Abstract
Cholera toxin (CT) increases intestinal secretion of water and electrolytes and modulates the mucosal immune response by stimulating cellular synthesis of arachidonic acid (AA) metabolites (e.g., prostaglandin E2), as well as the intracellular second messenger cyclic AMP (cAMP). While much is known about the mechanism of CT stimulation of adenylate cyclase, the toxin’s activation of phospholipase A2, which results in increased hydrolysis of AA from membrane phospholipids, is not well understood. To determine whether CT activation of AA metabolism requires CT’s known enzymatic activity (i.e., ADP-ribosylation of GSα), we used native CT and a mutant CT protein (CT-2*) lacking ADP-ribose transferase activity in combination with S49 wild-type (WT) and S49 cyc− murine Theta (Th)1.2-positive lymphoma cells deficient in GSα. The experimental results showed that native CT stimulated the release of [3H[AA from S49 cyc− cells at a level similar to that for S49 WT cells, indicating that GSα is not essential for this process. Further, levels of cAMP in the CT-treated cyc− cells remained the same as those in the untreated control cells. The ADP-ribosyltransferase-deficient CT-2* protein, which was incapable of increasing synthesis of cAMP, displayed about the same capacity as CT to evoke the release of [3H]AA metabolites from both S49 WT and cyc− cells. We concluded that stimulation of arachidonate metabolism in S49 murine lymphoma cells by native CT does not require enzymatically functional CT, capable of catalyzing the ADP-ribosylation reaction. These results demonstrated for the first time that stimulation of adenylate cyclase by CT and stimulation of AA metabolism by CT are not necessarily coregulated. In addition, the B subunits purified from native CT and CT-2* both simulated the release of [3H]AA from S49 cyc− cells and murine monocyte/macrophage cells (RAW 264.7), suggesting a receptor-mediated cell activation process of potential importance in enhancing immune responses to vaccine components.
The mechanism of action of cholera toxin (CT) has continued to intrigue investigators ever since it was discovered that this protein toxin stimulates adenylate cyclase activity (8, 38, 39) and chloride ion transport (12, 13). CT is an enzyme with dual nonlethal effects on eukaryotic cells (i.e., NAD+ glycohydrolase and ADP-ribosyltransferase activities) (26). New information about additional molecular events involving the synthesis and/or release of other potent mediators (e.g., prostaglandins [PGs] and 5-hydroxytryptamine) in cells exposed to CT has emerged (3, 15, 27, 29–32, 40). The physiological effects of these signals have been the target of intense investigation in several laboratories (3, 27, 29, 40). CT’s most important physiological effect is in evoking the hypersecretion of water and electrolytes from the small intestines of patients with cholera, an infectious disease limited to humans, who acquire Vibrio cholerae from contaminated food or water. The precise series of molecular events initiated after CT binds to its membrane receptor (GM1 ganglioside) depends on the hydrolysis of CT’s substrate, NAD+, and the transfer of ADP-ribose to a target protein(s). One such well-studied target protein is GSα, a GTP-binding regulatory protein (G protein) that normally increases the catalytic activity of membrane-bound adenylate cyclase, which in turn converts ATP to cyclic AMP (cAMP) (6, 21–23, 26).
Patients with cholera have increased amounts of cAMP in their small-intestinal mucosae (8, 9). In addition, intestinal fluids from patients with acute cholera contain increased levels of PGE2, which can affect the secretion of water and electrolytes (41). These observations are supported by numerous studies, with both animal models and cultured cells, indicating that CT increases eicosanoid synthesis (3, 7, 25, 29, 30, 37). Soon after exposure of cells to CT, the synthesis of eicosanoids (e.g., PGs and leukotrienes) (30, 31) and other lipid metabolites (e.g., platelet-activating factor [PAF]) increases (17). Although Burch et al. (7) demonstrated that CT stimulates phospholipase A2 (PLA2) activity in a murine monocyte/macrophage cell line (RAW 264.7) and Peterson et al. (33) recently reported that CT induces expression of a gene encoding the PLA2-activating protein PLAP) in several types of cells, the precise mechanism by which CT stimulates arachidonic acid (AA) metabolism is unclear. It was not known whether CT’s effect on AA metabolism emanated from the increased cAMP levels in cells (26) or occurred by another mechanism. Membrane-permeable cAMP derivatives have been shown to stimulate eicosanoid synthesis in eukaryotic cells (30), and PGE2, in turn, stimulates adenylate cyclase activity (24, 31). In clinical cholera, a combination of these events provides an enhancement loop that heightens and prolongs the secretory response.
Studies separating the cellular effects of CT on the adenylate cyclase system from AA metabolism have been fraught with difficulties. Early studies with drugs that block the cyclooxygenase pathways and PG synthesis revealed that indomethacin reduced the secretory effect of CT in the small intestine of the rabbit (16). For example, indomethacin exerted a suppressive effect on jejunal secretion in V. cholerae-infected patients (44), although the clinical benefit of indomethacin in the treatment of patients with severe cholera was disappointing (35). While this collective information supported a role for PGs in cholera, it was well known that indomethacin was not always specific in its inhibitory effect on cyclooxygenase (28). Indeed, indomethacin reduced adenylate cyclase activity, thereby reducing the level of cAMP accumulation in the intestine during experimental cholera (28). Further, reports showed that the local arterial concentration of exogenous PGE2 required for half-maximal stimulation of fluid secretion was 2 orders of magnitude below the concentration required for stimulation of adenylate cyclase in vitro (2).
Consequently, we designed experiments to determine whether the ADP-ribosylating activity of CT (26) for GSα was necessary for CT to stimulate AA metabolism. In these studies, we used both native CT and a mutant CT protein, known as CT-2* (19), which contains two amino acid substitutions in the active site of the A subunit, rendering it ADP-ribosyltransferase inactive (19). To determine the importance of CT-induced levels of cAMP on the capacity of CT to stimulate AA metabolism, we selected S49 wild-type (WT) murine lymphoma cells and a mutant S49 cell line, cyc−, in which the gene for GSα had been deleted (21). In cyc− cells, GSα was not produced, which rendered them insensitive to stimuli that normally increase cAMP levels (e.g., CT) (21). These experiments examined the molecular mechanism by which CT stimulates arachidonate metabolism and determined whether increases in cAMP levels are essential for this stimulation to occur. These results are important in determining the mechanism by which CT stimulates intestinal secretion as well as the mucosal immune response.
MATERIALS AND METHODS
Sources of CT and mutant CT-2* protein.
Native CT was purchased from Sigma Chemical Co. (St. Louis, Mo.), and native CT-B subunit was from List Laboratories, Inc. (Campbell, Calif.); after hydration, both were maintained at 4°C without agitation. CT-2* is a CT analog in which two codon substitutions altered the CT-A subunit (Arg7→Lys and Glu112→Gln) and eliminated the toxin’s ADP-ribosylation activity (19). The CT-2* protein was purified to homogeneity from V. cholerae CVD103[CT-2*] culture filtrates, as described and characterized previously (4). Briefly, the modified CT-2*-encoding gene was subcloned into V. cholerae CVD103 (ctxA ctxB+), and a recombinant strain that secreted inactive CT, referred to as CT-2*, was selected (4). The basic elements of purification included concentration of proteins from the culture medium by sodium hexametaphosphate precipitation (36), affinity purification on galactose-agarose (43), and Sephadex G75 gel filtration chromatography (14). Both native CT and CT-2* preparations were diluted in Dulbecco’s modified Eagle’s medium (DMEM) prior to performance of the assay.
Sources of cell lines.
S49 WT cells are a subclone of S49 murine lymphoma cells (10), and S49 cyc− is a subclone known to lack the guanine nucleotide-binding regulatory component GSα of adenylate cyclase (5). The S49 WT and cyc− cell lines were obtained from the Cell Culture Facility at the University of California at San Francisco. These cell lines were maintained as stationary suspension cultures in flasks containing DMEM plus 10% fetal calf serum (FCS), supplemented with penicillin (100 U/ml), streptomycin (100 μg/ml), and gentamicin (50 μg/ml), at 37°C in an atmosphere of 5% CO2. The Th1.2-positive S49 cells (American Type Culture Collection, Rockville, Md.) were negative for CD3, CD4, and CD8 surface antigens, as determined by flow cytometry (performed by G. Klimpel, University of Texas Medical Branch, Galveston), and produced little or no interleukin-2 (IL-2) (50 pg/ml) with or without exposure to CT or CT-2*, as determined by enzyme-linked immunosorbent assay (Perseptive Diagnostics, Cambridge, Mass.). The murine monocyte/macrophage cell line (RAW 264.7) was purchased from the American Type Culture Collection, and cultures were grown in the same medium and under the same conditions as the S49 cell lines.
[3H]AA release assay.
The phospholipids in the S49 cells (2 × 105/ml) were labeled by a modification of a procedure used previously (37) and consisted of adding [5, 6, 8, 9, 11, 12, 14, 15-3H(N)]arachidonic acid ([3H]AA; American Radiolabeled Chemicals, Inc., St. Louis, Mo.) to a final concentration of 1 μCi/ml in DMEM containing 10% FCS and subsequently incubating overnight at 37°C in an atmosphere of 5% CO2. The cells were centrifuged at 300 × g and washed three times with fresh DMEM containing 0.1% fatty acid-free bovine serum albumin in lieu of FCS. The cell suspension was dispensed into 35-mm-diameter culture dishes in quadruplicate and incubated for 30 min before addition of native CT or CT-2* (1 μg/ml) (18). After 2 h or 4 h, the suspension cultures were centrifuged (300 × g), and the radioactivity in aliquots of the supernatants was determined with a liquid scintillation counter.
Assays for cAMP and PGE2.
S49 WT and cyc− cells (2 × 105/ml) were dispensed into duplicate 35-mm-diameter plates and incubated with CT or CT-2* (1 μg/ml) for 2 to 6 h at 37°C in an atmosphere of 5% CO2. Radioimmunoassay kits for measuring cAMP and PGE2 were purchased from Perseptive Diagnostics. Extraction and assay of cAMP and PGE2 were performed in duplicate as recommended by the manufacturer.
Separation of [3H]AA metabolites by high-performance liquid chromatography (HPLC).
Separation of 3H-labeled PGs was accomplished essentially as described previously (25). Briefly, the culture media (2 ml) from triplicate 35-mm-diameter culture dishes containing S49 WT or cyc− cells that had been exposed to native CT or CT-2* were pooled and lyophilized. Subsequently, the samples were each hydrated with 1 ml of water and mixed with 3 ml of cold acetone to precipitate proteins. Two milliliters of petroleum ether was added to each of the supernatants, the tube contents were shaken, and the top layer (petroleum ether) was discarded. All samples were acidified to pH 3 with HCl, and then 3 ml of ethyl acetate was added to each. After sample agitation and phase separation, the top (organic) phase was collected and evaporated to dryness under a vacuum. Each sample was hydrated in 100 μl of 27% acetonitrile in 0.1% trifluoroacetic acid (TFA) and chromatographed through a C18 reverse-phase column (4.6 by 250 mm, 5 μm; Serva Biochemicals, Westbury, N.Y.) equilibrated with the same solvent (25). Fractions (1 ml) were evaporated under a vacuum, and their radioactivity levels were determined with a liquid scintillation counter. PG standards were purchased from Sigma Chemical Co., and their elution profiles were determined by monitoring the eluant at 192 nm.
Rabbit intestinal-loop assay.
Ten-centimeter segments were constructed with OO silk suture in the small intestines of two New Zealand albino rabbits as described previously (29, 31). One milliliter of CT or CT-2* (1 μg/ml) diluted in phosphate-buffered saline (PBS) was injected into the lumen of the intestinal loop in triplicate in each animal. After 16 h, the rabbits were euthanized, the lengths of the loops were determined, and luminal fluid that had accumulated was collected and measured. When no fluid was present, 10 ml of cold PBS was injected and gently used as a lavage fluid. The concentration of PGE2 in the intestinal fluid samples was determined by radioimmunoassay as specified by the manufacturer (Perseptive Diagnostics).
Statistical analysis.
We used the Student’s t test or Dunnett’s multiple group comparison test to establish the significance of differences between experimental and control groups.
RESULTS
cAMP responses of S49 WT and cyc− cells to CT and CT-2*.
The cAMP content of S49 Cyc− cells should not be affected by native CT because of the deletion of the gene encoding GSα (21), and CT-2* should not increase cAMP levels of S49 WT cells because of the mutations in the active site of the A subunit that eliminate the toxin’s ADP-ribosylation activity (26). The data shown in Table 1 validate these assumptions. In fact, the amount of cAMP extracted from the S49 cyc− cells, as well as from the culture medium, after exposure to native CT for 4 h was unchanged (P > 0.05). In contrast, cAMP levels in S49 WT cells exposed to native CT increased 2.4-fold (P < 0.01), and the amount of cAMP released from the cells into the medium increased by 2.6-fold (P < 0.01). Exposure of the S49 WT cells to the mutant CT-2* protein did not increase the amount of cAMP in the cells or in the medium compared to the controls. Rather, a small decrease in cAMP level was observed in both the cells and the medium. In general, the cAMP content of the S49 WT control cells was 5.8-fold higher than that of the S49 cyc− cells, while the cAMP level in the medium of the S49 WT control cells was 1.4-fold higher than that of the S49 cyc− cells. Further, the amount of cAMP in the S49 WT control cells was 2.9-fold less than that present in the culture medium, while the cAMP content of the S49 cyc− control cells was 12-fold less than that detected in the culture medium.
TABLE 1.
Effect of native CT and CT-2* on total cAMP levels in cultures of S49 WT and S49 cyc− cellsa
| Treatment group | Mean amt of cAMP ± SD (pmol) for:
|
|||
|---|---|---|---|---|
| S49 WT
|
S49 cyc−
|
|||
| Cells | Medium | Cells | Medium | |
| Control | 6.9 ± 1.3 | 20.0 ± 0.5 | 1.2 ± 0.3 | 14.8 ± 3.1 |
| CT, 1 μg/ml | 16.6 ± 1.1b | 51.7 ± 7.8b | 1.3 ± 0.2d | 15.3 ± 2.7d |
| CT-2*, 1 μg/ml | 5.0 ± 0.3c | 16.9 ± 0.9b | 1.4 ± 0.3d | 13.1 ± 1.5d |
Cells growing in duplicate 35-mm-diameter plates were treated with native CT or mutant CT-2* for 4 h at 37°C in an atmosphere of 5% CO2. cAMP was extracted from the cells, as well as the culture medium, and the mean amounts of cAMP (± the standard deviation) in duplicate aliquots from each sample were estimated by radioimmunoassay.
P < 0.01 relative to the respective control, Student’s t test.
P < 0.05 relative to the respective control, Student’s t test.
P > 0.05 relative to the respective control, Student’s t test.
Release of [3H]AA metabolites from S49 WT and cyc− cells.
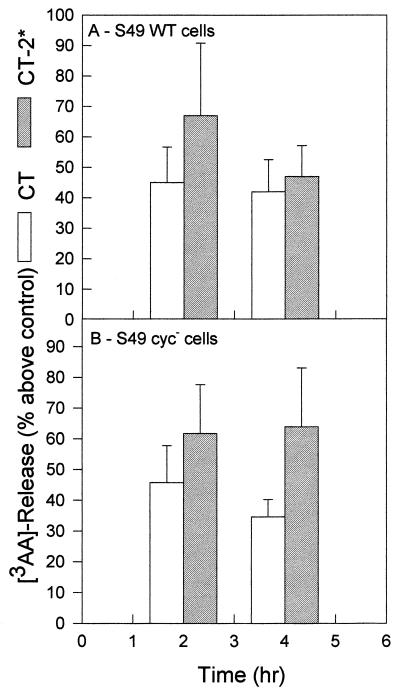
The native-CT-induced [3H]AA release response (24) of S49 WT cells should be similar to that of other cells (7, 25, 29, 30, 37); however, the response of S49 cyc− cells was not predictable. The data in Fig. 1 illustrate the mean responses (± standard error) of S49 WT (Fig. 1A) and cyc− (Fig. 1B) cells that had been loaded with [3H]AA before exposure to native CT or the mutant CT-2* protein. The percentage increases in release of [3H]AA metabolites from the cells into the culture medium relative to the controls after either 2- or 4-h incubation periods with the stimuli were plotted (Fig. 1). After a 2-h exposure to native CT, the percentages of [3H]AA metabolites released from the S49 WT and cyc− cells were similar (45% ± 12% and 46% ± 12%, respectively), and the amounts of CT-induced [3H]AA released from both cell types were significantly higher than those of the controls (P < 0.01) (Fig. 1). In comparison, the percentages of [3H]AA metabolites released in 2 h from the two cell types after exposure to the mutant CT-2* were approximately the same (67% ± 24% and 62% ± 16%, respectively) (Fig. 1), and as with CT, the CT-2*-induced responses were significant (P < 0.01) compared to those of the respective cell controls. By 4 h, the overall increase in percentage release of [3H]AA metabolites remained essentially unchanged from that seen at 2 h (Fig. 1).
FIG. 1.
Release of [3H]AA from S49 WT (A) and S49 cyc− (B) cells after exposure of the cells to native CT or CT-2* for a period of 2 or 4 h. The percentage of [3H]AA released into the culture supernatants, exceeding that of the respective untreated control cells, was plotted for seven experiments in which cells were plated in quadruplicate. The standard error is indicated above and below each mean. The average amount of [3H]AA released in response to native CT or CT-2* was significantly more than the amount spontaneously released from untreated control cells, as determined by Student’s t test (P < 0.01).
Since mutant CT caused the release of [3H]AA metabolites from S49 cyc− cells, we wanted to determine whether CT-B subunit alone would evoke a similar response. Table 2 summarizes results from four experiments in which CT-B from native CT or CT-2* was tested for its capacity to evoke the release of [3H]AA from either S49 cyc− cells or RAW 264.7 cells. Clearly, CT-B from either CT or CT-2* stimulated AA metabolism, as evidenced by increased [3H]AA release (Table 2). Irrespective of cell type, the amount of [3H]AA release evoked by CT-B was usually about 50% of that caused by the respective parent molecule (CT or CT-2*); the B subunit from CT-2* was the exception on S49 cyc− cells (Table 2).
TABLE 2.
Responses of S49 cyc− cells and RAW 264.7 murine monocyte/macrophage cells to the CT-B subunits from native CT and mutant CT, in comparison to CT and CT-2*
| Elicitor | % of [3H]AA metabolites released bya:
|
|
|---|---|---|
| cyc− S49 cells | RAW 264.7 cells | |
| Native CT | 35.9 ± 9.1 | 54.6 ± 15.4 |
| CT-B subunit | 18.2 ± 12.2b | 28.5 ± 9.8c |
| CT-2* | 25.4 ± 13.9b | 27.0 ± 8.5c |
| CT-2* B subunit | 28.8 ± 15.3b | 13.6 ± 8.4c |
The values shown, which are percentages above the respective controls, are the means ± standard deviations of data from four experiments in which the release of [3H]AA metabolites from cells was measured 6 h after stimulation.
P > 0.05 relative to the respective CT control, Dunnett’s multiple comparison test.
P < 0.05 relative to the respective CT control, Dunnett’s multiple comparison test.
HPLC separation of [3H]AA metabolites released from S49 WT and cyc− cells.
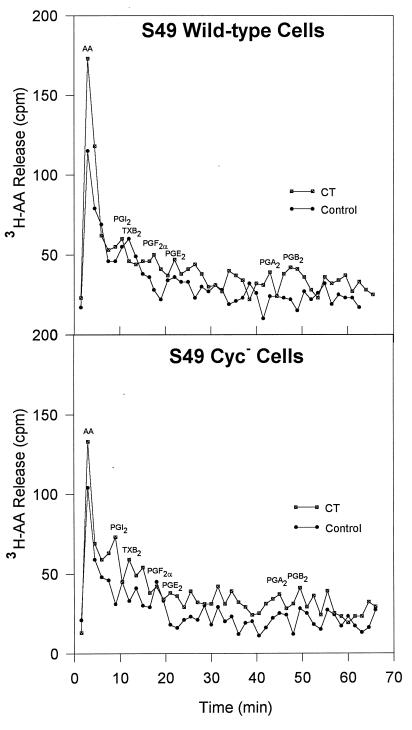
In an attempt to identify the [3H]AA metabolites released from the S49 WT and cyc− cells after native-CT treatment, PGs were extracted from the culture supernatants and chromatographed on a C18 reverse-phase column, using an isocratic gradient of 27% acetonitrile in 0.1% TFA. Figure 2 shows the positions at which selected PG standards eluted. The data illustrated in Fig. 2 indicate that minimal amounts of PGs were formed by the S49 WT and cyc− cells after exposure to native CT. Rather, most of the 3H radiolabel was associated with the AA peak, with minimal incorporation into eicosanoids. The chromatographic results shown in Fig. 2 were extended by testing culture supernatants of S49 WT and cyc− cells exposed to native CT for PGE2 by a radioimmunoassay for PGE2, but the levels of PGE2 were too low for reliable quantitation (data not shown).
FIG. 2.
HPLC separation of [3H]AA metabolites released from S49 WT and S49 cyc− cells exposed to native CT. After extraction of eicosanoids from the culture supernatants of three cultures, samples were chromatographed through a C18 reverse-phase column, using an isocratic gradient of 27% acetonitrile in 0.1% TFA. All fractions were evaporated to dryness in a vacuum centrifuge and hydrated with 50% acetonitrile, and their levels of radioactivity were then measured in a liquid scintillation counter. The positions of selected PGs are indicated on the profiles.
Effect of CT and CT-2* in rabbit intestinal loops.
The results summarized in Table 3 indicate that CT, but not CT-2*, evoked a secretory response when injected into rabbit intestinal segments. Further, only CT increased the concentration of PGE2 detectable in the luminal fluid.
TABLE 3.
Rabbit intestinal responses to CT and CT-2* after 16 ha
| Treatment group | Fluid accumulation (ml/cm) | PGE2 concn (ng/ml) |
|---|---|---|
| PBS control | 0 | 9.1 ± 0.9b |
| CT, 1 μg/ml | 1.9 ± 0.17 | 33.1 ± 5.0c |
| CT-2*, 1 μg/ml | 0 | 8.4 ± 1.7bd |
The values shown are mean responses from three intestinal loops constructed in each of two rabbits, ± the standard deviations.
Reflects the concentration of PGE2 in 10 ml of lavage fluid.
P < 0.05 relative to the respective control, Student’s t test.
P > 0.05 relative to the respective control, Student’s t test.
DISCUSSION
Distinguishing between CT-induced effects on the synthesis of cAMP and AA metabolites has been challenging because both molecular events occur simultaneously in virtually all cells. Therefore, it has been difficult to define the independent physiological or immunological impact of cAMP and PGs on the small intestinal mucosa. Indeed, prior to this study, it was uncertain whether CT-induced eicosanoid synthesis (3, 29, 30) resulted simply from the CT-induced increase in cAMP concentration in intestinal epithelial cells. Since no intestinal cell line that contained a mutation in a gene encoding a protein component required for the synthesis of PGs or cAMP was available, we selected the S49 murine lymphoma cyc− cell line, which, unlike its isogenic Th1.2-positive S49 WT parent cell line, lacks GSα. By comparing the effects of CT on AA metabolism in these cells, we were able to separate CT’s stimulatory effect on [3H]AA release from its effect on cAMP synthesis. Since the cyc− cells lack the gene encoding GSα, the cells do not produce an effective protein target that could be ADP-ribosylated by native CT. Consequently, CT could not upregulate the enzymatic activity of adenylate cyclase. The data summarized in Table 1 were consistent with an earlier report that the S49 cyc− cells are deficient in GSα (21), since cAMP levels increased only in the S49 WT cells and not in the S49 cyc− cells exposed to native CT. Basal cAMP levels were approximately fivefold lower in the S49 cyc− cells than in the S49 WT cells. The lower level of cAMP was likely due to the absence of the GSα in the S49 cyc− cells, which diminished the responsiveness of these cells to stimuli requiring this G protein.
When the native CT-induced [3H]AA release responses of S49 cyc− cells were compared with those of the S49 WT cells, it was clear that the S49 cyc− cells released essentially the same percentage of [3H]AA as did the S49 WT cells after 2 or 4 h of incubation (Fig. 1). Therefore, an increased amount of cAMP, which was not present in the native-CT-treated S49 cyc− cells, was not essential for the mechanism by which native CT stimulated PLA2 activity. Although these data showed no correlation between CT-induced release of [3H]AA and cAMP synthesis in S49 cyc− cells, dibutyryl cAMP could stimulate [3H]AA release in other cell lines (e.g., Chinese hamster ovary cells and murine monocyte/macrophage cells) (7, 25, 30, 37). We also determined that addition of dibutyryl cAMP (10 mM) to the culture media of S49 WT and cyc− cells plated in quadruplicate resulted in release of 40% ± 6% and 37% ± 6% [3H]AA, respectively, in a 2-h period (data not shown). After 4 h, dibutyryl cAMP-induced [3H]AA release diminished to 28% ± 5% and 25% ± 4% above control levels in S49 WT and cyc− cells, respectively. These data indicated that increases in cAMP in S49 cells indeed could cause release of [3H]AA metabolites; however, the S49 cyc− cells showed no increase in cAMP levels after exposure to native CT (Table 1). Thus, the low levels of cAMP had a permissive effect but were not needed for the [3H]AA metabolite release response to CT (Fig. 1). It is also interesting that CT-2* significantly decreased cAMP levels in S49 WT cells and their culture medium compared to the control levels (Table 1). The basis for this effect could have been due to the PGE2 formed in response to CT-2*. Thielman et al. (42) observed that addition of PGE2 to Chinese hamster ovary cell cultures caused a modest increase in cAMP within 10 min but that the cAMP level decreased between 1 and 4 h. The mechanism of this effect is not known.
If cAMP is not required for CT-induced release of [3H]AA from S49 cyc− cells, what can be concluded about the mechanism by which CT stimulates AA metabolism in these cells? To provide some answers to this question, we used CT-2*, which lacks the enzymatic capacity to catalyze the ADP-ribosylation reaction (26). The data in Fig. 1 indicate that the capacity of the mutant CT protein (CT-2*) to induce [3H]AA release in both S49 WT and cyc− cells was not noticeably diminished by the mutation in the active site of the CT-A subunit (Arg7→Lys and Glu112→Gln) (19). Thus, the capacity to catalyze ADP-ribosylation was not essential for CT to evoke the release of [3H]AA from S49 WT and cyc− cells, and GSα was not the target of ADP-ribosylation that led to [3H]AA release from S49 cyc− cells. Alternatively, CT could have stimulated AA metabolism in the S49 cells by a direct mechanism of PLA2 activation. Recently, we reported that CT induced the expression of the gene encoding PLAP (33). The mechanism by which the PLAP gene is induced by CT was not clear, but increased PLA2 activity occurred within minutes to hours depending on the cell type (33). The cellular response to the PLA2-catalyzed hydrolysis of membrane phospholipids and AA release resulted in a generalized enhancement of cyclooxygenase activity, e.g., production of PGE2. The latter eicosanoid is known to exert potent stimulatory effects on ion transport. Indeed, the results in Table 3 indicate that PGE2 production correlated with CT-induced fluid accumulation in rabbit intestinal segments; however, CT-2* neither evoked intestinal fluid loss nor increased PGE2 synthesis in the small intestine. Our in vitro results indicated that S49 murine lymphoma cells and RAW 264.7 cells responded to CT-2* (Table 2) but the intestinal mucosa in vivo (Table 3) did not. The reasons for this apparent discrepancy are not clear, but there are several variables that are different. For example, the cell types used in vitro were T lymphocytes and macrophages, because of their potential role in the immune response. The in vivo results were derived with intestinal segments, which contain many cell types. Further, the cell culture experiments were performed within 4 to 6 h, while the intestinal loops were examined after 16 h. Importantly, we should consider the possibility that CT stimulates AA metabolism, leading to PGE2 synthesis, by at least two different mechanisms. The results observed here with murine lymphocyte and macrophage cell lines suggest that the CT-B subunit and the mutant CT (CT-2*) activate receptor-mediated signal transduction, which results in PGE2 formation. While this effect may be occurring in lymphoid cells in vivo, such a direct effect does not appear to occur in enterocytes involved in water and electrolyte transport. On the other hand, CT catalyzes the ADP-ribosylation of GSα of adenylate cyclase, which stimulates cAMP formation. Since cAMP can elicit PGE2 synthesis, it appears from our results that the PGE2 in the intestinal loops could be secondary to cAMP formation. In effect, the secretory response during cholera likely results from a combination of cAMP and cAMP-induced PGE2, while adjuvant effects of CT-B subunit preparations likely involve activation of AA metabolism in macrophages and lymphocytes (1, 20, 34, 45). It is not clear why in this study (Table 1) S49 cells exposed to CT-B or CT-2* evoked just as much [3H]AA release as did CT. It is possible that cAMP formed in the CT-treated cells is downregulating the amount of [3H]AA released, since we observed earlier that addition of dibutyryl cAMP to rabbit intestinal loops decreased the amount of PGE2 formed in response to CT (31).
In this study, we observed that AA metabolism of murine S49 murine lymphoma cells was stimulated as readily by CT-2* as it was by native CT (Fig. 1) and CT-B (Table 2). Similarly, CT-2* and CT-B evoked the release of [3H]AA from murine monocytes/macrophages (RAW 264.7 cells) (Table 2). Reflecting on the possible immunological significance of these observations, stimulation of AA metabolism in macrophages could activate the cells and enhance antigen processing and presentation to lymphocytes. Earlier studies have determined that addition of AA or PGE2 to pristane-elicited murine macrophages increases IL-6 production (45). Indeed, PGE2 serves as an important stimulus for IL-6 production (1, 20, 34), and IL-6 is an important cytokine in antibody production (20). The fact that S49 murine lymphoma cells are T lymphocytes (Th1.2) could also be important in explaining the immunomodulatory properties of mutant CT proteins that lack enzymatic activity (46, 47). The observation that AA metabolism in these T cells is enhanced by CT-2* and CT-B suggests a mechanism of lymphocyte activation by mutant CT (46, 47). Our results indicate that CT-B stimulated the release of [3H]AA from S49 cells, usually at a level approximately one-half that of the respective CT or CT-2* molecule. This apparent difference could be important, since some reports indicated that mutant CT, but not recombinant CT-B, enhanced antibody responses to tetanus toxoid, ovalbumin, and influenza virus (46, 47). In response to vaccination, cells involved in antigen processing could be stimulated by mutant CT, or possibly by its B subunit under some conditions. Using identical assay procedures with S49 cyc− cells, we recently tested the mutant CT and matching B subunit used by Yamamoto et al. (46, 47) to assess the adjuvant effect of these recombinant proteins. Both their mutant CT and mutant CT-B subunit evoked [3H]AA release comparable to that of CT-2* and the CT-2* B subunit. Based on these observations, the adjuvant activity described for mutant CT or its B subunit may involve a combination of receptor-mediated signaling and/or eicosanoid formation, but it does not require the enzymatic activity of the CT-A subunit.
In conclusion, CT’s stimulatory effect on AA metabolism in S49 cyc− cells constitutes a novel molecular event, independent of GSα or cAMP synthesis. Several other receptor-mediated stimuli (e.g., bradykinin, N-methyl-d-aspartate, and 5-hydroxytryptamine [11]) are known to stimulate PLA2 activity in other cells. In S49 cells, CT-2* clearly did not require the enzymatic activity of the CT-A subunit to stimulate AA metabolism, and a similar mechanism could stimulate other types of cells. Stimulation of AA metabolism in lymphocytes and macrophages may be important in the mechanism of the effect of CT or the CT-B subunit on immune modulation, but additional studies are needed to define its precise role.
ACKNOWLEDGMENTS
We acknowledge H. R. Bourne for supplying S49 WT and the S49 cyc− cell line, which does not synthesize GSα. We thank Jerry McGhee for discussions of the adjuvant activity of mutant CT and for the opportunity to test the McGhee Lab CT proteins in our system.
This research was supported by research grants R01 AI18401, R01 AI21463 (both to J.W.P.), and R01 AI17312 (to R.A.F.) from the National Institute of Allergy and Infectious Diseases.
REFERENCES
- 1.Anderson G D, Hauser S D, McGarity K L, Bremer M E, Isakson P C, Gregory S A. Selective inhibition of cyclooxygenase (COX)-2 reverses inflammation and expression of COX-2 and interleukin 6 in rat adjuvant arthritis. J Clin Investig. 1996;97:2672–2679. doi: 10.1172/JCI118717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beubler E, Bukhave K, Rask-Madsen J. Significance of calcium for protstaglandin E2-mediated secretory response to 5-hydroxytryptamine in the small intestine of the rat in vivo. Gastroenterology. 1986;90:1972–1977. doi: 10.1016/0016-5085(86)90269-6. [DOI] [PubMed] [Google Scholar]
- 3.Beubler E, Kollar G, Saria A, Bukhave K, Rask-Madsen J. Involvement of 5-hydroxytryptamine, prostaglandin E2, and cyclic adenosine monophosphate in cholera toxin-induced fluid secretion in the small intestine of the rat in vivo. Gastroenterology. 1989;96:368–376. doi: 10.1016/0016-5085(89)91560-6. [DOI] [PubMed] [Google Scholar]
- 4.Boesman-Finkelstein M, Peterson J W, Thai L S, Finkelstein R A. A nontoxic cholera enterotoxin (CT) analog is chimeric with regard to both epitypes of CT-B subunits, CT-B-1 and CT-B-2. Infect Immun. 1996;64:346–348. doi: 10.1128/iai.64.1.346-348.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bourne H R, Coffino P, Tomkins G M. Selection of a variant lymphoma cell deficient in adenylate cyclase. Science. 1975;187:750–752. doi: 10.1126/science.163487. [DOI] [PubMed] [Google Scholar]
- 6.Bourne H R, Landis C A, Masters S B. Hydrolysis of GTP by alpha-chain of Gs and other GTP binding proteins. Proteins. 1989;6:222–230. doi: 10.1002/prot.340060304. [DOI] [PubMed] [Google Scholar]
- 7.Burch R M, Jelsema C, Axelrod J. Cholera toxin and pertussis toxin stimulate prostaglandin E2 synthesis in a murine macrophage cell line. J Pharmacol Exp Ther. 1988;244:765–773. [PubMed] [Google Scholar]
- 8.Chen L C, Rohde J E, Sharp G W. Intestinal adenyl-cyclase activity in human cholera. Lancet. 1971;i:939–941. doi: 10.1016/s0140-6736(71)91443-7. [DOI] [PubMed] [Google Scholar]
- 9.Chen L C, Rohde J E, Sharp G W. Properties of adenyl cyclase from human jejunal mucosa during naturally acquired cholera and convalescence. J Clin Investig. 1972;51:731–740. doi: 10.1172/JCI106867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coffino P, Bourne H R, Tomkins G M. Somatic genetic analysis of cyclic AMP action: selection of unresponsive mutants. J Cell Physiol. 1975;85:603–610. doi: 10.1002/jcp.1040850312. [DOI] [PubMed] [Google Scholar]
- 11.Felder C C, Kanterman R Y, Ma A L, Axelrod J. Serotonin stimulates phospholipase A2 and the release of arachidonic acid in hippocampal neurons by a type 2 serotonin receptor that is independent of inositolphospholipid hydrolysis. Proc Natl Acad Sci USA. 1990;87:2187–2191. doi: 10.1073/pnas.87.6.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Field M. Intestinal secretion: effect of cyclic AMP and its role in cholera. N Engl J Med. 1971;284:1137–1144. doi: 10.1056/NEJM197105202842008. [DOI] [PubMed] [Google Scholar]
- 13.Field M, Fromm D, al-Awqati Q, Greenough W B. Effect of cholera enterotoxin on ion transport across isolated ileal mucosa. J Clin Investig. 1972;51:796–804. doi: 10.1172/JCI106874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finkelstein R A, LoSpalluto J J. Pathogenesis of experimental cholera. Preparation and isolation of choleragen and choleragenoid. J Exp Med. 1969;130:185–202. doi: 10.1084/jem.130.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujita T, Osaka M, Yanatori Y. Granule release of enterochromaffin (EC) cells by cholera enterotoxin in the rabbit. Arch Histol Jpn. 1974;36:367–378. doi: 10.1679/aohc1950.36.367. [DOI] [PubMed] [Google Scholar]
- 16.Gots R E, Formal S B, Giannella R A. Indomethacin inhibition of Salmonella typhimurium, Shigella flexneri, and cholera-mediated rabbit ileal secretion. J Infect Dis. 1974;130:280–284. doi: 10.1093/infdis/130.3.280. [DOI] [PubMed] [Google Scholar]
- 17.Guerrant R L, Fang G D, Thielman N M, Fonteles M C. Role of platelet activating factor in the intestinal epithelial secretory and Chinese hamster ovary cell cytoskeletal responses to cholera toxin. Proc Natl Acad Sci USA. 1994;91:9655–9658. doi: 10.1073/pnas.91.20.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haga T, Ross E M, Anderson H J, Gilman A G. Adenylate cyclase permanently uncoupled from hormone receptors in a novel variant of S49 mouse lymphoma cells. Proc Natl Acad Sci USA. 1977;74:2016–2020. doi: 10.1073/pnas.74.5.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Häse C C, Thai L S, Boesman-Finkelstein M, Mar V L, Burnette W N, Kaslow H R, Stevens L A, Moss J, Finkelstein R A. Construction and characterization of recombinant Vibrio cholerae strains producing inactive cholera toxin analogs. Infect Immun. 1994;62:3051–3057. doi: 10.1128/iai.62.8.3051-3057.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hinson R M, Williams J A, Shacter E. Elevated interleukin 6 is induced by prostaglandin E2 in a murine model of inflammation: possible role of cyclooxygenase-2. Proc Natl Acad Sci USA. 1996;93:4885–4890. doi: 10.1073/pnas.93.10.4885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson G L, Kaslow H R, Bourne H R. Genetic evidence that cholera toxin substrates are regulatory components of adenylate cyclase. J Biol Chem. 1978;253:7120–7123. [PubMed] [Google Scholar]
- 22.Jones D T, Masters S B, Bourne H R, Reed R R. Biochemical characterization of three stimulatory GTP-binding proteins. The large and small forms of Gs and the olfactory-specific G-protein, Golf. J Biol Chem. 1990;265:2671–2676. [PubMed] [Google Scholar]
- 23.Kaslow H R, Groppi V E, Abood M E, Bourne H R. Cholera toxin can catalyze ADP-ribosylation of cytoskeletal proteins. J Cell Biol. 1981;91:410–413. doi: 10.1083/jcb.91.2.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kimberg D V, Field M, Johnson J, Henderson A, Gershon E. Stimulation of intestinal mucosal adenyl cyclase by cholera enterotoxin and prostaglandins. J Clin Investing. 1971;50:1218–1230. doi: 10.1172/JCI106599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liang Y, Peterson J W, Jackson C A, Reitmeyer J C. Chloroquine inhibition of cholera toxin. FEBS Lett. 1990;275:143–145. doi: 10.1016/0014-5793(90)81459-2. [DOI] [PubMed] [Google Scholar]
- 26.Moss J, Vaughan M. Cholera toxin and E. coli enterotoxins and their mechanisms of action. In: Hardegree M C, Tu A T, editors. Handbook of natural toxins. 4. Bacterial toxins. New York, N.Y: Marcel Dekker, Inc.; 1988. pp. 39–87. [Google Scholar]
- 27.Nilsson O, Cassuto J, Larsson P A, Jodal M, Lidberg P, Ahlman H, Dahlstrom A, Lundgren O. 5-Hydroxytryptamine and cholera secretion: a histochemical and physiological study in cats. Gut. 1983;24:542–548. doi: 10.1136/gut.24.6.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peterson J W, Berg W D, Ochoa L G. Indomethacin inhibits cholera toxin-induced cyclic AMP accumulation in Chinese hamster ovary cells. FEMS Microbiol Lett. 1988;49:187–192. [Google Scholar]
- 29.Peterson J W, Cantu J, Duncan S, Chopra A K. Molecular mediators formed in the small intestine in response to cholera toxin. J Diarrhoeal Dis Res. 1993;11:227–234. [PubMed] [Google Scholar]
- 30.Peterson J W, Jackson C A, Reitmeyer J C. Synthesis of prostaglandins in cholera toxin-treated Chinese hamster ovary cells. Microb Pathog. 1990;9:345–353. doi: 10.1016/0882-4010(90)90068-2. [DOI] [PubMed] [Google Scholar]
- 31.Peterson JW, Lu Y, Duncan S, Cantu J, Chopra A K. Interactions of intestinal mediators in the mode of action of cholera toxin. J Med Microbiol. 1994;41:3–9. doi: 10.1099/00222615-41-1-3. [DOI] [PubMed] [Google Scholar]
- 32.Peterson J W, Ochoa L G. Role of prostaglandins and cAMP in the secretory effects of cholera toxin. Science. 1989;245:857–859. doi: 10.1126/science.2549637. [DOI] [PubMed] [Google Scholar]
- 33.Peterson J W, Saini S S, Dickey W D, Klimpel G R, Bomalaski J S, Clark M A, Xu X-J, Chopra A K. Cholera toxin induces synthesis of phospholipase A2-activating protein. Infect Immun. 1996;64:2137–2143. doi: 10.1128/iai.64.6.2137-2143.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Portanova J P, Zhang Y, Anderson G D, Hauser S D, Masferrer J L, Seibert K, Gregory S A, Isakson P C. Selective neutralization of prostaglandin E2 blocks inflammation, hyperalgesia, and interleukin 6 production in vivo. J Exp Med. 1996;184:883–891. doi: 10.1084/jem.184.3.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rabbani G H, Butler T. Indomethacin and chloroquine fail to inhibit fluid loss in cholera. Gastroenterology. 1985;89:1035–1037. doi: 10.1016/0016-5085(85)90205-7. [DOI] [PubMed] [Google Scholar]
- 36.Rappaport R S, Rubin B A, Tint H. Development of a purified cholera toxoid. I. Purification of toxin. Infect Immun. 1974;9:294–303. doi: 10.1128/iai.9.2.294-303.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reitmeyer J C, Peterson J W. Stimulatory effects of cholera toxin on arachidonic acid metabolism in Chinese hamster ovary cells. Proc Soc Exp Biol Med. 1990;193:181–184. doi: 10.3181/00379727-193-43022. [DOI] [PubMed] [Google Scholar]
- 38.Schafer D E, Lust W D, Sircar B, Goldberg N D. Elevated concentration of adenosine 3′,5′-cyclic monophosphate in intestinal mucosa after treatment with cholera toxin. Proc Natl Acad Sci USA. 1970;67:851–856. doi: 10.1073/pnas.67.2.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sharp G W G, Hynie S. Stimulation of intestinal adenyl cyclase by cholera toxin. Nature. 1971;229:266–269. doi: 10.1038/229266a0. [DOI] [PubMed] [Google Scholar]
- 40.Sjöqvist A, Cassuto J, Jodal M, Lundgren O. Actions of serotonin antagonists on cholera toxin-induced intestinal fluid secretion. Acta Physiol Scand. 1992;145:229–237. doi: 10.1111/j.1748-1716.1992.tb09360.x. [DOI] [PubMed] [Google Scholar]
- 41.Speelman P, Rabbani G H, Bukhave K, Rask-Madsen J. Increased jejunal prostaglandin E2 concentrations in patients with acute cholera. Gut. 1985;26:188–193. doi: 10.1136/gut.26.2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thielman N M, Marcinkiewicz M, Sarosiek J, Fang G D, Guerrant R L. Role of platelet-activating factor in Chinese hamster ovary cell responses to cholera toxin. J Clin Investig. 1997;99:1999–2004. doi: 10.1172/JCI119368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Uesaka Y, Otsuka Y, Lin Z, Yamasaki S, Yamaoka J, Kurazono H, Takeda Y. Simple method of purification of Escherichia coli heat-labile enterotoxin and cholera toxin using immobilized galactose. Microb Pathog. 1994;16:71–76. doi: 10.1006/mpat.1994.1007. [DOI] [PubMed] [Google Scholar]
- 44.Van Loon F P, Rabbani G H, Bukhave K, Rask-Madsen J. Indomethacin decreases jejunal fluid secretion in addition to luminal release of prostaglandin E2 in patients with acute cholera. Gut. 1992;33:643–645. doi: 10.1136/gut.33.5.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Williams J A, Shacter E. Regulation of macrophage cytokine production by prostaglandin E2. J Biol Chem. 1997;272:25693–25699. doi: 10.1074/jbc.272.41.25693. [DOI] [PubMed] [Google Scholar]
- 46.Yamamoto S, Kiyono H, Yamamoto M, Imaoka K, Fujihashi K, Van Ginkel F W, Noda M, Takeda Y, McGhee J R. A nontoxic mutant of cholera toxin elicits Th2-type responses for enhanced mucosal immunity. Proc Natl Acad Sci USA. 1997;94:5267–5272. doi: 10.1073/pnas.94.10.5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamamoto S, Takeda Y, Yamamoto M, Kurazono H, Imaoka K, Yamamoto M, Fujihashi K, Noda M, Kiyono H, McGhee J R. Mutants in the ADP-ribosyltransferase cleft of cholera toxin lack diarrheagenicity but retain adjuvanticity. J Exp Med. 1997;185:1203–1210. doi: 10.1084/jem.185.7.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]