Abstract
Rationale
Demographic characteristics of pulmonary arterial hypertension (PAH) patients have changed over time, but the effects of cardiovascular risk factors on risk status and pulmonary vascular resistance (PVR) reduction with initial oral combination therapy are not known. Therefore, we tested the relevance of cardiovascular comorbidities in this setting.
Methods
The study enrolled 181 treatment-naive PAH patients with a 6-month (IQR 144–363 days) right heart catheterisation and risk assessment after initial oral combination therapy.
Results
Group A included 96 (53.0%) patients without cardiac comorbidities; Group B included 54 (29.8%) patients with one cardiac comorbidity; Group C included 31 (17.1%) patients with two cardiac comorbidities or more. Group C patients were older with a balanced sex distribution. There was a significant difference in PVR reduction moving from the absence to one or at least two cardiac comorbidities, respectively: median −45.0%, −30.3%, −24.3%. A European Respiratory Society/European Society of Cardiology low-risk status was present at first follow-up in 50 (52.0%) patients in Group A, 19 (35.1%) in Group B and 9 (29.0%) in Group C; a REVEAL 2.0 low-risk status was present at first follow-up in 41 (42.0%) patients in Group A, 15 (27.7%) in Group B and 7 (22.6%) in Group C. Group A patients were 2.3 times more likely to achieve/maintain a low-risk status compared with Group B and C (OR 2.27, 95% CI 1.15–4.54, p=0.02). No significant difference was observed between patients with non-cardiac comorbidities and those without comorbidities.
Conclusion
Initial oral combination therapy seems associated with a less effective response for patients with cardiovascular comorbidities compared with the others, related to the magnitude of treatment-induced decrease in PVR.
Short abstract
In patients with pulmonary arterial hypertension, initial oral combination therapy is associated with a less effective response for those with cardiovascular comorbidities, related to the magnitude of treatment-induced decrease in PVR. https://bit.ly/3QB0Gd5
Introduction
It is now recognised that in Western countries the demographic characteristics of pulmonary arterial hypertension (PAH) patients have changed over time [1–4]. Despite meeting the haemodynamic criteria and accepted definition for PAH, patients in real-life registries have older age and an increasing number of comorbidities, especially cardiovascular risk factors for left ventricular diastolic dysfunction (comorbidities).
After the World Symposium of Pulmonary Hypertension held in Nice in 2018, the treatment algorithm was updated with recommendation of initial combination of oral therapies targeting different pathways according to European Respiratory Society/European Society of Cardiology risk assessment [5]. Although initial oral combination therapy is recommended for the majority of PAH patients at low and intermediate risk, some discussion still persists for those patients with cardiovascular risk factors that may potentially overlap with Group 2 patients of the World Health Organization (WHO) pulmonary hypertension classification [6].
There is currently no consensus on how to define PAH patients with comorbidities, but efforts have been made to better understand their characteristics and response to PAH-targeted therapies. The AMBITION (AMBrIsentan and Tadalafil in Patients with Pulmonary Arterial HypertensION) trial showed that a more aggressive strategy with initial combination of the endothelin receptor antagonist (ERA) ambrisentan and the phosphodiesterase-5 inhibitor (PDE-5i) tadalafil versus either drug alone was associated with a 50% decreased relative risk of clinical deterioration at 6 months [7]. This study provided rationale for the updated treatment algorithm in PAH [5, 8]. However, a pre-specified additional analysis of the AMBITION trial showed a more attenuated treatment response and higher rates of clinical failure events for patients with multiple risk factors for left ventricular diastolic dysfunction treated with initial combination therapy [9].
It has been recently clarified that the efficacy of initial oral combination therapy is related to decreased pulmonary vascular resistance (PVR), allowing right ventricular (RV) afterload reduction and patients’ shift to a low-risk status [10, 11]. The aim of the present study was to investigate the relevance of comorbidities on the haemodynamic and clinical response to initial oral combination therapy in the same cohort of incident PAH patients previously evaluated.
Methods
Population and study design
181 consecutive patients with idiopathic (I) PAH or PAH related to connective tissue disease (CTD) or congenital heart disease with closed shunt, treated with initial double oral combination, were retrospectively analysed. Patients were diagnosed between January 2013 and December 2018 in 11 centres of the Italian Pulmonary Hpertension NETwork (iPHNET). The centres participating in the iPHNET share a common database for the prospective follow-up of PAH patients [12]. The diagnostic workup of PAH conformed to the European guidelines [5] with the typical haemodynamic profile of precapillary pulmonary hypertension, defined by a mean pulmonary artery pressure (mPAP) ≥25 mmHg, a pulmonary artery wedge pressure (PAWP) <15 mmHg, and a PVR>3 Wood units (WU), and the use of an algorithm including respiratory function tests (total lung capacity >70%, forced expiratory volume in 1 s (FEV1) >70%), perfusion lung scan, computed tomography scan and echocardiography. All patients were non-responders to acute vasodilator testing with nitric oxide at the time of diagnosis. Similar therapeutic strategies have been evolved over the years in the same way in each centre as part of the Italian networking. The study complied with the Declaration of Helsinki and was approved by the Institutional Review Board for human studies of the Policlinico Umberto I – Sapienza University of Rome (Protocol n. 42412).
All the patients were followed up by outpatient clinic visits every 3–6 months or when necessary. A complete assessment, including clinical examination, 6-min walk tests, echocardiography, right heart catheterisation, the ERS/ESC guidelines-derived risk assessment and the REVEAL 2.0 score, was collected from the patients’ medical records at baseline and between 3 and 12 months of follow-up.
For the study purposes the following were considered as cardiovascular comorbidities: systemic hypertension, hyperlipidaemia, obesity (mass index ≥30 kg·m−2), diabetes mellitus, peripheral artery disease, coronary artery disease (myocardial infarction, previous coronary angioplasty or coronary artery bypass grafting) not associated with left heart systolic dysfunction and previous atrial fibrillation. Of note, in the AMBITION trial [9] risk factors for left ventricular diastolic dysfunction included body mass index ≥30 kg·m−2, history of systemic hypertension, diabetes mellitus or historical evidence of significant coronary disease.
All clinical conditions not affecting heart function were considered non-cardiac comorbidities, based on clinical judgement.
Measurements
Right heart catheterisation was performed in accordance with the European guidelines [1], with zero reference levelled at mid chest in the supine position and cardiac output (CO) measured by thermodilution. PVR was calculated as (mPAP − PAWP)/CO. Baseline echocardiographic data were acquired using commercially available equipment and standard views, in accordance with international guidelines [13], as previously reported [10]. To avoid missing data, only a limited number of simple parameters and derived measures were considered in the analysis: RV end-diastolic area to left ventricular end-diastolic area ratio (RVEDA/LVEDA) in the four-chamber view (qualitative assessment), tricuspid annular plane systolic excursion (TAPSE) and presence of pericardial effusion. Tricuspid regurgitation was semi-quantitatively graded as mild, moderate or severe. We previously reported on interobserver variabilities of these measurements among the Italian Pulmonary Hypertension Network [14]: RVEDA intra-observer 0.18±0.66 (95% confidence interval (CI): −1.09–1.45), interobserver 0.15±1.08 (95% CI: −2.07–2.37); LVEDA intra-observer 0.06±0.79 (95% CI: −1.52–1.64), interobserver −0.07±0.76 (95% CI: −1.63–1.49); TAPSE intra-observer 0.20±0.63 (95% CI: −1.03–1.43), interobserver 0.00±0.67 (95% CI: −1.06–1.06).
Risk assessment was based on a simplified version of the ERS/ESC guidelines score, with incorporation of WHO functional class, 6-min walk distance (6MWD), right atrial pressure (RAP) and cardiac index [15], and on the REVEAL score 2.0, which incorporates aetiology, age, sex, WHO functional class, systolic blood pressure, heart rate, right atrial pressure, PVR, 6MWD, lung diffusing capacity for carbon monoxide, brain natriuretic peptide levels, renal function, echocardiography of pericardial effusion and previous hospitalisation [16].
As European guidelines recommend the achievement of a low-risk status as a treatment goal [5], we considered as treatment failure the persistence of either ERS/ESC or REVEAL 2.0 intermediate/high risk score after 6 months of initial combination of oral drugs.
Statistical analysis
Continuous data are expressed as mean±SD, and categorical data are expressed as counts and proportions. Two-group comparisons were done with unpaired or paired, two-tailed t-tests for means if the data were normally distributed or with Wilcoxon's rank-sum tests if the data were not normally distributed. Comparisons among the disease group were made by using ANOVA for variables normally distributed. If significant differences were found, post hoc comparisons (Duncan's multiple range test, Scheffé test) were used to determine the statistical significance among groups. The Kruskal–Wallis test was used for non-parametric comparison. Chi square or Fisher's exact tests were used to analyse the categorical data. Chi square or Fisher's exact tests were used to analyse the categorical data.
The proportional-hazards assumption was tested using log-minus-log plots for categorical variables and the Schoenfeld residuals plots for continuous variables.
Linear regression analysis was performed to assess the relationship between variables and expressed as a Pearson correlation coefficient.
Multivariate regression analysis was performed to assess the relationship between age, sex, cardiovascular comorbidities and PVR reduction.
Logistic regression analysis was used to measure the likelihood of achieving a low-risk status.
All statistical analyses were performed using SPSS software (version 25.0, IBM).
Results
Patient population
Overall patients’ characteristics have been previously reported [10]. There were 120 women and 61 men, aged 53±16 years, time from onset of symptoms to diagnosis 11±13 months, 146 idiopathic PAH (IPAH), 28 CTD PAH and seven corrected cardiac shunt-PAH, most of them in WHO class III with reduced exercise capacity (table 1). Patients’ comorbidities were: systemic hypertension (24, 13.2%), depression (21, 11.6%), hyperlipidaemia (21, 11.6%), thyroid diseases (14, 7.7%), obesity (17, 9.3%), diabetes mellitus (15, 8.2%), previous coronary angioplasty not associated with left heart systolic dysfunction (11, 6.0%), peripheral artery disease (8, 4.4%), previous atrial fibrillation (6, 3.3%) and other non-cardiac comorbidities (25, 13.8%).
TABLE 1.
Clinical, haemodynamic and echocardiographic characteristics of the study population based on the number of cardiovascular comorbidities
| Group A | Group B | Group C | Baseline | 6 months FU | ||||||||||||||
| Baseline | 6 months FU | Δ | p-value | Baseline | 6 months FU | Δ | p-value | Baseline | 6 months FU | Δ | p-value | 1 versus 2 | 1 versus 3 | 2 versus 3 | 1 versus 2 | 1 versus 3 | 2 versus 3 | |
| p-value | p-value | p-value | p-value | p-value | p-value | |||||||||||||
| Age years | 49.7±15 | 59.9±14 | 61.8±13 | 0.000 | 0.000 | ns | ||||||||||||
| Sex, female, n (%) | 65 (67.7) | 37 (68.5) | 18 (58.0) | ns | <0.01 | <0.01 | ||||||||||||
| DLCO % pred | 54±11 | 53±13 | 51±3 | ns | ns | ns | ||||||||||||
| NYHA | 2.8±0.4 | 2.2±0.6 | −0.5±0.6 | 0.0001 | 2.9±0.3 | 2.4±0.6 | −0.4±06 | 0.0001 | 2.6±0.5 | 2.5±0.6 | −0.1±0.4 | ns | ns | ns | 0.02 | ns | ns | ns |
| Class I–II, n (%) | 20 (20.8) | 65 (67.7) | 9 (16.7) | 31 (57.4) | 8 (25.8) | 11 (35.5) | ||||||||||||
| Class III, n (%) | 67 (69.8) | 31 (32.3) | 40 (74.0) | 21 (38.9) | 20 (64.5) | 13 (41.9) | ||||||||||||
| Class IV, n (%) | 9 (9.4) | 0 (0) | 5 (9.3) | 2 (3.7) | 3 (9.7) | 7 (22.6) | ||||||||||||
| 6MWT m | 337±96 | 392±103 | +55±71 | 0.0001 | 313±108 | 352±129 | +39±104 | 0.005 | 321±103 | 343±109 | +22±88 | 0.04 | ns | ns | ns | ns | ns | ns |
| Heart rate, beats·min−1 | 78±17 | 73±13 | −5±16 | 0.001 | 79±19 | 76±12 | −3±20 | ns | 78±13 | 71±25 | −7±24 | ns | ns | ns | ns | ns | ns | ns |
| NT-proBNP pg·mL−1 | 944±708 | 465±359 | −478±517 | 0.0001 | 824±574 | 391±198 | −433±521 | 0.003 | 1268±846 | 579±501 | −689±839 | 0.01 | ns | ns | 0.009 | ns | ns | ns |
| Haemodynamics | ||||||||||||||||||
| RAP mmHg | 9.0±4.4 | 6.8±3.8 | −2.1±4.3 | 0.0001 | 8.3±4.9 | 7.6±4.2 | −0.7±4.5 | ns | 8.0±5.5 | 5.9±4.9 | −2.2±3.2 | 0.02 | ns | ns | ns | ns | ns | ns |
| mPAP mmHg | 51±12 | 43±16 | −7.5±13 | 0.0001 | 49±12 | 43±9 | −6.5±10 | 0.0001 | 49±11 | 44±13 | −4.9±9 | ns | ns | ns | ns | ns | ns | ns |
| WP mmHg | 9.7±3.2 | 8.8±3.8 | 10.9±3.2 | 10.2±3.5 | 10±3.5 | 10±3.3 | ns | ns | ns | ns | ns | ns | ||||||
| CI L·min·m−2 | 2.3±0.9 | 3.1±0.9 | +0.8±0.9 | 0.0001 | 2.3±0.6 | 2.8±0.6 | +0.5±0.6 | 0.0001 | 2.3±0.6 | 2.8±0.9 | +0.5±0.6 | 0.002 | ns | ns | ns | ns | ns | ns |
| PVR WU | 12.4±6.5 | 7.4±4.2 | −4.8±5.6 | 0.0001 | 10.3±4.4 | 7.2±2.8 | −3.1±3.4 | 0.0001 | 10.6±4.9 | 8.7±3.9 | −2.0±2.6 | 0.01 | ns | ns | ns | ns | ns | ns |
| Echocardiography | ||||||||||||||||||
| RV/LV ratio | <0.01 | <0.01 | <0.01 | ns | ns | ns | <0.01 | <0.01 | ns | |||||||||
| <1, n (%) | 3 (3.1) | 22 (22.9) | 3 (5.6) | 8 (14.8) | 0 (0) | 4 (12.9) | ||||||||||||
| =1, n (%) | 15 (15.6) | 18 (18.7) | 7 (13) | 9 (16.7) | 5 (16.1) | 4 (12.9) | ||||||||||||
| >1, n (%) | 78 (81.3) | 56 (58.2) | 44 (81.5) | 37 (68.5) | 26 (83.9) | 23 (74.2) | ||||||||||||
| TAPSE, mm | 16.8±4 | 19.5±4 | +2.7±3 | 0.0001 | 16.2±4 | 17.6±4.7 | +1.4±4 | 0.01 | 16.7±3 | 18.2±4 | +1.5±3 | 0.01 | ns | ns | ns | 0.04 | ns | ns |
| TR, grade | <0.01 | <0.01 | <0.01 | ns | <0.01 | ns | ns | <0.01 | <0.01 | |||||||||
| Mild, n (%) | 48 (50.0) | 59 (61.5) | 25 (46.3) | 28 (51.8) | 12 (38.7) | 12 (38.7) | ||||||||||||
| Moderate, n (%) | 33 (34.3) | 29 (30.2) | 18 (33.3) | 21 (38.9) | 10 (32.3) | 15 (48.3) | ||||||||||||
| Severe, n (%) | 15 (15.7) | 8 (8.3) | 11 (20.3) | 5 (9.3) | 9 (29.0) | 4 (13.0) | ||||||||||||
| Pericardial effusion, n (%) | 20 (20.8) | 9 (9.3) | 12 (22.2) | 5 (9.2) | 6 (19.3) | 5 (16.1) | ||||||||||||
Group A: patients without cardiovascular comorbidities; Group B: patients with one cardiovascular comorbidity; Group C: patients with at least two cardiovascular comorbidities. Results are expressed as mean±sd unless indicated otherwise. FU: follow-up; ns: nonsignificant; DLCO: diffusing capacity of the lung for carbon monoxide; NYHA: New York Heart Association; 6MWT: non-encouraged 6-min walk test; RAP: mean right atrial pressure; mPAP: mean pulmonary arterial pressure; WP: wedge pressure; CI: cardiac index; PVR: pulmonary vascular resistance; RV: right ventricular end-diastolic area; LV: left ventricular end-diastolic area; TAPSE: tricupid anular plane systolic excursion; TR: tricuspid regurgitation.
According to the number of cardiovascular comorbidities, patients were divided into three groups: Group A, 96 (53.0%) patients without cardiac comorbidities; Group B, 54 (29.8%) patients with one cardiac comorbidity; Group C, 31 (17.2%) patients with at least two cardiac comorbidities.
The clinical characteristics of the three groups are shown in table 1. Patients with at least two comorbidities (Group C) were older and showed a trend for a more balanced sex distribution. Despite similar clinical and haemodynamic characteristics between the three groups, patients with at least two comorbidities (Group C) had higher N-terminal pro-brain natriuretic peptide (NT-proBNP) values compared with the others. A trend for lower values of PVR was also present in the two groups with comorbidities compared with patients without comorbidities. The echocardiography at baseline showed similar RV dilation, decreased TAPSE, severe tricuspid regurgitation and pericardial effusion rates among the three groups of patients.
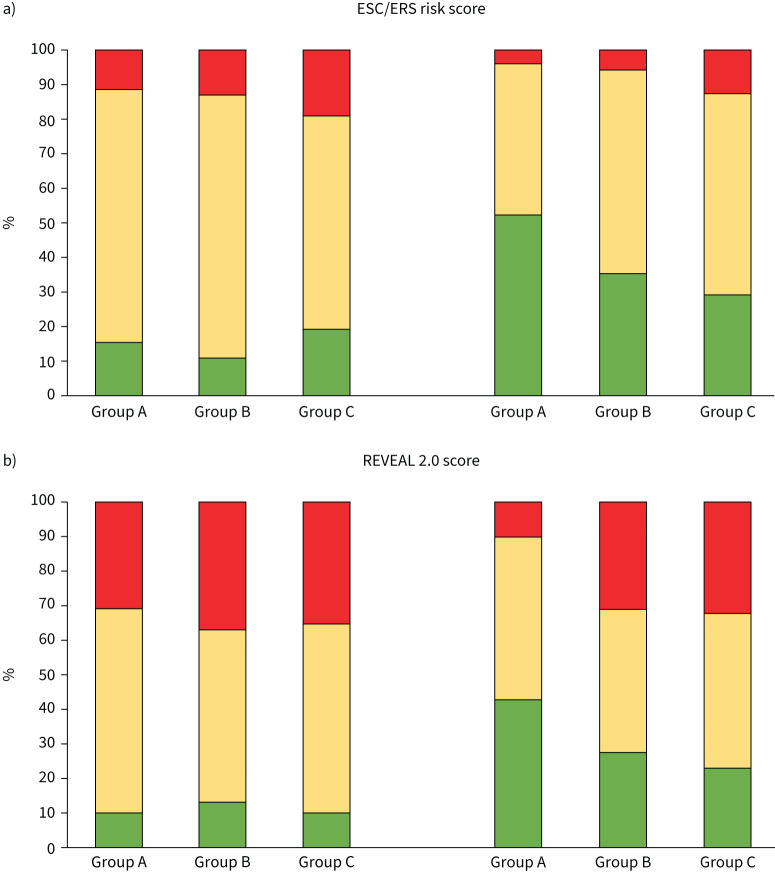
Patients’ risk distribution is shown in figure 1 and table 2. According to the ERS/ESC risk assessment, at the time of diagnosis a low-risk status was present in 15 (15.6%) patients in Group A, 6 (11.1%) patients in Group B and 6 (19.3%) patients in Group C (Group A versus Group B, p=NS; Group A versus Group C, p= NS; Group B versus Group C, p= NS). According to the REVEAL 2.0 score a low-risk status was present in 9 (9.3%) patients in Group A, 7 (12.9%) patients in Group B and 3 (9.6%) patients in Group C (Group A versus Group B, p= NS; Group A versus Group C, p= NS; Group B versus Group C, p= NS). The majority of patients were at intermediate risk whether considering the ERS/ESC or the REVEAL 2.0 score.
FIGURE 1.
a) Histogram reporting per cent changes in ESC/ERS score from diagnosis to last observation, according to groups based on the number of cardiovascular comorbidities. b) Histogram reporting per cent changes in REVEAL 2.0 score from diagnosis to last observation, according to groups based on the number of cardiovascular comorbidities. Group A: patients without cardiovascular comorbidities; Group B: patients with one cardiovascular comorbidity; Group C: patients with at least two cardiovascular comorbidities. Low risk: green; intermediate risk: yellow; high risk: red. ESC: European Society of Cardiology; ERS: European Respiratory Society, REVEAL: United States Registry to Evaluate Early and Long-Term PAH Disease Management registry.
TABLE 2.
Patients’ risk distribution at the time of diagnosis
| Overall | Group A | Group B | Group C | p-value | |
| Patients n | 181 | 96 | 54 | 31 | |
| ERS/ESC score, n (%) | |||||
| Low | 27 (14.9) | 15 (15.6) | 6 (11.1) | 6 (19.3) | NS |
| Intermediate | 130 (71.8) | 70 (72.9) | 41 (75.9) | 19 (61.4) | |
| High | 24 (13.3) | 11 (11.5) | 7 (13.0) | 6 (19.3) | |
| REVEAL 2.0, n (%) | |||||
| Low (<7) | 19 (10.5) | 9 (9.4) | 7 (13.0) | 3 (9.7) | NS |
| Intermediate (7–8) | 100 (55.2) | 56 (58.3) | 27 (50.0) | 17 (54.8) | |
| High (>8) | 62 (34.3) | 31 (32.3) | 20 (37.0) | 11 (35.5) | |
Group A: patients without cardiovascular comorbidities; Group B: patients with one cardiovascular comorbidity; Group C: patients with at least two cardiovascular comorbidities. Results are expressed as n (%). ERS/ESC: European Respiratory Society/European Society of Cardiology; ns: nonsignificant.
The majority of patients were started on ambrisentan–tadalafil combination therapy (62%). All but 11 out of 113 patients reached the full dose of ambrisentan and tadalafil (10 and 40 mg daily, respectively) within 2 months. Other initial PDE-5i and ERA combinations were previously described [10]. Drug-related adverse events occurred with similar frequency between groups with most frequent side-effects reported as peripheral oedema (Group A, 27 patients – 28%; Group B, 16 patients – 30%; Group C, 10 patients – 32%; p= NS), headache (Group A, 37 patients – 38%; Group B, 19 patients – 35%; Group C, 11 patients – 35%; p=NS) and nasal congestion (Group A, 20 patients – 21%; Group B, 12 patients – 22%; Group C, 6 patients – 19%; p=NS). None of the patients discontinued dual oral combination therapy due to severe side-effects.
Changes in pulmonary vascular resistance according to the presence of comorbidities
All the patients survived after a median of 180 days follow-up (IQR 144–363; minimum 79; maximum 394). The median PVR reduction obtained with double oral initial therapy was −40.4% (IQR −25.8– −45.3) (−2.9 WU, IQR −4.8– −1.7 WU).
At first follow-up the cumulative effect of cardiovascular comorbidities on PVR reduction with double oral initial therapy is shown in figure 2. There was a significant difference in treatment effect moving from the absence to one or at least two cardiac comorbidities, respectively, median −45.0% (IQR −26– −55%; mean −38.7%), −30.3% (IQR −15– −45%; mean −30.2%), −24.3% (IQR +2– −32%; mean –19.2%) (Group A versus Group B, p=NS; Group A versus Group C, p=0.003). No significant difference was observed between the presence of one or at least two comorbidities (Group B versus Group C, p=NS).
FIGURE 2.
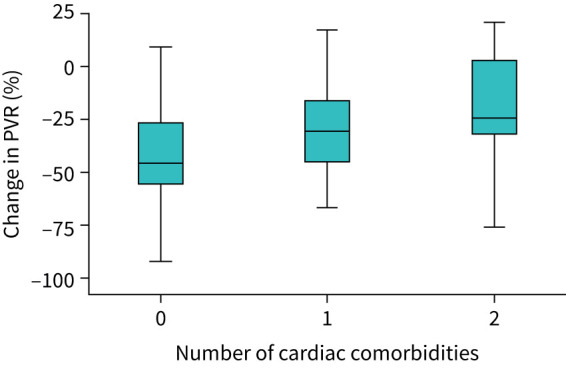
Boxplots of PVR reduction (%) with double oral initial therapy according to the number of cardiovascular comorbidities. PVR: pulmonary vascular resistance.
Multivariate regression analysis including age, sex and the presence of cardiovascular comorbidities showed male sex and the presence of cardiovascular comorbidities as independently associated with PVR reduction (age: B 0.12, 95% CI –0.13–0.18, p=NS; male sex: B 1.32, 95% CI 0.10–2.21, p=0.009; cardiovascular comorbidities: B 2.47, 95% CI 0.79–4.15, p=0.004).
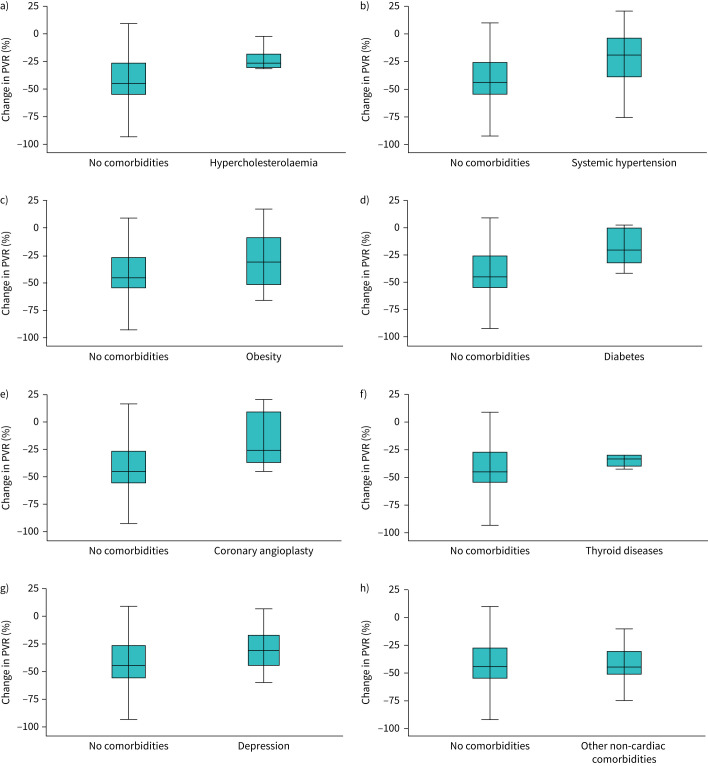
For those patients with one comorbidity figure 3 shows the median reduction in PVR based on different kind of comorbidities. Patients with hypercholesterolaemia, systemic hypertension, obesity, previous coronary angioplasty, peripheral artery disease, previous atrial fibrillation and diabetes mellitus had a lower decrease in PVR compared with those patients without comorbidities, respectively, −27.4% (IQR −18– −30%; mean –25.0%), −19.5% (IQR +3– −42%; mean –22.1%), −30.2% (IQR −8– −51%; mean –29.6%), −25.6% (IQR +12– −38%; mean –17.6%), −13.7 (IQR +15– −39%; mean –12.4%), −25.4 (IQR +1– −40%; mean –21.1%) and −19.8% (IQR 0– −33%; mean –17.9%) versus −45.0% median reduction at follow-up (respectively, p=0.05, p=0.02, p=NS, p=0.02, p=0.01, p=0.02, p=0.04). No significant difference was observed between patients with thyroid diseases, depression, other non-cardiac comorbidities and those without comorbidities in terms of PVR reduction, respectively, −33.1% (IQR −29– −40%; mean –34.3%), −30.3% (IQR −18– −46%; mean –30.8%) and −35.8% (IQR −1– −52%; mean –30.2%) versus −45.0% (respectively, p=NS, p=NS, p=NS).
FIGURE 3.
Boxplots of PVR reduction (%) with double oral initial therapy in patients with one comorbidity. Patients with a) hypercholesterolaemia, b) systemic hypertension, c) obesity, d) diabetes mellitus, e) previous coronary angioplasty, f) thyroid diseases, g) depression and h) other non-cardiac comorbidities. PVR: pulmonary vascular resistance.
Changes in risk scores according to the presence of comorbidities
As shown in table 1, all patients had significant improvements in WHO functional class, 6MWD, NT-proBNP and invasive haemodynamics. However, in patients with more than one cardiovascular comorbidity (Group C) clinical improvement was not associated with significant changes in echo-determined RV systolic function, with patients showing a higher proportion of RV dilation and severe tricuspid regurgitation compared with the others.
While the number of cardiovascular comorbidities increases, patients were less likely to achieve or maintain a low-risk status at second evaluation, especially when considering the ERS/ESC scoring system.
According to the ERS/ESC score, a low-risk status was present at second assessment in 50 (52.0%) patients in Group A, 19 (35.1%) patients in Group B and 9 (29.0%) patients in Group C (Group A versus Group B, p<0.05; Group A versus Group C, p<0.05; Group B versus Group C, p=NS) (figure 1). On the other hand, according to the REVEAL 2.0 score a low-risk status was present in 41 (42.0%) patients in Group A, 15 (27.7%) patients in Group B and 7 (22.6%) patients in Group C (Group A versus Group B, p<0.05; Group A versus Group C, p<0.05; Group B versus Group C, p=NS) (figure 1).
Patients without cardiovascular comorbidities were 2.3 times more likely to achieve or maintain a low-risk status compared with those with cardiovascular comorbidities (OR 2.27, 95% CI 1.15–4.54, p=0.02), corresponding to a 66.6% reduction in risk of treatment failure (OR 0.44, 95% CI 0.22–0.88, p=0.02). On the other hand, the presence of at least two cardiac comorbidities (Group C) was associated with a 3.8-fold increased likelihood of treatment failure compared with the absence of comorbidities (OR 3.76, 95% CI 1.14–12.3, p=0.02). There was no significant increased risk of treatment failure for patients with one cardiac comorbidity compared with those patients without cardiac comorbidity (OR 1.96, 95% CI 0.94–3.98, p=0.07).
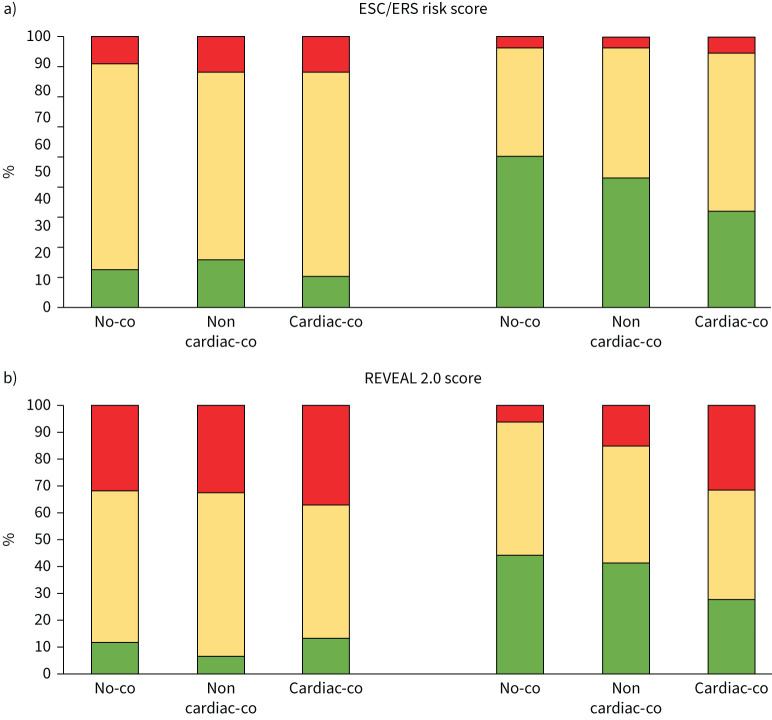
Considering patients with one comorbidity (Group B) a significant difference was observed between the 54 patients with cardiac comorbidities and the 46 patients with non-cardiac comorbidities. Indeed, at second evaluation a similar rate of low-risk status was observed among patients without comorbidities and with non-cardiac comorbidities, while a lower rate was observed among patients with cardiac comorbidities, respectively 28 (56.0%), 22 (47.8%), and 19 (35.1%) patients, according to the ERS/ESC score (without comorbidities versus with non-cardiac comorbidities, p=NS; without comorbidities versus with cardiac comorbidities, p<0.05; with non-cardiac comorbidity versus with cardiac comorbidity, p<0.05), and respectively, 22 (44.0%), 19 (41.3%) and 15 (27.7%) patients, according to the REVEAL 2.0 score (without comorbidities versus with non-cardiac comorbidities, p=ns; without comorbidities versus with cardiac comorbidities, p<0.05; with non-cardiac comorbidities versus with cardiac comorbidities, p<0.05) (figure 4).
FIGURE 4.
a) Histogram reporting per cent changes in ESC/ERS score from diagnosis to last observation, according to groups based on the presence of comorbidities. b) Histogram reporting per cent changes in REVEAL 2.0 score from diagnosis to last observation, according to groups based on the presence of comorbidities. Group A: patients without cardiovascular comorbidities; No-co: patients without comorbidities. Non-cardiac-co: patients with non-cardiac comorbidities. Cardiac-co: patients with cardiac comorbidities. Low risk: green; intermediate risk: yellow; high risk: red. ESC: European Society of Cardiology; ERS: European Respiratory Society, REVEAL: United States Registry to Evaluate Early and Long-Term PAH Disease Management registry.
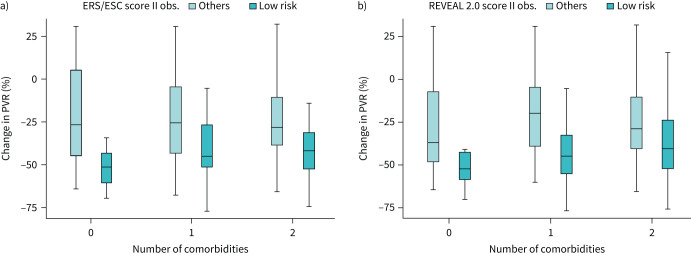
The tight relationship between PVR reduction under initial oral therapy and the presence of a low-risk status at last observation is confirmed in the presence of comorbidities (figure 5). Indeed, the achievement of a low-risk status is clearly a function of PVR reduction, with a more heterogeneous response in the presence of at least one cardiac comorbidity (Group B and Group C) compared with the absence of cardiac comorbidities (Group A).
FIGURE 5.
a) Boxplots of PVR reduction (%) versus number of cardiovascular comorbidities, based on the presence of an ERS/ESC low-risk status at last observation (II obs.). b) Boxplots of PVR reduction (%) versus number of cardiovascular comorbidities, based on the presence of a REVEAL 2.0 low-risk status at last observation. Box edges represent the 25th (Q1) and 75th (Q3) quantiles, respectively. Outliers are defined as values >1.5 times the interquartile range above Q3 or below Q1. Therefore, the upper whisker is drawn at the greatest value smaller than 1.5 IQR above the third quartile, while the lower whisker is drawn at the smallest value >1.5 IQR below the first quartile. ESC: European Society of Cardiology; ERS: European Respiratory Society, PVR: pulmonary vascular resistance, REVEAL: United States Registry to Evaluate Early and Long-Term PAH Disease Management registry.
Discussion
The present results show that an initial combination of ERA and PDE-5i drugs in PAH improves functional class, exercise capacity and NT-proBNP at 6 months assessment with a more attenuate response to treatment for patients with cardiovascular risk factors for left ventricular diastolic dysfunction compared with the others, in agreement with the results of the AMBITION trial [9]. These favourable effects seem accompanied by a lower rate of low-risk status at 6 months while the number of cardiovascular risk factors increases, by either the ERS/ESC or the REVEAL 2.0 score. The presence of non-cardiac comorbidities is not associated with reduced treatment effect after initial oral combination therapy.
Our results also show that the treatment response appears related to the magnitude of treatment-induced decrease in PVR.
The AMBITION trial demonstrated that the ex-primary analysis set patients, characterised by the inclusion of patients in spite of the presence of cardiovascular risk factors for left ventricular diastolic dysfunction, experienced a higher rate of clinical failure events compared with those without cardiovascular risk factors (primary analysis set patients cohort). Specifically, the latter cohort was associated with 50% reduction in risk of clinical failure compared with pooled monotherapy, either ERA or PDE-5i. On the other hand, the ex-primary analysis cohort was associated with a 30% reduction in risk of clinical failure, considered in the same direction but statistically not significant compared with the primary analysis set. The present study expands these results showing, independently of the number of cardiovascular comorbidities, that patients without comorbidities are associated with up to 66.6% reduction in risk of treatment failure compared with the former, considering a definition close to the updated guidelines treatment goal [5, 8, 17, 18], as the persistence of either ERS/ESC or REVEAL 2.0 intermediate/high risk score after 6 months of initial combination of oral drugs.
Our results bring also further insights on the chance of achieving or maintaining a low-risk status for patients with cardiovascular risk factors receiving initial combination therapy. While in the AMBITION trial [9] patients without cardiovascular risk factors had a higher rate of satisfactory clinical response compared with those receiving pooled monotherapy, defined as a ≥10% increase from baseline in 6MWD with a reduction or maintenance of WHO functional class I/II, we found patients without cardiovascular comorbidities to be 2.3 times more likely to achieve or maintain a low-risk status compared with those with cardiovascular comorbidities. The different behaviour of patients with and without cardiovascular comorbidities supports the attempt to distinguish the former population, potentially close to a heart failure with preserved ejection fraction phenotype, from the “classical” PAH vasculopathy. Indeed, a comprehensive cluster analysis of the Comparative, Prospective Registry of Newly Initiated Therapies for Pulmonary Hypertension (COMPERA) identified three distinct phenotypes of IPAH patients, two of them characterised by the presence of cardiovascular risk factors with potential reduced treatment response to targeted therapy compared with patients without cardiovascular comorbidities [19]. Our findings expand these results showing different haemodynamic response and rates of low-risk status achievement for different phenotypes of PAH patients based on the presence of cardiovascular comorbidities, treated with the updated standard of care recommended by guidelines. We also confirmed the analysis of the COMPERA registry showing that the presence of at least one cardiac comorbidity is sufficient to significantly affect treatment response, reducing the likelihood of achieving a low-risk status, and questioning the AMBITION strategy to arbitrarily introduce three cardiovascular risk factors as the best fit to identify a clean PAH population (i.e. “classical” PAH vasculopathy) with enhanced treatment response. Indeed, when considering the impact of add-on Selexipag in the GRIPHON trial in patients with cardiovascular comorbidities, the treatment effect was confirmed irrespective of patient comorbidity status for the morbi/mortality end-point, while the partition of patients based on the presence of at least three cardiac comorbidities was not able to show a consistent effect across subgroups for improvement in 6MWD and WHO functional class, with improvement limited in NT-proBNP for patients with less than three comorbidities [20].
Of note, whether considering the presence of cardiovascular comorbidities or the “classical PAH” phenotype without comorbidities, the achievement/maintenance of a low-risk status at second evaluation is clearly a function of treatment-induced PVR reduction with initial oral combination, on average −45% without cardiovascular comorbidities, −30% with one comorbidity and −24% with at least two comorbidities (figure 2). The reason for such a different treatment effect in the presence of cardiovascular comorbidities is not clear but might be related to a potentially less responsive pulmonary vasculature in patients with cardiovascular comorbidities. The relationship between treatment response and the magnitude of treatment-induced decrease in PVR reinforces the concept that the major driven mechanism of the disease is represented by RV afterload mismatch, even in the presence of cardiovascular comorbidities, and brings in favour of a common effort to correctly identify those patients with greater decrease in PVR among patients with cardiovascular comorbidities, which would benefit from an initial oral combination therapy. Although some discussion still persists on the opportunity of an initial combination therapy for those patients with cardiovascular risk factors [21], we observed that a not negligible proportion of patients with one comorbidity showed a considerable haemodynamic and clinical response to therapy, as 25% of patients had >51% reduction in PVR and up to 35% of patients achieved a low-risk status.
The importance of PVR reduction has been recently recognised by increasing evidence showing that a significant decrease in PVR is needed to induce RV reverse remodelling and functional recovery [14, 22–28]. Patients with more than one cardiovascular comorbidity showed a less important reduction in PVR associated with a higher proportion of RV dilation and severe tricuspid regurgitation compared with the others. Since the association between PVR reduction and RV reverse remodelling follows a sigmoid relationship, the likelihood of RV recovery is high for >50% decrease in PVR [22, 29]. We have recently clarified that the efficacy of initial oral combination in an unselected real-life population of consecutive PAH patients is related to decreased PVR, allowing RV unloading and increased rates of low-risk status achievement [10]. This argues in favour of PVR- and/or imaging-directed therapeutic strategies for a significant proportion of patients with one cardiovascular comorbidity. This issue will become of particular clinical meaning in the near future as the picture of PAH has changed over the past two decades, at least in developed countries, showing a change in demographic and epidemiology in favour of patients with advanced age and cardiovascular comorbidities [1–4].
For patients with multiple cardiovascular comorbidities a safer approach might be desirable considering monotherapy as a first step for sequential combination, as the present results show a poor haemodynamic response in terms of PVR reduction, on average −24%, very close to the 25± 8% mean decrease that emerged from a pooled metanalysis of 16 randomised controlled trials of mostly monotherapies, including a total of 2353 PAH patients [30]. Similar results of oral monotherapies on PVR have been observed in real-life observational studies [14, 26]. Although none of the patients in our study needed to withdraw combination therapy because of severe side-effects, the randomised controlled AMBITION trial identified an increased proportion of patients with adverse events leading to treatment discontinuation in patients with a higher number of cardiac comorbidities [9]. The higher rate of treatment-related side-effects in spite of very few patients (no more than 15% according to our findings) with >40% reduction in PVR would definitely be in favour of a less aggressive approach at the time of diagnosis in case of multiple cardiac comorbidities. Further studies are needed to explore whether an initial monotherapy with a rapid escalation would be preferable to a double oral initial approach in PAH patients with multiple cardiovascular comorbidities.
Limitations
This study has limitations as being retrospective and with a relatively small number of patients with cardiovascular comorbidities. The prevalence of comorbidities in the present cohort is relatively low compared with other European and US registries potentially related to the younger age of our cohort and a higher proportion of idiopathic PAH compared with the others. The majority of patients of the Italian network was still started on initial oral monotherapy in the study period, in accordance with all international registries, representing a potential selection bias. However, we believe that our findings are convincing as the study was multicentric with original haemodynamic data and updated risk scores. Data were collected with robust methodology [12] and analysed with rigorous statistics, in line with current literature, showing patients’ risk distribution similar to the multinational AMBITION study [31].
Conclusions
Initial combination of ERA and PDE-5i drugs in PAH improves functional class, exercise capacity and NT-proBNP at 6 months assessment with a less effective response to treatment for patients with cardiovascular comorbidities compared with the others. These favourable effects seem to be accompanied by a lower rate of low-risk status at 6 months while the number of cardiovascular comorbidities increases. Our findings also show that the treatment response appears related to the magnitude of decrease in PVR.
Finally, the presence of non-cardiac comorbidities is not associated with reduced treatment effect after initial oral combination therapy.
Further data are still needed to expand the growing body of evidence on the treatment effect of PAH-targeted therapies in PAH patients with cardiovascular comorbidities. Until further evidence-based data become available, the overall profile of the patient should be considered to identify the best fit for patient's phenotyping.
Footnotes
Provenance: Submitted article, peer reviewed.
The Italian Pulmonary Hypertension Network group: Carlo Albera, SC Pneumologia U Ospedale Molinette, Turin; Davide Barbisan, Azienda Sanitaria Universitaria Integrata of Trieste (ASUITS), University of Trieste, Cardiovascular Department, Cattinara Hospital, Trieste; Vincenzo Bellomo, Ecclesiastical Entity General Regional Hospital, Department of cardiology, Acquaviva delle Fonti; Marta Beretta, Pulmonology Unit, IRCCS – Istituto Mediterraneo Trapianti e Terapie ad Alta Specializzazione (ISMETT), Palermo; Marco Biolo, Pulmonology Unit, Heart-Thorax-Vessels Department, University Hospital of Cattinara, Trieste; Federico Biondi, Cardiovascular Department, Azienda Sanitaria Universitaria Giuliano Isontina, Trieste; Andrea Bonelli, Department of Medical and Surgical Specialties, Radiological Sciences, and Public Health, University and Civil Hospital; Brescia; Renato Carignola, SCDU Malattie dell'Apparato Respiratorio 2 AOU San Luigi Gonzaga Regione Gonzole, Orbassano; Francesco Cassadonte, Cardiology Unit, Hospital “Pugliese-Ciaccio”, Catanzaro; Maria Alberta Cattabiani, UO Cardiologia AOU di Parma – Ospedale maggiore di Parma, Parma; Vincenzo Antonio Ciconte, Cardiology Unit, Hospital “Pugliese-Ciaccio”, Catanzaro; Paola Confalonieri, Pulmonology Unit, Heart-Thorax-Vessels Department, University Hospital of Cattinara, Trieste; Chiara Cresci, SO Pneumologia e Fisiopatologia Toraco-Polmonare AOU Careggi, Florence; Alessandra Cuomo, Department of Translational Medical Sciences, Federico II University of Naples, Naples; Raffaele De Caterina, UOC Cardiologia 1 AOU Piasana, Pisa; Elisabetta De Tommasi, Cardiology Department, University Hospital Policlinico Consorziale, Bari; Gabriele Di Gesaro, Pulmonology Unit, IRCCS – ISMETT, Palermo; Stefania Farina, UO Scompenso, Cardiologia Clinica e Cardiologia Riabilitativa Centro cardiologico Monzino IRCCS, Milan; Francesco Fedele, Department of Cardiovascular and Respiratory Sciences, Sapienza University of Rome, Rome; Martino Fortunato, Cardiology Department, Ospedali Riuniti University Hospital, Foggia; Giulia Gagno, ASUITS, University of Trieste, Cardiovascular Department, Cattinara Hospital, Trieste; Pietro Geri, Pulmonology Unit, Heart-Thorax-Vessels Department, University Hospital of Cattinara, Trieste; Daniele Ghiraldin, Department of Medical and Surgical Specialties, Radiological Sciences, and Public Health, University and Civil Hospital, Brescia; Daniela Giannazzo, Dipartimento Cardio-Toraco-Vascolare AOU Policlinico Vittorio Emanuele Presidio Ferrarotto, Catania; Giorgio Giardina, Cardiology Unit, Azienda Ospedaliera “G. Brotzu” San Michele, Cagliari; Paolo Golino, Cardiologia Università L. Vanvitelli, Naples; Maria Chiara Grimaldi, Cardiology Unit, S. Andrea Hospital, Rome, Italy; Ludovico Lanfranchi, Dipartimento Cardio-Toraco-Vascolare – Clinica Pneumologica AO San Gerardo, Monza; Mariangela Lattanzio, Cardiologia Ospedale di Circolo e Fondazione Macchi, Varese; Claudio Lunardi, Clinical Immunology and Allergology, Department of Medicine, University of Verona – I, Verona; Antonella Mannarini, Cardiology Department, University Hospital Policlinico Consorziale, Bari; Lavinia Martino, Pulmonology Unit, IRCCS –ISMETT, Palermo; Pierluigi Merella, ATS Sardegna-ASSL Nuoro, San Francesco Hospital Nuoro, Nuoro; Beatrice Pezzuto, UO Scompenso, Cardiologia Clinica e Cardiologia Riabilitativa Centro cardiologico Monzino IRCCS, Milan; Cristina Piccinino, Cardiologia 1 AOU Maggiore della carità di Novara Corso Mazzini, Novara, Francesca Rampini, Department of Cardiovascular and Respiratory Sciences, Sapienza University of Rome, Rome; Imma Romanazzi, Dipartimento Cardio-Toraco-Vascolare AOU Policlinico Vittorio Emanuele –Presidio Ferrarotto, Catania; Susanna Sciomer, Department of Cardiovascular and Respiratory Sciences, Sapienza University of Rome, Rome; Gianmarco Scoccia, Department of Cardiovascular and Respiratory Sciences, Sapienza University of Rome, Rome; Piermario Scuri, Unità di Cardiologia Ospedale Papa Giovanni XXIII Centro Ospedaliero, Bergamo; Gianfranco Sinagra, Direttore Dipartimento Cardiovascolare Azienda Ospedaliero Universitaria “Ospedali Riuniti”, Trieste; Corrado Tamburino, Dipartimento Cardio-Toraco-Vascolare AOU Policlinico Vittorio Emanuele – Presidio Ferrarotto, Catania; Lucia Tricarico, Cardiology Department, Ospedali Riuniti University Hospital, Foggia; Sara Uras, ATS Sardegna-ASSL Nuoro, San Francesco Hospital Nuoro, Nuoro; Francesco Salton, Pulmonology Unit, Heart-Thorax-Vessels Department, University Hospital of Cattinara, Trieste; Rosalinda Madonna, University of Pisa, Pisa; Giovanna Manzi, Dept. of Cardiovascular and Respiratory Sciences – Sapienza University of Rome, Rome; Annalisa Caputo, Department of Cardiovascular and Respiratory Sciences, Sapienza University of Rome, Rome; Giorgia Serino, Department of Cardiovascular and Respiratory Sciences, Sapienza University of Rome, Rome; Francesca Adamo, Department of Cardiovascular and Respiratory Sciences, Sapienza University of Rome, Rome; Jean Pierre Jabour, Department of Cardiovascular and Respiratory Sciences, Sapienza University of Rome, Rome; Giulia Manguso, Department of Cardiovascular and Respiratory Sciences, Sapienza University of Rome, Rome.
Conflict of interest: R. Badagliacca reports personal fees from UT, Dompè, Ferrer, Bayer, MSD and AOP Orphan Pharmaceuticals, outside the submitted work. M. D'Alto reports personal fees from Bayer, Dompè, GSK, MSD and Ferrer, outside the submitted work. C.D. Vizza reports personal fees from GSK, UT, Dompè, Bayer and MSD, outside the submitted work. The other authors have nothing to disclose.
Contributor Information
Collaborators: The Italian Pulmonary Hypertension NETwork (iPHNET), Albera Carlo, Davide Barbisan, Vincenzo Bellomo, Marta Beretta, Marco Biolo, Federico Biondi, Andrea Bonelli, Renato Carignola, Francesco Cassadonte, Maria Alberta Cattabiani, Vincenzo Antonio Ciconte, Paola Confalonieri, Chiara Cresci, Alessandra Cuomo, Raffaele De Caterina, Elisabetta De Tommasi, Gabriele Di Gesaro, Stefania Farina, Francesco Fedele, Martino Fortunato, Giulia Gagno, Pietro Geri, Daniele Ghiraldin, Daniela Giannazzo, Giorgio Giardina, Paolo Golino, Maria Chiara Grimaldi, Ludovico Lanfranchi, Mariangela Lattanzio, Claudio Lunardi, Antonella Mannarini, Lavinia Martino, Pierluigi Merella, Beatrice Pezzuto, Cristina Piccinino, Imma Romanazzi, Susanna Sciomer, Gianmarco Scoccia, Piermario Scuri, Gianfranco Sinagra, Corrado Tamburino, Lucia Tricarico, Francesco Salton, Rosalinda Madonna, Giovanna Manzi, Annalisa Caputo, Giorgia Serino, Francesca Adamo, Jean Pierre Jabour, and Giulia Manguso
References
- 1.Hoeper MM, Huscher D, Pittrow D. Incidence and prevalence of pulmonary arterial hypertension in Germany. Int J Cardiol 2016; 203: 612–613. doi: 10.1016/j.ijcard.2015.11.001 [DOI] [PubMed] [Google Scholar]
- 2.Ling Y, Johnson MK, Kiely DG, et al. Changing demographics, epidemiology, and survival of incident pulmonary arterial hypertension: results from the pulmonary hypertension registry of the United Kingdom and Ireland. Am J Respir Crit Care Med 2012; 186: 790–796. doi: 10.1164/rccm.201203-0383OC [DOI] [PubMed] [Google Scholar]
- 3.Radegran G, Kjellstrom B, Ekmehag B, et al. Characteristics and survival of adult Swedish PAH and CTEPH patients 2000–2014. Scand Cardiovasc J 2016; 50: 243–250. doi: 10.1080/14017431.2016.1185532 [DOI] [PubMed] [Google Scholar]
- 4.Frost AE, Badesch DB, Barst RJ, et al. The changing picture of patients with pulmonary arterial hypertension in the United States: how REVEAL differs from historic and non-US Contemporary Registries. Chest 2011; 139: 128–137. doi: 10.1378/chest.10-0075 [DOI] [PubMed] [Google Scholar]
- 5.Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J 2015; 46: 903–975. doi: 10.1183/13993003.01032-2015 [DOI] [PubMed] [Google Scholar]
- 6.Hussain N, Charalampopoulos A, Ramjug S, et al. Pulmonary hypertension in patients with heart failure and preserved ejection fraction: differential diagnosis and management. Pulm Circ 2016; 6: 3–14. doi: 10.1086/685021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galiè N, Barberà JA, Frost AE, et al. Initial use of ambrisentan plus tadalafil in pulmonary arterial hypertension. N Engl J Med 2015; 373: 834–844. doi: 10.1056/NEJMoa1413687 [DOI] [PubMed] [Google Scholar]
- 8.Galiè N, Channick RN, Frantz RP, et al. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur Respir J 2019; 53: 1801889. doi: 10.1183/13993003.01889-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McLaughlin VV, Vachiery JL, Oudiz RJ, et al. Patients with pulmonary arterial hypertension with and without cardiovascular risk factors: results from the AMBITION trial. J Heart Lung Transplant 2019; 38: 1286–1295. doi: 10.1016/j.healun.2019.09.010 [DOI] [PubMed] [Google Scholar]
- 10.Badagliacca R, D'Alto M, Ghio S, et al. Risk reduction and hemodynamics with initial combination therapy in pulmonary arterial hypertension. Am J Respir Crit Care Med 2021; 203: 484–492. doi: 10.1164/rccm.202004-1006OC [DOI] [PubMed] [Google Scholar]
- 11.D'Alto M, Badagliacca R, Lo Giudice F, et al. Hemodynamics and risk assessment 2 years after the initiation of upfront ambrisentan‒tadalafil in pulmonary arterial hypertension. J Heart Lung Transplant 2020; 39: 1389–1397. doi: 10.1016/j.healun.2020.08.016 [DOI] [PubMed] [Google Scholar]
- 12.Poscia R, Ghio S, D'Alto M, et al. ‘Real-life' information on pulmonary arterial hypertension: the iPHnet Project. Curr Med Res Opin 2014; 30: 2409–2414. doi: 10.1185/03007995.2014.960514 [DOI] [PubMed] [Google Scholar]
- 13.Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography. Endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010; 23: 685–713. doi: 10.1016/j.echo.2010.05.010 [DOI] [PubMed] [Google Scholar]
- 14.Badagliacca R, Raina A, Ghio S, et al. Influence of various therapeutic strategies on right ventricular morphology, function and hemodynamics in pulmonary arterial hypertension. J Heart Lung Transplant 2018; 37: 365–375. doi: 10.1016/j.healun.2017.08.009 [DOI] [PubMed] [Google Scholar]
- 15.Boucly A, Weatherald J, Savale L, et al. Risk assessment, prognosis and guideline implementation in pulmonary arterial hypertension. Eur Respir J 2017; 50: 1700889. doi: 10.1183/13993003.00889-2017 [DOI] [PubMed] [Google Scholar]
- 16.Benza RL, Gomberg-Maitland M, Elliott CG, et al. Predicting survival in patients with pulmonary arterial hypertension: the REVEAL Risk Score Calculator 2.0 and comparison with ESC/ERS-based risk assessment strategies. Chest 2019; 156: 323–337. doi: 10.1016/j.chest.2019.02.004 [DOI] [PubMed] [Google Scholar]
- 17.Weatherald J, Boucly A, Sahay S, et al. The low risk profile in pulmonary arterial hypertension. Time for a paradigm shift to goal-oriented clinical endpoints? Am J Respir Crit Care Med 2018; 197: 860–868. doi: 10.1164/rccm.201709-1840PP [DOI] [PubMed] [Google Scholar]
- 18.Preston IR, Suissa S, Humbert M. New perspectives in long-term outcomes in clinical trials of pulmonary arterial hypertension. Eur Respir Rev 2013; 22: 495–502. doi: 10.1183/09059180.00006413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoeper MM, Pausch C, Grünig E, et al. Idiopathic pulmonary arterial hypertension phenotypes determined by cluster analysis from the COMPERA registry. J Heart Lung Transplant 2020; 39: 1435–1444. doi: 10.1016/j.healun.2020.09.011 [DOI] [PubMed] [Google Scholar]
- 20.Rosenkranz S, Channick R, Chin KM, et al. The impact of comorbidities on selexipag treatment effect in patients with pulmonary arterial hypertension: insights from the GRIPHON study. Eur J Heart Fail 2022; 24: 205–214. doi: 10.1002/ejhf.2369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Humbert M, Lau EMT. Should initial combination therapy be the standard of care in pulmonary arterial hypertension? Yes. Chest 2019; 156: 1039–1042. doi: 10.1016/j.chest.2019.07.005 [DOI] [PubMed] [Google Scholar]
- 22.Sanz J, Sánchez-Quintana D, Bossone E, et al. Anatomy, function, and dysfunction of the right ventricle: state-of-the-art review. J Am Coll Cardiol 2019; 73: 1463–1482. doi: 10.1016/j.jacc.2018.12.076 [DOI] [PubMed] [Google Scholar]
- 23.Sitbon O, Jais X, Savale L, et al. Upfront triple combination therapy in pulmonary arterial hypertension: a pilot study. Eur Respir J 2014; 43: 1691–1697. doi: 10.1183/09031936.00116313 [DOI] [PubMed] [Google Scholar]
- 24.D'Alto M, Badagliacca R, Argiento P, et al. Risk reduction and right heart reverse remodeling by upfront triple combination therapy in pulmonary arterial hypertension. Chest 2020; 157: 376–383. doi: 10.1016/j.chest.2019.09.009 [DOI] [PubMed] [Google Scholar]
- 25.Badagliacca R, Poscia R, Pezzuto B, et al. Prognostic relevance of right heart reverse remodeling in idiopathic pulmonary arterial hypertension. J Heart Lung Transplant 2018; 37: 195–205. [DOI] [PubMed] [Google Scholar]
- 26.Badagliacca R, Papa S, Matsubara H, et al. The importance of right ventricular evaluation in risk assessment and therapeutic strategies: raising the bar in pulmonary arterial hypertension. Int J Cardiol 2020; 301: 183–189. doi: 10.1016/j.ijcard.2019.10.043 [DOI] [PubMed] [Google Scholar]
- 27.van de Veerdonk MC, Huis In T Veld AE, Marcus JT, et al. Upfront combination therapy reduces right ventricular volumes in pulmonary arterial hypertension. Eur Respir J 2017; 49: 1700007. doi: 10.1183/13993003.00007-2017 [DOI] [PubMed] [Google Scholar]
- 28.Vizza CD, Lang IM, Badagliacca R, et al. Aggressive afterload lowering to improve the RV: a new target for medical therapy in PAH? Am J Respir Crit Care Med 2021; 205: 751–760. doi: 10.1164/rccm.202109-2079PP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Badagliacca R, Papa S, Manzi G, et al. Usefulness of adding echocardiography of the right heart to risk assessment scores in prostanoid-treated pulmonary arterial hypertension. JACC Cardiovasc Imaging 2020; 13: 2054–2056. doi: 10.1016/j.jcmg.2020.04.005 [DOI] [PubMed] [Google Scholar]
- 30.Savarese G, Musella F, D'Amore C, et al. Haemodynamics, exercise capacity and clinical events in pulmonary arterial hypertension. Eur Respir J 2013; 42: 414–424. doi: 10.1183/09031936.00123712 [DOI] [PubMed] [Google Scholar]
- 31.Frost AE, Hoeper MM, Barberá JA, et al. Risk-stratified outcomes with initial combination therapy in pulmonary arterial hypertension: application of the REVEAL risk score. J Heart Lung Transplant 2018; 37: 1410–1417. doi: 10.1016/j.healun.2018.07.001 [DOI] [PubMed] [Google Scholar]