectively by professional data abstractors who collect and enter comprehensive demographic, clinical, and pathologic data with multiple data reviews to ensure accurate and complete data. Patients who underwent concurrent IVC thrombectomy for pathologically proven RCC (pT3b-c) were included. Tumor thrombus level was further characterized via the Mayo Clinic RCC tumor thrombus classification system [11]. Patients with unavailable pathology reports for review were excluded from this analysis; those with bilateral tumors or multiple synchronous primary tumors were also excluded.
RCC histology was retrieved from pathology reports, and the study cohort was stratified into two groups based on the presence of clear cell versus non–clear cell histology. Data on patient demographics (age, sex, and race), clinical comorbidities (body mass index, diabetes mellitus, hypertension, and tobacco use), oncologic characteristics (pathologic stage, grade, size, lymphovascular invasion, sarcomatoid/rhabdoid differentiation, tumor necrosis, surgical margin status, caval wall invasion, preoperative systemic therapy, and metastasis), surgical approach, and perioperative outcomes (duration of surgery, estimated blood loss, length of hospital stay, in-hospital mortality, and intra- and postoperative complications) were obtained; surgical complications were further characterized using the Clavien-Dindo classification system [12]. Additionally, the date of recurrence was captured for patients who had a confirmed disease recurrence on axial imaging. Survival data (date of last follow-up, vital status, and cause of death) were also obtained.
2.2. Statistical analyses
Fisher’s exact and Pearson’s chi-square tests were used to compare categorical variables between patients with IVC thrombus arising from ccRCC versus nccRCC. Kruskal-Wallis tests were used to evaluate differences in continuous variables between the two groups.
The Kaplan-Meier method and the log-rank test were used to estimate and compare overall (OS) and cancer-specific (CSS) survival probabilities between ccRCC and nccRCC patients. Similarly, in the subset of patients with nonmetastatic (M0) disease, differences in OS, CSS, and recurrence-free survival (RFS) probabilities were compared between the two groups. Oncologic outcomes were measured from the date of surgery, with censoring performed at the date of last follow-up. Univariable and multivariable Cox regression analyses were performed to assess the impact of RCC histology on CSS, OS, and, in M0 patients, RFS. Significant predictors on univariable analyses were included in the multivariable models.
All tests were two sided, and p ≤ 0.05 was considered statistically significant. Statistical analyses were performed using STATA v.15.0 (2017; STATA Corp, College Station, TX, USA).
3. Results
3.1. Study cohort
A total of 103 patients with IVC tumor thrombus arising from RCC were identified, including 82 (79.6%) with ccRCC and 21 (20.4%) with nccRCC. The baseline patient and tumor characteristics are shown in Table 1. Among patients with nccRCC, the most common histology was pRCC (N = 11, 52.4%) followed by unclassified RCC (N = 7, 33.4%). Six patients in our total cohort (5.8%) received preoperative systemic therapy. Of note, patients with nccRCC were found to have more regional lymph node involvement than patients with ccRCC (42.9% vs 20.7%, p = 0.037). There were no other significant differences in clinicopathologic characteristics, including tumor thrombus level, sarcomatoid/rhabdoid differentiation, and caval wall invasion, between the two groups (Table 1).
Table 1.
Baseline clinical and tumor characteristics of patients who underwent radical nephrectomy with thrombectomy for clear cell and non–clear cell renal cell carcinoma with tumor extension into the inferior vena cava
| Characteristics |
N (%) |
p value | ||
|---|---|---|---|---|
| Total | Clear cell RCC | Non–clear cell RCC | ||
| Number of patients | 103 (100) | 82 (79.6) | 21 (20.4) | |
| Age (yr), median (IQR) | 63 (55–72) | 63 (54–72) | 64 (57–69) | 0.92 |
| Sex | ||||
| Male | 79 (76.7) | 63 (76.8) | 16 (76.2) | 0.95 |
| Female | 24 (23.3) | 19 (23.2) | 5 (23.8) | |
| Race | ||||
| White | 89 (86.4) | 73 (89.0) | 16 (76.2) | 0.07 |
| Black | 11 (10.7) | 6 (7.3) | 5 (23.8) | |
| Other | 3 (2.9) | 3 (3.7) | 0 (0) | |
| BMI (kg/m2), median (IQR) | 28 (25–34) | 28 (25–32) | 30 (25–35) | 0.55 |
| History of tobacco use | ||||
| No | 51 (49.5) | 39 (47.6) | 12 (57.1) | 0.43 |
| Yes | 52 (50.5) | 43 (52.4) | 9 (42.9) | |
| Diabetes mellitus | ||||
| No | 80 (77.7) | 61 (74.4) | 19 (90.5) | 0.11 |
| Yes | 23 (22.3) | 21 (25.6) | 2 (9.5) | |
| Hypertension | ||||
| No | 38 (36.9) | 28 (34.2) | 10 (47.6) | 0.25 |
| Yes | 65 (63.1) | 54 (65.8) | 11 (52.4) | |
| Tumor laterality | ||||
| Left | 28 (27.2) | 22 (26.8) | 6 (28.6) | 0.87 |
| Right | 75 (72.8) | 60 (73.2) | 15 (71.4) | |
| RCC histology | ||||
| Clear cell | 82 (79.6) | 82 (100) | – | – |
| Papillary | 11 (10.7) | – | 11 (52.3) | |
| Chromophobe | 1 (1.0) | – | 1 (4.8) | |
| Mixed clear papillary | 1 (1.0) | – | 1 (4.8) | |
| PNET | 1 (1.0) | – | 1 (4.8) | |
| Unclassified | 7 (6.7) | – | 7 (33.3) | |
| Pathologic tumor size (cm), median (IQR) | 10.5 (7.2–14.0) | 9.8 (7.0–13.5) | 12.5 (10.0–14.0) | 0.13 |
| Pathologic tumor stage | ||||
| pT3b | 76 (73.8) | 61 (74.4) | 15 (71.4) | 0.78 |
| pT3c | 27 (26.2) | 21 (25.6) | 6 (28.6) | |
| Pathologic nodal involvement | ||||
| pN0/pNX | 77 (74.8) | 65 (79.3) | 12 (57.1) | 0.037 |
| pN1 | 26 (25.2) | 17 (20.7) | 9 (42.9) | |
| Metastatic disease | ||||
| M0 | 74 (71.8) | 58 (70.7) | 16 (76.2) | 0.62 |
| M1 | 29 (28.2) | 24 (29.3) | 5 (23.8) | |
| Pathologic grade | ||||
| Low (1–2) | 14 (13.6) | 12 (14.6) | 2 (9.5) | 0.73 |
| High (3–4) | 89 (86.4) | 70 (85.4) | 19 (90.5) | |
| Tumor thrombus grading | 0.57 | |||
| Level I | 10 (9.7) | 7 (8.5) | 3 (14.3) | |
| Level II–III | 73 (70.9) | 60 (73.2) | 13 (61.9) | |
| Level IV | 20 (19.4) | 15 (18.3) | 5 (23.8) | |
| Caval wall invasion | ||||
| Absent | 93 (90.3) | 74 (90.2) | 19 (90.5) | 0.97 |
| Present | 10 (9.7) | 8 (9.8) | 2 (9.5) | |
| Lymphovascular invasion | ||||
| Absent | 34 (33.0) | 28 (34.2) | 6 (28.6) | 0.63 |
| Present | 69 (67.0) | 54 (65.8) | 15 (71.4) | |
| Sarcomatoid/rhabdoid differentiation | ||||
| Absent | 84 (81.5) | 65 (79.3) | 19 (90.5) | 0.24 |
| Present | 19 (18.5) | 17 (20.7) | 2 (9.5) | |
| Tumor necrosis | ||||
| Absent | 65 (63.1) | 51 (62.2) | 14 (66.7) | 0.71 |
| Present | 38 (36.9) | 31 (37.8) | 7 (33.3) | |
| Preoperative systemic therapy | ||||
| No | 97 (94.2) | 77 (93.9) | 20 (95.2) | 0.82 |
| Yes | 6 (5.8) | 5 (6.1) | 1 (4.8) | |
BMI = body mass index; IQR = interquartile range; PNET= primitive neuroectodermal tumor; RCC = renal cell carcinoma.
Thrombus level is classified using the Mayo Clinic RCC tumor thrombus convention.
3.2. Perioperative outcomes
Perioperative outcomes are shown in Table 2. We did not encounter any increased technical difficulty or tumor-related changes in patients with nccRCC compared with those with ccRCC: when comparing ccRCC with nccRCC patients, there were no significant differences in the median duration of surgery (5.6 vs 5.9 h, p = 0.69), median estimated blood loss (1.5 vs 2.3 l, p = 0.6), median length of hospital stay (7 vs 5 d, p = 0.32), and intraoperative (12.2% vs 4.8%, p = 0.45) or postoperative (36.6% vs 47.6%, p = 0.35) complication rates.
Table 2.
Perioperative outcomes of patients who underwent radical nephrectomy with thrombectomy for clear cell and non–clear cell renal cell carcinoma with tumor extension into the inferior vena cava
| Outcome |
N (%) |
p value | ||
|---|---|---|---|---|
| Total | Clear cell RCC | Non–clear cell RCC | ||
| Number of patients | 103 (100) | 82 (79.6) | 21 (20.4) | |
| Radical nephrectomy approach | ||||
| Open | 92 (89.3) | 74 (90.2) | 18 (85.7) | 0.89 |
| Laparoscopic/robotic | 11 (10.7) | 8 (9.8) | 3 (14.3) | |
| Duration of surgery (h), median (IQR) | 5.7 (4.1–7.6) | 5.6 (4.1–7.6) | 5.9 (4.6–7.2) | 0.69 |
| Estimated blood loss (l), median (IQR) | 1.7 (0.8–2.9) | 1.5 (0.8–2.5) | 2.3 (0.5–3.5) | 0.6 |
| Length of hospital stay (d), median (IQR) | 7 (4.5–12) | 7 (5–12) | 5 (3–11) | 0.32 |
| In-hospital mortality | ||||
| No | 97 (94.2) | 79 (96.3) | 18 (85.7) | 0.097 |
| Yes | 6 (5.8) | 3 (3.7) | 3 (14.3) | |
| Intraoperative complication | ||||
| No | 92 (89.3) | 72 (87.8) | 20 (95.2) | 0.45 |
| Yes | 11 (10.7) | 10 (12.2) | 1 (4.8) | |
| Postoperative complication | ||||
| No | 63 (61.2) | 52 (63.4) | 11 (52.4) | 0.35 |
| Yes | 40 (38.8) | 30 (36.6) | 10 (47.6) | |
| Postoperative complication by Clavien-Dindo scale | ||||
| Grade I | 7 (17.5) | 6 (20.0) | 1 (10.0) | 0.17 |
| Grade II | 22 (55.0) | 18 (60.0) | 4 (40.0) | |
| Grade IIIa | 1 (2.5) | 0 (0) | 1 (10.0) | |
| Grade IIIb | 4 (10.0) | 3 (10.0) | 1 (10.0) | |
| Grade IVa | 0 (0) | 0 (0.0) | 0 (0) | |
| Grade IVb | 0 (0) | 0 (0.0) | 0 (0) | |
| Grade V | 6 (15.0) | 3 (10.0) | 3 (30.0) | |
IQR = interquartile range; RCC = renal cell carcinoma.
Complications were characterized using the Clavien-Dindo classification system.
In-hospital mortality rates were nearly four times higher for nccRCC than for ccRCC, although this difference was not statistically significant (three deaths in each group, 3.7% vs 14.3%, p = 0.097). Notably, four of these patients (67%) had level IV thrombus extending above the diaphragm. All six patients died postoperatively; there were no intraoperative deaths. Three patients (one ccRCC and two nccRCC) experienced refractory hemodynamic instability requiring maximum inotropic and respiratory support followed by multiorgan failure. The third nccRCC death resulted from a spontaneous cardiovascular arrest on postoperative day 1. The remaining two ccRCC patients’ deaths were precipitated by pulmonary embolism, with one patient suffering a large cerebrovascular accident after receiving anticoagulation. Among these six deaths, only one patient (ccRCC) had evidence of metastatic disease.
3.3. Survival outcomes
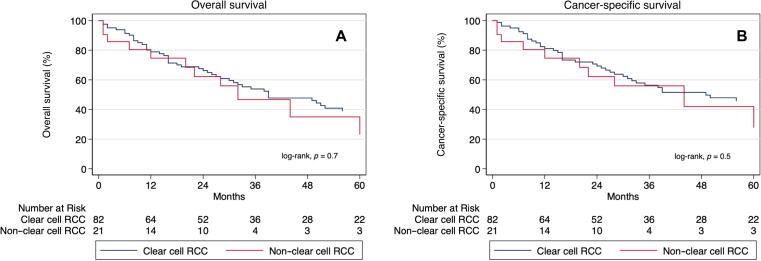
The median follow-up duration for the study cohort was 30 mo (interquartile range: 12–56 mo). Overall, 64 (62.1%) patients died. There was no difference in median OS between patients with ccRCC and those with nccRCC (39 vs 32 mo, p = 0.7; Fig. 1A). In univariable Cox regression analysis, tumor histology was not significantly predictive of all-cause mortality (nccRCC vs ccRCC hazard ratio [HR] = 1.16, 95% confidence interval [CI]: 0.60–2.23, p = 0.65); however, presence of hypertension (HR = 1.69, 95% CI: 1.04–2.78, p = 0.035), nodal involvement (HR = 2.10, 95% CI: 1.19–3.70, p = 0.010), and metastatic disease (HR = 2.13, 95% CI: 1.25–3.62, p = 0.005) were shown to be independently and significantly associated with an increased risk of all-cause mortality (Supplementary Table 1). Adjusting for these significant variables in a multivariable model, there was still no significant association between tumor histology and the risk of all-cause mortality (HR = 0.93, 95% CI: 0.46–1.89, p = 0.84; Supplementary Table 1).
Fig. 1.
Kaplan-Meier survival curves for clear cell and non–clear cell renal cell carcinoma in the setting of tumor thrombus comparing (A) overall survival and (B) cancer-specific survival probabilities between groups. RCC = renal cell carcinoma.
There were 54 deaths attributable to RCC. No difference in median CSS was noted between ccRCC and nccRCC (49 vs 44 mo, p = 0.5; Fig. 1B). While nodal involvement (HR = 2.57, 95% CI: 1.43–4.62, p = 0.002) and metastatic disease (HR = 2.09, 95% CI: 1.18–3.71, p = 0.012) were significant predictors of death due to RCC, nccRCC histology was not significantly associated with worse CSS compared with ccRCC (HR = 1.23, 95% CI: 0.62–2.46, p = 0.55) in univariable Cox regression analysis (Supplementary Table 2). This nonsignificant association persisted in the multivariable analysis (HR = 1.01, 95% CI: 0.48–2.12, p = 0.97) after controlling for nodal involvement and metastatic disease (Supplementary Table 2).
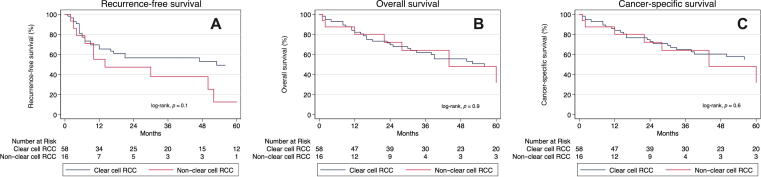
Among patients with nonmetastatic disease (N = 74), 34 (45.9%) developed disease recurrence. There was no significant difference in median RFS in patients with ccRCC versus nccRCC (53 vs 30 mo, p = 0.1; Fig. 2A). Moreover, comparing ccRCC with nccRCC, there was no significant difference in the median OS (56 vs 44 mo, p = 0.9) or median CSS (68 vs 44 mo, p = 0.6) among patients with nonmetastatic disease (Fig. 2B and 2C). None of the patient or tumor characteristics, including tumor histology (HR = 1.85, 95% CI: 0.88–3.88, p = 0.10), were significantly predictive of disease recurrence in our cohort upon univariable analysis (Supplementary Table 3). However, hypertension was a significant predictor of overall (HR = 2.28, 95% CI: 1.22–4.26, p = 0.010) and cancer-specific (HR = 2.14, 95% CI: 1.07–4.30, p = 0.032) mortality among patients with nonmetastatic disease in multivariable analysis (Supplementary Tables 4 and 5, respectively).
Fig. 2.
Kaplan-Meier survival curves for patients with nonmetastatic (M0) clear cell and non–clear cell renal cell carcinoma in the setting of tumor thrombus comparing (A) recurrence-free survival, (B) overall survival, and (C) cancer-specific survival probabilities between groups. RCC = renal cell carcinoma.
4. Discussion
RCC with venous tumor thrombus extension exhibits a unique biology and poses challenges in its surgical management [2], [13]. Herein, we uniquely and comprehensively characterized clinicopathologic, perioperative, and oncologic differences among patients with tumor thrombus arising from different histologic subtypes of RCC. We have shown that a sizable proportion of IVC tumor thrombi can arise from nccRCC, and these patients exhibit similar baseline characteristics and outcomes to those with ccRCC. Our results demonstrate that the surgical appropriateness and outcomes for RCC patients are not impacted by histologic subtype and that surgical resection remains an appropriate standard of care for patients with IVC tumor thrombus in nccRCC.
There has been an abundance of studies investigating the impact of histology on oncologic outcomes in RCC, although the data remain mixed [14]. However, few studies have looked at the prognostic impact of histologic subtype for RCC patients with IVC tumor thrombus. In one study, patients with pRCC with venous thrombus had lower 5-yr CSS than their ccRCC counterparts (35% vs 66%, p = 0.012) [15]. However, significance was lost on multivariable analysis. Additionally, prognostic factors such as papillary subtype, tumor necrosis, and lymphovascular invasion were not examined in all patients. Wagner et al [16] illustrated similar results, finding that histologic type of pT3b and pT3c RCCs failed to show a statistically significant impact on OS in multivariable analysis. In another study of 1774 patients with RCC with tumor thrombus who underwent radical nephrectomy and tumor thrombectomy (89.9% ccRCC and 8.5% pRCC), pRCC was associated with significantly worse 5-yr CSS compared with ccRCC (37% vs 55%, p < 0.001) [9]. However, there were no differences in outcomes between chromophobe RCC and ccRCC. Notably, type II papillary histology contributed to most cases of pRCC with IVC thrombus and was associated with worse outcomes than ccRCC patients with IVC thrombus [8]. These studies did not evaluate surgical outcomes such as duration of surgery, estimated blood loss, and complications.
For locally advanced RCC, radical nephrectomy with IVC thrombectomy remains the gold standard approach and the only potentially curative treatment option, with survival spanning from 40% to 60% at 5 yr following complete resection [13]. Although some studies have shown no significant difference in survival based on the level of tumor thrombus, the anatomic level of tumor thrombus continues to play an imperative role in surgical planning. Complete removal of thrombus, including resection of invaded caval wall, as seen in some pT3c tumors, is mandatory [4], [17], [18], [19]. It is critical that these complex surgeries be performed in high-volume, experienced centers to optimize outcomes [20].
There is an emerging role for multimodal treatment strategies in patients with locally advanced, nonmetastatic RCC, including those with IVC tumor thrombus extension. While targeted therapies have shown efficacy in ccRCC, clinical responses have notably remained low in patients with nccRCC [21], [22], [23]. With the advent of novel immune checkpoint inhibitors, we are witnessing unparalleled rates of complete pathologic response in the primary tumors of patients undergoing nephrectomy following receipt of systemic immunotherapy, ranging from 10% to 20% [24], [25], [26]. Some groups have reported cases of complete pathologic response in the tumor thrombus itself [27], [28], [29]. These impressive responses are, however, largely reported in ccRCC and remarkably extend to patients with sarcomatoid features as well [30], [31]. However, the benefits of immune checkpoint inhibition in nccRCC have yet to be defined further [32], and ongoing trials are underway to further investigate the safety and efficacy of novel immunotherapy combinations in advanced nccRCC. A recent safety lead-in phase II trial even demonstrated the safety and feasibility of administering neoadjuvant stereotactic radiation prior to surgical resection of IVC tumor thrombus arising from RCC [33]. Nevertheless, the consensus remains that the use of neoadjuvant therapy to reduce venous thrombus involvement is considered experimental and should not be used beyond clinical trials [34].
There is a pressing need to further understand the underlying biology of IVC tumor thrombus in RCC in order to better guide personalized therapeutic strategies [33]. Recently, Kim et al [10] undertook a comprehensive molecular and pathologic evaluation of 83 patients undergoing surgical resection of a renal mass with IVC tumor thrombus extension, including 73 with ccRCC, nine with nccRCC (one pRCC, one chromophobe, and seven unclassified), and one patient with leiomyosarcoma. Using geospatially resolved multiregional sampling, the investigators elucidated tropic drivers of vascular invasion and metastatic competence. Interestingly, their work uncovered that the grade of invasive subclones tended to be the main determinants of metastatic competence, but the most aggressive, highest-grade subclones did not necessarily drive venous invasion. Their findings hold prognostic implications and may even help inform multimodal strategies.
Our study is limited by its retrospective nature and inherent selection bias. We reported a higher rate of pathologic lymph node metastases among patients with nccRCC, which may reflect a potential selection bias; in particular, we do not report on clinical node stage, which may conceivably impact multimodal treatment strategies for RCC. However, systemic therapies for metastatic nccRCC have shown less efficacy than that for metastatic ccRCC, and so this difference in pN1 rates may alternatively reflect a more surgically aggressive approach for nccRCC patients or perhaps differential biology in lymphogenous dissemination of tumors by histologic type. Furthermore, statistical analyses are likely limited by the relatively small sample size, particularly in the nccRCC group, although patient numbers are relatively robust for a single-institution series of surgically resected IVC tumor thrombi from RCC. Validation of our findings in a larger multi-institutional consortium would be informative. Additionally, the subset of patients with nccRCC histology is comparable with expected rates of nccRCC reported previously. Our work is strengthened by its rigorous evaluation of perioperative parameters in an experienced, tertiary referral center and long-term oncologic follow-up.
5. Conclusions
Our study comprehensively characterizes perioperative and oncologic outcomes among patients with IVC tumor thrombus extension arising from RCC, stratified by histologic subtype. We note that nccRCC histology did not worsen perioperative outcomes or morbidity compared with patients with ccRCC. Likewise, oncologic outcomes were similar between groups, suggesting that surgical resection remains an appropriate standard of care for patients with IVC tumor thrombus in nccRCC. With the encouraging clinical and pathologic outcomes seen in patients receiving novel immunotherapy approaches for locally advanced RCC, including nccRCC, future studies will need to emphasize the role of personalized, multimodal strategies to optimize outcomes in this understudied cohort.
Author contributions: Nirmish Singla had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Cheaib, Patel, Metcalf, Allaf, Singla.
Acquisition of data: Singla, Rabinowitz, Patel.
Analysis and interpretation of data: Singla, Cheaib, Alam.
Drafting of the manuscript: Rabinowitz, Esfandiary.
Critical revision of the manuscript for important intellectual content: Metcalf, Enikeev, Pierorazio, Ged, Singla.
Statistical analysis: Cheaib, Rabinowitz.
Obtaining funding: None.
Administrative, technical, or material support: Singla.
Supervision: Singla.
Other: None.
Financial disclosures: Nirmish Singla certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
Associate Editor: M. Carmen Mir
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.euros.2022.07.001.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 2.Topaktaş R., Ürkmez A., Tokuç E., Kayar R., Kanberoğlu H., Öztürk M.İ. Surgical management of renal cell carcinoma with associated tumor thrombus extending into the inferior vena cava: a 10-year single-center experience. Turk J Urol. 2019;45:345–350. doi: 10.5152/tud.2019.95826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park H, Jeong CW, Yuk H, et al. Influence of tumor thrombus on occurrence of distant venous thromboembolism and survival in patients with renal cell carcinoma after surgery. Clin Appl Thromb Hemost 2019;25:1076029618823288. [DOI] [PMC free article] [PubMed]
- 4.Psutka S.P., Leibovich B.C. Management of inferior vena cava tumor thrombus in locally advanced renal cell carcinoma. Ther Adv Urol. 2015;7:216–229. doi: 10.1177/1756287215576443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reese A.C., Whitson J.M., Meng M.V. Natural history of untreated renal cell carcinoma with venous tumor thrombus. Urol Oncol. 2013;31:1305–1309. doi: 10.1016/j.urolonc.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 6.Vuong L., Kotecha R.R., Voss M.H., Hakimi A.A. Tumor microenvironment dynamics in clear-cell renal cell carcinoma. Cancer Discov. 2019;9:1349–1357. doi: 10.1158/2159-8290.CD-19-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonsib S.M. Renal veins and venous extension in clear cell renal cell carcinoma. Mod Pathol. 2007;20:44–53. doi: 10.1038/modpathol.3800726. [DOI] [PubMed] [Google Scholar]
- 8.Kim K.H., You D., Jeong I.G., et al. Type II papillary histology predicts poor outcome in patients with renal cell carcinoma and vena cava thrombus. BJU Int. 2012;110(11 Pt B):E673–E678. doi: 10.1111/j.1464-410X.2012.11498.x. [DOI] [PubMed] [Google Scholar]
- 9.Tilki D., Nguyen H.G., Dall’Era M.A., et al. Impact of histologic subtype on cancer-specific survival in patients with renal cell carcinoma and tumor thrombus. Eur Urol. 2014;66:577–583. doi: 10.1016/j.eururo.2013.06.048. [DOI] [PubMed] [Google Scholar]
- 10.Kim K., Zhou Q., Christie A., et al. Determinants of renal cell carcinoma invasion and metastatic competence. Nat Commun. 2021;12:5760. doi: 10.1038/s41467-021-25918-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quencer K.B., Friedman T., Sheth R., Oklu R. Tumor thrombus: incidence, imaging, prognosis and treatment. Cardiovasc Diagn Ther. 2017;7(Suppl 3):S165–S177. doi: 10.21037/cdt.2017.09.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clavien P.A., Barkun J., de Oliveira M.L., et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187–196. doi: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- 13.Master V.A., Ethun C.G., Kooby D.A., Staley C.A., Maithel S.K. The value of a cross-discipline team-based approach for resection of renal cell carcinoma with IVC tumor thrombus: a report of a large, contemporary, single-institution experience. J Surg Oncol. 2018;118:1219–1226. doi: 10.1002/jso.25271. [DOI] [PubMed] [Google Scholar]
- 14.Patard J.J., Leray E., Rioux-Leclercq N., et al. Prognostic value of histologic subtypes in renal cell carcinoma: a multicenter experience. J Clin Oncol. 2005;23:2763–2771. doi: 10.1200/JCO.2005.07.055. [DOI] [PubMed] [Google Scholar]
- 15.Margulis V., Tamboli P., Matin S.F., Swanson D.A., Wood C.G. Analysis of clinicopathologic predictors of oncologic outcome provides insight into the natural history of surgically managed papillary renal cell carcinoma. Cancer. 2008;112:1480–1488. doi: 10.1002/cncr.23322. [DOI] [PubMed] [Google Scholar]
- 16.Wagner B., Patard J.J., Méjean A., et al. Prognostic value of renal vein and inferior vena cava involvement in renal cell carcinoma. Eur Urol. 2009;55:452–459. doi: 10.1016/j.eururo.2008.07.053. [DOI] [PubMed] [Google Scholar]
- 17.Blute M.L., Leibovich B.C., Lohse C.M., Cheville J.C., Zincke H. The Mayo Clinic experience with surgical management, complications and outcome for patients with renal cell carcinoma and venous tumour thrombus. BJU Int. 2004;94:33–41. doi: 10.1111/j.1464-410X.2004.04897.x. [DOI] [PubMed] [Google Scholar]
- 18.Neves R.J., Zincke H. Surgical treatment of renal cancer with vena cava extension. Br J Urol. 1987;59:390–395. doi: 10.1111/j.1464-410x.1987.tb04832.x. [DOI] [PubMed] [Google Scholar]
- 19.Edge S, Byrd D, Frits A. AJCC cancer staging handbook. http://link.springer.com/book/9780387884424.
- 20.Freifeld Y., Woldu S.L., Singla N., et al. Impact of hospital case volume on outcomes following radical nephrectomy and inferior vena cava thrombectomy. Eur Urol Oncol. 2019;2:691–698. doi: 10.1016/j.euo.2018.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aizer A.A., Urun Y., McKay R.R., Kibel A.S., Nguyen P.L., Choueiri T.K. Cytoreductive nephrectomy in patients with metastatic non-clear-cell renal cell carcinoma (RCC) BJU Int. 2014;113:E67–E74. doi: 10.1111/bju.12442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choueiri T.K., Plantade A., Elson P., et al. Efficacy of sunitinib and sorafenib in metastatic papillary and chromophobe renal cell carcinoma. J Clin Oncol. 2008;26:127–131. doi: 10.1200/JCO.2007.13.3223. [DOI] [PubMed] [Google Scholar]
- 23.Marchetti A., Rosellini M., Mollica V., et al. The molecular characteristics of non-clear cell renal cell carcinoma: what’s the story morning glory? Int J Mol Sci. 2021;22:6237. doi: 10.3390/ijms22126237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singla N., Elias R., Ghandour R.A., et al. Pathologic response and surgical outcomes in patients undergoing nephrectomy following receipt of immune checkpoint inhibitors for renal cell carcinoma. Urol Oncol. 2019;37:924–931. doi: 10.1016/j.urolonc.2019.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singla N, Margulis V. Re: Geraldine Pignot, Antoine Thiery-Vuillemin, Jochen Walz, et al. Nephrectomy after complete response to immune checkpoint inhibitors for metastatic renal cell carcinoma: a new surgical challenge? Eur Urol. In press. https://doi.org/10.1016/j.eururo.2019.12.018: The next surgical frontier in kidney cancer: nephrectomy after immune checkpoint inhibition. Eur Urol 2020;78:e79–80. [DOI] [PubMed]
- 26.Singla N, Hutchinson RC, Ghandour RA, et al. Improved survival after cytoreductive nephrectomy for metastatic renal cell carcinoma in the contemporary immunotherapy era: an analysis of the National Cancer Database. Urol Oncol 2020;38:604.e9–17. [DOI] [PMC free article] [PubMed]
- 27.Labbate C., Hatogai K., Werntz R., et al. Complete response of renal cell carcinoma vena cava tumor thrombus to neoadjuvant immunotherapy. J Immunother Cancer. 2019;7:66. doi: 10.1186/s40425-019-0546-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hara T., Terakawa T., Hyodo T., Jinbo N., Nakano Y., Fujisawa M. Pathological complete response of renal cell carcinoma with vena cava tumor thrombus to neoadjuvant TKI/IO combination therapy. Urol Case Rep. 2021;39:101800. doi: 10.1016/j.eucr.2021.101800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okada T., Hamamoto S., Etani T., et al. Complete response of renal cell carcinoma with an inferior vena cava tumor thrombus and lung metastases after treatment with nivolumab plus ipilimumab. Int Cancer Conf J. 2020;9:88–91. doi: 10.1007/s13691-020-00403-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bakouny Z., Braun D.A., Shukla S.A., et al. Integrative molecular characterization of sarcomatoid and rhabdoid renal cell carcinoma. Nat Commun. 2021;12:808. doi: 10.1038/s41467-021-21068-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Motzer R.J., Banchereau R., Hamidi H., et al. Molecular subsets in renal cancer determine outcome to checkpoint and angiogenesis blockade. Cancer Cell. 2020;38:803–817.e4. doi: 10.1016/j.ccell.2020.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee C.H., Voss M.H., Carlo M.I., et al. Phase II trial of cabozantinib plus nivolumab in patients with non-clear-cell renal cell carcinoma and genomic correlates. J Clin Oncol. 2022 doi: 10.1200/JCO.21.01944. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Margulis V., Freifeld Y., Pop L.M., et al. Neoadjuvant SABR for renal cell carcinoma inferior vena cava tumor thrombus-safety lead-in results of a phase 2 trial. Int J Radiat Oncol Biol Phys. 2021;110:1135–1142. doi: 10.1016/j.ijrobp.2021.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Escudier B., Porta C., Schmidinger M., et al. Renal cell carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30:706–720. doi: 10.1093/annonc/mdz056. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.