Abstract
The phenotype of tumor-associated macrophages may be critical for tumor immunity, angiogenesis, and clinical disease outcome. Here, we elucidated the prognostic significance of the neovasculature and macrophages in colorectal cancer. We analyzed the effect of M2 macrophage density on the clinical behavior of 151 primary colorectal carcinomas using CD206 as a marker for type 2 macrophages. Triple immunohistochemical staining (ERG, SMA, and podoplanin) was used for microvessel evaluation. We found that M2 macrophages in colorectal cancer did not have a direct association with metastatic behavior. However, high numbers of CD206 tumor-associated macrophages correlated positively with recurrence-free interval duration (P=0.005). Fewer macrophages in the tumor microenvironment resulted in insufficient coverage of newly formed vessels by pericytes (P=0.011), and a high number of pericyte-impaired microvessels correlated with metastatic behavior (P<0.001). These results suggested that type 2 macrophages had a role in limiting the metastatic process by affecting vascular maturity and normalization. These findings contribute to understanding complex interactions in the tumor microenvironment and the clinical behavior of colorectal cancer.
Keywords: Colorectal cancer, alternatively activated macrophages, M2, angiogenesis, tumor microenvironment, prognosis
Introduction
More than 100 years have passed since Ilya Mechnikov discovered the phenomenon of phagocytosis and the phagocytes with which his name will always be associated. However, knowledge about these cells, now called macrophages, remains incomplete. In the 1980s, researchers discovered that compared to interferon (IFN)-γ and lipopolysaccharide, the cytokine interleukin (IL)-4, has differential effects on macrophage gene expression [1]. In contrast to the classical activation of macrophages by IFN-γ, macrophage phenotypes induced by IL-4 are described as undergoing “alternative activation” [2,3]. In 2000, Mills et al. proposed a new classification of macrophages as M1 (classical activation) versus M2 (alternative activation). This terminology is based on finding differential arginine metabolism of macrophages in different strains of mice with T helper type 1 (Th1) or T helper type 2 (Th2) backgrounds. Th1 mice with T cells that mainly produce IFN-γ have macrophage activation that produces nitric oxide (NO) from arginine. In contrast, ornithine production occurs in Th2 mice with T cells that produce IL-4 [4]. This finding led to the current paradigm that M1 macrophages have inflammatory and anti-tumor activity; M2 macrophages have anti-inflammatory and pro-tumor functions [5,6]. Although the M1/M2 classification of macrophages is now considered an oversimplified approach that inadequately describes the spectrum of macrophage populations, it is commonly used in pathology in the context of tumor-associated macrophages (TAMs) [7]. Macrophages are often ascribed a prometastatic function because their absence leads to pronounced metastatic failure [8,9]. Increased TAM numbers are associated with worse clinical outcomes for most malignancies, including breast, cervical, ovarian, prostate, and thyroid cancers, Hodgkin’s lymphoma, hepatocellular carcinoma, lung carcinoma, and cutaneous melanoma. Despite many studies on the pro-tumor functions of TAMs in several tumor types, their role in colorectal cancer (CRC) is controversial [10]. Some results suggest that in CRC, macrophages have anti-tumor activity and are associated with improved disease-free survival [11,12]. Forssell et al. found that a dense macrophage infiltrate at the tumor front positively affects prognosis in patients with CRC and that direct macrophage-to-tumor cell contact is required to manifest anti-tumor activity [11]. Consistent with these results, Zhou et al. found that a high TAM density at the invasive front is associated with a lower rate of hepatic metastasis and improved prognosis in patients with CRC [13]. In contrast, other studies found that massive macrophage infiltration correlates with tumor progression, growth, and disease aggressiveness. Kang et al. found in vitro that TAMs increase CRC cell invasiveness and migration [14]. A recent study found that during the removal of apoptotic colon cancer cells TAMs change their own phenotype and have increased expression of TGFβ and IL-6. The latter has a crucial role in colon tumorigenesis and putatively contributes to the angiogenic process [15]. In summary, the role of TAMs in CRC remains inconclusive.
One key feature of TAMs is the ability to support tumor angiogenesis [16,17]. M2 macrophages promote angiogenesis and tissue regeneration, whereas M1 macrophages are anti-angiogenic, although this strict categorization is controversial [18]. Because tumor cell numbers can only increase to one million cells before new blood vessels are required, cancers rely on M2 macrophages to provide angiogenic factors [19,20]. They share these functions with M2-like macrophages induced in a physiological context during the vascular and matrix remodeling necessary for damage repair [21]. In this study, we examined the relationship between infiltration of type 2 macrophages in tumor tissue and metastasis of localized CRCs. We also evaluated the properties of the microvasculature in metastasizing and non-metastasizing carcinomas and analyzed correlations between type 2 macrophage infiltration, characteristics of the newly formed vessels, and metastases.
Materials and methods
Colorectal cancer sample analysis
We retrospectively analyzed 151 samples of CRC tumor tissue from patients who underwent surgical resection at St. Petersburg Clinical Research and Practical Center for Specialized Oncological Care between 2012 and 2015. Corresponding clinicopathologic records were obtained from the database. The tumors were located in different parts of the colon and rectum. Cases with different depths of invasion, from T1 to T4b, were included. In all cases, no regional lymph nodes were involved (N0). Approximately one-third of the samples were cases of synchronous metastasis (n=53), one-third with metachronous metastasis to distant organs (n=45), and one-third (n=53) without distant metastasis at the time of diagnosis and within at least a 5-year follow-up period after surgical resection (minimum 64 months, maximum 92 months). “Synchronous metastasis” was defined as the diagnosis of distant metastasis together with or within a 3-month interval after the diagnosis of primary colon cancer. “Metachronous” was defined as an occurrence after a 3-month postoperative period. Distant metastases were diagnosed using clinical imaging and confirmed using biopsy. Tumors were classified into one of three groups, according to location: right-sided colon carcinomas (including those in the cecum, ascending colon, and transverse colon), left-sided colon carcinomas (from splenic flexure to sigmoid), and rectal carcinomas. Cases of multiple primary tumors and unknown locations were excluded.
The study was approved by local ethics committee at “Military medical academy of S.M. Kirov” (No. 255 from October 26, 2021).
Immunohistochemistry
Triple immunohistochemical staining was performed to evaluate microvascular density (MVD) and pericyte-impaired microvessels (PIMs). Nuclear staining with ERG identified endothelial cells of blood and lymphatic vessels. Lymphatic vessels were differentiated using selective podoplanin immunoreactivity. Pericytes were considered present if α-Smooth Muscle Actin (α-SMA) immunoreactivity was visible anywhere along the vessel perimeter. Normal blood vessels showed ERG-expression and α-SMA; immature tumor microvessels lacked α-SMA immunoreactivity. MVD and PIMs were evaluated per square millimeter using a high-power microscopic field (400x).
Type 2 TAMs were identified using immunohistochemical staining with an antibody against the macrophage mannose receptor (CD206). The number of positive cells per square millimeter of tumor stroma was measured using a high-power microscopic field (400 x). Human placenta tissue was used as a positive tissue control because the functional profile of this cell population most closely resembles M2 macrophages [22]. Villous stromal macrophages (Hofbauer cells) demonstrated intense staining, in contrast to negative placental trophoblast cell staining.
Cut sections (2 μm) of formalin-fixed, paraffin-embedded tissue were used for the immunohistochemical studies. Heat-induced epitope retrieval, incubation with antibody, and chromogen visualization were performed using a Ventana BenchMark XT. The antibodies and staining conditions used are presented in Table 1.
Table 1.
Sources and dilutions of the immunohistochemical markers used in the study
| Antigen | Distributor | Clone | Dilution |
|---|---|---|---|
| CD206 | Sigma-Aldrich | polyclonal | 1:1500 |
| ERG | Epitomics | EP111 | 1:150 |
| α-SMA | Dako | 1A4 | 1:200 |
| podoplanin | CellMarque | D2-40 | 1:50 |
Statistical analysis
Statistical analysis was performed using StatTech v. 2.1.0 (Developer-StatTech LLC, Russia). Quantitative variables were tested for normality using Shapiro-Wilk tests (if the number of subjects was less than 50) or Kolmogorov-Smirnov tests (if the number of subjects was more than 50). Quantitative variables that were not normally distributed were described using median (Me) and lower and upper quartiles (Q1-Q3). Mann-Whitney U tests were used to compare two groups with respect to a quantitative variable whose distribution deviated from the normal distribution. Comparisons of three or more groups during analysis of a quantitative variable whose distribution deviated from the normal distribution were performed using Kruskal-Wallis tests and Dunn’s criterion with Holm’s correction as a post-hoc method. The direction and strength of association between two quantitative variables were estimated using Spearman correlation coefficient analysis (when at least one variable was not normally distributed). Predictive modeling of the conditioning of quantitative variables on other quantitative variables was performed using simple or multivariable linear regression analysis.
Results
Immunohistochemical staining with CD206 revealed the presence of macrophages in the tumor stroma in all cases (Figure 1). Analysis of the subset of M2 macrophages in the tumor microenvironment found only a slight difference between metastasizing and non-metastasizing CRCs (Figure 2). The median value of CD206 positive cells for metastasizing CRC was 63, compared with 76 for non-metastasizing tumors (Table 2). A comparison of the dependence of metastasis on macrophages in those groups found the difference was not statistically significant (P=0.144).
Figure 1.
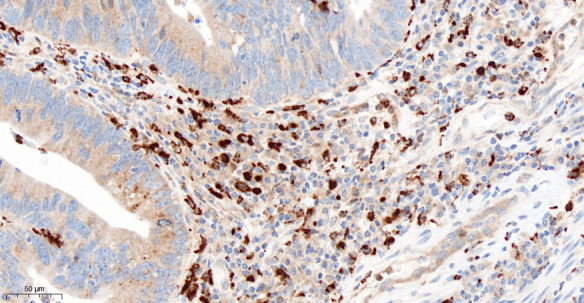
Immunohistochemical detection of macrophages in colorectal cancer. Stromal M2 macrophages are clearly indicated by strong cytoplasmic staining with anti-CD206 antibody, 400 x.
Figure 2.
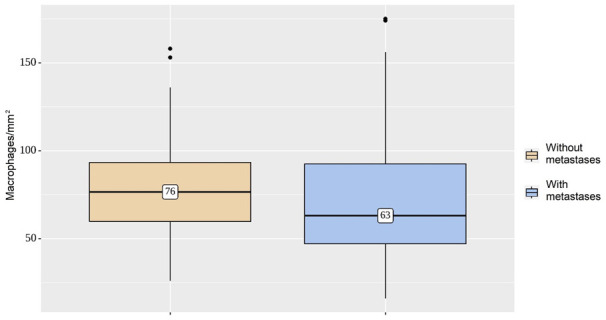
Density of macrophage infiltration per square millimeter. Mean numbers of M2 macrophages in the metastasizing and non-metastasizing groups are not different (P=0.144).
Table 2.
Analysis of macrophages in groups with different metastatic behaviors
| Group | Macrophages/mm2 | n | p* | |
|---|---|---|---|---|
|
| ||||
| Me | Q1-Q3 | |||
| CRC without metastases | 76 | 60-93 | 53 | 0.144 |
| CRC with metastases | 63 | 47-92 | 98 | |
Mann-Whitney U-test.
CRC: Colorectal Cancer.
When the group with metastasis was divided into two subgroups based on metachronous versus synchronous dissemination, a difference was revealed (Figure 3). The cases of synchronous metastasis had significantly lower densities of CD206 positive stromal cells - 56/mm2, compared to metachronous disease or local CRC, 74/mm2 and 76/mm2, respectively (Table 3). The results indicated that the differences in the numbers of M2 macrophages in tumor stroma in the compared groups were significant (P=0.029).
Figure 3.
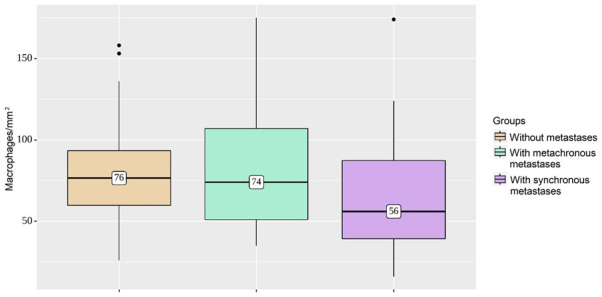
Comparison of macrophage infiltration in three groups. The cases with synchronous metastasis have significantly lower M2 macrophage densities, compared to metachronous disease or local colorectal cancer (P=0.029).
Table 3.
Analysis of macrophages in groups with different metastatic behavior
| Group | Macrophages/mm2 | n | p* | |
|---|---|---|---|---|
|
| ||||
| Me | Q1-Q3 | |||
| Without metastasis | 76 | 60-93 | 53 | 0.029 |
| With metachronous metastasis | 74 | 51-107 | 45 | |
| With synchronous metastasis | 56 | 39-87 | 53 | |
Kruskal-Wallis test.
The subsequent correlation analysis found a significant association between the number of macrophages in the CRC tumor microenvironment and the time to cancer recurrence (Table 4). The observed dependence of time to relapse from macrophages was described using a linear regression model:
Table 4.
Results of correlation analysis of the association between macrophages and time to relapse
| Variable | Correlation characteristic | ||
|---|---|---|---|
|
| |||
| ρ Spearman | Strength of association assessed using the Chaddock scale | p | |
| Macrophages-Time to relapse | 0.340 | Moderate | 0.005 |
Y Time to relapse = 0.195 × X Macrophages + 1.221
The model indicated that with an increase in the number of macrophages in the tumor stroma by 10/mm2, the recurrence-free time would increase by 1.95 months (P=0.005) (Figure 4).
Figure 4.
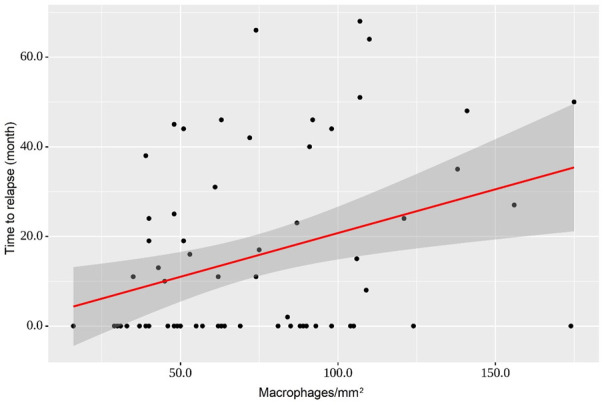
Regression curve characterizing the dependence of time to relapse from a stromal density of macrophages. With an increase in macrophage numbers in the tumor microenvironment in CRC by 10/mm2, it is expected that the recurrence-free time will increase by 1.95 months (P=0.005).
The results of triple immunohistochemical staining of normal intestinal wall and CRC samples are presented in Figure 5.
Figure 5.
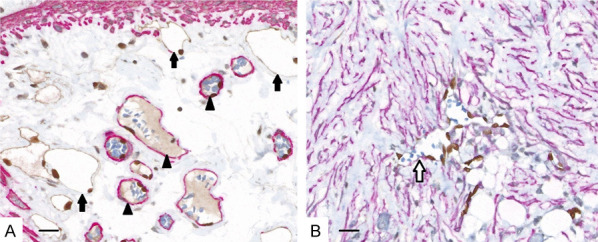
Triple immunohistochemical staining of microvessels in normal colon (A) and colorectal cancer (B). Nuclei of endothelial cells of the blood (arrowheads) and lymphatic capillaries (black arrows) are stained brown with ERG. Blood vessel contours appear red due to the presence of pericytes stained with α-SMA. Podoplanin staining defines the brown cytoplasm of endothelial cells of lymphatic vessels. Newly formed malformed blood vessels (white arrow) in the tumor stroma are devoid of pericytes and retain only the outline of their endothelial cells, which are recognizable by nuclei stained with brown anti-ERG. Note the striking desmoplastic change in the stroma in the tumor, with numerous activated myofibroblasts, detected using α-SMA. Scale bar = 200 μm, 400 x.
Assessment of the microvasculature revealed that the average density of microvessels between groups did not differ significantly; it was 9/mm2 in the group without metastases and 10-11/mm2 in the group with metastases (P=0.158) (Figure 6).
Figure 6.
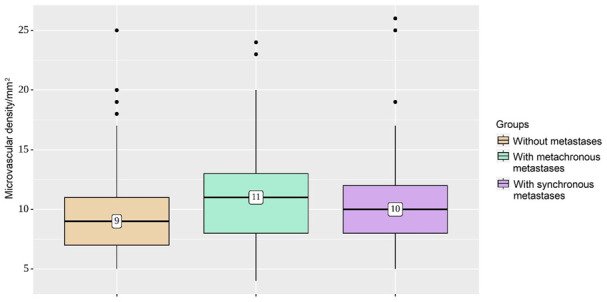
Microvascular density groups. The average density of microvessels in the group without metastases, compared with metastasizing tumors, is not significantly different (P=0.158).
In tumors with metachronous and synchronous metastases, capillaries without pericytes were more common among the newly formed vessels (i.e., median 7/mm2 and 8/mm2, respectively). At 2/mm2, PIMs were quite low in non-metastasizing tumors (P<0.001) (Figure 7).
Figure 7.
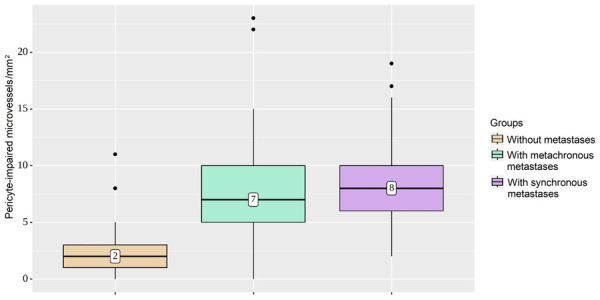
Characterization of pericyte-impaired microvessel densities in groups. In tumors with metachronous and synchronous metastases, capillaries without pericytes were more common among newly formed vessels, compared with quite low pericyte-impaired microvessels in non-metastasizing tumors (P<0.001).
A summary of results based on an analysis of microvascular characteristics in the studied groups of patients is presented in Table 5.
Table 5.
Microvascular characteristics of groups
| Variable | Group | Values | p* | ||
|---|---|---|---|---|---|
|
| |||||
| Me | Q1-Q3 | n | |||
| MVD | Without metastasis | 9 | 7-11 | 53 | 0.158 |
| With metachronous metastasis | 11 | 8-13 | 45 | ||
| With synchronous metastasis | 10 | 8-12 | 53 | ||
| PIMs | Without metastasis | 2 | 1-3 | 53 | <0.001 |
| With metachronous metastasis | 7 | 5-10 | 45 | ||
| With synchronous metastasis | 8 | 6-10 | 53 | ||
Kruskal-Wallis test.
MVD: Microvascular Density; PIMs: Pericyte-Impaired Microvessels.
We then assessed the relationship between TAM density and microvasculature characteristics (Table 6). A relationship between M2 stromal presentation and overall MVD was not revealed in this group of samples (P=0.110). There was an inverse correlation between M2 macrophages and PIMs (P=0.011). A linear regression model described the observed variation in PIMs with respect to macrophage density:
Table 6.
Results of correlation analysis of association between macrophages and microvasculature
| Variable | Correlation characteristic | ||
|---|---|---|---|
|
| |||
| ρ Spearman | Strength of the association assessed using Chaddock scale | p | |
| Macrophages-MVD | -0.147 | Weak | 0.110 |
| Macrophages-PIMs | -0.232 | Weak | 0.011 |
MVD: Microvascular Density; PIMs: Pericyte-Impaired Microvessels.
Y PIMs = -0.025 × X Macrophages + 7.969
An increase in the number of macrophages by 100/mm2 was accompanied by a decrease in PIMs by 2.5/mm2 (Figure 8).
Figure 8.
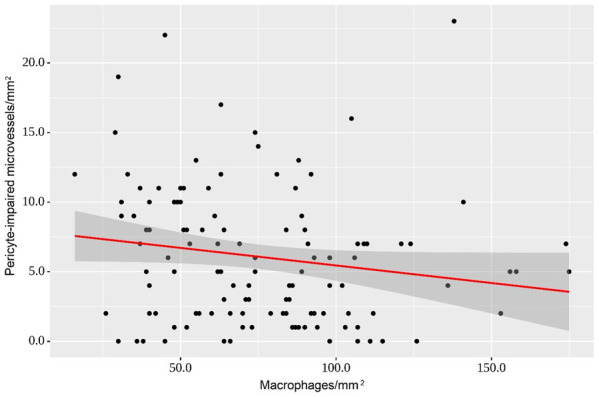
Regression curve demonstrates the observed variation in pericyte-impaired microvessels with respect to macrophage density. An increase in the number of macrophages of 100/mm2 is accompanied by a decrease in pericyte-impaired microvessels of 2.5/mm2 (P=0.011).
Discussion
Two distinct pathways link inflammation and cancer. Inflammatory conditions can promote the development of malignancies (extrinsic pathway). Activation of various oncogenes can promote an inflammatory tumor microenvironment (intrinsic pathway) [23]. TAMs are key players in cancer-related inflammation, and increased TAM density correlates with a poor prognosis and disease progression in most (but not all) human tumor types [24]. TAMs are included in the list of “corrupted” activated/recruited stromal cells that constitute the tumorigenic microenvironment and contribute to the hallmarks of cancer [25]. Plasticity is a well-known property of macrophages. They can integrate signals from the microenvironment and acquire distinct phenotypes (i.e., classical M1 or M2 macrophages) [26,27]. According to the classical paradigm, M1 macrophages are involved in tumor suppression and immunostimulation. M2 macrophages promote immunosuppression, tumor growth, metastasis, and a more invasive phenotype [10]. In CRC, it was reported that an IFN-γ-driven tumor microenvironment exerts a better effect on patient survival and outcome than an IFN-α-driven microenvironment or a microenvironment without an IFN-associated immune response [28]. However, whether TAM exerts anti- or pro-tumor activity in CRC remains controversial. For example, Koelzer et al. found that high infiltration of CD163+ M2 macrophages is associated with fewer lymph node metastases and better clinical outcomes; iNOS+ M1 macrophages is not [29]. This difference indicates that there is heterogeneity in macrophage subsets and that full functional characterization is needed to predict clinical effects in CRC.
In the past, tumor angiogenesis was thought to be regulated mainly by cancer cells expressing proangiogenic factors. However, ample evidence now indicates that stromal cells in the tumor microenvironment are responsible for activating and maintaining chronic angiogenesis in many tumor types [25]. Conflicting results have been reported regarding associations between TAMs and tumor angiogenesis or lymphangiogenesis. TAMs express factors that are directly or indirectly involved in new vessel formation and sprouting. These factors include TGF-β, VEGF, PDGF, MMP-9, thymidine phosphorylase, and chemokines [28]. Hypoxia is an important stimulator of angiogenesis, and a hypoxic tumor microenvironment changes recruited macrophages into the M2 phenotype [30]. M1 macrophages secrete factors that initiate the process of angiogenesis. For example, VEGF stimulates endothelial cell sprouting and proliferation. Although induction of angiogenesis initially provides the tumor with more oxygen and nutrients, the ultimate response is poor because the continuously remodeled tumor vasculature is leaky and tortuous, causing irregular blood flow [31]. We found no difference in overall microvascular density between non-metastasizing and metastasizing CRC tumors; the average density of microvessels was 9/mm2 and 10-11/mm2, respectively. This result indicated that in CRC, the neoangiogenesis remained at the same level regardless of metastatic potential. However, we found that in metastasizing tumors, there was a significant predominance of the numbers of PIMs. In tumors with metastases, the median density of capillaries without pericytes was 7/mm2, compared with 2/mm2 in non-metastasizing tumors (P<0.001). This result suggested that factors were present that slowed the rate of maturation of blood vessels in the colorectal carcinomas prone to metastasis. We found no direct association between microvascular density in CRC with the number of CD206+ TAMs (P=0.110). However, we found a direct correlation between macrophages and PIM density (P=0.011). A high total number of macrophages detected using the CD206 marker appeared to be beneficial for maturation of growing blood vessels and remodeling of the vascular network by promoting pericyte recruitment. M2 macrophages secrete factors involved in later stages of angiogenesis, particularly PDGF-B and high levels of MMP-9, which suggests they have a role in vascular remodeling [18]. Recent study findings suggest tumor-type-specific involvement of multiple signaling pathways during recruitment of pericytes to the tumor vasculature. Spiller et al. found that supporting pericytes are recruited through PDGF-B secreted by M2 macrophages to stabilize the sprouts and develop into mature vessels [18]. Our results indicated that a higher proportion of PIMs in the total microvessel density was directly associated with tumor progression (P<0.001). Interestingly, tumors with high M2 density were associated with prolonged recurrence-free survival (P=0.005). Every 100 macrophages per one square millimeter of the tumor stroma postponed the manifestation of metastases by almost 2.0 months. This phenomenon can be explained by “vascular normalization”, in which stabilized and mature vessels do not allow tumor cells to intravasate into the vasculature. Fewer circulating tumor cells reduce the likelihood that some will survive in the bloodstream and colonize distant organs.
In this study, we found that the presence and density of PIMs and the total number of M2 TAMs correlated with the clinical behavior of CRC. This finding was consistent with published studies that described a tendency toward a more favorable outcome in patients with CRC with increasing counts of M2 macrophages in the tumor microenvironment [29,32].
High macrophage infiltration in colorectal tumors often correlates with a poor prognosis.
The opposite relationship has also been described, but the mechanism behind this phenomenon remains unclear. Here, we sought to reveal the role of alternatively activated TAMs in CRC progression. We found that M2 macrophages in CRC did not have a straightforward association with metastatic behavior. However, they had a direct relationship to the duration of the interval between tumor resection and the occurrence of metastasis. We also found a correlation between CD206+ TAM density and characteristics of the local microvasculature, namely, the degree of microvessel maturity. A lower number of macrophages in the tumor microenvironment was associated with insufficient coverage of the neovasculature by pericytes. Therefore, one hypothesis is that M2 TAMs have a role in limiting the metastatic process by affecting vessel maturation. Because metastasis is a critical prognostic factor, these findings partly explain why some studies found that increased TAM density is associated with an improved prognosis in patients with CRC. These results could assist in obtaining a deeper understanding of the mechanisms of the complex effects of macrophages on metastasis.
Acknowledgements
We acknowledge Alexei Gratchev from N.N. Blokhin Russian Cancer Research Center, Moscow, Russia for the project/research collaboration.
Disclosure of conflict of interest
None.
References
- 1.Nathan CF, Murray HW, Wiebe ME, Rubin BY. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983;158:670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6:6–13. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stein M, Keshav S, Harris N, Gordon S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J Exp Med. 1992;176:287–292. doi: 10.1084/jem.176.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000;164:6166–6173. doi: 10.4049/jimmunol.1701141. [DOI] [PubMed] [Google Scholar]
- 5.Boutilier AJ, Elsawa SF. Macrophage polarization states in the tumor microenvironment. Int J Mol Sci. 2021;22:6995. doi: 10.3390/ijms22136995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roszer T. Understanding the mysterious M2 macrophage through activation markers and effector mechanisms. Mediators Inflamm. 2015;2015:816460. doi: 10.1155/2015/816460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soldevilla B, Carretero-Puche C, Gomez-Lopez G, Al-Shahrour F, Riesco MC, Gil-Calderon B, Alvarez-Vallina L, Espinosa-Olarte P, Gomez-Esteves G, Rubio-Cuesta B, Sarmentero J, La Salvia A, Garcia-Carbonero R. The correlation between immune subtypes and consensus molecular subtypes in colorectal cancer identifies novel tumour microenvironment profiles, with prognostic and therapeutic implications. Eur J Cancer. 2019;123:118–129. doi: 10.1016/j.ejca.2019.09.008. [DOI] [PubMed] [Google Scholar]
- 8.Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, Coussens LM, Gabrilovich DI, Ostrand-Rosenberg S, Hedrick CC, Vonderheide RH, Pittet MJ, Jain RK, Zou W, Howcroft TK, Woodhouse EC, Weinberg RA, Krummel MF. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med. 2018;24:541–550. doi: 10.1038/s41591-018-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Majidpoor J, Mortezaee K. Steps in metastasis: an updated review. Med Oncol. 2021;38:3. doi: 10.1007/s12032-020-01447-w. [DOI] [PubMed] [Google Scholar]
- 10.Wang H, Tian T, Zhang J. Tumor-associated macrophages (TAMs) in colorectal cancer (CRC): from mechanism to therapy and prognosis. Int J Mol Sci. 2021;22:8470. doi: 10.3390/ijms22168470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forssell J, Oberg A, Henriksson ML, Stenling R, Jung A, Palmqvist R. High macrophage infiltration along the tumor front correlates with improved survival in colon cancer. Clin Cancer Res. 2007;13:1472–1479. doi: 10.1158/1078-0432.CCR-06-2073. [DOI] [PubMed] [Google Scholar]
- 12.Nagorsen D, Voigt S, Berg E, Stein H, Thiel E, Loddenkemper C. Tumor-infiltrating macrophages and dendritic cells in human colorectal cancer: relation to local regulatory T cells, systemic T-cell response against tumor-associated antigens and survival. J Transl Med. 2007;5:62. doi: 10.1186/1479-5876-5-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou Q, Peng RQ, Wu XJ, Xia Q, Hou JH, Ding Y, Zhou QM, Zhang X, Pang ZZ, Wan DS, Zeng YX, Zhang XS. The density of macrophages in the invasive front is inversely correlated to liver metastasis in colon cancer. J Transl Med. 2010;8:13. doi: 10.1186/1479-5876-8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang JC, Chen JS, Lee CH, Chang JJ, Shieh YS. Intratumoral macrophage counts correlate with tumor progression in colorectal cancer. J Surg Oncol. 2010;102:242–248. doi: 10.1002/jso.21617. [DOI] [PubMed] [Google Scholar]
- 15.Popovic ZV, Sandhoff R, Sijmonsma TP, Kaden S, Jennemann R, Kiss E, Tone E, Autschbach F, Platt N, Malle E, Grone HJ. Sulfated glycosphingolipid as mediator of phagocytosis: SM4s enhances apoptotic cell clearance and modulates macrophage activity. J Immunol. 2007;179:6770–6782. doi: 10.4049/jimmunol.179.10.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh N, Baby D, Rajguru JP, Patil PB, Thakkannavar SS, Pujari VB. Inflammation and cancer. Ann Afr Med. 2019;18:121–126. doi: 10.4103/aam.aam_56_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Y, Song Y, Du W, Gong L, Chang H, Zou Z. Tumor-associated macrophages: an accomplice in solid tumor progression. J Biomed Sci. 2019;26:78. doi: 10.1186/s12929-019-0568-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spiller KL, Anfang RR, Spiller KJ, Ng J, Nakazawa KR, Daulton JW, Vunjak-Novakovic G. The role of macrophage phenotype in vascularization of tissue engineering scaffolds. Biomaterials. 2014;35:4477–4488. doi: 10.1016/j.biomaterials.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Folkman J, Klagsbrun M. Angiogenic factors. Science. 1987;235:442–447. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- 20.Fu LQ, Du WL, Cai MH, Yao JY, Zhao YY, Mou XZ. The roles of tumor-associated macrophages in tumor angiogenesis and metastasis. Cell Immunol. 2020;353:104119. doi: 10.1016/j.cellimm.2020.104119. [DOI] [PubMed] [Google Scholar]
- 21.Parisi L, Gini E, Baci D, Tremolati M, Fanuli M, Bassani B, Farronato G, Bruno A, Mortara L. Macrophage polarization in chronic inflammatory diseases: killers or builders? J Immunol Res. 2018;2018:8917804. doi: 10.1155/2018/8917804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reyes L, Golos TG. Hofbauer cells: their role in healthy and complicated pregnancy. Front Immunol. 2018;9:2628. doi: 10.3389/fimmu.2018.02628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mantovani A, Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol. 2010;22:231–237. doi: 10.1016/j.coi.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 24.Cassetta L, Pollard JW. Targeting macrophages: therapeutic approaches in cancer. Nat Rev Drug Discov. 2018;17:887–904. doi: 10.1038/nrd.2018.169. [DOI] [PubMed] [Google Scholar]
- 25.Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 26.Ricketts TD, Prieto-Dominguez N, Gowda PS, Ubil E. Mechanisms of macrophage plasticity in the tumor environment: manipulating activation state to improve outcomes. Front Immunol. 2021;12:642285. doi: 10.3389/fimmu.2021.642285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Locati M, Curtale G, Mantovani A. Diversity, mechanisms, and significance of macrophage plasticity. Annu Rev Pathol. 2020;15:123–147. doi: 10.1146/annurev-pathmechdis-012418-012718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mantovani A. Macrophages, neutrophils, and cancer: a double edged sword. New Journal of Science. 2014;2014:1–14. [Google Scholar]
- 29.Koelzer VH, Canonica K, Dawson H, Sokol L, Karamitopoulou-Diamantis E, Lugli A, Zlobec I. Phenotyping of tumor-associated macrophages in colorectal cancer: impact on single cell invasion (tumor budding) and clinicopathological outcome. Oncoimmunology. 2016;5:e1106677. doi: 10.1080/2162402X.2015.1106677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen XJ, Wu S, Yan RM, Fan LS, Yu L, Zhang YM, Wei WF, Zhou CF, Wu XG, Zhong M, Yu YH, Liang L, Wang W. The role of the hypoxia-Nrp-1 axis in the activation of M2-like tumor-associated macrophages in the tumor microenvironment of cervical cancer. Mol Carcinog. 2019;58:388–397. doi: 10.1002/mc.22936. [DOI] [PubMed] [Google Scholar]
- 31.Shah AA, Kamal MA, Akhtar S. Tumor angiogenesis and VEGFR-2: mechanism, pathways and current biological therapeutic interventions. Curr Drug Metab. 2021;22:50–59. doi: 10.2174/1389200221666201019143252. [DOI] [PubMed] [Google Scholar]
- 32.Algars A, Irjala H, Vaittinen S, Huhtinen H, Sundstrom J, Salmi M, Ristamaki R, Jalkanen S. Type and location of tumor-infiltrating macrophages and lymphatic vessels predict survival of colorectal cancer patients. Int J Cancer. 2012;131:864–873. doi: 10.1002/ijc.26457. [DOI] [PubMed] [Google Scholar]


